Abstract
Soils underpin terrestrial ecosystem functions, but they face numerous anthropogenic pressures. Despite their crucial ecological role, we know little about how soils react to more than two environmental factors at a time. Here we show experimentally that increasing the number of simultaneous global change factors (up to 10) caused increasing directional changes in soil properties, soil processes and in microbial communities, though there was greater uncertainty in predicting the magnitude of change. Our study provides a blueprint for addressing multi-factor change with an efficient, broadly applicable experimental design for studying the impacts of global environmental change.
Global environmental change is a multifactorial phenomenon, and the concurrent action of multiple factors gives rise to large uncertainty in predicting effects (sensu (1)). Soils are affected by multiple factors, but we do not know the effects of these factors when they act in concert. Understanding soils is important, since they provide a range of ecosystem functions, including carbon storage, and are central to agriculture and sustainable management. To address the impact of multiple drivers of global change, ecologists have used many tools, including observational approaches, such as studying complex environmental gradients (2), long-term time series (3), as well as modeling (4). However, in the canon of ecological approaches, experiments occupy a key role because they help establish causality between drivers and response (5).
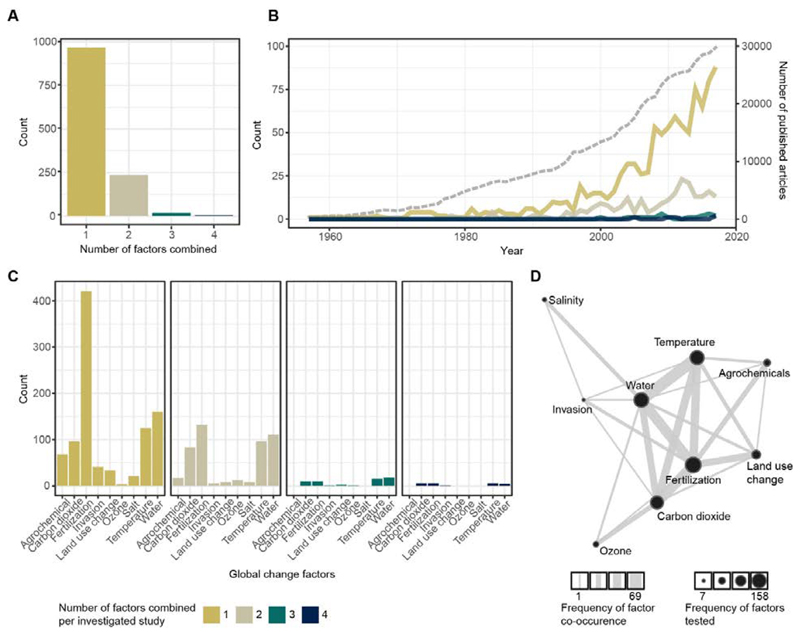
Given the multiple pressures that soils experience, we first asked how ecologists have experimentally approached the study of global change in the context of soils. We conducted a systematic survey (Fig. 1), screening the literature on experimental studies of how global change factors affect soil biota and processes. For each paper in our survey (1228 papers matched our inclusion criteria out of 4202 papers screened; (6)), we counted the number of global change factors included in the experiment. About 80% of studies examined only a single factor, 19% looked at the interaction of two factors, and only very few papers (<2%) tested the effects of 3 or more factors.
Fig. 1. Results from a literature survey on the number of global change factors included in soil ecology experiments, covering the years 1957 to 2017.
A. Frequency distribution of the number of factors of global change included in experimental studies. For the 19 studies testing 3-way interactions, we counted overall 38 investigated responses (across studies and variables), and in 21.05% of these authors reported a significant interaction. We found 5 studies including 4-way interactions, and in none of these was the 4-way interaction term significant. B. Number of experimental studies including a given number of factors over the last 50 years. For comparison, the dashed grey line (right y-axis) represents the number of published articles per year for the Web of Knowledge category “Ecology”. C. Number of papers including a given global change factor, for studies with 1-4 combined factors. D. Network graph depicting co-occurrence of global change factors in experimental studies, where circle size represents the frequency with which the driver was included in the studies and line thickness represents the frequency with which the drivers were tested as combinations.
Thus, although global change involves many factors, soil ecologists typically have conducted experiments varying only one or two factors at a time (Fig. 1A), a pattern that shows no signs of change over time (Fig. 1B), and that is unlikely to be unique to the study of soils (7). These studies are dominated by certain factors (e.g., fertilization, likely due to ease of application; Fig. 1C), and by certain factor combinations, to the exclusion of others (Fig. 1D). The reasons for these patterns, which shape our present knowledge of soil and ecosystem ecology, include logistical limitations and cost, but it is also clear that the main tool for addressing the interaction of multiple factors, the factorial experiment, starts to break down because of the combinatorial explosion problem, i.e. the rapid increase in possible combinations with the number of factors (8).
While examining a particular factor combination may provide mechanistic insights, we propose that it is also useful to ask how soils might change when exposed to an increasing number of factors. Here, we experimentally show that an increasing number of global change factors causes directional change in soil properties, processes and microbial communities, but that predicting the magnitude of the changes remains challenging.
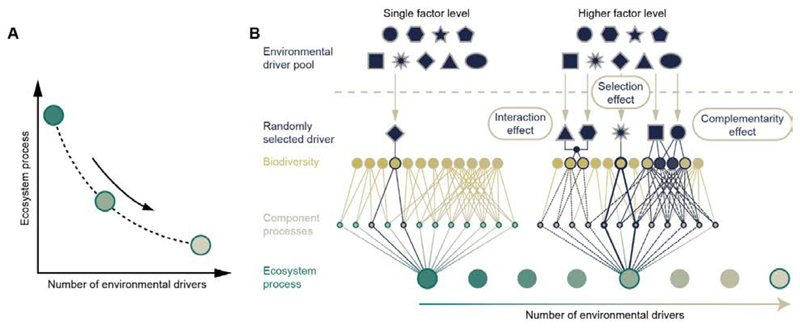
We examined the effects of an increasing number of these factors in combination using a design that takes inspiration from studies of biodiversity-effects on ecosystem function (6, 9). In these designs, species are randomly selected from a pool along a gradient of species number (richness) in order to draw general conclusions about how changes in species number would affect ecosystem functions overall, regardless of species identity. Analogously, we here use a pool of ten global change factors, from which we randomly selected a gradient of increasing factor number (also see (10)), namely the levels 2, 5, 8 and 10 factors, each replicated 10 times, thus testing if patterns of biodiversity and ecosystem processes show a consistent directional trend along the number of factors (Fig. 2). We tested abiotic factors (including temperature), resource availability, chemical toxicants and compounds (inorganic and synthetic organic), and an agent of physical change (microplastics). A system would rarely encounter all 10 factors simultaneously, but it may encounter many of them, e.g., in intensive farming systems. Each replicate in these ‘factor richness’ levels had a different, randomly determined combination of factors.
Fig. 2. Diagrams expressing the idea that the number of global change factors alone might predict general trends in changes of biodiversity and ecosystem processes.
A. We hypothesize that biodiversity and ecosystem processes display a consistent directional change (this could be either an increase or decline, concave or convex; in the panel we only show a decline and only one possible curve shape) along the number of environmental factors. B. The rationale behind this prediction is that with an increasing number of factors there is an increased chance of including an influential factor (selection effect), that factors may increasingly affect different components (complementarity effect), and that factors may interact with each other, so strengthening their effect (factor interaction effect).
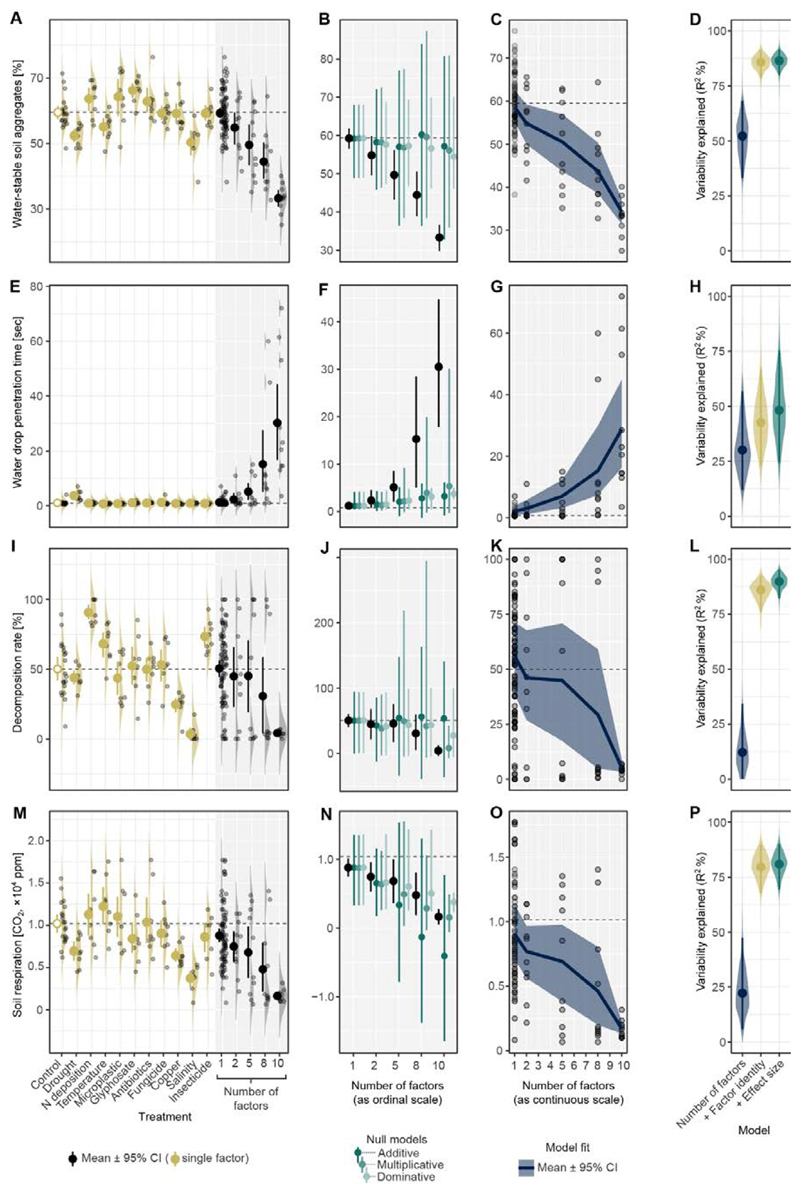
At the single factor level, some factors had neutral, negative or positive effects on a number of key responses, which included soil aggregation (a key component of soil structure), soil water repellency (water drop penetration time), decomposition, and soil respiration (Fig. 3, A, E, I and M). Therefore, predictions that combine single factor effects often had broad confidence intervals (Fig. 3, B, F, J and N; see (6) for how effects were combined). Soil aggregation and soil water repellency changed strongly with 8 or more factors, and effects deviated from predictions, indicating synergistic interactions. Nonetheless, in agreement with our prediction (Fig. 2), the changes in all response variables showed a consistent directional change with the number of factors included (Fig. 3, C, G, K and O; R2=13–52%, using random forest machine learning modeling). Knowing factor identity increased the amount of variability explained compared to just knowing the number of factors, but not for water repellency (Fig. 3, D, H, L and P; fig. S1).
Fig. 3. Effects on soil properties of global change factors applied singly and using different numbers of factors (2, 5, 8, 10 factors).
For each measured soil property (each row), single factor effects were estimated (1st column) and then used to predict multi-factor effects based on three different assumptions on how to combine multiple effect sizes (2nd column). An ideal prediction should have a small bias (accuracy) and narrow confidence interval (precision), but for the 1st and 2nd rows, predictions were neither accurate nor precise, regardless of the assumption used. The predictions are made difficult because the single factor effects have large variability and/or because there are strong factor interactions. The direction of the treatment effects were consistent with an increasing number of factors for all properties (3rd column). These curves were estimated using a random forest machine learning, and their predictability is shown in the 4th column (dark blue). Predictability was improved by adding factor identity information (0/1 for each factor; dark yellow) or effect size information (predicted values based on three assumptions; dark green) to the models as predictors (4th column), but predictability did not always improve (see H). Water stable soil aggregates (A-D); soil water repellency measured as water drop penetration time (E-H); decomposition rate (I-L); and, soil respiration (M-P). Replicates are represented by dots with density ridgeline plots. Horizontal dashed lines represent mean values of the control.
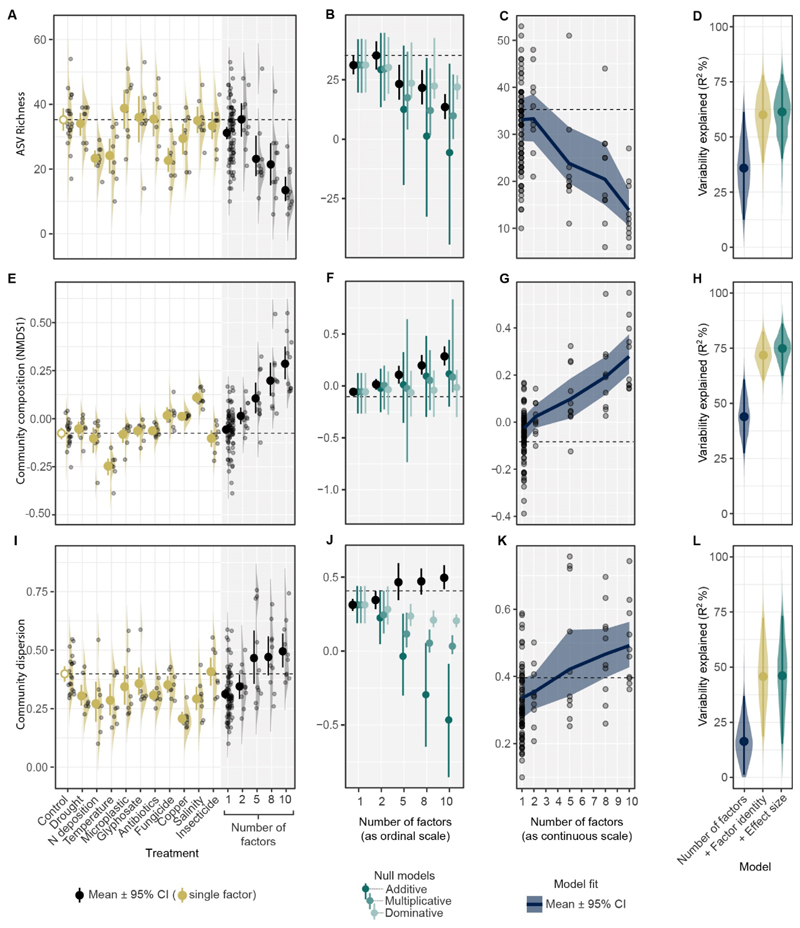
We also examined effects on soil biodiversity as measured by richness, community composition, and its dispersion. Here, we focus on soil fungi, which are strongly related to the processes we measured (e.g. (11)). We assessed communities of soil fungi using high-throughput sequencing (Illumina MiSeq), identifying 346 amplicon sequence variants (ASVs). A detailed description and analysis of the dataset is available in the Supplementary Materials (6). As in the results for soil properties, we find directional changes related to the number of factors, namely a reduction in ASV richness and a shift in community composition and dispersion (Fig. 4, D, H and L; fig. S2, fig. S3). For community dispersion, the magnitude of the changes was unpredictable from the single factor effects (Fig. 4J). ASV-poor communities were a subset of those in ASV-rich communities (temperature=7.3, SES=-8.3, p=0.001). When exposed to more factors, communities became species poorer (being mostly composed of generalist stress tolerant fungi, and losing mainly Basidiomycota; figs. S2 and S3).
Fig. 4. Effects on the soil fungal community of different global change factors applied singly and using different numbers of factors (2, 5, 8, 10 interacting factors).
For each biodiversity property (each row), single factor effects were estimated (1st column) and then used to predict multi-factor effects based on certain assumptions (2nd column). As with soil functions (Fig. 3), the effect directions were consistent along the number of factors for all properties as predicted using random forest machine learning (3rd column). The model predictability is shown in the 4th column (dark blue). Adding factor identity (dark yellow) or single factor effect size information (dark green) to the model improved predictability only for community composition, indicating that factor interactions exist (4th column). Fungal diversity is represented by ASV richness (A-D), community composition (E-H) and community dispersion (I-L). Community composition is represented by the 1st axis of an unconstrained multivariate ordination (NMDS) of the Bray-Curtis sample pairwise dissimilarities.
Our study expands understanding of the effects of multiple global change factors on soils, and shows that an increasing number of global change factors cause directional changes in soil properties, processes and microbial communities; however, predicting the magnitude of change was not always straightforward (fig. S1). We found that there were ‘ecological surprises’ that only became apparent at higher levels (especially 8 and 10) of factor interaction. This was best illustrated by the soil property ‘water repellency’, which was barely affected at the single-factor level, but greatly affected at the multi-factor level. Such phenomena clearly render predictions of effects of global change more challenging, but our results emphasize that simply projecting the direction of change and recognizing unpredictable impacts in the first place is important, and a step towards achieving better predictions.
Factors of global change will not be equally strongly expressed in all situations, and in reality, different combinations of factors may be at work. Nevertheless, our results offer general insights into system responses along such gradients of multiple global changes. As well as showing that factor number is important, data distributions also suggest that system responses (measured by combining data from all response variables after standardization at a given factor combination level) tended to develop in a bimodal-like pattern (fig. S4), and that this contributes to the observed unpredictability of the interactions. Such a bimodal pattern has been used to infer the occurrence of regime shifts (12). Regime shifts may be triggered by increasing diversity of drivers, but most experimental work on regime shifts has focused on single factors and has therefore overlooked the possibly greater effects of multiple factors (13, 14). Responses converge again at the highest level (10 factors), but this is likely to be an artefact because factor level 10 is by necessity a unique factor combination. It remains to be established if intermediate levels of factor richness have less predictable effects, and how this depends on the particular composition of factors.
Our experimental design was such that it could reveal trajectories for important aspects of ecosystem and biodiversity properties using a logistically feasible number of replicates (i.e. 140). Therefore, the approach is applicable to more complex systems including plants and soil fauna, possibly even in the field (table S2). Our literature survey and experimental results suggest the need to rethink global change biology with a focus on the number of factors and their higher-order interactions, and such a shift in focus would also benefit many other fields in which concurrent multiple factors are common (10, 15–17).
Supplementary Material
One sentence summary.
The number of global change factors can predict trends of ecosystem reactions, soil properties and microbial communities.
Acknowledgements
We thank the following for help with the experiment: P. Yakubovskaya, M. Ballhausen, G. Erzigkeit. Students in the M.Sc. course ‘Plant Ecology’ helped with conceptual design. We thank J. Antonovics for comments, and Forschungsstation Linde (Zwillenberg-Tietz Foundation, M. Wicke) for providing the soil.
Funding: MCR acknowledges funding from an ERC Advanced Grant (Gradual_Change) and from BMBF for the project ‘Bridging in Biodiversity Sciences (BIBS)’. MR acknowledges funding by the Grant-in-Aid for JSPS Overseas Research Fellowships.
Footnotes
Author contributions: MCR designed the study and set up the experiment, with the help of AL, GY, SB and AW. CAAT contributed respiration measurements. AL measured soil aggregation and conducted the literature synthesis. AI helped with the literature synthesis. AW measured water repellency and decomposition. JR carried out molecular ecology analyses. MR visualized the concept and carried out statistical analyses. GY designed the warming system. MCR wrote the first draft, and all authors contributed to the writing of the paper.
Competing interests: The authors confirm that there are no competing interests.
Data and materials availability: Data for this paper have been deposited to https://figshare.com/s/30b479473025f366013d. Molecular data are available under ENA study accession number: PRJEB34737. R code for effect size estimates is deposited at https://github.com/masahiroryo/joint-ES-estimate.
References
- 1.Lehmann J, Rillig M. Distinguishing variability from uncertainty. Nature Climate Change. 2014;4:153. [Google Scholar]
- 2.Grimm NB, et al. The changing landscape: ecosystem responses to urbanization and pollution across climatic and societal gradients. Frontiers in Ecology and the Environment. 2008;6:264–272. [Google Scholar]
- 3.Fischer M, et al. Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic and Applied Ecology. 2010;11:473–485. [Google Scholar]
- 4.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 5.Boyd PW, et al. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—A review. Global Change Biology. 2018;24:2239–2261. doi: 10.1111/gcb.14102. [DOI] [PubMed] [Google Scholar]
- 6.See supplementary materials.
- 7.Gunderson AR, Armstrong EJ, Stillman JH. Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Annual Review of Marine Science. 2016;8:357–378. doi: 10.1146/annurev-marine-122414-033953. [DOI] [PubMed] [Google Scholar]
- 8.Katzir I, Cokol M, Aldridge BB, Alon U. Prediction of ultra-high-order antibiotic combinations based on pairwise interactions. PLOS Computational Biology. 2019;15:e1006774. doi: 10.1371/journal.pcbi.1006774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. [Google Scholar]
- 10.Brennan G, Collins S. Growth responses of a green alga to multiple environmental drivers. Nature Climate Change. 2015;5:892. [Google Scholar]
- 11.Lehmann A, Zheng W, Rillig MC. Soil biota contributions to soil aggregation. Nature Ecology & Evolution. 2017;1:1828–1835. doi: 10.1038/s41559-017-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakos V, Carpenter SR, van Nes EH, Scheffer M. Resilience indicators: prospects and limitations for early warnings of regime shifts. Philosophical Transactions of the Royal Society B-Biological Sciences. 2015;370 [Google Scholar]
- 13.Conversi A, et al. A holistic view of marine regime shifts. Philosophical Transactions of the Royal Society B-Biological Sciences. 2015;370 [Google Scholar]
- 14.Moellmann C, Diekmann R. In: Advances in Ecological Research, Vol 47: Global Change in Multispecies Systems, Pt 2. Woodward G, Jacob U, Ogorman EJ, editors. Vol. 47. 2012. pp. 303–347. [Google Scholar]
- 15.Altenburger R, Backhaus T, Boedeker W, Faust M, Scholze M. Simplifying complexity: Mixture toxicity assessment in the last 20 years. Environmental Toxicology and Chemistry. 2013;32:1685–1687. doi: 10.1002/etc.2294. [DOI] [PubMed] [Google Scholar]
- 16.Rillig MC, Lehmann A. Exploring the agricultural parameter space for crop yield and sustainability. New Phytologist. 2019;0 doi: 10.1111/nph.15744. [DOI] [PubMed] [Google Scholar]
- 17.Tekin E, et al. Prevalence and patterns of higher-order drug interactions in Escherichia coli. npj Systems Biology and Applications. 2018;4:31. doi: 10.1038/s41540-018-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team. R: A language and environment for statistical computing. 2014 [Google Scholar]
- 19.Wickham H. ggplot2: Elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 20.IPCC. AR5 Synthesis report: Climate change. A contribution of working groups I, II, and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: 2014. [Google Scholar]
- 21.Vitousek PM, et al. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications. 1997;7:737–750. [Google Scholar]
- 22.Mountford JO, Lakhani KH, Kirkham FW. Experimental assessment of the effects of nitrogen addition under hay-cutting and aftermath grazing on the vegetation of meadows on a Somerset peat moor. Journal of Applied Ecology. 1993:321–332. [Google Scholar]
- 23.IPCC. TAR Climate change 2001: Synthesis report. A contribution of working groups I, II, and III to the third assessment report of the intergovernmental panel on climate change. Cambridge, United Kingdom, and New York, USA: 2001. [Google Scholar]
- 24.Lehner B, Döll P, Alcamo J, Henrichs T, Kaspar F. Estimating the impact of global change on flood and drought risks in Europe: A continental, integrated analysis. Climatic Change. 2006;75:273–299. [Google Scholar]
- 25.de Vries FT, Brown C, Stevens CJ. Grassland species root response to drought: consequences for soil carbon and nitrogen availability. Plant and Soil. 2016;409:297–312. [Google Scholar]
- 26.Ballabio C, et al. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Science of The Total Environment. 2018;636:282–298. doi: 10.1016/j.scitotenv.2018.04.268. [DOI] [PubMed] [Google Scholar]
- 27.Machado AAD, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biology. 2018;24:1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environmental Science & Technology. 2012;46:6453–6454. doi: 10.1021/es302011r. [DOI] [PubMed] [Google Scholar]
- 29.Machado AAD, et al. Impacts of microplastics on the soil biophysical environment. Environmental Science & Technology. 2018;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millennium Ecosystem Assessment. Ecosystems and human well-being: Synthesis report. Washington, DC: 2005. [Google Scholar]
- 31.Daliakopoulos IN, et al. The threat of soil salinity: A European scale review. Science of The Total Environment. 2016;573:727–739. doi: 10.1016/j.scitotenv.2016.08.177. [DOI] [PubMed] [Google Scholar]
- 32.Bernhardt ES, Rosi EJ, Gessner MO. Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment. 2017;15:84–90. [Google Scholar]
- 33.Ratcliff AW, Busse MD, Shestak CJ. Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Applied Soil Ecology. 2006;34:114–124. [Google Scholar]
- 34.Zhu Y-G, et al. Microbial mass movements. Science. 2017;357:1099–1100. doi: 10.1126/science.aao3007. [DOI] [PubMed] [Google Scholar]
- 35.Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators. 2008;8:1–13. [Google Scholar]
- 36.Wood TJ, Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environmental Science and Pollution Research. 2017;24:17285–17325. doi: 10.1007/s11356-017-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 38.Lang M, Cai Z. Effects of chlorothalonil and carbendazim on nitrification and denitrification in soils. Journal of Environmental Sciences. 2009;21:458–467. doi: 10.1016/s1001-0742(08)62292-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Wang F, Zhang Q, Liang W. Individual and combined effects of tebuconazole and carbendazim on soil microbial activity. European Journal of Soil Biology. 2016;72:6–13. [Google Scholar]
- 40.Bradford MA, Watts BW, Davies CA. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Global Change Biology. 2010;16:1576–1588. [Google Scholar]
- 41.Kemper WD, Rosenau RC. In: Methods of Soil Analysis. Part I - Physical and Mineralogical Methods. Lute A, editor. SSSA; Madison, USA: 1986. pp. 425–443. [Google Scholar]
- 42.Hallett PD. An introduction to soil water repellency. In: Gaskin RE, editor. 8th International Symposium on Adjuvants for Agrochemicals (ISAA), International Scociety for Agrochemical Adjuvants (ISAA); Columbus, Ohio, USA. 2007. [Google Scholar]
- 43.Ihrmark K, et al. New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiology Ecology. 2012;82:666–677. doi: 10.1111/j.1574-6941.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- 44.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods. 2016;13:581. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oksanen J, et al. vegan: Community Ecology Package. 2018 [Google Scholar]
- 46.Rodriguez-Girones MA, Santamaria L. A new algorithm to calculate the nestedness temperature of presence-absence matrices. Journal of Biogeography. 2006;33:924–935. [Google Scholar]
- 47.Claridge-Chang A, Assam PN. Estimation statistics should replace significance testing. Nature Methods. 2016;13:108–109. doi: 10.1038/nmeth.3729. [DOI] [PubMed] [Google Scholar]
- 48.Cumming G. The new statistics: Why and how. Psychological Science. 2014;25:7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- 49.Wasserstein RL, Lazar NA. The ASA's statement on p-values: Context, process, and purpose. American Statistician. 2016;70:129–131. [Google Scholar]
- 50.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond "p < 0.05". American Statistician. 2019;73:1–19. [Google Scholar]
- 51.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statist Sci. 1986;1:54–75. [Google Scholar]
- 52.Efron B. Better bootstrap confidence-intervals. Journal of the American Statistical Association. 1987;82:171–185. [Google Scholar]
- 53.Banjanovic ES, Osborne JW. Confidence intervals for effect sizes: Applying bootstrap resampling. Practical Assessment, Research & Evaluation. 2016;21:1–20. [Google Scholar]
- 54.Schafer RB, Piggott JJ. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Global Change Biology. 2018;24:1817–1826. doi: 10.1111/gcb.14073. [DOI] [PubMed] [Google Scholar]
- 55.Fraser DAS. The p-value function and statistical inference. American Statistician. 2019;73:135–147. [Google Scholar]
- 56.Greenland S. Valid p-values behave exactly as they should: Some misleading criticisms of p-values and their resolution with s-values. American Statistician. 2019;73:106–114. [Google Scholar]
- 57.Thompson PL, MacLennan MM, Vinebrooke RD. An improved null model for assessing the net effects of multiple stressors on communities. Global Change Biology. 2018;24:517–525. doi: 10.1111/gcb.13852. [DOI] [PubMed] [Google Scholar]
- 58.Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 59.Wilke CO. ggridges: Ridgeline plots in 'ggplot2'. 2018 [Google Scholar]
- 60.Strobl C, Boulesteix A-L, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhn M. Classification and regression training. CRAN Repository. 2015 [Google Scholar]
- 62.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson RH, et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Research. 2019;47:D259–D264. doi: 10.1093/nar/gky1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- 65.Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006;62:245–253. doi: 10.1111/j.1541-0420.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 66.G. R. Warnes et al. (2019).
- 67.Basu S, Kumbier K, Brown JB, Yu B. Iterative random forests to discover predictive and stable high-order interactions. Proceedings of the National Academy of Sciences. 2018;115:1943–1948. doi: 10.1073/pnas.1711236115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryo M, Harvey E, Robinson CT, Altermatt F. Nonlinear higher order abiotic interactions explain riverine biodiversity. Journal of Biogeography. 2018;45:628–639. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.