Abstract
Several gut commensals have been shown to modulate host immune response. Recently, many food derived microbes have also been reported to affect the immune system. However, a mechanism to identify immunostimulatory and immunoregulatory microbes is needed. Here, we successfully established an in vitro screening system and identified an immunoregulatory bacterium, Lactobacillus pentosus KF340 (LP340), present in various fermented foods. LP340 induced a regulatory phenotype in mice Ag presenting cells which, in turn, induced IL-10 and IFN-γ producing Type 1 regulatory T cells (Tr1 cells) from naïve CD4+ T cells. Naïve CD4+ T cells co-cultured with LP340 treated dendritic cells highly expressed cytokine receptor IL-27R and were CD49b and lymphocyte-activation gene 3 double positive. Oral administration of LP340 in mice with atopic dermatitis reduced cellular infiltration in affected ear lobes and serum IgE levels, thus, ameliorating the disease symptoms. This suggests a systemic immunoregulatory effect of LP340. These findings demonstrate that LP340, a bacterium derived from food, prevents systemic inflammation through the induction of IL-10 producing Tr1 cells.
Keywords: Tr1 cells, Lactobacillus pentosus, Interleukin-10, IL-10, Atopic dermatitis
INTRODUCTION
Several investigations into human and animal microbiome have established that microbial population in the gut, skin, lungs and other organs of body are just not an innocuous presence, rather they actively communicate with the host. Microbes not only help in various physiological processes of the human body but also influence the immune system. Commensals help in development as well as education of the host immune system in calibrating immune response against pathogens and pathobionts along with establishing immune tolerance towards innocuous and beneficial commensals (1). This can be appreciated by studies on germ free mice, whose immune system is both structurally and functionally compromised (2).
Microbes found in various sites of host body not only regulate the local immune response but are equally effective in modulating a systemic reaction. It has been shown how maternal microbes affect the immune development of fetus (3). External bacteriotherapy has been reported to ameliorate various autoimmune diseases in mice (4) by generating regulatory dendritic cells (DCs) and food allergy in human infants by reestablishing retinoic acid receptor-related orphan receptor gamma t expression in gut Tregs via a myeloid differentiation primary response 88 dependent pathway (5).
Recently, it has been reported that human microbiome is largely dependent on environmental factors like household, diet, etc. (6). Thus, probiotics of food origin provide a unique opportunity as they can be administered and established inside the human body without any major alteration in homeostasis. Probiotics have been shown to aid in inflammatory bowel disease in humans (7). Probiotic Lacobacillus reuteri has been shown to ameliorate experimental autoimmune encephalitis (8), arthritis (9), neuropsychiatric disorders like autism etc. in mice (10).
Type 1 regulatory T cells (Tr1 cells) are an immunosuppressive subset of CD4+ T cells which regulate an inflammatory response by higher IL-10 secretion. Tr1 cells are forkhead box protein 3 (FOXP3)− and produce IL-10 as well as IFN-γ (11). They are characterized by cell surface expression of both lymphocyte-activation gene 3 (LAG3) and CD49b (12). IL-27, a heterodimeric cytokine of IL-12 family, produced by Ag presenting cells (APCs) is the primary cytokine helping in differentiation of Tr1 cells (13). These cells have been reported to be important regulatory T cell type in various contact and atopic dermatites (14) and central nervous system diseases (15).
In this study, we screened several food derived bacteria to select immunoregulatory probiotic strains. We found that Lactobacillus pentosus KF340 (LP340) induced higher IL-10 production from APCs. Earlier, it has been reported that LP340 induced IL-10 production from peyer's patch DCs. These DCs induced regulatory splenic B10 cells which were protective in house dust mite (HDM) induced dermatitis in mice (16). However, here, we report that LP340 induces both local and systemic APCs to produce IL-27 and IL-10 which propels differentiation of naïve CD4+ T cells into IL-10 producing Tr-1 cells. These cells expressed high IL-27R and both CD49b and LAG3 on the cell surface. Functionally, these cells produced IL-10 and IFN-γ to regulate the inflammation. We further demonstrate therapeutic importance of LP340 mediated Tr1 cells in a mouse model of atopic dermatitis (AD). These findings establish that food derived probiotic LP340 induces tolerogenic APCs which in turn generate immunosuppressive Tr1 cells to moderate an inflammatory immune response.
MATERIALS AND METHODS
Screening of probiotics
All mice were maintained under pathogen-free condition at Pohang University of Science and Technology animal facility and experimental procedures were approved by Institutional Animal Care and Use Committee (IACUC, Approval No. POSTECH-2014-009) of POSTECH. Probiotics used in this study were provided by Korea Food Research Institute (Korea). Mesenteric lymph nodes (mLNs) from C57BL/6 mice were isolated and total lymphocytes were harvested by mechanical disruption. Cells were suspended in RPMI (Welgene, Gyeongsan, Korea) supplemented with 10% FBS (Hyclone, Smithfield, Australia), 10mM HEPES (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin (Sigma-Aldrich) and 100 U/ml streptomycin (Sigma-Aldrich) and 0.05mM 2-beta-mercaptoethanol (Sigma-Aldrich). The 2×105 cells were plated in 96 well plate and co-cultured with probiotic strains in 1:10 (cells:probiotic colony forming unit [CFU]) ratio for 72 h in presence of 150 μg/ml Gentamicin (Gibco; Thermo Fisher Scientific, Waltham, MA, USA). Supernatant was collected and used for IL-10 & IL-12 ELISA (eBioscience, San Diego, CA, USA), as per manufacturer's protocols.
Isolation and in vitro culture of cells
Splenic CD11c+ DCs or F4/80+ macrophages were isolated using magnetic beads (Miltenyi Biotec, Auburn, CA, USA), according to the manufacturer's protocol. Naïve CD4+ T-cells were isolated by negative selection (Stemcell technologies, Vancouver, Canada), as per manufacturer's instructions. For intestinal lamina propria DCs (LPDCs), small intestines were cleared of fat tissues and peyer's patches, cut longitudinally and washed with PBS. Intestines were then cut into pieces of approximately 1 cm length and incubated for 20 min at 37°C in PBS containing 10mM EDTA. Epithelial cells were strained out after vigorous shaking and tissue fragments were then minced and digested with 400 U/ml collagenase D (Roche, Basel, Switzerland) and 100 μg/ml DNase I (Roche) at 37°C for 45 min. LPDCs were enriched using density gradient centrifugation in 40% and 75% (v/v) Percoll (GE Healthcare, Chicago, IL, USA). For preparation of CD11c+ DCs, total cells were sorted by MoFlo Astrios (Beckman Coulter, Brea, CA, USA) as CD11b− CD11c+ MHCII+ DCs.
Splenic DCs (spDCs) or macrophages were incubated with LP-340 for 12–14 h at a ratio of 1:10 or 1:100 (cells:CFU), in presence of GM-CSF (10 ng/ml) and gentamicin (150 μg/ml). Probiotics were washed out after incubation and naïve CD4+ T-cells were added in fresh media containing anti-CD3 (0.01 μg/ml) and IL-2 (2 U/ml). Human TGF-β@0.1 ng/ml or 1 ng/ml or rIL-27@1 ng/ml (R&D systems, Minneapolis, MN, USA) were added wherever required. After 72 h, supernatant was collected, and cells were re-stimulated with PMA (50 ng/ml), ionomycin (2 μM) in presence of GolgiStop (BD biosciences, San Jose, CA, USA) for 5 h to detect cytokines by flow cytometry. For analysis of cytokine production by APCs, CD11c+ APCs were incubated with probiotics at 1:10 (cells:CFU) ratio in the presence of GM-CSF and gentamicin for 12 h or 48 h for quantitative RT-PCR (qRT-PCR) or ELISA, respectively. FACS Abs used were CD11b (M1/70), CD11c (N418), CD4 (RM4-5), CD8 (53-6.7), CD45 (30-F11), CD86 (GL-1), F4/80 (BM8), MHCII (M5/114.15.2), Gr1 (RB6-8C5), and CD49b (DX5) from Biolegend; LAG3 (eBioC9B7W), and FOXP3 (FJK-16s) were purchased from eBiosciences. To determine cytokine production, collected supernatants were used for detection of IFN-γ, IL-10, IL-12, IL-17a, and IL-27 using eBiosciences ELISA kits, according to manufacturer's protocols. IgE levels were determined in serum using IgE ELISA kits (BD Biosciences).
Quantitative real-time PCR
To examine the level of transcripts, total RNAs were isolated using TRIzol reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer's protocol. For reverse transcription, 500 ng to 1 μg of total RNA was used for cDNA was synthesis using the Improm-II reverse transcription system (Promega, Madison, WI, USA). The cDNA was subjected to quantitative real-time PCR using SYBR Premix Ex Taq (Takara, Kusatsu, Japan), primers and DNA Engine with Rotor-gene Q (Qiagen, Germantown, MD, USA). The data were normalized using the expression level of hypoxanthine phosphoribosyltransferase. The sequences of primers used for qRT-PCR are listed in Table 1.
Table 1. Primer sequences for qRT-PCR.
| Gene | Primer sequence (5′ → 3′) | |
|---|---|---|
| Hprt | F | TTATGGACAGGACTGAAAGAC |
| R | GCTTTAATGTAATCCAGCAGGT | |
| Cd80 | F | ACCCCCAACATAACTGAGTCT |
| R | TTCCAACCAAGAGAAGCGAGG | |
| Cd86 | F | TGTTTCCGTGGAGACGCAAG |
| R | CAGCTCACTCAGGCTTATGTTTT | |
| H2Ab | F | CACTCTGGTCTGTTCGGTGAC |
| R | CCTCTCCCTGATGAGGGGTC | |
| Cd274 | F | GGAATTGTCTCAGAATGGTC |
| R | GTAGTTGCTTCTAGGAAGGAG | |
| Ilt3 | F | ATGGGCACAAAAAGAAGGCTAA |
| R | CTGTGTCCTGATGCACAACTG | |
| Ido | F | GCTTTGCTCTACCACATCCAC |
| R | CAGGCGCTGTAACCTGTGT | |
| Socs3 | F | CCCTTGCAGTTCTAAGTTCAACA |
| R | ACCTTTGACAAGCGGACTCTC | |
| Cox2 | F | TGGCTGCAGAATTGAAAGCCCT |
| R | AAAGGTGCTCGGCTTCCAGTAT | |
| Il10 | F | ATAACTGCACCCACTTCCCA |
| R | TCATTTCCGATAAGGCTTGG | |
| Il27 | F | CACCTCCGCTTTCAGGTGC |
| R | AGGTATAGAGCAGCTGGGGC | |
| Tgfb1 | F | CTCCCGTGGCTTCTAGTGC |
| R | GCCTTAGTTTGGACAGGATCTG | |
| Il12p40 | F | GGAAGCACGGCAGCAGAATA |
| R | AACTTGAGGGAGAAGTAGGAATGG | |
Induction of AD
For the induction of AD in BALB/c mice, tape stripping was performed on both ear lobes for disruption of skin barrier. After stripping, 20 μl of 1.5% 2, 4-dinitrochlorobenzene (DNCB) (Sigma-Aldrich) dissolved in acetone/olive oil solution (acetone/olive oil, 3:1, v/v) was applied on each ear. After 3 days, 20 μl of HDM extract (10 mg/ml, Dermatophagoides farinae, GREER source materials; GREER Inc., Cambridge, MA, USA) was re-painted after tape stripping. Challenge of DNCB and mite extract was repeated once a week alternatively for 3–4 wk. Clinical symptoms were monitored every other day. Isolated immune cells from ears and spleen were stimulated with HDM extract (10 μg/ml) for 48–96 h. Cytokine secretion was examined by ELISA. For testing probiotics in this model, probiotics, 5×108 CFU suspended in 100 μl sterile PBS, were administered 5 times per week orally. Clinical condition and symptoms of mice were evaluated by histological analysis with H&E, toluidine blue staining, and immunohistochemistry (IHC). Briefly, collected tissues were fixed in 4% formaldehyde for 24 h, embedded in paraffin blocks, sectioned at 6–8 μm thickness and stained with hematoxylin (Sigma-Aldrich) and eosin (Sigma-Aldrich). Sectioned tissues were stained with toluidine blue solution (Sigma-Aldrich) as per manufacturer's protocol. For assessing neutrophil infiltration by IHC, peroxidase activity in tissue sections was blocked using 0.01% H2O2 blocking solution, washed with PBS and stained with neutrophil marker Ab (6A608; Santa Cruz Biotechnology, Dallas, TX, USA) and subsequently with goat-anti-rat-HRP.
Statistical analysis
Data are displayed as mean±SD. All statistical analyses were performed using Graph Pad Prism (v8.3) software (GraphPad Software Inc., San Diego, CA). Statistical significance was determined by a 2-tailed student's t-test/1-way ANOVA/repeated measures 2-way ANOVA followed by multiple comparison post hoc tests (*<0.05, **<0.005, ***<0.001). Significance is indicated only when p value <0.05.
RESULTS
LP340 induces IL-10 production from mouse mLN cells
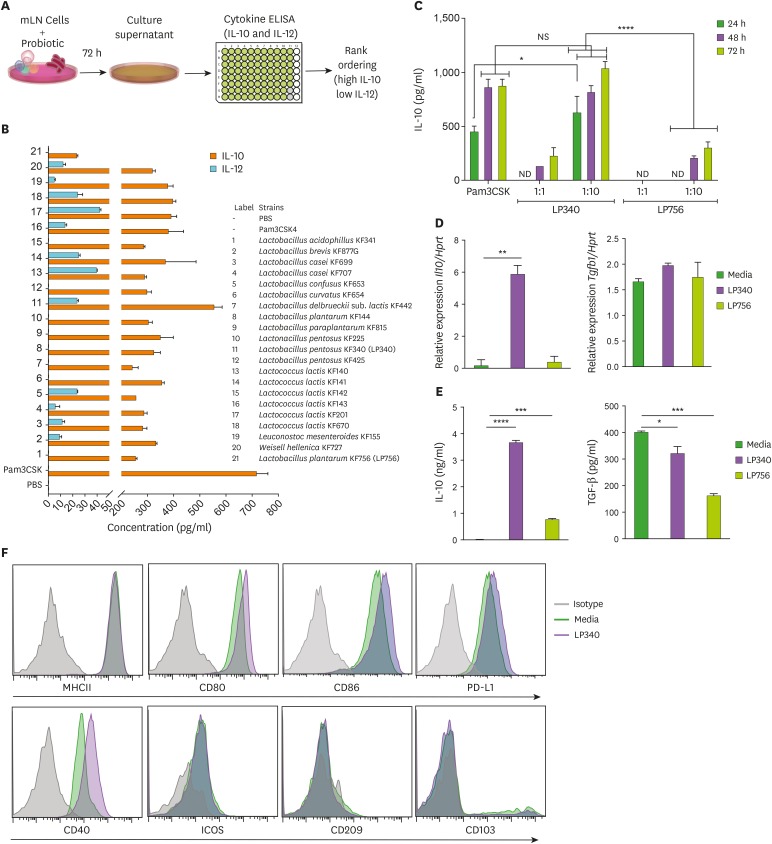
Several fermented foods including yogurts, naturally pickled vegetables as kimchi etc. harbor many bacteria with immunomodulatory properties. However, it is still a challenge to identify bacteria with peculiar immunoregulatory or immunostimulatory properties. To identify the bacterial strains (probiotics) in fermented foods with immunoregulatory properties, we devised a screening method (Fig. 1A) based on secretory cytokine profile of mouse total mLN cells stimulated ex vivo with various bacteria as described earlier (4). IL-10 is a general anti-inflammatory cytokine secreted by various leukocytes which suppresses inflammatory response from both innate and adaptive arms of immune system (17). On the other hand, IL-12, a heterodimeric proinflammatory cytokine, is primarily responsible for Th1 and Th17 mediated immunity (18,19,20). Thus, we designated high IL-10 and low IL-12 producing probiotics as probable immunoregulatory probiotics. A comprehensive screening resulted in identification of LP340 as most prominent immunoregulatory strain tested (Fig. 1B). The IL-10 levels produced by LP340 stimulated mLN cells were comparable with those of synthetic TLR-1/2 ligand PAM3CSK4 known for high induction of IL-10 secretion (21). Production of IL-12 was distinctly lower. Similarly, a relatively non-immunoregulatory strain Lactobacillus plantarum KF756 (LP756) was selected as control bacteria owing to its low IL-10 and IL-12 production. LP340's effect on IL-10 production by mLN cells was dose dependent and peaked around 72 h post incubation (which was the final time point for supernatant collection) (Fig. 1C). LP340 also induced IL-12 production albeit at very low levels (Supplementary Fig. 1A). As gut bacteria not only modulate the local gut immune response but can also affect the systemic immunity (22), we tested the induction of cytokines upon incubation of LP340 with splenic CD11c+ DCs. Quantitative PCR analysis of DCs revealed higher transcripts of Il10 while Tgfb1 transcripts were not different compared to PBS control (Fig. 1D). Also, there was about a 4-fold increase in inhibitory/tolerogenic genes PD-L1, Socs-3, and Ido while H2Ab (MHCII) gene was not upregulated (Supplementary Fig. 1B). This gene expression profile is indicative of immature regulatory DCs (23). Costimulatory genes CD80 and CD86 were also significantly upregulated suggestive of regulatory/tolerogenic phenotype. Further, we analyzed the culture supernatant for cytokines and like total mLN cells, spDCs also produced significantly high amount of IL-10 and lower amount of TGF-β1 compared to PBS control (Fig. 1E). These findings were further confirmed by flowcytometry which revealed that LP340 incubated spDCs did not upregulate MHCII expression on their surface, while inhibitory PD-L1 expression was increased. Costimulatory proteins CD40, CD80, and CD86 were also increased while other activation markers like inducible T-cell costimulator (CD278), CD103, and DCSIGN (CD209) were unchanged (Fig. 1F).
Figure 1. LP340 preferentially skews the immune microenvironment to immunoregulatory phenotype, both with total mLN cells and APCs. (A-C) Total mLN cells were co-cultured with probiotics and supernatant was used for ELISA, as described in (A) schematics of primary screening method (B) cytokines, IL-10 and IL-12 levels (C) cells were treated with LP340 and LP756 at different concentrations and incubation times followed by measurement of cytokines. (D-F) enriched splenic CD11c+ cells were treated with LP340 or LP756 and (D) qRT-PCR analysis of Il10 and Tgfb1 gene expression (E) cytokine analysis by ELISA and (F) flow cytometry analysis of MHCII, CD80, CD86, PD-L1, CD40, ICOS, CD209, and CD103.
All data are representative of 3 independent experiments and are presented as mean±SD.
NS, not significant; ND, not detectable; ICOS, inducible T-cell costimulator.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
LP340 primed DCs induce IL-10 production from CD4+ T cells
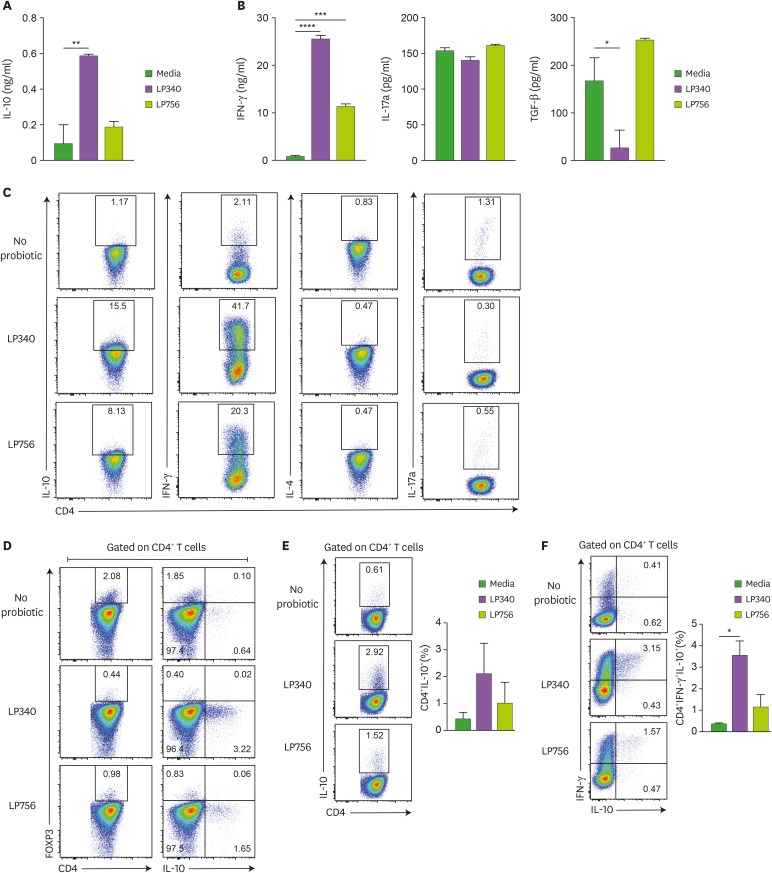
As APCs primarily direct and modulate the CD4+ T cell activation and differentiation program we investigated the effect of LP340 primed splenic CD11c+ DCs on naïve CD4+ T cells. When co-cultured with LP340 primed DCs, CD4+ T cells also produced several folds higher IL-10 than no bacteria and LP756 controls (Fig. 2A) which was dose-dependent to LP340 CFU (Supplementary Fig. 2A). To test whether bacteria can have any direct effect on CD4+ T cells, we cultured them together with LP340 but without APCs. In this scenario, CD4+ T cells failed to secrete IL-10 in the media, which suggests that IL-10 production by CD4+ T cells was dependent on APCs (Supplementary Fig. 2B). As APC educated CD4+ T cells can differentiate into different CD4+ T cell subsets we further analyzed production of CD4+ Th subset specific cytokines. Surprisingly, LP340 primed DC educated CD4+ T cells also produced IFN-γ while there was no significant difference in IL-17a and TGF-β production (Fig. 2B). IFN-γ production was also LP340 concentration dependent (Supplementary Fig. 2C) and could not be elicited upon direct culture without DCs (Supplementary Fig. 2D). We could not detect any IL-4 production in DC-CD4+T cell co-culture settings. To ascertain that source of these cytokines were indeed CD4+T cells, we analyzed co-cultured CD4+ T cells by flow cytometry. Indeed, CD4+ T cells were producing both IL-10 and IFN-γ, while no conspicuous IL-17a production was evident (Fig. 2C). As CD4+FOXP3+ Tregs are an established source of IL-10 (24,25), we wanted to confirm whether the excess IL-10 production is due to generation of induced Treg (iTreg) cells, however, neither the frequency of FOXP3+CD4+ Tregs was higher than controls nor the IL-10 production from Tregs was enhanced (Fig. 2D). In fact, under Treg skewing culture conditions, LP340 primed DCs rather suppressed the iTreg generation (data not shown). Thus, IL-10 production wasn't due to LP340 mediated iTreg conversion. Surprisingly, significantly higher frequency of CD4+IL-10+ T cells were also IFN-γ+ (Fig. 2E and F). Thus, LP340 primed DCs induced naïve CD4+T cells to differentiate into IL-10 and IFN-γ double positive cells. We also investigated effect of LP340 on splenic macrophages, which like spDCs induced IL-10 and IFN-γ secretion from CD4+T cells (Supplementary Fig. 2E and F). Since, most probiotics are administered via oral route, we also tested effect of LP340 priming on sorted intestinal LPDCs and just like spDCs and macrophages, they also generated more IL10+IFN-γ+ T cells and produced significantly higher IL-10 upon co-culture with naïve CD4+ T cells (Supplementary Fig. 2G and H). Thus, in vitro co-culture with LP340 induced a regulatory phenotype in intestinal and systemic APCs.
Figure 2. DCs exposed to LP340 induce CD4+ T-cells to produce IL-10. (A-D) Splenic CD11c+ cells were co-cultured with LP340 and LP756, then primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. Culture supernatants were used for ELISA of cytokines (A) IL-10 (B) IFN-γ, IL-17A, and TGF-β. (C) CD4+ T-cells were stimulated with PMA/Ionomycin and used for flow cytometry analysis of cytokines IL-10, IFN-γ, IL-4 and IL-17A (D) CD4+ T-cells were also analyzed by flow cytometry for FOXP3 expression and IL-10 expression in CD4+ T-cells. (E) CD4+IL-10+, and (F) CD4+ IFN-γ+ IL-10+ T-cells were analyzed by flow cytometry.
All data are representative of 3 independent experiments and presented as mean±SD.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
LP340 induced IL-10+IFN-γ+CD4+ T cells have a phenotype like Tr-1 cells
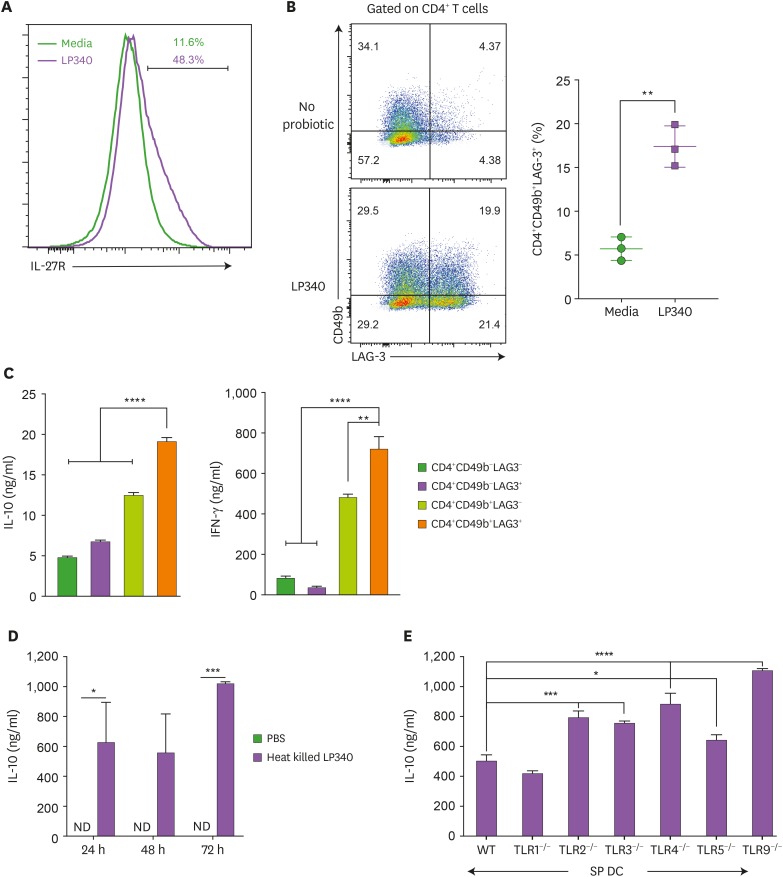
Other than Tregs, CD4+FOXP3− Tr1 cells are a major IL-10 producing cell type (26,27). As LP340 primed DC-T cell co-culture wasn't generating CD4+FOXP3+ Tregs, and IL-10 producing cells were also IFN-γ positive, we hypothesized that IL-10 producing cells might be Tr1 cells. In accordance, LP340 primed spDCs were expressing higher IL-27 mRNA (Supplementary Fig. 3A) and protein (Supplementary Fig. 3B), a crucial factor to induce Tr1 cell generation (28). On the other hand, CD4+ T cells incubated with LP340 primed DCs were highly expressing cognate IL-27 receptor (IL-27R) (Fig. 3A). Also, these cells were double positive for CD49b and LAG3 (Fig. 3B), which are consensus surface markers for Tr1 cells (12). Functionally, CD4+CD49b+LAG3+ T cells were producing high amount of both IL-10 and IFN-γ (Fig. 3C). To establish that only CD4+CD49b+LAG3+ cells are primary source of IL-10 we sorted and re-stimulated CD49b and LAG3 double positive, double negative and CD49b and LAG3 single positive cells. Indeed, only CD49b+LAG3+ cells were significantly expressing both IL-10 and IFN-γ (Supplementary Fig. 3C). Further, to ascertain the role of IL-27, we co-cultured CD4+ T cells with LP340 primed spDCs in presence of recombinant IL-27. And, as expected, IL-27 treatment resulted in generation of higher frequency of LAG3+CD49b+ and IFN-γ+IL-10+ double positive cells upon LP340 treatment (Supplementary Fig. 3D). Similarly, culturing CD4+ T cells with LP340 primed DCs in presence of anti-mouse IL-27 Ab reduced the generation of CD4+IL-10+IFN-γ+ T cells (Supplementary Fig. 3E).
Figure 3. LP340 induce a Tr-1 cell like phenotype in CD4+ T-cells. (A-E) Splenic CD11c+ cells were co-cultured with LP340 and primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. (A) Cells were analyzed for IL-27R and (B) CD49b+ LAG-3+ expression, gated on total CD4+ T-cells. The 4 populations sorted by expression of CD49b & LAG-3 were cultured and cell supernatants were used for (C) IL-10 & IFN-γ cytokine ELISA (D) Heat killed LP340 was used for priming the DCs and IL-10 ELISA was performed with supernatant (E) Splenic CD11c+ cells from TLR knock-out mice (TLR1−/−, TLR2−/−, TLR3−/−, TLR4−/−, TLR5−/−, and TLR9−/−) were treated with LP340 and IL-10 cytokine ELISA was performed.
Data are representative of 3 (A-D) and 2 (E) independent experiments and presented as mean±SD.
IL-27R, IL-27 receptor; ND, not detectable; WT, wild type.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
LP340 modulates spDCs independent of TLRs
To understand the mechanism by which LP340 modulates the APCs to generate Tr1 cells in vitro, first we used heat-killed bacteria for APC priming. However, heat killed bacteria were equally efficient at inducing IL-10 production by these cells suggesting that bacterial structural components instead of secretary molecules or metabolites are involved in Tr1 cell generation (Fig. 3D). Further, we primed various TLR deficient DCs with LP340, but, co-culture of these cells with naïve CD4+T cells did not result in reduced IL-10 production in the supernatant. In fact, except for TLR1, deficiency of all other TLRs (i.e., TLR2, TLR3, TLR4, TLR5, and TLR9) rather resulted in increase in IL-10 secretion in the supernatant (Fig. 3E). This is indicative of that LP340 modulates the DCs in a TLR independent fashion to generate Tr1 cells from CD4+T cells.
LP340 induced Tr-1 cells improve HDM induced AD in mice
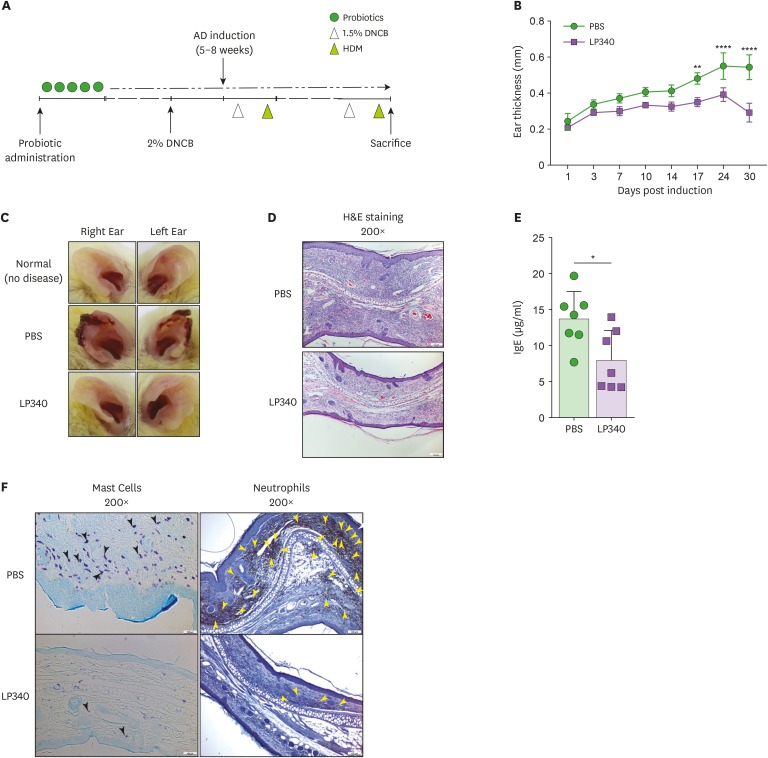
To ascertain the functional competence and importance of LP340 induced Tr1 cells, we developed HDM mediated AD in BALB/c mice as depicted in Fig. 4A. Prophylactic LP340 feeding in mice, significantly reduced the ear lobe thickness and lesions (Fig. 4B and C) upon induction of AD. Histologically, there was reduced cellular infiltration in ear lobes of mice treated with LP340 (Fig. 4D). Most conspicuously, neutrophil infiltration was significantly down in ear lobes of animals treated with LP340 (Supplementary Fig. 4A). Further, IgE is an important inflammatory factor in AD. In accordance, IgE concentration was significantly decreased in serum of LP340 treated animals (Fig. 4E). To confirm these findings, we performed immunocytochemistry analyses which established lower mast cell and neutrophil infiltration in the ear lobes (Fig. 4F). Re-stimulation of cells isolated from ear lobe skin and spleens with HDM and subsequent ELISA for measuring IL-10 levels revealed that indeed the LP340 treated animal cells were producing more IL-10 (Supplementary Fig. 4B and C). These results prove that LP340 induced IL-10 producing Tr1 cells are functional and are protective in mouse model of AD.
Figure 4. LP340 ameliorates HDM induced AD. (A-F) Mice orally fed with PBS or LP340 were immunized with DNCB and subsequently were challenged with HDM as depicted in (A) experimental scheme. (B) Ear thickness was measured till 30 days post induction and (C) gross ear lesions were observed (D) Inflammation and cellular infiltration in ear tissue was analyzed by histology, H&E staining (E) Serum IgE levels were measured by ELISA (F) Mast cell (left panel, black arrowheads) and neutrophil (right panel, yellow arrowheads) infiltration was observed by IHC of ear tissue.
Data are representative of 3 independent experiments and presented as mean±SD.
*p<0.05, **p<0.01, ****p<0.0001.
DISCUSSION
In the present study, we show that food derived probiotic LP340 induces differentiation of CD4+ T cells into IL-10 producing Tr1 cells in vitro. Various APCs like DCs, macrophages and intestinal LPDCs induce development of T cells into Tr1 cells upon priming with the LP340. LP340 modulates the APCs towards a regulatory phenotype by enhanced production of IL-27 and IL-10 from them. We have shown that structural components of LP340 are mainly involved in the priming of APCs. In fact, several reports have suggested the immune cell modulation by bacterial structural components. Polysaccharide A from Bacteroides fragilis cell wall has been shown to induce FOXP3+ Treg development (29). Similarly, cell surface polysaccharide of Bifidobacterium bifidum also induce FOXP3+ Treg development in colon (30). Both bacterial components were reported to be working through toll like pattern recognition receptors (PRRs) on DCs. However, DCs interact with LP340 in a TLR independent way. Certain studies have demonstrated IL-10 producing Tr1 cells in vitro and in vivo in mice (31,32,33). It has been shown that intestinal bacterium Bifidobacterium breve can induce local Tr1 cells (34). However, there are no reports of systemic effect of food derived bacteria producing Tr1 cells. This study clearly demonstrates that a food derived probiotic can protect against non-local inflammation like AD by modulating systemic APCs to promote development of IL-10 producing CD4+FOXP3- Tr1 cells.
To take advantage of diversity of microbiota at our disposal, it is important to develop a screening method to identify immunostimulatory and immunoregulatory bacteria. We have successfully utilized our cytokine screening paradigm to identify a bacterium with significant IL-10 production in a culture of total mLN lymphocytes. At the same time, we were able to identify a rather innocuous bacterium which did not induce IL-10. Further, LP340 being sourced from food and inducing a dose dependent IL-10 production by mLN cells suggests the therapeutic potential of it. That LP340 treated DCs produced IL-10 and were highly expressing regulatory genes like PD-L1, Socs3, and Ido without increased expression of H2Ab suggests their tolerogenic phenotype. However, costimulatory molecules like CD80 and CD86 were also upregulated in these cells. It has been reported that generation of Tr1 cells requires excess B7 signaling (35,36), which might be the reason for higher expression of these molecules. Also, this indicates that LP340 primed DCs were more of a semi-mature phenotype.
Tr1 cells, a distinct type of regulatory T cells, are identified by their unique cytokine production profile, surface markers and lack of expression of Treg transcription factor FOXP3 (11). This study clearly demonstrates that LP340 primed DCs when co-cultured with naïve CD4+ T cells, differentiated those into IL-10 and IFN-γ producing T cells. There was no significant induction of FOXP3, and all the IL-10 production was from FOXP3− T cells. Also, the TGF-β1 secretion was low which might be because the Tr1 conversion wasn't complete. That, CD4+T cells couldn't initiate the differentiation program upon culture with LP340 directly, suggests an unequivocal need of APCs to direct Tr1 differentiation.
Two factors which help in generation of Tr1 cells are IL-10 and IL-27 (37,38). Here, we demonstrated that LP340 primed DCs were not only producing IL-10 but also secreting IL-27. In turn, the co-cultured CD4+ T cells started higher expression of IL27R on the surface. The heat killed LP340 also primed DCs to generate IL-10 producing Tr1 cells indicating that bacterial structural components are major driver of this phenotype. However, despite TLRs being major PRRs, we could not find suppression of IL-10 production in CD4+ T cells co-cultured with various TLR deficient DCs which were primed with LP340. There was only a modest decrease with TLR1 but other TLR deficiencies rather increased the IL-10 production. The differential ability of DCs to produce IL-10 upon TLR activation might be a reason (39). The TLR deficient DCs used in our experiments were deficient in only one of the TLR out of several tested. It's probable that use of DCs deficient in 2 or more TLRs, such as the ones which often form functional heterodimers, could provide different results. Further, many reports have suggested that specific feedback signaling loop exist which can regulate IL-10 expression subsequent to TLR signaling. Villagra et al. (40) have shown that TLR4 ligand LPS induces IL-10 expression early on after ligand receptor binding. This leads to a rebound expression of HDAC11 which is a transcriptional repressor of Il10. Also, IFN-γ can alter TLR2 induced signaling cascade leading to reduced IL-10 production (41). Thus, in absence of TLR signaling these feedback loops might get abolished leading to enhanced IL-10 production. Other PRRs like C-type lectin receptors DC-SIGN (42) and Dectin-1 (43,44) can enhance IL-10 production. CD40 ligation in collaboration with TLRs can also enhance IL-10 production in DCs (45). Intracellular microbial PRRs like nucleotide-binding oligomerization domain-containing protein (NOD) 1 has been reported to enhance IL-10 signaling in DCs (46,47). Also, NOD-2 has been shown to induce IL-27 production in DCs in collaboration with TLR7 (48). Further studies on components of LP340 as well as receptors and mechanisms by which it is inducing regulatory DCs and, in turn, Tr1 cells will be an interesting future endeavor.
Our in vitro experiments clearly establish that LP340 induces a Tr1 phenotype on CD4+T cells via APCs. It has been shown that probiotic derived local Tr1 cells can suppress colitis in mice (34). However, to assess whether probiotics can be effective at systemic amelioration of pathology, we investigated effect of LP340 on HDM induced AD in mice. Tr1 cells and IL-10 have been reported to be instrumental in suppression of AD (49,50,51,52). The suppression of atopic lesions as well as cellular and immunoglobulin infiltration in such lesions clearly establishes beneficial effect of LP340 induced Tr1 cells. Tr1 cells and IL-10 have been implicated in reduced neutrophil (53) and mast cell recruitment (54). Also, IL-10 suppresses mast cell mediated IgE response (54). Here, we have demonstrated that both recruitment of neutrophils and mast cells in inflamed skin as well as IgE levels in serum are significantly reduced in mice fed with LP340.
Thus, in present study we have shown that LP340 a food derived bacterium can induce IL-10 producing Tr1 cells and thereby, suppress inflammatory response like AD. Therapeutic potential of food derived probiotic instead of one isolated from human gut provides an innate advantage of food consumption-based therapy. Further analysis of molecular components of LP340 and extension of their application in other disease models will certainly expand the immunomodulatory role of this probiotic.
ACKNOWLEDGEMENTS
This work was supported by research grant to Shin HS from the Korea Food Research Institute (E0170400-03).
Abbreviations
- AD
atopic dermatitis
- APCs
Ag presenting cells
- CFU
colony forming unit
- DC
dendritic cell
- DNCB
2, 4-dinitrochlorobenzene
- FOXP3
forkhead box protein 3
- HDM
house dust mite
- IHC
immunohistochemistry
- iTreg
induced Treg
- LAG3
lymphocyte-activation gene 3
- LP340
Lactobacillus pentosus KF340
- LP756
Lactobacillus plantarum KF756
- LPDC
lamina propria dendritic cell
- mLN
mesenteric lymph node
- NOD
nucleotide-binding oligomerization domain-containing protein
- PRR
pattern recognition receptor
- spDC
splenic dendritic cell
- Tr1 cell
type 1 regulatory T cell
- qRT-PCR
quantitative RT-PCR
Footnotes
Conflict of Interest: Kim JE is an employee of SK Biopharmaceuticals. Im SH is a major shareholder of Immunobiome Inc. Other authors declare no potential conflicts of interest.
- Conceptualization: Sharma G, Im SH.
- Data curation: Kim JE, Sharma G.
- Formal analysis: Sharma A, Sharma G, Im SH.
- Funding acquisition: Lee SY, Shin HS, Im SH.
- Investigation: Kim JE.
- Methodology: Sharma G.
- Project administration: Shin HS.
- Resources: Lee SY.
- Supervision: Im SH.
- Writing - original draft: Sharma A, Sharma G.
- Writing - review & editing: Rudra D, Im SH.
SUPPLEMENTARY MATERIALS
LP340 preferentially skews the immune microenvironment to immunoregulatory phenotype. (A) Total mLN cells were co-cultured with probiotics and supernatant was used for IL-12 cytokine ELISA (B) enriched splenic CD11c+ cells were treated with LP340 and LP756 for qRT-PCR analysis of PD-L1, Socs3, Ido, H2-Ab, CD80, and CD86.
IL-10 production in CD4+ T-cells by LP340 is mediated by DCs (A-B) Splenic CD11c+ cells were co-cultured with LP340 and LP756 in different concentrations, then primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. Culture supernatants were used for ELISA of cytokines (A) IL-10 (B) IFN-γ (C-D) LP340 was co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. Culture supernatants were used for ELISA of cytokines (C) IL-10 and (D) IFN-γ. (E-F) magnetic bead enriched F4-80+ macrophages and (G-H) FACS sorted lamina propria DCs CD11b− CD11c+ MHCII+ were treated with LP340 and co-cultured with naive CD4+ T cells like spDCs and analyzed for (E, G) CD4+IFN-γ+IL-10+ T-cells by flow cytometry and (F, H) IL-10 and IFN-γ cytokines by ELISA.
LP340 induces IL-27 mediated Tr-1 cells like phenotype in CD4+ T-cells. (A, B) Splenic CD11c+ cells were co-cultured with LP340 and (A) subjected to qPCR for IL-27 gene expression and (B) cell supernatants were used for testing IL-27 cytokine levels by ELISA. (C, D) Splenic CD11c+ cells were co-cultured with LP340 and primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of rIL-27, anti-CD3, and IL-2. Cells were analyzed by flowcytometry for IFN-γ and IL-10 (C) gated on total CD4+ T-cells (D) gated on CD4+CD49b+LAG-3+. (E) LP340 primed splenic CD11c+ DC were cocultured with CD4+ T cells in presence or absence of 0.05 μg/mL anti-mouse IL-27 Ab.
LP340 reduces inflammation in HDM induced AD. (A) Cellular infiltration in ear tissue was analyzed by flow cytometry for total immune cells (CD45+), T-cells (CD45+CD4+, CD45+CD8+), DCs (CD45+CD11c+MHCIIhi), Macrophages (CD45+F4/80+CD11b+) and neutrophils (CD45+F4/80+CD11b+Gr-1+). Cells isolated from ear (B) and spleen (C) were restimulated with mite and cytokine levels were measured by ELISA.
References
- 1.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosconi I, Geuking MB, Zaiss MM, Massacand JC, Aschwanden C, Kwong Chung CK, McCoy KD, Harris NL. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 2013;6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 3.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, Wang L, Li N, Crestani E, Spielman S, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med. 2019;25:1164–1174. doi: 10.1038/s41591-019-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 7.Coqueiro AY, Raizel R, Bonvini A, Tirapegui J, Rogero MM. Probiotics for inflammatory bowel diseases: a promising adjuvant treatment. Int J Food Sci Nutr. 2019;70:20–29. doi: 10.1080/09637486.2018.1477123. [DOI] [PubMed] [Google Scholar]
- 8.He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol. 2019;10:385. doi: 10.3389/fimmu.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Zeng B, Zhang J, Li W, Mou F, Wang H, Zou Q, Zhong B, Wu L, Wei H, et al. Role of the gut microbiome in modulating arthritis progression in mice. Sci Rep. 2016;6:30594. doi: 10.1038/srep30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng QX, Loke W, Venkatanarayanan N, Lim DY, Soh AYS, Yeo WS. A systematic review of the role of prebiotics and probiotics in autism spectrum disorders. Medicina (Kaunas) 2019;55:E129. doi: 10.3390/medicina55050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol. 2015;12:566–571. doi: 10.1038/cmi.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 13.Chihara N, Madi A, Karwacz K, Awasthi A, Kuchroo VK. Differentiation and characterization of Tr1 cells. Curr Protoc Immunol. 2016;113:3.27.1–3.27.10. doi: 10.1002/0471142735.im0327s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biedermann T, Skabytska Y, Kaesler S, Volz T. Regulation of T cell immunity in atopic dermatitis by microbes: the yin and yang of cutaneous inflammation. Front Immunol. 2015;6:353. doi: 10.3389/fimmu.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo L, Cunha AP, Madi A, Beynon V, Yang Z, Alvarez JI, Prat A, Sobel RA, Kobzik L, Lassmann H, et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain. 2016;139:1939–1957. doi: 10.1093/brain/aww113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae MJ, Kim HK, Lim S, Lee SY, Shin HS, Kim JE, Im SH, Kim S. Lactobacillus pentosus KF340 alleviates house dust mite-induced murine atopic dermatitis via the secretion of il-10-producing splenic b10 cells. J Funct Foods. 2016;26:258–267. [Google Scholar]
- 17.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 18.Tait Wojno ED, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50:851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 20.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funderburg NT, Jadlowsky JK, Lederman MM, Feng Z, Weinberg A, Sieg SF. The Toll-like receptor 1/2 agonists Pam(3) CSK(4) and human β-defensin-3 differentially induce interleukin-10 and nuclear factor-κB signalling patterns in human monocytes. Immunology. 2011;134:151–160. doi: 10.1111/j.1365-2567.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Shi B. Tolerogenic dendritic cells and their applications in transplantation. Cell Mol Immunol. 2015;12:24–30. doi: 10.1038/cmi.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brockmann L, Soukou S, Steglich B, Czarnewski P, Zhao L, Wende S, Bedke T, Ergen C, Manthey C, Agalioti T, et al. Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat Commun. 2018;9:5457. doi: 10.1038/s41467-018-07581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andolfi G, Fousteri G, Rossetti M, Magnani CF, Jofra T, Locafaro G, Bondanza A, Gregori S, Roncarolo MG. Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4(+) T cells. Mol Ther. 2012;20:1778–1790. doi: 10.1038/mt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H, Tamura T, Yoshida H, Charnay P, Yamamoto K. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. Eur J Immunol. 2013;43:1063–1073. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 29.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 30.Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, Byun S, Lee CG, Kang HJ, Kim GC, et al. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of foxp3+ regulatory T cells. Sci Immunol. 2018;3:eaat6975. doi: 10.1126/sciimmunol.aat6975. [DOI] [PubMed] [Google Scholar]
- 31.Collins FL, Rios-Arce ND, Schepper JD, Jones AD, Schaefer L, Britton RA, McCabe LR, Parameswaran N. Beneficial effects of Lactobacillus reuteri 6475 on bone density in male mice is dependent on lymphocytes. Sci Rep. 2019;9:14708. doi: 10.1038/s41598-019-51293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia H, Ren S, Wang X. Heat-killed probiotic regulates the body's regulatory immunity to attenuate subsequent experimental autoimmune arthritis. Immunol Lett. 2019;216:89–96. doi: 10.1016/j.imlet.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Lim SK, Kwon MS, Lee J, Oh YJ, Jang JY, Lee JH, Park HW, Nam YD, Seo MJ, Roh SW, et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci Rep. 2017;7:40040. doi: 10.1038/srep40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F, Zhang J, Chen H, Wu C. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett. 2011;136:21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Vasanthakumar A, Kallies A. IL-27 paves different roads to Tr1. Eur J Immunol. 2013;43:882–885. doi: 10.1002/eji.201343479. [DOI] [PubMed] [Google Scholar]
- 39.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol. 2006;177:7551–7558. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 40.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards AD, Manickasingham SP, Spörri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 46.Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuper T, Ellwanger K, Schwarz H, Kufer TA, Duschl A, Horejs-Hoeck J. NOD1 modulates IL-10 signalling in human dendritic cells. Sci Rep. 2017;7:1005. doi: 10.1038/s41598-017-00691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patin EC, Jones AV, Thompson A, Clement M, Liao CT, Griffiths JS, Wallace LE, Bryant CE, Lang R, Rosenstiel P, et al. IL-27 induced by select candida spp. Via TLR7/NOD2 signaling and IFN-beta production inhibits fungal clearance. J Immunol. 2016;197:208–221. doi: 10.4049/jimmunol.1501204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med. 2005;202:1459–1463. doi: 10.1084/jem.20052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W, Solouki S, Koylass N, Zheng SG, August A. ITK signalling via the Ras/IRF4 pathway regulates the development and function of Tr1 cells. Nat Commun. 2017;8:15871. doi: 10.1038/ncomms15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyman O, Werfel T, Akdis CA. The suppressive role of IL-10 in contact and atopic dermatitis. J Allergy Clin Immunol. 2012;129:160–161. doi: 10.1016/j.jaci.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 52.Volz T, Skabytska Y, Guenova E, Chen KM, Frick JS, Kirschning CJ, Kaesler S, Röcken M, Biedermann T. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol. 2014;134:96–104. doi: 10.1038/jid.2013.291. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. doi: 10.1186/1479-5876-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo . J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LP340 preferentially skews the immune microenvironment to immunoregulatory phenotype. (A) Total mLN cells were co-cultured with probiotics and supernatant was used for IL-12 cytokine ELISA (B) enriched splenic CD11c+ cells were treated with LP340 and LP756 for qRT-PCR analysis of PD-L1, Socs3, Ido, H2-Ab, CD80, and CD86.
IL-10 production in CD4+ T-cells by LP340 is mediated by DCs (A-B) Splenic CD11c+ cells were co-cultured with LP340 and LP756 in different concentrations, then primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. Culture supernatants were used for ELISA of cytokines (A) IL-10 (B) IFN-γ (C-D) LP340 was co-cultured with naïve CD4+ T-cells in presence of anti-CD3 and IL-2. Culture supernatants were used for ELISA of cytokines (C) IL-10 and (D) IFN-γ. (E-F) magnetic bead enriched F4-80+ macrophages and (G-H) FACS sorted lamina propria DCs CD11b− CD11c+ MHCII+ were treated with LP340 and co-cultured with naive CD4+ T cells like spDCs and analyzed for (E, G) CD4+IFN-γ+IL-10+ T-cells by flow cytometry and (F, H) IL-10 and IFN-γ cytokines by ELISA.
LP340 induces IL-27 mediated Tr-1 cells like phenotype in CD4+ T-cells. (A, B) Splenic CD11c+ cells were co-cultured with LP340 and (A) subjected to qPCR for IL-27 gene expression and (B) cell supernatants were used for testing IL-27 cytokine levels by ELISA. (C, D) Splenic CD11c+ cells were co-cultured with LP340 and primed CD11c+ cells were co-cultured with naïve CD4+ T-cells in presence of rIL-27, anti-CD3, and IL-2. Cells were analyzed by flowcytometry for IFN-γ and IL-10 (C) gated on total CD4+ T-cells (D) gated on CD4+CD49b+LAG-3+. (E) LP340 primed splenic CD11c+ DC were cocultured with CD4+ T cells in presence or absence of 0.05 μg/mL anti-mouse IL-27 Ab.
LP340 reduces inflammation in HDM induced AD. (A) Cellular infiltration in ear tissue was analyzed by flow cytometry for total immune cells (CD45+), T-cells (CD45+CD4+, CD45+CD8+), DCs (CD45+CD11c+MHCIIhi), Macrophages (CD45+F4/80+CD11b+) and neutrophils (CD45+F4/80+CD11b+Gr-1+). Cells isolated from ear (B) and spleen (C) were restimulated with mite and cytokine levels were measured by ELISA.