Abstract
Adipose tissue possesses the remarkable capacity to control its size and function in response to a variety of internal and external cues, such as nutritional status and temperature. The regulatory circuits of fuel storage and oxidation in white adipocytes and thermogenic adipocytes (brown and beige adipocytes) play a central role in systemic energy homeostasis, whereas dysregulation of the pathways is closely associated with metabolic disorders and adipose tissue malfunction, including obesity, insulin resistance, chronic inflammation, mitochondrial dysfunction, and fibrosis. Recent studies have uncovered new regulatory elements that control the above parameters and provide new mechanistic opportunities to reprogram fat cell fate and function. In this Review, we provide an overview of the current understanding of adipocyte metabolism in physiology and disease and also discuss possible strategies to alter fuel utilization in fat cells to improve metabolic health.
The human body has the remarkable ability to adapt to internal and external changes, including nutritional status, environmental temperature, infection, circadian rhythm, ageing, and more. These adaptations involve dynamic reprogramming of cellular metabolism in the peripheral tissues. For example, caloric restriction promotes mitochondrial biogenesis and fatty acid oxidation, thereby shifting cellular metabolism preferentially toward fatty acid oxidation rather than glucose oxidation in the mitochondria1,2. Similarly, cold exposure is associated with metabolic adaptation in skeletal muscle and adipose tissue by inducing a switch from carbohydrate metabolism to fatty acid oxidation3,4. From an evolutionary viewpoint, such metabolic shifts are considered to be protective mechanisms to secure enough glucose for the brain, while other fuels, such as fatty acids and amino acids, are allocated to the peripheral metabolic organs.
A main player during such adaptive processes, adipose tissue is a highly dynamic organ that can rapidly remodel in response to environmental inputs. White adipose tissue quickly expands by increasing lipid storage (hypertrophy) and by increasing the number of adipocytes (hyperplasia) that can be observed within days after initiation of an obesogenic diet, whereas involution of adipose tissue occurs within 24 hours of fasting5,6. On the other hand, thermogenic fat cells, such as brown adipocytes and beige adipocytes, swiftly adapt to utilize systemic fuels to drive thermogenesis. Systemic cues, such as norepinephrine released from the sympathetic nerve system (SNS), rapidly activate thermogenic fuel utilization. Moreover, beige adipocytes can transform into energy-storing white adipocytes within days after external stimuli are withdrawn7,8. These observations indicate that adipocytes are extraordinarily adaptive to the environment, as would be expected of cells that coordinate fuel storage and futile oxidation of stored energy.
By contrast, maladaptation in adipose tissue is tightly associated with the development of metabolic disorders, including insulin resistance, dyslipidaemia, hepatic steatosis, and type 2 diabetes. Chronic inflammation in white adipose tissue is induced by excess caloric intake, leading to insulin resistance and subsequent fat cell dysfunction9,10. Dysregulation of mitochondrial biogenesis and oxidative phosphorylation (OXPHOS), excess accumulation of extracellular matrix (ECM), and altered adipokines and lipid profile are strongly associated with adipose tissue malfunction and metabolic disorders. Here we review the current understanding of adipocyte metabolism and discuss strategies to reprogram fat cell fate and metabolism.
Regulatory pathways of adipocyte metabolism
Regulation of lipogenesis.
Adipocytes are integrated into systemic lipid metabolism with factors that control the uptake of free fatty acids from the circulation. Circulating chylomicronand very-low-density lipoprotein (VLDL)-bound triacylglycerols (TAG) interact with lipoprotein lipase (LPL), which hydrolyses circulating TAG to free fatty acids and monoacylglycerols that are taken up by adipocytes. CD36 (also known as fatty acid translocase (FAT)) is the principal fatty acid transporter in adipocytes that facilitates uptake, which depending on circumstance, can be either catabolized or stored11. Fat cells are also equipped with machinery capable of synthesizing new lipids from circulating carbohydrates through a process known as de novo lipogenesis (DNL). DNL relies on two key enzymes that are abundant in adipocytes: fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), which are transcriptionally regulated by sterol response element binding protein 1c (SREBP1c) and carbohydrate response element binding protein (ChREBP)12,13 (Fig. 1a).
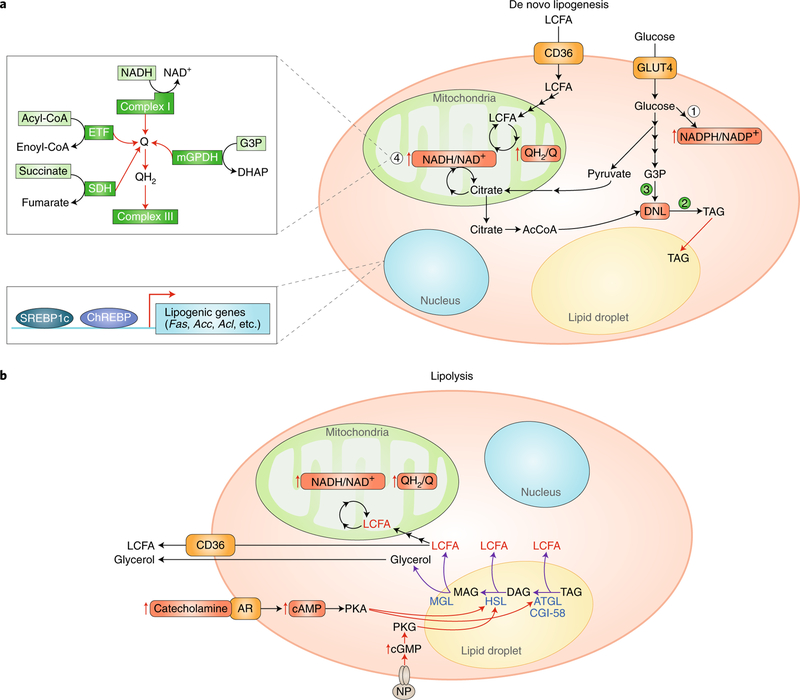
Fig. 1 |. Control of fatty acid storage and oxidation.
a, Adipocytes access long-chain fatty acids (LCFAs) and glucose from the circulation. Fatty acids can be oxidized by mitochondrial beta-oxidation, which elevates the reduction state of the mitochondrial ubiquinone and NADH/NAD+ pool. Imported glucose drives maintenance of an elevated NADPH/NADP+ ratio (1), which is essential for supporting both TAG synthesis (2) and thiol redox state in the cell. Oxidized glucose also generates G3P, which is required to initiate DNL as the glycerol backbone (3). Citrate, generated by either glucose or fatty-acid-linked metabolism is exported by mitochondria to form cytosolic Acetyl-CoA, which is the building block of the elongated acyl chains of TAG upon DNL. Importantly, high mitochondrial Q and NADH/NAD+ reduction will feedback to inhibit mitochondrial citrate metabolism, and these may act as a metabolic signal to promote citrate export to the cytosol and DNL. Additionally, a high Q reduction state will inhibit oxidation of cytosolic G3P by the mitochondrial glycerophosphate dehydrogenase and similarly may promote DNL (2). An expanded schematic on the left (4) summarizes the major metabolic pathways that share electrons with the mitochondrial Q pool and that therefore are subject to feedback regulation on the basis of its reduction state (that is, the QH2/Q ratio). These include mitochondrial complex I-mediated oxidation of mitochondrial NADH; mitochondrial oxidation of fatty acids through the electron transferring flavoprotein (ETF); succinate oxidation by succinate dehydrogenase (SDH); and oxidation of cytosolic G3P by the mitochondrial glycerol-phosphate dehydrogenase (mGPDH). In the nucleus, the transcription factors ChREBP and SREBP1c control genes involved in DNL. b, Adipocytes are engaged to generate high levels of LCFAs locally via triglyceride lipolysis. This process is initiated by external stimulation, for example by catecholamines followed by elevation in cytosolic cAMP and PKA activation, or by NP followed by elevation in cGMP and PKG signalling. PKA or PKG signalling stimulates liberation of free fatty acids from TAG through endogenous lipases, such as ATGL, HSL, and MGL. Upon liberation, LCFAs are either oxidized locally or released into the circulation to supply fuel to other cells.
Compared with lipid accumulation that relies on dietary fat, DNL is energetically costly. The adipocyte relies on two tracks of glucose metabolism to accomplish DNL. Glucose is oxidized to generate mitochondrial acetyl-CoA to fuel mitochondrial citrate synthesis (although citrate can also be synthesized using acetyl-CoA from other carbon sources). Mitochondrial citrate is then exported to the cytosol, where adenosine triphosphate (ATP)-citrate lyase and ACC drive malonyl-CoA production. In parallel, glucose-6-phosphate is also shunted to the pentose phosphate pathway to generate reducing equivalents in the form of NADPH14. This energy is required to fuel the fatty acid synthesis reactions that utilize malonyl-CoA to generate long-chain fatty acids for TAG synthesis. The metabolic intermediates of DNL act as acute regulators of fatty acid utilization in the adipocyte. Cytoplasmic malonyl-CoA is a potent inhibitor of mitochondrial β-oxidation via its inhibition of carnitine palmitoyltransferase 1 (CPT1)15, and citrate allosterically activates ACC to stimulate malonyl-CoA formation16. The NADPH/NADP+ ratio in the adipocyte likely plays a vital role in controlling fatty acid utilization, as will be discussed below.
Fatty acids taken up from the circulation or synthesized de novo are most often directed toward TAG synthesis for storage. This process requires a series of acyltransferases that facilitate stepwise esterification of a glycerol molecule to three free fatty acid molecules. TAG synthesis rates depend on both the levels of free fatty acids and glycerol-3-phosphate, which acts as the precursor for the TAG glycerol backbone17. As such, adipocyte TAG synthesis critically relies on enzymes that produce glycerol-3-phosphate (G3P). To this end, G3P can be generated by glycerol kinase, whereby free glycerol from the circulation or TAG hydrolysis is phosphorylated. However, the dominant pathway in adipocytes is to shunt glucose to produce G3P, which depends on the cytosolic glycerophosphate dehydrogenase (GPD1)18. Interestingly, G3P production by this enzyme is controlled by the cytosolic NADH/NAD+ ratio, while its consumption is controlled by competition between TAG synthesis and mitochondrial oxidation by the mitochondrial glycerophosphate dehydrogenase (GPD2). Since GPD2-mediated oxidation of G3P is inhibited by a reduced mitochondrial respiratory chain, that is, a high ubiquinol/ubiquinone (QH2/Q) ratio, it is reasonable to speculate that rates of mitochondrial fuel utilization regulate G3P levels and thereby promote TAG synthesis under conditions of nutrient excess.
An essential regulator of DNL is the mammalian target of rapamycin mTORC2, which controls ChREBP-β-dependent transcription of the major enzymes that coordinate fatty acid synthesis19,20. This transcriptional program is initiated by mTORC2-dependent glucose flux into adipocytes, which can elevate ChREBP-β expression via glucose-dependent activation of ChREBP-α. This systemic integration between glucose metabolism and adipocyte DNL appears to be a key nutrient-sensing mechanism for coordination of fuel storage and release.
Regulation of lipolysis.
On the opposing side of DNL and TAG synthesis is the cellular machinery for fatty acid liberation and oxidation. The dominant mode of free fatty acid liberation in adipocytes is through PKA-dependent lipolysis that is triggered by fasting or cold exposure via increased release of norepinephrine from the SNS21,22. Cold stimulus engages cold-sensitive thermoreceptors that transmit afferent signals to the hypothalamus and brain stem, leading to the release of norepinephrine from postganglionic sympathetic nerves that innervate adipocytes23,24. Norepinephrine acts on adrenoreceptors on the adipocyte plasma membrane, which ultimately results in the release of free fatty acids from stored triglycerides. The best-characterized effectors of this cascade are β-adrenergic receptors (β-ARs), which elevate intracellular cAMP to drive PKA-dependent phosphorylation of lipases within the adipocyte. The sequential hydrolysis of TAG by critical lipases, including adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL), mediates the conversion of TAGs to diacylglycerols (DAG), monoacylglycerols (MAG), and eventually fully liberated glycerol and free fatty acids25. Following liberation, the fate of free fatty acids depends on fat cell types. From white adipocytes, liberated fatty acids enter the bloodstream and supply peripheral tissues with reducing equivalents for mitochondrial respiration (Fig. 1b). In contrast, thermogenic brown and beige adipocytes can utilize proximal liberated fatty acids to drive uncoupled respiration, as discussed in the next section.
Recent studies also demonstrate that activation of lipolysis by catecholamine release from the SNS can be antagonized by competitive uptake and consumption of the activating molecules. Sympathetic-neuron-associated macrophages (SAMs) possess specialized transporters and monoamine oxidase A (MAOA), which effectively sequester and catabolize local extracellular norepinephrine26,27. These negative regulators of adipocyte lipolysis increase in content and activity with age and obesity, while genetic ablation of this pathway is sufficient to elevate adipocyte lipolysis and thermogenic activity.
In addition to cold temperature, other environmental perturbations that elevate local catecholamine levels can affect adipocyte lipolysis and have substantial effects on systemic metabolism. For instance, elevated energy expenditure in response to high-calorie diets (diet-induced thermogenesis) appears to involve local adrenergic signalling in thermogenic adipocytes. In fact, genetically engineered mouse models with defects in β-ARs are predisposed to obesity and exhibit reduced metabolic rate28. Moreover, individuals who are predisposed to obesity exhibit decreased adrenergic dependent thermogenic capacity29, suggesting that this impairment may be relevant in the context of human weight gain.
Regulation of adipose tissue thermogenesis
In contrast to white adipocytes, thermogenic fat cells—brown adipocytes and beige adipocytes—house mitochondria with the distinct capacity to rapidly oxidize fatty acids at extraordinary rates. Their highly active cellular metabolism is the result of unique protein machinery that allows thermogenic adipocytes to engage in futile cycling of metabolites that would typically be stored. The thermogenic oxidation machinery in brown and beige adipocytes is under the regulation of transcriptional factors and co-factors, such as PR domain containing 16 (PRDM16) and peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), which cooperate with DNA-binding transcription factors to coordinately induce mitochondrial biogenesis and fatty acid oxidation that require thermogenesis (Fig. 2a). Transcriptional regulation of brown and beige fat thermogenesis has been recently reviewed30,31.
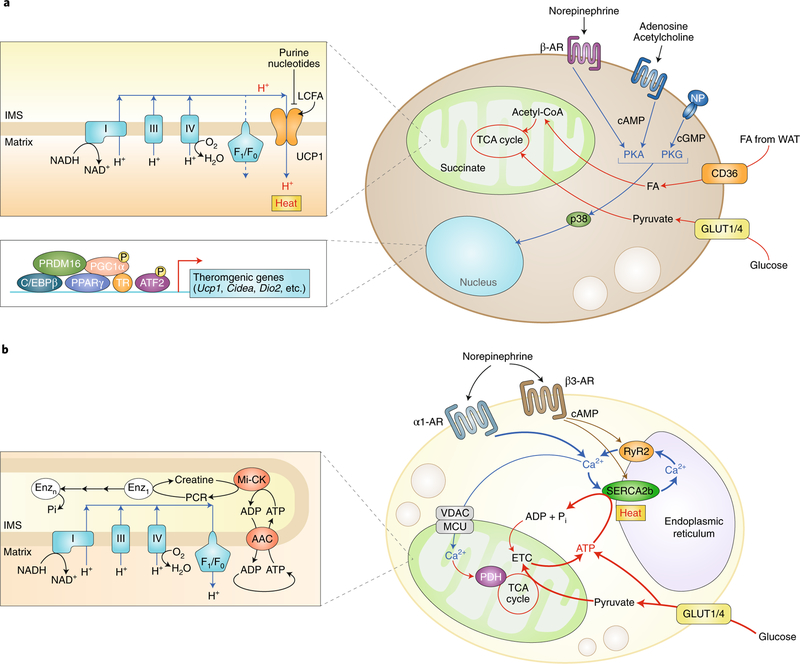
Fig. 2 |. Cellular metabolism in thermogenic fat cells.
a, UCP1-dependent thermogenesis in brown and beige adipocytes involves active free fatty acid and glucose oxidation. In response to thermogenic stimuli, such as norepinephrine, adenosine, acetylcholine, or NP, the intracellular cAMP or cGMP level is elevated, which triggers PKA or PKG signalling, respectively. p38MAPK (p38) phosphorylates transcriptional regulators, including PGC1a and ATF2, and promote the expression of BAT-specific thermogenic genes (Ucp1, Cidea, and Dio2) and mitochondrial biogenesis. Free fatty acid and glucose are actively imported from the circulation through CD36 and glucose transporter 1 or 4 (Glut1/4), respectively, and are oxidized in the mitochondria for UCP1-dependent thermogenesis. UCP1 activity is inhibited by purine nucleotides, while long-chain free fatty acids bind to UCP1 and stimulate proton uncoupling. b, UCP1-independent thermogenesis in beige adipocytes involves Ca2+ cycling through the SERCA2b–RyR2 pathway in the sarco/endoplasmic reticulum and subsequent activation of PDH in the mitochondria. In the mitochondria, creatine substrate cycling also generates heat independently of UCP1. Both pathways require active ATP synthesis in the mitochondria (ATP-dependent thermogenesis) and glucose oxidation. FA, fatty acid; P, phosphorylation; Pi, inorganic phosphate.
Mechanisms of canonical thermogenesis.
In response to cold exposure, norepinephrine from the SNS primarily acts on β1-AR and β3-AR, leading to the activation of PKA and p38MAPK signalling. Natriuretic peptides (NP; for example, atrial NP (ANP) and brain NP (BNP)) also activate adipose tissue thermogenesis through PKG signalling32. A new study showed that acetylcholine acts on the cholinergic receptor (CHRNA2) and activates beige fat biogenesis via the cAMP–PKA pathway33. The PKA–cAMP signalling pathway is known to activate many thermogenic genes in the nucleus, including Ucp1 and Pgc1a34.
The best-characterized mechanism of adipose thermogenesis is via uncoupling protein 1 (UCP1), a mitochondrial inner membrane protein that can dissipate the proton gradient across the mitochondrial lipid bilayer. The result is that the mitochondrial membrane potential can no longer be used for ATP synthesis. This uncoupling alleviates the inhibition of respiration that typically results from an elevation of the ATP/adenosine diphosphate (ADP) ratio in most cells. The result is a substantial elevation in the rate of respiration, substrate oxidation, and release of heat.
A well-characterized regulatory circuit for UCP1-dependent respiration is the competitive interaction between fatty acids and purine nucleotides. UCP1 is bound by purine nucleotides, the dominant species in the adipocytes being ATP. At concentrations that exist in the cell, this interaction appears to be sufficient to inhibit UCP1 proton current substantially. However, elevated local concentrations of long-chain fatty acids (longer than eight carbon atoms) overcome purine nucleotide inhibition and drive H+ leak through a fatty acid/H+ symport mechanism35. This competitive interaction between long-chain fatty acids and purine nucleotides occurs upon adrenergic stimulation and elevation of free fatty acid levels in thermogenic adipocytes, although structural insight into this competition remains unclear. Of note, a recent study has found that purine nucleotide pools in thermogenic adipocytes are dynamic, and modulation of these levels could additionally modify UCP1 activity36. This study showed that pharmacological inhibitors of the purinenucleotide-breakdown pathway significantly inhibit β-adrenergic signal-induced respiration in brown adipocytes and that overexpression of guanosine monophosphate reductase (GMPR) depletes guanosine triphosphate pools and potentiates fatty-acid-dependent respiration through UCP1. These findings are consistent with the fact that purine nucleotides competitively inhibit fatty-acid-dependent leak through UCP1; depleting ATP and ADP pools may alleviate this inhibition. These observations also suggest that modulation of purine nucleotide pool size in thermogenic adipocytes is an important mode of regulation of UCP1-dependent thermogenesis.
Another mode of regulation of adipocyte metabolism that has emerged more recently is through modification of cellular redox status. Genetic or pharmacological elevation of adipocyte reactive oxygen species (ROS) levels or resulting oxidation of cellular thiols (the major sensitive targets to ROS) can drive elevated thermogenesis37. Moreover, activation of thermogenesis in mouse BAT by applying either thermal stress (for example, cold exposure) or β-adrenergic stimulus results in elevated levels of multiple forms of ROS that play a role in supporting maximal thermogenesis, as evidenced by the fact that pharmacological depletion of these species compromises acute induction of thermogenic respiration37. A thermogenic action of mitochondrial ROS is likely mediated through cysteine modification of target proteins, including a regulatory cysteine residue (Cys253) on UCP1 itself38. More generally, the role of reversible thiol oxidation as an effector of thermogenic ROS signalling is important in the context of triggering thermogenic gene expression and mitochondrial biogenesis, as well as in acute control of thermogenic respiration. Continued investigation of protein cysteine targets of redox signalling in the context of thermogenesis is likely to yield additional modes of regulation of thermogenic gene expression and thermogenic function. Redox metabolism is also a noteworthy link between adipocyte DNL and thermogenesis, considering that two major adipocyte processes compete for the same NADPH pool: thiol antioxidant machinery, which maintains a reduced state for cellular thiols, and the above-described FAS-dependent DNL. Interestingly, genetic and pharmacological perturbations of thiol antioxidant systems and the NADPH/NADP+ ratio have substantial effects on both lipid accumulation and activation of thermogenesis in adipocytes. Elevation of the NADPH/NADP+ ratio thus appears to drive the adipocyte to a lipogenic phenotype, while oxidation of the NADPH/NADP+ drives cellular thiol oxidation and enhanced thermogenesis in BAT.
Mechanisms of non-canonical fat thermogenesis in beige fat.
As described above, UCP1 plays a central role in non-shivering thermogenesis via BAT. Because beige fat residing within WAT expresses lower levels of UCP1 than BAT, the contribution of beige fat to the regulation of whole-body energy homeostasis was thought to be marginal39. However, this view has been revised with the discovery of non-canonical (UCP1-independent) thermogenic mechanisms in beige fat involving Ca2+ cycling and creatine-dependent substrate cycling40,41 (Fig. 2b).
In the mitochondria-associated membrane (MAM) of beige adipocytes, ATP-dependent Ca2+ cycling by sarco/endoplasmic reticulum Ca2+-ATPase 2b (SERCA2b) and ryanodine receptor 2 (RyR2) is enhanced in the absence of UCP1, serving as a robust thermogenic mechanism in beige fat40. Furthermore, creatine and creatine-mediated ATP hydrolysis stimulate the futile cycling of ATP production and consumption within the mitochondria41. Notably, UCP1-negative beige adipocytes exist under physiological conditions; murine subcutaneous adipose tissue may contain ~10% or more of multilocular adipocytes that lack UCP1 expression42. Importantly, these UCP1-independent thermogenic mechanisms are active in humans, at least in cultured adipocytes40,41.
A particular interest related to Ca2+ cycling thermogenesis is fuel utilization in beige adipocytes. In contrast to thermogenesis driven by free fatty acid oxidation, Ca2+ cycling thermogenesis actively utilizes glucose as a primary substrate in the absence of UCP1. UCP1-deficient mice with increased beige fat mass (fat-specific PRDM16 transgenic mice in a Ucp1 KO background) display high respiratory exchange ratio (~0.9) and are protected from diet-induced obesity and glucose intolerance40. Mechanistically, this preferential glucose oxidation by UCP1-negative beige fat is due to increased activity of the pyruvate dehydrogenase (PDH) complex, the gatekeeper of glucose oxidation by transforming pyruvate into acetyl-CoA. As PDH activity is potentiated by Ca2+ entry into the mitochondria43, enhanced Ca2+ influx in the mitochondria likely leads to increased PDH activity, thereby promoting glucose oxidation in the absence of UCP1. It remains unknown, however, if enhanced Ca2+ flux in fat cells is sufficient to improve glucose homeostasis.
Besides Ca2+ and creatine cycling, a triacylglycerol (TG) futile cycle has been proposed as a thermogenic process in fat cells. In this context, glycerol derived from lipolysis is recycled back to TG by glycerol kinase (GyK). Intriguingly, this pathway is highly induced by thiazolidinediones (TZDs) or genetic knockout of NRIP1 (also known as receptor-interacting protein 140 (RIP140)), a transcriptional repressor of thermogenic genes44,45. However, the biological significance of TG futile cycling in humans is unclear46. The metabolic adaptation that occurs in conjunction with the futile cycle requires future investigation.
Fuel source of fat thermogenesis.
For many years, fatty acids generated within brown adipocytes were thought to be primarily used for BAT thermogenesis, given that brown adipocytes actively engage in de novo lipolysis47. However, recent studies demonstrate that adipocyte-specific deletion of adipose triglyceride lipase (ATGL) by a tamoxifen-inducible Cre recombinase under the control of the Adipoq gene promoter (Adiponectin-Cre) causes a defect in BAT thermogenesis, whereas BAT-specific deletion of ATGL or the ATGL-activating protein comparative gene identification-58 (CGI-58) with a Ucp1-Cre driver does not compromise cold-induced BAT thermogenesis in vivo48,49. This suggests that, within brown adipocytes, fatty acids derived from DNL are dispensable for BAT thermogenesis. Instead, circulating fatty acids generated through WAT lipolysis appear to be taken up by brown adipocytes via CD36 and are subsequently used as fuel by mitochondria and for activation of UCP1.
At a cellular level, mitochondrial free fatty acid oxidation is required for thermogenesis because fat-specific loss of carnitine palmitoyltransferase 2 (CPT2), essential for long-chain fatty acid oxidation in the mitochondria, leads to a substantial decline in BAT thermogenesis in mice50. Furthermore, genetic deletion of CD36, an essential membrane transporter of long-chain fatty acids, causes hypothermia in mice51,52. These results align well with the notion that thermogenic fat cell metabolism generally depends on free fatty acid oxidation in the mitochondria and subsequent activation of UCP1-mediated proton uncoupling. As discussed above, however, beige adipocytes have high metabolic plasticity, enabling them to shift between fuel sources depending on their availability and on internal thermogenic mechanisms, such as glucose-driven thermogenesis in the absence of UCP1.
Are all beige adipocytes the same?
Thermogenic adipocytes respond to β-AR stimulation by the SNS. When β-AR signalling is impaired (for example, as a result of aging) or pharmacologically blocked (for example, with β-blockers), brown fat thermogenesis is highly suppressed. Genetic studies have also demonstrated that mice lacking all the three forms of β-adrenergic receptors (β1−/−β2−/−β3−/−; also known as ‘β-less mice’) exhibit a defect in BAT thermogenesis and quickly develop hypothermia upon acute cold exposure28. However, if slowly acclimated to a cold environment, β-less mice can tolerate it for over 1 month because of enhanced beige fat biogenesis and thermogenesis. This observation suggests that compensatory pathways must exist that stimulate beige fat thermogenesis in the absence of β-AR signalling.
How is fat thermogenesis induced in the absence of β-AR signalling? This question is important because extensive efforts to develop selective β3-AR agonists for human obesity have so far been unsuccessful. This failure is partly because of poor bioavailability of available agonists and the relatively low expression levels of β3-AR in human adipocytes compared with murine adipocytes53. A potential side effect that β3-AR agonists will have to circumvent in the clinic is that pharmacological activation of β3-AR potently increases heart rate and blood pressure in humans54, which constitutes a major risk factor for the development of cardiovascular diseases.
One potential mechanism to activate brown and beige fat thermogenesis in the absence of β-AR signalling is mediated by adenosine55,56. An alternative mechanism, which does not conflict with the earlier studies, is the compensatory activation of a previously uncharacterized form of beige fat, the development and thermogenic regulation of which is independent of the β-AR pathway. A recent study57 identified a subset of adipose progenitors that express MyoD, which emerges in subcutaneous WAT when β-AR signalling is blocked. Unexpectedly, MyoD+ progenitors in WAT retain cellular plasticity and give rise to a unique population of beige adipocytes that express not only UCP1 but also key enzymes involved in glucose oxidation, such as enolase (ENO1) and an isoenzyme of the glycolytic enzyme pyruvate kinase (PKM2). Their gene expression profile and cellular metabolism are distinct from that of the ‘conventional’ beige adipocytes that emerge in response to β-AR activation (for example, synthetic β3-AR agonists) in that they possess high glucose uptake and oxidation capacities; accordingly, these thermogenic fat cells are termed ‘glycolytic beige fat’ (g-beige fat)57. Although these glycolytic beige adipocytes represent only a relatively small amount (~15%) of all UCP1+ beige adipocytes, glucose uptake and oxidation of g-beige fat makes a significant contribution to systemic glucose homeostasis because depletion of this cell type, either by selectively deleting peroxisome proliferator-activated receptor-γ (PPARγ) or expressing Diphtheria toxin receptor in MyoD+ progenitors, leads to glucose intolerance when β-AR signalling is blocked57. These results suggest that cellular metabolism and fuel utilization within adipose tissue are heterogeneous and that highly specialized subtypes of adipocytes exist.
Regulation of metabolite flux in the adipose tissue
The extraordinary metabolic plasticity of adipocytes extends to their integration into systemic metabolism. Brown, beige, and white adipocytes rapidly alter fuel uptake and consumption and the production of various metabolite classes in response to systemic cues, including the SNS-driven stimuli. In many cases, these acute changes in metabolite flux are critical upstream regulators of adipocyte function. As discussed below in detail, recent studies have demonstrated that exposure to cold temperatures has profound effects on metabolite flux in adipocytes. Notably, manipulation of some metabolites sufficiently alters the functional properties of adipocytes.
Cold-induced cellular metabolites.
An example of cold-induced changes in metabolite flux is purine nucleotide metabolism in brown adipocytes, where the total purine nucleotide pool size is dynamic, and ATP and ADP pools are substantially depleted upon β-adrenergic stimulation36. Depletion of guanosine triphosphate pools is sufficient to potentiate fatty-acid-dependent respiration through UCP1, suggesting that modulation of purine nucleotide pool size in thermogenic adipocytes by rapid catabolism is an essential mode of regulation of UCP1-dependent thermogenesis. Because additional thermogenic cycles rely on futile utilization of ATP, for example, via SERCA and creatine metabolism, it will be interesting to determine the extent to which β-AR-dependent effects on purine nucleotide pools modulate futile cycling of ATP in adipocytes.
In addition to the unusual dynamics of high-energy purine nucleotide phosphates, the utilization of creatine and creatine phosphate in thermogenic adipocytes differs from other cells in the body. The phosphorylation and dephosphorylation of creatine in most cells occur in 1:1 stoichiometry with the ATP/ADP couple. However, mitochondria in thermogenic adipocytes appear to liberate a molar excess of ADP with respect to creatine, as inferred by observing the respiratory response to creatine under ADP-limited conditions41,58. This observation suggests that creatine phosphorylation and dephosphorylation cycles futilely in these mitochondria to drive thermogenic respiration. Alternatively, creatine could stimulate spontaneous hydrolysis of newly generated ATP without cycling itself. In either case, this highly unusual type of creatine utilization suggests that thermogenic adipocytes possess the cellular machinery for atypical phosphotransfer reactions involving ATP and creatine, or perhaps a novel class of metabolite phosphatase that facilitates this thermogenic process. Detailed examination of the metabolites and proteins involved in this process will likely reveal novel mechanisms of thermogenesis in these cells.
Recent unbiased investigations of the adipocyte metabolome have also revealed additional distinct metabolic cascades that have substantial effects on thermogenic adipocyte function. Upon cold exposure, thermogenic adipocytes can selectively accumulate the mitochondrial tricarboxylic acid (TCA) cycle metabolite succinate, which drives activation of thermogenic respiration in these cells59. This accumulation can be achieved by sequestering succinate from the circulation, which suggests that this metabolite can coordinate thermogenic adipocyte function via a systemic release of succinate from other tissues or the diet. Mechanistically, succinate stimulates mitochondrial thermogenic respiration via the production of ROS, which is essential for its thermogenic role, as discussed above. Importantly, systemic administration of succinate can be potently thermogenic, which requires competent thermogenic adipose tissue59. The study further supports the notion that manipulation of cellular metabolites sufficiently alters adipose tissue function in vivo.
Analysis of metabolomic changes in thermogenic fat cells has identified rapid adaptations in metabolites that control redox tone. Rapid oxidation and depletion of the reduced glutathione pool, concomitant with a rapid elevation in numerous reactive metabolite intermediates and ROS, occurs early upon activation of adipocyte thermogenesis by exposure to cold38,60,61. Succinate sequestration and oxidation appears to be a critical ROS-producing pathway in this context, as it has been demonstrated that the production of thermogenic ROS depends on succinate metabolism by succinate dehydrogenase, while the adrenergic cascade may also play a potentiating role38,59. The production of these reactive molecules and consequent oxidation of cellular thiols appear to be a mode of thermogenic regulation via post-translational modification of key thermogenic proteins, including UCP1. The extent to which succinate–ROS metabolism initiates thermogenesis via UCP1 compared with other effectors such as creatine and Ca2+ metabolism will be an interesting area of future research.
Thermogenic fat as a metabolic sink.
Positron emission tomography-computed tomography (PET/CT) with 18F-fluorodeoxyglucose (18FDG) and the fatty acid tracer 18F-fluoro-thiaheptadecanoic acid (18FTHA)62–68 have demonstrated the remarkable capacity of BAT to take up fuels—thus BAT is often referred to as a ‘metabolic sink’ for glucose and free fatty acids. BAT fuel uptake is tightly coupled with its ability to increase whole-body energy expenditure and with improvement in systemic glucose homeostasis, lipid metabolism, and insulin sensitivity, both in rodents and adult humans. For example, cold promotes the uptake of triglyceride-rich lipoproteins in BAT, thereby contributing to triglyceride clearance and improvement in systemic lipid metabolism69,70. Cold acclimation also improves systemic glucose homeostasis and insulin sensitivity in adult humans71–73. Of note, glucose uptake per volume or mg of BAT is substantially higher than that of other metabolic organs; however, total glucose uptake and utilization by skeletal muscle tissue contributes to a greater extent to whole-body glucose homeostasis than BAT when the relative tissue mass is taken into consideration74.
It is worth mentioning that active glucose uptake by brown and beige fat is independent of UCP1. This somewhat unexpected observation is based on the studies showing that glucose uptake in brown and beige fat, as assessed by 18FDG-PET scans, remains unaffected in Ucp1 KO mice40,75. Because fuel uptake into fat cells can be uncoupled from its fuel oxidation capacity under certain conditions, measuring both fuel uptake and oxidation is essential for the critical assessment of thermogenic function in adipose tissue. Recent findings also suggest that creatine energetics play an important role in human adipose tissue thermogenesis. 18FDG-PET studies in humans have demonstrated that renal creatinine clearance is a significant predictor of total activated human BAT76. Since creatinine is a direct product of phosphocreatine metabolism, these results are consistent with the activation of UCP1-independent thermogenesis by creatine substrate cycling in human BAT.
Going forward, it will be interesting to determine whether thermogenic adipocytes can sequester and utilize any circulating metabolites other than glucose, fatty acids, and succinate. Such investigations may reveal that thermogenic fat is systemically integrated to metabolize many classes of metabolites. While the contribution of the human BAT to diet-induced thermogenesis was once in question77, recent studies clearly demonstrate that human BAT is metabolically active even under normal or warm ambient temperatures with higher blood flow than WAT78 and that glucose uptake in BAT is highly activated after food intake, a phenomenon known as diet-induced thermogenesis79,80. Hence, BAT might control the clearance of specific metabolites in the circulation, and such metabolites could serve as predictors of BAT activity.
Metabolic maladaptation in the adipose tissue
Cellular mechanisms of adipose tissue expansion.
In response to excess caloric intake, adipose tissue has the incredible capacity to expand its tissue mass by increasing adipose cell size (hypertrophy) and cell number (hyperplasia). In humans, adipose cell number is reportedly the primary determinant for the adipose tissue mass; adipocyte number is determined during childhood and adolescence, and maintained in adults even after substantial weight loss81.
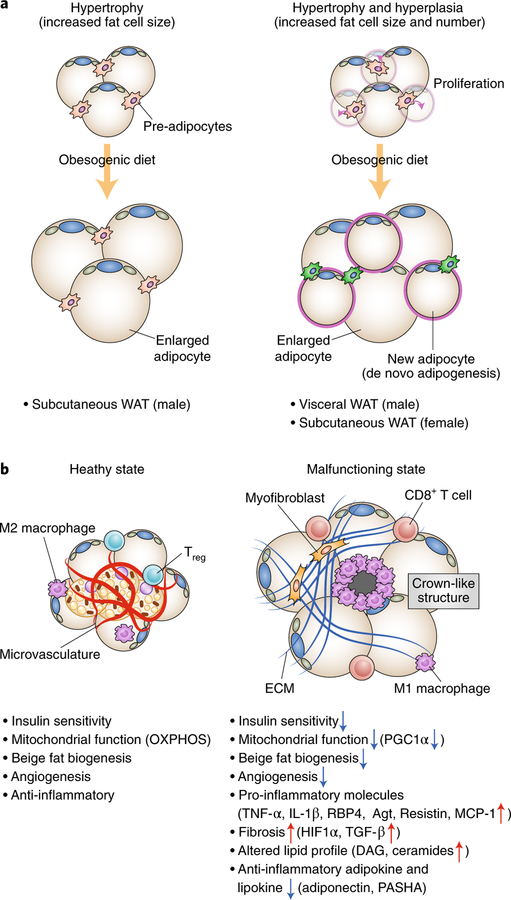
Recent advances in tracing of fat-cell lineage in mice have provided precise information about the regulation of adipose tissue expansion in a location- and gender-selective manner (Fig. 3a). For instance, expansion of epididymal WAT, a major visceral WAT depot in mice, involves both hypertrophy and hyperplasia5,82. Adipocyte precursors in the visceral WAT that are marked by Lin−:CD29+:CD34+:Sca1+ cells actively proliferate within 1 week after onset of high-fat diet feeding and undergo adipogenesis5. Moreover, perivascular preadipocytes that express zinc finger protein 423 (ZFP423) and platelet derived growth factor receptor-β (PDGFRβ) contribute to WAT hyperplasia in diet-induced obesity by increasing the cell number and by de novo differentiation83. On the other hand, inguinal WAT, a subcutaneous adipose depot in mice, expands exclusively through hypertrophy in male mice, although high-fat diet feeding in female mice can promote de novo adipogenesis and hyperplasia in the inguinal WAT84. Intriguingly, diet-induced hyperplasia depends on the activation of Akt2 signalling because high-fat diet feeding rapidly increases phosphorylation of Akt2 (S474) in adipocyte progenitors, and Akt2-null mice fail to stimulate diet-induced adipocyte progenitor proliferation. Since these observations were made in mice on the C57BL/6 genetic background, it would be intriguing to examine possible variations among mouse strains.
Fig. 3 |. Adaptation and maladaptation in adipose tissue.
a, Adipose tissue expansion occurs through hypertrophy and hyperplasia. Left, Adipose tissue hypertrophy involves increased cell size of existing adipocytes. Subcutaneous WAT in mice is increased in mass by hypertrophy. Right, Adipose tissue hyperplasia involves an increase in the number of adipocytes by adipocyte precursor proliferation and de novo adipogenesis. Visceral WAT of mice and subcutaneous WAT of female mice increase in mass through the combination of hypertrophy and hyperplasia. b, Hallmarks of healthy adipose tissue (left) and malfunctioning adipose tissue (right). Healthy adipose tissues are insulin sensitive with active mitochondrial biogenesis and OXPHOS. They also possess thermogenic beige adipocytes, increased angiogenesis, and anti-inflammatory M2 macrophages and regulatory T cells (Tregs). By contrast, malfunctioning ‘unhealthy’ adipose tissues are insulin resistant, and mitochondrial biogenesis and OXPHOS, beige adipocyte biogenesis, and angiogenesis are impaired. Adipose tissue expresses many pro-inflammatory cytokines (M1 macrophages and CD8+ T cells) and produces excess ECM from myofibroblasts, leading to the recruitment of pro-inflammatory immune cells and the formation of adipose tissue fibrosis and crown-like structures. Adipocytes also display altered lipid and adipokine and lipokine profiles, including increased DAG and ceramides as well as reduced secretion of adiponectin and palmitic acid hydroxystearic acids (PASHA).
Mechanisms of adipose tissue remodelling.
Besides hypertrophy and hyperplasia, emerging evidence suggests that ECM proteins regulate adipose tissue expansion in response to excess caloric intake. ECM proteins—including collagens (for example, collagen I, III, IV, VI, etc.), fibronectin, and elastin—ensure physical integrity of adipose tissue during expansion. On the other hand, excessive deposition of the ECM proteins is associated with infiltration of pro-inflammatory immune cells, such as M1 macrophages. Clinical studies show a strong correlation between increased adipose tissue fibrosis in the subcutaneous WAT and insulin resistance and type 2 diabetes85–89. Furthermore, the degree of adipose tissue fibrosis in the subcutaneous WAT is inversely correlated with the effectiveness of weight loss following gastric bypass surgery in humans90. These observations suggest that adipose tissue fibrosis is a morphological hallmark of adipose tissue malfunction that is coupled with increased pro-inflammatory responses and reduced angiogenesis (Fig. 3b).
Which cell types within the adipose tissue contribute to the accumulation of ECM proteins? A recent study suggests that myofibroblasts expressing PDGFRα and high levels of CD9 are profibrogenic and drive adipose tissue fibrosis91. It has also been suggested that the majority of ECM-producing myofibroblasts in the dermal WAT originate from Adiponectin-expressing adipocytes that go through adipocyte–myofibroblast transition92. Additionally, macrophages and mature adipocytes produce pro-fibrotic collagens, fibronectin, and tenascin-C in WAT that contribute to the accumulation of ECM93,94. Hence, multiple cell types likely contribute to the accumulation of ECM in the adipose tissue. Of note, the degree of adipose tissue fibrosis and its relationship to metabolic disorders appears to be dependent on adipose tissue depots and may be different between rodents and humans. In rodents, visceral WAT fibrosis is prominent in obese mice, whereas subcutaneous WAT fibrosis correlates well with metabolic dysfunction in humans. Therefore, the cell types responsible for the accumulation of ECM proteins and the development of adipose tissue fibrosis in humans warrant further investigation.
A key player in the initiation of adipose tissue fibrosis is transforming growth factor-β (TGF-β). TGF-β levels in the circulation and adipose tissues are highly elevated in obese humans and mice95,96. Adipose tissue hypoxia and subsequent accumulation of hypoxia-inducible factor 1α (HIF1α) lead to the activation of HIF1α-dependent gene transcription of pro-fibrotic genes in the adipose tissue97–99. Whereas mouse models with ectopic activation of HIF1α in adipose tissues induce fibrosis and systemic glucose intolerance100, treatment with PX-478, the HIF1α-selective inhibitor, potently ameliorates adipose tissue fibrosis, inflammation, and glucose intolerance101. Intriguingly, cold acclimation potently represses adipose tissue fibrosis—such repression in adipose tissue fibrosis is independent of UCP1-dependent thermogenesis and body-weight loss because cold acclimation inhibits adipose tissue fibrosis even in Ucp1 KO mice94.
Is excess ECM accumulation merely an epiphenomenon of adipose tissue malfunction? Recent work suggests that adipose tissue fibrosis is more than an epiphenomenon and functionally contributes to the regulation of adipose tissue homeostasis and systemic glucose metabolism. Col6-deficient mice, which lack the major adipose tissue ECM protein collagen VI (Col6), can expand adipose tissue with reduced levels of inflammation and exhibit high glucose tolerance and insulin sensitivity on a high-fat diet102. In another study, transgenic mice expressing general transcription factor II-I repeat domain-containing protein (GTF2IRD1), a cold-inducible transcription factor, have repressed pro-fibrotic gene expression in the adipose tissue94. The adipose tissue of GTF2IRD1 transgenic mice contains significantly less fibrosis relative to control mice on a high-fat diet, and these mice are protected from diet-induced insulin resistance and glucose intolerance although their body weight remains unaffected94. Furthermore, antibody-based blockade of endotrophin, a C-terminal cleavage product of Col6α3 that drives adipose tissue fibrosis, prevents diet-induced adipose tissue fibrosis and inflammation and ameliorates diet-induced insulin resistance103. These results hint at the exciting possibility that adipose tissue fibrosis can be a therapeutic target for the improvement of systemic glucose homeostasis and insulin sensitivity, suggesting adipose tissue fibrosis is not simply a consequence of an unhealthy metabolic state.
Mitochondrial dysfunction in adipose tissue.
Independent lines of evidence in mice and humans suggest that obesity is associated with reduced mitochondrial function in the adipose tissue. For example, mitochondrial OXPHOS and biogenesis in human subcutaneous WAT are compromised under obese and diabetic states104–106. Such a decline occurs independently of genetic background; comparisons between monozygotic twin pairs with discordant body mass index (BMI) found that mitochondrial biogenesis and OXPHOS in the subcutaneous WAT are downregulated in acquired obesity and associated with metabolic abnormalities107,108. Conversely, therapeutic interventions that improve insulin sensitivity, such as exercise and treatment with synthetic PPARγ agonists, potently increase mitochondrial biogenesis and OXPHOS in adipocytes109–113. Intriguingly, expression of genes that control mitochondrial function—such as OXPHOS, the TCA cycle, and free fatty acid oxidation pathways—is significantly higher in the subcutaneous WAT of individuals who successfully lost weight than in those who re-gained weight after a dietary-based weight loss intervention, suggesting that mitochondrial function is associated with success in weight loss114.
Is reduced mitochondrial function simply a metabolic consequence of obesity, or is it involved in the development of adipose tissue malfunction? Genetic studies in mice indicate a causal link between reduced mitochondrial function in adipocytes and the development of insulin resistance. For instance, fat-specific deletion of mitochondrial transcription factor A (TFAM) causes adipocyte death, adipose tissue inflammation, and systemic insulin resistance115. Similarly, fat-specific deletion of PGC1α leads to a decline in mitochondrial biogenesis in the subcutaneous WAT and systemic insulin resistance116. Conversely, improvement of mitochondrial function in adipocytes sufficiently prevents adipose tissue inflammation, fibrosis, and insulin resistance. Fat-specific overexpression of mitoNEET (CDGSH iron sulfur domain 1), an outer mitochondrial membrane containing iron-sulfur (Fe-S) clusters, ameliorates obesity-associated adipose tissue dysfunction, and thereby mice are protected from insulin resistance and display a ‘healthy obese’ phenotype117,118. Similarly, fat-specific overexpression of mitochondrial thiosulfate sulfurtransferase, a mitochondrial protein that controls Fe-S clusters, enhances insulin sensitivity and glucose tolerance119. These results suggest that impaired mitochondrial function, that is, mitochondrial OXPHOS and biogenesis, is an index of adipose tissue health and that reduced mitochondrial mass and/or function is an indicator of adipose tissue malfunction (Fig. 3b).
Altered adipokine and lipid profiles.
Adipocytes constitute a significant source of secretory cytokines, also known as adipokines, and adipokine profile is dynamically changed in response to nutritional cues. Generally speaking, expression and secretion of many pro-inflammatory adipokines, such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), angiotensinogen, monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), and resistin, are elevated in obesity, and each factor contributes to the activation of chronic inflammatory state in the adipose tissue through interaction with pro-inflammatory immune cells120. By contrast, adiponectin expression is closely correlated with anti-inflammatory states of adipose tissue in rodents and humans. Administration of adiponectin increases free fatty acid oxidation and insulin sensitivity in metabolic organs, including liver and skeletal muscle, through its receptors Adipo-R1 and Adipo-R2 (ref. 121). It is also notable that lipidomics studies have identified unique lipid species that are secreted from brown and white adipocytes, often referred to as lipokines. For instance, C16:1n7-palmitoleate is a lipokine that stimulates insulin sensitivity in skeletal muscle, while it suppresses hepatosteatosis in mice122. Palmitic acid hydroxystearic acids (PAHSAs) are recently identified lipokines with levels in circulation that are significantly lowered in the insulin-resistant state in mice and humans123. PAHSA treatment reduces adipose tissue inflammation and enhances insulin-stimulated glucose uptake through G-protein-coupled receptor 120 (GPR120). PAHSAs also stimulate intracellular Ca2+ flux through GPR40 and augment insulin and glucagon-like peptide (GLP-1) secretion124, although a recent paper has challenged this observation125. Circulating levels of 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) are increased in response to cold exposure in mice and humans, which enhances fatty acid uptake by thermogenic adipocytes126, while 12,13-diHOME is also synthesized in nervous tissue and causes thermal pain hypersensitivity via transient receptor potential cation channel subfamily V member 1 (TRPV1) in the sensory neurons127. Genetic manipulation of the biosynthesis enzymes will determine the physiological roles of these lipokines in the regulation of systemic energy homeostasis and insulin sensitivity in vivo. Given the recent advances in lipidomic profiling technology, new classes of lipokines will likely be identified, some of which may mediate inter-organ communication between adipose tissue and other metabolic organs.
Another change in fat cells that is associated with adipose tissue maladaptation is increased free fatty acid flux as well as increased levels of diacylglycerol (DAG) and ceramides. The ‘spillover’ of lipids from adipocytes and ectopic deposition in liver and skeletal muscle are considered to cause mitochondrial dysfunction, insulin resistance, and cardiometabolic diseases (that is, lipotoxicity)128. Recently, sphingolipids (for example, ceramide and its metabolites) have gained much attention as prominent lipotoxic lipids that induce insulin resistance. Circulating levels of many ceramide species are elevated in insulin-resistant subjects129, and pharmacological inhibitors of ceramide biosynthesis effectively alleviate obesity-induced inflammation, increase energy expenditure, and improve insulin sensitivity in rodent models130–134. Importantly, identification of key enzymes responsible for ceramide biosynthesis (CerS1–6) has allowed for genetic manipulation of de novo ceramide synthesis and establishment of a causal relationship between ceramides and metabolic disorders in vivo. For instance, CerS6 expression and C16:0 ceramides are elevated in adipose tissue of obese humans, whereas CerS6-knockout mice displayed reduced C16:0 ceramides and are protected from diet-induced obesity and glucose intolerance135. Furthermore, fat-specific deletion of serine palmitoyltransferase (Sptlc), the first enzyme in the sphingolipid biosynthesis pathway, induces beige adipocyte biogenesis and mitochondrial function that is associated with reduced inflammation and increased insulin sensitivity136. Notably, adiponectin potently reduces ceramide levels through its receptors AdipoR1 and AdipoR2, which possess ceramidase activity137,138. These observations are exciting because the ceramide biosynthesis pathway may form a part of the underlying mechanism by which dysregulated lipids causes adipose tissue malfunction and insulin resistance.
Future perspectives
One may presume that metabolites are equally distributed within cells, but some metabolites are compartmentalized, and the locally produced metabolites have unique cellular functions. A recent study shows that NAD+ accumulates in the nucleus of preadipocytes and inhibits the transcriptional activity of CCAAT/enhancer binding protein-β (C/EBPβ), a critical initiation factor of adipogenesis. When the expression of nicotinamide nucleotide adenylyltransferase 2 (NMNAT2), the cytosolic form of the NAD+ synthesis enzyme, is elevated, cytosolic NMNAT2 depletes the supply of NAD precursors to the nucleus, leading to the activation of CEBPβ and adipogenesis139. This study elegantly explains how a compartmentalized metabolite NAD+ controls the signal-dependent regulation of adipogenesis. Although stable chemical or protein indicators that allow accurate monitoring of intracellular metabolite flux are currently limited (for example, GCaMP6 for intracellular Ca2+), many metabolites other than NAD+ might be similarly compartmentalized in specific organelles.
At the tissue level, it will be intriguing to explore the mechanisms by which metabolic tissues communicate with each other to coordinate their fuel utilization. For instance, promoting glycolysis in hepatocytes by overexpressing glucokinase in the liver attenuates BAT thermogenesis and increases adiposity by suppressing sympathetic nerve activity140. Also, certain cancer cells, such as those in triple-negative breast cancers, actively utilize fatty acids to maximize cell growth and proliferation, and their metabolic activity heavily depends on the nutritional microenvironment141. A better understanding of the upstream regulators of fuel utilization will eventually allow for selective manipulation of cellular metabolism and function.
Lastly, cellular heterogeneity within adipose tissues needs further exploration. Although functional and developmental differences between visceral WAT and subcutaneous WAT have been recognized, more subtypes of adipocytes likely exist. Even among thermogenic adipocytes, multiple subtypes of these cells of distinct developmental origin exist, and each subtype likely has a unique biological role, depending on the nature of external stimuli, such as β-AR activation, exercise, bariatric surgery, cancer cachexia, bile acids, or PPARγ agonists. One case in point is g-beige fat, a new subtype of beige adipocytes whose developmental regulation is distinct from that of conventional beige adipocytes and whose thermogenic program can be induced even in the absence of β-AR signalling57. Another example is that pharmacological inhibition of cyclin-dependent kinase5 (CDK5) promotes the formation of beige adipocytes that exhibit a distinct molecular profile that is different from β3-AR agonist-induced beige adipocytes142. We envision rapid advances in cellular mapping studies because single-cell RNA sequencing analyses of adipose tissues indicate the existence of diverse progenitor cell populations143–145. In addition, a FACS-based approach for the isolation of mature adipocytes for single-cell analyses has become available146. Studies at this single-cell resolution will provide new insights into functional fat-cell heterogeneity in physiology and disease.
Acknowledgements
We apologize for being unable to cite papers that have contributed to the progress of this field owing to space limitations. This work was supported by the National Institutes of Health (DK97441, DK112268, and DK108822); the Edward Mallinckrodt, Jr. Foundation to S.K.; and the Claudia Adams Barr Program to E.T.C.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guarente L Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM & Weindruch R Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab 21, 134–141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajimura S, Spiegelman BM & Seale P Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22, 546–559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepa-Kishi DM, Sotoudeh-Nia Y, Iqbal A, Bikopoulos G & Ceddia RB Cold acclimation causes fiber type-specific responses in glucose and fat metabolism in rat skeletal muscles. Sci. Rep 7, 15430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffery E, Church CD, Holtrup B, Colman L & Rodeheffer MS Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol 17, 376–385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleemann R et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS One 5, e8817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenwald M, Perdikari A, Rülicke T & Wolfrum C Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol 15, 659–667 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Altshuler-Keylin S et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab 24, 402–419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenk S, Saberi M & Olefsky JM Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest 118, 2992–3002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czech MP Insulin action and resistance in obesity and type 2 diabetes. Nat. Med 23, 804–814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepino MY, Kuda O, Samovski D & Abumrad NA Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr 34, 281–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman MA et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strable MS & Ntambi JM Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol 45, 199–214 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J et al. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol. Cell. Biol 25, 5146–5157 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster DW Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J. Clin. Invest 122, 1958–1959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DB & Vagelos PR The mechanism of tricarboxylic acid cycle regulation of fatty acid synthesis. J. Biol. Chem 237, 1787–1792 (1962). [PubMed] [Google Scholar]
- 17.Reshef L et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem 278, 30413–30416 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Postic C & Girard J Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest 118, 829–838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CM et al. Rictor/mTORC2 loss in the Myf5 lineage reprograms brown fat metabolism and protects mice against obesity and metabolic disease. Cell Rep 8, 256–271 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun 7, 11365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arner P Human fat cell lipolysis: biochemistry, regulation and clinical role. Best. Pract. Res. Clin. Endocrinol. Metab 19, 471–482 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Braun K, Oeckl J, Westermeier J, Li Y & Klingenspor M Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol 221(Suppl 1), jeb165381 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K & Morrison SF Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. (Lond.) 589, 3641–3658 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayner DV The sympathetic nervous system in white adipose tissue regulation. Proc. Nutr. Soc 60, 357–364 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E & Sul HS Regulation of lipolysis in adipocytes. Annu. Rev. Nutr 27, 79–101 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camell CD et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirzgalska RM et al. Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med 23, 1309–1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachman ES et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Jung RT, Shetty PS, James WP, Barrand MA & Callingham BA Reduced thermogenesis in obesity. Nature 279, 322–323 (1979). [DOI] [PubMed] [Google Scholar]
- 30.Inagaki T, Sakai J & Kajimura S Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol 17, 480–495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harms M & Seale P Brown and beige fat: development, function and therapeutic potential. Nat. Med 19, 1252–1263 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Bordicchia M et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest 122, 1022–1036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun H et al. An immune–beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat. Med 24, 814–822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins S β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol 2, 102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedorenko A, Lishko PV & Kirichok Y Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fromme T et al. Degradation of brown adipocyte purine nucleotides regulates uncoupling protein 1 activity. Mol. Metab 8, 77–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han YH et al. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes 65, 2639–2651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouchani ET et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedergaard J & Cannon B UCP1 mRNA does not produce heat. Biochim. Biophys. Acta 1831, 943–949 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Ikeda K et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med 23, 1454–1465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazak L et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitali A et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res 53, 619–629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denton RM Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 1787, 1309–1316 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Guan HP et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med 8, 1122–1128 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Kiskinis E et al. RIP140 represses the “brown-in-white” adipocyte program including a futile cycle of triacylglycerol breakdown and synthesis. Mol. Endocrinol 28, 344–356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan GD et al. A “futile cycle” induced by thiazolidinediones in human adipose tissue? Nat. Med 9, 811–812; author reply 812 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Schreiber R et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26, 753–763.e757 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin H et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab 26, 764–777.e765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Ellis JM & Wolfgang MJ Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep 10, 266–279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson CM et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 10, 505–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putri M et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem. Biophys. Res. Commun 457, 520–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arch JR Challenges in β(3)-adrenoceptor agonist drug development. Ther. Adv. Endocrinol. Metab 2, 59–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cypess AM et al. Activation of human brown adipose tissue by a β 3-adrenergic receptor agonist. Cell Metab 21, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razzoli M et al. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol. Metab 5, 19–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnad T et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Chen Y et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 10.1038/s41586-018-0801-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazak L et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab 26, 660–671.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills EL et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chouchani ET, Kazak L & Spiegelman BM Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J. Biol. Chem 292, 16810–16816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X et al. The early metabolomic response of adipose tissue during acute cold exposure in mice. Sci. Rep 7, 3455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Marken Lichtenbelt WD et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Cypess AM et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouellet V et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest 122, 545–552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virtanen KA et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Nedergaard J, Bengtsson T & Cannon B Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab 293, E444–E452 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Saito M et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labbé SM et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29, 2046–2058 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Bartelt A et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med 17, 200–205 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Chondronikola M et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23, 1200–1206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee P et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanssen MJ et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med 21, 863–865 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Chondronikola M et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blondin DP et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J. Physiol. (Lond.) 593, 701–714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsen JM et al. β3-Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Mol. Metab 6, 611–619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerngross C, Schretter J, Klingenspor M, Schwaiger M & Fromme T Active brown fat during 18F-FDG PET/CT imaging defines a patient group with characteristic traits and an increased probability of brown fat redetection. J. Nucl. Med 58, 1104–1110 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Kozak LP Brown fat and the myth of diet-induced thermogenesis. Cell Metab 11, 263–267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weir G et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab 27, 1348–1355.e1344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hibi M et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int. J. Obes. (Lond.) 40, 1655–1661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mueez UD et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis.Cell Metab 28, 207–216 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Spalding KL et al. Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Wang QA, Tao C, Gupta RK & Scherer PE Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vishvanath L et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23, 350–359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeffery E et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab 24, 142–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lackey DE et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol. Endocrinol. Metab 306, E233–E246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muir LA et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: ccorrelations with diabetes in humanobesity. Obes. (Silver Spring). 24, 597–605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Divoux A et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reggio S et al. Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J. Clin. Endocrinol. Metab 101, 2578–2587 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Henegar C et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9, R14 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun K, Tordjman J, Clément K & Scherer PE Fibrosis and adipose tissue dysfunction. Cell Metab 18, 470–477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcelin G et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab 25, 673–685 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Marangoni RG et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67, 1062–1073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keophiphath M et al. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol 23, 11–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hasegawa Y et al. Repression of adipose tissue fibrosis through aprdm16–gtf2ird1 complex improves systemic glucose homeostasis. Cell Metab 27, 180–194.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yadav H et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14, 67–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samad F, Yamamoto K, Pandey M & Loskutoff DJ Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med 3, 37–48 (1997). [PMC free article] [PubMed] [Google Scholar]
- 97.Rausch ME, Weisberg S, Vardhana P & Tortoriello DV Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond. ) 32, 451–463 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Ye J, Gao Z, Yin J & He Q Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab 293, E1118–E1128 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Hosogai N et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Halberg N et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol 29, 4467–4483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun K, Halberg N, Khan M, Magalang UJ & Scherer PE Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol 33, 904–917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan T et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol 29, 1575–1591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun K et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun 5, 3485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choo HJ et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49, 784–791 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Yin X et al. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab 99, E209–E216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schöttl T, Kappler L, Fromme T & Klingenspor M Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol. Metab 4, 631–642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heinonen S et al. impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64, 3135–3145 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Heinonen S et al. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia 60, 169–181 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Ohno H, Shinoda K, Spiegelman BM & Kajimura S PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15, 395–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trevellin E et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63, 2800–2811 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Bogacka I, Xie H, Bray GA & Smith SR Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54, 1392–1399 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Wilson-Fritch L et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest 114, 1281–1289 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rong JX et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56, 1751–1760 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Jokinen R et al. Adipose tissue mitochondrial capacity associates with long-term weight loss success. Int. J. Obes. (Lond.) 42, 817–825 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Vernochet C et al. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J 28, 4408–4419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kleiner S et al. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc. Natl. Acad. Sci. USA 109, 9635–9640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kusminski CM et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med 18, 1539–1549 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kusminski CM, Park J & Scherer PE MitoNEET-mediated effects on browning of white adipose tissue. Nat. Commun 5, 3962 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morton NM et al. Genetic identification of thiosulfate sulfurtransferase as an adipocyte-expressed antidiabetic target in mice selected for leanness. Nat. Med 22, 771–779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ouchi N, Parker JL, Lugus JJ & Walsh K Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol 11, 85–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kadowaki T et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest 116, 1784–1792 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao H et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yore MM et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Syed I et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab 27, 419–427.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pflimlin E et al. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab 28, 217–227.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Lynes MD et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med 23, 631–637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zimmer B et al. The oxidized linoleic acid metabolite 12,13-DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 669–678 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Shulman GI Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med 371, 1131–1141 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Summers SA Could ceramides become the new cholesterol? Cell Metab 27, 276–280 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Kurek K et al. Inhibition of ceramide de novo synthesis ameliorates diet induced skeletal muscles insulin resistance. J. Diabetes Res 2015, 154762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ussher JR et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang G et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab 297, E211–E224 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holland WL et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5, 167–179 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Blachnio-Zabielska AU, Chacinska M, Vendelbo MH & Zabielski P The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell. Physiol. Biochem 40, 1207–1220 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Turpin SM et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20, 678–686 (2014). [DOI] [PubMed] [Google Scholar]
- 136.Chaurasia B et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab 24, 820–834 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Holland WL et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17, 55–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vasiliauskaité-Brooks I et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryu KW et al. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 360, eaan5780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsukita S et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab 16, 825–832 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Camarda R et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med 22, 427–432 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H, Liu L, Lin JZ, Aprahamian TR & Farmer SR Browning of white adipose tissue with roscovitine induces a distinct population of UCP1+ adipocytes. Cell Metab 24, 835–847 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burl RB et al. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab 28, 300–309.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwalie PC et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Hepler C et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hagberg CE et al. Flow cytometry of mouse and human adipocytes for the analysis of browning and cellular heterogeneity. Cell Rep 24, 2746–2756.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]