Abstract
Background:
The prevalence of IgE-mediated food allergy (FA) is rising worldwide but the underlying mechanisms are poorly understood.
Objective:
To examine the role of maternal lipidomic profiles in offspring risk of FA development; and to investigate the potential modification effects by timing of first solid food introduction.
Methods:
This report included 1,068 mother-child dyads from the Boston Birth Cohort. Maternal lipid metabolites in plasma were assessed using liquid chromatography tandem mass spectrometry. Food sensitization (FS) was defined as specific IgE ≥ 0.35 kU/L to any of the 8 common food allergens using ImmunoCAP. FA was defined based on FS, clinical symptoms and food avoidance. Logistic regression was applied to analyze the associations between maternal metabolites and offspring risk of FS and FA, and to explore the potential effect modifications.
Results:
Of the 1,068 children, 411 had FS and 132 had FA. Among the 209 metabolites, maternal triacylglycerols (TAGs) of shorter chain carbons and fewer double bonds were associated with higher risk of FA, whereas TAGs of longer chain carbons and more double bonds were significantly associated with lower risk of FA in offspring. These associations were stronger in children with delayed solid food introduction (≥7 months of age) than those with earlier solid food introduction (P=0.010 for interaction between the maternal TAG score and timing of solid food introduction). No significant association was found for FS.
Conclusion:
This is the first study to demonstrate a link between maternal TAGs and offspring risk of FA and potential risk modification by timing of solid food introduction.
Keywords: Food allergy, Lipidomics, Triacylglycerol, Timing of first solid food introduction
Capsule summary
We identified a maternal triacylglycerol signature associated with offspring risk of developing food allergy. The adverse impact of maternal triacylglycerols may be partly alleviated by early introduction of solid foods to at-risk children.
Introduction
Food Allergy (FA), a condition arising from a specific adverse immune response that occurs reproducibly upon exposure to a given food, has emerged as a major clinical and public health problem worldwide, given its increasing prevalence1-3 and the adverse medical, psychosocial and economic consequences of this potentially life-threatening condition.4-6 Current treatment for FA relies heavily on food avoidance and emergency preparedness.7 Unfortunately, accidental food ingestions are common,8 and allergic reactions can be severe and even fatal.9 Thus, identification of early-life modifiable factors is crucial for early prediction and intervention for FA.
Many hypotheses have been proposed for the recent increase in FA prevalence, including Gideon Lack’s dietary fat hypothesis.10 One intriguing observation is the parallel rise in the prevalence of FA and metabolic diseases such as obesity, diabetes and metabolic syndrome across all age groups, including pregnant women. This raises a question about whether maternal metabolic disorders may increase offspring risk of FA. Indeed, there is increasing interest in exploring the role of lipids and related metabolic factors in affecting allergy development, with some plausible evidence.11-17 Previous studies, including ours in Chinese twin cohorts, have demonstrated that dyslipidemia may be associated with an increased risk of allergy development.14, 16 However, some other studies have reported conflicting results.18, 19 Plasma lipids are composed of dozens of distinct molecules, which may have varying influence on disease development. A recent study in mice reported that medium-chain but not long-chain triglycerides can promote allergic sensitization and anaphylaxis to peanut protein, by affecting antigen absorption and by stimulating TH2-skewing cytokines.14 This animal study not only provides insight into mechanical pathways through which dietary medium-chain triglycerides affect FA risk, it also indicates that the standard clinical measurement of a lipid panel may be insufficient to capture the influence of different lipid molecules on FA risk. This notion has been further supported by findings from the Framingham Heart Study, in which, hundreds of plasma lipid molecules from distinct classes were quantified using the newly developed liquid chromatography tandem mass spectrometry (LC-MS) technologies but with diabetes as the endpoint.20 To our knowledge, no such study has been conducted to systematically investigate the role of maternal lipid molecules in FA development.
By leveraging cutting-edge biological technology, this study aimed to examine whether maternal lipid metabolites (that reflect fetal intra-utero exposure) are associated with offspring risk of food sensitization (FS) and FA in the Boston Birth Cohort (BBC), a U.S. urban, low-income, minority population with a high prevalence of maternal metabolic disorders and child atopic diseases including FA. We were also interested in identifying early life nutritional factors (including breastfeeding and timing of solid food introduction) that can modulate the associations between maternal lipid metabolites and offspring FA development..
Methods
Study population
The study participants were enrolled from the BBC, an ongoing prospective urban, low-income minority birth cohort enrolled and followed at the Boston Medical Center (BMC), Boston, MA, as described previously.21 All eligible mothers were enrolled within 1-3 days after delivery, and were interviewed using a standard questionnaire and had a blood draw. Starting in 2003, infants who sought primary or specialist care at BMC were invited to participate in a follow-up study. Follow-up visits were scheduled at 6 -12 months, 2 years, 4 years, and 6 years, respectively. At each visit, mothers were interviewed using postnatal health questionnaires, and a medical record abstraction form was used to obtain the child’s clinical data. The study protocol was approved by the Institutional Review Boards (IRBs) of BMC and Johns Hopkins Bloomberg School of Public Health.
As of July 2018, 3,163 mothers-child dyads have been enrolled and followed in the BBC. Among them, 2,830 dyads enrolled from 1998-2012 were the target population of the current study. After removing 1,040 mother-child dyads with no IgE measurement to food allergens, and 728 dyads with no data on maternal plasma lipid metabolites, the current study was focused on 1,068 mother-child dyads.
Assessments of maternal plasma lipidomics
Lipidomic assessment in maternal plasma (collected within 1-3 days after delivery) was conducted at the Broad Institute Metabolite Profiling Laboratory using LC-MS techniques.22-24 Details of the lab protocols, sample processing and quality control steps have been reported previously.25, 26 Briefly, maternal plasma specimens were sent to the laboratory in a blinded fashion. A pooled study reference sample, composed from all of the study plasma samples, was randomly distributed throughout the study samples (per 20-30 study samples). Coefficients of variation (CV) for each metabolite was calculated by analyzing the study reference sample that was repeatedly measured 77 times.
Using high-resolution full scan MS data acquisition, 216 lipid metabolites from multiple classes (including cholesterol esters, diacylglycerols, lysophosphatidylcholine, lysophosphatidylethanolamines, phosphatidylcholine, phosphatidylethanolamines (PEs), and triacylglycerol (TAGs)), were identified in maternal plasma. These metabolites were further classified based on the number of total acyl chain carbon atoms and double bond contents and annotated as [lipid class] [number of acyl chain carbon atoms]:[number of double bonds in fatty acid moieties], accordingly. After removing 6 metabolites with CVs >20%, and 1 metabolite that was unquantifiable in >10% of samples, 209 metabolites were available for next-step analyses.
Definition of outcomes
Plasma specific IgE (sIgE) for the eight common food allergens (including egg white, cow’s milk, peanut, soy, shrimp, walnut, wheat and cod) in early childhood was measured using ImmunoCAP® (Thermo-Fisher/Phadia) at Quest Diagnostics, according to the manufacturer’s prescribed protocol. FS was defined as having a sIgE ≥ 0.35 kUA/L to any of the eight food allergens, which is similar to other publications.27, 28
At each follow-up visit, mothers were interviewed about history of physician-diagnosed FA in the index child, clinical allergic symptoms (and/or anaphylaxis) that the child ever experienced upon ingestion of each of the eight allergenic foods, and the timing and treatment of these reactions (if there was any). In addition, a dietary history was recorded at each visit, in which parents were asked if, and how often, the child consumed these allergenic foods. Based on these available data, we categorized children into five groups for each visit: Group 5 (Confirmed/Convincing FA) was defined if the child met all the following criteria: 1) sIgE ≥0.35 kUA/L to a specific food; 2) having convincing clinical allergic symptoms within 2 hours upon ingestion of that food; and 3) avoidance of the food based on the food frequency questionnaire; Group 4 (Probable FA) was defined as 1) sIgE ≥0.35 kUA/L to a specific food and having convincing clinical allergic symptoms upon ingestion of that food, but without information on food avoidance; or 2) sIgE ≥ 95% positive predictive values (PPVs);17 Group 3 (Possible FA) was defined as 1) having sIgE within 0.35 kUA/L and 95% PPVs to a specific food and having food avoidance, but without reported clinical allergic symptoms on food ingestion; or 2) having a parental report of clinical allergic symptoms or having physician-diagnosed FA, but with sIgE < 0.35 kUA/L to the specific food. Group 2 (asymptomatic) was defined if the child had sIgE ≥ 0.35 kUA/L but reported consumption of the specific food without clinical allergic symptoms. Group 1 (Not Sensitized and Not Allergic) was defined as: 1) all sIgEs < 0.35 kU/L; 2) no reported clinical allergic symptoms on food ingestion; and 3) no history of parental-reported physician-diagnosed FA. For the purpose of analyses, each child was placed in the highest FA category he/she attained over the period of postnatal follow-up, and those who were grouped into the “convincing or confirmed FA” or the “probable FA” category were defined as having FA, and those who were either “asymptomatic” or “not sensitized and not allergic” were defined as non-allergic controls. Those with possible FA were removed from the analyses when FA was analyzed as the outcome.
Other allergic diseases, including allergic dermatitis, allergic rhinitis and asthma were defined if the child had one or more physician-diagnosis of the relevant disease, respectively, based on archived ICD-9 and ICD-10 codes from the electronic medical record (EMR).
Major covariates
At enrollment, each mother was interviewed using a postnatal health questionnaire to collect health characteristics and exposures during pregnancy. Maternal pre-pregnancy body mass index (BMI) was calculated as self-reported pre-pregnancy weight in kilograms divided by self-reported height in meters squared. Maternal diabetes status was classified as nondiabetic, pre-existing and gestational diabetes.21 Maternal history of atopy was defined if the mother ever had any of the physician-diagnosed conditions including FA, allergic dermatitis, allergic rhinitis, or asthma. Information regarding delivery mode was abstracted from the EMR.
During the follow-up visit at age < 2 years, each mother was asked “At what age did you first introduce solid food to your child?” The options were “not yet”, “never”, “unsure”, or detailed years and months when the common solid foods were first introduced. Timing of first solid food introduction was then classified as “early” (within the first 3 months of age),”suggested” (within 4–6 months of age) or “delayed” (7 months of age or later). Infant breastfeeding history (“bottle-fed exclusively”, “both bottle-fed and breastfed”, or “breastfed exclusively”) was also assessed at follow-up visits, and grouped into “ever” versus “never” breastfeeding, as reported previously.29
Statistical analysis
For each lipid metabolite (with a missing rate < 10%), we first imputed any missing values with one-half of the limit of detection, and then performed inverse normal transformation (which were scaled to per standard deviation (SD)) to render the distributions approximately Gaussian. Pearson correlations were applied to examine the inter-correlation among lipid metabolites. Multiple logistic regression models were used to investigate the associations between each lipid metabolite and FA outcomes by estimating odds ratios (ORs) with 95% confidence intervals (CIs), with adjustment of known or suspected confounders, including maternal race/ethnicity, maternal history of atopy, delivery mode and infant’s sex. We also adjusted for maternal pre-pregnancy BMI, which is significantly associated with maternal lipid profiles30, 31. Lipid metabolites were analyzed both as continuous and categorical (using tertiles) variables. We applied the Benjamini-Hochberg false discovery rate (FDR)32 to adjust for multiple testing, with FDR < 0.05 as the significance threshold. For the identified associations with FA, a sensitivity test was performed on the FA definitions using more stringent criteria to define FA cases. We further performed stratified analyses by the timing of food sIgE measurement (< 2 years vs. ≥ 2 years).
To estimate the combined effects of multiple TAG metabolites that were individually associated with FA outcomes, the maternal weighted TAG score was calculated as follows: score = ∑n k=1 βk × TAGk, where n is the total number of selected TAGs to be analysed (n=2 in this study, representing TAGs 48:1 and 58:10), and βk is the corresponding regression coefficient for TAGk in association with FA risk as estimated from the individually fitted regression models. The weighted TAG score did not include the interaction term between the TAGs (or TAG 48:1 and 58:10 in this study), which was not statistically significant. The associations between the weighted TAG score (or tertiles) and FA outcomes were then tested using the logistic regression models.
To minimize the residual confounding effects, we further performed propensity score-matched sensitivity analyses33 to investigate the effect of high (or the 3rd tertile) vs. low (or the 1st tertile) maternal TAG score on risk of FA. The propensity score for exposure to a high maternal TAG score was first calculated using the multivariate logistic regression model, with maternal ethnicity, maternal history of atopy, maternal pre-pregnancy BMI, delivery mode, and infant’s sex as the covariates. One-to-one pair matching on the propensity score was applied. Using the “nearest neighbor” technique with caliper=0.1 in the R package MatchIt, 177 (out of 311) children with a high maternal TAG score were successfully matched to 177 children with a low maternal TAG score. Next, between these 177 pairs of children, risk of FA was compared using both the chi-square test and logistic regression.
To explore potential effect modification by timing of solid food introduction, we first analyzed the associations between maternal weighted TAG score and risk of FA, stratified by timing of solid food introduction. Specifically, we observed that the identified associations were comparable in children with solid food introduced within 3 months of age and in children with solid food introduced at 4–6 months of age, thus, we reclassified timing of solid food introduction to “0–6 months of age“ (or early) versus “ ≥7 months of age” (or delayed) in the subsequent analyses. The joint effects between maternal weighted TAG score (analyzed as tertiles) and timing of solid food introduction were analyzed in the logistic regression models, with the adjustment of confounders as described above. The interaction effects between maternal weighted TAG score and timing of solid food introduction were then estimated by adding their interaction term into the logistic regression models. Similar analyses were performed to explore the modification effect of breastfeeding status. All analyses were performed in R 3.5.1 version.
Results
As shown in Table E1, demographic characteristics of the 1,068 mother-child dyads enrolled in this study were largely comparable with that of the total target population. Among the 1,068 children, food sIgE was measured at an average of 2.5±2.3 years, and about 44.7% of the children with sIgE were measured at >2 years. There were 411 (38.5%) children with FS, and 132 (12.4%) with FA to at least one of the eight major food allergens tested. As expected, children with FS were more likely to be male (P=0.001) than those without FS (Table 1). A similar gender difference was also observed for FA. In addition, children with FA were more likely to have delayed solid food introduction (≥ 7 months) than those without (P=0.011, Table 1).
Table 1.
Population characteristics of the 1,068 study mother-child dyads, stratified by food sensitization and food allergy status
| Variable | Food Sensitizatio (n=1,068) |
Food Allergy (n=913) a |
||||
|---|---|---|---|---|---|---|
| No | Yes | Pb | No | Yes | Pb | |
| n | 657 | 411 | 781 | 132 | ||
| Maternal age (years) | 28.4±6.5 | 28.8±6.8 | 0.407 | 28.5±6.5 | 28.9±6.9 | 0.499 |
| Maternal race | ||||||
| Black | 400 (60.9) | 272 (66.2) | 0.062 | 483 (61.8) | 95 (72.0) | 0.113 |
| White | 39 (5.9) | 11 (2.7) | 37 (4.7) | 3 (2.3) | ||
| Hispanic | 130 (19.8) | 79 (19.2) | 158 (20.2) | 23 (17.4) | ||
| Other | 88 (13.4) | 49 (11.9) | 103 (13.2) | 11 (8.3) | ||
| Maternal history of atopy | ||||||
| No | 415 (63.2) | 231 (56.2) | 0.075 | 495 (63.4) | 70 (53.0) | 0.075 |
| Yes | 168 (25.6) | 123 (29.9) | 199 (25.5) | 44 (33.3) | ||
| Unknown | 74 (11.2) | 57 (13.9) | 87 (11.1) | 18 (13.7) | ||
| Maternal education, | ||||||
| College or above | 231 (35.2) | 139 (33.8) | 0.547 | 269 (34.4) | 39 (29.5) | 0.478 |
| Maternal BMI (kg/m2) | ||||||
| < 25 | 288 (43.8) | 190 (46.2) | 0.630 | 345 (44.2) | 65 (49.2) | 0.423 |
| 25-29.9 | 189 (28.8) | 104 (25.3) | 225 (28.8) | 29 (22.0) | ||
| ≥ 30 | 152 (23.1) | 101 (24.6) | 177 (22.7) | 31 (23.5) | ||
| Unknown | 28 (4.3) | 16 (3.9) | 34 (4.3) | 7 (5.3) | ||
| Smoking during pregnancy | 55 (8.4) | 26 (6.3) | 0.290 | 60 (7.7) | 11 (8.3) | 0.923 |
| Caesarean (C/S) delivery | 232 (35.3) | 149 (36.3) | 0.796 | 267 (34.2) | 58 (43.9) | 0.057 |
| Maternal diabetes | ||||||
| No | 580 (88.3) | 363 (88.3) | 0.977 | 695 (89.0) | 111 (84.1) | 0.234 |
| Gestational diabetes | 48 (7.3) | 29 (7.1) | 54 (6.9) | 12 (9.1) | ||
| Pre-existing diabetes | 29 (4.4) | 19 (4.6) | 32 (4.1) | 9 (6.8) | ||
| Child’s sex, male | 301 (45.8) | 230 (56.0) | 0.001 | 378 (48.4) | 81 (61.4) | 0.006 |
| Preterm birth | 166 (25.3) | 102 (24.8) | 0.869 | 189 (24.2) | 36 (27.3) | 0.449 |
| Child’s age at sIgE measurement (years) | 2.4±2.2 | 2.7±2.4 | 0.031 | 2.4±2.2 | 2.7±2.7 | 0.255 |
| Child’s age at the last visit (years) | 5.6±3.4 c | 5.7±3.3 c | 0.447 | 5.5±3.3 | 5.9±3.8 | 0.295 |
| Never breastfed | 164 (25.0) | 92 (22.4) | 0.463 | 196 (25.1) | 31 (23.5) | 0.926 |
| Timing of solid food introduction | ||||||
| Early (1- 3 months) | 137 (20.9) | 81 (19.7) | 0.903 | 165 (21.1) | 15 (11.4) | 0.011 |
| Suggested (4-6 months) | 435 (61.2) | 276 (67.2) | 518 (66.3) | 92 (69.7) | ||
| Delayed ( ≥7 months) | 85 (12.9) | 54 (13.1) | 98 (12.6) | 25 (18.9) | ||
Mean±SD and n (%) is shown for continuous and categorical variables, respectively.
Approximately 155 children were classified as “possible FA” but did not meet the criteria for FA, and thus were removed from the analysis for FA.
The difference in each population characteristics between FS and non-FS children or between FA and non-allergic children was assessed based on t-test and chi-square test for continuous and categorical variables, respectively.
Lipidomic profiles identified a significant lipid signature for FA
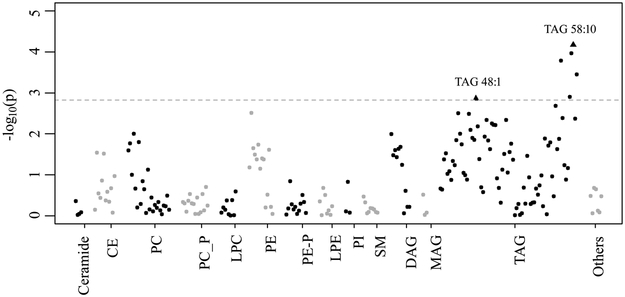
Table E2 summarizes the 209 maternal metabolites studied in this study, among which 23 metabolites had missing values (18 with a missing rate of <1.0% and 5 with a missing rate of 1.0–4.0%) that were imputed using one-half of the limit of detection for next-step analyses. Figure E1 and Figure 1 illustrate the associations between the 209 maternal lipid metabolites and offspring risk of having FS or FA, respectively. With FDR <0.05 as the cut-off, 6 out of the 78 TAG metabolites, including TAG 48:1 (P=0.001), 56:8 (P=1.6×10−4), 58:8 (P=0.001), 58:9 (P=1.1×10−4), 58:10 (P=6.8×10−5) and 60:12 (P=3.6×10−4), were significantly associated with an altered risk of FA, which were independent of other maternal metabolic factors (maternal overweight/obesity and maternal preexisting/gestational diabetes, data not shown). As for the metabolites in the other lipid classes, only maternal PE 32:1 showed a borderline significant association with offspring risk of FA (P=0.003, FDR=0.067). In comparison, no significant associations were found for FS (Figure E1).
Figure 1. Manhattan plot for associations of 209 maternal lipid metabolites with offspring risk of food allergy in 1,068 mother-child dyads from the BBC,
adjusted for maternal ethnicity, maternal history of atopy, maternal pre-pregnancy BMI category, delivery mode, and sex. Each dot represents a lipid metabolite in a specific class. The dotted line represents the FDR-significance cut-off. Triangles represent the two metabolites of interest in this study. CE: Cholesterol esters; PC: Phosphatidylcholine; PC_P: Phosphatidylcholine plasmalogen; LPC: Lysophosphatidylcholine; PE: Phosphatidylethanolamine; PE_P: Phosphatidylethanolamine plasmalogen; LPE: Lysophosphatidylethanolamine; PI: Phosphatidylinositol; SM: Sphingomyelin; DAG: Diacylglycerol; MAG: monoacylglycerol; TAG: Triacylglycerol.
Maternal TAG profile and offspring risk of FA outcomes
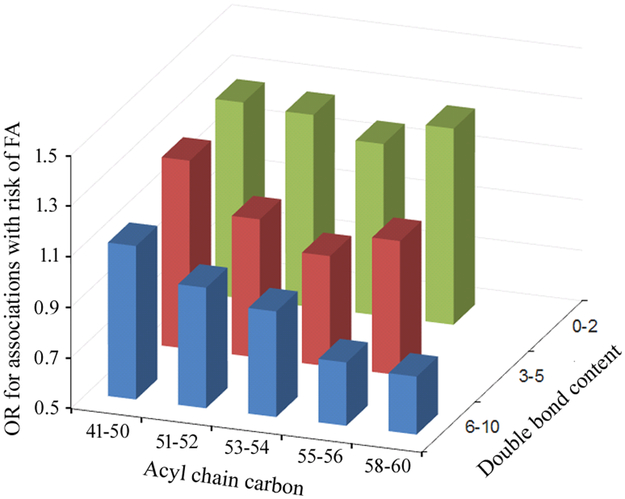
Figure E2 presents the correlation matrix for all 78 maternal TAG metabolites analyzed in this study: TAGs of shorter acyl chain carbons (i.e., 41–52) with relatively fewer double bonds (i.e., 0–4) were positively correlated with each other; TAGs of longer chain carbons (i.e., 56–60) with relatively more double bonds (5–12) were also positively correlated with each other; however, these two groups of TAGs were inversely correlated. The associations between maternal TAGs and offspring risk of FA also varied by the number of acyl chain carbons and double bonds (Figure 2): TAGs of shorter acyl chain carbons and fewer bonds (TAG 48:1 as the top significant metabolite of this group) were associated with an increased risk for FA with an OR > 1; In comparison, TAGs of longer acyl chain carbons and more double bonds (TAG 58:10 as the top significant metabolite of this group) were associated with an decreased risk for FA with an OR < 1.
Figure 2. The average odds ratios for the maternal TAG – offspring risk of food allergy associations, according to the number of total acyl chain carbon atoms (41-50, 51-52, 53-54, 55-56, and 58-60) and double bond content (0-1, 2-4, 5+).
Y axis is the odds ratio for having food allergy per standard deviation increment of each type of TAG, X axis is the number of acyl chain carbon atoms, and Z axis is the number of double bond content.
Given the significant correlations between the top metabolites (TAGs 48:1 and 58:10) and other FA-associated TAGs, we focused on TAGs 48:1 and 58:10 in the subsequent analyses (Table 2): Each SD increment of maternal TAG 48:1 was associated with 1.4-fold (95%CI=1.2–1.8, P=0.001) increased risk of FA in the offspring, while each SD increment of maternal TAG 58:10 was associated with a reduction in FA risk by 36% (OR=0.6, 95%CI=0.5–0.8, P=6.8×10−5). In the sensitivity tests, we observed that the estimated ORs for TAG 48:1 and TAG 58:10 remained comparable when more stringent FA definitions were applied (Table E3), and these associations did not significantly vary by child’s age when food sIgE was measured (Table E4).
Table 2.
Associations of maternal lipid metabolites with offspring risk of food sensitization and food allergy status in 1,068 mother-child dyads from the BBC
| Metabolite a | Food Sensitization | Food Allergy | ||||||
|---|---|---|---|---|---|---|---|---|
| FS (%) | OR | 95%CI | Pb | FA (%) | OR | 95%CI | Pb | |
| Maternal TAG 48:1 | ||||||||
| Continuous (1SD) | 1,068 (38.5) | 1.08 | 0.94-1.24 | 0.294 | 913 (14.5) | 1.42 | 1.15-1.76 | 0.001 |
| Tertile | ||||||||
| Low (T1) | 353 (34.8) | 1.00 | ref | ref | 301 (10.3) | 1.00 | ref | ref |
| Medium (T2) | 362 (41.4) | 1.41 | 1.02-1.94 | 0.036 | 309 (17.2) | 2.34 | 1.42-3.88 | 9.1×10−4 |
| High (T3) | 353 (39.1) | 1.41 | 1.00-1.99 | 0.049 | 303 (15.8) | 2.68 | 1.55-4.63 | 4.3×10−4 |
| Maternal TAG 58:10 | ||||||||
| Continuous (1SD) | 1068 (38.5) | 0.94 | 0.82-1.09 | 0.402 | 913 (14.5) | 0.64 | 0.51-0.80 | 6.8×10−5 |
| Tertile | ||||||||
| Low (T1) | 353 (38.5) | 1.00 | ref | ref | 312 (17.6) | 1.00 | ref | ref |
| Medium (T2) | 362 (39.2) | 0.97 | 0.71-1.33 | 0.870 | 301 (15.6) | 0.77 | 0.49-1.21 | 0.254 |
| High (T3) | 353 (37.7) | 0.92 | 0.65-1.29 | 0.616 | 300 (10.0) | 0.40 | 0.23-0.67 | 6.0×10−4 |
| Maternal weighted TAG score | ||||||||
| Continuous (1SD) | 1068 (38.5) | 1.12 | 0.91-1.37 | 0.285 | 913 (14.5) | 1.92 | 1.41-2.61 | 3.6×10−5 |
| Tertile | ||||||||
| Low (T1) | 353 (35.4) | 1.00 | ref | ref | 304 (10.5) | 1.00 | ref | ref |
| Medium (T2) | 362 (40.3) | 1.28 | 0.93-1.77 | 0.123 | 302 (14.9) | 1.88 | 1.13-3.13 | 0.016 |
| High (T3) | 353 (39.7) | 1.38 | 0.97-1.95 | 0.073 | 307 (17.9) | 2.94 | 1.71-5.05 | 9.4×10−5 |
Abbreviation: CI: confidence interval; FA: food allergy; FS: food sensitization; OR: odds ratio; SD: standard deviation; T1: 1st tertile; T2: 2nd tertile; T3: 3rd tertile.
Two individual metabolites that were significantly associated with FA: one (TAG 48:1) having the strongest positive association, and the other (TAG 58:10) having the strongest negative association, are shown in the table as illustrations.
Adjusted for maternal race/ethnicity, maternal history of atopy, maternal pre-pregnancy BMI category, delivery mode and infants’ sex.
The weighted score for the two maternal TAGs (referred to as “the maternal TAG score”, which correlated positively with TAG 48:1 but inversely with 58:10) was then analyzed to reflect the combined effect, for which each SD increment was associated with a 1.9-fold (95%CI=1.4–2.6, P=3.6×10−5) increased risk of having FA in the offspring. Similarly, children in the 3rd tertile of the maternal TAG score were at a 2.9 (95%CI=1.7–5.1, P=9.4×10−5) times higher risk of having FA than children in the 1st tertile (Table 2). In propensity score-matched sensitivity analyses, there were 177 pairs of matched children with comparable covariates (Table E5). Among these 177 pairs, we further observed that children in the 3rd tertile of maternal TAG score were at a 2.4-fold increased risk (95%CI=1.4–4.4, P=0.008) of having FA compared to those in the 1st tertile of maternal TAG score.
When other allergic phenotypes were analyzed, similar trends were observed between maternal TAG 58:10 and offspring risk of asthma (or hay fever), which, however, were no longer significant after FDR correction for multiple testing (Table E6).
Modification effects by timing of solid food introduction in infancy
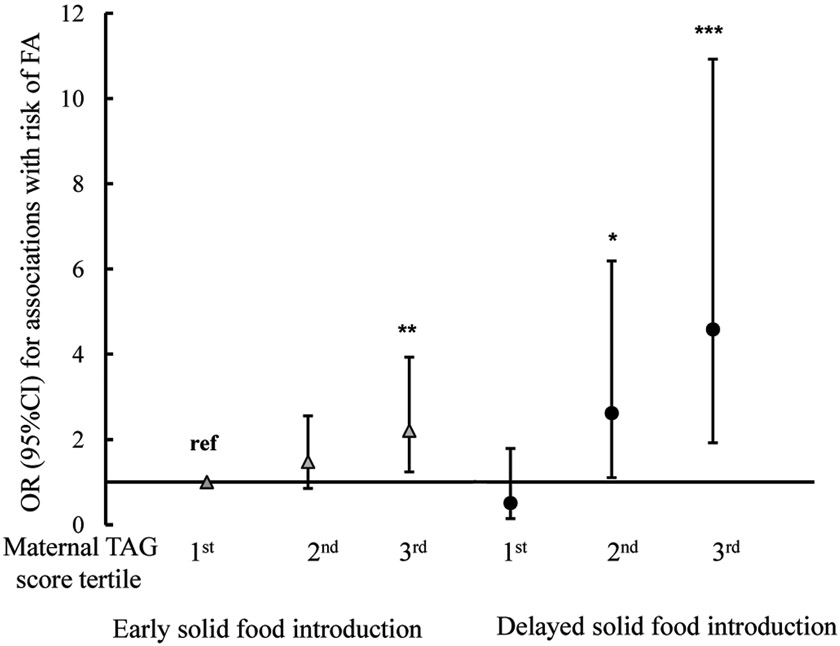
In this cohort, children with delayed solid food introduction were more likely to have FA (P=0.010, Table 1) than children with early solid food introduction, although their maternal TAGs level were comparable (P=0.24). The joint associations between the maternal TAG score and timing of solid food introduction (“early: 0–6 months” versus “delayed: ≥7 months of age”; see Methods for details) on risk of FA are presented in Figure 3. Using children in the 1st tertile of the maternal TAG score and with early solid food introduction as the reference group, the risk of having FA was 1.5 (95%CI=0.9–2.6, P=0.166) and 2.2 (95%CI=1.2–3.9, P=0.007) times higher in their counterparts exposed to the 2nd and 3rd tertiles of maternal TAG score, respectively; And the risk was the highest (OR=4.6, 95%CI=1.9–10.9, p=0.0006) in children in the 3rd tertile of maternal TAG score and with delayed solid food introduction. There was a significant interaction (P=0.011) between the maternal TAG score (analyzed as a continuous variable) and timing of solid food introduction. In comparison, no significant interaction was observed between maternal TAGs and breastfeeding status (data not shown).
Figure 3. The joint associations between tertiles of the maternal weighted TAG score and timing of first solid food introduction on risk of food allergy in 913 mother-child dyads from the Boston Birth Cohort.
Y axis reflects the odds ratio (OR) and 95% confidence interval (CI) of FA risk for each subgroup stratified by tertiles of the maternal weighted TAG score and timing of first solid food introduction, using children in the 1st tertile of maternal TAG score and with early solid food introduction as the reference group. *P < 0.05; ** P < 0.01; *** P < 0.001, when compared with the reference group.
Discussion
This is the first study to link high-throughput “snapshots” of maternal lipidomics with offspring FA development in a prospective minority birth cohort. We showed that maternal TAG levels of longer acyl chain carbons and more double bonds may protect against FA development in the offspring, while maternal TAG levels of shorter acyl chain carbons and fewer double bonds were associated with an increased risk of FA. These identified associations may be modified by the timing of first solid food introduction, for which, the effect sizes were stronger if the children had delayed introduction of solid foods. These findings, if validated in another independent cohort, may facilitate the identification of high-risk children and suggest that early solid food introduction may alleviate the risk for FA in this high-risk group.
Dyslipidemia was reported as a risk factor for allergy development by our study in a Chinese twin cohort16 and by others,15, 34 although some other studies have reported inconsistent findings.18, 19 Because most of these studies employed a cross-sectional study design, the temporal relationship was unclear. Also, among the existing studies, plasma lipid level was measured using the standard clinical measurement, which may have obscured the diversity of the lipid molecules from distinct classes. In comparison, by analyzing 209 maternal lipid molecules from different classes, we clearly demonstrated that the maternal TAG profile (rather than other lipid classes) was associated with offspring risk of developing FA, and its association directions varied by the numbers of acyl chain carbons and double bonds of the specific TAGs. Integrating the positive risk captured by a TAG of relatively shorter chain carbons and fewer double bonds (TAG 48:1) with the negative risk captured by a TAG of relatively longer chain carbons and more double bonds (TAG 58:10) may slightly improve FA prediction.
Several lines of evidence demonstrate that the identified associations between maternal TAGs and offspring FA risk are biologically plausible. An animal study has suggested that medium-chain triglycerides, but not long-chain triglycerides, may increase intestinal-epithelial mRNA expression of three TH2-biasing cytokines including thymic stromal lymphopoitein, interleukin (IL)-25, and IL33, providing a potential pathway for FA development.14 Consistently, our study supported that maternal TAGs of shorter acyl carbon chain were associated with an increased FA risk in the offspring. TAGs constitute the largest source of different types of dietary fatty acids, which potentially have different impacts on the immune system. TAGs with shorter chain carbon and fewer double bonds are predominantly composed of saturated (i.e., 16:0 palmitic acid and 18:0 stearic acid) and monounsaturated (e.g., 18:1 oleic acid) fatty acids.20 Saturated fatty acids such as palmitic acids may induce pro-inflammatory signaling via activation of the Toll-like receptor 4,35, 36 and thus may promote allergy development. In comparison, TAGs with longer chain carbon and more double bonds, which may protect against development of FA in the offspring as observed in this study, are predominantly composed of polyunsaturated fatty acids (PUFAs, i.e., 18:2 linoleic acid, 18:3 a-linolenic acid, and 22:5 docosahexaenoic acid). Maternal PUFAs, especially n-3 PUFAs, may regulate functions of the fetal immune system through several anti-inflammatory mechanisms and/or result in a reduced TH2 response, thus protecting against allergy.37-39 In addition, our findings are also consistent with a recent study which demonstrated that epidermal PUFAs and saturated short-chain fatty acids may affect allergy development differently, via altering skin barrier function.17 Alternatively, another plausible explanation for the identified associations is that there is a common cause (such as maternal diets / environmental exposures during pregnancy, and genetic factors) shared by maternal TAG levels and offspring FA development that leads to indirect associations between these two traits, which is worth further investigation.
Of note, these identified FA-associated TAGs showed no significant associations with offspring risk of FS (Table 2) after adjusting for multiple testing. FS and FA are known to be two different phenotypes. Although immediate-type FAs are mediated by IgE, the presence of IgE is insufficient for developing clinical allergic reaction on exposure. For example, in our cohort, only one third of sensitized children developed allergic symptoms on food exposure. We hypothesized that the FA-associated TAGs identified in this study may influence the manifestation of clinical symptoms, and/or oral tolerance in sensitized children, rather than affect IgE production, which, however, await further validation.
Previous studies40-42 have reported that early solid food introduction was associated with decreased FA risk. This finding indicates that dietary factors during infancy may modulate a child’s risk of FA, although the underlying mechanism is unclear. Given the potential role of maternal dietary and/or metabolic factors in regulating the fetal immune system14 and on FA development as reported previously,11, 17 we were motivated to test potential interactions between maternal TAGs (a prenatal nutritional factor) and timing of solid food introduction (a postnatal nutritional factor), and observed that the adverse impact of maternal TAGs on offspring risk of FA was much stronger if the children had delayed solid food introduction. Alternatively, early solid food introduction may partly reduce the risk of developing FA in high-risk children exposed to a higher maternal TAG score, although the mechanisms are still unclear. Our findings, if proved to be true, will provide new insight into the biological pathways of FA and a new avenue for early risk assessment and interventions for FA.
This birth cohort study design allowed us to clearly assess the temporal relationship between the in-utero metabolic environment and FA development. Compared to most available studies that have primarily focused on European populations, this study was conducted in high-risk but far less-studied inner-city minority (Black and Hispanic) populations. We expect that our findings have direct relevance to understanding and reducing FA in minority populations. Meanwhile, several limitations of this study also warrant discussion. First, the enrolled children did not undergo double-blind, placebo-controlled food challenges (the gold standard for FA diagnosis), and food-specific IgE level was measured only at one time point. Our FA definition, similar to other published studies,27 is based on the available robust clinical, serologic and dietary information. We further showed that the identified associations between maternal TAGs and risk of FA were robust when more stringent FA definitions were applied. It is likely that, if misclassification of FA existed in this study, it was random and did not substantially influence our findings. Taking together, our findings may be regarded as hypothesis generating, and replication studies in other independent cohorts, especially with FA defined by food challenge testing, are warranted. Second, our study population was comprised of predominantly urban, low-income minorities living in the US, an under-studied population. However, caution is needed when generalizing our findings to other populations. Third, the LC-MS technology applied in this study does not provide absolute quantitation of lipid metabolites, nor can it identify the specific fatty acids that constitute the TAG metabolites of interest. Fourth, we focused on the timing of introduction of any solid food as opposed to allergenic food introduction. We did not have sufficient data to further investigate whether our findings remained consistent when the timing of allergenic solid foods introduction was analyzed, which awaits future studies. We also cannot fully exclude the possibility that the identified interaction between maternal TAGs and timing of solid food introduction on FA risk was a chance finding, which calls for further replication in other independent cohorts.
In conclusion, we are the first to link maternal lipidomics with offspring FA development, and demonstrate that the identified associations between maternal TAG profiles and offspring FA risk could be modified by the timing of solid food introduction, which, if further validated in other cohorts, will have important clinical implications for personalized FA prevention and intervention. Overall, our analyses provide novel evidence revealing the role of maternal TAGs (but not other lipid classes) and the underlying biology they represent in FA development. Future studies of this kind are needed to validate and extend our findings.
Supplementary Material
Key Messages:
Maternal lipids of different classes may have different associations with offspring risk of food allergy.
Maternal triacylglycerols of shorter chain carbons and fewer double bonds were associated with an increased risk of FA, while triacylglycerols of longer chain carbons and more double bonds may protect against FA development in the offspring.
There was a significant interaction between maternal triacylglycerol levels and timing of solid food introduction in predicting offspring risk of food allergy.
Acknowledgements
We gratefully acknowledge the individuals who participated in the studies and contributed to this work. We also thank Ms. Tami R. Bartell for her English editing.
Funding resource
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605), and the National Institutes of Health (NIH) grants (R21ES011666, R01HD041702). The follow-up study is supported in part by grants from the Bunning family and their family foundations, Food Allergy Research and Education (FARE), and the NIH grants (U01AI090727, R21AI079872, R21HD085556, R01HD041702 and R01HD086013). Dr Hong is supported by NICHD (PI: Hong X, R03HD096136). We would like to acknowledge Linda Rosen and the Clinical Data Warehouse (CDW) for the assistance in obtaining relevant clinical information; CDW service is supported by the Boston University’s Clinical and Translational Institute and NIH CTSA grant U54-TR001012.
Abbreviations used
- BBC
Boston Birth Cohort
- BMI
body mass index
- CE
cholesterol ester
- CI
confidence interval
- CV
coefficients of variation
- DAG
diaceylglycerol
- EMR
electronic medical record
- FS
food sensitization
- FA
food allergy
- FDA
false discovery rate
- IgE
immunoglobulin E
- IRB
Institutional Review Boards
- IL
interleukin
- LC-MS
liquid chromatography tandem mass spectrometry
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- OR
odds ratios
- PE
phosphatidylethanolamine
- PUFA
polyunsaturated fatty acid
- SD
standard deviation
- TAG
triacylglycerol
Footnotes
Declaration of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics 2009; 124:1549–55. [DOI] [PubMed] [Google Scholar]
- 2.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 2010; 126:798–806 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 2010; 125:1322–6. [DOI] [PubMed] [Google Scholar]
- 4.Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet 2013; 382:1656–64. [DOI] [PubMed] [Google Scholar]
- 5.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126:S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol 2011; 128:110–5 e5. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–307; quiz 8. [DOI] [PubMed] [Google Scholar]
- 8.Cherkaoui S, Ben-Shoshan M, Alizadehfar R, Asai Y, Chan E, Cheuk S, et al. Accidental exposures to peanut in a large cohort of Canadian children with peanut allergy. Clin Transl Allergy 2015; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkins D, Bock SA. Fatal anaphylaxis to foods: epidemiology, recognition, and prevention. Curr Allergy Asthma Rep 2009; 9:179–85. [DOI] [PubMed] [Google Scholar]
- 10.Lack G Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008; 121:1331–6. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, Wang G, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. The Journal of allergy and clinical immunology 2009; 124:1031–8 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donovan SM, OBH J, Murray DM, Kenny LC, Khashan AS, Chaoimh CN, et al. Neonatal adiposity increases the risk of atopic dermatitis during the first year of life. J Allergy Clin Immunol 2016; 137:108–17. [DOI] [PubMed] [Google Scholar]
- 13.Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol 2018; 141:2094–106. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wang Y, Tang L, de Villiers WJ, Cohen D, Woodward J, et al. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J Allergy Clin Immunol 2013; 131:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinding RK, Stokholm J, Chawes BLK, Bisgaard H. Blood lipid levels associate with childhood asthma, airway obstruction, bronchial hyperresponsiveness, and aeroallergen sensitization. J Allergy Clin Immunol 2016; 137:68–74 e4. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang F, Kumar R, Pongracic J, Story RE, Liu X, Wang B, et al. Adiposity, serum lipid levels, and allergic sensitization in Chinese men and women. J Allergy Clin Immunol 2009; 123:940–8 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baurecht H, Ruhlemann MC, Rodriguez E, Thielking F, Harder I, Erkens AS, et al. Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J Allergy Clin Immunol 2018; 141:1668–76 e16. [DOI] [PubMed] [Google Scholar]
- 18.Schafer T, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Doring A, et al. Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin Exp Allergy 2003; 33:1360–7. [DOI] [PubMed] [Google Scholar]
- 19.Pesonen M, Ranki A, Siimes MA, Kallio MJ. Serum cholesterol level in infancy is inversely associated with subsequent allergy in children and adolescents. A 20-year follow-up study. Clin Exp Allergy 2008; 38:178–84. [DOI] [PubMed] [Google Scholar]
- 20.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011; 121:1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA : the journal of the American Medical Association 2014; 311:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med 2010; 2:33ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaham O, Slate NG, Goldberger O, Xu Q, Ramanathan A, Souza AL, et al. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci U S A 2010; 107:1571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clin Chem 2013; 59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted metabolomics. Curr Protoc Mol Biol 2012; Chapter 30:Unit 30 2 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, et al. Influence of early-life exposures on food sensitization and food allergy in an inner-city birth cohort. J Allergy Clin Immunol 2015; 135:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan EC, Peng RD, Salo PM, Zeldin DC, Keet CA. Changes in Food-Specific IgE Over Time in the National Health and Nutrition Examination Survey (NHANES). J Allergy Clin Immunol Pract 2016; 4:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol 2011; 128:374–81 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauschert S, Uhl O, Koletzko B, Kirchberg F, Mori TA, Huang RC, et al. Lipidomics Reveals Associations of Phospholipids With Obesity and Insulin Resistance in Young Adults. J Clin Endocrinol Metab 2016; 101:871–9. [DOI] [PubMed] [Google Scholar]
- 31.Weir JM, Wong G, Barlow CK, Greeve MA, Kowalczyk A, Almasy L, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res 2013; 54:2898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 1995; 57:289–300. [Google Scholar]
- 33.Austin PC, Stuart EA. Estimating the effect of treatment on binary outcomes using full matching on the propensity score. Stat Methods Med Res 2017; 26:2505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeever TM, Lewis SA, Smit H, Burney P, Britton J, Cassano PA. Serum nutrient markers and skin prick testing using data from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 2004; 114:1398–402. [DOI] [PubMed] [Google Scholar]
- 35.Jin J, Zhang X, Lu Z, Perry DM, Li Y, Russo SB, et al. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am J Physiol Endocrinol Metab 2013; 305:E853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 2009; 29:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc 2010; 69:373–80. [DOI] [PubMed] [Google Scholar]
- 38.Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids 2007; 42:801–10. [DOI] [PubMed] [Google Scholar]
- 39.Krauss-Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Del Carmen Ramirez-Tortosa M, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol 2008; 121:464–70 e6. [DOI] [PubMed] [Google Scholar]
- 40.Nwaru BI, Erkkola M, Ahonen S, Kaila M, Haapala AM, Kronberg-Kippila C, et al. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics 2010; 125:50–9. [DOI] [PubMed] [Google Scholar]
- 41.Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. JAMA 2016; 316:1181–92. [DOI] [PubMed] [Google Scholar]
- 42.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015; 372:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.