Abstract
Rheumatoid arthritis (RA) is an autoimmune disease that primarily affects the synovial joints and can lead to bone erosion and cartilage damage. One hallmark of RA is anti-citrullinated protein autoantibodies (ACPA) and memory citrulline-specific B-cells, which have been implicated in RA pathogenesis. While depletion of B-cells with Rituximab improves clinical responses in RA patients, this treatment strategy leaves patients susceptible to infections. Therefore, using Siglec-engaging Tolerance-inducing Antigenic Liposomes (STALs) to selectively target the citrulline-specific B-cells may be beneficial. ACPA production from purified human RA patients’ B-cells in vitro was achieved through a set stimulation conditions, which includes: BAFF, anti-CD40, IL-21, and LPS. In vivo generation of citrulline specific B-cells and ACPA production was accomplished by antigenic liposomes consisting of monophosphoryl lipid A (MPLA) and a cyclic citrullinated peptide (CCP) administered to SJL/J mice. We show that STALs that co-display a high affinity CD22 glycan ligand and synthetic citrullinated antigen (CCP STALs) can prevent ACPA production from RA patients’ memory B-cells in vitro. These CCP STALs were also effective in inducing tolerance to citrullinated antigens in SJL/J mice. The results demonstrate that tolerization of the B-cells responsible for ACPA can be achieved by exploiting the inhibitory receptor CD22 with high affinity glycan ligands. Such a treatment strategy could be beneficial in the treatment of RA.
Keywords: rheumatoid arthritis, anti-citrullinated protein antibodies, liposomes, antigen specific tolerance, auto-antibodies
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease affecting the synovium of the joints, characterized by joint inflammation, bone erosion, loss of bone density, and articular cartilage damage leading to limited mobility1-3. Inflammation and disease pathology are mediated by pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) released by infiltrating immune cells within the synovial tissue4-6. Current therapies to control inflammation use non-steroidal anti-inflammatory (NSAIDs) or disease modifying anti-rheumatic drugs (DMARDs)7-9. However, increased understanding of disease progression has opened doors for novel disease modifying biologic therapies that target anti-inflammatory cytokines (e.g. anti-TNF-α, anti-IL-6), or prevent immune responses within the B- and T-cell compartments10-13.
Increased knowledge of disease progression has also provided diagnostic tools that identify patients who are at high risk of developing RA. Genetic and serological screens reveal that HLA-DRB1 alleles are highly associated with RA incidence and are strongly associated with the development of anti-citrullinated protein autoantibodies (ACPA)14. The association between HLA-DRB1 and ACPA stems from HLA-DRB1 accommodating peptides bearing post-translational modifications of antigens associated with RA, such as citrullination, leading to a break in tolerance and autoantibody formation15. Conversion of arginine to citrulline is catalyzed by peptidylarginine deiminases (PAD) enzymes generating the epitope for ACPAs16. ACPA are highly correlated with RA disease burden and are predictive of disease onset17-20.
The discovery of autoantibodies, such as ACPA and rheumatoid factor (RF) and the associated genetic alleles, has changed the way RA is classified. RA patients that develop ACPA or RF are defined as seropositive, while those who do not are seronegative 2. Seropositive cases represents the majority of patients (~70%)21, present with more severe bone and joint destruction22, and respond better to disease modifying biologics such as B-cell depletion (Rituximab)23 or co-stimulation blockade (CTLA4-Ig)24. Disease progression in seropositive patients is also well understood due to longitudinal studies tracking patient pathology25. The discovery of ACPA came from a systematic analysis of autoantibodies in RA patients, which defined a common epitope containing a citrullinated motif that occurred in many different proteins like fibrinogen, vimentin, and type II collagen25-30. Currently, ACPAs are detected in RA sera using synthetic cyclic citrullinated peptides (CCP) that broadly cover antibodies that recognize the citrullinated epitope31-34. CCP-reactive antibodies are specific for RA, not found in healthy individuals, and found only at low levels in other autoimmune inflammatory conditions such as type 1 diabetes35 and multiple sclerosis36.
RA patients undergoing B-cell depletion therapy with Rituximab exhibit improved clinical responses and reduced circulating IgM RF and ACPA, supporting a pathological role for ACPA and strong rationale for targeting ACPA reactive B-cells in RA37. Rituximab only targets naïve and memory B-cells, due to the loss of CD20 expression upon differentiation into plasmablasts and plasma cells, yet targeting these populations is sufficient to reduce circulating ACPA and achieve therapeutic efficacy in RA patients, suggesting a plausible approach of RA treatment by depleting antigen-specific memory B-cells. Further support for the role of ACPA comes from preclinical studies evaluating the contribution of ACPA and citrulline-specific B-cells to disease pathology38-41. Although the mechanisms that underlie ACPA induction of RA pathology remains under investigation38, 42-45, there is emerging consensus that citrulline post-translational modifications and ACPA play a significant role in RA disease progression and severity.
As an alternative to depletion of B-cells with Rituximab, more selective targeting of citrulline-specific B-cells could have a similar therapeutic profile for RA without compromising broad B-cell directed immunity37, 46. To this end, we have examined the potential of exploiting Siglec-engaging Tolerance-inducing Antigenic Liposomes (STALs) as an approach to deplete the citrulline-specific B-cells. STALs contain both an antigen of interest and a high affinity ligand for the inhibitory B-cell Siglec, CD22. The STALs exploit the inhibitory properties of CD22 by driving clustering of CD22 with the BCR that binds the antigen47, suppressing B-cell activation, and deleting the antigen-specific B-cells from the B-cell repertoire, leading to antigen-specific tolerance48-52. Here, we have used a synthetic cyclic citrullinated peptide, CCP, as the antigen since it is recognized by a broad range of RA pathogenic citrulline-specific B-cells, to generate tolerizing STALs - referred to throughout as CCP STALs - to test the hypothesis that CCP STALs can selectively deplete citrullinated protein-specific B-cells. We show that RA patients have normal expression patterns of CD22 on various B-cell subsets, and that CCP STALs can induce in vitro tolerance of citrullinated protein-specific memory B-cells from RA patients via depletion mechanisms. Importantly, these tolerizing effects occur in an antigen-specific manner. Similarly, CCP STALs induce antigen-specific tolerance in SJL/J mice, leading to impaired ACPA responses. Our results demonstrate that selectively silencing the potential pathogenic B-cells in RA patients with high ACPA titers, using STALs, could be beneficial in treating this autoimmune disease.
RESULTS AND DISCUSSION
RA and Healthy Donors Show Similar Profile of B-Cell Subsets and CD22 Expression.
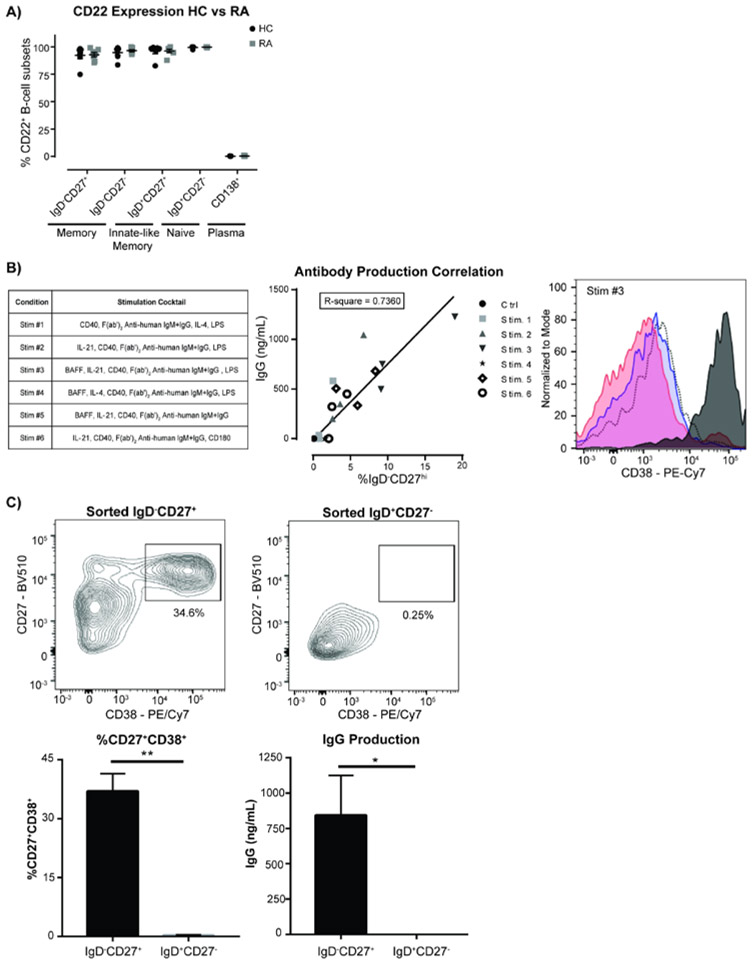
A small cohort of RA patients selected for elevated levels of ACPA (≥ 20 units) were documented for sex, age, rheumatoid factor levels (RF), ACPA titers, health assessment questionnaire (HAQ) score, and current medications (Supplemental Figure 1A). While several reports document small differences in the naïve B-cell compartment in RA patients 53-56, no statistically significant differences in B-cell subsets were found in this cohort compared to healthy controls, including the naïve (CD20+IgD+CD27−), innate memory (CD20+IgD+CD27+), and memory (CD20+IgD−CD27int/+) and plasma (CD138+) B-cells (Supplemental Figure 1B). CD22 expression on B-cells of RA patients was similar to healthy controls across all B-cell subsets, with strong expression in naïve and memory subsets and very low levels in plasma B-cells as documented previously57, 58 (Figure 1A and Supplemental Figure 1C).
Figure 1.
CD22 expression on B-cell subsets in rheumatoid arthritis patients and human memory B-cells are the major source of antibody secreting cells in an in vitro differentiation assay. a) CD22 expression in different B-cell subsets from HC and RA blood was shown. (N=7 individual donors for RA and 7 matched HC donors, unpaired t-test). b) Total IgG antibody production from in vitro B cell differentiation under different stimulation conditions as listed in the table was determined in culture supernatants, with each symbol representing a different healthy control sample. (N=3 independent experiments from 3 individual healthy control donors, linear regression). c) Generation of plasmablasts and production of IgG from naïve or memory B-cells were tested in the B cell in vitro differentiation assay. Sorted IgD+CD27− naïve B cells and IgD−CD27+ memory B cells from healthy donor blood were stimulated (Stim #3) and expression of B cell markers in cells after 7-day cultures were assessed by flow cytometry. Supernatants from day 7 stimulated B cell cultures were collected for measurement of total IgG production by ELISA (N=3 independent experiments with 3 individual healthy donors, unpaired t-test).
B-cell Antibody Production Correlates with Plasmablast Differentiation In Vitro.
To assess ACPA production from RA patient blood samples, an in vitro antibody production assay was developed. Various stimulation conditions were evaluated for their effects on B-cell proliferation, differentiation, and antibody production (Supplemental Figure 2A and Figure 1B). IgG antibody production correlated with the differentiation of B-cells to IgD−CD27hi cells (R-square=0.7360, Figure 1B), but had no correlation with differentiation to other B-cell subsets (e.g. IgD+CD27hi, IgD−CD27−, and IgD+CD27−) or B-cell proliferation (Supplemental Figure 3). The stimulation condition with anti-human CD40, BAFF, anti-human IgG/IgM, IL-21, and LPS in Stim-3 consistently produced the highest level of total IgG across multiple healthy controls, is comprised of mediators that mimic signals from T-follicular helper cells in vivo 59.
To further characterize the IgD−CD27hi B-cells that correlate with IgG antibody production, purified B-cells were incubated under Stim-3 condition and assessed for well-established markers of antibody-producing B-cell lineages and plasmablast/plasma cells. The cells expressed high levels of CD38 and CD95, and low levels of CD22 (Figure 1B and Supplemental Figure 2B). CD138 expression was not detected in these B-cell cultures (Supplemental Figure 4) and it is likely that this time point is too early for plasma cell generation and thus CD138 expression60, 61. Based on these markers, IgD−CD27hi B-cells can be classified as antibody-secreting cells (ASC), resembling plasmablasts, whose appearance strongly correlates with production of high levels of human IgG antibodies.
Memory B-cells are Responsible for the Generation of Plasmablasts.
Memory B-cells are thought to play an important role in RA disease pathogenesis, being responsible for the local generation of ACPA producing ASCs in the synovium62, 63. To assess the role of memory B-cells in the production of IgG and plasmablasts in our in vitro assay, B-cells were purified from healthy controls and sorted for naïve (CD20+CD27−IgD+) or memory (CD20+CD27+IgD−) cells (Supplemental Figure 5). As expected, sorted naïve B-cells had no IgG surface expression and were almost entirely surface IgM+, while sorted memory B-cells had a higher percentage of IgG+ cells. These B-cell subsets were cultured under Stim-3 condition without BCR stimulation (anti-human IgG/IgM) for 7 days to evaluate the ability of the two different B-cell types to differentiate into ASC. Plasmablasts were found to be generated only in the cultures of sorted memory B-cells and correlated with production of significant amounts of IgG in culture supernatants (Figure 1C). We conclude that memory B-cells are primarily responsible for the generation of IgG producing plasmablasts in this assay.
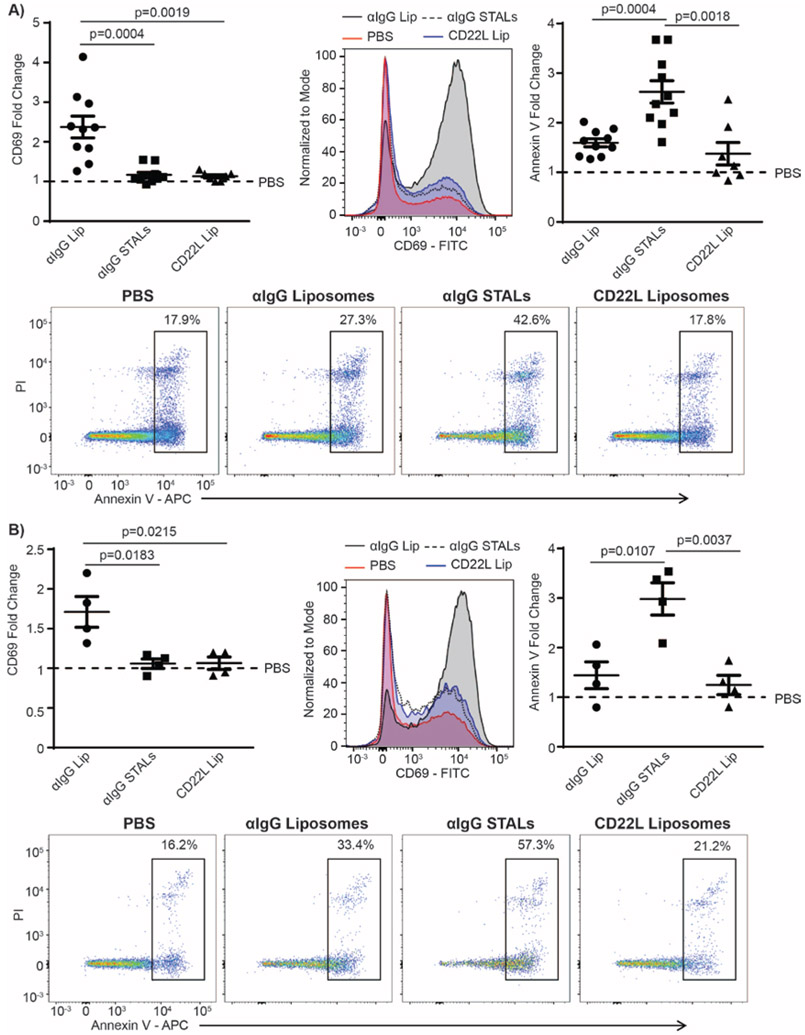
Memory RA B-cells are Depleted using Anti-human IgG-STALs.
To selectively target human memory B-cells, we formulated STALs with a synthetic hCD22 ligand, 6’MBP-5F-Neu5Ac (hCD22L) and anti-human IgG Fab fragments to bind to the IgG memory B-cell BCR as a surrogate antigen (αIgG-STALs). Purified B-cells were cultured for 24hr under one of the following conditions: PBS, liposomes decorated with only anti-human IgG (αIgG-liposomes), liposomes decorated with CD22L only (CD22L-liposomes.) or αIgG-STALs presenting both αIgG and CD22L. Cells were then assessed for activation and apoptosis by measuring upregulation of CD69 and PI/Annexin V, respectively. Memory B-cells treated with αIgG-liposomes had greatly increased CD69 expression and negligible increased apoptosis relative to the PBS controls (Figure 2A). In contrast, αIgG-STALs caused no activation of memory B-cells while significantly increasing apoptosis. This effect was dependent on co-presentation of αIgG and CD22 since CD22L-liposomes alone had no effect on either B-cell activation or cell death. In contrast to the impact of αIgG-STALs on IgG-memory B-cells, they had no effect on IgM-naïve B-cells (Supplemental Figure 6). Soluble antigens and autoantibodies may be present in circulation in RA, which could potentially compete with or bind the antigen on STALs to mask its ability to engage the antigen specific B-cells. Therefore, we monitored the effect of STALs in the presence of soluble antigen (purified human IgG) and found that it did not affect the ability of anti-human IgG liposomes to cause B-cell activation or anti-human IgG STALs to inhibit activation and induce apoptosis of memory B-cells (Figure 2B).
Figure 2.
IgG+ expressing B-cells are depleted by anti-human IgG STALs even in the presence of soluble IgG antibody. a) Human memory B-cells were isolated from healthy control PBMCs and incubated under one of the following conditions: unstimulated (PBS), anti-human IgG-liposomes (αIgG Lip), anti-human IgG+hCD22L (αIgG STALs), or CD22L liposomes alone (CD22L Lip) for 24 hrs. After 24hrs, cells were stained for markers of activation (CD69) and cell death (Annexin V/PI) (N=10 for PBS, αIgG, and αIgG STAL, N=7 for CD22L Lip, each independent experiments, pooled data, one-way ANOVA with multiple comparisons). c) B-cells were cultured under the same conditions as above, except that soluble IgG (5 ng/mL) was added to the wells prior to the addition of PBS or the liposomes (N=4 for PBS, αIgG, αIgG STALS, and CD22L Lip, each an independent experiment, pooled data, one-way ANOVA with multiple comparisons). Representative FACS analysis data on activation marker CD69 and cell death markers annexin V and PI uptake from one healthy donor are shown.
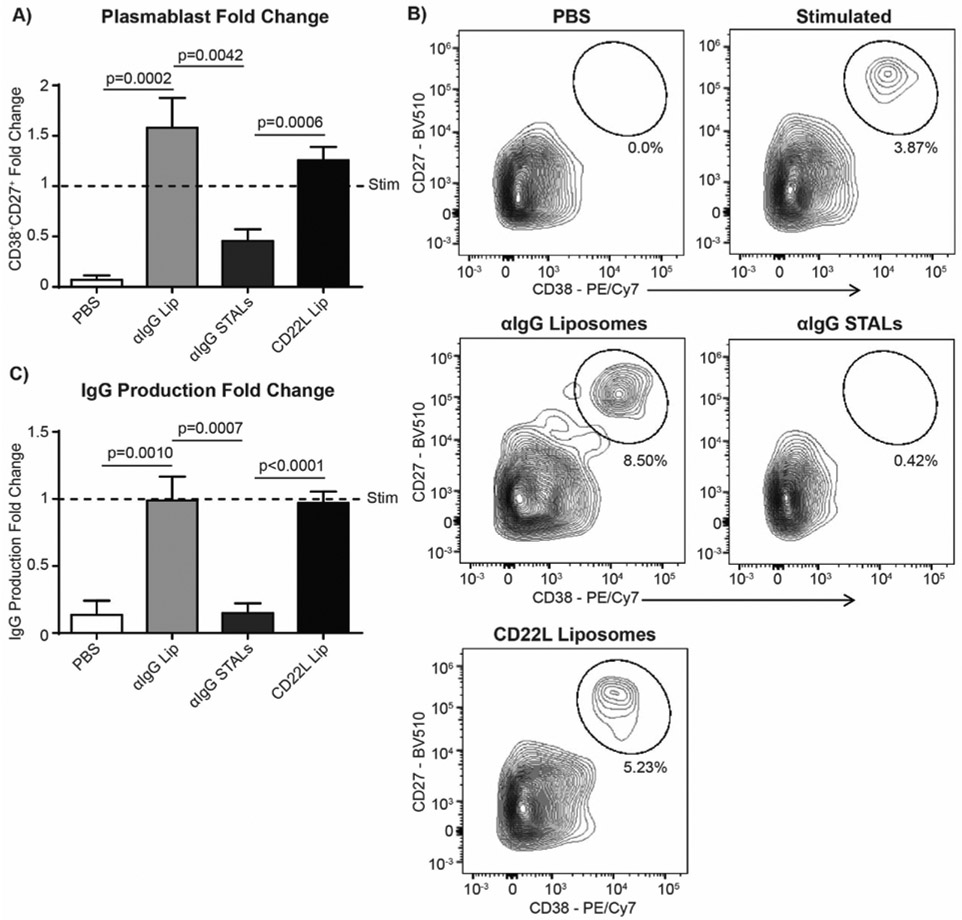
αIgG-STALs Prevent Differentiation of Stimulated Memory B-cells to Plasmablasts.
The inflammatory conditions in the RA synovium produces a variety of cytokines that can potentially provide a secondary signal that abrogates the effect of STALs 49, 50. To assess the impact of such stimulatory conditions on αIgG-STALs, purified B-cells were left unstimulated (PBS) or cultured under Stim-3 conditions for 24hrs, followed by addition of αIgG-Liposomes, αIgG-STALs, or CD22L-Liposomes. Under these conditions, anti-IgG STALs significantly blocked the differentiation of memory cells to plasmablasts, while αIgG-Liposomes or CD22L-Liposomes had no effect on differentiation (Figure 3A, B). The decreased differentiation of plasmablasts also correlated with a near complete abrogation of IgG production relative to the unstimulated control (Figure 3C). Notably, αIgG-STALs had no effect when added 5 days after cells were stimulated (Supplemental Figure 7), consistent with the timeframe in which down regulation of CD22 occurs when B-cells are differentiated into plasmablasts. This data demonstrates that STALs maintain their tolerizing effects on memory B-cells even in the presence of some inflammatory mediators.
Figure 3.
Generation of plasmablasts is inhibited using anti-human IgG STALs. a) Human B-cells were isolated from healthy control PBMCs and plated at 1×105 stimulated under Stim #3 conditions for 24hr or PBS, followed by 7 days under one of the following conditions: unstimulated (PBS), anti-human IgG-liposomes (αIgG Lip), anti-human IgG+hCD22L (αIgG STALs), or CD22L liposomes alone (CD22L Lip) for the entirety of the study. After 7 days, each well was harvested for flow cytometry analysis of B-cell subsets (N=8 each an independent experiment, pooled data, one-way ANOVA with multiple comparisons). B) Representative plots are shown in b) and c) supernatant from each experiment was assessed for total IgG (N=8 each an independent experiment, pooled data, one-way ANOVA with multiple comparisons).
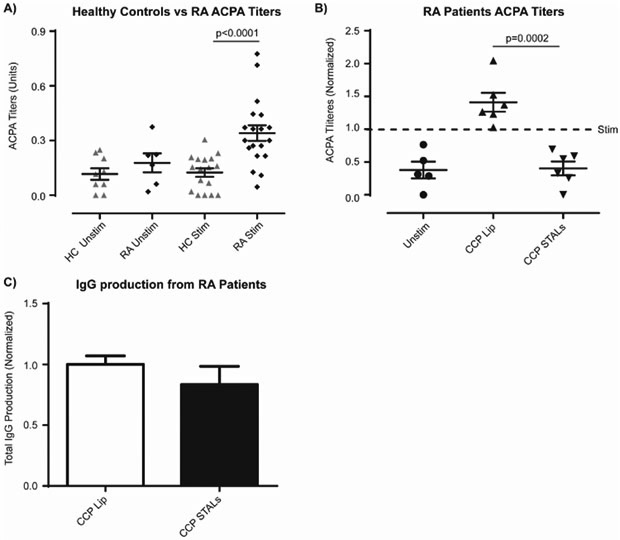
CCP STALs Abrogate ACPA Production by RA Patient B-cells In Vitro.
We next formulated CCP-STALs to directly assess the impact on ACPA-generating B-cells in RA blood60, 64-66. Under the optimized Stim-3 conditions, B-cells from RA patients produced detectable ACPA relative to background levels set by B-cells from healthy controls (Figure 4A). Although the ACPA titers are significantly lower than those in human sera, this likely relates to the low frequency of ACPA producing B-cells. There are, on average, only one ACPA-generating B-cell per 2×104 B-cells64, which translates to ~5 citrulline-specific B-cells per well. Nevertheless, these in vitro ACPA responses were consistent and sufficient to test the effect of CCP STALs on ACPA-producing B-cells. Direct detection of ACPA B-cells was tested exhaustively, however one complication is that the same peptide used to detect ACPA-secreting cells is used for ACPA B-cell STAL targeting and tolerization. Therefore, interpreting results due to competition of binding and detection of ACPA B-cells by ELiSpot or flow cytometry may not be valid.
Figure 4.
CCP STALs prevent ACPA production from rheumatoid arthritis patients in vitro. a) Human B-cells were isolated from healthy control (N=4 each an independent experiment, pooled data, one-way ANOVA with multiple comparisons) or rheumatoid arthritis (N=4 each an independent experiment, pooled data, one-way ANOVA with multiple comparison) patients’ PBMCs. RA patients were pre-screened for high titers of ACPA. B-cells were plated and either left unstimulated (PBS), or stimulated under standard stimulating conditions for 7 days, and ACPA titers in individual wells are shown. b) B-cells were isolated from RA patient PBMCs and left unstimulated or stimulated in accordance with the above conditions with CCP decorated-liposomes (CCP Lip) or CCP+hCD22L decorated liposomes (CCP STALs) for the entirety of the study. Supernatants from the wells were assessed for ACPA titers and normalized titers of individual patient are shown (N=6 each an independent experiment with an individual RA patient sample, unstim. N=5 (one patient did not have enough B-cells for all conditions), Stim. N=6, CCP Lip N=6, CCP STALs N=6, pooled data, one-way ANOVA with multiple comparisons). c) Total IgG titers in supernatants was measured (N=4 for CCP Lip and CCP STALs, each an independent experiment with an individual RA patient sample, pooled data, one-way ANOVA with multiple comparisons).
Accordingly, RA patient B-cells were left unstimulated or stimulated, as described above, with the addition of liposomes decorated with CCP peptides (CCP-Lip) or CCP-STALs thirty minutes later. As shown in Figure 4B, CCP-STALs significantly reduced ACPA production to levels in the unstimulated control. The depletion was consistent across multiple donors and repeated data (Figure 4C). In contrast, CCP-Lip increased the production of ACPA, as expected due to crosslinking of the BCR with the citrulline-reactive B-cells. It is also notable that while CCP-STALs prevented ACPA production from RA patient samples, they did not affect total IgG production under the same conditions from the same wells as tested for ACPA (Figure 4D). This is consistent with the previous findings of affecting only the specific antigen attached to the liposome 50 and this shows tolerization of B-cells that recognize CCP, while other memory B-cells are unaffected.
CCP-STALs Induce B-cell Tolerance to ACPA Production In Vivo.
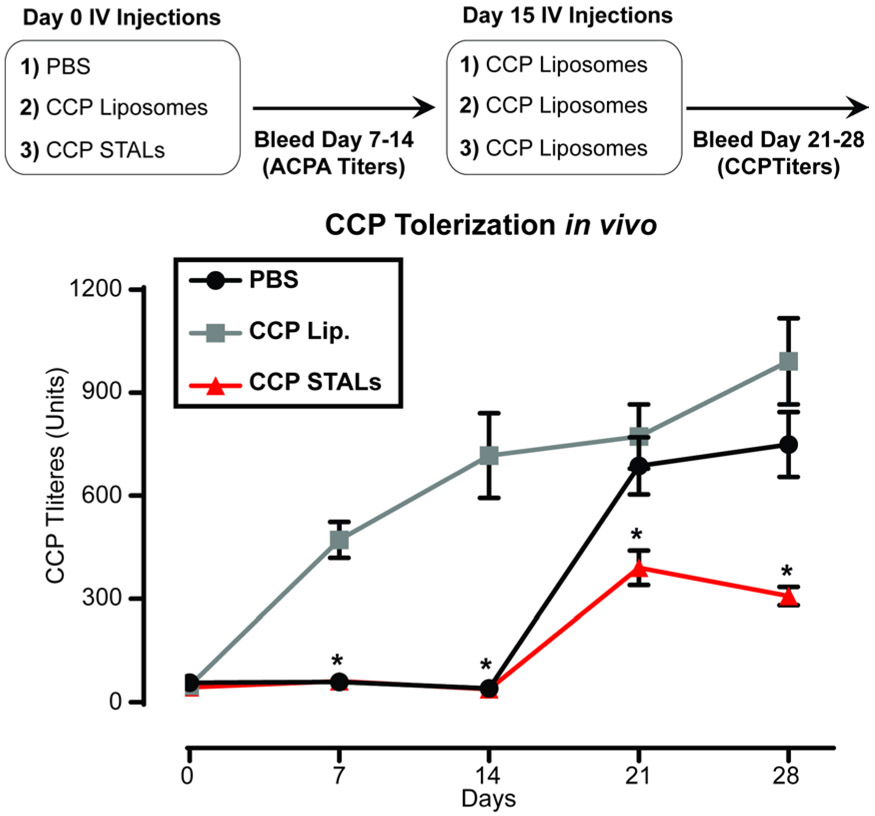
SJL/J mice are known to generate antibody responses against citrullinated antigens67. Therefore, we tested the ability of CCP-Lip to generate ACPA-like responses in SJL/J mice. Since CCP-Lip is considered a T-independent type II antigen, we added a TLR4 ligand, MPLA, to CCP-Lip as an adjuvant. Indeed, CCP-Lip with MPLA generated a robust ACPA antibody response relative to CCP-Lip without MPLA (Supplemental Figure 8). For the experiment, SJL/J mice initially treated with PBS, CCP-STALs, or CCP-Lip containing MPLA. Two weeks after the initial injection, all groups of mice were challenged with CCP-Lip containing MPLA, and ACPA titers were measured the following 2 weeks. Mice treated initially with CCP-STALs had significantly reduced total ACPA titers at all time points compared to the group that was treated immunized with two doses of CCP-Lip, and significantly reduced titers at day 21 and day 28 compared to mice that received CCP-Lip only at day 14 (Figure 5). These data demonstrate that liposomes decorated with a cyclic citrullinated peptide and a high affinity ligand for CD22 can tolerize CCP-specific B-cells in ACPA production in vivo.
Figure 5.
ACPA production in SJL/J mice are tolerized upon STAL treatment in vivo. a) SJL/J mice were treated with indicated conditions on Day 0, followed by immunization on Day 14 with CCP liposomes containing MPLA. ACPA titers were measured once a week for 4 weeks (N=16 mice from 2 independent experiments, pooled data). Significance was measured between CCP Lip and CCP STALs at all time points (*p<0.0001, unpaired t-test) or between CCP STALs and PBS on days 21 and 28 (*p=<0.001, unpaired t-test).
Discussion.
B-cells have gained attention as therapeutic targets in RA, largely due to positive clinical and radiological responses seen in patients receiving Rituximab for B-cell depletion therapy. Rituximab targets CD20 on naïve and memory B-cells, causing depletion of these CD20-expressing cells primarily through complement-dependent cytotoxicity68. Other monoclonal antibodies targeting B-cells, such as CD19 and CD22 conjugated to toxins or radionuclides are currently being developed and tested37. Prolonged B-cell depletion, however, limits the use of these therapies due to loss of protective humoral immunity and increased risk for infection37. In principle, a more selective therapy that targets B-cells directly involved in the pathology of RA would be more tolerated and maintains a functional humoral immunity for protection from infections.
B-cells that recognize citrullinated proteins are of interest for RA given evidence for ACPA levels correlated with disease onset and pathogenesis, and the potential of these B-cells to secrete cytokines and present antigen to T-cells, playing a pathogenic role beyond antibody production. Memory B-cells are the major cytokine producers and T-cell activators in RA patients, and are enriched in the synovial fluid, synovial tissues, and intra-tissue like lymphoid structures in the joints69-72. Memory B-cells specific for citrullinated antigens in the peripheral blood express both IgA and IgG isotypes64, and clinical responses correlate with the number of these cells in the blood73-75. Transplantation of lymphoid tissues from joints in RA joints into SCID mice results in production of class-switched ACPA63, 76. Together, these data suggest that memory B-cells specific for citrullinated antigens are key progenitors of the ASCs that secrete ACPA, making them a logical population for targeted B-cell therapy.
Cyclic citrullinated peptides were originally developed to mimic epitopes on citrullinated proteins recognized by ACPA and could be used diagnostically to detect ACPA in RA patient sera. We took advantage of CCP to therapeutically target ACPA B-cells using CCP-STALs. We showed here that treating B-cells from RA patients in vitro with CCP-liposomes induced ACPA formation, while CCP-STALs suppressed formation of ACPA. Furthermore, mice treated with CCP-STALs had an impaired ability to mount an antibody response upon a subsequent challenge with CCP antigenic liposomes. The results suggest the potential for use of CCP-STALs for inducing tolerance and reducing ACPA formation. Although CCP does not recognize 100% of ACPAs, second and third generation CCPs have been shown to cover > 95% of ACPAs33, providing an opportunity to create CCP-STALs that recognize nearly all ACPAs.
STALs met the expectation for antigen-specific targeting of B-cells since CCP-STALs suppressed ACPA, but did not impact induction of non-ACPA (total) antibody50. Similarly, αIgG-STALs targeted memory B-cells (IgG+) but not naïve B-cells (IgM+). Moreover, we found that the αIgG-STALs inhibitory effects on memory B-cells was not abrogated when exogenous IgG F(ab’)2 was added. This could be due to the fact that the soluble antigen used is divalent while the large number of IgG F(ab) molecules decorating the liposomes surface is highly multimeric, increasing avidity to the surface IgG BCRs on the B-cell. ACPA in patient serum, amounting to 10-200 μg/ml77, could also potentially interfere with CCP-STALs and would be important to address in the context of an ongoing disease where such antibodies would be present.
In summary, we have shown that ACPA producing B-cells from RA patients can be selectively suppressed in vitro using CCP-STALs, as illustrated in Figure 6. Depletion of antigen-specific memory B-cells which develop into ACPA-producing ASCs, may have multiple benefits in RA patients that present with high ACPA titers, including: depleting the local and systemic pool of ACPA-producing B-cells, reducing ACPA titers and their ability to antagonize the immune system through forming immune complexes, removal of the citrulline-specific B-cells that can capture and present citrullinated antigens to T-cells, and reducing inflammatory cytokine secretion. Since STALs do not directly deplete long-lived plasma cells, even though they may indirectly affect this population through depleting the memory B-cells, a combination therapy that depletes both the long-lived plasma cells and the antigen-specific naïve and memory B-cells may ultimately be the most efficacious while keeping protective humoral immunity. Such a targeted immunotherapy provides an attractive alternative to current treatments for RA.
Figure 6.
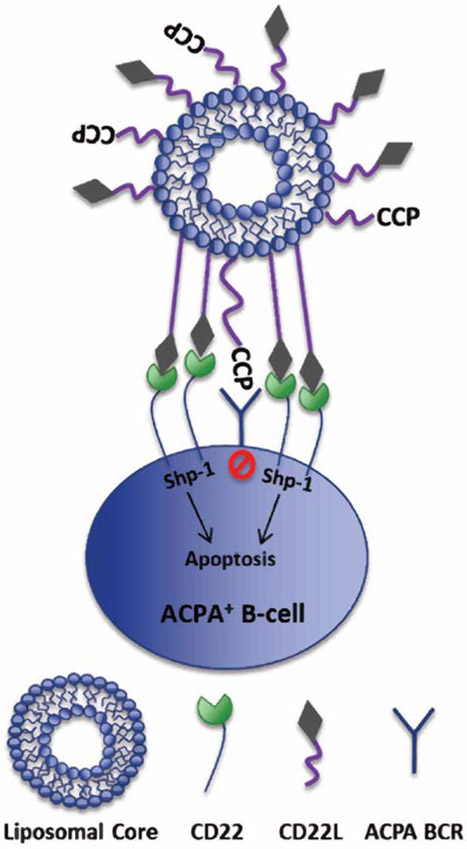
Proposed mechanism of targeting citrulline-specific B-cells using a CCP-STAL. CCP on STAL targets citrulline protein-specific B cells by binding to citrulline-specific BCR. Human CD22 synthetic ligand (CD22L) on STAL binds CD22 on B cells, causing clustering of CD22 to BCR and SHP-1 recruitment in BCR signaling pathway. This clustering and SHP-1 recruitment causes antigen-specific B cell tolerization possibly through an apoptotic mechanism.
Conclusions.
Memory B-cells are responsible for plasmablast generation in vitro. Targeting memory B-cells using STALs is able to prevent the activation of memory B-cells and ultimately resulting in reduction of antibodies. Targeting ACPA memory B-cells using STALs decorated with a cyclic citrullinated peptide was able to reduce ACPA production in vitro. SJL/J are able to produce ACPA after injection with an antigenic liposome. STALs can significantly reduce ACPA production in vivo. These results demonstrate that STALs could be an effect therapy for the depletion of ACPA B-cells.
METHODS
B-cell Purification and Staining.
Frozen peripheral blood mononuclear cells (PBMCs) were obtained through AllCells (Alameda, CA) or BioreclamationIVT (Chestertown, MD) as per institutional protocols for human sample acquisition. Cells were thawed in accordance with the manufacture’s protocol. B-cells were purified by negative enrichment (B-cell isolation kit II, Miltenyi), resulting in 91-98% pure CD20+ B-cells. B-cells were suspended in FACS Buffer (0.2% BSA, BD Bioscience) containing Human Fc Block (BD Bioscience) on ice for 10 minutes. Primary antibodies were added at a concentration directed by the manufacturer (Biolegend) and left for 1hr on ice. The following antibodies directly toward the human antigens were used (clone; fluorophore): CD20 (SJ25C1; APC-Cy7), CD27 (CM-T271; Brilliant Violet 510, APC), IgD (IA6-2; FITC), IgM (MHM-88; Pacific Blue), CD38 (HB-7; PE-Cy7), CD138 (DL-101; APC), IgG (M1310G05; Percp/Cy5.5), CD22 (HIB22; PE), CD95 (DX2; Brilliant Violet 421), and CD69 (FN50; FITC)). B-cells were centrifuged (300 RCF, 5 min), washed 3x with FACS buffer, and were stored on ice until analyzed by flow cytometry. For Annexin V staining, the cells were stained in accordance with the manufacturer’s protocol (BD Biosciences). Flow cytometry data was obtained on a CantoII flow cytometer (BD Biosciences) with up to ten fluorochromes and analyzed using FlowJo software (TreeStar).
B-cell Proliferation and IgG Antibody Production.
Purified B-cells were cultured with DMEM containing: 10% FBS, 2% Penicillin and Streptomycin, 1% L-glutamine, MEM Non-Essential Amino Acids (NEAA), and sodium pyruvate (ThermoFisher), described below as standard culturing media, in a 96-well U-bottom plate at 2×104 cells per/well in a volume of 200 μl. Combinations of additional factors were added for 7 days, including: F(ab’)2 anti-human IgG/IgM (Jackson ImmunoResearch, 100 ng/mL), B-cell activating factor (BAFF; eBioscience, 100 ng/mL), anti-human CD40 (eBioscience, 100 ng/mL), IL-4 (eBioscience, 10 ng/mL), IL-21 (eBioscience, 50ng/mL), LPS (Invivogen, 1 ng/mL), and CD180 (eBioscience, 100 ng/mL). After 7 days, plates were spun down (300 RCF, 5 min) and the supernatant analyzed for total human IgG antibody (Ready-Set-Go total human IgG ELISA Kit, Invitrogen). B-cell proliferation was measured using CellTiter-Glo (Promega).
B-Cell Sorting and in vitro Stimulation.
B-cells were sorted on a FacsAria (BD Biosciences) cell sorter for memory (CD20+CD27+IgD−) or naïve B-cells (CD20+CD27−IgD+) using a 70μm nozzle. After-sorting, purity of each B-cell subset population was determined and cells were cultured in standard culturing media in a 96-well U-bottom plate at 1×105 cells per/well in 200 μL. All wells were stimulated with the following cocktail for 7 days: BAFF (100 ng/mL), anti-human CD40 (100 ng/mL), IL-21 (50 ng/mL), and LPS (1ng/mL), referred to below as the standard stimulation conditions or Stim-3 below. After 7 days, the plate was spun down (300 RCF, 5min) and the supernatant was harvested and total human IgG determined by ELISA. The remaining cells were also stained for specific B-cell markers to look at subsets.
In Vitro B-Cell Activation and Evaluation of Cell Death.
B-cells from healthy controls were cultured in standard culturing media in a 96-well U-bottom plate at 1×105 cells per/well in 200 μl. Following plating of B-cells, one of the following treatments was added into the well for 24 hours: PBS, anti-human IgG Liposomes (αIgG Lip), STALs decorated with anti-human IgG and hCD22L (αIgG STALs), or liposomes decorated with hCD22L alone (CD22L Lip). The final concentration of liposomes used in these assays was 40 μM, based on total lipids. Following 24hrs, the plate was spun down (300 RCF, 5min) and stained as described above for the B-cell markers labeled in each figure. All results were normalized to the unstimulated control (PBS) and fold change was determined for both activation (CD69+) and cell death (PI+ and Annexin V+). For the addition of soluble protein, B-cells were cultured in identical conditions, as stated above, with soluble human IgG (5 ng/mL, Sigma) added into the cultures of all the wells prior to the addition of liposomes.
In Vitro B-Cell Activation and Analysis of Plasmablast Generation.
B-cells were cultured in standard culturing media in a 96-well U-bottom plate at 1×105 cells per/well in 200 μL. Cells were cultured with standard stimulation conditions starting on day 0 for 7 days. The following treatments were initiated on day 1: (a) PBS, (b) αIgG Lip, (c) αIgG STALs, or (d) CD22L Lip. On day 7, the plates were spun down at (300 RCF, 5min) and supernatants were harvested from each well for the quantitation of total human IgG. B-cells were stained for plasmablast-like differentiation markers indicated in each of the figures.
In Vitro RA and Healthy Donors B-Cell Activation and Measurement of ACPA Production.
B-cells from healthy donors or RA patients were cultured in standard culturing media in a 96-well U-bottom plate at 1×105 cells per/well in 200 μl. Cells were cultured with standard stimulation conditions starting on day 0 for 7 days. On day 7, the plates were spun down (300 RCF, 5 min) and supernatants were collected and tested for anti-citrullinated protein antibodies (ACPA titer) in accordance with the protocol from Inova Diagnostics CCP3 IgG kit. polyethyleneglycol-distearoyl phosphoethanolamine (PEG-DSPE, Avanti Polar Lipids) was conjugated to a peptide known to bind ACPA producing B-cells, CFFCP (HSTKRGHAKSRPV-Cit-GHQ-(CHQEST-Cit-GRSRGRC)-GRSGS-OH)78, herein called CCP. DSPE-PEG-CCP was synthesized by an outside vendor (Peptide International) to 99% purity and was decorated onto the liposomes as described below. B-cells from RA patients were cultured in standard culturing media in a 96-well U-bottom plate at 1×105 cells per/well in 200 μl. The B-cells were either left unstimulated or stimulated with standard stimulation conditions for 7 days with liposomes decorated with CCP (CCP Liposome), or liposomes decorated with CCP and hCD22L (CCP STALs). Plates were spun down (300 RCF, 5min) and ACPA production was measured from the supernatants. All results were normalized to the stimulated condition and assessed for fold changed differences in ACPA titers. From the same supernatant, total human IgG production (Invitrogen) was assessed to determine the effect of CCP liposomes or CCP STALs on overall IgG production.
Immunization of Mice with CCP Liposomes or CCP STALs.
Janssen Pharmaceutical, LLC Institutional Animal Care and Use Committee approved all experimental procedures involving mice. Female SJL/J mice were obtained from Jackson laboratory at 6 weeks of age. Whole blood (50 μl) was collected via tail vein bleeds to obtain the serum after centrifugation (17,000 RCF, 1 min). Serum was aliquoted and stored at −20°C. PBS or liposomes were delivered via the lateral tail vein (I.V.) in a volume of 100 μl/mouse. Briefly, mice were injected on day 0 with PBS, CCP liposomes with 5% (mol %) MPLA (Invivogen) as an adjuvant (100 μM of liposomes were given, based on total lipid), or CCP STALs (100 μM of liposomes were given, based on total lipid). All groups were challenged on day 15 with CCP liposomes with 5% (mol %) MPLA (250 μM of liposomes were given, based on total lipid). Mice were bled weekly for serum and sacrificed two weeks after CCP liposome challenge.
Measurement of ACPA Titers from in vivo Animal Models.
Neutravidin high-binding plates were coated (O/N, 4°C) with a biotinylated CCP peptide (Peptide International) (Biotin-HSTKRGHAKSRPV-Cit-GHQ-(CHQEST-Cit-GRSRGRC)-GRSGS-OH) at 100 μl/well of 10 μg/ml stock solution in PBS. The following day, plates were washed 6x with PBS-T (0.1% Tween 20) and blocked (2 hour, RT) with PBS with 0.1% Tween 20 and 1% BSA, and mouse sera were applied at 1:1000 dilution. Plates were incubated (2 hour, RT) with samples (100 μl/well), washed 6x with PBS-T, and incubated (1 hour, RT) with the appropriate HRP-conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology). Following 6 washes in PBS-T, plates were developed (15 minutes, RT) in 75μl/well of TMB substrate (ThermoFisher) and quenched with 75μl/well of 2N H2SO4. Absorbance was measured at 450 nm, and the numerical titer was based on the standard from the Inova Diagnostics CCP3 kit.
Generation of High Affinity Murine and Human CD22 Ligands.
The synthesis of the high affinity mCD22L, described as BPANeu5Gca2-6Galb1-4GlcNAc-lipid, and high affinity hCD22L, described as MPBNeu5Aca2-6Galb1-4GlcNAc-lipid, were prepared as described previously48, 49.
Liposomes and Protein-lipid Conjugation.
A detailed protocol for conjugation of proteins to PEG-DSPE and liposomal preparation can be found in detail elsewhere50, 79, 80. Preparation of CCP-PEG-DSPE was commercially prepared (Peptide International; Louisville, KY). Briefly, all liposomes were composed of distearoyl phosphatidylcholine (DSPC, Avanti Polar Lipids), cholesterol (SigmaAldrich), PEG-DSPE, pegylated lipids in a 60:35:5 molar ratio and were extruded through an 800 nm, 200 nm, and finally 100 nm filter a minimum of 20 times each, followed by running the liposomes on a CL-4B column to remove any unconjugated protein or ligand. Liposomal compositions used in these studies consisted of 0.03% anti-human IgG Fab fragment or 0.1% CCP as the antigens, with or without 1.5% CD22L (mouse- or human-specific ligands), all of which were linked to PEG-DSPE. Care was taken to ensure that the total mol% of PEG was 5% in all liposomes.
Statistics.
GraphPad Prism was used to determine statistical significance; test used for statistical significance is indicated in each figure legend.
Supplementary Material
Acknowledgements
Funding
This work was supported by grants from the Department of Defense (W81XWH-16-1-0303 to M.S.M.) and the National Institute for Allergy and Infectious Diseases (R01 AI099141 and R01 AI050143 to J.C.P.)
Footnotes
Conflict-of-Interest
The authors have no conflict-of-interests to declare
Ethics Approval and Consent Participate
Human Samples: Frozen peripheral blood mononuclear cells (PBMCs) were obtained through AllCells (Alameda, CA) or BioreclamationIVT (Chestertown, MD) as per institutional protocols for human sample acquisition. Janssen Pharmaceutical, LLC Institutional Animal Care and Use Committee approved all experimental procedures involving mice.
Supporting Information Available: This material is available free of charge via the Internet
References
- 1.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, and et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis, Arthritis and rheumatism 31, 315–324. [DOI] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, and Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative, Arthritis and rheumatism 62, 2569–2581. [DOI] [PubMed] [Google Scholar]
- 3.Banal F, Dougados M, Combescure C, and Gossec L (2009) Sensitivity and specificity of the American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis according to disease duration: a systematic literature review and meta-analysis, Annals of the rheumatic diseases 68, 1184–1191. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan FM, and Maini RN (1996) Role of cytokines in rheumatoid arthritis, Annual review of immunology 14, 397–440. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M, Brennan FM, and Maini RN (1996) Rheumatoid arthritis, Cell 85, 307–310. [DOI] [PubMed] [Google Scholar]
- 6.Scott DL, Wolfe F, and Huizinga TW (2010) Rheumatoid arthritis, Lancet 376, 1094–1108. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, and Taylor RS (2008) Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation, Health technology assessment 12, 1–278, iii. [DOI] [PubMed] [Google Scholar]
- 8.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL Jr., Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O’Dell J, Turkiewicz AM, Furst DE, and American College of, R. (2008) American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis, Arthritis and rheumatism 59, 762–784. [DOI] [PubMed] [Google Scholar]
- 9.Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, Hansen RA, Morgan LC, and Lohr KN (2008) Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis, Annals of internal medicine 148, 124–134. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, and Quintana A (2008) Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety, BMC musculoskeletal disorders 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell LJ, and Singh JA (2010) Abatacept for rheumatoid arthritis: a Cochrane systematic review, The Journal of rheumatology 37, 234–245. [DOI] [PubMed] [Google Scholar]
- 12.Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, Proudlove C, Kennedy T, Moots R, Williamson P, and Dickson R (2009) Rituximab for the treatment of rheumatoid arthritis, Health technology assessment 13 Suppl 2, 23–29. [DOI] [PubMed] [Google Scholar]
- 13.An MM, Zou Z, Shen H, Zhang JD, Cao YB, and Jiang YY (2010) The addition of tocilizumab to DMARD therapy for rheumatoid arthritis: a meta-analysis of randomized controlled trials, European journal of clinical pharmacology 66, 49–59. [DOI] [PubMed] [Google Scholar]
- 14.Huizinga TW, Amos CI, van der Helm-van Mil AH, Chen W, van Gaalen FA, Jawaheer D, Schreuder GM, Wener M, Breedveld FC, Ahmad N, Lum RF, de Vries RR, Gregersen PK, Toes RE, and Criswell LA (2005) Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins, Arthritis and rheumatism 52, 3433–3438. [DOI] [PubMed] [Google Scholar]
- 15.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, and Cairns E (2003) Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule, Journal of immunology 171, 538–541. [DOI] [PubMed] [Google Scholar]
- 16.Vossenaar ER, Zendman AJ, van Venrooij WJ, and Pruijn GJ (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease, BioEssays : news and reviews in molecular, cellular and developmental biology 25, 1106–1118. [DOI] [PubMed] [Google Scholar]
- 17.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, and van Venrooij WJ (2003) Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis, Arthritis and rheumatism 48, 2741–2749. [DOI] [PubMed] [Google Scholar]
- 18.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, and Dijkmans BA (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors, Arthritis and rheumatism 50, 380–386. [DOI] [PubMed] [Google Scholar]
- 19.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, Drijfhout JW, Huizinga TW, Toes RE, and Pruijn GJ (2010) Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis, Annals of the rheumatic diseases 69, 1554–1561. [DOI] [PubMed] [Google Scholar]
- 20.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, and Robinson WH (2012) Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis, PloS one 7, e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payet J, Goulvestre C, Biale L, Avouac J, Wipff J, Job-Deslandre C, Batteux F, Dougados M, Kahan A, and Allanore Y (2014) Anticyclic citrullinated peptide antibodies in rheumatoid and nonrheumatoid rheumatic disorders: experience with 1162 patients, The Journal of rheumatology 41, 2395–2402. [DOI] [PubMed] [Google Scholar]
- 22.van der Linden MP, van der Woude D, Ioan-Facsinay A, Levarht EW, Stoeken-Rijsbergen G, Huizinga TW, Toes RE, and van der Helm-van Mil AH (2009) Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis, Arthritis and rheumatism 60, 2232–2241. [DOI] [PubMed] [Google Scholar]
- 23.Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Gabay C, van Riel PL, Nordstrom DC, Gomez-Reino J, Pavelka K, Tomsic M, Kvien TK, and van Vollenhoven RF (2011) Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries, Annals of the rheumatic diseases 70, 1575–1580. [DOI] [PubMed] [Google Scholar]
- 24.Gottenberg JE, Courvoisier DS, Hernandez MV, Iannone F, Lie E, Canhao H, Pavelka K, Hetland ML, Turesson C, Mariette X, and Finckh A (2016) Brief Report: Association of Rheumatoid Factor and Anti-Citrullinated Protein Antibody Positivity With Better Effectiveness of Abatacept: Results From the Pan-European Registry Analysis, Arthritis & rheumatology 68, 1346–1352. [DOI] [PubMed] [Google Scholar]
- 25.Malmstrom V, Catrina AI, and Klareskog L (2017) The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting, Nature reviews. Immunology 17, 60–75. [DOI] [PubMed] [Google Scholar]
- 26.Nienhuis RL, and Mandema E (1964) A New Serum Factor in Patients with Rheumatoid Arthritis; the Antiperinuclear Factor, Annals of the rheumatic diseases 23, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young BJ, Mallya RK, Leslie RD, Clark CJ, and Hamblin TJ (1979) Anti-keratin antibodies in rheumatoid arthritis, British medical journal 2, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, Durieux JJ, and Serre G (1995) The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies, The Journal of clinical investigation 95, 2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, and van Venrooij WJ (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies, The Journal of clinical investigation 101, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessiere C, Jolivet-Reynaud C, Jolivet M, and Serre G (1999) The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues, Journal of immunology 162, 585–594. [PubMed] [Google Scholar]
- 31.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, and van Venrooij WJ (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide, Arthritis and rheumatism 43, 155–163. [DOI] [PubMed] [Google Scholar]
- 32.van Venrooij WJ, Hazes JM, and Visser H (2002) Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis, The Netherlands journal of medicine 60, 383–388. [PubMed] [Google Scholar]
- 33.Szekanecz Z, Szabo Z, Zeher M, Soos L, Danko K, Horvath I, and Lakos G (2013) Superior performance of the CCP3.1 test compared to CCP2 and MCV in the rheumatoid factor-negative RA population, Immunologic research 56, 439–443. [DOI] [PubMed] [Google Scholar]
- 34.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, Gilliland W, Edison JD, Buckner JH, Mikuls TR, O’Dell JR, Keating RM, Gregersen PK, Norris JM, Holers VM, and Deane KD (2013) Performance of anti-cyclic citrullinated Peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease, Arthritis and rheumatism 65, 2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rondas D, Crevecoeur I, D’Hertog W, Ferreira GB, Staes A, Garg AD, Eizirik DL, Agostinis P, Gevaert K, Overbergh L, and Mathieu C (2015) Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes, Diabetes 64, 573–586. [DOI] [PubMed] [Google Scholar]
- 36.Bradford CM, Ramos I, Cross AK, Haddock G, McQuaid S, Nicholas AP, and Woodroofe MN (2014) Localisation of citrullinated proteins in normal appearing white matter and lesions in the central nervous system in multiple sclerosis, Journal of neuroimmunology 273, 85–95. [DOI] [PubMed] [Google Scholar]
- 37.Bluml S, McKeever K, Ettinger R, Smolen J, and Herbst R (2013) B-cell targeted therapeutics in clinical development, Arthritis research & therapy 15 Suppl 1, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, and Holers VM (2006) Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis, The Journal of clinical investigation 116, 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, Lee DM, Hueber W, Robinson WH, and Cairns E (2008) Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice, The Journal of experimental medicine 205, 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, and Holers VM (2011) N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis, Journal of immunology 186, 4396–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis VC, Banda NK, Cordova KN, Chandra PE, Robinson WH, Cooper DC, Lugo D, Mehta G, Taylor S, Tak PP, Prinjha RK, Lewis HD, and Holers VM (2017) Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis, Clinical and experimental immunology 188, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, Ytterberg AJ, Engstrom M, Fernandes-Cerqueira C, Amara K, Magnusson M, Wigerblad G, Kato J, Jimenez-Andrade JM, Tyson K, Rapecki S, Lundberg K, Catrina SB, Jakobsson PJ, Svensson C, Malmstrom V, Klareskog L, Wahamaa H, and Catrina AI (2016) Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss, Annals of the rheumatic diseases 75, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes R, Scherer HU, Catrina AI, Klareskog L, Jurdic P, and Schett G (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin, The Journal of clinical investigation 122, 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokolove J, Zhao X, Chandra PE, and Robinson WH (2011) Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor, Arthritis and rheumatism 63, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, and Kaplan MJ (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis, Science translational medicine 5, 178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozsgay J, Szekanecz Z, and Sarmay G (2017) Antigen-specific immunotherapies in rheumatic diseases, Nature reviews. Rheumatology 13, 525–537. [DOI] [PubMed] [Google Scholar]
- 47.Macauley MS, Crocker PR, and Paulson JC (2014) Siglec-mediated regulation of immune cell function in disease, Nature Reviews Immunology 14, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rillahan CD, Macauley MS, Schwartz E, He Y, McBride R, Arlian BM, Rangarajan J, Fokin VV, and Paulson JC (2014) Disubstituted Sialic Acid Ligands Targeting Siglecs CD33 and CD22 Associated with Myeloid Leukaemias and B Cell Lymphomas, Chemical science 5, 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, and Nemazee D (2010) Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo, The Journal of experimental medicine 207, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, and Paulson JC (2013) Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis, The Journal of clinical investigation 123, 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macauley MS, and Paulson JC (2014) Siglecs induce tolerance to cell surface antigens by BIM-dependent deletion of the antigen-reactive B cells, Journal of immunology 193, 4312–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres RM, Law CL, Santos-Argumedo L, Kirkham PA, Grabstein K, Parkhouse RM, and Clark EA (1992) Identification and characterization of the murine homologue of CD22, a B lymphocyte-restricted adhesion molecule, Journal of immunology 149, 2641–2649. [PubMed] [Google Scholar]
- 53.Moura RA, Weinmann P, Pereira PA, Caetano-Lopes J, Canhao H, Sousa E, Mourao AF, Rodrigues AM, Queiroz MV, Souto-Carneiro MM, Graca L, and Fonseca JE (2010) Alterations on peripheral blood B-cell subpopulations in very early arthritis patients, Rheumatology 49, 1082–1092. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma L, and Jiang Y (2013) High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis, Clinical and experimental immunology 174, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, Fleisher TA, Wilson M, Goldbach-Mansky R, and Lipsky PE (2009) Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor, Arthritis research & therapy 11, R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sellam J, Rouanet S, Hendel-Chavez H, Abbed K, Sibilia J, Tebib J, Le Loet X, Combe B, Dougados M, Mariette X, and Taoufik Y (2011) Blood memory B cells are disturbed and predict the response to rituximab in patients with rheumatoid arthritis, Arthritis and rheumatism 63, 3692–3701. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Andres M, Santiago M, Almeida J, Mateo G, Porwit-MacDonald A, Bjorklund E, Valet G, Kraan J, Gratama JW, D’Hautcourt JL, Merle-Beral H, Lima M, Montalban MA, San Miguel JF, Orfao I, European Working Group on Clinical Cell, A., and Spanish Network on Multiple, M. (2004) Immunophenotypic approach to the identification and characterization of clonal plasma cells from patients with monoclonal gammopathies, Journal of biological regulators and homeostatic agents 18, 392–398. [PubMed] [Google Scholar]
- 58.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, Kovarova L, Lioznov M, Mateo G, Morilla R, Mylin AK, Omede P, Pellat-Deceunynck C, Perez Andres M, Petrucci M, Ruggeri M, Rymkiewicz G, Schmitz A, Schreder M, Seynaeve C, Spacek M, de Tute RM, Van Valckenborgh E, Weston-Bell N, Owen RG, San Miguel JF, Sonneveld P, Johnsen HE, and European Myeloma, N. (2008) Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders, Haematologica 93, 431–438. [DOI] [PubMed] [Google Scholar]
- 59.Qi H (2016) T follicular helper cells in space-time, Nature reviews. Immunology 16, 612–625. [DOI] [PubMed] [Google Scholar]
- 60.Kerkman PF, Kempers AC, van der Voort EI, van Oosterhout M, Huizinga TW, Toes RE, and Scherer HU (2016) Synovial fluid mononuclear cells provide an environment for long-term survival of antibody-secreting cells and promote the spontaneous production of anti-citrullinated protein antibodies, Annals of the rheumatic diseases 75, 2201–2207. [DOI] [PubMed] [Google Scholar]
- 61.Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, and Klein B (2009) An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization, Blood 114, 5173–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, Engstrom M, Snir O, Israelsson L, Catrina AI, Wardemann H, Corti D, Meffre E, Klareskog L, and Malmstrom V (2013) Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition, The Journal of experimental medicine 210, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, and Pitzalis C (2009) Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium, PLoS medicine 6, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerkman PF, Fabre E, van der Voort EI, Zaldumbide A, Rombouts Y, Rispens T, Wolbink G, Hoeben RC, Spits H, Baeten DL, Huizinga TW, Toes RE, and Scherer HU (2016) Identification and characterisation of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis, Annals of the rheumatic diseases 75, 1170–1176. [DOI] [PubMed] [Google Scholar]
- 65.Kerkman PF, Rombouts Y, van der Voort EI, Trouw LA, Huizinga TW, Toes RE, and Scherer HU (2013) Circulating plasmablasts/plasmacells as a source of anticitrullinated protein antibodies in patients with rheumatoid arthritis, Annals of the rheumatic diseases 72, 1259–1263. [DOI] [PubMed] [Google Scholar]
- 66.Pozsgay J, Babos F, Uray K, Magyar A, Gyulai G, Kiss E, Nagy G, Rojkovich B, Hudecz F, and Sarmay G (2016) In vitro eradication of citrullinated protein specific B-lymphocytes of rheumatoid arthritis patients by targeted bifunctional nanoparticles, Arthritis research & therapy 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho PP, Lee LY, Zhao X, Tomooka BH, Paniagua RT, Sharpe O, BenBarak MJ, Chandra PE, Hueber W, Steinman L, and Robinson WH (2010) Autoimmunity against fibrinogen mediates inflammatory arthritis in mice, Journal of immunology 184, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitashny M, and Shoenfeld Y (2005) B cell depletion in autoimmune rheumatic diseases, Autoimmunity reviews 4, 436–441. [DOI] [PubMed] [Google Scholar]
- 69.Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, Fitzgerald O, Bresnihan B, Caporali R, Montecucco C, Uguccioni M, and Pitzalis C (2005) Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis, European journal of immunology 35, 1347–1359. [DOI] [PubMed] [Google Scholar]
- 70.Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, Buckley CD, Montecucco C, and Pitzalis C (2007) CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis, The American journal of pathology 171, 1549–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, and Weyand CM (2001) Lymphoid neogenesis in rheumatoid synovitis, Journal of immunology 167, 1072–1080. [DOI] [PubMed] [Google Scholar]
- 72.Weyand CM, Braun A, Takemura S, and Goronzy JJ (2001) Lymphoid microstructures in rheumatoid synovitis, Current directions in autoimmunity 3, 168–187. [DOI] [PubMed] [Google Scholar]
- 73.Roll P, Dorner T, and Tony HP (2008) Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment, Arthritis and rheumatism 58, 1566–1575. [DOI] [PubMed] [Google Scholar]
- 74.Roll P, Palanichamy A, Kneitz C, Dorner T, and Tony HP (2006) Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis, Arthritis and rheumatism 54, 2377–2386. [DOI] [PubMed] [Google Scholar]
- 75.Pelzek AJ, Gronwall C, Rosenthal P, Greenberg JD, McGeachy M, Moreland L, Rigby WFC, and Silverman GJ (2017) Persistence of Disease-Associated Anti-Citrullinated Protein Antibody-Expressing Memory B Cells in Rheumatoid Arthritis in Clinical Remission, Arthritis & rheumatology 69, 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manzo A, Bombardieri M, Humby F, and Pitzalis C (2010) Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling, Immunological reviews 233, 267–285. [DOI] [PubMed] [Google Scholar]
- 77.Willemze A, Shi J, Mulder M, Stoeken-Rijsbergen G, Drijfhout JW, Huizinga TW, Trouw LA, and Toes RE (2013) The concentration of anticitrullinated protein antibodies in serum and synovial fluid in relation to total immunoglobulin concentrations, Annals of the rheumatic diseases 72, 1059–1063. [DOI] [PubMed] [Google Scholar]
- 78.Sanmarti R, Graell E, Perez ML, Ercilla G, Vinas O, Gomez-Puerta JA, Gratacos J, Balsa A, Gomara MJ, Larrosa M, Canete JD, and Haro I (2009) Diagnostic and prognostic value of antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis, Arthritis research & therapy 11, R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, and Paulson JC (2012) Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169, PloS one 7, e39039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, and Paulson JC (2010) In vivo targeting of B-cell lymphoma with glycan ligands of CD22, Blood 115, 4778–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.