Abstract
Dopaminergic dysfunction has long been connected to the development of HIV infection in the CNS. Our previous data showed that dopamine increases HIV infection in human macrophages by increasing the susceptibility of primary human macrophages to HIV entry through stimulation of both D1-like and D2-like receptors. These data suggest that, in macrophages, both dopamine receptor subtypes may act through a common signaling mechanism. To define better the mechanism(s) underlying this effect, this study examines the specific signaling processes activated by dopamine in primary human monocyte-derived macrophages (hMDM). In addition to confirming that the increase in entry is unique to dopamine, these studies show that dopamine increases HIV entry through a PKA insensitive, Ca2+ dependent pathway. Further examination demonstrated that dopamine can signal through a previously defined, non-canonical pathway in human macrophages. This pathway involves both Ca2+ release and PKC phosphorylation, and these data show that dopamine mediates both of these effects and that both were partially inhibited by the Gq/11 specific inhibitor YM-254890. Studies have shown that Gq/11 preferentially couples to the D1-like receptor D5, indicating an important role of the D1-like receptors in mediating these effects. These data indicate a role for Ca2+ flux in the HIV entry process, and suggest a distinct signaling mechanism mediating some of the effects of dopamine in macrophages. Together, the data indicate that targeting this alternative dopamine signaling pathway might provide new therapeutic options for individuals with elevated CNS dopamine suffering from NeuroHIV.
Keywords: HIV associated neurocognitive disease, dopamine signaling, macrophage, calcium signaling, HIV viral entry
1. Introduction
In addition to its role in cognition, reward and motor control, dopamine has significant immunomodulatory properties (1-3). Dopamine receptors are present on many types of immune cells, including macrophages, T-lymphocytes and neutrophils (4), and have been shown to regulate an array of different immune functions (1, 2, 5), in both the CNS and the periphery (6). Dopamine has also been shown to have a significant effect on the etiology of a number of neurologic diseases, including HIV-associated neuropathologies (7, 8). Dopaminergic dysfunction, elevated pathology, infection and atrophy in dopaminergic brain regions and aberrant dopamine metabolism have been observed in infected individuals since early in the epidemic (8-18). While the regional pathology associated with NeuroHIV has shifted in the cART era, with less overt neuronal death and damage (19, 20), there is still significant dopaminergic involvement, with increased neuropathology and inflammation in dopaminergic regions, as well as disruption in cognitive processes initiated in those areas (19, 21-33).
Despite these studies, the precise relationship between dopamine and HIV neuropathogenesis remains unclear. The primary targets for HIV in the CNS are myeloid lineage cells such as macrophages and microglia (34-38). These cells are central to the development of HIV-associated neuropathology, as infected cells release virus, inflammatory mediators and neurotoxic viral proteins, spreading infection and inducing chronic neuroinflammation and subsequent neuropathology (19). Our previous data demonstrate that dopamine increases HIV infection in primary human monocyte derived macrophages (hMDM) (39), by increasing the susceptibility of the target cells to HIV entry (40). These data correlate with studies showing elevated HIV mRNA (16, 17) and greater neuropathology in dopaminergic brain regions such as the basal ganglia and substantia nigra (SbN) (9, 41). This suggests that the impact of dopamine signaling on macrophages could contribute significantly to the development and exacerbation of NeuroHIV.
Dopamine acts primarily through activation of its cognate G-protein coupled receptors, the dopamine receptors. The 5 dopamine receptors are divided into 2 subgroups; the D1-like receptors (D1 and D5) and the D2-receptors (D2, D3, D4). The D1-like receptors mainly couple to Gαs and increase intracellular cyclic AMP (cAMP), while the D2-like receptors primarily couple Gαi, inhibiting cAMP production (42-44). We have previously demonstrated that the dopamine-mediated increase in HIV entry could be induced by activation of either D1-like or D2-like receptors (40). Although D1-like receptors and D2-like receptors conventionally have opposing effects, the involvement of both dopamine receptor sub-types in increasing HIV entry suggests a common, non-canonical activation (42, 44-46). Interestingly, dopamine has also been shown to signal via an alternative mechanism involving Gq/11 or Gβγ activation of PLC, followed by activation of IP3 and diacylglycerol, release of intracellular Ca2+ from the endoplasmic reticulum, and increased activation of PKC (44, 47-54). Rodent studies have shown these signaling cascades can be mediated by the activation of either D5 coupling to Gq/11 (50, 55), or by D2 acting through Gβγ (49, 54). Increases in Ca2+ have previously been associated with the HIV entry process, and several studies have demonstrated that binding of the viral Env protein gp120 to the CCR5 coreceptor initiates a similar series of signaling events mediated by Gq/11 (56-58). This suggests that activation of this alternate signaling pathway could be one mechanism by which dopamine increases HIV entry into macrophages. However, the connection of this pathway to HIV entry, and the presence and activity of this signaling cascade in macrophages is unclear.
Within the CNS, the cells targeted by HIV are exposed to variable levels of dopamine. In dopaminergic regions of the CNS such as the striatum and SbN, the amount of dopamine to which macrophages can be exposed depends on the stimulus initiating dopamine release (59-61). Further, because dopamine signaling is mediated by volume transmission (59, 62), stimuli that increase dopamine concentrations will increase the brain volume and consequently the number of cells exposed to dopamine (62, 63). To effectively model the range of dopamine concentrations to which CNS macrophages could be exposed under both homeostatic conditions and in response to the use of drugs and therapeutics, this study uses a range of dopamine concentrations (10−6M to 10−9M). While the precise dopamine concentrations in the human CNS remain undetermined, estimates place tonic concentrations in the low nanomolar range (6, 60). However, drug abuse and the use of dopaminergic therapeutics, can increase dopamine concentrations in the brains of primates to the low micromolar range (60, 64, 65), and potentially increase the area of the brain and number of cells exposed to dopamine. In addition, macrophages in peripheral tissues can also be exposed to high levels of dopamine, potentially impacting infection in those regions as well (6). Therefore, there is a critical need to understand the relationship between dopamine signaling and viral infection in macrophages to address infection in individuals with elevated levels of dopamine.
These studies address this knowledge gap by examining the activity of this alternative dopamine signaling pathway in myeloid cells and determining whether it is involved in the effects of dopamine on the HIV entry process. We assessed the ability of dopamine to induce calcium release and activate PKC in human macrophages and evaluated the importance of this signaling in modulating the effects of dopamine on viral entry. We demonstrate that the dopamine-mediated increase in HIV entry requires Ca2+ release but not activation of PKA, suggesting that this process is mediated by the non-canonical signaling pathway. The data also demonstrate that this signaling mechanism is active in hMDM, as dopamine was shown to increase calcium release and phosphorylate PKC in a Gq/11-dependent manner. Overall, these studies suggest new therapeutic targets which could ameliorate the progression of NeuroHIV and propose a novel paradigm for dopamine signaling in myeloid immune cells.
2. Materials and Methods
2.1. Reagents
RPMI-1640 and penicillin-streptomyocin (P/S) from Invitrogen (Carlsbad, CA). Human AB serum and fetal bovine serum (FBS) from Mediatech (Tewksbury, MA). HEPES solution from Fisher Scientific (Fair Lawn, NJ). Dantrolene sodium salt, serotonin hydrochloride, isoproterenol hydrochloride, NKH 477, forskolin and phorbol 12-myristate 13-acetate (PMA) from Tocris Biosciences (Minneapolis, MN). Isoproterenol hydrochloride from R&D Systems (Minneapolis MN). Dopamine and H89 dihydrochloride from Sigma-Aldrich (St. Louis, MO). 3-Isobutyl-1-methylxanthine (IBMX) from MP Biomedicals (Santa Ana, CA). RO-20-1724 from EMD Millipore (Temecula, CA). YM-254,890 from Focus Biomolecules (Plymouth Meeting, PA). Dopamine and the beta-adrenergic receptor agonist Isoproterenol were resuspended in distilled H2O to make stock concentrations of 10 mM, then diluted into media as needed. RO-20-1724 (200 mM), IBMX (100 mM), Forskolin (10 mM), YM-254,890 (10 mM) and PMA (100 μM) were resuspended in DMSO to the indicated concentrations, then diluted into media as indicated. Human macrophage colony stimulating factor (M-CSF) was from Peprotech (Rocky Hill, NJ) and resuspended in 100 μL distilled H2O.
2.2. Cell Isolation and Culture
Human peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Paque (GE Healthcare, Piscataway, NJ) gradient centrifugation from blood obtained by healthy individuals (New York Blood Center, Long Island City, New York; and BioreclamationIVT Collection Center, Miami, Florida). The percentage of monocytes in the PBMC from each donor was determined by isolating monocytes from 20 × 106 PBMC using a Pan Monocyte Isolation Kit (Miltenyl Biotech, Auburn, CA). PBMC were then plated at 100,000 monocytes per cm2 in tissue culture dishes (Corning, NY). Monocyte-derived macrophages (hMDMs) were obtained through adherence isolation by maturing the monocytes in macrophage media (RPMI-1640 with 10% FBS, 5% human AB serum, 10mM HEPES, 1% P/S, and 10 ng/mL M-CSF). The cells were washed after 3 days and then cultured for an additional 3 - 4 days. After 6 days in culture, cells were considered mature MDM. All experiments were performed on day 6 or day 7 post-isolation.
2.3. Quantitative RT-PCR
Isolation of mRNA from hMDMs was accomplished using the RNeasy Mini Plus kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Preparations were treated with DNase cocktail composed of 70 μl buffer RDD, 10 μL DNase I (RNase Free DNase Kit, Qiagen, Hilden, Germany) and 1 μL Turbo DNase (Turbo DNase Kit, Thermo Fisher Scientific, Waltham, MA). The concentration and purity of RNA was determined using a NanodropOne spectrophotometer (Nanodrop Technologies, Wilmington, DE). To generate cDNA, 500 ng/μl of RNA was run in a High-Capacity cDNA Reverse Transcriptase kit (Applied Biosystems, Foster City, CA). Human brain total RNA (Life Technologies, Carlsbad, CA) was used as a positive control, and reactions containing no sample was used as a negative control. Taqman 20x gene expression assays for human dopamine receptor 1 (DRD1, Hs00265245_s1), dopamine receptor 2 (DRD2, Hs00241436_m1), dopamine receptor 3 (DRD3, Hs00364455_m1), dopamine receptor 4 (DRD4, Hs00609526_m1), and dopamine receptor 5 (DRD5, Hs00361234_s1) (Thermo Fisher Scientific, Waltham, MA) were used to determine expression levels of the dopamine receptors. All reactions were performed in triplicate using a Taqman 20x gene expression assay. 18s (Hs99999901_s1, Thermo Fisher Scientific, Waltham, MA) was used as an internal control. Expression of DR in total human brain RNA (ThermoFisher Scientific) was used as a positive control for DR expression. All cycle threshold values above 37 cycles were considered to be negative, and were set to 37 cycles prior to data analysis. All values were transformed for analysis using the equation 2−ΔCt.
2.4. Analysis of cAMP production
At T = −30 minutes, hMDM cultured in 12-well plates were treated with the phosphodiesterases IBMX (500 μM) and RO-20-17,24 (100 μM) to prevent cAMP breakdown. At T= 0, plates were treated with dopamine as indicated. At 5, 10, 15 or 30 minutes, cells were washed with cold PBS, and 150 μL of lysis buffer from the Direct cAMP EIA kit (Ann Arbor Assays, Ann Arbor, MI) was added. Cells were incubated for 10 minutes RT, lysate was collected, spun down at 600 × g for 15 minutes and supernatant was aliquoted and stored at - 20° C. Cyclic AMP assays were performed using the Direct cAMP EIA kit according to the manufacturer’s instructions. Briefly, standards were prepared at 150 pM/mL, 50 pM/mL, 16.67 pM/mL, 5.56 pM/mL, 1.85 pM/mL, and 0.617 pM/mL. Plate was prepared by adding 25 μL of plate primer, then 50 μL of either freshly thawed sample or standard, 25 μL of DetectX cAMP Conjugate and 25 μL of DetectX cAMP antibody. Diluent alone was used as a negative control. Samples were shaken for 2 hours at RT, washed and developed using 100 μL of TMB substrate. After an additional 30 minutes of shaking, 50 μL stop solution was added and plates were analyzed using an Enspire Multimode Plate Reader (Perkin-Elmer, Waltham, MA).
2.5. Signaling Assays and Protein Lysate preparation
hMDM cultured in 6-well dishes were fed with fresh macrophage media 3-18 hours prior to all experiments. Cells were treated with the indicated concentrations of dopamine for 1, 2.5, 5, or 10 minutes, immediately washed in 1X PBS and then lysed in M-PER Mammalian extraction reagent (Thermo Fisher Scientific, Waltham, MA), with 1% Halt Protease and Phosphatase Inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA). Phorbol myristic acid (PMA, 10−6 M) was added to one well 30 minutes prior to dopamine treatment as a positive control. For assays examining the involvement of Gq/11, some cells were pretreated for 30 minutes with the Gq/11 inhibitor YM-254,890 prior to dopamine treatment. After lysing, cells were sonicated with a Q125 sonicator (Qsonica, Newtown, CT) at 25% power for 5 seconds and then spun down at 13,000 RPM for 10 minutes at 4° C. Lysates were then stored at 4°C until the protein concentration could be quantified by Bicinchoninic acid assay (BCA) using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific).
2.6. Western Blotting
Protein lysates (20 μg) were run on a Bolt Bis-Tris Plus 10% precast gel (Life Technologies, Carlsbad CA) in MOPS/SDS running buffer in a mini gel tank (Life Technologies) for 50 minutes at 200V. Gels were then transferred onto an Immobilon PVDF membrane from EMD Millipore (Temecula, CA) at 25V for 40 minutes. Total protein stain was performed using Revert Total Protein Stain (LI-COR Biosciences, Lincoln, NE) according to the manufacturer’s instructions. After addition of Reversal Solution, (LI-COR Biosciences, Lincoln, NE), the membranes were blocked in 50% Odyssey Blocking Buffer (TBS) for 1 hour (Licor Biosciences, Lincoln, NE). Blots were then incubated overnight at 4°C on a shaker with pan-phospho-PKC Ser660 (Cell Signal Technology, Danvers, MA) diluted in primary antibody solution (TBS - 0.2% Tween) at 1:1000. The next day, blots were washed 3x in TBS-0.1% Tween. Blots were stained with IRDye 800CW Donkey Anti-Rabbit IgG secondary antibody diluted in secondary antibody solution (TBS-0.2% Tween + SDS 0.01%) at a 1:20,000 dilution and membranes were incubated in the dark at room temperature for 1 hour. Membranes were again washed three times and imaged using the Odyssey Fc Imaging System (Licor Biosciences, Lincoln, NE).
Blots were analyzed using Image Studio Lite (Licor Biosciences, Lincoln, NE). Phospho-PKC signals were normalized to the total protein stain, then each condition was compared to the untreated control to determine fold-change in phosphorylation. For experiments examining the effects of YM-254,890 on PKC phosphorylation, only donors who showed above a 1.3-fold increase in response to dopamine were included.
2.7. HIV Entry Assay
Entry assays were performed using stocks of HIVBaL containing Vpr-β-lactamase (β-lac HIV), and stocks were prepared as previously described (40). Entry assays were run using the Geneblazer in vivo detection kit (Thermo Fisher Scientific, Waltham, MA), optimized for use in primary macrophages as previously described (40). hMDM are cultured in flat, clear bottom 96 well black plates (Thermo Fisher Scientific, Waltham, MA) were infected with β-lac HIV at an MOI of 0.01, which is equivalent to approximately 10 ng•p24/mL, for 2.5 hours at 37°C with 5% CO2. Dopamine (10−6M or 10−9M) was added concurrently with HIV. These concentrations of dopamine were used as representative of pharmacologic and physiologic concentrations of dopamine, although prior studies in the lab show no differences between the magnitude of the effect of dopamine at 10−5M, 10−6M, 10−7M and 10−8M (40). The PKA inhibitor H89 (10−5M) and the calcium inhibitor dantrolene (10−4M) were added 60 minutes prior to inoculation and maintained in culture through the end of the inoculation period. NKH 477 (10−5M) was added concurrently with dopamine.
After the 2.5 hr inoculation, hMDM were washed and incubated in the dark at RT for 6 hours in 100 μl of phenol red free macrophage media and 20 μL of 6X CCF2-AM loading solution. Due to a lab relocation during this project, two different systems were used to image these infections. For all but 5 of these donors, 12 images of each infection condition were obtained on a Zeiss IX70 inverted microscope (Zeiss) with an attached Olympus E-620 Live View DSLR (Olympus, Shinjuku, Tokyo, Japan). Volocity (Perkin-Elmer, Waltham, MA) was used to enumerate the number of infected (blue) and uninfected (green) cells in each condition. For the remaining 5 donors, images were taken using the CellInsight CX7 High Content Screening (HSC) Platform. Twenty-five images per well were acquired using this automated platform and the HCS Studio software was used to determine the total number of cells per well. The number of infected (blue) cells were determined for each image using ImageJ software. Susceptibility to HIV infection, and sensitivity to dopamine and the inhibitors used varied among donors, therefore the number hMDM infected with HIV varied substantially between experiments. To evaluate changes in HIV entry induced by each condition, we determined the fold change in infection for each donor relative to the cultures infected with HIV alone by normalizing to the mean of the HIV alone condition.
2.8. Calcium Imaging
Macrophages were cultured on 12 mm round cover slips (#1.5) and incubated with 5 × 10−6M Oregon Green Bapta-488 (OGB 488, Thermo Fisher Scientific, Waltham MA) for 1 hour at room temperature in the dark. Following incubation, cover slips were washed and allowed to rest in 1x external solution (130 mM NaCl, 10 mM HEPES, 34 mM Dextrose, 1.3 mM KH2PO4, 1.5 mM CaCl2•2H2O, 0.5 mM MgSO4•7H2O) for 15 minutes. Imaging experiments were conducted at 37°C. Macrophages on cover slips were placed into an imaging chamber (Warner Instruments, RC-26G, Hamden, CT) and washed with 1X external solution by brief perfusion. Perfusion throughout the experiments was accomplished using a Warner VC-8 automated perfusion system (Warner Instruments, RC-26G, Hamden, CT) with the flow rate approximately 3 mL/min. All experiments were performed on a Nikon FN-1 upright microscope (Nikon Instruments, Melville, NY) using a Nikon 40× 0.8NA NIR APO objective. A phase contrast image was taken prior to and following each experiment to ensure good cell health. Throughout the experiment, a SpectraX Light Engine (Lumencor, Beaverton OR) was used to emit a 480nm excitation laser at 5% power passed through a quadruple bandpass filer (Chroma Technology Corp., Bellows Falls VT). Images were acquired every 3 seconds with 300ms exposure with an Andor Zyla 4.2 PLUS camera (Andor Technology, Belfast, UK). A 1-minute baseline was acquired during perfusion with 1x external solution, after which drug was perfused. Fluorescence signals were measured for each cell using post-hoc regions of interest in Nikon NIS Elements software (Nikon Instruments, Melville NY) and normalized to each cell’s fluorescence baseline. The results were reported as normalized fluorescence.
2.9. Statistical analysis
All data were normalized to the mean of the control condition, and then outlier analysis was performed using the ROUT method with Q set at 1% for all data sets with 5 or more values. After outlier analysis, data were evaluated using the D’Agostino-Pearson Omnibus Normality test and by analysis of skewness to determine whether the data were parametric. Normally distributed data were analyzed using a paired t-test, one-way ANOVA or 2way ANOVA, using repeated measures as appropriate. Data were analyzed using a mixed effects model in cases where a condition was removed as an outlier or due to technical error in the experimental process. Data that were not parametrically distributed were analyzed using a Wilcoxon test, Kruskal-Wallis test or Friedmans’ test as appropriate. For ANOVA analyses, post-hoc analysis was performed using Tukeys’ multiple comparisons’ test if the distribution was normal, Dunn’s multiple comparisons test if it was not. All data analysis was performed using GraphPad Prism 8.0 (Graphpad, La Jolla, CA), with p < 0.05 was considered significant.
3. Results
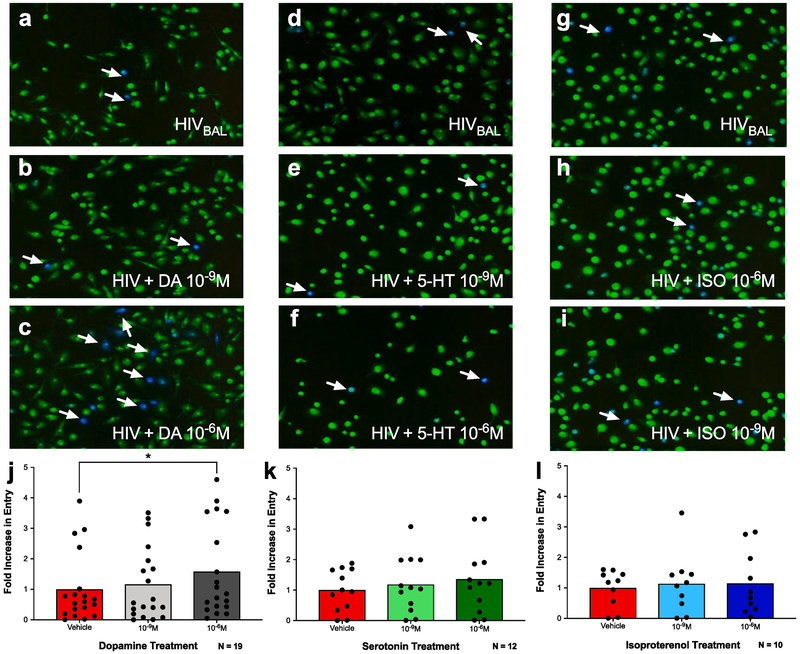
3.1. Activation of dopamine receptors, but not serotonin or norepinephrine receptors, increases HIV entry into macrophages
Our previous data show that exposure to dopamine increases HIV replication in primary human monocyte-derived macrophages (hMDM) (39). This is due to an increase in the entry of HIV into these cells, mediated by activation of both D1-like and D2-like dopamine receptors and induced by dopamine concentrations of 10−8M dopamine or higher (40). However, many drugs that increase dopamine, such as psychostimulants and anti-depressants can also activate the receptors for other biogenic amines (61, 65-67). Therefore, an entry assay was used to examine whether activation of other biogenic amine receptors could increase HIV entry (40). In this assay, hMDM are infected with HIVBaL containing an active β-lactamase enzyme (β-lac HIV), which causes infected cells to fluoresce blue, while uninfected cells remain green, enabling quantification of viral entry. This assay has an advantage over the use of fluorescently labeled viruses in that it enables rapid visualization of viral entry as early as 8 hours post infection, in contrast to other systems using fluorescent virus or luciferase reporters, which can take up to 2 days before enough virus accumulated to enable visualization(68). Therefore, this assay allows for rapid examination of the entry process after a single round of infection.
Cells were infected with β-lac HIV (MOI 0.01) in the presence of either dopamine, serotonin or isoproterenol, a β-adrenergic receptor agonist (Figure 1; representative images of dopamine 1A – C, serotonin 1D – F, isoproterenol 1G – I). Analysis showed a main effect of dopamine on HIV entry into macrophages, and post-hoc analysis showed that 10−6M but not 10−9M dopamine significantly increased entry [1J, n = 19, RM one-way ANOVA, F (1.888, 33.98) = 5.169, *p = 0.0122. Post-hoc analysis with Tukey’s, HIVBAL vs. HIV + DA (10−6M), *p=0.0261]. These effects were specific to dopamine receptor activation, as neither serotonin [1K, n = 12, RM one-way ANOVA, F (1.477, 16.25) = 2.495, p = 0.1242] nor isoproterenol [1L, n = 10, RM one-way ANOVA, F (1.932, 17.39) = 0.2429, p = 0.779] increased viral entry. These data confirm our previous findings (40) that dopamine increases viral entry into human macrophages and indicate that this effect is specific to dopamine receptors.
Figure 1 -. Activation of dopamine receptors, but not serotonin or norepinephrine receptors, increases HIV entry into macrophages.
Human monocyte-derived macrophages (MDM) were infected with β-lac HIV in the presence or absence of dopamine (DA, A-C), serotonin (5-HT, D-F), or isoproterenol (Iso, G-H) at 10−9 M or 10−6 M. (A-H) show representative images of each condition, with infected cells (blue) indicated by white arrows. Dopamine at 10−6 M, but not 10−9 M significantly increased HIV entry over the HIV alone control (J, dopamine gray columns, HIV red columns). In contrast, neither serotonin (K, brown bars) nor isoproterenol (L, blue bars) increased HIV entry over the HIV alone control (red columns) at either concentration used. Data are represented as fold change compared to the mean of the vehicle treated control. *p<0.05.
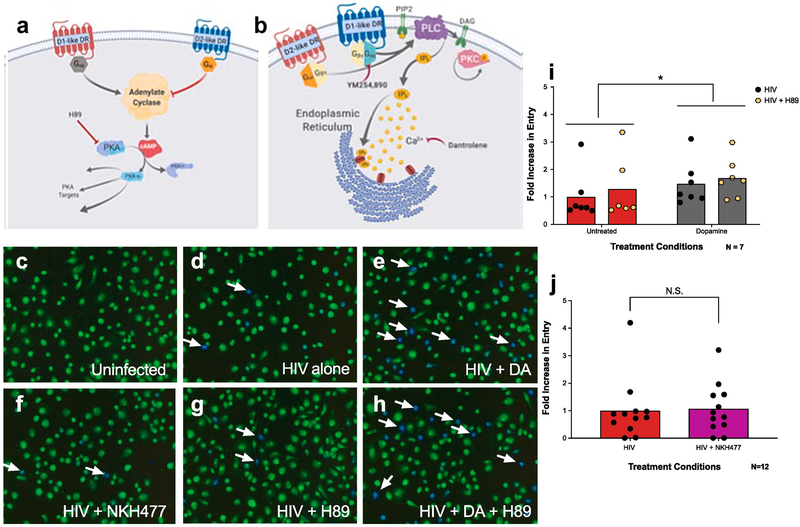
3.2. Dopamine mediated increase in HIV entry is abolished by blocking calcium release but not by inhibiting canonical dopamine signaling
Dopamine receptor activation is associated with several signaling pathways, the most prominent of which is mediated by cAMP as a second messenger (Fig. 2A). Another, less studied signaling mechanism uses Ca2+ released from the endoplasmic reticulum though activation of IP3 receptors as a second messenger (Fig. 2B). The canonical, cAMP-mediated pathway is activated by D1-like dopamine receptors (D1 and D5) coupling to Gαs, and inhibited by activation of D2-like dopamine receptors (D2, D3, D4) coupled to Gαi. In contrast, the alternative, Ca2+ mediated pathway is triggered by activation of both dopamine receptor subtypes, through either D5 receptors coupled to Gq/11 or D2 receptors acting through Gβγ (47, 49-55, 69, 70). In macrophages, the signaling activity of dopamine is undefined, but previous data showed that activation of both dopamine receptor subtypes enhances HIV entry, supporting the hypothesis that dopamine modulation of HIV entry is mediated by a common pathway (40).
Figure 2 -. Dopamine-mediated increase in HIV entry in macrophages is independent of canonical dopamine signaling.
(A, B) Cartoon depicting the dopaminergic signaling pathways. Canonically (A) D1-like receptors (D1 and D5, blue) couple to Gαs, which activates adenylate cyclase, leading to production of cyclic AMP (cAMP), and activation of PKA and further downstream targets. In contrast, D2-like receptors (D2, D3, and D4, red) couple to Gαi to inhibit adenylate cyclase and oppose D1-like signaling. In the non-canonical pathway (B), the D1-like receptor D5 (blue) couples to Gq/11 while the D2-like receptor D2 (red) couples to Gβγ to activate PLC, which hydrolyzes PIP2 to form IP3 and diacyglycerol (DAG). These second messengers then induce IP3-mediated calcium release from the endoplasmic reticulum, and DAG-mediated PKC activation. Inhibitors used throughout this paper to examine these pathways are depicted by red arrows. To examine whether activation of the canonical pathway modulates the effect of dopamine on HIV entry into MDM, human monocyte derived macrophages (MDM) were pretreated with either vehicle (C-F) or H89 (10−6 M, G, H) for 60 min, then infected with β-lac HIV in the presence or absence of dopamine (10−6 M), or NKH 477 (10−5 M) for 2.5 hours. MDM were then incubated in the dark for 6 hrs and HIV entry was assessed. Representative images are depicted in (C-H). Representative infected cells (blue) are indicated by white arrows. Dopamine treatment had a main effect on HIV entry into MDM (gray columns) relative to cells infected with HIV alone (red columns), while treatment with H89 (yellow hexagons) had no effect on HIV entry of the effect of dopamine (I). Increasing cAMP concentrations using NKH477 (pink column) also did not alter HIV entry into MDM relative to cells infected with HIV alone (red column) (J). Data are represented as fold change compared to the mean of the vehicle treated control *p<0.05
Therefore, the role of both the canonical and alternative dopamine signaling pathways in modulating viral entry in macrophages was examined. To study the canonical pathway, hMDM were infected with β-lac HIV either after pretreatment with H89 (10−5M), a PKA inhibitor (71), or concurrently with NKH477, a water-soluble adenylyl cyclase activator (72) (Figure 2C-H). Analysis of the infections pretreated with H89 showed a main effect of dopamine [Figure 2I, 2way ANOVA, F(1,6) = 9.443, *p = 0.0219], with no effect of H89 [(F(1,6) = 4.192, p = 0.0865)] and no dopamine x H89 interaction [(F(1,6) = 0.7539, p = 0.4186)]. Treatment with NKH477 also had no effect on HIV entry (Figure 2J, Wilcoxon signed rank test, p=0.6221). The ability of NKH 477 to increase cAMP in human macrophages was confirmed using a cAMP assay (Supplemental Figure 1). Together, these data indicate that changes in cAMP do not alter the dopamine-mediated increase in HIV entry, and suggest that dopamine’s effects on viral entry are not mediated through the canonical Gαs signaling pathway.
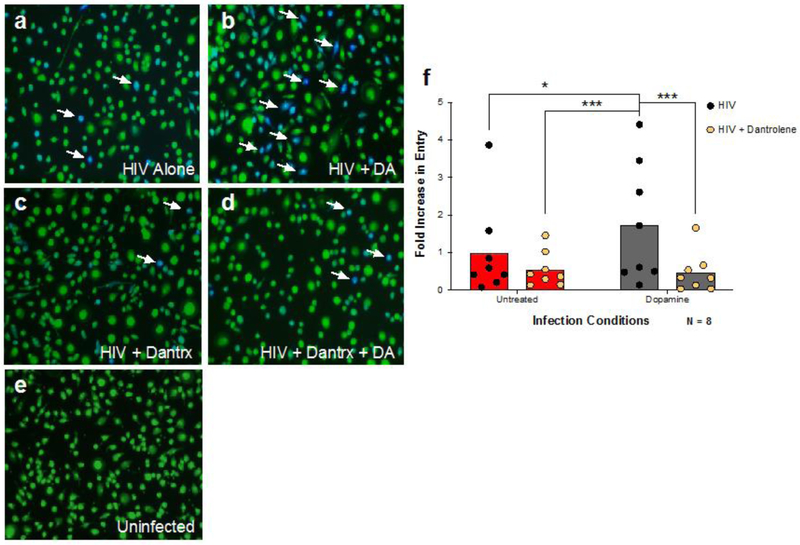
To determine whether the alternative dopamine signaling pathway was involved in the increased HIV entry, hMDM were infected in the presence of dopamine after pretreatment with dantrolene (10−4M), an inhibitor of both IP3 and RyR1 mediated Ca2+ release (73, 74) (Figure 3A - D). Analysis of these experiments showed a significant dantrolene × dopamine interaction [Figure 3F, 2way ANOVA, n = 8, F (1, 7) = 12.88, ** p = 0.0089] with no main effect for either dantrolene and dopamine [F (1,7) = 5.125, p=0.0580 and F (1,7) = 4.547, p = 0.0704]. Post-hoc analysis showed that dopamine significantly increased HIV entry, and that treatment with dantrolene, alone or in the presence of dopamine, significantly reduced entry relative to the effect of dopamine alone (Tukeys’, HIV + DA vs. HIV alone, * p = 0.0108, HIV + DA vs. HIV + Dantrolene, *** p = 0.0007, HIV + DA vs. HIV + DA + Dantrolene, *** p = 0.0005). These data indicate that the dopamine-induced increase in the HIV entry process involves Ca2+ release, suggesting that the effects of dopamine on HIV infection maybe induced by activation of the alternative, Ca2+ mediated signaling pathway.
Figure 3 -. Dopamine-mediated calcium flux is required to increase HIV entry into macrophages.
To examine whether activation of the non-canonical pathway modulates the effect of dopamine on HIV entry into MDM, cells were pretreated with either vehicle (A and B) or Dantrolene (10−4M, Dantrx) (C and D) for 60 min, then infected with β-lac HIV in the presence or absence of dopamine (10−6M) for 2.5 hours. MDM were incubated in the dark for 6 hrs and HIV entry was assessed. Representative images are depicted in (A-E). Representative infected cells (blue) are indicated by white arrows. There was a significant interaction between dopamine and dantrolene, and post-hoc analyses showed dopamine treatment increased HIV entry into macrophages (gray columns) relative to cells infected with HIV alone (red columns). Inhibition of calcium with dantrolene (yellow hexagons) significantly attenuated DA-mediated HIV entry into macrophages (F). Data are represented as fold change compared to the mean of the vehicle treated control *p<0.05, ***p<0.001
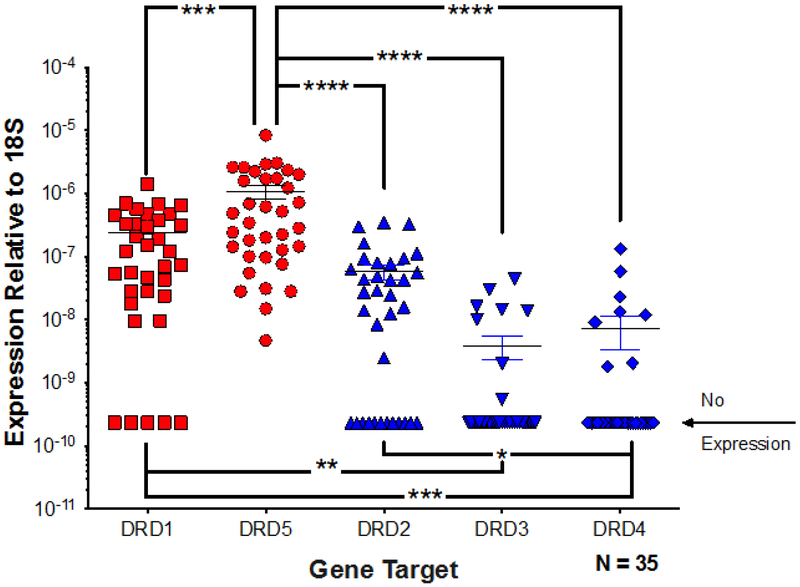
3.3. Primary human macrophages express significantly more D1-like dopamine receptors than D2-like receptors
To better understand why changes in the canonical dopamine signaling pathway were not affecting the dopamine-mediated increase in HIV entry, experiments were performed to define the dopamine signaling pathway in hMDM. While all five dopamine receptor subtypes have been shown to be present in human macrophages (33, 75), the relative expression levels of each subtype have not been closely examined. Therefore, expression of dopamine receptor mRNA was quantified in hMDM RNA from 35 donors using quantitative real-time PCR (qRT-PCR). Due to the unreliability of dopamine receptor antibodies, quantification of mRNA was used (75). While dopamine receptor expression varied significantly among donors (Figure 4, Friedman test, n = 30, Friedman statistic 88.23, p < 0.0001), the relative mean expression of the different subtype transcripts indicated DRD5 > DRD1 ≫ DRD2 > DRD3 > DRD4. This confirms our previously published data showing an identical trend in hMDM from a different group of donors (33). Post hoc analysis showed expression of DRD5 was significantly greater than expression of transcripts for DRD1 and D2-like receptors across all donors (Figure 4, Dunn’s multiple comparisons, DRD5 vs. DRD2, DRD3 or DRD4 **** p > 0.0001, DRD1 vs. DRD3, ** p = 0.0052, and *** DRD1 vs. DRD4, ***p > 0.0005, DRD5 vs DRD1 ***p=0.0009). Transcripts for DRD5 were detected in all hMDM analyzed, whereas DRD1 was undetectable in 16.7% of donors. For the D2-like receptors, DRD2 was the most common, being undetectable in 26.7% of donors, while DRD3 and DRD4 were undetectable in 73.3% of donors. These data indicate D1-like receptors are the predominant dopamine receptors expressed on hMDM and suggest that the majority of dopamine signaling in these cells is mediated by D1-like receptors, specifically D5.
Figure 4 -. Human macrophages express significantly more D1-like receptors than D2-like receptors.
mRNA from human MDM was analyzed for expression of DRD1, DRD2, DRD3, DRD4,DRD5, and 18s through qRT-PCR. Expression of all receptors was normalized to 18s for each donor. Analysis using a one-way ANOVA showed the D1-like receptors DRD1 and DRD5 (red dots) were expressed at significantly higher levels than the D2-like receptors, DRD2, DRD3, and DRD4 (blue dots). *p<0.05, **p<0.01, ****p<0.0001
3.4. Dopamine mediates calcium release in human macrophages
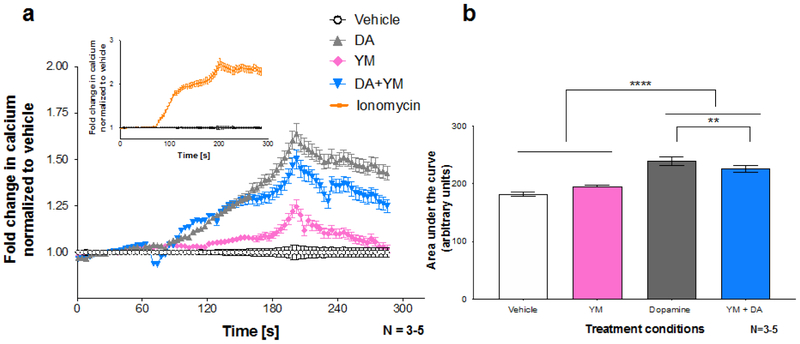
Our previous study showed that dopamine is able to potentiate Ca2+ flux in HEK293 cells (40), but the precise effects of dopamine on intracellular Ca2+ concentrations in human macrophages are unclear. Since our data suggested the dopamine-mediated increase in HIV entry into hMDM was Ca2+-dependent, we further investigated the ability of dopamine to stimulate intracellular Ca2+ release in human macrophages. Although both D1-like and D2-like receptors are proposed to stimulate Ca2+ release via PLC-mediated mechanisms (47-55, 69), these experiments specifically focused on the pathway mediated by the D1-like receptors, specifically D5, coupling to Gq/11 (50, 55). This was due both to the relatively high expression of the D1-like receptors compared to the D2-like receptors, as well as the proposed importance of Gq/11 signaling in HIV entry in other cell types (58). To this end, these assays utilized the compound YM-254,890 (YM), a specific Gq/11 inhibitor (76), to assess the role of Gq/11 in dopamine’s effects on Ca2+ release. Primary hMDM were matured on glass coverslips and loaded with the fluorescent Ca2+ indicator dye Oregon Green Bapta-488 (OGB) to enable real-time assessment of Ca2+ release. Changes in Ca2+ were examined in response to treatment with either vehicle (H2O), dopamine or YM-254,890. Fluorescence intensity was measured throughout perfusion (Figure 5A). Ionomycin perfusion was used as a positive control (Figure 5A, Inset). Analysis of the area under the curve generated after treatment showed a significant main effect for dopamine [Figure 5B, 2way ANOVA, F (1, 10) = 168.9, **** p < 0. 0001] and a significant dopamine × YM-254,890 interaction [2way ANOVA, F (1, 10) = 16.37, ** p = 0.0023], with no significant effect of YM-254,890 alone [2way ANOVA, F (1,10) = 0.007851, p = 0.9311]. These data indicate that in primary human macrophages, dopamine induces Ca2+ release, and this Ca2+ flux is, at least partially, mediated by coupling of dopamine receptors to Gq/11.
Figure 5 -. Activation of the Gq/11-linked dopamine receptor stimulates calcium release in human macrophages.
Human MDM were loaded with the fluorescent calcium indicator dye Oregon Green Bapta-488 for an hour, followed by treatment with the indicated drug. A represents calcium traces over the time period whereas B shows the area under the curve analysis. Dopamine (gray) significantly increased calcium release over the vehicle control (white line). Treatment with the Gq/11 inhibitor YM-254,890 partially attenuated this dopamine mediated increase (blue). YM alone (pink) had no effect on calcium flux compared to the vehicle control. Ionomycin (inset, orange line) was used as a positive control. All data was normalized to baseline, and the vehicle control was set to one. Each N represents an average of 75-110 cells from 3 - 5 individual donors. **p<0.01, **** p<0.0001
3.5. Dopamine increases PKC phosphorylation through a Gq/11 mediated pathway
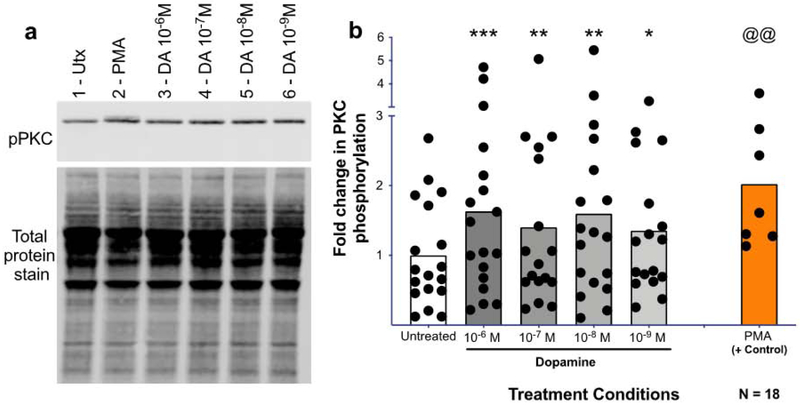
The role of Ca2+ in the dopamine-mediated increase in HIV entry, and the ability of dopamine to increase Ca2+ on its own suggest that dopamine activates the alternative Ca2+ mediated signaling pathway in macrophages. In this pathway, Gq/11 couples to D5 receptors and Gβγ couples to D2 receptors, both of which activate PLC and induce phosphorylation of PKC and Ca2+ release (Fig. 2B) (44, 47-55, 69). To confirm the activity of this signaling mechanism in human macrophages, the ability of dopamine to activate PKC was examined. MDM were treated with vehicle or dopamine (10−6 to 10−9 M) for 1 minute and examined by Western blot for changes in PKC phosphorylation (Fig. 6A). Relative to vehicle, dopamine significantly increased PKC phosphorylation [Fig 6B, mixed effects model, n = 17 - 18, F(4, 66) = 5.993, ***p = 0.0004. Post-hoc analysis with Tukey’s, veh. vs. 10−6M, *** p = 0.0006, veh. vs. 10−7M, ** p = 0.0093, veh. vs. 10−8M, **p = 0.0014, veh. vs. 10−9M, *p = 0.0110]. In a subset of donors, PMA was additionally used as a positive control to ensure the assay and antibody worked and we were picking up the correct band (Fig 6B, n = 7, @@p = 0.0015, t=5.518, df=6). Additional experiments at later time points suggested that these events occur rapidly, as the dopamine-mediated increase in phosphorylation was not seen in MDM treated with dopamine for 2.5, 5 and 10-minutes (Supplemental Fig. 2A-C), although the results trended towards significance at 5 minutes.
Figure 6 -. Dopamine induces PKC phosphorylation in human macrophages.
Human MDMs were treated with dopamine for 1 minute and protein was isolated, run on a western blot, and probed for phospho-PKC (bII Ser660). A representative western blot is shown in (A, 1-Untx, 2-PMA 10−6 M, 3-DA 10−6 M, 4-DA 10−7 M, 5-DA 10−8 M, 6-DA 10−9 M)). Dopamine significantly increased PKC phosphorylation at 10−6M, 10−7M, 10−8M, and 10−8M after 1 minute relative to the untreated control (B, DA, gray column, Utx, white column). Phospho-PKC signal was normalized to total protein for all donors, and analyzed using a one-way ANOVA. *p<0.05, **p<0.01, ***p<0.005. PMA (orange) was used as a positive control and was analyzed separately using a Paired T-test. @@ p<0.01. Data is expressed as fold change compared to the mean of the untreated.
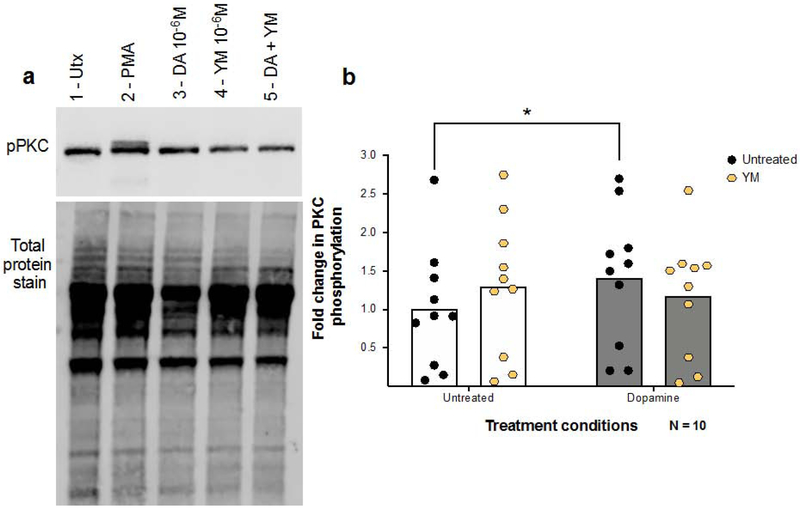
The Ca2+ imaging experiments in hMDM suggest that dopamine signaling in macrophages is at least partially mediated through D1-like receptors coupling to Gq/11. To examine whether this pathway also modulates the effects of dopamine on PKC activation, phosphorylation of PKC was examined in hMDM pretreated with either vehicle or YM-254,890. After pretreatment, hMDM were treated with vehicle or dopamine (10−6M) for 1 minute (Figure 7A) and analyzed for phosphorylation of PKC. Analysis showed a significant interaction of dopamine × YM-254890 [Figure 7B, 2way rmANOVA, n = 10, F(1,9) = 12.10, **p = 0.0070] and no main effect of dopamine [F(1,9) = 3.147, p = 0.1098] or YM-254,890 treatment [F(1,9) = 0.05719, p = 0.8163]. Post-hoc analysis showed that dopamine significantly increased PKC phosphorylation in untreated [Tukey’s, UT vs. UT + DA, *p = 0.0196], but not YM treated hMDM. This suggests the dopamine-induced phosphorylation of PKC in hMDM is mediated, at least in part, by a Gq/11-coupled receptor. As the only dopamine receptor suggested to couple to Gq/11 is D5 (50, 55), together with our data indicating that DRD5 is the highest-expressed receptor on hMDM, these results suggest that the D5 receptor is being activated in this pathway.
Figure 7 -. Inhibition of Gq/11 modulates PKC phosphorylation in macrophages.
MDM were pre-treated with the Gq/11 inhibitor YM-254890 for thirty minutes followed by treatment with vehicle or 10−6M DA for one minute (A, B). Cells were lysed and analyzed for phospho-PKC via Western Blot. A representative western blot is shown in (A, 1-Untx, 2-PMA 10−6 M, 3-DA 10−6 M, 4-YM-254890 10−6 M, 5-YM+DA). Analysis using a 2way ANOVA showed a significant interaction between dopamine and YM-254890, and post hoc analyses showed that dopamine alone (B, gray columns) significantly increased PKC phosphorylation over the untreated vehicle (white columns), while this effect was not present in MDM treated with YM-254,890. *p<0.05 Data are represented as fold change compared to the mean of the vehicle treated control.
3.6. Dopamine does not stimulate cAMP production in human macrophages.
The above data suggest that the effects of dopamine on HIV entry are not mediated through the canonical dopamine pathway, but rather through a Ca2+-dependent, non-canonical mechanism. They further indicate that dopamine activates this non-canonical signaling pathway to induce Ca2+ release and PKC activation, and this is partially mediated by D1-like receptor coupling to Gq/11. However, in the majority of neuronal cells, D1-like signaling is largely associated with activation of adenylyl cyclase and increases in cAMP (42, 44, 77, 78). Therefore, dopaminergic stimulation of cAMP production in human MDM was examined to assess whether dopamine can activate the canonical D1-like pathway in human macrophages.
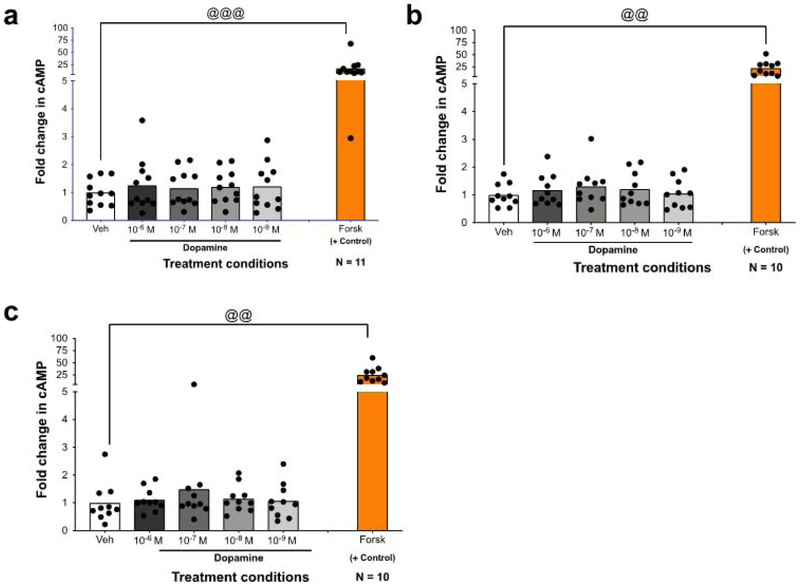
Human macrophages were treated with vehicle (H2O) or different concentrations of dopamine (10−6 M to 10−9M) for 5 minutes and assayed for cAMP. After 5 minutes, none of the tested dopamine concentrations increased cAMP relative to vehicle [Figure 8A, one-way rmANOVA, n = 11, F (2.079, 20.79) = 1.199, p = 0.3229]. To ensure that the effects of dopamine were being examined at the correct time point, production of cAMP at 10 minutes [figure 8B, one-way rmANOVA, n = 10, F (2.987, 26.88) = 0.9176, p=0.4453] and 15 minutes [figure 8C, one-way rmANOVA, n = 10, f (1.545, 13.90) = 0.8886, p=0.4077] was examined. As with a 5 minute treatment, dopamine had no effect on cAMP production at any concentration for these time points. One explanation for this is a dysfunction in adenylyl cyclase, but forskolin, a direct activator of adenylyl cyclase that was used as a positive control, still significantly increased cAMP at all time points indicating adenylyl cyclase is active (figure 8A, 5 minutes, Wilcoxon signed rank test, n = 11, veh vs. Forsk, @@@ p = 0.001; for figure 8B, 10 minutes, T-test, n=10, veh vs. Forsk, @@ p = 0.001; for figure 8C, inset, 15 minutes, Wilcoxon signs rank test, n=10, veh vs. Forsk, @@p=0.002). This suggests that adenylyl cyclase is functional in these cells. It is possible that the lack of change in cAMP is due to the action of the D2-like receptors, which can counteract the actions of the D1-like receptors signaling through Gαs via activation of GαI (44). However, our data indicate that D1-like receptors are signaling via Gαq. Therefore, these results indicate that dopamine does not act on cAMP in hMDM, and that activation of the canonical D1-like pathway may not represent the dominant dopamine signaling mechanism in these cells.
Figure 8 -. Dopamine receptor activation does not increase cAMP production in MDM.
To determine if dopamine signals through the canonical D1-like pathway, stimulation of cAMP was measured. The indicated concentrations of dopamine were added to MDMs for 5 (A), 10 (B), or 15 (C) minutes, after which cells were washed, lysed, and analyzed for production of cAMP. Analysis by one-way ANOVA showed that not dopamine concentration assayed (gray column) increased cAMP production compared to the vehicle treated control (white column). This pattern held true at all time points measured. Forskolin, a direct activator of adenylyl cyclase, was used as a positive control and was analyzed separately using a paired T-test (orange bar). @@ p<0.01, @@@ p<0.005 Data are represented as fold change compared to the mean of the vehicle treated control.
4. Discussion
Research over the past few decades has shown that dopamine is central to neuroimmune communication, and to the development of several neurological disease (1, 2, 79), including NeuroHIV (7, 8, 10). Prior to the development of combination anti-retroviral therapy (cART), prominent neuropathology and high levels of HIV RNA were common in dopamine-rich brain regions such as the basal ganglia, SbN and prefrontal cortex (PFC) (9, 16-18, 41, 80). With the use of cART, the CNS damage associated with NeuroHIV has shifted, but infected individuals still show subcortical atrophy, striatal dysfunction, inflammation in the basal ganglia, neuronal damage and aberrant metabolite ratios indicating neuronal injury in basal ganglia and frontal cortex (21, 22, 26, 81). While the precise connection between dopamine and NeuroHIV remains undefined, our research shows that dopamine can increase HIV replication and entry into macrophages (39, 40). These cells are primary targets for HIV in the CNS, and infection of myeloid populations creates a viral reservoir and promotes chronic, ongoing neuroinflammation (15, 38, 82, 83).
This study specifically examines the signaling pathways mediating the effects of dopamine on HIV entry into human macrophages and evaluates the activity of those pathways in these cells. The results show only activation of dopamine receptors, and not other biogenic amine receptors, increases HIV entry. Previous studies have demonstrated that activation of both D1-like and D2-like dopamine receptors can enhance the entry process (40). The two subtypes of dopamine receptors canonically have opposing effects on cAMP production and PKA activation, suggesting that dopamine could use a different signaling pathway to mediate its effects on HIV entry. These data support this hypothesis, demonstrating that this increase seems to be mediated through a mechanism involving increased intracellular Ca2+ levels, and not through the canonical cAMP mediated signaling pathway. Further, the data show that dopamine exposure increases Ca2+ release but does not trigger the formation of cAMP, indicating that a non-canonical signaling pathway may be involved (44).
Previous studies in rodents have shown that activation of some dopamine receptors can induce Ca2+ release via IP3 receptor activation, and our data suggest this pathway could also be active in human macrophages. In this pathway, activation of phospholipase C (PLC) catalyzes the cleavage of PIP2 into IP3 and diacylglycerol. The IP3 then binds to IP3 receptors (IP3R) on the endoplasmic reticulum (ER), mediating both Ca2+ release from the ER and the activation of PKC. This pathway can be activated by either D5 receptors coupling to Gq/11 proteins, or D2 receptors through the release of Gβγ proteins (47, 49-54, 69). These data show that dopamine treatment of macrophages increases both intracellular Ca2+ flux and PKC activation, and that these effects were reduced or inhibited by blocking Gq/11 with YM-254,890. Calcium release induced by this non-canonical pathway mediates the dopamine-induced increase in HIV infection, as blockade of Ca2+ flux using dantrolene abrogates increased entry. The importance of Ca2+ in the entry process is supported by previous experiments showing that both HIV co-receptors, CCR5 and CXCR4, can initiate Ca2+ flux when bound by the viral envelope protein gp120 (84). In response to CCR5 binding, a critical interaction in the HIV entry process, Ca2+ is released through the same IP3R activation pathway described above (84, 85). Further, this same Gq/11-mediated intracellular Ca2+ release mechanism was necessary for HIV entry in CCR5 transfected astrocytes (86), and phospholipase C, a key component of this pathway, has been shown to be an important factor in HIV infection (87). Taken together, these studies emphasize the importance of Ca2+ signaling in the HIV entry process, and suggest that the impact of dopamine on entry is mediated through D5 activation of IP3 mediated Ca2+ release.
Our previous study indicated that the dopamine-mediated increase in Ca2+ could result from dopaminergic potentiation of an existing Ca2+ flux (40), such as that initiated by coreceptor binding. However, the data presented here indicate that, at least in macrophages, dopamine receptor activation alone can also initiate Ca2+ release, thereby potentially enhancing the HIV entry process directly. While Ca2+ is also involved in the entry mechanism of Herpes simplex virus (HSV) (88, 89) and a number of other viruses also stimulate Ca2+ flux during infection (90), it is not clear if these processes are mediated through the same Gq/11 pathway, or if the other mechanisms could be involved. However, these findings highlight the importance of Ca2+ release and the need to define Ca2+ dynamics in the context of viral entry.
Both subtypes of dopamine receptors can mediate Ca2+ release (40, 52, 54, 91, 92), but only D5 couples to Gq/11 (50, 55), suggesting that Ca2+ induced by activation of this receptor is central to mediating the effects of dopamine on HIV entry. This is supported by studies in brain slices from D1 knock-out mice, showing dopamine and SKF38,393, a D1-like receptor agonist, did not induce a cAMP response but did diminish the PLC-IP3 response, suggesting that D5 was responsible for the PLC-IP3 activity (50). In D5 knock-out mice, dopamine and D1-like agonists did not induce IP3 or diacylglycerol messengers after stimulation, while they were able to do so in wild-type animals (55). Our data here, as well as that in our recently published study (33) show that in human macrophages, transcripts for the D1-like receptors, D1 and D5, were expressed significantly more than those for the D2 receptors, with DRD5 showing the highest mean expression. Additionally, DRD5 was expressed in all donors, whereas DRD1 and DRD2, DRD3, and DRD4 were more variable across the population. Our data show inhibition of Gq/11 with YM-254,890 is sufficient to block both dopamine-induced PKC phosphorylation and Ca2+ release. Taken together with the higher level of D5 expression in hMDM and the role of Gq/11 in D5 mediated signaling, our data suggest that dopamine acts on HIV infection via D5-mediated Ca2+ release.
While these data suggest that dopamine signaling in macrophages is mediated largely through the D5 - Gq/11 mechanism, the D2-like receptors may also play a significant role. Multiple studies have demonstrated that activation of D2 receptors that releases Gβγ has also been linked to PKC activation and IP3 mediated Ca2+ release (49, 53, 54). These data show that blocking Gq/11 with YM-254,890 significantly reduced but did not eliminate dopamine induced Ca2+ release, suggesting that a non-Gq/11-coupled receptor could also play a role. Further, the potential involvement of both D5 and D2 receptors in this pathway is supported by our previous data showing that activation of both D1-like and D2-like receptors enhances the viral entry process (40). It is possible that convergent pathways through both D2 and D5 receptors allow for an enhanced Ca2+ response or greater sensitivity to low dopamine concentrations. Future experiments using specific D1-like and D2-like agonists for both PKC activation and Ca2+ release, as well as experiments utilizing cells in which specific dopamine receptors have been knocked down, are necessary to define the input of both receptor subtypes on dopamine signaling in macrophages.
In addition to demonstrating dopamine activation of a non-canonical signaling mechanism, these data also show that dopamine did not increase cAMP production in human macrophages from 11 different donors. This was surprising as dopamine mediated regulation of cAMP is a central process in the canonical D1-like pathway (78). The lack of activity across many donors suggests the effect is not donor-dependent. Dopamine has a higher affinity for D5 than D1 (93), and therefore, the lack of canonical D1-like signaling could be due to the levels of dopamine being too low to activate the D1 receptors. As macrophages can take on different phenotypes, another possible explanation is that culture conditions could promote an activation state that biases signaling through the non-canonical pathway. Experiments examining the impact of different dopamine concentrations on myeloid cells in different activation states are necessary to resolve these questions. However, the data here raise the possibility that, in macrophages, dopamine signaling is preferentially mediated through a non-canonical pathway.
While these studies focus on primary human monocyte-derived macrophages (hMDM), it is important to note that this type of mononuclear phagocyte is just one of several distinct populations of myeloid cells present in the human brain (94). Infection of these populations, particularly microglia and perivascular macrophages, is thought to be a central component in the development of HIV neuropathology (34-37). While microglia are distinct from hMDM (95), they express all five dopamine receptor subtypes (4, 96) as hMDM do, and dopamine has been demonstrated to alter the cytokine profile of these cells (1, 2, 5, 32). This may also be the case for other types of CNS macrophages, as CNS myeloid populations are transcriptionally related (97). However, our data strongly suggest that dopamine signaling mechanisms may vary from between cell types, and therefore it is critical to specifically define the dopamine signaling pathways active in human microglia and other CNS macrophages. Such studies are hampered by the logistical and ethical challenges involved in obtaining primary human myeloid cells from the CNS , as well as the lack of reliable human cell lines that can accurately model human microglia in vitro (95, 98). Moreover, as mice and rats cannot be infected with HIV (99, 100), it is not practical to use rodent microglia for studies examining dopaminergic signaling in the context of viral infection. As studies into the dopamine signaling pathways in human microglia are an important step in further defining the full effects of dopamine on viral infection in the CNS, future work will need to use either adult human microglia, which are difficult to obtain, or develop and optimize the use of new microglial cell lines and culture systems such as microglia developed from inducible pluripotent stem-cells (iPSC).
Despite this limitation, the use of human monocyte-derived macrophages is advantageous as they model not only the hMDM present in the brain, but are also similar to numerous types of macrophages found in peripheral tissues such as the lungs, gut and kidneys, in which dopamine plays a critical role in tissue function. The macrophages present in all of these tissues are exposed to concentrations of dopamine high enough to induce changes in HIV entry (6, 40), adding to the importance of understanding the impact of dopamine on CNS and peripheral HIV infection in human macrophages. Thus, the effects of dopamine on macrophages is important to both neuropathogenesis of HIV, but also their potential role in mediating peripheral HIV infection. Within the CNS, the impact of changes in dopaminergic tone would be most pronounced in dopamine-rich regions, where dopamine would increase the susceptibility of these myeloid cells to infection. Elevated dopaminergic tone in the striatum and substantia nigra would enable the infection to spread more rapidly, resulting in more infected cells, a larger viral reservoir, and a greater release of neurotoxic and inflammatory factors in these areas. These effects could also damage dopaminergic neurons in these regions, dysregulating dopaminergic neurotransmission and exposing the resident, uninfected myeloid cells to greater aberrancy in dopamine concentrations. As dopamine can directly increase production of inflammatory cytokines from macrophages (32, 101), the initial insult would be amplified, sustaining and expanding the neuroinflammation resulting in further damage and inflammatory responses across a larger area. This process could be exacerbated by exogenous increases in CNS dopamine due to substance abuse (102) or legal therapeutics that affect the dopaminergic system, such as those for depression (66), a common comorbidity among HIV-infected individuals (20). In addition, as the HIV-infected population ages, the use of dopaminergic therapeutics that treat age-related disorders, such as Alzheimer’s, Parkinson’s, diabetes and some cancers (103-106) could further impact the development of NeuroHIV. Together, these data suggest that the bidirectional interaction between macrophages and the dopaminergic system may be central to HIV neuropathogenesis, and exogenous elevation of dopamine may increase the susceptibility of vulnerable populations to neurological damage.
These data support and expand on the hypothesis that changes in dopaminergic tone could play a significant role in the development and exacerbation of NeuroHIV by increasing macrophage susceptibility to HIV entry. Together with previous studies, these data indicate that the increased entry is exclusively mediated by activation of dopamine receptors, particularly D5 receptors coupled to Gq/11, and activation of these receptors induces a dopamine-mediated Ca2+ release that is critical to the effects of dopamine. Further, the mechanism underlying this process is an alternative signaling pathway and not the canonical, cAMP-mediated dopamine signaling cascade. In addition to demonstrating a critical role for Ca2+ in the HIV infection process, these data suggest that therapeutic strategies targeting dopamine signaling could be used to reduce the adverse effects of illicit drugs and dopaminergic therapeutics in NeuroHIV. These data also suggest that dopamine signaling in macrophages may be biased toward a non-canonical signaling mechanism, and that immunologic signaling pathways mediating the actions of neurotransmitters may be distinct from those found in neurons. Overall, these data further define a novel mechanism by which dopamine could substantially impact the development of HIV and other diseases, and highlight the importance of understanding dopamine signaling in immune cells under both physiological and pathological conditions.
Supplementary Material
Highlights.
Dopamine increases HIV entry into human macrophages via Ca2+ dependent mechanism
Dopamine stimulates Ca2+ release and PKC activation in macrophages
D1-like receptors coupling to Gq/11 partially mediates effects on PKC and Ca2+
Dopamine doesn’t increase cAMP in human macrophages
Acknowledgements
We would like to thank all the members of Dr. Peter J. Gaskill’s lab at the Drexel University College of Medicine, especially Kimberly Bonar, for their help and advice on this project. We would also like to thank Doug Miller from the Khoshbouei lab and Dr. Jacqueline Barker at Drexel, for their invaluable input regarding statistical analysis. We would like to acknowledge our funding from the National Institutes of Drug Addiction, DA029476 and DA039005 (PJG), DA026947, DA026947S1, and DA043895 (HK), the National Institutes of Neurological Disease and Stroke, NS071122 (HK), the Office of the Director at the NIH OD020026 (HK), the Brody Family Medical Trust Fund (SMM) and the Department of Pharmacology and Physiology at Drexel University College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. Journal of Neuroimmunology. 2000;102(2):113–24. doi: 10.1016/S0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24(4):525–8. Epub 2009/11/10. doi: 10.1016/j.bbi.2009.10.015. PubMed PMID: 19896530; PMCID: PMC2856781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco R, Pacheco R, Lluis C, Ahern GP, O'Connell PJ. The emergence of neurotransmitters as immune modulators. Trends Immunol. 2007;28(9):400–7. Epub 2007/08/11. doi: 10.1016/j.it.2007.07.005. PubMed PMID: 17689291. [DOI] [PubMed] [Google Scholar]
- 4.McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. Journal of Neuroimmunology. 2002;132(1):34–40. doi: 10.1016/S0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- 5.Arreola R, Alvarez-Herrera S, Perez-Sanchez G, Becerril-Villanueva E, Cruz-Fuentes C, Flores-Gutierrez EO, Garces-Alvarez ME, de la Cruz-Aguilera DL, Medina-Rivero E, Hurtado-Alvarado G, Quintero-Fabian S, Pavon L. Immunomodulatory Effects Mediated by Dopamine. J Immunol Res. 2016;2016:3160486. Epub 2016/11/01. doi: 10.1155/2016/3160486. PubMed PMID: 27795960; PMCID: PMC5067323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matt SM, Gaskill PJ. Where Is Dopamine and how do Immune Cells See it?: Dopamine-Mediated Immune Cell Function in Health and Disease. J Neuroimmune Pharmacol. 2019. Epub 2019/05/12. doi: 10.1007/s11481-019-09851-4. PubMed PMID: 31077015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H. HIV, Tat and dopamine transmission. Neurobiol Dis. 2017;105:51–73. Epub 2017/05/02. doi: 10.1016/j.nbd.2017.04.015. PubMed PMID: 28457951; PMCID: PMC5541386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. Journal of psychopharmacology (Oxford, England). 2000;14(3):214–21. Epub 2000/12/06. doi: 10.1177/026988110001400304. PubMed PMID: 11106299. [DOI] [PubMed] [Google Scholar]
- 9.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43(10):2099–104. Epub 1993/10/01. PubMed PMID: 8413973. [DOI] [PubMed] [Google Scholar]
- 10.Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta neuropathologica. 2004;107(3):216–26. Epub 2004/01/09. doi: 10.1007/s00401-003-0801-3. PubMed PMID: 14712399. [DOI] [PubMed] [Google Scholar]
- 11.Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Molecular neurobiology. 2011;44(1):102–10. Epub 2011/07/01. doi: 10.1007/s12035-011-8195-z. PubMed PMID: 21717292. [DOI] [PubMed] [Google Scholar]
- 12.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Annals of neurology. 1986;19(6):525–35. Epub 1986/06/01. doi: 10.1002/ana.410190603. PubMed PMID: 3014994. [DOI] [PubMed] [Google Scholar]
- 13.Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta neurologica Scandinavica. 1993;88(2):112–8. Epub 1993/08/01. PubMed PMID: 8213054. [DOI] [PubMed] [Google Scholar]
- 14.Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1991;82(1):39–44. Epub 1991/01/01. PubMed PMID: 1950477. [DOI] [PubMed] [Google Scholar]
- 15.Kure K, Llena JF, Lyman WD, Soeiro R, Weidenheim KM, Hirano A, Dickson DW. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Human pathology. 1991;22(7):700–10. Epub 1991/07/01. PubMed PMID: 2071114. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16(3):146–52. Epub 1997/12/09. PubMed PMID: 9390565. [DOI] [PubMed] [Google Scholar]
- 17.Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain pathology (Zurich, Switzerland). 1998;8(2):277–84. Epub 1998/04/18. PubMed PMID: 9546286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson M, Hagberg L, Forsman A, Norkrans G. Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. Journal of neuroscience research. 1991;28(3):406–9. Epub 1991/03/01. doi: 10.1002/jnr.490280313. PubMed PMID: 1856886. [DOI] [PubMed] [Google Scholar]
- 19.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS reports. 2011;8(1):54–61. Epub 2010/12/31. doi: 10.1007/s11904-010-0070-4. PubMed PMID: 21191673; PMCID: 3035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nature reviews Neurology. 2016;12(4):234–48. Epub 2016/03/12. doi: 10.1038/nrneurol.2016.27. PubMed PMID: 26965674; PMCID: PMC4937456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM, Multicenter ACS. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5(2):77–85. Epub 2011/01/26. doi: 10.1007/s11682-011-9113-8. PubMed PMID: 21264551; PMCID: PMC3082694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, Taylor M, Singer E, Algers J, Zhong J, Brown M, McMahon D, So YT, Mi D, Heaton R, Robertson K, Yiannoutsos C, Cohen RA, Navia B, Consortium HIVN. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. Journal of neurovirology. 2013;19(3):209–18. Epub 2013/04/25. doi: 10.1007/s13365-013-0162-1. PubMed PMID: 23613008; PMCID: 3740160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford KM, Samboju V, Cobigo Y, Milanini B, Marx GA, Hellmuth JM, Rosen HJ, Kramer JH, Allen IE, Valcour VG. Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Over Age 60. J Acquir Immune Defic Syndr. 2017. doi: 10.1097/QAI.0000000000001489. PubMed PMID: 28650401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Current HIV research. 2014;12(2):97–110. PubMed PMID: 24862332; PMCID: 4152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.du Plessis S, Vink M, Joska JA, Koutsilieri E, Bagadia A, Stein DJ, Emsley R. Prefrontal cortical thinning in HIV infection is associated with impaired striatal functioning. Journal of neural transmission (Vienna, Austria : 1996). 2016;123(6):643–51. Epub 2016/05/14. doi: 10.1007/s00702-016-1571-0. PubMed PMID: 27173383. [DOI] [PubMed] [Google Scholar]
- 26.du Plessis S, Vink M, Joska JA, Koutsilieri E, Bagadia A, Stein DJ, Emsley R. HIV Infection Is Associated with Impaired Striatal Function during Inhibition with Normal Cortical Functioning on Functional MRI. Journal of the International Neuropsychological Society : JINS. 2015;21(9):722–31. Epub 2015/10/06. doi: 10.1017/s1355617715000971. PubMed PMID: 26435417. [DOI] [PubMed] [Google Scholar]
- 27.Bairwa D, Kumar V, Vyas S, Das BK, Srivastava AK, Pandey RM, Sharma SK, Jagannathan NR, Sinha S. Case control study: magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol. 2016;16:99. Epub 2016/07/14. doi: 10.1186/s12883-016-0628-x. PubMed PMID: 27405321; PMCID: PMC4942893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ann HW, Jun S, Shin NY, Han S, Ahn JY, Ahn MY, Jeon YD, Jung IY, Kim MH, Jeong WY, Ku NS, Kim JM, Smith DM, Choi JY. Characteristics of Resting-State Functional Connectivity in HIV-Associated Neurocognitive Disorder. PLoS One. 2016;11(4):e0153493. Epub 2016/04/23. doi: 10.1371/journal.pone.0153493. PubMed PMID: 27104345; PMCID: PMC4841538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, Rabiner EA, Kalk N, Bishop C, Gunn RN, Matthews PM, Winston A. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology. 2016;86(15):1425–32. Epub 2016/02/26. doi: 10.1212/wnl.0000000000002485. PubMed PMID: 26911637; PMCID: PMC4831035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb KM, Mactutus CF, Booze RM. The ART of HIV therapies: dopaminergic deficits and future treatments for HIV pediatric encephalopathy. Expert review of anti-infective therapy. 2009;7(2):193–203. Epub 2009/03/04. doi: 10.1586/14787210.7.2.193. PubMed PMID: 19254168; PMCID: PMC3704143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Ananthan S, Zhan CG. The role of human dopamine transporter in NeuroAIDS. Pharmacol Ther. 2017. Epub 2017/10/11. doi: 10.1016/j.pharmthera.2017.10.007. PubMed PMID: 28987321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan R, Gaskill PJ. The role of catecholamines in HIV neuropathogenesis. Brain Res. 2018. Epub 2018/05/01. doi: 10.1016/j.brainres.2018.04.030. PubMed PMID: 29705605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan RA, Muir R, Runner K, Haddad EK, Gaskill PJ. Role of Macrophage Dopamine Receptors in Mediating Cytokine Production: Implications for Neuroinflammation in the Context of HIV-Associated Neurocognitive Disorders. J Neuroimmune Pharmacol. 2018. Epub 2018/12/07. doi: 10.1007/s11481-018-9825-2. PubMed PMID: 30519866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74(5):635–41. Epub 2003/09/10. doi: 10.1189/jlb.0503204. PubMed PMID: 12960232. [DOI] [PubMed] [Google Scholar]
- 35.Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4(4):430–47. Epub 2009/09/22. doi: 10.1007/s11481-009-9174-2. PubMed PMID: 19768553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. Mechanisms of HIV Entry into the CNS: Increased Sensitivity of HIV Infected CD14(+)CD16(+) Monocytes to CCL2 and Key Roles of CCR2, JAM-A, and ALCAM in Diapedesis. PLoS ONE. 2013;8(7):e69270. doi: 10.1371/journal.pone.0069270. PubMed PMID: PMC3724935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. Journal of the Neurological Sciences. 2002;202(1):13–23. doi: 10.1016/S0022-510X(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 38.Rappaport J, Volsky DJ. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol. 2015;21(3):235–41. Epub 2015/05/03. doi: 10.1007/s13365-015-0346-y. PubMed PMID: 25933548; PMCID: PMC4445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175(3):1148–59. Epub 2009/08/08. doi: 10.2353/ajpath.2009.081067. PubMed PMID: 19661443; PMCID: PMC2731133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One. 2014;9(9):e108232. Epub 2014/10/01. doi: 10.1371/journal.pone.0108232. PubMed PMID: 25268786; PMCID: PMC4182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta neuropathologica. 2000;99(4):376–84. Epub 2000/04/29. PubMed PMID: 10787036. [DOI] [PubMed] [Google Scholar]
- 42.Kebabian JW, Greengard P. Dopamine-Sensitive Adenyl Cyclase: Possible Role in Synaptic Transmission. Science. 1971;174(4016):1346. [DOI] [PubMed] [Google Scholar]
- 43.Undieh AS. Pharmacology of signaling induced by dopamine D(1)-like receptor activation. Pharmacol Ther. 2010;128(1):37–60. Epub 2010/06/16. doi: 10.1016/j.pharmthera.2010.05.003. PubMed PMID: 20547182; PMCID: PMC2939266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. Epub 2011/02/10. doi: 10.1124/pr.110.002642. PubMed PMID: 21303898. [DOI] [PubMed] [Google Scholar]
- 45.Beaulieu J-M, Espinoza S, Gainetdinov RR. Dopamine receptors – IUPHAR Review 13. British Journal of Pharmacology. 2015;172(1):1–23. doi: 10.1111/bph.12906. PubMed PMID: PMC4280963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enjalbert A, Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Molecular Pharmacology. 1983;23(3):576. [PubMed] [Google Scholar]
- 47.Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. Journal of Pharmacology and Experimental Therapeutics. 1989;248:171–5. [PubMed] [Google Scholar]
- 48.Jin L-Q, Wang H-Y, Friedman E. Stimulated D1 dopamine receptors couple to multiple Gα proteins in different brain regions. Journal of neurochemistry. 2001;78(5):981–90. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 49.Di Marzo V, Vial D, Sokoloff P, Schwartz JC, Piomelli D. Selection of alternative G-mediated signaling pathways at the dopamine D2 receptor by protein kinase C. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(11):4846–53. Epub 1993/11/01. PubMed PMID: 7693893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51(1):6–11. Epub 1997/01/01. PubMed PMID: 9016340. [DOI] [PubMed] [Google Scholar]
- 51.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol. 1995;48(6):988–94. Epub 1995/12/01. PubMed PMID: 8848015. [PubMed] [Google Scholar]
- 52.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253(3):987–92. Epub 1990/06/01. PubMed PMID: 1972756. [PubMed] [Google Scholar]
- 53.Yan Z, Feng J, Fienberg AA, Greengard P. D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci U S A. 1999;96(20):11607–12. Epub 1999/09/29. PubMed PMID: 10500224; PMCID: PMC18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(24):8987–95. Epub 2000/01/11. PubMed PMID: 11124974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75(3):447–53. Epub 2008/12/03. doi: 10.1124/mol.108.053017. PubMed PMID: 19047479; PMCID: PMC2684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Corno M, Liu QH, Schols D, de Clercq E, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98(10):2909–16. Epub 2001/11/08. PubMed PMID: 11698270. [DOI] [PubMed] [Google Scholar]
- 57.Freedman BD, Liu Q-H, Del Corno M, Collman RG. HIV-1 gp120 chemokine receptor-mediated signaling in human macrophages. Immunologic Research. 2003;27(2):261–76. doi: 10.1385/IR:27:2-3:261. [DOI] [PubMed] [Google Scholar]
- 58.Harmon B, Ratner L. Induction of the G q Signaling Cascade by the Human Immunodeficiency Virus Envelope Is Required for Virus Entry. Journal of virology. 2008;82(18):9191–205. doi: 10.1128/jvi.00424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87(5):1284–95. Epub 2003/11/19. doi: 10.1046/j.1471-4159.2003.02109.x. PubMed PMID: 14622108. [DOI] [PubMed] [Google Scholar]
- 60.Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS. Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosensors & bioelectronics. 2010;25(5):1179–85. Epub 2009/11/10. doi: 10.1016/j.bios.2009.10.008. PubMed PMID: 19896822; PMCID: 2818289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei C, Han X, Weng D, Feng Q, Qi X, Li J, Luo M. Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA. Cell discovery. 2018;4:60. Epub 2018/11/13. doi: 10.1038/s41421-018-0060-z. PubMed PMID: 30416749; PMCID: PMC6218454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuxe K, Agnati LF, Marcoli M, Borroto-Escuela DO. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem Res. 2015;40(12):2600–14. Epub 2015/04/22. doi: 10.1007/s11064-015-1574-5. PubMed PMID: 25894681. [DOI] [PubMed] [Google Scholar]
- 63.Fox ME, Wightman RM. Contrasting Regulation of Catecholamine Neurotransmission in the Behaving Brain: Pharmacological Insights from an Electrochemical Perspective. Pharmacol Rev. 2017;69(1):12–32. Epub 2017/03/08. doi: 10.1124/pr.116.012948. PubMed PMID: 28267676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28(3):653–61. Epub 1989/01/01. PubMed PMID: 2710338. [DOI] [PubMed] [Google Scholar]
- 65.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. Epub 1988/07/01. PubMed PMID: 2899326; PMCID: PMC281732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D'Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405(1–3):365–73. Epub 2000/10/18. PubMed PMID: 11033341. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cellular and molecular neurobiology. 1999;19(4):467–89. Epub 1999/06/24. PubMed PMID: 10379421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitsuki Y-y, Yamamoto T, Mizukoshi F, Momota M, Terahara K, Yoshimura K, Harada S, Tsunetsugu-Yokota Y. A novel dual luciferase assay for the simultaneous monitoring of HIV infection and cell viability. Journal of Virological Methods. 2016;231:25–33. doi: 10.1016/j.jviromet.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Medvedev IO, Ramsey AJ, Masoud ST, Bermejo MK, Urs N, Sotnikova TD, Beaulieu JM, Gainetdinov RR, Salahpour A. D1 dopamine receptor coupling to PLCbeta regulates forward locomotion in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(46):18125–33. Epub 2013/11/15. doi: 10.1523/JNEUROSCI.2382-13.2013. PubMed PMID: 24227722; PMCID: PMC3828465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin L-Q, Cai G, Wang H-Y, Smith C, Friedman E. Characterization of the Phosphoinositide-Linked Dopamine Receptor in a Mouse Hippocampal-Neuroblastoma Hybrid Cell Line. Journal of neurochemistry. 1998;71(5):1935–43. doi: 10.1046/j.1471-4159.1998.71051935.x. [DOI] [PubMed] [Google Scholar]
- 71.Ernens I, Bousquenaud M, Lenoir B, Devaux Y, Wagner DR. Adenosine stimulates angiogenesis by up-regulating production of thrombospondin-1 by macrophages. Journal of leukocyte biology. 2015;97(1):9–18. Epub 2014/11/13. doi: 10.1189/jlb.3HI0514-249RR. PubMed PMID: 25387836. [DOI] [PubMed] [Google Scholar]
- 72.Hosono M, Takahira T, Fujita A, Fujihara R, Ishizuka O, Tatee T, Nakamura K. Cardiovascular and adenylate cyclase stimulant properties of NKH477, a novel water-soluble forskolin derivative. J Cardiovasc Pharmacol. 1992;19(4):625–34. Epub 1992/04/01. PubMed PMID: 1380607. [DOI] [PubMed] [Google Scholar]
- 73.Isomura M, Kotake Y, Masuda K, Miyara M, Okuda K, Samizo S, Sanoh S, Hosoi T, Ozawa K, Ohta S. Tributyltin-induced endoplasmic reticulum stress and its Ca(2+)-mediated mechanism. Toxicology and applied pharmacology. 2013;272(1):137–46. Epub 2013/06/08. doi: 10.1016/j.taap.2013.05.026. PubMed PMID: 23743301. [DOI] [PubMed] [Google Scholar]
- 74.Cornelius G, Gebauer G, Techel D. Inositol trisphosphate induces calcium release from Neurospora crassa vacuoles. Biochemical and biophysical research communications. 1989;162(2):852–6. Epub 1989/07/31. PubMed PMID: 2527035. [DOI] [PubMed] [Google Scholar]
- 75.Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation. 2012;9:203. Epub 2012/08/21. doi: 10.1186/1742-2094-9-203. PubMed PMID: 22901451; PMCID: PMC3488577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M. A novel Galphaq/11-selective inhibitor. The Journal of biological chemistry. 2004;279(46):47438–45. doi: 10.1074/jbc.M408846200. PubMed PMID: 15339913. [DOI] [PubMed] [Google Scholar]
- 77.Prasad KN, Gilmer KN. Demonstration of dopamine-sensitive adenylate cyclase in malignant neuroblastoma cells and change in sensitivity of adenylate cyclase to catecholamines in "differentiated" cells. Proc Natl Acad Sci U S A. 1974;71(6):2525–9. Epub 1974/06/01. PubMed PMID: 4366772; PMCID: PMC388492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kebabian JW. Multiple classes of dopamine receptors in mammalian central nervous system: the involvement of dopamine-sensitive adenylyl cyclase. Life sciences. 1978;23(5):479–83. Epub 1978/08/07. PubMed PMID: 357876. [DOI] [PubMed] [Google Scholar]
- 79.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. Journal of neuroinflammation. 2012;9:155. Epub 2012/07/04. doi: 10.1186/1742-2094-9-155. PubMed PMID: 22747919; PMCID: PMC3410819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Annals of neurology. 1995;38(5):755–62. Epub 1995/11/01. doi: 10.1002/ana.410380510. PubMed PMID: 7486867. [DOI] [PubMed] [Google Scholar]
- 81.Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances B, Collins DL. Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-infected Patients in the HAART era. J Acquir Immune Defic Syndr. 2017. doi: 10.1097/QAI.0000000000001294. PubMed PMID: 28129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65(2):736–42. Epub 1991/02/01. PubMed PMID: 1702842; PMCID: PMC239813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellano P, Prevedel L, Eugenin EA. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Scientific reports. 2017;7(1):12866. Epub 2017/10/11. doi: 10.1038/s41598-017-12758-w. PubMed PMID: 28993666; PMCID: PMC5634422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melar M, Ott DE, Hope TJ. Physiological levels of virion-associated human immunodeficiency virus type 1 envelope induce coreceptor-dependent calcium flux. J Virol. 2007;81(4):1773–85. Epub 2006/11/24. doi: 10.1128/JVI.01316-06. PubMed PMID: 17121788; PMCID: PMC1797554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu QH, Williams DA, McManus C, Baribaud F, Doms RW, Schols D, De Clercq E, Kotlikoff MI, Collman RG, Freedman BD. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc Natl Acad Sci U S A. 2000;97(9):4832–7. Epub 2000/04/12. doi: 10.1073/pnas.090521697. PubMed PMID: 10758170; PMCID: PMC18318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harmon B, Ratner L. Induction of the Galpha(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J Virol. 2008;82(18):9191–205. Epub 2008/07/18. doi: 10.1128/JVI.00424-08. PubMed PMID: 18632858; PMCID: PMC2546909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cecchetti S, Spadaro F, Gessani S, Podo F, Fantuzzi L. Phospholipases: at the crossroads of the immune system and the pathogenesis of HIV-1 infection. J Leukoc Biol. 2017;101(1):53–75. Epub 2016/11/03. doi: 10.1189/jlb.3RU0316-148RR. PubMed PMID: 27803127. [DOI] [PubMed] [Google Scholar]
- 88.Cheshenko N, Del Rosario B, Woda C, Marcellino D, Satlin LM, Herold BC. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol. 2003;163(2):283–93. Epub 2003/10/22. doi: 10.1083/jcb.200301084. PubMed PMID: 14568989; PMCID: PMC2173509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, Herold BC. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J. 2013;27(7):2584–99. Epub 2013/03/20. doi: 10.1096/fj.12220285. PubMed PMID: 23507869; PMCID: PMC3688744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46(1):1–17. Epub 2009/06/19. doi: 10.1016/j.ceca.2009.05.005. PubMed PMID: 19535138; PMCID: PMC3449087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai R, Ali MK, Lezcano N, Bergson C. A crucial role for cAMP and protein kinase A in D1 dopamine receptor regulated intracellular calcium transients. Neurosignals. 2008;16(2–3):112–23. Epub 2008/02/07. doi: 10.1159/000111557. PubMed PMID: 18253052. [DOI] [PubMed] [Google Scholar]
- 92.Fregeau MO, Carrier M, Guillemette G. Mechanism of dopamine D2 receptor-induced Ca(2+) release in PC-12 cells. Cellular signalling. 2013;25(12):2871–7. Epub 2013/09/24. doi: 10.1016/j.cellsig.2013.08.021. PubMed PMID: 24055909. [DOI] [PubMed] [Google Scholar]
- 93.Gaskill PJ, Calderon TM, Coley JS, Berman JW. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol. 2013;8(3):621–42. Epub 2013/03/05. doi: 10.1007/s11481-013-9443-y. PubMed PMID: 23456305; PMCID: PMC4303241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid Cells in the Central Nervous System. Immunity. 2017;46(6):943–56. Epub 2017/06/22. doi: 10.1016/j.immuni.2017.06.007. PubMed PMID: 28636961; PMCID: PMC5657250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–43. Epub 2013/12/10. doi: 10.1038/nn.3599. PubMed PMID: 24316888; PMCID: PMC4066672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005;29(1):128–38. Epub 2005/05/04. doi: 10.1016/j.mcn.2005.01.003. PubMed PMID: 15866053. [DOI] [PubMed] [Google Scholar]
- 97.Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805. Epub 2016/05/03. doi: 10.1038/ni.3423. PubMed PMID: 27135602; PMCID: PMC4968048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia-Mesa Y, Jay TR, Checkley MA, Luttge B, Dobrowolski C, Valadkhan S, Landreth GE, Karn J, Alvarez-Carbonell D. Immortalization of primary microglia: a new platform to study HIV regulation in the central nervous system. J Neurovirol. 2017;23(1):47–66. Epub 2016/11/23. doi: 10.1007/s13365-016-0499-3. PubMed PMID: 27873219; PMCID: PMC5329090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10(12):852–67. Epub 2012/11/17. doi: 10.1038/nrmicro2911. PubMed PMID: 23154262; PMCID: PMC4334372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bieniasz PD, Cullen BR. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol. 2000;74(21):9868–77. Epub 2000/10/12. doi: 10.1128/jvi.74.21.9868-9877.2000. PubMed PMID: 11024113; PMCID: PMC102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. Journal of neuroinflammation. 2012;9(1):203. Epub 2012/08/21. doi: 10.1186/1742-2094-9-203. PubMed PMID: 22901451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30(2):215–38. Epub 2005/08/16. doi: 10.1016/j.neubiorev.2005.04.016. PubMed PMID: 16099045. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, Zhou FM, Dani JA. Cholinergic drugs for Alzheimer's disease enhance in vitro dopamine release. Molecular pharmacology. 2004;66(3):538–44. Epub 2004/08/24. doi: 10.1124/mol.104.000299. PubMed PMID: 15322245. [DOI] [PubMed] [Google Scholar]
- 104.Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, Weiner DM, Tison F, Bezard E. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011;10(5):377–93. Epub 2011/05/03. doi: 10.1038/nrd3430. PubMed PMID: 21532567. [DOI] [PubMed] [Google Scholar]
- 105.Petrossians P, Thonnard AS, Beckers A. Medical treatment in Cushing's syndrome: dopamine agonists and cabergoline. Neuroendocrinology. 2010;92 Suppl 1:116–9. Epub 2010/09/21. doi: 10.1159/000317716. PubMed PMID: 20829631. [DOI] [PubMed] [Google Scholar]
- 106.Valiquette G Bromocriptine for diabetes mellitus type II. Cardiology in review. 2011;19(6):272–5. Epub 2011/10/11. doi: 10.1097/CRD.0b013e318229d2d2. PubMed PMID: 21983314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.