Abstract
Cellular damage produced by conditions generating oxidative stress have far-reaching implications in human disease that encompass, but are not restricted to aging, cardiovascular disease, type 2 diabetes, airway inflammation/asthma, cancer, and metabolic syndrome including visceral obesity, insulin resistance, fatty liver disease, and dyslipidemia. Although there are numerous sources and cellular targets of oxidative stress, this review will highlight literature that has investigated downstream consequences of oxidatively-induced DNA damage in both nuclear and mitochondrial genomes. The presence of such damage can in turn, directly and indirectly modulate cellular transcriptional and repair responses to such stressors. As such, the persistence of base damage can serve as a key regulator in coordinated gene-response cascades. Conversely, repair of these DNA lesions serves as both a suppressor of mutagenesis and by inference carcinogenesis, and as a signal for the cessation of ongoing oxidative stress. A key enzyme in all these processes is 8-oxoguanine DNA glycosylase (OGG1), which, via non-catalytic binding to oxidatively-induced DNA damage in promoter regions, serves as a nucleation site around which changes in large-scale regulation of inflammation-associated gene expression can occur. Further, the catalytic function of OGG1 can alter the three-dimensional structure of specialized DNA sequences, leading to changes in transcriptional profiles. This review will concentrate on adverse deleterious health effects that are associated with both the diminution of OGG1 activity via population-specific polymorphic variants and the complete loss of OGG1 in murine models. This mouse model displays diet-and age-related induction of metabolic syndrome, highlighting a key role for OGG1 in protecting against these phenotypes. Conversely, recent investigations using murine models having enhanced global expression of a mitochondrial-targeted OGG1 demonstrate that they are highly resistant to diet-induced disease. These data suggest strategies through which therapeutic interventions could be designed for reducing or limiting adverse human health consequences to these ubiquitous stressors.
Keywords: base excision repair, metabolic syndrome, mitochondrial DNA repair
DNA damage as an intracellular sentinel of prior environmental and endogenous exposures and initiator of transcriptional signaling cascades.
Beyond the role of DNA in providing the genetic blueprint to guide replication, transcription, differentiation, and development for cells and organisms, DNA is also a long-lived molecule in which structural damages can be persistent and can regulate many transcriptional networks. In the absence of repair, DNA damage serves as a cumulative signature of endogenous and exogenous stressors, including but not limited to those caused by chemical and various radiation exposures, as well as those formed spontaneously within the intracellular environment. In addition to apoptotic responses initiated by formation of double-strand breaks, replication stalling, and transcriptional blockage, unrepaired DNA can elicit cellular and organism-wide transcriptional and biological cascades. One of the best characterized of these cascades is ultraviolet light (UV)-induced immune suppression in which the persistence of cyclobutane pyrimidine dimers (CPDs) in genomic DNA initiates complex cascades that modulate cellular function, migration, antigen presentation, and immune surveillance (reviewed in (1–3)). In this example, residual DNA damage was definitively shown to be one of the initiating factors, since experimental designs that resulted in enhancing the cells’ capacity to repair CPDs by introducing T4 pyrimidine dimer DNA glycosylase, greatly abrogated or eliminated the UV-induced immune suppression cascade (reviewed in (4,5)).
In addition to residual unrepaired DNA damage being interpreted by cells as having sustained stable DNA damage, the presence of DNA repair intermediates can influence transcription and flux through stress-induced pathways. An example of this phenomenon was discovered in the repair of DNA alkylation damage in Escherichia coli. Although there are many sites in DNA that can be alkylated, methylation of the exocyclic oxygen at position six in guanine is one of the primary sites of base damage for many chemicals. This lesion is repaired through a direct-reversal mechanism by the product of the Ada gene, O6-methyl DNA methyl transferase in which the methyl group is transferred to Cys321 in the carboxy-terminal domain of the protein. Beyond this suicide reaction, this protein also carries out the removal of methyl-phosphotriesters at Cys38 in the amino-terminal portion of the protein. Although methyl-phosphotriesters are not considered either genotoxic or mutagenic, transfer of this methyl group activates the dead-end protein into a positive transcriptional activator, including its own gene expression (reviewed in (6)).
Another potential example comes from the literature investigating nucleotide excision repair in E. coli in which following extensive DNA damage by UV irradiation or bulky chemical modifications, dual DNA backbone incisions created by the concerted activities of the UvrABC proteins result in release of single-stranded DNA (ssDNA) 12-mers containing the bulky damage. This signal, along with replication stalling that creates regions of ssDNA and dNTP pool fluctuations, all contribute to the initiation and amplification of the SOS pathway (reviewed in (7–9)). Many of the genes up-regulated in this pathway are associated with DNA replication, repair, and recombination, and thus promote survival over death until the initiating signals are brought back to steady-state levels.
These brief examples serve to highlight how DNA damage and its repair, function to not only “alert” a cell that it has experienced a significant stressor, but also alter gene expression profiles to respond to the stressor.
Oxidatively-induced DNA damage and its repair as a transcriptional modulator.
Reactive oxygen species (ROS)-induced DNA damage. The examples described above establish two key fundamental mechanisms through which cells use residual DNA damage and repair intermediates to activate (and suppress) cellular processes. As will be described below, these principles are also operative for DNA damage formed by reaction with ROS and the 8-oxoguanine DNA glycosylase (OGG1) through which damage-specific binding and catalytic activity and end-product binding properties minimize mutagenesis and regulate transcription in both nuclear and mitochondrial genomes. Recently, there have been comprehensive reviews published on the roles of this enzyme in modulating: i) innate and adaptive immunity under conditions of pulmonary inflammation (10); ii) lung and other cancers (11); and iii) metabolic pathways (12).
All major cellular components, including lipids, proteins, DNA, RNA, and their nucleotide pools, are subject to damage caused by the production of ROS, including superoxide anions, hydrogen peroxide, and hydroxyl-radicals that are routinely generated directly or as by-products of mitochondrial oxidative respiration, heavy metal-mediated Fenton chemistry, ionizing and UV radiation, chemical oxidants, and chemotherapeutic drugs. However, for purposes of this review, we will limit our focus and discussion to hydroxyl-radical-induced damage to guanine. Due to its low redox potential, guanine undergoes preferential oxidation, with its hydroxyl-radical intermediate undergoing either a one electron oxidation to yield 7,8-dihydro-8-oxo-2´-guanine (8-oxoG) or a one electron reduction to generate 2–6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) (reviewed in (13)). Additionally, 8-oxoG can be directly formed via singlet oxygen-induced damage. The distribution of these lesions is not random within the genome, with these lesions most abundant within guanine-rich regions that are commonly associated with promoters and key transcription factor binding sites. Within runs of guanines, the preferential site of oxidation is the 5′ guanine, a process which is governed by long-distance electron tunneling through the hydrophobic core of duplex DNA (reviewed in (14,15)). The nonrandom distribution of 8-oxoG has been demonstrated using next generation sequencing of DNAs enriched by selective-capture methodologies using antibodies against 8-oxoG (16–18). Additionally, high resolution mapping of 8-oxoG in the mouse genome has been reported using covalent capture techniques that are based on selective oxidation of 8-oxoG that can be trapped through an imine intermediate (19–21). These data reveal selective induction of 8-oxoG in promoter-rich regions. These preferential modifications of DNA structure form the equivalent of a transient mark in DNA that can be used as a scaffold on which OGG1 can potentially bind and either interfere with transcription factor binding or facilitate DNA bending and transcriptional activation (see below for an expanded discussion of this concept).
Both 8-oxoG and FapyG are substrates for OGG1 in duplex DNA, but are not excised in ssDNA (reviewed in (22)). Although OGG1 was initially characterized as a DNA glycosylase with a concomitant abasic (AP) site lyase activity that is associated with bifunctional DNA glycosylases using either primary or secondary amines as the C1´ deoxyribose attacking nucleophile (reviewed in (23–25)), elegant structure-function analyses demonstrated that the glycosylase and AP lyase activities were uncoupled and enzymes containing site-specifically altered amino acids would possess one or the other activity (26). Thus, following scanning of duplex DNA via electrostatic and hydrophobic interactions (reviewed in (27–30)), processive nucleotide flipping, and specific damage recognition through extrahelical binding, OGG1 utilizes an activated water molecule to catalyze glycosyl bond scission. Due to slow turnover rates, the resulting AP site is subject to β-elimination chemistry that can be trapped as a covalent intermediate by reduction with sodium borohydride. The result is the release of 8-oxoG or FapyG and a single-stranded break that is subject to downstream base excision repair (BER) pathways in both the nucleus and mitochondria. Although biochemical reactions generally capture both glycosylase and lyase reactions, data have been presented which suggest that within an intracellular environment, the glycosylase reaction dominates over the combined activities (26,31).
Post-translational modification of OGG1. Although the majority of studies characterizing the biochemical properties of OGG1 have utilized an unmodified recombinant DNA produced enzyme, OGG1 is subject to a variety of post-translational modifications that either reduce or enhance its activity. These include inactivation through cysteine oxidation (32,33) and O-linked-N-acetylglucosamine acylation of hydroxyl groups on serine and threonine residues (34). Inactivation via cysteine oxidation is a reversible reaction that is regulated by the intracellular redox such that under pro-oxidant conditions, OGG1 is inactivated, and upon the re-establishment of redox homeostasis, activity is restored O-GlcNAcylation of mitochondrial OGG1 (mtOGG1) under conditions of oxidative stress resulted in increased mitochondrial DNA (mtDNA) damage and dysfunction. Both these results raise interesting questions regarding the function of OGG1 under conditions of sustained oxidative stress, but unequivocally indicate that at least two post-translational modifications can lead to catalytic inactivation that potentially do not interfere with damage-specific binding. This opens a regulatory mechanism by which substrate-specific binding can result in DNA damage-specific binding and DNA bending and result in recruitment of transcription factors (see below for further discussion).
In addition to modifications leading to inactivation, the catalytic efficiency of OGG1 can be enhanced via phosphorylation by the Cdk4 serine/threonine kinase (35) and by cyclic AMP response element-binding protein/p300-mediated lysine (K338 and K341) acetylation (36). Interestingly, mitochondrial NAD+-dependent deacetylase, SIRT3, increases the half-life of mtOGG1, decreases mtDNA damage and improves mitochondrial function (37). Beyond these post-translational modifications, OGG1 catalytic activity is stimulated via protein-protein interactions with AP endonuclease (APE1) (38,39), XRCC1 (40,41) and the cut homeobox 1 and 2 (42,43).
Overall, there is an extraordinarily complex set of modifications of nuclear and mtOGG1 that can modulate its DNA binding and catalytic properties. Many of these are dictated by changes in the intracellular environment. This further sets the stage for dual roles of OGG1 in transcriptional modulation and DNA repair activities.
Non-catalytic transcriptional regulation. As alluded to above, an increase in intracellular ROS is likely to result in post-translational modifications in OGG1 that result in a loss of catalytic function. Although this decrease in activity for the repair of ROS-induced DNA damage may seem counterintuitive, these conditions establish frameworks around which large-scale transcriptional changes can occur. To illustrate this concept, we will draw upon multiple investigations from the Boldogh laboratory and others who have provided the fundamental framework from which to understand and rationalize the non-catalytic functions of OGG1 as a transcriptional modulator. Early indications of the transcriptional regulatory role of OGG1 came from analyses of blunted gene expression in airway inflammation studies of OGG1-knockout mice in which these mice displayed a decreased Th1 response to bacterial infection and increased resistance to lipopolysaccharide- as well as tumor necrosis factor alpha (TNF-α)-induced inflammation (44,45) and a decreased inflammatory response to various allergens (46,47). These data suggested that in the absence of OGG1, normal pro-inflammatory gene activation cascades were compromised.
In addition to OGG1-deficiency conferring resistance to airway inflammation, similar protective effects have also been measured in models of gastric inflammation using Helicobacter pylori infection (45). In contrast, OGG1-deficient mice are significantly more susceptible to dextran-sulfate sodium-induced ulcerative colitis than wild-type control mice (48). Although there has not been an adequate explanation for the differential organ-specific sensitivities to oxidant stressors, these data highlight the critical nature of OGG1 in mediating cellular and organ-specific responses to these conditions.
Consistent with preferential formation of 8-oxoG lesions in gene regulatory regions and a critical role of OGG1 in mediating these responses, chromatin immunoprecipitation analyses following TNF-α mediated challenge revealed a substantial overlap between sites of OGG1 binding and NF-κB binding (49). This interdependent relationship between common sites of OGG1 and NF-κB binding was reinforced by studies demonstrating that knockdown of OGG1 significantly down-regulated NF-κB-dependent gene expression profiles (46,50). These and multiple other investigations are highly consistent with a model in which ROS-induced 8-oxoG lesions in and in close proximity to NF-κB binding sites recruit a catalytically inactive OGG1, which in turn, sharply bends DNA thus facilitating the recruitment of NF-κB and a transcription initiation complex. It is envisioned that 8-oxoG lesions formed in ssDNA that are transcriptionally competent could be bound by either active or inactive OGG1, but in this structure, no incision would occur. Thus, the inflammatory gene expression cascades can be directly linked to the non-catalytically productive binding of OGG1 to genes controlled by these master regulators. Re-establishing redox homeostasis would result in the reactivation of the catalytic function of OGG1, lesion excision, and repair. This would not only minimize the potential mutagenic effect of replicating DNAs with 8-oxoG and FapyG lesions, but also take away the promoter-rich damage binding structures for OGG1. These conclusions are strongly reinforced by recent data from the Boldogh and Helleday laboratories in which a small molecule inhibitor of OGG1 was shown to efficiently suppress inflammation and pro-inflammatory gene expression (51).
OGG1 incision of DNA as an activator of transcription. To further expand the potential role of OGG1 in modulating transcriptional activities, several laboratories have investigated DNA structural alterations in response to oxidatively-induced damage and repair initiation in regions of potential G-quadruplex (G4) forming, potential Z DNA forming, and i-motif-forming sequences (16,52–62). Investigations from the laboratory of Dr. Cynthia Burrows have demonstrated that promoter and 5′ untranslated regions of many DNA repair genes contain multiple potential G4 DNA forming sequences and that at least a subset of these modulate transcriptional activity upon the introduction and processing of 8-oxoG. The Burrows group has clearly demonstrated that there are strong strand-specific effects relative to coding versus non-coding strands. Their investigations have also made it clear that there can be a series of structural alterations in DNA that reflect transitions between the native, undamaged DNA, those containing site-specific base damage, those processed by the DNA glycosylase activity of OGG1, and those following incision by APE1.
As an example, oxidation of guanine residues in the coding strand within the VEGF promoter and subsequent OGG1-mediated glycosylase activity generating an AP site, induced the formation of G4 and transcriptional activation. Scenarios in which damage was introduced into the template strand decreased transcription. Thus, the site and strand specificity of the damage and subsequent initiation of repair can be key modulators of transcription binding sites. Additionally, formation of the G4 structure with an AP site in a stem-loop structure rendered the site refractory to APE1 incision, thus sustaining the signal (55). In a conceptually similar manner, upon oxidation of guanines within potential Z DNA forming sequences, the equilibrium between Z and B DNA structures still favored Z DNA but upon OGG1-mediated base excision in the coding strand, this incision resulted in a shift from a Z DNA structure to a cruciform-like structure, with the resulting AP site extruded into an extended loop (56). Overall, these examples further reinforce to extend the repertoire of gene regulation that can be mediated by OGG1.
OGG1 as a modulator of GDP/GTP exchange factors – a post-catalytic regulatory mechanism. An additional mechanism through which OGG1 exerts transcriptional control was made by the discovery that OGG1 is able to bind the free base 8-oxoG and subsequently modulate Ras and Rac signaling pathways (63–65) and reviewed in (66)). The origin of this discovery came through the analyses of transcriptional profiling of OGG1-proficient cells in which the free base, 8-oxoGua was added to the cells and pathway analyses revealed that 8 out of the 10 induced pathways involved the G protein, Ras. Further, it was demonstrated that GTP-bound Ras increased with the addition of 8-oxoG, but that cells depleted of OGG1 did not display this change (64). Using changes in OGG1 intrinsic tryptophan fluorescence, it was demonstrated that OGG1 tightly binds 8-oxoG and that the OGG1–8-oxoG bound complex specifically binds H-Ras and K-Ras. This H-Ras binding was dependent on OGG1 binding of the free base and these interactions were competed in the presence of GTP. These studies went on to demonstrate that the OGG1–8-oxoGua bound complex facilitated H-Ras/GDP to H-Ras/GTP exchange. The resulting activation of Ras led to phosphorylation of mitogen-activated kinases, MEK1,2 and ERK1,2 (64).
These investigations have been extended to demonstrate that the OGG1–8-oxoG bound complex interacts with guanine nucleotide-free and GDP-bound Rac1 and that this interaction efficiently results in GDP to GTP exchange (65). Further adding to the complexity of this post-catalytic transcriptional regulation via the OGG1–8-oxoG bound complex, studies have also demonstrated that this complex modulated differential expression of 84 miRNAs that are associated with antigen-driven allergic inflammation (63).
Enhanced mitochondrial DNA repair of ROS-induced damage.
Relative to nuclear DNA, the mitochondrial genome has been shown to be significantly more prone to oxidatively-induced base damage (67–69). The importance of these data is highlighted by investigations involving organ transplant of pancreatic islets and lungs (reviewed in (70). Under such conditions, it is anticipated that the mtDNA repair capacity of damaged cells would be overwhelmed, leading to mitochondrially-driven cell death pathways. To address this hypothesis, a series of experimental strategies have been implemented to introduce mtOGG1 into cells as either a purified recombinant enzyme or via transgenic expression (71–80). Collectively, these studies consistently report significant improvement in cellular function in response to acute, pro-oxidant stressors, and thus provide a rational basis from which to design pharmacologic interventions under chronic and acute pro-inflammatory environments. In this regard, the laboratory of Dr. Vilhelm Bohr has reported the first small molecule agonists of OGG1(81). Although these compounds were not anticipated to have appropriate pharmacological properties, they establish a proof-of-principle that such pharmacologic interventions could have significant clinical value.
Disease consequences associated with the major human polymorphic variant of OGG1: Ser326Cys.
Given the potential functions of OGG1 in modulating gene expression profiles, with murine models of OGG1-deficiency revealing both protective and sensitization phenotypes, characterization of naturally-occurring polymorphic variants of OGG1 is likely to reveal changes in odds-ratios of various human disease susceptibilities. In this regard, there is one predominant allelic variant of OGG1, Ser326Cys (S326C) (rs1052133) that is present in 40–60 % and 25–40 % in Asian and Caucasian populations, respectively (82). Characterization of this enzyme variant has revealed decreased activity and the potential to dimerize through cysteine-cysteine disulfide bond formation (81,83,84). In contrast to wild-type (WT) OGG1 stimulation by APE1, the S326C variant was resistant to APE1-mediated increased turnover (84). The potential adverse health effects associated with individuals carrying monoallelic Ser/Cys and biallelic Cys/Cys variants have been analyzed in many human disease cohorts including, but not limited to i) various cancers including colorectal (85), breast (86), oropharyngeal squamous cell carcinoma (87), acute myeloid leukemia relapse (88), endometrial (89), prostate (90,91), and lung (92), ii) obesity and type 2 diabetes (93,94), iii) Parkinson’s disease (95), iv) Alzheimer’s disease (96), v) age-related cataract formation (97,98), vi) infertility (99), vii) cardiovascular disease (100), and viii) dry, age-related macular degeneration (101). The trend of the conclusions of these investigations suggest that the S326C variant has wide-spread negative health implications. This variant of OGG1 has been reported to significantly increase incidence of obesity, type II diabetes, and death due to cardiovascular disease (94,100,102). Characterization of the rarer allelic variants of OGG1 have lagged far behind and disease associations, if any have yet to be established.
Knockout of OGG1 results in delayed onset of metabolic syndrome.
Novel roles for the DNA repair glycosylase, OGG1, in the development of obesity and metabolic syndrome.
While OGG1-deficient animals have been available in multiple labs and studied extensively with regard to cancer phenotypes, no association between OGG1 and metabolism or body weight regulation had been reported until recently. We observed that with increasing age, Ogg1−/− mice tended to be significantly heavier than WT counterparts, such that by a year of age, ad libitum chow-fed Ogg1−/− mice were not only heavier, but also fatter than WT mice (103). Given the role for OGG1 excising oxidatively-induced lesions, we asked the question of whether the obesogenic phenotype of OGG1 deficiency could be exacerbated via a metabolic stress that would increase production of ROS. To do this, we placed age-matched mice on a high-fat diet (HFD) for 10 weeks. Following this acute stress in relatively young animals (HFD from 12–18 weeks of age), Ogg1−/− mice became significantly heavier and accumulated more adipose tissue than WT counterparts (103). These studies were conducted using a diet that derives approximately 60% of its calories from fat and is commonly used as a model to study extreme metabolic stress. More recently, we have carried out similar feeding studies using a more moderate and physiologically relevant high-fat diet, deriving approximately 45% of its calories from fat. Even upon being subjected to this more moderate stress, Ogg1−/− mice become heavier and fatter than WT counterparts within six weeks of feeding (Sharma & Sampath, unpublished). Concomitant with increased adiposity, HFD-fed Ogg1−/− mice develop significant fatty livers, relative to WT mice (103). This is accompanied by persistent hyperinsulinemia and significantly impaired glucose clearance in these mice, indicating the development of insulin resistance and a pre-diabetic state in HFD-fed Ogg1−/− mice.
Food intake and voluntary physical activity do not differ in the OGG1-deficient cohort. We also did not observe overall changes in energy expenditure that would explain the obesity-proneness of this strain. However, indirect calorimetry measurements revealed that during the resting phase, HFD-fed Ogg1−/− mice had a significantly elevated respiratory exchange ratio (RER) (103). The RER value, obtained by dividing VCO2 by VO2, serves as a proxy for substrate utilization in vivo, with an RER close to 1.0 representing carbohydrate utilization, while an RER closer to 0.7 represents sole reliance on fatty acids for energy production. Thus, an elevated RER in HFD-fed Ogg1−/− mice indicated a shift away from fat oxidation towards carbohydrate utilization in these animals, consistent with their obese phenotype. Further measures of hepatic fat oxidation, including expression of key genes regulating fat oxidation, as well as fasting levels of plasma ketones, a measure of hepatic fat oxidation, were all reduced in Ogg1−/− mice. Notably, expression of the transcriptional co-activator, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which regulates mitochondrial biogenesis and fatty acid oxidation, was significantly lower in Ogg1−/− mice (103).
Altered skeletal muscle function in Ogg1−/− mice.
While the studies described above were primarily focused on hepatic alterations underlying the increased adiposity and fatty liver phenotype of Ogg1−/− mice, subsequent studies that focused on skeletal muscle of these mice revealed a similar increase in muscle triglyceride (TG) deposition in Ogg1−/− mice (104). Intramuscular TG (IMTG) can be associated both with beneficial alterations in muscle endurance as seen in trained athletes as well as with pathological skeletal muscle decline, as observed in the obese state (105). In Ogg1−/− mice, increased IMTGs were associated with a reduction in treadmill endurance and muscle grip strength. This increase in IMTG was mediated by an increase in lipid uptake in skeletal muscle in Ogg1−/− mice, but was not accompanied by an increase in levels of 8-oxoG (104). Given the importance of mitochondrial function to skeletal muscle function, and the known localization of OGG1 in mitochondria, we carried out measures of mitochondrial health in the muscle of Ogg1−/− mice. There were no appreciable differences in mitochondrial content in these animals. However, measures of mitochondrial dynamics indicated a significant increase in genes regulating mitochondrial fission in Ogg1−/− mice. Levels of dynamin related protein-1 (Drp1) and Fission-1 (Fis1) levels were significantly increased in muscle of Ogg1−/− mice. Similar increases in mitochondrial fragmentation, associated with decreased mitochondrial function, have been reported in obese, insulin-resistant muscle. Importantly, in our study, these changes were observed in chow-fed mice and preceded alterations in body weight and adiposity. Thus, the temporal nature of these changes is suggestive of OGG1 deficiency altering mitochondrial dynamics, favoring fission, potentially to dilute DNA damage and thereby causing mitochondrial fragmentation (104). Similar to our findings, a study using a culture cardiomyocyte model (106) indicated that adenoviral OGG1 overexpression reduced DRP1 and FIS1 in these cells, even upon exposure to oxidative stress. Interestingly, in studies using mice with transgenic overexpression of OGG1, we have observed a reciprocal mitochondrial elongation phenotype (73), as discussed in greater detail in the next section.
Overexpression of a mitochondrially-targeted OGG1 significantly protects from diet-induced obesity.
Mitochondria play an integral role in regulating energy balance in the cell and the whole organism. To test the specific role of mtOGG1 activity on whole body energy balance, we utilized a transgenic OGG1 model (OGG1Tg) developed in the lab of Dr. Lars Eide. OGG1Tg mice constitutively express the human OGG-1a gene downstream of a mitochondrial targeting sequence derived from the manganese superoxide dismutase (MnSOD) gene. Using this strategy, we and others have observed an approximately 2-fold increase in mtOGG1 activity (73), without any measurable increases in nuclear activity.
Following 12 weeks of hypercaloric high-fat diet consumption, body weight and composition analyses revealed that OGG1Tg mice were significantly protected from increased body weight and fat mass after HFD consumption, relative to WT animals (73). This reduction in fat mass was also accompanied by an almost complete protection from ectopic lipid accumulation in the livers of these HFD-fed transgenic mice. In contrast, Ogg1−/− mice are largely prone to development of fatty livers on a similar feeding regimen (Supplemental Fig. S1). This protection from diet-induced obesity was associated with reduced plasma insulin and enhanced glucose tolerance in OGG1Tg mice (73). While food intake and voluntary physical activity were not significantly altered, OGG1Tg mice had significantly increased whole body energy expenditure across both day and light cycles. To elucidate mechanisms mediating obesity-resistance in OGG1Tg mice, we examined expression of genes regulating lipid and energy metabolism in various metabolically-active tissues, including liver, gastrocnemius (gastroc), heart, and various adipose depots, including brown adipose tissue (BAT), epididymal white adipose tissue (eWAT), and inguinal adipose tissue (iWAT). Interestingly, we found that genes regulating fatty acid oxidation were significantly upregulated in eWAT from OGG1Tg mice, indicating increased lipid catabolism in eWAT of OGG1Tg mice. Importantly, we did not observe similar changes in gene expression in other tissues examined. Furthermore, expression of PGC-1α, which we previously found to be largely reduced in Ogg1−/− tissues (103), was significantly increased in eWAT of OGG1Tg mice. A similar increase was not evident in liver, heart, muscle or other adipose depots such as BAT or iWAT (73).
To determine if mtOGG1 alters mitochondrial parameters, we measured mitochondrial content, structure, and function in several metabolically important organs. Overall mtDNA content was unchanged in any tissue examined (73). Interestingly, however, mitochondrial protein content, particularly of proteins involved in oxidative phosphorylation, was increased in eWAT of HFD-fed OGG1Tg mice. A similar increase in mitochondrial protein was not observed in liver of transgenic mice. Concomitant with the increase in mitochondrial proteins, in the context of unchanged mtDNA content, OGG1Tg mice had a measurable increase in a majority of the thirteen mitochondrially-encoded genes, as well as in levels of mitochondrial transcription factor A (Tfam), a nuclear-encoded master regulator of mitochondrial transcription. These alterations are suggestive of OGG1 activity in the mitochondria being important to maintaining mitochondrial transcription rates. In agreement with this hypothesis and as discussed above, studies have demonstrated that the presence of even a single 8-oxoG site in the promoter regions of genes (31), as well as presence of further oxidized forms of 8-oxoG, including the spiroiminodihydantoin and 5-guanidinohydantoin lesions (107) exert a transcriptional silencing effect via reduced bypass by RNA polymerase II. Similarly, studies using mitochondrial RNA polymerase (108) have demonstrated that 8-oxoG exerts a significant, but not complete, transcriptional block. In this regard, our studies suggest that 8-oxoG content is increased in eWAT of Ogg1−/− mice (Supplemental Fig. S2) and tends to be reduced in eWAT of OGG1Tg mice, after HFD feeding.
In addition to changes in mitochondrial protein and transcript abundance, mitochondrial ultrastructure was visualized by transmission electron microscopy in fixed liver and adipose tissue from chow- and HFD-fed animals. No genotypic effects were observed in liver (Komakula and Sampath, unpublished). However, in HFD-fed eWAT samples, mitochondrial length was significantly increased in OGG1Tg animals, relative to WT controls (73). This was accompanied by a significant increase in levels of mitofusin-2, a key regulator of mitochondrial fusion. It is notable that this phenotype is reciprocal to the mitochondrial fragmentation that we (103), and others (106), have reported in the OGG1-deficient state. Together, these data indicate a novel role for mtOGG1 in regulating mitochondrial dynamics. Importantly, this increase in mitochondrial length was associated with increased mitochondrial respiration in OGG1Tg eWAT, correlating with their increased energy expenditure and protection from diet-induced obesity (73).
The multiple effects of mtOGG1 on mitochondrial structure and function were functionally translated into beneficial alterations in adipocyte metabolic health. Importantly, OGG1Tg mice had increased circulating levels of the adipose-derived hormone, adiponectin (73), which is associated with increased energy expenditure and improved metabolic health (109). Further, markers of inflammation in adipose tissue, including TNF-α and NF-ĸB levels, which are correlated with worsened metabolic outcomes (110), were all significantly attenuated in OGG1Tg mice, even upon HFD-feeding. In contrast, adipose tissue inflammation was significantly increased in Ogg1−/− mice that are prone to diet-induced obesity.
Future directions.
Taken together, our findings of increased body weight in Ogg1−/− mice and protection from diet-induced obesity in OGG1Tg animals have cemented a role for OGG1 in body weight regulation and energy balance. Our studies in OGG1Tg mice have indicated unexpected, but critical roles for DNA damage and repair in the adipocyte that warrant further investigation in and of themselves. These data also point to the need to study the DNA damage response in other animal models with perturbations in adipose tissue redox balance or mitochondrial function. For instance, adipose-specific depletion of TFAM was associated with significant increases in adipose tissue O2 consumption, along with increased whole-body energy expenditure and protection from HFD-induced weight gain (111). Interestingly, in this model, levels of 8-oxoG were significantly increased in Tfam−/− mice, but OGG1 levels were not measured, and the significance of the elevated 8-oxoG levels was not discussed. However, given the role of OGG1 on 8-oxoG repair, it is likely that such a measurable increase in 8-oxoG would elicit a compensatory induction in OGG1 levels, suggesting that modulation of OGG1 may be a mechanism mediating obesity resistance in adipose-specific Tfam−/− mice. In yet another example of adipose-specific modulation of ROS resulting in global changes in energy metabolism, animals with adipose-specific depletion of MnSOD were reported to be significantly protected from diet-induced obesity (112). Deletion of the mitochondrial SOD was associated with increased superoxide levels in these mice. Functional consequences of increasing superoxide, including measurements of DNA damage and repair, were not reported (112). However, it is likely that elevated ROS production in adipose MnSod−/− mice would result in elevations in mtOGG1 activity to repair ROS-induced lesions. Such an effect would again link elevated mtOGG1 activity, specifically in adipose tissue, with protection from diet-induced obesity.
The studies described in our initial reports on the whole-body physiology of mtOGG1 enhancement were focused on epididymal adipose tissue. However, subcutaneous adipose tissue is an important additional source of circulating adiponectin levels as well as a regulator of systemic energy balance. Similarly, BAT, a tissue that is critical to energy balance and expresses a high concentration of mitochondria, may also be expected to be sensitive to changes in mtDNA damage. Therefore, ongoing studies are focused on delineating differences in the DNA damage response in different adipose depots, especially following metabolic perturbation by dietary and genetic means. Further, given the important role of skeletal muscle in systemic glucose handling, combined with our prior findings of altered skeletal muscle function in Ogg1−/− mice, ongoing studies are also aimed at delineating the impact of mtOGG1 enhancement on skeletal muscle function.
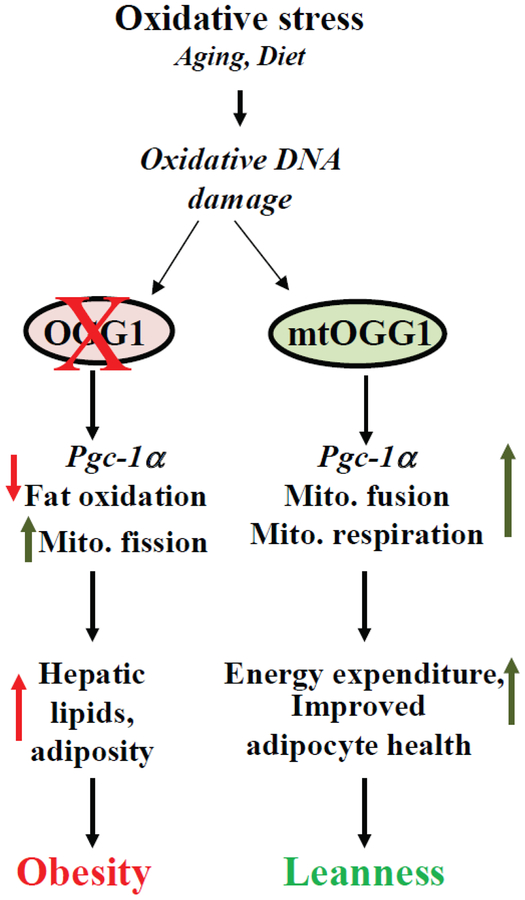
Our studies have begun elucidating the basic mechanisms by which defects in BER render animals susceptible to metabolic disease (Fig. 1). These data which are corroborated by studies in human cohorts, have started revealing that the relatively common OGG1 polymorphism, Ser326Cys, is associated with increased incidence of obesity, type II diabetes, and death due to cardiovascular disease (94,100,102). Therefore, further studies should be designed with the goal of precisely defining the role of the BER pathway in the etiology of obesity and related diseases, and designing potential targeted therapeutics for clinical management of metabolic syndrome.
Fig. 1.
Effects of OGG1-mediated repair on body weight and metabolism.
Concluding perspectives.
The data summarized in this review highlight the extreme complexity of the potential roles of OGG1 through both its non-catalytic, damage-specific promoter binding and subsequent transcriptional modulation and its catalytic role in altering DNA structure and repair of pre-mutagenic and apoptotic-inducing base damage. It is clear that the role of OGG1 in both nuclear and mitochondrial organelles play distinct, but complementary functions in response to oxidative stress. In this regard, there is renewed interest in understanding nuclear/mitochondrial crosstalk and its role in aging, neurodegeneration, cancer, and metabolism (reviewed in (113–116). Especially critical in defining the relative contributions and interplay between these organelles are investigations in the laboratory of Dr. Scott Ballinger using mitochondrial-nuclear eXchange mice in which isolated embryonic pronuclei from one mouse strain are introduced into an enucleated embryo of a different species (117). Their data definitively establish that transcriptionally-mediated crosstalk between the mitochondria and nucleus is a two-way exchange in which the transcriptional events in both organelles, contribute to overall metabolic outcomes in the resulting progeny. Such models may be ideal candidates for testing differential repair capacity for oxidatively-induced DNA damage. Finally, given the importance of mitochondrial repair in the initiation, maintenance, and progression of many diseases, significant effort should be invested in the development of mitochondrially-targeted small molecule agonists of OGG1 and potentially other mitochondrial DNA glycosylases that function on oxidatively-induced DNA damage.
Supplementary Material
Acknowledgements.
We wish to thank Dr. Vladimir Vartanian who has made invaluable contributions to this work. We also wish to thank Drs. Amanda K. McCullough, Steve Boldogh, Cindy Burrows, and Scott Ballinger for their insightful comments and recommendations on this review. These investigations were supported by grants from the NIH, R01 DK075974, DK100640. Generous additional support has been provided by the Oregon Institute for Occupational Health Sciences and the New Jersey Institute for Food, Nutrition, and Health at Rutgers University.
Abbreviations:
- OGG1
8-oxoguanine DNA glycosylase
- UV
ultraviolet light
- CPDs
cyclobutane pyrimidine dimers
- ssDNA
single-stranded DNA
- ROS
reactive oxygen species
- 8-oxoG
7,8-dihydro-8-oxo-2´-guanine
- FapyG
2–6-diamino-4-hydroxy-5-formamidopyrimidine
- AP
abasic site
- BER
base excision repair
- mtOGG1
mitochondrial OGG1
- mtDNA
mitochondrial DNA
- APE1
AP endonuclease
- G4
G-quadruplex
- S326C
Ser326Cys
- HFD
high-fat diet
- WT
wild-type
- RER
respiratory exchange ratio
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- TG
triglyceride
- IMTG
intramuscular TG
- OGG1Tg
transgenic OGG1 model
- MnSOD
manganese superoxide dismutase
- BAT
brown adipose tissue
- eWAT
epididymal white adipose tissue
- iWAT
inguinal adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Strickland FM and Kripke ML (1997) Immune response associated with nonmelanoma skin cancer. Clin Plast Surg, 24, 637–647. [PubMed] [Google Scholar]
- 2.Vink AA, Yarosh DB and Kripke ML (1996) Chromophore for UV-induced immunosuppression: DNA. Photochem Photobiol, 63, 383–386. [DOI] [PubMed] [Google Scholar]
- 3.Yu SH, Bordeaux JS and Baron ED (2014) The immune system and skin cancer. Adv Exp Med Biol, 810, 182–191. [DOI] [PubMed] [Google Scholar]
- 4.Yarosh DB (2002) Enhanced DNA repair of cyclobutane pyrimidine dimers changes the biological response to UV-B radiation. Mutat Res, 509, 221–226. [DOI] [PubMed] [Google Scholar]
- 5.Yarosh DB and Klein J (1996) DNA repair enzymes in prevention of photocarcinogenesis. Photochem Photobiol, 63, 445–447. [DOI] [PubMed] [Google Scholar]
- 6.Sedgwick B and Lindahl T (2002) Recent progress on the Ada response for inducible repair of DNA alkylation damage. Oncogene, 21, 8886–8894. [DOI] [PubMed] [Google Scholar]
- 7.Indiani C and O’Donnell M (2013) A proposal: Source of single strand DNA that elicits the SOS response. Front Biosci (Landmark Ed), 18, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslowska KH, Makiela-Dzbenska K and Fijalkowska IJ (2019) The SOS system: A complex and tightly regulated response to DNA damage. Environ Mol Mutagen, 60, 368–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton MD, Smith BT, Godoy VG and Walker GC (2000) The SOS response: recent insights into umuDC-dependent mutagenesis and DNA damage tolerance. Annu Rev Genet, 34, 479–497. [DOI] [PubMed] [Google Scholar]
- 10.Ba X and Boldogh I (2018) 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol, 14, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlahopoulos S, Adamaki M, Khoury N, Zoumpourlis V and Boldogh I (2019) Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer. Pharmacol Ther, 194, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P and Sampath H (2019) Mitochondrial DNA Integrity: Role in Health and Disease. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boiteux S, Coste F and Castaing B (2017) Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic Biol Med, 107, 179–201. [DOI] [PubMed] [Google Scholar]
- 14.Genereux JC and Barton JK (2010) Mechanisms for DNA charge transport. Chem Rev, 110, 1642–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley SO and Barton JK (1999) Radical migration through the DNA helix: chemistry at a distance. Met Ions Biol Syst, 36, 211–249. [PubMed] [Google Scholar]
- 16.Pastukh V, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, Borchert GM and Gillespie MN (2015) An oxidative DNA “damage” and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am J Physiol Lung Cell Mol Physiol, 309, L1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Sturla SJ, Burrows CJ and Fleming AM (2019) Impact of DNA Oxidation on Toxicology: From Quantification to Genomics. Chem Res Toxicol, 32, 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshihara M, Jiang L, Akatsuka S, Suyama M and Toyokuni S (2014) Genome-wide profiling of 8-oxoguanine reveals its association with spatial positioning in nucleus. DNA Res, 21, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Y, Fleming AM and Burrows CJ (2017) Sequencing the Mouse Genome for the Oxidatively Modified Base 8-Oxo-7,8-dihydroguanine by OG-Seq. J Am Chem Soc, 139, 2569–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming AM, Ding Y and Burrows CJ (2017) Sequencing DNA for the Oxidatively Modified Base 8-Oxo-7,8-Dihydroguanine. Methods Enzymol, 591, 187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosford ME, Muller JG and Burrows CJ (2004) Spermine participates in oxidative damage of guanosine and 8-oxoguanosine leading to deoxyribosylurea formation. J Am Chem Soc, 126, 9540–9541. [DOI] [PubMed] [Google Scholar]
- 22.Dizdaroglu M, Coskun E and Jaruga P (2017) Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics. Mutat Res, 771, 99–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodson ML and Lloyd RS (2002) Mechanistic comparisons among base excision repair glycosylases. Free Radic Biol Med, 32, 678–682. [DOI] [PubMed] [Google Scholar]
- 24.McCullough AK, Dodson ML and Lloyd RS (1999) Initiation of base excision repair: glycosylase mechanisms and structures. Annu Rev Biochem, 68, 255–285. [DOI] [PubMed] [Google Scholar]
- 25.Verdine GL and Norman DP (2003) Covalent trapping of protein-DNA complexes. Annu Rev Biochem, 72, 337–366. [DOI] [PubMed] [Google Scholar]
- 26.Dalhus B, Forsbring M, Helle IH, Vik ES, Forstrom RJ, Backe PH, Alseth I and Bjoras M (2011) Separation-of-function mutants unravel the dual-reaction mode of human 8-oxoguanine DNA glycosylase. Structure, 19, 117–127. [DOI] [PubMed] [Google Scholar]
- 27.Howard MJ and Wilson SH (2018) DNA scanning by base excision repair enzymes and implications for pathway coordination. DNA Repair (Amst), 71, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwahara J, Zandarashvili L, Kemme CA and Esadze A (2018) NMR-based investigations into target DNA search processes of proteins. Methods, 148, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd RS (2001) Processivity of DNA repair enzymes. Methods Mol Biol, 160, 3–14. [DOI] [PubMed] [Google Scholar]
- 30.Verdine GL and Bruner SD (1997) How do DNA repair proteins locate damaged bases in the genome? Chem Biol, 4, 329–334. [DOI] [PubMed] [Google Scholar]
- 31.Allgayer J, Kitsera N, Bartelt S, Epe B and Khobta A (2016) Widespread transcriptional gene inactivation initiated by a repair intermediate of 8-oxoguanine. Nucleic Acids Res, 44, 7267–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravard A, Campalans A, Vacher M, Gouget B, Levalois C, Chevillard S and Radicella JP (2010) Inactivation by oxidation and recruitment into stress granules of hOGG1 but not APE1 in human cells exposed to sub-lethal concentrations of cadmium. Mutat Res, 685, 61–69. [DOI] [PubMed] [Google Scholar]
- 33.Bravard A, Vacher M, Gouget B, Coutant A, de Boisferon FH, Marsin S, Chevillard S and Radicella JP (2006) Redox regulation of human OGG1 activity in response to cellular oxidative stress. Mol Cell Biol, 26, 7430–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cividini F, Scott BT, Dai A, Han W, Suarez J, Diaz-Juarez J, Diemer T, Casteel DE and Dillmann WH (2016) O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J Biol Chem, 291, 26515–26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, Imam SZ, Hashiguchi K, de Souza-Pinto NC and Bohr VA (2005) Phosphorylation of human oxoguanine DNA glycosylase (alpha-OGG1) modulates its function. Nucleic Acids Res, 33, 3271–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK and Mitra S (2006) Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol Cell Biol, 26, 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y, Ren X, Gowda AS, Shan Y, Zhang L, Yuan YS, Patel R, Wu H, Huber-Keener K, Yang JW et al. (2013) Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis, 4, e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill JW, Hazra TK, Izumi T and Mitra S (2001) Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair. Nucleic Acids Res, 29, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saitoh T, Shinmura K, Yamaguchi S, Tani M, Seki S, Murakami H, Nojima Y and Yokota J (2001) Enhancement of OGG1 protein AP lyase activity by increase of APEX protein. Mutat Res, 486, 31–40. [DOI] [PubMed] [Google Scholar]
- 40.Campalans A, Moritz E, Kortulewski T, Biard D, Epe B and Radicella JP (2015) Interaction with OGG1 is required for efficient recruitment of XRCC1 to base excision repair and maintenance of genetic stability after exposure to oxidative stress. Mol Cell Biol, 35, 1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsin S, Vidal AE, Sossou M, Menissier-de Murcia J, Le Page F, Boiteux S, de Murcia G and Radicella JP (2003) Role of XRCC1 in the coordination and stimulation of oxidative DNA damage repair initiated by the DNA glycosylase hOGG1. J Biol Chem, 278, 44068–44074. [DOI] [PubMed] [Google Scholar]
- 42.Pal R, Ramdzan ZM, Kaur S, Duquette PM, Marcotte R, Leduy L, Davoudi S, Lamarche-Vane N, Iulianella A and Nepveu A (2015) CUX2 protein functions as an accessory factor in the repair of oxidative DNA damage. J Biol Chem, 290, 22520–22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramdzan ZM, Vadnais C, Pal R, Vandal G, Cadieux C, Leduy L, Davoudi S, Hulea L, Yao L, Karnezis AN et al. (2014) RAS transformation requires CUX1-dependent repair of oxidative DNA damage. PLoS Biol, 12, e1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH and Szabo C (2005) Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J, 19, 290–292. [DOI] [PubMed] [Google Scholar]
- 45.Touati E, Michel V, Thiberge JM, Ave P, Huerre M, Bourgade F, Klungland A and Labigne A (2006) Deficiency in OGG1 protects against inflammation and mutagenic effects associated with H. pylori infection in mouse. Helicobacter, 11, 494–505. [DOI] [PubMed] [Google Scholar]
- 46.Ba X, Bacsi A, Luo J, Aguilera-Aguirre L, Zeng X, Radak Z, Brasier AR and Boldogh I (2014) 8-oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors. J Immunol, 192, 2384–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Yuan K, Yan C, Fox J 3rd, Gaid M, Breitwieser W, Bansal AK, Zeng H, Gao H and Wu M (2012) 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic Biol Med, 52, 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao J, Seril DN, Lu GG, Zhang M, Toyokuni S, Yang AL and Yang GY (2008) Increased susceptibility of chronic ulcerative colitis-induced carcinoma development in DNA repair enzyme Ogg1 deficient mice. Mol Carcinog, 47, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Mitra A, Dojer N, Fu S, Rowicka M and Brasier AR (2013) A probabilistic approach to learn chromatin architecture and accurate inference of the NF-kappaB/RelA regulatory network using ChIP-Seq. Nucleic Acids Res, 41, 7240–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan L, Zhu B, Hao W, Zeng X, Vlahopoulos SA, Hazra TK, Hegde ML, Radak Z, Bacsi A, Brasier AR et al. (2016) Oxidized Guanine Base Lesions Function in 8-Oxoguanine DNA Glycosylase-1-mediated Epigenetic Regulation of Nuclear Factor kappaB-driven Gene Expression. J Biol Chem, 291, 25553–25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visnes T, Cazares-Korner A, Hao W, Wallner O, Masuyer G, Loseva O, Mortusewicz O, Wiita E, Sarno A, Manoilov A et al. (2018) Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science, 362, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An N, Fleming AM and Burrows CJ (2016) Human Telomere G-Quadruplexes with Five Repeats Accommodate 8-Oxo-7,8-dihydroguanine by Looping out the DNA Damage. ACS Chem Biol, 11, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming AM and Burrows CJ (2013) G-quadruplex folds of the human telomere sequence alter the site reactivity and reaction pathway of guanine oxidation compared to duplex DNA. Chem Res Toxicol, 26, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleming AM, Ding Y and Burrows CJ (2017) Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc Natl Acad Sci U S A, 114, 2604–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleming AM, Zhu J, Ding Y and Burrows CJ (2017) 8-Oxo-7,8-dihydroguanine in the Context of a Gene Promoter G-Quadruplex Is an On-Off Switch for Transcription. ACS Chem Biol, 12, 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming AM, Zhu J, Ding Y, Esders S and Burrows CJ (2019) Oxidative Modification of Guanine in a Potential Z-DNA-Forming Sequence of a Gene Promoter Impacts Gene Expression. Chem Res Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fleming AM, Zhu J, Ding Y, Visser JA, Zhu J and Burrows CJ (2018) Human DNA Repair Genes Possess Potential G-Quadruplex Sequences in Their Promoters and 5’-Untranslated Regions. Biochemistry, 57, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omaga CA, Fleming AM and Burrows CJ (2018) The Fifth Domain in the G-Quadruplex-Forming Sequence of the Human NEIL3 Promoter Locks DNA Folding in Response to Oxidative Damage. Biochemistry, 57, 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C and Avvedimento EV (2008) DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science, 319, 202–206. [DOI] [PubMed] [Google Scholar]
- 60.Redstone SCJ, Fleming AM and Burrows CJ (2019) Oxidative Modification of the Potential G-Quadruplex Sequence in the PCNA Gene Promoter Can Turn on Transcription. Chem Res Toxicol, 32, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers RA, Fleming AM and Burrows CJ (2018) Rapid Screen of Potential i-Motif Forming Sequences in DNA Repair Gene Promoters. ACS Omega, 3, 9630–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu J, Fleming AM and Burrows CJ (2018) The RAD17 Promoter Sequence Contains a Potential Tail-Dependent G-Quadruplex That Downregulates Gene Expression upon Oxidative Modification. ACS Chem Biol, 13, 2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguilera-Aguirre L, Hao W, Pan L, Li X, Saavedra-Molina A, Bacsi A, Radak Z, Sur S, Brasier AR, Ba X et al. (2017) Pollen-induced oxidative DNA damage response regulates miRNAs controlling allergic inflammation. Am J Physiol Lung Cell Mol Physiol, 313, L1058–L1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK and Mitra S (2012) Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J Biol Chem, 287, 20769–20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajas G, Bacsi A, Aguilera-Aguirre L, Hegde ML, Tapas KH, Sur S, Radak Z, Ba X and Boldogh I (2013) 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular signaling via the activation of the small GTPase Rac1. Free Radic Biol Med, 61, 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choudhary S, Boldogh I and Brasier AR (2016) Inside-Out Signaling Pathways from Nuclear Reactive Oxygen Species Control Pulmonary Innate Immunity. J Innate Immun, 8, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA et al. (2000) Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res, 86, 960–966. [DOI] [PubMed] [Google Scholar]
- 68.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A and Bohr VA (2001) Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res, 61, 5378–5381. [PubMed] [Google Scholar]
- 69.Yakes FM and Van Houten B (1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A, 94, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan YB, Mulekar S, Gorodnya O, Weyant MJ, Zamora MR, Simmons JD, Machuka T and Gillespie MN (2017) Pharmacologic Protection of Mitochondrial DNA Integrity May Afford a New Strategy for Suppressing Lung Ischemia-Reperfusion Injury. Ann Am Thorac Soc, 14, S210–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN and Parker JC (2013) Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol, 304, L287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, Weitzman S, Bohr VA and Kamp DW (2014) Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J Biol Chem, 289, 6165–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komakula SSB, Tumova J, Kumaraswamy D, Burchat N, Vartanian V, Ye H, Dobrzyn A, Lloyd RS and Sampath H (2018) The DNA Repair Protein OGG1 Protects Against Obesity by Altering Mitochondrial Energetics in White Adipose Tissue. Sci Rep, 8, 14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GR, Schumacker PT, Weitzman SA et al. (2009) Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic Biol Med, 47, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP and Wilson GL (2002) Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem, 277, 44932–44937. [DOI] [PubMed] [Google Scholar]
- 76.Rachek LI, Thornley NP, Grishko VI, LeDoux SP and Wilson GL (2006) Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes, 55, 1022–1028. [DOI] [PubMed] [Google Scholar]
- 77.Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjoras M and Eide L (2011) Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci, 31, 9746–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuzefovych LV, Kahn AG, Schuler MA, Eide L, Arora R, Wilson GL, Tan M and Rachek LI (2016) Mitochondrial DNA Repair through OGG1 Activity Attenuates Breast Cancer Progression and Metastasis. Cancer Res, 76, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuzefovych LV, LeDoux SP, Wilson GL and Rachek LI (2013) Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One, 8, e83349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuzefovych LV, Solodushko VA, Wilson GL and Rachek LI (2012) Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology, 153, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baptiste BA, Katchur SR, Fivenson EM, Croteau DL, Rumsey WL and Bohr VA (2018) Enhanced mitochondrial DNA repair of the common disease-associated variant, Ser326Cys, of hOGG1 through small molecule intervention. Free Radic Biol Med, 124, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung RJ, Hall J, Brennan P and Boffetta P (2005) Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol, 162, 925–942. [DOI] [PubMed] [Google Scholar]
- 83.Bravard A, Vacher M, Moritz E, Vaslin L, Hall J, Epe B and Radicella JP (2009) Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity. Cancer Res, 69, 3642–3649. [DOI] [PubMed] [Google Scholar]
- 84.Hill JW and Evans MK (2006) Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase. Nucleic Acids Res, 34, 1620–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kabzinski J, Walczak A, Dziki A, Mik M and Majsterek I (2018) Impact of the Ser326Cys polymorphism of the OGG1 gene on the level of oxidative DNA damage in patients with colorectal cancer. Pol Przegl Chir, 90, 13–15. [DOI] [PubMed] [Google Scholar]
- 86.Alanazi M, Pathan AAK, Shaik JP, Alhadheq A, Khan Z, Khan W, Al Naeem A and Parine NR (2017) The hOGG1 Ser326Cys Gene Polymorphism and Breast Cancer Risk in Saudi Population. Pathol Oncol Res, 23, 525–535. [DOI] [PubMed] [Google Scholar]
- 87.Costa EF, Santos ES, Liutti VT, Leal F, Santos VC, Rinck-Junior JA, Mariano FV, Coutinho-Camillo CM, Altemani A, Lima CS et al. (2016) Association between polymorphisms in genes related to DNA base-excision repair with risk and prognosis of oropharyngeal squamous cell carcinoma. J Cancer Res Clin Oncol, 142, 1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gotoh N, Saitoh T, Takahashi N, Kasamatsu T, Minato Y, Lobna A, Oda T, Hoshino T, Sakura T, Shimizu H et al. (2018) Association between OGG1 S326C CC genotype and elevated relapse risk in acute myeloid leukemia. Int J Hematol, 108, 246–253. [DOI] [PubMed] [Google Scholar]
- 89.Smolarz B, Michalska MM, Samulak D, Wojcik L and Romanowicz H (2018) Studies of Correlations Between Single Nucleotide Polymorphisms of DNA Repair Genes and Endometrial Cancer in Polish Women. Anticancer Res, 38, 5223–5229. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Elahi A, Pow-Sang J, Lazarus P and Park J (2003) Association between polymorphism of human oxoguanine glycosylase 1 and risk of prostate cancer. J Urol, 170, 2471–2474. [DOI] [PubMed] [Google Scholar]
- 91.Xu J, Zheng SL, Turner A, Isaacs SD, Wiley KE, Hawkins GA, Chang BL, Bleecker ER, Walsh PC, Meyers DA et al. (2002) Associations between hOGG1 sequence variants and prostate cancer susceptibility. Cancer Res, 62, 2253–2257. [PubMed] [Google Scholar]
- 92.Le Marchand L, Donlon T, Lum-Jones A, Seifried A and Wilkens LR (2002) Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev, 11, 409–412. [PubMed] [Google Scholar]
- 93.Anderson SM, Naidoo RN, Ramkaran P, Asharam K, Muttoo S and Chuturgoon AA (2018) OGG1 Ser326Cys polymorphism, HIV, obesity and air pollution exposure influences adverse birth outcome susceptibility, within South African Women. Reprod Toxicol, 79, 8–15. [DOI] [PubMed] [Google Scholar]
- 94.Thameem F, Puppala S, Lehman DM, Stern MP, Blangero J, Abboud HE, Duggirala R and Habib SL (2010) The Ser(326)Cys polymorphism of 8-oxoguanine glycosylase 1 (OGG1) is associated with type 2 diabetes in Mexican Americans. Hum Hered, 70, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanders LH, Paul KC, Howlett EH, Lawal H, Boppana S, Bronstein JM, Ritz B and Greenamyre JT (2017) Editor’s Highlight: Base Excision Repair Variants and Pesticide Exposure Increase Parkinson’s Disease Risk. Toxicol Sci, 158, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sliwinska A, Kwiatkowski D, Czarny P, Toma M, Wigner P, Drzewoski J, Fabianowska-Majewska K, Szemraj J, Maes M, Galecki P et al. (2016) The levels of 7,8-dihydrodeoxyguanosine (8-oxoG) and 8-oxoguanine DNA glycosylase 1 (OGG1) - A potential diagnostic biomarkers of Alzheimer’s disease. J Neurol Sci, 368, 155–159. [DOI] [PubMed] [Google Scholar]
- 97.Liu XC, Guo XH, Chen B, Li ZH and Liu XF (2018) Association between the 8-oxoguanine DNA glycosylase gene Ser326Cys polymorphism and age-related cataract: a systematic review and meta-analysis. Int Ophthalmol, 38, 1451–1457. [DOI] [PubMed] [Google Scholar]
- 98.Wu X, Lai W, Lin H and Liu Y (2017) Association of OGG1 and MTHFR polymorphisms with age-related cataract: A systematic review and meta-analysis. PLoS One, 12, e0172092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia-Rodriguez A, de la Casa M, Serrano M, Gosalvez J and Roy Barcelona R (2018) Impact of polymorphism in DNA repair genes OGG1 and XRCC1 on seminal parameters and human male infertility. Andrologia, 50, e13115. [DOI] [PubMed] [Google Scholar]
- 100.Corella D, Ramirez-Sabio JB, Coltell O, Ortega-Azorin C, Estruch R, Martinez-Gonzalez MA, Salas-Salvado J, Sorli JV, Castaner O, Aros F et al. (2018) Effects of the Ser326Cys Polymorphism in the DNA Repair OGG1 Gene on Cancer, Cardiovascular, and All-Cause Mortality in the PREDIMED Study: Modulation by Diet. J Acad Nutr Diet, 118, 589–605. [DOI] [PubMed] [Google Scholar]
- 101.Synowiec E, Blasiak J, Zaras M, Szaflik J and Szaflik JP (2012) Association between polymorphisms of the DNA base excision repair genes MUTYH and hOGG1 and age-related macular degeneration. Exp Eye Res, 98, 58–66. [DOI] [PubMed] [Google Scholar]
- 102.Daimon M, Oizumi T, Toriyama S, Karasawa S, Jimbu Y, Wada K, Kameda W, Susa S, Muramatsu M, Kubota I et al. (2009) Association of the Ser326Cys polymorphism in the OGG1 gene with type 2 DM. Biochem Biophys Res Commun, 386, 26–29. [DOI] [PubMed] [Google Scholar]
- 103.Sampath H, Vartanian V, Rollins MR, Sakumi K, Nakabeppu Y and Lloyd RS (2012) 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS One, 7, e51697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vartanian V, Tumova J, Dobrzyn P, Dobrzyn A, Nakabeppu Y, Lloyd RS and Sampath H (2017) 8-oxoguanine DNA glycosylase (OGG1) deficiency elicits coordinated changes in lipid and mitochondrial metabolism in muscle. PLoS One, 12, e0181687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X, Li Z, Zhao M, Nie Y, Liu P, Zhu Y and Zhang X (2019) Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torres-Gonzalez M, Gawlowski T, Kocalis H, Scott BT and Dillmann WH (2014) Mitochondrial 8-oxoguanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am J Physiol Cell Physiol, 306, C221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolbanovskiy M, Chowdhury MA, Nadkarni A, Broyde S, Geacintov NE, Scicchitano DA and Shafirovich V (2017) The Nonbulky DNA Lesions Spiroiminodihydantoin and 5-Guanidinohydantoin Significantly Block Human RNA Polymerase II Elongation in Vitro. Biochemistry, 56, 3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakanishi N, Fukuoh A, Kang D, Iwai S and Kuraoka I (2013) Effects of DNA lesions on the transcription reaction of mitochondrial RNA polymerase: implications for bypass RNA synthesis on oxidative DNA lesions. Mutagenesis, 28, 117–123. [DOI] [PubMed] [Google Scholar]
- 109.Lee B and Shao J (2014) Adiponectin and energy homeostasis. Rev Endocr Metab Disord, 15, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee BC and Lee J (2014) Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta, 1842, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vernochet C, Mourier A, Bezy O, Macotela Y, Boucher J, Rardin MJ, An D, Lee KY, Ilkayeva OR, Zingaretti CM et al. (2012) Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab, 16, 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han YH, Buffolo M, Pires KM, Pei S, Scherer PE and Boudina S (2016) Adipocyte-Specific Deletion of Manganese Superoxide Dismutase Protects From Diet-Induced Obesity Through Increased Mitochondrial Uncoupling and Biogenesis. Diabetes, 65, 2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bray AW and Ballinger SW (2017) Mitochondrial DNA mutations and cardiovascular disease. Curr Opin Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL and Bohr VA (2016) Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol, 17, 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL and Bohr VA (2015) DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb Perspect Med, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saki M and Prakash A (2017) DNA damage related crosstalk between the nucleus and mitochondria. Free Radic Biol Med, 107, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunham-Snary KJ, Sandel MW, Sammy MJ, Westbrook DG, Xiao R, McMonigle RJ, Ratcliffe WF, Penn A, Young ME and Ballinger SW (2018) Mitochondrial - nuclear genetic interaction modulates whole body metabolism, adiposity and gene expression in vivo. EBioMedicine, 36, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.