Abstract
Pancreatic beta (β) cell dysfunction results in compromised insulin release and, thus, failed regulation of blood glucose levels. This forms the backbone of the development of diabetes mellitus (DM), a disease that affects a significant portion of the global adult population. Physiological calcium (Ca2+) signaling has been found to be vital for the proper insulin-releasing function of β-cells. Calcium dysregulation events can have a dramatic effect on the proper functioning of the pancreatic β-cells. The current review discusses the role of calcium signaling in health and disease in pancreatic β-cells and provides an in-depth look into the potential role of alterations in β-cell Ca2+ homeostasis and signaling in the development of diabetes and highlights recent work that introduced the current theories on the connection between calcium and the onset of diabetes.
Keywords: β-cells, Ca2+ signaling, insulin secretion, diabetes, mitochondria
1. Introduction
Under physiological conditions, blood glucose levels only transiently increase following intake of food [1]. The observed rise in blood glucose is controlled through the body’s employment of insulin, a hormone generated by the beta (β) cells of the pancreatic islets of Langerhans. Insulin’s primary function is to regulate the body’s metabolism of carbohydrates, protein, and fats. It does so by fostering the absorption of carbohydrates (primarily glucose) out of the blood into skeletal muscle, adipose tissue and the liver [2].
The islets of Langerhans are structures found throughout the exocrine pancreas, and together they form the endocrine part of this gland. They contain primarily β, alpha (α), gamma (γ), and delta (δ) cells. Each of these cell types secretes a separate hormonal product (insulin, glucagon, pancreatic polypeptide, and somatostatin) and they are evident in different proportions across species [3]. These cell types are all able to communicate with each other through paracrine signals, integral to the intricate web of signaling and maintenance of β-cell health [4].
Key to the role of insulin in maintaining homeostatic blood glucose levels is the sensitivity of β-cells to the glucose itself [5]. For example, when blood glucose increases following food intake, β-cells sense this change in concentration and subsequently secrete insulin into the blood [6]. On the other hand, when blood glucose levels are low, such as following a prolonged fasting period, the release of insulin from β-cells is inhibited [7]. Importantly, insulin’s effect on glucose metabolism directly counteracts those of multiple hormones, such as glucagon [8]. The interplay between the activity of hormones like insulin and glucagon, therefore, regulates overall glucose homeostasis in the body.
Pathophysiological conditions arise with insulin dysregulation; declines in either insulin’s release or its actual production by β-cells characterizes the main features of diabetes mellitus (DM) development. Diabetes is a significant health problem: according to the International Diabetes Federation close to 10% of the worldwide adult population is affected by this disease [9]. Type 1 diabetes (T1DM) is the result of decreased insulin production by the pancreas due to destroyed β-cells. This typically occurs as a result of an autoimmune reaction in the body and results in insulin not being able to be synthesized or subsequently secreted into the blood [10]. Type 2 (T2DM), in comparison, is characterized by less β-cell destruction than its type 1 counterpart, as cells are not destroyed through an autoimmune response [11]. This form of diabetes is observed when remaining functional β-cells are unable to produce insulin in sufficient amounts to compensate for the body’s insulin resistance [12].
Calcium (Ca2+) has a significant role to play in physiological insulin release from islet β-cells. Glucose, acting as the main stimulus of these cells, actually serves to control Ca2+ concentrations across many compartments of the cell, including the endoplasmic reticulum (ER), mitochondria, nucleus, and cytosol, among others. Ca2+ dysregulation in β-cells also has been observed in disease states, including type 2 diabetes. The present review discusses previously known, as well as recently emerged aspects of Ca2 dynamics in pancreatic β-cells, and how this new information may relate to the pathophysiological development of T2DM.
2. The Role of Ca2+ Signaling in Pancreatic β-cells: The Basics
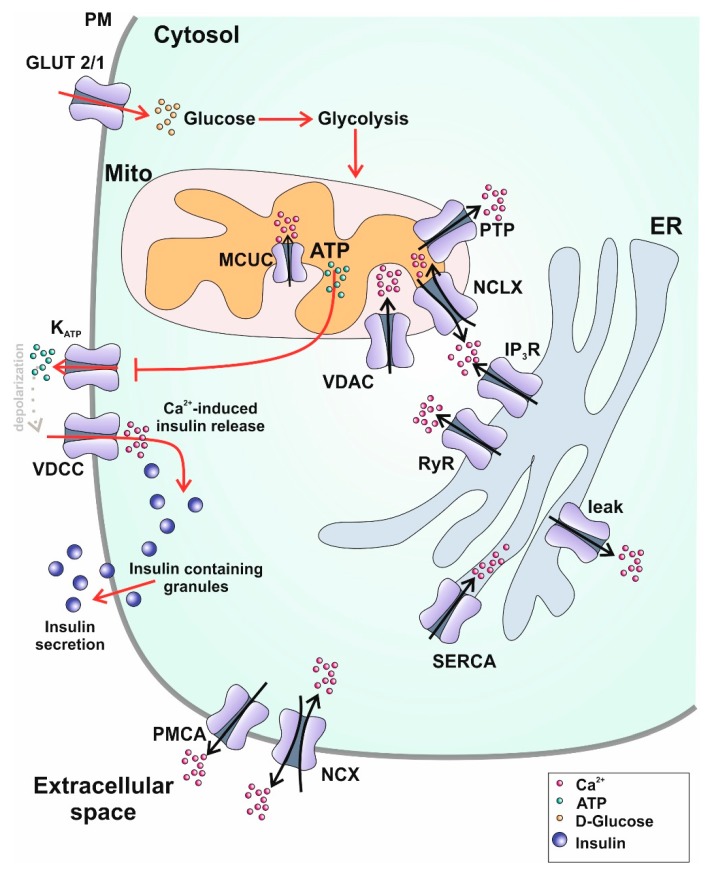
The movement of Ca2+ is vital to the function of any cell type but has a special role to play in pancreatic β-cells due to its importance in the process of insulin release. Insulin production and subsequent release from these cells is controlled by multiple players, including glucose, neurotransmitters, peptide hormones, and other compounds [13,14]. Briefly, the elevation in blood glucose levels that follows food intake is sensed by these cells, which subsequently take glucose up from the blood and metabolize it to more fuel for the mitochondria to shunt towards ATP production, increased levels of which result in the inhibition of the cell’s KATP channels. This ultimately leads to depolarization at the plasma membrane (PM), an electrical change that functions to activate L-type Ca2+ channels, which allows an influx of Ca2+ into the cell. Finally, this wave of Ca2+ triggers the release of secretory granules containing insulin, to be released from the cell by exocytosis (Figure 1).
Figure 1.
Schematic representation of glucose stimulated insulin secretion (GSIS) and subcellular Ca2+ dynamics in pancreatic β-cells. Ca2+ transporters within a pancreatic β-cell responsible for balancing Ca2+ homeostasis are the following: in the plasma membrane: PMCA: plasma membrane Ca2+-ATPase, NCX: Na+/Ca2+ exchanger; within the endoplasmic reticulum: SERCA: sarco/endoplasmic reticulum Ca2+-ATPase, RyR: ryanodine receptor, IP3R: inositol 1,4,5-trisphsphate receptor, VDAC: voltage dependent anion-selective channel; within mitochondria: NCLX: mitochondrial Na+/Ca2+ exchanger, PTP: permeability transition pore, MCUC: mitochondrial Ca2+ uniporter complex. Red arrows depict the process of glucose-stimulated insulin secretion. After uptake of glucose via GLUT2/1 mitochondrial ATP production is boosted leading to a closing of plasma membrane located ATP-sensitive K+ channels (KATP). The resulting shift in membrane potential activates PM voltage-dependent Ca2+ channels (VDCC) stimulating Ca2+-induced Ca2+ release which ultimately leads to exocytosis of insulin-containing granules.
Previous work has also put forth that glucose may directly affect the activity of voltage-dependent Ca2+ and K+ channels [15]. Recent investigation has also shown that cellular insulin secretion stimulated by glucose is tied to mitochondrial Ca2+ dynamics. Namely, the proper functioning of mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial calcium uniporter (MCU) proteins is necessary for the physiological function of β-cells [16].
The well-established insulin release-triggering pathway outlined above is not the only mechanism wherein glucose stimulates insulin release. Another, as yet not completely understood pathway is also evident, though it depends on the KATP-dependent mechanism due to the need for high cytosolic Ca2+ levels. Therefore, it is poignant to consider the fact that any of β-cells’ channels that may affect membrane potential could also have an effect on Ca2+ movement into the cytosol and thus insulin secretion.
Importantly, β-cells have a wide array of channels involved in Ca2+ influx across their plasma membranes. The placement of these excitable cells’ voltage-dependent channels has been observed to be erratically distributed across the PM, allowing for micro-domains containing high levels of Ca2+ to form [17]. In certain species, the electrical activity of the β-cells contained within an islet as a whole is synchronized due to coupling through the presence of gap junctions [18]. This leads to synchronized Ca2+ oscillations between the cells as well, which subsequently induce insulin secretion waves observable at the level of a single islet. This does not apply fully to human islets, because while there is some coupling apparent between adjacent β-cells, there is a more heterogeneous spread of the many cell types present in each islet, thus affecting the ability of β-cells separated by other cell types to behave synchronously [18].
The extrusion of Ca2+ from β-cells does not differ significantly from the majority of other cell types. Ca2+ in the cytosol is removed through a combination of Na+/Ca2+ exchanger(s) (NCX) and plasma membrane Ca2+–ATPase(s) (PMCA) activity [19,20,21,22]. As each of these transporters have complementary characteristics (PMCA has a high affinity for Ca2+ and a low extrusion capacity, while NCX displays exactly the opposite traits [23]), each exhibits different levels of activity under cellular conditions. Namely, NCX acts as the main Ca2+ extrusion pathway at high cytosolic Ca2+ concentrations, while PMCA takes over most extrusion activity when low levels are observed [24].
ER Ca2+ homeostasis is vital to proper function in most cell types [25]. In the case of β-cells, Ca2+ is taken up by the ER through SERCA activity, specifically isoforms SERCA2b and SERCA3, which are expressed ubiquitously and only in β-cells found within pancreatic islets, respectively [26]. Insofar as Ca2+ release from the ER goes, this organelle is known to exhibit considerable Ca2+ leakage into the cytosol, which must ultimately be corrected for by SERCA pump activity [27]. A likely mechanism for the ER Ca2+ leak in β-cells was recently outlined to involve presenilin-1 (PS 1) [28].
As in various other cell types, ER Ca2+ release in β-cells is mediated by inositol 1,4,5-triphosphate (IP3R) and ryanodine receptors (RyR) [29]. The latter can be activated by (local) Ca2+ elevations representing the so-called “calcium-induced calcium release” (CICR) processes [30,31]. In regard to the Ca2+ release from the ER in pancreatic β-cells, it is unclear whether they exhibit CICR as triggered by RyRs on the ER membrane. These cells show expression of all three of IP3R isoforms, but their expression and the overall function of RyR is reported as being underwhelming [32,33]. It has been discussed that RyR expression in β-cells is much lower than in most other tissues. Nevertheless, it is possible that lower expression of RyR may be sufficient to allow β-cells’ to accomplish CICR, because of their large-conductance capacity [34].
Another very important aspect of pancreatic β-cell Ca2+ signaling is the great role of stromal interaction molecules 1 (STIM1/2) and its pairing with Orai1/2/3. Generally speaking, Orais are ion channels that are selective for Ca2+, and are activated following this ion’s depletion from intracellular Ca2+ stores [35,36]. The depletion of ER Ca2+ is first sensed by the ER STIM1 protein, which is then induced to oligomerize at ER/plasma membrane junctions. STIM1 subsequently activates the Orai1 Ca2+-release-activated channel (CRAC) through direct protein interaction, thus inducing store-operated Ca2+ entry (SOCE) [37]. STIM has been observed to have two highly homologous isoforms, STIM1 and STIM2. Despite their degree of similarity, the two isoforms have differing functions. Namely, STIM1 serves as the main activator of SOCE channels, while STIM2 serves as a feedback regulator maintaining ER and cytosolic Ca2+ concentrations within a narrow range [38,39]. The Orai protein also exists in various forms: Orai1, Orai2, and Orai3. Orai2 and 3 are not as thoroughly characterized as Orai1, but they have been shown to have similar roles in modifying SOCE across different cell types [40,41,42].
Since the discovery of its mechanism in the early 2000s, the STIM/Orai Ca2+-signaling pair has been found to play a distinct role in pancreatic β-cell insulin secretion. It has been demonstrated in rat β-cells that a complex is formed by Orai1, STIM1, and TRPC1 proteins in response to ER Ca2+ depletion [43]. Blocking the activity of Orai1 or TRPC1 was shown to impair GSIS in these cells [44], indicating that Orai1 and TRCP1 are vital to the formation of store-operated Ca2+ channels (SOCs), which, combined with their activation by STIM1, are necessary for the physiological response of cells to acetylcholine (Ach) in insulin secretion. Furthermore, the group postulated that the effective activation of these SOCs may be reduced in T2DM, thus confirming the importance of Orai1 to the pancreatic cell’s optimal functionality [44]. Notably, the importance of basal mitochondrial Ca2+ entry, possibly via a TRPC-mediated mechanism has been postulated to be fundamental for the responsiveness to increased glucose [28].
Beyond the basic mechanisms involved in β-cell Ca2+ signaling, the bigger picture must of course also be considered. What kind of signals are these channels and pumps propagating? What effects can they exert on the cell? Ca2+ sparks, the creation of Ca2+ microdomains, the potential effect of Ca2+ on processes ranging from channel activity to gene expression are but a few of the many ways that this ion exerts its effect on β-cell function. Oscillations are also observable in β-cells, as well as almost universally across cell types, including cells performing substantially varied functions, such as fibroblasts and astrocytes [45,46,47]. Importantly, glucose-stimulated insulin release has been associated with transient changes to these cells’ cytosolic Ca2+ concentration. This increase in Ca2+ levels, and the subsequently observed oscillations are induced by cellular glucose metabolism [48]. It has been determined that in primary mouse islets, for example, the addition of glucose to cells can induce the generation of IP3. Every time that cytoplasmic Ca2+ increased, so did IP3, indicating clearly that these processes are related [45].
Cellular Ca2+ oscillations have also been linked to effects on gene expression. For example, Dolmetsch and colleagues [49], demonstrated that cytoplasmic Ca2+ oscillations effectively reduced the Ca2+ threshold required for the activation of a specific set of transcription factors. Whether this holds true in β-cells is as yet unclear, though other breakthroughs in the role of Ca2+ in β-cell gene expression are evident in the literature. It was discussed that the signals resulting from glucose flux into the cells are transmitted through the insulin enhancer, activation of which could result from direct protein modification along the lines of phosphorylation or similar processes, or through changes in local co-factor concentrations through Ca2+ signals [50]. Others have shown that extended exposure of β-cells to glibenclamide, an ATP-sensitive K+-channel inhibitor, which depolarizes the β-cells and thereby stimulates the cells to secrete insulin (often enlisted as a treatment tool for diabetes) [51], causes a prolonged increase in the cells’ basal insulin production, and showed that this was a Ca2+-dependent effect [52]. Ultimately, it was determined that extended exposure of the cells to this insulin secretagogue activated protein translation through Ca2+-regulated signaling pathways mTOR, MEK, and PKA [52].
The important role of Ca2+ in β-cells’ general function, indicates that dysregulation of this cation likely contributes strongly to the development of pathophysiological conditions.
3. The Role of Ca2+ Signaling in Pancreatic β-Cells: A Closer Look
3.1. Ca2+ in β-Cell Proliferation
β-cells can adapt to increased metabolic demands to ensure balanced euglycemia by boosting their proliferation rates. Several studies demonstrate that besides elevated glucose levels also Ca2+ signaling is a prerequisite for inducing augmented β-cell replication [53,54,55]. Porat et al. could show that Ca2+ influx is essentially contributing to β-cell replication since blocking membrane depolarization with diazoxide drastically reduced β-cell proliferation rates [56]. Moreover, treating rat β-cells with the L-VGCC agonist BayB8644, thus, augmenting intracellular Ca2+ concentrations, stimulates β-cell proliferation [57,58].
Intracellular Ca2+ elevations promote β-cell proliferation amongst others by Ca2+/calmodulin dependent kinase 4 (CaMK4) and calcineurin/nuclear factor of activated T-cells (NFAT)-dependent mechanisms. In β-cells CaMK4 is activated by elevated glucose levels and increased intracellular Ca2+ levels [59]. Inhibition of CaMK activity or alterations in its expression influences glucose-mediated increase in β-cell proliferation. Liu et al. [60] demonstrated that CaMK inhibition or expression of a dominant-negative CaMK result in abolished β-cell proliferation rates whereas overexpression of a constitutive active version of CaMK leads to the opposite effect. The calcineurin/NFAT axis controls islet responses by promoting the expression of cell cycle regulators and increased β-cell proliferation rates and mass. NFAT family members control cell cycle regulators on the transcriptional level in β-cell which play a role in β-cell proliferation. Blocking NFAT inhibitory kinases such as DYRK1A and GSK3β supports and boosts β-cell proliferation [57,58]. Overexpression of NFATC2 in mice leads to 2-fold increased proliferation rates [61], an effect that has also been detected when the expression of NFATC1, NFATC3, and NFATC4 is increased [62]. β-cell-specific deletions of the Ca2+-activated calcineurin phosphatase regulatory subunit, calcineurin b1 (Cnb1), in mice results in the development of age-dependent diabetes characterized by reduced β-cell proliferation and mass, reduced pancreatic insulin content and hypoinsulinaemia [61]. Recent human data show that the ability of β-cells to replicate is highest in infancy, with their ability to regeneration declining substantially soon after this period of life [63]. This age-related decrease in proliferation of β-cells has also been corroborated in rodent models [64,65]. This, of course, begs the question: what is the expected life span of human β-cells? In vitro work has demonstrated that in culture, these cells are capable of releasing insulin for more than 9 months at a time [66].
3.2. Ca2+ in β-cell Survival
Ca2+ signals play a vital role in the induction of cellular apoptosis, a trigger event that occurs in response to a wide range of cellular conditions or particular intracellular agents. On the other hand, it is also possible for Ca2+ signals to function as a cell’s saving grace by ensuring cell survival [67]. Isolated mouse islet β-cells and MIN6 insulinoma cells exhibit 3-fold reduced apoptosis rates when cultured with high glucose medium (15 mM or 25 mM) compared to low-glucose medium (2 mM or 5 mM, respectively). The high glucose concentration increases survival-required depolarization and subsequent Ca2+ influx. These pro-survival effects get lost when depolarization is inhibited with diazoxide or Ca2+ influx with nifedipine [68]. As for β-cell proliferation, also β-cell survival is mediated by calcineurin and CaMK-pathways. Calcineurin inhibition has toxic effects on β-cells and promotes β-cell failure after transplantation by blunting β-cell replication [69] and apoptosis induction [70]. Simultaneous calcineurin inhibition and treatment with the mTOR inhibitor rapamycin impair β-cell regeneration during recovery from diphtheria toxin-induced β-cell death [71]. Conversely, calcineurin inhibition has been shown to be beneficial after treatment with cytokines [72] and corticosteroids [73]. These conflicting data prove that effected down-stream pathways are diverse, but the crucial involvement of Ca2+ in β-cell survival stays unchallenged. Furthermore, the ER Ca2+ homeostasis has been shown to impact β-cell survival. Several groups provide evidence that ER Ca2+ depletion results in ER stress and β-cell apoptosis [74,75,76]. This ER Ca2+ depletion occurs due to inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) and the resultant reduced ER Ca2+ uptake in the situation when low glucose exposure is paralleled with low intracellular Ca2+ levels [77].
3.3. Ca2+ Oscillations for Proper β-Cell Function
The cycling variations of circulating insulin are reflected by the pulsatile release of the hormone from the pancreas [78].These insulin pulses occur over 4–13 min [79,80] whereas the rise of plasma glucose levels is preceding the rise in plasma insulin levels in average two minutes [81]. Loss of these regular insulin oscillations is an early phenomenon in the development of insulin [82] and non-insulin dependent [83] diabetes mellitus. Glucose stimulates an increase in cytosolic Ca2+ concentration in β-cells due to cellular depolarization mediated by closure of the ATP-sensitive K+-channels and subsequent opening of VDCC. This rise in [Ca2+]i is not a steady-state increase of the ion, but rather consists of periodic Ca2+ oscillations [84,85]. These Ca2+ waves are synchronized with β-cell bursting electrical activity [86]. The basis for a proper insulin secretion upon increased glucose levels is a tightly controlled inter-islet regulation, which are coupled by gap junctions. Islet β-cells are comprised of functionally heterogeneous cell populations, whereas a special subpopulation termed “hubs” is crucial for mediating the islets response to high glucose levels. Inhibition of these hubs abolished the coordinated and tightly regulated Ca2+ oscillations necessary for driving insulin secretion [87]. Controlled by these hubs, the islets behave as functional syncytium in response to glucose and therefore homogenizing the actual heterogeneous cell population [88]. Diverse oscillation patterns are existing: the fast, the slow (approx. 5 min) and the mixed oscillations [48]. Slow activated Ca2+-sensitive K+-channels and cyclic cytoplasmic Na+ changes are responsible for the fast and the slow oscillations, respectively. The ATP/ADP ratio and the ER Ca2+ levels are supposed to serve as pacemakers for fast bursts and cytosolic Ca2+ oscillations [89]. The total secretory activity is determined by the number of β-cells recruited into the active phases [90]. Several physiologic advantages of the glucose-induced Ca2+ oscillations within β-cells should be mentioned. As these Ca2+ oscillations are the basis for the fluctuating plasma insulin signals, a down-regulation of peripheral receptors is prevented. This is substantiated by the finding that the recycling time for these receptors is shorter than the periods for plasma insulin pulses [91]. Furthermore, the intracellular Ca2+ pulses prevent the desensitization of the secretory machinery of the β-cells. Jones et al. [92] could show that prolonged exposure to high Ca2+ concentrations in permeabilized β-cells makes the machinery refractory to Ca2+ signals. A steady-state rise of cytosolic Ca2+ concentrations in stimulated β-cells could be responsible for the loss of the pulsatile insulin signals, a process occurring at the onset of diabetes [82,83]. Sustained Ca2+ rise may activate autolytic processes by stimulating phospholipases, proteases and endonucleases [93] deteriorating primary lesions of a diabetic β-cell [90].
3.4. Ca2+ in Biphasic Insulin Secretion
Nowadays, it is well established that insulin secretion follows a biphasic pattern which was clearly described for the first time in perfused rat pancreas in 1968 [94] and one year later in humans [95]. The early peak is starting 3–5 min after glucose stimulation lasting for approximately ten minutes. This rapid insulin peak is followed by a sustained period of slowly increasing insulin levels lasting as long as the glucose level is elevated [96]. In patients diagnosed with T2DM this initial peak is absent and the second phase reduced or delayed [97,98,99,100]. The early phase is pivotal for human physiology due to several processes initiated by this first rise in insulin such as suppression of hepatic glucose production [101,102], suppression of lipolysis [102] and preparation of target cells i.e., liver, muscle, and adipocytes—to the action of insulin [103]. The reduction of the first-phase of insulin secretion occurs early in disease. The involvement of Ca2+ in the process of (biphasic) insulin secretion is unchallenged but early studies on its contribution showed a great extent of controversy: Wollheim et al. [104] stated that the first phase insulin response is independent of glucose-stimulated uptake of extracellular Ca2+ but crucially depends on intracellular Ca2+ handling. Contrary findings were presented by Henquin et al. [105] showing that withdrawal of extracellular Ca2+ or inhibition of Ca2+ influx abolishes glucose-stimulated insulin secretion postulating that extracellular Ca2+ influx plays an essential role for insulin secretion. The current model of Ca2+ signaling in insulin secretion attributes important functions to both theories. Ca2+ influx through voltage-dependent Ca2+ channels is a prerequisite for proper insulin exocytosis [106] but also intracellular Ca2+ flux is necessary for physiological insulin secretion. The latter recently has been outlined by our group as we demonstrated a pivotal engagement of a β-cell-specific permanent presenilin-1-mediated ER-Ca2+ leak in the initiation of first-phase insulin secretion. Inhibition of presenilin-1 activity or reduction of its expression or in other words a reduction of the ER Ca2+ leak leads to abrogation of the first phase of insulin release [107].
4. Ca2+ on Subcellular Level
Cytoplasmic Ca2+ homeostasis in β-cells is tightly regulated by a complex interplay between Ca2+ uptake and extrusion across the plasma membrane as well as uptake and release into/from internal Ca2+ stores. Since alterations in this equilibrium result in drastic changes of Ca2+ signaling in β-cells ultimately affecting insulin secretion, it is of crucial importance to keep cellular Ca2+ fluxes balanced. In the next paragraph, the most important contributors to β-cell Ca2+ homeostasis are discussed.
4.1. Plasma Membrane
As described above, Ca2+ transporters regulating Ca2+ extrusion from the cell—such as NCX and PMCA—located within the plasma membrane, function as essential players in maintaining a balanced Ca2+ homeostasis in β-cells. Notably, the activity of the forward-mode (i.e., 1Ca2+ out/3Na+ in) of the NCX in the pancreatic β-beta-cell is sensitive to long-chain acyl-coenzyme and alterations in intracellular acyl-CoA levels may cause negatively control Ca2+-mediated exocytosis and insulin secretion [108]. Moreover, the forward mode of the NCX1 splice variants of that is expressed in the pancreatic β-cells (NCX1.3 and NCX1.7) exhibit an unusually high sensitivity to KB-R7943 [109] that rather selectively inhibits the reversed mode of the NCX (i.e., 1Ca2+ in/3Na+ out) in most other cell types. Consequently, the pharmacological inhibition of the NCX1 with KB-R7943 results in a considerable augmentation in glucose-stimulated increases in cytosolic Ca2+ concentrations and insulin secretion in mouse and human β-cell islets, while KB-R7943 was without effect under non-stimulated conditions. [109]. Heterozygous inactivation of PMCA isoform 2 (PMCA2) in mice also leads to cytosolic Ca2+ accumulation and to a 1.5-fold increase in glucose-induced insulin release. Furthermore, PMCA2 inhibition results in increased β-cell mass, proliferation, and viability [110].
4.2. Mitochondria
Cellular energy demand and mitochondrial activation are tightly linked especially in states of energy-consuming processes. Increased energy demand especially observed during stimulation of a cell—comes along with enhanced ATP hydrolysis which must be compensated for by accelerated ATP synthesis. Examples for such an increased energy demand where mitochondria are involved in balancing the energetic homeostasis are increased workload of the heart [111], activation of specific brain areas [112] and insulin production in pancreatic β-cells after glucose stimulus. Upon glucose stimulation, β-cell mitochondria augment oxidative metabolism [113], respiration [114,115] and mitochondrial ATP synthesis rate [116] to match the increased energy need. Hormones and extracellular messengers that stimulate ATP-requiring processes through rises of cytosolic Ca2+ concentrations regulate oxidative metabolism whereas the Ca2+ concentration needed for proper stimulation of the downstream processes ranges from 0.1–10 µM. The citric acid cycle (i.e., the Krebs cycle) is the major energy-producing metabolic pathway in mitochondria and generally in cells, providing enormous amounts of reduction-equivalents which subsequently are oxidized in the electron transport chain to yield ATP. Ca2+ was shown to regulate three key dehydrogenases of the citric acid cycle i.e., NAD+-isocitrate dehydrogenase (NAD-ICDH), 2-oxoglutarate dehydrogenase (OGDH) and pyruvate dehydrogenase (PDH) [117,118,119]. Notably, because of its stimulatory effect on the dehydrogenases of the citric acid cycle [120], mitochondrial Ca2+ increase is known to serve as a key trigger for insulin release in β-cells [121]. This offers some contradiction to the current concept of at exactly what point in the process of GSIS mitochondrial Ca2+ is involved. In fact, the current concept suggests that mitochondrial Ca2+ increase occurs upon Ca2+ entry due to the opening of the L-type Ca2+ channels, thus, already downstream from mitochondrial activation by glycolysis-derived pyruvate and ATP production. However, increases in mitochondrial Ca2+ are thought to be essential for stimulation of Ca2+-dependent dehydrogenases of the citric acid cycle in order to establish suitable enzyme activity to handle the substrate overflow upon elevated blood D-glucose [122]. In particular, the responsiveness and proper D-glucose sensing of β-cells to elevated blood D-glucose is determined by the cells’ pace of producing ATP when exposed to elevated glucose [123]. Data of our group show strongly enhanced basal respiratory activity and elevated mitochondrial ATP levels in mitochondria of clonal β-cell lines in comparison to non-β-cell lines pointing to such pre-stimulation of mitochondria in β-cells [28]. These data are in line with the new perspective of GSIS that involves a continuous priming of β-cells based on a weak stimulation of β-cell mitochondria to ensure proper responsiveness to elevated blood D-glucose sensing [124].
4.3. Endoplasmic Reticulum
The endoplasmic reticulum as preserver of Ca2+ homeostasis in β-cells plays an important role in β-cell physiology and insulin secretion. ER stress caused by ER Ca2+ depletion, has been shown to contribute to β-cell dysfunction and the development of type 2 diabetes [125]. The Ca2+-sensor protein sorcin, which is a 22 kDa protein of the penta EF-hand family, is involved in maintaining ER Ca2+ stores by inhibiting RyR-activity and playing a role in the termination of CICR [126]. Sorcin is strongly expressed in primary mouse islets [127]. Knock-down in MIN6 insulinoma β-cells reduces ER Ca2+ stores and inhibits GSIS [128]. Elevated Ca2+ levels lead to a conformational change of the sorcin protein, a subsequent translocation from the cytoplasm to membranes (amongst others to the ER) where it interacts with target proteins including RYR [126], thereby, inhibiting RYR [126,129] and activating SERCA pumps [130].
Binding of insulin to the insulin receptor at the cell surface leads to autophosphorylation of tyrosyl residues of the IR-β-subunit and the phosphorylation of insulin receptor substrates 1 and 2 (IRS1 and 2). The subsequent cascade initiates the translocation of the insulin-responsive glucose transporter GLUT4 in muscle cells and adipocytes [131]. In β-cells IRS-1 is able to translocate to several intracellular locations dependent on upstream signals, which is also true for intracellular membranes, in particular, the ER [132]. The β-cell-selective insulin receptor (IR) knockout and IRS-1 knockout result in reduced glucose-induced insulin secretion while overexpression of IR and IRS-1 result in augmented insulin secretion and increased cytosolic Ca2+ levels due to inhibition of the ER-located SERCA. In β-cells the SERCA2 and SERCA3 are expressed [26]. Loss of SERCA activity and reduced SERCA3b expression in β-cells are associated with diabetes development in db/db mice [20]. SERCA3 gene mutations (Gln108→His, Val648→Met, Arg674→Cys and Ile753→Leu) in type 2 diabetes patients provide a link to a genetic susceptibility to T2DM development [133].
The above-mentioned ER Ca2+ depletion and subsequent ER Ca2+ stress can be explained—at least partially—by the existence of ER Ca2+ leak channels. Cassel and Ducreux [27] could show that translocon-mediated ER Ca2+ leak in murine MIN6 insulinoma β-cells and human islets is influencing lipotoxicity. Furthermore, translocon inhibition resulted in reduced ER stress and a restoration of insulin secretion [27]. Two recent studies of our group demonstrated that a presenilin-1-mediated ER Ca2+ leak crucially contributes to β-cell physiology and insulin secretion. The presenilin-1-mediated ER Ca2+ leak is directly sequestered by mitochondria, leading to increased basal matrix Ca2+ levels that yield enhanced resting activity of mitochondria in the pancreatic β-cells due to pre-stimulation the Ca2+-dependent dehydrogenases of the citric acid cycle. Upon elevation of glucose, glucose is metabolized and the pre-activated citric cycle in the mitochondria efficiently converts glucose metabolism to activation of the respiratory chain (OXPHOS) and, subsequently, fast ATP production, thus, ensuring a fast, initial insulin secretion within 10 min of exposure to elevated glucose [28,107].
4.4. The Golgi-Apparatus
Another intracellular Ca2+ storage important for a balanced Ca2+ homeostasis in mammalian cells and also in β-cells is the golgi apparatus. IP3 receptors are expressed at the surface of the golgi apparatus, mediating Ca2+ release from these IP3-sensitive pools [134]. Early measurements of intracellular Ca2+ demonstrate that upon cellular stimulation with IP3-generating agonists such as histamine, the golgi Ca2+ concentration rapidly decreases, presenting the golgi apparatus as IP3-sensitive Ca2+ pool [135]. However, in cell types that exhibit a high expression of RyR (such as cardiac myocytes), the Ca2+ extrusion of the golgi apparatus is mediated by these receptors [136]. The ATP-sensitive Ca2+ pump responsible for fueling the golgi apparatus with Ca2+ from the cytoplasm is the secretory pathway Ca2+-ATPase Ca2+ pump (SPCA1) [134]. Two main isoforms of this Ca2+ pump exist i.e., SPCA1 and SPCA2, whereas they show a tissue-specific expression. In mammals, SPCA1 is expressed in all tissues [137] whereas SPCA2 is expressed only in a limited set of tissues [138]. SPCA1 has been identified as being the main regulator of golgi Ca2+ homeostasis [139], which is also true for pancreatic β-cells [140]. Bone et al. [140] demonstrated a crucial role of SPCA1 in β-cell physiology. On the one hand, SPCA1 expression is reduced in patients suffering from T1DM and T2DM and on the other hand SPCA1 knock-out β-cells show increased rates of apoptosis, augmented cytosolic Ca2+ levels and significantly reduced GSIS (bone), highlighting the importance of the Ca2+ homeostatic function of the golgi apparatus.
5. Ca2+ in the Development of T2DM
As described in parts three and four of this review, Ca2+ is a crucial factor for β-cell survival, proliferation and function as well as for a proper insulin secretion on the one hand and is tightly regulated among diverse intracellular compartments within β-cells on the other hand. Therefore, an association with the development and progression of diabetes is obvious. Furthermore, deregulated Ca2+ signaling has been associated with the development of one of the key characteristics of T2DM i.e., insulin resistance [141,142,143]. Deregulated Ca2+ homeostasis has been implicated in a vast range of disease conditions. The case is not different when considering T2DM. In fact, it seems likely that there can be multiple degrees of separation between an original Ca2+ dysregulation and the eventual development of T2DM. In this section some issues of how Ca2+ deregulation can contribute to diabetes pathophysiology by highlighting some Ca2+-associated aspects on the cellular level but also in the human body.
Problems with pancreatic β-cell function and a loss of sensitivity to insulin are often significant factors in the development of T2DM [144]. Whether patients deal with T1 or T2DM has little consequence on the host of complications that they are likely to encounter as a result of this cellular dysfunction, including cardiomyopathy, hypertension, cataracts, and more [145,146]. It has been discussed whether the significant variability in the severity of these clinical outcomes of diabetes could be due to Ca2+ dysregulation. Decades ago, Levy and colleagues [147] stated that in the majority of tissues observed (i.e., heart, erythrocytes, platelets, skeletal muscle, kidney, hepatocytes, aorta, adipocytes, liver and osteoblasts), whether involving human diabetes patients or animal models for the disease, exhibited increased intracellular Ca2+ concentrations often accompanied by decreased Ca2+-ATPase activity. These findings highlight that deregulated Ca2+ homeostasis is a fundamental disorder in the diabetic state.
Much of the literature investigating a potential role for the ion in diabetes focuses on dietary Ca2+, taken either alone or in consideration with other important components of a healthy diet, such as magnesium (Mg2+) or vitamin D [148,149,150]. Increased dietary Mg2+ intake, for example, was found to correlate with a decreased risk of T2DM in subjects, while increased consumption of Ca2+ appeared not to show any association with risk [148]. It is well-outlined, for example, that vitamin D functions in the maintenance of phosphorus and Ca2+ homeostasis, thus promoting the healthy mineralization of bone. Recently, however, vitamin D and Ca2+ together have begun to be considered as factors affecting an individual’s risk of diabetes [149]. Namely, the existence of a link between vitamin D deficiency and downstream irregularities in glucose-induced release of insulin, a Ca2+-dependent process [151], appear to be connected by the ion itself. Similarly, a project investigating the regulation of blood sugar control in T2DM patients demonstrated that the addition of vitamin D, with or without supplemental Ca2+, improved glycemic status [150]. Vitamin D deficiency is causing β-cell death and, thus, is contributing to the development of β-cell dysfunction and β-cell death as onset and progression of diabetes. This vitamin not only maintains normal resting levels of Ca2+ and reactive oxygen species (ROS) [152]—both factors which are deregulated in diabetes [147,153,154]—but also prevents DNA hypermethylation of gene promoter regions regulating the expression of diabetes related genes [155].
Such research implies, therefore, that if Ca2+ is involved in the pathophysiology of T2DM, then it is not a matter of maintaining a certain level of dietary intake of the mineral, but rather has to do with an ingrained physiological signaling role of the ion. Indeed, it has been determined that normal Ca2+ homeostasis is impaired in patients with T2DM. Irregularities have been observed in a range of cell types, including platelets, cardiomyocytes, and pancreatic β-cells, among many others [156,157,158]. The widespread observance of altered steady-state Ca2+ levels indicates that this serves as a consistent factor in diabetic conditions.
Ca2+’s proper functioning across multiple signaling pathways is vital in all cells but is also important in some circumstances due to a tissue-specific role. Also important is the tight interconnectivity of Ca2+ signals across different cellular pathways and mechanisms, wherein a disturbance in one could affect a host of others. A dysregulation in such a signaling web oftentimes makes diagnoses of the original problem difficult, if not impossible. Undeniably, multiple changes to normal Ca2+ signals and regulation have been described in diabetes patients and animal models, hitting all of the main players in Ca2+ movement, such as SERCA and NCX, across tissues [147,156]. Thus far, the most agreed-upon abnormality in Ca2+ homeostasis in diabetes appears to be an increase in steady-state cytosolic Ca2+ levels across different cell types [147].
More specifically, in β-cells, the Ca2+ dysregulation evident in diabetes is so far better characterized in animal and cell-level models of the disease. Changes to normal Ca2+ homeostasis include increased voltage-dependent Ca2+ channel activity in diabetic rodents as well as decreased SERCA activity [157,159]. Considering the bigger picture, it is known that glucose homeostasis is achieved predominantly through many pathways that are regulated by Ca2+, including glycolysis, gluconeogenesis, and others [160]. These vital processes are thus derailed if some form of Ca2+ dysregulation event occurs, which could be the end result of changes to normal Ca2+ channel or pump activity. For example, as mentioned, an increased intracellular Ca2+ concentration is evident in diabetes which results in the inhibition of glycogen synthase, ultimately causing, through a disrupted series of activations and phosphorylation events, glucose resistance [147].
As the vigilant reader of the manuscript might have already realized, a new and for some maybe pugnacious finding concerning β-cell responsiveness and regulation of insulin secretion is presented in several sections of the manuscript—namely, the crucial role of a presenilin-1-mediated ER Ca2+ leak for proper β-cell function [28,107], data which are graphically summarized in Figure 2.
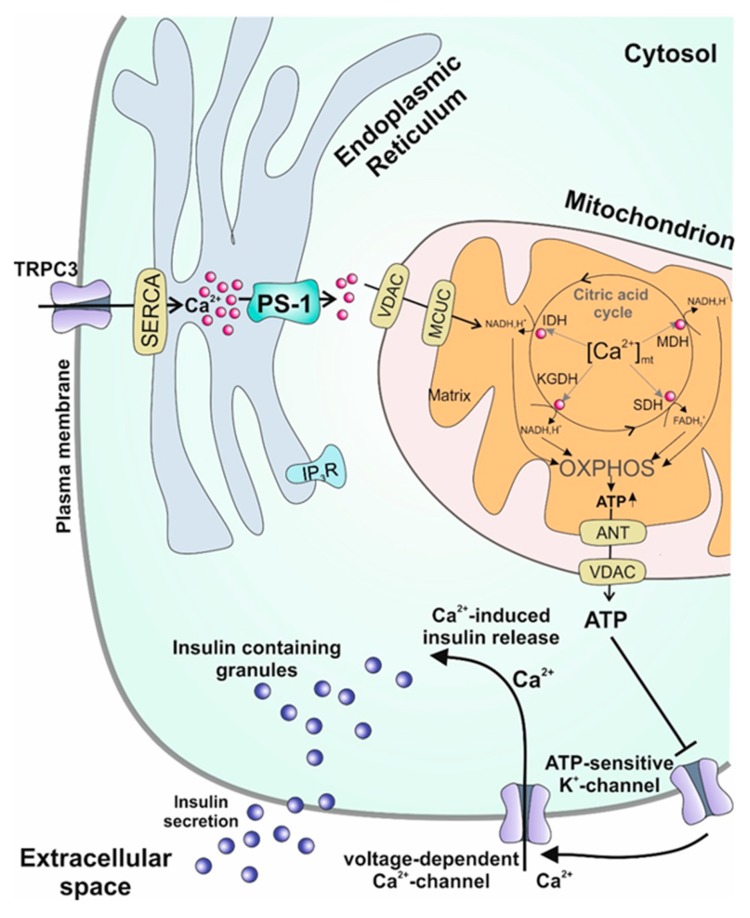
Figure 2.
Graphical summary of the consequences of the β-cell-specific presenilin-1-mediated ER Ca2+ leak. Ca2+ leaking out of the ER is directly sequestered to mitochondria, leading to increased basal matrix Ca2+ levels, where it pre-stimulates the Ca2+-dependent dehydrogenases of the citric acid cycle, augmenting resting organelle ATP levels. ATP-sensitive K+-channels are inhibited leading to cellular depolarization. This electrochemical shift triggers Ca2+ uptake via L-type Ca2+ channels. As a result, Ca2+-induced Ca2+ release is initiated, promoting insulin exocytosis into the extracellular space.
Our work demonstrates that this leak is regulating many steps in the cascade ultimately leading to insulin secretion. Reducing the ER-Ca2+ leak by either knocking down presenilin-1 or inhibiting its activity by blocking the PS1-regulatory kinase GSK3β causes several important effects in β-cells: i. reduction of mitochondrial respiration; ii. decreased mitochondrial ATP levels; iii. reduction of glucose-stimulated Ca2+ oscillation and delayed onset of these oscillations; iv. abrogation of the first-phase of insulin secretion. Several studies have already demonstrated that a loss of the first phase insulin response [95] which is resulting in delayed insulin secretion [161,162,163,164] is an irregularity occurring in the early stages of T2DM. The loss of this first phase is worsening post-prandial hyperglycemia yielding a progression of the pathologic condition and ultimately leading to clinically relevant hyperglycemia [165]. Therefore, this loss of first-phase insulin secretion is a predictive marker for the risk of developing T2DM [166,167], highlighting the importance of a regulated biphasic insulin response. The results of our study demonstrate that a functional ER-mitochondrial Ca2+ transfer mediated by presenilin-1 is pivotal for an adequate insulin response [28,107].
6. Presenilin-1, the Missing Link between Diabetes and Alzheimer’s Disease?: Excursus
In this section, we would like to draw the attention to a circumstantiated but sometimes underestimated correlation of two diseases where both presenilin-1 and Ca2+ are known contributors to disease development namely T2DM and Alzheimer’s disease (AD). Interestingly, there is a profound epidemiologic and experimental correlation between these two devastating diseases. Several studies show that T2DM may lead to cognitive impairment [168,169]. Until recently the amyloid theory which states that β-amyloid plaques cause apoptotic cell death of neuronal cells was the most supported theory as a causative trigger for AD development [170,171,172]. This theory finds support by several studies demonstrating the occurrence of increased Aβ42/Aβ40 ratios in the early stages of AD development [173]. At this point the presenilin-1 comes into play: together with PEN-2, APH1 and nicastrin, presenilin-1 forms the y-secretase complex [174,175] responsible for the production of β-amyloids. Since β-amyloid plaque formation is considered a hallmark of AD, y-secretase function is implicated in the pathogenesis of AD [176]. New discoveries challenge this hypothesis [176,177,178] by demonstrating that mutations of presenilin-1—which is pivotal to Aβ production—do not increase the activity of the protein but rather decrease it. A phenomenon observed in vitro and in cells [179,180,181,182,183] and also enzymatic inhibition fails to reduce symptoms of AD patients [184]. Moreover, as β-amyloid plaques are also found in healthy individuals [185,186] and patients suffering from front temporal dementia (FTD), a disease with similar functional defects but a lack of plaque formation [187,188,189], the correlation between the occurrence of plaques with the emergence and severity of AD is only weak. All these studies indicate that the y-secretase/amyloid theory is not the only causative factor for AD development. At this point the Ca2+ comes into play: the more than 219 AD-associated mutations in the presenilin-1 gene [190] are the most common cause of the inherited form of AD, namely familial AD (FAD) that can onset already early in life. In contrast, the more common form of AD, from those a possible influence of presenilin mutations has not established so far, develop later in life. Notably, all so far tested presenilin-1 mutations also influence in some aspect intracellular Ca2+ handling [191,192,193]. Depending on the mutation(s) present both possible functions of presenilin-1 can be altered i.e., y-secretase activity and Ca2+ handling. Examples, therefore, are the following: ΔE9 affects y-secretase function, M146V disturbs ER Ca2+ handling, L166P affects both [194]. Accordingly, deranged Ca2+ homeostasis and β-amyloid toxicity due to mutations of presenilin-1 might act as independent causative factors for developing FAD. Importantly, the known crucial involvement of presenilin-1 in physiological amyloid degradation [195] and its essential function for β-cell responsiveness [28,107] might explain the high incidence of T2DM in AD patients. This possible link could connect the high incidence of the non-inherited version of AD and T2DM. Concluding this paragraph, changes in Ca2+ homeostasis due to alterations in presenilin-1 function could be the missing link for the causative factors of diabetes and AD development and could shed some light into the correlation of these two diseases. The exact regulation as well as additional mechanisms still need to be further elucidated but these data justify more intensive studies on this topic.
7. Discussion and Conclusions
Considering the implications of the intracellular changes discussed here, it is clear that further investigation into the Ca2+ signaling and other machinery of β-cells is required to truly gain insight into how they relate to the pathophysiology of diabetes. Researchers are constantly innovating new techniques to accomplish exactly this. For example, the concept of synthetic cells has been around for decades [196,197], but recent work has made significant strides. Groups have so far reported using vesicles seeded with integrated proteins for various purposes, and nanoparticles covered in cell-membrane materials, among other creative cell facsimiles [198,199] to mimic vital and cell-specific processes. The development of synthetic β-cells, in particular, could prove to be a promising technique for gaining an in-depth understanding of the dynamics of insulin secretion and other physiological processes. The concept, as is it has so far been developed, involves using synthetic materials in order to mimic glucose-dependent secretion of insulin, an application that could prove important for making strides in diabetes research. Chen and colleagues [200] have recently made headway with this notion, using the structure of ‘vesicles-inside-a-vesicle’ with additional features that allow for glucose metabolism and the ability of membranes to fuse. These artificial cells have so far been shown to differentiate between varied experimental glucose conditions. When glucose is high, the artificial β-cells exhibit high glucose uptake and increased oxidation, resulting in a decreased pH within the compartment. Low pH subsequently induces mechanical changes within the cells’ that result in a fusion of inner and outer vesicle membranes, thus simulating insulin release through exocytosis. While still a rudimentary system, such work could ultimately contribute to the emergence of cell therapy as a potential course of action in diabetes; countering the worst of the limitations of the current practice of islet donation.
While promising concepts are constantly emerging, it is vital to continue diabetes investigation with the tried and tested cellular and animal models available to all. As we have outlined here, recent work has helped identify specific mechanisms of intra- and inter-cellular communication that allow for the complicated insulin-secreting function of β-cells, particularly considering the importance of Ca2+ to such processes. Despite now knowing of the intricate roles that this ion plays in pancreatic cell-specific function, there is a significant knowledge gap between early questions posed on Ca2+’s importance to the development of diabetes and current understanding of the same query.
The research discussed here reveals a significant phenomenon in pancreatic β-cells that sheds further light on the mechanism by which these cells exhibit a response to increased glucose levels. Dysregulation of this mechanism could therefore potentially contribute to the pathophysiological development of T2DM or could be the missing link to other diseases.
Abbreviations
| AD | Alzheimer’s Disease |
| ATP | Adenosine triphosphate |
| CaMK | Ca2+/calmodulin dependent kinase |
| CICR | Calcium induced calcium release |
| DM | Diabetes mellitus |
| ER | Endoplasmic reticulum |
| GLUT | Glucose transporter |
| GSIS | Glucose stimulated insulin secretion |
| GSK3β | Glycogen synthase kinase 3 β |
| IP3 | Inositol trisphosphate |
| IP3R | IP3 receptor |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate |
| MCU | Mitochondrial calcium uniporter |
| MICU | Mitochondrial calcium uptake 1 |
| NCX | Na+/Ca2+ exchanger |
| NCLX | Mitochondrial Na+/Ca2+ exchanger |
| NFAT | Nuclear factor of activated T-cells |
| PM | Plasma membrane |
| PMCA | Plamsa membrane Ca2+-ATPase |
| PTP | Permeability transition pore |
| RyR | Ryanodine receptor |
| SERCA | Sarco/endoplasmic reticulum Ca2+-ATPase |
| T1/2DM | Type 1 or type 2 diabetes mellitus |
| VDCC | Voltage dependent calcium channels |
Author Contributions
Conceptualization, W.F.G., C.K. and G.Z.; writing—original draft preparation, C.K., G.Z., R.M., M.P. and W.F.G.; writing—review and editing, C.K., G.Z., R.M., M.P. and W.F.G.
Funding
G.Z. was supported by the Austrian Science Fund (FWF) (DK-MCD W1226).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mayer J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 2.Defronzo R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Diaz R., Molano R.D., Weitz J.R., Abdulreda M.H., Berman D.M., Leibiger B., Leibiger I.B., Kenyon N.S., Ricordi C., Pileggi A., et al. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metab. 2018;27:549–558. doi: 10.1016/j.cmet.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mari A., Tura A., Natali A., Laville M., Laakso M., Gabriel R., Beck-Nielsen H., Ferrannini E., Investigators R. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53:749–756. doi: 10.1007/s00125-009-1647-6. [DOI] [PubMed] [Google Scholar]
- 6.German M.S. Glucose sensing in pancreatic islet beta cells: The key role of glucokinase and the glycolytic intermediates. Proc. Natl. Acad. Sci. USA. 1993;90:1781–1785. doi: 10.1073/pnas.90.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosboom R.S., Zweens J., Bouman P.R. Effects of feeding and fasting on the insulin secretory response to glucose and sulfonylureas in intact rats and isolated perfused rat pancreas. Diabetologia. 1973;9:243–250. doi: 10.1007/BF01221849. [DOI] [PubMed] [Google Scholar]
- 8.Vergari E., Knudsen J.G., Ramracheya R., Salehi A., Zhang Q., Adam J., Asterholm I.W., Benrick A., Briant L.J.B., Chibalina M.V., et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat. Commun. 2019;10:139. doi: 10.1038/s41467-018-08193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IDF . DF Diabetes Atlas. 8th ed. International Diabetes Federation; Brussels, Belgium: 2017. [(accessed on 23 October 2019)]. Available online: http://www.diabetesatlas.org. [Google Scholar]
- 10.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 11.Kahn S.E., Zraika S., Utzschneider K.M., Hull R.L. The beta cell lesion in type 2 diabetes: There has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renstrom E., Ding W.G., Bokvist K., Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron. 1996;17:513–522. doi: 10.1016/S0896-6273(00)80183-X. [DOI] [PubMed] [Google Scholar]
- 14.Alfa R.W., Park S., Skelly K.R., Poffenberger G., Jain N., Gu X., Kockel L., Wang J., Liu Y., Powers A.C., et al. Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 2015;21:323–334. doi: 10.1016/j.cmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henquin J.C., Nenquin M., Ravier M.A., Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes. Metab. 2009;11(Suppl. 4):168–179. doi: 10.1111/j.1463-1326.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- 16.Alam M.R., Groschner L.N., Parichatikanond W., Kuo L., Bondarenko A.I., Rost R., Waldeck-Weiermair M., Malli R., Graier W.F. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J. Biol. Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutter G.A., Tsuboi T., Ravier M.A. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551. doi: 10.1016/j.ceca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Sherman A., Rinzel J. Model for synchronization of pancreatic beta-cells by gap junction coupling. Biophys. J. 1991;59:547–559. doi: 10.1016/S0006-3495(91)82271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaustein M.P., Lederer W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 20.Philipson K.D., Nicoll D.A. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Strehler E.E., Treiman M. Calcium pumps of plasma membrane and cell interior. Curr. Mol. Med. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 22.Prasad V., Okunade G.W., Miller M.L., Shull G.E. Phenotypes of SERCA and PMCA knockout mice. Biochem. Biophys. Res. Commun. 2004;322:1192–1203. doi: 10.1016/j.bbrc.2004.07.156. [DOI] [PubMed] [Google Scholar]
- 23.Carafoli E. Biogenesis: Plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993–1002. doi: 10.1096/fasebj.8.13.7926378. [DOI] [PubMed] [Google Scholar]
- 24.Noble D., Herchuelz A. Role of Na/Ca exchange and the plasma membrane Ca2+-ATPase in cell function. Conference on Na/Ca exchange. EMBO Rep. 2007;8:228–232. doi: 10.1038/sj.embor.7400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagur R., Hajnoczky G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravier M.A., Daro D., Roma L.P., Jonas J.C., Cheng-Xue R., Schuit F.C., Gilon P. Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic beta-cells: Interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes. 2011;60:2533–2545. doi: 10.2337/db10-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassel R., Ducreux S., Alam M.R., Dingreville F., Berle C., Burda-Jacob K., Chauvin M.A., Chikh K., Paita L., Al-Mawla R., et al. Protection of Human Pancreatic Islets from Lipotoxicity by Modulation of the Translocon. PLoS ONE. 2016;11:e0148686. doi: 10.1371/journal.pone.0148686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klec C., Madreiter-Sokolowski C.T., Stryeck S., Sachdev V., Duta-Mare M., Gottschalk B., Depaoli M.R., Rost R., Hay J., Waldeck-Weiermair M., et al. Glycogen Synthase Kinase 3 Beta Controls Presenilin-1-Mediated Endoplasmic Reticulum Ca2+ Leak Directed to Mitochondria in Pancreatic Islets and beta-Cells. Cell. Physiol. Biochem. 2019;52:57–75. doi: 10.33594/000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luciani D.S., Gwiazda K.S., Yang T.L., Kalynyak T.B., Bychkivska Y., Frey M.H., Jeffrey K.D., Sampaio A.V., Underhill T.M., Johnson J.D. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–432. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bootman M.D., Cheek T.R., Moreton R.B., Bennett D.L., Berridge M.J. Smoothly graded Ca2+ release from inositol 1,4,5-trisphosphate-sensitive Ca2+ stores. J. Biol. Chem. 1994;269:24783–24791. [PubMed] [Google Scholar]
- 31.Isenberg G., Han S. Gradation of Ca2+-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. Pt 3J. Physiol. 1994;480:423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee B., Jonas J.C., Weir G.C., Laychock S.G. Glucose regulates expression of inositol 1,4,5-trisphosphate receptor isoforms in isolated rat pancreatic islets. Endocrinology. 1999;140:2173–2182. doi: 10.1210/endo.140.5.6738. [DOI] [PubMed] [Google Scholar]
- 33.Johnson J.D., Kuang S., Misler S., Polonsky K.S. Ryanodine receptors in human pancreatic beta cells: Localization and effects on insulin secretion. FASEB J. 2004;18:878–880. doi: 10.1096/fj.03-1280fje. [DOI] [PubMed] [Google Scholar]
- 34.Sabatini P.V., Speckmann T., Lynn F.C. Friend and foe: Beta-cell Ca2+ signaling and the development of diabetes. Mol. Metab. 2019;21:1–12. doi: 10.1016/j.molmet.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 36.Yeromin A.V., Zhang S.L., Jiang W., Yu Y., Safrina O., Cahalan M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liou J., Kim M.L., Heo W.D., Jones J.T., Myers J.W., Ferrell J.E., Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soboloff J., Spassova M.A., Hewavitharana T., He L.P., Xu W., Johnstone L.S., Dziadek M.A., Gill D.L. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr. Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 39.Brandman O., Liou J., Park W.S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motiani R.K., Abdullaev I.F., Trebak M. A novel native store-operated calcium channel encoded by Orai3: Selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J. Biol. Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inayama M., Suzuki Y., Yamada S., Kurita T., Yamamura H., Ohya S., Giles W.R., Imaizumi Y. Orai1-Orai2 complex is involved in store-operated calcium entry in chondrocyte cell lines. Cell Calcium. 2015;57:337–347. doi: 10.1016/j.ceca.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Vaeth M., Yang J., Yamashita M., Zee I., Eckstein M., Knosp C., Kaufmann U., Karoly Jani P., Lacruz R.S., Flockerzi V., et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017;8:14714. doi: 10.1038/ncomms14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y., Erxleben C., Abramowitz J., Flockerzi V., Zhu M.X., Armstrong D.L., Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. USA. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabourin J., Le Gal L., Saurwein L., Haefliger J.A., Raddatz E., Allagnat F. Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat beta-Cells. J. Biol. Chem. 2015;290:30530–30539. doi: 10.1074/jbc.M115.682583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamarina N.A., Kuznetsov A., Rhodes C.J., Bindokas V.P., Philipson L.H. Inositol (1,4,5)-trisphosphate dynamics and intracellular calcium oscillations in pancreatic beta-cells. Diabetes. 2005;54:3073–3081. doi: 10.2337/diabetes.54.11.3073. [DOI] [PubMed] [Google Scholar]
- 46.Harootunian A.T., Kao J.P., Paranjape S., Adams S.R., Potter B.V., Tsien R.Y. Cytosolic Ca2+ oscillations in REF52 fibroblasts: Ca(2+)-stimulated IP3 production or voltage-dependent Ca2+ channels as key positive feedback elements. Cell Calcium. 1991;12:153–164. doi: 10.1016/0143-4160(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 47.Pasti L., Volterra A., Pozzan T., Carmignoto G. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunemaker C.S., Bertram R., Sherman A., Tsaneva-Atanasova K., Daniel C.R., Satin L.S. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys. J. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolmetsch R.E., Xu K., Lewis R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 50.German M.S., Moss L.G., Rutter W.J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J. Biol. Chem. 1990;265:22063–22066. [PubMed] [Google Scholar]
- 51.Luzi L., Pozza G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol. 1997;34:239–244. doi: 10.1007/s005920050081. [DOI] [PubMed] [Google Scholar]
- 52.Wang Q., Heimberg H., Pipeleers D., Ling Z. Glibenclamide activates translation in rat pancreatic beta cells through calcium-dependent mTOR, PKA and MEK signalling pathways. Diabetologia. 2008;51:1202–1212. doi: 10.1007/s00125-008-1026-8. [DOI] [PubMed] [Google Scholar]
- 53.Stamateris R.E., Sharma R.B., Kong Y., Ebrahimpour P., Panday D., Ranganath P., Zou B., Levitt H., Parambil N.A., O’Donnell C.P., et al. Glucose Induces Mouse beta-Cell Proliferation via IRS2, MTOR, and Cyclin D2 but Not the Insulin Receptor. Diabetes. 2016;65:981–995. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alonso L.C., Yokoe T., Zhang P., Scott D.K., Kim S.K., O’Donnell C.P., Garcia-Ocana A. Glucose infusion in mice: A new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitt H.E., Cyphert T.J., Pascoe J.L., Hollern D.A., Abraham N., Lundell R.J., Rosa T., Romano L.C., Zou B., O’Donnell C.P., et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54:572–582. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porat S., Weinberg-Corem N., Tornovsky-Babaey S., Schyr-Ben-Haroush R., Hija A., Stolovich-Rain M., Dadon D., Granot Z., Ben-Hur V., White P., et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Shen W., Taylor B., Jin Q., Nguyen-Tran V., Meeusen S., Zhang Y.Q., Kamireddy A., Swafford A., Powers A.F., Walker J., et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat. Commun. 2015;6:8372. doi: 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W., Walker J.R., Wang X., Tremblay M.S., Lee J.W., Wu X., Schultz P.G. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc. Natl. Acad. Sci. USA. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X., Murao K., Sayo Y., Imachi H., Cao W.M., Ohtsuka S., Niimi M., Tokumitsu H., Inuzuka H., Wong N.C., et al. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes. 2004;53:1475–1481. doi: 10.2337/diabetes.53.6.1475. [DOI] [PubMed] [Google Scholar]
- 60.Liu B., Barbosa-Sampaio H., Jones P.M., Persaud S.J., Muller D.S. The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of beta-cells. PLoS ONE. 2012;7:e45711. doi: 10.1371/journal.pone.0045711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heit J.J., Apelqvist A.A., Gu X., Winslow M.M., Neilson J.R., Crabtree G.R., Kim S.K. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 62.Dai C., Hang Y., Shostak A., Poffenberger G., Hart N., Prasad N., Phillips N., Levy S.E., Greiner D.L., Shultz L.D., et al. Age-dependent human beta cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J. Clin. Investig. 2017;127:3835–3844. doi: 10.1172/JCI91761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier J.J., Butler A.E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R.A., Butler P.C. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McEvoy R.C. Changes in the volumes of the A-, B-, and D-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes. 1981;30:813–817. doi: 10.2337/diab.30.10.813. [DOI] [PubMed] [Google Scholar]
- 65.Finegood D.T., Scaglia L., Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen J.H., Brunstedt J., Andersson A., Frimodt-Moller C. Preservation of beta cell function in adult human pancreatic islets for several months in vitro. Diabetologia. 1979;16:97–100. doi: 10.1007/BF01225457. [DOI] [PubMed] [Google Scholar]
- 67.Rong Y., Distelhorst C.W. Bcl-2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 68.Srinivasan S., Bernal-Mizrachi E., Ohsugi M., Permutt M.A. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2002;283:E784–E793. doi: 10.1152/ajpendo.00177.2002. [DOI] [PubMed] [Google Scholar]
- 69.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 70.Soleimanpour S.A., Crutchlow M.F., Ferrari A.M., Raum J.C., Groff D.N., Rankin M.M., Liu C., De Leon D.D., Naji A., Kushner J.A., et al. Calcineurin signaling regulates human islet {beta}-cell survival. J. Biol. Chem. 2010;285:40050–40059. doi: 10.1074/jbc.M110.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nir T., Melton D.A., Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Investig. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.del Castillo J.M.B., Arias-Diaz J., Garcia Martin M.C., Vives-Pi M., Garcia Perez J.C., Cantero Cid R., Vara Ameigeiras E., Balibrea Cantero J.L. Cytoprotective effect of low-dose tacrolimus on islets of Langerhans in cultures subjected to stimulation by acute rejection cytokines. Cirugía Española. 2010;87:372–377. doi: 10.1016/j.ciresp.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Ranta F., Avram D., Berchtold S., Dufer M., Drews G., Lang F., Ullrich S. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes. 2006;55:1380–1390. doi: 10.2337/db05-1220. [DOI] [PubMed] [Google Scholar]
- 74.Hara T., Mahadevan J., Kanekura K., Hara M., Lu S., Urano F. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology. 2014;155:758–768. doi: 10.1210/en.2013-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oyadomari S., Takeda K., Takiguchi M., Gotoh T., Matsumoto M., Wada I., Akira S., Araki E., Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc. Natl. Acad. Sci. USA. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gwiazda K.S., Yang T.L., Lin Y., Johnson J.D. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am. J. Physiol. Endocrinol. Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 77.Moore C.E., Omikorede O., Gomez E., Willars G.B., Herbert T.P. PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic beta-cells. Mol. Endocrinol. 2011;25:315–326. doi: 10.1210/me.2010-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lefebvre P.J., Paolisso G., Scheen A.J., Henquin J.C. Pulsatility of insulin and glucagon release: Physiological significance and pharmacological implications. Diabetologia. 1987;30:443–452. doi: 10.1007/BF00279610. [DOI] [PubMed] [Google Scholar]
- 79.Goodner C.J., Walike B.C., Koerker D.J., Ensinck J.W., Brown A.C., Chideckel E.W., Palmer J., Kalnasy L. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977;195:177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy R.T., Kauri L.M., Dahlgren G.M., Jung S.K. Metabolic oscillations in beta-cells. Diabetes. 2002;51(Suppl. 1):S152–S161. doi: 10.2337/diabetes.51.2007.S152. [DOI] [PubMed] [Google Scholar]
- 81.Lang D.A., Matthews D.R., Peto J., Turner R.C. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 1979;301:1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 82.Bingley P.J., Matthews D.R., Williams A.J., Bottazzo G.F., Gale E.A. Loss of regular oscillatory insulin secretion in islet cell antibody positive non-diabetic subjects. Diabetologia. 1992;35:32–38. doi: 10.1007/BF00400849. [DOI] [PubMed] [Google Scholar]
- 83.O’Rahilly S., Turner R.C., Matthews D.R. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N. Engl. J. Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 84.Grapengiesser E., Gylfe E., Hellman B. Glucose-induced oscillations of cytoplasmic Ca2+ in the pancreatic beta-cell. Biochem. Biophys. Res. Commun. 1988;151:1299–1304. doi: 10.1016/S0006-291X(88)80503-5. [DOI] [PubMed] [Google Scholar]
- 85.Hellman B., Gylfe E., Grapengiesser E., Lund P.E., Berts A. Cytoplasmic Ca2+ oscillations in pancreatic beta-cells. Biochim. Biophys. Acta. 1992;1113:295–305. doi: 10.1016/0304-4157(92)90003-S. [DOI] [PubMed] [Google Scholar]
- 86.Santos R.M., Rosario L.M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflug. Arch. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- 87.Lei C.L., Kellard J.A., Hara M., Johnson J.D., Rodriguez B., Briant L.J.B. Beta-cell hubs maintain Ca2+ oscillations in human and mouse islet simulations. Islets. 2018;10:151–167. doi: 10.1080/19382014.2018.1493316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valdeolmillos M., Nadal A., Soria B., Garcia-Sancho J. Fluorescence digital image analysis of glucose-induced [Ca2+]i oscillations in mouse pancreatic islets of Langerhans. Diabetes. 1993;42:1210–1214. doi: 10.2337/diab.42.8.1210. [DOI] [PubMed] [Google Scholar]
- 89.Fridlyand L.E., Tamarina N., Philipson L.H. Bursting and calcium oscillations in pancreatic beta-cells: Specific pacemakers for specific mechanisms. Am. J. Physiol. Endocrinol. Metab. 2010;299:E517–E532. doi: 10.1152/ajpendo.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hellman B., Gylfe E., Bergsten P., Grapengiesser E., Lund P.E., Berts A., Tengholm A., Pipeleers D.G., Ling Z. Glucose induces oscillatory Ca2+ signalling and insulin release in human pancreatic beta cells. Diabetologia. 1994;37(Suppl. 2):S11–S20. doi: 10.1007/BF00400821. [DOI] [PubMed] [Google Scholar]
- 91.Goodner C.J., Sweet I.R., Harrison H.C., Jr. Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes. 1988;37:1316–1323. doi: 10.2337/diab.37.10.1316. [DOI] [PubMed] [Google Scholar]
- 92.Jones P.M., Persaud S.J., Howell S.L. Ca2+-induced insulin secretion from electrically permeabilized islets. Loss of the Ca2(+)-induced secretory response is accompanied by loss of Ca2+-induced protein phosphorylation. Pt 3Biochem. J. 1992;285:973–978. doi: 10.1042/bj2850973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orrenius S., McConkey D.J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol. Sci. 1989;10:281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- 94.Curry D.L., Bennett L.L., Grodsky G.M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- 95.Porte D., Jr., Pupo A.A. Insulin responses to glucose: Evidence for a two pool system in man. J. Clin. Investig. 1969;48:2309–2319. doi: 10.1172/JCI106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henquin J.C., Ishiyama N., Nenquin M., Ravier M.A., Jonas J.C. Signals and pools underlying biphasic insulin secretion. Diabetes. 2002;51(Suppl. 1):S60–S67. doi: 10.2337/diabetes.51.2007.S60. [DOI] [PubMed] [Google Scholar]
- 97.Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Eur. J. Endocrinol. 1967;55:278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- 98.Fujita Y., Herron A.L., Jr., Seltzer H.S. Confirmation of impaired early insulin response to glycemic stimulus in nonobese mild diabetics. Diabetes. 1975;24:17–27. doi: 10.2337/diab.24.1.17. [DOI] [PubMed] [Google Scholar]
- 99.Metz S.A., Halter J.B., Robertson R.P. Paradoxical inhibition of insulin secretion by glucose in human diabetes mellitus. J. Clin. Endocrinol. Metab. 1979;48:827–835. doi: 10.1210/jcem-48-5-827. [DOI] [PubMed] [Google Scholar]
- 100.Pfeiffer B., Sarrazin W., Weitzel G. Insulin-like effects of agmatine derivatives in vitro and in vivo (author’s transl) Hoppe-Seyler’s Z. Physiol. Chemie. 1981;362:1331–1337. doi: 10.1515/bchm2.1981.362.2.1331. [DOI] [PubMed] [Google Scholar]
- 101.Luzi L., DeFronzo R.A. Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am. J. Physiol. 1989;257:E241–E246. doi: 10.1152/ajpendo.1989.257.2.E241. [DOI] [PubMed] [Google Scholar]
- 102.Rebrin K., Steil G.M., Mittelman S.D., Bergman R.N. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J. Clin. Investig. 1996;98:741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Getty L., Hamilton-Wessler M., Ader M., Dea M.K., Bergman R.N. Biphasic insulin secretion during intravenous glucose tolerance test promotes optimal interstitial insulin profile. Diabetes. 1998;47:1941–1947. doi: 10.2337/diabetes.47.12.1941. [DOI] [PubMed] [Google Scholar]
- 104.Wollheim C.B., Kikuchi M., Renold A.E., Sharp G.W. The roles of intracellular and extracellular Ca++ in glucose-stimulated biphasic insulin release by rat islets. J. Clin. Investig. 1978;62:451–458. doi: 10.1172/JCI109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henquin J.C. Relative importance of extracellular and intracellular calcium for the two phases of glucose-stimulated insulin release: Studies with theophylline. Endocrinology. 1978;102:723–730. doi: 10.1210/endo-102-3-723. [DOI] [PubMed] [Google Scholar]
- 106.Boyd A.E., 3rd The role of ion channels in insulin secretion. J. Cell. Biochem. 1992;48:235–241. doi: 10.1002/jcb.240480303. [DOI] [PubMed] [Google Scholar]
- 107.Klec C., Madreiter-Sokolowski C.T., Ziomek G., Stryeck S., Sachdev V., Duta-Mare M., Gottschalk B., Depaoli M.R., Rost R., Hay J., et al. Presenilin-1 Established ER-Ca2+ Leak: A Follow Up on Its Importance for the Initial Insulin Secretion in Pancreatic Islets and beta-Cells upon Elevated Glucose. Cell. Physiol. Biochem. 2019;53:573–586. doi: 10.33594/000000158. [DOI] [PubMed] [Google Scholar]
- 108.Hamming K.S., Riedel M.J., Soliman D., Matemisz L.C., Webster N.J., Searle G.J., MacDonald P.E., Light P.E. Splice variant-dependent regulation of beta-cell sodium-calcium exchange by acyl-coenzyme As. Mol. Endocrinol. 2008;22:2293–2306. doi: 10.1210/me.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamming K.S., Soliman D., Webster N.J., Searle G.J., Matemisz L.C., Liknes D.A., Dai X.Q., Pulinilkunnil T., Riedel M.J., Dyck J.R., et al. Inhibition of beta-cell sodium-calcium exchange enhances glucose-dependent elevations in cytoplasmic calcium and insulin secretion. Diabetes. 2010;59:1686–1693. doi: 10.2337/db09-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pachera N., Papin J., Zummo F.P., Rahier J., Mast J., Meyerovich K., Cardozo A.K., Herchuelz A. Heterozygous inactivation of plasma membrane Ca(2+)-ATPase in mice increases glucose-induced insulin release and beta cell proliferation, mass and viability. Diabetologia. 2015;58:2843–2850. doi: 10.1007/s00125-015-3745-y. [DOI] [PubMed] [Google Scholar]
- 111.Balaban R.S., Kantor H.L., Katz L.A., Briggs R.W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986;232:1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- 112.Lin A.L., Fox P.T., Hardies J., Duong T.Q., Gao J.H. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc. Natl. Acad. Sci. USA. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hellman B., Idahl L.A., Lernmark A., Sehlin J., Taljedal I.B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem. J. 1974;138:33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sweet I.R., Cook D.L., DeJulio E., Wallen A.R., Khalil G., Callis J., Reems J. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401–409. doi: 10.2337/diabetes.53.2.401. [DOI] [PubMed] [Google Scholar]
- 115.Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C.W., Schoonjans K., Pozzan T., Spat A., Wollheim C.B. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011;13:601–611. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 116.Wiederkehr A., Park K.S., Dupont O., Demaurex N., Pozzan T., Cline G.W., Wollheim C.B. Matrix alkalinization: A novel mitochondrial signal for sustained pancreatic beta-cell activation. EMBO J. 2009;28:417–428. doi: 10.1038/emboj.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Denton R.M., McCormack J.G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980;119:1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- 118.Denton R.M., McCormack J.G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol. 1985;249:E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- 119.Hansford R.G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev. Physiol. Biochem. Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- 120.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 121.Wiederkehr A., Wollheim C.B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Rutter G.A., McCormack J.G., Midgley P.J., Denton R.M. The role of Ca2+ in the hormonal regulation of the activities of pyruvate dehydrogenase and oxoglutarate dehydrogenase complexes. Ann. N. Y. Acad. Sci. 1989;573:206–217. doi: 10.1111/j.1749-6632.1989.tb14998.x. [DOI] [PubMed] [Google Scholar]
- 123.Maechler P., Wang H., Wollheim C.B. Continuous monitoring of ATP levels in living insulin secreting cells expressing cytosolic firefly luciferase. FEBS Lett. 1998;422:328–332. doi: 10.1016/S0014-5793(97)01618-9. [DOI] [PubMed] [Google Scholar]
- 124.Komatsu M., Takei M., Ishii H., Sato Y. Glucose-stimulated insulin secretion: A newer perspective. J. Diabetes Investig. 2013;4:511–516. doi: 10.1111/jdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasnain S.Z., Prins J.B., McGuckin M.A. Oxidative and endoplasmic reticulum stress in beta-cell dysfunction in diabetes. J. Mol. Endocrinol. 2016;56:R33–R54. doi: 10.1530/JME-15-0232. [DOI] [PubMed] [Google Scholar]
- 126.Marmugi A., Parnis J., Chen X., Carmichael L., Hardy J., Mannan N., Marchetti P., Piemonti L., Bosco D., Johnson P., et al. Sorcin Links Pancreatic beta-Cell Lipotoxicity to ER Ca2+ Stores. Diabetes. 2016;65:1009–1021. doi: 10.2337/db15-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kone M., Pullen T.J., Sun G., Ibberson M., Martinez-Sanchez A., Sayers S., Nguyen-Tu M.S., Kantor C., Swisa A., Dor Y., et al. LKB1 and AMPK differentially regulate pancreatic beta-cell identity. FASEB J. 2014;28:4972–4985. doi: 10.1096/fj.14-257667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noordeen N.A., Meur G., Rutter G.A., Leclerc I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca2+ ions in pancreatic beta-cells. Diabetes. 2012;61:574–585. doi: 10.2337/db10-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lokuta A.J., Meyers M.B., Sander P.R., Fishman G.I., Valdivia H.H. Modulation of cardiac ryanodine receptors by sorcin. J. Biol. Chem. 1997;272:25333–25338. doi: 10.1074/jbc.272.40.25333. [DOI] [PubMed] [Google Scholar]
- 130.Matsumoto T., Hisamatsu Y., Ohkusa T., Inoue N., Sato T., Suzuki S., Ikeda Y., Matsuzaki M. Sorcin interacts with sarcoplasmic reticulum Ca(2+)-ATPase and modulates excitation-contraction coupling in the heart. Basic Res. Cardiol. 2005;100:250–262. doi: 10.1007/s00395-005-0518-7. [DOI] [PubMed] [Google Scholar]
- 131.Cushman S.W., Wardzala L.J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 132.Borge P.D., Moibi J., Greene S.R., Trucco M., Young R.A., Gao Z., Wolf B.A. Insulin receptor signaling and sarco/endoplasmic reticulum calcium ATPase in beta-cells. Diabetes. 2002;51(Suppl. 3):S427–S433. doi: 10.2337/diabetes.51.2007.S427. [DOI] [PubMed] [Google Scholar]
- 133.Varadi A., Lebel L., Hashim Y., Mehta Z., Ashcroft S.J., Turner R. Sequence variants of the sarco(endo)plasmic reticulum Ca(2+)-transport ATPase 3 gene (SERCA3) in Caucasian type II diabetic patients (UK Prospective Diabetes Study 48) Diabetologia. 1999;42:1240–1243. doi: 10.1007/s001250051298. [DOI] [PubMed] [Google Scholar]
- 134.Micaroni M., Mironov A.A. Roles of Ca and secretory pathway Ca-ATPase pump type 1 (SPCA1) in intra-Golgi transport. Commun. Integr. Biol. 2010;3:504–507. doi: 10.4161/cib.3.6.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pinton P., Pozzan T., Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lissandron V., Podini P., Pizzo P., Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gunteski-Hamblin A.M., Clarke D.M., Shull G.E. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- 138.Vanoevelen J., Dode L., Van Baelen K., Fairclough R.J., Missiaen L., Raeymaekers L., Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- 139.Micaroni M., Perinetti G., Berrie C.P., Mironov A.A. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic. 2010;11:1315–1333. doi: 10.1111/j.1600-0854.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 140.Bone R.N., Kono T., Evans-Mollina C. Reduced ß Cell SPCA1 Leads to Impaired Calcium Oscillations and Decreased Autophagy. Diabetes. 2018;67 doi: 10.2337/db18-194-OR. [DOI] [Google Scholar]
- 141.Sun G., Vasdev S., Martin G.R., Gadag V., Zhang H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes. 2005;54:3336–3339. doi: 10.2337/diabetes.54.11.3336. [DOI] [PubMed] [Google Scholar]
- 142.Wu X., Han T., Gao J., Zhang Y., Zhao S., Sun R., Sun C., Niu Y., Li Y. Association of Serum Calcium and Insulin Resistance With Hypertension Risk: A Prospective Population-Based Study. J. Am. Heart Assoc. 2019;8:e009585. doi: 10.1161/JAHA.118.009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ozcan L., Tabas I. Calcium signalling and ER stress in insulin resistance and atherosclerosis. J. Intern. Med. 2016;280:457–464. doi: 10.1111/joim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weyer C., Bogardus C., Mott D.M., Pratley R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Klein B.E., Klein R., Moss S.E. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92:1191–1196. doi: 10.1016/S0161-6420(85)33877-0. [DOI] [PubMed] [Google Scholar]
- 146.Sowers J.R., Epstein M., Frohlich E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.HYP.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 147.Levy J., Gavin J.R., 3rd, Sowers J.R. Diabetes mellitus: A disease of abnormal cellular calcium metabolism? Am. J. Med. 1994;96:260–273. doi: 10.1016/0002-9343(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 148.van Dam R.M., Hu F.B., Rosenberg L., Krishnan S., Palmer J.R. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care. 2006;29:2238–2243. doi: 10.2337/dc06-1014. [DOI] [PubMed] [Google Scholar]
- 149.Pittas A.G., Lau J., Hu F.B., Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nikooyeh B., Neyestani T.R., Farvid M., Alavi-Majd H., Houshiarrad A., Kalayi A., Shariatzadeh N., Gharavi A., Heravifard S., Tayebinejad N., et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: A randomized clinical trial. Am. J. Clin. Nutr. 2011;93:764–771. doi: 10.3945/ajcn.110.007336. [DOI] [PubMed] [Google Scholar]
- 151.Borissova A.M., Tankova T., Kirilov G., Dakovska L., Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 2003;57:258–261. [PubMed] [Google Scholar]
- 152.Berridge M.J. Vitamin D deficiency and diabetes. Biochem. J. 2017;474:1321–1332. doi: 10.1042/BCJ20170042. [DOI] [PubMed] [Google Scholar]
- 153.Kaneto H., Katakami N., Matsuhisa M., Matsuoka T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Volpe C.M.O., Villar-Delfino P.H., Dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pereira F., Barbachano A., Singh P.K., Campbell M.J., Munoz A., Larriba M.J. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle. 2012;11:1081–1089. doi: 10.4161/cc.11.6.19508. [DOI] [PubMed] [Google Scholar]
- 156.Roe M.W., Philipson L.H., Frangakis C.J., Kuznetsov A., Mertz R.J., Lancaster M.E., Spencer B., Worley J.F., 3rd, Dukes I.D. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J. Biol. Chem. 1994;269:18279–18282. [PubMed] [Google Scholar]
- 157.Levy J., Zhu Z., Dunbar J.C. The effect of glucose and calcium on Ca2+-adenosine triphosphatase in pancreatic islets isolated from a normal and a non-insulin-dependent diabetes mellitus rat model. Metabolism. 1998;47:185–189. doi: 10.1016/S0026-0495(98)90218-9. [DOI] [PubMed] [Google Scholar]
- 158.Raggi P., Shaw L.J., Berman D.S., Callister T.Q. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J. Am. Coll. Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 159.Kato S., Ishida H., Tsuura Y., Okamoto Y., Tsuji K., Horie M., Okada Y., Seino Y. Increased calcium-channel currents of pancreatic beta cells in neonatally streptozocin-induced diabetic rats. Metabolism. 1994;43:1395–1400. doi: 10.1016/0026-0495(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 160.Rasmussen H. The calcium messenger system (2) N. Engl. J. Med. 1986;314:1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- 161.Porte D., Jr. Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 162.Davies M.J., Rayman G., Grenfell A., Gray I.P., Day J.L., Hales C.N. Loss of the first phase insulin response to intravenous glucose in subjects with persistent impaired glucose tolerance. Diabet. Med. 1994;11:432–436. doi: 10.1111/j.1464-5491.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 163.Gerich J.E. Pathogenesis and treatment of type 2 (noninsulin-dependent) diabetes mellitus (NIDDM) Horm. Metab. Res. 1996;28:404–412. doi: 10.1055/s-2007-979828. [DOI] [PubMed] [Google Scholar]
- 164.Bergstrom R.W., Wahl P.W., Leonetti D.L., Fujimoto W.Y. Association of fasting glucose levels with a delayed secretion of insulin after oral glucose in subjects with glucose intolerance. J. Clin. Endocrinol. Metab. 1990;71:1447–1453. doi: 10.1210/jcem-71-6-1447. [DOI] [PubMed] [Google Scholar]
- 165.Pratley R.E., Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia. 2001;44:929–945. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- 166.Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia. 2003;46(Suppl. 1):M2–M8. doi: 10.1007/s00125-002-0930-6. [DOI] [PubMed] [Google Scholar]
- 167.Del Prato S., Marchetti P., Bonadonna R.C. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;51(Suppl. 1):S109–S116. doi: 10.2337/diabetes.51.2007.S109. [DOI] [PubMed] [Google Scholar]
- 168.Reaven G.M., Thompson L.W., Nahum D., Haskins E. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- 169.Perlmuter L.C., Hakami M.K., Hodgson-Harrington C., Ginsberg J., Katz J., Singer D.E., Nathan D.M. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am. J. Med. 1984;77:1043–1048. doi: 10.1016/0002-9343(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 170.Pike C.J., Burdick D., Walencewicz A.J., Glabe C.G., Cotman C.W. Neurodegeneration induced by beta-amyloid peptides In Vitro: The role of peptide assembly state. J. Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gschwind M., Huber G. Apoptotic cell death induced by beta-amyloid 1-42 peptide is cell type dependent. J. Neurochem. 1995;65:292–300. doi: 10.1046/j.1471-4159.1995.65010292.x. [DOI] [PubMed] [Google Scholar]
- 172.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 173.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Francis R., McGrath G., Zhang J., Ruddy D.A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M.C., et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev. Cell. 2002;3:85–97. doi: 10.1016/S1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 175.Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y.Q., Rogaeva E., Chen F., Kawarai T., et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 176.Sun L., Zhou R., Yang G., Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc. Natl. Acad. Sci. USA. 2017;114:E476–E485. doi: 10.1073/pnas.1618657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 178.Pimplikar S.W., Nixon R.A., Robakis N.K., Shen J., Tsai L.H. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J. Neurosci. 2010;30:14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chavez-Gutierrez L., Bammens L., Benilova I., Vandersteen A., Benurwar M., Borgers M., Lismont S., Zhou L., Van Cleynenbreugel S., Esselmann H., et al. The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bentahir M., Nyabi O., Verhamme J., Tolia A., Horre K., Wiltfang J., Esselmann H., De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J. Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 181.Shimojo M., Sahara N., Murayama M., Ichinose H., Takashima A. Decreased Abeta secretion by cells expressing familial Alzheimer’s disease-linked mutant presenilin 1. Neurosci. Res. 2007;57:446–453. doi: 10.1016/j.neures.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Cacquevel M., Aeschbach L., Houacine J., Fraering P.C. Alzheimer’s disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified gamma-secretase complexes. PLoS ONE. 2012;7:e35133. doi: 10.1371/journal.pone.0035133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heilig E.A., Xia W., Shen J., Kelleher R.J., 3rd A presenilin-1 mutation identified in familial Alzheimer disease with cotton wool plaques causes a nearly complete loss of gamma-secretase activity. J. Biol. Chem. 2010;285:22350–22359. doi: 10.1074/jbc.M110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Doody R.S., Raman R., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., He F., Sun X., Thomas R.G., et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 185.Nordberg A. Amyloid plaque imaging In Vivo: Current achievement and future prospects. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(Suppl. 1):S46–S50. doi: 10.1007/s00259-007-0700-2. [DOI] [PubMed] [Google Scholar]
- 186.Villemagne V.L., Fodero-Tavoletti M.T., Pike K.E., Cappai R., Masters C.L., Rowe C.C. The ART of loss: Abeta imaging in the evaluation of Alzheimer’s disease and other dementias. Mol. Neurobiol. 2008;38:1–15. doi: 10.1007/s12035-008-8019-y. [DOI] [PubMed] [Google Scholar]
- 187.Raux G., Gantier R., Thomas-Anterion C., Boulliat J., Verpillat P., Hannequin D., Brice A., Frebourg T., Campion D. Dementia with prominent frontotemporal features associated with L113P presenilin 1 mutation. Neurology. 2000;55:1577–1578. doi: 10.1212/WNL.55.10.1577. [DOI] [PubMed] [Google Scholar]
- 188.Tang-Wai D., Lewis P., Boeve B., Hutton M., Golde T., Baker M., Hardy J., Michels V., Ivnik R., Jack C., et al. Familial frontotemporal dementia associated with a novel presenilin-1 mutation. Dement. Geriatr. Cogn. Disord. 2002;14:13–21. doi: 10.1159/000058328. [DOI] [PubMed] [Google Scholar]
- 189.Dermaut B., Kumar-Singh S., Engelborghs S., Theuns J., Rademakers R., Saerens J., Pickut B.A., Peeters K., van den Broeck M., Vennekens K., et al. A novel presenilin 1 mutation associated with Pick’s disease but not beta-amyloid plaques. Ann. Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 190.Cruts M., Theuns J., Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum. Mutat. 2012;33:1340–1344. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.F., Hao Y.H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nelson O., Tu H., Lei T., Bentahir M., de Strooper B., Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Investig. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Supnet C., Bezprozvanny I. Presenilins function in ER calcium leak and Alzheimer’s disease pathogenesis. Cell Calcium. 2011;50:303–309. doi: 10.1016/j.ceca.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bezprozvanny I. Presenilins and calcium signaling-systems biology to the rescue. Sci. Signal. 2013;6:pe24. doi: 10.1126/scisignal.2004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xia W., Ostaszewski B.L., Kimberly W.T., Rahmati T., Moore C.L., Wolfe M.S., Selkoe D.J. FAD mutations in presenilin-1 or amyloid precursor protein decrease the efficacy of a gamma-secretase inhibitor: Evidence for direct involvement of PS1 in the gamma-secretase cleavage complex. Neurobiol. Dis. 2000;7:673–681. doi: 10.1006/nbdi.2000.0322. [DOI] [PubMed] [Google Scholar]
- 196.Szostak J.W., Bartel D.P., Luisi P.L. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Y., Ruder W.C., LeDuc P.R. Artificial cells: Building bioinspired systems using small-scale biology. Trends Biotechnol. 2008;26:14–20. doi: 10.1016/j.tibtech.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 198.Molinaro R., Corbo C., Martinez J.O., Taraballi F., Evangelopoulos M., Minardi S., Yazdi I.K., Zhao P., De Rosa E., Sherman M.B., et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D., Nguyen P., Angsantikul P., Wen C.H., Kroll A.V., et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen Z., Wang J., Sun W., Archibong E., Kahkoska A.R., Zhang X., Lu Y., Ligler F.S., Buse J.B., Gu Z. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 2018;14:86–93. doi: 10.1038/nchembio.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]