Abstract
Virus assembly constitutes a key phase of the HIV-1 replication cycle. The assembly process is initiated by the synthesis of the Gag precursor protein, Pr55Gag, in the cytosol of the infected cell. After its synthesis, Pr55Gag is rapidly transported in most cell types to the plasma membrane (PM) where it associates with the inner leaflet of the lipid bilayer. Gag-Gag interactions lead to the assembly of an electron-dense patch of Gag proteins at the membrane. The viral envelope (Env) glycoproteins associate with Gag during the assembly process. The highly multimerized Gag complex begins to bud outwardly from the PM and eventually pinches off from the cell surface. Concomitant with release, the viral protease cleaves Pr55Gag to the mature Gag proteins matrix, capsid, nucleocapsid and p6, leading to core condensation. The mature infectious virus particle is now able to initiate a new round of infection in a fresh target cell. Techniques have been developed in many laboratories to study each of the distinct phases of the HIV-1 assembly and release pathway. A number of these techniques are described in detail in this chapter.
Keywords: Virus assembly, Pr55Gag, membrane binding, lipid raft association, multimerization, epitope exposure, release rescue assay, punctate staining, virus budding, virus release
1. Introduction
Retroviral Gag proteins are synthesized as polyprotein precursors in the cytoplasm of the infected cell and direct the assembly of nascent virus particles. While the Env glycoproteins and the pol-encoded enzymes are required for the generation of infectious virions, expression of Gag proteins alone is generally sufficient for the assembly and release of noninfectious, virus-like particles (VLPs). The HIV-1 Gag precursor, Pr55Gag, is composed of matrix (MA), capsid (CA), nucleocapsid (NC), and p6 domains, as well as spacer peptides SP1 and SP2 (Fig. 12.1). Discrete regions have been identified within Pr55Gag that orchestrate the major steps in virus assembly and release: the MA domain mediates targeting of Pr55Gag to the plasma membrane (PM) and directs the incorporation of the Env glycoproteins into virions; the C-terminal portion of CA, SP1, and the N-terminal domain of NC promote Gag multimerization, in part via interactions between NC and the viral genomic RNA; and the p6 domain functions late in the assembly pathway to stimulate virus release from the PM. The mature Gag proteins are generated during virus release upon cleavage of Pr55Gag by the viral protease (PR). PR-mediated Gag processing leads to virus maturation, which is, in effect, a reassembly of CA, NC, and genomic RNA to form the mature conical core (1).
Fig. 12.1.
Domain organization of Pr55Gag. The membrane-binding domain (M) is composed of the N-terminal myristate (Myr) and the highly basic region of MA (+++). The interaction domain (I) spans the C-terminal portion of CA, SP1, and the N-terminal domain of NC. The late domain (L) consists primarily of the PTAP motif near the N-terminus of p6. Spacer peptides are shown as SP1 and SP2.
In most cell types, HIV-1 assembly takes place primarily at the PM (2, 3), although in some cells, including the physiologically relevant macrophage, assembly may occur in intracellular compartments known as multivesicular bodies (MVBs) (3, 4). PM assembly appears to take place predominantly in cholesterol-and glycosphingolipid-enriched microdomains known as lipid rafts (5). Lipid rafts can be isolated as detergent-resistant membrane (DRM) by TX-100 treatment at low temperature, followed by equilibrium flotation centrifugation. Recent findings from our lab indicate that phosphatidylinositol-(4, 5)-bisphosphate [PI(4, 5)P2], a member of the phosphoinositide family of lipids, directs Pr55Gag to the PM (6), probably via a direct interaction between MA and PI(4, 5)P2 (7–9). The HIV-1 assembly process can be subdivided into a series of discrete steps, which include: (1) Gag targeting to the PM; (2) membrane/lipid raft binding; (3) Env incorporation, (4) Gag multimerization; and (5) virus particle budding and release (Fig. 12.2). In this chapter we describe the methods to study these steps of the HIV-1 assembly and release pathway.
Fig. 12.2.
Schematic representation of steps involved in virus assembly and release. After its synthesis, Pr55Gag is directed to the plasma membrane by its membrane targeting signal in the MA domain. At the plasma membrane, Gag associates with cholesterol- and sphingolipid-rich domains (lipid rafts). Gag–Gag interactions lead to the assembly of viral particles; the viral Env glycoproteins are incorporated during the assembly process. The highly multimerized Gag complex begins to bud outwardly from the plasma membrane and ultimately pinches off from the cell surface. The particle undergoes maturation upon cleavage of Gag and GagPol precursors by the viral protease.
A number of full-length, infectious HIV-1 molecular clones are available for the study of HIV-1 assembly. Gag expression constructs can also be used to generate noninfectious VLPs. Our work has been based predominantly on the full-length HIV-1 molecular clone pNL4-3 (10) as well as a large number of pNL4-3 derivatives. These include Env-defective and PR-defective clones. Assembly-deficient mutants often serve as important controls in virus assembly assays. Particularly useful are the myristylation-defective MA mutant, which is unable to bind membrane; assembly-deficient CA and NC mutants; and budding-deficient p6 mutants. Epitope-tagged Gag derivatives are also very useful, particularly for the study of Gag–Gag interactions. For this purpose, we have generated HA and FLAG-tagged derivatives.
2. Materials
2.1. Plasmids
Full-length HIV-1 molecular clone pNL4-3 (10).
Env-defective clone pNL4-3/KFS contains a frameshift mutation at nucleotide 6343, which eliminates Env expression (11).
PR-defective clone pNL4-3/PR− contains a PR active-site mutation (Asp to Asn) at PR amino acid 25 (12).
Myristylation-deficient mutant pNL4-3/1GA contains a Gly-to-Ala change at MA amino acid 1 (13). This mutant is defective in Gag binding to membrane.
CA dimerization mutant pNL4-3/WM184,185AA contains a two amino acid substitution at the CA dimer interface, thereby inducing a defect in Gag multimerization (14–16).
NC basic amino acid substitution mutant pNLHX15A, in which all 15 basic residues of HIV-1 NC were mutated to alanine, is defective in higher-order Gag multimerization (15, 17).
p6-mutant derivative pNL4-3/PTAP− contains mutations in all four residues of the PTAP motif in the p6 domain of Gag (12). The PTAP motif is involved in virus release and mutations in this motif result in tethering of particles at the PM (12).
HA and FLAG-tagged derivatives pNL4-3-55HA and pNL4-3-55FLAG, in which the HA or FLAG epitope tags are fused to the C-terminus of Gag (18, 19).
NL4-3-based GagPol expression vector pCMVNLGagPolRRE (20).
The vesicular stomatitis virus G glycoprotein (VSV-G) expression vector pHCMV-G (21).
2.2. Cell Culture and Transfection Reagents
Dulbecco’s modified Eagle’s medium (DMEM) or RPMI (Cambrex, Walkersville, MD) supplemented with antibiotics and 5% or 10% fetal bovine serum (FBS, HyClone, Logan, UT).
Solution of trypsin (0.25%) and ethylenediamine tetraacetic acid (EDTA) (1 mM) from Gibco.
- Calcium-phosphate transfection reagents.
- 1X HEPES buffer containing 138 mM NaCl, 5 mM KCl, 1 mM Na2HPO4. 7H2O, 5.5 mM dextrose, and 5 mM HEPES, pH adjusted to 7.05 (filtered and stored at 4 ◦C).
- 15% glycerol in 1X HEPES (stored at 4 ◦C).
- 1.25 M CaCl2 in double-distilled water (DDW) (filtered and stored at 4 ◦C).
- Lipofectamine™2000 transfection reagents.
- Lipofectamine™2000 (Invitrogen).
- OptiMEM-I reduced serum medium from Invitrogen to dilute Lipofectamine ™2000 and nucleic acids before complexing.
- Antibiotic-free DMEM-5 or DMEM-10.
- 5. ExGen500 transfection reagents.
- ExGen 500 (Fermentas) is a sterile solution of linear polyethylenimine (PEI) molecules (22 kDa) in DDW at a concentration of 5.47 mM in terms of nitrogen residues.
- 0.15 M NaCl (endotoxin-free, sterile) solution for dilution of DNA.
2.3. Metabolic Labeling and Immunoprecipitation
RPMI-1640 medium lacking Met and Cys [Chemicon International (Temecula, CA)], with or without 2% FBS.
[35S]Met/Cys [Perkin-Elmer (Wellesley, MA)].
Cell lysis buffer: 300 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.5% Triton X-100 (TX-100), 10 mM iodoacetamide, and 10 tablets of “Complete protease inhibitor” cocktail tablets (Boehringer Mannheim).
2X radioimmunoprecipitation assay (RIPA) buffer: 280 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4, 2% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 20 mM iodoacetamide, and protease inhibitors.
Protein A agarose beads (Invitrogen).
HIV-1 IgG obtained from the AIDS Research and Reference Reagent Program of the National Institutes of Health, purified from the serum of HIV-1-infected patients.
Triton wash buffer: 300 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.5% TX-100.
SDS/DOC wash buffer: 300 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.1% SDS, 0.1% deoxycholic acid.
2.4. SDS-PAGE, Autoradiography and Western Blotting
29% acrylamide/1% acrylaide gel (Cambrex, Walkersville, MD) reagent for SDS-PAGE, followed by fluorography.
30% acrylamide/0.8% bisacrylamide solution for SDS-PAGE, followed by Western blotting.
Lower gel buffer (4X): 1.5 M Tris-HCl, pH 8.7, 0.4% SDS.
Upper gel buffer (4X): 0.5 M Tris-HCl, pH 6.8, 0.4 % SDS.
10% ammonium persulfate (APS) in DDW
N, N, N, N′-tetramethyl-ethylenediamine (TEMED) [Bio-Rad (Hercules, CA)].
Running buffer (10X): 250 mM Tris, 1.92 M glycine, 1% (w/v) SDS.
Prestained molecular weight markers (Invitrogen).
Fixative: 40% methanol and 10% acetic acid in DDW.
1 M sodium salicylate in DDW.
Transfer buffer: 25 mM Tris, 0.19 M glycine, and 20% methanol.
Tris-bufferred saline containing 0.05% Tween 20 (TBS-T).
Blocking buffer: 5% (w/v) nonfat dry milk in TBS-T.
The Western lightning® chemiluminescence reagent Plus [Perkin-Elmer (Wellesley, MA)].
2.5. Membrane Flotation, DRM Isolation, Virion Purification
Teflon cell lifters (Fisher).
TE + Complete (hypotonic buffer): 10 mM Tris-HCl (pH 7.5), 4 mM EDTA, Complete protease inhibitor cocktail.
4X TNE: 100 mM Tris-HCl (pH 7.5), 600 mM NaCl, 16 mM EDTA.
TNE + 0.5% [v/v] TX-100.
Sucrose solutions of the following concentrations (w/v) prepared in TNE: 85.5%, 65%, 60%, 50%, 40%, 30%, 20%, and 10%.
2.6. Immunofluorescence Microscopy
Lab-Tek eight-well glass chamber slides [Nalge Nunc (Naperville, IL)], and microscope cover slips (22 × 40 × 0. 15 mm) [Fisher (Pittsburgh, PA)].
Fresh 4% paraformaldehyde (w/v) solution in PBS.
Permeabilization solution: Methanol stored at −20 °C or 0.1% TX-100 in PBS.
Quench solution: 0.1 M glycine in PBS.
Antibody dilution buffer: 3% (w/v) BSA in PBS.
Secondary antibody: Alexa-488/594 [Molecular Probes (Eugene, OR)].
Nuclear stain: 300 nM DAPI (4.6-diamidino-2-phenylindole) in DDW.
Mounting medium: Antifade [Molecular Probes (Eugene, OR)].
2.7. Electron Microscopy
2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, prepared fresh.
0.1 M, 0.2 M, 0.4 M sodium cacodylate buffer, pH 7.2–7.4 (adjusted with 0.1 M HCl).
2% osmium tetroxide in 0.2 M sodium cacodylate.
0.1 N sodium acetate buffer, pH 4.5 (adjusted with acetic acid).
35%, 50%, 70%, 90%, and 100% ethanol.
Propylene oxide (1,2 epoxy propane).
Epoxy resin (Epon-812 based): Mix 53.3 g of Poly/Bed®R812 resin, 20.7 g of dodecenyl succinic anhydride (E.M. grade), 26 g of nadic methyl anhydride (E.M. grade), and 1.4 mL of 2,4,6-Tri(Dimethylaminoethyl)phenol (DMP-30) in a plastic disposable beaker using a wooden spatula.
Uranyl acetate: 0.5% uranyl acetate in DDW (can be used for 3 months if stored in a brown glass bottle).
Reynold’s lead citrate: Add 1.33 g lead nitrate, 1.76 g sodium citrate to 30 mL of DDW. Shake for 1 min and allow to stand for 30 min shaking occasionally. Add 8 mL of 1 M NaOH, mix and dilute to 50 mL with DDW, adjust pH to 12 (can be used for up to 6 months).
3. Methods
3.1. Preparation of Virus Stocks
Virus stocks are prepared to infect target cells and evaluate various aspects of the HIV-1 replication cycle. Virus stocks prepared by pseudotyping with VSV-G can be used to infect a wide range of target cells, including those not readily amenable to transfection or electroporation. Infectious virus stocks can be prepared as described below.
Plate HeLa or 293T cells (106 cells/60-mm dish)
Next day, transfect with HIV-1 molecular clone (e.g., pNL4-3 or its derivative) alone or with Gag-Pol expression vector pCMVNLGagPolRRE and VSV-G expression plasmid (pHCMV-G), using either of the transfection methods described below (see Note 1).
3.1.1. Calcium-Phosphate Transfection
Add 20 μg of DNA (total) to 0.45 mL of 1X HEPES and mix. Add 50 μL of CaCl2, shake well and leave for 30 min at room temperature. Add the mixture to the cells and incubate overnight at 37 °C in a CO2 incubator. The next morning, remove supernatant, add 4 mL of fresh DMEM-5.
3.1.2. Lipofectamine™ 2000 Transfection
Dilute 8 μg of DNA into 0.5 mL of OptiMEM-I reduced serum medium. Dilute 20 μL of Lipofectamine™2000 in 0.5 mL of OptiMEM-I medium. Incubate for 5 min at room temperature. Combine diluted DNA with diluted Lipofectamine™2000. Mix gently and incubate for 20 min at room temperature. Remove growth medium from cells and replace with 4 mL of antibiotic-free medium. Add 1 mL of the DNA-Lipofectamine complexes to 60-mm dish containing cells and mix gently. Incubate the cells at 37 °C in a CO2 incubator for 4–8 h; medium may be replaced with fresh medium.
3.1.3. ExGen500 Transfection Reagent
Dilute 5 μg of DNA in 500 μL of 0.15 M NaCl and vortex gently. Add the 20 μL ExGen 500 and vortex the solution immediately for 10 s. Incubate for 10 min at room temperature. Add 500 μL of the ExGen 500/DNA mixture to 60-mm dish.
Incubate cells at 37 °C in a CO2 incubator for 24–48 h.
Collect supernatant, pass through a 0. 45-μm filter, aliquot into cryotubes, freeze on dry ice and store at −80 °C. Reserve a small aliquot for reverse transcriptase or p24 assay.
3.2. Infection and Transfection
Target cells are infected with virus stocks prepared as described in Section 3.1 with appropriate input inoculum depending on the cell types used for study (discussed below). Viral proteins are often expressed by transient transfection with molecular clones as detailed in Section 3.1. However, infection is less cytotoxic than transfection and represents a more physiological method for transduction of the viral genome.
3.2.1. Infection of Hela cells
Plate HeLa cells (0. 4 × 106 cells/well of six-well plate).
The next morning, remove supernatant and add pseudotyped virus (amount used will depend on the purpose of the assay). Add DMEM-5 to a final volume of 0.8 mL and incubate for 6–10 h at 37 °C.
Remove supernatant and add 2 mL of fresh DMEM-5. Incubate for 2 days before biochemical analysis.
3.2.2. Infection of T cells
Place 2 × 106 cells per sample in a 15-mL tube. Spin at 260 × g for 5 min; remove the supernatant.
Resuspend cells in a small amount of RPMI-10 (typically 0.3–0.5 mL) containing an appropriate amount of virus and incubate cells for 6–10 h in the CO2 incubator.
Add 2 mL RPMI-10, spin cells, remove supernatant and resuspend cells in fresh RPMI-10 and transfer to T-25 flash and incubate for 2 days before biochemical analysis (see Note 2).
3.2.3. Transfection
Plate HeLa cells (0. 4 × 106 cells/well in six-well plate).
The next day, transfect with approximately 3 μg DNA/well (ExGen500) or 6 μg/well (Lipofectamine) or 10 μg/well (calcium-phosphate) (detailed in Section 3.1).
3.3. Virus Release Assay
Comparing the virus release efficiency of WT vs. mutant molecular clones has provided detailed information concerning the domains in Pr55Gag responsible for mediating virus assembly and release. These assays can also be used to measure the effect of assembly/release inhibitors, siRNA depletion of cellular proteins, or other disruption of host cell machinery on virus particle production.
3.3.1. Cell Labeling
One day post-transfection or two days postinfection, starve the cells by rinsing with RPMI Cys−/Met− and adding 1 mL of RPMI Cys−/Met−. Incubate for 30 min at 37 °C.
Rinse cells again and add 1 mL of RPMI Cys−/Met− containing 2% FBS.
Add 250 μCi [35 S]Met/Cys; incubate at 37 °C, 2 h-overnight (Note 3).
3.3.2. Pelleting Released Virions
Collect tissue culture supernatant or cell suspension; spin down at 2,000 rpm for 3 min in Sarstedt screw-cap tubes to pellet cells or remove cell debris.
Remove supernatant without disturbing the cell pellet and pass through 0. 45-μm filter.
Ultracentrifuge the filtered supernatant at 35,000 rpm (100, 000 × g) for 45 min at 4 °C in a Sorvall ultracentrifuge using a TH-660 rotor or use comparable ultracentrifuge and rotor (e.g., Beckman SW50.1).
Remove supernatant by decanting, then wipe the inside wall of tube with rolled paper towel to remove as much supernatant as possible (do not touch the bottom of the tube).
Add 0.2 mL of PBS (to further purify the viral particles on sucrose gradient) or 0.1 mL of TX-100 cell lysis buffer. Leave the tube at room temperature for approximately 10–15 min, and pipette several times to resuspend virus pellet.
Meanwhile, add 0.5 mL of lysis buffer to each well, leave for 10–15 min, and transfer cell and viral lysates to separate Sarstedt screw-cap tubes (see Note 3).
3.4. Purification of Viral Particles
Virus preparations obtained by pelleting tissue culture supernatants at 100, 000 × g may include contaminants such as soluble viral proteins, cell membrane-derived lipid microvesicles, and cell debris that may interfere with subsequent analysis. To increase the purity of the particle preparations, the pelleted virions can be subjected to equilibrium gradient centrifugation. A detailed protocol for purification of viral particles on sucrose gradients is described below. Other types of gradients (e.g., OptiPrep) can also be used.
Layer virion-containing supernatants prepared in step 5 of Section 3.3 onto a 20% sucrose cushion; centrifuge at 35,000 rpm for 2 h at 4 °C in ultracentrifuge. Resuspend the viral pellet in 0.2 mL of PBS.
Layer 70, 65, 50, 40, 30, 20% [w/v] sucrose in TNE from bottom of tube (0.64 mL each) with pipetman and load 0.2 mL virus suspension on top (10%) sucrose layer.
Ultracentrifuge at 35,000 rpm, 16 h, at 4 ◦C (with slow acceleration/deceleration).
Remove fractions (~ 0. 4 mL each) from the top of the gradient with a pipetman (try to place the tip of the pipetman just beneath the surface of the solution during fractionation). While taking the last fraction (Fr. 10), pipette several times to resuspend the (invisible) pellet.
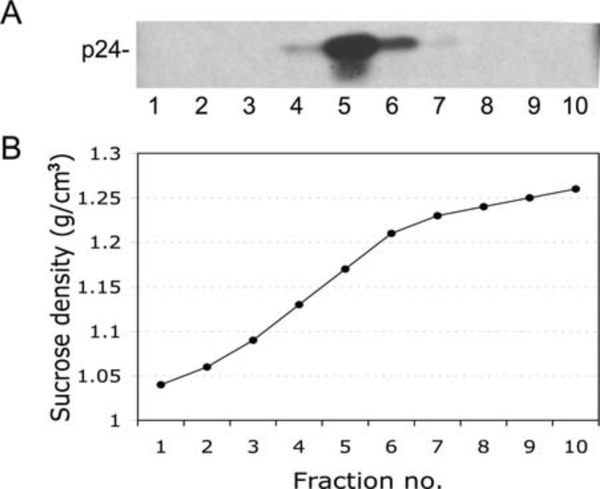
Mix a portion of each fraction with sample buffer and analyze by Western blotting; store remaining material at −20 °C. An example of virion purification on a sucrose density gradient is shown in Fig. 12.3.
Pool the fractions that are enriched in viral proteins (p24CA or Pr55Gag), dilute with TNE and spin down at 35,000 rpm for 1 h. Suspend the pellet in appropriate buffer depending on further use (see Note 4).
Fig. 12.3.
Purification and density determination of viral particles. A, Western blot analysis of virions with HIV-Ig. Virions were concentrated by ultracentrifugation and were resuspended in 200 μL of PBS and layered onto a 20–70% (w/v) linear sucrose density gradient and subjected to ultracentrifugation in a TH-660 rotor (Sorvall) at 100, 000 × g for 16 h. Ten fractions were collected and analyzed by Western blotting. B, The density of sucrose is plotted against each fraction.
3.5. Immunoprecipitation and Fluorography
To evaluate the efficiency of virus production, the Gag in the viral particles produced from the metabolically labeled cells is quantified by immunoprecipitation followed by fluorography. Since accurate quantification of released particles depends on the amount of viral proteins made in the producer cells, it is essential to quantify the amount of cell-associated viral protein expression. Therefore, immunoprecipitation of viral proteins from both viral and cell lysates is carried out using HIV-Ig or other appropriate anti-HIV antiserum.
3.5.1. IP of Cell-Associated Material
Spin cell lysate for 1 min at 4 °C and transfer 50 μL of supernatant to Sarstedt tube containing 50 μL of protein A agarose beads (Gibco-BRL) and 50 μL 1% BSA in 1 mL TX-100 wash buffer.
Rotate for 1 h or more on an orbital rotor at 4 °C and spin for 1 min at full-speed in an Eppendorf microfuge (supernatant is the precleared lysate).
At the same time, prepare antibody-bead complex by combining 1 mL PBS, 0. 8 μL HIV-Ig or appropriate amount of other anti-HIV antiserum, 50 μL protein A, and 30 μL 1% BSA (per sample) in 15 or 50 mL tube (depending on the number of samples).
Rotate for 1 h or more on an orbital rotor at 4 °C. Spin for 5 min at 2,500 rpm and remove supernatant. Add 0.5 mL PBS/sample; aliquot 0.5 mL of this suspension to each Sarstedt tube, spin for 1 min at full speed in microfuge and remove supernatant.
Combine antibody-bead complex with precleared lysate and rotate for 1 h or more on an orbital rotor at 4 °C.
3.5.2. IP of Virion-Associated Material
Prepare antibody–bead complex as above and add half of the virion sample to antibody–bead complex. Raise volume to 1 mL with TX-100 lysis buffer.
Rotate for 1 h or more on an orbital rotor at 4 °C.
3.5.3. Washing of Beads
After incubations, wash three times with 1 mL of TX-100 wash buffer and once with 1 mL of SDS/DOC wash buffer.
Resuspend samples in 60 μL 1X sample buffer and boil the samples for 5 min. Mix the supernatant with 5 μL 0.1% bromophenol blue (BPB) and load samples onto acrylamide/acrylaide gel.
3.5.4. SDS-PAGE and Fluorography
Prepare lower gel by mixing 14 mL 30% acrylamide/0.8% bisacrylaide solution with 11 mL DDW, 9.9 mL 4X lower Tris buffer, 0.5 mL 2% APS and 40 μl TEMED. Pour the gel, leaving space for staking gel, and overlay with water-saturated isobutanol. Polymerization takes place in about 30 min.
Pour off the isobutanol and rinse the top of the gel with water.
Prepare stacking gel by mixing 2.7 mL 30% acrylamide/0.8% bisacrylamide solution with 10 mL DDW, 5 mL 4X lower Tris buffer, 0.2 mL 2% APS and 20 μL TEMED. Pour the gel and insert the comb.
Once the stacking gel has polymerized ( ~ 30 min ), remove the comb and wash the wells with 1X running buffer.
Add 1X running buffer to the upper and lower chambers of the gel unit and load samples in wells. Also load prestained molecular weight markers in one or more wells.
Electrophorese until the dye front reaches the bottom of the gel.
Remove gel from plate and fix in 40% methanol, 10% acetic acid in water for 2 h. Rinse for 15 min in water and incubate in 250 mL of “Enhancer” (1 M salicylic acid + 5 mL glycerol) for 2 h. Place gel on paper towels to absorb excess liquid and dry in 100 °C oven for ~ 1. 5 h if using AcrylAide gel, or dry in gel dryer if using conventional acrylamide gel.
Expose dried gel to X-ray film or phosphorimager screen for quantification. An example of a virus release assay performed with wild-type and mutant pNL4-3 molecular clone is shown in Fig. 12.4 (see Note 5).
Fig. 12.4.
Mutations in Gag lead to defects in virus release. HeLa cells transfected with WT pNL4-3 or the indicated Gag mutants that are defective in virus release or Env expression were metabolically labeled with [35S]Met/Cys, and virions were pelleted by ultracentrifugation. Labeled viral proteins in cell and virion lysates were immunoprecipitated with HIV-Ig and analyzed by SDS-PAGE. Mutant clones are as follows: 1GA, nonmyristylated Gag mutant; WM184,185AA, CA C-terminal domain dimer interface mutant; 15A, clone in which all basic residues in NC were mutated to Ala; PTAP−, mutant bearing substitutions in all four PTAP late domain residues; KFS, Env-minus molecular clone.
3.6. Western Blotting
In cases where metabolic labeling and immunoprecipitation are not required the detection of viral proteins can be carried out by Western blotting. For example, detecting p24 in equilibrium sucrose gradients to check the density of viral particles does not require radiolabeling and quantification. Also, cases in which antibodies are not available for efficient immunoprecipitation of a specific viral protein, Western blotting detection and quantification by digital imaging system can be used. In rescue assays in which epitope-tagged Gags are used, cell and virus lysates can be subjected to immunoblotting with anti-tag antibodies followed by quantification.
SDS-PAGE for Western blotting is performed as described in Section 3.4. Proteins separated by SDS-PAGE are transferred electrophoretically to PVDF membrane by using a semi-dry transfer apparatus.
3.6.1. Electrotransfer
Remove gel from glass plates and soak in transfer buffer.
Prepare a piece of PVDF membrane of appropriate size and soak in methanol for more than 30 s. Cut eight to 10 pieces of 3 mm Whatmann paper of the same size and soak in transfer buffer. Cut gel to the same size as PVDF membrane.
Place four to five pieces of 3 mm Whatmann paper, then PVDF membrane, then gel, then four to five pieces of 3 mm Whatmann paper in electroblot transfer apparatus. Avoid bubbles between PVDF membrane and gel.
Pour enough transfer buffer to prevent drying of the membrane and electrotransfer for 1–2 h at constant current, 1 mA/cm2 membrane.
3.6.2. Immunoblotting
Incubate PVDF membrane with 50 mL of blocking buffer at room temperature for 1 h to reduce nonspecific binding.
Discard the blocking buffer and incubate the PVDF membrane in primary antibody appropriately diluted (2,000–10,000) with TBS-T or blocking buffer for 1 h.
Remove the primary antibody and wash three times for 5 min each with 50 mL of TBS-T.
Freshly prepare the secondary antibody (HRP-conjugated) for each experiment appropriately diluted in TBS-T or blocking buffer and add to the membrane and incubate for 1 h at room temperature.
Discard the secondary antibody and wash the membrane four times for 5 min each with 50 mL of TBS-T.
During washing, warm the Western lightning® ch milumi-nescence reagent Plus separately to room temperature, mix together and then immediately add to the membrane and incubate for 1 min.
Remove the membrane from the chemiluminescence reagent, wrapped with Saran wrap, and expose to X-ray film in dark for a few min. An example of Western blotting detection of Pr55Gag (WT or 1GA) in equilibrium flotation gradients is shown in Fig. 12.5.
Fig. 12.5.
Gag membrane binding, DRM association, and multimerization assay. A. Membrane binding of Gag. HeLa cells were transfected with pNL4-3/PR− or pNL4-3/1GA/PR−, PNS were prepared and subjected to membrane flotation centrifugation. The gradient fractions were subjected to Western blotting for detecting Pr55Gag. M/DRM denotes membrane or DRM fractions; NM/DS denotes non-membrane bound or detergent soluble fractions. B. Schematic representation of epitope masking. When Gag is immunoprecipitated with anti-Gag antibodies not all the Gag is immunoprecipitated due to epitope masking resulting from higher-order multimerization. Upon denaturation with sample buffer, the multimerized Gag is converted to monomeric Gag, and the masked epitopes are exposed, increasing the efficiency of Gag immunoprecipitation. C. HeLa cells expressing either WT pNL4-3 or p6-mutant (pNL4-3/PTAP−) Pr55Gag were pulse-labeled for 5 min and chased for 15 min. PNSs were incubated in the absence or presence of 0.25% TX-100 (final concentration) on ice for 30 min before membrane flotation. Fractions were treated with RIPA buffer and membrane (M) and nonmembrane (NM) fractions were pooled. Labeled Pr55Gag in each pooled fraction was recovered by immunoprecipitation either without ( − ) or with ( + ) prior denaturation.
4. Assays for Membrane Binding and DRM Association
As mentioned in the Introduction, in most cell types HIV-1 assembly takes place primarily at the PM. Specific cholesterol-and glycosphingolipid-enriched PM microdomains known as lipid rafts play an important role in HIV-1 assembly. Lipid raft-associated proteins can be isolated in DRM fractions based on the resistance of lipid rafts to solubilization in certain detergents (e.g., TX-100) at low temperature (22). The analysis of membrane binding and DRM association of viral proteins thus provides important information about the late stages of the virus assembly/release process. A detailed protocol for measuring the membrane binding and DRM association of HIV-1 Gag is provided below.
Plate HeLa cells (106 cells/60-mm dish) and infect or transfect as described in Section 3.2.
One day posttransfection or two days postinfection, rinse cells with RPMI Cys−/Met− and add 1 ml of RPMI Cys−/Met−; incubate for 30 min at 37 °C.
Rinse the cells again and add 1 ml of RPMI Cys−/Met− containing 2% FBS.
Add 500 μCi [35S]Cys/Met to the medium and incubate at 37 °C for 5 min in a CO2 incubator.
Rinse the cells with DMEM-5, add the same medium, and incubate for 15 min at 37 °C in a CO2 incubator.
Rinse the cells with cold PBS once and scrape with cell lifters in 1 mL cold PBS.
Transfer the cells to Sarstedt tubes and spin at 2,000 rpm for 2 min in microfuge.
Remove the PBS and resuspend the cells in 0.2 mL of TE + Complete protease inhibitor cocktail. Disrupt the cells by sonication or dounce-homogenization. Examine cells microscopically to verify complete disruption.
Spin the homogenate at 2,000 rpm for 3 min in a microfuge to remove the unbroken cells. The supernatant is the postnu-clear supernatant (PNS).
Aliquot the PNS into two Sarstedt tubes (90 μL each) and add 2. 7 μL of 5 M NaCl (final concentration 150 mM).
To the PNS, add 90 μL of either TNE or TNE + 0. 5% TX-100 (prechilled), and incubate for 20–40 min on ice.
While samples are being solubilized, place 0.8 mL of TNE + 85. 5% sucrose in each centrifuge tube. After incubation, add 160 μL of detergent-treated sample directly into the ultracentrifuge tube containing 85.5% sucrose and vortex carefully to mix the PNS (TX-100 untreated or treated) completely with sucrose (now the concentration of sucrose is 73%) (see Note 6).
Above the bottom 73% sucrose layer, overlay 2.4 mL of 65% sucrose in TNE without disturbing the interface, then overlay with 0.8 mL of 10% sucrose in TNE. Carefully place the tubes in the ultracentrifuge buckets and spin at 35,000 rpm for 16 h at 4 °C
After ultracentrifugation, remove the tubes carefully, collect 10 fractions of 0.41 mL each from the top of each tube, and mix with 0.4 mL of 2X RIPA buffer.
Subject gradient fractions to immunoprecipitation and fluorography as described in Section 3.5 or detect by Western blotting as detailed in Section 3.6. An example of Gag distribution in membrane and DRM fractions is shown in Fig. 12.5 (see Note 7).
5. Gag Multimerization Assay
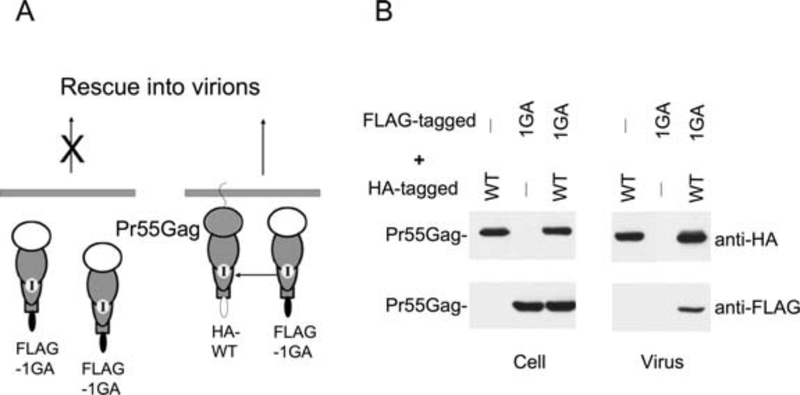
Membrane-bound Pr55Gag undergoes multimerization through Gag-Gag interactions that results in the assembly of viral particles. Gag mutants that are defective for Gag–Gag interactions do not multimerize and are thus defective in virus assembly and release. To study Gag multimerization, Pr55Gag derivatives bearing different C-terminal epitope tags have been constructed (18, 19). FLAG-tagged nonmyristylated (1 GA) Gag is unable to bind membrane and does not assemble into VLPs when expressed alone. However, upon coexpression with HA-tagged WT Gag it is incorporated into virions via Gag–Gag interactions (Fig. 12.6). The extent to which the nonmyristylated mutant Gag protein is recovered in VLP fractions provides a measure of Gag–Gag interactions. Rescue of the mutant (FLAG-tagged) Gag protein into VLPs by the WT (HA-tagged) Gag protein can be monitored by quantitative immunoblotting using anti-HA and anti-FLAG antibodies. To monitor homotypic Gag multimerization in cells, our lab recently developed an assay based on multimerization-induced epitope masking (15). When immunoprecipitation is carried out using anti-Gag antibodies, only a fraction of Gag is immunoprecipitated due to poor immunoprecipiation of highly assembled Gag complexes. However, when the samples are denatured prior to immunoprecipitation, Gag recovery is significantly increased (Fig. 12.5B,C). The difference in Gag recovery before and after denaturation thus provides a measure of higher-order Gag multimerization.
Fig. 12.6.
In vivo rescue assay. (A) Schematic representation of in vivo rescue assay. The nonmyristylated Gag (FLAG-tagged) that is deficient in membrane binding does not produce virus particles. However, when coexpressed with HA-tagged WT Gag, the nonmyristylated FLAG-tagged Gag is incorporated into virions through Gag–Gag interactions. (B) Coexpression of WT and mutant Gag proteins. HeLa cells were transfected with HA-tagged WT or FLAG-tagged Gag mutant alone or in combination at a 1:1 DNA. Virions were pelleted by ultracentrifugation, cell and virion lysates were analyzed by Western blotting with anti-HA or anti-FLAG antibodies. Reprinted from Joshi et al., 2006, (16) with permission. Copyright 2006, The American Society for Microbiology.
5.0.3. “Rescue-Based” Gag Multimerization Assay (16, 18, 23)
Cotransfected HeLa cells with various combinations of clones expressing epitope-tagged versions of WT Pr55Gag and Gag mutants (for example, HA-tagged WT Gag and a FLAG-tagged version of pNL4-3/1GA).
Perform virus release assay as described in Section 3.3.
Subject cell and virion lysates to SDS-PAGE and immunoblot with anti-HA and anti-FLAG antibodies; quantify levels of VLP-associated Gag. An example of rescue of 1GA mutant Gag into VLPs upon coexpression with WT Gag is shown in Fig. 12.6.
5.0.4. Gag Multimerization Assay Based on “Epitope Masking” (15)
Pool 200 μL each from upper (Frs. 1–5) and lower (Frs. 6–10) fractions from a sucrose gradient (see Section 4.) to 1.5 mL Sarstedt tubes; divide pooled fractions into two (0. 5 ml × 2).
Add 28. 5 μL 2X sample buffer to one of the tubes and boil for 5 min; transfer the contents to 15-mL tubes.
Add 3.2 mL TX-100 wash buffer to the 15 mL tubes and add 50 μL 1% BSA and 50 μL protein A agarose beads; incubate for 1 h at 4 ◦C.
Spin for 5 min at 2,500 rpm and collect supernatant for immunoprecipitation (this is the precleared lysate).
At the same time, combine 1 mL PBS, 0. 8 μL HIV-Ig, 50 μL protein A agarose beads, and 30 μL 1% BSA per sample in 15 or 50 mL tube (depending on the number of samples) and incubate for 1 h at 4 °C on an orbital rotor.
Spin for 5 min at 1, 120 × g, remove supernatant, add 0.5 mL PBS per sample, and aliquot 0.5 mL of the suspension to Sarstedt tubes and spin for 1 min at full speed in a microfuge.
Remove supernatant and combine the HIV-Ig-bound protein A agarose beads with precleared lysates in 15 mL tube and incubate overnight at 4 °C.
Spin for 5 min at 2,500 rpm, remove supernatant and wash three times with 1 mL TX-100 wash buffer, then wash once with 1 mL SDS/DOC wash buffer.
Resuspend samples in 60 μL of 1X sample buffer and boil the samples for 5 min. Mix the supernatant with 5 μL 0.1% BPB and subject to SDS-PAGE and fluorography as described in Section 3.5. An example of the use of this assay to monitor Gag multimerization in membrane and DRM fractions is shown in Fig. 12.5.
5.1. Immunofluoresence Microscopy
Assembling virus particles have been observed either at the PM or in MVBs, depending on the cell type in which Gag is expressed. Further, we and others have described mutations that redirect Gag to intracellular sites (13, 18, 24). The membrane binding assays described in Section 4 do not distinguish Gag associated with PM from Gag bound to intracellular membrane. Thus, to identify the subcellular site(s) of virus assembly it is often highly informative to visualize Gag localization by immunofluorescence microscopy with a deconvolution or confocal microscope. The following steps are carried out for this purpose.
Plate HeLa cells in chamber slide. Cells should be 60–80% confluent on day 3 after plating.
One day after plating, transfect with appropriate amounts of DNA using one of the transfection reagents described in Section 3.1.
One day posttransfection, rapidly rinse cells once with PBS.
Add 4% paraformaldehyde for 10 min at room temperature to fix the cells, remove the upper plastic housing, leaving the gasket on the slide, and further incubate for 20 min. (slides can now be removed from HIV containment if using infectious material).
Discard the paraformaldehyde and wash the cells three to four times with PBS.
Permeabilize the cells by incubation in methanol (stored at −20 °C until use) for 4 min at −20 °C or with 0.1% TX-100 in PBS for 5 min at room temperature, with intermittent shaking; rinse three to four times with PBS.
Incubate the cells with 0.1 M glycine for 10 min, then with 3% BSA in PBS for 30 min at room temperature to block nonspecific binding.
Remove the blocking solution and incubate with primary antibody in 3% BSA at an appropriate dilution (1:100–1:250) for 1 h at room temperature or at 4 °C overnight.
Remove the primary antibody and wash the sample three times for 5 min each with PBS. Place the sample under aluminum foil and dim the lights for subsequent steps.
Prepare the fluorescently conjugated secondary antibody at 1:250 in 3% BSA and add to the sample for 1 h at room temperature.
Discard the secondary antibody and add DAPI for 10 min at room temperature to stain the DNA and identify the nuclei.
Wash the samples four times for 5 min each with PBS, then aspirate the excess PBS from one corner (avoid complete drying).
Mount the samples with mounting medium and a cover slip, seal with nail varnish and store at 4 °C for up to a month.
View the slides under a fluorescent/confocal microscope using appropriate excitation wavelengths. Excitation at 350 nm induces DAPI fluorescence (blue emission); excitation at 488 nm induces the FITC fluorescence (green emission); excitation at 543 nm induces Texas red fluorescence (red emission). Typical data are shown in Fig. 12.7.
Fig. 12.7.
Immunofluorescence staining showing the assembly of viral particles. HeLa cells expressing either WT or 15A-mutant Pr55Gag in the presence of active PR were immunostained either with monoclonal anti-p17 or -p24 antibody prelabeled with Zenon One Alexa Fluor reagent. The punctate staining pattern represents sites of virus assembly. Reprinted with permission from Ono et al., 2005, (15). Copyright 2005, The American Society for Microbiology.
5.2. Electron Microscopy
The fluorescence microscopy method described in Section 5.1 provides information as to the site of Gag localization, but does not distinguish assembled from nonassembled Gag. To directly visualize the state of particle assembly, electron microscopy can be used. EM analysis also provides information crucial to discerning the morphology of assembled particles and the state of virion maturation.
Plate HeLa cells (0. 4 × 106 cells/well of six-well plate).
One day post-plating, transfect with approximately 3 μg DNA/well (ExGen500), 6 μg/well (Lipofectamine), or 10 μg/well (calcium-phosphate) by using the transfection reagents detailed in Section 3.1. To study virus assembly and release in T-cell lines or in primary cells, infect the cells with VSV-G-pseudotyped virus prepared as described above (Section 3.1).
One day transfection Wash the cells once with PBS, and fix with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at room temperature, for a minimum of 2 h.
Remove the glutaraldehyde and wash the cells twice with 0.1 M cacodylate buffer for 10 min each time.
Postfix the cells in 1% osmium tetroxide in 0.2 M buffer for 1 h, and rinse twice in 0.2M cacodylate buffer for 10 min.
Wash the cells once with 0.1 N acetate buffer for 10 min.
Incubate the cells with 0.5% uranyl acetate for 1 h.
Wash cells twice with 0.1 N acetate buffer for 10 min.
Dehydrate the cells in 35%, 50%, and 70% ethanol twice for 10 min each (samples may be stored overnight in 70% ethanol if absolutely necessary), then treat twice in 90% ethanol for 10 min, and three times with 100% ethanol for 10 min. Steps 10–13 are performed only with pelleted samples. For plated samples, go directly to step 14.
After dehydration, treat the cell or virus pellets twice with propylene oxide (1,2 epoxy propane) for 10 min.
Treat the samples with propylene oxide/epoxy resin mixture (50/50) for 1 h.
Add epoxy resin to the cell or virus pellets and leave the samples uncovered in the hood overnight to allow any remaining propylene oxide to evaporate.
Embed the samples in labeled capsules with freshly prepared resin and polymerize at 55 °C for 48 h.
Remove 100% ethanol from cells, add resin and leave the samples uncovered in the hood overnight to completely evaporate the remaining ethanol.
Add freshly prepared resin to the cells and polymerize at 55 °C for 48 h.
Cut sections of 100-nm thickness using a diamond knife with a ultramicrotome and the sections are picked up on carbon-coated 300 mesh copper grids.
Stain the sections with uranyl acetate and lead citrate, wash in DDW, and examine under an electron microscope. Typical electron micrographs of WT HIV-1 and p6-mutant derivative are shown in Fig. 12.8 (see Note 8).
Fig. 12.8.
Morphology of WT and p6-deleted HIV-1. HeLa cells transfected with WT pNL4-3 efficiently release mature virions (left panel), whereas cells expressing a p6-mutant molecular clone display numerous particles tethered to the plasma membrane (right panel). Images are visualized by transmission EM. Bar represents 100 nm (micro-graphs kindly provided by Kunio Nagashima. Reprinted from Freed and Martin (25)) Fields Virology 5th edition, copyright Lippincott Williams & Wilkins, with permission).
6. Notes
pCMVNLGagPolRRE is included in the transfection if the full-length molecular clone expresses an assembly-deficient Gag mutant or defective Pol enzymes. The VSV-G expression vector is included to increase the infectious titer of the virus stock and to allow infection of a greater range of cell types than is achievable by using the HIV-1 Env.
Amount of virus used in the infection will depend on the purpose of the assay and on the target cell line. For highly susceptible T-cell lines (e.g., MT-4), a smaller amount of input virus is needed relative to that used for less-susceptible lines (e.g., H9).
In experiments in which Env glycoproteins need to be quantified, carry out labeling with [35S]Cys for more efficient Env labeling.
The sucrose equilibrium gradient described above is also used to measure the density of the viral particles. Densities of the fractions are determined by weighing 100 μl of each fraction on an analytical balance or by using a refractometer. The density of the viral particles corresponds to the density of the sucrose in which it is concentrated ( ~ 1. 15–1. 17 g/mL).
Virion-associated p24(CA) is normalized to the volume of cell lysate taken for quantification. Virus release efficiency is calculated as the amount of released p24(CA) divided by the total Gag (virion p24-CA + cell Pr55Gag + cell p41Gag + cell p24-CA). Since many mutations or treatments will affect the levels of cell-associated Gag, calculation of virus release efficiency based only on quantification of released p24-CA will often lead to incorrect conclusions.
Ultraclear (polypropylene, from Beckman) tubes are best for this application as they allow good visibility during collection of the fractions.
The optimal amount of TX-100 depends on the number of cells. 2X RIPA buffer preserves the multimerized Gag and inactivates any infectious particles in PNS. In cases where the fractions are subjected to Western blotting alone, samples can be treated directly with 2X sample buffer.
All steps must be performed in a fume cupboard. Osmium tetroxide, propylene oxide and propylene oxide/resin waste should be collected in bottles for safe disposal.
References
- 1.Freed EO (1998) HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251, 1–15. [DOI] [PubMed] [Google Scholar]
- 2.Jouvenet N, Neil SJ, Bess C, et al. (2006) Plasma Membrane Is the Site of Productive HIV-1 Particle Assembly. PLoS Biol 4, e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono A, Freed EO (2004) Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J Virol 78, 1552–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelchen-Matthews A, Raposo G, Marsh M. (2004) Endosomes, exosomes and Trojan viruses. Trends Microbiol 12, 310–316. [DOI] [PubMed] [Google Scholar]
- 5.Ono A, Freed EO (2005) Role of lipid rafts in virus replication. Adv Virus Res 64, 311–358. [DOI] [PubMed] [Google Scholar]
- 6.Ono A, Ablan SD, Lockett SJ, et al. (2004) Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA 101, 14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shkriabai N, Datta SA, Zhao Z, et al. (2006) Interactions of HIV-1 Gag with assembly cofactors. Biochemistry 45, 4077–4083. [DOI] [PubMed] [Google Scholar]
- 8.Saad JS, Miller J, Tai J, et al. (2006) Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci USA 103, 11364–11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed EO (2006) HIV-1 Gag: flipped out for PI(4,5)P(2). Proc Natl Acad Sci USA 103, 11101–11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi A, Gendelman HE, Koenig S, et al. (1986) Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed EO, Martin MA (1995) Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol 69, 1984–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Orenstein JM, Martin MA, et al. (1995) p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J Virol 69, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freed EO, Orenstein JM, Buckler-White AJ, et al. (1994) Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J Virol 68, 5311–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Schwedler UK, Stray KM, Garrus JE, et al. (2003) Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J Virol 77, 5439–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono A, Waheed AA, Joshi A, et al. (2005) Association of human immunodeficiency virus type 1 gag with membrane does not require highly basic sequences in the nucleocapsid: use of a novel Gag multimerization assay. J Virol 79, 14131–14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi A, Nagashima K, Freed EO (2006) Mutation of dileucine-like motifs in the human immunodeficiency virus type 1 capsid disrupts virus assembly, gag-gag interactions, gag-membrane binding, and virion maturation. J Virol 80, 7939–7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimarelli A, Sandin S, Hoglund S, et al. (2000) Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J Virol 74, 3046–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono A, Orenstein JM, Freed EO (2000) Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J Virol 74, 2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono A, Freed EO (1999) Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J Virol 73, 4136–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono A, Freed EO (2001) Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA 98, 13925–13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee JK, Friedmann T, Burns JC (1994) Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 43, 99–112. [DOI] [PubMed] [Google Scholar]
- 22.Brown DA (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21, 430–439. [DOI] [PubMed] [Google Scholar]
- 23.Cimarelli A, Luban J. (2000) Human immunodeficiency virus type 1 virion density is not determined by nucleocapsid basic residues. J Virol 74, 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facke M, Janetzko A, Shoeman RL, et al. (1993) A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol 67, 4972–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freed EO, Martin MA (2007) HIVs and their replication in Fields Virology, Fourth Edition, Knipe and Howley, eds. Lippincott Williams & Wilkins. [Google Scholar]