Abstract
Background
People with diabetes manage their condition by monitoring the amount of glucose (a type of sugar) in their blood, typically using a method called self-monitoring of blood glucose. Flash glucose monitoring is another method of assessing glucose levels; it uses a sensor placed under the skin and a separate touchscreen reader device. We conducted a health technology assessment of flash glucose monitoring for people with type 1 or type 2 diabetes, which included an evaluation of effectiveness and safety, the budget impact of publicly funding flash glucose monitoring, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Cochrane risk-of-bias tool for randomized controlled trials and the Cochrane ROBINS-I tool for nonrandomized studies, and we assessed the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search, and we analyzed the net budget impact of publicly funding flash glucose monitoring in Ontario for people with type 1 diabetes and for people with type 2 diabetes requiring intensive insulin therapy who are eligible for coverage under the Ontario Drug Benefit program. To contextualize the potential value of flash glucose monitoring, we spoke with adults with diabetes and parents of children with diabetes.
Results
Six publications met the eligibility criteria for the clinical evidence review. Compared with self-monitoring of blood glucose, people who used flash glucose monitoring spent on average 1 hour more in the target glucose range (95% confidence interval [CI] 0.41–1.59) and 0.37 hours (22 minutes) less in a high glucose range (95% CI −0.69 to −0.05) (GRADE: Moderate). Among adults with well-controlled type 1 diabetes, flash glucose monitoring was more effective than self-monitoring of blood glucose in reducing glucose variability (GRADE: Moderate). Flash glucose monitoring was more effective than self-monitoring of blood glucose in reducing the average time spent in hypoglycemia (−0.47 h [95% CI −0.73 to −0.21]) and the average number of hypoglycemia events (−0.16 [95% CI −0.29 to −0.03]) among adults with type 2 diabetes requiring intensive insulin therapy (GRADE: Moderate). Our certainty in the evidence for the effectiveness of flash glucose monitoring for other clinical outcomes, such as quality of life and severe hypoglycemia events, is low or very low. We identified no studies on flash glucose monitoring that included pregnant people, people with diabetes who did not use insulin, or children younger than 13 years of age.
We identified two studies for the economic evidence review: one cost analysis and one cost–utility analysis. The cost analysis study, conducted from the perspective of United Kingdom's National Health Service, found that flash glucose monitoring reduced costs when self-monitoring of blood glucose was performed 10 times daily but was more expensive when self-monitoring of blood glucose was performed 5.6 times daily. The cost–utility analysis had methodological limitations and was not applicable to the context of Ontario's health care system.
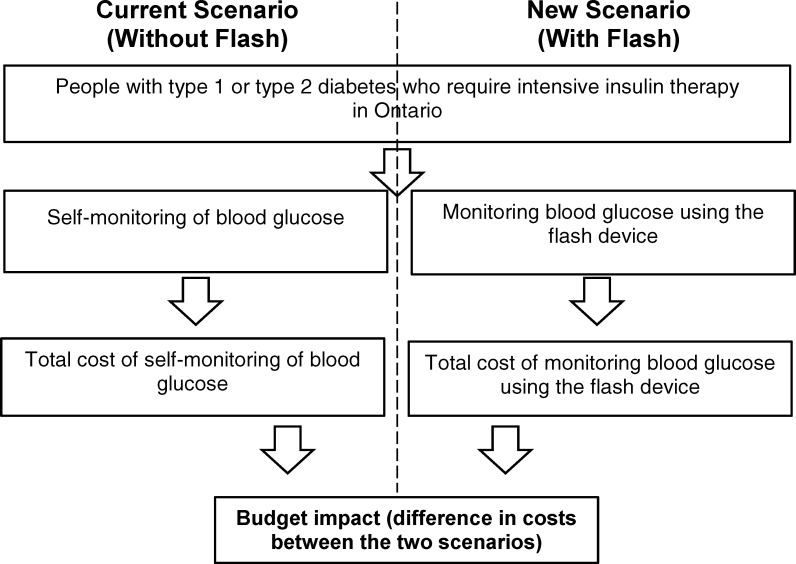
Our 5-year budget impact analysis found that flash glucose monitoring may lead to a net budget increase ranging from $14.6 million ($2.9 million for type 1 diabetes and $11.7 million for type 2 diabetes) in year 1, at an uptake rate of 15%, to $38.6 million ($7.7 million for type 1 diabetes and $30.9 million for type 2 diabetes) in year 5, at an uptake rate of 35%. In this analysis, we assumed that people with type 1 diabetes who self-monitor their blood glucose levels would perform six blood glucose tests daily and that people with type 2 diabetes would perform four blood glucose tests daily. For people switching from self-monitoring of blood glucose using the maximum number of blood glucose test strips for reimbursement (3,000 strips yearly) to flash glucose monitoring, the net budget impact of using flash glucose monitoring is likely to be small.
Adults with diabetes and parents of children with diabetes with whom we spoke reported positively on their experiences with flash glucose monitoring, reporting they believed that flash glucose monitoring helped them control their blood glucose levels, resulting in physical, social, and emotional benefits. The cost of flash glucose monitoring was the largest barrier to its use.
Conclusions
Based on an assessment of several glycemic outcomes, moderate-quality evidence shows that flash glucose monitoring improves diabetes management among adults with well-controlled type 1 diabetes and adults with type 2 diabetes requiring intensive insulin therapy. We estimate that publicly funding flash glucose monitoring in Ontario for people with type 1 diabetes and for people with type 2 diabetes requiring intensive insulin therapy who are eligible for coverage under the Ontario Drug Benefit program would result in additional costs of between $14.6 million and $38.6 million annually over the next 5 years. Adults with diabetes and parents of children with diabetes with whom we spoke reported that flash glucose monitoring helped them or their children control their blood glucose levels, resulting in physical, social, and emotional benefits.
BACKGROUND
Objective
This health technology assessment evaluates the effectiveness and safety of flash glucose monitoring for people with type 1 or type 2 diabetes. It also evaluates the budget impact of publicly funding flash glucose monitoring and the experiences, preferences, and values of people with type 1 or type 2 diabetes.
Health Condition
Diabetes is a group of metabolic disorders in which the body cannot produce insulin, cannot produce sufficient insulin, or cannot properly use the insulin it produces.1 The three principal types of diabetes are type 1 diabetes, type 2 diabetes, and gestational diabetes. Type 1 diabetes develops when the immune system mistakenly attacks and kills special cells in the pancreas (beta cells) that are responsible for releasing insulin, a hormone that causes cells to take in glucose (a type of sugar) and use it as energy or store it as fat.1 Type 2 diabetes occurs when cells fail to respond to insulin properly (called insulin insensitivity)1 or beta cells are unable to produce sufficient insulin (this primarily affects those with family history of diabetes).2 Gestational diabetes develops during pregnancy.3 Major risk factors for type 2 diabetes include being overweight, older age, and socio-economic status.4 Type 1 diabetes usually develops in childhood or adolescence but can also start in adulthood.4
Clinical Need and Target Population
In 2014, there were about 422 million diabetes cases worldwide,5 of which 90% were type 2.6 The prevalence of diabetes in Canada was estimated at 9.3% in 2015 and is projected to reach 12.1% in 2025.7 In 2016, the prevalence in Ontario was estimated at 10.5%.8 People with diabetes have an increased risk of being hospitalized with cardiovascular disease, being hospitalized with end-stage kidney disease, and requiring nontraumatic lower limb amputation compared with the general population.4 The risk of blindness in people with diabetes is up to 25 times higher than in those without diabetes.4 There is a disproportionate prevalence of type 2 diabetes among Indigenous peoples.9 Other groups of people who are at higher risk of developing diabetes include people of South Asian, Chinese, and African descent.10
Conventional Method of Glucose Monitoring
Regular testing of blood glucose is critical to effectively manage type 1 diabetes and type 2 diabetes requiring intensive insulin therapy (i.e., multiple daily insulin injections or a continuous subcutaneous insulin infusion) to keep their blood glucose levels in the target range.
Traditionally, people with diabetes have monitored their glucose levels using finger-prick meters. This method was introduced in the 1970s,11 is commonly known as self-monitoring of blood glucose, and is currently the standard method for monitoring blood glucose in Ontario. Diabetes Canada12 recommends that blood glucose be kept below a level of glycated hemoglobin (abbreviated as “A1C”; this is a form of hemoglobin to which glucose is bound) of 7% for effective diabetes management. For people whose glucose levels are not well controlled (A1C > 7%) and who require insulin, self-monitoring of blood glucose is required throughout the day, with measurements taken before meals, after meals, before and after physical activity, before driving, and during the night.13,14 Periodic self-monitoring of blood glucose is needed for some adults with type 2 diabetes who use oral anti-diabetes drugs.15 Self-monitoring of blood glucose has drawbacks, including the pain of finger prick (usually done four to six times a day when using insulin) and less comprehensive glycemic data.16
To overcome these limitations, sensor-based glucose monitoring systems were introduced in 1999 and have since continued to evolve.11,17 Sensor-based systems measure glucose levels in the interstitial fluid (fluid found in the spaces between cells), rather than in the blood, every few minute and are either calibrated at home (by a person with diabetes or a caregiver) or pre-calibrated by the device manufacturer to measure glucose levels in whole blood. For home-calibrated systems, about two finger-prick measurements per day are required. This requirement is generally not applicable to factory-calibrated systems, although occasional finger-prick measurements are necessary in certain cases (see subsequent sections for details). There are two major classes of sensor-based system: continuous glucose monitoring18 and flash glucose monitoring (introduced in 2014).11 Although both types of system measure glucose levels every few minutes, continuous glucose monitoring devices display results on a continuous basis, whereas flash glucose monitoring devices show results when prompted by the user. This review focuses on the flash glucose monitoring system.
Health Technology Under Review
A flash glucose monitoring system consists of a sensor inserted subcutaneously on a person's upper arm and a separate touchscreen reader device.19 The sensor's working electrode is coated with an enzyme (glucose oxidase) and mediator molecules (an osmium complex), which interact with glucose in the interstitial fluid to free electrons from the glucose molecules and direct them to the sensor's electrode. The freed electrons generate an electric current the magnitude of which is proportional to the concentration of glucose molecules in the interstitial fluid. When the reader is scanned on the sensor, the sensor transmits information (coded in the form of radio waves) about the instantaneous glucose level and a graph of the most recent 8-hour trend to the reader. This allows users to obtain current blood glucose readings and trend information.19 The previous flash glucose monitoring system does not have hypoglycemia or hyperglycemia alarms.19 The new version has an option for hypoglycemia and hyperglycemia alarms and a feature that notifies the user of signal loss (i.e., when the sensor is not communicating with the reader). Both versions of the flash glucose monitoring system display 8-hour trend data graphically only when the reader is scanned.
The Abbott FreeStyle Libre is the only brand of flash glucose monitoring device on the market designed for nonhospital use. Abbott also manufactures the FreeStyle Libre Pro for professional (i.e., health care provider) use, but this device it is out of scope for this health technology assessment. Flash glucose monitoring does not require a finger prick for calibration, but occasional finger pricks might be needed during times of rapidly changing glucose levels, if symptoms do not match the device reading, or to confirm hypoglycemia or impending hypoglycemia.19,20 The sensor is worn for 14 days before being replaced, and the reader can store data for up to 90 days. People with diabetes or their caregivers can insert these sensors on the forearm by following instructions provided in a manual.
Regulatory Information
The FreeStyle Libre system is licensed by Health Canada as a Class III device (licence number 99351)21 for adults aged 18 years and older who have at least 2 years of experience of self-managing their diabetes. A class 3 device is defined by Health Canada as a non-invasive device intended for modifying the biological or chemical composition of blood or other body fluids, or liquids, for the purpose of introduction into the body by means of infusion or other means of administration.22
Ontario Context
In Ontario, the FreeStyle Libre system is not currently publicly funded. The cost may be covered through private insurance23,24; otherwise, people must pay out of pocket for the device. Financial support for other blood glucose monitors is available for people who qualify for the Ontario Disability Support Program and the Mandatory Special Necessities benefit.25 Applicants must be at least 18 years of age, be an Ontario resident, be in financial need, and meet the program's definition of a person with a disability, or be a member of a prescribed class.26 (Prescribed classes are specific categories of people who do not have to go through the disability adjudication process to qualify for Ontario Disability Support Program income support.26) Currently, the yearly maximum number of blood glucose test strips reimbursed by the Ministry of Health and Long-Term Care (through the Ontario Drug Branch) per person is 3,000 for those managing diabetes with insulin, 400 for those managing diabetes with non-insulin anti-diabetes medication with a high risk of causing hypoglycemia, 200 for those managing diabetes using non-insulin anti-diabetes medication with a low risk of causing hypoglycemia, and 200 for those managing diabetes through diet and lifestyle only (without insulin or anti-diabetes medications).27
Canadian and International Context
The flash glucose monitoring system is not currently publicly funded anywhere in Canada. According to Abbott,28 most private insurers are reimbursing the cost of FreeStyle Libre under their formulary system. In the United States, the cost for FreeStyle Libre is covered under Medicare for beneficiaries with diabetes who have intensive insulin regimens.29 In the United Kingdom, the cost for FreeStyle Libre is mainly paid out of pocket, although qualified patients are covered by the National Health Service.20 The qualifications include type 1 diabetes, age of at least 4 years, and receiving specialist care for type 1 diabetes using multiple daily injections or insulin pump therapy. In Japan, the FreeStyle Libre has been approved only to supplement self-monitoring of blood glucose.30 In Japan, it is contraindicated for use among pregnant people, people receiving dialysis, and children younger than 6 years of age.30
Expert Consultation
We engaged with experts in the specialty area of endocrinology to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42018098975), available at https://www.crd.york.ac.uk/PROSPERO.
CLINICAL EVIDENCE
Research Questions
What is the effectiveness of flash glucose monitoring compared with self-monitoring of blood glucose in managing blood glucose levels among people with type 1 or type 2 diabetes?
Is flash glucose monitoring associated with more adverse events than self-monitoring of blood glucose?
Methods
We developed the research questions in consultation with patients, health care providers, clinical experts, and other health system stakeholders, including the Canadian Agency for Drugs and Technologies in Health.
Clinical Literature Search
We performed a clinical literature search on April 6, 2018, to retrieve studies published from January 1, 2014, until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategy using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.31
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
We included:
English-language full-text publications
Studies published between January 1, 2014 (the year the Freestyle Libre system was introduced and the first two studies of the system were launched),32,33 and April 6, 2018
Randomized controlled trials, observational cohort studies (before-after or parallel groups designs)
We excluded:
Animal and in vitro studies
Editorials, commentaries, case reports, conference abstracts, and letters
Studies focusing exclusively on device accuracy, such as error grid analyses
Participants
We included studies that recruited people of any age diagnosed with type 1 or type 2 diabetes
Interventions
We included studies that evaluated flash glucose monitoring devices designed for use by patients compared with self-monitoring of blood glucose. We excluded studies that either evaluated flash glucose monitoring devices designed for use by health care providers or compared flash glucose monitoring devices with continuous glucose monitoring devices.
Outcome Measures
We included studies reporting the following outcomes:
Time spent in the target glucose range (3.9–10.0 mmol/L). This range conforms to the consensus report developed by several diabetes organizations.34 It is often reported in clinical studies but is not systematically incorporated into the clinical practice guidelines35
Time spent in hypoglycemia (< 3.9 mmol/L)
Hypoglycemia events (< 3.9 mmol/L)
Quality of life, as measured using the following tools: Pediatric Quality of Life Inventory (PedsQL), Hypoglycemia Fear Survey (HFS), Diabetes Distress Scale (DDS), Diabetes Quality of Life (DQoL), and World Health Organization Five Well-Being Index (WHO-5)
Glucose variability, as measured using the following scales: Mean Amplitude of Glycemic Excursions (MAGE), Coefficient of Variation (CV), Blood Glucose Risk Index (BGRI), Low Glucose Risk Index (LGRI), standard deviation (SD), and Continuous Overall Net Glycemic Action (CONGA)
Glycated hemoglobin (A1C) values
Severe hypoglycemic events (hypoglycemia that requires assistance from another person to treat)
Device-related adverse events
Literature Screening
Three reviewers (two clinical epidemiologists and one clinical epidemiology student) independently conducted an initial screening of titles and abstracts using DistillerSR36 and then obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. The reviewers then examined the full-text articles independently and selected studies that were eligible for inclusion. Any disagreement among reviewers was resolved through a consensus-based discussion. A study was selected if at least two reviewers reached consensus.
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information about the following:
Source (e.g., citation information, contact details, study type)
Characteristics of patients, intervention, and comparator
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, and reporting of outcomes)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, and time points at which the outcome was assessed)
Statistical Analysis
We did not conduct meta-analyses because population characteristics varied across the included studies. Wherever possible, we reported effect sizes, along with 95% confidence intervals, and computed these values if they were unreported. To account for any potential inaccuracy in flash readings, we performed quantitative bias analysis37 (Appendix 3) and used the findings to fill the risk-of-bias tables where appropriate (Appendix 4). We used R, version 3.5.0,38 for our analysis.
Critical Appraisal of Evidence
We assessed risk of bias using the Cochrane risk-of-bias tool for randomized controlled trials and the Cochrane ROBINS-I tool39 for nonrandomized studies (Appendix 4).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.40 The body of evidence was assessed on the basis of the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality score reflects our assessment of the certainty of the evidence.
Our interpretation of results from the included studies took into consideration the recommended cut-off points for the clinically meaningful effects,41–44 National Institute for Health and Care Excellence (NICE) criteria for funding continuous glucose monitoring,45 NHS criteria for funding Freestyle Libre,46 and suggestions from clinical experts. Studies were determined imprecise if the confidence interval crossed the null value and at least one of the confidence limits exceeded the minimum recommended clinically meaningful effect.
Results
Literature Search
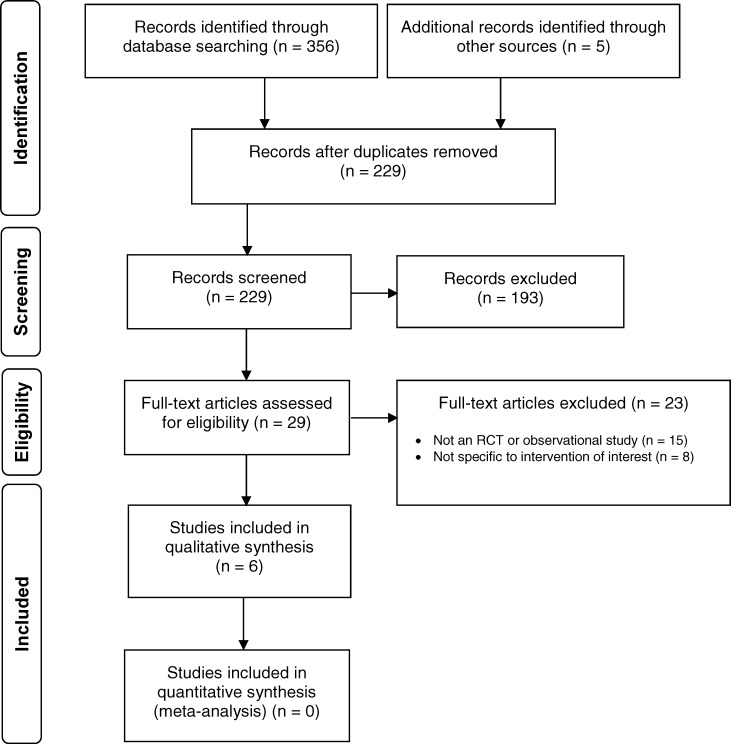
The literature search yielded 229 citations published between January 1, 2014, and April 6, 2018, after removing duplicates. Five studies32,33,47–49 (two randomized controlled trials, of which one also published a separate subgroup analysis,49 and two observational studies) met the inclusion criteria. One additional observational study50 was identified from other sources and added for a total of six eligible studies.
Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search. Table 1 describes the characteristics of the included studies.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.51
Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT, randomized controlled study.
Table 1:
Summary of Included Studies
| Author, Year, Country | Study Design | Inclusion Criteria | No. of Observations | ||||
|---|---|---|---|---|---|---|---|
| Length of Follow-Up | Age | Diabetes Diagnosis | Baseline Glucose | Other Criteria | |||
| Bolinder et al, 201632,a | RCT | 6 mo | ≥ 18 y | Type 1, > 5 y | A1C < 7.5% | Not hypoglycemia unaware Not having diabetic ketoacidosis or MI in the last 6 months No known allergy to medical-grade adhesives Not using CGM within the last 4 months Not currently using sensor-augmented insulin pumps Not pregnant, nor planning to become pregnant Not receiving oral steroid therapy |
120/121 (flash/SMBG) |
| Haak et al, 201652,53,b | RCT | 6 mo | > 18 y | Type 2 | A1C 7.5%-12.0% | Not pregnant Using insulin for ≥ 6 months (prandial only or prandial and basal intensive insulin therapy or insulin pump therapy) Not having a total daily dose of insulin ≥ 1.75 U/kg at study entry Not having severe hypoglycemia Not having diabetic ketoacidosis Not having hyperosmolar-hyperglycemic state in the last 6 months No known allergy to medical-grade adhesives Not using CGM within the last 4 months Not receiving steroid therapy |
149/75 (flash/SMBG) |
| Mitsuishi et al, 2017,48 Japan | Observational cohort (before-after) | Not reported | 18–80 y | Type 1 and type 2 | Mean A1C 7.8% | Not pregnant, nor likely to become pregnant Not receiving dialysis Not allergic to medical adhesives Not using insulin pumps equipped with CGM |
80/80 (SMBG/flash) |
| Oskarsson et al, 201749,a | RCT, subgroup analysis | 6 mo | > 18 y | Type 1 | A1C < 7.5% | Not hypoglycemia unaware No diabetic ketoacidosis or MI in the last 6 months No known allergy to medical-grade adhesives Not using CGM within the last 4 months Not receiving steroid therapy |
82/81 (flash/SMBG) |
| Al Hayek et al, 2017,47 Saudi Arabia | Observational cohort (before–after) | 3 mo for flash but not reported for SMBG | 13–19 y | Type 1 | Not reported | Not diagnosed with dermatological disorders within the last 6 months No severe or unstable medical conditions No severe hypoglycemia that requires third-party assistance No diabetic ketoacidosis, nor hyperosmolar-hyperglycemic state |
47/47 (SMBG/flash) |
| Moreno-Fernandez et al, 2018,50 Spain | Observational cohort (parallel groups) | 6 mo | 18–65 y | Type 1 | A1C ≤ 7.8% | Diagnosed with type 1 diabetes for at least 6 months Not pregnant or planning pregnancy Not breastfeeding Naïve to flash glucose monitoring |
18/18 (SMBG/flash) |
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; MI, myocardial infarction; RCT, randomized controlled trial; SMBG, self-monitoring of blood glucose.
Involves diabetes centres from Sweden, Austria, Germany, Spain, and The Netherlands.
Involves diabetes centres from France, Germany, and the United Kingdom.
Type 1 Diabetes
For type 1 diabetes, we identified three main studies, a randomized controlled trial by Bolinder et al32 and two observational studies by Al Hayek et al47 and Moreno-Fernandez et al.50 We also identified a substudy by Oskarsson et al,49 which restricted the analysis in Bolinder et al32 to people using multiple daily injections of insulin. Bolinder et al recruited adults 18 years of age and older who had well-controlled glucose levels (A1C < 7.5%), whereas Al Hayek et al47 and Moreno-Fernandez et al50 recruited patients of 13 to 19 years of age and 18 to 65 years of age, respectively (Table 1).
In addition to the overall analysis, AI Hayek et al47 also reported the subgroup analysis by multiple daily injections of insulin and continuous subcutaneous insulin infusion.
Details on the outcomes assessed are given below.
Time in Target Glucose Range
Bolinder et al32 reported a statistically significant increase in time spent in the target glucose range (3.9–10.0 mmol/L) for flash glucose monitoring compared with self-monitoring of blood glucose (Table 2). When Oskarsson et al49 restricted the analysis to people using multiple daily injections of insulin, the authors' conclusion remained unchanged (Table 3). We rated the certainty of evidence for this outcome as moderate (Table A3), downgrading for indirectness because we are uncertain whether this increase translates to an improvement in clinical outcomes.
Table 2:
Time Within, Below, and Above Target Glucose Range, Hypoglycemia Events, Glucose Variability for Type 1 Diabetes
| Group Mean | Difference Between Flash and SMBG, % Change | Difference in Adjusted Means Between Flash and SMBG (95% CI)a | P Value | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Hours in target glucose range (3.9–10.0 mmol/L) within 24 hours | ||||||
| 15.0 | 15.8 | 14.8 | 14.6 | NA | 1.0 (0.41 to 1.59) | .0006 |
| Hours above target glucose range (> 13.3 mmol/L) within 24 hours | ||||||
| 1.84 | 1.67 | 1.91 | 2.06 | −19.1% | −0.37 (−0.69 to −0.05) | .0247 |
| Hours in hypoglycemia (< 3.9 mmol/L) within 24 hours | ||||||
| 3.38 | 2.03 | 3.44 | 3.27 | −38.0% | −1.24 (−1.71 to −0.77) | < .0001 |
| Hours in hypoglycemia at night (11 pm-6 am) within 7 hours | ||||||
| 1.32 | 0.68 | 1.48 | 1.23 | −39.8% | −0.47 (−0.70 to −0.24) | < .0001 |
| Mean no. of hypoglycemia events < 3.9 mmol/L (70 mg/dL) within 24 hours | ||||||
| 1.81 | 1.32 | 1.67 | 1.69 | −25.8% | −0.45 (−0.62 to −0.28) | < .0001 |
| Mean amplitude of glucose excursionb | ||||||
| 142 | 132 | 144 | 141 | NA | −8.0 (−13.88 to −2.12) | .0004 |
| Blood glucose risk index, mg/dLb | ||||||
| 8.2 | 7.3 | 8.3 | 8.4 | NA | −0.90 (−1.41 to −0.39) | .0004 |
| Coefficient of variation in glucose, %b | ||||||
| 43.0 | 37.6 | 42.5 | 41.8 | NA | −4.4 (−5.62 to −3.18) | < .0001 |
| Low blood glucose risk indexb | ||||||
| 2.7 | 1.8 | 2.7 | 2.6 | NA | −0.8 (−1.11 to −0.49) | < .0001 |
| Standard deviation of glucose, mg/dLb | ||||||
| 60.6 | 55.0 | 60.1 | 59.7 | NA | −5.0 (−7.27 to −2.73) | < .0001 |
| Continuous overlapping net glycemic action, 2 hours, mg/dLb | ||||||
| 56 | 49 | 56 | 58 | NA | −9 (−11.55 to −6.45) | < .0001 |
| Continuous overlapping net glycemic action, 6 hours, mg/dLb | ||||||
| 71 | 61 | 69 | 72 | NA | −12 (−18.66 to −5.34) | .0004 |
Abbreviations: CI, confidence interval; NA, not applicable; SMBG, self-monitoring of blood glucose.
Confidence intervals were computed by the authors of this health technology assessment using standard errors reported in the primary study.
A scale for measuring glucose variability.
Source: Bolinder et al.32
Table 3:
Subgroup Analyses of Time Spent in Glucose Range and Hypoglycemia Among People with Type 1 Diabetes who Use Multiple Daily Injections
| Group Mean | Difference Between Flash and SMBG (% Change) | Difference in Adjusted Means Between Flash and SMBG (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Hours in target glucose range (3.9–10.0 mmol/L) within 24 hours | ||||||
| 15.0 | 15.7 | 14.3 | 14.3 | 6.5% | 0.9 (0.2 to 1.7) | .011 |
| Hours in hypoglycemia (< 3.9 mmol/L) within 24 hours | ||||||
| 3.44 | 1.86 | 3.73 | 3.66 | −46.0% | −1.65 (−2.21 to −1.09) | < .0001 |
| Hours in hypoglycemia at night (11 pm-6 am) within 7 hours | ||||||
| 1.20 | 0.61 | 1.41 | 1.28 | −46.6% | −0.57 (−0.81 to −0.34) | < .0001 |
| Mean hypoglycemia events < 3.9 mmol/L (70 mg/dL) within 24 hours | ||||||
| 1.80 | 1.23 | 1.72 | 1.78 | −32.8% | −0.59 (−0.78 to −0.40) | < .0001 |
| Hours above target glucose range (> 13.3 mmol/L) within 24 hours | ||||||
| 1.77 | 1.78 | 2.05 | 2.10 | −9.2% | −0.19 (−0.58 to 0.21) | .36 |
| Mean amplitude of glucose excursiona | ||||||
| 7.9 | 7.5 | 8.2 | 8.0 | −3.9% | −0.31 (−0.72 to 0.11) | .14 |
| Blood glucose risk indexa | ||||||
| 8.1 | 7.4 | 8.7 | 8.6 | −9.4% | −0.8 (−1.4 to −0.1) | .017 |
| Coefficient of variation in glucose, %a | ||||||
| 43.2 | 37.8 | 43.4 | 42.6 | −11.1% | −4.7 (−6.2 to −3.2) | < .0001 |
| Low blood glucose risk indexa | ||||||
| 2.70 | 1.61 | 2.87 | 2.77 | −39.3% | −1.07 (−1.42 to −0.72) | < .0001 |
| Standard deviation of glucose, (mmol/L)a | ||||||
| 3.36 | 3.10 | 3.41 | 3.36 | −6.9% | −0.23 (−0.39 to −0.07) | .0051 |
| Continuous overlapping net glycemic action, 2 hours, mg/dLa | ||||||
| 3.2 | 2.8 | 3.2 | 3.3 | −14.8% | −0.48 (−0.66 to −0.30) | < .0001 |
| Continuous overlapping net glycemic action, 6 hours, mg/dLa | ||||||
| 4.0 | 3.7 | 4.0 | 4.1 | −9.7% | −0.39 (−0.85 to 0.06) | .089 |
Abbreviations: CI, confidence interval; SMBG, self-monitoring of blood glucose.
A scale for measuring glucose variability
Source: Oskarsson et al.49
Time Above Target Glucose Range
Bolinder et al32 reported a statistically significant decrease in time spent above the target glucose range (> 13.3 mmol/L) for flash glucose monitoring compared with self-monitoring of blood glucose (Table 2). We rated the certainty of evidence for this outcome as moderate (Table A3) downgrading for indirectness, because, although evidence54 on how acute hyperglycemic spikes affect onset of diabetes complications has been published, we are unsure whether the reported increase is large enough to affect clinical outcomes.
Time in Hypoglycemia
Bolinder et al32 reported a statistically significant reduction in the time spent in hypoglycemia (< 3.9 mmol/L) with flash glucose monitoring compared with self-monitoring of blood glucose (Table 2). We downgraded the certainty of evidence for this outcome to moderate (Table A3) for risk of bias for the following reasons. First, although recurrent hypoglycemia can impair awareness,55 and any single episode of severe hypoglycemia can be dangerous,56 we could not determine from the study how low the glucose level dropped after crossing the hypoglycemia threshold. Second, the authors reported imputing missing values for this outcome by carrying forward the last observation, potentially introducing bias.
Hypoglycemia Events
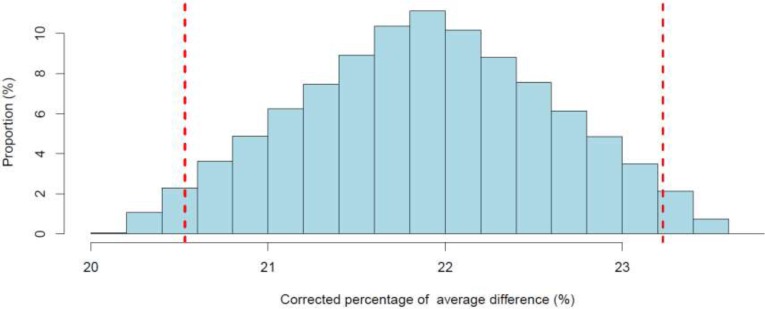
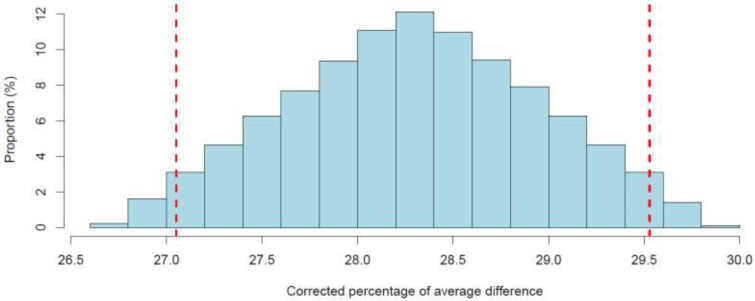
Bolinder et al32 reported a statistically significant decrease in the daily mean number of hypoglycemia events (< 3.9 mmol/L) for flash glucose monitoring compared with self-monitoring of blood glucose (Table 2). We noticed that the observed percentage reduction in hypoglycemia (25.5%) was slightly lower than the minimum intended by the authors (30%). However, when Oskarsson et al49 performed a subgroup analysis, they observed a larger percentage reduction (46%) among people using multiple daily injections of insulin (Table 3). After performing the quantitative bias analysis to account for plausible flash reading errors (Appendix 2), we noted that the reduction in the point estimates remained above 20% (Figure A1), implying that reading errors were too small to entirely explain the observed reduction. Given uncertainty about the importance of this reduction (since the observed reduction is slightly lower than the minimum threshold set by the authors), we downgraded the certainty of evidence for this outcome to moderate (Table A3).
Glucose Variability
Bolinder et al32 reported a statistically significant improvement in glucose variability for flash glucose monitoring compared with self-monitoring of blood glucose (Table 2) on all seven scales of variability used in the study. Although none of the scales is considered the gold standard for glucose variability,57 results were consistent across scales. We were unable to determine the clinically important cut-off points for other scales of glucose variability. Given the uncertainty in the interpretation of scales for glucose variability, owing to indirectness, we downgraded the certainty of evidence for this outcome to moderate (Table A3).
Quality of Life
Al Hayek et al47 reported a statistically significant improvement in the quality of life for people using flash glucose monitoring compared with self-monitoring of blood glucose (Table 4), which exceeded the recommended minimum clinically important difference (i.e., 1 unit in the PEDsQL scale).42 The effect persisted among people using multiple daily injections of insulin, but diminished and was imprecise among people using continuous subcutaneous insulin infusion (Table 5). Bolinder et al32 reported a statistically nonsignificant difference in the quality of life between flash glucose monitoring and self-monitoring of blood glucose, but reported results of only per-protocol analysis (mean difference in the DQoL scale was −0.08, 95% CI −0.16 to 0.00; P = .0524). The observed difference was also much lower than the minimum recommended clinically important difference (i.e., 3–4 units in the DQoL scale).58 Mitsuishi et al48 reported a statistically significant increase in quality for life for flash glucose monitoring compared with self-monitoring of blood glucose (mean difference in the WHO-5 scale was 2.1, 95% CI 0.45–3.75; P < .0001), but the results fell well short of the recommended cut-off point for clinically important difference (i.e., 10 units in the WHO-5 scale).41 Based on several limitations including inconsistency of results and potential reporting bias, we rated the certainty of evidence for this outcome as very low (Table A3).
Table 4:
Behaviour, Fear of Hypoglycemia, and Quality of Life
| Variable | Baseline (After Using SMBG) | 3 Months After Using Flash | Difference (95% CI)a | P Valuea |
|---|---|---|---|---|
| Behaviourb | 1.91 | 2.1 | 0.19 (0.11–0.27) |
.0001 |
| Worry (fear of hypoglycemia)b | 1.95 | 1.81 | −0.06 (−0.09 to −0.03) |
.0001 |
| Quality of lifec | 45.9 | 49.3 | 3.4 (1.31–5.49) |
.0020 |
Abbreviations: CI, confidence interval; SMBG, self-monitoring of blood glucose.
Confidence intervals and P values were computed by the authors of this health technology assessment using information reported in the primary studies.
Assessed through the Hypoglycemia Fear Survey–Child subscale. Lower scores are better.
Assessed through the PedsQL DM Questionnaire, version 3.0. Higher scores are better.
Source: Al Hayek et al.47
Table 5:
Subgroup Analyses of Behaviour, Fear of Hypoglycemia, and Quality of Life for People With Type 1 Diabetes Treated With Multiple Daily Injections and Insulin Pump Therapy
| Variable | Type 1 Diabetes Treated With MD | Type 1 Diabetes Treated With Insulin Pump | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Using SMBG | 3 Mo After Using Flash | Difference (95% CI)a | P Valuea | Baseline Using SMBG | 3 Mo After Using Flash | Difference (95% CI)a | P Valuea | |
| Behaviour | 1.97 | 2.1 | 0.13 (0.07 to 0.19) |
.0001 | 1.8 | 2.09 | 0.29 (0.16 to 0.42) |
.0001 |
| Worry (fear of hypoglycemia) | 2.02 | 1.8 | −0.22 (−0.32 to −0.12) |
.0001 | 1.88 | 1.75 | −0.13 (−0.19 to −0.07) |
.0001 |
| Quality of life | 44.7 | 50.6 | 5.9 (2.20 to 9.60) |
.0030 | 46.8 | 48.2 | 1.4 (−1.44 to 4.25) |
.3342 |
Abbreviations: CI, confidence intervals; MDI, multiple daily injections of insulin; SMBG, self-monitoring of blood glucose.
Confidence intervals and P values were computed by the authors of this health technology assessment using information reported in the primary studies.
Source: Al Hayek et al.47
Fear of Hypoglycemia
Al Hayek et al47 reported a statistically significant reduction in the fear of hypoglycemia when patients switched from self-monitoring of blood glucose to flash glucose monitoring (Table 4). However, the reduction was below the recommended threshold for clinical significance (i.e., 3.4–3.6 in the current version of HFS). Bolinder et al32 did not find a difference in the fear of hypoglycemia between flash glucose monitoring and self-monitoring of blood glucose (mean difference in the HFS scale 0.0, 95% CI −1.41 to 1.41). Results remained similar when Oskarsson et al49 analyzed results from people using multiple daily injections of insulin. We rated the certainty of evidence (that there is no effect) as moderate (Table A3), noting that Bolinder et al32 excluded people who would be most likely to experience the fear of hypoglycemia (e.g., those with hypoglycemia unawareness), making it unlikely to observe an effect.
Severe Hypoglycemia Events
Moreno-Fernandez et al50 found no difference in the change from baseline in the mean number of severe hypoglycemia events between flash glucose monitoring and self-monitoring of blood glucose (mean difference 0.0, 95% CI −0.01 to 0.01; P = 1.00). However, we noted that the number of events in either group was too small to draw any conclusion. Bolinder et al32 reported two events of severe hypoglycemia in the flash group and four events in the self-monitoring group, but the number of events again was too small to draw any conclusion (risk difference −0.02, 95% exact CI −0.07 to 0.04). Because of sample size limitations, we rated down the certainty of evidence for imprecision to very low (Table A3).
Glycated Hemoglobin Levels
Bolinder et al32 did not find a difference in A1C levels between flash glucose monitoring and self-monitoring of blood glucose after 6 months of follow-up (Table 6). However, as the authors pointed out, A1C levels were well controlled at baseline. Thus, the observed reduction in hypoglycemia in the flash group accompanied by no change in A1C level suggests that the hypoglycemia reduction did not alter the already impressive A1C levels. The results were not substantially altered when Oskarsson et al49 restricted the data in Bolinder et al32 to people using multiple daily injections of insulin (Table 7).
Table 6:
Glycated Hemoglobin Levels for Type 1 Diabetes Cases
| Author, Year | Mean A1C (%) | End-of-Study Means Difference Between Flash and SMBG (95% CI)a | P Value | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Bolinder et al, 201632 | 6.79 | 6.94 | 6.78 | 6.95 | 0.0 (−0.12 to 0.12)b | .9556 |
| Al Hayek et al, 201747 | NA | 7.84 | NA | 8.5 | −0.66 (−1.14 to −0.18) | .008 |
Abbreviations: A1C, glycated hemoglobin; CI, confidence interval; NA, not applicable; SMBG, self-monitoring of blood glucose.
Confidence intervals were computed by the authors of this health technology assessment using information reported in the primary studies.
Adjusted for baseline A1C, centre, and mode of insulin intake.
Table 7:
Subgroup Analyses of Glycated Hemoglobin Levels for Type 1 Diabetes Cases
| Author, Year | Mean A1C (%) | End-of-Study Mean Difference Between Flash and SMBG (95% CI) | P Valuea | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Multiple daily in injections of insulin | ||||||
| Oskarsson et al, 201749 | 6.80 | 7.00 | 6.71 | 6.91 | 0.02 (−0.13 to 0.18)a,b |
.77 |
| Al Hayek et al, 201747 | NA | 7.4 | NA | 8.69 | −1.29 (−2.30 to −0.28) | .014 |
| Continuous subcutaneous insulin infusion | ||||||
| Al Hayek et al, 201747 | NA | 8.15 | NA | 8.33 | −0.18 (−1.12 to 0.76) | .7075 |
Abbreviations: A1C, glycated hemoglobin; CI, confidence interval; NA, not applicable; SMBG self-monitoring of blood glucose.
Confidence intervals or P values were computed by the authors of this health technology assessment using information reported in the primary studies.
Adjusted for baseline A1C, study centre, and mode of insulin intake.
Al Hayek et al47 reported a statistically significant decrease in A1C for flash glucose monitoring compared with self-monitoring of blood glucose (Table 6), which exceeded the recommended threshold of 0.5%34 for a minimum clinically important difference. The effect persisted when confined to people using multiple daily injections of insulin but was small and imprecise among those using continuous subcutaneous insulin infusion (Table 7). Moreno-Fernandez et al50 reported a statistically nonsignificant decrease from baseline in A1C for flash glucose monitoring but not for self-monitoring of blood glucose (difference in mean change from baseline −0.50, 95% CI −1.05 to 0.05; P = .07). We noted that the results were imprecise with the confidence interval covering the null (zero) value, as well as values that are in favour of both flash glucose monitoring and self-monitoring of blood glucose, by a magnitude that exceeds the minimum recommended clinically important difference.34
Because of inconsistency in results across studies and differing interpretations of A1C results, we rated the certainty of evidence as very low (Table A3).
Device-Related Adverse Events
Thirteen device-related adverse events were reported in Bolinder et al32 (a subset of these was also reported in Oskarsson et al49). These were allergy, itching, rash, insertion-site symptom, and edema. None of the adverse events contributed to severe hypoglycemia or hospitalization. No adverse events were reported related to self-monitoring of blood glucose. We were unable to perform a comparative safety assessment because of sparse events. Given this limitation we rated the certainty of the evidence for this outcome as very low (Table A3).
Type 2 Diabetes
We identified one randomized controlled trial (Haak et al, 2017)33 and one observational study (Mitsuishi et al, 2018)48 that compared the effectiveness of glucose monitoring to that of self-monitoring of blood glucose in managing type 2 diabetes. Haak et al33 enrolled adults aged 18 years and older who had A1C levels in the range of 7.5% to 12% and were using intensive insulin therapy. The authors reported both overall results and a subgroup analysis by age. Mitsuishi et al48 enrolled adults 18 years of age and older who were using insulin treatment. The following outcomes were reported.
Time in Target Glucose Range
Haak et al33 reported a statistically nonsignificant increase in the time spent in the target glucose range for flash glucose monitoring compared with self-monitoring of blood glucose, but the results appear imprecise (Table 8). Results were even more imprecise when the analysis was partitioned by age group (Table 9). Because of imprecision, we rated the certainty of evidence for this outcome as low (Table A4).
Table 8:
Time in Target Glucose Range and Hypoglycemia and Glucose Variability for People With Type 2 Diabetes
| Group Means | Difference Between Flash and SMBG (% Change) | Difference in End-of-Study Adjusted Means Between Flash and SMBG (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Hours in target glucose range (3.9–10.0 mmol/L) within 24 hours | ||||||
| 13.9 | 13.6 | 13.5 | 13.2 | 1.1% | 0.2 (−0.94, 1.34) | .7925 |
| Hours above target glucose range (> 13.3 mmol/L) within 24 hours | ||||||
| 3.1 | 3.5 | 3.9 | 3.9 | 2.1% | 0.1 (−0.80 to 1.00) | .8729 |
| Hours in hypoglycemia (< 3.9 mmol/L) within 24 hours | ||||||
| 1.3 | 0.59 | 3.44 | 3.27 | −38.0% | −1.24 (−1.71 to −0.77) | < .0001 |
| Hours in hypoglycemia at night (11 pm-6 am) within 7 hours | ||||||
| 0.55 | 0.23 | 0.49 | 0.51 | −54.3% | −0.29 (−0.45, −0.13) | .0001 |
| Mean hypoglycemia events < 3.9 mmol/L (70 mg/dL) within 24 hours | ||||||
| 0.64 | 0.38 | 0.63 | 0.53 | 27.7% | −0.16 (−0.29 to −0.03) | .0164 |
| Hours in hyperglycemia (> 13.3 mmol/L) within 24 hours | ||||||
| 3.1 | 3.5 | 3.9 | 3.9 | 2.1% | 0.1 (−0.80, 1.00) | .8729 |
| Mean amplitude of glucose excursiona | ||||||
| 128 | 125 | 131 | 131 | NA | −4.0 (−10.47 to −2.47) | .1909 |
| Blood glucose risk indexa | ||||||
| 9.5 | 9.9 | 10.4 | 10.5 | NA | 0.0 (−1.37 to 1.37) | .9431 |
| Coefficient of variation in glucose, %a | ||||||
| 34.1 | 31.4 | 33.1 | 33.0 | NA | −2.26 (−3.65 to −0.868) | .0017 |
| Low blood glucose risk indexa | ||||||
| 1.1 | 0.60 | 1.0 | 0.90 | NA | −0.30 (−0.52 to −0.08) | .0029 |
| Standard deviation of glucose, mg/dLa | ||||||
| 56 | 54 | 56 | 56 | NA | −1.67 (−4.51 to −1.17) | .2538 |
| Continuous overall net glycemic action, 2 hours, mg/dLa | ||||||
| 49 | 47 | 50 | 51 | NA | −3.0 (−5.55 to −0.45) | .0385 |
| Continuous overall net glycemic action, 4 hours, mg/dLa | ||||||
| 61 | 57 | 61 | 64 | NA | −5.0 (−9.31 to −0.69) | .0133 |
| Continuous overall net glycemic action, 6 hours, mg/dLa | ||||||
| 63 | 58 | 62 | 65 | NA | −8.0 (−13.88 to −2.12) | .0046 |
Abbreviations: CI, confidence interval; NA, not applicable; SMBG, self-monitoring of blood glucose.
A scale for measuring glucose variability.
Source: Haak et al.33
Table 9:
Subgroup Analyses of Time in Target Glucose Range and Hypoglycemia Among People With Type 2 Diabetes
| Author, Year | Group Means | Difference Between Flash and SMBG (% Change) | Difference in End-of-Study Adjusted Means Between Flash and SMBG (95% CI)a | P Value | |||
|---|---|---|---|---|---|---|---|
| Flash | SMBG | ||||||
| Baseline | Study End | Baseline | Study End | ||||
| Hours in target glucose range (3.9–10.0 mmol/L) within 24 hours | |||||||
| Haak et al, 201733 (< 65 years) | 13.3 | 13.3 | 12.7 | 12.7 | NA | 0.3 (−1.19 to 1.79) | .6777 |
| Haak et al, 201733 (≥ 65 years) | 14.9 | 14.2 | 14.9 | 14.0 | NA | 0.3 (−1.44 to 2.04) | .7476 |
| Hours in hypoglycemia (< 3.9 mmol/L) within 24 hours | |||||||
| Haak et al, 201733 (< 65 years) | 1.17 | 0.64 | 0.98 | 0.96 | −35.4% | −0.37 (−0.70 to −0.04) | .0279 |
| Haak et al, 201733 (≥ 65 years) | 1.53 | 0.49 | 1.26 | 1.03 | −55.9% | −0.60 (−1.03 to −0.17) | .0083 |
| Hours in hyperglycemia (> 13.3 mmol/L) within 24 hours | |||||||
| Haak et al, 201733 (< 65 years) | 3.5 | 3.7 | 4.4 | 4.2 | 1.9% | −0.1 (−1.33 to 1.13) | .9063 |
| Haak et al, 201733 (≥ 65 years) | 2.40 | 3.20 | 3.0 | 3.4 | 3.7% | 0.1 (−1.29 to 1.49) | .8791 |
Abbreviations: CI, confidence interval; NA, not applicable; SMBG, self-monitoring of blood glucose.
Confidence intervals were computed by the authors of this health technology assessment using the standard error reported in the primary study.
Time Above Target Glucose Range
Haak et al33 reported a statistically nonsignificant increase in the time spent in the target glucose range for flash glucose monitoring compared with self-monitoring of blood glucose, but the results appear imprecise (Table 8). Because of imprecision, we rated the certainty of evidence for this outcome as low (Table A4).
Time Spent in Hypoglycemia
Haak et al33 reported a statistically significant reduction in the time spent in hypoglycemia for flash glucose monitoring compared with self-monitoring of blood glucose (Table 8). The results remain similar when a subgroup analysis was done for people 65 years of age and younger and for people older than 65 years of age (Table 9). We rated the certainty of evidence as moderate (Table A4) because we could not determine from the study how low the glucose level dropped after crossing the hypoglycemia threshold, even though it is well documented that recurrent hypoglycemia can impair awareness55 and any single episode of severe hypoglycemia can be life-threatening.56
Hypoglycemia Events
Haak et al33 reported a statistically significant reduction in the daily mean number of hypoglycemia events for flash glucose monitoring compared with self-monitoring of blood glucose (Table 8). The observed percentage reduction in hypoglycemia (27.7%) was comparable to the minimum value that the study by Bolinder et al32 was designed to detect (30%). When we accounted for plausible flash reading errors through a quantitative bias analysis (Appendix 2), all corrected point estimates were above 25%. Because of uncertainty in the importance of this reduction (which is slightly lower than the minimum threshold set in the study by Bolinder et al32), we rated the certainty of evidence as moderate (Table A4).
Glucose Variability
Haak et al33 evaluated the effect of flash glucose monitoring compared with self-monitoring of blood glucose on glucose variability. There was inconsistency in results across scales of glucose variability (Table 8). Given the lack of a gold standard for the measures of glucose variability, we were unable to determine whether flash glucose monitoring is more effective than self-monitoring of blood glucose in reducing glucose variability. Because of this limitation we rated the certainty of the evidence as low (Table A4).
Severe Hypoglycemia Events
Haak et al33 reported four serious hypoglycemia events, three in studies of flash glucose monitoring and one in a study of self-monitoring of blood glucose (risk difference −0.03, 95% exact CI −0.10 to 0.01). However, we noted that the number of events was very small and that the study excluded people with a history of severe hypoglycemia at baseline. Because of imprecision and indirectness, we rated the certainty of evidence as very low (Table A4).
Glycated Hemoglobin Levels
Haak et al33 did not find a difference in A1C levels between flash glucose monitoring and self-monitoring of blood glucose (mean difference 0.03, 95% CI −0.19 to 0.25). However, when they partitioned the study population by age (< 65 and ≥ 65 years of age), they observed statistically significant results that favoured flash glucose monitoring among those younger than 65 years of age and that favoured self-monitoring of blood glucose among those 65 years of age and older (Table 10), with point estimates in both groups exceeding the recommended cut-off point for clinically meaningful difference (i.e., 0.5%).34 Given that generalizability of these results depends on the age distribution of the target population and that the authors reported imputing missing values for this outcome in a way that could introduce biases, we rated the certainty of evidence as low (Table A4).
Table 10:
Subgroup Analysis of Glycated Hemoglobin Levels by Age for People With Type 2 Diabetes
| Author, Year | Mean A1C, % | Difference in Adjusted Means Between Flash and SMBG (95% CI)a | P Value | |||
|---|---|---|---|---|---|---|
| Flash | SMBG | |||||
| Baseline | Study End | Baseline | Study End | |||
| Haak et al, 201733 (< 65 years) | 8.81 | 8.38 | 8.93 | 8.60 | −0.33 (−0.65 to −0.01) | .0301 |
| Haak et al, 201733 (≥ 65 years) | 8.36 | 8.36 | 8.44 | 7.90 | 0.44 (0.12 to 0.76) | .0081 |
Abbreviations: CI, confidence interval; A1C, glycated hemoglobin; SMBG, self-monitoring of blood glucose.
Confidence intervals were computed by the authors of this health technology assessment using information reported in the primary studies.
Quality of Life
Haak et al33 reported that there was no statistically significant increase in quality of life for people using flash glucose monitoring versus self-monitoring of blood glucose; however, the authors presented only the P value and a graph with confidence bars (without clearly discernable values). Nonetheless, it is clear from the graph that any difference between flash glucose monitoring and self-monitoring of blood glucose was negligible.58 Mitsuishi et al48 reported a statistically nonsignificant increase in quality of life for flash glucose monitoring compared with self-monitoring of blood glucose (mean difference in the WHO-5 scale was 1.0, 95% CI −1.16 to 3.16; P = .218). Neither the point estimate nor the confidence limits exceeded the recommended threshold for a clinically important difference of 10 units.41 Because some questions in the surveys did not seem to relate to the use of flash glucose monitoring or self-monitoring of blood glucose and because how these questions contributed to the overall score is unknown, we rated the certainty of evidence for this outcome as low (Table A4).
Device-Related Adverse Events
All six device-related adverse events reported by Haak et al33 were related to flash glucose monitoring and were primarily treated with topical preparations. Too few events made comparison of the safety of flash glucose monitoring versus self-monitoring of blood glucose both uninformative and unlikely to reflect a difference in safety levels (or lack thereof) in the target population. Because of imprecision and indirectness, we rated the certainty of the evidence as very low (Table A4).
Combined Type 1 and Type 2 Diabetes
We identified only one study (a before-after trial by Mitsuishi et al48) that compared the effectiveness of flash glucose monitoring with that of self-monitoring of blood glucose in improving the quality of life in a mixed population of people with type 1 or type 2 diabetes treated with insulin therapy. The findings are presented below.
Quality of Life
Mitsuishi et al48 reported a statistically significant increase in the quality of life for flash glucose monitoring compared with self-monitoring of blood glucose (mean difference in the WHO-5 scale was 1.7, 95% CI 0.35–3.05; P = .014); however, the difference was below the recommended threshold for clinical importance.41 We rated the certainty of evidence as very low (Table A5) because the authors did not report the duration of follow-up; hence, we could not determine whether it was comparable between intervention groups.
Device-Related Adverse Events
Mitsuishi et al48 reported 34 adverse events related to the use of flash glucose monitoring. These were itching, scar at the insertion site, erythema, bruising, bleeding, epidermolysis, pain, and subcutaneous bleeding. No adverse events were reported as related to self-monitoring of blood glucose. We were unable to compare safety of flash glucose monitoring versus self-monitoring of blood glucose because data on self-monitoring of blood glucose were missing. Given these limitations we rated the certainty of the evidence as very low (Table A5).
Discussion
Patient characteristics varied greatly across studies in age, baseline A1C measurements, and propensity for severe hypoglycemia. Our evidence appraisal took this variation into account. For example we noted that one randomized controlled trial32 reported no difference in the reduction in fear of hypoglycemia between flash glucose monitoring and self-monitoring of blood glucose, but excluded people with a history of severe hypoglycemia. Given that these people have a high risk of developing fear of hypoglycemia, we downgraded the quality of evidence (another study47 reporting the same outcome did not exclude severe hypoglycemia but quality of its finding was downgraded for other reasons explained in the Results section). Further, none of the studies recruited pregnant people, children younger than 13 years of age, or patients who did not use insulin. Consequently, the conclusions of this health technology assessment might not apply to these patients. Similarly, no study assessed how flash glucose monitoring affected people with uncontrolled type 1 diabetes. As treatment adherence can be a challenge in these patients (especially adolescents),59 our findings might not apply.
We further noted that the target glucose ranges used by the studies in this review were adopted from a consensus report written by several diabetes organizations,34 which differ somewhat from the targets set by Diabetes Canada.12 Nonetheless, the optimal range remains to be determined.12
Our interpretation of results took into account clinical relevance. We encountered several instances where authors' interpretation of results based on P values conflicted with what is deemed as clinically relevant. In these cases, our evidence appraisal weighed the fact that authors' interpretation might not reflect empirical evidence.60 However, validation studies from which clinical relevance is determined can sometimes be controversial.61 When in doubt we consulted our clinical experts.
We identified three other English-language health technology assessments that compared the effect of flash glucose monitoring with that of self-monitoring of blood glucose.62–64 All three focused on the two randomized controlled trials by Bolinder et al32 and Haak et al.33 There were some differences in the way evidence was appraised across these assessments, but overall, the authors concluded that the quality of evidence is either low or very low. In contrast, our rating of evidence varied from very low to moderate depending on the outcome assessed. For the outcomes rated as moderate, we noted some limitations but concluded that the effect of these limitations was too small to alter the conclusions of the reported results. For example, instead of downgrading results for an outcome outright because of lack of blinding, we assessed its impact on the reported effect size.
Limitations
All studies reviewed were conducted with the first generation of flash glucose monitoring system. In addition to being less reliable during times of rapid glucose variability, this flash glucose monitoring system lacks an alarm to alert users when the glucose level is too low, which is important for hypoglycemia unawareness. This might explain the exclusion of people with a history of hypoglycemia unawareness at baseline in one randomized controlled trial,32 as well as the noted instances of finger-prick use (an average of 0.5 per day) in randomized controlled trials32,65 among subjects who were otherwise randomized to receive flash glucose monitoring. As device calibration errors cannot be ruled out, several studies16,66–73 evaluated the accuracy of flash glucose monitoring through error grid analysis. We used results from these studies to quantify the uncertainty in the observed percentage reduction in mean hypoglycemic events in the randomized controlled trials32,33 that could be caused by flash reading errors. In these trials, results for hypoglycemia in the self-monitoring group were obtained using a flash glucose monitoring device; thus, in the analysis it was important to account for the possibility that some of the hypoglycemia results in either treatment group could be due to flash reading errors. We did not observe any important deviations of the reported results after correcting for potential flash reading errors (Appendix 2).
We identified several further limitations. First, missing data for the primary outcome in the randomized controlled trials32,33,49 were imputed by carrying forward the last observation. This approach could have underestimated random errors and induced misclassification errors.74 We were unable to determine the extent of this imputation bias. Second, we were unable to accurately compare the safety of the two technologies given too few events reported in the studies and the failure of one study48 to report events with self-monitoring of blood glucose. Finally, we did not find studies that assessed the effect of switching from self-monitoring of blood glucose to flash glucose monitoring on vascular complications. This reflects the fact that flash glucose monitoring is still new and diabetes complications take many years to develop.75,76
Ongoing Studies
Via ClinicalTrials.gov, we identified three ongoing randomized trials (identifiers NCT03522870, NCT03570138, NCT02776007) and one randomized controlled trial that has been completed but the results of which have not yet been published (identifier NCT03182842). These studies compare the effectiveness of flash glucose monitoring versus self-monitoring of blood glucose.
Conclusions
Based on moderate certainty of evidence, we found that, compared with self-monitoring of blood glucose, flash glucose monitoring reduces the mean time spent in hypoglycemia and mean hypoglycemia events in adults with well-controlled type 1 diabetes and adults with type 2 diabetes who use intensive insulin therapy. Also based on moderate certainty of evidence, flash glucose monitoring is more effective than self-monitoring of blood glucose in increasing time in the target glucose range, reducing time above the target glucose range, and reducing glucose variability among adults with well-controlled type 1 diabetes. The certainty of evidence on the effectiveness of flash glucose monitoring in other clinical outcomes is either low or very low.
Findings from this health technology assessment are not generalizable to pregnant people, people with diabetes who do not use insulin, and children younger than 13 years of age.
ECONOMIC EVIDENCE
Research Questions
What is the cost-effectiveness of flash glucose monitoring compared with self-monitoring of blood glucose for people with type 1 diabetes requiring intensive insulin therapy?
What is the cost-effectiveness of flash glucose monitoring compared with self-monitoring of blood glucose for people with type 2 diabetes requiring intensive insulin therapy?
Methods
Economic Literature Search
We performed an economic literature search on April 10, 2018, to retrieve studies published from January 1, 2014, until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms. We later added one health technology assessment published in July 2018.64
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and performed further assessment for eligibility.
Inclusion Criteria
English-language, individual-level economic evaluations conducted alongside randomized controlled trials (trial-based), economic analyses based on decision analytic models (model-based), costing studies
Studies published between January 1, 2014, and April 10, 2018
Studies of type 1 diabetes or type 2 diabetes requiring intensive insulin therapy
Studies comparing flash glucose monitoring to self-monitoring of blood glucose
Exclusion Criteria
Editorials, case reports, or commentaries
Studies of people with hypoglycemic unawareness
Outcomes of Interest
Costs and effects (e.g., quality-adjusted life-years [QALYs])
Incremental costs or cost of interventions
Incremental effectiveness outcomes (e.g., incremental QALYs)
Incremental net benefit
Incremental cost-effectiveness ratios (ICERs)
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, and ICER)
Study Applicability
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform development of NICE's clinical guidelines.77 We modified the wording of the questions to remove references to guidelines and to make them Ontario-specific. For studies with full text, we assessed the applicability of each study to the research question (directly, partially, or not applicable). Our findings are summarized in Appendix 4.
Results
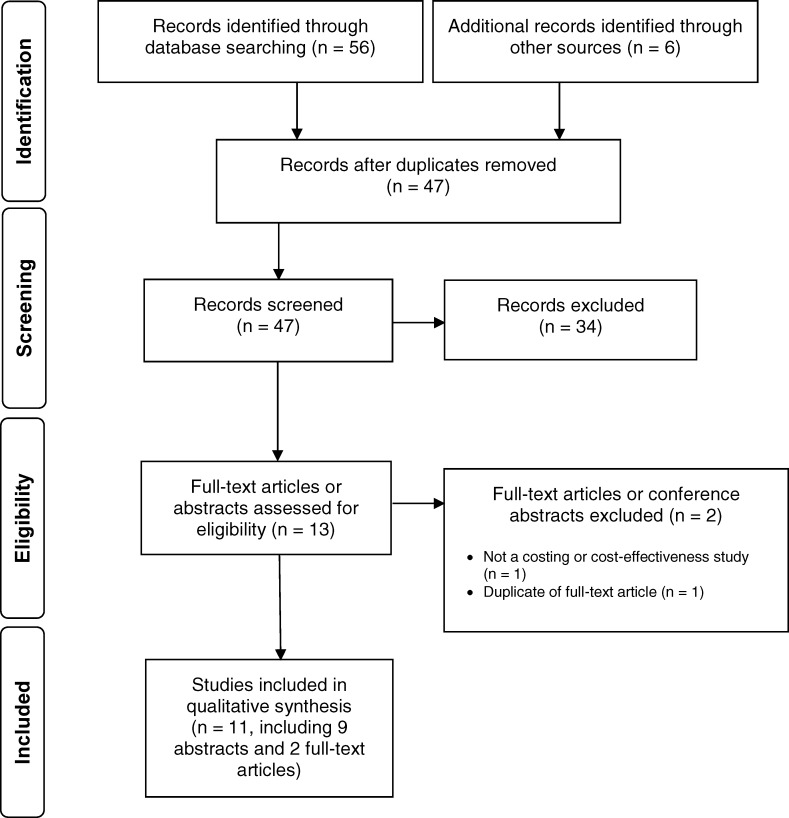
Literature Search
The literature search yielded 47 citations published between January 1, 2014, and April 10, 2018, after removing duplicates. We excluded a total of 34 articles based on information in the title and abstract. We then obtained the full-text articles or conference abstracts of 13 potentially relevant citations for further assessment. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.51
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Eleven studies (seven cost–utility studies64,78–83 and four costing studies84–87) met the inclusion criteria. All but two were conference abstracts.64,87 We hand-searched the reference lists of the single full-text article and health technology assessment websites and did not identify any additional studies.
Review of Included Economic Studies
Table 11 summarizes the included studies, organized by diabetes subtype. There were seven cost–utility analyses, six of which were published as conference abstracts only. The one full-text cost–utility analysis was part of a health technology assessment from Scotland.64 All six conference abstracts on cost–utility analyses used the IMS CORE diabetes model.88 Of four costing and budget impact studies, three were published as conference abstracts only.84–87
Table 11:
Results of Economic Literature Review
| Name, Year, Country, Publication Type | Study Design and Perspective | Population | Intervention/Comparator | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| Type 1 diabetes | ||||||
| Bilir et al, 2016,80 United States, conference abstract |
Economic analysis: CUA Study design: decision analytic model Perspective: NR Time horizon: 50 y Discount rate: NR |
People with type 1 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: NR | Currency, cost year: Euro (€), Swedish krona (SEK), Australian dollar (ÄD), 2015 Total costs: NR | Base case ICERs (cost/additional QALY): 240,909 SEK, $24,621 AUD, €22,099 (Germany), €22,503 (Spain), €16,008 (Italy), €31,887 (France), €19,445 (Portugal), €14,209 (Netherlands) |
| Bilir et al, 2017,79 United States, conference abstract | Economic analysis: CUA Study design: decision analytic model Perspective: NR Time horizon: 50 y Discount rate: NR |
People with type 1 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: 12.6 for SMBG, 13.6 for flash | Currency, cost year: Swedish krona, 2016 Flash: 1,786,017 SEK SMBG: 1,681,620 SEK |
Base case ICERs (cost/additional QALY): 97,468 SEK |
| Billir et al, 2017,78 United States, conference abstract |
Economic analysis: CUA Study design: decision analytic model Perspective: NR Time horizon: 50 y Discount rate: NR |
People with type 1 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: NR | Currency, cost year: GBP (€), NR Base case: NR |
Base case ICER (cost/additional QALY): €25,045 |
| Hellmund et al, 2018,87 United States, full-text journal article |
Economic analysis: cost analysis Study design: costing model Perspective: UK public health care system (NHS) Discount rate: NR |
People with type 1 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
NR | Currency, cost year: GBP, 2015 Base case (10 SMBG/d) Flash: €970 SMBG: €1,205 Scenario 1 (5.6 SMBG/d) SMBG: €675 Flash: €970 Scenario 2 (including costs of severe hypoglycemia) Flash: €1,191 SMBG: €1,103 Scenario 3 (16 SMBG/d) Flash: €970 SMBG: €1,927 |
NR |
| Type 2 diabetes | ||||||
| Hellmund, 2016,86 United States, conference abstract | Economic analysis: budget impact analysis Study design: NR Perspective: German health care system Time horizon: 3 y Discount rate: NR |
People with type 2 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG | NR | Currency, cost year: Euro, NR Total costs Flash: €2,210 SMBG: €2,484 |
NR |
| Khan-Miron et al, 2017,85 Spain, conference abstract | Economic analysis: cost analysis Study design: NR Perspective: NR Time horizon: NR Discount rate: NR |
People with type 2 diabetes who require insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
NR | Currency, cost year: Euro, NR Base case Flash: €1,592 SMBG: €433 |
NR |
| Li et al, 2014,83 United States, conference abstract | Economic analysis: CUA Study design: decision analytic model Perspective: NR Time horizon: 40 y Discount rate: NR |
People with type 2 diabetes who require insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: NR | Currency, cost year; GBP, 2013 Base case: NR |
Base case ICER (cost/additional QALY): €10,034-€29,068 |
| Li et al, 2016,82 United States, conference abstract |
Economic analysis: CUA Study design: decision analytic model Perspective: NR Time horizon: 40 y Discount rate: NR |
People with type 2 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: NR | Currency, cost year: Euro, Swedish krona, 2015 Total costs: NR Discount rate: NR |
Base case ICERs (cost/additional QALY): 317,038 SEK, €29,672 (Germany), €28,745 (Spain), €20,968 (Italy), €29,008 (France), €28,369 (Portugal), €21,105 (Netherlands) |
| Type 1 and type 2 diabetes | ||||||
| Curto et al, 2017,84 Italy, conference abstract | Economic analysis: cost analysis Study design: NR Perspective: NR Time horizon: 1 y Discount rate: NR |
People with type 1 or type 2 diabetes living in Veneto, Italy Age: NR Male (%): NR |
Intervention: flash | NR | Currency, cost year: Euro, NR Yearly cost: €936 |
NR |
| Vellopoulou et al, 2017,81 Greece, conference abstract | Economic model: CUA Study design: decision analytic model Perspective: Greek payer Time horizon: lifetime Discount rate: NR |
People with type 1 or type 2 diabetes who require intensive insulin treatment Age: NR Male (%): NR |
Intervention: flash Control: SMBG |
Total QALYs: NR Incremental QALYs: Type 1: 0.567 Type 2: 0.317 |
Currency, cost year: Euro, NR Base case incremental cost Type 1: €8,225 Type 2: €6,236 3-year net budget impact Type 1: €5,114,658 Type 2: €614,473 Both: €5,729,131 |
Base case ICER (cost/additional QALY): Type 1: €14,567 Type 2: €19,703 |
| Healthcare Improvement Scotland, 2018,64 health technology assessment | Economic model: cost-utility study Study design: decision analytic model Perspective: NHS Time horizon: lifetime Discount rate: 3.5% annually |
People with type 1 or type 2 diabetes who require intensive insulin treatment Age (years): 43.7 for type 1 diabetes, 59.2 for type 2 diabetes Male (%): 56.9 for type 1 diabetes, 67% for type 2 diabetes |
Intervention: flash Control: SMBG | Total QALYs: Type 1 Flash: 9.73 SMBG: 7.61 Type 2 Flash: 6.14 SMBG: 5.04 Incremental QALYs: Type 1: 2.12 Type 2: 1.09 |
Currency, cost year: GBP, year of costing NR Total costs: Type 1 Flash: €18,074 SMBG: €12,860 Type 2 Flash: €10,450 SMBG: €5,535 Incremental costs: Type 1: €5,214 Type 2: €4,916 Budget impact, flash: Year 1: €8.8 million Year 2: €18.2 million |
Base case ICERs (cost/additional QALY): Type 1: €2,459 Type 2: €4,498 |
Abbreviations: AUD, Australian dollars; CUA, cost–utility analysis; flash, flash glucose monitoring; GBP, Great British pound; ICER, incremental cost-effectiveness ratio; NHS, National Health Service; NR, not reported; QALY, quality-adjusted life-year; SEK, Swedish krona; SMBG, self-monitoring of blood glucose.
All conference abstracts either provided insufficient information on the study modelling approach (e.g., time horizon, costing perspective, characteristics of target population) or had not reported disaggregated results (e.g., total costs, total outcome). Therefore, in this review, we provide greater details below for the full-text studies.
Type 1 Diabetes
Four studies included only people diagnosed with type 1 diabetes; three were cost–utility analyses,78–80 and one was a full-text costing study.87
The full-text costing study estimated the annual cost of using flash glucose monitoring from the perspective of the UK National Health Service.87 The annual cost of flash glucose monitoring per person was £970.23, where the reader and sensor costs were £910.00 and additional lancet and test strips for self-monitoring of blood glucose were £60.23. Compared with self-monitoring of blood glucose, flash glucose monitoring resulted in annual cost savings of £234.28 if patients tested glucose levels 10 times per day (£1,204.50 per year) but in a higher annual cost of £295.71 if patients tested glucose levels five to six times per day (£674.52 per year).
All cost–utility analyses used the IMS CORE model,88 and cohort characteristics were based on the IMPACT study.32 None of the studies reported the costing perspective or the discount rate. The reported base case ICERs ranged from €14,209 to €31,887 per QALY across seven western European countries (Germany, Greece, Spain, Italy, France, Portugal, and the Netherlands).78–80 Two studies by Bilir et al, which used the same methodology, reported an ICER of 97,468 Swedish kronor (SEK) per QALY in one80 and 240,909 SEK per QALY in the other.79 The studies concluded that flash glucose monitoring could be considered cost-effective compared with self-monitoring of blood glucose on the basis of published willingness-to-pay thresholds but did not identify the thresholds.78–80
Type 2 Diabetes
Two cost–utility analyses,82,83 one costing study,85 and one budget impact analysis86 included only people with type 2 diabetes. Both cost–utility analyses were conducted by the same research team and used the IMS CORE model88 with a lifetime horizon and cohort characteristics based on the REPLACE study.33 The reported ICERs ranged from €19,703 to €29,672 across seven European countries.82 One study, conducted from the German health care system perspective, reported an annual cost of €2,210 per person for flash glucose monitoring, with €1,635 attributed to flash glucose monitoring, €104 to health care use following non-severe hypoglycemic episodes, and €472 for other health services.86 Another costing study, based in Spain, reported similar costs for flash glucose monitoring: €1,592 per year.85
Type 1 and Type 2 Diabetes
Three studies included people with either type 1 or type 2 diabetes: one full-text cost–utility analysis within a health technology assessment conducted in Scotland and two conference abstracts.64,81,84
The cost–utility analysis from the perspective of Scotland's health care system reported the ICER of glucose monitoring versus self-monitoring of blood glucose at £2,459 and £4,498 per QALY gained for type 1 and type 2 diabetes, respectively.64 However, we identified important limitations related to the critical input parameter assumptions, which likely overestimated the cost-effectiveness of flash glucose monitoring. We provide further details of these limitations in the Applicability and Critical Appraisal section of this review. In the health technology assessment from Scotland, the authors reported that the budget impact of flash glucose monitoring versus self-monitoring of blood glucose ranged from £8.8 million to £18.2 million, assuming an increase in adoption rate from 30% in year 1 to 50% in year 5.
Another cost–utility analysis from the perspective of the Greek health care system reported an ICER of €14,567 per QALY for type 1 diabetes and €19,703 per QALY for type 2 diabetes.81 The study also estimated that the 3-year budget was €5,114,658 for type 1 diabetes, €614,473 for type 2 diabetes, and €5,729,131 for both types of diabetes.81
The last of the three studies was a costing study from the perspective of the health care system in Italy. The authors estimated that the annual cost of flash glucose monitoring was €1,277 per person: €936 for by the flash glucose monitoring system (including sensors, glucose test strips, lancets, needles), €185 for patient training, and €156 for distribution costs.84
Applicability and Limitations of Included Studies
We used the checklist to assess applicability for the two full-text studies.64,87
The costing study was conducted from the perspective of the UK health care system, which is comparable to that of Canada. However, we took into consideration that the costs associated with management of diabetes might not be the same in both jurisdictions.87 For this reason, results of the costing study were only partially applicable to our research question.
We also carefully reviewed the cost utility analysis conducted by Healthcare Improvement Scotland.64 That analysis concluded that flash glucose monitoring was associated with a gain of 2.12 and 1.09 QALYs for type 1 and type 2 diabetes, respectively. In consultation with experts, we decided that there is a high likelihood of these gains being overestimates, because of several limitations in the analysis (e.g., the estimated disutility due to non-severe hypoglycemic events, and the reduced risk of severe hypoglycemic events with flash glucose monitoring compared with self-monitoring of blood glucose).
Information from abstracts was limited, so we were unable to assess their applicability using the checklist. However, as none of these studies were conducted in Canada, they were not considered directly applicable to our research question. Further, the abstracts had limited information regarding the methodology used. It was therefore difficult to assess the quality of the economic evidence and the reliability of the findings.
Discussion
Our evidence review identified seven cost–utility studies (six conference abstracts and one full-text article64) that compared flash glucose monitoring with self-monitoring of blood glucose for people with type 1 diabetes or type 2 diabetes who require intensive insulin therapy. All of these studies concluded that flash glucose monitoring could be considered cost-effective as compared with self-monitoring of blood glucose, given that the reported ICERs (i.e., the additional QALYs gained per additional cost) were below published willingness-to-pay thresholds. However, there was a large variation in the ICERs depending on the country of the target population, and all studies made several assumptions based on extrapolations from the evidence.
In terms of cost, whether flash glucose monitoring could be considered cost-saving largely depends on the number of glucose tests carried out in routine blood glucose monitoring. As the costing perspective was not stated by most studies, we could not evaluate whether the studies included all cost items relevant to the Ontario context. Only one costing study87 included the cost of resource use associated with flash glucose monitoring, and another84 included the cost of training that could be required for people using flash glucose monitoring. All other studies estimated the annual cost from summing the cost of various glucose monitoring supplies.
Conclusions
We identified one full-text cost–utility analysis that compared flash glucose monitoring with self-monitoring of blood glucose in people with type 1 diabetes or people with type 2 diabetes requiring intensive insulin therapy. Although this study reported that flash glucose monitoring represented good value for money, the study was associated with methodological limitations and may have overestimated the benefits and cost-effectiveness of flash glucose monitoring versus self-monitoring of blood glucose.
PRIMARY ECONOMIC EVALUATION
The highest-quality evidence for the flash glucose monitoring system, reported on in the clinical evidence section of this report, comes from two open-label randomized controlled trials with adult study populations: Bolinder et al32 for well-controlled type 1 diabetes and Haak et al33 for type 2 diabetes with intensive insulin therapy. From these randomized controlled trials, flash glucose monitoring reduced the time spent with glucose levels below 3.9 mmol per litre (i.e., in hypoglycemia) and increased the convenience for patients compared with self-monitoring of blood glucose. As yet, there is insufficient evidence from randomized trials that flash glucose monitoring improves other clinical outcomes (e.g., glycated hemoglobin level, frequency of severe hypoglycemia), or improves health-related quality-of-life outcomes that are typically used in economic models that assess the cost-effectiveness of interventions used in diabetes. For these reasons, it would be difficult to conduct a cost–utility analysis (i.e., using QALYs as the measure), and a cost-effectiveness analysis (e.g., using hypoglycemic time as the measure) would be difficult to interpret. The major long-term diabetes models (which were developed before continuous glucose monitoring and flash glucose monitoring) do not often consider the effect of time spent in hypoglycemia or of non-severe hypoglycemic events.88,89
In addition to being associated with reduced hypoglycemia time, flash glucose monitoring has been associated with higher patient satisfaction32,33 and greater convenience of use.90 However, these outcomes are not typically included in economic models.
For these reasons, and in consultation with external experts, we decided to forgo conducting a primary economic evaluation in this health technology assessment.
BUDGET IMPACT ANALYSIS
Research Question
What is the potential annual budget impact for the Ontario Ministry of Health and Long-Term Care of publicly funding flash glucose monitoring for people with type 1 diabetes and for people with type 2 diabetes requiring intensive insulin therapy who are eligible for the Ontario Drug Benefit program over 5 years?
Methods
Analytic Framework
We estimated the budget impact of flash glucose monitoring using the cost difference between two scenarios: the current scenario of using self-monitoring of blood glucose, and the new scenario of using flash glucose monitoring as the primary method of glucose monitoring. Figure 3 presents the model schematic.
Figure 3: Budget Impact Model Schematic.
The current standard approach to monitor blood glucose in Ontario is using self-monitoring test systems that require patients to prick their finger with a lancet and apply the blood sample to a test strip. The test strip will then be inserted into a reflectance photometer to measure blood glucose levels. The frequency of testing varies depending on the type of diabetes, pharmacological regimen, and history of glucose management. According to the 2018 Diabetes Canada clinical practice guidelines, self-monitoring of blood glucose should be performed at least three times daily for type 1 diabetes or for type 2 diabetes with intensive insulin therapy.91 In practice, clinicians often recommend more frequent monitoring of blood glucose, such as six or more times daily for type 1 diabetes and four or more times daily for people with type 2 diabetes requiring intensive insulin therapy.
We conducted a reference case analysis and various scenario analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our scenario analyses explored how the results are affected by varying model assumptions.
Key Assumptions
To simplify the analysis, we made the following assumptions for the reference case:
All participants would remain on the same method of blood glucose monitoring during the budget year (e.g., no crossover with 100% compliance)
Pediatric patients would experience the same clinical benefits from flash glucose monitoring as those reported for adults in the clinical evidence review of this health technology assessment
During an episode of hypoglycemia depicted by the flash glucose monitoring system, or when experiencing symptoms suggestive of hypoglycemia not detected by the device and after correcting hypoglycemia, blood glucose level might be checked using self-monitoring of blood glucose
The reimbursement policy for self-monitoring of blood glucose would not change in the next 5 years
There would be no introduction of new methods of monitoring blood glucose in the next 5 years that would alter the uptake of self-monitoring of blood glucose or flash glucose monitoring for our target population
If the flash glucose monitoring sensors fail, they would be replaced without additional cost
The cost of flash glucose monitoring sensors and self-monitoring of blood glucose would be constant over 5 years
Target Population
We introduced the target population in the reference case, and the populations in various scenario analyses can be found in the Analysis section below. The target population was people with type 1 diabetes or with type 2 diabetes undergoing intensive insulin therapy who use self-monitoring of blood glucose to assess their blood glucose levels and are covered by the Ontario Drug Benefit program. Currently, all people in Ontario younger than 25 years of age or older than 65 years of age are covered by the Ontario Drug Benefit program. For Ontarians between 25 and 64 years of age, the Ontario Drug Benefit program covers about 11.2% who are enrolled in the Ontario Disability Support Program, the Ontario Works program, Ontario's home care program, or the Trillium Drug Program.92 Note that 75.7% of people in Ontario between 25 and 64 years of age are enrolled in private drug plans.92
We assumed that all patients with type 1 diabetes would receive intensive insulin therapy in the reference case. For patients with type 2 diabetes, we included only those who receive multiple (i.e., more than one) daily insulin injections or continuous subcutaneous insulin infusion.
We excluded people with hypoglycemic unawareness who are at high risk for glycemic variability. This is because continuous monitoring of glucose, with alerts to prevent high or low blood glucose levels, would be more suitable for these people.
We included both adults and children (supervised by their parents) in the budget impact analysis, in the likelihood that Health Canada would approve use in the pediatric age groups (Dr. Celine Huot, email communication, August 25, 2018). Experts suggest that it is increasingly prescribed for children, but off label, in Canada. We estimate the target population in Table 12. Further details of the process of estimating the target population are provided below.
Table 12:
Expected Target Population for Flash Glucose Monitoring
| Measure | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Ontario population, n | 14,004,100 | 14,081,900 | 14,154,600 | 14,222,100 | 14,284,300 |
| Projected prevalence of diabetes in Ontario, % | 11.12 | 11.43 | 11.74 | 12.05 | 12.36 |
| Projected Ontario population with diabetes, n | 1,557,256 | 1,609,561 | 1,661,750 | 1,713,763 | 1,765,539 |
| Projected type 1 diabetes (6%), n | 93,435 | 96,574 | 99,705 | 102,826 | 105,932 |
| Type 1 diabetes suitable for flash glucose monitoring (65%), na | 60,733 | 62,773 | 64,808 | 66,837 | 68,856 |
| 0–24 years of age | 16,702 | 17,263 | 17,822 | 18,380 | 18,935 |
| 25–64 years of age | 36,257 | 37,475 | 38,691 | 39,902 | 41,107 |
| ≥ 65 years of age | 7,774 | 8,035 | 8,295 | 8,555 | 8,814 |
| Suitable type 1 diabetes covered by ODB program, nb | 28,537 | 29,495 | 30,450 | 31,404 | 32,353 |
| Uptake rate, % | 15 | 20 | 25 | 30 | 35 |
| Target population with type 1 diabetes, n | 4,281 | 5,899 | 7,613 | 9,421 | 11,324 |
| Projected type 2 diabetes (94%), n | 1,463,821 | 1,512,987 | 1,562,045 | 1,610,937 | 1,659,607 |
| Type 2 diabetes treated with intensive insulin therapy (10%), n | 146,382 | 151,299 | 156,205 | 161,094 | 165,961 |
| Type 2 diabetes suitable for flash glucose monitoring (80%), na | 117,106 | 121,039 | 124,964 | 128,875 | 132,769 |
| 0–24 years of age | 1,757 | 1,816 | 1,874 | 1,933 | 1,992 |
| 25–64 years of age | 62,886 | 64,998 | 67,106 | 69,206 | 71,296 |
| 65+ years of age | 52,463 | 54,225 | 55,984 | 57,736 | 59,481 |
| Suitable type 2 diabetes covered by ODB program, nb | 61,263 | 63,321 | 65,374 | 67,420 | 69,458 |
| Uptake rate, % | 15 | 20 | 25 | 30 | 35 |
| Target population with type 2 diabetes, n | 9,189 | 12,664 | 16,344 | 20,226 | 24,310 |
| Total target population in reference case, n | 13,470 | 18,563 | 23,957 | 29,647 | 35,634 |
Abbreviation: ODB, Ontario Drug Benefit.
We excluded people with hypoglycemic unawareness and people at high risk for glycemic variability who were not suitable for flash glucose monitoring (i.e., 35% type 1 diabetes and 20% type 2 diabetes treated with intensive insulin therapy).
The Ontario Drug Benefit program covers all people younger than 25 or older than 65 years of age and 11.2% of patients between 25 and 64 years of age.92
In our previous health technology assessment on continuous monitoring of glucose for type 1 diabetes,18 we projected that the prevalence of both type 1 and type 2 diabetes would increase by 0.31% annually, from 11.12% in year 1 to 12.36% in year 5. We projected that the Ontario population would also slightly increase over 5 years from 2018 to 2022. Further, we assumed that 6% of people with diabetes would be type 1,18 and that the remaining 94% would be type 2.
In Canada, around 15% of all people with type 2 diabetes are not treated with either insulin or medication.93 Of those who are treated, 20% to 24% are prescribed insulin.94 Given these estimates, we assumed that 10% of all people with type 2 diabetes require intensive insulin therapy in Ontario.
The published literature suggests that around 20% to 30% of people with type 1 diabetes and 10% of people with type 2 diabetes who require insulin therapy have hypoglycemic unawareness.18,95 However, estimating the proportion of people at high risk of glycemic variability is not straightforward, and we have not found the prevalence of this condition in the literature. There is no established cut-off value for determining high risk of glycemic variability. In addition, a high risk of glycemic variability is associated with severe hypoglycemia. Consequently, we arbitrarily assumed that around 15% of patients with intensive insulin therapy are at high risk of glycemic variability. After accounting for the overlap of patients with hypoglycemic unawareness who also have a high risk for glycemic variability, we estimated that 35% of patients with type 1 diabetes and 20% of patients with type 2 diabetes undergoing intensive insulin therapy would not be suitable candidates for flash glucose monitoring.
Since the population distribution of age groups (e.g., ages ≤ 24 or ≥ 65 years versus ages 25–64 years) varies for type 1 and type 2 diabetes, we estimated the target population in these age groups separately by diabetes type. According to Statistics Canada, approximately 44.8% of people with diabetes were 65 years of age or older in Ontario.96 However, no breakdown distinguishing between type 1 or type 2 diabetes was provided in these data.96 Because most people with diabetes have type 2, we assumed that the proportion of people with type 2 diabetes who are 65 years or older would be similar to the proportion of people with either type of diabetes (e.g., 44.8%). The prevalence of type 2 diabetes in children and young adults (≤ 24 years of age) is low, and we have not found any data sources that provide a reliable estimate. Therefore, we estimated that 1.5% of people with type 2 diabetes were 24 years of age or younger. Given unavailable data sources for type 1 diabetes in Ontario, and based on the estimates from England, we estimated that 27.5%, 59.7%, and 12.8% were in the age groups 0 to 24 years, 25 to 64 years, and 65 years and older, respectively.97
Based on input from the manufacturer, we estimated that the uptake of replacing self-monitoring of blood glucose with flash glucose monitoring would be 15% in year 1, gradually increasing to 35% in year 5. The total number of people in the target population would then be 13,470 in year 1 and 35,634 in year 5.
Uptake of New Intervention
In the reference case, we estimated that the uptake of flash glucose monitoring would increase over time, from 15% in year 1 to 35% in year 5. This estimate was established based on information received from the manufacturer, based on the experiences of other countries that fund flash glucose monitoring systems (written communication, Abbott Diabetes Care, September 2018).
Resources and Costs
We estimated the medical costs of using flash glucose monitoring or self-monitoring of blood glucose that would be incurred by the Ministry of Health and Long-Term Care if flash glucose monitoring were publicly funded. Table 13 lists the cost items, unit cost, resource use, and data source for both the current scenario and the new scenario. The randomized controlled trials showed that both flash and self-monitoring patients had similar doses of insulin during the study periods.32,33 Further, no evidence suggested any difference in routine physician visits and diabetes-related complications between flash glucose monitoring and self-monitoring of blood glucose. Note: mild hypoglycemia generally can be solved by patients' self-management without requiring physician visits. Thus, we excluded the costs of insulin therapy, oral diabetic agents, physician visits, and diabetes-related complications and focused on the cost for monitoring blood glucose only. In addition, since the cost of the blood glucose meter is relatively low compared with that of the flash sensor (e.g., the cost of the FreeStyle Libre reader is $49 for 3 years98) and is incurred by both the flash and self-monitoring groups, for simplicity, we excluded this cost item from the analysis.
Table 13:
Annual Total Cost of Glucose Monitoring
| Variable | Unit Cost ($)a | Quantity/Yearb | Total Cost/Year ($)a | Publicly Funded Amounta | Reference |
|---|---|---|---|---|---|
| Self-monitoring of blood glucose (current scenario) | |||||
| Testing strips | 0.74 | – | – | 100% | Health Quality Ontario, 201818 |
| Lancets | 0.1 | – | – | 75% | Amazon.ca, 2018100 |
| Total: T1D | 0.84 (0.74 + 0.1) | 2,196 (6 × 365) | 1,840 (0.84 × 2,196) | $1,785/y | |
| Total: T2D | 0.84 (0.74 + 0.1) | 1,460 (4 × 365) | 1,226 (0.84 × 1,460) | $1,190/y | |
| Flash glucose monitoring (new scenario) | |||||
| Flash sensor | 89 | 26 (1 sensor for 14 days, 365/14) | 2,314 | $2,314 (100%) | Abbott, 201898 |
| SMBG for T1D or T2D | 0.84 (0.74 + 0.1) | 183 (0.5 × 365) | 153 | $149 (100% for strips, 75% for lancets) | Health Quality Ontario, 2018; Amazon, 201818,100 |
| Total: T1D or T2D | – | – | 2,467 | $2,463/y | |
Abbreviations: SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
All costs are in 2018 CAD.
Numbers may appear inexact because of rounding.
The costs of self-monitoring of blood glucose included the cost of blood glucose test strips and lancets (used to prick fingers to draw blood). The mean frequency of self-testing was around two times daily for insulin users older than 65 years of age in Ontario.99 However, based on our expert consultations (Dr. Bruce Perkins, email communication, August 24, 2018) and the Canadian Clinical Practice Guidelines of Monitoring Glycemic Control,91 the frequency of self-monitoring of blood glucose should be higher. In the reference case, we estimated that the frequency of self-testing for type 1 and type 2 diabetes were six and four times daily, respectively. These estimates were close to those reported in the two randomized controlled trials by Bolinder et al and Haak et al above.32,33 The cost of testing strips was obtained from our earlier health technology assessment on continuous monitoring of glucose for type 1 diabetes,18 and cost of lancets was from the top-selling product (EasyTouch Twist Lancets) on Amazon.ca.100 The cost of strips reimbursed by the Ministry of Health and Long-Term Care ranges from $0.40 per strip to $0.77 per strip. The unit cost of strips ($0.74) in our reference case generally reflected the most popular and most commonly used brands among Ontario Drug Benefit program recipients in Ontario. We also considered the cheaper strip ($0.40) in the scenario analysis.
The cost of flash glucose monitoring sensors ($89 for 2 weeks) was taken from the Abbott Laboratories Ltd. website.98 Flash glucose monitoring device users also occasionally use self-monitoring of blood glucose to confirm hypoglycemic readings; we assumed patients with type 1 or type 2 diabetes using flash glucose monitoring would also use self-monitoring of blood glucose about once every 2 days. According to the user manual for flash glucose monitoring devices, users are directed to use self-monitoring of blood glucose to check the readings of the flash glucose monitoring device under some circumstances, such as during times of rapidly changing glucose levels and biochemical hypoglycemia reported by the flash sensor.101
Currently, the Ontario Ministry of Health and Long-Term Care funds self-monitoring of blood glucose mainly through the Ontario Drug Benefit program and the Ontario Monitoring for Health program.102,103 Patients who are eligible for the Ontario Drug Benefit program are reimbursed for up to 3,000 blood glucose test strips yearly for diabetes with insulin therapy. The cost of lancets can be submitted to the Ontario Monitoring for Health program for 75% reimbursement up to a maximum of $920 per year. We estimate the total cost and the publicly funded amount of flash glucose monitoring for Ontarians in Table 13. All costs are expressed in 2018 Canadian dollars.
Analysis
The budget impact was calculated as the cost difference between the new scenario (using flash glucose monitoring) and the current scenario (using self-monitoring of blood glucose) for people with type 1 diabetes or people with type 2 diabetes requiring intensive insulin therapy. The total cost in each scenario is calculated using the average cost per patient multiplied by the target population per year. We calculated the annual budget impact for the next 5 years. We reported the budget impact for type 1 and type 2 diabetes separately and also reported the total budget impact for both types of diabetes.
We also conducted the following scenario analyses:
Scenario 1: includes all patients with type 1 or type 2 diabetes who require intensive insulin therapy, regardless of Ontario Drug Benefit program eligibility
Scenario 2: assumes that the target population uses the maximum number of blood glucose test strips reimbursed at 3,000 strips yearly (i.e., 8 strips daily) in the self-monitoring group
Scenario 3: the target population self-tests blood glucose as previously reported for Ontario at two strips daily99
Scenario 4: includes all patients with type 1 and type 2 diabetes who require insulin therapy (in this scenario, the type 1 diabetes target population remained the same as that in the reference case, and the target population of type 2 diabetes would double that in the reference case)
Scenario 5: includes only adults with diabetes who require intensive insulin therapy (i.e., we excluded patients younger than 18 years of age)
Scenario 6: includes people at high risk of glycemic variability (this population was not considered in the reference case analysis) but excludes those having hypoglycemic unawareness
Scenario 7 and 8: assumes that four and eight flash sensors were funded yearly, which corresponds to the use of flash glucose monitoring at 8 and 16 weeks yearly (i.e., one sensor for 2 weeks), respectively (i.e., assuming that patients continue using self-monitoring of blood glucose in the remaining weeks of the year)
Scenario 9: includes all costs associated with monitoring blood glucose (i.e., accounting for all medical costs of monitoring blood glucose beyond those reimbursed by the Ontario Ministry of Health and Long-Term Care)
Scenario 10: assumes a lower price of one flash sensor ($70 vs. $89 in the reference case)
Scenario 11: assumes a higher uptake rate, from 50% in year 1 to 70% in year 5
Scenario 12: uses a lower-cost strip ($0.40 per test strip)
We provided various estimates of the target population in Scenarios 1, 4, 5, 6, and 11 in Table A7 (Appendix 5).
In Scenario 1, we included all patients with type 1 or type 2 diabetes who require intensive insulin therapy. However, it is challenging to provide accurate estimates of the budget impact of funding flash glucose monitoring for these patients, as various public and private health insurance plans currently subsidize the cost of diabetes treatment and management. The assumptions we made are that people between 25 and 64 years of age who are ineligible for the Ontario Drug Benefit program receive (1) no public funding if they have private insurance, and (2) up to $920 of funding through the Ontario Monitoring for Health Program if they are without private insurance in the current scenario (self-monitoring group).92,103 In Table A8 in Appendix 5, we provided a publicly funded amount per patient by population breakdown between those who have private insurance and those who do not.
In Scenario 6, the cost for the Ontario Ministry of Health and Long-Term Care is $2,445 per year for insulin users who use 3,000 strips yearly. In this scenario, the cost of self-monitoring of blood glucose is almost the same as the cost of flash glucose monitoring.
In Scenarios 7 and 8, the cost of using flash glucose monitoring partially during a year can be considered the weighted cost by time of fully using flash glucose monitoring and fully using self-monitoring of blood glucose.
We conducted the budget impact analysis using Microsoft Excel.104
Results
Reference Case
Table 14 presents the projected total costs of flash glucose monitoring and self-monitoring of blood glucose at an increased uptake rate for type 1 and type 2 diabetes separately. It also shows the expected net budget impact in the next 5 years. At an uptake rate of 15% in year 1, the net budget increase of adoption of flash glucose monitoring for type 1 diabetes and type 2 diabetes was $2.9 million and $11.7 million, respectively. The total budget impact of flash glucose monitoring, including both type 1 and type 2 diabetes, was $14.6 million in year 1. The total budget impact increased to $38.6 million (type 1 diabetes: $7.7 million; type 2 diabetes: $30.9 million) in year 5, assuming the uptake rate of 35%.
Table 14:
Budget Impact of Adopting Flash Glucose Monitoring, Reference Case
| Variable | Budget Impact, $a | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Type 1 diabetes | |||||
| SMBG (current scenario) | 7,640,943 | 10,528,830 | 13,588,063 | 16,815,072 | 20,211,641 |
| Flash (new scenario) | 10,542,979 | 14,527,689 | 18,748,821 | 23,201,450 | 27,888,039 |
| Net budget impact of T1D | 2,902,036 | 3,998,858 | 5,160,758 | 6,386,378 | 7,676,398 |
| Type 2 diabetes | |||||
| SMBG (current scenario) | 10,933,991 | 15,068,894 | 19,447,726 | 24,066,917 | 28,926,469 |
| Flash (new scenario) | 22,630,095 | 31,188,108 | 40,250,982 | 49,811,329 | 59,869,149 |
| Net budget impact of T2D | 11,696,104 | 16,119,214 | 20,803,256 | 25,744,411 | 30,942,680 |
| Net budget impact of T1D and T2D | 14,598,140 | 20,118,072 | 25,964,014 | 32,130,789 | 38,619,078 |
Abbreviations: SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
Numbers may be inexact because of rounding.
Scenario Analyses
Table 15 presents the results of the scenario analyses (for Scenarios 1 through 12). Compared with the reference case, flash glucose monitoring led to a greater budget increase when the target population was expanded (Scenarios 1, 4, 6, and 11) and a smaller budget increase when the target population was reduced (Scenario 5). For instance, if flash glucose monitoring were publicly funded for all diabetes cases requiring intensive insulin therapy (with or without Ontario Drug Benefit program coverage), then the net budget impact would increase between $44 million in year 1 and $115 million in year 5 (Scenario 1). If the target population uses 3,000 strips per patient yearly (the maximum number of strips reimbursed) in the self-monitoring group (Scenario 2), then the budget impact of flash glucose monitoring would be around $240,000 in year 1 and $630,000 in year 5. If funding is capped at four or eight flash sensors each year (Scenarios 7 and 8, respectively), then the net budget impact of flash glucose monitoring would be much smaller than the reference case, where we assumed an annual funding of 26 sensors per patient.
Table 15:
Budget Impact of Adopting Flash Glucose Monitoring, Scenario Analyses
| Scenario | Budget Impact, $a | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Scenario 1: All patients who require intensive insulin therapy, with or without ODB coverageb | |||||
| SMBG, T1D | 8,295,983 | 11,432,270 | 14,753,703 | 18,257,632 | 21,945,841 |
| Flash, T1D | 21,823,186 | 30,075,733 | 38,812,217 | 48,032,490 | 57,732,074 |
| Net budget impact, T1D | 13,527,203 | 18,643,463 | 24,058,514 | 29,774,859 | 35,786,232 |
| SMBG, T2D | 12,071,111 | 16,635,654 | 21,469,886 | 26,569,317 | 31,933,949 |
| Flash, T2D | 42,198,312 | 58,154,224 | 75,051,879 | 92,879,113 | 111,631,298 |
| Net budget impact, T2D | 30,127,201 | 41,518,570 | 53,581,993 | 66,309,795 | 79,697,349 |
| Net budget impact, T1D and T2D | 43,654,405 | 60,162,033 | 77,640,507 | 96,084,654 | 115,483,581 |
| Scenario 2: Assumes that target population uses 3,000 strips per year (8 per day) in SMBG groupc | |||||
| SMBG, T1D | 10,467,045 | 14,423,055 | 18,613,785 | 23,034,345 | 27,687,180 |
| Net budget impact, T1D | 75,934 | 104,634 | 135,036 | 167,105 | 200,859 |
| SMBG, T2D | 22,467,105 | 30,963,480 | 39,961,080 | 49,452,570 | 59,437,950 |
| Net budget impact, T2D | 162,990 | 224,628 | 289,902 | 358,759 | 431,199 |
| Net budget impact, T1D and T2D | 238,924 | 329,261 | 424,937 | 525,864 | 632,058 |
| Scenario 3: Assumes that target population uses 730 strips per year (2 per day) in SMBG groupc | |||||
| SMBG, T1D | 2,546,981 | 3,509,610 | 4,529,354 | 5,605,024 | 6,737,214 |
| Net budget impact, T1D | 7,995,998 | 11,018,078 | 14,219,466 | 17,596,426 | 21,150,826 |
| SMBG, T2D | 5,466,996 | 7,534,447 | 9,723,863 | 12,033,459 | 14,463,235 |
| Net budget impact, T2D | 17,163,099 | 23,653,661 | 30,527,119 | 37,777,870 | 45,405,914 |
| Net budget impact, T1D and T2D | 25,159,098 | 34,671,739 | 44,746,585 | 55,374,296 | 66,556,740 |
| Scenario 4: All patients with type 2 diabetes who require any type of insulin therapy (including, but not limited to, intensive insulin therapy)b,d | |||||
| SMBG, T2D | 21,867,982 | 30,137,787 | 38,895,451 | 48,133,835 | 57,852,938 |
| Flash, T2D | 45,260,190 | 62,376,215 | 80,501,963 | 99,622,657 | 119,738,297 |
| Net budget impact, T2D | 23,392,208 | 32,238,428 | 41,606,512 | 51,488,823 | 61,885,359 |
| Net budget impact, T1D and T2D | 26,294,244 | 36,237,287 | 46,767,270 | 57,875,201 | 69,561,757 |
| Scenario 5: Adults with diabetes (type 1 and type 2) who require intensive insulin therapyb | |||||
| SMBG, T1D | 4,472,834 | 6,163,087 | 7,955,076 | 9,845,233 | 11,831,771 |
| Flash, T1D | 6,171,620 | 8,503,833 | 10,976,421 | 13,584,460 | 16,325,487 |
| Net budget impact, T1D | 1,698,786 | 2,340,746 | 3,021,345 | 3,739,227 | 4,493,716 |
| SMBG, T2D | 10,830,470 | 14,924,916 | 19,260,911 | 23,837,267 | 28,650,412 |
| Flash, T2D | 22,415,837 | 30,890,116 | 39,864,332 | 49,336,020 | 59,297,794 |
| Net budget impact, T2D | 11,585,367 | 15,965,201 | 20,603,421 | 25,498,754 | 30,647,381 |
| Net budget impact, T1D and T2D | 13,284,153 | 18,305,946 | 23,624,765 | 29,237,981 | 35,141,098 |
| Scenario 6: Includes patients at high risk of glycemic variabilityb | |||||
| SMBG, T1D | 8,817,159 | 12,149,474 | 15,678,122 | 19,401,320 | 23,320,850 |
| Flash, T1D | 12,165,923 | 16,763,854 | 21,632,686 | 26,769,957 | 32,178,128 |
| Net budget impact, T1D | 3,348,764 | 4,614,380 | 5,954,564 | 7,368,637 | 8,857,278 |
| SMBG, T2D | 12,301,186 | 16,952,505 | 21,878,691 | 27,074,985 | 32,542,575 |
| Flash, T2D | 25,459,780 | 35,086,621 | 45,282,354 | 56,037,129 | 67,353,408 |
| Net budget impact, T2D | 13,158,594 | 18,134,116 | 23,403,663 | 28,962,144 | 34,810,833 |
| Net budget impact, T1D and T2D | 16,507,358 | 22,748,496 | 29,358,227 | 36,330,782 | 43,668,111 |
| Scenario 7: Funding is capped at 4 flash sensors per patient per yeare | |||||
| Flash, T1D | 8,087,410 | 11,144,039 | 14,382,026 | 17,797,592 | 21,392,626 |
| Net budget impact, T1D | 446,467 | 615,209 | 793,963 | 982,520 | 1,180,984 |
| Flash, T2D | 12,733,392 | 17,548,773 | 22,648,227 | 28,027,596 | 33,686,881 |
| Net budget impact, T2D | 1,799,401 | 2,479,879 | 3,200,501 | 3,960,679 | 4,760,412 |
| Net budget impact, T1D and T2D | 2,245,868 | 3,095,088 | 3,994,464 | 4,943,198 | 5,941,397 |
| Scenario 8: Funding is capped at 8 flash sensors per patient per yeare | |||||
| Flash, T1D | 8,533,877 | 11,759,248 | 15,175,988 | 18,780,111 | 22,573,610 |
| Net budget impact, T1D | 892,934 | 1,230,418 | 1,587,925 | 1,965,039 | 2,361,969 |
| Flash, T2D | 14,532,792 | 20,028,652 | 25,848,727 | 31,988,275 | 38,447,294 |
| Net budget impact, T2D | 3,598,801 | 4,959,758 | 6,401,002 | 7,921,357 | 9,520,825 |
| Net budget impact, T1D and T2D | 4,491,735 | 6,190,176 | 7,988,927 | 9,886,397 | 11,882,793 |
| Scenario 9: Includes all costs associated with monitoring blood glucose (i.e., beyond those reimbursed) | |||||
| SMBG, T1D | 7,875,328 | 10,851,800 | 14,004,875 | 17,330,872 | 20,831,630 |
| Flash, T1D | 10,562,511 | 14,554,603 | 18,783,555 | 23,244,433 | 27,939,705 |
| Net budget impact, T1D | 2,687,184 | 3,702,802 | 4,778,680 | 5,913,562 | 7,108,075 |
| SMBG, T2D | 11,269,390 | 15,531,130 | 20,044,282 | 24,805,166 | 29,813,784 |
| Flash, T2D | 22,672,020 | 31,245,887 | 40,325,551 | 49,903,610 | 59,980,063 |
| Net budget impact, T2D | 11,402,630 | 15,714,758 | 20,281,270 | 25,098,443 | 30,166,279 |
| Net budget impact, T1D and T2D | 14,089,814 | 19,417,560 | 25,059,950 | 31,012,005 | 37,274,354 |
| Scenario 10: Assumes a lower price of flash sensors ($70 vs. $89 in the reference case) | |||||
| Flash, T1D | 8,428,165 | 11,613,583 | 14,987,999 | 18,547,476 | 22,293,983 |
| Net budget impact, T1D | 787,222 | 1,084,752 | 1,399,936 | 1,732,404 | 2,082,342 |
| Flash, T2D | 18,090,729 | 24,932,092 | 32,177,046 | 39,819,685 | 47,860,009 |
| Net budget impact, T2D | 7,156,738 | 9,863,198 | 12,729,320 | 15,752,767 | 18,933,540 |
| Net budget impact, T1D and T2D | 7,943,960 | 10,947,950 | 14,129,256 | 17,485,171 | 21,015,882 |
| Scenario 11: Assumes a higher uptake rate, at 50% in year 1 to 70% in year 5b | |||||
| SMBG, T1D | 25,468,025 | 28,953,837 | 32,609,210 | 36,434,143 | 40,421,498 |
| Flash, T1D | 35,140,801 | 39,950,528 | 44,994,214 | 50,271,861 | 55,773,616 |
| Net budget impact, T1D | 9,672,777 | 10,996,691 | 12,385,005 | 13,837,718 | 15,352,118 |
| SMBG, T2D | 36,449,017 | 41,440,647 | 46,672,638 | 52,144,988 | 57,854,128 |
| Flash, T2D | 75,438,575 | 85,769,759 | 96,598,416 | 107,924,545 | 119,740,760 |
| Net budget impact, T2D | 38,989,558 | 44,329,112 | 49,925,778 | 55,779,558 | 61,886,632 |
| Net budget impact, T1D and T2D | 48,662,335 | 55,325,803 | 62,310,783 | 69,617,275 | 77,238,750 |
| Scenario 12: Uses lower-priced strips, $0.4 per strip | |||||
| SMBG, T1D | 4,453,310 | 6,136,435 | 7,919,423 | 9,800,195 | 11,779,791 |
| Flash, T1D | 10,277,343 | 14,161,656 | 18,276,434 | 22,616,877 | 27,185,385 |
| Net budget impact, T1D | 5,824,033 | 8,025,221 | 10,357,011 | 12,816,682 | 15,405,594 |
| SMBG, T2D | 6,372,572 | 8,782,484 | 11,334,564 | 14,026,731 | 16,858,985 |
| Flash, T2D | 22,059,917 | 30,402,307 | 39,236,837 | 48,556,305 | 58,360,713 |
| Net budget impact, T2D | 15,687,346 | 21,619,823 | 27,902,273 | 34,529,574 | 41,501,728 |
| Net budget impact, T1D and T2D | 21,511,379 | 29,645,043 | 38,259,283 | 47,346,256 | 56,907,322 |
Abbreviations: ODB, Ontario Drug Benefit; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
Numbers might be inexact because of rounding.
Estimation of target population in Appendix 5.
Cost in flash group was same as cost in the reference case.
Budget impact for type 1 diabetes was same as for reference case.
Flash group combined use of flash glucose monitoring for a given duration and use of self-monitoring of blood glucose for the remaining time. Total cost of self-monitoring of blood glucose was the same as total cost in the reference case.
Discussion
Our analysis shows that flash glucose monitoring would lead to an annual budget increase of $14.6 to $38.6 million over 5 years for type 1 and type 2 diabetes with intensive insulin therapy, assuming an uptake rate of 15% in year 1 and 35% in year 5. The main factors affecting the net budget include the number of people using flash glucose monitoring, the frequency of self-testing, and the price of flash glucose monitoring.
If the target population is expanded to include people who are ineligible for coverage under the Ontario Drug Benefit program, then the net budget impact would be substantially greater. Because people with type 1 diabetes self-test more frequently than those with type 2 diabetes, the difference in the cost between flash glucose monitoring and self-monitoring of blood glucose per person is smaller in type 1 diabetes. If insulin users use the maximum number of blood glucose test strips that are allowable for reimbursement at 3,000 per year, then the annual cost of self-monitoring of blood glucose and flash glucose monitoring would be very similar (at $2,445 for self-monitoring of blood glucose and $2,463 for flash glucose monitoring). If funding is limited to a small number of flash sensors per patient yearly (e.g., for times when it is inconvenient to use self-monitoring of blood glucose, such as during travel), then the net budget impact would be much smaller.
Target Population
Flash glucose monitoring can be used in almost all cases of diabetes, with the exception of a few conditions.101 There are more than 1 million persons with diabetes in Ontario, and the potential cost of adopting flash glucose monitoring for all who have diabetes could reach billions of dollars a year. Published randomized controlled trials have evaluated flash glucose monitoring for type 1 diabetes with relatively stable disease conditions or type 2 diabetes with intensive insulin therapy who were using self-monitoring of blood glucose multiple times daily.32,33 We expected that people using intensive insulin therapy are likely to gain the greater benefit from flash glucose monitoring than any other population subgroup (e.g., people managing their diabetes through diet and exercise or people with hypoglycemic unawareness). The associated budget impact of funding flash glucose monitoring for people using intensive insulin therapy only is expected to be much smaller than that for all people with diabetes.
Conclusions
We estimate that publicly funding flash glucose monitoring for people with type 1 diabetes or with type 2 diabetes requiring intensive insulin therapy who are eligible for coverage under the Ontario Drug Benefit program would lead to an annual net budget increase ranging from $14.6 million in year 1 to $38.6 million in year 5.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, preferences, priorities, and values of people with type 1 or type 2 diabetes.
Background
Patient, caregiver, and public engagement provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the patient, the patient's family and other caregivers, and the patient's personal environment. It also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., sometimes typical outcome measures do not reflect what is important to those with lived experience).105–107 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in published literature, we contact and speak directly with people who live with a given health condition, including those who have experience with the intervention we are exploring.
Diabetes affects both the young and old, requires daily management, and lasts for a lifetime. To truly understand its effect on quality of life, we heard from people with diabetes and their families. For children with diabetes, we spoke to their parents about the effect of the disease, and several youth participated in a focus group.
Because the flash glucose monitoring device can be used to monitor glucose levels in both type 1 and type 2 diabetes, we spoke to participants with both types of the disease. Many participants had experience with managing their diabetes using a variety of devices, including test strips and glucometer, continuous glucose monitoring, and flash glucose monitoring. Understanding and appreciating the experience of day-to-day diabetes management helps to contextualize the potential value of these devices from the perspective of people affected by the condition.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with type 1 or type 2 diabetes, including their experience with the management of this disease using different techniques, such as the flash glucose monitoring device.
We used a variety of approaches, including qualitative interviews, focus groups, and online surveys. This permitted the participation of a diverse group of affected Ontarians. Our main task in interviewing is to understand what people tell us and understand the story behind their experiences.108 The survey allowed for a greater number and range of participants to respond, providing value in the volume of experiences shared. The focus groups allowed for valuable in-depth discussion to explore themes and perspectives around diabetes management.
Participant Recruitment
We used an approach called purposive sampling,109–112 which involves actively reaching out to patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, health clinics, diabetes support associations, and patient groups to spread the word about this engagement activity and to make contact with patients, families, and caregivers with experience of diabetes management and the flash glucose monitoring device.
Inclusion Criteria
We sought to speak with patients with diabetes and their families, who may have used flash glucose monitoring to manage their diabetes treatment. Participants were not required to have direct experience with flash glucose monitoring.
We sought a broad geographic, cultural, and socioeconomic representation to elicit possible equity issues in accessing and using these devices.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
We conducted interviews and focus groups with 37 people. We interviewed twenty-five people with diabetes and family members one on one, either in person or over the phone, and we conducted two six-person focus groups. We also conducted an Ontario-wide survey, through which we heard from 344 people. Participants included adults with diabetes as well as parents of children with diabetes. Participants were recruited from across Ontario. Most participants had direct experience with the flash glucose monitoring device. Since no patients interviewed received a flash glucose monitoring device immediately upon diagnosis of diabetes, patients were able to compare management of diabetes both with and without the aid of flash glucose monitoring.
Approach
At the beginning of the interviews and focus groups, we explained the role of Health Quality Ontario, the purpose of the health technology assessment, the risks of participation, and how personal health information would be protected. We gave this information to participants both verbally and in a letter of information if requested (Appendix 6). We then obtained each participant's verbal consent before starting the interview or focus group. With participants' consent, we audio-recorded the phone interviews and then had the recordings transcribed.
Interviews lasted 20 to 60 minutes, while the focus groups each lasted between 1 and 2 hours. Sessions were loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.113 Questions focused on how diabetes affected the patients' and families' quality of life, experiences with treatments, the effect of having access or not having access to a flash glucose monitoring device; and perceived benefits, limitations, and barriers to using flash glucose monitoring for managing diabetes. See Appendix 7 for our patient interview guide.
Data Extraction and Analysis
We used a modified grounded-theory method to analyze interview transcripts, focus groups transcripts, and written input from the surveys. The grounded-theory approach allowed us to organize and compare information across participants. This method consisted of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.114,115 We used the NVivo qualitative data analysis software program116 to identify and interpret patterns in interview, focus group, and survey data. The patterns we identified then allowed us to highlight how health conditions and treatments affected the patients, family members, and caregivers we interviewed.
Results
Lived Experience of Diabetes
Participants in interviews, focus groups, and the online survey consistently commented on the daily burden of managing diabetes. For people with type 1 diabetes, regular monitoring and adjustment of glucose levels in the blood through administration of insulin is required. This involves regular calculations and medical decisions regarding dosages and food intake for themselves or a loved one. Participants emphasized that, while these calculations and injections might eventually become routine, they are of vital medical importance and errors can have grave health consequences in both the short term and long term. Participants with type 2 diabetes shared the burden of having to constantly think about their diabetes: testing their blood glucose, thinking carefully about what they eat and when they eat, and timing physical activity appropriately.
Diagnosis
When discussing their lived experience with either type 1 or type 2 diabetes, participants often began with their diagnosis. The diagnosis of diabetes was often described as a traumatic, emotional event, especially for parents when applied to their young children. Acute medical care occasionally prompted the unexpected diagnosis. Often, both adult patients and parents had no previous experience and limited knowledge of diabetes and reported feeling overwhelmed and scared by the implications of the diagnosis. Participants recounted the steep learning curve they faced in learning about the disease and how it was to be managed on a day-to-day basis.
It's been a constant struggle since I was diagnosed. I went undiagnosed for months before I was diagnosed.
We are 5 years in, so you do take a little while to get on your feet after diagnosis, and it is overwhelming just learning about type 1 and about the basics of type 1, the carbs, and the insulin, and the dosing. So that took about a year.
When we were diagnosed, … our paediatrician sent us to the emergency. … And it's because my doctor did a urine test in the office and sent us straight to the hospital. If he didn't, [my son] would have been sick.
Adult patients and parents of newly diagnosed children (both type 1 and type 2) reported quickly learning about the daily management of diabetes. This included the daily injections of insulin or other medications, adjustments to diet, and the effect that activities could have on blood glucose levels. All of these factors had to be learned and adjusted for to ensure good health, which could be overwhelming.
I am now an insulin-dependent diabetic and test my blood four times a day and require [pancreatic enzymes] to digest food.
So at first we tried to manage it with diet; that worked for a short period of time. And then we went onto the [medication] and that worked for a little while. And then I increased the dose of that and then eventually I went on to short-acting insulin and then I went on to long-acting insulin.
As I said, I can always do better with the fruit and vegetables. I can always, you know, stay better away from the chips and cookies. But I have a much healthier diet than I did before being diagnosed.
There are different carbs. Pizza and french fries, for instance, they're not the greatest choice of food nutritionally, but it can be a late high. It can take a while to show that high blood sugar. And there are adjustments on the pump that allow you to account for those sugars, and they teach us that in diabetes management.
Well, after [my] diagnosis, I just avoided sugar completely. At first it was really strict but then I gradually once in a while I kind of, you know, just feel too much stress to avoid all sweets, and also I eat way less starchy food and go to gym twice a week or three times a week if I can and that really helped, really helped.
Beyond the overwhelming information and learning required, participants often reported that the diagnosis of diabetes had an emotional impact. This was especially true for parents of newly diagnosed children with type 1 diabetes. Parents often reported feeling overwhelmed, inadequate, and fearful of their own inability to keep their children healthy. This emotional burden was not limited to parents but could affect the entire family.
I think, I mean obviously initially it's a bit of a roller coaster ride [emotionally]. It's not a diagnosis anybody wants to hear. We knew a little bit about type 1 because we have friends that have type 1, but that didn't give any, you know, … what we thought we knew was nowhere near what we needed to know to raise a boy that has it, especially a boy that had already secured his spot as an athlete. It was tough.
We started off with the four finger pokes like the hospital suggested, and we very quickly went to 8 to 10 a day, but it was heart-wrenching for all of us.
Day-to-Day Impact of Diabetes
Participants emphasized that the diagnosis of diabetes, whether type 1 or type 2, led to a profound change in their daily activities, affected their families, and reduced their quality of life. Day-to-day diabetes management required constant awareness and monitoring of glucose and diet, influencing many aspects of their lives. Participants often reported struggling to understand the fundamentals of the disease at first, including how to control glucose levels and understanding why glucose levels go up or down at particular times.
And it's not mismanagement that causes those lows always; it can be lack of attention, but sometimes there's just no rhyme or reason.
Illness is way harder, like my son was not sick physically looking at him but his blood sugar was 32. So you crack, crack, crack, fix, fix, fix, and then a week later he got a cold, but it's stuff like that. You can't see or predict or know why [his levels are] high. …
The daily management of diabetes was also reported to affect the social life of patients and families and to reduce their freedom to perform many common activities. Common examples raised during interviews were challenges with driving long distances, challenges with work activities, and restrictions on physical activities.
As a type 1 there is the issue of driving, I have to [have a level of 5] to drive. So if I need to go somewhere and my blood sugar comes out at 4.9 with the finger poke, I have to figure out how am I going to deal with that to get it to a 5 so I can drive.
I quit full time and I went part time so I could be more flexible with appointments and again school trips or if the school needs us to pick them up. One of us is home, but we do shift work and it's the opposite. So, again, we don't really see each other because we're on opposite [shifts] so one of us is home with them or can be available for the school.
One challenge raised by many parents was navigating the school system and finding supports for their children with diabetes while in the classroom. This was described as a particularly difficult, frustrating, and emotional challenge, as many school staff or other adult supervisors were hesitant to care for a child with type 1 diabetes.
And when I dropped her off at school, I sat in that parking lot because—after the education I'd been given about low blood sugars, high blood sugars, complications, things that can happen—after the education I'd been given, they wanted me to drop this little 7-year-old in grade 2 off who wasn't poking her own finger and just hope for the best.
So the school system has a policy in place where if his sugars are 6 or less they won't allow him to leave the school unsupervised. We live in an area where he walks home from school, so a couple of days a week I'm getting the panic call, well you know, the teacher and the office staff have to leave, he's 4.2 so you know, we've got to come and pick him up. …They'd rather he'd run at 14 because then they're not worrying about him having a low, you know? They don't understand the long-term effects that that 14 is going to have on him.
When speaking of diabetes and its effect, whether in their day-to-day lives or on social activities, participants often reflected on the daily emotional burden of the illness. Fear, frustration, and anxiety were emotions commonly expressed and could last for many years. This was especially true for parents of children with type 1 diabetes. These parents related their ongoing fears and anxiety in caring for their child. The daily burden of keeping their child healthy was often accompanied by a strong fear of failure, especially for younger children. For older children, parents reported struggling with maintaining the balance of giving their children independence to manage their diabetes and of trying to keep them safe. This emotional burden had a large impact on both on the parents, their children, and extended families.
We're exhausted. But we still have to get up and go to work. And then, like I don't know, I feel bad complaining; they have to live with it. It's emotional; I'm sorry. And it's all day every day. What activities do we have tonight, or what are we going to eat?
So, … that's one thing that weighs on us as a family, that we're always watching. And constant—like it's a major mental … tiredness. You get just so tired because you're always mentally on.
So he's mouthy to me because his sugars are high and then when I say to him, “You know, maybe we should check” and then he's mouthy back—you know, it just is a struggle. It's definitely a struggle.
People with type 1 get “diabetes burnout” after years of having to manage this condition 24 hours a day. The [flash glucose monitoring device] got her excited about testing. As a young adult, discretion around testing is very important. Finger pokes are painful and messy and wear on you after so many years of doing them so many times a day.
Cost of Diabetes
When asked about the impact of diabetes, a common comment from participants was the cost burden of the disease. Whether adult or child with type 1 or type 2 diabetes, the burden of cost associated with diabetes was mentioned as a significant hardship. The effect varied, however, as some participants reported having some private insurance coverage for glucometer test strips, for example, while others did not. In particular, participants who were retired and on a fixed income often mentioned the challenges they face in paying for their diabetes management supplies. Additionally, some participants reported that they expected these costs to rise as long-term diabetes resulted in future medical complications.
It's a lot of money that I miss out during the month when I could be putting that toward something else that's needed, you know, for my life. It gets quite expensive, and then on top of it you have to pay for your insulin and stuff like that, too, so it's not a cheap disease, that's for sure.
I guess I am at a bit of an advantage in that I do have the benefits in my retirement, so my test strips are covered 100%. And 600 test strips is $430, I think.
Well, as you age with diabetes, it gets more of a concern and you certainly run into more side effects. … I've always had excellent blood pressure and good cholesterol control. I'm not on any medication for either of those, but I do see the blood pressure rising with my age, … but also not so good on the [low-density lipoprotein cholesterol]. That one's climbing as well. So I can see that, you know, there's going to be a cost involved in having to take more medications.
Other Effects of Diabetes
Managing diabetes also resulted in changes to sleep patterns for either the patient or family members. People with diabetes sometimes report waking up at night to check their blood glucose levels. For parents of children with diabetes, this would often necessitate waking up several times a night to test their children's blood glucose levels. Knowing the risks of hypoglycemia, parents reported that this was a nightly requirement without fail, which had a large impact on themselves and their child.
And then … I wake up every night, a minimum of one time to check him while he's sleeping.
So we have sort of a rule of thumb: If [my son] is under 8.0, we'll get up and test in 2 hours. If he's over 8.0 going to bed, we sleep through the night. [He] feels his lows so he'll wake up. He knows it. And traditionally he's up once a night going to the bathroom anyway. When he gets up, he tests.
Yes, we check every 2 hours. So we just get up, … but it is exhausting. [My son] is pretty active in hockey and lacrosse, so we find he kind of goes low around 2:00 or 3:00 in the morning and then just when they've been sick over March (they've both been sick, so they've needed more insulin) we got up to [check blood sugar levels] and then treated.
Diabetes Management
Many of those interviewed had been dealing with diabetes for several years and had managed their condition in various ways. Often, participants expressed frustration at the traditional methods of diabetes management and wished for more convenience and more accuracy. Self-monitoring of blood glucose was perceived as a burden.
It's very difficult to live with something where every single day everything you eat has to be managed and monitored and controlled. And if you've got something that can keep you focused and functioning other than poking your finger 10 times a day, it sure would be a blessing.
The relatively recent development of medical devices such as continuous glucose monitors and their expansion into Canada has allowed many of those interviewed the opportunity to manage and accurately monitor their diabetes without using self-monitoring of blood glucose. Most interviewees had direct experience with continuous glucose monitoring devices and were able to compare flash glucose monitoring with those devices, as well as with self-monitoring of blood glucose. A previous health technology assessment conducted by Health Quality Ontario provides details of the perceived benefits and drawbacks of continuous glucose monitoring devices from the perspectives of adults with diabetes and parents of children with diabetes.18
Flash Glucose Monitoring
Perceived Costs
Compared with continuous glucose monitoring devices, participants generally reported feeling that the flash glucose monitoring device was more affordable and more accessible financially. The level of financial commitment was not as large as for some continuous glucose monitoring devices but could still be challenging for patients and families on a fixed income. When online survey respondents were asked what they disliked about the flash glucose monitoring device, cost was the most frequent (60%) response.
For the most part, participants reported that the cost of a flash glucose monitoring device was approximately equivalent to paying for test strips for their glucometer, necessary for self-monitoring of blood glucose. However, test strips were often covered by insurance or paid for by the province, whereas the flash glucose monitoring device was not. Additionally, participants reported preferring the flexibility of the flash glucose monitoring device sensors versus continuous glucose monitoring device transmitters. Flash sensors are used for 2 weeks at a time and can thus be purchased in 2-week installments, whereas continuous glucose monitoring transmitters last for 3 months and are more expensive.
Don't get me wrong; I love [continuous glucose monitoring]. You'll have to take that from my cold, dead hands, but once you start a [continuous glucose monitoring] transmitter, you're committed for that 3 months, so it's not like I can take it off and try it, and put it on again, right? That transmitter starts and the battery dies. If you don't use it: so sad, too bad. But with the [flash glucose monitoring device], she can wear it off and on. It's just a matter of putting another sensor on her. The wand is the wand, and nothing expires, and batteries don't die. So I like that you can use it at your leisure when you want it based on your needs as opposed to [continuous glucose monitoring where] you're kind of committed [for] those 3 months if you buy into the system.
The [flash glucose monitoring device] also works out to be cheaper than the test strips that my supplemental insurance covers. I tried to explain this to my insurance company, but all they could say was it wasn't covered.
I find it difficult to pay for and feel bad for those who cannot at all afford it.
I think $90 [for] a 14-period monitoring strip is too expensive. I think something in the order of $25 makes sense. I pay for it, as my current insurance does not cover preexisting conditions and I have [had] diabetes for over 25 years. If you can subsidize this for people like me, I can use it throughout the year. Now I use it intermittently based on my cash flow.
Perceived Medical Benefits
Participants reported believing that the flash glucose monitoring device was of medical benefit to them because it allowed for better glucose control. These perceived benefits align with the findings reported in the Health Quality Ontario health technology assessment of continuous glucose monitoring devices.18 Stabilizing glucose levels to avoid dangerous highs and lows is the goal of those with diabetes, and participants thought that the flash glucose monitoring device was more useful in achieving this than self-monitoring of blood glucose. Participants thought that this was achieved mostly through the ability of the flash glucose monitoring device to provide a continuous reading of their glucose levels over several days, rather than simply a single point of reference as observed using traditional finger pricks. This is consistent with the reports of medical benefits of continuous glucose monitoring devices.
Additionally, several participants reported that the ease of use of flash glucose monitoring led them to check their glucose levels more often; it was simply easier and less painful to check glucose using the flash glucose monitoring device than using traditional finger pricks. In the online survey, when asked what they liked about the flash glucose monitoring device, the two most common responses were the reduction in finger pricks (96%) and the ability to see blood glucose trends (92%).
This device has improved my A1C [levels] by being able to see how my blood sugars are trending and correcting a high before it gets even higher. Testing by finger poke captures only that moment in time, and you can't tell where it has been or where it is heading. [Flash glucose monitoring] helps with this.
I started using [flash glucose monitoring] 2 months ago, and because of it my blood sugars have been greatly improved, I feel better, and I am no longer worried about going low or high during exercise, because I have immediate feedback from [the flash glucose monitoring device] and I can adjust my insulin, or my food intake, to stay within acceptable parameters. My diabetes management has become efficient, less stressful, and my long-term outcomes have greatly improved.
I started using [flash glucose monitoring] at the end of February 2018, and it has completely changed my type 1 diabetes care management. My [glycated hemoglobin level] has dropped almost 1%, which is incredible. I have tighter control on my glucose, and it's actually so much easier than the finger poking I have been doing for the past 25 years. What it has done to my quality of life is immeasurable. Checking my glucose is so much faster and with less fuss, and the [flash glucose monitoring device] shows me predictions of where my glucose is trending so I can act on trends faster (i.e., if it's going up or down).
Participants reported that the perceived medical benefits of being able to monitor glucose levels (whether through a continuous glucose monitor or with flash glucose monitoring) resulted in decreased stress and anxiety concerning their condition. These devices were seen as providing both physical and emotional benefits.
I have been a diabetic 53 years, and with [flash glucose monitoring], I have a huge psychological stress removed from my life.
When she learned to poke her finger, I breathed a sigh of relief because now I knew at least she could test herself. That all would be eliminated with [flash glucose monitoring]. All gone. All that stress, and worry, and dramatic impact on her life and my life all would've been gone with the [flash glucose monitoring device].
It certainly gives me more peace of mind, enabling me to check levels no matter where I am and take appropriate action.
This ability to improve blood glucose levels through more timely and accurate information was dependent on flash glucose monitoring providing accurate readings of blood glucose levels. When participants thought that the device was not providing accurate readings, they were more inclined to discontinue using the device and use self-monitoring of blood glucose. Often, participants indicated that it would take a little time for the device to provide accurate data and they often double-checked the readings through manual finger pricks. However, once accuracy was consistently established, participants reported that they would reduce the number of finger pricks and trust the readings on their devices.
I find that the accuracy of [flash glucose monitoring] can be somewhat off at first, by 1 or
2 mmol per litre, but after a day or two it reads the same as my [glucometer].
It's critical and essential, as I found that the value detected by the [flash] sensor is always 2 mmol higher or lower than my actual glucose value obtained by finger pricking. If that inaccuracy gap can be closed up by the calibration, the users can then really trust the [flash] sensor and no need [for] always finger pricking to verify if the value shown on [the flash glucose monitoring device] is accurate. Accurate glucose value is exceptionally crucial to anyone with insulin-dependent diabetes.
Perceived Usability and Social Benefits
Some participants thought that the flash glucose monitoring device was slightly easier to use and maintain than continuous glucose monitors. Participants appreciated that it could be easily worn on the arm in a location that was not too obvious and did not fall off easily. However, this was not universally reported. Others who were interviewed mentioned having to spend extra on tape and adhesives to keep the device on or that it would fall off if the arm bumped into something. This was mentioned particularly as a challenge for young, active children.
I help out with the grandkids a lot, and that's when I find I tend to test less, because I have to get out my gear and, you know, I'm busy with them or whatever. But if I had the [flash glucose monitoring device], I could just wave my arm and it would be hidden, like inconspicuous.
My brother [has type 1 diabetes], … and he raves about [flash glucose monitoring]. We just took a trip to Florida in November, him and I, and we were on the road for 2 days and he was just waving his arm with his reader. And I was able to hand him a candy if he thought he was going to be going a little low or whatever.
We tried the continuous glucose monitors when we first got the pump, but he lasted only a week on that. He just didn't like having another gadget attached to him in that area, like it had to be on his belly area because he's very lean, so he didn't want to. It wasn't his thing. … We actually borrowed [a flash glucose monitoring device] from another diabetic family that had an extra, … just to do a trial for the first 14 days. Within 2 days he said, “You know, you can go online and order it because I'm keeping it.” He made a decision pretty quickly. It's on the inside of his arm where it's a little bit more discreet; … he just found it less intrusive, too.
The other [drawback] would be the ability to keep it on. You know, we spend the extra money and buy the special tapes and the stickers to keep it on there because 12-year-old boys move around a lot, and he's at the point where he actually likes to shower now, too, so that's kind of nice. Kind of want to keep it on there.
Adult patients and parents of children both expressed the opinion that flash glucose monitoring devices were less socially obtrusive than continuous glucose monitors and that both devices were an improvement over traditional finger pricking. These advantages were apparent in social settings and during physical activities. By reducing barriers to checking blood glucose levels, many participants thought that devices actually keep them or their family members safer.
My teenage daughter had stopped checking herself in school because of the attention she got when taking out her strips and meter; now she just scans and goes on with her activities.
The [flash glucose monitoring device] is so much easier to carry around versus my old glucometer. Having to check my glucose in social settings is not as much of a burden and embarrassment for me, therefore keeping me safer.
You know the coaches can quickly scan his arm when he's just passing by them as opposed to making a whole display out of taking his gloves off and finger poke and all that stuff.
Tobogganing, we just went through that. Like it was fantastic, just kept it in your pocket and pulled it out and okay we're good to go. Swimming in the summer, like I'm looking forward to, again not poking the finger and you know, get the stuff out, by the side of the pool scan him and away he goes. So it's easier.
The fact that the sensor could stay in place for 14 days was seen as an advantage over continuous glucose monitors, which might not last as long and sometimes have to be re-sited every few days. Some participants found the insertion of the flash sensors easier than the continuous glucose monitoring sensors, though this varied; other participants thought both types were equally easy to insert and remove.
I have tried the combination insulin pumps and [continuous glucose monitors]. I found them impractical (given my activity levels), imprecise, and very cumbersome and embarrassing in gym showers. Also, costs were higher than [flash glucose monitoring] or purely test strips and injections. Also, I continuously had infections at the probe sites.
Pros of [flash glucose monitoring]: the flash sensor can last for 2 weeks (14 days), while [the continuous glucose monitoring] sensor can last for 10 days maximum (sometimes 7 days only). [The continuous glucose monitoring] sensor gives me skin irritation while [the flash] sensor hasn't given me any yet. Hopefully it'll never happen.
Continuous glucose monitoring does the same thing but is more expensive and not covered yet by a lot of plans. [The device] also has to be changed more frequently. The 14-day lifespan of the [flash] sensor means that you are not changing sites as often.
Disadvantages of Flash Glucose Monitoring Devices
Many participants reported appreciating the ability of continuous glucose monitoring devices to set an alarm for low blood glucose levels. This was considered an extremely valuable feature, especially for parents concerned about the glucose levels of young children. Unfortunately, this is not a common feature of the flash glucose monitoring device, which was cited as a disadvantage by several participants.
So … the only part that I don't like about the [flash glucose monitoring device] is that there's no way [it can alert] me like the [continuous glucose monitor] does. So there are pros and cons to both of them, I guess. I mean … I'm on a lot of social media groups, so I know there are ways to get the [flash glucose monitoring device to sound an] alarm, but you have to put, like, this whole other … thing on top of the sensor, like—and it's a lot of technical stuff, which is difficult for me sometimes.
There are only three things I dislike: There is no alarm. Most of the time, this is fine for me because I feel my lows, but it would be useful to have an alarm for nighttime lows.
It's close but not perfect. Additional application sites would be helpful and an alarm to my phone for nights.
We love the device but really wish it could [set off an] alarm or communicate with the pump. We may end up having to go back to using continuous glucose monitoring for the sake of alarms.
Additionally, participants who were familiar with the benefits and drawbacks of both continuous glucose monitors and flash glucose monitoring devices often lamented that the latter did not include a feature that allowed for remote monitoring or connection to smart phones. Newer versions of continuous glucose monitors have this feature, where a parent could monitor a child's glucose levels while the child was with a friend, away at school, or sleeping (for example). This was the most common disadvantage mentioned when participants compared flash glucose monitoring devices to continuous glucose monitoring devices.
She still forgets to text me her numbers, though; an app with a caregiver login would be very beneficial.
You know, he doesn't love wearing the [continuous glucose monitor] because, you know, I look at it on my phone constantly and [when] he's at school, I'll send him a text message. Like you know, check your [monitor], your [alarm] is going off and it bothers him, right? So [he], as a diabetic, prefers the [flash glucose monitoring device] 100%. For me as a parent, [I] prefer the [continuous glucose monitor] just because I can see it.
I really wish that there was an app for the meter. As a parent of a [child with type 1 diabetes], following on an app would give me peace of mind when he is sleeping, at school, or out with friends.
Hopefully one day it will be linked with a pump system to make it more convenient than it already is.
The flash blood glucose monitoring system is relatively new to Canada, and participants reported that it isn't widely available. Many participants reported having to order the system and its components online; it wasn't available locally. This created access issues and caused frustration when the device needed to be serviced or if there were issues with delivery.
We have been using this device for about 6 months. We have found it very difficult to get refills. Phone line is always busy with message that the wait is up to 32 minutes. The company has not contacted the doctor's office regarding refills as they stated they had.
One concern I have is the sole sourcing of this product in Ontario. I found the company not very customer focused. I would prefer to use my pharmacy, as I do with all my other prescriptions.
My experience from the beginning was a bit of frustration, as they were not readily available through our regular drug store where I purchase all my medications and supplies. However, once I did manage to obtain them from the online supplier, I have nothing but praise for this system.
Discussion
Participation in engagement for this topic was extensive, and patients and caregivers shared their experiences enthusiastically. Many patients with type 1 and type 2 diabetes participated in each of our methods of engagement; through one-on-one interviews, focus groups, or online surveys. Family members of those with diabetes also participated greatly in this engagement, especially parents of children with type 1 diabetes.
Many of those who participated had direct experience with multiple methods of diabetes management, including using diet and exercise, multiple daily injections, continuous glucose monitors, and the flash glucose monitoring system. This familiarity with multiple methods of management allowed for extensive discussion and varied perspectives on the advantages and disadvantages of each. Many participants were able to express clearly the perceived benefits of the flash glucose monitoring device compared with other methods of diabetes management. Similarly, these participants were able to describe the barriers and drawbacks to this technology.
Overwhelmingly, participants reported support for flash glucose monitoring. To clearly emphasize and illustrate the benefits of these devices, many participants spoke of the burden and challenges of diabetes and its daily management. Participants reported passionately on the negative impact diabetes, whether type 1 or type 2, could have on their daily activities and quality of life. Participants then described how devices such as continuous glucose monitors and the flash glucose monitoring device could reduce these negative impacts, in multiple ways. Often, participants categorized these benefits as medical, social, and emotional and provided many examples of each.
While specific types of continuous glucose monitors were not compared as part of patient engagement, most participants were able to compare continuous glucose monitors with the flash glucose monitoring device. Participants generally believed that there were benefits to each and that preference for a particular device would be based on a person's unique preferences and needs. Overall, however, participants expressed the view that flash glucose monitoring was more affordable and therefore there were fewer barriers to accessing flash glucose monitoring devices than continuous glucose monitoring devices. This reduced cost allowed for greater freedom to try using the device on a limited basis, without the large commitment needed to try continuous glucose monitoring.
Despite mostly positive reports on both continuous glucose monitors and flash glucose monitoring devices, those who participated were also able to identify concerns about and drawbacks to these devices. Such concerns included cost, accuracy, comfort, and specific features of each type of device. However, the overwhelming opinion among participants was that these concerns were relatively minor compared with the benefits these devices could provide. Many participants currently using the flash glucose monitoring device stated that it is essential to their diabetes management and that they would never want to return to previous methods of managing their diabetes.
Conclusions
Participants reported on the positive impact that the flash glucose monitoring system had on their diabetes management or that of their family members. Participants with diabetes and parents of children with diabetes reported that flash glucose monitoring helped them control their blood glucose levels or their children's, resulting in physical, social, and emotional benefits. Many participants were able to compare different methods of diabetes management. The cost of flash glucose monitoring was the largest barrier to its use.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
Based on a moderate certainty of evidence, we found that, compared with self-monitoring of blood glucose, flash glucose monitoring reduces the mean time spent in hypoglycemia and mean hypoglycemia events in adults with well-controlled type 1 diabetes and adults with type 2 diabetes who use intensive insulin therapy. Also based on a moderate certainty of evidence, flash glucose monitoring is more effective than self-monitoring of blood glucose in increasing time in the target glucose range, reducing time above the target glucose range, and reducing glucose variability among adults with well-controlled type 1 diabetes. The certainty of evidence on the effectiveness of flash glucose monitoring in other clinical outcomes is either low or very low. Findings from this health technology assessment are not generalizable to pregnant people, people with diabetes who do not use insulin, and children younger than 13 years of age.
We identified one full-text cost–utility analysis that compared flash glucose monitoring with self-monitoring of blood glucose among people diagnosed with type 1 diabetes or with type 2 diabetes requiring intensive insulin therapy. Although this study reported that flash glucose monitoring represented good value for money, the study was associated with major methodological limitations and likely overestimated the benefits and cost-effectiveness of flash glucose monitoring versus self-monitoring of blood glucose.
We were unable to conduct a primary economic evaluation for the Ontario setting owing to the unavailability of necessary clinical outcomes.
Our budget impact analysis found that publicly funding flash glucose monitoring for people with type 1 diabetes or with type 2 diabetes requiring intensive insulin therapy who are eligible for coverage under the Ontario Drug Benefit program would lead to an annual net budget increase ranging from $14.6 million in year 1 to $38.6 million in year 5.
Participants reported on the positive impact that flash glucose monitoring had on their diabetes management or that of their family members. Participants reported finding that flash glucose monitoring helped them or their children control their blood glucose levels, resulting in medical, social, and emotional benefits. The cost of flash glucose monitoring was seen as the largest barrier to its use.
Acknowledgments
This report was developed by a multidisciplinary team from Health Quality Ontario, which is now the Quality business unit at Ontario Health, and the Canadian Agency for Drugs and Technologies in Health (CADTH). From Health Quality Ontario, the lead clinical epidemiologist was Conrad Kabali, who was assisted by clinical epidemiology student Selena Hussain; the lead health economist was Xuanqian Xie; the secondary health economist was Olga Gajic-Veljanoski; the health economics associate was Jennifer Guo; the patient and public partnership analysts were Jenny Gilbert and David Wells; and the medical librarian was Melissa Walter.
From CADTH, Manager of program development Teo Quay and program development officers Michel Boucher and Jeff Mason provided input on the scope of the review and reviewed the draft report. Research information specialist Melissa Severn provided peer review for the literature search strategy.
The medical editor was Elizabeth Jean Betsch. Others involved in the development and production of this report were Doug Willcocks, Claude Soulodre, Kara Cowan, Saleemeh Abdolzahraei, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to the following experts for lending their expertise to the development of this report: Alice Cheng (St. Michael's Hospital), Céline Huot (University of Montreal), Margaret Lawson (Children's Hospital of Eastern Ontario), Lorraine Lipscome (Women's College Hospital), Karen McAssey (McMaster Children's Hospital), Daria O'Reilly (McMaster University), Bruce Perkins (University of Toronto), Christine Richardson (Children's Hospital of Eastern Ontario), David Saleh (Queen's University), Baiju Shah (Sunnybrook Health Sciences Centre), Sandi Williams (St. Michael's Hospital), and Vincent Woo (University of Manitoba).
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those consulted.
ABBREVIATIONS
- A1C
Glycated hemoglobin
- BGRI
Blood Glucose Risk Index
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CI
Confidence interval
- DDS
Diabetes Distress Scale
- DQoL
Diabetes Quality of Life
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HFS
Hypoglycemia Fear Survey
- ICER
Incremental cost-effectiveness ratio
- LGRI
Low Glucose Risk Index
- MAGE
Mean Amplitude of Glycemic Excursions
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- Peds QL
Pediatric Quality of Life Inventory
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY
Quality-adjusted life-year
GLOSSARY
- A1C
A1C is glycated hemoglobin, a form of hemoglobin measured primarily to identify a person's 3-month average blood glucose concentration.
- Adverse event
An adverse event is any unexpected problem that happens during or as a result of treatment, regardless of the cause or severity.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Blood Glucose Risk Index (BGRI)
The Blood Glucose Risk Index (BGRI) is a measure of overall glucose variability when assessing the relationship between glucose variability and the risk for hypoglycemia and hyperglycemia.
- Confidence interval
Where a value (e.g., the number of people in Ontario with a particular health condition) is estimated based on a sample of the population (e.g., the number of people in a particular region of Ontario with the health condition), the true value for the entire population may fall above or below the estimated value. The confidence interval shows the range of values likely to include the true value and is usually given at 95%, meaning that there is a 95% chance that the true value falls within the given range around the estimated value.
- Continuous monitoring of blood glucose
Continuous monitoring of blood glucose is a way to measure a person's blood glucose levels that involves measuring the blood glucose level every few minutes via a sensor inserted under the skin.
- Continuous subcutaneous insulin infusion
Continuous subcutaneous insulin infusion is a type of insulin therapy that involves wearing a device called an insulin pump that provides a steady stream of insulin into a person's body.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- Fear of hypoglycemia
Fear of hypoglycemia consists of anxiety-like symptoms that a person with diabetes may experience resulting from a fear of experiencing hypoglycemia.
- Glucose variability
Glucose variability consists of fluctuations in blood glucose levels that occur throughout the day; it may include periods of hypoglycemia (low blood glucose) and hyperglycemia (high blood glucose).
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Hyperglycemia
Hyperglycemia is high blood glucose. If not managed effectively, hyperglycemia can lead to long-term diabetes complications such as kidney disease, heart disease, stroke, nerve damage, and damage to the eyes, leading to blindness.
- Hypoglycemia
Hypoglycemia is low blood glucose. It may lead to loss of consciousness, seizure, or coma.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Intensive insulin therapy
Intensive insulin therapy is a method of controlling blood glucose levels that involves multiple daily insulin injections or a continuous subcutaneous insulin infusion.
- Low Blood Glucose Risk Index (LBGR)
The Low Blood Glucose Index (LBGR) is a measure that summarizes the number and extent of hypoglycemia events experienced by a person with diabetes.
- Mean amplitude of glycemic excursions (MAGE)
The mean amplitude of glycemic excursions (MAGE) is an arithmetic average of either the upward or downward movement of all glycemic excursions exceeding the standard deviation of blood glucose obtained from all blood glucose concentrations within a 24-hour period.
- Mean difference
Also known as “difference in means,” the mean difference is the difference between the average values of two different groups (e.g., treatment group versus control group).
- Minimum clinically important difference
The minimum clinically important difference is a patient-derived score on a measurement scale that reflects a change resulting from a clinical intervention that is meaningful for the patient.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Quality of life
Quality of life is a standard of health, wellness, and happiness experienced by a person or group.
- Randomized controlled trial
A randomized controlled trial is a type of study in which participants are randomly assigned to different groups, with one group receiving the treatment under study and the other group(s) receiving a different treatment or a placebo (no treatment) in order to determine the effectiveness of one approach compared with the other(s).
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Risk difference
Risk difference is the difference in the risk of an outcome occurring between one health care intervention and an alternative intervention.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Severe hypoglycemia
Severe hypoglycemia is hypoglycemia that requires assistance from another person to treat.
- Systematic review
A systematic review is a process used to answer a research question by methodically identifying and assessing all available studies that evaluate the research question. The systematic review process is designed to be transparent and objective and is aimed at reducing bias in determining the answer to a research question.
- Time in hypoglycemia
Time in hypoglycemia is the time a person with diabetes spends outside the target glucose range (< 3.9 mmol/L).
- Time in target glucose range
Time in target glucose range is the time a person with diabetes spends within the target glucose range (3.9–10.0 mmol/L).
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: April 6, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2018>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to April 04, 2018>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 14>, Ovid MEDLINE(R) ALL <1946 to April 04, 2018>
Search Strategy:
-
1
Blood Glucose Self-Monitoring/ (21466)
-
2
flash.ti,ab,kf. (39069)
-
3
1 and 2 (174)
-
4
((freestyle or free style) adj3 (libre∗ or flash)).ti,ab,kf. (195)
-
5
(libre adj3 flash).ti,ab,kf. (49)
-
6
(Abbott adj3 (flash or libre∗)).ti,ab,kf. (54)
-
7
((flash or intermittent or wearable or patch or patches or factory calibrat∗ or scan or scans or scanned or scanning or disc or discs or disk or disks or noninvasive∗ or non invasive∗ or minimal∗ invasive∗) adj3 (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (1016)
-
8
((sensor based or sensorbased) adj3 (glucose monitor∗ or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (26)
-
9
((upper arm or FGM or FGMs or FMS) and (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (133)
-
10
or/3–9 (1138)
-
11
exp Animals/ not Humans/ (14285663)
-
12
10 not 11 (898)
-
13
Case Reports/ or Comment.pt. or Editorial.pt. or (letter not (letter and randomized controlled trial)).pt. or Congresses.pt. (4914680)
-
14
12 not 13 (863)
-
15
limit 14 to english language [Limit not valid in CDSR; records were retained] (828)
-
16
limit 15 to yr=“2014 -Current” (417)
-
17
16 use medall,coch,cctr,clhta,cleed (193)
-
18
FreeStyle Libre/ (14)
-
19
flash glucose monitoring/ (13)
-
20
blood glucose monitoring/ (20494)
-
21
flash.tw,kw,dv. (40770)
-
22
20 and 21 (170)
-
23
(flash and glucose).dq. (30)
-
24
((freestyle or free style) adj3 (libre∗ or flash)).tw,kw,dv. (235)
-
25
(libre adj3 flash).tw,kw,dv. (57)
-
26
(Abbott adj3 (flash or libre∗)).tw,kw,dv. (90)
-
27
((flash or intermittent or wearable or patch or patches or factory calibrat∗ or scan or scans or scanned or scanning or disc or discs or disk or disks or noninvasive∗ or non invasive∗ or minimal∗ invasive∗) adj3 (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (1028)
-
28
((sensor based or sensorbased) adj3 (glucose monitor∗ or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (26)
-
29
((upper arm or FGM or FGMs or FMS) and (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (138)
-
30
or/18–19,22–29 (1184)
-
31
(exp animal/ or nonhuman/) not exp human/ (10360323)
-
32
30 not 31 (1116)
-
33
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (9746617)
-
34
32 not 33 (827)
-
35
limit 34 to english language [Limit not valid in CDSR; records were retained] (785)
-
36
limit 35 to yr=“2014 -Current” (351)
-
37
36 use emez (163)
-
38
17 or 37 (356)
-
39
38 use medall (166)
-
40
38 use coch (0)
-
41
38 use cctr (25)
-
42
38 use clhta (2)
-
43
38 use cleed (0)
-
44
38 use emez (163)
-
45
remove duplicates from 38 (230)
Economic Evidence Search
Search date: April 10, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2018>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to April 04, 2018>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 15>, Ovid MEDLINE(R) ALL <1946 to April 09, 2018>
Search Strategy:
-
1
Blood Glucose Self-Monitoring/ (21633)
-
2
flash.ti,ab,kf. (39219)
-
3
1 and 2 (182)
-
4
((freestyle or free style) adj3 (libre∗ or flash)).ti,ab,kf. (208)
-
5
(libre adj3 flash).ti,ab,kf. (53)
-
6
(Abbott adj3 (flash or libre∗)).ti,ab,kf. (55)
-
7
((flash or intermittent or wearable or patch or patches or factory calibrat∗ or scan or scans or scanned or scanning or disc or discs or disk or disks or noninvasive∗ or non invasive∗ or minimal∗ invasive∗) adj3 (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (1030)
-
8
((sensor based or sensorbased) adj3 (glucose monitor∗ or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (27)
-
9
((upper arm or FGM or FGMs or FMS) and (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).ti,ab,kf. (139)
-
10
or/3–9 (1161)
-
11
economics/ (256540)
-
12
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (803833)
-
13
economics.fs. (402628)
-
14
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (793726)
-
15
exp “costs and cost analysis”/ (553128)
-
16
(cost or costs or costing or costly).ti. (242789)
-
17
cost effective∗.ti,ab,kf. (285283)
-
18
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (187441)
-
19
models, economic/ (11229)
-
20
markov chains/ or monte carlo method/ (72118)
-
21
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (36728)
-
22
(markov or markow or monte carlo).ti,ab,kf. (115103)
-
23
quality-adjusted life years/ (35050)
-
24
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (61388)
-
25
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (100050)
-
26
or/11–25 (2360051)
-
27
10 and 26 (102)
-
28
exp Animals/ not Humans/ (14293560)
-
29
27 not 28 (83)
-
30
Case Reports/ or Comment.pt. or Editorial.pt. or (letter not (letter and randomized controlled trial)).pt. (4860966)
-
31
29 not 30 (80)
-
32
limit 31 to english language [Limit not valid in CDSR; records were retained] (77)
-
33
limit 32 to yr=“2014 -Current” (49)
-
34
33 use medall,coch,cctr,clhta (19)
-
35
limit 10 to english language [Limit not valid in CDSR; records were retained] (1118)
-
36
limit 35 to yr=“2014 -Current” (566)
-
37
36 use cleed (0)
-
38
34 or 37 (19)
-
39
FreeStyle Libre/ (15)
-
40
flash glucose monitoring/ (14)
-
41
blood glucose monitoring/ (20656)
-
42
flash.tw,kw,dv. (40926)
-
43
41 and 42 (178)
-
44
(flash and glucose).dq. (31)
-
45
((freestyle or free style) adj3 (libre∗ or flash)).tw,kw,dv. (248)
-
46
(libre adj3 flash).tw,kw,dv. (61)
-
47
(Abbott adj3 (flash or libre∗)).tw,kw,dv. (91)
-
48
((flash or intermittent or wearable or patch or patches or factory calibrat∗ or scan or scans or scanned or scanning or disc or discs or disk or disks or noninvasive∗ or non invasive∗ or minimal∗ invasive∗) adj3 (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (1043)
-
49
((sensor based or sensorbased) adj3 (glucose monitor∗ or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (27)
-
50
((upper arm or FGM or FGMs or FMS) and (glucose monitor∗ or glucose sensor∗ or glucose sensing or glucose test∗ or (diabet∗ adj3 monitor∗))).tw,kw,dv. (144)
-
51
or/39–40,43–50 (1208)
-
52
Economics/ (256540)
-
53
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (130907)
-
54
Economic Aspect/ or exp Economic Evaluation/ (428334)
-
55
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (818372)
-
56
exp “Cost”/ (553128)
-
57
(cost or costs or costing or costly).ti. (242789)
-
58
cost effective∗.tw,kw. (296284)
-
59
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (195029)
-
60
Monte Carlo Method/ (57906)
-
61
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (40489)
-
62
(markov or markow or monte carlo).tw,kw. (120090)
-
63
Quality-Adjusted Life Years/ (35050)
-
64
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (65167)
-
65
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (119478)
-
66
or/52–65 (2002056)
-
67
51 and 66 (102)
-
68
(exp animal/ or nonhuman/) not exp human/ (10370093)
-
69
67 not 68 (102)
-
70
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) (6887772)
-
71
69 not 70 (96)
-
72
limit 71 to english language [Limit not valid in CDSR; records were retained] (93)
-
73
limit 72 to yr=“2014 -Current” (57)
-
74
73 use emez (37)
-
75
38 or 74 (56)
-
76
75 use medall (16)
-
77
75 use emez (37)
-
78
75 use coch (0)
-
79
75 use cctr (3)
-
80
75 use clhta (0)
-
81
75 use cleed (0)
-
82
remove duplicates from 75 (41)
Grey Literature Search
Performed: March 7–April 9, 2018
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: FreeStyle Libre, flash, glucose monitor, glucose monitoring
Results (included in PRISMA): 4
Ongoing clinical trials: 27 (ClinicalTrials.gov)
Ongoing HTAs: 2 (PROSPERO/EUnetHTA)
Appendix 2: Quantitative Bias Analysis
Steps to perform quantitative bias analysis to evaluate the uncertainty in point estimates for the reported percentage reduction in hypoglycemia after accounting for potential flash reading errors in Haak et al33 and Bolinder et al32:
-
1.
Determine flash reading error rate. This information is sought from accuracy studies in the literature (the focus is on the proportion of values that fall in the C, D, and E regions of the Clarke error grid).
-
2.
Derive the sum of hypoglycemic events in intervention arms from the reported average number of hypoglycemic events.
-
3.
Using the error rate reported in each accuracy study, estimate the number of misclassified events in Haak et al and Bolinder et al. This is computed as the product of reported events in Haak et al and Bolinder et al and the error rate in accuracy studies. Error rates are randomly drawn from a probability distribution that reflect their plausibility of occurrence. In our case we used the uniform distribution because no information in the literature suggests whether certain levels of misclassification errors are more likely than others.
-
4.
For each randomly selected error value, compute the corrected number of hypoglycemic events in Haak et al and Bolinder et al by subtracting the reported number of events in each intervention arm from the misclassified number of events.
-
5.
Compute the average number of corrected hypoglycemic events in each arm by dividing the corrected number of events in step 4 by the sample size.
-
6.
Compute the corrected percentage average difference by dividing the difference in the average number of corrected hypoglycemic events between the self-monitoring of blood glucose and flash glucose monitoring groups with the average number of corrected hypoglycemic events in the self-monitoring group, then multiply by 100.
-
7.
Iterate steps 4 through 6 100,000 times to obtain several plausible values for the percentage average difference.
-
8.
Draw the histogram of the distribution of the corrected percentage average difference and 95% simulation limits (the limits that cover 95% of the values that are closest to the simulated mean).
R Code for Performing Quantitative Bias Analysis
############################################################################
#
#This R code performs a quantitative bias analysis to evaluate uncertainty #
#in the reported percentage average difference in hypoglycemia events #
#between SMBG and Flash, reported as #28% in Haak et al. and 26% in #
#Bolinder et al. The procedure uses reading error rate in Flash #
# reported in Clarke Error Grid analyses. The errors of interest are #
# those falling in regions C, D, and E, which are combined to estimate #
#the overall misclassification rate. The misclassification rates reported #
#in the published literature range from 0% to 2.2% #
############################################################################
#
#Sampling sensitivity/bias parameters for Flash from the uniform distribution set.seed(400) sensit.flash<-runif(100000, min = 0, max = 0.022)
#Sampling sensitivity/bias parameters for SMBG from the uniform distribution set.seed(12)
sensit.smbg<-runif(100000, min = 0, max = 0.022)
par(cex=1.3)
quantbias <- function(nflash,nsmbg, avgflash,avgsmbg) {
#Derive the observed number of hypoglycemia events from the average number of hypoglycemia events
n.flash<-nflash
n.smbg<-nsmbg
ysum.flash.obs<-avgflash∗n.flash
ysum.smbg.obs<-avgsmbg∗n.smbg
#Initializing the variables that represent the simulated number of misclassified hypoglycemia events
ymis.flash<-numeric()
ymis.smbg<-numeric()
#Initializing the variables that represent that represent the “corrected” Flash and SMBG values for hypoglycemia events
correct.avg.flash<-numeric()
correct.avg.smbg<-numeric()
correct.percent.diff<-numeric()
#Initializing the variables that will be used to concatenate the corrected percentage average difference in hypoglycemic events
vector.correct.percentage.diff<-numeric()
#Generating the corrected percentage of hypoglycemia events in each intervention arm for (i in 1:length(sensit.flash)){
ymis.flash[i]<-ysum.flash.obs∗sensit.flash[i]
ymis.smbg[i]<-ysum.smbg.obs∗sensit.smbg[i]
correct.avg.flash[i]<-(ysum.flash.obs-ymis.flash[i])/n.flash
correct.avg.smbg[i]<-(ysum.smbg.obs-ymis.smbg[i])/n.smbg
correct.percent.diff[i]<-((correct.avg.smbg[i]-correct.avg.flash[i])/correct.avg.smbg[i])∗100 }
#Compute 95% simulated interval for corrected percentage of average difference quantile(correct.percent.diff,probs=c(0.025,0.975))
mean(correct.percent.diff)
#Draw histogram of corrected percentage of average difference and 95% simulation limits h<-hist(correct.percent.diff)
h$density = h$counts/sum(h$counts)∗100
plot(h,freq=FALSE,col=“lightblue”,ylab=“Proportion (%)”,xlab=“Corrected percentage of average difference (%)”,main=” “)
abline(v=as.vector(quantile(correct.percent.diff,probs=c(0.025,0.975))), col=c(“red”, “red”), lty=c(2,2), lwd=c(3, 3))
}
#Haak
quantbias(149,75,0.38,0.53)
#Bolinder
quantbias(119,119,1.32,1.69)
Figure A1: Histogram of Corrected Percentage of Average Reduction in Hypoglycemic Episodes and 95% Simulation Limits in Bolinder et al32 (dashed red lines).
Figure A2: Histogram of Corrected Percentage of Average Reduction in Hypoglycemic Episodes and 95% Simulation Limits in Haak et al33 (dashed red lines).
Appendix 3: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk of Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Bolinder et al, 201632 | Low risk; participants were randomized using a computer random number generator | Low risk; there is no indication that intervention allocations could have been foreseen before or during enrolment | Low risk; although participants were not blinded on intervention, it is unlikely that lack of blinding affected treatment adherence or resulted in differential care | High risk; missing data were imputed by the last observation carried forward for the primary endpoint. This could have induced outcome misclassification and underestimation of random errors | Unknown risk | Low risk for the influence of flash inaccuracy on hypoglycemic outcomes based on the findings from quantitative bias analysis |
| Haak et al, 201633 | Low risk; participants were randomized using a computer random number generator | Low risk; there is no indication that intervention allocations could have been foreseen before or during enrolment | Low risk; although participants were not blinded on intervention, it is unlikely that lack of blinding affected treatment adherence or resulted in differential care | High risk; missing data were imputed by the last observation carried forward for the primary endpoint. This could have induced outcome misclassification and underestimation of random errors | Unknown risk | Low risk for the influence of flash inaccuracy on hypoglycemic outcomes based on the findings from quantitative bias analysis |
Possible risk-of-bias levels: low, high, and unclear.
Table A2:
Risk of Biasa Among Nonrandomized Trials (ROBINS-I Tool)
| Author, Year | Pre-intervention | At Intervention | Postintervention | ||||
|---|---|---|---|---|---|---|---|
| Confounding | Study Participation Selection | Classification of Interventions | Deviations from Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results | |
| Mitsuishi et al, 201848 | Low to moderate risk; length of follow-up not provided. There could be bias if length of follow-up affects outcomes differently between intervention groups. Also unknown confounders cannot be ruled out | Low risk; before-after study makes it unlikely that selection factors were associated with type of intervention | Low risk; nothing indicates that SMBG users would be misclassified as flash users or vice versa | Low risk; nothing indicates that intended SMBG users would use flash or vice versa | Low risk; nothing suggests that data were missing | Low risk; there could be subjectivity in the quality-of-life measurements, but all reported quality-of-life scales have been validated | Unknown risk; no information to assess unknown risks |
| Al Hayek et al, 201747 | Low to moderate risk; unknown duration of SMBG use. Potential for bias if length of follow-up differs between intervention groups. Also unknown confounders cannot be ruled out | Low risk; before-after study makes it unlikely that selection factors were associated with type of intervention | Low risk; nothing indicates that SMBG users would be misclassified as flash users or vice versa | Low risk; nothing indicates that intended SMBG users would use flash or vice versa | Low risk; nothing suggests that data were missing | Low risk; there could be subjectivity in the quality of life measurements, but all reported quality of life scales have been validated | Unknown risk; no information to assess unknown risks |
| Moreno-Fernandez et al, 201850 | Low to moderate risk; unknown confounders cannot be ruled out | Low risk; nothing indicates that interventions or other selection factors dictated who would participate in study | Low risk; nothing indicates that SMBG users would be misclassified as flash users or vice versa | Low risk; nothing indicates that intended SMBG users would use flash or vice versa | Low risk; nothing suggests that data were missing | Low risk; nothing suggests systematic errors in outcome measurements | Unknown risk; no information to assess unknown risks |
Abbreviations: ROBINS-I, Risk of Bias in Non-randomized Studies—of Interventions; SMBG, self-monitoring of blood glucose.
Possible risk-of-bias levels: low, moderate, serious, critical, and no information.
Table A3:
GRADE Evidence Profile for Comparison of Flash Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Managing Type 1 Diabetes
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Glucose variability | |||||||
| 1 (RCT, adults)32 | No serious limitations | Unknown | Serious limitations (−1)a | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Time in target glucose range (3.9–10.0 mmol/L [70–180 mg/dL]) in 24 hours | |||||||
| 1 (RCT, adults)32 | No serious limitations | Unknown | Serious limitations (−1)b | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Time above the target glucose range (> 13.3 mmol/L [70 mg/dL]) in 24 hours | |||||||
| 1 (RCT, adults)32 | No serious limitations | Unknown | Serious limitations (−1)b | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Time in hypoglycemia (< 3.9 mmol/L [70 mg/dL] within 24 hours) | |||||||
| 1 (RCT, adults)32 | Serious limitations (−1)c | Unknown | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Time in hypoglycemia (< 3.9 mmol/L [70 mg/dL] at night [11 pm-6 am] within 7 hours) | |||||||
| 1 (RCT, adults)32 | Serious limitations (−1)c | Unknown | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Hypoglycemia events (<3.9 mmol/L (70mg/dL) within 24 hours | |||||||
| 1 (RCT, adults)32 | No serious limitations | Unknown | Serious limitations (−1)b | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Quality of life | |||||||
| 1 (observational, adolescents)47,48 | Serious limitations (−1)d | Serious limitations (−1)f | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| 1 (RCT, adults)32 | Very serious limitations (−2)e | No serious limitations | No serious limitations | Undetected | None | ||
| Fear of hypoglycemia | |||||||
| 1 (observational, adolescents)47 | Serious limitations (−1)g | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderatel |
| 1 (RCT, adults)32 | No serious limitations | Serious limitations (−1)h | No serious limitations | Undetected | None | ||
| Glycated hemoglobin levels | |||||||
| 1 (RCT, adults)32 | No serious limitations | Serious limitations (−1)g | Very serious limitations (−2)i | No serious limitations | Undetected | None | ⊕ Very lowl |
| 2 (observational, adolescents, adults)47,50 | Serious limitations1)d | No serious limitations | Serious limitations1)j | Undetected | None | ||
| Severe hypoglycemia events | |||||||
| 1 (observational, adults)50 | No serious limitations | Unknown | No serious limitations | Serious limitations (−1)k | Undetected | None | ⊕ Very lowl |
| 1 (RCT, adults)32 | No serious limitations | Serious limitations (−1)m | Very serious limitations (−2)k | Undetected | None | ||
| Device-related adverse events | |||||||
| 1 (RCT, adults)32 | No serious limitations | Unknown | Serious limitations (−1)n | Very serious limitations (−2)k | Undetected | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Results for standard deviation and coefficient of variation are reported as statistically significant, but standard deviation and coefficient of variation at the end of follow-up for both flash glucose monitoring and self-monitoring of blood glucose is larger than the maximum recommended clinical threshold.
Uncertainty if the difference is large enough to affect hard clinical outcomes.
Imputed data using the last observation carried forward.
Duration of self-monitoring of blood glucose in AI Hayek et al43 was not given. Potential for bias if effectiveness depends on duration of device use.
Only per-protocol results were shown. Unclear why intent-to-treat results were not reported or why some participants did not adhere to protocol.
Inconsistency in findings across all three studies.
Inconsistency in findings across studies.
Certain populations that are likely to fear hypoglycemia (e.g., those with hypoglycemia unawareness) were excluded.
Null glycated hemoglobin results were desirable, but we wanted to assess situations where null results are undesirable.
Large confidence intervals covering effect sizes that are consistent with null effects, favouring flash glucose monitoring, and favouring self-monitoring of blood glucose.
Very small number of events.
Conclusion on the certainty of evidence is based on the best quality study.
Certain populations that are likely to have severe hypoglycemia (e.g., those with hypoglycemia unawareness) were excluded.
A very small information size makes it difficult to generalize results beyond the sample in the study.
Table A4:
GRADE Evidence Profile for Comparison of Flash Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Managing Type 2 Diabetes
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Glucose variability | |||||||
| 1 (RCT, adults)33 | No serious limitations | Very serious limitations (−2)a | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Time in target glucose range (3.9–10.0 mmol/L [70–180 mg/dL]) in hours, within 24 hours | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | No serious limitations | Very serious limitations (−2)b | Undetected | None | ⊕⊕ Low |
| Time above target glucose range (> 13.3 mmol/L) in hours, within 24 hours | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | No serious limitations | Very serious limitations (−2)b | Undetected | None | ⊕ Low |
| Time in hypoglycemia (< 3.9 mmol/L [70 mg/dL] within 24 hours) | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | Serious limitations (−1)c | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Time in hypoglycemia (< 3.9 mmol/L [70 mg/dL] at night [2300–0600h] within 7 hours) | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | Serious limitations (−1)c | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Hypoglycemia events (< 3.9 mmol/L [70 mg/dL] within 24 hours) | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | Serious limitations (−1)c | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Severe hypoglycemia events | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | Serious limitations (−1)c | Very serious limitations (−2)d | Undetected | None | ⊕ Very low |
| Quality of life | |||||||
| 1 (RCT, adults)33 | No serious limitations | No serious limitations | Very serious limitations (−2)j | No serious limitations | Undetected | None | ⊕⊕ Lowh |
| 1 (observational, adults)48 | Serious limitations (−1)e | No serious limitations | No serious limitations | Undetected | None | ||
| Glycated hemoglobin levels | |||||||
| 1 (RCT, adults)33 | Serious limitations (−1)f | No serious limitations | Serious limitations (−1)g | No serious limitations | Undetected | None | ⊕⊕ Low |
| Device-related adverse events | |||||||
| 1 (RCT, adults)33 | No serious limitations | Unknown | Serious limitations (−1)i | Very serious limitations (−2)d | Undetected | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Inconsistency across several scales of glucose variability.
Confidence intervals are too wide, covering values that are consistent with null, in favour of flash glucose monitoring, and in favour of self-monitoring of blood glucose.
Uncertainty on whether the difference is big enough to affect clinical outcomes.
Data too sparse. No meaningful comparative safety inference can be made.
Duration of study not given. Potential for bias if length of follow-up differs.
Data imputed using the last observation carried forward.
Generalizability depends on the age distribution of target population.
Conclusion on certainty of evidence is based on the better-quality study.
Sample size for assessing outcome is too small. Findings might not reflect target population.
Some questions in the survey do not seem to relate to use of flash glucose monitoring or self-monitoring of blood glucose.
Table A5:
GRADE Evidence Profile for Comparison of Flash Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Managing Combined Type 1 and Type 2 Diabetes
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| 1 (observational, adults)48 | Serious limitations (−1)a | Unknown | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Device-related adverse events | |||||||
| 1 (observational, adults)48 | Serious limitations (−1)a | Unknown | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very low |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Duration of follow-up not reported. Potential for bias if length of follow-up differed between flash glucose monitoring and self-monitoring of blood glucose.
Data on self-monitoring of blood glucose were missing.
Appendix 4: Results of Applicability for Studies Included in the Economic Literature Review
Table A6:
Applicability of Studies Assessing Cost-Effectiveness of Flash Glucose Monitoring
| Author, Year | Is study population similar to that in our question? | Are interventions similar to those in our question? | Is health care system in which study was conducted sufficiently similar to current Ontario context? | Were perspectives clearly stated? | Are estimates of relative treatment effect from best available source? |
|---|---|---|---|---|---|
| Healthcare Improvement Scotland, 2018,64 | Yes | Yes | No | Yes | No |
| Hellmund et al, 201887 | Yes | Yes | No | Yes | Yes |
| Author, Year | Are all future costs and outcomes discounted? | Is value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgment (directly applicable/partially applicable/not applicable) |
|---|---|---|---|---|
| Healthcare Improvement Scotland, 201864 | Yes | Yes | No | NAa |
| Hellmund et al, 201887 | No | No | No | Partially applicable |
Abbreviation: NA, not applicable.
Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA.”
Appendix 5: Target Population Estimates in Scenario Analyses
Table A7:
Target Population Estimates in Scenario Analyses
| Variables | Target Population | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Scenario 1: All patients requiring intensive insulin therapy, with or without ODB coverage | |||||
| Type 1 diabetes | 9,110 | 12,555 | 16,202 | 20,051 | 24,100 |
| Have ODB coverage | 4,281 | 5,899 | 7,613 | 9,421 | 11,324 |
| Do not have ODB coverage or private insurance | 712 | 982 | 1,267 | 1,568 | 1,885 |
| Do not have ODB coverage, but have private insurance | 4,117 | 5,674 | 7,322 | 9,062 | 10,891 |
| Type 2 diabetes | 17,566 | 24,208 | 31,242 | 38,663 | 46,469 |
| Have ODB coverage | 9,189 | 12,664 | 16,344 | 20,226 | 24,310 |
| Do not have ODB coverage or private insurance | 1,236 | 1,703 | 2,198 | 2,720 | 3,269 |
| Do not have ODB coverage, but have private insurance | 7,141 | 9,841 | 12,700 | 15,717 | 18,890 |
|
Scenario 4: All patients with type 1 and type 2 diabetes requiring any type of insulin therapy (including, but not limited to, intensive insulin therapy) We assumed that patients with type 2 diabetes requiring any type of insulin therapy would be double the population of patients with type 2 diabetes requiring intensive insulin therapy only | |||||
| Type 2 diabetes | 18,378 | 25,328 | 32,688 | 40,452 | 48,620 |
|
Scenario 5: Adults with diabetes (type 1 or type 2) who require intensive insulin therapy We assumed that 29% (i.e., [7 ÷ 24] × 100%) of people with type 1 diabetes are adults < 25 years of age and 67% of people with type 2 diabetes are adults < 25 years of age | |||||
| Type 1 diabetes | 2,506 | 3,453 | 4,457 | 5,516 | 6,629 |
| Type 2 diabetes | 9,102 | 12,543 | 16,187 | 20,033 | 24,078 |
|
Scenario 6: Includes people at high risk of glycemic variability (not including those with hypoglycemic unawareness for both type 1 and type 2 diabetes) We excluded 25% and 10% of type 1 diabetes and type 2 diabetes with intensive insulin therapy, respectively | |||||
| Type 1 diabetes | 4,940 | 6,807 | 8,784 | 10,870 | 13,066 |
| Type 2 diabetes | 10,338 | 14,247 | 18,387 | 22,754 | 27,349 |
| Scenario 11: Assumed a higher uptake rate, at 50% in year 1 to 70% in year 5 | |||||
| Type 1 diabetes | 14,269 | 16,222 | 18,270 | 20,413 | 22,647 |
| Type 2 diabetes | 30,632 | 34,827 | 39,224 | 43,823 | 48,621 |
| Other scenarios: the target population is the same as in the reference case | |||||
Abbreviation: ODB, Ontario Drug Benefit.
Table A8:
Per-Patient Annual Funding of Glucose Monitoring in Scenario 1
| Glucose Monitoring | Funding for Patients With ODB Coverage ($/y) | Funding for Patients Without ODB Coverage or Private Insurance ($/y) | Funding for Patients Without ODB Coverage, but With Private Insurance ($/y) |
|---|---|---|---|
| Self-monitoring of blood glucose (current scenario) | |||
| Total: T1D | 1,785 | 920 | 0 |
| Total: T2D | 1,190 | 920 | 0 |
| Flash glucose monitoring (new scenario) | |||
| Total: T1D or T2D | 2,463 | 2,463 | 2,314 |
Abbreviations: ODB, Ontario Drug Benefit; T1D, type 1 diabetes; T2D, type 2 diabetes.
Appendix 6: Letter of Information
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of Flash glucose monitoring device for patients with Type 1 or Type 2 diabetes. The purpose is to understand whether these devices should be more broadly funded in Ontario.
An Important part of this review Involves speaking to patients and families of those who have experience with diabetes, who may or may not have used a Flash device. Our goal is to make sure the experiences of patients and caregivers are considered in the funding recommendations for the Flash device.
WHAT DO YOU NEED FROM ME?
-
✓
20–40 minutes of your time for a phone or ln-person Interview to share your story
-
✓
Permission to audio- (not video-) record the interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an Interview with Health Quality Ontario staff The interview will likely last 20–40 minutes. It will be held in a private location or over the telephone. With your consent, the interview will be audio-taped. The interviewer will ask you questions about you or your loved one's condition and your perspectives about diabetes and treatment options in Ontario. If you or your loved one have used Flash glucose monitoring device, the interviewer will ask some additional questions surrounding the device.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect the care you receive.
CONFIDENTIALITY
All information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying Information will be released or published Any records containing information from your Interview will be stored securely.
RISKS TO PARTICIPATION
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
If you are interested in participating, please contact Health Quality Ontario staff:
Appendix 7: Interview Guide
Interview for Flash Glucose Monitoring HTA
Intro
Explain HQO purpose, HTA process, and purpose of interview
History of Type 1 or Type 2 Diabetes diagnosis and various treatments (general only)
Lived Experience
Day-to-day routine
What is the impact on family? Adverse events?
Impact on parent if child with Type 1 diabetes (if applicable)
Decision-Making
What glucose management options are available?
(any equity issues with regard to treatment options? Cost/inconveniences?)
How to choose among treatment options? Factors that influence those decisions?
(bow to choose for you child, if applicable?)
Role of family in decision-making? Physician? Other sources of information (Internet)?
Emotion (anxiety, worry) of decision-making?
Glucose Management Devices
Experience with glucose monitoring devices, past v present
Explore CGM and Flash devices – any equity issues
Cost? Access? Health Literacy (i.e., training to be comfortable)
Safety?
Specific comparison between the devices – advantages and disadvantages of each
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario, which is now the Quality business unit at Ontario Health, and the Canadian Agency for Drugs and Technologies in Health (CADTH).
From Health Quality Ontario, the lead clinical epidemiologist was Conrad Kabali, who was assisted by clinical epidemiology student Selena Hussain; the lead health economist was Xuanqian Xie; the secondary health economist was Olga Gajic-Veljanoski; the health economics associate was Jennifer Guo; the patient and public partnership analysts were Jenny Gilbert and David Wells; and the medical librarian was Melissa Walter.
From CADTH, clinical research officer Kwakye Peprah reviewed the protocol, performed a second review of titles, abstracts, and full-text articles, performed a second review of the data extracted, and reviewed the draft clinical report.
KEY MESSAGES
What Is This Health Technology Assessment About?
Diabetes is a health condition in which the pancreas cannot produce any insulin, the pancreas cannot produce enough insulin, or the body cannot properly use the insulin the pancreas does produce. Insulin is a hormone that helps the body's cells use glucose (a type of sugar) for energy. Without insulin, glucose builds up in the blood and can cause serious damage to the body. Type 1 diabetes occurs when the pancreas produces little or no insulin. Type 2 diabetes occurs when the pancreas does not produce enough insulin or when the body does not respond to insulin properly. People with diabetes have a greater risk of being sent to hospital with life-threating conditions, such as cardiovascular and kidney disease, than people without diabetes.
People with type 1 diabetes and people with type 2 diabetes that requires insulin therapy (daily injections of insulin or a continuous infusion of insulin under the skin) must test their blood glucose levels regularly. The standard way to do this is to prick a finger to obtain a drop of blood, apply the drop of blood to a blood glucose test strip, and insert the strip into a device called a blood glucose meter. This is called self-monitoring of blood glucose. Flash glucose monitoring is a new method of measuring blood glucose levels. It uses a small sensor inserted under the skin of a person's upper arm and a separate touchscreen reader device.
This health technology assessment looked at how safe and effective flash glucose monitoring is for people with type 1 or type 2 diabetes, the budget impact of publicly funding flash glucose monitoring, and the experiences, preferences, and values of people with type 1 or type 2 diabetes.
What Did This Health Technology Assessment Find?
People with type 1 diabetes and people with type 2 diabetes requiring insulin therapy who used flash glucose monitoring spent more time in the target blood glucose range and experienced fewer episodes of low blood glucose than people who used self-monitoring of blood glucose.
We estimate that publicly funding flash glucose monitoring in Ontario over the next 5 years for people with type 1 diabetes and for people with type 2 diabetes requiring intensive insulin therapy who are eligible for coverage under the Ontario Drug Benefit program would cost about $15 million to $39 million annually.
Adults with diabetes and parents of children with diabetes with whom we spoke reported that they thought using flash glucose monitoring helped them better control their blood glucose levels or their children's, resulting in physical, social, and emotional benefits. The cost of flash glucose monitoring was the largest barrier to its use.
Contributor Information
Ontario Health (Quality):
Conrad Kabali, Selena Hussain, Kwakye Peprah, Xuanqian Xie, Olga Gajic-Veljanoski, Jennifer Guo, Jenny Gilbert, David Wells, and Melissa Walter
About Us
This health technology assessment was produced by Health Quality Ontario, which is now the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Health Quality Ontario, visit hqontario.ca.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1).World Health Organization. About diabetes [Internet]. Geneva (Switzerland): World Health Organization; 2014. [cited 2018 Mar 7]. Available from: https://web.archive.org/web/20140331094533/http://www.who.int/diabetes/action_online/basics/en/ [Google Scholar]
- (2).Madhu SV, Munichoodappa C. Clinical features of type 2 diabetes mellitus. In: Tripathy DD, Chandalia HB, Das AK, Rao PV, Madhu SV, Mohan V, editors. RRSDI textbook of diabetes mellitus. 2nd ed. New Dehli, India: Jaypee Brothers Medical Publishers (P) LTD; 2012. p. 374–80. [Google Scholar]
- (3).National Institute of Diabetes and Digestive and Kidney Diseases. Gestational diabetes [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [cited 2018 Mar 7]. Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/gestational [Google Scholar]
- (4).Diabetes in Canada: Facts and figures from a public health perspective [Internet]. Ottawa: Public Health Agency of Canada; 2011. [cited 2018 Feb 20]. Available from: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/cd-mc/publications/diabetes-diabete/facts-figures-faits-chiffres-2011/pdf/facts-figures-faits-chiffres-eng.pdf [Google Scholar]
- (5).World Health Organization. Global report on diabetes [Internet]. France: World Health Organization; 2016. [cited 2018 Feb 20]. Available from: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf [Google Scholar]
- (6).Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Diabetes Canada. Diabetes statistics in Canada [Internet]. Toronto (ON): Diabetes Canada; 2018. [cited 2018 Mar 7]. Available from: http://www.diabetes.ca/how-you-can-help/advocate/why-federal-leadership-is-essential/diabetes-statistics-in-canada [Google Scholar]
- (8).Canadian Diabetes Association. Diabetes in Ontario [Internet]. Toronto (ON): The Association; 2016. [cited 2018 Mar 7]. Available from: https://www.diabetes.ca/getmedia/73485544-2d42-4fd8-a834-b80b13ed231e/diabetes-charter-backgrounder-on-2016-09.pdf.aspx [Google Scholar]
- (9).Crowshoe L, Dannenbaum D, Green M, Henderson R, Hayward MN, Toth E. Type 2 diabetes and Indigenous peoples. Can J Diabetes. 2018;42 Suppl 1:S296–S306. [DOI] [PubMed] [Google Scholar]
- (10).Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabet Med. 2018. [DOI] [PubMed] [Google Scholar]
- (12).Diabetes Canada. Clinical practice guidelines. Can J Diabetes. 2018;42:A6–A16. [Google Scholar]
- (13).Ruiz Gracia T, Garcia de la Torre Lobo N, Duran Rodriguez Hervada A, Calle Pascual AL. Structured SMBG in early management of T2DM: Contributions from the St Carlos study. World J Diabetes. 2014;5(4):471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41(1):57–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Canadian Agency for Drugs and Technologies in Health. Self-monitoring of blood glucose [Internet]. Ottawa (ON): The Agency; 2018. [cited 2018 Aug 27]. Available from: https://www.cadth.ca/self-monitoring-blood-glucose [Google Scholar]
- (16).Ajjan RA, Cummings MH, Jennings P, Leelarathna L, Rayman G, Wilmot EG. Accuracy of flash glucose monitoring and continuous glucose monitoring technologies: implications for clinical practice. Diab Vasc Dis Res. 2018;5(3):175–84. [DOI] [PubMed] [Google Scholar]
- (17).Hoss U, Budiman ES, Liu H, Christiansen MP. Feasibility of factory calibration for subcutaneous glucose sensors in subjects with diabetes. J Diabetes Sci Technol. 2014;8(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Health Quality Ontario. Continuous monitoring of glucose for type 1 diabetes: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2018 Feb;18(2):1–160. Available from: http://www.hqontario.ca/evidence-to-improve-care/journal-ontario-health-technology-assessment-series. [PMC free article] [PubMed] [Google Scholar]
- (19).DiaTribe Learn. Flash glucose monitoring [Internet]. San Francisco: DiaTribe Foundation; 2018. [cited 2018 Mar 7]. Available from: https://diatribe.org/flash-glucose-monitoring [Google Scholar]
- (20).National Institute for Health and Care Excellence. FreeStyle Libre for glucose monitoring [Internet]. London: The Institute; 2017. [cited 2018 Mar 7]. Available from: https://www.nice.org.uk/advice/mib110/chapter/The-technology [Google Scholar]
- (21).Device name results summary [Internet]. Ottawa (ON): Health Canada; c2018. [updated 2018 Dec 11; cited 2018 Dec 12]. Available from: https://health-products.canada.ca/mdall-limh/dispatch-repartition.do?type=active [Google Scholar]
- (22).Health Canada. Guidance document—guidance on the risk-based classification system for non-in vitro diagnostic devices (non-IVDDs) [Internet]. Ottawa (ON): Health Canada; 2015. [cited 2018 Mar 22]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/guidance-document-guidance-risk-based-classification-system-non-vitro-diagnostic.html [Google Scholar]
- (23).Manulife. New FreeStyle Libre Flash glucose monitoring system [Internet]. Toronto (ON): Manufacturers Life Insurance Company; 2018. [cited 2018 Mar 7]. Available from: https://www.manulife.ca/for-business/group-benefits/learn/employee-benefits-news/new-freestyle-libre-flash-glucose-monitoring-system.html [Google Scholar]
- (24).Great-West Life plan members to have more coverage options to help manage insulin-dependent diabetes [Internet]. Winnipeg: Great-West Life; c2018. [updated 2018 Jul 05 cited 2018 Aug 28]. Available from: https://www.greatwestlife.com/common/news/news-releases/-great-west-life-plan-members-to-have-more-coverage-options-to-h.html [Google Scholar]
- (25).Ontario Ministry of Children, Community and Social Services. Ontario Disability Support Program [Internet]. Toronto (ON): Queen's Printer for Ontario; 2018. [cited 2018 Mar 7]. Available at: https://www.mcss.gov.on.ca/en/mcss/programs/social/odsp/. [Google Scholar]
- (26).Ontario Ministry of Children, Community and Social Services. Prescribed classes [Internet]. Toronto (ON): Queen's Printer for Ontario; 2018. [cited 2018 Mar 7]. Available from: https://www.mcss.gov.on.ca/en/mcss/programs/social/odsp/income_support/PrescribedClasses.aspx. [Google Scholar]
- (27).Ontario Ministry of Health and Long-Term Care. Ontario public drug programs: reimbursement levels for blood glucose test strips [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2018 Mar 19]. Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/teststrips/bg_teststrips.aspx [Google Scholar]
- (28).Abbott Laboratories Limited. FreeStyle Libre [Internet]. Mississauga (ON): Abbott Laboratories Limited; 2018. [cited 2018 Aug 27]. Available from: https://myfreestyle.ca/en/products/libre [Google Scholar]
- (29).Tucker ME. Medicare to cover FreeStyle Libre glucose monitoring system [Internet]. New York: MedScape; 2018. [cited 2018 Nov 27]. Available from: https://www.medscape.com/viewarticle/890879 [Google Scholar]
- (30).Murata T, Sakane N, Kato K, Tone A, Toyoda M. The current intermittent-scanning CGM device situation in Japan: only adjunctive use to SMBG is approved and the latest health insurance coverage details. J Diabetes Sci Technol. 2017;12(3):729–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016 Jul;75:40–6. [DOI] [PubMed] [Google Scholar]
- (32).Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–63. [DOI] [PubMed] [Google Scholar]
- (33).Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Imran M, Hafeez U, Akhtar M, Sial MA, Ahmed E, Durr ES, et al. Monitoring of glucose, salt and pure water in human whole blood: An in vitro study. Pak J Pharm Sci. 2016;29(4):1237–42. [PubMed] [Google Scholar]
- (36).DistillerSR systematic review and literature review software. Evidence Partners. Ottawa (ON). Available at: https://www.evidencepartners.com/.
- (37).Greenland S, Lash TL. Bias analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd ed. Philadelphia: Lippincott; 2008. p. 345–80. [Google Scholar]
- (38).R statistical computing software. R Foundation. Vienna. Available at: http://www.R-project.org.
- (39).Sterne JC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Internet]. [Google Scholar]
- (41).Topp CW, Østergaard SD, S⊘ndergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–76. [DOI] [PubMed] [Google Scholar]
- (42).Hilliard ME, Lawrence JM, Modi AC, Anderson A, Crume T, Dolan LM, et al. Identification of minimal clinically important difference scores of the PedsQL in children, adolescents, and young adults with type 1 and type 2 diabetes. Diabetes Care. 2013;36(7):1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Lenters-Westra E, Schindhelm RK, Bilo HJ, Groenier KH, Slingerland RJ. Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Neth J Med. 2014;72(9):462–6. [PubMed] [Google Scholar]
- (44).Hajós TRS, Polonsky WH, Pouwer F, Gonder-Frederick L, Snoek FJ. Toward defining a cutoff score for elevated fear of hypoglycemia on the hypoglycemia fear survey worry subscale in patients with type 2 diabetes. Diabetes Care. 2014;37(1):102–8. [DOI] [PubMed] [Google Scholar]
- (45).National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management [Internet]. London: The Institute; 2015. [cited 2018 Aug 27]. Available from: https://www.nice.org.uk/guidance/ng17/chapter/1-Recommendations#blood-glucose-management-2 [Google Scholar]
- (46).National Health Service London Procurement Partnership. Implementation of FreeStyle Libre prescribing guidance across the NHS in London [Internet]. London: National Health Service; 2018. [cited 2018 Aug 27]. Available from: http://www.londonscn.nhs.uk/wp-content/uploads/2018/05/dia-London-FreeStyle-Libre-Implementation-Guidance-for-London052018.pdf [Google Scholar]
- (47).Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clinical Medicine Insights. 2017;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Mitsuishi S, Nishimura R, Harashima SI, Kawamura T, Tsujino D, Koide K, et al. The effect of novel glucose monitoring system (flash glucose monitoring) on mental well-being and treatment satisfaction in Japanese people with diabetes. Adv Ther. 2018;35(1):72–80. [DOI] [PubMed] [Google Scholar]
- (49).Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Moreno-Fernandez J, Pazos-Couselo M, Gonzalez-Rodriguez M, Rozas P, Delgado M, Aguirre M, et al. Clinical value of flash glucose monitoring in patients with type 1 diabetes treated with continuous subcutaneous insulin infusion. Endocrinologia, diabetes y nutricion. 2018;65(10):556–63. [DOI] [PubMed] [Google Scholar]
- (51).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Haak T, Hanaire H, Ajjan RA, Hermanns N, Riveline J, Rayman G. Use of novel flash glucose-sensing technology to optimise glucose control in individuals with type 2 diabetes on intensive insulin therapy. Diabetes Technology and Therapeutics Conference: 9th International Conference on Advanced Technologies and Treatments for Diabetes. 2016;18:A28–A9. [Google Scholar]
- (53).Haak T, Hanaire H, Ajjan RA, Hermanns N, Riveline JP, Rayman G. The impact on quality of life, glucose monitoring frequency and safety of novel glucose-sensing technology used by individuals with type 2 diabetes on intensive-insulin therapy. Diabetes Technology and Therapeutics Conference: 9th International Conference on Advanced Technologies and Treatments for Diabetes. 2016;18:A73. [Google Scholar]
- (54).Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193(2):328–34. [DOI] [PubMed] [Google Scholar]
- (55).Henriksen MM, Andersen HU, Thorsteinsson B, Pedersen-Bjergaard U. Hypoglycemic exposure and risk of asymptomatic hypoglycemia in type 1 diabetes assessed by continuous glucose monitoring. J Clin Endocrinol Metab. 2018;103(6):2329–35. [DOI] [PubMed] [Google Scholar]
- (56).Lee AK, Warren B, Lee CJ, McEvoy JW, Matsushita K, Huang ES, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2017;41(1):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Le Floch JP, Kessler L. Glucose Variability: Comparison of Different Indices During Continuous Glucose Monitoring in Diabetic Patients. J Diabetes Sci Technol. 2016;10(4):885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Huang IC, Liu JH, Wu AW, Wu MY, Leite W, Hwang CC. Evaluating the reliability, validity and minimally important difference of the Taiwanese version of the diabetes quality of life (DQOL) measurement. Health and quality of life outcomes. 2008;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2011;22(4):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).American Statistical Association. American Statistical Association releases statement on statistical significance and P-values: provides principles to improve the conduct and interpretation of quantitative science [Internet]. Alexandria (VA): The Association; 2016. [cited 2018 Nov 27]. Available from: https://www.amstat.org/asa/files/pdfs/P-ValueStatement.pdf [Google Scholar]
- (61).Clark NG, Pawlson G. Change in HbA1c as a measure of quality of diabetes care. Diabetes Care. 2006;29(5):1184–5. [DOI] [PubMed] [Google Scholar]
- (62).Bidonde J, Fagerlund BC, Fronsdal KB, Lund UH, Robberstad B. A single-technology assessment: FreeStyle Libre flash glucose self-monitoring system [Internet]. Oslo (Norway): Norwegian Institute of Public Health; 2017. [cited 2018 Nov 27]. Available from: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2017/freestyle-libre-flash-glucose-self-monitoring-system-a-single-technology-assessment-rapport-2017-v3.pdf [PubMed] [Google Scholar]
- (63).Agency for Quality and Accreditation in Health Care and Social Welfare, Main Association of Austrian Social Security Institutions, Norwegian Institute of Public Health. Continuous glucose monitoring (CGM real-time) and flash glucose monitoring (FGM) as personal, standalone systems in patients with diabetes mellitus treated with insulin: joint assessment [Internet]. Zagreb (Croatia): European Network for Health Technology Assessment; 2018. [cited 2018 Nov 27]. Available from: https://www.eunethta.eu/wp-content/uploads/2018/07/OTJA08_CGM-real-time-and-FGM-aspersonal2c-standalone-systems-in-patients-with-diabetes-mellitus-treatedwith-insulin.pdf [Google Scholar]
- (64).Healthcare Improvement Scotland. What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin therapy? [Internet]. Edinburgh (Scotland): Healthcare Improvement Scotland; 2018. [cited 2018 Nov 27]. Available from: https://www.healthcareimprovementscotland.org/his/idoc.ashx?docid=ccdbf92d-2c39-4b61-971d-33479051f739&version=-1 [Google Scholar]
- (65).Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8(3):573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Alsaffar H, Turner L, Yung Z, Didi M, Senniappan S. Continuous flash glucose monitoring in children with congenital hyperinsulinism; first report on accuracy and patient experience. Int J Pediatr Endocrinol. 2018;2018:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ancona P, Eastwood GM, Lucchetta L, Ekinci EI, Bellomo R, Martensson J. The performance of flash glucose monitoring in critically ill patients with diabetes. Crit Care Resusc. 2017;19(2):167–74. [PubMed] [Google Scholar]
- (68).Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Charleer S, Mathieu C, Nobels F, Gillard P. Accuracy and precision of flash glucose monitoring sensors inserted into the abdomen and upper thigh compared with the upper arm. Diabetes Obes Metab. 2018;20(6):1503–7. [DOI] [PubMed] [Google Scholar]
- (70).Fokkert MJ, Van Dijk PR, Edens MA, Abbes S, De Jong D, Slingerland RJ, et al. Performance of the freestyle libre flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Olafsdottir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Scott EM, Bilous RW, Kautzky-Willer A. Accuracy, user acceptability, and safety evaluation for the FreeStyle Libre flash glucose monitoring system when used by pregnant women with diabetes. Diabetes Technol Ther. 2018;20(3):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Mancini G, Berioli M, Santi E, Rogari F, Toni G, Tascini G, et al. Flash glucose monitoring: a review of the literature with a special focus on type 1 diabetes. Nutrients. 2018;10(8):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Molnar FJ, Hutton B, Fergusson D. Does analysis using “last observation carried forward” introduce bias in dementia research? Can Med Assoc J. 2008;179(8):751–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Shahbazian H, Rezaii H. Diabetic kidney disease; review of the current knowledge. J Renal Inj Prev. 2013;2(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003;26(suppl 1):s99–s102. [DOI] [PubMed] [Google Scholar]
- (77).National Institute for Health and Care Excellence. Process and methods guides. Appendix I quality appraisal checklist–economic evaluations [Internet]. London: The Institute; 2012. [cited 2018 May 8]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (78).Bilir SP, Li H, Wehler EA, Hellmund R, Munakata J. Cost effectiveness analysis of a flash continuous glucose monitoring system for type 1 diabetes (T1DM) patients receiving intensive insulin treatment in the UK. Value Health. 2017;20 (5):A245. [Google Scholar]
- (79).Bilir SP, Wehler EA, Hellmund R, Munakata J. Cost-effectiveness of a flash glucose monitoring system based on real-world usage for type 1 diabetes (T1DM) patients using intensive insulin: a Swedish perspective. Value Health. 2017;20 (9):A583. [Google Scholar]
- (80).Bilir SP, Li H, Wehler EA, Hellmund R, Munakata J. Cost effectiveness analysis of a flash glucose monitoring system for type 1 diabetes (T1DM) patients receiving intensive insulin treatment in Europe and Australia. Value Health. 2016;19 (7):A697–8. [Google Scholar]
- (81).Vellopoulou K, Kourlaba G, Doupis J, Maniadakis N. Economic evaluation of flash glucose monitoring compared to self-monitoring of blood glucose for the management of patients receiving intensive insulin with diabetes type 1 and type 2 in Greece. Value Health. 2017;20 (9):A585. [Google Scholar]
- (82).Li H, Bilir SP, Wehler EA, Hellmund R, Munakata J. Cost effectiveness analysis of a flash glucose monitoring system for type 2 diabetes (T2DM) patient receiving intensive insulin treatment in Europe. Value Health. 2016;19:A347–A766. [Google Scholar]
- (83).Li H, Bilir SP, Donga P, Samiian A, Munakata J. Cost effectiveness analysis of flash glucose monitoring for type 2 diabetes patients receiving insulin treatment in the UK. Value Health. 2014;17(7):A351. [DOI] [PubMed] [Google Scholar]
- (84).Curto A, Torbol M, Cavazzana A, Andretta M, Scroccaro G. A cost analysis of flash glucosemonitoring systems in Veneto region. Int J Technol Assess Health Care. 2017;33 (Suppl 1):235–6. [Google Scholar]
- (85).Khan-Miron A, Sanchez-Iriso E, Cabases-Hita JM. Costs analysis of novel flash glucose monitoring technology in adults with type 2 diabetes mellitus (T2DM) under insulin treatment in Spain. Value Health. 2017;20 (9):A579. [Google Scholar]
- (86).Hellmund R. Budget impact analysis of a flash glucose monitoring system for patients with diabetes who are using intensive insulin. Value in Health. 2016;19 (7):A690–A1. [Google Scholar]
- (87).Hellmund R, Weitgasser R, Blissett D. Cost calculation for a flash glucose monitoring system for UK adults with type 1 diabetes mellitus receiving intensive insulin treatment. Diabetes Res Clin Pract. 2018;138:193–200. [DOI] [PubMed] [Google Scholar]
- (88).Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–26. [DOI] [PubMed] [Google Scholar]
- (89).Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33. [DOI] [PubMed] [Google Scholar]
- (90).Matza LS, Stewart KD, Davies EW, Hellmund R, Polonsky WH, Kerr D. Health state utilities associated with glucose monitoring devices. Value Health. 2017;20(3):507–11. [DOI] [PubMed] [Google Scholar]
- (91).Diabetes Canada Clinical Practice Guidelines Expert Committee, Berard LD, Siemens R, Woo V. Monitoring glycemic control. Can J Diabetes. 2018;42(Suppl 1):S47–S53. [DOI] [PubMed] [Google Scholar]
- (92).Sutherland G, Dinh T. Understanding the gap: a pan-Canadian analysis of prescription drug insurance coverage [Internet]. Ottawa (ON): Conference Board of Canada; 2017. [cited 2018 Nov 27]. Available from: http://innovativemedicines.ca/wp-content/uploads/2017/12/20170712-understanding-the-gap.pdf [Google Scholar]
- (93).Public Health Agency of Canada. Chapter 2: Diabetes in Canada–facts and figures from a public health perspective [Internet]. Ottawa (ON): The Agency; 2011. [cited 2018 Jul 26]. Available from: https://www.canada.ca/en/public-health/services/chronic-diseases/reports-publications/diabetes/diabetes-canada-facts-figures-a-public-health-perspective/chapter-2.html [Google Scholar]
- (94).Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1):e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87(1):64–8. [DOI] [PubMed] [Google Scholar]
- (96).Statistics Canada. Table 13-10-0096-07: Diabetes, by age group [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 Aug 9]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009607&pickMembers%5B0%5D=1.7&pickMembers%5B1%5D=3.1 [Google Scholar]
- (97).Statista. Percentage of people registered with type 1 diabetes in England in 2017, by age [Internet]. New York: Statista, Inc.; 2018. [cited 2018 Aug 8]. Available from: https://www.statista.com/statistics/387322/individuals-with-type-1-diabetes-by-age-in-england-and-wales/ [Google Scholar]
- (98).Bayshore Specialty Rx Ltd. Product catalogue [Internet]. Markham (ON): Bayshore Specialty Rx Ltd.; 2018. [cited 2018 Apr 29]. Available from: https://libre.myfreestyle.ca/?_ga=2.217637334.1963900320.1525034970-210899828.1524154548 [Google Scholar]
- (99).Gomes T, Martins D, Tadrous M, Paterson JM, Shah BR, Juurlink DN, et al. Self-monitoring of blood glucose levels: evaluating the impact of a policy of quantity limits on test-strip use and costs. Can J Diabetes. 2016;40(5):431–5. [DOI] [PubMed] [Google Scholar]
- (100).Lancets [Internet]. Vancouver (BC): Amazon.ca; c2008–2018. [cited 2018 Aug 8]. Available from: https://www.amazon.ca/s/ref=nb_sb_noss_1?url=search-alias%3Daps&field-keywords=lancets [Google Scholar]
- (101).Abbott Laboratories Limited. FreeStyle Libre flash glucose monitoring system: user's manual [Internet]. Oxford (UK): Abbot Laboratories Limited; 2014. [cited 2018 Sep 15]. Available from: https://freestylediabetes.co.uk/images/uploads/documents/FreeStyle_Libre_Manual.pdf [Google Scholar]
- (102).Ontario Ministry of Health and Long-Term Care. Ontario Public Drug Programs: reimbursement levels for blood glucose test strips [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2018 Aug 8]. Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/teststrips/bg_teststrips.aspx [Google Scholar]
- (103).Diabetes Canada. Ontario monitoring for health program [Internet]. Toronto (ON): Diabetes Canada; 2018. [cited 2018 Aug 8]. Available from: https://www.diabetes.ca/in-your-community/local-programs-events/regional-events-programs/ontario-events/ontario-monitoring-for-health-program [Google Scholar]
- (104).Excel spreadsheet software. Microsoft Corporation. Redmond (WA). Available at: http://www.products.office.com/Microsoft/Excel.
- (105).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (106).Whelan ME, Kingsnorth AP, Orme MW, Sherar LB, Esliger DW. Sensing interstitial glucose to nudge active lifestyles (SIGNAL): feasibility of combining novel self-monitoring technologies for persuasive behaviour change. BMJ Open. 2017;7(10):e018282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto: Queen's Printer for Ontario; 2015. [cited 2017 Dec]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (108).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (109).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (110).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (111).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (112).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (113).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton: Health Technology Assessment International; 2015. [cited 2017 Dec]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (114).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (115).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (116).NVivo qualitative data analysis software. QSR International. Doncaster, Victoria (Australia) Available at: https://www.qsrinternational.com/nvivo/home. [Google Scholar]