Abstract
Three new 11-hydroxyburnamine (1) and rauvoyunnanines A–B (2–3), and fourteen known (4–17) monoterpenoid indole alkaloids were isolated from the total alkaloids extract of Rauvolfia yunnanensis, which exhibited promising immunosuppressive activity on T cell proliferation in preliminary screening. Their structures were determined by analysis of high-resolution electrospray ionization mass (HRESIMS), ultraviolet (UV) and nuclear magnetic resonance (NMR) data, and by comparison with the literature. All the alkaloids were evaluated for inhibitory activity on T cell proliferation. Among them, one new compound (1) and reserpine (6) exhibited moderate immunosuppressive activity, with IC50 values of 5.9 μM and 5.0 μM, respectively.
Keywords: Rauvolfia yunnanensis, monoterpenoid indole alkaloid, 11-hydroxyburnamine, rauvoyunnanine A, rauvoyunnanine B, immunosuppressive activity
1. Introduction
Abnormal T cell proliferation plays a very important role in the development of T-cell mediated organ transplantation rejection and autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [1,2]. It is crucial to find immunosuppressive agents on T cell proliferation for this kind of immunopathogenesis. Some clinical immunosuppressants inhibit T cell proliferation by different modes of action [3]. For example, cyclosporine and tacrolimus are calcineurin-inhibitors [4]. Sirolimus and everolimus (Target-of-Rapamycin Inhibitors) inhibit nucleotide synthesis and azathioprine [5,6], and mycophenolic acid inhibits inosine monophosphate dehydrogenase and de novo GTP biosynthesis [7]. However, their side effects, such as infections and toxicities, prompt scientists to look for new immunosuppressants constantly. Medicinal plants are also useful sources of immunosuppressive agents. Triptolide obtained from the Chinese herbal plant Tripterygium wilfordii Hook F is widely used in East Asia for the treatment of SLE, RA, etc. [8]. Sinomenine from Sinomenium acutum (Thunb.) Rehd. et Wils has been used for patients with autoimmune diseases as it possesses immunosuppressive activity [9].
Rauvolfia yunnanensis Tsiang (Apocynaceae) is a kind of shrub, mainly distributed in the south of China, such as Yunnan, Guizhou, and Guangxi Provinces. It is a medicinal plant of Dai Nationality in Yunnan Province, and its roots have been used for the treatment of hypertension, fever, sore throat, hepatitis, nephritis, and snakebite [10]. Until now, more than twenty indole alkaloids have been obtained from R. yunnanensis in previous studies [11,12,13]. The most famous one is reserpine, which possesses a significant antihypertension effect [14]. It was also reported that reserpine inhibited delayed hypersensitivity and contact sensitivity responses [15]. Yohimbine in combination with berberine has an immunoregulatory effect [16]. In our ongoing search for immunosuppressive compounds from medicinal plants [17], the total alkaloid extracts of whole R. yunnanensis plants exhibited promising immunosuppressive activity on T cell proliferation. Therefore, a comprehensive phytochemical investigation on the total alkaloids was carried out. The isolation, structural elucidation, and immunosuppressive activity of the isolated alkaloids are described herein.
2. Results and Discussion
2.1. Identification of New Compounds
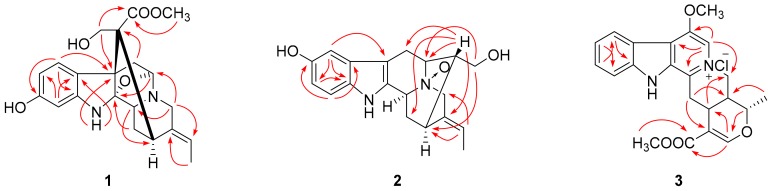
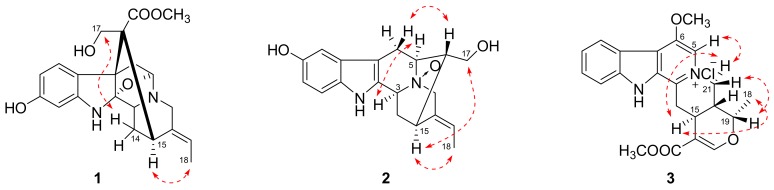
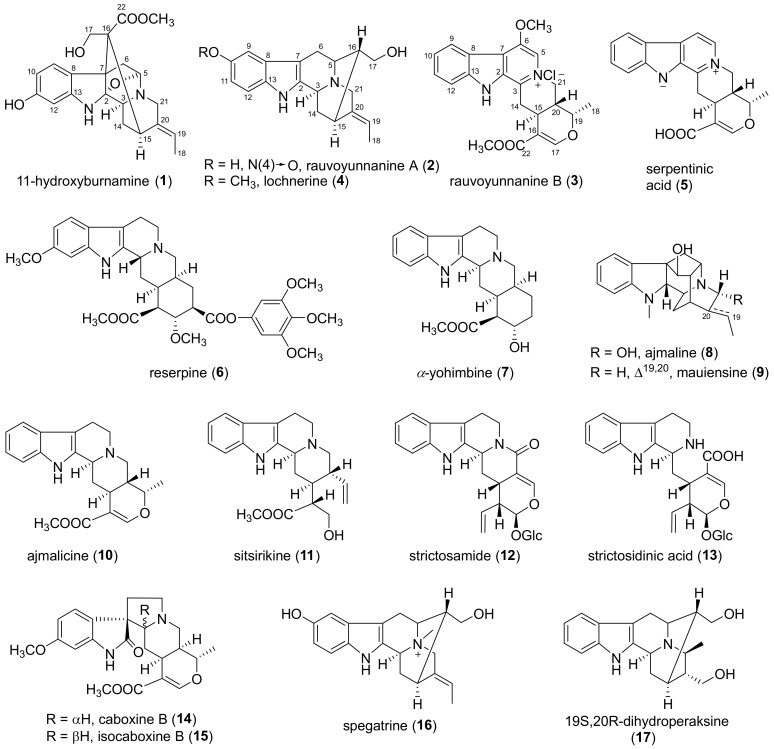
Compound 1 was isolated as a yellowish, amorphous powder with [α]20D − 117.5 (MeOH, c 0.04). Its molecular formula was determined to be C21H24N2O5 by positive HRESIMS at m/z 385.1766 [M + H]+ (calcd 385.1758), corresponding to 11 degrees of unsaturation. Its UV spectrum showed absorption maxima at 207 and 293 nm, which is characteristic of a hydroindole/alkylaniline chromophore [18]. The 1H NMR spectrum (Table 1) exhibited an ABX spin system at δH 7.61 (1H, d, J = 8.1 Hz), 6.79 (1H, d, J = 1.8 Hz), and 6.71 (1H, dd, J = 8.1, 1.8 Hz), an ethylidene at δH 5.28 (1H, m) and 1.64 (3H, d, J = 6.5 Hz), and a methoxyl group at δH 3.70 (3H, s). The 13C NMR spectrum (Table 1) displayed 21 carbon signals including one methyl, one methoxyl, four methylenes, seven methines (four sp2 and three sp3), and eight quaternary carbons (four sp2, three sp3, and one carbonyl). The above spectroscopic evidences, along with the previously isolated alkaloid structures from this plant indicated that compound 1 was a monoterpenoid alkaloid [11]. The key HMBC correlations (Figure 1) between H-5 and C-2, H2-17 and C-7, H2-6 and C-16 suggested that compound 1 possessed a picraline-type skeleton, similar to 11-methoxyburnamine [19]. The HMBC correlations from H2-21 to C-19 indicated the presence of an ethylidine at C-20. In addition, the correlations of H-9 to C-7 and C-11 implied the location of a hydroxyl group at C-11. The presence of a methoxyl group at C-22 was also proved by the HMBC experiment of δH 3.70 to δC 175.6. The NOESY correlation between H3-18 and H-15 suggested that the configuration of C-19 should be E. Meanwhile, the NOESY correlations from H2-17 to H-14β indicated that the C-16 configuration is R (Figure 2). Finally, compound 1 was elucidated as 11-hydroxyburnamine.
Table 1.
1H and 13C NMR spectroscopic data of compounds 1–3. 1 in C5H5N-d5, 2 and 3 in MeOH-d4.
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 2 | 108.7 | 132.7 | 135.7 | |||
| 3 | 53.0 | 3.89, m | 66.8 | 5.02, d (9.8) | 133.1 | |
| 5 | 87.8 | 4.92, d (1.6) | 70.7 | 3.74, m | 133.2 | 8.24, s |
| 6 | 46.5 | 3.44, d (13.6) 2.62, dd (13.6, 2.1) |
25.0 | 3.37, brd (12.2) 2.95, d (16.1) |
139.5 | |
| 7 | 53.6 | 102.0 | 130.8 | |||
| 8 | 125.6 | 128.5 | 121.0 | |||
| 9 | 128.0 | 7.61, d (8.1) | 103.6 | 6.87, br s | 125.4 | 8.36, d (8.2) |
| 10 | 108.1 | 6.71, dd (8.1, 1.8) | 152.3 | 123.5 | 7.50, m | |
| 11 | 159.8 | 113.5 | 6.74, d (7.7) | 132.4 | 7.79, overlap | |
| 12 | 100.1 | 6.79, d (1.8) | 113.2 | 7.21, d (8.5) | 114.1 | 7.79, overlap |
| 13 | 153.1 | 133.5 | 145.3 | |||
| 14 | 22.9 | 2.28, d (14.0) 1.97, m |
34.7 | 2.59, m 2.17, br d (9.7) |
31.6 | 4.68, m 3.14, m |
| 15 | 33.6 | 3.85, m | 27.5 | 3.05, br s | 26.0 | 3.07, m |
| 16 | 59.1 | 46.1 | 2.27, m | 107.2 | ||
| 17 | 64.6 | 4.22, d (11.6) 4.01, d (11.6) |
63.8 | 3.55, d (7.1) | 156.3 | 7.72, d (1.2) |
| 18 | 13.8 | 1.64, d (6.5) | 13.2 | 1.70, d (6.5) | 14.2 | 1.36 d (6.7) |
| 19 | 119.7 | 5.28, m | 122.9 | 5.62, m | 72.9 | 4.77, m |
| 20 | 140.1 | 129.8 | 38.5 | 2.70, m | ||
| 21 | 47.5 | 3.90, d (17.8) 3.17, d (17.8) |
69.8 | 4.66 d (14.0) 4.36 d (14.0) |
57.4 | 4.87, m 4.61, t (13.0) |
| 22 | 175.6 | 168.5 | ||||
| COOCH3 | 51.7 | 3.70, s | 51.9 | 3.82, s | ||
| OCH3 | 64.3 | 3.60, s | ||||
Figure 1.
Selected HMBC correlations of compounds 1–3.
Figure 2.
Selected NOESY correlations of compounds 1–3.
The positive HRESIMS of compound 2 showed an ion peak at m/z 327.1676 [M + H]+, which assigned its molecular formula as C19H22N2O3. An ABX spin system at δH 7.21 (1H, d, J = 8.5 Hz), 6.87 (1H, br s), and 6.74 (1H, d, J = 7.7 Hz) in the downfield of 1H NMR spectrum (Table 1) implied a one-substituted indole ring. Signals of an ethylidene group were present at δH 5.62 (1H, m) and 1.70 (3H, d, J = 6.5 Hz). These two substructures corresponded to ten sp2 carbon signals at δC 102.0–152.3 and one methyl signal at δC 13.2 (Table 1). On the basis of HSQC experiment, the left eight carbon signals were comprised of four methines and four methylenes. These NMR data of compound 2 were similar to those of lochnerine (4) [20], except that the methoxyl group in 4 was displaced by a hydroxyl group in 2. The HMBC correlations of H-9 to C-7, C-11, and C-13, H-11 to C-9 and C-13, and H-12 to C-8 and C-10 confirmed this hydroxyl group was at C-10. Further careful comparison of the 13C NMR data between 2 and 4 demonstrated that the chemical shifts of C-3 (δ 66.8), C-5 (δ 70.7), and C-21 (δ 69.8) were remarkably downfield shifted, which indicated that 2 was an N-oxide. The HMBC correlations from H-16, 17 to C-5, and H-19 to C-21 confirmed this deduction (Figure 1). Although no HMBC correlations were observed for C-3, the chemical shift at δC 66.8 was the only left nitrogenized methine, which should belong to C-3. The relative configuration of 2 was the same as that of 4, which was verified by a NOESY experiment (Figure 2). Thus, the structure of compound 2 was identified as shown in Figure 3 and named rauvoyunnanine A.
Figure 3.
Structures of compounds 1–17.
The characteristic UV maxima absorption bands of compound 3 were at 252 (4.38), 307 (4.07), and 367 (3.44) nm, almost identical to those of serpentinic acid (5). Moreover, the 1H and 13C NMR data of 3 (Table 1) were similar to those of 5 [21], but two more methoxyl groups in 3. In the HMBC spectrum (Figure 1), one of the methoxyl group (δH 3.82) correlated to the carbonyl group at δC 168.5. The other one (3.60, δC 64.3) was located at C-6, which was supported by HMBC correlations from H-5 (δH 8.24, 1H, s) to C-3, C-6, C-7, and C-21. On the basis of HSQC, HMBC, and NOESY spectra, other substructures and the relative configuration of compound 3 were assigned as the same with compound 5. As its molecular formula was predicted as C22H23N2O4Cl from an ion peak at m/z 437.1274 [M + Na]+ in HRESIMS (calcd C22H23N2O4ClNa, 437.1239), compound 3 was a chloride salt. Finally, the structure of compound 3 was determined as shown in Figure 3, and named rauvoyunnanine B.
The known compounds 4–17 were identified as lochnerine (4) [20], serpentinic acid (5) [21], reserpine (6) [13], α-yohimbine (7) [22], ajmaline (8) [22], mauiensine (9) [23], ajmalicine (10) [24], sitsirikine (11) [25], strictosamide (12) [26], strictosidinic acid (13) [27], caboxine B (14) [28], isocaboxine B (15) [28], spegatrine (16) [29], and 19(S),20(R)-dihydroperaksine (17) [30] by comparing their MS, 1H and 13C NMR data with those reported in the literature, respectively.
2.2. Immunosuppressive Activity Assay
All isolated compounds were evaluated for the inhibitory activity against human T cell proliferation according to reported protocols [17]. Compounds 1 and 6 showed immunosuppressive activity on human T cell proliferation, with IC50 values of 5.9 μM and 5.0 μM, respectively. Other compounds with IC50 > 50 μM were inactive (Table 2).
Table 2.
IC50 values of compounds 1–17 (μM) and total alkaloids (µg/mL) from R. yunnanensis against T cell proliferation.
| Sample | IC50 |
|---|---|
|
1
2–5 |
5.9 ± 0.8 >50 |
|
6
7–17 |
5.0 ± 0.5 >50 |
| Total alkaloids | 18.5 ± 0.8 |
3. Experimental
3.1. General Experimental Procedures
A PerkinElmer 341 digital polarimeter was used for optical rotations. UV spectra were measured on a PerkinElmer Lambda 35 UV/VIS spectrometer (PerkinElmer, Waltham, MA, USA). NMR experiments were performed on Bruker Avance 600 MHz NMR spectrometer (Bruker Biospin Gmbh, Rheinstetten, Germany). A BioTOF-Q mass spectrometer (Bruker Daltonics, Billerica, MA, USA) was used to record HRESIMS spectra. Column chromatography (CC) was performed through glass columns packed with silica gel (200–300 mesh, Qingdao Marine Chemistry Co. Ltd., Qingdao, China), Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), reversed-phase silica gel (octadecylsilyl (ODS), 50 μm, YMC Co. Ltd., Kyoto, Japan), macroporous resin D-101 (Chengdu Kelong Chemical Co. Ltd., Chengdu, China), and NH-gel (MB 100–40/75, Fuji Silysia Chemical Ltd., Kasugai, Japan). Analytical (1 mL/min) and semipreparative (3 mL/min) HPLC (Waters Corporation, Milford, MA, USA) were carried out on a Waters 2695 apparatus corresponding to a Kromasil 100–5C18 (5 μm, 250 × 4.6 mm) and a Waters XTerra RP18 (10 μm, 250 ×10 mm) columns, respectively. An Agilent 1260 apparatus coupling to an Agilent Pursuit XRs 5 C18 (5 μm, 250 × 21.2 mm) column was used for preparative (10 mL/min) HPLC (Agilent Corporation, Waldbronn, Germany). Thin layer chromatography (TLC) was performed on silica gel GF254 glass plates (Qingdao Marine Chemistry Co. Ltd., Qingdao, China). Spots were visualized by UV light (254 nm) and Wagner’s reagent. All solvents were analytical and HPLC grade.
3.2. Plant Material
The whole plants of R. yunnanensis were collected in October 2009, from Mengla County (21.08°–22.36° N latitude, 99.56°–101.50° E longitude, 900–1300 m.a.s.l.), XishuangBanna, Yunnan Province, China, and authenticated by Dr. Yu-Lan Peng, Chengdu Institute of Biology, Chinese Academy of Sciences. A voucher specimen (LMRY0904) was deposited at School of Pharmacy, Southwest University for Nationalities (Chengdu, China).
3.3. Extraction, Isolation, and Purification Procedures
The air-dried and powdered whole plants of R. yunnanensis (8.5 kg) were extracted as described before to yield CHCl3 and n-BuOH extractions [17]. The CHCl3 extraction (79.6 g) was subjected to silica gel CC eluted with a step gradient CHCl3–MeOH (50:1 to 0:1) system. After being analyzed by TLC, fractions A–E were obtained. Fraction A (3.0 g) was fractionated by silica gel CC eluted with step gradient n-hexane-aectone (8:1 to 1:1) eluents to obtain alkaloid-containing fractions A1 and A2. Compound 10 (71 mg) was precipitated from A1 in MeOH. The mother liquor (335 mg) was separated by Sephadex LH-20 (MeOH) to afford compounds 10 (27 mg) and 14 (80 mg), and a mixture, which was further purified by semipreparative HPLC eluted with 35% MeOH–H2O containing 0.1% trifluoroacetic acid (TFA) to give compound 11 (13 mg, tR 17.5 min). The purification of fraction A2 (650 mg) through Sephadex LH-20 (acetone) provided two subfractions, A21–A22. A21 (150 mg) was isolated on silica gel CC eluted with CHCl3–MeOH (10:1) to yield compound 6 (15 mg). Compounds 7 (9 mg, tR 25.2 min) and 15 (6 mg, tR 16.5 min) were purified from A22 by semipreparative HPLC with 20% MeCN–H2O (+0.1% TFA) as an eluent. Compound 8 (1.8 g) was precipitated from fraction B. The left mother liquor (13.5 g) was performed CC on ODS eluted with a step gradient MeOH–H2O (10% to 50%) to obtain two alkaloid-containing parts B1 and B2. Compounds 8 (320 mg) and 9 (50 mg) were precipitated from B1 and B2, respectively. Fractions C (3.1 g), D (3.9 g), and E (9.8 g) were isolated by ODS CC with a step gradient MeOH–H2O (10% to 60%) eluent, respectively. Compound 1 (103 mg) was precipitated from the 30% MeOH fraction of C in MeOH. The following 40% MeOH fraction of D was further purified by semipreparative HPLC using 25% MeCN–H2O (+0.1% TFA) as eluent to give compound 12 (32 mg, tR 22.6 min). Compound 4 (300 mg) was crystalized from the 30% MeOH fraction of D in MeOH. Subfractions E1 (830 mg) and E2 (310 mg) from fraction E were first separated by Sephadex LH-20 (MeOH) to afford E1A, E1B, and E2A, respectively. Compounds 17 (3 mg, 20% MeOH–H2O, +0.1% TFA, tR 18.1 min), 2 (15 mg, 10% MeCN–H2O, +0.1% TFA, tR 23.5 min), and 3 (4 mg, 25% MeCN–H2O, +0.1% TFA, tR 20.7 min) were further purified from E1A, E1B, and E2A by semipreparative HPLC, respectively.
The n-BuOH (60.5 g) extraction was applied to macroporous resin D-101 CC eluted with a step gradient of EtOH-H2O (0 to 100%) solution. TLC analysis showed only the 20% EtOH fraction included alkaloids. Therefore, the 20% EtOH fraction (10.8 g) was submitted to NH-gel CC eluted with a gradient of CHCl3–MeOH (6:1 to 1:1) to give fractions F–I. Fraction F (1.2 g) was first isolated by Sephadex LH-20 (MeOH) and then purified by preparative HPLC (40% MeOH–H2O, +0.1% TFA) to yield compound 5 (520 mg, tR 20.3 min). Fraction C (1.5 g) was chromatographed through an ODS column with a step gradient of MeOH–H2O (10% to 30%) to afford a mixture, which was further purified by preparative HPLC (15% MeCN–H2O, +0.1% TFA) to give compounds 13 (371 mg, tR 18.3 min) and 16 (152 mg, tR 11.6 min), respectively. NMR spectra of compounds 1−3 can be found in the Supplementary Materials.
11-Hydroxyburnamine (1): yellowish, amorphous powder; − 117.5 (MeOH, c 0.04); UV (MeOH) λ max (log ɛ) 207 (4.75), 293 (3.68) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z 385.1766 [M + H]+ (calcd for C21H25N2O5, 385.1758).
Rauvoyunnanine A (2): yellowish, amorphous powder; + 74 (MeOH, c 0.1); UV (MeOH) λ max (log ɛ) 203 (4.44), 275 (3.85) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z 327.1676 [M + H]+ (calcd for C19H23N2O3, 327.1703).
Rauvoyunnanine B (3): yellowish, amorphous powder; + 151 (MeOH, c 0.1); UV (MeOH) λ max (log ɛ) 252 (4.38), 307 (4.07), 367 (3.44) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z 437.1274 [M + Na]+ (calcd for C22H23N2O4ClNa, 437.1239).
3.4. Assay for Inhibitory Activity on T Cell Proliferation [17]
3.4.1. T Cell Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from three healthy donors by density-gradient centrifugation using Lymphoprep. The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% FBS. The T cells were isolated using the Pan T Cell Isolation Kit II Human with negative selection. Then, the T cells were stained using the PE-anti-CD3 antibody (BD PharMingen, San Diego, CA, USA), and the number of stained cells was determined using flow cytometry (Acurri C6, Becton Dickinson, San Jose, CA, USA). The T cells were at 95% purity during the following experiments.
3.4.2. Cell Proliferation Assay
Flow cytometry was used to probe T cell proliferation using 5-carboxyfluorescein diacetate succinimide ester (CFSE, Molecular Probes, Eugene, OR, USA)-labeling. Briefly, human naïve T cells or PBMCs (106 cells/mL) were stained with 2.5 μM CFSE at 37 °C for 10 min, washed with PBS twice and re-suspended in RPMI 1640 medium containing 10% FBS. Then, the labeled naïve T cells (106 cells/mL) were activated by plate-bound anti-CD3 (2 μg/mL, HIT3a clone) and soluble anti-CD28 (1 μg/mL, CD28.2 clone, BD PharMingen). The labeled PBMCs (106 cells/mL) were activated with equal numbers of PBMCs irradiated with 3000 rad from another person. Subsequently, the proliferation of the T cells activated by the anti-CD3/anti-CD28 antibodies or alloantigen was measured by flow cytometry after stimulation for 72 h incubated with or without different concentrations of isolated compounds. The cells without a stimulation or drug served as the negative control, and the positive control was the cells with the stimulation but without the drug.
3.4.3. Statistical Analysis
The inhibitory concentrations of the compounds that reduced cell proliferation by 50% (IC50) values were calculated using GraphPad Prism 6 (GraphPad, San Diego, CA, USA). One-way analysis of variance (ANOVA) with Dunnett comparisons on post-tests were used to analyze data and compare groups. The results are expressed as the mean ± S.E.M. P < 0.05 was considered to be statistically significant.
4. Conclusions
In this study, a new picraline-type alkaloid (1), a new sarpagine-type alkaloid (2), and a new serpentine-type alkaloid (3) were obtained from the whole plants of R. yunnanensis. Their structures were extensively elucidated by HRESIMS, 1D and 2D NMR, and UV analysis. Compounds 1 and 6 showed moderate immunosuppressive activity on T cell proliferation. Previous bioactivity studies of reserpine (6) mainly focused on antihypertension [14]. Although reserpine induced suppression on delayed hypersensitivity and contact sensitivity [15], its immunosuppressive activity on T cell proliferation was reported for the first time. The discovery of these new compounds enriched the chemical diversity of monoterpene indole alkaloids and also provided a potential immunosuppressant for further mechanism study.
Supplementary Materials
Supplementary data (NMR spectra of compounds 1−3) to this article can be found online.
Author Contributions
L.-M.L. contributed in the identification of all the isolated compounds and writing the manuscript. S.-D.S. performed the isolation of the compounds. Y.L. accomplished the immunosuppressive activity assay. Q.Z. reviewed the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31870341).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–17 are available from the authors.
References
- 1.Sakaguchi S., Sakaguchi N., Shimizu J., Yamazaki S., Sakihama T., Itoh M., Kuniyasu Y., Nomura T., Toda M., Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Monteleone G., Pallone F., Macdonald T.T., Chimenti S., Costanzo A. Psoriasis: from pathogenesis to novel therapeutic approaches. Clin. Sci. 2011;120:1–11. doi: 10.1042/CS20100163. [DOI] [PubMed] [Google Scholar]
- 3.Stucker F., Ackermann D. Immunosuppressive drugs–how they work, their side effects and interactions. Umsch. 2011;68:679–686. doi: 10.1024/0040-5930/a000230. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Farmer J.D., Lane W.S., Friedman J., Weissman I., Schreiber S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-H. [DOI] [PubMed] [Google Scholar]
- 5.Brown E.J., Albers M.W., Shin T.B., Ichikawa K., Keith C.T., Lane W.S. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman A.C. Immunosuppressive medications. Clin. J. Am. Soc. Nephro. 2016;11:332–343. doi: 10.2215/CJN.08570814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senda M., DeLustro B., Eugui E., Natsumeda Y. Mycophenolic acid, an inhibitor of IMP dehydrogenase that is also an immunosuppressive agent, suppresses the cytokine-induced nitric oxide production in mouse and rat vascular endothelial cells. Transplantation. 1995;60:1143–1148. doi: 10.1097/00007890-199511270-00015. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Ma X.J. Triptolide inhibits IL-12/IL-23 expression in APCs via CCAAT/enhancer-binding protein alpha. J. Immunol. 2010;184:3866–3877. doi: 10.4049/jimmunol.0903417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.W., Yang C.Y., Jin N.S., Xie Z.Y., Fei L., Jia Z.C., Wu Y.Z. Sinomenine promotes differentiation but impedes maturation and co-stimulatory molecule expression of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2007;7:1102–1110. doi: 10.1016/j.intimp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.State Administration of Traditional Chinese Medicine . Zhonghua Bencao. Volume 6. Shanghai Science & Technology Press; Shanghai, China: 1999. p. 308. [Google Scholar]
- 11.Hu X.J., He H.P., Zhou H., Di Y.T., Yang X.W., Hao X.J. New indole alkaloids from Rauvolfia yunnanensis. Helv. Chim. Acta. 2006;89:1344–1350. doi: 10.1002/hlca.200690134. [DOI] [Google Scholar]
- 12.Wang H.B., Liu X.K. Novel indole alkaloid from the flowers of Rauvolfia yunnanensis. Chem. J. Chin. U. 2010;31:2005–2009. [Google Scholar]
- 13.Geng C.A., Liu X.K. Indole alkaloids from the leaves of Rauvolfia yunnanensis. Chem. J. Chin. U. 2010;31:731–735. doi: 10.1016/j.fitote.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Hanna B., Slim M.D., Henry R., Black M.D., Paul D., Thompson M.D. Older blood pressure medications-do they still have a place? Am. J. Cardiol. 2011;108:308–316. doi: 10.1016/j.amjcard.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Mekori Y.A., Weitzman G.L., Galli S.J. Reevaluation of reserpine-induced suppression of contact sensitivity. J. Exp. Med. 1985;162:1935–1953. doi: 10.1084/jem.162.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H.D., Lu D.X., Qi R.B. Therapeutic strategies targeting the LPS signaling and cytokines. Pathophys. 2009;16:291–296. doi: 10.1016/j.pathophys.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Zeng T., Wu X.Y., Yang S.X., Lai W.C., Shi S.D., Zou Q., Liu Y., Li L.M. Monoterpenoid indole alkaloids from Kopsia officinalis and the immunosuppressive activity of rhazinilam. J. Nat. Prod. 2017;80:864–871. doi: 10.1021/acs.jnatprod.6b00697. [DOI] [PubMed] [Google Scholar]
- 18.Ndongo J.T., Mbing J.N., Monteillier A., Tala M.F., Rütten M., Mombers D., Cuendet M., Pegnyemb D.E., Dittrich B., Laatsch H. Carbazole-, aspidofractinine-, and aspidocarpamine-type alkaloids from Pleiocarpa pycnantha. J. Nat. Prod. 2018;81:1193–1202. doi: 10.1021/acs.jnatprod.7b00958. [DOI] [PubMed] [Google Scholar]
- 19.Boğa M., Kolak U., Topçu G., Bahadori F., Kartal M., Farnsworth N.R. Two new indole alkaloids from Vinca herbacea L. Phytochem. Lett. 2011;4:399–403. doi: 10.1016/j.phytol.2011.07.008. [DOI] [Google Scholar]
- 20.Abaul J., Philogène E., Bourgeois P., Mérault G., Poupat C., Ahond A., Potier P. Alcaloïdes indoliques de Rauvolfia biauriculate. J. Nat. Prod. 1986;49:829–832. doi: 10.1021/np50047a011. [DOI] [Google Scholar]
- 21.Schlittler E., Schwarz H. The alkaloid serpentine from Rauwolfia serpentine. Helv. Chim. Acta. 1950;33:1463–1477. doi: 10.1002/hlca.19500330609. [DOI] [Google Scholar]
- 22.Staerk D., Lemmich E., Christensen J., Kharazmi A., Olsen C.E., Jaroszewski J.W. Leishmanicidal, antiplasmodial and cytotoxic activity of indole alkaloids from Corynanthe pachyceras. Planta. Med. 2000;66:531–536. doi: 10.1055/s-2000-8661. [DOI] [PubMed] [Google Scholar]
- 23.Christiane K., Pierre P., Kan S.K., Jokela R., Lounasma M. Indole alkaloids from Rauvolfia media. Phytochemistry. 1986;25:1783–1784. [Google Scholar]
- 24.Wenkert E., Chang C.J., Chawla H.P.S., Cochran D.W., Hagaman E.W., King J.C., Orito K. General methods of synthesis of indole alkaloids. 14. Short routes of construction of yohimboid and ajmalicinoid alkaloid systems and their 13C nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1976;98:3645–3655. doi: 10.1021/ja00428a044. [DOI] [Google Scholar]
- 25.Nunes D.S., Koike L., Taveira J.J., Reis F.A.M. Indole alkaloids from Aspidosperma pruinosum. Phytochemistry. 1992;31:2507–2511. [Google Scholar]
- 26.Zhang Z.Z., Elsohly H.N., Jacob M.R., Pasco D.S., Walker L.A., Clark A.M. New indole alkaloids from the bark of Nauclea orientalis. J. Nat. Prod. 2000;64:1001–1005. doi: 10.1021/np010042g. [DOI] [PubMed] [Google Scholar]
- 27.Arbain D., Putra D.P., Sargent M.V. The alkaloids of Ophiorrhiza filistipula. Aust. J. Chem. 1993;46:977–985. doi: 10.1071/CH9930977. [DOI] [Google Scholar]
- 28.Reina M., Ruiz-Mesia W., Ruiz-Mesia L., Martínez-Díaz R., González-Coloma A. Indole alkaloids from Aspidosperma rigidum and A. schultesii and their antiparasitic effects. Z. Nat. 2011;C 66:225–234. doi: 10.1515/znc-2011-5-605. [DOI] [PubMed] [Google Scholar]
- 29.Lin M., Yang B.Q., Yu D.Q. Studies on the quaternary alkaloids of Rauvolfia verticillata (Lour.) Baill var. hainanensis Tsiang. Acta. Pharm. Sin. 1986;21:114–118. [PubMed] [Google Scholar]
- 30.Sheludko Y., Gerasimenko I., Kolshorn H., Stöckigt J. New alkaloids of the sarpagine group from Rauvolfia Serpentine hairy root culture. J. Nat. Prod. 2002;65:1006–1010. doi: 10.1021/np0200919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.