Abstract
We previously demonstrated that atherogenic Ldlr−/−Apobec1−/− (LDb) double knockout mice lacking both low-density lipoprotein receptor (LDLR) and apolipoprotein B mRNA-editing catalytic polypeptide-1 (Apobec1) had increased serum IL-17 levels, with T cell programming shifted towards Th17 cells. In this study, we assessed the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in T cell programming and atherogenesis. We deleted the Pcsk9 gene from LDb mice to generate Ldlr−/−Apobec1−/−Pcsk9−/− (LTp) triple knockout mice. Atherosclerosis in the aortic sinus and aorta were quantitated. Lymphoid cells were analyzed by flow cytometry, ELISA and real-time PCR. Despite of dyslipidemia, LTp mice developed barely detectable atherosclerotic lesions. The IL-17, was very low in plasma and barely detectable in the aortic sinus in the LTp mice. In the spleen, the number of CD4+CD8− cells and splenocytes were much lower in the LDb mice than LTp mice, whereas, the IL-17-producing cells of γδTCR+ T cells and effector memory CD4+ T cells (CD44hiCD4+) in the spleen were significantly higher in the LDb mice than in the LTp mice. The Rorc mRNA expression levels were elevated in LDb mice compared to LTp mice. When re-stimulated with an anti-CD3 Ab, CD44hiCD4+ T cells from LDb mice secreted more IL-17 than those from LTp mice. T cells from LDb mice (with PCSK9) produce more IL-17 at basal and stimulated conditions when compared with LTp mice (without PCSK9). Despite the dyslipidemic profile and the lack of LDLR, atherogenesis is markedly reduced in LTp mice. These results suggest that PCSK9 is associated with changes in T cell programming that contributes to the development of atherosclerosis.
Keywords: PCSK9, IL-17, Atherosclerosis, T-cells
INTRODUCTION
Atherosclerosis is characterized by the interplay between modified lipoprotein particles and the immune system in the vascular wall resulting in the initiation and progression of the disease development (1,2). Although aggressive lipid lowering therapeutics aimed to reduce low-density lipoprotein (LDL) cholesterol is currently the standard practice of care in both primary and secondary prevention of atherosclerotic cardiovascular disease (3), addressing the underlying immune regulation of atherosclerosis development may offer an alternative therapeutic approach.
Various studies have demonstrated the essential role of both innate and adaptive immunity in the pathogenesis of atherosclerosis (4). Immune cells, such as macrophages, activated T lymphocytes and dendritic cells (DCs) are either recruited, or locally activated, resulting in their proliferation in the atherosclerotic lesions. This in turn, induces an intense inflammatory response, contributing to the development of vascular atherosclerosis (4,5,6). We and others have shown that Th17 cells are present in atherosclerotic lesions in hyperlipidemic mice and humans (7,8,9,10). However, the effects of Th17 responses on atherogenesis remain debatable (8,11,12).
Proprotein convertase subtilisin/kexin type 9 (PCSK9) interacts with the low-density lipoprotein receptor (LDLR). This induces the lysosomal degradation of LDLR and results in an increase in plasma LDL cholesterol levels (13,14). However, PCSK9 may also affect atherosclerosis development via other non-LDLR-dependent mechanisms. Our laboratory has demonstrated that PCSK9 interacts directly with cytoplasmic apolipoprotein B (apoB) and prevents its degradation via the autophagosome/lysosome pathway, resulting in increased levels of very low-density lipoprotein (VLDL) and LDL production, which contributes to atherosclerosis development irrespective of the LDLR (15,16). Moreover, the LDLs generated from the PCSK9 expressing Ldlr−/−Apobec1−/− (LDb) double knockout mice (15) induce elevated levels of proinflammatory gene expression in endothelial cells compared to the LDLs obtained from PCSK9 deficient Ldlr−/−Apobec1−/−Pcsk9−/− (LTp) triple knockout mice. These observations provide further evidence that PCSK9 modulates atherosclerosis development independent of LDLR.
Since we have demonstrated that our hyperlipidemic double knockout LDb mice have increased Th17 cells in the atherosclerotic lesions (7), we have decided to assess the role of PCSK9 in the Th17 response and atherogenesis. We deleted the Pcsk9 gene from LDb mice to generate a triple knockout LTp mouse model. We report here that LTp triple knockout mice have significantly reduced atherosclerosis, virtually undetectable IL-17 in the atherosclerotic lesions, significantly reduced circulating IL-17 levels and reduced differentiation of IL-17-producing cells when compared with LDb mice. Thus, this study suggests that PCSK9 may play a role in modulating the inflammatory response and the development of atherosclerosis.
MATERIALS AND METHODS
Animal studies
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Double knockout LDb (Ldlr−/−Apobec1−/−) and triple knockout LTp (Ldlr−/−Apobec1−/−Pcsk9−/−) mice were generated in our laboratory as described (15,16). All mice were bred and maintained in a specific pathogen-free barrier facility in vivarium with a 12:12 h dark-light cycle, and maintained on a standard laboratory chow diet. All animal experiments were conducted in accordance with the guidelines of the Animal Protocol Review Committee of the University of Texas Health Science Center at Houston (IACUC approval No. AWC 18-0157).
Flow cytometry
Lymphoid cells were obtained from the thymus or spleen of wild-type (WT) (C57BL/6J), LDb or LTp mice at 5 months of age. For surface staining, we stained with PE or PerCp-Cy5.5 anti-CD4 (GK1.5), PerCp-Cy5.5 anti-CD8a (53-6.7), APC anti-CD44 (IM7), or Pacific Blue anti-γδTCR (UC7-13D5). For intracellular cytokine staining, isolated lymphoid cells were stimulated with PMA (100 ng/ml, Sigma-Aldrich, St. Louis, MO, USA) and ionomycin (1 μM, Sigma-Aldrich) in the presence of Brefeldin A and Monensin (eBioscience, San Diego, CA, USA) for 4 h, followed by surface staining. The cells were then resuspended in permeabilization buffer (eBioscience) for 30 min at 4°C, followed by staining with PE-conjugated anti-IL-17A (TC11-18H10.1), and Alexa488-conjugated anti-IFN-γ (clone: XMG1.2). The stained cells were analyzed by FACSAria II flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
ELISA
To measure the levels of IL-17A in the circulation, serum was collected from 5 months old of WT (C57BL/6J), LDb or LTp mice. Mouse IL-17A were measured from serum using mouse IL-17A ELISA kit according to the manufacturer's instruction (BioLegend, San Diego, CA, USA).
To measure the levels of IL-17A in CD4+CD44+ T cells, FACS sorted 2×105 of CD4+CD44+ T cells were re-stimulated with different concentration of anti-CD3 (2C11, 0 μg/ml, 0.5 μg/ml, and 5 μg/ml) for 3 days and then the supernatant was harvested. Mouse IL-17A were measured from the collected samples using a mouse IL-17A ELISA kit.
Quantitative real-time RT-PCR
FACS sorted CD4+CD44+ T cells were stimulated with anti-CD3 (1 μg/ml) for 4 h then total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using amfiRivert reverse transcriptase (GenDepot, Baker, TX, USA) according to the manufacturer's protocol. Gene expression was measured with iTaq-SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and the ABI-PRISM 7900 detection system (Applied Biosystems, Foster City, CA, USA). Data were normalized to expression of the β-actin gene. The primer pairs used in quantitative RT-PCR are described in our previous study (7).
Quantification of atherosclerotic lesions
Atherosclerotic lesions were quantified by 2 methods: en face and aortic root cross-section. We analyzed 10 male littermates per group (LDb and LTp) at 5-months of age for en face and 5 males per group for aortic root cross-section. The en face method measures atherosclerotic lesions throughout the whole aortic tree from the aortic root to the bifurcation of the iliac arteries. The aortic root cross section quantifies atherosclerotic lesions at the aortic root. Both methods were described in detail by others (17,18,19,20) and have been used routinely in our laboratory (7,15,16,21,22,23,24).
En face atherosclerotic lesions: Briefly, mouse aorta was dissected and cleaned to remove adventitial tissues. The aorta was then cut opened longitudinally, pinned and fixed overnight in 10% neutrally buffered formalin. The aorta was stained with freshly prepared filtered Oil Red O solution (1.56 mg/ml in methanol). The image of the whole aorta and atherosclerotic lesions was captured and scanned. We used SigmaScan Pro 4.0 imaging software (SPSS Inc., Chicago, IL, USA) to quantify the total area of the aorta and the area of atherosclerotic lesions. The results are presented as the ratio of lesions (mm2) divided by the total surface area of the aorta (mm2) expressed as a percentage.
Aortic root cross-section: The base of the heart containing the aortic sinus in each mouse was embedded in optimal cutting temperature compound at −80°C. The aortic sinus or aortic root was sequentially sectioned using a cryostat. Once all three aortic valves appeared, serial sections were collected at 5 μm/section. We collected 2 sections/slide and 9–10 slides/aorta until intact valves were no longer seen. Usually, approximately 18 sections were collected. We fixed and stained every other slide with Oil Red O. Six sections per aortic root were used for atherosclerotic lesion measurements. The images were captured by a Zeiss D1M microscope at 100× to cover the whole aortic root section. We carefully drew along the lesion areas which were measured using AxioVision Rel 4.8 software (Zeiss USA, Peabody, MA, USA). The results are presented as area of atherosclerotic lesions (μm2).
Immunohistochemical analysis on IL-17
Mouse aortic sinus tissue slides were fixed in 4% paraformaldehyde and were incubated with goat anti-IL-17 (sc-6077; Santa Cruz Biotechnology, Dallas, TX, USA) or isotype control. The slides were further incubated with Alexa594-conjugated anti-goat IgG (Invitrogen) and were mounted with mounting medium containing DAPI (vector). The slides were examined with a Zeiss Axio Observer.D1M fluorescence microscope with DAPI or Texas Red filters. The intensity was determined using NIH ImageJ software.
Statistical analysis
Comparison between two groups was performed using 2-tailed unpaired t-tests with Welch correction (GraphPad Prism Software, version7; Graphpad Software, San Diego, CA, USA). A 2-tailed p<0.05 was considered to be statistically significant. Comparison of three groups (C57BL/6J, LDb and LTp) was also analyzed by 1-way ANOVA.
RESULTS
Deletion of PCSK9 decreases atherosclerosis under hyperlipidemia condition
We have demonstrated that atherogenic LDb double knockout mice have elevated LDL cholesterol and develop atherosclerosis spontaneously (7,15,16,21,22,23,24). These mice also have increased plasma levels of IL-17 with increased numbers of Th17 cells in lymphoid organs (7). PCSK9 is a new gene recently been shown to regulate LDL cholesterol levels and modulate atherosclerosis development in humans and mice. PCSK9 was also shown to have immunological effects on activation and maturation of DCs and plaque T cells by oxidized low-density lipoprotein (oxLDL) (25). Thus, we proposed that PCSK9 could modulate IL-17 producing T cell differentiation in atherosclerosis development.
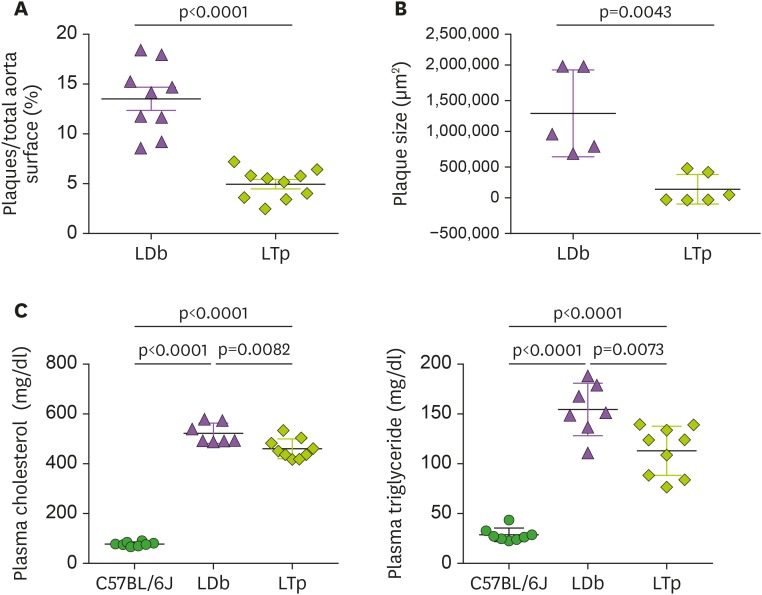
To understand the role of PCSK9 in the development of atherosclerosis, we deleted Pcsk9 gene from LDb mice to generate the triple knockout LTp mice. We have reported that triple knockout LTp mice have significantly lower total plasma cholesterol and triglyceride levels and markedly reduced atherosclerosis in the aorta when compared with LDb mice (15), which is confirmed in the current study. Using en face quantitation of the aortic lesions, the percentage of plaque to total aortic surface area was significantly lower in the LTp mice than in the LDb mice (LTp vs. LDb, 4.95%±0.47% vs. 13.52%±1.20%; p<0.0001) (Fig. 1A). Moreover, the total plaque area in the aortic sinus was approximately 8-fold less in the LTp mice than in the LDb mice (p=0.0043; Fig. 1B). Of note, although the plasma cholesterol and triglyceride levels in the LTp mice were lower than those in the LDb mice (plasma cholesterol of C57BL/6J, LTp, and LDb; 78±8.1 mg/dl, 460±40 mg/dl, 522±41 mg/dl, respectively, p<0.0001; plasma triglyceride of C57BL/6J, LTp, and LDb; 29±6.8 mg/dl, 113±25 mg/dl, 155±26 mg/dl, respectively, p<0.0001; see Fig. 1C), their levels were still more than 5-fold higher than those in the C57BL/6J mice. Thus, despite the markedly elevated lipid levels in the LTp mice relative to those seen in the WT animals, LTp mice had markedly reduced atherosclerosis.
Figure 1. The effect of PCSK9 on atherosclerosis development and on the levels of plasma cholesterol and triglyceride in LDb and LTp male mice at 5 months of age.
(A) The atherosclerotic lesions using en face quantification method on the aorta of LDb (n=10) and LTp (n=10) mice are expressed as % lesions, which were calculated as the ratio of aortic surface covered by plaques (in square millimeters) divided by the total surface of the whole aorta (in square millimeters). The results of % plaques of each aorta and mean±SD are shown. (B) The plaque area (in square microns) of each aortic sinus (LDb=5 and LTp=6) were quantified using AxioVision release 4.8 software (Zeiss). Each data point represents an average of 6 sections of aortic sinus for 1 animal. The plaque size (µm2) of each animal and mean±SD are shown. We used 2-tailed unpaired t-test with Welch's correction to analyze the difference between LDb versus LTp mice. The p values are shown. (C) The concentrations (mg/dl) of plasma cholesterol and triglyceride in C57BL/6J (n=7), LDb(n=7), and LTp (n=7) are presented.The mean±SD of each group is shown. Statistical analysis were performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. One-way ANOVA was also used to analyze the results.
PCSK9 is associated with alterations in CD4+ T cell populations in the thymus and spleen
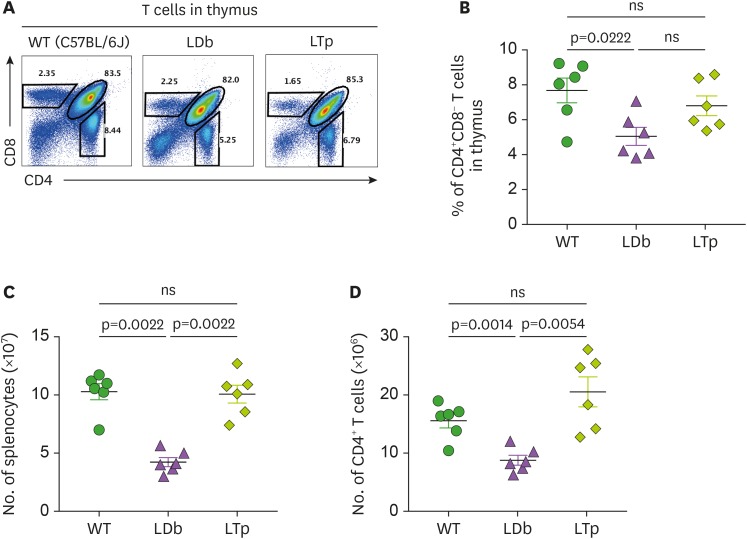
In view of our previous study demonstrating that increased IL-17 producing T cells were associated with atherosclerosis development in the LDb double knockout mice (7), we assessed the influence of deficiency of PCSK9 in T cell populations in the thymus and spleen. We first addressed frequencies of T cell population in the thymus. Using flow cytometry, we compared the frequencies of CD4+CD8− T cells in the thymus of C57BL/6J, LDb and LTp mice (Fig. 2A and B). LDb mice had significantly fewer CD4+CD8− cells, compared to WT C57BL/6J mice (LDb vs. C57BL/6J, 5.05%±0.51% vs. 7.68%±0.71%; p=0.0222). In contrast, LTp mice had increased levels of CD4+CD8− cells (LTp, 6.80%±0.57%) similar to those in the WT mice, but the increment was not significant, compared with LDb mice or C57BL/6J WT.
Figure 2. Analysis of T cell in the thymus and the spleen of C57BL/6J, LDb and LTp mice.
(A) A representative result of flow cytometry analysis showing the frequencies of T cell populations in the thymus of WT (C57BL/6J), LDb and LTp mice is shown. (B) The frequencies of CD4+CD8− T cells from the thymus of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. (C) The total number of splenocytes in the spleen of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. (D) The total number of CD4+ T cells in the spleen of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. Statistical analysis of results was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly.
ns, not significant.
Next, we compared the T cell population in the spleen of C57BL/6J, LDb and LTp mice. Similar to those described above in the thymus, in the spleen LDb mice had significantly fewer splenocytes, compared to WT C57BL/6J mice and LTp mice (LDb, C57BL/6J, and LTp; 4.23±0.95×107, 10.3±1.69×107, and 10.1±1.87×107, respectively; p=0.022 for LDb vs. C57BL/6J and p=0.022 for LDb vs. LTp; see Fig. 2C). LDb mice also had fewer CD4+ cells due to their poor cellularity (LDb, C57BL/6J, and LTp; 8.78±2.07×106, 15.57±3.01×106, and 20.28±6.32×106, respectively; p=0.0014 for LDb vs. C57BL/6J and p=0.0054 for LDb vs. LTp; see Fig. 2D). Notably, LTp mice showed normal splenic cellularity and recovered the levels of CD4+ cells to that of C57BL/6J mice. Together, LTp mice showed comparable levels of CD4+ T cells in both thymus and spleen to those in the WT animals, whereas LDb mice had fewer CD4+ T cells in either the thymus or the spleen.
Deficiency of PCSK9 reduces IL-17 levels in plasma and atherosclerotic lesions and decreases IL-17 producing cells
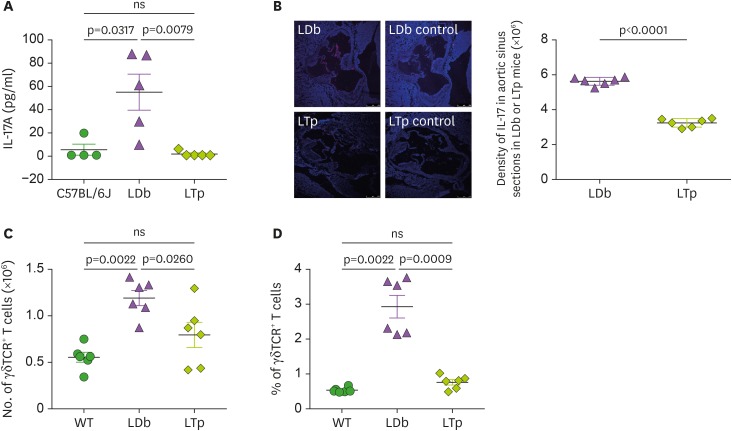
Next, we studied the effect of deficiency of PCSK9 on IL-17 producing cells. Plasma IL-17 levels were markedly reduced in the LTp mice to levels lower than in WT C57BL/6J mice (C57BL/6J, LTp, and LDb; 4.75±4.75 pg/ml, 1.07±1.07 pg/ml, and 54.3±15.5 pg/ml, respectively; Fig. 3A). Importantly, LTp mice also had markedly reduced levels of IL-17 in the lesions in the aortic sinus when compared with LDb mice (Fig. 3B). We, then, studied γδTCR+ T cells, one of the major sources of IL-17 (26,27). Using flow cytometry, we evaluated the total number of γδTCR+ T cells (Fig. 3C) and the percentage of γδTCR+ T cells (Fig. 3D) in the spleen of C57BL/6J, LDb and LTp mice. LTp mice had similar levels of γδTCR+ T cells as those in the C57BL/6J mice, whereas LDb mice had significantly higher levels of γδTCR+ T cells (LTp, C57BL/6J, and LDb; 0.80±0.14×106, 0.56±0.05×106, 1.19±0.08×106, respectively). Together, LTp mice have reduced levels of γδTCR+ T cells in the spleen.
Figure 3. The effect of PCSK9 on IL-17 in C57BL/6J (WT), LDb and LTp mice.
(A) LTp mice had barely detectable plasma levels of IL-17. IL-17 levels in serum obtained from C57BL/6J (n=4), LDb (n=5), and LTp (n=5) mice were measured by ELISA and expressed as pg/ml. (B) LTp mice had barely detectable expression of IL-17 in the aortic sinus. Aortic sinus sections obtained from LDb (n=6) and LTp (n=6) mice were stained with isotype control or polyclonal anti-IL-17 (red) and DAPI (blue). We quantified the intensity of immunofluorescence images expressing IL-17 from each section. LDb mice show increased expression of IL-17 in the aortic sinus, compared to LTp mice. The densities of the expressions of IL17 were determined. (C). LTp mice had decreased numbers of γδTCR+ T cells, compared to LDb mice. The absolute numbers of γδTCR+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice were analyzed by flow cytometry. (D) LTp mice had decreased % of γδTCR+ T cells, compared to LDb mice. The frequency of γδTCR+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice were analyzed by flow cytometry. All results are presented as mean±SD in this figure. Statistical analysis of the esults was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly.
ns, not significant.
Deficiency of PCSK9 in the atherogenic mice affects T cell differentiation
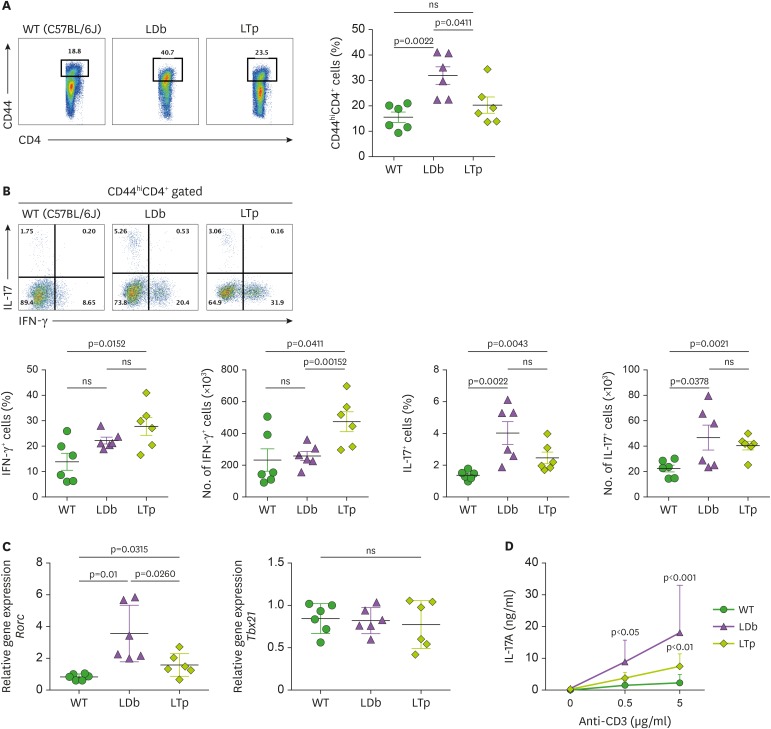
Next, we addressed whether the deficiency of PCSK9 in the atherogenic condition affects T cell differentiation. By flow cytometry, we analyzed the effector-memory CD4+ T cells (CD44hiCD4+ T cells) in the spleen (Fig. 4A). LDb mice had the highest percentage of CD44hiCD4+ T cells, compared to C57BL/6J or LTp mice (LDb, C57BL/6J, LTp; 31.9%±3.4%, 15.6%±2.0%, and 20.3%±3.2%, respectively; p=0.0022 for LDb vs. C57BL/6J and p=0.0411 for LDb vs. LTp; Fig. 4A). Among them, LTp mice had significantly higher percentage and number of IFN-γ producing cells when compared to those of WT mice (LTp vs. C57BL/6J, 28%±8.7% vs. 14%±8.2%, p=0.0152; LTp vs. C57BL/6J, 473×103±154 vs. 232×103±172, p=0.0411). But, there was no significant difference between LDb versus C57BL/6J mice or LDb versus LTp mice. Thus, there were no differences in the Th1 cells.
Figure 4. Deficiency of PCSK9 reduces the expression and secretion of IL-17 from Th subset cells (CD44hiCD4+) obtained from the spleens of C57BL/6J (WT), LDb, and LTp mice.
(A) The frequencies of CD44hiCD4+ T cells from the spleen of WT (C57BL/6J), LDb, and LTp mice were analyzed using flow cytometry. The results of the frequencies of CD44hiCD4+ T cells and mean±SD are shown on the right. (B) The frequencies of IFN-γ and IL-17 expressing cells from CD44hiCD4+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice are analyzed by flow cytometry. The frequencies and total number of IFN-γ and IL-17 expressing cells from CD44hiCD4+ T cells are presented and mean±SD are also shown. (C) The relative expression levels of mRNA transcripts of Rorc and Tbx21 genes from the flow cytometry sorted CD44hiCD4+T cells of the spleen of WT (C57BL/6J), LDb, and LTp mice (n=6 from each strain) were determined using real-time qPCR. The sorted CD44hiCD4+T cells were stimulated with anti-CD3 (1 μg/ml) for 4 h, followed by RNA extraction and qPCR determination. The RNA levels from each animal and the mean±SD are shown. Statistical analyses of results in figures A to C was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly. ns, not significant. (D) Re-stimulated CD44hiCD4+ T cells from LDb mice secret more IL-17. The flow cytometry sorted CD44hiCD4+ T cells (2×105 cells) from the spleen of WT (C57BL/6J), LDb, and LTp mice were re-stimulated with different concentrations of anti-CD3 (0, 0.5, and 5 µg/ml) for 3 days. The IL-17 (ng/ml) secreted to the media was measured using ELISA. The results are presented as mean±SD at each concentration. Data represents 3 independent experiments. Statistical analysis was performed using 2-way ANOVA. The p values are listed.
ns, not significant.
Importantly, LTp mice had fewer IL-17 producing cells than LDb mice, but the difference did not reach significance levels (LTp vs. LDb, 40±8.2×103 vs. 46±24×103; LTp vs. LDb, 2.4%±0.9% vs. 4.0%±1.8%; p values were not significant) (Fig. 4B). We also quantified the effector-memory T cells' master transcription factor expression levels in CD44hiCD4+ sorted T cells from the spleen (Fig. 4C). These cells have been stimulated with anti-CD3 at 1 µg/ml for 4 h. LDb mice had the highest relative levels of Rorc, Th17 cells master transcription factor, mRNA expression when compared to those in LTp or WT C57BL/6J mice (LDb, LTp, and C57BL/6J; 3.56±0.1.78, 1.58±0.71, and 0.83±0.18, respectively; p=0.0260 for LDb vs. LTp and p=0.01 for LDb vs. C57BL/6J; Fig. 4C). The mRNA levels of Tbx21, Th1 cells master transcription factor, were similar among the WT C57BL/6J, LDb, and LTp mice (C57BL/6J, LDb, and LTp; 0.85±0.17, 0.82±0.15, and 0.77±0.28, respectively; Fig. 4C). Thus, the gene expression levels corroborated the T cell populations data.
To further evaluate the production of IL-17 from CD44hiCD4+ T cells in C57BL/6J, LDb, and LTp mice, we re-stimulated these CD44hiCD4+ T cells with plate-bound anti-CD3 Ab. Significantly more IL-17A was produced from LDb mice when compared to LTp or C57BL/6J WT mice. (LDb, LTp, and C57BL/6J; 8.9±6.8 ng/ml, 3.8±1.8 ng/ml, and 1.5±1.3 ng/ml at 0.5 µg/ml, respectively; LDb, LTp, and C57BL/6J; 18±15 ng/ml, 7.5±3.9 ng/ml, and2.3±2.6 ng/ml at 5 µg/ml, respectively; Fig. 4D). Collectively, these results demonstrate that deletion of PCSK9 in LTp mice results in decreased the IL-17 in the circulation and reduced Th17 cell populations in the spleen. These results indicate that the deficiency of PCSK9 in the proatherogenic condition reverts the immune phenotypes to normal conditions when compared to WT mice.
DISCUSSION
In this study, we confirmed that atherogenesis could be modified by PCSK9 in the absence of LDLR. Even though LTp mice had lower lipid levels than LDb double knockout mice (the parental strain), LTp mice still have plasma lipid levels at 5- to 6-fold higher levels than the WT C57BL/6J animals. Yet, in the setting of dyslipidemia, LTp mice had virtually no plaque formation in the aortic sinus or the whole aorta, while LDb mice had extensive atherosclerosis. Since the atherogenic LDb mice had increased serum IL-17 levels and T cell programming shifted towards Th17 cells (7), we examined the effect of PCSK9 on IL-17 production in this study. We demonstrated that PCSK9 expression is associated with changes in T cell programming and IL-17 production. LTp mice were found to have reduced plasma and tissue levels of IL-17 when compared with LDb mice. The reduced IL-17 levels in LTp mice were associated with changes in major cellular sources of IL-17, namely Th17 cells and γδTCR+ T cells. In the LTp mice, both the number and proportions of Th17 cells and γδTCR+ T cells were decreased. In addition, Rorc expression level was decreased in the LTp mice. Furthermore, re-stimulated CD44hiCD4+ T cells harvested from these animal models demonstrated different levels of IL-17 secretion, namely LTp mice had much lower levels of IL-17 secretion than the atherogenic LDb mice. In conclusion, the expression of PCSK9 is associated with changes in the polarization of IL-17-producing cells independent of its known effects on LDLR. This study represents the first study to demonstrate that PCSK9 can regulate atherogenesis and this is in part, mediated by changes in T cell programming and IL-17 production.
PCSK9 is best known for its effect on LDLR turnover, and its inhibition has resulted in dramatic reduction in plasma LDL cholesterol levels. In addition to its LDLR-dependent mechanisms, PCSK9 also has other LDLR-independent functions. Studies have shown that PCSK9 can directly interact with other receptors, such as the apoE receptor (28), LDLR-related protein 1 (29), VLDL receptor (30), CD36 (31), CD81 (32), and epithelial Na+ channel (33). We have shown that PCSK9 can directly control hepatic lipoprotein production via its interaction with cytoplasmic apoB which subsequently inhibits the degradation of apoB in the hepatocytes and results in increased secretion of proatherogenic VLDL and LDL (15).
In this study, we specifically studied the association between PCSK9 and the adaptive immune response in T cells. We observed that the expression of PCSK9 had a profound effect on the expansion of IL-17-producing cells. A recent study also demonstrated that PCSK9 overexpression resulted in T cell-mediated inflammatory response in the lung and liver, disrupted T cell homeostasis and induced T cell expansion (34). Increased γδTCR+ T cells were found in hypercholesterolemic animals with atherosclerosis (35) and our study supports the mechanism that a reduction of γδTCR+ T cells may attenuate atherogenesis. Studies using anti-PCSK9 Ab in BALB/c mice (36) or siRNA in human T cells (25) to inhibit PCSK9 show lower production of IFN-γ- and IL-17-producing cells. In comparison to C57BL/6J WT mice in our study, the CD44hiCD4+ T cells from LTp mice had increased IFN-γ- and IL-17-producing cells, but lower production of IL-17 cells than that of LDb mice. These suggest that hyperlipidemia play a role in activation of these effector cells. Th1 and Th17 lineages is complex and could be counter-regulate each other (37), it is possible by inhibiting one target may cause an exacerbation of the other. Thus, this could explain that in CD44hiCD4+ T cells in LTp mice we observed increased IFN-γ- and IL-17-producing cells, compared to C57BL/6J WT mice.
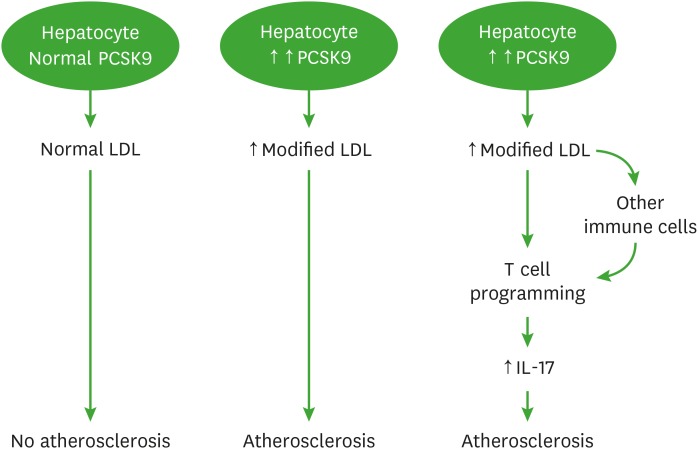
When stimulated with anti-CD3 Abs, the amount of IL-17A secreted by CD44hiCD4+ T cells was different between LDb and LTp mice, suggesting increased number of Th17 cells in LDb derived T cells. Our in vivo results confirmed that LDb mice have increased numbers of Th17 cells. Since dyslipidemia is present in both LDb and LTp mice, the question is whether PCSK9 alone could affect T cells differentiation. We have shown that PCSK9 alters VLDL and LDL metabolism in hepatocytes, producing proatherogenic LDLs which contain more cholesterol esters and phospholipids (15). Human subjects treated with a PCSK9 inhibitor, produced lipoprotein particles with significant reduction in sphingolipids, cholesterol esters and free cholesterol levels (38). Thus, the effects of PCSK9 on compositional changes in LDL may affect their likelihood of oxidation. Indeed, PCSK9 affects oxLDL-induced DC maturation (25). oxLDL skews T cells differentiation to proatherogenic conditions (7). Ceramide, has also been shown to alter T cell biology (39). Collectively, changes in lipid composition in LDL in the setting of dyslipidemia may alter T cell response via cholesterol enrichment of T cell membranes that promotes Th1 differentiation of CD4+ T cells (40) or nanoclustering of T cell receptors in the cholesterol-enriched cell membrane that subsequently enhances the interactions between T cell receptors and Ag (41). We hypothesized that PCSK9 influences LDL compositions in the liver to produce modified LDLs, and these modified LDLs, in turn, could act on T cells to alter T cell biology. It is also possible that the modified LDLs stimulated cytokine production from Ag presenting cells (APCs) such as DCs, macrophages, B cells, or non-professional APC, the different cytokines produced from the APCs could direct T cells into particular subtypes (42). We present a schematic diagram (Fig. 5) to illustrate the potential mechanism of this study.
Figure 5. A schematic diagram illustrates the role of PCSK9 mediates atherosclerosis via immune cells.
This diagram displays the role of PCSK9 in atherosclerosis and immune response mediated via IL-17 cells and other immune cells including Th1, Treg and Tfh cells. Under normal lipidemic condition with low PCSK9 levels, the hepatocytes produce and secrete normal LDLs, these LDLs do not influence the development of atherosclerosis. Under hyperlipidemia condition with increased PCSK9 levels, the hepatocytes produce increased amounts of modified LDLs, which are cholesterol ester and phospholipid enriched. These modified LDLs contribute to the development of atherosclerosis by possibly altering T cells programing shifted towards IL-17 producing T cells to increase IL-17 production or via induce cytokines production to modulating immune cells including Th1, Treg or Tfh T cells to influence IL-17 production. Increased IL-17 contributes to the development of atherosclerosis.
Given that plasma and tissue IL-17 levels were elevated in the LDb mice and suppressed in the LTp mice, we explored the cell types and signaling pathways responsible for such changes. In LDb mice, the expression of PCSK9 was associated with the expansion of IL-17-producing cells. The production of IL-17 was driven by the master transcription switch, Rorc. A previous study showed that hyperlipidemia promoted Rorc expression in the adipose tissues (43), but it is yet to be determined if enhanced Rorc expression is related to other factors, such as TGF-β (44). IL-17 is a pro-inflammatory cytokine that functions via mesenchymal and myeloid cells to induce secretion of diverse cytokines, chemokines, anti-microbial peptides and matrix metalloproteinases. IL-17 promotes endothelial cell activation via the p38MAPK pathway (45); increased expression of IL-17A receptor in apoE knockout mice reduces atherosclerotic lesions (46). IL-17 also works synergistically with hypoxia and promotes migration and invasion of pro-inflammatory cells (47). In addition, IL-17 is a pro-angiogenic factor (48), which may promote neovascularization in complex atherosclerotic lesions. Despite these multiple mechanisms that might explain the pro-atherogenic properties of IL-17, the role of IL-17 in atherosclerosis remains controversial. Inhibition of IL-17A by anti-IL-17A Abs (49), by the RORα/γ inverse agonist (50) and by IL-17A ablation (49) were effective in attenuating atherosclerotic lesion development in apoE mice. However, there is concern that the rat origin of the anti-IL-17A Abs may promote a Th2 response and may in part be responsible for the anti-atherogenic effects of the neutralizing Ab. In fact, studies using mouse-derived anti-mouse IL-17 Ab (51) and disruption of IL-17 expression in bone marrow-derived cells (52) did not alter atherosclerosis.
The presence of both LDLR and PCSK9 in apoE mice may confound the interpretation of the contribution of IL-17 in atherogenesis. This was taken into consideration in the current study by utilizing LTp mice with deletion of both LDLR and Pcsk9 genes. However, other studies suggested that IL-17 may stabilize plaque morphology via enhanced TGF-β signaling and collagen production by vascular smooth muscle cells (11,53). It is highly likely that the effects of IL-17 may be dependent on the inflammatory context. For instance, in the presence of anti-inflammatory combination of TGF-β1 and IL-6, there may be increased formation of IL-10-producing regulatory Th17 which may be protective against pathogenic Th1 cell differentiation (8). Conversely, in the presence of pro-inflammatory IFN-γ, a concomitant increase in IL-17 may be pro-atherogenic (9).
Finally, our study suggests that PCSK9 cross-links hyperlipidemia, atherosclerosis and immune responses mediated via IL-17-producing cells. Different subtypes of T cells yield different effects on atherogenesis. For example, studies in mice have provided that Th1 cells are atherogenic (25,54), while Th2 cells and Treg are atheroprotective (55,56,57); the role of Th17 is controversial (12,58), but our previous (7) and current studies provided evidence that Th17 is atherogenic. Moreover, recent studies demonstrate that T follicular helper (Tfh) cells promote atherosclerosis under a dyslipidemic condition (59,60,61). What is the role of PCSK9 in all these different T cells subtypes? There is no evidence of direct role of PCSK9 on T cells; most studies examined PCSK9 in T cells are showing association effects of PCSK9 on activation of T cells by either through increasing cholesterol or oxLDL. Thus, all those studies support our hypothesis that PCSK9 influences the production of modified LDL, which affect T cell homeostasis.
It is evident from our study that PCSK9 attenuates the development of atherosclerosis and this phenomenon may not be dependent on the well-known interactions between PCSK9 and LDLR. We have also demonstrated that the pro-atherogenic effects of hyperlipidemia could be modified when PCSK9 is absent and this may be explained by altered T cell programming and IL-17 secretion by T cells in our LTp mouse model. Future studies will need to elucidate if the programming of other T cell subsets may also be altered in the setting of dyslipidemia and PCSK9 expression.
ACKNOWLEDGEMENTS
We thank Hua Sun from Research Center for Human Genetics, UTHealth for his excellent techniques for this study. The Flow Cytometry Core Facility is supported in part by the shared instrumentation grant (RP 110776) from the Cancer Prevention and Research Institute of Texas. The work was supported by research grant funded by the National Institute of Health (grant No. R01 HL118361-01A). We thank the editorial support of Dr. Eva Zsigmond from Institute of Molecular Medicine at UTHealth.
Abbreviations
- APC
antigen presenting cell
- apo
apolipoprotein
- DC
dendritic cell
- LDb
Ldlr−/−Apobec1−/−
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LTp
Ldlr−/−Apobec1−/−Pcsk9−/−
- ns
not significant
- oxLDL
oxidized low-density lipoprotein
- PCSK9
proprotein convertase subtilisin/kexin type 9
- Tfh
T follicular helper
- VLDL
very low-density lipoprotein
- WT
wild-type
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Teng BB.
- Formal analysis: Kim YU, Teng BB.
- Investigation: Kim YU.
- Methodology: Kim YU, Kee P, Danila D.
- Writing - original draft: Kim YU.
- Writing - review & editing: Kee P, Kim YU, Danila D, Teng BB.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16:1978–1990. doi: 10.1111/j.1582-4934.2012.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012;28:631–641. doi: 10.1016/j.cjca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014;40:153–165. doi: 10.1016/j.immuni.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 9.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 11.Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 12.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci U S A. 2005;102:2069–2074. doi: 10.1073/pnas.0409736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 15.Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res. 2018;59:207–223. doi: 10.1194/jlr.M078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng BB. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 2012;32:1585–1595. doi: 10.1161/ATVBAHA.112.250043. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009;573:1–15. doi: 10.1007/978-1-60761-247-6_1. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–138. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 20.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 21.Dutta R, Singh U, Li TB, Fornage M, Teng BB. Hepatic gene expression profiling reveals perturbed calcium signaling in a mouse model lacking both LDL receptor and Apobec1 genes. Atherosclerosis. 2003;169:51–62. doi: 10.1016/s0021-9150(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 22.Mak S, Sun H, Acevedo F, Shimmin LC, Zhao L, Teng BB, Hixson JE. Differential expression of genes in the calcium-signaling pathway underlies lesion development in the LDb mouse model of atherosclerosis. Atherosclerosis. 2010;213:40–51. doi: 10.1016/j.atherosclerosis.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh U, Zhong S, Xiong M, Li TB, Sniderman A, Teng BB. Increased plasma non-esterified fatty acids and platelet-activating factor acetylhydrolase are associated with susceptibility to atherosclerosis in mice. Clin Sci (Lond) 2004;106:421–432. doi: 10.1042/CS20030375. [DOI] [PubMed] [Google Scholar]
- 24.Nischal H, Sun H, Wang Y, Ford DA, Cao Y, Wei P, Teng BB. Long-term expression of apolipoprotein B mRNA-specific hammerhead ribozyme via scAAV8.2 vector inhibits atherosclerosis in mice. Mol Ther Nucleic Acids. 2013;2:e125. doi: 10.1038/mtna.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu A, Frostegård J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med. 2018;284:193–210. doi: 10.1111/joim.12758. [DOI] [PubMed] [Google Scholar]
- 26.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepanova H, Mensikova M, Chlebova K, Faldyna M. CD4+ and γδTCR+ T lymphocytes are sources of interleukin-17 in swine. Cytokine. 2012;58:152–157. doi: 10.1016/j.cyto.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kysenius K, Muggalla P, Mätlik K, Arumäe U, Huttunen HJ. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol Life Sci. 2012;69:1903–1916. doi: 10.1007/s00018-012-0977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canuel M, Sun X, Asselin MC, Paramithiotis E, Prat A, Seidah NG. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1) PLoS One. 2013;8:e64145. doi: 10.1371/journal.pone.0064145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Commun. 2008;375:69–73. doi: 10.1016/j.bbrc.2008.07.106. [DOI] [PubMed] [Google Scholar]
- 31.Demers A, Samami S, Lauzier B, Des Rosiers C, Ngo Sock ET, Ong H, Mayer G. PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol. 2015;35:2517–2525. doi: 10.1161/ATVBAHA.115.306032. [DOI] [PubMed] [Google Scholar]
- 32.Labonté P, Begley S, Guévin C, Asselin MC, Nassoury N, Mayer G, Prat A, Seidah NG. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology. 2009;50:17–24. doi: 10.1002/hep.22911. [DOI] [PubMed] [Google Scholar]
- 33.Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9) J Biol Chem. 2012;287:19266–19274. doi: 10.1074/jbc.M112.363382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proto JD, Doran AC, Subramanian M, Wang H, Zhang M, Sozen E, Rymond CC, Kuriakose G, D'Agati V, Winchester R, et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J Clin Invest. 2018;128:2370–2375. doi: 10.1172/JCI97785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momtazi-Borojeni AA, Jaafari MR, Badiee A, Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. 2019;283:69–78. doi: 10.1016/j.atherosclerosis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilvo M, Simolin H, Metso J, Ruuth M, Öörni K, Jauhiainen M, Laaksonen R, Baruch A. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis. 2018;269:159–165. doi: 10.1016/j.atherosclerosis.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Adam D, Heinrich M, Kabelitz D, Schütze S. Ceramide: does it matter for T cells? Trends Immunol. 2002;23:1–4. doi: 10.1016/s1471-4906(01)02091-9. [DOI] [PubMed] [Google Scholar]
- 40.Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS One. 2012;7:e38733. doi: 10.1371/journal.pone.0038733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krauzová E, Kračmerová J, Rossmeislová L, Mališová L, Tencerová M, Koc M, Štich V, Šiklová M. Acute hyperlipidemia initiates proinflammatory and proatherogenic changes in circulation and adipose tissue in obese women. Atherosclerosis. 2016;250:151–157. doi: 10.1016/j.atherosclerosis.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 45.Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, et al. Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J Biol Chem. 2016;291:4939–4954. doi: 10.1074/jbc.M115.690081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α pathway. Mol Immunol. 2013;53:227–236. doi: 10.1016/j.molimm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 49.Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, Haller H, von Vietinghoff S. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor a and are normalized by inhibition of interleukin-17A. JACC Basic Transl Sci. 2018;3:54–66. doi: 10.1016/j.jacbts.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billon C, Sitaula S, Burris TP. Inhibition of RORα/γ suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab. 2016;5:997–1005. doi: 10.1016/j.molmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 52.Ge S, Hertel B, Koltsova EK, Sörensen-Zender I, Kielstein JT, Ley K, Haller H, von Vietinghoff S. Increased atherosclerotic lesion formation and vascular leukocyte accumulation in renal impairment are mediated by interleukin-17A. Circ Res. 2013;113:965–974. doi: 10.1161/CIRCRESAHA.113.301934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Östberg T, Yan ZQ, Kuchroo VK, Hansson GK, Wahren-Herlenius M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res. 2018;114:158–167. doi: 10.1093/cvr/cvx181. [DOI] [PubMed] [Google Scholar]
- 54.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation. 2018;138:1130–1143. doi: 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016;13:167–179. doi: 10.1038/nrcardio.2015.169. [DOI] [PubMed] [Google Scholar]
- 57.Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018;49:666–677.e6. doi: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095. doi: 10.1038/s41467-018-03493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nus M, Sage AP, Lu Y, Masters L, Lam BY, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med. 2017;23:601–610. doi: 10.1038/nm.4315. [DOI] [PubMed] [Google Scholar]
- 61.Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, Ahn CW, Kim YC, Kim WU, Lee SH, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol. 2018;19:583–593. doi: 10.1038/s41590-018-0102-6. [DOI] [PubMed] [Google Scholar]