Manganese homeostasis is primarily regulated at the level of transport. Bacillus subtilis MntR serves as a Mn(II)-activated repressor of importer genes (mntH and mntABC) and an activator of efflux genes (mneP and mneS). Elevated intracellular Mn(II) also binds to Mn-sensing riboswitches to activate transcription of yybP and ykoY, which encodes a TerC family member. Here, we demonstrate that two TerC family proteins, YceF and YkoY, help prevent Mn(II) intoxication. TerC family proteins are widespread in bacteria and may influence host-pathogen interactions, but their effects on Mn(II) homeostasis are unclear. Our results suggest that TerC proteins work by Mn(II) export under Mn(II) overload conditions to help alleviate toxicity.
KEYWORDS: Bacillus subtilis, TerC family, manganese, metal resistance, metalloregulation, regulation, riboswitch
ABSTRACT
Manganese (Mn) is an essential element and is required for the virulence of many pathogens. In Bacillus subtilis, Mn(II) homeostasis is regulated by MntR, a Mn(II)-responsive, DNA-binding protein. MntR serves as both a repressor of Mn(II) uptake transporters and as a transcriptional activator for expression of two cation diffusion facilitator Mn(II) efflux pumps, MneP and MneS. Mutants lacking either mntR or both mneP and mneS are extremely sensitive to Mn(II) intoxication. Using transposon mutagenesis to select suppressors of Mn(II) sensitivity, we identified YceF, a TerC family membrane protein, as capable of providing Mn(II) resistance. Another TerC paralog, YkoY, is regulated by a Mn(II)-sensing riboswitch and is partially redundant in function with YceF. YkoY is regulated in parallel with an unknown function protein YybP, also controlled by a Mn(II)-sensing riboswitch. Strains lacking between one and five of these known or putative Mn(II) tolerance proteins (MneP, MneS, YceF, YkoY, and YybP) were tested for sensitivity to Mn(II) in growth assays and for accumulation of Mn(II) using inductively coupled plasma mass spectrometry. Loss of YceF and, to a lesser extent, YkoY, sensitizes cells lacking the MneP and MneS efflux transporters to Mn(II) intoxication. This sensitivity correlates with elevated intracellular Mn(II), consistent with the suggestion that TerC proteins function in Mn(II) efflux.
IMPORTANCE Manganese homeostasis is primarily regulated at the level of transport. Bacillus subtilis MntR serves as a Mn(II)-activated repressor of importer genes (mntH and mntABC) and an activator of efflux genes (mneP and mneS). Elevated intracellular Mn(II) also binds to Mn-sensing riboswitches to activate transcription of yybP and ykoY, which encodes a TerC family member. Here, we demonstrate that two TerC family proteins, YceF and YkoY, help prevent Mn(II) intoxication. TerC family proteins are widespread in bacteria and may influence host-pathogen interactions, but their effects on Mn(II) homeostasis are unclear. Our results suggest that TerC proteins work by Mn(II) export under Mn(II) overload conditions to help alleviate toxicity.
INTRODUCTION
Manganese (Mn) is an important nutrient metal ion critical for growth and is used to support many cellular processes, including detoxification of reactive oxygen species (ROS) and the production of deoxynucleoside triphosphates for DNA replication, and in enzymes of central carbon metabolism (1–3). Escherichia coli is considered to have an iron-centric metabolism and conditionally imports Mn(II) in response to oxidative stress (4). Some enzymes that use Fe(II) as a cofactor may be inactivated by oxidative processes or iron limitation, and binding of Mn(II) may help to sustain enzyme function (5–8). Alternatively, Fe(II)-dependent enzymes may be functionally replaced by Mn(II)-dependent isozymes under conditions of iron limitation (9–11).
In contrast to E. coli, Bacillus subtilis and many pathogenic Firmicutes have a more manganese-centric physiology and require comparatively high levels of Mn(II) for growth (2). Mammalian hosts may limit the bioavailability of Mn(II) for use by pathogens, as part of the innate immune mechanism known as nutritional immunity (12, 13). The immune effector protein calprotectin is an important mediator of the Mn(II)-withholding response and is produced in high concentrations at sites of infection to limit bacterial growth (14). The essential roles of Mn(II) in cell physiology likely vary between organisms and depend on growth conditions. In B. subtilis the sole ribonucleotide reductase and the glycolytic enzyme phosphoglycerate mutase are both Mn(II)-dependent enzymes (15, 16). In Staphylococcus aureus, a Mn(II)-dependent phosphoglycerate mutase is sensitive to inhibition by calprotectin, and the cell responds by production of a Mn(II)-independent isozyme (3). Nevertheless, Mn(II) can act as a limiting factor for infection, and simply increasing dietary Mn(II) can exacerbate S. aureus infections (17, 18).
As a result of host-imposed restrictions on metal ion availability, high-affinity Mn(II) uptake systems are important virulence determinants for many pathogens (1, 19–23). However, high-affinity Mn(II) import systems impose their own risks, since increased Mn(II) availability may lead to Mn(II) intoxication. Balancing intracellular Mn(II) levels therefore requires pathways both for uptake and efflux. Indeed, Mn(II) efflux systems have recently been described as critical for virulence in diverse pathogenic bacteria (24–29).
Bacillus subtilis displays an absolute requirement for Mn(II) (30) and has long served as a model system for bacterial manganese homeostasis (2, 31–33). The B. subtilis MntR regulatory protein and its orthologs serve as the central regulator of Mn(II) homeostasis in many bacteria (1, 33–36). A role for MntR in Mn(II) homeostasis was inferred when it was found that null mutants were exquisitely sensitive to Mn(II) (31). Whereas B. subtilis normally grows well at up to 1 mM extracellular Mn(II), an mntR-null mutant is unable to grow with 10 μM Mn(II) (31, 37). This high sensitivity is due, in part, to derepression of Mn(II) import in the mntR mutant. Subsequent studies revealed that Mn(II)-bound MntR represses expression of both a proton-coupled importer of the NRAMP family (MntH) and an ABC transporter (MntABC) (32). However, derepression of uptake is insufficient to explain the high sensitivity of an mntR mutant to manganese: MntR also serves as a required activator for the expression of two, Mn(II)-inducible cation diffusion facilitator (CDF) efflux pumps, MneP and MneS (37).
Mn(II) also regulates gene expression through the metal-sensing yybP-ykoY riboswitch, named after the two genes associated with this regulatory element in B. subtilis (38–40). In response to elevated cytosolic levels of Mn(II), the B. subtilis yybP-ykoY riboswitches adopt an altered RNA structure that precludes formation of a transcription termination structure (38, 39). However, the functions of the riboswitch-regulated proteins, YybP and YkoY, in manganese homeostasis are unclear. YybP is a predicted membrane protein of unknown function, whereas YkoY is a member of the widespread TerC family of membrane proteins, named for their original association with tellurium resistance (41). Recently, Alx, an E. coli TerC homolog also regulated by a Mn(II)-sensing riboswitch, was suggested to be involved in facilitating Mn(II) import (42).
Here, we sought to better understand the molecular basis for Mn(II) intoxication by selecting mutations that increase tolerance in cells lacking the two known Mn(II) efflux pumps, MneP and MneS. Suppression of the Mn-sensitive phenotype of an mneP mneS parent strain was conferred by mutations that upregulated the expression of YceF, a TerC protein family member. Consistently, mutation of yceF in the mneP mneS parent strain further increased Mn(II) sensitivity and led to a rise in intracellular Mn(II). YceF is a paralog of the Mn-inducible YkoY protein, and we demonstrate that these two TerC proteins have partially overlapping functions in manganese homeostasis, likely by contributing to a secondary pathway for Mn(II) efflux.
RESULTS
Elevated expression of YceF increases Mn(II) resistance in an efflux-deficient strain.
To identify genes that are involved in Mn(II) resistance, we took advantage of the high Mn(II) sensitivity of an mneP mneS double efflux mutant (37). We introduced a plasmid carrying the mariner transposon (mTn) into the mneP mneS strain and selected for growth on plates containing elevated levels of Mn(II) (100 and 150 μM). Subsequently, the location of the mariner insertion was identified by DNA sequencing. Multiple independent insertions were found across the yceC operon, including two insertions in yceC and one in yceE (Fig. 1a). In addition, we recovered a single insertion disrupting yhdP, encoding a putative Mg(II) efflux pump homologous to the S. aureus mpfA gene (43, 44). Genomic DNA was isolated for each insertion and backcrossed into the parental mneP mneS strain. All reconstructed strains maintained resistance to Mn(II) as judged by zone of inhibition assays. The mneP mneS yceC::mTn and mneP mneS yceE::mTn strains were as resistant to Mn(II) intoxication as the wild type (WT) (Fig. 1b).
FIG 1.
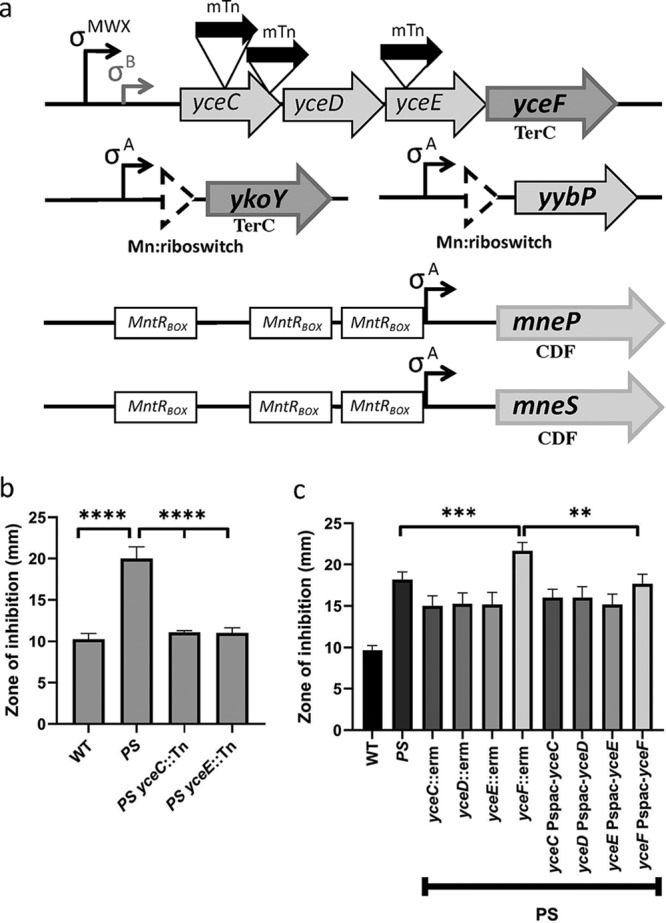
Transposon insertions in a mneP mneS mutant background confer resistance to Mn(II). (a) Schematic illustration of genes implicated in Mn(II) tolerance. The yceC operon is under complex transcriptional control and encodes the TerC homolog, YceF. The ykoY and yybP genes are Mn(II) inducible by virtue of a Mn(II)-sensing riboswitch, and YkoY is a paralog of YceF. MntR activates expression of two cation diffusion facilitator (CDF) family Mn(II) efflux pumps. The locations of the mariner transposon (mTn) insertions in the yceC operon are shown. (b) Disk diffusion tests were performed to measure sensitivity to Mn(II). PS indicates the mneP mneS mutant strain. Mid-logarithmic cells (OD600, ∼0.4) were plated on LB agar plates and overlaid with 10 μl of 100 mM MnCl2 on a filter paper disk. The zone of growth inhibition was measured after overnight growth. Data represent the means ± the SD for at least three biological replicates. (c) Mn(II) sensitivity as measured using zone-of-inhibition assays. PS indicates an mneP mneS efflux defective strain. Complementation experiments indicate that the Mn(II) sensitivity of deletions in the yceC operon can be decreased to wild-type levels only by induction of ectopic copies of YceF integrated at the amyE locus. Data represent means ± the SD for three biological replicates. Experiments were performed with IPTG added to 100 μM. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined using an unpaired two-tailed Student t test).
The yceC operon includes yceCDEF-yceGH, with one transcriptional terminator after yceF and a second after yceH (45). This operon is under complex transcriptional control by multiple stress-responsive σ factors, including the σW, σM, and σX extracytoplasmic function (ECF) σ factors (46–49) and the general stress σ factor, σB (50). The yceCDEF loci are homologous to sequences in other organisms originally associated with tellurium resistance, but their precise functions are unknown (51). The YceCDE proteins are all related to TerD (the UniProt CAPAB TerDEXZ family), whereas YceF is related to TerC. In contrast, yceGH are not related to defined tellurium resistance genes. Although originally associated with an ability to reduce extracellular tellurite and thereby confer resistance, the physiological role of these tellurite resistance genes has remained obscure (51).
To test whether inactivation of the yceC, yceD, and yceE genes contributed to Mn(II) resistance, null mutations were generated in the mneP mneS mutant background using the BKE collection of strains carrying erythromycin resistance (erm) cassettes replacing the corresponding coding regions (52). In contrast to the original mariner insertions, the strains with integrated erm cassettes had only a modestly increased Mn(II) resistance relative to the mneP mneS parent strain, and this effect could not be complemented by expression of the inactivated gene from an ectopic locus (Fig. 1C). Furthermore, when the erm cassettes were removed to leave behind unmarked, in-frame deletions (52), the increased resistance to Mn(II) was lost (data not shown). These results led us to hypothesize that the increased Mn(II) resistance in the original transposon insertion mutants was due to a polar effect in which the integrated transposon led to an increase in expression of the downstream gene yceF.
To test the role of yceF in Mn(II) resistance, we introduced a yceF::erm null mutation into the mneP mneS mutant background. This led to a small but reproducible increase in Mn(II) sensitivity, and this effect was complemented by the ectopic expression of yceF integrated at the amyE locus (Fig. 1c). These results suggest that YceF increases Mn(II) resistance, and the original selection of yceC::mTn and yceE::mTn transposants reflected the upregulation of this minor resistance determinant.
Next, we tested the phenotypes of yceF in an otherwise WT background. For these studies, we removed the erm cassette from the yceF::erm strain to generate a mutant strain with a clean deletion at yceF (ΔyceF). Sensitivity to Mn(II), Fe(II), and Zn(II) was tested with zone of inhibition assays. A yceF mutation in a WT background has a small but statistically significant sensitivity to Mn(II), and a yceF mutation exacerbates the Mn(II) sensitivity of the mneP mneS strain (Fig. 2). The ΔyceF mutant is also slightly more sensitive to Fe(II) intoxication in the WT but not in the mneP mneS mutant background. Interestingly, the mneP mneS strain also displayed a modest increase in Fe(II) sensitivity relative to WT. This suggests that perhaps MneP and/or MneS may also have efflux activity with respect to Fe(II). Finally, yceF did not confer Zn(II) sensitivity in a WT and mneP mneS mutant background (Fig. 2). This suggests that yceF has activity primarily related to Mn(II) resistance.
FIG 2.
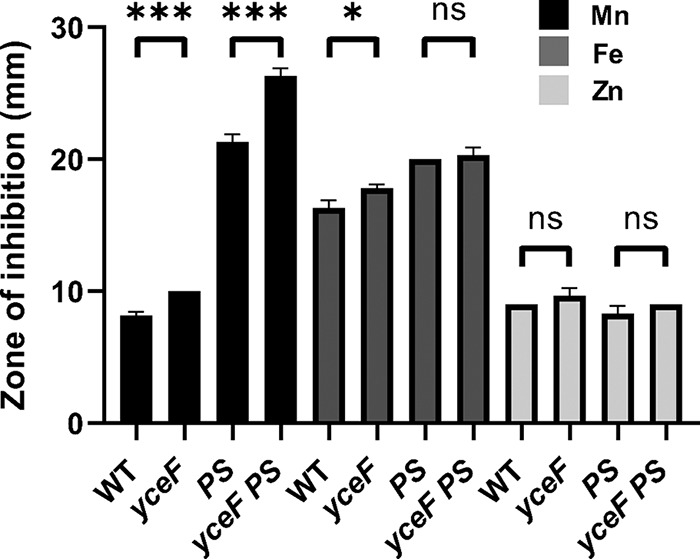
YceF confers increased Mn(II) tolerance, but has little effect on sensitivity to other tested metals. yceF mutant sensitivity to Mn(II) (10 μl of 100 mM MnCl2/1 M FeSO4/100 mM ZnCl2). PS indicates the mneP mneS background. Mid-logarithmic cells (OD600, ∼0.4) were plated on LB agar plates and overlaid with 10 μl of each metal on a filter paper disk. The zone of growth inhibition was measured after overnight growth. Data represent the means ± the SD for at least three biological replicates. *, P < 0.05; ***, P < 0.001; ns, no statistical significance (as determined using an unpaired two-tailed Student t test).
TerC family proteins YceF and YkoY are partially redundant.
YceF is one of three TerC family proteins in Bacillus subtilis, the others being YkoY and YjbE. YjbE is a sporulation-specific protein, expressed as part of the σE regulon (53), and there is little if any expression of yjbE during vegetative growth (45). Therefore, we focused our attention here on the two paralogs expressed during growth, YceF and YkoY. The ykoY leader region contains a Mn(II)-responsive riboswitch (39, 40), consistent with a role for YkoY under conditions of high Mn(II). A homologous riboswitch precedes the yybP gene, encoding a predicted membrane protein of unknown function, and both ykoY and yybP are induced by Mn(II) (39). We hypothesized that there may be genetic redundancy between the yceF, ykoY, and yybP genes. Therefore, we constructed an array of mutant strains lacking one or more of these genes. These mutants were assayed using zones of inhibition with Mn(II) on the filter disk. In a WT background, no single deletion strain displayed an increased Mn(II) sensitivity (Fig. 3). However, both the yceF ykoY double mutant and the yceF ykoY yybP triple mutant showed a statistically significant increase in Mn(II) sensitivity (Fig. 3). Thus, there is redundancy between the YceF and YkoY TerC family proteins, and their role in Mn(II) resistance can be revealed even in a strain able to express the two efflux pumps, MneP and MneS.
FIG 3.
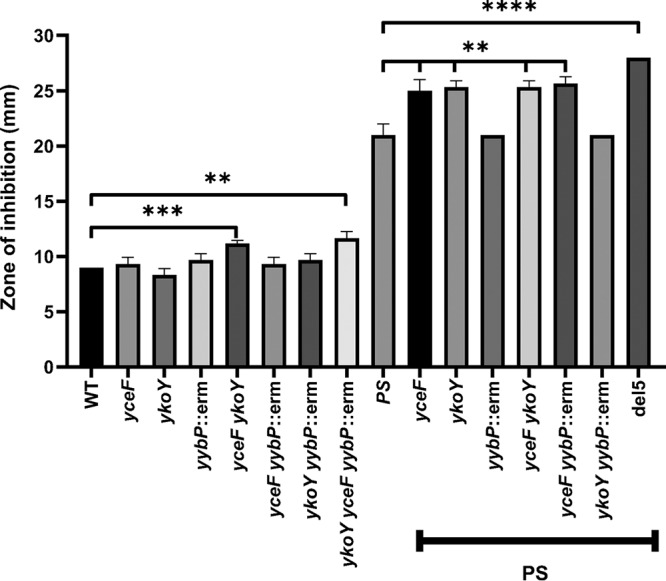
Mn(II) tolerance results from an additive effect of multiple genes. Mn(II) sensitivity was measured by using zone-of-inhibition assays. PS indicates the mneP mneS background. The del5 strain (ΔmneP ΔmneS ΔykoY ΔyceF yybP::erm) is described in Table S1 in the supplemental material. Data represent means ± the SD for three biological replicates. Multipronged significance bars indicate the statistical significance for a given strain in reference to mneP and mneS. **, P < 0. 01; ***, P < 0.001; ****, P < 0.0001 (as determined using an unpaired two-tailed Student t test).
We also tested the role of these genes in the mneP mneS mutant background, which is already sensitized to Mn(II) intoxication (Fig. 3). In this case, the yceF mutation, but not the ykoY mutation, increased Mn(II) sensitivity. It is not obvious why the yybP gene appeared to contribute to Mn(II) resistance when combined with a ykoY single mutant in a WT background but not in the mneP mneS mutant background (Fig. 3). Finally, we note that in both WT and mneP mneS mutant backgrounds, the combined inactivation of yybP, ykoY, and yceF led to the highest level of Mn(II) sensitivity.
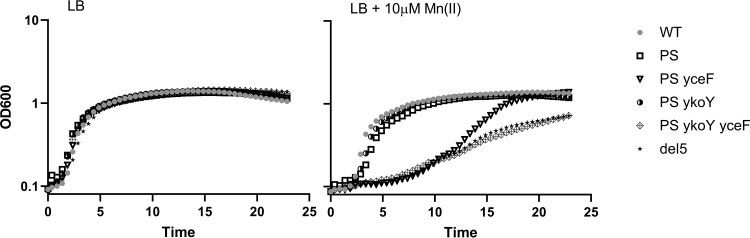
We next measured growth curves of these strains with increasing levels of MnCl2 (Fig. 4; see also Fig. S1 in the supplemental material). Again, mutation of yceF conferred an increase in sensitivity in the mneP mneS mutant background. This can be seen as an increased lag phase during subculture in lysogeny broth (LB) supplemented with 5 to 10 μM Mn(II) (Fig. 4; see also Fig. S1). A ykoY mutation had no noticeable effect on growth in the mneP mneS mutant background but exacerbated the growth defect when combined with the yceF mutation (the mneP mneS yceF ykoY mutant versus the corresponding mneP mneS yceF and mneP mneS ykoY triple mutants [Fig. 4]). This contrasts with the lack of an apparent additive effect in zone of inhibition assays (Fig. 3).
FIG 4.
Growth curves highlighting the role of TerC family proteins in Mn(II) homeostasis. Strains were grown in a Bioscreen multiwell growth analyzer in LB broth amended or not with 10 μM MnCl2, and cell growth was determined as a function of cell density (OD600) over the course of 25 h. PS indicates the mneP mneS mutations. All curves were monitored at least three times with consistent results. The yceF and ykoY single mutants grew as well as the wild type under these conditions. Complete results, including a range of Mn(II) concentrations, are provided in Fig. S1 in the supplemental material.
Cells lacking YceF and YkoY accumulate manganese.
Since YceF and YkoY contribute to Mn(II) resistance in a mneP mneS mutant background, we next tested whether the loss of these proteins affects the intracellular accumulation of Mn(II). Cells growing in LB medium were shocked by addition of 100 μM MnCl2 and intracellular metal ion levels were measured using inductively coupled plasma mass spectrometry (ICP-MS) before shock and 30 min after shock. The Mn(II) levels of all strains were similar before shock, and WT cells maintained Mn(II) homeostasis even after shock (Fig. 5). In contrast, the mneP mneS strain accumulated 12-fold more Mn(II), as reported previously (37). In a mneP mneS ykoY mutant a similar increase was noted, whereas in a yceF mneP mneS mutant Mn(II) levels increased ∼26-fold. In the yceF ykoY mneP mneS quadruple mutant, Mn(II) accumulation increased slightly above that of a yceF mneP mneS mutant, although this difference did not reach our defined level of statistical significance (P < 0.05). The intracellular levels of Fe(II), Zn(II), and Cu(II) stayed relatively stable for all strains despite the Mn(II) shock (Fig. S2). These results suggest that YceF and YkoY are important for adaptation to excess Mn(II) in mneP mneS strains and that in their absence intracellular Mn(II) accumulates to even higher levels than that seen with the mneP mneS mutant alone.
FIG 5.
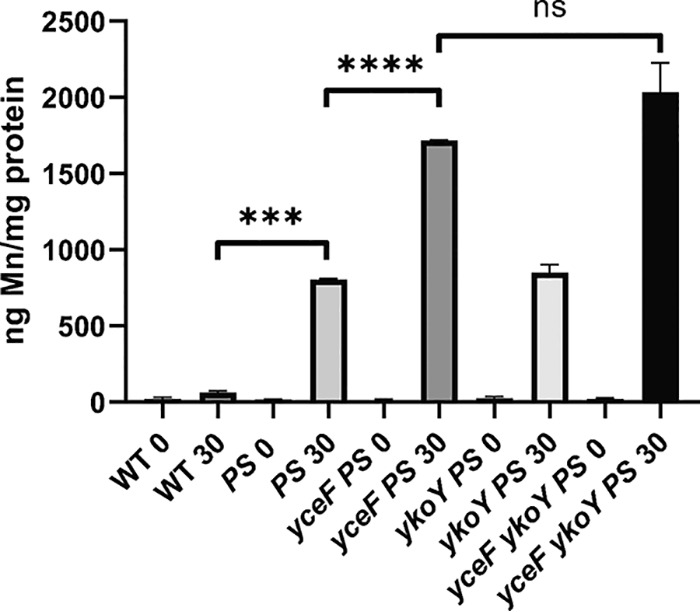
yceF and ykoY mutants in a mneP mneS background (PS) confer Mn(II) tolerance by reducing internal Mn(II) accumulation. ICP-MS analysis was used to measure intracellular Mn(II) accumulation after Mn(II) shock. Wild-type and mutant cells were grown to mid-logarithmic phase (OD600, ∼0.4), 100 μM MnCl2 was added to the LB medium, and samples were collected before shock (“0”) and 30 min after shock (“30”). Mn(II) levels were normalized against total protein. Data represent means for two biological replicates performed at different times, and error bars indicate the ranges. ***, P < 0.001; ****, P < 0.0001; ns, no statistical significance (as determined using an unpaired two-tailed Student t test).
Transcription of the yceC operon is not strongly regulated by Mn(II).
Since mneP and mneS are both transcriptionally induced by Mn(II) acting through the MntR transcription factor (37), and ykoY and yybP are regulated by a Mn(II)-sensing riboswitch (39), we wanted to explore whether transcription of the yceC operon might also be induced by Mn(II). We generated promoter-lacZ fusion strains for yybP, ykoY, and the yceC operon and compared their expression with mneP and mneS promoter fusions 30 min after resuspension of washed, mid-logarithmic-phase cells in LB medium amended with various concentrations of Mn(II) (Fig. 6). As reported, mneP and mneS are responsive to Mn(II) (37), with maximal induction achieved at 300 μM Mn(II) for mneP and at 400 μM Mn(II) for mneS. The yybP and ykoY riboswitches were also responsive to Mn(II) concentration [maximal induction by ∼200 μM Mn(II)], although in both cases there was a substantial basal level of expression (∼50% full induction). Expression of the yceC operon was constitutive and did not appear to respond to Mn(II) under these conditions. However, prior studies suggest that this operon may be induced by a shift of cells to conditions of elevated Mn(II), which can lead to a transient spike in intracellular Mn(II) levels (32).
FIG 6.
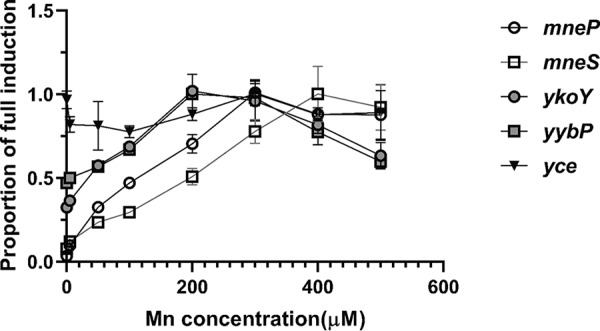
Expression of ykoY, yybP, mneS, and mneP and the yceC operon as a function of Mn(II) concentration. Promoter activity was monitored using β-galactosidase assays for mneP-lacZ, mneS-lacZ, yceC-lacZ, yybP-lacZ, and ykoY-lacZ transcriptional fusions in the WT as a function of added Mn(II). Cells were grown to mid-logarithmic phase (OD600, ∼0.4) with Mn(II) added to the specified concentration. A proportion of 1 is equal to the fully activated value in Miller units (21.2 for yceC, 32 for mneP, 8.3 for mneS, 3.5 for ykoY, and 4.3 for yybP). The results shown are representative of experiments performed at least three different times. Open shapes represent MntR-regulated loci, gray shapes are riboswitch regulated, and the triangle represents uncharacterized regulation. Data represent means ± the SD for three biological replicates.
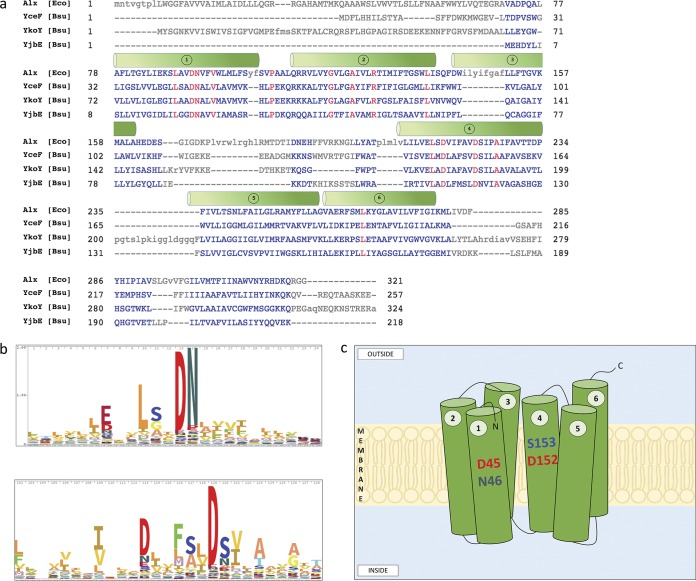
YceF and YkoY share conserved sequence features with other TerC homologs.
A previous study aligned TerC family proteins from various bacterial species to define conserved motifs, although B. subtilis was not included (42). Here, we aligned the three TerC family proteins encoded in B. subtilis (YceF, YkoY, and YjbE) with the Escherichia coli homolog (Alx) (Fig. 7a). All four proteins share six predicted transmembrane segments with conserved aspartic acid residues present in segments 1 and 4, as noted previously (42). These conserved acidic motifs (Fig. 7b) are predicted to be located within the membrane, and were previously postulated to form part of an ion transport channel (42). This is consistent with the observations here that cells lacking YceF accumulate elevated levels of Mn(II) after shock (Fig. 5). In contrast with previous results (42), this suggests a likely function in Mn(II) export.
FIG 7.
Alignment of TerC family proteins and putative structure of YceF. (a) Multisequence alignment of Alx (E. coli), YceF (B. subtilis), YkoY (B. subtilis), and YjbE (B. subtilis) protein sequences derived from NCBI. Proteins were aligned using COBALT with default parameters and “identity” conservation settings. Conserved residues across species are in red. Transmembrane helices (green cylinders) are depicted as previously described (42). (b) Sequence logos showing the degree of residue conservation within the TerC superfamily of transporters. The two sequences shown here align with sections within helix 1 and helix 4 as shown in panel a. Sequence logos were obtained from Pfam. (c) Depiction of B. subtilis YceF. Six transmembrane helices are represented with the conserved DN and DS residues in helices 1 and 4.
DISCUSSION
Cells rely on essential transition metals as cofactors for an estimated 30% of enzymes. Managing the acquisition, storage, and efflux of metal ions is critical for the health of the cell and helps to sustain cytosolic metals at levels needed to metallate critical enzymes, while avoiding toxicity associated with metal ion overload (33). Mn(II) is an essential element for B. subtilis and related bacteria, and sequestration of Mn(II) by the mammalian innate immune protein calprotectin is important in the ability of hosts to prevent infection (1, 14). Because of the wide variation and the dynamic nature of Mn(II) pools, both in the host and in the environment, mechanisms for sustaining Mn(II) homeostasis are critical for cell function.
In B. subtilis, the MntR metalloregulatory protein is the central regulator of Mn(II) homeostasis (31). The homodimeric MntR protein binds two Mn(II) ions per protomer, with the critical sensing site displaying Mn(II)-selective, heptacoordinate ligation (54). Interestingly, the Mn(II) selective riboswitches of the yybP-ykoY family also bind and respond to Mn(II) with high selectivity (40), and this also involves a heptacoordinate sensing site (55). Once activated by Mn(II), MntR represses two operons encoding Mn(II) import systems: the ATP-dependent MntABC system and the proton-coupled NRAMP family protein MntH (32). In addition, MntR serves as a transcriptional activator for two cation diffusion facilitator (CDF) proteins, MneP and MneS, that function to reduce intracellular Mn(II) levels (37). The ykoY and yybP genes are both regulated by the eponymous Mn(II)-sensing riboswitch, as documented for B. subtilis, Lactobacillus, and E. coli (39, 40). However, the functions of YkoY and YybP in Mn(II) homeostasis remain undefined.
Here, we used a forward genetics approach to identify mutations that can alleviate the toxicity associated with growth of cells lacking Mn(II) efflux systems in medium with excess Mn(II). Analysis of the resulting transposants identified upregulation of the unknown function YceF protein as the key determinant of increased Mn(II) resistance. We were intrigued by the observation that YceF is a TerC homolog, and therefore a paralog of the YkoY protein known to be regulated by a Mn(II)-sensing riboswitch. We postulated that YceF and YkoY have overlapping functions in Mn(II) homeostasis. To test this idea, we generated strains lacking various Mn(II) resistance determinants.
Our data indicate that YceF functions to help maintain Mn(II) homeostasis as revealed in cells lacking the two Mn(II) exporting CDF proteins, MneP and MneS (the mneP mneS mutant). Cells lacking mneP mneS and additionally lacking YceF accumulate much greater amounts of Mn(II) after shift to excess Mn(II) conditions (Fig. 5). The yceF gene is cotranscribed with poorly understood genes encoding TerD family proteins as part of the yceC operon. This operon is under complex transcriptional control that likely involves the general stress response regulator σB (50) and the extracytoplasmic function factors σW and σM (46, 47). Interestingly, the σB regulon has been previously shown to be responsive to changes in Mn(II) availability (32). Indeed, the yceC operon is modestly induced by a shift of Mn(II) limited cells [grown with 50 nM Mn(II)] to replete conditions [2.5 μM Mn(II)], and this induction is much more dramatic in an mntR-null mutant (32). However, the yceC operon did not seem to respond to elevated Mn(II) under the steady-state growth conditions monitored here (Fig. 6).
We suggest that YceF has a more general function in cellular physiology and that this function may involve export of Mn(II) from the cytosol as part of its mechanism. This function appears to be at least partly overlapping with the role of the paralogous protein YkoY, as revealed by the additive effect of yceF and ykoY mutations in growth studies (Fig. 4). Thus, these two TerC homologs may be involved in pathways involved in translocation of Mn(II) across the membrane. Indeed, it was recently noted that TerC proteins are distantly related to known Mn(II) transporters, as part of the LysE superfamily, and are frequently associated with Mn(II)-sensing riboswitches (42). This superfamily of transporters includes documented Mn(II) efflux pumps of the MntP family, a cadmium resistance transporter (Cad), UPF0016 proteins, and iron-lead transporters (ILT), as well as TerC family proteins (42). These transporters display a conserved transmembrane architecture with conserved acidic residues (Asp) in two transmembrane segments (42). This architecture is conserved for the YceF and YkoY proteins (Fig. 7). However, in contrast with this prior work, we here show that the loss of TerC homologs leads to an increase in intracellular Mn(II), suggestive of a role in Mn(II) efflux.
The YkoY TerC homolog is regulated by a Mn(II)-sensing riboswitch, and this regulation is consistent with a role related to helping sustain optimal cytosolic Mn(II) levels when Mn(II) levels are in excess. However, both YkoY and YceF are expressed even in the absence of overt Mn(II) stress (45). We hypothesize that TerC homologs function in Mn(II) transport across the cell membrane for the purpose of metallating secreted or cell surface-associated metalloenzymes. This model is suggested, in part, from the broad conservation of TerC proteins (42, 56). For example, TerC is found in plants where it is critical for proper assembly of photosystem II, a complex that requires Mn(II) for function. In Arabidopsis thaliana, TerC (AtTerC) is localized to the thylakoid membrane of the chloroplast and interacts with the photosystem II assembly factor ALB3 (57). More generally, TerC proteins are related to the larger UPF0016 family of proteins, which includes the plant PAM71 protein, postulated to translocate Mn(II) into the chloroplast lumen for photosystem assembly (58). Similarly, the yeast UPF0016 protein Gdt1p translocates Mn(II) and Ca(II) from the cytosol to the Golgi compartment, where it contributes to activities required for protein glycosylation (59). It is interesting to note that when expressed in the bacterium Lactococcus lactis, yeast Gdt1p mediated Mn(II) influx. A similar role has been ascribed to the mammalian homolog, TMEM165 (60). Although we favor a model in which bacterial TerC homologs function physiologically in Mn(II) export, perhaps coupled to metallation of secreted or membrane-localized enzymes, they will likely function in import if inserted in the membrane in the wrong orientation. This could explain the impact of expressing a heterologous yeast protein in L. lactis (59) and may also account for the reported increase in cytosolic Mn(II) when the E. coli Alx protein was overproduced (42). Further studies will be required to test this general hypothesis regarding the possible roles of TerC proteins in B. subtilis physiology.
In conclusion, the studies presented here expand our view of the set of proteins affecting cellular Mn(II) homeostasis. In addition to MntR-regulated importers (MntABC and MntH) and efflux proteins (MneP and MneS), the two TerC homologs (YceF and YkoY) appear to play overlapping functions related to Mn(II) export. For YkoY, this export function may provide a backup pathway for detoxifying the cytosol, as suggested by its regulation by a Mn(II)-inducible riboswitch. Alternatively, it may be involved in metallating a secreted or membrane-associated protein that is only metallated when Mn(II) is abundant in the cell. The role of YceF may also be associated with metallation of exoenzymes, as suggested by its ability to reduce the accumulation of intracellular Mn(II), the lack of strong regulation by Mn(II), and by analogy with UPF0016 members from Eukarya. The role of the YybP protein in Mn(II) homeostasis was not apparent under our tested conditions. Further studies will be required to define how these and other factors help cells adapt to the changing availability of essential metal ions.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains were used in this study as described in Table S1 in the supplemental material. B. subtilis was grown in lysogeny broth (LB). Ampicillin (100 mg ml−1) was used for selection in Escherichia coli strains. Erythromycin (1 mg ml−1) was used for the selection of the B. subtilis strains. Techniques for B. subtilis transformation were as performed as described previously (61). Clean deletions were generated using the Cre recombinase in the pDR244 plasmid (52).
To make promoter-lacZ transcriptional fusions, promoters were synthesized from CU1065 genomic DNA using the primers in Table S2, digested with EcoRI/HindIII or HindIII/BamHI, and cloned into pDG1663 (62). Constructs were then introduced into CU1065 B. subtilis. For complementation, gene products were amplified from CU1065 genomic DNA, digested with HindIII/BglII or HindIII/XbaI, and cloned into pPL82 (63). The new plasmids, which allow IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression of genes, were linearized with PstI and transformed into the B. subtilis chromosome at the amyE locus.
Mariner transposon mutagenesis.
Mariner transposon mutagenesis procedure was carried out in the mneP mneS mutant strain (PS strain) using the pMarA mariner (mTn) delivery plasmid as described previously (64). Cells were grown at 30°C until stationary phase to maintain pMarA and were then selected for growth on LB plates containing elevated levels of Mn(II) (100 and 150 μM) at 42°C. Colonies that grew overnight were inoculated in LB supplemented with kanamycin (15 μg ml−1) at 37°C to ensure the integration of the transposon. Genomic DNA was isolated and then subjected to TaqαΙ restriction enzyme digestion, followed by a ligation of sticky ends to generate circular DNA. An inverse PCR using primers annealing to the ends of the mTn was performed, followed by sequence analysis to identify the orientation and location of the mTn insertions.
Zone of inhibition assays.
Cells were grown in LB with shaking at 37°C to an optical density at 600 nm (OD600) of ∼0.4. A 100-μl aliquot of cultures was mixed with 4 ml of prewarmed LB soft agar (0.75% agar), poured onto LB agar plates (containing 15 ml of 1.5% LB agar), and allowed to solidify at room temperature for 10 min. Filter paper disks (7 mm) were placed on top of the soft agar, 10 μl of 100 mM MnCl2 was added to the disks, and the plates were incubated at 37°C overnight. For IPTG induction, IPTG was added to both the soft agar and the plates to a final concentration of 0.1 mM. The data shown represent the averages and standard deviations (SD) of three biological replicates.
Bioscreen assay.
Strains were grown at 37°C to an OD600 of ∼0.4, from which 2 μl of cells was inoculated to 200 μl in a Bioscreen 100-well microtiter plate. Growth was monitored spectrophotometrically (OD600) every 15 min for 25 h using a Bioscreen C growth analyzer (Growth Curves USA, Piscataway, NJ) at 37°C with continuous shaking. Data shown are representative growth curves (or cell density at a fixed time), and experiments were repeated at least three times.
ICP-MS.
Cells were grown in LB medium to an OD600 of ∼0.4 and then shocked with 100 μM MnCl2. Samples (4 ml) were collected immediately before shock and at 30 min after shock. Samples were prepared and analyzed by ICP-MS as previously described (37). Cells were washed twice with phosphate-buffered saline (PBS) buffer containing 0.1 M EDTA, followed by two Chelex-treated PBS-buffer-only washes. Cells were resuspended in 500 μl of Chelex-treated PBS buffer with 1% Triton X-100 and containing 75 mM NaN3, followed by incubation at 37°C for 90 min. The samples were centrifuged, and 10 μl of the supernatant was used to measure the total cell protein concentration by Bradford assay. Then, 750 μl of 5% HNO3 with 0.1% (vol/vol) Triton X-100 was added to the rest of the supernatant, which was boiled at 95°C for 30 min. Samples were centrifuged, and then the supernatant was diluted with 1% HNO3. Metal levels were measured by ICP-MS (Perkin-Elmer ELAN DRC II using ammonia as the reaction gas and gallium as an internal standard) and normalized against the total cell protein concentration. Data represent means ± ranges of two separate experiments.
β-Galactosidase assays.
Strains containing promoter-lacZ fusions were grown in LB to an OD600 of ∼0.4 with a gradient of concentrations of MnCl2 added to the media. β-Galactosidase assays were performed as described previously (65), except that cells were lysed with lysozyme at 0.1 mg ml−1 for 30 min at 37°C instead of chloroform.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institutes of Health (R35GM122461) to J.D.H. S.P. was supported by undergraduate grants from the Cornell University College of Agriculture and Life Sciences and the Cornell Institute of Host-Microbe Interactions and Disease.
We thank members of the Helmann lab for helpful comments. We also thank Chris Furman for the construction of yybP and ykoY overexpression strains and Daniel Kim for preliminary ICP-MS data.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Juttukonda LJ, Skaar EP. 2015. Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol 97:216–228. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmann JD. 2014. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem 289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radin JN, Kelliher JL, Solorzano PKP, Grim KP, Ramezanifard R, Slauch JM, Kehl-Fie TE. 2019. Metal-independent variants of phosphoglycerate mutase promote resistance to nutritional immunity and retention of glycolysis during infection. PLoS Pathog 15:e1007971. doi: 10.1371/journal.ppat.1007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutfilz CR, Wang NE, Hoff CA, Lee JA, Hackert BJ, Courcelle J, Courcelle CT. 2019. Manganese is required for the rapid recovery of DNA synthesis following oxidative challenge in Escherichia coli. J Bacteriol doi: 10.1128/JB.00426-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imlay JA. 2014. The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobota JM, Gu M, Imlay JA. 2014. Intracellular hydrogen peroxide and superoxide poison 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J Bacteriol 196:1980–1991. doi: 10.1128/JB.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobota JM, Imlay JA. 2011. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A 108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JE, Imlay JA. 2011. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol 80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant SS, Helmann JD. 2012. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Physiol 60:91–210. doi: 10.1016/B978-0-12-398264-3.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini S, Imlay JA. 2015. The induction of two biosynthetic enzymes helps Escherichia coli sustain heme synthesis and activate catalase during hydrogen peroxide stress. Mol Microbiol 96:744–763. doi: 10.1111/mmi.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez CA, Skaar EP. 2018. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23:737–748. doi: 10.1016/j.chom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer LD, Skaar EP. 2016. Transition metals and virulence in bacteria. Annu Rev Genet 50:67. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zygiel EM, Nolan EM. 2018. Transition metal sequestration by the host-defense protein calprotectin. Annu Rev Biochem 87:621–643. doi: 10.1146/annurev-biochem-062917-012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Stubbe J. 2011. Bacillus subtilis class Ib ribonucleotide reductase is a dimanganese(III)-tyrosyl radical enzyme. Biochemistry 50:5615–5623. doi: 10.1021/bi200348q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chander M, Setlow B, Setlow P. 1998. The enzymatic activity of phosphoglycerate mutase from gram-positive endospore-forming bacteria requires Mn2+ and is pH sensitive. Can J Microbiol 44:759–767. doi: 10.1139/cjm-44-8-759. [DOI] [PubMed] [Google Scholar]
- 17.Juttukonda LJ, Berends ETM, Zackular JP, Moore JL, Stier MT, Zhang Y, Schmitz JE, Beavers WN, Wijers CD, Gilston BA, Kehl-Fie TE, Atkinson J, Washington MK, Peebles RS, Chazin WJ, Torres VJ, Caprioli RM, Skaar EP. 2017. Dietary manganese promotes staphylococcal infection of the heart. Cell Host Microbe 22:531–542.e8. doi: 10.1016/j.chom.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez CA, Skaar EP. 2018. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23:737–748. doi: 10.1016/j.chom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colomer-Winter C, Flores-Mireles AL, Baker SP, Frank KL, Lynch AJL, Hultgren SJ, Kitten T, Lemos JA. 2018. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog 14:e1007102. doi: 10.1371/journal.ppat.1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si M, Zhao C, Burkinshaw B, Zhang B, Wei D, Wang Y, Dong TG, Shen X. 2017. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc Natl Acad Sci U S A 114:E2233–E2242. doi: 10.1073/pnas.1614902114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eijkelkamp BA, McDevitt CA, Kitten T. 2015. Manganese uptake and streptococcal virulence. Biometals 28:491–508. doi: 10.1007/s10534-015-9826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do H, Makthal N, Chandrangsu P, Olsen RJ, Helmann JD, Musser JM, Kumaraswami M. 2019. Metal sensing and regulation of adaptive responses to manganese limitation by MtsR is critical for group A streptococcus virulence. Nucleic Acids Res 47:7476–7493. doi: 10.1093/nar/gkz524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin JN, Zhu J, Brazel EB, McDevitt CA, Kehl-Fie TE. 2019. Synergy between nutritional immunity and independent host defenses contributes to the importance of the MntABC manganese transporter during Staphylococcus aureus infection. Infect Immun 87:e00642-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. 2015. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunenwald CM, Choby JE, Juttukonda LJ, Beavers WN, Weiss A, Torres VJ, Skaar EP. 2019. Manganese detoxification by MntE is critical for resistance to oxidative stress and virulence of Staphylococcus aureus. mBio 10:e02915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsrude MJ, Pitzer JE, Martin DW, Roop RM II. 2019. The cation diffusion facilitator family protein EmfA confers resistance to manganese toxicity in Brucella abortus 2308 and is an essential virulence determinant in mice. J Bacteriol doi: 10.1128/JB.00357-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilhen C, Taha MK, Veyrier FJ. 2013. Role of transition metal exporters in virulence: the example of Neisseria meningitidis. Front Cell Infect Microbiol 3:102. doi: 10.3389/fcimb.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. 2009. Role of the manganese efflux system mntE for signaling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol 72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veyrier FJ, Boneca IG, Cellier MF, Taha MK. 2011. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog 7:e1002261. doi: 10.1371/journal.ppat.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh YK, Freese E. 1976. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J Bacteriol 127:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 32.Guedon E, Moore CM, Que Q, Wang T, Ye RW, Helmann JD. 2003. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA, and σB regulons. Mol Microbiol 49:1477–1491. doi: 10.1046/j.1365-2958.2003.03648.x. [DOI] [PubMed] [Google Scholar]
- 33.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey R, Russo R, Ghanny S, Huang X, Helmann J, Rodriguez GM. 2015. MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol 98:1168–1183. doi: 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merchant AT, Spatafora GA. 2014. A role for the DtxR family of metalloregulators in Gram-positive pathogenesis. Mol Oral Microbiol 29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Shin J-H, Pinochet-Barros A, Su TT, Helmann JD. 2017. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol 103:253–268. doi: 10.1111/mmi.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. 2004. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci U S A 101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G. 2015. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell 57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price IR, Gaballa A, Ding F, Helmann JD, Ke A. 2015. Mn2+-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol Cell 57:1110–1123. doi: 10.1016/j.molcel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DE. 1999. Bacterial tellurite resistance. Trends Microbiol 7:111–115. doi: 10.1016/s0966-842x(99)01454-7. [DOI] [PubMed] [Google Scholar]
- 42.Zeinert R, Martinez E, Schmitz J, Senn K, Usman B, Anantharaman V, Aravind L, Waters LS. 2018. Structure-function analysis of manganese exporter proteins across bacteria. J Biol Chem 293:5715–5730. doi: 10.1074/jbc.M117.790717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trachsel E, Redder P, Linder P, Armitano J. 2019. Genetic screens reveal novel major and minor players in magnesium homeostasis of Staphylococcus aureus. PLoS Genet 15:e1008336. doi: 10.1371/journal.pgen.1008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armitano J, Redder P, Guimaraes VA, Linder P. 2016. An essential factor for high Mg2+ tolerance of Staphylococcus aureus. Front Microbiol 7:1888. doi: 10.3389/fmicb.2016.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, et al. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 46.Cao M, Kobel PA, Morshedi MM, Wu MF, Paddon C, Helmann JD. 2002. Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J Mol Biol 316:443–457. doi: 10.1006/jmbi.2001.5372. [DOI] [PubMed] [Google Scholar]
- 47.Eiamphungporn W, Helmann JD. 2008. The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Mol Microbiol 67:830–848. doi: 10.1111/j.1365-2958.2007.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asai K, Yamaguchi H, Kang CM, Yoshida K, Fujita Y, Sadaie Y. 2003. DNA microarray analysis of Bacillus subtilis sigma factors of extracytoplasmic function family. FEMS Microbiol Lett 220:155–160. doi: 10.1016/S0378-1097(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 49.Luo Y, Asai K, Sadaie Y, Helmann JD. 2010. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function sigma factors. J Bacteriol 192:5736–5745. doi: 10.1128/JB.00826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersohn A, Brigulla M, Haas S, Hoheisel JD, Volker U, Hecker M. 2001. Global analysis of the general stress response of Bacillus subtilis. J Bacteriol 183:5617–5631. doi: 10.1128/JB.183.19.5617-5631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chasteen TG, Fuentes DE, Tantalean JC, Vasquez CC. 2009. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev 33:820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- 52.Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann AB, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst 4:291–305 e7. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eichenberger P, Jensen ST, Conlon EM, van Ooij C, Silvaggi J, Gonzalez-Pastor JE, Fujita M, Ben-Yehuda S, Stragier P, Liu JS, Losick R. 2003. The σE regulon and the identification of additional sporulation genes in Bacillus subtilis. J Mol Biol 327:945–972. doi: 10.1016/s0022-2836(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 54.Kliegman JI, Griner SL, Helmann JD, Brennan RG, Glasfeld A. 2006. Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis. Biochemistry 45:3493–3505. doi: 10.1021/bi0524215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachas ST, Ferré-D’Amaré AR. 2018. Convergent use of heptacoordination for cation selectivity by RNA and protein metalloregulators. Cell Chem Biol 25:962–973 e5. doi: 10.1016/j.chembiol.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demaegd D, Colinet AS, Deschamps A, Morsomme P. 2014. Molecular evolution of a novel family of putative calcium transporters. PLoS One 9:e100851. doi: 10.1371/journal.pone.0100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider A, Steinberger I, Strissel H, Kunz HH, Manavski N, Meurer J, Burkhard G, Jarzombski S, Schunemann D, Geimer S, Flugge UI, Leister D. 2014. The Arabidopsis tellurite resistance C protein together with ALB3 is involved in photosystem II protein synthesis. Plant J 78:344–356. doi: 10.1111/tpj.12474. [DOI] [PubMed] [Google Scholar]
- 58.Hoecker N, Leister D, Schneider A. 2017. Plants contain small families of UPF0016 proteins, including the photosynthesis affected mutant 71 transporter. Plant Signal Behav 12:e1278101. doi: 10.1080/15592324.2016.1278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thines L, Deschamps A, Sengottaiyan P, Savel O, Stribny J, Morsomme P. 2018. The yeast protein Gdt1p transports Mn2+ ions and thereby regulates manganese homeostasis in the Golgi. J Biol Chem 293:8048–8055. doi: 10.1074/jbc.RA118.002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potelle S, Morelle W, Dulary E, Duvet S, Vicogne D, Spriet C, Krzewinski-Recchi MA, Morsomme P, Jaeken J, Matthijs G, De Bettignies G, Foulquier F. 2016. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum Mol Genet 25:1489–1500. doi: 10.1093/hmg/ddw026. [DOI] [PubMed] [Google Scholar]
- 61.Gryczan TJ, Contente S, Dubnau D. 1978. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol 134:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerout-Fleury AM, Frandsen N, Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 63.Quisel JD, Burkholder WF, Grossman AD. 2001. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol 183:6573–6578. doi: 10.1128/JB.183.22.6573-6578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Breton Y, Mohapatra NP, Haldenwang WG. 2006. In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72:327–333. doi: 10.1128/AEM.72.1.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.