Abstract
Simple Summary
Resistance has developed against almost all the main classes of antibiotics, and finding efficient alternatives to these antibiotics is urgently required. Based on previous research, three types of probiotic bacteria were chosen to be administrated in a laying hen diet. We found that the performance, egg quality, and gut health of laying hens were improved after probiotic treatment.
Abstract
With recent bans on the growth-promoting use of antibiotics, alternative strategies are needed to improve the performance of agricultural animals. Here, the effects of dietary supplementation with Clostridium butyricum and a combination of Saccharomyces boulardii and Pediococcus acidilactici were assessed on laying performance, egg quality, oxidative status, and gut health in laying hens. A total of 8208 Lohmann pink laying hens were divided into 3 treatment groups, with each group replicated 12 times (n = 228). Hens in the control group (CON) were provided a basic diet devoid of added antibiotics and probiotics. Treatment group 1 (T1) received the same base diet supplemented with 0.5 g/kg C. butyricum, and the diets of treatment group 2 (T2) supplemented with S. boulardii (0.05 g/kg) and P. acidilactici (0.1 g/kg) for the entirety of the 5-week trial. The data indicated that C. butyricum supplementation resulted in a significant reduction in ADFI, a significant increase in feed conversion, eggshell strength, and the CP% of albumen (dry matter, DM) relative to CON. The probiotic-treated hens exhibited decreased reactive oxygen species (ROS) levels in ileum and cecum, and reduced malondialdehyde (MDA) in serum. In conclusion, dietary supplementation with C. butyricum may be beneficial with respect to hen performance, egg quality, and gut health.
Keywords: C. butyricum, S. boulardii, P. acidilactici, laying performance, egg quality, gut health, laying hens
1. Introduction
Antibiotics have been recognized as one of the most effective therapies in medicine [1,2]. However, due to the growing number of antibiotic-resistant bacteria, the agricultural use of antibiotics as growth promoters in livestock has being banned in many countries worldwide. Antibiotic growth promoters (AGPs) have been banned in the European Union since 2006 [3]. In spite of the prohibition, a large number of AGPs are being administered to laying hens, through individual treatments or water and diet supplementation, in an effort to reduce the occurrence of diseases and improve the performance of laying hens [4,5]. Not only can overuse of antibiotics at subtherapeutic doses lead to bacterial resistance [6,7,8], but they are also detectable in eggs. This poses significant concerns regarding the potential effects on human health [9]. Therefore, alternatives to antibiotics are urgently needed.
It has been documented that probiotics are an attractive alternative to antibiotics which have been demonstrated to improve intestinal health, increase the stability of the gut flora, and suppress the colonization of pathogens [10,11]. Previous reports have indicated that dietary supplementation with probiotics is not only capable of increasing egg production, but also improving feed conversion efficiency [12], promoting hen performance, and eggshell quality [13]. Moreover, it was reported that probiotics could also regulate symbiotic bacteria colonization [14], increase the number of intestinal goblet cells [15], and stimulate intestinal T-cell immunity [16]. These findings provide intriguing evidence that probiotics may have a beneficial effect on laying hens. Clostridium butyricum is an anaerobic bacillus, which has been approved as a feed additive for broilers and weaned piglets in the European Union since 2003. In addition, C. butyricum was also approved as a feed additive for laying hens. In mouse studies, C. butyricum supplementation increased the numbers of Treg cells in the intestinal lamina propria, promoted the secretion of the key anti-inflammatory cytokine IL-10, and improved the early development of intestinal immune tolerance [17]. Of particular interest is a study by Zhang et al. (2014), in which the authors demonstrated that C. butyricum supplementation was able to improve the growth performance, immune function, and cecal microflora in broiler chickens following treatment with E. coli K88 [18]. However, studies examining the effects of C. butyricum on laying hens are limited.
Previous studies have demonstrated that dietary yeast products could improve intestinal immunity [19,20,21] and minimize coccidial infection in layer chickens [22]. S. boulardii, a relative of Saccharomyces cerevisiae, is a recognized probiotic fungus. As a facultative anaerobic fungus, S. boulardii can provide nutrients for the host, improve the activity of beneficial intestinal bacteria, inhibit the growth of pathogens, and improve the immune function of the intestinal mucosa [23,24]. P. acidilactici was reported to produce specific lactic acid products, which was widely used in improving productive performance of laying hens [13,25]. As P. acidilactici is a strict anaerobic probiotic, S. boulardii may improve the function of P. acidilactici by accelerating intestinal oxygen consumption.
The objectives of this study were to (1) evaluate the effects of C. butyricum and a combination of S. boulardii and P. acidilactici on the performance, egg quality, morbidity, and mortality of laying hens, and (2) to study the effect of probiotics on the gut microbiota and the intestinal structure and function of laying hens.
2. Materials and Methods
2.1. Probiotic Strains
Three pure probiotic strains were used in this study. C. butyricum was provided by Hubei Lvxue Biotechnology Co., Ltd. (Xianning, Hubei, China) Powdered C. butyricum is provided. S. boulardii I-1079 and P. acidilactici MA18/5M were provided by Beijing Hilink International Biotechnology Co., Ltd. (Beijing, China) The dose of dietary probiotic supplement was added with reference to the companies’ commercial recommendations.
2.2. Experimental Design
The protocol for the animal experimental procedures was approved by Institutional Animal Care and Use Committee of Huazhong Agricultural University (Wuhan, China). The ethical number of this study is HZAUCH-2017-009.
A total of 8208 Lohmann pink laying hens (180 days) were randomly assigned to 3 groups with 12 replicates of 228 birds each (2736 laying hens per group). The 3 groups were divided into one control group (CON) and 2 treatment groups (T1 and T2). The diets for T1 consisted of a standard feed supplemented with C. butyricum (0.5 g/kg), and that of T2 was supplemented with both S. boulardii (0.05 g/kg) and P. acidilactici (0.1 g/kg).
2.3. Birds, Diet and Management
This trial was carried out in Xian Ning, Hubei Province, during the month of January. Three birds were housed in individual cages, under a 16 h light/8 h dark cycle (lights on at 5:00, lights off at 21:00) and a constant temperature of 9 ± 2 °C. All birds were acclimated to a basal diet for 1 week. laying hens were caged in 3-tier battery cages, 3 hens per cage. The basal diets provided to the hens are presented in Table 1. The temperature, humidity, and light conditions in different chicken coops were similar. Water and feed were provided ad libitum during the 5-week study period.
Table 1.
Composition and nutrition levels of the based diet 1.
| Items | Content | Items | Content |
|---|---|---|---|
| Ingredients | % | Nutrient levels | |
| Corn | 63 | ME/(MJ/kg) | 10.92 |
| Soybean meal | 24 | Crude protein, % | 15.73 |
| Limestone | 8 | Lysine, % | 0.82 |
| Premix 2 | 5 | Methionine, % | 0.41 |
| Total | 100 | Calcium, % | 3.32 |
1 Values are expressed on an air-dried basis. 2 The premix provided the following per kg of diets: 17.0 × 104 IU VA; 5.04 × 104 to 10.0 × 104 IU VD3; 366 mg DL-α-tocopheryl acetate; 48.0 mg menadione nicotinamide bisulfite (MNB, vitamin K3); 172 mg pantothenic; 32.1 mg thiamine nitrate; 97.2 mg vitamin B2; 425 mg nicotinamide; 144 mg Cu; 640 mg Fe; 1620 mg Mn; 1520 mg Zn.
2.4. Sample Collection and Analytical Determination
2.4.1. Samples
Health condition, mortality, feed intake, laying performance, and egg quality were assessed daily. At the end of the trial, a single healthy laying hen was selected from each group replicate, 12 in each group, for a total of 48 hens, which were then sacrificed. Blood and intestinal samples were collected for examination. Blood samples were collected from the axillary vein into vacuum tubes (10 mL) containing coagulant. The blood was then centrifuged at 3000× g for 10 min at 4 °C. Serum was collected and stored at −80 °C until the time of further analysis. The gut of each hen was removed immediately after sacrifice, and segments of ileum and cecum were identified and ligated prior to excision. Digesta of the ileum and cecum were collected and stored at −80 °C until analysis. Intestinal tissues were fixed in 10% phosphate-buffered formalin and stored at room temperature (Huang et al. 2014) for histological examination.
2.4.2. Laying Performance
Daily feed intake, egg production, and egg weights were measured throughout the experimental period. The actual daily feed intake per group replicate was measured and used to calculate the average daily feed intake (ADFI). Laying rate is expressed as average hen-day production, calculated from the total number of eggs divided by the total number of days. Feed conversion ratio was expressed as grams of feed consumed per grams of eggs produced.
2.4.3. Egg Quality
Egg quality was evaluated at the end of trial. A total of 72 eggs were randomly collected from each treatment (n = 72, 6 per replicate) in order to evaluate egg quality. Egg weight, Haugh unit (HU), yolk color, shell strength (measured with an eggshell strength tester), shell thickness (measured with a spiral micrometer), and shape index (determined using Vernier calipers) were evaluated. Crude protein, water, crude fat, ash, calcium, phosphorus, and the cholesterol levels of the eggs were also evaluated.
2.4.4. Antioxidative Stress
Reactive oxygen species (ROS), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-PX) activity, and malondialdehyde (MDA) concentrations in either ileum or cecum were measured to estimate the oxidative status. To assess ROS, chemiluminescence (CL) derived from luminol was measured as an indicator of radical formation. Determination of T-SOD, GSH-Px, and MDA levels was accomplished using an assay kit from the Nanjing Jiancheng Bioengineering Institute. The specific procedures were followed according to the manufacturer’s instructions.
2.4.5. Gut Histological Analysis
Ileum and cecum were fixed in 10% phosphate-buffered formalin, and embedded in paraffin blocks. The samples were then sectioned to a thickness of 5 mm, and stained with hematoxylin and eosin. Villus height, villus width, and crypt depth were measured on the stained sections using a light microscope fitted with an image analyzer (Image Pro Plus 6.0, Media Cybernetics, Bethesda, MD, USA).
2.5. Statistical Analysis
All results were statistically compared with an ANOVA test using SAS 9.4. A one-way ANOVA, followed by Duncan’s multiple comparison test, was used to evaluate different means among treatments. Differences were considered to be significant at p < 0.05.
2.6. Ethical Statement
This study is approved by the Scientific Ethic Committee of Huazhong Agricultural University (ethical approval number is HZAUCH-2017-009).
3. Results
3.1. Laying Performance
Laying performance data are summarized in Table 2. Dietary supplementation with C. butyricum (T1) not only reduced ADFI, but also significantly increased feed conversion (p = 0.0341). However, there was no apparent effect on the mortality of hens during the experimental period (p = 0.3445). In addition, laying rate (p = 0.4777), average egg weights (p = 0.4923), and average cracked egg percentage (p = 0.0957) were not influenced by dietary probiotic supplementation compared with the CON group.
Table 2.
Different probiotics effect on laying performance of laying hens.
| Item | CON | T1 | T2 | p-Value |
|---|---|---|---|---|
| ADFI | 105.5 ± 1.80 a | 104.1 ± 1.14 b | 105.2 ± 1.61 ab | 0.083 |
| Average egg weights (g) | 57.2 ± 0.42 | 57.1 ± 0.46 | 57.3 ± 0.34 | 0.4923 |
| Feed conversion (g of feed/g of egg) | 1.97 ± 0.04 a | 1.92 ± 0.03 b | 1.95 ± 0.03 ab | 0.0341 |
| Laying rate (%) | 93.8 | 94.4 | 94.5 | 0.4777 |
| Mortality (%) | 2.01 | 1.83 | 2.44 | 0.3445 |
| Average cracked egg percent/% | 0.10 | 0.07 | 0.09 | 0.0957 |
a,b Means without a common superscript with a row differ significantly (p < 0.05). CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation. ADFI = average daily feed intake.
3.2. Egg Quality
The external and internal egg qualities for layers are presented in Table 3. Compared with CON, there were no significant differences in egg quality in T2. In T1, dietary C. butyricum supplementation decreased eggshell strength (p = 0.0044) and yolk color (p = 0.055), and increased the CP% of albumen (DM) (p = 0.0809), but no statistically significant changes were noted in any of the other egg quality traits examined.
Table 3.
Effect of different probiotics on the egg quality of laying hens.
| Item | CON | T1 | T2 | p-Value |
|---|---|---|---|---|
| Egg shape index | 77.81 ± 1.15 | 76.44 ± 3.01 | 77.07 ± 0.90 | 0.234 |
| Eggshell strength, kg/cm2 | 4.77 ± 0.27 a | 4.41 ± 0.33 b | 4.86 ± 0.37 a | 0.0044 |
| Haugh unit | 83.20 ± 5.94 | 82.65 ± 5.82 | 82.98 ± 4.09 | 0.9678 |
| Albumen height, mm | 7.08 ± 0.89 | 6.93 ± 0.78 | 6.95 ± 0.58 | 0.8791 |
| Yolk color | 7.33 ± 0.26 a | 7.07 ± 0.27 b | 7.11 ± 0.29 ab | 0.055 |
| Eggshell thickness, um | 346.42 ± 8.16 | 347.57 ± 11.66 | 344.68 ± 8.28 | 0.7569 |
| Yolk percentage, % | 25.80 ± 0.72 | 26.46 ± 1.53 | 26.14 ± 0.93 | 0.3553 |
| Yolk CP%/DM | 30.53 ± 0.55 | 31.49 ± 1.48 | 30.57 ± 1.48 | 0.3479 |
| Albumen CP%/DM | 81.06 ± 1.63 b | 82.65 ± 0.91 a | 82.27 ± 0.57 ab | 0.0809 |
| Yolk Fat%/DM | 55.97 ± 1.89 | 54.52 ± 1.77 | 55.77 ± 1.64 | 0.1091 |
| Cholesterol content of yolk, % | 3.16 ± 0.65 | 2.90 ± 0.17 | 2.94 ± 0.21 | 0.3046 |
a,b Means without a common superscript with a row differ significantly (p < 0.05). CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation. CP = crude protein, DM = dry matter.
3.3. Antioxidative Stress
Table 4 shows the antioxidative stress status of the serum, ileum, and cecum of laying hens. The data indicate that dietary S. boulardii and P. acidilactici (T2) supplementation did not have an effect on the biomarkers of antioxidative stress. However, C. butyricum supplementation (T1) decreased ROS levels in both ileum (p < 0.01) and cecum (p < 0.01), as well as reduced serum MDA (p < 0.05). No statistically significant differences were observed for T-SOD and GSH-PX in laying hens (p > 0.05).
Table 4.
Different probiotics effect on antioxidative status in serum, ileum, and cecum of laying hens.
| Item | CON | T1 | T2 | p | |
|---|---|---|---|---|---|
| Serum | ROS (U/mL) | 5.64 ± 1.25 | 6.60 ± 2.39 | 6.09 ± 3.28 | 0.7313 |
| T-SOD (U/mL) | 80.37 ± 12.01 | 81.79 ± 8.09 | 82.83 ± 10.8 | 0.4654 | |
| MDA (nmol/mL) | 16.82 ± 4.57 a | 13.40 ± 3.86 b | 15.80 ± 5.56 ab | 0.0386 | |
| Ileum | ROS (U/mL) | 4.12 ± 0.47 a | 3.57 ± 0.34 b | 3.94 ± 0.48 a | 0.0017 |
| T-SOD (U/mL) | 101.27 ± 13.4 | 88.04 ± 23.69 | 111.56 ± 22.96 | 0.3091 | |
| GSH-PX (U/mL) | 132.00 ± 103.96 | 159.34 ± 103.71 | 67.75 ± 42.2 | 0.1613 | |
| Cecum | ROS (U/mL) | 3.81 ± 0.41 a | 3.08 ± 0.24 b | 3.64 ± 0.34 a | 0.0009 |
| T-SOD (U/mL) | 57.87 ± 16.16 | 52.09 ± 15.97 | 55.00 ± 14.44 | 0.5541 | |
| GSH-PX (U/mL) | 117.08 ± 74.91 | 57.74 ± 30.00 | 143.12 ± 160.82 | 0.3833 |
a,b Means without a common superscript with a row differ significantly (p < 0.05). CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation. ROS = reactive oxygen species, T-SOD = total superoxide dismutase, GSH-PX = glutathione peroxidase, MDA = malondialdehyde.
3.4. Histological Analysis of the Gut
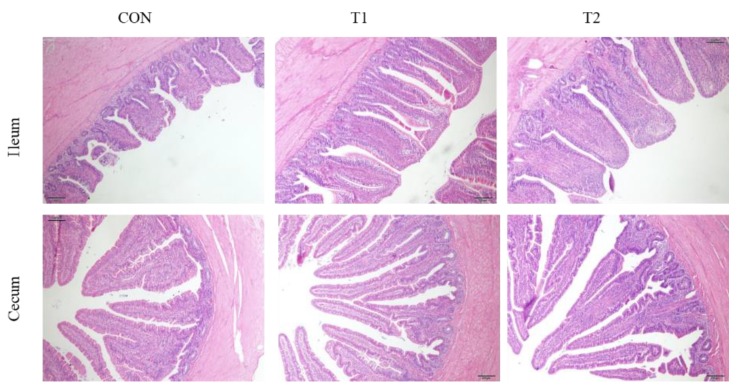
As presented in Figure 1 and Table 5, dietary probiotic supplementation improved the health and microscopic structure of the ileum and cecum. The villi were observed to be short and thin in the ileum of the CON, whereas they were taller and a greater proportion were intact as observed in the ileum of groups A and B alike. Similarly, the villi in the cecum of hens from groups A and B were higher than in CON. Furthermore, villus height and villus height/crypt depth ratio (villus/crypt) were significantly increased in groups A and B compared with CON in the ileum and cecum (p < 0.05) (Table 5).
Figure 1.
Effect of different probiotics on ileum and cecum morphology in laying hens. CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation.
Table 5.
Effect of different probiotics on ileum and cecum morphology in laying hens.
| Intestinal Segment | Item | CON | T1 | T2 | p-Value |
|---|---|---|---|---|---|
| ileum | villus height | 488.51 ± 92.18 b | 650.60 ± 169.29 a | 649.47 ± 191.44 a | <0.0001 |
| crypt depth | 187.10 ± 37.92 b | 196.18 ± 49.84 ab | 207.74 ± 54.222 a | 0.0008 | |
| villus width | 317.38 ± 115.76 | 310.19 ± 77.30 | 354.11 ± 109.75 | 0.508 | |
| villus/crypt | 2.77 ± 0.78 b | 3.58 ± 1.33 a | 3.24 ± 1.01 a | 0.0026 | |
| cecum | villus height | 1178.39 ± 187.56 c | 1531.50 ± 287.69 a | 1310.61 ± 238.16 b | <0.0001 |
| crypt depth | 197.16 ± 38.93 | 186.64 ± 36.45 | 193.73 ± 36.09 | 0.3794 | |
| villus width | 322.65 ± 80.66 a | 282.21 ± 91.54 b | 293.17 ± 85.75 ab | 0.0338 | |
| villus/crypt | 6.20 ± 1.60 b | 8.76 ± 2.38 a | 7.06 ± 2.14 b | <0.0001 |
a–c Means without a common superscript with a row differ significantly (p < 0.05). CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation.
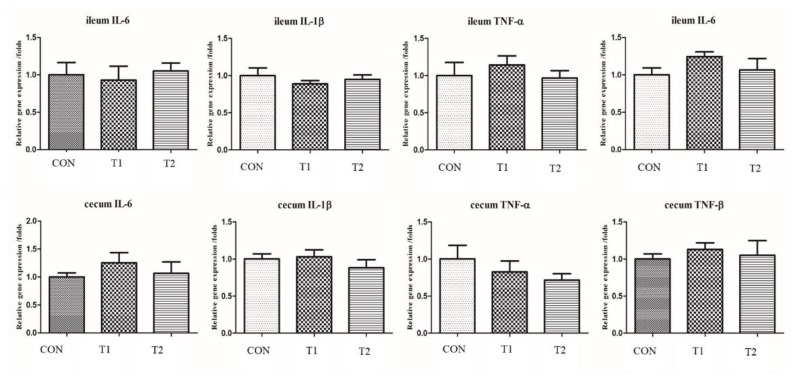
3.5. mRNA Levels of Pro-Inflammatory Cytokines in the Intestinal Tract
Gene expression levels of four major inflammatory cytokines involved in intestinal tissues were measured. Included in the assays were interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α and/β (TNF-α/β). There were no statistically significant differences in gene expression observed between T1, T2, or CON.
4. Discussion
In recent years, the demand for eggs is no longer an issue of quantity, but has become increasingly focused on egg quality. The extensive use of antibiotics in poultry farms has not only increased the safety risks associated with egg consumption, but has also decreased egg quality. Moreover, microbial antibiotic resistance, caused by the overuse of antibiotics, is one of the biggest threats to global health, food security, and development [26]. Therefore, alternatives to antibiotics are critically needed. An important alternative to antibiotics, probiotics have been receiving more and more attention in the field of animal husbandry. Previous studies have suggested that C. butyricum and S. boulardii could improve growth performance of broiler chickens [18,27,28,29]. A few studies have also demonstrated beneficial effects of dietary C. butyricum supplementation on the laying performance of hens [11,30]. However, there are very limited studies examining the effects of S. boulardii on laying hens. Conversely, several studies have shown that the addition of butyric acid bacteria to the diet has no significant effect on improving the performance of agricultural animals [31].
In the present study, diets supplemented with C. butyricum or a combination of S. boulardii and P. acidilactici were examined. Diets supplemented with C. butyricum reduced ADFI and increased feed conversion, which is similar to the results in broilers in Mervat et al. [32]. However, the combination of probiotics had no effect on laying performance. Compared with other studies, the scale of the animal experiments performed here was lager, lending confidence in our conclusion that C. butyricum exerts positive effects on laying performance in hens. The significant increase in the crude protein content of albumen means the egg quality of C. butyricum-treated laying hen was improved.
Antioxidative stress is one of the important factors affecting the performance and egg quality of laying hens. Reactive oxygen species are widely accepted to be detrimental for health [33,34]. Studies have indicated that dietary probiotics are effective in counteracting the adverse influences of antioxidative stress [35], and promoting the activities of antioxidant enzymes [36]. Here, it was observed that C. butyricum significantly decreased the levels of ROS in both the ileum and cecum. Although the major antioxidant enzymes (T-SOD and GSH-PX) were apparently unaffected by probiotic supplementation, the levels of MDA, a cellular endproduct of lipid peroxidation and an important indicator of stress [37], were significantly decreased. These results may indicate that C. butyricum is capable of reducing oxidative stress in the intestines of the laying hens by reducing the degree of intestinal lipid oxidation as opposed to by accelerating the depletion of ROS.
The health status of the mucosa and the microscopic structure can be good indicators of the response of the intestinal tract to active substances in feed [38]. Previous studies have indicated that probiotics may be useful for improving the gut health of laying hens [35] and broiler chickens [16]. Here, it was observed that probiotic supplementation was able to significantly improve the intestinal histological morphology of laying hens (Figure 1 and Table 5). In our study, we found that dietary probiotic supplementation increased the villus height and villus height/crypt depth ratio in both ileum and cecum, which may reflect better epithelial growth after probiotic supplement [39,40].
The levels of pro-inflammatory cytokines in the intestinal tract were within normal ranges (Figure 2). These results indicate that all hens were in good health, which would explain the lack of difference in the observed mortality (p = 0.3445, Table 2). Deng et al. (2012) demonstrated that the level of serum TNF-α in hens was reduced if probiotics were included in the diet under heat stress [35]. As this study was conducted during winter, none of the hens were under heat stress. In addition, our results also show that not all layers were experiencing oxidative stress.
Figure 2.
Effect of different probiotics on the mRNA levels of pro-inflammatory cytokines. CON = basal diet (BD), T1 = BD plus 0.5 g/kg diet C. butyricum preparation, T2 = BD plus 0.1 g/kg diet P. acidilactici and 0.05 g/kg S. boulardii preparation.
5. Conclusions
According to our findings, we concluded that the use of probiotics containing both C. butyricum and a combination of S. boulardii and P. acidilactici in the laying hen diet was beneficial in enhancing the development of the intestine. In particular, dietary C. butyricum has significant beneficial effects on the performance of laying hens, and contributes to the improvement of normal gut morphology. However, the beneficial effects on the production performance of treatment with S. boulardii and P. acidilactici were not detected during the five-week experimental period, and this may be due to the experimental time being too short, but this result also gives us some useful guidance, as not all probiotics can be used in laying hen production, and the right choice for effective and safe probiotics is critical, and more comprehensive research needs to be performed in the future.
Author Contributions
J.P. was in charge of the whole trial; Q.X. analyzed experimental data and wrote the manuscript; Q.X. and C.W. for animal feeding and sample collections; Q.X., H.Z. and W.L. assisted with laboratory analyses. All authors read and approved the final manuscript.
Funding
This research was supported by the National Key Research and Development Project of China (NO. 2017YFD0502004). The authors declare that they have no conflict of interest.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Davies J., Davies D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanan L., Adriano D., Chand W., Zaidi A.K.M., Wertheim H.F.L., Nithima S., Erika V., Gabriel Levy H., Gould I.M., Herman G. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 3.Millet S., Maertens L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011;187:143–144. doi: 10.1016/j.tvjl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Singer R.S., Hofacre C.L. Potential impacts of antibiotic use in poultry production. Avian Dis. 2006;50:161–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- 5.Wongsuvan G., Wuthiekanun V., Hinjoy S., Day N.P., Limmathurotsakul D. Antibiotic use in poultry: A survey of eight farms in Thailand. Bull. World Health Organ. 2018;96:94–100. doi: 10.2471/BLT.17.195834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., Goossens H., Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A., Laxminarayan R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 9.Donoghue D.J. Antibiotic residues in poultry tissues and eggs: Human health concerns? Poult. Sci. 2003;82:618–621. doi: 10.1093/ps/82.4.618. [DOI] [PubMed] [Google Scholar]
- 10.Callaway T.R., Edrington T.S., Anderson R.C., Harvey R.B., Genovese K.J., Kennedy C.N., Venn D.W., Nisbet D.J. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health Res. Rev. 2008;9:217–225. doi: 10.1017/S1466252308001540. [DOI] [PubMed] [Google Scholar]
- 11.Gaggia F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Yörük M.A., Gül M., Hayirli A., Laçin E. Laying Performance and Egg Quality of Hens Supplemented with Humate and Sodium Bicarbonate during the Late Laying Period. J. Appl. Anim. Res. 2004;26:17–21. doi: 10.1080/09712119.2004.9706498. [DOI] [Google Scholar]
- 13.Mikulski D., Jankowski J., Naczmanski J., Mikulska M., Demey V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult. Sci. 2012;91:2691–2700. doi: 10.3382/ps.2012-02370. [DOI] [PubMed] [Google Scholar]
- 14.Coillie E., Van Goris J., Cleenwerck I., Grijspeerdt K., Botteldoorn N., Immerseel F., Van Buck J., De Vancanneyt M., Swings J., Herman L. Identification of lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J. Appl. Microbiol. 2010;102:1095–1106. doi: 10.1111/j.1365-2672.2006.03164.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahdavi A.H., Rahmani H.R., Pourreza J. Effect of probiotic inclusion in different levels of barley substitution for corn diets on laying hen’s histological changes of duodenum; Proceedings of the European Symposium on Poultry Nutrition; Gdańsk, Poland. 10–13 June 2019. [Google Scholar]
- 16.Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- 17.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Cao G.T., Zeng X.F., Zhou L., Ferket P.R., Xiao Y.P., Chen A.G., Yang C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2014;93:46–53. doi: 10.3382/ps.2013-03412. [DOI] [PubMed] [Google Scholar]
- 19.Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J., Gao Y.P., Qi G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- 20.Higgins S.E., Higgins J.P., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Tellez G., Hargis B. Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella enteritidis in neonatal broiler chicks. Poult. Sci. 2008;87:27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- 21.Liu N., Wang J.Q., Jia S.C., Chen Y.K., Wang J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018;97:477–484. doi: 10.3382/ps/pex342. [DOI] [PubMed] [Google Scholar]
- 22.Markazi A.D., Perez V., Sifri M., Shanmugasundaram R., Selvaraj R.K. Effect of whole yeast cell product supplementation (CitriStim(R)) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017;96:2049–2056. doi: 10.3382/ps/pew482. [DOI] [PubMed] [Google Scholar]
- 23.De Avila L.F., Conceicao F.R., Telmo Pde L., Dutra G.F., de los Santos D.G., Martins L.H., Berne M.E., da Silva P.E., Scaini C.J. Saccharomyces boulardii reduces infection intensity of mice with toxocariasis. Vet. Parasitol. 2012;187:337–340. doi: 10.1016/j.vetpar.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Gedek B., Kirchgessner M., Eidelsburger U., Wiehler S., Bott A., Roth F.X. Influence of formic acid on the microflora in different segments of the gastrointestinal tract, 5: Investigations about the nutritive efficacy of organic acids in the rearing of piglets. Med. Wieku Rozw. 1992;13:260–269. [Google Scholar]
- 25.Quarantelli A., Righi F., Agazzi A., Invernizzi G., Ferroni M., Chevaux E. Effects of the administration ofPediococcus Acidilacticito laying hens on productive performance. Vet. Res. Commun. 2008;32:359–361. doi: 10.1007/s11259-008-9148-5. [DOI] [PubMed] [Google Scholar]
- 26.Boeckel T.P.V., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365 doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X., Guo Y., Guo S., Tan J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl. Microbiol. Biotechnol. 2013;97:6477–6488. doi: 10.1007/s00253-013-4970-2. [DOI] [PubMed] [Google Scholar]
- 28.Liao X.D., Ma G., Cai J., Fu Y., Yan X.Y., Wei X.B., Zhang R.J. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015;94:662–667. doi: 10.3382/ps/pev038. [DOI] [PubMed] [Google Scholar]
- 29.Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Yu D.Y., Li W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- 30.Zhan H.Q., Dong X.Y., Li L.L., Zheng Y.X., Gong Y.J., Zou X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019;98:896–903. doi: 10.3382/ps/pey436. [DOI] [PubMed] [Google Scholar]
- 31.Amerah A.M., Quiles A., Medel P., Sánchez J., Lehtinen M.J., Gracia M.I. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim. Feed. Technol. 2013;180:55–63. doi: 10.1016/j.anifeedsci.2013.01.002. [DOI] [Google Scholar]
- 32.Abdel-Latif M.A., Abd El-Hack M.E., Swelum A.A., Saadeldin I.M., Elbestawy A.R., Shewita R.S., Ba-Awadh H.A., Alowaimer A.N., Abd El-Hamid H.S. Single and Combined Effects of Clostridium butyricum and Saccharomyces cerevisiae on Growth Indices, Intestinal Health, and Immunity of Broilers. Animals. 2018;8:184. doi: 10.3390/ani8100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi Z., Xiang Q., Wang J., Wei H., Jian P. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest. Sci. 2016;192:33–38. [Google Scholar]
- 34.Zou Y., Wei H.K., Xiang Q.H., Wang J., Zhou Y.F., Peng J. Protective effect of quercetin on pig intestinal integrity after transportstress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016;78:1487–1494. doi: 10.1292/jvms.16-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng W., Dong X.F., Tong J.M., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- 36.Sanders M.E. Summary of conclusions from a consensus panel of experts on health attributes of lactic cultures: Significance to fluid milk products containing cultures. J. Dairy Sci. 1993;76:1819–1828. doi: 10.3168/jds.S0022-0302(93)77514-1. [DOI] [PubMed] [Google Scholar]
- 37.Gaweł S., Wardas M., Niedworok E., Wardas P. [Malondialdehyde (MDA) as a lipid peroxidation marker] Wiad. Lek. 2004;57:453–455. [PubMed] [Google Scholar]
- 38.Munyaka P.M., Echeverry H., Yitbarek A., Camelo-Jaimes G., Sharif S., Guenter W., House J.D., Rodriguez-Lecompte J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012;91:2164–2172. doi: 10.3382/ps.2012-02306. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.S., Ingale S.L., Kim Y.W., Kim K.H., Sen S., Ryu M.H., Lohakare J.D., Kwon I.K., Chae B.J. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J. Anim. Physiol. Anim. Nutr. 2012;96:618–626. doi: 10.1111/j.1439-0396.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- 40.Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]