Abstract
Background
Circulating levels of endothelin-1 (ET1) are elevated in heart failure and predict poor prognosis; however, it is not clear whether ET1 elevation is an adaptive response, maladaptive response, or an epiphenomenon of heart failure. In the current study, we evaluated relationships between ET1, cardiac morphology, and incident heart failure or cardiovascular death in participants with no evidence of clinical cardiovascular disease at the time ET1 was measured.
Methods and Results
ET1 was measured 1,361 participants in the Multi-Ethnic Study of Atherosclerosis Angiogenesis Sub-Study. As suggested by linear regression, participants with lower circulating ET1 levels tended to be older, non-white, more likely to have smoked heavily, and less likely to report intentional exercise. Participants with higher ET1 levels had smaller left ventricular end-diastolic volumes (8.9 mL smaller per log increase in ET1, 95% CI 17.1 to 0.7, p=0.03) with an increased left ventricular ejection fraction (2.8% per log increase in ET1, 95% CI 0.5 to 5.2%, p=0.02). As suggested by Cox Proportional Hazards estimates, participants with higher ET1 levels had a lower risk for the composite outcome of heart failure or cardiovascular death in models that were unadjusted or had limited adjustment (p=0.03 and 0.05 respectively). Lower risk for heart failure could not be clearly shown in a model including health behaviors.
Conclusions
These results suggest, but do not confirm that elevated levels of circulating ET1 are associated with a more favorable cardiac phenotype. The relationship between ET1 and outcomes was not fully independent of one or more covariates.
Subject terms: Endothelin-1, heart failure, cardiac morphology
Introduction
Endothelin signaling is a treatment target in pulmonary arterial hypertension.1 Endothelin-receptor antagonists lower pulmonary vascular resistance, improve right heart function, and reduce morbidity and mortality in patients with pulmonary arterial hypertension.2–4 Endothelin signaling has also been hypothesized to be a marker or mediator of combined pre- and post- capillary pulmonary hypertension and elevated levels of Endothelin-1 (ET1) are associated with worse morbidity and mortality in individuals with left heart disease.5–10 Despite this observation and in counterpoint to individuals with pulmonary arterial hypertension, endothelin-receptor antagonism in patients with left heart failure has not shown benefit and may be harmful.11–13 Furthermore, some animal models suggest endothelin-receptor agonism may benefit the heart in the absence of excessive right ventricular afterload and antagonism may therefore be harmful.14–17
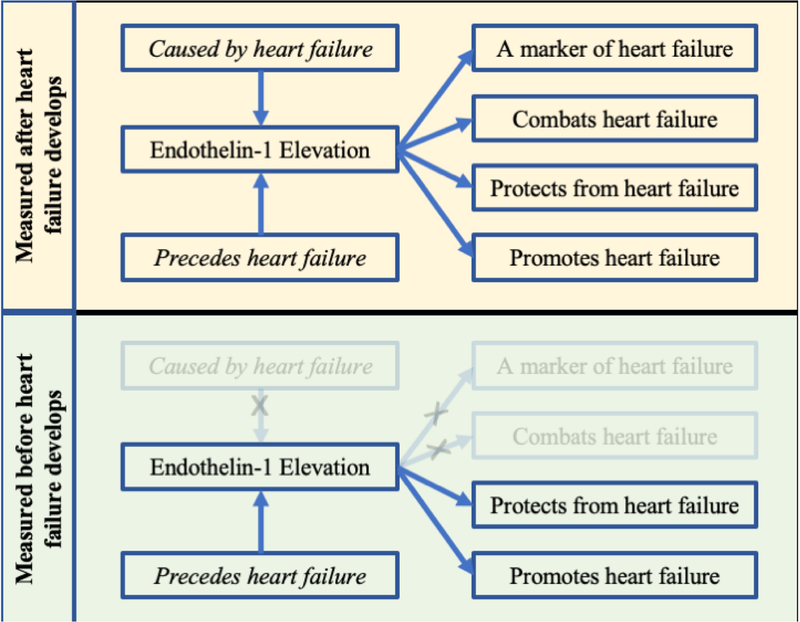
As such, while perturbed endothelin signaling is commonly observed in heart failure, the casual role of endothelin signaling in the pathogenesis and progression of heart failure is less clear. ET1 elevation in patients with extant heart failure could represent an adaptive response, a maladaptive or contributing factor to disease progression, or an epiphenomenon with no causal role in disease (Figure 1). Measurement before disease onset may improve our ability to understand the underlying role of a biomarker in disease pathogenesis.
Figure 1. Timing of endothelin-1 measurement.
If endothelin-1 is associated with heart failure, there are fewer possible explanations if endothelin-1 is measured before the development of disease in a prospective cohort design.
We examined relationships between ET1, cardiac morphology, and incident heart failure or cardiovascular death in a multi-ethnic cohort of adults free of clinical cardiovascular disease at baseline. We hypothesized that increased ET1 levels at baseline would be associated with right ventricular dilation and decreased function. In addition, we hypothesized that ET1 would be associated with increased risk for incident heart failure or cardiovascular death over follow-up.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a cohort study sponsored by the National Heart, Lung, and Blood Institute and designed to investigate subclinical cardiovascular disease. From 2000 to 2002, MESA recruited participants without clinical cardiovascular disease aged 45–84 years old from six US communities.
The current study included participants in the MESA-Angiogenesis sub-study. Of 6,814 participants enrolled in MESA, 4,204 with interpretable right ventricular morphology on cardiac magnetic resonance imaging (cMRI) were included in the MESA-Right Ventricle Study (MESA-RV). As previously reported participants in MESA-RV were slightly younger and had slightly lower body mass indices than the parent study.18 A random subset of the total MESA-RV cohort was randomly selected as the MESA-Angiogenesis cohort with event rates and characteristics similar to the parent MESA-RV cohort. The American Heart Association funded measurement of biomarkers including endothelin-1, angiopoietin, and vascular endothelial growth factor in the MESA-Angiogenesis sub-study. Institutional Review Boards of participating institutions approved MESA protocols. All participants provided informed consent.
Cardiac magnetic resonance imaging
cMRI was obtained at the baseline examination for all participants in MESA-RV. The cMRI protocol and interpretation of LV and RV parameters in MESA have been previously described.19–21 Briefly, endocardial and epicardial borders of the RV and LV were traced on MRI short axis fast gradient recalled (FGRE) cine images using a semi-automated method at end-systole and end-diastole from (MASS 4.2, Medic, Leiden, the Netherlands). The outflow tract was included in RV volume. Papillary muscles and trabeculae were included in volumes and excluded from mass in both the RV and LV. End-systolic and end-diastolic volumes were calculated using Simpson’s rule by summation of areas multiplied by the sum of slice thickness and image gap. Difference between epicardial and endocardial volumes of the RV or LV free wall at end-diastole multiplied by the specific gravity of the heart (1.05g/mL) was used to estimate mass. Ejection fraction was calculated by dividing stroke volume by end-diastolic volume.
Endothelin-1 Assay
ET1 was measured using EMD Millipore’s MILLIPLEX MAP Human Angiogenesis Growth Factor Magnetic Bead Panel 1 (Lot# 2802970). Seventy-eight samples were assayed in duplicate. The reliability coefficient was 0.83 and inter-assay coefficient of variation was 4.8% on control samples in this study. There is another commercially available assay (R&D systems ELISA assay; used in the Jackson Heart Study7). To place our results in context with these other results: each method is reliable; however, the absolute level is discrepant. When both methods were used on the same pool of 20 control participants by the MESA coordinating center, the mean level using the R&D Elisa was 1.6 pg/mL (standard deviation 0.6 pg/mL) and the Millipore assay was 46.6 pg/mL (standard deviation 6.3 pg/mL). ET1 was log-transformed for all regression analyses.
Ascertainment of events
Full details of event ascertainment and definition are available in MESA’s manual of procedures (MOP).22 Briefly, clinical outcomes were assessed at MESA study examinations and by telephone interview every 9 to 12 months. Records were obtained for approximately 99% of hospitalizations and 97% of outpatient cardiovascular diagnostic encounters through calendar year 2014. Incident heart failure required heart failure symptoms, a physician diagnosis of heart failure and an objective feature of heart failure. Cardiovascular death was any death adjudicated as related to cardiac or vascular disease. If a participant developed incident heart failure and subsequently died, the time to first event (heart failure) was used for analyses. Two physicians from the MESA events committee independently reviewed all medical records for classification and dating of events. If reviewers disagreed, they adjudicated differences. If disagreement persisted, the full events committee made the final classification.
Statistical Analysis
We used linear regression to estimate associations between ET1 levels and cardiac morphology or predictors of ET1 level at the baseline exam. Covariates were assessed at the initial MESA exam and chosen a priori.22 In limited models, we adjusted for age, sex, race, height, weight, and study site. In adjusted models, we included participants’ education and cardiovascular risk factors including smoking status, pack-years of smoking, hypertension, diabetes mellitus, and cholesterol. In exploratory models, we further adjusted for estimated glomerular filtration rate (GFR), co-medication use, or intentional exercise.
Cox proportional hazards was used to estimate unadjusted and adjusted associations between ET1 level at the baseline exam and incident heart failure or cardiovascular death. Limited and adjusted models were evaluated. Because a test of Schoenfeld residuals in unadjusted analyses suggested a non-zero slope with relation to time (p=0.05), all models included a time varying covariate of ET1 level (interaction of time with ET1 level) to account for non-proportional hazards. Exploratory models also evaluated whether age, sex, or hypertension modified significant associations between ET1 and clinical outcomes. Parsimonious models are included in the online supplement and were created using sequential backward elimination of the least significant covariate until are included adjustments were statistically significant (threshold of significance, p-value <0.05) to evaluate whether coefficients were stable and whether pre-specified primary models were overly adjusted. Analyses were performed using STATA 15.1 (StataCorp, College Station, TX, USA).
Results
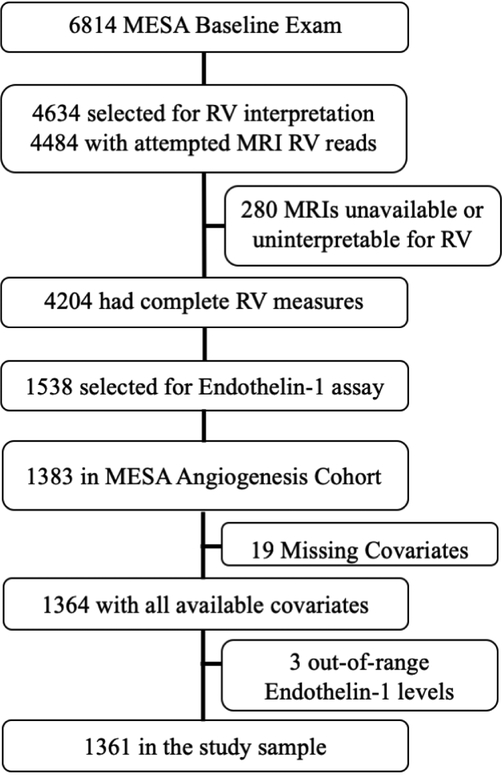
1,538 participants had ET1 sampled as part of the MESA-Angiogenesis case-cohort study. Of these, 1,383 participants were in the MESA-Angiogenesis cohort and 1,364 had all available covariates. Three additional participants were excluded from primary analyses (but included in analyses in the online supplement) given concern for measurement error since these individuals had measured ET1 levels of 348.8 pg/mL, 368.4pg/mL, and 621.1pg/mL. These values were >30 standard deviations above the mean (>14 standard deviations when these values were included) and >16 standard deviations from the closest observation (> 8 standard deviations when these observations were included). The final study sample included 1,361 participants of whom 1,280 participants did not have an event during follow-up, 53 participants had incident heart failure over follow-up, and 28 participants suffered cardiovascular death during follow-up (Figure 2). The mean age of the sample was 61.2 years, 52.4% were women, and 40.3% were white. The average ET1 level was 51.3 ± 9.7 pg/mL (Table 1). Individuals with higher ET1 levels tended to be younger, more likely to be non-white, more likely to exercise regularly and were less likely to have smoked heavily (Table 2 and Table E1). Median follow-up was 13.1years, maximum follow-up was 14.5 years, and total follow-up was 16,212 person-years. The incidence of heart failure or cardiovascular death among members of the cohort was 5.0 events per 1,000 person-years.
Figure 2.
Study Design
Table 1.
Characteristics of the MESA Angiogenesis Cohort
| n=1,361 | |
|---|---|
| Mean Endothelin-1 (pg/mL) | 51.3 ± 9.7 |
| Endothelin-1 range (pg/mL) | 34.6 − 189.1 |
| Age (years) | 61 ± 10 |
| Female (%) | 52 |
| Race (%) | |
| White | 40 |
| Chinese | 12 |
| African-American | 27 |
| Hispanic | 21 |
| Height (cm) | 166 ± 10 |
| Weight (kg) | 77 ± 16 |
| Body mass index (kg/m2) | 28 ± 5 |
| Educational attainment (%) | |
| No high school degree | 16 |
| High school degree | 19 |
| Some college or certificate | 28 |
| Bachelor’s Degree | 17 |
| Higher than bachelor’s degree | 20 |
| Insurance Status (%) | |
| No insurance | 7 |
| Medicare | 33 |
| Private insurance | 76 |
| Cigarette smoking status (%) | |
| Never | 53 |
| Former | 36 |
| Current | 11 |
| Pack-years | 10 ± 21 |
| Metabolic Syndrome (%) | 33 |
| Hypertension (%) | 43 |
| Systolic blood pressure (mmHg) | 125 ± 21 |
| Diabetes mellitus (%) | 13 |
| Glucose (mg/dL) | 97 ± 31 |
| Cholesterol (mg/dL) | 194 ± 33 |
| NT-ProBNP (pg/mL) | 93 ± 147 |
| Medications (%) | |
| NSAIDs | 42 |
| Beta-blockers | 9 |
| ACE-inhibitors/ARBs | 17 |
| Any diuretic | 12 |
Data presented as mean ± standard deviation or percentage as appropriate Abbreviations: pg=picogram, mL=milliliter, cm=centimeters, kg=kilograms, m2=meters squared, mmHg=millimeters of mercury, mg=milligram, dL=deciliter, NT-ProBNP=amino-terminal fragment of pro-B-type natriuretic peptide NSAIDs=non-steroidal anti-inflammatory medications, ACE-inhibitors= angiotensin converting enzyme inhibitors, ARBs=angiotensin II receptor blockers
Table 2.
Unadjusted and multivariable linear regression estimating associations of demographic and cardiac risk factors with endothelin-1 levels (n=1,361)
| Difference in Endothelin-1 Level (pg/mL) | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Difference | (95% CI) | p-value | Difference | (95% CI) | p-value | |
| Age (per 10 years) | −0.8 | (−1.3, −0.3) | 0.002 | −0.8 | (−1.4, −0.2) | 0.007 |
| Female | 0.1 | (−0.9, 1.1) | 0.84 | −0.2 | (−1.2, 0.9) | 0.75 |
| Body mass index (per 5 kg/m2) | −0.2 | (−0.7, 0.3) | 0.50 | −0.2 | (−0.8, 0.4) | 0.46 |
| Race | ||||||
| White | Referent | Referent | ||||
| Chinese | −1.5 | (−3.3, 0.2) | 0.07 | −3.3 | (−5.5, −1.2) | 0.002 |
| African-American | −0.6 | (−1.9, 0.7) | 0.36 | −0.8 | (−2.3, 0.6) | 0.26 |
| Hispanic | −0.7 | (−2.1, 0.7) | 0.35 | −2.3 | (−4.1, −0.6) | 0.008 |
| Educational attainment | ||||||
| < high school degree | Referent | Referent | ||||
| High school degree | 0.9 | (−0.9, 2.7) | 0.31 | 0.4 | (−1.4, 2.2) | 0.67 |
| Some college | 0.5 | (−1.1, 2.1) | 0.53 | −0.6 | (−2.2, 1.2) | 0.54 |
| Bachelor’s Degree | −0.7 | (−2.5, 1.0) | 0.42 | −1.9 | (−3.9, 0.0) | 0.06 |
| ≥ bachelor’s degree | 0.1 | (−1.7, 1.8) | 0.93 | −1.6 | (−3.5, 0.4) | 0.12 |
| Exercise (per 500 MET/min/week) | 0.1 | (0.0, 0.2) | 0.02 | 0.1 | (0.0, 0.2) | 0.06 |
| Pack-years (per 10 years) | −0.4 | (−0.6, −0.1) | 0.003 | −0.4 | (−0.6, −0.1) | 0.003 |
| GFR (per 10 mL/min/1.73m2) | 0.2 | (−0.2, 0.5) | 0.32 | 0.1 | (−0.2, 0.5) | 0.46 |
| Hypertension | −0.6 | (−1.7, 0.4) | 0.24 | 0.2 | (−1.0, 1.3) | 0.79 |
| Diabetes mellitus | −1.4 | (−2.9, 0.2) | 0.09 | −1.0 | (−2.6, 0.6) | 0.22 |
Multivariate model included all listed variables and also included study site.
Data presented as mean ± standard deviation or percentage as appropriate
Abbreviations: pg=picogram, mL=milliliter, CI= confidence interval, kg=kilograms, m2=meters squared, MET/min/week= Metabolic equivalents per minute per week
A parsimonious backward elimination multivariate model of predictors of endothelin-1 level included only age (−0.8 pg/mL per 10 additional years of age, p=0.004), intentional exercise (0.1 pg/mL per 500 additional MET/min/week, p=0.02), and pack-years of smoking (−0.3 pg/mL per 10 pack-years, p=0.008).
After adjustment for covariates, higher levels of ET1 were associated with a smaller LV end-diastolic volume (−8.9 mL smaller per log increase in ET1, 95% CI 17.1 to 0.7, p=0.03), and an increased LV ejection fraction (2.8% per log increase in ET1, 95% CI 0.5 to 5.2%, p=0.02)(Table 3; shown graphically in Figures E1 and E2 of the online supplement). Relationships between ET1 and the RV were sensitive to adjustment and were less compelling. Bi-ventricular relationships were similar with further adjustment for co-medication use, when accounting for renal function, or when accounting for intentional exercise (Table E2 in the supplemental material).
Table 3.
Multivariable linear regression estimating associations between endothelin-1 level and cardiac structure and function (n=1,361)
| Per log increase in Endothelin-1 | |||
|---|---|---|---|
| Difference | (95% CI) | p-value | |
| Right Ventricle | 0.5 | (−1.1,2.0) | 0.57 |
| RV mass, g (Unadjusted) | |||
| RV mass, g (Limited model*) | −0.8 | (−1.9,0.4) | 0.18 |
| RV mass, g (Full Model†) | −0.8 | (−2.0, 0.3) | 0.17 |
| RVEDV, mL (Unadjusted) | 1.9 | (−8.7, 12.5) | 0.72 |
| RVEDV, mL (Limited model) | −6.0 | (−13.2, 1.2) | 0.10 |
| RVEDV, mL (Full Model) | −6.9 | (−14.0, 0.4) | 0.06 |
| RVEF, % (Unadjusted) | 0.0 | (−2.2, 2.3) | 0.99 |
| RVEF, % (Limited model) | 0.2 | (−1.9,2.3) | 0.85 |
| RVEF, % (Full Model) | −0.1 | (−2.0, 2.2) | 0.91 |
| Left Ventricle | |||
| LV mass, g (Unadjusted) | −1.8 | (−15.5,11.8) | 0.79 |
| LV mass, g (Limited model*) | −3.0 | (−12.5, 6.6) | 0.53 |
| LV mass, g (Full Model†) | −1.9 | (−11.0,7.1) | 0.67 |
| LVEDV, mL (Unadjusted) | −0.1 | (−10.8, 10.6) | 0.98 |
| LVEDV, mL (Limited model) | −8.2 | (−16.4,0.0) | 0.05 |
| LVEDV, mL (Full Model) | −8.9 | (−17.1,−0.7) | 0.03 |
| LVEF, % (Unadjusted) | 2.5 | (0.0,5.1) | 0.05 |
| LVEF, % (Limited model) | 2.8 | (0.5, 5.2) | 0.02 |
| LVEF, % (Full Model) | 2.8 | (0.5, 5.2) | 0.02 |
Abbreviations: SD=standard deviation, CI=confidence interval, RV=right ventricular,
GFR=glomerular filtration rate, EDV=end-diastolic volume, and EF=ejection fraction, LV=left ventricular, g=grams, mL=milliliters
Limited model: age, sex, race/ethnicity, height and weight, study site
Full model: Limited + education, smoking status, pack-years, hypertension, systolic blood pressure, diabetes, and cholesterol
Strata of participants by ET1 level were created. Fourteen participants with the lowest ET1 levels (<44 pg/mL) had incident heart failure or cardiovascular death over 2,123 person years (6.6 per 1,000 person-years), 36 participants with medium-low ET1 levels (44–50 pg/mL) experienced an event over 6,338 person-years (5.7 per 1,000 person-years), 19 participants with medium-high ET1 levels (50–56 pg/mL) had incident heart failure or cardiovascular death over 4,566 person-years of follow-up (4.2 per 1,000 person-years), and 12 participants with the highest ET1 levels (>56 pg/mL) had incident heart failure or cardiovascular death over 3,185 person-years of follow-up (3.8 per 1,000 person-years). This suggests a risk difference between the highest and lowest cohort of 2.8 episodes of heart failure or cardiovascular death per 1,000 person-years or a relative risk of 0.58.
In unadjusted models using cox proportional hazards, a log increase in ET1 level was associated with decreased hazard of heart failure or cardiovascular death (hazard ratio 0.09, 95% CI 0.01 to 0.73, p=0.03). The association was similar with limited adjustment (hazard ratio 0.06, 95% CI 0.00 to 1.03, p=0.05) and slightly less strong with full adjustment (hazard ratio 0.07, 95% CI 0.00 to 1.30, p=0.08) where results were not statistically significant (Table 4). Although the trend was similar, associations between ET1 and heart or cardiovascular death separately were not statistically significant in these smaller groups. There was no suggestion of a relationship between ET1 and non-cardiovascular death (Table 4).
Table 4.
Cox proportional hazard regression estimating the relationship of log of endothelin-1 level at the baseline exam with clinical outcomes (n=1,361)
| Hazard Ratio per log increase in endothelin-1 | level | (95% CI) | p-value |
|---|---|---|---|
| Hazard of heart failure or cardiovascular death | |||
| Unadjusted | 0.09 | (0.01, 0.73) | 0.03 |
| Limited model* | 0.06 | (0.00, 1.03) | 0.05 |
| Full Model† | 0.07 | (0.00, 1.30) | 0.08 |
| Hazard of heart failure | |||
| Unadjusted | 0.04 | (0.00, 1.20) | 0.06 |
| Limited model* | 0.10 | (0.01, 1.90) | 0.13 |
| Full Model† | 0.14 | (0.01, 2.91) | 0.20 |
| Hazard cardiovascular death | |||
| Unadjusted | 0.02 | (0.00, 2.09) | 0.10 |
| Limited model* | 0.06 | (0.00, 3.01) | 0.16 |
| Full Model† | 0.07 | (0.00, 3.21) | 0.17 |
| Hazard of non-cardiovascular death | |||
| Unadjusted | 0.37 | (0.02, 6.54) | 0.50 |
| Limited model* | 0.60 | (0.07, 5.09) | 0.64 |
| Full Model† | 0.76 | (0.08, 6.83) | 0.80 |
Definition of abbreviations: CI-confidence interval
Because initial models suggested a non-proportional hazard, all models accounted included a term accounting for the possibility of a time-varying relationship between endothelin-1 and the hazard of heart failure or death. Three influential endothelin-1 outliers were excluded from the primary analysis. Inclusion of these outlier strengthened the association with mortality
Limited model: age, sex, race/ethnicity, height and weight, study site
Full model: Limited + education, smoking status, pack-years, hypertension, systolic blood pressure, diabetes, and cholesterol
In exploratory analyses, there was no suggestion that age or sex modified relationships between ET1 and incident heart failure or cardiovascular death (p-value for the interactions= 0.63 & 0.75). Systemic hypertension may modify the association between ET and incident heart failure or cardiovascular death with a stronger relationship among individuals with systemic hypertension (p-value for the interaction= 0.03; hazard ratio in participants with hypertension was 0.01 per log increase in ET1, 95% CI 0.00 to 0.53, p=0.02; hazard ratio in participants WITHOUT hypertension was 0.76, 95% CI 0.02 to 28.2, p=0.88). Inclusion of the three participants with markedly elevated ET1 levels did not impact results in any analysis (Tables E3 & E4) and parsimonious models for all relationships were similar to the full models (Table E5).
Discussion
We observed associations between higher ET1 levels, increased ejection fraction, and reduced risk for incident heart failure or cardiovascular death. We did not observe a clear relationship between ET1 and incident heart failure or cardiovascular death in the fully adjusted model. This suggests the relationship between ET1 and outcomes was not fully independent of one or more covariates. ET1 levels appeared to be associated with age, race/ethnicity, smoking, and intentional exercise.
These results are different than we expected, but not necessarily incongruous with previous studies. In participants with existing heart disease, several studies have shown higher ET1 levels are associated with worse outcomes.6,8–10 ET1 is a pulmonary vasoconstrictor and we anticipated higher ET1 levels would be associated with right ventricular dilation. Although they did not report on right ventricular dilation, this hypothesis was reinforced by the Jackson Heart Study, which observed that elevated ET1 was associated with increased pulmonary artery systolic pressure, heart failure and death.7 The key difference between previous studies and the current analysis is the absence of clinical cardiovascular disease at the time ET1 was assayed.
MESA participants in the current study were enrolled with the intent of excluding individuals with clinically detectable cardiovascular disease at the time baseline bloodwork was measured.23 The absence of overt cardiovascular disease is likely important. By measuring ET1 levels in the absence of clinical cardiovascular disease, the potential for reverse causation to cause associations is diminished.24,25 Ideally, previous observational studies in individuals with heart failure would have evaluated associations between ET1 and outcomes in individuals with otherwise similar heart failure severity; however, in practice such a comparison is difficult and residual confounding may exist despite adjustment. This is especially true when the marker of interest itself may reflect severity.
Adaptive response, maladaptive response, and epiphenomena all increase in the setting of active disease and can be proportional to disease severity. Because of this, even beneficial responses can be associated with increased mortality if they are measured in the setting of more severe disease. In heart failure this paradigm has been seen with IL-33. Increased IL-33 is associated with heart failure severity, but is actually likely to be cardioprotective.26,27 Allegorically, while the number of firefighters at a fire are certainly associated with the severity of the fire, no one doubts that the firefighters are there to help. We intuitively understand the causal pathway in this example, but cannot be as certain for observed biologic pathways in disease.
Endothelin signaling is already a target in pulmonary arterial hypertension where elevation is harmful and blockade is clearly beneficial. This benefit is predominantly thought to be related to the action of endothelin-receptor blockade in the diseased pulmonary vasculature. This is different than left heart failure where endothelin-receptor blockade remains an active area of investigation, the pulmonary vasculature is not the primary problem, and results are less encouraging.11 The MELODY-1 trial of endothelin receptor-blockade in individuals with significant pre and post capillary disease was recently published and did not suggest benefit. Instead, there was worse volume retention in treated individuals and no significant impact on pre-capillary parameters.11,28 Some have speculated that endothelin-receptor blockade may be similar to beta-blockade where short-term worsening may be offset by long-term disease stability and improved mortality.29–32 None of the participants in the current study are known to have pulmonary arterial hypertension and our results, suggesting high levels of ET1 may prevent heart failure, cautiously argue against the idea that reduced ET1 levels or ET1 blockade will lead to a long term benefit in the absence of pulmonary arterial hypertension.
The possibility that low ET1 levels can lead to heart failure and higher ET1 levels may be cardioprotective is supported by animal models. Acutely, endothelin-receptor antagonism results in negative inotropy in animal models, which agrees with our observation that lower ET1 levels were associated with reduced LV ejection fraction.14,17,28 To evaluate chronic impacts of ET1 difference, genetically modified mice with variable ET1 expression (20%, 65%, wild-type, and 350%) have been studied. Mice with lower ET1 expression develop a dilated cardiomyopathy and die more quickly than wild-type mice (20% expression- death at 560 days; 65% expression- death at 632 days; wild-type/100% expression- death at 841 days). Mice with over-expression of ET1 (350%) had slightly more cardiac hypertrophy, normal cardiac function, and lived 876 days.16 It may be possible to have too much ET1 as mice with 1,500% over-expression of ET1 developed heart failure in a separate study.33 This pre-clinical research agrees with our observation that lower ET1 levels (in a population with few extreme values) are associated with increased incidence of heart failure and cardiovascular death compared to higher levels.
The mechanism by which elevated ET1 levels may offer benefit is unknown. Though both pulmonary arterial and venous remodeling may occur in PH due to left heart disease, in most instances the majority of the pre-capillary component is functional in nature, as evidenced by the rapid reduction of pre-capillary parameters after left ventricular assist device implantation and LV unloading.34–36 This functional component may in part be mediated by elevated ET1 levels.37 This paradigm may be supported by our observation that increased ET1 levels are associated with smaller LV volumes and a higher LV ejection fraction. One might speculate that modest elevations in pulmonary vascular resistance could in fact “protect” the diseased left ventricle from further elevations in preload. Alternatively, there may be a direct action of ET1 on cardiac myocytes contributing to a greater tolerance for physiologic stress.16
This study has limitations. Our results do not definitively inform mechanism. It is noteworthy that statistical significance in adjusted models was not consistent, which increases the possibility for residual or unmeasured confounding to explain associations between elevated ET1 and cardiovascular outcomes. Alternatively the loss of significance could suggest that ET1 could mediate some of the effects of age, exercise, or cigarette smoking on cardiac health. Other causal explanations are also possible and would need to be investigated in mechanistic studies. For instance, elevated ET1 levels could be a compensation for poorly functioning endothelin-receptors. Our description of circulating levels of ET1 do not necessarily reflect differences in endothelin signaling at the cellular level. Our results in isolation may raise more questions than they answer; however, our observations reinforce mechanistic studies in mice and align with recent tepid results in the randomized trials of endothelin receptor antagonists in left heart disease. The questions raised are important as we consider whether endothelin-receptor antagonism has a role in the treatment of left heart disease.
Summary
Our results also suggest, but do not confirm that higher ET1 levels measured in the absence of cardiovascular disease may be associated with a lower risk for incident heart failure or cardiovascular death. This agrees with previous results in pre-clinical animal models that suggest ET1 prevents heart failure. Understanding whether ET1 elevation is adaptive, maladaptive, or merely a bystander response in left heart failure is important because there are ongoing plans to target this pathway in diseases of left heart failure.
Supplementary Material
Acknowledgements/Sources of Funding:
This research was supported by an American Heart Association Clinical and Population Research Award (MESA-Angiogenesis). The parent MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclosures: Dr. Leary reports grants from American Heart Association, CHEST Foundation, and NHLBI. He is a site investigator for industry sponsored studies and registries in pulmonary hypertension sponsored by United Therapeutics, Actelion, and Bayer. Dr. Kawut reports non-financial support from the American Thoracic Society and grants from Actelion, United Therapeutics, Gilead, Lung Biotech, Mallinkrodt, and Bayer. He has received grants and non-financial support from Cardiovascular Medical Research and Education Fund and Pulmonary Hypertension Association. He has served in an advisory capacity (for grant review and other purposes) for United Therapeutics, Akros Pharmaceuticals, Glaxo SmithKline, and Complexa without financial support or in-kind benefits. Dr. Tedford reports research support from Actelion, Merck, Abiomed, and Abbott. Dr. Jenny died recently before the submission, but her colleagues believe she had nothing to disclose relevant to this work. All other authors report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galiè N, et al. 2015. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119. [DOI] [PubMed] [Google Scholar]
- 2.Rubin L Effect of Macitentan on Morbidity and Mortality in Pulmonary Arterial Hypertension (PAH): Results From the SERAPHIN Trial. Chest. 2012;142:1026A. [Google Scholar]
- 3.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani H-A, Jansa P, Jing Z-C, Le Brun F-O, Mehta S, Mittelholzer CM, Perchenet L, Sastry BKS, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G, SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Barberà JA, Frost AE, Ghofrani H-A, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grunig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JHN, Langley J, Rubin LJ. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015;373:834–844. [DOI] [PubMed] [Google Scholar]
- 5.Meoli DF, Su YR, Brittain EL, Robbins IM, Hemnes AR, Monahan K. The transpulmonary ratio of endothelin 1 is elevated in patients with preserved left ventricular ejection fraction and combined pre- and post-capillary pulmonary hypertension. Pulm Circ. 2017;8:204589321774501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivier A, Girerd N, Michel JB, Ketelslegers JM, Fay R, Vincent J, Bramlage P, Pitt B, Zannad F, Rossignol P, EPHESUS Investigators. Combined baseline and one-month changes in big endothelin-1 and brain natriuretic peptide plasma concentrations predict clinical outcomes in patients with left ventricular dysfunction after acute myocardial infarction: Insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Int J Cardiol. 2017;241:344–350. [DOI] [PubMed] [Google Scholar]
- 7.Jankowich MD, Wu W-C, Choudhary G. Association of Elevated Plasma Endothelin-1 Levels With Pulmonary Hypertension, Mortality, and Heart Failure in African American Individuals: The Jackson Heart Study. JAMA Cardiol. 2016;1:461–469. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C-L, Xie S, Qiao X, An Y-M, Zhang Y, Li L, Guo X-B, Zhang F-C, Wu L-L. Plasma endothelin-1-related peptides as the prognostic biomarkers for heart failure. Medicine. 2017;96:e9342–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaggin HK, Truong QA, Gandhi PU, Motiwala SR, Belcher AM, Weiner RB, Baggish AL, Januzzi JL Jr. Systematic Evaluation of Endothelin 1 Measurement Relative to Traditional and Modern Biomarkers for Clinical Assessment and Prognosis in Patients With Chronic Systolic Heart Failure. Am J Clin Pathol. 2017;147:461–472. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb SS, Harris K, Todd J, Estis J, Christenson RH, Torres V, Whittaker K, Rebuck H, Wawrzyniak A, Krantz DS. Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem. 2015;48:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vachiery JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, Papadakis K, Rubin LJ. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51. [DOI] [PubMed] [Google Scholar]
- 12.Packer M, McMurray JJV, Krum H, Kiowski W, Massie BM, Caspi A, Pratt CM, Petrie MC, DeMets D, Kobrin I, Roux S, Swedberg K, ENABLE Investigators and Committees. Long-Term Effect of Endothelin Receptor Antagonism With Bosentan on the Morbidity and Mortality of Patients With Severe Chronic Heart Failure: Primary Results of the ENABLE Trials. JACC: Heart Failure. 2017;5:317–326. [DOI] [PubMed] [Google Scholar]
- 13.Handoko ML, de Man FS, Vonk Noordegraaf A. The rise and fall of endothelin receptor antagonists in congestive heart failure. Eur Resp J. 2011;37:484–485. [DOI] [PubMed] [Google Scholar]
- 14.Smyrnias I, Goodwin N, Wachten D, Skogestad J, Aronsen JM, Robinson EL, Demydenko K, Segonds-Pichon A, Oxley D, Sadayappan S, Sipido K, Bootman MD, Roderick HL. Contractile responses to endothelin-1 are regulated by PKC phosphorylation of cardiac myosin binding protein-C in rat ventricular myocytes. J Mol Cell Cardiol. 2018;117:1–18. [DOI] [PubMed] [Google Scholar]
- 15.Brunner F Cardiac tissue endothelin-1 levels under basal, stimulated, and ischemic conditions. J Cardiovasc Pharmacol. 1995;26 Suppl 3:S44–6. [PubMed] [Google Scholar]
- 16.Hathaway CK, Grant R, Hagaman JR, Hiller S, Li F, Xu L, Chang AS, Madden VJ, Bagnell CR, Rojas M, Kim H-S, Wu B, Zhou B, Smithies O, Kakoki M. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc Natl Acad Sci USA. 2015;112:5141–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelso EJ, Geraghty RF, McDermott BJ, Trimble ER, Nicholls DP, Silke B. Mechanical effects of ET-1 in cardiomyocytes isolated from normal and heart-failed rabbits. Mol Cell Biochem. 1996;157:149–155. [DOI] [PubMed] [Google Scholar]
- 18.Kawut SM, Lima JAC, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JAC, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:65. [DOI] [PubMed] [Google Scholar]
- 20.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawut SM, Barr RG, Lima JAC, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, Bluemke DA. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)--right ventricle study. Circulation. 2012;126:1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MESA Manual of Operations: Field Center and Laboratory Procedures [Internet]. mesa-nhlbi.org [cited 2014 Mar 17];Available from: http://www.mesa-nhlbi.org/manuals.aspx
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 24.Cobb LK, Anderson CAM, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ, American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. [DOI] [PubMed] [Google Scholar]
- 25.Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JPA, Kirsch-Volders M, Matullo G, Phillips DH, Schoket B, Stromberg U, Vermeulen R, Wild C, Porta M, Vineis P. STrengthening the Reporting of OBservational studies in Epidemiology--Molecular Epidemiology STROBE-ME: an extension of the STROBE statement. J Clin Epidemiol. 2011;64:1350–1363. [DOI] [PubMed] [Google Scholar]
- 26.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhillon OS, Narayan HK, Khan SQ, Kelly D, Quinn PA, Squire IB, Davies JE, Ng LL. Pre-discharge risk stratification in unselected STEMI: is there a role for ST2 or its natural ligand IL-33 when compared with contemporary risk markers? Int J Cardiol. 2013;167:2182–2188. [DOI] [PubMed] [Google Scholar]
- 28.Hsu S, Tedford RJ. Will we be singing a different tune on combined post- and pre-capillary pulmonary hypertension? Eur Respir J. 2018;51:1702589. [DOI] [PubMed] [Google Scholar]
- 29.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 30.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–1161. [DOI] [PubMed] [Google Scholar]
- 31.Nagendran J, Sutendra G, Paterson I, Champion HC, Webster L, Chiu B, Haromy A, Rebeyka IM, Ross DB, Michelakis ED. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res. 2013;112:347–354. [DOI] [PubMed] [Google Scholar]
- 32.Taguchi K, Hattori Y. Unlooked-for Significance of Cardiac Versus Vascular Effects of Endothelin-1 in the Pathophysiology of Pulmonary Arterial Hypertension. Circ Res. 2013;112:227–229. [DOI] [PubMed] [Google Scholar]
- 33.Yang LL, Gros R, Kabir MG, Sadi A, Gotlieb AI, Husain M, Stewart DJ. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation. 2004;109:255–261. [DOI] [PubMed] [Google Scholar]
- 34.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction. Circulation. 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikus E, Stepanenko A, Krabatsch T, Loforte A, Dandel M, Lehmkuhl HB, Hetzer R, Potapov EV. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg. 2011;40:971–977. [DOI] [PubMed] [Google Scholar]
- 36.Masri SC, Tedford RJ, Colvin MM, Leary PJ, Cogswell R. Pulmonary Arterial Compliance Improves Rapidly after Left Ventricular Assist Device Implantation. ASAIO J. 2016;:1. [DOI] [PubMed] [Google Scholar]
- 37.Barnett CF, De Marco T. Pulmonary hypertension associated with left-sided heart disease. Heart Fail Clin. 2012;8:447–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.