Rosai-Dorfman disease (RDD) is a rare histiocytosis characterized by infiltration of tissue by CD68+ S100+CD1a− histiocytes with large nuclei and abundant lesions of emperipolesis.1 The first clinical observations of RDD were of cervical lymphadenopathy,2 but later extranodal locations3,4 were reported as well (sinonasal, skin, bone, soft tissue, respiratory tract, eye or brain). Gain-of-function mutations of genes of the MAP kinase-signaling pathway, including BRAF, NRAS, KRAS, MAP2K1, and ARAF have been reported in some patients with RDD.5,6
The diagnosis of Erdheim-Chester disease (ECD) is based on a clinical/radiological presentation along with compatible histology.7 In contrast to RDD, histology is not always specific for the diagnosis of ECD, although biopsy is mandatory to rule out other diagnoses, confirm infiltration by histiocytes and detect somatic mutations.8 Typically, ECD involves long bones (in over 95% of cases), perinephric fat (“hairy-kidney”) and adventitia of blood vessels7,9 (“coated aorta”). More than half of ECD patients have BRAF(V600E) mutations, and more than 80% have mutations in MAPK pathway genes.10
Overlapping forms of ECD and Langerhans cell histiocytosis have been observed to occur in approximately 10-15% of patients with ECD, and there is a very high frequency of BRAF(V600E) mutations in this mixed histiocytosis.11 Here we present the clinical and mutational observations of overlapping ECD and RDD, a previously uncharacterized entity.
Databases from the Internal Medicine Department of Pitié-Salpêtrière Hospital (Paris, France), Memorial Sloan Kettering Cancer Center (MSKCC; New York, NY, USA) and Mayo Clinic (Rochester, MN, USA) were reviewed. Clinical records from patients with ECD from Pitié-Salpêtrière Hospital (n=168), MSKCC (n=96) and Mayo Clinic (n=89) between January 1998 and April 2018 were retrieved. This study was conducted in accordance with the institutional review boards of all institutions. Two patients referred from Germany and Greece were also included from the database of Ambroise Paré pathology department.
All patients with ECD had a compatible clinical/imaging presentation and at least one biopsy of an involved tissue. Infiltration of involved tissue by CD68+ S100+ CD1a− histiocytes, with large nuclei and nucleoli, and abundant lesions of emperipolesis were mandatory for the diagnosis of RDD. Biopsy samples were investigated for mutations of genes of the MAP kinase pathway using target-capture next-generation sequencing or whole exome sequencing. (Online Supplementary Data).
Thirteen patients with typical ECD and at least one site with RDD histology were included in this analysis, of whom 11 (3.1%) were from the Pitié-Salpêtrière, MSKCC, and Mayo Clinic referral centers out of the total of 353 ECD patients identified in those centers.
In all three cohorts, the occurrence of RDD histology in ECD patients was a rare phenomenon with a frequency of 3.6% (6/168) in the French center, 3.1% (3/96) at MSKCC and 2.2% (2/89) in the Mayo Clinic. The median age at diagnosis of the overlapping forms was 68 years (range, 29-86). The median age at diagnosis in France was 71 years (range, 29-86), while American patients were younger with a median age of 44 years (range, 32-78). A 56-year old patient from Germany and a 68-year old woman from Greece also fulfilled the criteria fir inclusion in this study.
In the study cohort of 13 patients (12 males, 1 female), all had typical ECD findings. At the diagnosis of ECD, no patient had another myeloproliferative neoplasm. Apart from cases #8, #9 and #10, all patients had at least one biopsy of a tissue compatible with ECD histology at initial diagnosis. ECD histiocytes were negative for S100, except in cases #4 and #5 in whom focal positivity was observed. Typical RDD histology was observed on biopsies, which were usually performed during the course of the disease because a new location of involvement was revealed by imaging (Figure 1) except for three patients who did not have compatible histology of ECD and for one who had co-occurrence of both histiocytoses at diagnosis. Seven out of the 12 males had testicular infiltration by RDD. The diagnosis was suspected by abnormal radiotracer uptake on follow-up fluorodeoxyglucose positron emission tomography or bone scintigraphy. Doppler ultrasound or magnetic resonance imaging demonstrated nodules or increased volume of epididymis. The other RDD locations were tibia (n=1), cheek (n=1), peri-nephric region (n=1), optical nerve (n=1), pleura (n=1), and vocal cord (n=1). No abnormality on clinical/radiological presentation could predict RDD lesions and none of the patients had lymph nodes involved by RDD.
Figure 1.
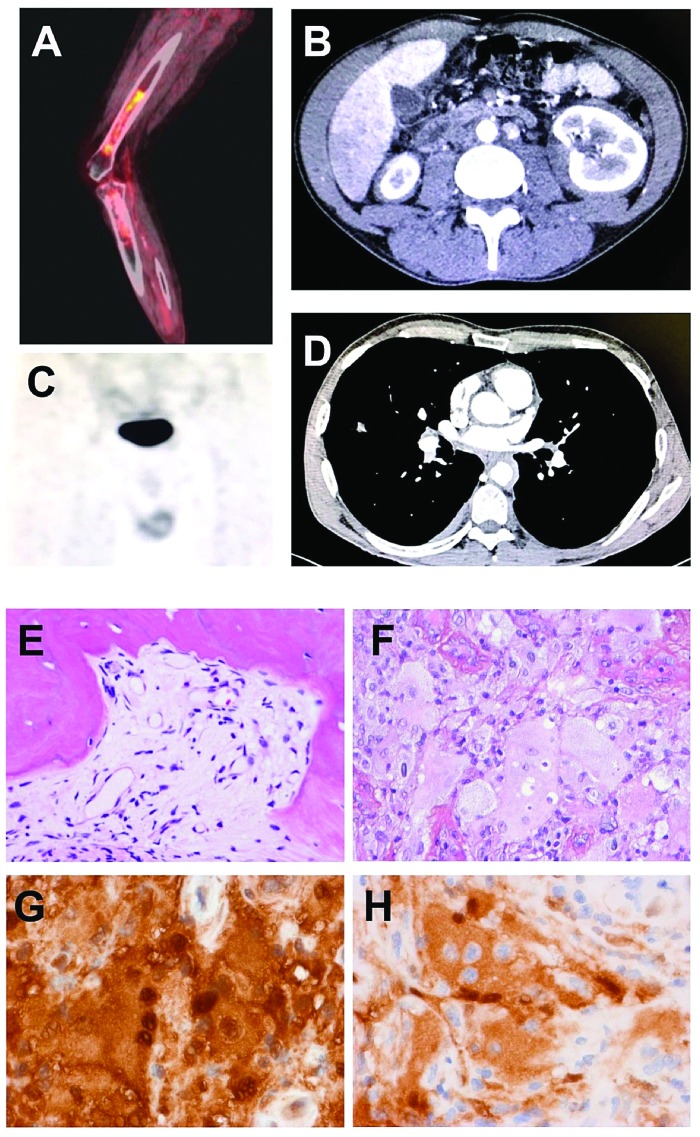
Imaging of Erdheim-Chester disease and histological features of Rosai-Dorfman disease. (A) Sagittal fused (18F) fluorodeoxyglucose positron emission tomography demonstrates radiotracer uptake in the meta-diaphysis of long bones in Erdheim-Chester disease (ECD), (B) Axial computed tomography (CT) scan of a patient demonstrates infiltration of perinephric fat, defined as “hairy-kidney”. (C) Bone scintigra-phy showing radiotracer uptake in the testis of a patient with ECD. (D) Axial CT scan of a patient showing circumferential sheathing of thoracic aorta, defined as “coated aorta”, (E) Bone biopsy of an Erdheim-Chester lesion, with replacement of bone marrow by fibrosis and a few histiocytes with small nuclei. Hematoxylin & eosin (H&E) staining, original magnification ×200). (F) Testicular involvement with Rosai-Dorfman histology showing large multinucleated histiocytes with large nuclei, abundant cytoplasm and lesions of emperipolesis (H&E, ×400). (G) Same sample with strong expression of S100 protein (brown staining) by the multinucleated histiocytes (immunohistochemistry, ×400). (H) Same sample with strong expression of phosphoERK by the histiocytes with abundant emperipolesis (immunohistochemistry, ×400)
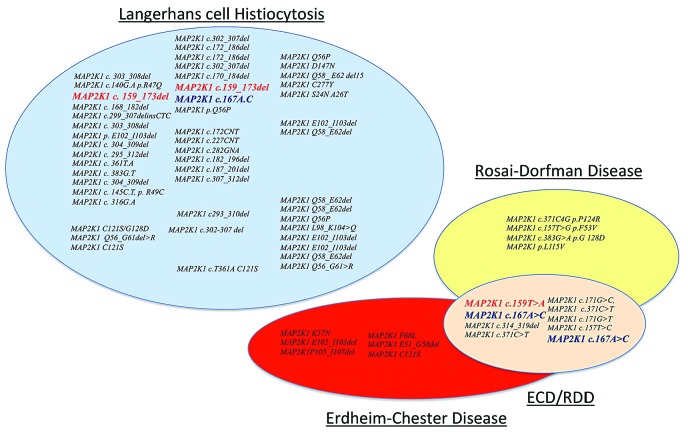
The BRAF(V600E) mutation was absent in all cases on tissues with ECD or RDD histology. By contrast nine cases had MAP2K1 mutations and one had a PIK3CA mutation on biopsies disclosing RDD lesions. Three patients harbored mutations already reported in Langerhans cell histiocytosis but none of the mutations has been reported in patients with ECD or RDD (Figure 2). Two patients had the same MAP2K1 mutation in their ECD and RDD samples.
Figure 2.
Representation of MAP2K1 gene mutations reported in histiocytoses. In red, mutations described in Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD)/Rosai-Dorfman disease (RDD) forms and in blue another mutation described in LCH and ECD/RDD forms.
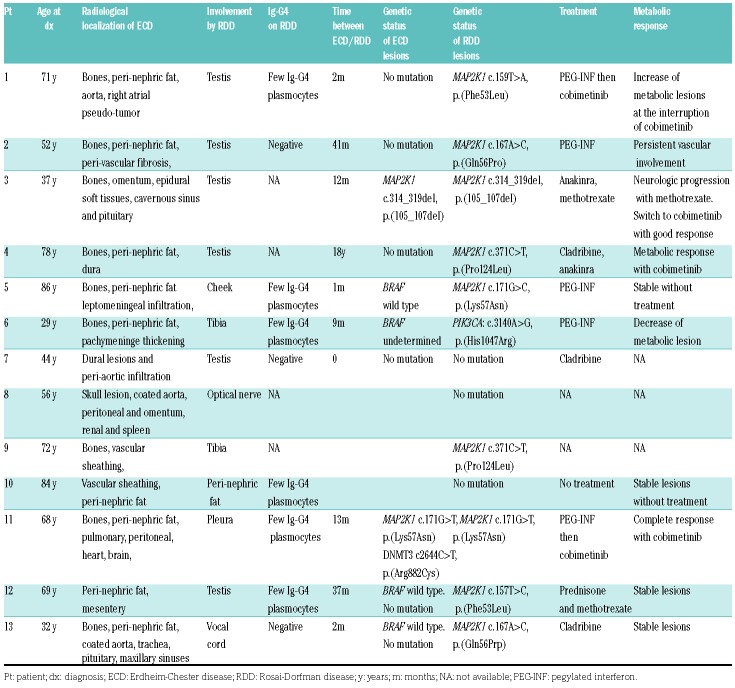
Patients received first-line conventional therapy for ECD (i.e., immunosuppressive treatment or chemotherapeutics) (Table 1). Four patients were given MEK inhibitor therapy, because conventional therapies were ineffective or intolerable, with favorable radiological responses.
Table 1.
Characteristics of patients with Erdheim-Chester disease and features of Rosai-Dorfman disease, including their molecular features and outcomes.
As far as we know, ECD/RDD overlap has not been reported previously, although clinical phenotypes consistent with ECD have been described in four reports of RDD patients.12,13 The median age of 68 years at diagnosis of overlapping forms in the present series is higher than that observed in ECD9,14 and RDD, which affect younger individuals.1
A key finding in our series is that RDD lesions frequently emerged in the setting of otherwise controlled systemic ECD. This suggests that repeat biopsy may be of merit in cases of apparent treatment failures for ECD, particularly in the context of patients with wildtype BRAF.
A unique phenotypic aspect of these cases is that all but one were male and that 7/12 males had testicular infiltration by RDD. Perturbations in the MEK/ERK pathway might underlie the development of the disease in men and especially in the testes, as this pathway has been shown to be involved with maintenance of a functional population of adult Leydig cells in mice.15 In these ECD patients, none had the BRAF(V600E) mutation, which is usually present in more than half of patients with ECD.11 The BRAF(V600E) mutation is also present in most patients with overlapping forms of ECD/Langerhans cell histiocytosis.11 These findings suggest that the co-occurrence of ECD and RDD is driven by MAP kinase (MAP2K1) but does not involve the BRAF gene. Additionally, a review of therapies received by this cohort suggests that MEK inhibition might be efficacious for inducing metabolic responses in both ECD-and RDD-involved locations and should be considered in those patients.
There are some limitations to this study. First of all, it is a retrospective series with data collection based on information available in the patients’ records. Moreover, biopsy of new lesions in ECD patients was at the discretion of the physicians in collaboration with radiologists and surgeons. With regards to the mutational findings, DNA quality and quantity were low, because the DNA was mostly obtained from small fresh-frozen paraffin-embedded biopsies, and exhaustive next-generation sequencing analysis could not be performed for some patients.
The major strength of this study is that the data were derived from three referral centers for ECD and RDD. Hence, the biopsy specimens were reviewed by expert pathologists and all known mutations of MAP kinase pathway genes were investigated.
RDD is a heterogeneous histological entity (more than a disease), which may be observed in several clinical inherited or sporadic conditions. Our study demonstrates that some patients with ECD may also have the histological lesions described by Destombes, Rosai and Dorfman. This subset of ECD/RDD overlap mainly affects older males, is almost always extranodal, frequently involves the testes, and is mainly driven by MAP2K1 gain-of-function mutations.
Acknowledgments
ELD would like to thank the Erdheim-Chester Global Alliance, the Frame Fund, and the Joy Family West Foundation.
Footnotes
Funding: this work was also funded in part through NIH/NCI Cancer Center Support Grant P30 CA008748.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosai J, Dorfman RF. Sinus histiocytosis with massive lym-phadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87(1):63–70. [PubMed] [Google Scholar]
- 3.Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-Dorfman disease isolated to the central nervous system: a report of 11 cases. Mod Pathol. 2001;14(3):172–178. [DOI] [PubMed] [Google Scholar]
- 4.Cohen Aubart F, Haroche J, Emile J-F, et al. [Rosai-Dorfman disease: diagnosis and therapeutic challenges]. Rev Med Interne. 2018; 39(8):635–640. [DOI] [PubMed] [Google Scholar]
- 5.Fatobene G, Haroche J, Hélias-Rodzwicz Z, et al. BRAF V600E mutation detected in a case of Rosai-Dorfman disease. Haematologica. 2018;103(8):e377–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garces S, Medeiros LJ, Patel KP, et al. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol. 2017;30(10):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haroche J, Cohen-Aubart F, Rollins BJ, et al. Histiocytoses: emerging neoplasia behind inflammation. Lancet Oncol. 2017;18(2):e113–e125. [DOI] [PubMed] [Google Scholar]
- 10.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. [DOI] [PubMed] [Google Scholar]
- 11.Hervier B, Haroche J, Arnaud L, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124(7):1119–1126. [DOI] [PubMed] [Google Scholar]
- 12.Wang C-C, Al-Hussain TO, Serrano-Olmo J, Epstein JI. Rosai-Dorfman disease of the genito-urinary tract: analysis of six cases from the testis and kidney. Histopathology. 2014;65(6):908–916. [DOI] [PubMed] [Google Scholar]
- 13.Del Gobbo A, Moltrasio F, Young RH, Rosai J. Involvement of the testis and related structures by Rosai-Dorfman disease: report of 2 new cases and review of the literature. Am J Surg Pathol. 2013; 37(12):1871–1875. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Aubart F, Emile J-F, Carrat F, et al. Phenotypes and survival in Erdheim-Chester disease: results from a 165-patient cohort. Am J Hematol. 2018;93(5):E114–E117. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita S, Tai P, Charron J, Ko C, Ascoli M. The Leydig cell MEK/ERK pathway is critical for maintaining a functional population of adult Leydig cells and for fertility. Mol Endocrinol. 2011; 25(7):1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]