Abstract
Clinical translation of novel therapeutics that improve the survival and quality of life of patients with neurological disease remains a challenge, with many investigational drug and device candidates failing in advanced stage clinical trials. Naturally occurring inherited and acquired neurological diseases, such as epilepsy, inborn errors of metabolism, brain tumors, spinal cord injury, and stroke occur frequently in companion animals, and many of these share epidemiologic, pathophysiologic and clinical features with their human counterparts. As companion animals have a relatively abbreviated lifespan and genetic background, are immunocompetent, share their environment with human caregivers, and can be clinically managed using techniques and tools similar to those used in humans, they have tremendous potential for increasing the predictive value of preclinical drug and device studies. Here, we review comparative features of spontaneous neurological diseases in companion animals with an emphasis on neuroimaging methods and features, illustrate their historical use in translational studies, and discuss inherent limitations associated with each disease model. Integration of companion animals with naturally occurring disease into preclinical studies can complement and expand the knowledge gained from studies in other animal models, accelerate or improve the manner in which research is translated to the human clinic, and ultimately generate discoveries that will benefit the health of humans and animals.
Keywords: Animal models, Epilepsy, Movement disorders, Neurodegenerative disease, Neuro-oncology, Spinal cord injury, Stroke
1. Introduction
The term “companion animal” has various medicolegal and psychosocial definitions that can incorporate numerous and diverse species. Here we define companion animals as domesticated animals whose behavioral, emotional, physical and social needs can be readily met as companions in the home or in close daily contact with humans (American Society for the Prevention of Cruelty to Animals, 2019). This review further and intentionally limits companion animals to dogs and cats, as the overwhelming majority of existing translational studies of spontaneous neurological disease involve these species.
1.1. Opportunities provided by companion animal models of neurological disease
The potential of naturally occurring animal models to serve as models for human disease was recognized nearly a century ago and has had a relatively recent resurgence with the One Health initiative (Krogh, 1929; Christopher, 2015). Hundreds of inherited disorders have been recognized in domesticated dogs and cats, and the majority of these have a human analog (Christopher, 2015; Starkey et al., 2005). The incidence of cancer in companion animals is also remarkably similar to humans, which may be partially explained by their shared environment and genetic traits (LeBlanc et al., 2016). Parallels in disease onset, phenotypes and genotypes, pathophysiology, and progression as well as therapeutic responses have also been reported for various human, canine and feline diseases, which may facilitate identification of epidemiologic risk factors, biomarkers, and the development of effective preventative and therapeutic strategies (Christopher, 2015; Starkey et al., 2005; LeBlanc et al., 2016; Parker et al., 2010). Many companion animal diseases are overrepresented in specific purebreeds, with founder effects resulting from several hundred years of intensely selective mating practices. As genetic heterogeneity is reduced and linkage disequilibrium orders of magnitudes higher in companion animals compared to humans, the study of disease in purebreeds is a robust tool for genomic mapping (Starkey et al., 2005; Parker et al., 2010). In addition, the lifespans of dogs and cats are 3–8 times shorter than to humans, which allows for the study of the natural history of disease in a compressed timeframe.
In industrialized nations, significant societal value is placed on companion animal ownership and healthcare (Einav et al., 2017; Pet industry market size & ownership statistics and American Pet Products Association, 2019). Nearly $70 billion was spent on pets in the United States in 2017, of which $16.6 billion was used for veterinary care, and there are an estimated 90 million pet dogs and 94 million cats residing in 68 % of US households (Einav et al., 2017). Similar to trends in human medical practice, a significant proportion of companion animal medical expenses are allocated to the management of chronic comorbidities and end-of-life healthcare.7 Maintenance of companion animal health is evident as growing populations of companion animals are available to participate in epidemiological and investigational agent or device trials.
Veterinary medical practice has evolved substantially in the past two decades, with rapid growth in the specialty practice sector. (Einav et al., 2017; Pet industry market size & ownership statistics and American Pet Products Association, 2019) Clinically ill companion animals are frequently managed with diagnostic and therapeutic approaches that are similar or identical to those used in human patients. In contemporary veterinary neurological and neurosurgical practice, this translates to the ability to thoroughly clinically characterize and annotate diseases using state-of-the-art electrophysiologic, genomic, molecular biologic, neuroimaging, and neuropathologic techniques (LeBlanc et al., 2016). In this review, we will highlight the use of traditional and emerging quantitative morphometric and functional neuroimaging methods as a nearly universal methodological tool employed in translational companion animal neurological research.
1.2. Obstacles to the use of companion animal models in neuroscience research
Despite the many advantages of companion animal models described herein, inherent limitations exist (Christopher, 2015; Starkey et al., 2005; LeBlanc et al., 2016; Parker et al., 2010). Although there are many physiologic and genetic similarities between dogs, cats and humans, fundamental differences exist that can have profound impacts on drug metabolism, efficacy and toxicity. Administration of anesthetics is generally needed to immobilize animals during acquisition of diagnostic quality neuroimages. As anesthetics inherently alter consciousness, cerebral blood flow, and oxygenation, this requirement has been a major impediment to the routine performance of blood oxygen level dependent functional MRI in companion animal neurology. Compared to rodent models and humans, the repertoire of validated reagents for molecular biological investigations is restricted for companion animals; this is particularly true for feline models. The current level of understanding of the genetic alterations that define companion animal diseases is also limited, and the identification of species-specific drivers of neurological disease is inevitable (LeBlanc et al., 2016; Connolly et al., 2018). Historically, the ability of translational studies in companion animals to truly inform human clinical trials has been hampered by a lack of statistical power or inclusion of controls that receive a validated standard of care treatment. Companion animal model research is also considerably more expensive and met with more public scrutiny and stringent ethical regulations compared to the use of rodents (Baneux et al., 2014). Such factors should not preclude the use of companion animal models in neuroscience research. Rather, these differences should highlight the need to incorporate rational and innovative study designs into companion animal model trials to improve the predictive value of preclinical research.
2. Spontaneous neurological diseases in companion animals
2.1. Epilepsy
Epilepsy is one of the most pervasive neurological disorders affecting mammals, with a prevalence in domestic dogs of ~0.6–1 %, and an estimated 1–3% prevalence in the human population (Kearsley-Fleet et al., 2013; Erlen et al., 2018; G.B.D. Epilepsy Collaborators, 2019). Spontaneous seizures in companion animals demonstrate good construct validity, with the etiologies of canine epilepsies being broad and representative of what is observed in humans. Brain tumors, encephalitides, neurodegenerative diseases, stroke, and traumatic brain injury are common causes of structural/metabolic epilepsies, and confirmed and suspected gene mutations have been observed in cases of idiopathic/genetic epilepsies (Blades Golubovic and Rossmeisl, 2017a; Ekenstedt et al., 2012). A robust body of literature has documented the etiologic, epidemiologic, pharmacologic, semiologic, and electrophysiologic similarities that exist between human and canine epilepsies (Blades Golubovic and Rossmeisl, 2017b; Grone and Baraban, 2015; Licht et al., 2002; Potschka et al., 2013; Patterson et al., 2015; Mariani, 2015). These studies indicate that canine epilepsies demonstrate considerable seizure phenotypic and electroencephalographic (EEG) face validity with humans, encompassing numerous focal and generalized epileptic syndromes. Emerging evidence also indicates that epileptic humans and dogs are afflicted by similar cognitive and behavioral comorbidities (Packer et al., 2018a; Watson et al., 2018). Although much less is known about the neuropathology of the majority of companion animal epilepsies, hallmark features of neuronal death, impaired neurogenesis, microglial activation, and blood-brain barrier compromise have been described (Matiasek et al., 2015; Troscher et al., 2017).
In humans and companion animals, the health burden of epilepsy is significant in terms of its association with chronic disability, premature death, and negative socioeconomic impacts (G.B.D. Epilepsy Collaborators, 2019; Blades Golubovic and Rossmeisl, 2017b; Packer et al., 2018a; Watson et al., 2018; Berendt et al., 2007). Nearly 60 % of epileptic dogs will experience one or more episodes of status epilepticus (SE) during their lives (Blades Golubovic and Rossmeisl, 2017b; Patterson et al., 2015). SE remains one of the most immediately life-threatening neurological conditions for humans and dogs, with associated mortality rates approaching 25 % for both species (Blades Golubovic and Rossmeisl, 2017b; Patterson et al., 2015). Treatment-refractory seizures are also a significant challenge, being reported in 33 % and 25 % of epileptic humans and dogs, respectively. Thus, epileptic dog populations offer a unique avenue to investigate the predictive validity of novel anticonvulsant drug, diet, or device candidates using safety, pharmacokinetic, or efficacy end-points in both acute emergent and chronic clinical settings (Licht et al., 2002; Potschka et al., 2013; Patterson et al., 2015).
In certain purebred dogs, the prevalence of epilepsy is significantly higher than that of the general population (Kearsley-Fleet et al., 2013). The comparatively limited genetic variation present in purebreeds as well as the common popular sire effects seen in inbred dogs offer tremendous opportunities to understand the genetics of complex diseases such epilepsy or cancer (Ekenstedt et al., 2012). Although to date there has not been a novel causative mutation for genetic epilepsy first identified in dogs that was later confirmed to be present in people, investigations of canine and human progressive myoclonic epilepsies (PME) have clearly illustrated that mutations in the same gene cause similar disease manifestations in different species. PME are phenotypically and genotypically heterogeneous metabolic diseases characterized by structural/metabolic epilepsy, myoclonus, and relentlessly progressive interictal neurological disability. Among the nine genes that have been identified in canine PME caused by Lafora disease and neuronal ceroid-lipofuscinoses (ARSG, ATP13A2, CLN5, CLN6, CLN8, CTSD, EPM2B, PPT1, and TPP1), eight are associated with orthologous mutations in humans that result in the same disease (Ekenstedt et al., 2012).
Identification of definitive mutations causing canine idiopathic/genetic epilepsies has been more challenging, supporting research that suggests that many human and canine epilepsies are multifactorial disorders caused by genetic, lifestyle, and environmental factors (Ekenstedt et al., 2012; Shearin and Ostrander, 2010; Ekenstedt et al., 2011). To date, only a single mutation causing a canine idiopathic/genetic epilepsy has been described, which is a truncation in LGI2 that causes benign familial epilepsy in Lagotto Romagnolo dogs that spontaneously remits by 4 months of age (Seppala et al., 2011). Through their interactions with a disintegrin and metalloproteinase (ADAM) receptors, LGI2 and its human ortholog LGI1, which is also a known epilepsy gene, are crucial for cell to cell adhesion, syntaptogenesis, and synaptic pruning in the developing brain. The recognition of LGI1 and LGI2 mutations provided novel insight into molecular mechanisms of epileptogenesis, being the first epilepsy mutations described that do not affect ion channels (Seppala et al., 2011).
Several quantitative MRI and functional neuroimaging techniques have been investigated in dogs with idiopathic/genetic epilepsy. Hippocampal atrophy (HA), similar to what is described in humans with mesial temporal lobe epilepsy (TLE) and hippocampal sclerosis, has been identified in up to 56 % of epileptic dogs in which volumetric MRI analyses are performed (Czerwick et al., 2018; Etsey et al., 2017). Studies that have obtained contemporaneous EEG on dogs with HA have also demonstrated that epileptiform discharges frequently localize to the hemisphere containing the HA (Czerwick et al., 2018). Interictal diffusion- and perfusion-weighted MRI abnormalities have also been identified in dogs with idiopathic/genetic epilepsy, including increased apparent diffusion coefficient values in the piriform lobe, amygdala, and centrum semiovale, as well decreased cerebral blood flow compared to healthy controls (Hartmann et al., 2018; Hartmann et al., 2017).
Clinical applications of radiotracer imaging in companion animal epilepsy remains in its infancy. The human experience has indicated that PET and SPECT can be highly valuable non-invasive tools that assist in the identification of the epileptogenic zone of patients undergoing comprehensive and multimodality presurgical diagnostic evaluations in consideration for resection of the seizure focus (Sarikaya, 2015). 18Fluoro-2-deoxyglucose (18F-FDG) PET imaging of brain glucose metabolism is the most widely utilized and available nuclear imaging technique in both humans and companion animals, and interictal 18F-FDG PET scans in humans typically reveal areas of hypometabolism associated with the epileptic focus. In Lagotto Romagnolo dogs with a focal genetic epilepsy, 18F-FDG PET scans also revealed reduced glucose metabolism in cortical brain regions associated with EEG abnormalities (Jokinen et al., 2014). Epileptic Finnish Spitz dogs have also demonstrated to have significantly lower standardized uptake 18F-FDG PET values in the hippocampus, occipital cerebral cortex, and cerebellum compared to healthy controls (Viitmaa et al., 2014).
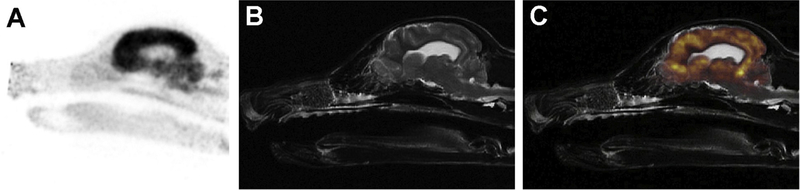
Performance of nuclear imaging in companion animal models provides several opportunities to advance epilepsy research. These include the characterization of mechanisms of epileptogenesis or anticonvulsant drug resistance, interrogation of the utility of novel radio-pharmaceuticals that are being developed for localization of epileptogenic foci, such as through the use of the GABA-A receptor ligands 11C-flumazenil and 18F-flumazenil, and to define biomarkers that are predictive of risk of the development of seizures after brain injuries, such as trauma, or for the attainment of seizure freedom after seizure focus resective surgery (Galovic et al., 2016). With image fusion capabilities and MRI-PET scanner availability increasing in veterinary medicine (Fig. 1), the ability to obtain robust quantitative morphologic and functional imaging datasets in dogs and cats with epilepsy also provides another avenue for potential future epilepsy research.
Fig. 1.
MRI-18F-FDG PET brain scan in a dog. Raw 18F-FDG PET sagittal image (A), T2W structural sagittal MRI image (B), and (C) MRI-PET fusion image. There is diffusely high 18F-FDG PET uptake by the brain, particularly in the gray matter.
Spontaneous epilepsies also exist in domestic cats, although compared to dogs, they are relatively poorly characterized and understood. However, there is evidence that a seizure phenotype with a worldwide distribution in cats, called feline complex partial seizures with orofacial involvement (FEPSO), is associated with pathologies that have striking parallels with human leucine-rich glioma-inactivated protein 1 autoimmune limbic encephalitis (LGI1-LE) and TLE with hippocampal sclerosis (Troscher et al., 2017; Pakozdy et al., 2014; Wagner et al., 2014). FEPSO manifests as focal facial motor seizures and various oral automatisms including lip smacking, chewing, licking, swallowing, and vocalization, with post-ictal aggression also frequently reported (Troscher et al., 2017; Pakozdy et al., 2014).Affected cats have brain magnetic resonance imaging (MRI) examinations that demonstrate bilateral and often symmetric signal changes in the hippocampi (Fig. 2), and a subset of these cats with LGI1-LE will have serum antibodies to the voltage gated potassium channel (VGKC) complex protein LGI1 (Pakozdy et al., 2014). Neuropathologic changes documented in the hippocampi of cats with LGI1-LE also resemble those seen in humans including neuronal degeneration and loss, astrogliosis, and neuroinflammation (Fig. 2) (Troscher et al., 2017; Wagner et al., 2014).
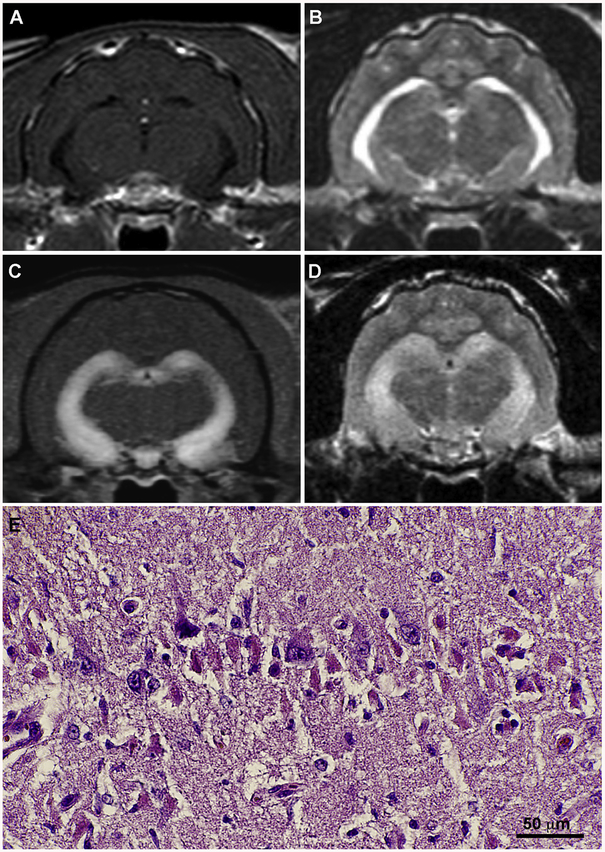
Fig. 2.
Brain magnetic resonance imaging (MRI) and neuropathologic features of feline LGI1-limbic encephalitis (LE). T1-weighted, post-contrast (A) and T2-weighted (B) images from an age-matched, healthy control cat with normal hippocampal morphology. MRI from a cat with LGI1-LE demonstrating bilaterally symmetric and marked contrast enhancement (C) and T2-hyperintensity (D) within the hippocampus. Hippocampal pyramidal cell degeneration in feline LGI1-LE (E) is characterized by acidophilic neuronal necrosis, H&E stain.
Limitations associated with the incorporation of companion animal models into translational epilepsy studies exist. These include the reliance on and reliability of owner-based seizure reporting and monitoring, the lack of harmonized terminology for seizure semiology, challenges associated with obtaining EEG recordings in companion animals, the undetermined and likely heterogeneous genetic etiologies of epilepsy in study populations that include diverse dog breeds, and differences in physiology and drug pharmacology between dogs, cats, and humans. To overcome some of these limitations, consensus statements modeled after those published by the International League Against Epilepsy, that address the genetics, classification, diagnostic approach to, and treatment of epilepsy in companion animals have been developed by joint panels of human and veterinary medical epilepsy experts and widely adopted by veterinarians (Berendt et al., 2015; DeRisio et al., 2015; Bhatti et al., 2015; Potschka et al., 2015). In addition, traditional barriers to the electrophysiologic characterization of companion animal seizure disorders are being overcome in parallel with improvements in medical device technology. The feasibilities of obtaining diagnostic EEG recording in awake dogs in a routine clinical setting using a wireless EEG system, as well as chronically in epileptic dogs with cortically implanted telemetric EEG devices have been demonstrated (James et al., 2017; Davis et al., 2011). These studies illustrate the potential value of companion animals with epilepsy for the advancement of quantitative EEG analyses, such as the development of seizure detection algorithms.
2.2. Stroke
The Global Burden of Diseases, Injuries, and Risk Factors Study ranked stroke as the second most common cause of death and the third most common cause of disability worldwide in 2010 (Lozano et al., 2012). Dogs with experimentally induced strokes have used been as models for decades, as they possess anatomic features including a large gyrencephalic brain as well as a ratio and distribution of gray and white matter that is more representative to that of humans than rodents (Casals et al., 2011). Given the recent evidence of the benefits of endovascular therapies for the treatment of stroke in humans, canine models will likely continue to be used because their cerebrovascular anatomy is amenable to minimally invasive, image-guided endovascular interventions (Jovin et al., 2016). This allows for both the induction of stroke and assessment of experimental therapeutics.
With widespread availability of MRI in veterinary specialty hospitals, cerebrovascular accidents (CVA) are being increasingly recognized in companion animals, especially dogs (Garosi and McConnell, 2005). Naturally occurring canine CVA share many similarities with the human condition, which makes them a relevant model for the global assessment of the safety and efficacy of novel treatments. Ischemic strokes are more common than hemorrhagic strokes in both species, and the majority of territorial and lacunar stroke topographies that humans experience with their associated clinical symptoms and MRI findings have been observed in dogs (Fig. 3) (Garosi and McConnell, 2005; Garosi et al., 2006; Rossmeisl et al., 2007). Several PET and quantitative imaging techniques have also been used in canine stroke models to determine the temporal effects of stroke and its treatment on cerebral blood flow and oxidative metabolism, as well as to investigate the use of novel radiotracer agents that provide insight into the pathogenesis of and recovery from stroke (Kuge et al., 2000; Weyne et al., 1987).
Fig. 3.
Canine ischemic and hemorrhagic strokes. T2-weighted (A) and fluid-attenuated inversion recovery (B) MRI of middle cerebral artery territorial ischemic infarction. T2-weighted (C) and T1-weighted (D) MRI of lacunar ischemic infarction of the caudate nucleus. T2* gradient echo (E) MRI and corresponding gross pathologic (F) specimen of hemorrhagic infarct in the frontal lobe with concurrent cerebral microbleeds (blue arrows).
Strokes often occur in dogs within age ranges (8–10 years) equivalent to those of geriatric humans, and affected dogs frequently have similar and heterogeneous comorbid risk factors for CVA, such as systemic hypertension, chronic kidney disease, atherosclerosis, and diabetes mellitus (Garosi et al., 2005a). The National Institutes of Health Stroke Scale, a clinical assessment instrument that has been shown to be predictive of stroke outcome in humans, has been modified and applied to canine models of stroke (Adams et al., 1999; Boulos et al., 2011). Spontaneous canine strokes also provide an avenue to explore mechanisms of neuroinflammation or liquid biopsy biomarkers without the confounding factor of a surgical or endovascular procedure in experimentally induced stroke that may itself cause or contribute to the inflammatory host response (Gredal et al., 2017). The neuropathologic features of large artery strokes in dogs are also similar to that humans, although mural pathologies described in the deep perforating arteries of humans with lacunar infarcts such as fibrinoid necrosis, lipohyalinosis, and segmental arteriolar disorganization have not been widely recognized in dogs (Thomsen et al., 2017; Mena et al., 2004). Additionally, ~25 % of dogs and humans die within the first few weeks of stroke onset, with stroke severity being associated with subacute mortality in both species (Garosi et al., 2005a).
Several disadvantages are associated with the spontaneous canine stroke model. There are considerable differences between humans and dogs regarding the blood supply to the brain. In dogs, the cerebral arterial circle receives a significant proportion of blood flow from numerous vascular anastomoses derived from branches of the external carotid artery; the vertebral arteries contribute more substantially to the total blood supply to the brain through perfusion of the rostral thalamus, hypothalamus and caudal cerebrum; and the canine middle cerebral artery receives more leptomeningeal circulation from the ipsilateral rostral and caudal cerebral arteries (Jewell, 1952; Symon, 1960). These collateral pathways serve to protect the canine brain against the effects of cerebral arterial occlusion, and result in canine infarct volumes that are relatively limited in size compared to those seen in humans. Relatively smaller infarct volumes and the dominance of the tegmentospinal tracts rather than the pyramidal system in the regulation of movement also contribute to the rapid and dramatic recovery of motor system dysfunction that is often observed in dogs with stroke (Rossmeisl et al., 2007; Garosi et al., 2005a; Symon, 1960; de Oliveira-Souza, 2017). In companion animals, it is also common for the clinical diagnosis of stroke to be delayed by several days after the appearance of neurological dysfunction, which significantly limits opportunities to deliver and evaluate therapeutics in the peracute “golden hours” after stoke onset (Rossmeisl et al., 2007; Garosi et al., 2005a).
2.3. Alzheimer’s disease and cognitive dysfunction syndrome
In companion animals and humans, age is a significant risk factor for cognitive impairment (Hebert et al., 2013; Alzheimer’s Association Report, 2018). The risk for developing Alzheimer’s disease (AD), the predominant cause of human dementia, increases dramatically with age. Approximately 3 % percent of people age 65–74, 17 % of people age 75–84, and 33 % of those older than 85 have AD (Hebert et al., 2013). Cognitive dysfunction syndrome (CDS) is a neurodegenerative disorder affecting geriatric dogs and cats characterized by age-related cognitive decline sufficient to affect daily activities, with specific neurobehavioral manifestations that cannot be attributed to other medical conditions. CDS is highly prevalent in companion animal populations, affecting ~25 % of dogs and cats 11–14-years of age and 50–66% of dogs and cats 15+ years of age (Neilson et al., 2001; Moffat and Landsberg, 2003).
Clinical signs of CDS in animals mirror those of AD and include disorientation, anxiety, loss of previously learned behaviors (i.e. housetraining or work-related tasks), disrupted circadian rhythms (excessive daytime somnolence with increased nocturnal aimless activity or vocalization), and diminished social interactions with human caregivers and other animals (Neilson et al., 2001). The neuropathology of CDS resembles AD, with the brains of dogs and cats affected with CDS demonstrating atrophy of cerebral cortex and basal ganglia secondary to neuronal loss, cerebral microbleeds, microgliosis, and the presence of extraneuronal and vascular β-amyloid (Aβ) plaques, particularly in the frontal lobes and hippocampus, and intraneuronal hyperphosphorylated tau pathologies (Schmidt et al., 2015; Head et al., 2005).
Traditional structural neuroimaging features of CDS are also similar to those seen in the later stages of AD, with cortical atrophy, particularly of the medial temporal lobes and hippocampi, a reduced size of the interthalamic adhesion, and ventriculomegaly being commonly observed (Trzepacz et al., 2014). Recent research in humans has demonstrated the utility of quantitative structural MRI and functional neuroimaging techniques such as 18F-FDG PET for cerebral metabolism and Pittsburg compound-B PET (PiB-PET) for amyloid imaging to demonstrate characteristic signatures in the brains of AD patients that my facilitate diagnosis prior to the onset of symptoms (Trzepacz et al., 2014). PiB-PET scans have been performed in dogs with CDS; however, studies performed to date indicate that PiB retention patterns in the canine brain are markedly different from those of humans with AD, and PiB retention does not appear correlate with histological distribution of Aβ pathology in dogs (Fast et al., 2013).
As is true for people with AD, there is no cure or currently effective preventative strategies for CDS, and the healthcare burden associated with cognitive decline is expected to grow significantly as the human and pet populations grow and age (Hebert et al., 2013). As there is only one FDA-approved drug, selegeline, for dogs with cognitive impairment, dogs are being used as models in the investigation of a spectrum of novel disease modifying approaches with potential human applications including nutritional, pharmacologic, immunotherapeutic, and behavioral interventions (Pan et al., 2010; Davis et al., 2017).
2.4. Lysosomal storage diseases
Lysosomal storage diseases (LSD) comprise a group of over 70 rare inherited metabolic diseases in humans, many of which have been identified in dogs and cats (Gurda et al., 2017; Sun et al., 2018; Skelly and Franklin, 2002). The majority of LSD in humans and companion animals are inherited in an autosomal recessive manner, and are caused by deficient activity of a single enzyme resulting in impaired catabolism of metabolic substrates within lysosomes. Accumulation of undegraded products within cells causes enlargement of cells, cellular dysfunction, and eventually cell death. As most lysosomal enzymes are nearly ubiquitously expressed in mammalian tissues, clinical manifestations of phenotypes can be quite heterogeneous and are frequently referable to involvement of multiple organ systems (Beck, 2001). Even among affected individuals carrying identical mutant alleles, remarkable phenotypic variability is often observed due to modifying genes and epigenetic factors, particularly in those LSD with a late (adult) onset (Beck, 2001). An additional mechanism of phenotypic heterogeneity is the variable levels of residual enzyme activity associated with different defective alleles that cause LSD (Beck, 2001). Despite the relatively protean clinical manifestations of LSD, symptoms reflective of central nervous or neuromuscular system dysfunction are found in most disease variants and are often the earliest apparent or most severely debilitating phenotypes of LSD (Gurda et al., 2017; Sun et al., 2018; Skelly and Franklin, 2002).
Numerous canine and feline LSD are highly faithful models of disease, with mutations involving orthologous genes resulting in the same pathological, diagnostic imaging, and biochemical abnormalities that are observed in human patients (Fig. 4]; Table 1]) (Berg et al., 1997; Skelly et al., 1996; Menon et al., 1992; He et al., 1999; Yogalingam et al., 2002; Yogalingam et al., 1996; Ray et al., 1998; Fyfe et al., 1999; Seppala et al., 2013; Gilliam et al., 2015; Victoria et al., 1996; Yamato et al., 2002; Sanders et al., 2013; Somers et al., 2003). These companion animal models of LSD have made substantial contributions to the understanding of disease pathophysiology including how specific genotypes produce disease phenotypes as well as to the molecular bases of the different levels of lysosomal enzymatic activity observed in patients (Yogalingam et al., 2002; Yogalingam et al., 1996; Ray et al., 1998; Fyfe et al., 1999; Seppala et al., 2013; Gilliam et al., 2015).
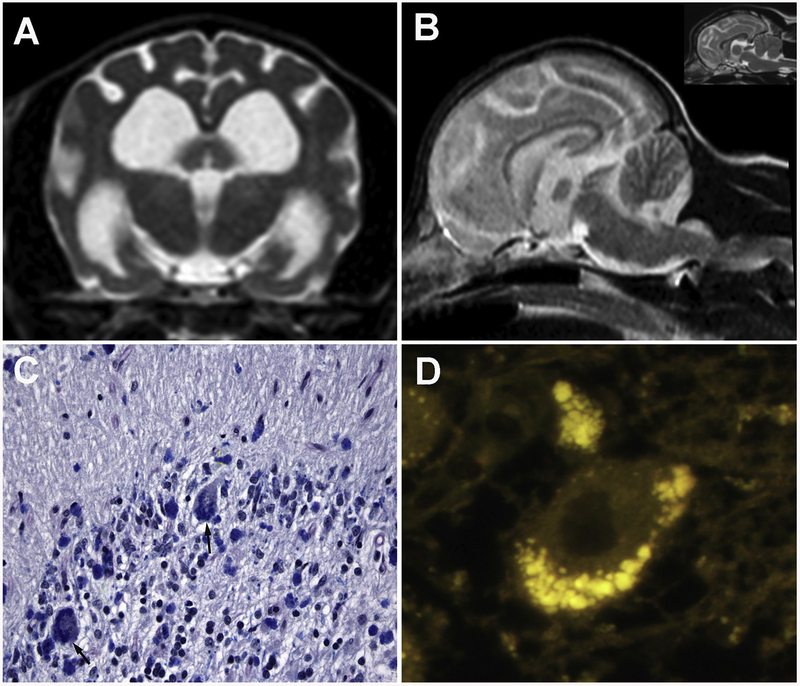
Fig. 4.
Neuronal ceroid lipofuscinosis in a 3-year-old beagle dog. Transverse (A) and sagittal (B) T2-weighted brain MRI demonstrating generalized brain atrophy characterized by shrunken gyri and cerebellar folia, widened sulci, and dilation of the ventricular system compared to an age-matched control (inset, B). Microscopic section of the cerebellar cortex (C) demonstrating Purkinje cell loss and Luxol fast blue-positive perikaryal cytoplasmic substrate accumulation visible within residual Purkinje cells (black arrows). Fluorescent micrograph (D) illustrating aggregates of autofluorescent storage bodies present in a Purkinje cell.
Table 1.
Companion Animal Models of Lysosomal Storage Diseases.
| Lysosomal Storage Disease Subgroup | Disease | Defective Gene | MutationRef | Affected Species; Breeds |
|---|---|---|---|---|
| Glycoproteinoses | ||||
| Alpha-mannosidosis | MAN2B1 | c.1748delCCAG (Berg et al., 1997) | Feline; DLH, DSH, Persian | |
| Fucosidosis | FUCA1 | c.379_392del14bp (Skelly et al., 1996) | Canine; English Springer Spaniel | |
| Mucopolysaccharidoses | ||||
| Mucopolysaccharidosis I | IDUA | c.155 + 1G > A (Menon et al., 1992)c.1107– 1109 del (He et al., 1999) | Canine; Plott Hound Feline; DSH | |
| Mucopolysaccharidosis IIIA | SGSH | c.708–709insA (Yogalingam et al., 2002) | Canine; New Zealand Huntaway dogs | |
| Mucopolysaccharidosis IIIB | NAGLU | Unpublished | Canine; Schipperke | |
| Mucopolysaccharidosis VI | ARSB | c.1427T > C (Yogalingam et al., 1996) | Feline; Siamese | |
| Mucopolysaccharidosis VII | GUSB | c.559 G > A (Ray et al., 1998)c.1074G > A (Fyfe et al., 1999) | Canine; Mixed breed Feline; DSH | |
| Oligosaccharidoses | ||||
| Pompe’s disease | GAA | c.2237 G > A (Seppala et al., 2013) | Canine; Swedish and Finnish Lapphunds | |
| Proteinoses | ||||
| Ceroid Lipofuscinosis | CLN5 | c.934_935delAG (Gilliam et al., 2015) | Canine; Golden Retriever | |
| Sphingolipidoses | ||||
| Globoid cell leukodystrophy | GALC | c.473A > C (Victoria et al., 1996) | Canine; West Highland White and Cairn terriers | |
| GM1-gangliosidosis | GLB1 | c.1647delC (Yamato et al., 2002) | Canine; Shiba Inu | |
| GM2-gangliosidosis | HEXA | c.967 G > A (Sanders et al., 2013) | Canine; Japanese Chin | |
| Niemann Pick Type C1 | NPC1 | c.2864 G > C (Somers et al., 2003) | Feline; DSH |
DLH = Domestic long-hair cat.
DSH = Domestic short-hair cat.
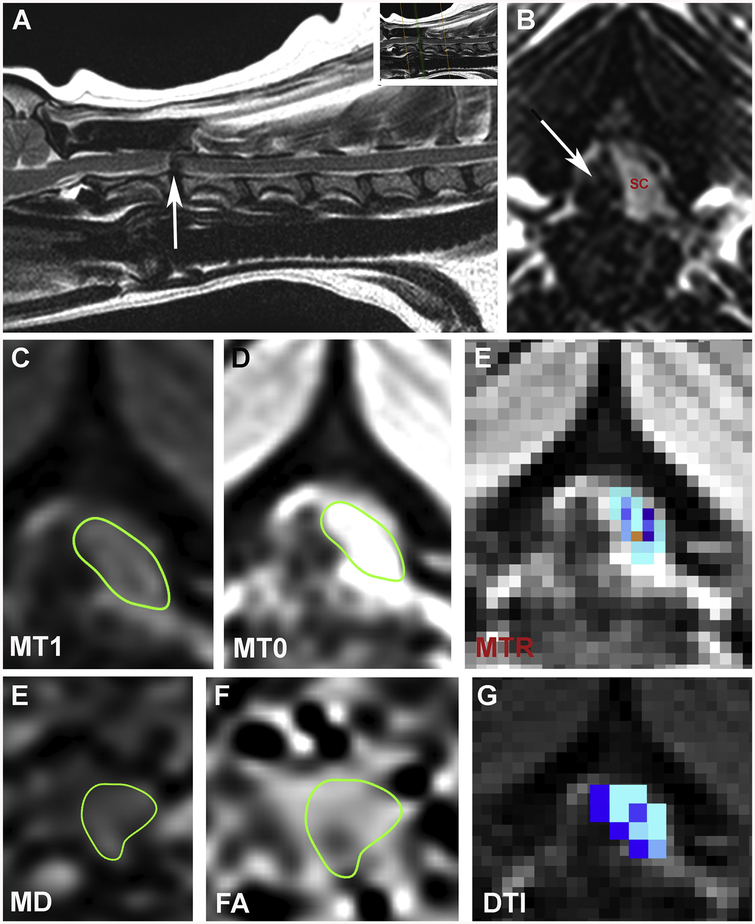
Quantitative neuroimaging techniques have been employed for decades to further characterize structural nervous system abnormalities, pathophysiological mechanisms, and biochemical aberrations associated with various companion animal LSD variants, as well as to catalog morphological and functional correlates of therapeutic interventions (Middleton et al., 2018; Choi et al., 2015; Li et al., 2018; Kumar et al., 2016; Vite et al., 2001; Vite et al., 2013; Magnitsky et al., 2010). High-field morphometric MRI scans are frequently used to longitudinally quantify grey and white matter volumetric changes that occur in LSD and their treatment (Vite et al., 2001; Vite et al., 2013). Diffusion tensor MR imaging (DTI), and magnetization transfer MR imaging (MTI) are widely used techniques that have proven beneficial for assessment of the structure, integrity, and organization of white matter tracts in canine and feline LSD, and in some cases have revealed useful imaging biomarkers for specific LSD variants (Middleton et al., 2018; Choi et al., 2015; Li et al., 2018; Kumar et al., 2016; Magnitsky et al., 2010). Proton magnetic resonance spectroscopy may also be employed to identify unique spectral signatures that correlate with accumulation of undigested metabolic substrates within tissues (Magnitsky et al., 2010).
Canine and feline models with orthologous mutations have also had important roles in advancing therapies for LSD, including hematopoietic stem cell transplantation, enzyme replacement therapy (ERT), and gene therapy (Gurda et al., 2017; Bradbury et al., 2015). Currently, a major obstacle to the treatment of central nervous system manifestations of LSD is the inability to deliver macromolecular therapeutic agents, such as systemically administered ERT, across the blood-brain barrier. Dog and cat models have been used extensively to demonstrate the feasibility, safety, and potential efficacy of intrathecal or intracerebrally delivered ERT or gene therapies, and have subsequently informed the rational design of comparative trials in humans (Kakkis et al., 2004; Kondagari et al., 2015). In aggregate, studies in companion animal models have demonstrated the potential for these therapeutic approaches to ameliorate clinical signs of disease, slow disease progression, reduce substrate accumulation within affected tissues, favorably influence biochemical disease markers, and improve neuropathological lesions (Bradbury et al., 2015; Kakkis et al., 2004; Kondagari et al., 2015; Taylor et al., 1992; Walkley et al., 1994; Richter et al., 2015).
2.5. Movement disorders
Companion animals, particularly dogs, display a broad phenotypic range of abnormal involuntary movements (Table 2) (Richter et al., 2015; Urkasemsin and Olby, 2014; Podell, 2004; Waln and Jankovic, 2015; Pancotto and Rossmeisl, 2017; Gilliam et al., 2014; Bhalerao et al., 2002; Quitt et al., 2018; Lowrie et al., 2018; Gill et al., 2012; Kolicheski et al., 2017; Geiger and Klopp, 2009; Gill et al., 2011). These movement disorders (MD) are classified using a scheme that parallels that of humans in which the abnormal movement is first defined as hyperkinetic or hypokinetic, followed by the frequency that the abnormal movements are observed (episodic/paroxysmal or constant/continual), and then a description of the cardinal phenotypic manifestation of the abnormal movement (dyskinesia, dystonia, myoclonus, myokymia/neuromyotonia, and myotonia) (Richter et al., 2015; Urkasemsin and Olby, 2014; Podell, 2004; Waln and Jankovic, 2015). Etiologies of MD in companion animals are equally diverse. Examples that recapitulate human conditions (Table 2) include autoimmune disorders (stiff-dog-syndrome (Pancotto and Rossmeisl, 2017), canine epileptoid cramping syndrome (Lowrie et al., 2018)), inherited glial (KCNJ10, spinocerebellar ataxia with myokymia, seizures, or both (Gilliam et al., 2014)) or sarcolemmal (CLCN1, myotonia congenita (Bhalerao et al., 2002; Quitt et al., 2018)) ion channelopathies, spinal cord serotonin deficiency (Scottish terrier cramp (Geiger and Klopp, 2009)), glycine transporter mutations (Startle disease (Gill et al., 2011)), and suspected neurodegenerative diseases of subcortical circuits and basal nuclei (Garosi et al., 2005b).
Table 2.
Companion Animal Models of Movement Disorders.
| Phenotype | Disease Name(s) | PathophysiologyRef | Phenotypic Trigger(s) | Affected Species; Breeds | Human Disease Analogue(s) |
|---|---|---|---|---|---|
| Hypertonicity Syndromes | |||||
| Stiff-dog-syndrome | Anti-glutamic acid decarboxylase antibodies (Pancotto and Rossmeisl, 2017) | Tactile stimulation | Canine; Beagle | Stiff-person syndrome | |
| Labrador hypertonicity syndrome | Unknown | Unknown | Canine; Labrador retriever | Stiff-person syndrome | |
| Myokymia/Neuromyotonia | |||||
| Spinocerebellar ataxia, with myokymia, seizures, or both | KCNJ10 mutation (Gilliam et al., 2014) (Kir4.1 glial potassium channel) | Stress, excitement | Canine; Jack Russel Terriers and related breeds Feline | EAST SeSAME | |
| Myotonia | |||||
| Myotonia congenita | CLCN1 mutation (Bhalerao et al., 2002; Quitt et al., 2018) (sarcolemmal chloride channel); autosomal recessive inheritance | Stiffness worse after rest; often remits after “warm up” | Canine; several breeds Feline | Myotonia congenita | |
| Paroxysmal dyskinesias | |||||
| PD; Canine epileptoid cramping syndrome | Gluten-sensitivity (Lowrie et al., 2018) | Waking, excitement, stress, heat or cold | Canine; Border terrier | Paroxysmal non-kinesigenic dyskinesia | |
| PD; Episodic falling syndrome | BCAN mutation (Gill et al., 2012); autosomal recessive inheritance | Exercise, stress, or excitement | Canine; Cavalier King Charles Spaniel | PD | |
| PD | PIGN mutation (Kolicheski et al., 2017); autosomal recessive inheritance | Stress or excitement | Canine; Wheaten Terrier | MCASH1 | |
| PD; Scottie cramps | Spinal cord serotonin deficiency (Geiger and Klopp, 2009); autosomal recessive inheritance | Exercise, stress, or excitement | Canine; Scottish terrier | Paroxysmal non-kinesigenic dyskinesia | |
| Startle disease | SLC6A5 mutation (Gill et al., 2011) (presynaptic glycine transporter); autosomal recessive inheritance | Tactile or acoustic stimulation | Canine; Irish Wolfhound | Hyperexplexia | |
| Postural myoclonus | |||||
| Orthostatic postural myoclonus | Unknown (Garosi et al., 2005b) | Standing or posturing to eat or eliminate | Canine; Great Dane and Scottish deerhound | Orthostatic tremor |
EAST = Epilepsy, ataxia, sensorineural deafness, and tubulopathy.
MCASH1 = Multiple congenital abnormalities- hypotonia-seizures syndrome 1.
PD=Paroxysmal dyskinesia.
SeSAME = Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalances.
The majority of canine MD are breed-associated conditions, which provides an additional opportunity for studies of homogeneous inbred dogs to facilitate the identification of the pathophysiologic mechanisms of and evaluate therapeutics for inherited diseases. For example, episodic falling syndrome in the Cavalier King Charles Spaniel, which is phenotypically similar to human hyperekplexia, is caused by a deletion in BCAN (Gill et al., 2012). BCAN encodes the brevican protein, an extracellular matrix (ECM) proteoglycan that is highly expressed in the central nervous system and is involved in perineuronal ECM network regulation of neuronal plasticity, axonal guidance, and cellular adhesion (Gill et al., 2012). Clinical signs in dogs with episodic falling can be ameliorated with acetazolamide or clonazepam therapies. Border terriers with gluten-sensitive epileptoid cramping syndrome and autoantibodies directed against transglutaminase and gliadin have provided evidence that the dog may also be a good model for the study of non-celiac gluten sensitivity (NCGS) (Lowrie et al., 2018). NCGS comprises a spectrum of disorders in people with diverse multisystemic symptoms affecting the gastrointestinal tract, skin, and nervous system that all result from an immune response triggered by the ingestion of gluten.117 In affected Border terriers, clinical manifestations of epileptoid cramping syndrome improve or resolve when dogs are fed a gluten-free diet (Lowrie et al., 2018). Gluten-free dietary modification has also been demonstrated to alleviate ataxia and parkinsonian symptoms in some humans with celiac disease or NCGS (Hadjivassiliou et al., 2003; Di Lazzaro et al., 2014).
Advanced neuroimaging plays a pivotal role in the management of individual patients with MD by excluding malformations, encephalitides, cerebrovascular events and neoplasms as potential etiologies for the observed phenotypic abnormality (Masalachi et al., 2012; Wu et al., 2011). The shaking (sh) pup, a companion animal model of the human X-linked leukodystrophy Pelizaeus-Merzbacher Disease (PMD), provides an excellent example of translational applications for quantitative MRI imaging studies. The sh pup phenotypically manifests with a juvenile-onset diffuse, action related myoclonus that results from a mutation in the myelin PLP gene. This inhibits oligodendrocyte development and subsequently causes severe and diffuse hypomyelination, although axons are mostly spared. DTI and MTI MR studies in sh pups have provided significant and translationally relevant insight into mechanisms of dysmyelination, and age-related brain white matter maturation patterns in this model (Samsonov et al., 2012; Sanmamed et al., 2016).
2.6. Neuro-oncology
Relevant pre-clinical in vivo research models are necessary to advance novel diagnostic and treatment regimens for primary CNS tumors in people. Historically, rodent models have been widely used for pre-clinical in vivo research, but inherent limitations preclude direct translation of any derived pre-clinical results to humans. Despite these limitations, rodent models are easy to obtain and economical, especially when a large number of subjects are needed for statistical significance (Sanmamed et al., 2016). Genetically engineered mice (GEM) have the advantage of an intact immune system, which helps generate a more physiologically relevant tumor microenvironment, providing an opportunity to evaluate response to immunotherapy, such as checkpoint inhibition (Sharpless and Depinho, 2006). Engineering these mice is time consuming and expensive, and may lead to variability in clinical presentation due to unexpected phenotypes (Lin, 2008; Talmadge et al., 2007). Patient derived xenograft (PDX) tumor models share the characteristic cellular complexity and histologic architecture of the tumor in its natural state, but lack an intact immune system, limiting their utility in research investigating immune responses (Williams et al., 2013; Candolfi et al., 2007).
Practically speaking, the small size of most rodent models makes serial brain tissue sample collection and other neurosurgical therapeutic manipulations challenging. Additionally, tumors are often experimentally induced and lack important histologic features of naturally- occurring tumors, such as endothelial cell proliferation (Candolfi et al., 2007; Stoica et al., 2004). Given these limitations, murine models may respond differently than human tumors to novel cancer therapies and accurate evaluation of such treatments in conjunction with surgical resection presents a significant challenge. The use of canines as a large animal pre-clinical model for human investigations may serve to overcome these limitations due to their larger size, presence of an intact immune system and similarities in tumor histologic, molecular and neuro-imaging characteristics (Stoica et al., 2004; Lipsitz et al., 2003; Sturges et al., 2008; Dobson et al., 2002). Canine brain tumors occur spontaneously, and since people and dogs are exposed to similar environments and factors capable of influencing tumorigenesis, dogs likely represent a more appropriate pre-clinical model.
Dogs and humans are the only species that frequently develop spontaneous brain tumors, and with similar incidence. The incidence of spontaneously arising primary brain tumors is approximately 14–20 per 100,000 dogs compared to an estimated 29.9 per 100,000 persons, and is likely underestimated in dogs due to owners electing humane euthanasia prior to obtaining a definitive diagnosis (Ostrom et al., 2018; Hu et al., 2015). The incidence of primary brain tumors is highest in middle-aged to older dogs, with a median age of 9–10 years, which correlates well with human disease, in which incidence is greatest in people over the age of 70 (Porter et al., 2010; Wiemels et al., 2010). Like humans, meningiomas are the most common primary brain tumors in dogs, followed by gliomas, of which glioblastoma is the most common malignant histologic subtype (Snyder et al., 2006; Platt et al., 2001).
Canine patients presenting with brain tumors often have significant clinical signs that can be objectively characterized using tools similar to those used in humans. These include neurologic examination, modified Glasgow Coma Scale, canine Karnofsky performance score, Engel seizure classification, and Modified Rankin Score (Rossmeisl et al., 2015a; Peus et al., 2013). Likewise, shared MRI neuroimaging diagnostic and treatment response assessment approaches are currently in use for primary brain tumors in both species, which may further facilitate translation of novel therapies between canine and human patients (Young et al., 2011; Packer et al., 2018b; Rossmeisl et al., 2014).
2.6.1. Malignant glioma
Malignant gliomas are the most common subtype of malignant primary brain tumors in humans and glioblastoma multiforme (GBM), in particular, are leading causes of cancer-related death in adults and children. This is due to the incredibly aggressive, invasive and neurologically destructive nature of these tumors, making complete surgical excision nearly impossible. Additionally, its ability to produce a highly immunosuppressive microenvironment and location behind the blood brain barrier (BBB) allow it to evade immune system detection and limit effective therapeutic options (Chen and Hambardzumyan, 2018; Stupp et al., 2005). Thus, these tumors carry a grave prognosis with median survival times of only 14–16 months following a combination of aggressive surgery, radiation therapy and chemotherapy (Dolecek et al., 2012).
Gliomas are also common in dogs, and as in humans, their risk increases with age (Hayes et al., 1975; Song et al., 2013). The median age at diagnosis is 8–9 years in canine patients, which corresponds to the median age at diagnosis in humans of 50–70 years (Stupp et al., 2005). In dogs, a significant breed predisposition has been identified in brachycephalic breeds, with Boston terriers, Boxers and Bulldogs being over-represented (Hu et al., 2015). This suggests glioma-associated genetic aberrations contribute to tumor development, some of which have previously been identified in canine and human glioma patients. An across breed genome-wide association study identified a significant locus on canine chromosome 26 containing single nucleotide variants most commonly associated with coding regions of CAMKK2, P2RX7 and DENR, thus these genes appear to influence glioma susceptibility (Truve et al., 2016). In both species, clinical presentation of affected patients may vary based on primary tumor size and location in proximity to intracranial structures. The most common clinical signs associated with canine gliomas are seizures and behavior changes, due to the fact that they arise from the forebrain in the majority of patients (Bagley et al., 1999). People often present with signs related to increased intracranial pressure, including headache and progressive neurologic deficits, with seizures occurring in 25–50% of patients (Davis, 2016).
The shared histologic and diagnostic imaging features of canine gliomas with their human counterparts facilitate comparative classification and grading of tumors using a revised classification system, and objective therapeutic response assessments using the Response Assessment in Neuro-Oncology (RANO) criteria (Young et al., 2011; Rossmeisl et al., 2014; Koehler et al., 2018). Magnetic resonance imaging (MRI) is considered the gold standard imaging technique for detection and classification of brain tumors in human and canine patients. MRI characteristics of canine gliomas have been described and share numerous features with their human counterpart (Young et al., 2011; Rodenas et al., 2011). In dogs, the majority of gliomas appear as intra-axial tumors associated with both gray and white matter located within the telencephalon or diencephalon in close contact with the lateral ventricle. In both species, most tumors appear hypointense on T1-weighted images and hyperintense on T2-weighted images (Pancotto and Rossmeisl, 2017; Gilliam et al., 2014). Common features include contrast-enhancement, ring-like contrast enhancement, intralesional cystic regions and hemorrhage (Young et al., 2011; Rodenas et al., 2011; Wisner et al., 2011; Stadler et al., 2017; Bentley et al., 2013). Similar imaging appearances have been described in humans (Pronin et al., 1997). Canine and human gliomas are frequency associated with marked edema, creating a moderate to severe mass effect. However, their infiltrative growth can limit distinction of tumor margins from surrounding normal brain parenchyma, resulting in misdiagnoses of non-neoplastic lesions. Gliomas appear to be the tumor type most commonly associated with intracranial hemorrhage in both canine and human patients (Wisner et al., 2011; Bentley et al., 2013; Pronin et al., 1997). Currently, no imaging features have been identified that reliably distinguish between glioma subtypes in neither canine nor human patients. Thus, histopathology remains the gold standard for definitively diagnosing intracranial neoplasia (Rossmeisl et al., 2015b).
Even though computed tomography (CT) is no longer considered the gold-standard diagnostic modality for diagnosing intracranial neoplasia, it is frequently used in veterinary medicine, as it is more widely available and less cost prohibitive (Stadler et al., 2017). CT continues to be used in companion animals for radiation therapy and stereotactic procedural planning, as well as image-guided neurosurgical interventions (Fig. 5) (Rossmeisl et al., 2015b; Kani et al., 2019). Similar to humans, brain biopsy is one of the most frequently performed stereotactic intracranial procedure performed in companion animals, and it has a reported diagnostic accuracy of 95 % for intracranial tumors in dogs (Rossmeisl et al., 2015b; Kani et al., 2019). Results from a study comparing mensuration and predictability of canine glioma type and grade between CT and MRI suggests both imaging modalities are equivalent for tumor mensuration. Overall accuracy of CT in predicting tumor type and grade was 46.7 % and 53.3 % respectively. Overall accuracy of MRI in predicting tumor type and grade was similar at 53.3 % and 60 % respectively (Stadler et al., 2017).
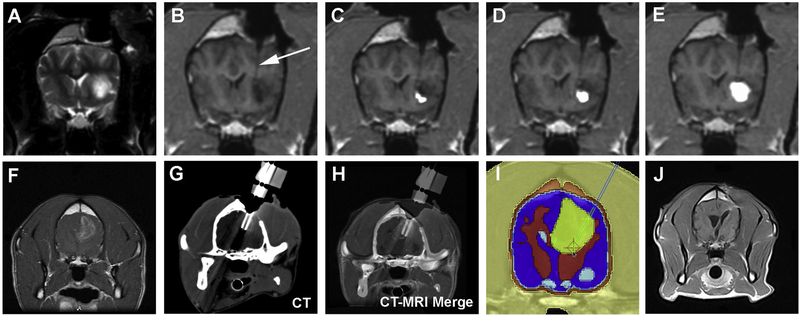
Fig. 5.
Image-guided treatments of canine malignant gliomas including convection enhanced drug delivery (CED; A–E) and irreversible electroporation (F–J) ablative treatment. Intraoperative transverse T2-weighted MRI image (A) of a canine high-grade oligodendroglioma in the frontal lobe of the cerebrum before convection enhanced delivery (CED) treatment with IL-13RA2 and EphA2 targeted bacterial cytotoxins. Sequential (B–E) 3DT1-weighted MRI intraoperative images showing intratumoral catheter placement (C, arrow), and progressive distribution of the gadolinium-labeled cytotoxins within the tumor mass during the CED infusion. Pretreatment transverse, post-contrast T1-weighted MRI image of a canine glioblastoma (F) in the right parietal lobe of the cerebrum. Following computed tomographic (CT)-guided stereotactic insertion of electrodes into the target (G, H), the tumor is ablated with irreversible electroporation according to the therapeutic plan (I). Follow-up MRI performed after treatment (J) demonstrates a significant reduction in the tumor volume.
Numerous functional and quantitative neuroimaging techniques have been employed in dogs and cats with brain tumors for several different indications (Fig. 6]). Diffusion and perfusion imaging techniques, such as dynamic contrast enhanced-CT and dynamic contrast enhanced-MRI, and proton magnetic resonance spectroscopy (MRS) have been primarily evaluated as non-invasive methods to discriminate inflammatory, neoplastic, and vascular brain lesions or to differentiate various histopathologic types or grades of brain tumors (MacLeod et al., 2009; Zhao et al., 2010; Sutherland-Smith et al., 2011; Stadler et al., 2014). The utility of perfusion imaging and MRS to discriminate tumor recurrence from reactive-treatment induced changes or necrosis are also being explored in dogs with brain tumors (Rossmeisl et al., 2014). 18F-FDG PET imaging of canine brain tumors has been performed and typically reveals areas of hypermetabolism corresponding to the presence of tumor (Fig. 6) (Kang et al., 2009). Other novel PET radiotracers designed to interrogate cellular proliferation (11C 2’-fluoro-5-methyl-1-beta-d-arabinofuranosyluracil [FMAU]) and apoptosis (18F-C-SNAT [caspase-sensitive nanoaggregation tracer]) have also been investigated in dogs with brain tumors as quantitative therapeutic response surrogates (Conti et al., 2008).
Fig. 6.
Neuro-oncological applications of quantitative and functional neuroimaging techniques in dogs. Two-dimensional diffusion tensor (DTI, A; left panel) and three-dimensional tractography (A; right panel) illustrating white matter anatomy and tracts to facilitate surgical approach corridors for surgical treatment of canine malignant glioma. Multivoxel proton magnetic resonance spectroscopy (B) in canine glioblastoma, with intratumoral region of interest (arrow) illustrating elevated choline and lactate peaks and lower N-acetylaspartate (NAA) and creatine compared to normal brain. Perfusion MRI imaging (C, D) with cerebral blood volume (CBV) map demonstrating elevated intratumoral CBV in a canine meningioma (C) and comparison of contrast-enhanced T1-weighted MRI (D; top left panel) and perfusion MRI (D; top right panel) in canine glioblastoma with superior spatial resolution of blood-brain barrier permeability distribution within same lesion evident on the perfusion imaging map. Contrast-enhanced T1-weighted MRI (E; left panels) and corresponding 18F-FDG PET-CT scans (E; right panels) of canine histiocytic sarcoma demonstrating hypermetabolic region in area of the tumor.
MRI neuronavigation and real-time intraoperative-MRI guided monitoring are also used clinically and in research settings to manage brain tumors in dogs (Fig. 5) (Wininger, 2014). These techniques may be combined with quantitative imaging sequences, such as DTI and tractography (Fig. 6), to facilitate tumor resection and minimize damage to eloquent brain regions or to confirm accurate delivery of macromolecular therapeutic payloads that require intratumoral delivery in order bypass the blood-brain-barrier.
Canine and human gliomas share the same histologic subtypes, however the overall frequency of each subtype differs between species. In dogs, 30–50% of all canine neuroepithelial tumors are classified as oligodendrogliomas, compared to only 10–15% of all human neuroepithelial tumors, however high grade oligodendrogliomas predominate in dogs (Koehler et al., 2018). The most frequent glioma subtype encountered in humans is astrocytoma, accounting for 60–70% of all neuroepithelial tumors, of which the majority are grade IV (glioblastoma) (Dolecek et al., 2012; Rossmeisl, 2017). Despite these differences, canine gliomas share a number of immunohistochemical characteristics with their human counterpart. Olig2 expression in grade II and III oligodendrogliomas has been described in both species (Herranz et al., 2016; Rhee et al., 2009). Expression of Olig2 and nestin appear highest in canine glioblastoma, which suggests the presence of neural cells increases with tumor grade (Herranz et al., 2016). Additionally, cells obtained from the center of the tumor, regardless of tumor type, have been shown to contain a higher proportion of progenitor cells compared to the periphery, suggesting these cells are capable of adapting to a hypoxic environment. Similar immunohistochemical patterns have been described for human gliomas, thus spontaneously arising canine gliomas may represent a clinically relevant model for investigation of novel therapies, in particular those specifically designed to target cancer stem cells (Herranz et al., 2016; Phillips et al., 2006).
One of the hallmarks of human and canine malignant glioma is the induction of an immunosuppressive tumor microenvironment, allowing it to evade immune system detection (Dix et al., 1999; Nduom et al., 2015). Increased intratumoral infiltration of CD4+, CD8+ and CD4+Foxp3+ T cells has been described in both species. The relative proportion of CD4+Foxp3+ regulatory T cells and plasmacytoid dendritic cells in peripheral blood appears greater in canine glioma patients compared to normal dogs. The presence of increased circulating regulatory T cells in both species suggests these cells share similar functions in glioma-induced immunosuppression (Filley et al., 2018; Berghoff et al., 2015; Kmiecik et al., 2013; El Andaloussi and Lesniak, 2006; Jacobs et al., 2009). Additionally, canine and human gliomas have been shown to express programmed death ligand 1 (PD-L1) with a greater proportion of associated T-lymphocytes expressing immune checkpoint receptors, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) (Filley et al., 2018; Berghoff et al., 2015). When bound to specific ligands expressed on the surface or either antigen presenting cells or tumor cells, these receptors inhibit T-cell immune function. Thus, activation of these immune checkpoint receptors in human gliomas via binding of PD-1 to PD-L1 further suppresses anti-tumor immune responses (Filley et al., 2018; Berghoff et al., 2015; El Andaloussi and Lesniak, 2006). The relative expression of these immune checkpoint receptors by regulatory T cells may vary between individuals of the same or different species. In one study, CTLA-4 expression by regulatory T cells was relatively low compared to the remaining lymphocyte population in normal patients, whereas regulatory T cells had relatively increased CTLA-4 expression in human glioma patients (Nduom et al., 2015; El Andaloussi and Lesniak, 2006). The introduction of immune checkpoint inhibitors has revolutionized cancer treatment by removing the brake on immune system activation and allowing immune-mediated destruction of cancer cells. Unfortunately, checkpoint inhibitors developed for human use lack utility in canine patients due to minimal cross reactivity between human and canine PD-1, PD-L1 and CTLA-4, as well as the potential for severe systemic side effects. This has led to the development of monoclonal antibodies specific to canine PD-1 and PD-L1 for further investigation into their potential efficacy in treating various canine cancers (Maekawa et al., 2017; Nemoto et al., 2018). Given the aforementioned similarities in immunologic signatures between canine and human gliomas, spontaneously arising canine glioma may provide a clinically relevant model for further investigation into these novel therapies.
Molecular and genetic profiling of brain tumors has led to the discovery of genetic mutations shared between canine and human malignant gliomas. Somatic mutations of the retinoblastoma transcriptional corepressor 1 (RB1), tumor protein p53 (TP53) and receptor tyrosine kinase/phosphoinositide 3-kinase (RTK/PI3K) signaling pathways have been identified in the majority of human gliomas. RB1 and TP53 are prototypical tumor suppressor genes that are essential for regulating cell division and preventing tumor formation. In contrast, the RTK/PI3K signaling pathway promotes cell growth and proliferation, however this pathway is regulated by activation of phosphatase and tensin homolog (PTEN). Inactivation of PTEN or activating mutations in PI3K itself have been identified in GBM and may contribute to tumorigenesis. Amplification of CDK4, a cyclin-dependent kinase associated with cell cycle progression, is a common mutation affecting the RB1 pathway in human gliomas and appears to also be upregulated in canine gliomas (Filley et al., 2018; Cancer Genome Atlas Research N, 2008). Alterations in p53 pathway signaling have been identified in human and canine gliomas, such as overexpression of the transcription factor, Olig2, which ultimately antagonizes p53 function and promotes tumor progression (Ligon et al., 2007; Mehta et al., 1995; York et al., 2012). Human gliomas frequently harbor activating mutations in epidermal growth factor receptor (EGFR) and platelet derived growth receptor alpha (PDGFRA), with PDGFRA amplification occurring in high-grade gliomas of canine patients as well (Brennan et al., 2013; Reifenberger and Collins, 2004). EGFR and PDGFR are receptor tyrosine kinases involved in the activation of cell signaling pathways that normally control cell division and survival, thus these activating mutations result in uncontrolled cell growth and likely contribute to the aggressive behavior of affected tumors.
Overexpression of numerous other proteins essential to cellular proliferation and regulation has been documented in gliomas from both species, including the following: receptor tyrosine kinases, ephrin type-A receptor 2 (EphA2), and c-Met (hepatocyte growth factor receptor), which regulate tissue homeostasis under normal conditions; the potent angiogenic factor, vascular endothelial growth factor (VEGF); alpha3-beta1 integrin, urokinase-type plasminogen activation receptor (uPAR) and matrix metallopeptidases 2 (MMP-2) and 9 (MMP-9), which are proteins that contribute to cellular migration, invasion and metastasis; insulin like growth factor binding protein 2 (IGFBP2), which is highly expressed in CNS tissue and contributes to tumor growth by prolonging the half-life and thus growth promoting effects of insulin growth factor; (IGF); and interleukin 13 receptor subunit alpha 2 (IL-13RA2), which binds IL-13 with high affinity, resulting in decreased tumor immunosurveillance and apoptosis (Verhaak et al., 2010; Boudreau et al., 2017; Dickinson et al., 2006; Pandya et al., 2012; Debinski et al., 2013). This suggests that human and canine gliomas arise from alterations of common pathways, and may therefore yield similar responses to treatment. The value of using spontaneously arising canine gliomas for investigation of novel therapies targeting these altered signaling pathways was demonstrated by a group that generated monoclonal antibodies against IL-13RA2 with cross reactivity between humans and dogs, as well as a recombinant bacterial cytotoxin to target this receptor, which is over-expressed in gliomas of both people and dogs.188 Successful inhibition of IL-13 signaling by blocking this receptor may boost anti-tumor immune responses and ultimately improve tumor control.
The utility of spontaneous canine gliomas as clinically relevant models has already been demonstrated in several pre-clinical trials investigating various therapeutic methods. A study demonstrating the safety and potential efficacy of dendritic cell vaccination in canine glioma patients with tumor cell lysates in combination with in situ adenoviral IFNg gene transfer led to its translation to a human clinical trial (Pluhar et al., 2010; Xiong et al., 2010; Castro et al., 2014). Clinical trials involving dogs with spontaneous gliomas have advanced the use of convection-enhanced delivery (CED) as a drug delivery platform for malignancies within the CNS through improved cannula design, therapeutic planning and real-time MRI monitoring (Fig. 5) (Saito and Tominaga, 2017; Dickinson et al., 2010). Irreversible electroporation (IRE) is a non-thermal ablation method that relies on short, intense, monopolar pulses to ablate tumor tissue (Rossmeisl, 2017; Garcia et al., 2012; Garcia et al., 2017; Ellis et al., 2011; Rossmeisl et al., 2013a). Transient disruption of the blood brain barrier outside the zone of ablation following IRE delivery provides an opportunity for therapeutic delivery to microscopic tumor cells extending beyond visible tumor margins, a major source of disease recurrence (Garcia et al., 2012; Hjouj et al., 2012). Safety and preliminary efficacy of IRE has been demonstrated in normal dogs and dogs with spontaneous gliomas (Fig. 5) (Rossmeisl et al., 2015a; Rossmeisl, 2017; Garcia et al., 2017; Ellis et al., 2011). These preliminary studies have led to the development of numerical treatment planning models that improve the accuracy of tumor ablation, resulting in a submillimeter line of demarcation between ablated and normal brain tissue (Garcia et al., 2017; Garcia et al., 2017; Ellis et al., 2011; Rossmeisl et al., 2013a). High-frequency irreversible electroporation (H-FIRE) represents the next generation of IRE as it is associated with fewer technical challenges than typical IRE, and appears to preferentially target malignant cells in vitro (Ivey et al., 2015; Miklovic et al., 2017). In contrast to IRE, H-FIRE uses ultra-short (1us), bipolar pulses, which minimizes nerve and muscle excitation, thus negating the need for cardiac synchronization and paralytics during treatment delivery (Arena et al., 2011; Siddiqui et al., 2016; Sano et al., 2018). Clinical trials evaluating the efficacy of H-FIRE in dogs with spontaneous brain tumors are currently ongoing, and could potentially lead to its translation into human patients (Latouche et al., 2018).
2.6.2. Meningioma
Meningiomas represent the most common subtype of primary CNS tumors in dogs, comprising about 30–45% of all primary CNS tumors (Hu et al., 2015; Snyder et al., 2006; Song et al., 2013). They are the most common benign intracranial tumor in people, but second most common subtype overall, comprising 27–33% of all primary CNS tumors (Ostrom et al., 2018; Wiemels et al., 2010). The median age at diagnosis in dogs is significantly older than other tumor types with a range of 10–11 years, which corresponds to the median age at diagnosis in humans of 65 years and older (Hu et al., 2015; Snyder et al., 2006; Song et al., 2013). Dogs > 15 kg have an increased risk of meningioma compared to dogs < 15 kg (Song et al., 2013). A breed predisposition exists with Boxers, Golden Retrievers, Miniature Schnauzers, Rat Terriers and dolichocephalic breeds in general being over-represented. The most common presenting clinical signs in canine meningioma patients are altered consciousness, seizures and vestibular dysfunction, due to compression, invasion and inflammation of adjacent CNS tissue (Snyder et al., 2006; Hicks et al., 2017; Motta et al., 2012). Likewise, the most common symptoms reported in human patients include alterations in cognitive and functioning behaviors, headaches, visual impairment, migraines and neuralgia (Tsikoudas and Martin-Hirsch, 1999). Many of the symptoms reported in humans may go undetected in canine patients, allowing these tumors to grow undetected for a longer duration.
The shared histologic and diagnostic imaging features of canine meningiomas with their human counterparts facilitate comparative classification and grading of tumors using the World Health Organization (WHO) criteria (Sturges et al., 2008; Montoliu et al., 2006). Meningiomas are extra-axial tumors located within the dura mater but outside the brain parenchyma, but direct parenchymal invasion can occur (Sturges et al., 2008; Motta et al., 2012). MRI has the greatest accuracy and sensitivity for diagnosing canine and human intracranial meningiomas based on imaging characteristics (Engelhard, 2001). Canine intracranial meningiomas typically appear as well-defined heterogenous, isointense solitary, broad-based extra-axial masses within the olfactory or frontal lobes, but may occur in other locations less commonly (Rodenas et al., 2011; Wisner et al., 2011). Homogenous contrast enhancement is noted on T1W image in approximately 60–70%, and most meningiomas are hyperintense or isointense to cortical gray matter on T2W images (Sturges et al., 2008; Wisner et al., 2011). Approximately 95 % of canine meningiomas are accompanied by edema, which improves visualization of the internal tumor margin in T2W images (Sturges et al., 2008). The dural tail sign, due to thickening of adjacent meninges, supports a diagnosis of an extraaxial lesion but is not pathognomonic for meningioma, especially in humans where it represents reactive meninges rather than tumor extension (Wisner et al., 2011; Griffin et al., 2016; Graham et al., 1998; Cherubini et al., 2005; Chen et al., 1992; Elster et al., 1989). In contrast to human intracranial meningiomas, correlations between tumor grade and any MRI features, tumor location, and pattern of edema have not been consistently demonstrated in dogs (Sturges et al., 2008; Chen et al., 1992; Elster et al., 1989). Likewise, an association between tumor subtype and these same variables appears to be lacking (Sturges et al., 2008). Thus, as with other primary brain tumors, histopathology is necessary to make a definitive diagnosis and may be obtained via CT-guided frame-based stereotactic biopsy as previously described, or following surgical removal (Kani et al., 2019).
Canine meningiomas can be divided into three major histologic groups based on the WHO classification system. The first group includes benign, slow growing tumors of various subtypes, the second group atypical tumors, and the final group anaplastic tumors (Sturges et al., 2008; Montoliu et al., 2006). According to the WHO classification system, approximately 56–63% of canine intracranial meningiomas are benign (grade I), 23–43% are atypical with invasion into the brain (grade II), and < 10 % are malignant (grade III) (Sturges et al., 2008). Regardless of grade, all canine meningiomas are capable of brain invasion, which can make surgical removal more challenging. Likewise, the incidence of atypical meningiomas in dogs is higher than their human counterparts, which may explain the difference in therapeutic response observed, as canine meningiomas tend to be more resistant to therapy (Sturges et al., 2008; Mariani et al., 2015; Suzuki et al., 1994). Canine meningiomas share many characteristic immunohistochemical patterns with their human counterpart, including those observed with vimentin and S100 labeling (Montoliu et al., 2006). In both species, high-grade meningiomas tend to display stronger levels of the glucose transporter, GLUT 1, expression. Decreased expression of the adhesion molecule, E-cadherin has been associated with more malignant meningiomas in both species (Montoliu et al., 2006; Ramos-Vara et al., 2010; Barnhart et al., 2002). Progesterone receptors, unlike estrogen receptors, are expressed by most canine meningiomas, where the number of progesterone receptors correlates inversely with tumor grade and survival (Roser et al., 2004; Baxter et al., 2014; Theon et al., 2000; Mandara et al., 2002; Adamo et al., 2003; Parker et al., 2010; Fakhrjou et al., 2012). Likewise, most human meningiomas express progesterone receptors rather than estrogen receptors, but an association between receptor expression and prognosis has not been made to date (Roser et al., 2004; Baxter et al., 2014; Theon et al., 2000; Mandara et al., 2002; Adamo et al., 2003; Parker et al., 2010; Fakhrjou et al., 2012). Cyclooxygenase-2 (COX-2) expression has been identified within canine, feline, and human meningiomas, but an association between level of expression and tumor grade and local invasion has only been identified in human meningiomas (Lee et al., 2014; Rossmeisl et al., 2009; Samarani et al., 2018). Thus, further evaluation into the role of COX-2 in tumorigenesis and the therapeutic potential of COX-2 inhibition is warranted, for which dogs and cats may serve as relevant spontaneous animal models.
Alterations in a number of cell signaling pathways are frequently shared between canine and human meningiomas. VEGF expression appears to be present in all canine meningiomas and correlates inversely with survival time (Dickinson et al., 2006; Platt et al., 2006a). Likewise, VEGF expression has been associated with tumor recurrence in humans, making it a potential therapeutic target in both species (Lee et al., 2014; Preusser et al., 2012). As in malignant gliomas, increased presence of interleukin IL13RA-2 compared to normal brain has been documented in canine and human meningiomas (Debinski et al., 2013). Thus, its potential use as a target for imaging and therapy parallels that described for malignant gliomas.
Despite the extensive number of shared characteristics between canine and human meningiomas, species-specific differences exist. The loss of a chromosome 22, secondary to mutations in the NF2 gene, has been identified in nearly 50 % of human meningiomas (Tabernero et al., 2013). NF2 expression in canine meningiomas does not appear to differ from normal canine tissues and chromosomal analysis failed to identify deletion of NF2 (Courtay-Cahen et al., 2008). CSF glutamate levels appear to be reduced in human patients with benign brain tumors in contrast to the elevated glutamate levels detected in the CSF of canine patients (el-Yazigi and Al-Mefty, 1985; Platt et al., 2006b). These distinct molecular markers may serve as molecular targets for future therapy.
Complete surgical resection is a primary mode of therapy for meningioma in canine and human patients (Sturges et al., 2008; Apra et al., 2018). The reported median survival time of canine patients following surgery alone is approximately 10.5 months (Hu et al., 2015; Motta et al., 2012; Axlund et al., 2002; Greco et al., 2006). The friable and infiltrative nature of meningiomas makes complete excision difficult in some cases, and differences in outcomes depend largely on tumor location and extent of surgery with radical complete resection yielding superior outcomes (Greco et al., 2006; Klopp and Rao, 2009; Klopp et al., 2000). In dogs, tumors located within the skullbase region or brainstem are not easily accessible, thus surgical resection is often associated with significant morbidity (Klopp et al., 2000; Niebauer et al., 1991). Hypofractionated radiation therapy and stereotactic radiosurgery offer alternative treatment options for canine meningioma with comparable median survival times of 10–17 months (Hu et al., 2015; Griffin et al., 2016; Mariani et al., 2015; Theon et al., 2000; Keyerleber et al., 2015; Bley et al., 2005). Likewise, adjuvant radiation therapy is typically reserved for atypical or anaplastic meningiomas in human patients (Apra et al., 2018). Multimodal therapy involving a combination of surgery followed by adjuvant radiation therapy appears to prolong survival times in canine patients with reported increases in median survival times to 17–30 months (Axlund et al., 2002; Keyerleber et al., 2015; Brearley et al., 1999). Chemotherapeutics used for treatment of meningioma in humans include hydroxyurea, sandostatin and IFN2b, of which the therapeutic potential for canine meningioma remains unknown (Hu et al., 2015; Moazzam et al., 2013). By comparison, palliative therapy for canine meningiomas patients has been associated with a survival time of about 2 months (Foster et al., 1988; Rossmeisl et al., 2013b).
The utility of spontaneously occurring canine meningiomas as a clinically relevant model has previously been demonstrated by a number of pre-clinical trials. A study demonstrating improvement in median survival of canine meningioma patients receiving an adjuvant autologous vaccine lysate/TLR ligands following tumor resection resulted in translation to human meningioma patients. Induction of antibody-dependent cell-mediated cytotoxicity to autologous and allogeneic tumor cells following vaccine delivery was documented in both species (Andersen et al., 2013). These findings suggested the presence of common antigens across species, which allowed for translation into human patients. Stem cells and recombinant viral vectors are currently being evaluated for their potential as gene therapy vectors in both humans and animal models (Bexell et al., 2013; Chauvet et al., 1998).
2.6.3. Secondary (metastatic) tumors
Brain metastases occur in 10–20% of human cancer patients, most commonly from primary lung tumors, followed by melanoma, renal, breast and colorectal cancers (Barnholtz-Sloan et al., 2004; Posner and Chernik, 1978). By comparison, the most common secondary intracranial neoplasia in dogs is hemangiosarcoma (29 %) followed by carcinoma (12 %) and lymphoma (12 %) (Snyder et al., 2008; Johnson et al., 2014). In both species, the majority of these tumors predominantly metastasize to the CNS via hematogenous spread, however spread along the cranial nerves has also been documented (Pekmezci and Perry, 2013). In general, metastatic tumors are typically located within the gray-white matter interface, a likely consequence of neoplastic emboli becoming lodged within the narrowed vessels present within this region (Wisner et al., 2011). Additionally, local extension of tumors originating outside of the CNS, such as nasal carcinomas, may occur via direct tumor invasion across the ethmoid turbinates and cribriform plate. Direct invasion of extracranial meningiomas via the optic foramen has been reported in canine patients (Johnson et al., 2014). Prognosis is generally poor in all patients, however newer approaches to individualized therapy have been capable of improving overall survival and quality of life (Lin and DeAngelis, 2015).
MRI characteristics of various secondary CNS tumors have been described, many of which are shared amongst human and canine patients. Metastatic hemangiosarcoma typically appears as multiple mass lesions with mixed signal intensity in T1W and T2W images. Contrast enhancement is variable but occurs most commonly along the tumor periphery. Intratumoral hemorrhage is common and peritumoral edema may be marked (Wisner et al., 2011). Likewise, features of metastatic carcinoma include multiple ill-defined mass lesions with contrast enhancement and peritumoral edema. Unlike metastatic hemangiosarcoma, intratumoral hemorrhage is not typically present in metastatic carcinoma lesions (Wisner et al., 2011). In people, CNS metastases from lung cancers and melanoma are more likely to be associated with multiple metastases compared to the solitary brain metastasis seen in breast, renal and colorectal carcinomas (Delattre et al., 1988; Nussbaum et al., 1996). Tumor associated hemorrhage in people occurs most often with metastases from melanoma and clear cell renal cell carcinoma (Wronski and Arbit, 2000; Wronski et al., 1996). Canine and human metastatic lesions share a number of macroscopic features, such as sharp demarcation with rare infiltration into the surrounding brain, and peri-tumoral edema. These similarities may help facilitate translation of novel therapies via direct comparison of therapeutic response.
The majority of CNS metastases share histologic features with the primary tumor in canine and human patients, however grade and differentiation may vary, necessitating immunohistochemistry for confirmation (Pekmezci and Perry, 2013). Additionally, differentiating primary from secondary CNS tumors in patients with only one or two lesions can be a diagnostic challenge. Various immunohistochemical markers have been used to further characterize CNS tumors and distinguish between primary and secondary tumors. Markers frequently used for identification of metastatic tumors include pancytokeratin, vimentin, CD31, melanA, S100 (melanoma, glioma) and lymphoma markers (Johnson et al., 2014). Gliomas are typically negative for these markers but positive for glial markers, such as GFAP, OLIG2 and SOX2, facilitating differentiation from metastatic tumors (Pekmezci and Perry, 2013).
Treatment of brain metastases in canine and human patients typically involves a combination of targeting the tumor itself and symptomatic therapy with steroids, antiepileptic drugs and anticoagulants. In people, the type of definitive therapy chosen is based on the number, size and location of metastatic lesions, primary tumor type and extent of systemic disease (Lin and DeAngelis, 2015). The majority of CNS metastases in humans affect the cerebrum, allowing for image-guided metastasectomy, typically via stereotactic radiosurgery, to control symptoms associated with disease and improve overall quality of life (Tan and Black, 2007; Lim et al., 2004). Likewise, the majority of metastases in dogs affect the cerebrum, but standard treatment typically involves some combination of whole brain radiation therapy, stereotactic radiosurgery and/or systemic chemotherapy, with surgical metastasectomy rarely performed (Snyder et al., 2008). Regardless, chemotherapy is the treatment of choice in patients with chemosensitive primary tumors, such as small-cell lung carcinoma in people and lymphoma in either species. Resistance to therapy is not uncommon due to acquired mutations of neoplastic cells comprising the metastases. This has been documented in human and canine patients, thus molecular and genetic profiling of the metastatic lesions could aid in identification of targets for investigation of novel therapies to delay or even prevent spread to the CNS (Campbell et al., 2010; Shah et al., 2009; Einav et al., 2017; Park et al., 2016).
2.7. Spinal cord injury
Traumatic spinal cord injury (SCI) is a significant source of morbidity, mortality, and healthcare expenditures in both humans and companion animals (World Health Organization, 2019; National Spinal Cord Injury Statistical Center, 2019; Kwon et al., 2015; Moore et al., 2016). SCI globally affects up to 500,000 people each year, and for those suffering severe and complete SCI, clinically meaningful recovery of neurological function rarely occurs (World Health Organization, 2019; National Spinal Cord Injury Statistical Center, 2019). The required care for the trauma and disabilities resulting from SCI are associated with costs of $1 M/per person in the first year after injury and up to $5 M/per person over their lifetime (National Spinal Cord Injury Statistical Center, 2019). Naturally occurring traumatic SCI is extremely prevalent within the pet dog population, with ~20,000 new cases evaluated by veterinary neurosurgeons annually (Moore et al., 2016), which is comparable to the ~18,000 new cases of human SCI seen in the United States each year.266 Thus, there exists a robust population of dogs to serve as translational models for the development of diagnostics and interventions for SCI, and an international canine SCI clinical trials consortium has been formed to facilitate this effort (Moore et al., 2017).
In dogs, naturally occurring SCI is primarily caused by intervertebral disc herniation (IVDH) secondary to degeneration of the nucleus pulposus or vertebral fracture/luxation. Both of these types of SCI result in a lesion that forms from a combination of spinal cord contusion and compression (Moore et al., 2017; Sharif-Alhoseini et al., 2017). IVDH is by far the predominant mode of SCI in dogs, and commonly affects the thoracolumbar spinal cord segments of small, chondrodystrophic breeds of dogs such as Dachshunds, French Bulldogs, Corgis, and Beagles (Olby et al., 2003). Dogs with SCI experience a full range of neurological disability that parallel those described in the human Abbreviated Injury Scale (AIS) (Maiman et al., 2018). Approximately 15 % of dogs with IVDH-SCI present with severe injuries equivalent to human AIS-A status (complete sensorimotor loss below the level of injury), and among those, 50 % will be permanently paralyzed and incontinent (Olby et al., 2003). Many owners are dedicated to the life-long care of dogs with SCI that fail to recover neurological functions, which also provides a substantial population available for study of interventions focused on chronic SCI, which has been identified as a critical need by the human medical community (Olby et al., 2003; van Middendorp et al., 2016).
Another advantage of the use of the dogs with SCI is that these cases will be clinically managed with approaches that closely parallel those used in humans. Multiple, quantitative end-point assessment instruments have been developed for canine SCI, and when implemented as a suite of tests could provide more global and clinically relevant measures of outcome. Ordinal gait scales have been validated and can statistically discriminate differences in neurological recovery in populations of dogs with SCI (Levine et al., 2009a; Olby et al., 2001). More complex computational evaluations of locomotor ability, such as kinematic and treadmill gait analyses, are surrogates that allow interrogation of the effects of an intervention on the integrity of specific neuronal circuits that contribute to limb movement (Hamilton et al., 2007). Magnetic resonance imaging (MRI) is routinely used in dogs to diagnose the etiology of SCI and plan for surgical spinal cord decompression and vertebral stabilization. As is observed in humans with SCI, the degree of intramedullary signal change observed on MRI correlates with the level of neurological impairment determined with ordinal gait scores (Levine et al., 2009b). Existing evidence demonstrates the potential utility of MRI as a prognostic biomarker, with several studies reporting that the severity and extent of T2-weighted hyperintensity within the spinal cord is negatively correlated with recovery of locomotor function (Levine et al., 2009b; Boekhoff et al., 2012). Emerging data also suggests that utilization of combinations of anatomical and quantitative MRI data, such as diffusion tensor or MTI (Fig. 7]), may improve neurobehavioral and electrophysiological outcome prediction in both humans and dogs with SCI (Griffin et al., 2013; D’Souza et al., 2017).
Fig. 7.
Anatomical and quantitative magnetic resonance imaging (MRI) of canine spinal cord injury secondary to cervical intervertebral disc herniation. Sagittal (A) and transverse (B) T2-weighted anatomical cervical spinal cord MRI demonstrating marked extradural spinal cord (SC, panel B) compression from herniated disc (arrows) between the second and third cervical vertebrae. Processing of quantitative magnetization transfer (MTI; C–E) and diffusion tensor (DTI; F–H) sequences, in which manual regions of interest delineate the spinal cord on the MT1 (C), MT0 (D), fractional mean diffusivity (MD; F) and fractional anisotropy (FA; G) and images, and the magnetization transfer ratio (MTR; E) and DTI (H) are subsequently calculated with commercial software.
The dog model of SCI is highly amenable to electrophysiological evaluations of spinal cord and urinary functions using equipment identical to that used in humans. Although not commonly used in clinical settings, spinal-evoked potentials and somatosensory evoked potentials (SSEPs) have been shown to correlate with clinical assessment of SCI severity in dogs (Shores et al., 1987; Poncelet et al., 1993). Severe SCI in dogs also leads to abnormalities of urine storage and voiding that require time intensive and chronic care (Freeman et al., 2013). Urodynamic assessments performed in dogs with SCI-induced neurogenic incontinence have revealed similar abnormalities as observed in humans including low bladder capacity and compliance, detrusor overactivity during filling, and detrusor-sphincter dyssynergia (Granger et al., 2013). Electrophysiological evaluations allow opportunity for more objective assessments of spinal cord integrity in animals models in the assessment of recovery following SCI, or the specific effects of interventions intended to improve urinary function, as was demonstrated in dogs with implanted sacral nerve root stimulators (Granger et al., 2013).
Quantitative and clinically relevant evaluations of sensory disturbances have been more challenging to develop, which is at least partly due to the difficulties with inferring neurobehavioral manifestations of pain in animal models. However mechanical sensory threshold measurements and thermal threshold testing have both been validated in dogs with SCI. Both of these techniques have been shown to discriminate healthy dogs from those with SCI, and normalization of the sensory threshold parallels locomotor recovery (Song et al., 2016; Gorney et al., 2016). The utility of quantitative sensory testing is also being investigated in dogs with spinal cord disorders associated with chronic neuropathic pain pathologies and phenotypes (Sparks et al., 2018).
There has been a long history of canine clinical trials contributing to the advancement of pharmaceuticals, biologicals, and medical devices intended for the treatment of acute and chronic SCI. A phase I trial in dogs with SCI was paramount to the translation of 4-aminopyridine to the human clinic, although late stage human trials with this agent failed to meet their primary endpoints (Blight et al., 1991; Cardenas et al., 2014). Trials in SCI have also been pivotal to the development of intravenous surfactant and oscillating electrical field therapies (Laverty et al., 2004; Borgens et al., 1999). More recently, rigorously designed and controlled canine clinical trials have been conducted to further investigate acute neuroprotective strategies and stem-cell therapeutics that showed promise in laboratory settings. A randomized, placebo-controlled clinical trial evaluated the effects of polyethylene glycol (PEG) and methylprednisolone sodium succinate (MPSS) on the locomotor outcome of dogs with complete SCI, but was terminated prematurely after interim analyses indicated that detecting a significant treatment effect would be futile (Olby et al., 2016). Another placebo-controlled trial investigated a metalloproteinase inhibitor (GM6001) in a population of dogs with acute SCI. Although significant biochemical inhibition of metalloproteinases was achieved in the treatment group, locomotor outcomes were not significantly different between dogs that received GM6001 or those which received vehicle (DMSO), indicating it was likely the vehicle which influenced recovery (Levine et al., 2014). Trials in dogs with SCI have also demonstrated the safety, feasibility, and potential utility of various types of cell-based regenerative strategies including autologous adipose derived stem cells, olfactory ensheathing cells, and bone-marrow derived mesenchymal stem cells (Granger et al., 2012; Penha et al., 2014; McMahill et al., 2015). Results of these studies have been reviewed elsewhere (McMahill et al., 2015).
3. Conclusions
Numerous naturally occurring neurological disorders that occur in companion animals, such as epilepsy, cognitive dysfunction, and brain cancer, faithfully recapitulate key clinical, neuroimaging, and pathobiological features of human disease, and remain a significant health burden to all affected species. In this review we have provided examples of successful multidisciplinary collaborations and experimental methods that illustrate the utility of companion animal models to expand our mechanistic understanding of human and animal neurological diseases, accelerate or improve the manner in which research discoveries are translated to the human clinic, and provide unique training opportunities to the next generation of neuroscientists and clinicians. We acknowledge that a single ideal model organism currently does not exist for any human neurological disease. However, integration of companion animals with spontaneous diseases into comprehensive multi-species research programs can complement and expand the aggregate knowledge obtained from studies in other animal models, and allow for the fluid and bidirectional flow of data between affected species that can ultimately benefit the health of humans and animals. There are many examples of how human healthcare discoveries have benefited veterinary medical practice. Following the introduction of the anticonvulsants fosphenytoin and levetiracetam to the human market, these drugs were rapidly assimilated into veterinary practice where they were subsequently shown to be safe and effective for the treatment of canine status epilepticus (Blades Golubovic and Rossmeisl, 2017b; Patterson et al., 2015). In addition, although the majority of translational trials in companion animals are not conducted specifically to attain approval for veterinary marketing, there is precedent that data from such studies provided proof-of-concept and impetus to conduct the trials that led to the approval of the same drugs as novel therapeutics for pets (Thamm et al., 2014; London et al., 2014).
Acknowledgements
Data used for generation of figures appearing in this manuscript are hosted and curated by the Large Animal Models Core and Central Nervous System Tissue Biorepository of the Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Blacksburg, VA, USA. The authors would like to thank Dr. Gregory B. Daniel, Dr. Richard Shinn, Dr. Krystina Stadler, Dr. Thomas Cecere and Mr. Jonathan Hinckley, Virginia-Maryland College of Veterinary Medicine, for assistance with figure preparation and neuropathological interpretations.
Funding
The Large Animal Models Core, Central Nervous System Tissue Biorepository, Dr. Partridge, Dr. Rossmeisl, and selected canine brain tumor trials referenced in the manuscript are supported by the National Institutes of Health (P30CA012197, P01CA207206, R01CA139099, and R01CA213423). The sponsor had no role in the design, preparation, or writing of the manuscript, or in the decision to submit the article for publication.
References
- Adamo PF, Cantile C, Steinberg H, 2003. Evaluation of progesterone and estrogen receptor expression in 15 meningiomas of dogs and cats. Am. J. Vet. Res 64, 1310–1318. [DOI] [PubMed] [Google Scholar]
- Adams HP Jr., et al. , 1999. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology 53, 126–131. 10.1212/WNL.53.1.126. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association Report, 2018. Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429. 10.1016/j.jalz.2018.02.001. [DOI] [Google Scholar]
- American Society for the Prevention of Cruelty to Animals Companion Animal Policy and Position Statement. https://www.aspca.org/about-us/aspca-policy-and-position-statements/definition-companion-aninal.
- Andersen BM, Pluhar GE, Seiler CE, et al. , 2013. Vaccination for invasive canine meningioma induces in situ production of antibodies capable of antibody-dependent cell-mediated cytotoxicity. Cancer Res. 73, 2987–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apra C, Peyre M, Kalamarides M, 2018. Current treatment options for meningioma. Expert Rev. Neurother 18, 241–249. [DOI] [PubMed] [Google Scholar]
- Arena CB, Sano MB, Rossmeisl JH Jr., et al. , 2011. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 10, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axlund TW, McGlasson ML, Smith AN, 2002. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002). J. Am. Vet. Med. Assoc 221, 1597–1600. [DOI] [PubMed] [Google Scholar]
- Bagley RS, Gavin PR, Moore MP, et al. , 1999. Clinical signs associated with brain tumors in dogs: 97 cases (1992–1997). J. Am. Vet. Med. Assoc 215, 818–819. [PubMed] [Google Scholar]
- Baneux PJR, et al. , 2014. Issues related to institutional animal care and use committees and clinical trials using privately owned animals. ILAR J. 55, 200–209. 10.1093/ilar/ilu005. [DOI] [PubMed] [Google Scholar]
- Barnhart KF, Wojcieszyn J, Storts RW, 2002. Immunohistochemical staining patterns of canine meningiomas and correlation with published immunophenotypes. Vet. Pathol 39, 311–321. [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. , 2004. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J. Clin. Oncol 22, 2865–2872. [DOI] [PubMed] [Google Scholar]
- Baxter DS, Orrego A, Rosenfeld JV, et al. , 2014. An audit of immunohistochemical marker patterns in meningioma. J. Clin. Neurosci 21, 421–426. [DOI] [PubMed] [Google Scholar]
- Beck M, 2001. Variable clinical presentation in lysosomal storage disorders. J. Inherit. Metab. Dis. Suppl 2, 47–51. 10.1023/A:1012463605992. [DOI] [PubMed] [Google Scholar]
- Bentley RT, Ober CP, Anderson KL, et al. , 2013. Canine intracranial gliomas: relationship between magnetic resonance imaging criteria and tumor type and grade. Vet. J 198, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M, et al. , 2007. Premature death, risk factors, and life patterns in dogs with epilepsy. J. Vet. Int. Med 21, 754–759. 10.1111/j.1939-1676.2007.tb03017.x. [DOI] [PubMed] [Google Scholar]
- Berendt M, et al. , 2015. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet. Res 11, 182 10.1186/s12917-015-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T, et al. , 1997. Purification of feline lysosomal alpha-mannosidase, determination of its cDNA sequence and identification of a mutation causing alpha-mannosidosis in Persian cats. Biochem. J 328, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS, Kiesel B, Widhalm G, et al. , 2015. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 17, 1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexell D, Svensson A, Bengzon J, 2013. Stem cell-based therapy for malignant glioma. Cancer Treat. Rev 39, 358–365. [DOI] [PubMed] [Google Scholar]
- Bhalerao DP, et al. , 2002. Detection of a genetic mutation for myotonia congenita among Miniature Schnauzers and identification of a common carrier ancestor. Am. J. Vet. Res 63, 1443–1447. [DOI] [PubMed] [Google Scholar]
- Bhatti SF, et al. , 2015. International Veterinary Epilepsy Task Force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet. Res 11, 176 10.1186/s12917-015-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blades Golubovic S, Rossmeisl JH, 2017a. Status epilepticus in dogs and cats, part 1: etiopathogenesis, epidemiology, and diagnosis. J. Vet. Emerg. Crit. Care (San Antonio) 27, 278–287. 10.1111/vec.12605. [DOI] [PubMed] [Google Scholar]
- Blades Golubovic S, Rossmeisl JH, 2017b. Status epilepticus in dogs and cats, part 2: treatment, monitoring, and prognosis. J. Vet. Emerg. Crit. Care (San Antonio) 27, 288–300. 10.1111/vec.12604. [DOI] [PubMed] [Google Scholar]
- Bley CR, Sumova A, Roos M, et al. , 2005. Irradiation of brain tumors in dogs with neurologic disease. J. Vet. Intern. Med 19, 849–854. [DOI] [PubMed] [Google Scholar]
- Blight AR, et al. , 1991. The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: a phase I clinical trial. J. Neurotrauma 8, 103–119. 10.1089/neu.1991.8.103. [DOI] [PubMed] [Google Scholar]
- Boekhoff TM, et al. , 2012. Quantitative magnetic resonance imaging characteristics: evaluation of prognostic value in the dog as a translational model for spinal cord injury. J. Spinal Disord. Technol 25, E81–E87. 10.1097/BSD.0b013e31823f2f55. [DOI] [PubMed] [Google Scholar]
- Borgens RB, et al. , 1999. An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J. Neurotrauma 16, 639–657. 10.1089/neu.1999.16.639. [DOI] [PubMed] [Google Scholar]
- Boudreau CE, York D, Higgins RJ, et al. , 2017. Molecular signalling pathways in canine gliomas. Vet. Comp. Oncol 15, 133–150. [DOI] [PubMed] [Google Scholar]
- Boulos AS, et al. , 2011. Tamoxifen as an effective neuroprotectant in an endovascular canine model of stroke. J. Neurosurg 114, 1117–1126. 10.3171/2010.8.JNS09352. [DOI] [PubMed] [Google Scholar]
- Bradbury AM, et al. , 2015. A review of gene therapy in canine and feline models of lysosomal storage disorders. Hum. Gene Ther. Clin. Dev 26, 27–37. 10.1089/humc.2015.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley MJ, Jeffery ND, Phillips SM, et al. , 1999. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival of 83 cases (1991–1996). J. Vet. Intern. Med 13, 408–412. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, et al. , 2013. The somatic genomic landscape of glioblastoma. Cell 155, 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, et al. , 2010. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N, 2008. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Nichols WS, et al. , 2007. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J. Neurooncol 85, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas DD, et al. , 2014. Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord 52, 70–76. 10.1038/sc.2013.137. [DOI] [PubMed] [Google Scholar]
- Casals JB, et al. , 2011. The use of animal models for stroke research: a review. Comp. Med 61, 305–313. [PMC free article] [PubMed] [Google Scholar]
- Castro MG, Candolfi M, Wilson TJ, et al. , 2014. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin. Biol. Ther 14, 1241–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet AE, Kesava PP, Goh CS, et al. , 1998. Selective intraarterial gene delivery into a canine meningioma. J. Neurosurg 88, 870–873. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hambardzumyan D, 2018. Immune microenvironment in glioblastoma subtypes. Front. Immunol 9, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Zee CS, Miller CA, et al. , 1992. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery 31, 1015–1021 discussion 1021–1012. [DOI] [PubMed] [Google Scholar]
- Cherubini GB, Mantis P, Martinez TA, et al. , 2005. Utility of magnetic resonance imaging for distinguishing neoplastic from non-neoplastic brain lesions in dogs and cats. Vet. Radiol. Ultrasound 46, 384–387. [DOI] [PubMed] [Google Scholar]
- Choi J, et al. , 2015. Predicting degree of myelination based on diffusion tensor imagining of canines with mucopolysaccharidosis type I. Neuroradiol. J 28, 562–573. 10.1177/1971400915609351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MM, 2015. One health, one literature: weaving together veterinary and medical research. Sci. Transl. Med 7, 303fs36 10.1126/scitranslmed.aab0215. [DOI] [PubMed] [Google Scholar]
- Connolly NP, et al. , 2018. Cross-species transcriptional analysis reveals conserved and host-specific neoplastic processes in mammalian glioma. Sci. Rep 8, 1180 10.1038/s41598-018-19451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti PS, et al. , 2008. In vivo measurement of cell proliferation in canine brain tumor using C-11-labeled FMAU and PET. Nucl. Med. Biol 35, 131–141. 10.1016/j.nucmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Courtay-Cahen C, Platt SR, De Risio L, et al. , 2008. Preliminary analysis of genomic abnormalities in canine meningiomas. Vet. Comp. Oncol 6, 182–192. [DOI] [PubMed] [Google Scholar]
- Czerwick A, et al. , 2018. Comparison of electroencephalographic findings with hippocampal magnetic resonance imaging volumetry in dogs with idiopathic epilepsy. J. Vet. Int. Med 32, 2037–2044. 10.1111/jvim.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MM, et al. , 2017. Diffusion tensor MR imaging in spinal cord injury. Injury 48, 880–884. 10.1016/j.injury.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Davis ME, 2016. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs 20, S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, et al. , 2011. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res. 96, 116–122. 10.1016/j.eplepsyres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PR, et al. , 2017. Aβ vaccination in combination with behavioral enrichment in aged beagles: effects on cognition, Aβ, and microhemorrhages. Neurobiol. Aging 49, 86–99. 10.1016/j.neurobiolaging.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, 2017. The clinicoanatomic uniqueness of the human pyramidal tract and syndrome. J. Neurol. Neuromed 2, 1–5. [Google Scholar]
- Debinski W, Dickinson P, Rossmeisl JH, et al. , 2013. New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS One 8, e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre JY, Krol G, Thaler HT, et al. , 1988. Distribution of brain metastases. Arch. Neurol 45, 741–744. [DOI] [PubMed] [Google Scholar]
- DeRisio L, et al. , 2015. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet. Res 11, 148 10.1186/s12917-015-0462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. , 2014. Dramatic improvement of parkinsonian symptoms after gluten- free diet introduction in a patient with silent celiac disease. J. Neurol 261, 443–445. 10.1007/s00415-014-7245-7. [DOI] [PubMed] [Google Scholar]
- Dickinson PJ, Roberts BN, Higgins RJ, et al. , 2006. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet. Comp. Oncol 4, 132–140. [DOI] [PubMed] [Google Scholar]
- Dickinson PJ, LeCouteur RA, Higgins RJ, et al. , 2010. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 12, 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix AR, Brooks WH, Roszman TL, et al. , 1999. Immune defects observed in patients with primary malignant brain tumors. J. Neuroimmunol 100, 216–232. [DOI] [PubMed] [Google Scholar]
- Dobson JM, Samuel S, Milstein H, et al. , 2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract 43, 240–246. [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Propp JM, Stroup NE, et al. , 2012. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. (14 Suppl 5), v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einav L, et al. , 2017. Is American pet health care (also) uniquely inefficient? Am. Econ. Rev 107, 491–495. 10.1257/aer.p20171087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekenstedt KJ, et al. , 2011. Candidate genes for idiopathic epilepsy in four dog breeds. BMC Genet. 12, 38 10.1186/1471-2156-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekenstedt KJ, et al. , 2012. Canine epilepsy genetics. Mamm. Genome 23, 28–39. 10.1007/s00335-011-9362-2. [DOI] [PubMed] [Google Scholar]
- El Andaloussi A, Lesniak MS, 2006. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 8, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TL, Garcia PA, Rossmeisl JH Jr., et al. , 2011. Nonthermal irreversible electroporation for intracranial surgical applications. Laboratory investigation. J. Neurosurg 114, 681–688. [DOI] [PubMed] [Google Scholar]
- Elster AD, Challa VR, Gilbert TH, et al. , 1989. Meningiomas: MR and histopathologic features. Radiology 170, 857–862. [DOI] [PubMed] [Google Scholar]
- el-Yazigi A, Al-Mefty O, 1985. Decreased cerebrospinal fluid glutamine levels in patients with benign brain tumors. J. Neurochem 45, 815–818. [DOI] [PubMed] [Google Scholar]
- Engelhard HH, 2001. Progress in the diagnosis and treatment of patients with meningiomas. Part I: diagnostic imaging, preoperative embolization. Surg. Neurol 55, 89–101. [DOI] [PubMed] [Google Scholar]
- Erlen A, et al. , 2018. Seizure occurrence in dogs under primary veterinary care in the UK: prevalence and risk factors. J. Vet. Int. Med 32, 1665–1676. 10.1111/jvim.15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etsey CM, et al. , 2017. A subset of dogs with presumptive idiopathic epilepsy show hippocampal asymmetry: a volumetric comparison with non-epileptic dogs using MRI. Front. Vet. Sci 4, 183 10.3389/fvets.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrjou A, Meshkini A, Shadrvan S, 2012. Status of Ki-67, estrogen and progesterone receptors in various subtypes of intracranial meningiomas. Pak. J. Biol. Sci 15, 530–535. [DOI] [PubMed] [Google Scholar]
- Fast R, et al. , 2013. PiB Fails to Map Amyloid Deposits in Cerebral Cortex of Aged Dogs with Canine Cognitive Dysfunction. Front. Aging Neurosci 5, 99 10.3389/fnagi.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley A, Henriquez M, Bhowmik T, et al. , 2018. Immunologic and gene expression profiles of spontaneous canine oligodendrogliomas. J. Neurooncol 137, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ES, Carrillo JM, Patnaik AK, 1988. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J. Vet. Intern. Med 2, 71–74. [DOI] [PubMed] [Google Scholar]
- Freeman P, et al. , 2013. Time requirement and effect on owners of home-based management of dogs with severe chronic spinal cord injury. J. Vet. Behav 8, 439–443. 10.1016/j.jveb.2013.08.004. [DOI] [Google Scholar]
- Fyfe JC, et al. , 1999. Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII. Genomics 58, 121–128. 10.1006/geno.1999.5825. [DOI] [PubMed] [Google Scholar]
- Epilepsy Collaborators GBD, 2019. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 357–375. 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovic M, et al. , 2016. Advances of molecular imaging in epilepsy. Curr. Neurol. Neurosci. Rep 16, 58 10.1007/s11910-016-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PA, Rossmeisl JH Jr., Robertson JL, et al. , 2012. 7.0-T magnetic resonance imaging characterization of acute blood-brain-barrier disruption achieved with intracranial irreversible electroporation. PLoS One 7, e50482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PA, Kos B, Rossmeisl JH Jr., et al. , 2017. Predictive therapeutic planning for irreversible electroporation treatment of spontaneous malignant glioma. Med. Phys 44, 4968–4980. [DOI] [PubMed] [Google Scholar]
- Garosi L, McConnell JF, 2005. Ischaemic stroke in dogs and humans: a comparative review. J. Sm. Anim. Pract 46, 521–529. 10.1111/j.1748-5827.2005.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Garosi L, et al. , 2005a. Results of diagnostic investigations and long-term outcome of 33 dogs with brain infarction (2000–2004). J. Vet. Int. Med 19, 725–731. 10.1111/j.1939-1676.2005.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Garosi L, et al. , 2005b. Primary orthostatic tremor in Great Danes. J. Vet. Int. Med 4, 606–609. 10.1111/j.1939-1676.2005.tb02736.x. [DOI] [PubMed] [Google Scholar]
- Garosi L, et al. , 2006. Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J. Vet. Int. Med 2, 311–321. 10.1111/j.1939-1676.2006.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Geiger KM, Klopp LS, 2009. Use of a selective serotonin reuptake inhibitor for treatment of episodes of hypertonia and kyphosis in a young adult Scottish Terrier. J. Am. Vet. Med. Assoc 235, 168–171. 10.2460/javma.235.2.168. [DOI] [PubMed] [Google Scholar]
- Gill LJ, et al. , 2011. Startle disease in Irish wolfhounds associated with a microdeletion in the glycine transporter GlyT2 gene. Neurobiol. Dis 43, 184–189. 10.1016/j.nbd.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JL, et al. , 2012. A canine BCAN microdeletion associated with episodic falling syndrome. Neurobiol. Dis 45, 130–136. 10.1016/j.nbd.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam D, et al. , 2014. A homozygous KCNJ10 mutation in Jack Russell Terriers and related breeds with spinocerebellar ataxia with myokymia, seizures, or both. J. Vet. Int. Med 28, 871–877. 10.1111/jvim.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam D, et al. , 2015. Golden Retriever dogs with neuronal ceroid lipofuscinosis have a two-base-pair deletion and frameshift in CLN5. Mol. Genet. Metab 115, 101–109. 10.1016/j.ymgme.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Gorney AM, et al. , 2016. Mechanical and thermal sensory testing in normal chondrodystrophoid dogs and dogs with spinal cord injury caused by thoracolumbar intervertebral disc herniations. J. Vet. Intern. Med 30, 627–635. 10.1111/jvim.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JP, Newell SM, Voges AK, et al. , 1998. The dural tail sign in the diagnosis of meningiomas. Vet. Radiol. Ultrasound 39, 297–302. [DOI] [PubMed] [Google Scholar]
- Granger N, et al. , 2012. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain 135, 3227–3237. 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger N, et al. , 2013. Use of an implanted sacral nerve stimulator to restore urine voiding in chronically paraplegic dogs. J. Vet. Intern. Med 27, 99–105. 10.1111/jvim.12011. [DOI] [PubMed] [Google Scholar]
- Greco JJ, Aiken SA, Berg JM, et al. , 2006. Evaluation of intracranial meningioma resection with a surgical aspirator in dogs: 17 cases (1996–2004). J. Am. Vet. Med. Assoc 229, 394–400. [DOI] [PubMed] [Google Scholar]
- Gredal H, et al. , 2017. Interleukin-6 is increased in the plasma and cerebrospinal fluid of community-dwelling domestic dogs with acute ischaemic stroke. NeuroReport 28, 134–140. 10.1097/WNR.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JF 4th, et al. , 2013. Thoracic and lumbar spinal cord diffusion tensor imaging in dogs. J. Magn. Reson. Imaging 37, 632–641. 10.1002/jmri.23862. [DOI] [PubMed] [Google Scholar]
- Griffin LR, Nolan MW, Selmic LE, et al. , 2016. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet. Comp. Oncol 14, e158–e170. [DOI] [PubMed] [Google Scholar]
- Grone BP, Baraban SC, 2015. Animal models in epilepsy research: legacies and new directions. Nat. Neurosci 18, 339–343. 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- Gurda BL, et al. , 2017. Canine and feline models of human genetic diseases and their contributions to advancing clinical therapies. Yale J. Biol. Med 90, 417–431. [PMC free article] [PubMed] [Google Scholar]
- Hadjivassiliou M, et al. , 2003. Dietary treatment of gluten ataxia. J. Neurol. Neurosurg. Psychiatry 74, 1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L, et al. , 2007. Development of a universal measure of quadrupedal forelimbhindlimb coordination using digital motion capture and computerised analysis. BMC Neurosci. 8, 77 10.1186/1471-2202-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, et al. , 2017. Diffusion-weighted imaging of the brains of dogs with idiopathic epilepsy. BMC Vet. Res 13, 338 10.1186/s12917-017-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, et al. , 2018. Quantitative analysis of brain perfusion parameters in dogs with idiopathic epilepsy by use of magnetic resonance imaging. Am. J. Vet. Res 79, 433–442. 10.2460/ajvr.79.4.433. [DOI] [PubMed] [Google Scholar]
- Hayes HM, Priester WA Jr., Pendergrass TW, 1975. Occurrence of nervous-tissue tumors in cattle, horses, cats and dogs. Int. J. Cancer 15, 39–47. [DOI] [PubMed] [Google Scholar]
- He X, et al. , 1999. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol. Genet. Metab 67, 106–112. 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- Head E, et al. , 2005. Beta- amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol. Aging 26, 749–763. 10.1016/j.neurobiolaging.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hebert LE, et al. , 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 Census. Neurology 80, 1778–1783. 10.1212/WNL.0b013e31828726f5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz C, Fernandez F, Martin-Ibanez R, et al. , 2016. Spontaneously arising canine glioma as a potential model for human glioma. J. Comp. Pathol 154, 169–179. [DOI] [PubMed] [Google Scholar]
- Hicks J, Platt S, Kent M, et al. , 2017. Canine brain tumours: a model for the human disease? Vet. Comp. Oncol 15, 252–272. [DOI] [PubMed] [Google Scholar]
- Hjouj M, Last D, Guez D, et al. , 2012. MRI study on reversible and irreversible electroporation induced blood brain barrier disruption. PLoS One 7, e42817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Barker A, Harcourt-Brown T, et al. , 2015. Systematic review of brain tumor treatment in dogs. J. Vet. Intern. Med 29, 1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey JW, Latouche EL, Sano MB, et al. , 2015. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep 5, 17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JF, Idema AJ, Bol KF, et al. , 2009. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 11, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James FMK, et al. , 2017. Diagnostic utility of wireless video- electroencephalography in unsedated dogs. Vet. Intern. Med 31, 1469–1476. 10.1111/jvim.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell PA, 1952. The anastomoses between internal and external carotid circulations in the dog. J. Anat 86, 83–94. [PMC free article] [PubMed] [Google Scholar]
- Johnson GC, Coates JR, Wininger F, 2014. Diagnostic immunohistochemistry of canine and feline intracalvarial tumors in the age of brain biopsies. Vet. Pathol 51, 146–160. [DOI] [PubMed] [Google Scholar]
- Jokinen TS, et al. , 2014. FDG-PET in healthy and epileptic Lagotto Romagnolo dogs and changes in brain glucose uptake with age. Vet. Radiol. Ultrasound 55, 331–341. 10.1111/vru.12129. [DOI] [PubMed] [Google Scholar]
- Jovin TG, et al. , 2016. Stroke treatment academic industry roundtable the next generation of endovascular trials. Stroke 47, 2656–2665. 10.1161/STROKEAHA.116.013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkis E, et al. , 2004. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab 83, 163–174. 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Kang BT, et al. , 2009. 18F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of primary intracranial histiocytic sarcoma in a dog. J. Vet. Med. Sci 71, 1397–1401. 10.1292/jvms.001397. [DOI] [PubMed] [Google Scholar]
- Kani Y, et al. , 2019. Diagnostic accuracy of stereotactic brain biopsy for intracranial neoplasia in dogs: comparison of biopsy, surgical resection, and necropsy specimens. J. Vet. Int. Med 33, 1384–1391. 10.1111/jvim.15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsley-Fleet L, et al. , 2013. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec 172, 338 10.1136/vr.101133. [DOI] [PubMed] [Google Scholar]
- Keyerleber MA, McEntee MC, Farrelly J, et al. , 2015. Three-dimensional conformal radiation therapy alone or in combination with surgery for treatment of canine intracranial meningiomas. Vet. Comp. Oncol 13, 385–397. [DOI] [PubMed] [Google Scholar]
- Klopp LS, Rao S, 2009. Endoscopic-assisted intracranial tumor removal in dogs and cats: long-term outcome of 39 cases. J. Vet. Intern. Med 23, 108–115. [DOI] [PubMed] [Google Scholar]
- Klopp LS, Simpson ST, Sorjonen DA, et al. , 2000. Ventral surgical approach to the caudal brain stem in dogs. Vet. Surg 29, 533–542. [DOI] [PubMed] [Google Scholar]
- Kmiecik J, Poli A, Brons NH, et al. , 2013. Elevated CD3+ and CD8+ tumor-in-filtrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol 264, 71–83. [DOI] [PubMed] [Google Scholar]
- Koehler JW, Miller AD, Miller CR, et al. , 2018. A revised diagnostic classification of canine glioma: towards validation of the canine glioma patient as a naturally occurring preclinical model for human glioma. J. Neuropathol. Exp. Neurol 77, 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolicheski AL, et al. , 2017. A homozygous PIGN missense mutation in Soft-Coated Wheaten Terriers with a canine paroxysmal dyskinesia. Neurogenetics 18, 39–47. 10.1007/s10048-016-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondagari GS, et al. , 2015. The effects of intracisternal enzyme replacement versus sham treatment on central neuropathology in preclinical canine fucosidosis. Orphanet J. Rare Dis 10, 143 10.1186/s13023-015-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, 1929. The progress of physiology. Science 70, 200–204. [DOI] [PubMed] [Google Scholar]
- Kuge Y, et al. , 2000. [1–11C]Octanoate as a PET tracer for studying ischemic stroke: evaluation in a canine model of thromboembolic stroke with positron emission tomography. Biol. Pharm. Bull 23, 984–988. 10.1248/bpb.23.984. [DOI] [PubMed] [Google Scholar]
- Kumar M, et al. , 2016. Diffusion tensor imaging for assessing brain gray and white matter abnormalities in a feline model of α-mannosidosis. J. Neuropathol. Exp. Neurol 75, 35–43. 10.1093/jnen/nlv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, et al. , 2015. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol 269, 154–168. 10.1016/j.expneurol.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Latouche EL, et al. , 2018. High-frequency irreversible electroporation for intracranial meningioma: a feasibility study in a spontaneous canine tumor model. Technol. Canc. Res. Treat 17, 1533033818785285 10.1177/1533033818785285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty PH, et al. , 2004. A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. J. Neurotrauma 21, 1767–1777. 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- LeBlanc AK, et al. , 2016. Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro. Oncol 18, 1209–1218. 10.1093/neuonc/now051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee YS, Hong YG, et al. , 2014. Significance of COX-2 and VEGF expression in histopathologic grading and invasiveness of meningiomas. APMIS 122, 16–24. [DOI] [PubMed] [Google Scholar]
- Levine GJ, et al. , 2009a. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med 89, 121–127. 10.1016/j.prevetmed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Levine JM, et al. , 2009b. Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J. Vet. Intern. Med 23, 1220–1226. 10.1111/j.1939-1676.2009.0393.x. [DOI] [PubMed] [Google Scholar]
- Levine JM, et al. , 2014. Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One 9, e96408 10.1371/journal.pone.0096408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. , 2018. Quantitative DTI metrics in a canine model of Krabbe disease: comparisons versus age-matched controls across multiple ages. Neuroradiol. J 31, 168–176. 10.1177/1971400917733431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht BG, et al. , 2002. Clinical presentations of naturally occurring canine seizures: similarities to human seizures. Epilepsy Behav. 3, 460–470. 10.1016/S1525-5050(02)00523-1. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, et al. , 2007. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron 53, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LC, Rosenthal MA, Maartens N, et al. , 2004. Management of brain metastases. Intern. Med. J 34, 270–278. [DOI] [PubMed] [Google Scholar]
- Lin JH, 2008. Applications and limitations of genetically modified mouse models in drug discovery and development. Curr. Drug Metab 9, 419–438. [DOI] [PubMed] [Google Scholar]
- Lin X, DeAngelis LM, 2015. Treatment of brain metastases. J. Clin. Oncol 33, 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz D, Higgins RJ, Kortz GD, et al. , 2003. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet. Pathol 40, 659–669. [DOI] [PubMed] [Google Scholar]
- London CA, et al. , 2014. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoS One 9 (2), e87585 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie M, et al. , 2018. Characterization of paroxysmal gluten-sensitive dyskinesia in Border Terriers using serological markers. J. Vet. Intern. Med 32, 775–781. 10.1111/jvim.15038(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, et al. , 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AG, Dickinson PJ, LeCouteur RA, et al. , 2009. Quantitative assessment of blood volume and permeability in cerebral mass lesions using dynamic contrast-enhanced computed tomography in the dog. Acad. Radiol 16 (10), 1187–1195. [DOI] [PubMed] [Google Scholar]
- Maekawa N, Konnai S, Takagi S, et al. , 2017. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep 7, 8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnitsky S, et al. , 2010. Magnetic resonance spectroscopy of the occipital cortex and the cerebellar vermis distinguishes individual cats affected with alpha-mannosidosis from normal cats. NMR Biomed. 23, 74–79. 10.1002/nbm.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiman D, et al. , 2018. AIS scores in spine and spinal cord trauma: epidemiological considerations. Traffic Inj. Prev 19, S169–S173. 10.1080/15389588.2017.1410144. [DOI] [PubMed] [Google Scholar]
- Mandara MT, Ricci G, Rinaldi L, et al. , 2002. Immunohistochemical identification and image analysis quantification of oestrogen and progesterone receptors in canine and feline meningioma. J. Comp. Pathol 127, 214–218. [DOI] [PubMed] [Google Scholar]
- Mariani CL, 2015. Terminology and classification of seizures and epilepsy in veterinary patients. Top. Comp. Anim. Med 28, 34–41. 10.1053/j.tcam.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Mariani CL, Schubert TA, House RA, et al. , 2015. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet. Comp. Oncol 13, 409–423. [DOI] [PubMed] [Google Scholar]
- Masalachi M, et al. , 2012. Movement disorders: role of imaging in diagnosis. J. Magn. Reson. Imaging 35, 239–256. 10.1002/jmri.22825. [DOI] [PubMed] [Google Scholar]
- Matiasek K, et al. , 2015. International veterinary epilepsy task force recommendations for systematic sampling and processing of brains from epileptic dogs and cats. BMC Vet. Res 11, 216 10.1186/s12917-015-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahill BG, et al. , 2015. Stem cells in canine spinal cord injury- promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev. 11, 180–193. 10.1007/s12015-014-9553-9. [DOI] [PubMed] [Google Scholar]
- Mehta RC, Pike GB, Haros SP, et al. , 1995. Central nervous system tumor, infection, and infarction: detection with gadolinium-enhanced magnetization transfer MR imaging. Radiology 195, 41–46. [DOI] [PubMed] [Google Scholar]
- Mena H, et al. , 2004. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 108, 524–530. 10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Menon KP, et al. , 1992. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics 14, 763–768. 10.1016/S0888-7543(05)80182-X. [DOI] [PubMed] [Google Scholar]
- Middleton DM, et al. , 2018. Diffusion tensor imaging findings suggestive of white matter alterations in a canine model of mucoploysaccharidosis type I. Neuroradiol. J 31, 90–94. 10.1177/1971400917715792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklovic T, Latouche EL, DeWitt MR, et al. , 2017. A comprehensive characterization of parameters affecting high-frequency irreversible electroporation lesions. Ann. Biomed. Eng 45, 2524–2534. [DOI] [PubMed] [Google Scholar]
- Moazzam AA, Wagle N, Zada G, 2013. Recent developments in chemotherapy for meningiomas: a review. Neurosurg. Focus 35, E18. [DOI] [PubMed] [Google Scholar]
- Moffat KS, Landsberg GM, 2003. An investigation of the prevalence of clinical signs of cognitive dysfunction syndrome (CDS) in cats. J. Am. Anim. Hosp. Assoc 39 (5), 512.abstract. [Google Scholar]
- Montoliu P, Anor S, Vidal E, et al. , 2006. Histological and immunohistochemical study of 30 cases of canine meningioma. J. Comp. Pathol 135, 200–207. [DOI] [PubMed] [Google Scholar]
- Moore SA, et al. , 2016. Practice patterns in the management of acute intervertebral disc herniation in dogs. J. Small Anim. Pract 57, 409–415. 10.1111/jsap.12496. [DOI] [PubMed] [Google Scholar]
- Moore SA, et al. , 2017. Targeting translational successes through CANSORT-SCI: Using pet dogs to identify effective treatments for spinal cord injury. J. Neurotrauma 34, 2007–2018. 10.1089/neu.2016.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta L, Mandara MT, Skerritt GC, 2012. Canine and feline intracranial meningiomas: an updated review. Vet. J 192, 153–165. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center, 2019. Spinal Cord Injury (SCI) Facts and Figures at a Glance. https://www.nscisc.uab.edu.
- Nduom EK, Weller M, Heimberger AB, 2015. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 17 (Suppl 7), vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson JC, et al. , 2001. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc 218, 1787–1791. 10.2460/javma.2001.218.1787. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Shosu K, Okuda M, et al. , 2018. Development and characterization of monoclonal antibodies against canine PD-1 and PD-L1. Vet. Immunol. Immunopathol 198, 19–25. [DOI] [PubMed] [Google Scholar]
- Niebauer GW, Dayrell-Hart BL, Speciale J, 1991. Evaluation of craniotomy in dogs and cats. J. Am. Vet. Med. Assoc 198, 89–95. [PubMed] [Google Scholar]
- Nussbaum ES, Djalilian HR, Cho KH, et al. , 1996. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78, 1781–1788. [PubMed] [Google Scholar]
- Olby NJ, et al. , 2001. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res 62, 1624–1628. [DOI] [PubMed] [Google Scholar]
- Olby NJ, et al. , 2003. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J. Am. Vet. Med. Assoc 222, 762–769. 10.2460/javma.2003.222.762. [DOI] [PubMed] [Google Scholar]
- Olby NJ, et al. , 2016. A placebo-controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J. Vet. Intern. Med 30, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Truitt G, et al. , 2018. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 20, iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RMA, et al. , 2018a. Cognitive dysfunction in naturally occurring canine idiopathic epilepsy. PLoS One 13, e0192182 10.1371/journal.pone.0192182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RA, et al. , 2018b. Consensus recommendations on standardized magnetic resonance imaging protocols for multicenter canine brain tumor clinical trials. Vet. Radiol. Ultrasound 59, 261–271. 10.1111/vru.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakozdy A, et al. , 2014. Suspected limbic encephalitis and seizure in cats associated with voltage‐gated potassium channel (VGKC) complex antibody. J. Vet. Int. Med 27, 212–214. 10.1111/jvim.12026. [DOI] [PubMed] [Google Scholar]
- Pan Y, et al. , 2010. Dietary supplementation with medium- chain TAG has long- lasting cognition- enhancing effects in aged dogs. Br. J. Nutr 103, 1746–1754. 10.1017/S0007114510000097. [DOI] [PubMed] [Google Scholar]
- Pancotto TE, Rossmeisl JH, 2017. A case of stiff dog syndrome associated with anti-glutamic acid decarboxylase antibodies. J. Clin. Movement Dis 4, 5 10.1186/s40734-017-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya H, Gibo DM, Garg S, et al. , 2012. An interleukin 13 receptor alpha 2-specific peptide homes to human Glioblastoma multiforme xenografts. Neuro Oncol. 14, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Withers SS, Modiano JF, et al. , 2016. Canine cancer immunotherapy studies: linking mouse and human. J. Immunother. Cancer 4, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HG, et al. , 2010. Man’s best friend becomes biology’s best in show: genome analyses in the domestic dog. Annu. Rev. Genet 44, 309–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson EE, et al. , 2015. Canine status epilepticus treated with fosphenytoin: a proof of principle study. Epilepsia 56, 882–887. 10.1111/epi.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekmezci M, Perry A, 2013. Neuropathology of brain metastases. Surg. Neurol. Int 4, S245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penha EM, et al. , 2014. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014, 437521 10.1155/2014/437521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pet industry market size & ownership statistics, American Pet Products Association, 2019. Pet Industry Market Size & Ownership Statistics. https://americanpetproducts.org/press_industrytrends.asp.
- Peus D, Newcomb N, Hofer S, 2013. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med. Inform. Decis. Mak 13, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, et al. , 2006. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173. [DOI] [PubMed] [Google Scholar]
- Platt SR, Radaelli ST, McDonnell JJ, 2001. The prognostic value of the modified Glasgow Coma Scale in head trauma in dogs. J. Vet. Intern. Med 15, 581–584. [DOI] [PubMed] [Google Scholar]
- Platt SR, Scase TJ, Adams V, et al. , 2006a. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J. Vet. Intern. Med 20, 663–668. [DOI] [PubMed] [Google Scholar]
- Platt SR, Marlin D, Smith N, et al. , 2006b. Increased cerebrospinal fluid uric acid concentrations in dogs with intracranial meningioma. Vet. Rec 158, 830. [DOI] [PubMed] [Google Scholar]
- Pluhar GE, Grogan PT, Seiler C, et al. , 2010. Anti-tumor immune response correlates with neurological symptoms in a dog with spontaneous astrocytoma treated by gene and vaccine therapy. Vaccine 28, 3371–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podell M, 2004. Tremor, fasciculations, and movement disorders. Vet. Clin. North Am. Small Anim. Pract 34, 1435–1452. 10.1016/j.cvsm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Poncelet L, et al. , 1993. Somatosensory potentials in dogs with naturally acquired thoracolumbar spinal cord disease. Am. J. Vet. Res 54, 1935–1941. [PubMed] [Google Scholar]
- Porter KR, McCarthy BJ, Freels S, et al. , 2010. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 12, 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner JB, Chernik NL, 1978. Intracranial metastases from systemic cancer. Adv. Neurol 19, 579–592. [PubMed] [Google Scholar]
- Potschka H, et al. , 2013. Canine epilepsy as a translational model? Epilepsia 54, 571–579. 10.1111/epi.12138. [DOI] [PubMed] [Google Scholar]
- Potschka H, et al. , 2015. International veterinary epilepsy task force consensus proposal: outcome of therapeutic interventions in canine and feline epilepsy. BMC Vet. Res 11, 177 10.1186/s12917-015-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser M, Hassler M, Birner P, et al. , 2012. Microvascularization and expression of VEGF and its receptors in recurring meningiomas: pathobiological data in favor of anti-angiogenic therapy approaches. Clin. Neuropathol 31, 352–360. [DOI] [PubMed] [Google Scholar]
- Pronin IN, Holodny AI, Petraikin AV, 1997. MRI of high-grade glial tumors: correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology 39, 348–350. [DOI] [PubMed] [Google Scholar]
- Quitt PR, et al. , 2018. Myotonia congenita in a Labrador Retriever with truncated CLCN1. Neuromuscul. Disord 28, 597–605. 10.1016/j.nmd.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Miller MA, Gilbreath E, et al. , 2010. Immunohistochemical detection of CD34, E-cadherin, claudin-1, glucose transporter 1, laminin, and protein gene product 9.5 in 28 canine and 8 feline meningiomas. Vet. Pathol 47, 725–737. [DOI] [PubMed] [Google Scholar]
- Ray J, et al. , 1998. Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics 48, 248–253. 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Collins VP, 2004. Pathology and molecular genetics of astrocytic gliomas. J. Mol. Med. (Berl) 82, 656–670. [DOI] [PubMed] [Google Scholar]
- Rhee W, Ray S, Yokoo H, et al. , 2009. Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia 57, 510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, et al. , 2015. Dystonia and paroxysmal dyskinesias: under- recognized movement disorders in domestic animals? A comparison with human dystonia/paroxysmal dyskinesias. Front. Vet. Sci 2, 65 10.3389/fvets.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas S, Pumarola M, Gaitero L, et al. , 2011. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet. J 187, 85–91. [DOI] [PubMed] [Google Scholar]
- Roser F, Nakamura M, Bellinzona M, et al. , 2004. The prognostic value of progesterone receptor status in meningiomas. J. Clin. Pathol 57, 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmeisl J, 2017. Maximizing local access to therapeutic deliveries in glioblastoma. Part v: clinically relevant model for testing new therapeutic approaches. In: De Vleeschouwer S (Ed.), Glioblastoma, Brisbane (AU). [PubMed] [Google Scholar]
- Rossmeisl JHJ, et al. , 2007. Presumed and confirmed striatocapsular brain infarctions in six dogs. Vet. Ophthalmol 10, 23–36. 10.1111/j.1463-5224.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Rossmeisl JH Jr., Robertson JL, Zimmerman KL, et al. , 2009. Cyclooxygenase-2 (COX-2) expression in canine intracranial meningiomas. Vet. Comp. Oncol 7, 173–180. [DOI] [PubMed] [Google Scholar]
- Rossmeisl JH Jr., Garcia PA, Roberston JL, et al. , 2013a. Pathology of non-thermal irreversible electroporation (N-TIRE)-induced ablation of the canine brain. J. Vet. Sci 14, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmeisl JH Jr., Jones JC, Zimmerman KL, et al. , 2013b. Survival time following hospital discharge in dogs with palliatively treated primary brain tumors. J. Am. Vet. Med. Assoc 242, 193–198. [DOI] [PubMed] [Google Scholar]
- Rossmeisl JH, et al. , 2014. Invited review-neuroimaging response assessment criteria for brain tumors in veterinary patients. Vet. Radiol. Ultrasound 55, 115–132. 10.1111/vru.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmeisl JH Jr., Garcia PA, Pancotto TE, et al. , 2015a. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J. Neurosurg 123, 1008–1025. [DOI] [PubMed] [Google Scholar]
- Rossmeisl JH, et al. , 2015b. Frame-based stereotactic biopsy of canine brain masses: technique and clinical results in 26 cases. Front. Vet. Sci 2, 20.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Tominaga T, 2017. Convection-enhanced delivery of therapeutics for malignant gliomas. Neurol. Med. Chir. (Tokyo) 57, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarani F, et al. , 2018. Immunohistochemical expression of cyclooxygenase-2 (COX-2) is not associated with tumor grade in feline meningiomas. Vet. J 241, 20–23. [DOI] [PubMed] [Google Scholar]
- Samsonov A, et al. , 2012. Quantitative MR imaging of two-pool magnetization transfer model parameters in myelin mutant shaking pup. Neuroimage. 62, 1390–1398. 10.1016/j.neuroimage.2012.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DN, et al. , 2013. GM2 gangliosidosis associated with a HEXA missense mutation in Japanese Chin dogs: a potential model for Tay Sachs disease. Mol. Genet. Metab 108, 70–75. 10.1016/j.ymgme.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, Chester C, Melero I, et al. , 2016. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann. Oncol 27, 1190–1198. [DOI] [PubMed] [Google Scholar]
- Sano MB, Fan RE, Cheng K, et al. , 2018. Reduction of muscle contractions during irreversible electroporation therapy using high-frequency bursts of alternating polarity pulses: a laboratory investigation in an ex vivo swine model. J. Vasc. Interv. Radiol 29 893–898 e894. [DOI] [PubMed] [Google Scholar]
- Sarikaya I, 2015. PET studies in epilepsy. Am J. Nuc. Med. Mol. Imaging 5, 416–430. [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, et al. , 2015. Detection and quantification of β-amyloid, pyroglutamil Aβ, and tau in aged canines. J. Neuropathol. Exp. Neurol 74, 912–923. 10.1097/NEN.0000000000000230. [DOI] [PubMed] [Google Scholar]
- Seppala EH, et al. , 2011. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genet. 7, e1002194 10.1371/journal.pgen.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala EH, et al. , 2013. A nonsense mutation in the acid α-glucosidase gene causes Pompe disease in Finnish and Swedish Lapphunds. PLoS One 8, e56825 10.1371/journal.pone.0056825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Morin RD, Khattra J, et al. , 2009. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461, 809–813. [DOI] [PubMed] [Google Scholar]
- Sharif-Alhoseini M, et al. , 2017. Animal models of spinal cord injury: a systematic review. Spinal Cord 55, 714–721. 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA, 2006. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discov 5, 741–754. [DOI] [PubMed] [Google Scholar]
- Shearin AL, Ostrander EA, 2010. Leading the way: canine models of genomics and disease. Dis. Model. Mech 3, 27–34. 10.1242/dmm.004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores A, et al. , 1987. Spinal-evoked potentials in dogs with acute compressive thoracolumbar spinal cord disease. Am. J. Vet. Res 48, 1525–1530. [PubMed] [Google Scholar]
- Siddiqui IA, Latouche EL, DeWitt MR, et al. , 2016. Induction of rapid, reproducible hepatic ablations using next-generation, high frequency irreversible electroporation (H-FIRE) in vivo. HPB (Oxford) 18, 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly BJ, Franklin RJM, 2002. Recognition and diagnosis of lysosomal storage diseases in the cat and dog. J. Vet. Int. Med 16, 133–141. 10.1111/j.1939-1676.2002.tb02344.x. [DOI] [PubMed] [Google Scholar]
- Skelly BJ, et al. , 1996. The molecular defect underlying canine fucosidosis. J. Med. Genet 33, 284–288. 10.1136/jmg.33.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JM, Shofer FS, Van Winkle TJ, et al. , 2006. Canine intracranial primary neoplasia: 173 cases (1986–2003). J. Vet. Intern. Med 20, 669–675. [DOI] [PubMed] [Google Scholar]
- Snyder JM, Lipitz L, Skorupski KA, et al. , 2008. Secondary intracranial neoplasia in the dog: 177 cases (1986–2003). J. Vet. Intern. Med 22, 172–177. [DOI] [PubMed] [Google Scholar]
- Somers KL, et al. , 2003. Mutation analysis of feline Niemann-Pick C1 disease. Mol. Genet. Metab 79, 99–103. 10.1016/S1096-7192(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Song RB, Vite CH, Bradley CW, et al. , 2013. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J. Vet. Intern. Med 27, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Song RB, et al. , 2016. Von Frey anesthesiometry to assess sensory impairment after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. Vet. J 209, 144–149. 10.1016/j.tvjl.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CR, et al. , 2018. Investigation of sensory thresholds in Cavalier King Charles Spaniels with and without Chiari-like malformations and syringomyelia. J. Vet. Intern. Med 32, 2021–2028. 10.1111/jvim.15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler KL, et al. , 2014. Multivoxel proton magnetic resonance spectroscopy of inflammatory and neoplastic lesions of the canine brain at 3.0 T. Am. J. Vet. Res 75, 982–989. 10.2460/ajvr.75.11.982. [DOI] [PubMed] [Google Scholar]
- Stadler KL, Ruth JD, Pancotto TE, et al. , 2017. Computed tomography and magnetic resonance imaging are equivalent in Mensuration and similarly inaccurate in grade and type predictability of canine intracranial gliomas. Front. Vet. Sci 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey MP, et al. , 2005. Dogs really are man’s best friend – canine genomics has applications in veterinary and human medicine!. Brief Funct. Genom. Proteom 4, 112–128. 10.1093/bfgp/4.2.112. [DOI] [PubMed] [Google Scholar]
- Stoica G, Kim HT, Hall DG, et al. , 2004. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet. Pathol 41, 10–19. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, et al. , 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Sturges BK, Dickinson PJ, Bollen AW, et al. , 2008. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J. Vet. Intern. Med 22, 586–595. [DOI] [PubMed] [Google Scholar]
- Sun A, et al. , 2018. Lysosomal storage disease overview. Ann. Trans. Med 6, 476 10.21037/atm.2018.11.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland-Smith J, et al. , 2011. Magnetic resonance imaging apparent diffusion coefficients for histologically confirmed intracranial lesions in dogs. Vet. Radiol. Ultrasound 52, 142–148. 10.1111/j.1740-8261.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Sugimoto T, Shibuya M, et al. , 1994. Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochir. (Wien) 129, 39–46. [DOI] [PubMed] [Google Scholar]
- Symon L, 1960. Observations on the leptomeningeal collateral circulation in dogs. J. Physiol 154, 1–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero M, Jara-Acevedo M, Nieto AB, et al. , 2013. Association between mutation of the NF2 gene and monosomy 22 in menopausal women with sporadic meningiomas. BMC Med. Genet 14, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Singh RK, Fidler IJ, et al. , 2007. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol 170, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Black PM, 2007. Image-guided craniotomy for cerebral metastases: techniques and outcomes. Neurosurgery 61, 349–356 discussion 356–347. [DOI] [PubMed] [Google Scholar]
- Taylor RM, et al. , 1992. Amelioration of clinical disease following bone marrow transplantation in fucosidase-deficient dogs. Am. J. Med. Genet 42, 628–632. 10.1002/ajmg.1320420439. [DOI] [PubMed] [Google Scholar]
- Thamm DH, et al. , 2014. GS-9219/VDC-1101–a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet Res Jan 25 (10), 30 10.1186/1746-6148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theon AP, Lecouteur RA, Carr EA, et al. , 2000. Influence of tumor cell proliferation and sex-hormone receptors on effectiveness of radiation therapy for dogs with incompletely resected meningiomas. J. Am. Vet. Med. Assoc 216 701–707, 684–705. [DOI] [PubMed] [Google Scholar]
- Thomsen BB, et al. , 2017. Spontaneous ischaemic stroke lesions in a dog brain: neuropathological characterization and comparison to human ischaemic stroke. Acta Vet. Scand 59, 7 10.1186/s13028-016-0275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troscher AR, et al. , 2017. Selective limbic blood-brain barrier breakdown in a feline model of limbic encephalitis with LGI1 antibodies. Front. Immunol 8, 1364 10.3389/fimmu.2017.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truve K, Dickinson P, Xiong A, et al. , 2016. Utilizing the dog genome in the search for novel candidate genes involved in glioma development-genome wide association mapping followed by targeted massive parallel sequencing identifies a strongly associated locus. PLoS Genet. 12, e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT, et al. , 2014. Alzheimer’s Disease Neuroimaging Initiative. Comparison of neuroimaging modalities for the prediction of conversion from mild cognitive impairment to Alzheimer’s dementia. Neurobiol. Aging 35, 143–151. 10.1016/j.neurobiolaging.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Tsikoudas A, Martin-Hirsch DP, 1999. Olfactory groove meningiomas. Clin. Otolaryngol. Allied Sci 24, 507–509. [DOI] [PubMed] [Google Scholar]
- Urkasemsin G, Olby NJ, 2014. Canine paroxysmal movement disorders. Vet. Clin. North Am. Small Anim. Pract 44, 1091–1102. 10.1016/j.cvsm.2014.07.006. [DOI] [PubMed] [Google Scholar]
- van Middendorp JJ, et al. , 2016. Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnership. Spinal Cord 54, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, et al. , 2010. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria T, et al. , 1996. Cloning of the canine GALC cDNA and identification of the mutation causing globoid cell leukodystrophy in West Highland White and Cairn terriers. Genomics 33, 457–462. 10.1006/geno.1996.0220. [DOI] [PubMed] [Google Scholar]
- Viitmaa R, et al. , 2014. Cerebral glucose utilization measured with high resolution positron emission tomography in epileptic Finnish Spitz dogs and healthy dogs. Vet. Radiol. Ultrasound 55, 453–461. 10.1111/vru.12147. [DOI] [PubMed] [Google Scholar]
- Vite CH, et al. , 2001. Histopathology, electrodiagnostic testing, and magnetic resonance imaging show significant peripheral and central nervous system myelin abnormalities in the cat model of alpha-mannosidosis. J. Neuropathol. Exp. Neurol 60, 817–828. 10.1093/jnen/60.8.817. [DOI] [PubMed] [Google Scholar]
- Vite CH, et al. , 2013. Features of brain MRI in dogs with treated and untreated mucopolysaccharidosis Type 1. Comp. Med 63, 163–173. [PMC free article] [PubMed] [Google Scholar]
- Wagner E, et al. , 2014. Hippocampal sclerosis in feline epilepsy. Brain Pathol. 24 10.1111/bpa.12147. 6-7-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley SU, et al. , 1994. Bone marrow transplantation corrects the enzyme defect in neurons of the central nervous system in a lysosomal storage disease. Proc. Natl. Acad. Sci. U. S. A 91, 2970–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waln O, Jankovic J, 2015. Paroxysmal movement disorders. Neurol. Clin 33, 137–152. 10.1016/j.ncl.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Watson F, et al. , 2018. A review of treatment options for behavioural manifestations of clinical anxiety as a comorbidity in dogs with idiopathic epilepsy. Vet. J 238, 1–9. 10.1016/j.tvjl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Weyne J, et al. , 1987. PET studies of changes in cerebral blood flow and oxygen metabolism after unilateral microembolization of the brain in anesthetized dogs. Stroke 18, 128–137. 10.1161/01.str.18.1.128. [DOI] [PubMed] [Google Scholar]
- Wiemels J, Wrensch M, Claus EB, 2010. Epidemiology and etiology of meningioma. J. Neurooncol 99, 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Anderson WC, Santaguida MT, et al. , 2013. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab. Invest 93, 970–982. [DOI] [PubMed] [Google Scholar]
- Wininger F, 2014. Neuronavigation in small animals: development, techniques, and applications. Vet. Clin. North Am. Small Anim. Pract 44, 1235–1248. 10.1016/j.cvsm.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Wisner ER, Dickinson PJ, Higgins RJ, 2011. Magnetic resonance imaging features of canine intracranial neoplasia. Vet. Radiol. Ultrasound 52, S52–61. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2019. Spinal Cord Injury. https://www.who.int/en/news-room/fact-sheets/detail/spinal-cord-injury.
- Wronski M, Arbit E, 2000. Surgical treatment of brain metastases from melanoma: a retrospective study of 91 patients. J. Neurosurg 93, 9–18. [DOI] [PubMed] [Google Scholar]
- Wronski M, Arbit E, Russo P, et al. , 1996. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology 47, 187–193. [DOI] [PubMed] [Google Scholar]
- Wu Y-C, et al. , 2011. High b-value and diffusion tensor imaging in a canine model of dysmyelination and brain maturation. Neuroimage 58, 829–837. 10.1016/j.neuroimage.2011.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Candolfi M, Liu C, et al. , 2010. Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial. PLoS One 5, e11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato O, et al. , 2002. A novel mutation in the gene for canine acid beta-galactosidase that causes GM1-gangliosidosis in Shiba dogs. J. Inherit. Metab. Dis 25, 525–526. [DOI] [PubMed] [Google Scholar]
- Yogalingam G, et al. , 1996. Feline mucopolysaccharidosis type VI. Characterization of recombinant N-acetylgalactosamine 4-sulfatase and identification of a mutation causing the disease. J. Biol. Chem 271, 27259–27265. [DOI] [PubMed] [Google Scholar]
- Yogalingam G, et al. , 2002. Identification of a mutation causing mucopolysaccharidosis type IIIA in New Zealand Huntaway dogs. Genomics 79, 150–153. 10.1006/geno.2002.6699. [DOI] [PubMed] [Google Scholar]
- York D, Higgins RJ, LeCouteur RA, et al. , 2012. TP53 mutations in canine brain tumors. Vet. Pathol 49, 796–801. [DOI] [PubMed] [Google Scholar]
- Young BD, Levine JM, Porter BF, et al. , 2011. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet. Radiol. Ultrasound 52, 132–141. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Lee S, Kent M, et al. , 2010. Dynamic contrast-enhanced magnetic resonance imaging of canine brain tumors. Vet. Radiol. Ultrasound 51 (2), 122–129. [DOI] [PubMed] [Google Scholar]