Abstract
While the fundamental mechanism by which cardiac cell therapy mitigates ventricular dysfunction in the post ischemic heart remains poorly defined, donor cell paracrine signaling is presumed to be a chief contributor to the afforded benefits. Of the many bioactive molecules secreted by transplanted cells, extracellular vesicles (EVs) and their proteinaceous, nucleic acid, and lipid rich contents, comprise a heterogeneous assortment of prospective cardiotrophic factors-whose involvement in the activation of endogenous cardiac repair mechanism(s), including reducing fibrosis and promoting angiogenesis, have yet to be fully explained. In the current study we aimed to interrogate potential mechanisms by which cardiac mesenchymal stromal cell (CMC)-derived EVs contribute to the CMC pro-angiogenic paracrine signaling capacity in vitro. Vesicular transmission and biological activity of human CMC-derived EVs was evaluated in in vitro assays for human umbilical vein endothelial cell (HUVEC) function, including EV uptake, cell survival, migration, tube formation, and intracellular pathway activation. HUVECs incubated with EVs exhibited augmented cell migration, tube formation, and survival under peroxide exposure; findings which paralleled enhanced activation of the archetypal pro-survival/pro-angiogenic pathways, STAT3 and PI3K-AKT. Cytokine array analyses revealed preferential enrichment of a subset of prototypical angiogenic factors, Ang-1 and Ang-2, in CMC EVs. Interestingly, pharmacologic inhibition of Tie2 in HUVECs, the cognate receptors of angiopoietins, efficiently attenuated CMC-EV-induced HUVEC migration. Further, in additional assays a Tie2 kinase inhibitor exhibited specificity to inhibit Ang-1-, but not Ang-2-, induced HUVEC migration. Overall, these findings suggest that the pro-angiogenic activities of CMC EVs are principally mediated by Ang-1-Tie2 signaling.
INTRODUCTION
The vascular system is essential for myocardial function. A defect in the function of the circulatory system causes decreased or absent myocardial blood flow, which leads to ischemic injury and death or dysfunction of the affected regions1. The most severe form of ischemic injury (myocardial infarction) recruits immune cells to remove the dead cells, activates the formation of granulation tissue with hyperproliferative, extracellular matrix-depositing stromal cells, and induces pro-angiogenic signaling to restore homeostatic tissue blood flow1–6. Vasculogenesis is a multistep process involving vascular basal membrane remodeling, endothelial cell proliferation, migration, sprouting, and branching, and tube formation3, 7–10. Newly-formed vessels undergo further maturation that makes them susceptible to mechanical stimulation by shear stress. Failure of timely or proper revascularization can lead to incomplete tissue recovery, non-resolving inflammation, adverse remodeling, and progressive failure of the pumping function of the heart1, 4, 7, 9–11.
Cell therapy emerged over two decades ago as a strategy to improve organ recovery after ischemic injury, including hindlimb ischemia, stroke, and myocardial infarction. Numerous cell types have been tested in animal models and clinical trials12; some of them show promise as a new treatment for myocardial repair. The initial promise to replace lost myocytes has not been delivered, but cell therapy has proven to be safe and to provide a modest improvement in cardiac function via anti-inflammatory effects, reduction of fibrosis, and enhanced angiogenesis. We have recently discovered a novel population of cardiac mesenchymal cells (CMCs) that have cardiac reparative properties and offer potential advantages for cardiac repair compared with other cell types13, 14. The mechanism of action of CMCs, like that of other stem/progenitor cells, is unclear.
Although cardiac function improves after cell therapy, long-term survival of the injected cells is negligible15, 16, implicating paracrine signaling as the mechanism underlying the effects of cell therapy17–20. It has been proposed that transplanted cells produce numerous paracrine factors that activate endogenous mechanisms of repair, including cytokines, bioactive lipids, and extracellular vesicles (EVs)17–26. EVs are heterogenous structures that include exosomes secreted from the endosomal compartment and from multivesicular bodies into the extracellular space, and membrane-derived vesicles that are shed from the cell membrane21, 23, 25, 27–29. EVs are small (80–250 nm), cup-shaped vesicles, which are surrounded by a lipid bilayer and contain bioactive lipids, membrane receptors, cytokines, transcription factors, but also various subtypes of RNA, i.e., mRNA, microRNA, lncRNA, and Y-RNA21, 23, 25, 27–29. Due to their complex composition, EVs can regulate the function of target cells via multiple mechanisms, including transfer of cell surface receptors from donor cells to the targeted cells, activation of targeted cells receptors through protein and lipid ligands in EVs, and horizontal transfer of RNAs and transcriptional factors21, 23, 25, 28, 29. Recent studies have demonstrated that EVs can reproduce the reparative effects of cell therapy23–25, 28, 30, 31.
EVs have strong pro-angiogenic effects both in vitro and in vivo21, 28, 32–35. Little is known regarding the mechanisms of the pro-angiogenic effects of EVs, and also regarding the mechanisms regulating packaging of EVs in donor cells. Here, we demonstrate that human MSCs secrete EVs with strong pro-angiogenic effects on HUVECs in vitro. We found that the pro-angiogenic effects of CMC-EVs were inhibited by a specific Tie-2 receptor receptor antagonist, but not by antagonists of other pro-angiogenic receptors such as VEGFR, CXCR4, and FGF2R. Moreover, we found that Ang1 and Ang2 are selectively packaged in CMC-EVs in contrast to FGF2 and VEGF. This suggests that EV protein packaging is not random, but the mechanism is not fully explored. Our data suggest that the pro-angiogenic effects of CMC-EVs are independent of horizontal transfer of RNAs and rely on EV packaged pro-angiogenic cytokines.
MATERIALS AND METHODS
Isolation of human cardiac mesenchymal cells (CMCs).
CMCs were isolated and cultured as described previously36. Briefly, human CMCs were isolated from discarded atrial appendage specimens collected from patients during routine coronary artery bypass surgery at Jewish Hospital (University of Louisville, Louisville, KY). De-identified right atrial appendage (RAA) specimens were acquired via written consent agreement according to the approved protocol by the Institutional Review Boards on human subject research (IRB number: 03.052J) at the University of Louisville. Freshly obtained RAA was cleaned off the connective tissue in ice-cold PBS and chopped into small pieces (< 1 mm3) using fine scissors. Minced tissue was enzymatically dissociated Collagenase II (5 μg/mL) in Ham’s F-12 media at 37°C with a slight agitation. After 2 h of incubation, undigested tissue pieces were removed by passing cell suspension through 30 μm sterile cell strainer and single cell suspension was washed with DMEM/F-12 medium. Cells were then plated in a 6-well plate in F-12 medium supplemented with 10% FBS (Seradigm AB premium grade), 20 ng/mL bFGF (Peprotech), 5 mU/mL EPO (Peprotech), 0.2 mM L-glutamine (Gibco), and 100 U/mL penicillin/streptomycin (Gibco). After 2 d, non-adherent cells were removed and adherent cells were expanded in a growth medium. CMC were passaged when reached 70–80% confluence. All experiments in the current study were performed on cells at passages 4–8.
Flow cytometry.
Cells were detached from culture dishes with 0.25% trypsin-EDTA and washed in PBS. After incubation for 30 min at 4°C with mAbs, cells were washed, suspended in 0.5 mL of PBS, and analyzed by flow cytometry on an LSRII system. The mAbs used for characterization of cell phenotype are summarized in Table 1.
Table 1.
Flow cytometry mAB.
| Antibody | Clone | Source |
|---|---|---|
| CD90 | 5E10 | eBioscience |
| CD34 | H11 | eBioscience |
| CD29 | TS2/16 | eBioscience |
| CD45 | 2D1 | eBioscience |
| CD31 | WM-59 | eBioscience |
| CD105 | SN6 | eBioscience |
| CD73 | AD2 | eBioscience |
Human Umbilical Vein Endothelial Cell (HUVEC) cultures.
HUVECs (ATCC) were maintained in EBM-2 medium with EGM-2™ BulletKit™ supplement (Clonetics Biowhittaker, Walkersville, MD). For all experiments in the current study we used HUVECs at passages 4–8.
Isolation of EVs.
CMC-derived EVs were isolated as previously described37–39. Briefly, CMCs were cultured in growth medium till 80% confluent; subsequently, the cell monolayer was washed in PBS and suspended in Ham’s F-12 media supplemented with 0.5% BSA. After 24 h, conditioned media was collected and centrifuged (3,000 × g, 15 min, 4°C) to pellet dead cells and cellular debris. The EV-containing supernatant was precipitated with one-third volume of polyethylene glycol (PEG) buffer (33.4% w/v PEG 4000, 50 mM HEPES [pH 7.4], 1 mM NaCl) overnight at 4°C, followed by centrifugation at 3000 × g for 30 min. The EV pellet was suspended in PBS. In some experiments, EVs were also isolated by differential centrifugation. Briefly, after centrifugation at 3,000 × g for 15 min, supernatants were centrifuged at 100,000 × g (Beckman Coulter Optima L-90K ultracentrifuge, Fullerton, CA) for 2 h at 4°C, washed in PBS and subjected to a second ultracentrifugation in the same conditions. The EV pellet was suspended in PBS. Protein concentration in EVs was measured with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific).
EV Size analysis.
EVs suspended in PBS were subjected to dynamic light-scattering measurements performed in a Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, UK). The analysis yields the z-average of the sample, which is the intensity weighted mean diameter of the bulk population, and the polydispersity index, which is a measure of the width of the size distribution. The measuring range of the Zetasizer is from 0.1 nm to 10 µm.
EV labeling with PKH26.
EVs were labeled with PKH26 according to the manufacturer’s protocol, with some modifications. Briefly, EV pellets were suspended in 1 mL Diluent C containing PKH26 (0.4%) and incubated for 10 min at room temperature. The labeling reaction was stopped by adding an equal volume of 1% BSA in PBS. Labeled EVs were then precipitated overnight in PEG buffer and centrifuged, as described in the previous section, and suspended in PBS.
EVs uptake by HUVECs.
HUVECs (5 × 104 per well) were seeded on a 12-well plate. After 24 h, cells were stimulated with 50 μg/mL of PKH26-labeled EVs for up to 24 h. At indicated time points HUVECs were detached with trypsin, washed in PBS, and analyzed with an LSRII flow cytometer (BD Bioscience). In the second set of experiments, HUVECs (5 ×103) were seeded on the chamber slide and stimulated with PKH26-labeled EVs as described above. Subsequently, cells were fixed with 4% paraformaldehyde, washed with PBS, and stained with DAPI and tubulin for 1 h at room temperature. Stained cells were washed in PBS, mounted with Vectashield, and imaged with confocal microscopy.
Cell proliferation assay.
Cell viability was evaluated using PrestoBlue® assay (Thermo Fisher Scientific). Briefly, HUVECs (2 × 104 cells in 100 µL) were seeded in a 96-well plate for 24 h and then stimulated with different concentrations of CMC-derived EVs or vehicle at 37°C. After 72 h, cell growth was tracked by measuring the formation of fluorescent product following the addition of a metabolically reducible substrate (PrestoBlue™ reagent, Invitrogen) according to the manufacturer’s instructions.
Transwell migration assay.
Chemotaxis assays were performed as described40, 41. Briefly, HUVECs (5 × 104 in 100 μL of EBM media supplemented with 0.5% BSA) were added to the Transwell insert of 24-well plates (6.5-mm diameter, 8 μm pores; Corning). EVs (in 650 uL of EGM 0.5% BSA media) were added to the bottom chamber of the plate. Cells were allowed to migrate for 18 h at 37°C in a humid atmosphere with 5% CO2. Non-migrated cells from the upper side of the transwell inserts were removed with cotton buds. Transmigrated cells were fixed with 4% paraformaldehyde (PFA), stained with 0.5% crystal violet dye, and enumerated using bright-field microscopic images at 40x magnification.
Endothelial cell tube formation.
HUVECs were serum-starved for 6 hours and seeded in a 96-well plate pre-coated with growth factor-reduced Matrigel (75 µl/well, BD Biosciences) at a density of 1.6 × 104 cells/well with or without CMC EVs. Eight to ten hours later, 1 µM Calcein AM (ThermoFisher Scientific) was added to each well and images of capillary-like tube structures were acquired by fluorescence microscope. The number of intact tubes per field was counted for each experimental condition.
Proteome Profiler™ Human Angiogenesis Array.
The expression of angiogenesis-related proteins in CMC-derived EVs was analyzed by a Proteome Profiler™ Human Angiogenesis Array Kit (R&D Systems), according to the manufacturer’s instructions. Briefly, CMCs and CMC EVs were lysed in RIPPA buffer containing a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific) for 30 min on ice followed by centrifugation at 14,000 × g for 10 min, 4°C. Protein concentration was measured with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Protein extracts (300 μg) were incubated with nitrocellulose membranes spotted with antibodies against pro-angiogenic cytokines and the intensity of the signal was visualized with a chemiluminescence substrate by acquiring images with a ChemiDoc Gel Imaging System (Thermo Fisher Scientific).
ELISA for SDF-1, bFGF, VEGF, angiopoietin-1, and angiopoietin-2.
CMCs and CMC-derived EVs protein extracts were evaluated for the expression of the pro-angiogenic cytokines SDF-1, bFGF, VEGF, angiopoietin-1, and angiopoietin-2 with ELISA kits according the manufacturer’s recommendations.
Western blot analysis.
CMC and CMC EV protein extracts were subjected to 4–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions followed by transfer onto PVDF membranes (Thermo Fisher Scientific). Membranes were blocked with 5% BSA in TBS-T and subsequently incubated overnight at 4°C with the primary antibodies specified in Table 2. Membranes were then probed with HRP-conjugated secondary anti-mouse (1 to 10,000, Cell Signaling) or anti-rabbit antibody (1:1000, Cell Signaling) and visualized with enhanced chemiluminescence substrate (Cell Signaling). Images were acquired with a ChemiDoc Gel Imaging System (Thermo Fisher Scientific). For signaling studies, HUVECs were cultured for 24 h in serum- and growth factor-free media to render them quiescent and then were incubated with EVs for 2, 5, 10, 20, and 30 min at 37°C before lysing for 10 min on ice in RIPPA buffer containing a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Subsequently, the extracted proteins were separated on 4–12% SDS-PAGE; the fractionated proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell), probed with primary antibodies (see Table 2) followed by corresponding secondary HRP-conjugated antibodies, and visualized with enhanced chemiluminescence substrate (Cell Signaling). Images were acquired with a ChemiDoc Gel Imaging System (Thermo Fisher Scientific).
Table 2.
Western blot Ab.
| Antibody | Cat # | Source |
|---|---|---|
| p42/44 MAPK | #9106 | Cell Signalling |
| 42/44 MAPK | #9102 | Cell Signalling |
| pAKT (S473) | #9272 | Cell Signalling |
| AKT | #9138 | Cell Signalling |
| pSTAT3 (Y705) | #12640 | Cell Signalling |
| STAT3 | #9138 | Cell Signalling |
| pSTAT6 (Y641) | ab54461 | Abcam |
| STAT6 | #9362 | Cell Signalling |
| p38 MAPK (T180/Y182) | #9211 | Cell Signalling |
| 38 MAPK | #8690 | Cell Signalling |
Statistical analysis.
Results are shown as mean ± SEM. Statistical analyses (GraphPad 7.0d) were performed with Student’s t-tests or one-way ANOVA followed by Student’s t-tests with Bonferroni correction, as appropriate. Differences were considered statistically significant if P<0.05.
RESULTS
CMC isolation and characterization.
Cells isolated from enzymatically dissociated human RAA samples adhere to the plastic dishes and have a spindle-like shape morphology typical of fibroblasts (Figure 1A). The cell surface marker characteristics assessed by flow cytometry are typical of mesenchymal cells, i.e., high expression of CD105, CD29, CD73, heterogeneous levels of CD90, and absence of endothelial CD31 and hematopoietic CD45 markers (Figure 1B and C). Together, these data indicate that the cells used in this study represent a cardiac population of mesenchymal cells.
Figure 1. CMC morphology and phenotype.
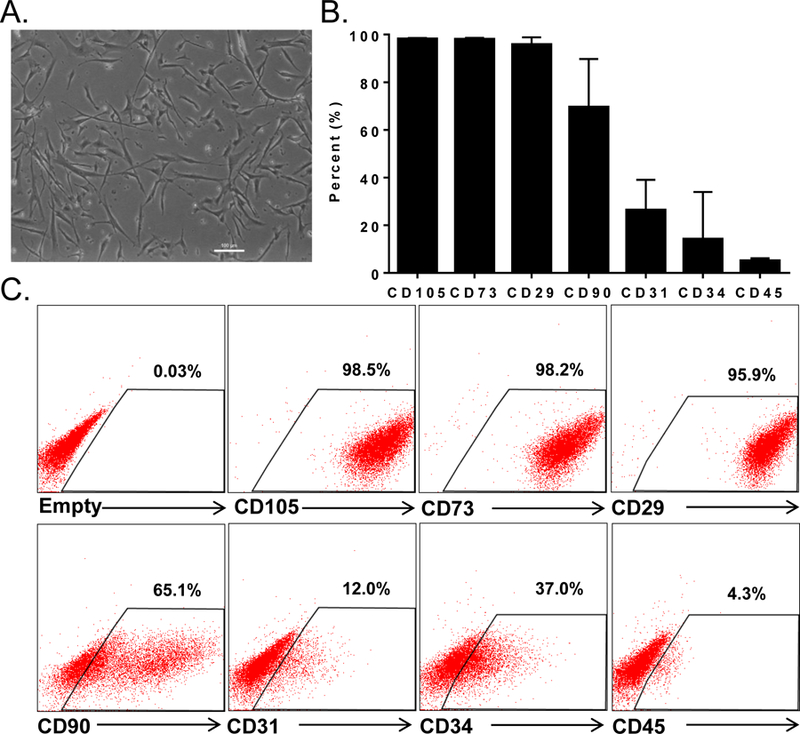
Brightfield microscopy image of human CMCs isolated from RAA; scale bar 100 μm (A); cell surface markers expression on CMCs (B); representative flow cytometry dot plots (C). Flow cytometric analysis was performed on CMCs isolated from three independent patents samples. Data are mean ± SEM.
CMCs secrete EVs.
Numerous studies have demonstrated that EVs, along with soluble factors, are a component of the cellular secretome with robust signalling functions22, 26, 27, 42. It has been demonstrated that virtually all mammalian cell types are capable of EV production, including embryonic stem cells, iPSCs, hematopoietic stem cells, various subtypes of immune cells, endothelial cells, and mesenchymal cells of various origin. Here, to show the capacity of EV secretion by CMCs, we have used the PEG buffer precipitation method on conditioned media incubated with CMCs for 24 h. The light scattering method confirmed heterogeneity in CMC-EV size, with two major particle populations, a smaller one peaking ~40 nm and a larger one at ~180 nm (Figure 2A). This suggests that our preparation of EVs contains a mixed population of exosomes and membrane-derived vesicles. To further confirm that the CMC conditioned media preparation contains EVs, we performed a series of Western blots. We found that CMC and CMC-derived EV protein extracts express CD63, flotillin, and HSP70, but organelle-characteristic markers such as calriteculin (endoplasmic reticulum), prohibitin (mitochondria), GM130 (Golgi apparatus), lamin B (nuclear membrane), PMP70 (peroxisome), and structural protein β-actin were present only in CMCs, not in EVs (Figure 2B). These data indicate that CMC-derived EVs isolated with the PEG buffer precipitation method contain a mixed population of exosomes and membrane-derived vesicles and are not contaminated with cell damage products such as organelles and dead cell membrane fragments. Next, we tested the signalling functions of EVs on endothelial cells.
Figure 2. Isolation and characterization of hCMC-derived EVs.
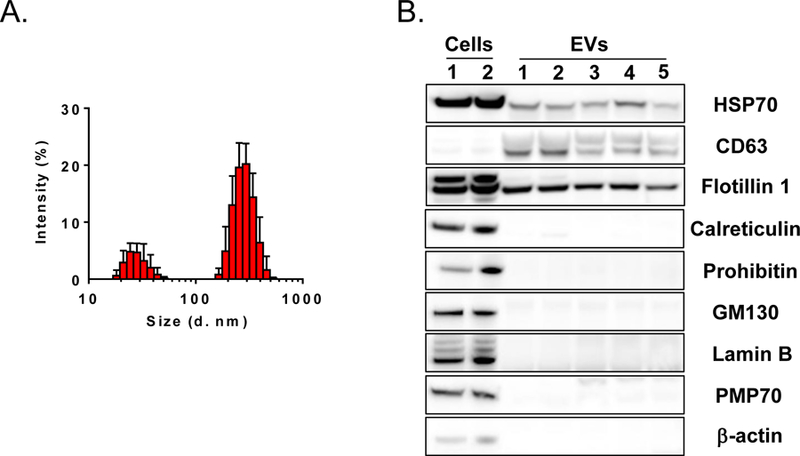
CMC-EV particle size distribution analysis with zeta-sizer (A); Western blot analysis of EV markers (B).
Endothelial cells bind and internalize EVs.
EVs can carry membrane receptors, growth factors, and various types of nucleic acids including mRNA, microRNA, lncRNA, and Y-RNA. Thus, EVs can affect targeted cells via activation of surface receptors, transfer of surface receptors, or transfer of nucleic acids27. We tested whether the composition of CMC-derived EVs allows their interaction with endothelial cells. HUVECs were stimulated with EVs labeled with PKH26 for 0.5–24 h and analyzed by flow cytometry. The percentage of PKF26 positive HUVECs increased rapidly to 60% and continued to increase to reach a plateau at 2 h after incubation (~92%) (Figures 3A and B). The same set of data was evaluated for mean fluorescent intensity (MFI) to determine the amount of EV uptake by HUVECs. MFI reached statistical significance at the 2-h time point and continued to grow to reach a maximum at 24 h (Figure 3 C). This shows that HUVECs have the ability to bind CMC-derived EVs in a time-dependent manner. To further confirm that EVs can be taken up by endothelial cells, we performed confocal microscopy. EVs were rapidly co-localized with the endothelial cell membrane (1–3 h), and subsequently the PHK26 signal was detected in the cytoplasm (6 h). At the final, 24 h time point, EV signals were detected in the perinuclear compartment (Figure 3D). These data suggest that EVs can activate and modulate endothelial cell function. To test this possibility, we performed a series of studies to elucidate the intracellular signaling pathways activated by EVs.
Figure 3. HUVECs bind and internalize CMC-derived EVs.
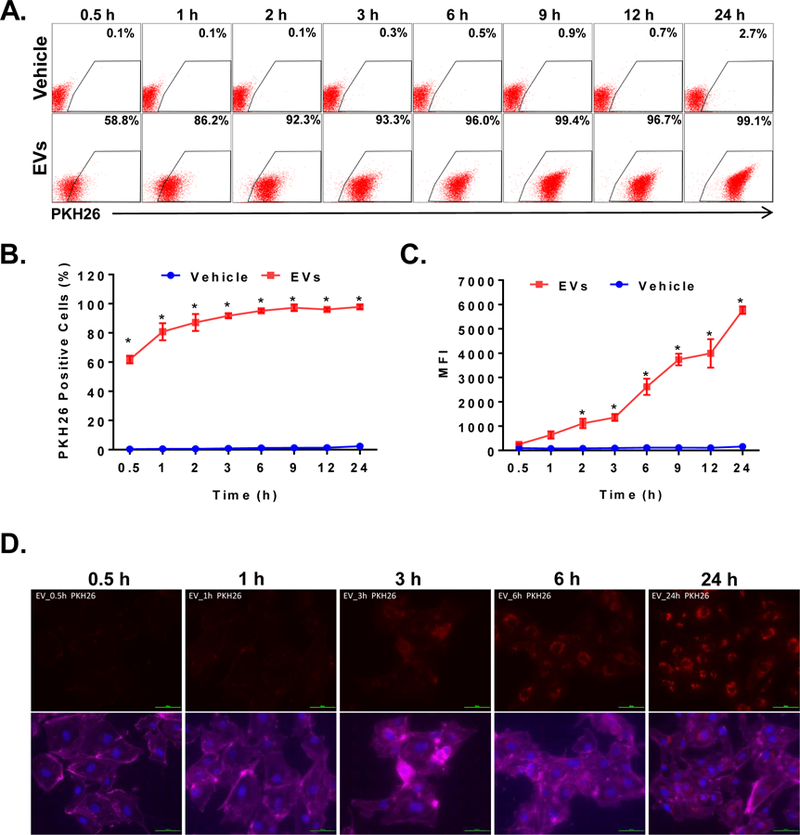
HUVECs were stimulated with PKH26 labeled EVs (50 μg/mL) or vehicle for up to 24 h. The EV-derived fluorescence was evaluated with flow cytometry and confocal microscopy. Representative flow cytometry dot plots of HUVECs stimulated with PKH26-labeled EVs of vehicle (A). Numerical representation of EV-uptake by HUVECs as a percentage of positive cells (B) or MFI measured in HUVECs (C). Representative fluorescent microscopy images of HUVECs stimulated with PKH26 labeled EVs (D). Data are mean ± SEM, n=4. *P<0.5 vs vehicle.
EVs activate intracellular signaling in endothelial cells.
Various signaling pathways including PI3K-AKT, MAPK, and JAK/STAT have been implicated in the activation of the pro-angiogenic function of endothelial cells. Accordingly, we tested CMC-EV-induced intracellular signaling in HUVECs. Serum and growth factor starved HUVECs were stimulated in a time- and dose-dependent manner. We found that serum-starved HUVECs stimulated with EVs exhibited a dose- and time-dependent activation of the MAPK pathway by phosphorylation of the 38, 42, and 44 proteins. We also found that EVs induce phosphorylation of STAT3 but not STAT6. EVs did not have any effect on the PI3K-AKT pathway as AKT was not phosphorylated (Figure 4). These pathways are involved in cell migration, proliferation, and survival, and in endothelial cell sprouting. Next, we investigated the effects of EVs on endothelial cell function.
Figure 4. CMC-EVs activate intracellular pathways in HUVECs.
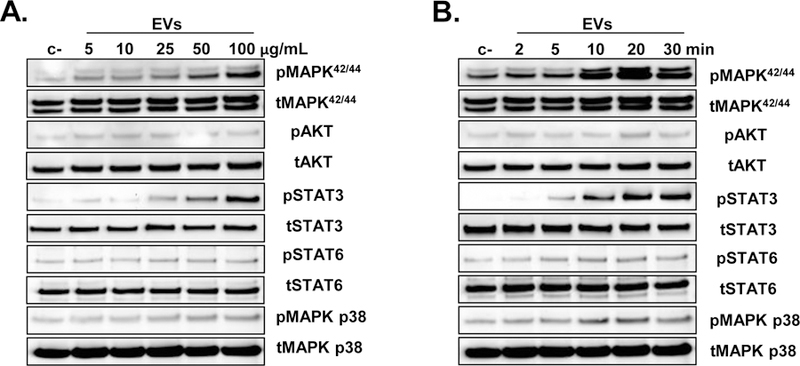
HUVECs were cultured in serum and growth factors free media to silence the intracellular signalling pathways. After 24 h cells were stimulated with escalating does of EVs for 10 min (A) or with 50 μg/mL of EVs for up to 30 min (B), and the cell lysates were evaluated for activation of MAPK, PI3K-AKT, and STAT pathways with Western blot. Representative Western blot images from three independent experiments are shown.
EVs stimulate chemotaxis, tube formation, and survival, but not proliferation of endothelial cells.
Angiogenesis is a multistep process that requires cell migration, proliferation, survival, and tube formation. We evaluated whether EVs stimulate in vitro angiogenesis. The migration assay with Boyden chambers showed that endothelial cells have no migratory activity toward basal media; however, as little as 1.25 µg/mL of EVs induced migration of HUVECs with a dose-dependent increase up to 50 µg/mL when it reached close to 90% of the maximum migratory response observed with full growth media containing FBS, which served as a positive control (Figure 5A). Similarly, EVs induced a dose-dependent increase in matrigel tube formation which plateaued at 10 µg/mL and reached close to 80% of the maximal tube formation induced by full growth media (used as a positive control) (Figure 5B). We also observed that EVs exert cytoprotective effects on endothelial cells toward increasing doses of H2O2 (Figure 5C) The EVs did show any effect on endothelial cell proliferation (not shown). These data suggest that EVs contain pro-angiogenic factors. Because EVs stimulated proangiogenic activity within hours after stimulation, it can be speculated that most likely EVs activate HUVECs receptors to promote angiogenesis.
Figure 5. CMC-EVs induce angiogenesis in vitro.
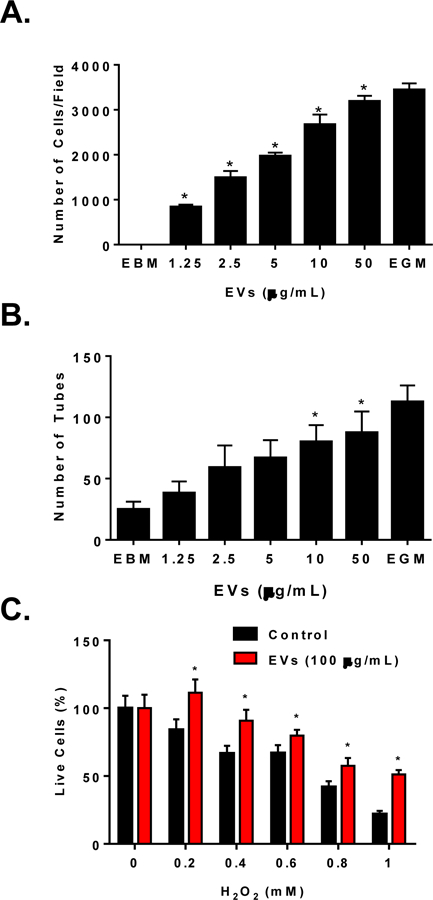
Dose dependent increase of HUVECs migration (A) and tube formation (B) in response to CMC-EVs; CMC-EVs (100 μg/mL) improve HUVECs survival in response to increasing doses of H2O2 challenge (C); Data are mean ± SEM, n=3. P<0.5 vs vehicle control or EBM control.
Ang-1 and Ang-2 in EVs, but not SDF-1, FGF-2, and VEGF, are responsible for the proangiogenic activity of CMC-EVs.
To test which pro-angiogenic cytokines can regulate the pro-angiogenic effects of CMC-EVs, we used a HUVEC migration assay and specific pro-angiogenic receptor inhibitors. We found that inhibition of Tie-2, which serves as a receptor for Ang-1 and Ang-2, inhibited HUVECs migration to CMC-EVs. HUVECs showed significantly improved migration to Ang-1, but not to Ang-2, which was abrogated when cells were pretreated with a Tie-2 kinase inhibitor (Figure 6). To test toxicity, we performed a migration assay on HUVECs to endothelial growth media (EGM). We found that the Tie-2 kinase inhibitor did not reduce HUVEC migration (Figure 6), suggesting that the Tie-2 kinase inhibitor specifically inhibits Ang-1-induced migration. We also tested migration of HUVECs to EVs in the presence of antagonists of CXCR4 (AMD3100), of the VEGF receptor (Vetalanib), and of the FGF receptor (FIIN1), and found that inhibition of these receptors had no effect on HUVEC migration to CMC-EVs (Figure 6). Taken together, these data suggest that Ang-1 is the primary pro-angiogenic cytokine in CMC-EVs that is responsible for migration of endothelial cells.
Figure 6. Pro-angiogenic effect of CMC-EV is mediated via Ang-1-Tie2 signalling.
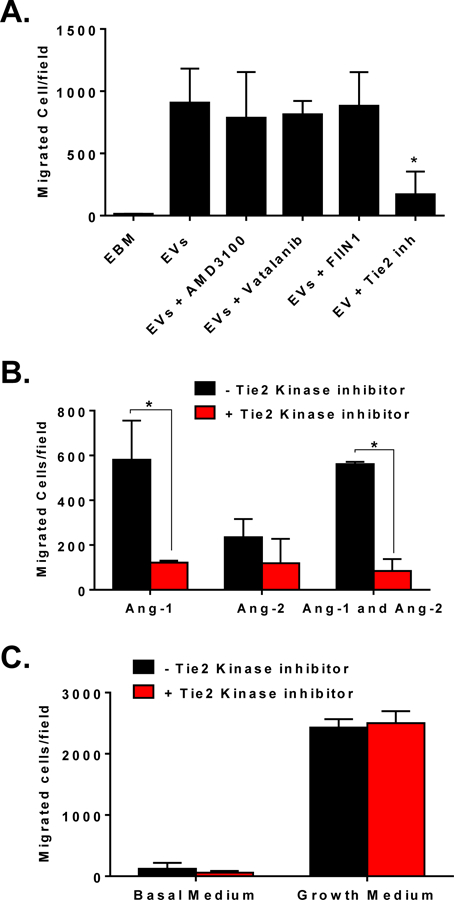
CMC-EVs induced HUVEC migration is inhibited with Tie2 kinase inhibitor, but not with AMD3100, Vetalanib, or FIIN1 (A); Tie2 kinase inhibitor specifically inhibit Ang-1 but not Ang-2 induced migration of HUVECs (B); Tie2 kinase inhibitor is ineffective in inhibition of HUVECs migration to endothelial growth medium (C). Data are mean ± SEM, n=3–4. *P<0.5 vs EVs (A) or vehicle control (B).
EVs are enriched in Ang1 and Ang2 but not in VEGF or FGF2.
To further characterize the EVs and identify proangiogenic protein content, CMCs, conditioned media, and EVs were subjected to a cytokine array. We detected numerous pro- and anti-angiogenic factors in CMCs, conditioned media, and EVs isolated from CMC-conditioned media. We found that Ang1 and Ang2 were highly upregulated in EVs compared with cell lysates or conditioned media. Other major factors regulating angiogenesis were unchanged. Still others, like FGF-1, FGF-2, FGF-7, IL-8, and thrombospondin-1, were reduced in EVs compared with cells lysates (Supplementary Figure 1). The enrichment of Ang-1 and Ang-2 was further confirmed by ELISA: Ang-1 and Ang-2 were ~65 and ~115-fold higher, respectively, in EVs than in CMCs (Figure 7). Moreover, compared with CMC, FGF2 and VEGF protein content was significantly reduced in EV preparations. These data suggest that EV packaging is not arbitrary, but rather that the EV content is tightly regulated via the mechanism that is not fully explored.
Figure 7. CMC-EVs are enriched in Ang-1 and Ang-2 but not in FGF-2 and VEGF.
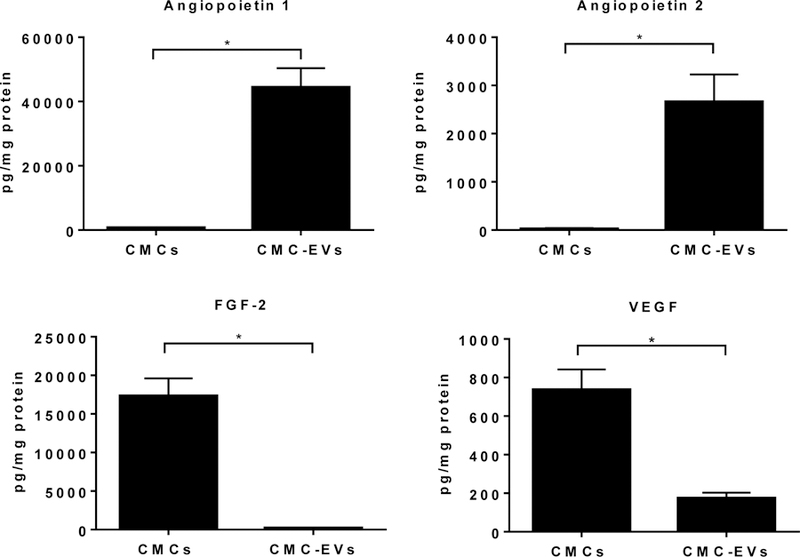
CMC and CMC-EVs protein extracts where evaluated for Ang-1, Ang-2, FGF-2, and VEGF expression by ELISA. Data are mean ± SEM, n=5. *P<0.5 vs CMCs.
DISCUSSION
The salient findings of this study are that human CMCs produce EVs that bind to endothelial cells and activate the pro-survival/pro-angiogenic pathways, STAT3 and PI3K-AKT. CMC-EVs have in vitro pro-angiogenic actions on HUVECs (migration, tube formation, and survival). Inhibition of Tie-2 kinase in endothelial cells blocks the proangiogenic activity of CMC-EVs, which is consistent with the observation that the Tie-2 ligand Ang-1 is highly enriched in CMC-EVs. This is the first demonstration that the CMC-EV pro-angiogenic effects rely on Ang-1 which binds the Tie-2 receptor on endothelial cells. Here, we have also reported the novel observation that the CMC-EV protein content is dependent on the specific packaging of proteins (Ang1, Ang2, VEGF) that are associated with the conventional ER-Golgi protein secretory pathways, but does not include proteins (FGF-2) that follow unconventional Golgi-independent secretory pathways. The present findings may help to understand the mechanism of the reparative actions of CMCs, but also identify specific components of EVs that are responsible for the reparative actions of EVs.
Cell therapy has evolved as a strategy to activate tissue reparative processes after injury including reparative angiogenesis19, 31, 43–45. To date, various cell types have been demonstrated to induce angiogenesis in vitro and in vivo19, 31, 43–46. These include bone marrow hematopoietic stem/progenitor cells47, bone marrow mesenchymal stromal cells (BM MSCs)47, 48, adipose derived mesenchymal stromal cells (AD-MSCs)49, endothelial progenitor cells (EPCs)50, 51, and cardiac progenitor cells (CPCs)52. Limited integration of these cells into vascular structures suggests paracrine actions as a potential mechanism of improved angiogenesis. Numerous studies have demonstrated that pro-angiogenic cytokines are secreted by these cells17, 19, 47, 53–55. Recently, it has been recognized that pro-angiogenic effects can be also mediated via secretion of EVs23, 26, 30, 33–35, 56. To date, it has been shown that several cell types produce EVs with pro-angiogenic potential, including BM CD34+ cells33–35, EPCs33–35, BM MSCs57, and CPCs58, 59. The complexity of the EV cargo suggests several mechanisms whereby EVs can induce reparative angiogenesis, such as activation of endothelial cells though transfer of cytokines or various micro-RNAs23–26, 35.
In the current study, we show that CMCs isolated from human atrial appendages have mesenchymal-like morphology and phenotype (Figure 1), similar to BM MSCs, and similarly to BM MSCs, CMCs secrete EVs that are a mixed population of exosomes and membrane-derived microvesicles (Figure 2). Because both of these vesicle populations are part of the cell secretome, we decided to use in our studies a mixture of both, without further purification with sucrose gradient to obtain exosomes. Particle size analysis showed that the CMC-EV preparation has two peaks that could potentially belong to smaller exosomes and larger membrane-derived vesicles (Figure 2); however, without further experiments, this conclusion is rather speculative. Discrimination between exosomes and membrane-derived vesicles based on their size is rather arbitrary, as systematic studies have not been performed to determine the size range for each of these vesicle populations. Moreover, the size of EVs also depends of the cell type and the method used to determine EV diameter. Currently, there are two methods to evaluate the size of EVs, light scattering and electron microscopy24, 34, 35, 56. The latter has been shown to underestimate EV size due to chemical processing related to fixation of EVs. In the future studies it would be important to determine which fraction of EVs is responsible for pro-angiogenic activities in HUVECs. We further characterized CMC-EVs by demonstrating that they contain HSP70, CD63, and Flotillin-1 proteins, which are known markers of exosomes and membrane-derived vesicles. In contrast, we did not detect cell damage markers such as calreticulin, prohibin, GM130, lamin B, PMP70, and beta-actin (Figure 2). Therefore, our preparations were not contaminated by cell fragments or apoptotic bodies.
EVs have been demonstrated to be a novel mechanism of the cell-cell communication and to be composed of lipids, proteins, and nucleic acid23, 26, 27, 56. These can activate cells receptors or be internalized and release their content in the targeted cells to alter their gene function through horizontal transfer of nucleic acids such as micro-RNAs or lncRNAs23, 26, 27, 56. We found that HUVECs bind and internalize CMC-EVs fluorescently labeled with PKH26 in a time-dependent manner (Figure 3). One of the potential limitations of this assay is that PKH26 is a lipophilic dye. Therefore, it tracks only lipid binding and uptake by the cells; this method cannot definitively determine whether the protein and RNA content of EVs is internalized by HUVECs. Further studies using protein and nucleic acid tagging may be able to address these issues in more detail.
Because of the complexity of EV composition, several potential mechanisms may account for their pro-angiogenic effects on endothelial cells. In this study, we examined the direct effects of EVs that change endothelial cell signaling and function within minutes to hours (migration, tube formation, proliferation, survival). These effects can be induced by cytokines packaged in EVs. One could speculate that horizontal transfer of nucleic acids may modulate gene expression or translation and alter cell functions related to angiogenesis. This mechanism would alter cell function several hours or days after treatment with EVs, because it would require changes in gene expression in the target cells. Since the observed effects of EVs on endothelial cells are more rapid, we speculated that they are due to proteins that activate receptors on endothelial cells. Indeed, we found that Ang-1 and Ang-1, but not FGF-2 and VEGF, are enriched in EVs and activate endothelial cell migration, tube formation, and survival via the Tie2 receptor. We do not exclude the possibility that CMC EVs also may contain bioactive lipids with pro-angiogenic activities, e.g. spingosine-1 phosphate, which perhaps need to be evaluated in the future studies. To exclude contributions of nucleic acids, further careful studies will be required to identify the nucleic acid content in CMC-EVs and investigate their effects on endothelial cells.
Little is known regarding the mechanisms that regulate packaging and secretion of EVs27, 56. It could be speculated that the packaging is related to protein secretory pathways. The conventional process of protein synthesis, segregation, and secretion occurs in the endoplasmic reticulum (ER) that is followed by transport to the Golgi apparatus where the secretory proteins undergo post-translational modifications and are further incorporated into secretory vesicles including multivesicular bodies. These are then transported to the cell membrane and secreted into the extracellular space60, 61. A second, unconventional pathway bypasses the Golgi apparatus and releases proteins directly into the cytoplasm from where the proteins are transported to the cell membrane and released through lipid channels in the cell membrane60, 61. In this study we found that Ang-1 and Ang-2, which follow the conventional secretory pathway, are packaged and enriched in EVs in multivesicular bodies. However, FGF-2, which bypasses the Golgi apparatus and is secreted though the unconventional secretory pathway, is not incorporated into the EV-CMCs62. We also found that VEGF is present in CMC-EVs but its expression is lower compared with CMCs. Although VEGF follows the conventional secretory pathway, it is possible that in the post-Golgi processing some of the alternatively spliced forms are not incorporated in EVs, which may explain the reduced expression of VEGF in EVs compared with CMCs. Further studies are necessary to clarify the packaging of VEGF alternatively spliced forms in EVs, which may reveal important mechanisms regulating EV packaging. Nevertheless, based on our data we can conclude that the conventional protein secretory pathway contributes to EV packaging, and that proteins that bypass the Golgi apparatus, like FGF-2, are not part of the EV packaging process. This hypothesis can be further tested in studies focused on the ER to Golgi transport. Such studies would illuminate the mechanisms of EV protein packaging in the health and disease and contribute to our understanding of physiological and pathological EV signaling.
In conclusion, human CMCs secrete heterogeneous population of EVs that are a mixture of exosomes and membrane derived vesicles with pro-angiogenic activity. Based on our results, we can conclude that the pro-angiogenic activities of CMC EVs are principally mediated via Ang-1-Tie-2 signaling. This data provide insight regarding the mechanism of CMC-induced myocardial repair in particular their pro-angiogenic effects via secretion of Ang-1 enriched EVs.
Supplementary Material
Supplementary Figure 1. Angiogenic protein array. CMC lysates, CMC-conditioned media (CM), and CMC-EVs (EVs) were evaluated for expression of pro- and anti-angiogenic factors with cytokine array membranes (Proteome Profiler Human Angiogenic Array Kit; R&D Systems, Minneapolis, MN). Proteome cytokine array heat map illustrating densitometric quantification or relative pixel density for each cytokine (A). Cytokine array membranes detected using enhanced chemiluminescence (B).
ACKNOWLEDGMENTS
This work was supported by NIH Grants P20 GM103492, P01 HL078825 (to RB and MW), UM1 HL113530 (to RB), and R01 HL141081 (to JBM).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
DISCLOSURE
The authors have nothing to disclose.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol 2015;5:1841–75. [DOI] [PubMed] [Google Scholar]
- 2.Chen B and Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation 2017;24. [DOI] [PubMed] [Google Scholar]
- 3.Christia P and Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest 2013;43:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol 2015;30:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong P, Christia P and Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhu SD and Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khurana R, Simons M, Martin JF and Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 2005;112:1813–24. [DOI] [PubMed] [Google Scholar]
- 8.Evans I In Vitro Angiogenesis Assays. Methods Mol Biol 2015;1332:143–50. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Maeda K, Takefuji M, Kikuchi R, Morishita Y, Hirashima M and Murohara T. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci Rep 2017;7:7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian X, Pu WT and Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circ Res 2015;116:515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henning RJ. Therapeutic angiogenesis: angiogenic growth factors for ischemic heart disease. Future Cardiol 2016;12:585–99. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee MN, Bolli R and Hare JM. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ Res 2018;123:266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysoczynski M, Guo Y, Moore JBt, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL and Bolli R. Myocardial Reparative Properties of Cardiac Mesenchymal Cells Isolated on the Basis of Adherence. J Am Coll Cardiol 2017;69:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q and Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 2017;112:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A and Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 2014;9:e96725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A and Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 2013;108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnecchi M, Zhang Z, Ni A and Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgkinson CP, Bareja A, Gomez JA and Dzau VJ. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res 2016;118:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M and Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 2011;50:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wysoczynski M, Khan A and Bolli R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res 2018;123:138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todorova D, Simoncini S, Lacroix R, Sabatier F and Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ Res 2017;120:1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garikipati VNS, Shoja-Taheri F, Davis ME and Kishore R. Extracellular Vesicles and the Application of System Biology and Computational Modeling in Cardiac Repair. Circ Res 2018;123:188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan M and Kishore R. Stem Cell Exosomes: Cell-FreeTherapy for Organ Repair. Methods Mol Biol 2017;1553:315–321. [DOI] [PubMed] [Google Scholar]
- 24.Kishore R, Garikipati VN and Gumpert A. Tiny Shuttles for Information Transfer: Exosomes in Cardiac Health and Disease. J Cardiovasc Transl Res 2016;9:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishore R and Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res 2016;118:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishore R and Khan M. Cardiac cell-derived exosomes: changing face of regenerative biology. Eur Heart J 2017;38:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A and Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006;20:1487–95. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, Silva AP, Fernandes R, Zuzarte M, Enguita FJ, Costa MC, Pinto-do OP, Pinto MT, Gouveia P, Ferreira L, Mason JC, Pereira P, Kwak BR, Nascimento DS and Girao H. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res 2017;113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 29.Sluijter JPG, Davidson SM, Boulanger CM, Buzas EI, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K and Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2018;114:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ and Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Johnson T and Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther 2017;8:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R and Losordo DW. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 2012;111:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ and Sahoo S. Angiogenic Mechanisms of Human CD34(+) Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ Res 2017;120:1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R and Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahoo S and Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res 2014;114:333–44. [DOI] [PubMed] [Google Scholar]
- 36.Moore JBt, Zhao J, Keith MC, Amraotkar AR, Wysoczynski M, Hong KU and Bolli R. The Epigenetic Regulator HDAC1 Modulates Transcription of a Core Cardiogenic Program in Human Cardiac Mesenchymal Stromal Cells Through a p53-Dependent Mechanism. Stem Cells 2016;34:2916–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G and Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 2013;431:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A and Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012;126:2601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL and Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 2015;192:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wysoczynski M, Liu R, Kucia M, Drukala J and Ratajczak MZ. Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling. Mol Cancer Res 2010;8:677–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wysoczynski M and Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer 2009;125:1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI, Stillitano F, Hare JM, Sahoo S, Hajjar RJ and Costa KD. Exosomal microRNA-21–5p Mediates Mesenchymal Stem Cell Paracrine Effects on Human Cardiac Tissue Contractility. Circ Res 2018;122:933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebson PR. Stem-cell angiogenesis and regeneration of the heart: review of a saga of 2 decades. Clin Cardiol 2015;38:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Fan Y, Zhou L, Zhu HY, Song YC, Hu L, Wang Y and Li QP. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int J Cardiol 2015;188:22–32. [DOI] [PubMed] [Google Scholar]
- 45.Moradi K, Abbasi M, Aboulhasani F, Abbasi N, Babatunde KA, Sargolzaeiaval F and Dehpour AR. Therapeutic angiogenesis promotes efficacy of human umbilical cord matrix stem cell transplantation in cardiac repair. Iran J Basic Med Sci 2015;18:563–70. [PMC free article] [PubMed] [Google Scholar]
- 46.Moore JBt, Zhao J, Fischer AG, Keith MCL, Hagan D, Wysoczynski M and Bolli R. Histone Deacetylase 1 Depletion Activates Human Cardiac Mesenchymal Stromal Cell Proangiogenic Paracrine Signaling Through a Mechanism Requiring Enhanced Basic Fibroblast Growth Factor Synthesis and Secretion. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baraniak PR and McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med 2010;5:121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, Saffrich R, Schubert M, Ho AD, Giese N, Buchler MW, Friess H, Buchler P and Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 2008;99:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suga H, Glotzbach JP, Sorkin M, Longaker MT and Gurtner GC. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg 2014;72:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shantsila E, Watson T and Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 2007;49:741–52. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X, Wu N and Huang L. Endothelial progenitor cells and spleen: new insights in regeneration medicine. Cytotherapy 2010;12:7–16. [DOI] [PubMed] [Google Scholar]
- 52.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P and Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010;121:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janowska-Wieczorek A, Majka M, Ratajczak J and Ratajczak MZ. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells 2001;19:99–107. [DOI] [PubMed] [Google Scholar]
- 54.Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M and Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012;26:1166–73. [DOI] [PubMed] [Google Scholar]
- 55.Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, Greco NJ, Tendera M and Ratajczak MZ. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells--implications for stem cell therapies in regenerative medicine. Stem Cells Dev 2013;22:422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beach A, Zhang HG, Ratajczak MZ and Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res 2014;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McBride JD, Rodriguez-Menocal L, Guzman W, Candanedo A, Garcia-Contreras M and Badiavas EV. Bone Marrow Mesenchymal Stem Cell-Derived CD63(+) Exosomes Transport Wnt3a Exteriorly and Enhance Dermal Fibroblast Proliferation, Migration, and Angiogenesis In Vitro. Stem Cells Dev 2017;26:1384–1398. [DOI] [PubMed] [Google Scholar]
- 58.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD and Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 2015;116:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S and Davis ME. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ Res 2017;120:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, Kirov A, Small D, Kathir KM, Rajalingam D and Kumar TK. Secretion without Golgi. J Cell Biochem 2008;103:1327–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prudovsky I Nonclassically Secreted Regulators of Angiogenesis. Angiol Open Access 2013;1:1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wegehingel S, Zehe C and Nickel W. Rerouting of fibroblast growth factor 2 to the classical secretory pathway results in post-translational modifications that block binding to heparan sulfate proteoglycans. FEBS Lett 2008;582:2387–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Angiogenic protein array. CMC lysates, CMC-conditioned media (CM), and CMC-EVs (EVs) were evaluated for expression of pro- and anti-angiogenic factors with cytokine array membranes (Proteome Profiler Human Angiogenic Array Kit; R&D Systems, Minneapolis, MN). Proteome cytokine array heat map illustrating densitometric quantification or relative pixel density for each cytokine (A). Cytokine array membranes detected using enhanced chemiluminescence (B).


