Abstract
Objectives
Useful Field of View training (UFOVt) is an adaptive computerized cognitive intervention that improves visual attention and transfers to maintained health and everyday functioning in older adults. Although its efficacy is well established, the neural mechanisms underlying this intervention are unknown. This pilot study used functional MRI (fMRI) to explore neural changes following UFOVt.
Method
Task-driven and resting-state fMRI were used to examine changes in brain activity and connectivity in healthy older adults randomized to 10 hr of UFOVt (n = 13), 10 hr of cognitively stimulating activities (CSA; n = 11), or a no-contact control (NC; n = 10).
Results
UFOVt resulted in reduced task-driven activity in the majority of regions of interest (ROIs) associated with task performance, CSA resulted in reduced activity in one ROI, and there were no changes within the NC group. Relative to NC, UFOVt reduced activity in ROIs involved in effortful information processing. There were no other significant between-group task-based differences. Resting-state functional connectivity between ROIs involved in executive function and visual attention was strengthened following UFOVt compared with CSA and NC.
Discussion
UFOVt enhances connections needed for visual attention. Together with prior work, this study provides evidence that improvement of the brain’s visual attention efficiency is one mechanism underlying UFOVt.
Keywords: Cognitive training, Speed of processing training, Cognitive intervention, Neural plasticity, Cognitively stimulating activities
There is a growing market for cognitive interventions ranging from brain teaser workbooks to commercially available computer games, which purport to maintain or improve cognition. Cognitive training programs are a promising subset of such interventions developed to improve the performance of specific cognitive domains, such as processing speed and memory. The cognitive and everyday functional transfer of one such program, Useful Field of View training (UFOVt; also known as “speed of processing training” and “divided attention training”), has been thoroughly investigated in 17 randomized controlled trials (Ball, Edwards, & Ross, 2007; Edwards, Fausto, Tetlow, Corona, & Valdés, 2018). UFOVt is a process-based computerized adaptive program focused on visual processing speed, divided attention, and selective attention that has repeatedly demonstrated real-world transfer to improved instrumental activities of daily living (Rebok et al., 2014; Wolinsky, Vander Weg, Howren, Jones, & Dotson, 2015), maintained driving mobility and safety (Ball, Edwards, Ross, & McGwin, 2010; Ross et al., 2016; Ross, Freed, Edwards, Phillips, & Ball, 2017), maintained health and reduced predicted health expenditures (Wolinsky et al., 2009, 2010), reduced risk of depression (Wolinsky et al., 2015), and maintained physical functioning in older adults (Ross, Sprague, Phillips, O’Connor, & Dodson, 2018; Smith-Ray, Makowski-Woidan, & Hughes, 2014) with effects persisting between 3 and 10 years.
Although such transfer effects have been found across multiple studies, a better understanding of the neural mechanisms underlying this training is clearly needed (see Lövdén, Bäckman, Lindenberger, Schaefer, & Schmiedek, 2010). To our knowledge, this pilot study is the first to explore the neural changes associated with UFOVt using both task-based and resting-state functional MRI (fMRI) in a sample of healthy older adults. As there has been some criticism of control groups used in cognitive training studies (Simons et al., 2016), this study included both a treatment-as-usual control group (no-contact control [NC]) and an active control condition that included complex cognitively stimulating activities (CSA). The CSA were chosen based on older adults’ reported expectations for activities that maintain brain health (Friedman et al., 2011, 2013; Kim, Sargent-Cox, & Anstey, 2015), as well as the complexity of activities included (Tranter & Koutstaal, 2008).
Neural Correlates of Useful Field of View Training
Several studies have examined neural changes associated with a wide range of cognitive training programs (Anguera et al., 2013; Erickson et al., 2007a; Scalf et al., 2007). However, to our knowledge, only two studies examined neural changes associated specifically with UFOVt in older adults. The first study (O’Brien et al., 2013) examined event-related potentials (ERPs) after 20 hr of Posit Science’s Insight training in a sample of healthy older adults (N = 22). Insight was a commercialized cognitive training program focused on visual processing that included UFOVt (i.e., Double Decision) as one of the available five exercises. Results indicated that N2pc and P3b component amplitudes, which typically decline with age and are involved in visual attention allocation and capacity (Lorenzo-López, Amenedo, & Cadaveira, 2008), increased after training as compared to controls. These results suggest that the Insight program may remediate age-related visual processing deficits associated with attention allocation and capacity. The second study (Lin et al., 2016) examined changes in resting-state connectivity in the default mode network in participants with amnestic mild cognitive impairment (MCI; N = 21) after Insight training. Results indicated that 24 hr of Insight training resulted in maintained connectivity as compared to an active control group of participants with MCI whose default mode network connectivity decreased over time.
Although both of these studies provide support that UFOVt directly affected neural functioning, no study has explored how UFOVt influences functional activity and connectivity of the brain regions that are recruited for a UFOV-type task in healthy older adults (i.e., fMRI Useful Field of View Imaging Task2 [fUFOV2], see Measures). This pilot study used fMRI to examine how UFOVt affects task-specific neural responses to a visual-attention task by using a region of interest (ROI) approach across two separate sets of analyses, namely, task-based functional activity and resting-state connectivity. Based on the previous work (Lin et al., 2016; Lustig, Shah, Seidler, & Reuter-Lorenz, 2009; O’Brien et al., 2013), we hypothesized that following UFOVt, there would be (a) reduced neural activity in brain regions involved in processing the visual-attention stimuli, as well as less reliance on regions involved in attention and cognitive control (see Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Scalf et al., 2007) in the task-based set of analyses, and (b) increased resting-state connectivity among regions involved in performing the task (Lin et al., 2016). We also predicted that there would be little change in the NC group. Finally, we predicted that given the complexity of the cognitively stimulating tasks (Tranter & Koutstaal, 2008), the CSA group could exhibit some reduced task-based activity and improved connectivity, although not to the same extent as the UFOVt group.
Method
Participants
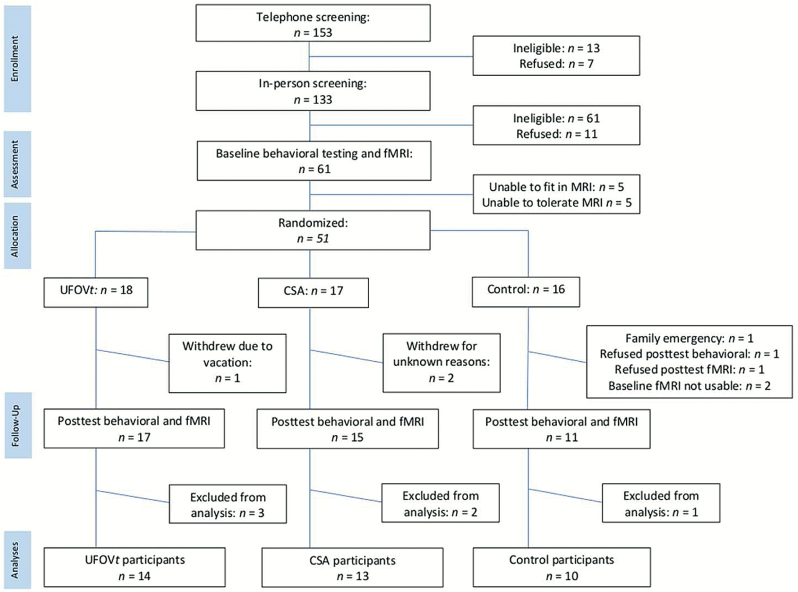
A total of 37 healthy community-dwelling older adults with complete data were included in these analyses. Potential participants were excluded based on the following criteria: less than 65 years of age; corrected far visual acuity worse than 20/40; less than 65% accuracy to complete fMRI task outside of scanner; evidence of potential dementia (cutoff of ≤21 on the Modified Telephone Interview of Cognitive Status [TICS-M]; de Jager, Budge, & Clarke, 2003); or self-reported history of dementia, multiple sclerosis, Parkinson’s disease, epilepsy, muscular dystrophy, cerebral palsy, stroke or transient ischemic attack, anxiety disorder, or drug/alcohol addiction. Due to fMRI safety considerations, additional exclusion criteria were as follows: weight greater than 300 pounds (or >60-inch waist), reported implanted ferromagnetic materials, claustrophobia, or other contraindications to fMRI. Participants who completed all assessments had a mean age of 70.5 years (SD = 4.42), reported mean of 15.5 (SD = 2.97) years of education, an average score of 28.8 (SD = 4.42) on the TICS-M, and were 48.6% female and 81.1% White. See Figure 1 for the CONSORT flowchart of participants.
Figure 1.
Study CONSORT flowchart of participants. Final participant numbers for task-based and resting-state analyses differed slightly due to participant movement in the scanner. CSA = cognitively stimulating activities; UFOVt = Useful Field of View training.
Procedures
The University IRB approved all study procedures, and all participants provided written informed consent. This convenience sample of older adults was recruited using flyers posted throughout the community and letters sent using a purchased mailing list. First, interested potential participants completed a telephone screening to determine eligibility criteria regarding age, self-reported medical conditions, standard self-reported fMRI safety questions, and TICS-M performance (see Participants). Those who were eligible then completed an in-lab screening to determine additional eligibility criteria of visual acuity, fMRI safety criteria, and ability to complete the scanner task with at least 65% accuracy (see Participants). If eligible, participants then stayed to complete the full in-person baseline behavioral assessment. Participants then returned on a separate visit for the baseline fMRI assessment. Certified testers administered all assessments throughout the study. After the baseline visits, participants were randomized using sealed envelopes to the UFOVt arm, a CSA arm that served as an active control or the NC (i.e., treatment as usual) arm. The UFOVt and CSA arms consisted of 10 hr of activities across 5 weeks and were conducted in groups of two participants in the lab. Two certified trainers and one back-up trainer oversaw both UFOVt and CSA. Standardized brief educational discussions (<5 min) were included in both groups, which focused on general issues related to healthy aging.
The UFOVt arm involved computerized adaptive training that increased in difficulty through both reductions in stimulus display duration and increases in task complexity. UFOVt is a lab-developed program that was used in the longitudinal Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE; Jobe et al., 2001; N = 2,802) randomized controlled trial. However, unlike the ACTIVE study that included 5 hr of nonadaptive and 5 hr of adaptive UFOVt, the present study included 10 hr of fully adaptive UFOVt because prior work has found this increases training effectiveness and transfer (Lampit, Valenzuela, & Gates, 2015; Lövdén et al., 2010). UFOVt is modeled after the UFOV test and focuses on processing speed, divided attention, and selective attention. Certified trainers follow a standardized training protocol to change the display duration, move the peripheral target closer or further from the central target, as well as alter colors and luminance of the targets and distractors based on participant performance. The amount of time spent on each task differs between participants and depends on ongoing performance. In terms of the broader literature, Posit Science’s Double Decision task was developed from the original lab-based UFOVt program used in the present study (also known as “speed of processing training”). The Double Decision task was included as part of Insight program (no longer available) and is now included as part of the Brain HQ programs. Please see Ball and colleagues (2007) for extensive details of the UFOVt training program.
The CSA involved complex and challenging paper-and-pencil activities specifically selected to target higher level reasoning, recall, and executive functioning (Seagull & Seagull, 2005). The CSA were standardized across participants in terms of the presentation of activities (e.g., sessions 1–10); however, participants could work at their own speed as they progressed through the activities. The CSA were chosen based on older adults’ reported expectations for activities that maintain brain health (Friedman et al., 2011, 2013; Kim et al., 2015) and the complexity of activities such as complex puzzles, brain teasers, and math problems (Tranter & Koutstaal, 2008).
Participants assigned to the NC group completed all baseline and post-test assessments but were not provided with training.
Within 3 weeks of training completion, participants returned to complete one behavioral post-test assessment and one fMRI post-test session. Importantly, post-test assessors were blinded to the study arm randomization of the participant, and participants in the UFOVt and CSA arms were blinded to the hypotheses of the study. See Figure 1 for the CONSORT flowchart of participants. Table 1 presents sample information and demographics for participants included in the analyses across the arms separated by analysis type (task-based functional and resting-state connectivity).
Table 1.
Sample Demographics
| Useful Field of View training | Cognitively stimulating activities | No-contact control | p | |
|---|---|---|---|---|
| Analysis set 1: Task driven | ||||
| Age in years, mean (SD) | 70.7 (5.19) | 70.1 (4.70) | 70.6 (3.92) | .95 |
| Education in years, mean (SD) | 14.9 (2.96) | 16.2 (2.96) | 16.0 (3.33) | .56 |
| Sex (% female) | 53.8 | 63.6 | 30.0 | .29 |
| White (%) | 92.3 | 81.8 | 70.0 | .38 |
| Baseline UFOV total | 858.8 (187.8) | 747.0 (119.8) | 718.0 (233.7) | .16 |
| Post-test UFOV total | 369.7 (135.1) | 567.5 (181.7) | 562.7 (189.0) | .01 |
| Change in UFOV total | 489.1 (229.5) | 179.5 (126.3) | 188.9 (235.6) | .001 |
| Total n | 13 | 11 | 10 | |
| Analysis set 2: Connectivity | ||||
| Age in years, mean (SD) | 70.6 (5.22) | 70.5 (4.59) | 70.6 (3.92) | .95 |
| Education in years, mean (SD) | 14.9 (2.96) | 15.6 (2.95) | 16.0 (3.33) | .56 |
| Sex (% female) | 53.8 | 45.5 | 30.0 | .29 |
| White (%) | 84.6 | 90.9 | 70 | .38 |
| Baseline UFOV total | 853.3 (195.1) | 726.0 (102.8) | 718.0 (233.7) | .16 |
| Post-test UFOV total | 358.5 (125.53) | 568.2 (180.5) | 562.7 (189.0) | .01 |
| Change in UFOV total | 497.8 (237.41) | 157.8 (145.4) | 188.9 (235.6) | .001 |
| Total n | 13 | 11 | 10 | |
Note: UFOV = Useful Field of View test. ANOVA and chi-square analyses revealed no group differences at baseline.
Measures
Useful Field of View
The UFOV test assessed cognitive processing speed, divided attention, and selective attention. There are four subtests that range in presentation duration between 17 and 500 ms with higher scores indicating worse (i.e., slower) performance (see Edwards et al., 2005). UFOV1 assessed processing speed through identification of a central target (presentation of a 2 cm × 1.5 cm car or truck in a 3 cm × 3 cm fixation box). UFOV2 assessed divided attention with the addition of a peripheral localization task (2 cm × 1.5 cm car) presented in one of eight potential radial locations in addition to the same central identification target from UFOV1. UFOV3 assessed selective attention by adding visual distractors to the UFOV2. UFOV4 assessed selective attention with discrimination by changing the central target, such that participants must identify if the central targets were the same or different, while also including the peripheral target (UFOV2) plus the distractors (UFOV3). The sum of the four UFOV subtests was computed for baseline and post-test and served as a confirmation that UFOVt was successful and in line with prior UFOVt studies (Ball et al., 2007; Edwards et al., 2018; Lin et al., 2016; O’Brien et al., 2013).
Functional MRI Useful Field of View Imaging Task2
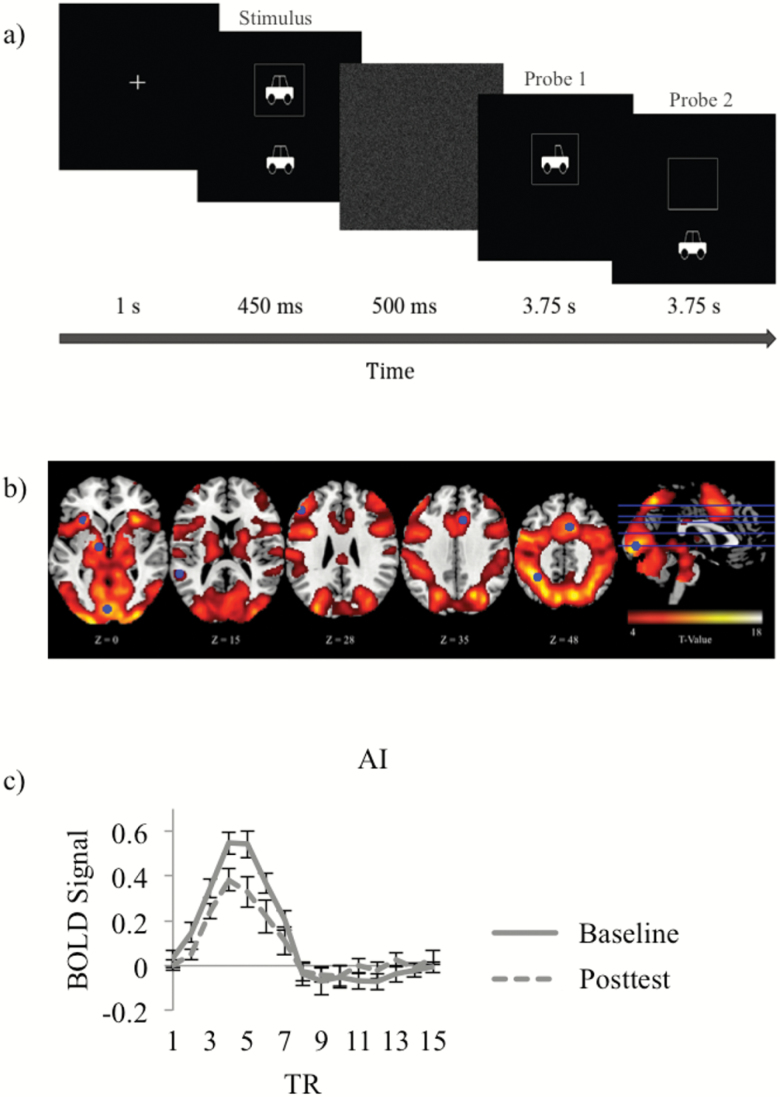
The fMRI task was modeled after UFOV2, as this task is independently predictive of future everyday functioning (Ball et al., 2006) and was also most adaptive for use in the MRI during pilot testing and development. fUFOV2 was designed to be easy to allow for high accuracy within the scanner environment. The task followed an event-related design (Rosen, Buckner, & Dale, 1998) and directed participants to focus on a central cross that was then followed by the stimulus for 450 ms. The stimulus included a central target (identification of a car or truck) and a peripheral localization target (a car presented in one of eight locations at 5 degrees eccentricity). The stimulus was followed by a white noise field for 500 ms and then two probe questions. The first probe presented a car or truck and asked participants if this was the same or different than the central target in the stimulus. The second probe presented a peripheral car and asked participants if the presented target was in the same (50%) or a different (50%) location as the peripheral stimulus. Participants had 4 s to answer each probe with a scanner-compatible button-box (see Figure 2a for general task design). Correct responses on both the central and peripheral task were needed for the trial to be considered accurate (percent correct). Stimulus display duration was consistent across trials as required for the fMRI, and 180 trials were conducted per participant per visit. A larger sample of pilot participants were screened during the development of the task (n = 129) and performed both the UFOV2 and the fUFOV2 in the lab. Correlation of scores on both these tasks revealed a moderate relationship (r = .56, p < .001), suggesting that the two tasks are similar.
Figure 2.
Functional MRI Useful Field of View Imaging Task2 (fUFOV2) scanner task and task-based responses. (a) The stimulus consisted of a central car (two windows) or truck (one window) with simultaneous presentation of a car in one of eight peripheral locations. This was followed by two probes to assess target identification and localization. (b) Whole-brain responses across baseline and post-test. (c) Example of average activation time course across Useful Field of View training (UFOVt) participants in a representative region of interest (anterior insula [AI]).
Data Analyses
Demographic and behavioral analyses
First, baseline differences in age, education, sex, race, or UFOV test performance between the study arms were assessed with chi-square and analysis of variance (ANOVA) tests. A repeated-measures ANOVA was used to assess if the UFOV test, a measure frequently used to quantify UFOVt efficacy (Ball et al., 2007; Lin et al., 2016; O’Brien et al., 2013), significantly changed with UFOVt. Significance was set at p < .05 for all tests. Then, Cohen’s d scores were calculated for significant training effects as change in the outcome/pooled SD.
Functional MRI analyses
We first identified regions associated with stimulus-driven fUFOV2 task performance across baseline and post-test in all participants. We then extracted ROIs based on the locations of the strongest peak values. Given our a priori hypotheses, analyses examining within-group and between-group effects (UFOVt vs. CSA; CSA vs. NC; and UFOVt vs. CSA) within these ROIs were conducted using one-tailed t tests (p < .05) across the task-based and resting-state functional connectivity analyses. The fMRI image acquisition and processing details, ROI selection, and details regarding both task-based functional and resting-state connectivity analyses are found in Supplemental Materials for fMRI Data Analyses. Similar to the behavioral analyses, Cohen’s d effect sizes were calculated for any significant training effects.
Results
Demographic and Behavioral Results
ANOVA and chi-square analyses revealed that training and control groups did not differ on demographics or baseline UFOV (see Table 1). As indicated above, this study used two sets of fMRI analyses (task-based and resting-state) to assess the study hypotheses. It is typical in fMRI experiments to lose some data due to participant movement (see Figure 1 and Supplemental Materials for fMRI Data Analyses). Participant movement during the task-based and/or resting-state portion of the scan resulted in a slight variation in samples (n = 6) across task-based and resting-state connectivity analyses. ANOVA and chi-square analyses revealed no baseline differences between the six participants who were unique between the two analyses compared with the remaining 31 participants common across the two sets of analyses on age, F(1, 35) ≤ 0.001, p = .99; education, F(1, 35) = 0.10, p = .76; sex, χ2(1) = 0.94, p = .41; or race, χ2(1) = 0.86, p = .32.
The repeated-measures ANOVA examining the UFOV test revealed a significant Group × Time interaction, F(1, 38) = 15.18, p < .001. Planned comparison analyses revealed that UFOVt resulted in improved UFOV scores compared with both the NC and the CSA (p’s < .05, Cohen’s d = 0.74 and .321, respectively). Changes in UFOV test performance from baseline to post-test did not differ between the NC and CSA groups (p > .05). See Table 1 for descriptive statistics on baseline, post-test, and change in UFOV scores.
Functional MRI Task Activation Results
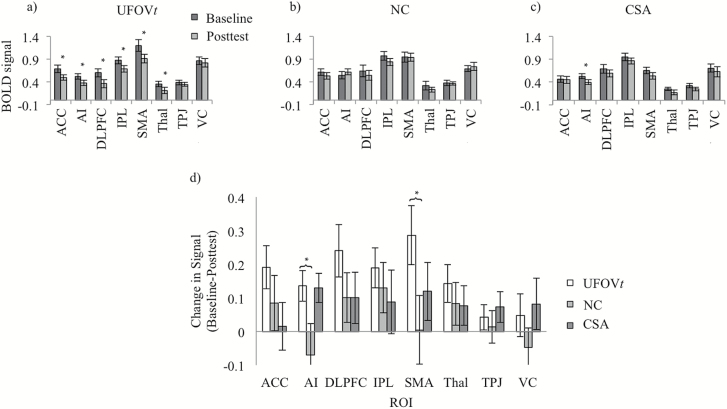
Figure 2b shows a whole-brain map indicating where there were strong responses to fUFOV2 trials. Figure 2c is an example of the hemodynamic response data in a representative ROI. Eight ROIs were defined based on the peak values of this map (Figure 2b and Supplemental Figure 1), namely, anterior cingulate cortex (ACC), anterior insula (AI), dorsolateral prefrontal cortex (DLPFC), inferior parietal lobe (IPL), supplemental motor area (SMA), thalamus, temporoparietal junction (TPJ), and visual cortex (VC). Because we had specific directional hypotheses of reduced activity following training (Lustig et al., 2009; O’Brien et al., 2013), one-tailed paired t tests (p < .05) were conducted to examine within-group differences from baseline to post-test within the eight ROIs. The UFOVt group showed reduced activity at post-test in all ROIs, ACC: t(12) = 2.98, p = .006, Cohen’s d = 0.62; AI: t(12) = 2.99, p = .006, Cohen’s d = 0.63; DLPFC: t(12) = 3.08, p = .005, Cohen’s d = 0.73; IPL: t(12) = 3.18, p = .004, Cohen’s d = 0.70; SMA: t(12) = 3.25, p = .004, Cohen’s d = 0.63; thalamus: t(12) = 2.55, p = .013, Cohen’s d = 0.64, except for the TPJ and the VC (p’s > .05; see Figure 3a). The CSA group showed reduced activity at post-test in AI, t(10) = 2.98, p = .007, Cohen’s d = 0.72, and the NC group showed no activation differences between testing sessions (see Figure 3b and c). Then, between-group analyses were conducted using t tests. Compared to the NC group, UFOVt resulted in significant reductions in activity in both AI, t(21) = 2.13, p = .023, Cohen’s d = 0.87, and SMA, t(21) = 2.10, p = .024, Cohen’s d = 0.70 (see Figure 3d). No other between-group differences were observed.
Figure 3.
Task-based functional MRI Useful Field of View Imaging Task2 (fUFOV2) training results. Within-group baseline and post-test activation for each selected region of interest (ROI) across (a) Useful Field of View training (UFOVt), (b) no-contact control (NC), and (c) cognitively stimulating activities (CSA). (d) Between-group difference scores in activation (baseline − post-test) for each selected ROI. Higher bar values represent greater decreases in neural signal as a result of training. *p < .05. ACC = anterior cingulate cortex; AI = anterior insula; DLPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobe; SMA = supplementary motor area; Thal = Thalamus; TPJ = temporoparietal junction; VC = visual cortex.
Resting-State Functional MRI Results
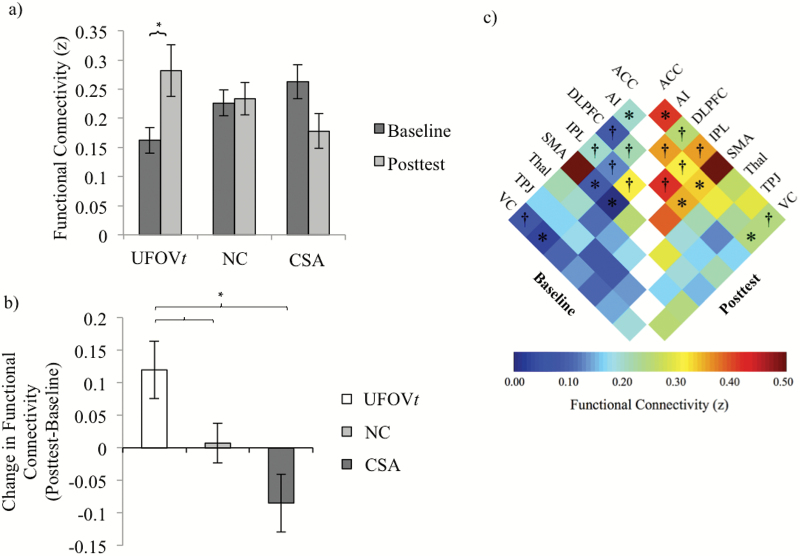
The effects of training on average network connectivity were examined within each group using one-tailed paired t tests given our a priori directional hypotheses that UFOVt connectivity would increase. Results revealed an expected significant and large increase in connectivity from baseline to post-test in the UFOVt group, t(12) = −2.73, p = .009, Cohen’s d = 0.92, and no significant increase in the either the CSA or NC groups (see Figure 4a). When comparing training-related changes between groups, results showed that UFOVt resulted in significantly greater network connectivity compared with either control group, NC: t(21) = 1.98, p = .031, Cohen’s d = −0.80; CSA: t(2) = 3.24, p = .002, Cohen’s d = −1.28; see Figure 4b.
Figure 4.
Resting-state functional connectivity training results. (a) Within-group baseline and post-test average network connectivity for Useful Field of View training (UFOVt), no-contact control (NC), and cognitively stimulating activities (CSA) groups. *Significant difference between baseline and post-test at p < .05. (b) Between-group change (post-test − baseline) in average network connectivity. Higher bars represent greater functional connectivity after training. *Significant difference between groups at p < .05. (c) Baseline and post-test functional connectivity of individual network connections in the UFOVt group. ACC = anterior cingulate cortex; AI = anterior insula; DLPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobe; SMA = supplementary motor area; Thal = Thalamus; TPJ = temporoparietal junction; VC = visual cortex. †Significant change in connectivity at uncorrected p < .05. *Significant change in connectivity at corrected p < .05.
Last, to investigate which of the 28 possible network connections were significantly strengthened (i.e., increased connectivity) following UFOVt, we conducted paired one-tailed t tests of baseline versus post-test connectivity for each network connection within the UFOVt group. We corrected for multiple comparisons by controlling the false discovery rate (FDR; alpha = 0.05) using the procedure by Benjamini and Hochberg (1995). After FDR correction, there were four network connections showing significant strengthening in the UFOVt group: AI and ACC (t(12) = 3.36, uncorrected p = .003, corrected p = .021), AI and VC (t(12) = 3.35, uncorrected p = .003, corrected p = .021), AI and SMA (t(12) = 3.73, uncorrected p = .001, corrected p = .021), and DLPFC and SMA (t(12) = 3.33, uncorrected p = .003, corrected p = .021). These results are summarized in Figure 4c.
Discussion
UFOVt has repeatedly demonstrated transfer to real-world outcomes across 3–10 years (Ball et al., 2010; Ross et al., 2016, 2017; Wolinsky et al., 2009, 2015). Despite the evidence of transfer, little is known about the neural mechanisms underlying UFOVt in older adults. This pilot study examined the effects of UFOVt on neural activity and connectivity of a set of brain regions that were involved in performing a task similar to the training paradigm. As predicted, UFOVt resulted in improved UFOV performance compared with both the NC and the CSA groups, a finding that is comparable to the UFOVt behavioral changes reported in the literature (Ball et al., 2007; Edwards et al., 2018). Importantly, our hypotheses regarding neural activity and connectivity changes in the UFOVt group were supported, as our results showed both reduced task-based neural activity (Figure 3a and d) and increased resting-state functional connectivity in several brain areas involved in task performance compared with the CSA and NC groups (Figure 4). Also, as hypothesized, the NC group demonstrated little change in either activity (Figure 3b and d) or connectivity (Figure 4a and b). However, our hypotheses regarding the CSA group were only partially supported. As expected, the CSA group did demonstrate some reduced task-based activity in the within-group analyses, although not to the same extent as the UFOVt (Figure 3c and d). However, there were no significant differences in patterns of activity or connectivity between the CSA and NC groups.
Together with previous work (Burge et al., 2013; Lin et al., 2016; O’Brien et al., 2013), these results indicate that UFOVt enhances connections needed for the allocation of attention and cognitive control during fUFOV2 task performance, thus decreasing the cognitive effort required to process stimuli. There was a trend for the majority of the ROIs to show decreases in activation after UFOVt (Figure 3a), which is consistent with prior training literature (Lustig et al., 2009). However, when comparing the trained and control groups, the strongest decreases were found in the AI and SMA regions (Figure 3d), which are generally found to be involved in effortful tasks and executive function/cognitive control. Although the specific role of the AI is debated, it has been consistently identified as part of a network-mediating general executive processes, and more specifically, in task-level control and focal attention (Nelson et al., 2010), as well as goal maintenance (Dosenbach et al., 2008).
The SMA is part of a dorsal frontoparietal network of regions supporting voluntary and stimulus-driven attentional shifts to spatial and nonspatial targets (Chung, Han, Jeong, & Jack, 2005; Stoppel et al., 2013) and has been identified as being important to a variety of different experimental paradigms, including those with motor control, spatial memory, and working memory demands. The SMA is very near to the dorsal aspect of the ACC, making the borders distinguishing the two regions unclear. In line with our results, a number of studies find both increased AI and ACC/SMA activity during effortful task performance, as well as decreased activity in these same regions following practice or training (Chein & Schneider, 2005; Erickson et al., 2007a; Weissman, Woldorff, Hazlett, & Mangun, 2002), indicating that AI and ACC/SMA probably play a role in the efficient allocation of executive control and monitoring of resources necessary to manage task demands. In addition, the training group showed training-related reduced activity in DLPFC, a region shown to support a host of executive functions. This result is in line with a study by Erickson and colleagues (2007b), which also found that older adults exhibited training-related reductions in DLPFC indicating a more efficient use of prefrontal support to engage in task coordination. Thus, given the prior work and functional roles of these regions, the current findings suggest that UFOVt results in a need for fewer executive resources to perform the task.
Resting-state functional connectivity results further contribute to this interpretation. Following UFOVt, functional connections among brain regions within the cognitive control network (Dosenbach et al., 2008) were strengthened, suggesting that neural systems of trained individuals became tuned to more efficiently utilize neural resources. A growing number of studies report increased functional connectivity between the cingulo-opercular and frontoparietal cognitive control regions (e.g., AI, ACC/SMA, and DLPFC) with learning/practice or following training (Martínez et al., 2013; Mohr et al., 2016). Taken together, these results indicate that more efficient utilization of neural resources may arise through increased connectivity of cognitive control regions. Moreover, we also propose that increased connection strength at all times, including during rest, may be one possible neural mechanism through which UFOVt achieves transfer to maintained cognition and everyday functioning. Interestingly, our results also showed increased connectivity between AI and VC following UFOVt (see Figure 4c). Prior work has demonstrated that top-down attention can modulate task-specific functional connectivity involving VC (Griffis, Elkhetali, Burge, Chen, & Visscher, 2015), perhaps serving as a mechanism to prioritize processing of task-relevant information. Furthermore, there is preferential connectivity between AI and parts of the VC involved in the part of peripheral vision that is trained in UFOVt (about 5–10 degrees eccentricity; Griffis et al., 2017). The current data demonstrate that UFOVt strengthened this known connection between AI and the VC, reducing neural resources needed to process the visual information used during the fUFOV2.
Further evidence of UFOVt resulting in reduced neural effort for task completion and improved resting-state functional connectivity is found in complementary work. Using EEG, O’Brien and colleagues (2013) demonstrated that N2pc and P3b ERP amplitudes increased after UFOVt, reflecting improved attention allocation and capacity. Along the same lines, using pupillometry in young adults, Burge and colleagues (2013) found that UFOVt improved the efficiency of effortful processing. Prior work also found that less than 5 hr of a functional field of view training, which shares some similar characteristics but differs from UFOVt, was associated with decreased activity in right inferior frontal gyrus, a region close to the current AI ROI (Scalf et al., 2007). Similarly, 5 hr of single- and dual-task training in younger adults resulted in decreased activation in brain regions involved with executive control (Erickson et al., 2007a). Together with these previous findings, the present study suggests that UFOVt results in a reduced reliance on, or effort required by, regions involved in attention, which are needed to complete the fUFOV2.
The concept of changes in efficiency with aging and with training has been the target of a great deal of inquiry. For example, several studies and reviews have suggested that neural efficiency decreases with aging (reviewed in Lustig et al., 2009). Efficiency is often measured as a decrease in neural activity associated with a particular task; however, this operationalization has been criticized as too simplistic, as potential mechanisms for activation decreases are rarely provided (Poldrack, 2015). The present study provides an indication of which resources may be more efficiently used after UFOVt and implies that increased connectivity may be one possible mechanism through which efficiency is improved. Importantly, we are not implying that increased connections equate to decreased cognitive effort; we suggest instead that enhanced connectivity could lead to a decreased need for cognitive effort to perform the task at a given level.
We did not explicitly query the participants about the cognitive effort they put forward to perform this task. That said, regardless of how it is measured, training such as UFOVt, has typically been shown to decrease the mental effort and reliance on brain regions required for a task (Bays, Visscher, Le Dantec, & Seitz, 2015; Lustig et al., 2009; Shenhav et al., 2017). Consistent with this, we observed improvements in performance and decreases in neural activations following training, which is consistent with a decrease in cognitive effort and increase in efficiency. Further work is needed to strengthen this point, for example, by explicitly measuring perceptions of effort.
Reductions in activity were generally most pronounced when the UFOVt was compared with the NC group rather than the CSA group. Given the sample size and the lack of prior work using CSA, the interpretation of the CSA data must be done cautiously. Although CSA did not result in behavioral UFOV improvements, there were some neural changes associated with the CSA, indicating that CSA may provide an incremental influence that is more than the NC group but less than the UFOVt (see Figure 3). For example, changes in AI are similar between baseline and post-test for UFOVt and CSA conditions suggesting that some of the improvements in neural efficiency may be shared among the UFOVt and CSA conditions. Interestingly, the AI has been shown to be involved in a broad range of effortful tasks (Nelson et al., 2010), indicating that this region might be influenced by training to perform a variety of different processes. At the same time, while interpreting the significant CSA effects, it is important to keep in mind that CSA (a) did not result in behavioral UFOV improvements, (b) importantly, did not significantly differ from the NC group in task-based activation changes, and (c) resulted in no significant increase in connectivity. In terms of the broader cognitive training literature, the CSA involved very challenging and complex activities, which should not be confused with simpler leisure activities such as crossword puzzles or reading. In terms of participant expectation, which is frequently cited as a possible mechanism of training transfer, it is important to note that older adults and physicians frequently advocate that simple cognitive activities (e.g., crosswords, puzzles, and reading) are protective of cognition (Friedman et al., 2011, 2013; Kim et al., 2015). However, the definition of cognitive stimulation in research differs between studies, such as the very complex and challenging activities used in the present study’s CSA group compared with much simpler activities such as reading, basic puzzles, and crosswords (see Friedman et al., 2015 for a review). Given the small sample size of the present study, further work is needed to examine how scientifically designed cognitive training programs, such as UFOVt, differ from complex CSA, from simple leisure activities (such as crosswords), and from treatment as usual conditions, are more reflective of daily life (e.g., NC).
A strength of the present study is the use of 10 hr of adaptive UFOVt, an evidence-based intervention that has been standardized and widely researched (see Edwards et al., 2018 for a recent review). In addition, the present study included both an NC (considered “treatment as usual”) and a demanding active control. The main limitation of this study is the small sample size that reduced the statistical power of the study and may limit its generalizability. Future work should investigate both behavioral and neural mechanisms of UFOVt in large representative samples that include everyday far transfer measures (e.g., driving, Instrumental Activities of Daily Living), biomarkers (e.g., brain-derived neurotrophic factor), and other markers of neuroplasticity. Inclusion of such measures will allow us to assess whether UFOVt mechanisms differ as a function of the outcome or combinations of factors. In addition, future work should examine the potential behavioral correlates or moderators, such as baseline cognitive functioning and participant beliefs and expectations, which may inform future training program designs and identification of individuals in need of early intervention.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by the University of Alabama at Birmingham’s Center for Clinical and Translational Sciences (UL1 TR000165S), Center for Aging, Roybal Center for Research on Applied Gerontology (National Institute of Aging, P30AG022838), and Vision Science Research Center (P30 EY003039). C. E. Webb was partially supported by a T32 National Institute on Aging Grant (AG049676) awarded to the Pennsylvania State University.
Conflict of Interest
None reported.
Acknowledgments
The authors thank the participants, funders, and research assistants from the Visual Integrity and Neural plasticity in the Elderly Study (VINES; L. A. Ross PI) as well as Karlene Ball for her guidance. We would especially thank Martha Graham, Wesley Burge, Benjamin McManus, and Ryan J. Vaden who volunteered their time to collect this data. J. M. Hicks is now at the Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA.
References
- Anguera J. A., Boccanfuso J., Rintoul J. L., Al-Hashimi O., Faraji F., Janowich J.,…Gazzaley A (2013). Video game training enhances cognitive control in older adults. Nature, 501, 97–101. doi:10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K., Edwards J. D., & Ross L. A (2007). The impact of speed of processing training on cognitive and everyday functions. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62, 19–31. doi:10.1093/geronb/62.special_issue_1.19 [DOI] [PubMed] [Google Scholar]
- Ball K., Edwards J. D., Ross L. A., & McGwin G. Jr (2010). Cognitive training decreases motor vehicle collision involvement of older drivers. Journal of the American Geriatrics Society, 58, 2107–2113. doi:10.1111/j.1532-5415.2010.03138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K. K., Roenker D. L., Wadley V. G., Edwards J. D., Roth D. L., McGwin G. Jr,…Dube T (2006). Can high-risk older drivers be identified through performance-based measures in a department of motor vehicles setting?Journal of the American Geriatrics Society, 54, 77–84. doi:10.1111/j.1532-5415.2005.00568.x [DOI] [PubMed] [Google Scholar]
- Bays B. C., Visscher K. M., Le Dantec C. C., & Seitz A. R (2015). Alpha-band EEG activity in perceptual learning. Journal of Vision, 15, 7. doi:10.1167/15.10.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. doi:10.2307/2346101 [Google Scholar]
- Burge W. K., Ross L. A., Amthor F. R., Mitchell W. G., Zotov A., & Visscher K. M (2013). Processing speed training increases the efficiency of attentional resource allocation in young adults. Frontiers in Human Neuroscience, 7, 684. doi:10.3389/fnhum.2013.00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J. M., & Schneider W (2005). Neuroimaging studies of practice-related change: FMRI and meta-analytic evidence of a domain-general control network for learning. Brain Research: Cognitive Brain Research, 25, 607–623. doi:10.1016/j.cogbrainres.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Chung G. H., Han Y. M., Jeong S. H., & Jack C. R. Jr (2005). Functional heterogeneity of the supplementary motor area. American Journal of Neuroradiology, 26, 1819–1823. [PMC free article] [PubMed] [Google Scholar]
- de Jager C. A., Budge M. M., & Clarke R (2003). Utility of TICS-M for the assessment of cognitive function in older adults. International Journal of Geriatric Psychiatry, 18, 318–324. doi:10.1002/gps.830 [DOI] [PubMed] [Google Scholar]
- Dosenbach N. U., Fair D. A., Cohen A. L., Schlaggar B. L., & Petersen S. E (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12, 99–105. doi:10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. D., Fausto B. A., Tetlow A. M., Corona R. T., & Valdés E. G (2018). Systematic review and meta-analysis of useful field of view cognitive training. Neuroscience and Biobehavioral Results, 84, 72–91. doi:10.1016/j.neubiorev.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Edwards J. D., Vance D. E., Wadley V. G., Cissell G. M., Roenker D. L., & Ball K. K (2005). Reliability and validity of useful field of view test scores as administered by personal computer. Journal of Clinical and Experimental Neuropsychology, 27, 529–543. doi:10.1080/13803390490515432 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Colcombe S. J., Wadhwa R., Bherer L., Peterson M. S., Scalf P. E.,…Kramer A. F (2007a). Training-induced functional activation changes in dual-task processing: An fMRI study. Cerebral Cortex, 17, 192–204. doi:10.1093/cercor/bhj137 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Colcombe S. J., Wadhwa R., Bherer L., Peterson M. S., Scalf P. E.,…Kramer A. F (2007b). Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiology of Aging, 28, 272–283. doi:10.1016/j.neurobiolaging.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Friedman D. B., Becofsky K., Anderson L. A., Bryant L. L., Hunter R. H., Ivey S. L.,…Lin S. Y (2015). Public perceptions about risk and protective factors for cognitive health and impairment: A review of the literature. International Psychogeriatrics, 27, 1263–1275. doi:10.1017/S1041610214002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., Laditka S. B., Laditka J. N., Wu B., Liu R., Price A. E.,…Sharkey J. R (2011). Ethnically diverse older adults’ beliefs about staying mentally sharp. International Journal of Aging & Human Development, 73, 27–52. doi:10.2190/AG.73.1.b [DOI] [PubMed] [Google Scholar]
- Friedman D. B., Rose I. D., Anderson L. A., Hunter R., Bryant L. L., Wu B.,…Tseng W (2013). Beliefs and communication practices regarding cognitive functioning among consumers and primary care providers in the United States, 2009. Preventing Chronic Disease, 10, E58; quiz 8–E58; quiz13. doi:10.5888/pcd10.120249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis J. C., Elkhetali A. S., Burge W. K., Chen R. H., Bowman A. D., Szaflarski J. P., & Visscher K. M (2017). Retinotopic patterns of functional connectivity between V1 and large-scale brain networks during resting fixation. Neuroimage, 146, 1071–1083. doi:10.1016/j.neuroimage.2016.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis J. C., Elkhetali A. S., Burge W. K., Chen R. H., & Visscher K. M (2015). Retinotopic patterns of background connectivity between V1 and fronto-parietal cortex are modulated by task demands. Frontiers in Human Neuroscience, 9, 338. doi:10.3389/fnhum.2015.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe J. B., Smith D. M., Ball K., Tennstedt S. L., Marsiske M., Willis S. L.,…Kleinman K (2001). ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials, 22, 453–479. doi:10.1016/S0197-2456(01)00139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Sargent-Cox K. A., & Anstey K. J (2015). A qualitative study of older and middle-aged adults’ perception and attitudes towards dementia and dementia risk reduction. Journal of Advanced Nursing, 71, 1694–1703. doi:10.1111/jan.12641 [DOI] [PubMed] [Google Scholar]
- Lampit A., Valenzuela M., & Gates N. J (2015). Computerized cognitive training is beneficial for older adults. Journal of the American Geriatrics Society, 63, 2610–2612. doi:10.1111/jgs.13825 [DOI] [PubMed] [Google Scholar]
- Lin F., Heffner K. L., Ren P., Tivarus M. E., Brasch J., Chen D. G.,…Tadin D (2016). Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: A pilot study. Journal of the American Geriatrics Society, 64, 1293–1298. doi:10.1111/jgs.14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-López L., Amenedo E., & Cadaveira F (2008). Feature processing during visual search in normal aging: Electrophysiological evidence. Neurobiology of Aging, 29, 1101–1110. doi:10.1016/j.neurobiolaging.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Lövdén M., Bäckman L., Lindenberger U., Schaefer S., & Schmiedek F (2010). A theoretical framework for the study of adult cognitive plasticity. Psychological Bulletin, 136, 659–676. doi:10.1037/a0020080 [DOI] [PubMed] [Google Scholar]
- Lustig C., Shah P., Seidler R., & Reuter-Lorenz P. A (2009). Aging, training, and the brain: A review and future directions. Neuropsychology Review, 19, 504–522. doi:10.1007/s11065-009-9119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez K., Solana A. B., Burgaleta M., Hernández-Tamames J. A., Alvarez-Linera J., Román F. J.,…Colom R (2013). Changes in resting-state functionally connected parietofrontal networks after videogame practice. Human Brain Mapping, 34, 3143–3157. doi:10.1002/hbm.22129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr H., Wolfensteller U., Betzel R. F., Mišić B., Sporns O., Richiardi J., & Ruge H (2016). Integration and segregation of large-scale brain networks during short-term task automatization. Nature Communications, 7, 13217. doi:10.1038/ncomms13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. M., Dosenbach N. U., Cohen A. L., Wheeler M. E., Schlaggar B. L., & Petersen S. E (2010). Role of the anterior insula in task-level control and focal attention. Brain Structure and Function, 214, 669–680. doi:10.1007/s00429-010-0260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. L., Edwards J. D., Maxfield N. D., Peronto C. L., Williams V. A., & Lister J. J (2013). Cognitive training and selective attention in the aging brain: An electrophysiological study. Clinical Neurophysiology, 124, 2198–2208. doi:10.1016/j.clinph.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. (2015). Is “efficiency” a useful concept in cognitive neuroscience?Developmental Cognitive Neuroscience, 11, 12–17. doi:10.1016/j.dcn.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok G. W., Ball K. K., Guey L. T., Jones R. N., Kim H.-Y., King J. W.,…Willis S. L (2014). Ten year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62, 16–24. doi:10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. R., Buckner R. L., & Dale A. M (1998). Event-related functional MRI: Past, present and future. Proceedings of the National Academy of Sciences of the United States of America, 95, 773–780. doi:10.1073/pnas.95.3.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. A., Edwards J. D., O’Connor M. L., Ball K. K., Wadley V. G., & Vance D. E (2016). The transfer of cognitive speed of processing training to older adults’ driving mobility across 5 years. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71, 87–97. doi:10.1093/geronb/gbv022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. A., Freed S. A., Edwards J. D., Phillips C. B., & Ball K (2017). The impact of three cognitive training programs on driving cessation across 10 years: A randomized controlled trial. The Gerontologist, 57, 838–846. doi:10.1093/geront/gnw143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. A., Sprague B. N., Phillips C. B., O’Connor M. L., & Dodson J. E (2018). The impact of three cognitive training interventions on older adults’ physical functioning across 5 years. Journal of Aging and Health, 30, 475–498. doi:10.1177/0898264316682916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalf P. E., Colcombe S. J., McCarley J. S., Erickson K. I., Alvarado M., Kim J. S.,…Kramer A. F (2007). The neural correlates of an expanded functional field of view. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62, 32–44. doi:10.1093/geronb/62.special_issue_1.32 [DOI] [PubMed] [Google Scholar]
- Seagull B., & Seagull S (2005). Mind your mind: A whole brain workout for older adults. New Paltz, NY: Attainment Company, Inc. [Google Scholar]
- Shenhav A., Musslick S., Lieder F., Kool W., Griffiths T. L., Cohen J. D., & Botvinick M. M (2017). Toward a rational and mechanistic account of mental effort. Annual Review of Neuroscience, 40, 99–124. doi:10.1146/annurev-neuro-072116-031526 [DOI] [PubMed] [Google Scholar]
- Simons D. J., Boot W. R., Charness N., Gathercole S. E., Chabris C. F., Hambrick D. Z., & Stine-Morrow E. A (2016). Do “brain-training” programs work?Psychological Science in the Public Interest, 17, 103–186. doi:10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- Smith-Ray R. L., Makowski-Woidan B., & Hughes S. L (2014). A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Education & Behavior, 41(Suppl. 1), 62S–69S. doi:10.1177/1090198114537068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel C. M., Boehler C. N., Strumpf H., Krebs R. M., Heinze H. J., Hopf J. M., & Schoenfeld M. A (2013). Distinct representations of attentional control during voluntary and stimulus-driven shifts across objects and locations. Cerebral Cortex, 23, 1351–1361. doi:10.1093/cercor/bhs116 [DOI] [PubMed] [Google Scholar]
- Tranter L. J., & Koutstaal W (2008). Age and flexible thinking: An experimental demonstration of the beneficial effects of increased cognitively stimulating activity on fluid intelligence in healthy older adults. Neuropsychology, Development, and Cognition, Section B: Aging, Neuropsychology and Cognition, 15, 184–207. doi:10.1080/13825580701322163 [DOI] [PubMed] [Google Scholar]
- Weissman D. H., Woldorff M. G., Hazlett C. J., & Mangun G. R (2002). Effects of practice on executive control investigated with fMRI. Brain Research: Cognitive Brain Research, 15, 47–60. doi:10.1016/S0926-6410(02)00215-X [DOI] [PubMed] [Google Scholar]
- Wolinsky F. D., Mahncke H. W., Kosinski M., Unverzagt F. W., Smith D. M., Jones R. N.,…Tennstedt S. L (2009). The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Services Research, 9, 109. doi:10.1186/1472-6963-9-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky F. D., Mahncke H., Vander Weg M. W., Martin R., Unverzagt F. W., Ball K. K.,…Tennstedt S. L (2010). Speed of processing training protects self-rated health in older adults: Enduring effects observed in the multi-site ACTIVE randomized controlled trial. International Psychogeriatrics, 22, 470–478. doi:10.1017/S1041610209991281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky F. D., Vander Weg M. W., Howren M. B., Jones M. P., & Dotson M. M (2015). The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: Results from the IHAMS randomized controlled trial. Journal of Aging and Health, 27, 334–354. doi:10.1177/0898264314550715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.