Abstract
Europe's growing awareness of gaps in its healthcare provision is not being matched by an increase in remedial action – despite the rich transformative potential of new approaches to data. The new availability of data offers policymakers tools that would allow Europe's huge investments in health to be far better spent, by being properly targeted. The result would be far better health for far more Europeans. But that requires a step that most European policymakers have not been ready to take. They need to cooperate so that the data can be shared and its full value realised. This paper explores the potential and the challenges that stand in the way of mobilising health data for wider health benefits. This paper goes on to summarise the results of a survey on how different components of the healthcare sector perceive the opportunities from mobilising data effectively, and the barriers to doing so. The responses demonstrated a widespread genuine will to promote research and innovation, and its take-up, for the betterment of healthcare. There was strong appreciation of the merits of data sharing and readiness – under the right circumstances – to share personal health data for research purposes and to undergo genetic sequencing. This paper also suggests the strategic direction that should influence policy formation. The solution can be found without changing the EU treaties, which already provide an adequate base for cooperation. Properly handled, the problems facing European healthcare can be turned into major assets for Europe and make it easier for citizens to have equal access to high-quality care through the meaningful use of digital innovations.
Keywords: Patient, Big data, Regulatory systems, Member states, Enablers, Innovation, Commission, EU, Genomics, Registry, Pan-cancer, Rare disease, Personalised healthcare, Molecular diagnostics, Therapeutics
Europe's Healthcare Dilemma
Normalising the widespread use of digital solutions for health and care can increase the well-being of millions of citizens and radically change the way health and care services are delivered to patients – but it is not happening.
The EU has repeatedly recognised the challenges it faces in its health policy and healthcare provision – those familiar acknowledgements of the resource implications of ageing populations, increased chronic disease, and rising expectations, intensified by the speed and scope of scientific and technological progress that increasingly outpaces regulatory systems. But Europe has not yet got around to providing much in the way of adequate answers.
A key document in 2018 (Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee, and the Committee of the Regions on enabling the digital transformation of health and care in the Digital Single Market; empowering citizens and building a healthier society [SWD(2018) 126 final]) said it all in the first paragraph: “Only by fundamentally rethinking our health and care systems can we ensure that they remain fit-for-purpose. This means systems which aim to continue to promote health, prevent disease and provide patient-centred care that meets citizens' needs. Health and care systems require reforms and innovative solutions to become more resilient, accessible and effective in providing quality care to European citizens” [1].
It was not a new thought. The radical concept of exploiting the data from new science through making use of new information technology – not least through personalised medicine – has been around for a decade. In 2017, the Commission declared its intention to take action to ensure “better data to advance research, disease prevention and personalised health and care” as well as to boost the development of “digital tools for citizen empowerment and person-centred care” [2].
The Council is on board too – in theory. In December 2017, it adopted Conclusions inviting Member States and the Commission to work together on a range of issues and seize the potential of digital technologies in health and care. The Conclusions also call specifically for the implementation in the health sector of existing EU legislation on the protection of personal data, electronic identification, and information security [3]. Even earlier, the Council's Conclusions on personalised medicine called on the Commission to help achieve the potential of “Big Data” [4].
In 2014, Council conclusions on innovation for the benefit of patients noted “a need to facilitate the translation of scientific advances into innovative medicinal products that meet regulatory standards, accelerate patients' access to innovative therapies” [5]. A seminal EU paper on -omics in 2013 noted, obviously but presciently, that “the amount of medically relevant data available electronically is increasing dramatically. The challenge is to organise electronic data and to make them usable for research and healthcare” [6].
On the threshold of the third decade of the 21st century, the essence of this dilemma is that normalising the widespread use of digital solutions for health and care can increase the well-being of millions of citizens and radically change the way health and care services are delivered to patients – but it is not happening anything like as much or as fast as it should.
The Potential of Digital Health: Filling the Gaps
Institutions and official organisations could ease the barriers to innovation and uptake by ensuring that health systems can access the information needed to optimise services, by countering market fragmentation, and by overcoming the current lack of interoperability.
All that opportunity is still out there – and the failure to grasp it risks wasting current and future opportunities. Digital health can support the reform of systems and their transition to new care models centred on people's needs and enable a shift from hospital-centred systems to more community-based and integrated care structures.
Digital tools can translate scientific knowledge into helping citizens remain in good health, thus helping to ensure that they do not turn into patients, a theme frequently addressed before as P4 medicine [7]. The potential from better use of health data in research and innovation is within reach to support personalised healthcare, better health interventions, and more effective health and social care systems. “Attainment of the broad health system goals, including quality, accessibility, efficiency and equity, are objectives against which to judge new digital health services. These goals are unaltered by the process of digitalisation. Evaluations should be designed and tailored in such a way as to capture all relevant changes in an adequate manner,” it concluded.
The European Parliament has consistently given strong backing to the European Union's attempts to take advantage of well-managed data sharing to improve healthcare for Europe's citizens. As recently as February 2019, it massively endorsed the EU's efforts to make a reality of its 2011 cross-border healthcare legislation – which also provides for cooperation on eHealth, rare diseases, and HTA. It called for greater efforts by the member states to ensure patients could benefit, and praised the progress made with the European Reference Networks that the legislation introduced, urging the setting up of specialised centres for rare diseases in the EU. It also “asked the Commission to further guarantee access to information, medicine and medical treatment for patients with rare diseases throughout the EU, and to strive for improved access to early and accurate diagnosis” [8]. It emphasised that interoperability of eHealth should be made a priority in order to improve global patient records and continuity of care and welcomed the creation of the EU-wide eHealth Digital Service Infrastructure designed to foster the cross-border exchange of health data.
But the pre-condition, as the EU acknowledged in its 2017 communication, is that the new systems are “designed purposefully and implemented in a cost-effective way.” This means tackling the current well-documented deficiencies: “Health systems lack key information to optimise their services, and providers find it hard to build economies of scale to offer efficient digital health and care solutions and to support cross-border use of health services.”
The same conviction of the need for cooperation was the background to the European Commission's release in early 2019 of an electronic health record exchange format, along with an invitation to related industries to embrace an interoperable data ecosystem and open, international standards and make data available to the healthcare sector. “The interoperability of electronic health records in the EU will make it easier for citizens to access their health data securely across borders,” said Commissioner Mariya Gabriel, “and medical professionals will be able to assist citizens more efficiently and effectively. The development of these systems will require support from all stakeholders” [9].
Progress remains blocked by that constant handicap of a still-splintered EU, the Parliament underlined: “Market fragmentation and lack of interoperability across health systems stand in the way of an integrated approach to disease prevention, care and cure better geared to people's needs.” Success “depends on the availability of vast amounts of high quality data and appropriate regulatory frameworks that will safeguard the rights of the individual and society as well as stimulating innovation” [10].
The expert panel was clear where gaps needed to be filled: “Further investment in the development of methodologies and a European repository for evaluation methods and evidence of digital health services is encouraged,” it said. It emphasised the importance of data interoperability, particularly in the context of collecting, sharing, and manipulation of data and recommended the use and development of international classifications and terminologies to increase interoperability. And above all, “governments could play a more active role in the further optimisation both of the process of decision-making (both at the central and decentralised level) and the related outcomes.”
“Digital transformation may sometimes lead to new care pathways and services, which may not be a good fit with current organisation of care, care pathways or financial structures. Lack of flexibility in those aspects may lead promising developments not to be used, simply because the organisational prerequisites for their use are not met,” it warned.
Making Disruptive Innovation the New Normal
Systemic change is required to create an environment that not only allows for the deployment of new technologies, but that promotes new attitudes among citizens, patients, and professionals.
The barriers and possibilities for the uptake of “disruptive” health services were discussed at length in an earlier report (Report on Disruptive Innovation, Expert Panel on Effective Ways of Investing in Health (EXPH), Brussels, 2016), which said: “Some of the most important barriers to keep in mind are: lack of engagement of patients/people; resistance of the health workforce and organisational/institutional structures; inadequate networks and processes; economic and legal factors; lack of political support, lack of coordinated actions across agents, and lack of knowledge and evaluations” [11].
The panel recommended creating an environment willing and able to adopt evidence-based innovations. The implication is that wholesale systemic change is required to achieve the avowed goal. The adoption and implementation of effective and cost-effective new digital health services requires having an environment that allows for this. This relates to the attitudes and training of citizens, patients, and professionals but also to overcoming organisational and financial barriers in adopting new technologies. “Removing such barriers and allowing the freedom to (controlled and evaluated) experiment with new technologies is needed. This can also facilitate decentralised innovation, which may contribute to the best services being developed and implemented and to continuous improvement of health services.”
The well-documented failure to implement European systems that can mobilise the potential for better care is a genuinely European issue. The organisation and delivery of health and social care remain – as always – the ultimate responsibility of the Member States, but it is also well-established EU doctrine that the Commission can promote public health and the prevention of disease and support cooperation between the Member States, for example, to improve the complementarity of their health services cross-border, or to stimulate innovation, economic growth, and the development of the Single Market.
This is why the Commission has been working – adventurously, for them, but so far somewhat timorously, for others – with the Member States, regional authorities, and other stakeholders both to tap into the potential of innovative solutions such as digital technologies and data analytics and to assist Member States in pursuing the reforms of their health and care systems. Funding and actions that promote policy cooperation and exchange of good practice have trickled in through programmes such as Horizon 2020, which has given some backing to research and innovation in digital health and care solutions, or support via the Connecting Europe Facility for infrastructure for cross-border exchange of patient summaries and electronic prescriptions.
There is nothing wrong with the current Commission vision in its digital health communication “to promote health, prevent and control disease, help address patients' unmet needs and make it easier for citizens to have equal access to high quality care through the meaningful use of digital innovations.” It is right to seek to “strengthen the resilience and sustainability of Europe's health and care systems,” and to declare that “by helping to maximise the potential of the digital internal market with a wider deployment of digital products and services in health and care, the proposed actions also aim to stimulate growth and promote the European industry in the domain.” But the bottom line is – as it admits in the same document – that “the uptake of digital solutions for health and care remains slow and varies greatly across Member States and regions (and) further action at EU level is crucial to accelerate the meaningful use of digital solutions in public health and healthcare in Europe.” And as long as the current careful – some might say leisurely – approach predominates, the prospects look slender for any real improvement commensurate with the evident needs.
Cooperation and Coordination Going Forward
So far, the progress has been minimal. Even on components as basic as electronic health records, it is hard to find much consensus emerging. Even in a country as advanced – and not large – as the Netherlands, it has proved impossible to adopt a single electronic health record format, and at EU level, the prospects for cooperation are currently dismayingly remote.
Take the Commission's meticulously calibrated plan for moving beyond the recent small success of some local transfers across borders of e-prescriptions and patient summaries: “The Commission sees a need to gradually extend these two use cases to also cover the interoperability of Member States' electronic health record systems by supporting the development and adoption of a European electronic health record exchange format. There is also a clear case to develop further effective methods for enabling the use of medical information for public health and research and to develop common identification and authentication measures, as laid down in Article 14(2) of Directive 2011/24/EU. Such changes will require reviewing the management and functioning of the eHealth network to ensure appropriate governance of the eHealth digital service infrastructure and its financial basis” [12].
Similarly, the Commission openly acknowledges that it is not cooperating sufficiently to advance personalised medicine. While its 2018 communication notes with evident gratification that now “several national and regional initiatives already support the pooling of genomic and other health data to advance research and personalised medicine,” it admits “we need to better coordinate these existing initiatives to reach the necessary critical mass at EU level and match similar initiatives in other world regions.”
The key lies in “linking Europe's fragmented resources through secure cross-border digital infrastructures.” But the lure of “European coordinated action in this field,” with all the expected “tangible benefits for citizens and health systems in the EU” in terms of tackling major health challenges, is manifestly not yet sufficient to prompt adequate European action to seize the fruits.
All the right words are there: “building on the European High Performance Computing initiative and the European Open Science Cloud infrastructure,” connecting national initiatives with “European networks of scientific and clinical expertise, such as the International Consortium for Personalised Medicine, the European Reference Networks, the European Research Infrastructures, the Human Brain Project and other relevant initiatives,” and “linking national and regional banks of -omics data, biobanks and other registries across the EU.” But when it comes to action, most of it – with the honourable exception of the take-up of EAPM's MEGA project [13], now EU 1 Million Genomes (EU1MG), in respect of reaching a cohort of one million genomes by 2022 – remains for the future: “It is paramount to agree on technical specifications for access and exchange of health data for research and public health purposes, addressing, for example, health data collection, storage, compression, processing and access across the EU,” said the Commission in 2018. But how much has happened so far? Hungary and Croatia exchanging a handful of electronic prescriptions. Europe is still at the stage of “pilot actions,” including the use of real-world data, which “will be developed with clinical associations, national competent authorities, health technology assessment bodies, research infrastructures, industry, the Innovative Medicines Initiative and relevant EU agencies” and “others may also be considered,” for instance on cancer or neurodegenerative diseases.
There is an aspirational rather than a determined tone to the current EU approach. It speaks almost dreamily of how “a critical mass of usable data will support vital knowledge generation and help improve prevention, diagnosis and treatment of patients,” and how “the Commission will explore with scientific representatives and clinical groups how best to stimulate demand for data aggregation, addressing incentives and concerns, such as safeguarding data protection compliance, for the further processing of health data.” It does not inspire much confidence to read that “additional support from the Member States will be encouraged to allow the pilots to reach their full potential,” or that “additional funding for this might be considered also under the next EU multi-annual financial framework to more closely link existing European resources to a world-leading health data and computation infrastructure able to effectively support scientific research and personalised medicine.”
Impatience has reached the highest levels of healthcare organisations in the EU. In January, no less a figure than EMA executive director Guido Rasi told the European Parliament that despite the “incredible opportunities” that new science offers for medicine, exploiting that potential is being held back by European Union legislation on data privacy. He expressed “deep concern” over limits imposed on secondary use of data by the EU's General Data Protection Regulation. The public health potential in translating the promise of precision medicine into real patient access is an avowed top priority for Rasi. He set out his view of how the approvals process should be redesigned around evidence, “facilitating patient access through data that serves the entire decision-making chain” [14].
Mariya Gabriel, in her role as European Commissioner for the Digital Economy and Society in the outgoing administration, looked forward to Digital Day 2018 “to bring the digital cooperation in Europe to a new level regarding eHealth,” insisting that “coordinated EU-level commitment and investments are much needed to tackle the challenges ahead,” and encouraging “all EU Member States and stakeholders to contribute to our efforts to keep Europe a global player in the digital age” [15].
Health Commissioner Vytenis Andriukaitis said the Commission is working towards cooperation on the sharing of genomic data because it will “contribute to the development of precision medicine and the improvement of clinical trials” [15].
Wolfgang Burtscher, the Commission's deputy director-general for research, has also called for the identification of common ground within the Member States' diverse approaches: breakthroughs in genomics “provide abundant potential for healthcare,” he said, recommending greater uptake of medical innovation: “We must increase our efforts in order to make the outcomes of the research translate into better patient treatment” [16].
And in a new bid to pull together on personalised medicine, Khalil Rouhana, deputy director-general in DG CONNECT, spoke of personalised medicine and genomics putting Europe “at a point of major transformation in healthcare over the next four to five years” at a Brussels conference on genomics.
Andrzej Rys, a Commission director for health, similarly spoke of the need to engage scientists, health systems, healthcare professionals and patients, and of the need to “take this innovation into the regulatory framework.” He asked: “Are society, patients, citizens ready for innovation?,” urging attention to promoting understanding of what personalised medicine is. That message was echoed by Kalle Killar, deputy secretary-general for innovation in the Estonian ministry of social affairs: “We have to keep everyone on board,” he said. “We need trust and cooperation to bring the real value for our patients from personalised medicine” [17].
Developing the Opportunities
Europe has the skills and resources – and data – to make a success in a rapidly developing new world of digital health. But the world market is highly competitive, and the leaders, the first to market, will reap the greatest benefits.
Europe's own analysis confirms the self-evident truth: “The use of patient-centred health data is still under-developed across the EU” (page 37 of the “State of Health in the EU ‘Companion Report 2017’”) [18].
The opportunities are there for the seizing. In aggregate, Europe has many of the skills and resources – and data – to make a success of the new future of personalised medicine. An overview of the different projects initiated within the boundaries of the European Union in the field of genomic medicine, sequencing, and personalised healthcare can be found in Table 1. In addition to the projects being undertaken at the European level, many countries also run programmes within their national borders. An overview of country-level projects appears in the appendix of this paper.
Table 1.
Overview of European projects in the field of genomic medicine and personalised healthcare
| Program/initiative/project | Website | Coordinating institution | Countries/Member States | Field |
|---|---|---|---|---|
| EU core activities | ||||
| 1. EATRIS | https://eatris.eu | EU | 13 | |
| 2. Bbmri-eric | http://bbmri-eric.eu | EU | 18 | Bioinformatics |
| 3. Elixir | EU | 21 | Bioinformatic | |
| 4. CancercoreEurope | EU | 7 | ||
| 5. Euengage | EU | Cancer care | ||
| 6. RD-Connect | EU | |||
| 7. Solve-RD | EU | |||
| 8. MEGA/EU1MG | EU | |||
| 9. EMBL | EU | 6 | ||
But success depends on learning to share those assets across its own internal borders so that it can profit from them in terms of better health and better innovation. A world market is developing all the time, but it is a competitive market, where the leaders, the first to market, will reap most of the benefits. The level of activity could be a positive signal for Europe's innovators and Europe's citizens. But Europe is not alone in pursuing this course. In the US, the heat may for the moment have gone out of the 21st Century Cures initiative that Barack Obama launched (and President Trump has not urged support for), and Joe Biden's “Moonshot” bid to tackle cancer has fallen into abeyance during his run for the Democrat nomination as its candidate for the presidency in 2020. But there are plenty of other efforts underway around the world, and the window of opportunity for Europe to exploit its potential is not going to be open indefinitely.
How true the conclusion that the EU draws in its main 2018 policy paper: “The swift deployment of innovative digital health solutions can best be achieved by working together at EU level” [19]. And how far Europe still is from either swift deployment or significant working together at EU level!
The Barriers That Stand in the Way of Access to Personalised Healthcare
There is nothing new in the statement that barriers exist in Europe. What is new is that the stakes are higher than ever, and the path to success is, in theory at least, now much clearer. Europe's healthcare systems are strained as never before. But new thinking – on training, on tools, on validation, on innovation – could drive a transformation of European healthcare.
The uptake of personalised healthcare approaches and the integration of genomics is halted by a certain number of barriers that slow down the process. Stark et al. [20] recently came up with a summary of these different barriers spanning different aspects. Table 2 lists these barriers and identifies corresponding stakeholders at the European level, as well as potential action points they could focus on in order to facilitate the uptake of genomic medicine approaches.
Table 2.
Summary of the barriers to the uptake of personalised healthcare with corresponding European stakeholders and potential action points (adapted from Stark et al. [20])
| Barriers to integration of genomics into healthcare | Stakeholders at the European level | Potential action points |
|---|---|---|
| Data integration and interpretation | EATRIS-ERIC, Elixir, BBMRI, bioinformatics research institutions | Integration, aggregation, and analysis of existing genomic datasets at the European level Oversight of the collection and analysis of new genomic data at the European level |
| Workforce capacity and capability | European medical universities, medical schools, academic hospitals | Integration of genomics and principles of personalised healthcare inside of the medical, nursing, and healthcare curriculums Introduction of career-long education programs regarding personalised healthcare and genomic medicine for practicing professionals |
| Public acceptability and government engagement | National, local, and regional governments at the member state levels | European Union Introduction of outreach methods for the general public |
| Paucity of evidence for clinical utility | Research centres and funding institutions | Support and grants for studies aimed at producing clinical evidence |
| Cost-effectiveness | HTA institutions, healthcare payers | More transparency, data sharing, and exchange of best practices to support reimbursement of personalised healthcare and genomic technologies |
| Stakeholder alignment and political engagement | European Alliance for Personalised Medicine | Development of frameworks for decision making and adoption |
| Ethical and legislative issues | EU and country-level legislators, patient organizations, medical-ethical committees | Development of model for ethical guidance and coordination of personalized healthcare activities Compilation of rigorous ethical standards and best practices to support roll-out of genomic technologies |
Against this background of inertia, the list of what still has to happen to win success, drawn up by the Commission in its 2013 -omics paper, takes on the character of a prophecy largely unfulfilled. The Commission then pointed out – now six years ago – that “a specific characteristic of personalised medicinal products is that, in addition to the need for prescribers of such medicines to have pharmacogenomic knowledge (requiring education and training), they also need to have adequate IT tools and systems at their disposal. Doctors will need to be trained in a number of disciplines in order to understand and to be able to use all the sophisticated tools that will be at their disposal for personalised medicine. And once trained, they should have access to diagnostic and treatment facilities to administer this care in line with the EU principle of health equality and universal access to medicine. This is a further challenge for national health systems.”
The Commission paper went on: “The effective uptake of personalised medicine approaches in a Member State will depend on acceptance of the medicinal products and the diagnostic tests by the payers, the public healthcare system and private health insurance. Both medicinal products and diagnostic tests, even if already authorised to be placed on the market, may thus be subject to rigorous evaluations of their cost and clinical effectiveness in comparison with other therapies available to treat the same disease.”
In parallel to more official health policy reflections relating to data exchange and personalised medicine, a huge constituency of involved stakeholders is constantly reflecting on the prospects, potential, and possibilities of putting this new opportunity at the service of healthcare. The multi-stakeholder EU Health Summit in November 2018 saw the healthcare industry, patient groups, and professional and academic organisations unite around an agenda that included some strong messages of support for progress in tackling the deficiencies in this domain. It explicitly backed setting up “a European Health Data Institute to produce a range of health data to inform the work of policymakers, researchers, industry and healthcare providers.” It said Member States, regions, payers, insurance companies, and data donors “should make available national data sets to facilitate the European Institute” and “the information produced by the Institute could help stimulate a European market in health informatics, research and analytics” [21]. There was similarly wide support for creating “a High Level Forum for better access to health innovation” – a multi-stakeholder forum that would discuss barriers and solutions to further innovation.
The Medtech sector as a whole called on all stakeholders to support digital health interoperability standards specified by the European Commission. The devices industry is also strongly supportive of further measures in this direction and took the opportunity of urging greater public investment in digital health infrastructures.
But COCIR secretary general Nicole Denjoy added: “The continuing slow deployment of interoperable digital health solutions in member states remains a barrier for scaling up integrated care and bringing its benefits to citizens. Member States need to deploy this recommendation at national and regional levels, to allow healthcare professionals and citizens to securely access and use relevant health data, including in cross-border exchange” [22].
The EAPM Survey
The call for change does not derive from comfortable theorising or commercial impatience. EAPM has taken a lead in assembling a wide coalition of practitioners from right across the healthcare sector who share the conviction that cooperation on data offers better ways of delivering for Europe's citizens – and who share an anxiety that policy discussions are still insufficiently informed of what needs to be done. EAPM has added to its own database of evidence by conducting surveys directly with the constituencies at the heart of Europe's healthcare – stakeholder groups representing basic science, translational research, the regulatory field, health systems, and patient perspectives.
Methodology
Literature Review. A narrative literature review was undertaken to develop an overview to contextualise the known barriers of access to personalised health and genome-based medicine; PubMed, Web of Science, and Medline were searched. Search terms were based on terminology used by, e.g., the European Alliance for Personalised Medicine and the authors' own experiences in the field and given to European strategic reports in the context of a data-driven economy, genomes, focussed on (synonyms of): “enablers” and “personalised healthcare” combined with “molecular diagnostics,” “treatment,” “Europe,” “patient,” “ethics,” “biological,” “clinical,” “public health,” “regulatory,” “legislation,” and “commercial.”
Only documents in English were included in the review. Specific attention was -omics, access, molecular diagnostics, legislation, European Parliament, Council, and Commission. The aim of gathering these results is to identify relevant fields, organisations, current initiatives, policies, and capacities related to personalised health, based on an inventory and synthesis of existing relevant information. The data from EAPM were combined with the results from the review of scientific literature, since it offers a broader scope on the knowledge available. A detailed survey was undertaken.
Stakeholder Survey and Mapping. Stakeholders were asked about their views on enablers in personalised healthcare implementation via an online survey and structured interviews. The involved stakeholder groups represented basic science, translational research, the regulatory field, health systems, and patient perspectives. The numbers of respondents and their affiliation is compiled in Table 3.
Table 3.
Overview of the stakeholders and number of participants in the EAPM survey
| Viewpoint | Stakeholder group | Amount |
|---|---|---|
| Basic science | 1. ICT | 67 |
| 2. Industry | ||
| Translational research | 1. HTA | 38 |
| 2. Insurance | ||
| Regulatory field | 1. Member states | 24 |
| Health systems | 1. Healthcare professionals | 236 |
| 2. Pathologists/oncologists | ||
| Patient perspective | 1. Patient groups | 71 |
| Total | 446 | |
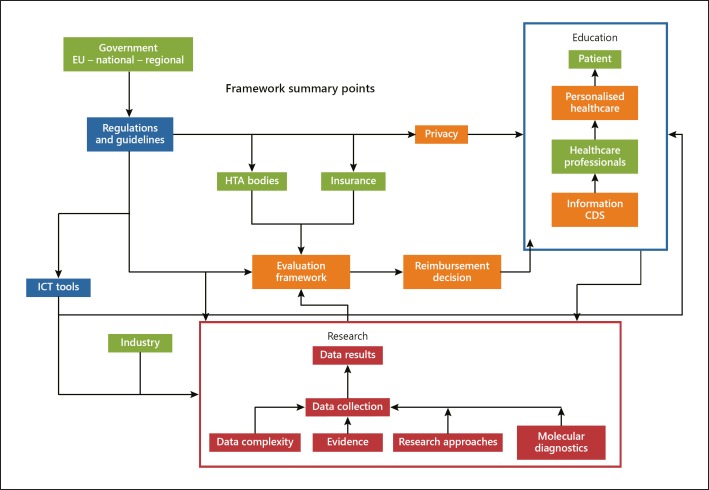
The data for the online survey was collected by the European Alliance for Personalised Medicine between April and June 2019. Each survey was divided into sections, partially identical among groups and partially tailored to the stakeholder group. In the invitation email, stakeholders received information about the study and the survey was briefly outlined, thus fully informing the stakeholders. The stakeholders, as well as the links between them, were interpreted based on the framework included in Figure 1.
Fig. 1.
Overview and links between the relevant stakeholders in the field of personalised healthcare.
Each survey consisted of close-ended questions to ensure comparability among the responses. For example, a section on general information included questions about current healthcare applications to facilitate genome-based healthcare and access to molecular diagnostics. Other sections focussed on cross-border initiatives (e.g., if they are supported by the government). Healthcare professionals and policymakers responded to a wide range of general and specific questions. The different stakeholders were contacted by mail and phone and were able to contribute on the EAPM website. Their responses, summarised by EAPM, provide valuable new insights both into personalised medicine and into perceptions of many distinct important stakeholder groups. The respondents originated from the United Kingdom, Germany, Italy, and other EU nations. In summary, a range of variables was included to achieve a nuanced exploration of possible barriers.
Ten structured interviews were conducted in June 2019. Participants from different Member States were selected based on their expertise in the field of personalised medicine. The interviews were conducted in a semi-structured fashion, based on interview headlines stemming from previous literature and policy research. Informed consent and willingness to participate were taken from all the study subjects. All of the interviews were recorded with the knowledge of the interviewee.
Each interview took a maximum of 30 minutes, and several interviewers carried out the interviews. To ensure comparability, the interviewers were informed beforehand and received an interview guide. Interviews were conducted and analysed according to statements. Some similar reflections have been captured in the survey conducted by EAPM on the future and on the potential impediments to adoption of personalised medicine (insurance companies, ICT experts, HDA experts, industry employees, pathologists, patient groups).
Results
The survey ranged across questions such as whether systems are in place to make the most of the rapid advances in the science relating to healthcare to more personal questions such as attitudes to screening or the sources of information for adopting opinions and taking decisions. The overall stance of the different stakeholders on the topics of personalised healthcare are in Table 3.
One of the most striking features of the responses demonstrates considerable uncertainty in many constituencies about the developmental, economic and regulatory processes for medicines. But there was evidence of a widespread genuine will to promote research and innovation – and its take-up – for the betterment of healthcare.
When oncologists, haematologists, and urologists were asked whether systems are in place to make the most of the rapid advances that science is making in healthcare, they displayed a very hands-on – if not always very well-informed – attitude. 90% of them believed that provision is insufficient and identified issues relating to data as the main impediments – such as the need for data warehouses or improvements in data sharing. The same group of specialised health professionals overwhelmingly felt that there was not sufficient incentive or resources for research in personalised medicine, as less than 10% of the respondents felt that the current incentives were sufficient.
Over 60% of researchers involved in the survey felt that the potential was undervalued or insufficiently understood beyond their own world, pinpointing to both insufficient structures and resources and the lack of profile among policymakers. And specialists were largely in favour of improving structures and processes for screening to reduce the possibility of false positive results and potential over-treatment.
Funding of personalised medicine is an area where opinions diverge widely. Half of the healthcare professionals interviewed consider that it was covered in health insurance and half said it was not. The majority of them considered that health insurance was a real restriction. But healthcare professionals are not convinced that incentives to business help in ensuring early patient access to innovations: about two-thirds of them (58.3%) were not sure about that, and the other third said incentives were of no use. They are all equally in the dark about whether the reimbursement model in their country takes account of issues such as value-based pricing, as 81% of the survey participants were not sure.
Three quarters of patients asked (75%) responded being worried that personalised medicine risks pushing up insurance premiums. But at the same time, half of them think that health insurance is restrictive when it comes to personalised medicine. They also suffer from a lack of information on the possibilities for clinical trials, as only about 25% of the respondents declare being sufficiently informed. The study participants, however, unequivocally (100%) declare that under the right circumstances, they would be happy to share their personal health data for research purposes.
Just under half of the patients (45%) had taken part in public health initiatives to promote early detection programmes such as screening, but 75% of the patients involved would be prepared to undergo full genetic sequencing as a future-proofing preventive measure against hereditary disease. There is also wide interest in having a test done that would allow patients and doctors to identify the chances of developing a particular condition even if there is not yet a treatment option. In fact, all of the patients asked (100%) responded in favour of that.
In contrast, if unsurprisingly, industry respondents expressed concern over the rising cost of development, the lack of incentives, and the threats to intellectual property protection – all of which served as discouragement, they said, for the heavy spending required in research and development. Industry respondents complained of delays in shifting innovations into patient use, suggesting as possible solutions more targeted approaches, faster patient recruitment to trials, greater reliance on post-approval data to monitor drug performance, and better modelling approaches using artificial intelligence. But medical specialists evinced some scepticism about the role of industry with suggestions that prices should be lower, pricing negotiations tougher, and assessments of new technology stricter.
Views on Regulation, Now and in the Future
Among specialists and healthcare professionals more broadly, there is wide awareness of the legal or regulatory framework that is currently in place for genetic testing and the use of genetic information. Half of the healthcare professionals interviewed considered that genetic data was just as important for analysis as general health research data and more than half of them felt that the decisions that they made based on molecular test results improved patient outcomes. They also overwhelmingly felt that patients should be encouraged to share their health data for research purposes as long as appropriate conditions are in place. But they demonstrated high levels of uncertainty over the extent to which security of patient data was a focus of attention in the regions where they worked.
Data privacy clearly remains a major issue for healthcare professionals and for researchers, and when they were asked to what extent the EU's general data protection regulation had affected their work, more than half of them said the impact had been negative and less than a third thought it had been positive. Areas of concern included the need for new solutions for big data projects and for secondary use of data, the brake effect the regulation's bureaucracy had on speed of work, accompanied by uncertainties over how it is implemented in different countries. Industry respondents were even more critical, and in much larger numbers, highlighting divergent interpretation and increased compliance requirements complicating international collaboration on the use of big data.
Half of the healthcare professionals surveyed (50%) also felt that current regulations can constitute barriers for the best possible use of the data, with another 40% of the respondents stating that additional barriers were in the pipeline. They cited data protection rules, lack of uniformity of European legislation and interpretation, and a focus on privacy at the expense of advancing science and healthcare. For healthcare professionals, the main barriers to the use of personalised medicine are lack of data, economic issues, lack of training and education, the disparity between diagnostics and related therapeutics, drug shortages, national guidelines, access to diagnostic testing, and clinical trial design.
More than half of the responding oncologists and pathologists (55%) found reimbursement to be the principal limiting factor for cancer patients to access medication and drug pricing came close as another major barrier, as it was named by 45% of the responding physicians.
Pursuing a European Genomic Federated Database with a GO-FAIR Principles Approach
There are multiple targets to be addressed to allow Europe to move forward effectively in realising the potential of sharing data, ranging from the political to the technical. The leap forward that the EU1MG project could provide, because it has backing from national governments, will be all the more valuable if it develops along agreed lines that can meet many of these multiple challenges, while respecting commonly agreed principles.
Europe's most conspicuous move so far towards cooperating on data is the EU1MG project. This was formalised in 2018 in the Declaration of Cooperation “towards access to at least 1 million sequenced genomes in the European Union by 2022,” and is now making progress towards its avowed goal [23]. The declaration, launched on April 10, 2018, has so far been signed by 19 EU Member States and is also open to all Member States of the European Economic Area and the European Free Trade Association. Beyond the initial goal of at least 1 million sequenced genomes in the EU by 2022, it envisages a larger prospective population-based cohort. The aim is to have, by 2025, at least 10 million records that integrate molecular profiling, diagnostic imaging, and lifestyle (in particular risk factors), as well as microbiological genomics and environmental data, all linked to electronic health records. It will also build on “digital patient” predictive approaches based on computer modelling, simulations, and artificial intelligence. Ultimately, the intention is to lay the foundation for developing a reference atlas of all human cells and to analyse human tissues and organs by state-of-the-art methodologies so as to compare and understand changes during disease.
This is a politically significant initiative because governments have committed themselves to cooperating in delivering cross-border access to genomic information. The declaration has the potential to maximise use of healthcare resources and advance the development of personalised medicine, especially in the oncology and rare disease field. However, constructing this new system requires attention to a series of essential requirements – not least the issue of data privacy.
More than nine-out-of-ten patients welcome the sharing of their genomic data for research purposes, according to a 2015 report – “Genome sequencing: what do patients think?” – by Genetic Alliance UK, which also found that patients consider a lack of genomic data sharing as a hindrance to scientific research progress, which in turn would be counter-intuitive to their hope for a better quality of life. Nonetheless, patients' willingness to share their data comes with its own specific conditions [24].
For most patients, even when willing to share their health data, it is important to control how and for which purpose the data will be shared. Being in favour of sharing data and calling for more control are not contradictory but parallel. Patients have made clear their need to be at the centre of data-driven innovation and to be recognised as active agents in data sharing initiatives in which they participate.
The current wider climate on data privacy – in the wake of reports of data breaches and abuse of social media – is an argument in favour of moving cautiously. Even if the EU's General Data Protection Regulation (Article 6 (4); Recital 502,018) allows organisations that process personal data for research purposes to avoid restrictions on secondary processing and on processing sensitive categories of data including health data, patients' requests for control over their data build the case for enabling patients to express preferences regarding the use of their data [25].
Ethical and responsible data sharing should be enabled through widespread implementation of the IRDiRC recognised resource, the international charter of principles for sharing bio-specimens and data, which provides guidance for effective legally and ethically grounded data sharing. Furthermore, several ongoing initiatives are testing the use of blockchain technology to protect personal data. Biotechnology companies are also using blockchain to share and protect genomic data.
Patients' views vary significantly by sociodemographic profile over sensitivity of data, preferences over frequency or methods of being informed, and even trust in stakeholders. Echoing these trends, dynamic systems have started to emerge as tools that would enable the provision of regular and accessible information to patients regarding the purpose and outcome of the projects whilst allowing patients to select and tailor their preferences related to when, how, and who can use their data, thus respecting individual preferences with the possibility to amend these. The concept of dynamic consent has been recently tested and reviewed offering additional potential for improving research outcomes and providing an adapted and flexible system corresponding to future technological and regulatory/legal changes in the European health systems.
In the longer term, ensuring patient trust and confidence in projects involving data sharing will help sustainable patient participation and increase the chance of successful outcomes – through mechanisms such as better technology standards, proper marketing of the benefits, an easy opt-out procedure, and dynamic consent processes [26]. Without that trust, projects can be at risk – as demonstrated by the collapse of NHS England's care data. Data sharing initiatives would also benefit from involving general practitioners and other healthcare professionals in management and communication. The FP7-funded project RD-Connect is considered a good example of practices for governance of the platform on which patients and researchers deposit data, with mandatory adherence to a Code of Conduct for access and a committee that reviews and supervises all access requests [27].
There is good evidence from surveys that rare-disease patients wish for access to information related to their disease, and it is considered important to enable this. The most pressing bottlenecks and shortcomings regarding data sharing as well as associated action points from the point of view of different stakeholders are summarised in Table 4: The rarer the disease, the greater the need for patients – already experts on their disease – to continue to build knowledge on every aspect of their disease and enable them to share updated information with their peers.
Table 4.
Current perspectives of the stakeholders involved in the EAPM survey
| Stakeholders | |
|---|---|
| Industry |
|
| Patients |
|
| HC professionals, physicians, and researchers |
|
Since scientists, clinicians, patients, industry, and policymakers concerned with progress in rare-disease research, healthcare, and policy share a similar goal of faster access to accurate diagnosis and improved healthcare, effective communication within the community is a valuable tool to break the silo-ed pattern inherent to rare-disease data and expertise.
Genomics is becoming more and more important in healthcare. But, as in any other aspect of healthcare, especially a fast-moving one, universally agreed good practices are required.
Standards and good practices should take account of the following elements:
1) In personalised medicine, the patient is (or certainly should be) at the heart of his or her own healthcare, consent and privacy are a vital component in this world of Big Data and genomics. Therefore, a patient or anyone who is a subject of research, should be asked to give consent for genomic analysis for use or linkage to health data. From a research point of view, consent in the relevant national EU languages should be sought for longitudinal data linkage and further findings down-the-line.
2) When acquiring genomic samples, the methods should be consistent across the EU, documented to an agreed standard, and be appropriate for clinical research, and potential dual-use purposes.
Storage should take place in an easily retrievable facility that also meets agreed standards, for example an approved biobank or similar. Interoperability/agreed format is essential.
3) Whole genome sequencing (WGS) and any DNA mining should also be carried out in an EU/industry-accredited facility, as above. Important are measures of evenness of sequencing across a genome. It is essential to agree at the percentage of any genome required in order to establish a minimum threshold for reliably calling variants.
4) We have existing databases through various national and international initiatives that have already been undertaken. Our genomes initiative should support moves to improve their clinical relevance.
5) There is a need for incentives to ensure that research initiatives can offer-up rapid evidence to support clinical validity and utility in respect of genome variants. Any delays in providing such evidence, for example through exclusivity or publication of findings, should be kept to an absolute minimum.
Sharing Is Caring
There is some hope of movement towards cooperation – if only rather slow at present – in other health-related areas. Recently, the European Commission released a recommendation to securely share electronic health records across Europe, building on existing programmes to share e-prescriptions and patient summaries. The Commission wants to create a framework for an EU-wide exchange platform where national systems would be able to exchange information. The potential impact of this recommendation will be entirely dependent on the willingness of countries to make the necessary investments in their national health IT infrastructure. In February 2019, it adopted a recommendation on a European Electronic Health Record exchange format – itself a useful small step. It invites industry sectors to embrace an interoperable data ecosystem and open, international standards and make data available to the healthcare sector. “The interoperability of electronic health records in the EU will make it easier for citizens to access their health data securely across borders,” said Commissioner Mariya Gabriel, “and medical professionals will be able to assist citizens more efficiently and effectively. The development of these systems will require support from all stakeholders” [28].
Electronic health records do not yet exist in most EU countries for a number of reasons including lack of interoperability, fragmentation, the large amount of unstructured data, and also to some extent a lack of trust in private companies to provide this kind of service. However, there is a trend in Europe showing an emerging political support from several countries to invest in health data hubs and electronic health records. Sharing of health data through the implementation of electronic health records across Europe will enable optimised use of health data to improve healthcare and outcomes for patients as well as promoting research. Based on the feedback from the relevant stakeholders, Table 5 pinpoints the potential action points that could be taken by the EU to facilitate the current situation. At bottom, the inescapable fact is that the Human Genome Project, completed in 2003, succeeded in mapping the human genome only through widespread international collaboration.
Table 5.
Overview of the action points for the implementation of personalised healthcare from the point of view of different stakeholders
| Stakeholders | Action points |
|---|---|
| Industry |
|
| Patients and the general public |
|
| Healthcare professionals, physicians, and researchers |
|
“Good Genomics Practice”
Ambition is fine. Europe frequently expresses ambition. But without action and the practical steps needed to make action possible, ambition remains just that: ambition. To help turn current concepts into realities that will improve the future of healthcare, serious work is being undertaken in many parts and places of Europe – and an excellent example of this is the document drafted in the framework of the EU1MG project, which aims to capture the necessities for the Initiative.
As suggested by expert interviews, “Good Genomics Practice” can be a guideline to maximise the benefits of sharing human genome sequencing data generated in the context of clinical applications” [29]. The objectives are to speed diagnosis and improve treatment and prevention, but the suggestion is rigorously practical. It identifies three medical areas where WGS or whole exome sequencing (WES) data could be of benefit: rare diseases, cancer, and prevention of common diseases.
On rare diseases, it suggests that trio analysis can help to pinpoint potentially causal gene variants. At present, this is uphill work: resolution rates hover below – often well below − 50%. The benefit of sharing is to increase the likelihood of identifying a causal variant – and consequently to improve the chances of rapid diagnosis in the future.
On cancer, WGS or WES of tumours (and comparative assessment of normal tissue) can identify lesions in the tumour genome, which opens the door wider to treatment options. Connecting genomic lesions with treatment outcomes will allow better decisions in the future.
On prevention of common diseases, the ability to inform individuals of their risk of succumbing to a certain disease can help future patients modify their behaviour to eliminate, decrease, or defer the onset of disease.
This presupposes effective awareness of the population-specific variation, which can be characterised by carrying out WGS on a subset of the population, imputing this characterisation to other individuals who have been genotyped with a whole genome genotyping array. But the common core lies in the framework requirements that the document sets out step-by-step. Top of the list is ensuring that legal, ethical, and societal issues are satisfactorily resolved so as to allow secure sharing of data and knowledge across borders, in a way that is both GDPR-compliant and flexible enough for any necessary future adaptations. That framework will support effective operations only, however, if it is backed by an adequate data governance structure: this will need to be both user-friendly and capable of surmounting any potential barriers raised over legal compliance (Table 6).
Table 6.
Bottlenecks in the data sharing and opportunities for EU action
| Stakeholders | Barriers | EU-level opportunities |
|---|---|---|
| Policymakers |
|
|
| Industry |
|
|
| Patients and general public |
|
|
| Healthcare professionals, physicians, and researchers |
|
|
There was a recent investigation of key Member States regarding the readiness of their healthcare systems to scale up data-sharing practices in the fields of genomic sequencing, personalised healthcare, and other key aspects. The countries could hence be classified in three tiers based on the existing structures and procedures in place. The results are summarised in Table 7. The results suggest that some countries are leading the way and can rely upon well-functioning structures for the use and sharing of data (tier 1), while some countries lack basic items in order to take part in the common efforts at European level (tier 3). Some countries have also taken up the task and are busy developing the necessary strategies in order to develop their capacities in the fields of genomics and personalised medicine; these countries are classified in tier 2.
Table 7.
Classification of countries based on their data sharing capacities
| Tier 1: functioning data sharing system | Tier 2: data sharing plans in the making | Tier 3: No existing data sharing structure |
|---|---|---|
| <I>UK</I>: – Effective data sharing frameworks and anonymisation strategy in place |
Greece: – Genomic medicine strategy in development with strict ethical framework, outreach strategies, and data sharing options in place |
Portugal: – Sequencing and genomic research activities performed in research and clinical practice without cooperation or oversight from the authorities; low awareness among the general public |
|
Estonia: – Established data pool with many participants included; numerous outreach strategies to the general in place |
Germany: – No functional data sharing system but the German Medical Informatics initiative is in the making |
Bulgaria: – Isolated projects on genomics but no country-/regionwide cooperation; severe lack of infrastructure and expertise |
| The Netherlands: – Functional links between academic medical centers for collaboration on sequencing efforts with close oversight of national authorities | ||
Interoperability is another priority need – not only in terms of the data itself, but at the technical and legal levels. So, standards will have to be established for sample procurement and storing and agreed for capturing phenotype and clinical information and for carrying out sequencing and variant calling. At the systems level, the to-do list includes establishing a federated data system that connects all participating sites – which will need to be accompanied by an agreed mechanism for querying across its entire breadth.
The conditions are essential – but it is not necessary to start from scratch with all of them, or re-invent the wheel, since already several individual initiatives have made their own progress towards an adequate framework, and the best elements of these could well be incorporated, the document suggests. The International Cancer Genome Consortium (ICGC) has established a harmonised consent form that can be used across many legislations and that allows sharing cancer genome data, for instance. The Global Alliance for Genomics and Health (GA4GH) has a working group on an adequate legal framework, and cooperation might be pursued there [30].
Standards for sample procurement and storing have been evolved by BBMRI-ERIC and are at the heart of the EU-funded projects SPIDIA and SPIDIA4P. GA4GH has a working group on capturing phenotypes – although much work will be needed to join phenotypes with electronic health records and longitudinal information. And a fully connected federated system as described by GA4GH would be helped by the inclusion of ELIXIR and systems such as the RD-Connect Genome Phenome Analysis Platform. Experts suggested that this could be a mechanism for analysis across the system and provide a systematic way to poll researchers on what kind of questions they would want to run if they had access to one million genomes including phenotypes.
The overarching recommendation is that genomic principles should be agreed by all signatories to the Declaration on defining the building blocks that will permit its full implementation. Some of these principles are outlined in the document – such as that rare in genomics should set thresholds and levels that describe a dataset considered fit-for-purpose according to the type of analysis and assay. Also, several initiatives from GA4GH like their Work-streams, Driver projects, and Toolkits are promising/encouraging developments [31]. Other suggestions cover aspects such as consent for genomic analysis to extending also to linkage with health and phenotypic data, validated methods for DNA extraction and preparation, and obligatory use of accredited facilities – clinical or research – with appropriate quality metrics. At its most technical, the document enters into details of desired minimum read depth and evenness or the genome standard reference to be used for alignment (currently GRCh38) or the preferable sequence variation annotation. But there are also broader and more policy-oriented recommendations – such as giving the one million genomes initiative a role in supporting national and international initiatives that aim to improve the clinical relevance of existing databases for specific populations.
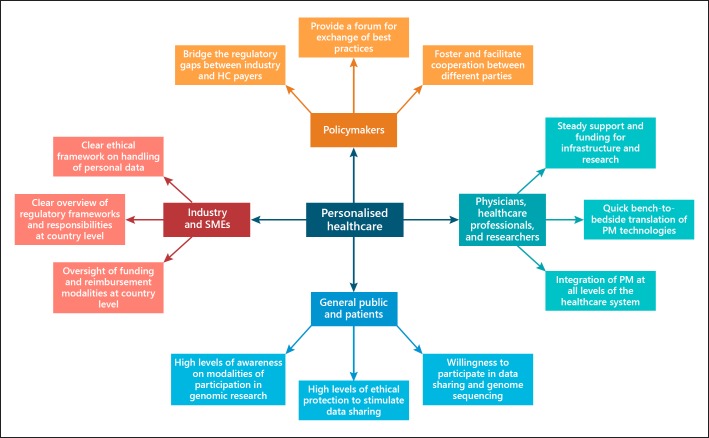
Based on the findings of the survey and mapping, EAPM puts forward the Personalised Healthcare Mosaic that summarises the best practices for a functioning personalised healthcare approach encompassing the different stakeholders involved in the process. These include patients, the industry and SME actors, physicians, researchers and healthcare professionals, and policymakers. The mosaic is included in Figure 2.
Fig. 2.
Personalized Healthcare Mosaic: model summarizing the aspects of importance when working and collaborating towards better personalised medicine and data sharing.
In some respects, the results from the interviews conducted for this paper indicate that Europe is halfway there. WGS is routinely carried out in more than half of the countries that responded to an EU survey in 2018, and there were plans in place for implementing it in the other countries. But halfway there is not the same as being there. WGS is a vital aspect of the progress towards personalised medicine, but it is part of the journey and not the destination. The results of the survey do, however, provide some mapping of the current situation – and thereby contribute to defining what still has to be done.
In most countries that took part in the survey and in the mapping exercise, access to genomic data is possible for sequenced individuals, and extracted data is exploitable for research purposes. Genomic sequencing has entered the research scene in all respondent countries and, in the majority of them, has also been implemented in clinical settings for the personalised treatment of cancer and rare diseases. But the respondents report that it is less frequently being used for population screening and prevention purposes.
The survey and mapping exercise participants report that the vast majority of the institutions carrying out WGS are public institutions, and they conduct their sequencing internally nearly half the time. Almost all of them share genomic data with other institutions, frequently across borders.
Methods, standards and interoperability show many common features. The results show that respondent countries use a number of common sequencing techniques and platforms. Data quality checks in reference databases are typically performed by applying metrics such as depth of coverage and base quality, whilst sample quality checks are most commonly performed via spectrophotometric and fluorimetric methods.
Use of standardised nomenclature for sequence variant description greatly facilitates possibilities for data sharing and interoperability. Most respondents and Member State actors have standards in place for the interpretation of sequenced genomes – and although there are usually no requirements for the use of specified methodologies, some funders expect sequencing to be carried out in accredited institutions by certified professionals and the generated data to be interoperable.
What sort of health data? Phenotypic data is collected via doctors and clinical care teams from medical centres and hospitals, and in some countries, anonymised genotype-phenotype databases are already being developed. Overall, most survey respondents link data from sequenced genomes to other types of health data – frequently demographic and occasionally patient registries, clinical reports, prescription data, and electronic health records as well as biobank, imaging, and -omics data. Attitudes still vary as to de-identification of data.
Based on the numbers provided for all three use cases (rare disease, cancer, and complex disease) from eleven member states included in the mapping efforts in this paper, the results state that it is clear that at present in general most genomes (WES and WGS combined) are sequenced in the UK, with the Netherlands as second best. Only for rare disease, the Dutch performed the most WES (ca. 82,000 exomes). Estonia and Spain are following, although in numbers approximately by a factor 10 less in numbers of genomes. The other seven countries are lagging behind, based on the numbers provided. The projections of the numbers of genomes to be sequenced towards 2021 are far from complete and probably not very accurate, but in general there is an increasingly positive attitude towards big genome projects as one of the tools to assist in personalised healthcare. Also, within these projections, the Netherlands and the UK are leading. For the UK, this is also supported by their announcements to scale up from 100,000 genomes to five million genomes within five years from 2018.
Training and Awareness Raising
Ultimately, for all the technological advances, the practice of medicine is an intensely personal matter – both for the patient and for the treating professionals. That is why a successful approach to making better use of health data depends on suitably skilled professionals and a public that understands how and why healthcare is evolving.
Training has been reported as a persistent issue in countries with low readiness among healthcare professionals to incorporate genomic data into medical practice, but the country stakeholders state that the culture has shifted from trial-and-error towards personalised medicine, and half of the respondent countries have certification systems for sequencing and interpreting genomes. Specialist training is provided for clinical geneticists and postgraduate degrees in -omics, bioinformatics, and systems biology as well as increased curriculum content in genomics for biomedical and life students and cross-disciplinary student exchange between medical/life science and mathematics/computer science departments. Where there is still no established competency profile for specialists, stakeholders from the interrogated Member States claim that the focus is tending towards core competencies shared by all healthcare professionals and additional competencies only where required by a particular job function.
National initiatives for citizen engagement in genomics are becoming more common, with the promotion of screening programmes, advocacy of participation in research projects or clinical trials, and encouragement of lay information about the merits of genomic research as reported by countries such as Greece, The United Kingdom, and Estonia in their response. Public information or public relations programmes also have a growing role in dispelling misconceptions and mistrust. But the type of information provided to patients or healthy citizens before involving them in genomic sequencing varies depending on the country and on whether the test is part of research studies, screening programmes, testing members of families with known genetic illnesses, or sample donations for biobanks.
Who Do We Sequence and How?
In view of the wide interest evinced by countries in sharing prospective and retrospective data for both research and clinical purposes, including public health and epidemiology, discussion will experts urged a boost for capacity building for sequence generation and analysis. Agreement is going to be needed on whom to sequence (healthy citizens vs. patients), for what purpose (improving disease understanding and identifying new drug targets; population screening for determining genetic predispositions to illnesses and predicting response to preventive measures; improving diagnoses; personalising treatments), and whether or not to focus on specific disease areas. This will help define inclusion and exclusion criteria as well as what minimum set of clinical, phenotypic, and other relevant health data should accompany the sequenced genomes (with further data made accessible depending on purpose and use).
Focus on Rare Disease
Responding to rare diseases has been the trigger both to scientific inquiry and to institutional action, with technological advances feeding into deeper understanding of pathologies and support provided in terms of funding, incentives, and research programmes. The scope for benefiting from greater cooperation in rare diseases is a perfect example both of the needs and of the advantages of a shift in European thinking.
Rare diseases starkly define many of the issues raised by the advance of medical science and the challenges facing healthcare systems. Although each disorder affects a small number of individuals, there are as many as seven thousand diseases already identified and classified as rare, with more constantly being identified as science and technology evolve – which makes the cumulative impact impressive, affecting 6% to 8% of the EU population. This means more than 30 million individuals, of which half are children. Many rare diseases are severe, chronic, and progressive, and for one-third of them, life expectancy is less than five years. So, this is a real European health issue – which offers both more possibilities of treatment from valuable innovations but intensely-focused clashes over treatment options and the associated regulatory and resources challenges [32].
The high prevalence of genetic mutations underlying these rare or ultra-rare diseases, coupled with the increased availability of next generation sequencing (NGS) facilities in many diagnostic laboratories is improving and speeding up the diseases' recognition and makes it possible to anticipate the process of taking them into care. In some ways, since rare disease research is so much at the cutting-edge of science, it is a sort of epitome of the dilemma faced by health systems in taking advantage of personalised medicine.
Both national and European policies, including the creation of centres of expertise equipped with NGS tools and the creation of reference centres, are contributing to get faster diagnoses and treatments for rare diseases, and to develop concerned actions and research projects, raising new hopes for patients and their families. The increasing range of networking research is widening the availability of large patients' registries, cohorts, and datasets and the collection of genomic profiles, thus improving knowledge about the natural history of these disorders. An overview of some of the important stakeholders and projects in the field of rare diseases is provided in Table 8. It should, however, be stated that this list is non-exhaustive and that many more actors are actively involved in the field of rare diseases, both in Europe and a global scale.
Table 8.
Overview of the stakeholders in the field of rare diseases
| Barriers to integration of genomics into healthcare | Stakeholders at the European level | Potential action points |
|---|---|---|
| Data integration and interpretation | Elixir, BBMRI, bioinformatics research institutions | Integration, aggregation, and analysis of existing genomic datasets at the European level Oversight of the collection and analysis of new genomic data at the European level |
| Workforce capacity and capability | European medical universities, medical schools, academic hospitals | Integration of genomics and principles of personalised healthcare inside of the medical, nursing and healthcare curriculums Introduction of career-long education programmes regarding personalised healthcare and genomic medicine for practicing professionals |
| Public acceptability and government engagement | National, local, and regional governments at the member state levels European Union |
Introduction of outreach methods for the general public |
| Paucity of evidence for clinical utility | Research centers and funding institutions | Support and grants for studies aimed at producing clinical evidence |
| Cost-effectiveness | HTA institutions, healthcare payers | More transparency, data sharing, and exchange of best practices to support reimbursement of personalised healthcare and genomic technologies |
| Ethical and legislative issues | EU and country-level legislators, patient organizations, medical-ethical committees | Development of model for ethical guidance and coordination of personalised healthcare activities Compilation of rigorous ethical standards and best practices to support roll-out of genomic technologies |
As part of the general goal of the cooperation towards access to at least one million sequenced genomes in the EU by 2022, efforts are now underway to provide access to existing and future databases dedicated to rare diseases in Europe through a federated environment that respects security and privacy. A working group formed as part of the EU1MG project is identifying current national or European pilot projects, with a view to proposing a database, EU tools, and additional pilot projects that can show the merits of sharing within the rare-disease area. It is taking account of the broadest range of needs: end-users/patients, research, healthcare, and industry stakeholders, including disease-gene discovery, mechanism study, diagnostics, therapy, prevention, and knowledge building.
An inventory of accessible rare-disease data is under construction, building on data generated through the mapping exercise, and indicating conspicuous gaps. At the same time, reflections are crystallising on how to work together with other initiatives, and to refine and constantly align goals and requirements. So far, the inventory comprises institutes/centres/hospitals contributing rare-disease genomic data, available clinical exomes, research WES and WGS in rare disease patients, a listing of the tools used for clinical phenotyping of patients, and a list of projects at national and European level.
The inventory currently (it is being constantly updated) displays information from a dozen countries, ranging from Bulgaria – with one institute contributing data and providing less than 100 WES – to Italy, where other institutes are contributing into the thousands in clinical exomes, research WES, and WGS, and where most clinical data are characterised by Human Phenotype Ontology. The picture that emerges is of only a limited number of WGS carried out in rare disease, with the exception of the UK, which has performed the 93% of WGS surveyed, according Genomics England. Most collected data refer to clinical exomes/WES, with heterogeneous depth of analysis, and clinical data collected using different tools [33].
Outstanding questions include how to link retrospectively genomic data collected without a specific consent for sharing and how to harmonise the heterogeneous clinical and molecular data. For each country, a provisional list of priority tasks has also been created, including issues such as centralising data storage, harmonisation across regional diversity, standardisation of consent procedures, and upgrading of IT infrastructure. Recommendations should also be produced for genome versus exome utility in diagnosis.
The key improvements that could result from European-level data sharing include speeding recognition and diagnosis (currently an average of five years in the EU), reducing misdiagnosis, advancing understanding of a disease, and managing clinical trials for rare disease individuals. The chief argument is that specificity of rare-disease research justifies a concerted action between different financing and management policies in order to optimise the use of funding, infrastructures, and technological platforms. There are important implications for industry, too: the link with rare diseases is significant in terms of the designation of orphan drugs and access to the regulatory advantages that the orphan status brings. A consensus forecast for the leading 500 pharmaceutical and biotechnology companies has estimated that the market for orphan drugs is expected to grow by more than 11% per year between 2017 and 2022 to USD 209 billion (a growth more than double that of the overall prescription drug market, which is set to grow by 5.3% over the same period). And orphan drugs are set to account for 21.4% of global prescription sales in 2022, excluding generics.
The work on rare disease is spread unevenly within the European territory. In fact, while some countries can make use of steadily increasing numbers of sequenced genomes and exomes, some others still have a lot of work ahead of them. The leaders in the field of rare-diseases research are the Netherlands and the United Kingdom, who have sequenced tens of thousands of genomes for rare diseases research. Some smaller European countries – including Slovenia and Estonia – contribute each at the level of several thousands of sequenced genomes. Many countries have, however, not initiated programmes aimed at producing data for rare diseases research. This may be interpreted as differences in policies, capabilities, or infrastructures.
Discussion
Molecular profiling has made it possible to advance the understanding of disease and to contemplate radical shifts in the approaches to screening, diagnosis, and treatment. The potential is compellingly demonstrated by the elucidation of cancer-driving genomic alterations, many of which are rare and not exclusive to a specific-tumour type. But real progress depends on access to data. The classic methods of generating data from large randomised clinical trials is challenging in this setting, and supplementary mechanisms and sources are necessary. It is now increasingly recognised that real-world data could play a major role in identifying patient characteristics and treatment effectiveness. But this cannot happen under current circumstances. This should be accompanied by data governance (with a strong focus on privacy and security) and the definition and adoption of common interoperable standards for cross-border sample transport, sequencing, data annotation, data processing, and data interpretation.
There are at present difficult barriers to tackle so as to tap into and combine existing real-world data sources to deliver a large enough dataset for clinicians, payers, and regulators to benefit from in informed clinical decision-making. Differing data standards, variables, data quality, and data-sharing regulations and incentives stand firmly in the way.
The answer is not hard to see: large-scale cross-country initiatives are needed that collect high-quality real-world data from diagnosis to treatments and patient outcomes in a standardised way. Europe, equipped with tools and processes for data sharing, would establish a learning loop on good clinical decision-making in terms of data, the relevant operational factors, the appropriate partners, and how to ensure genomic signatures of tumours influence good patient outcomes. Such initiatives would at the same time identify more clearly problems of data privacy or data sharing and suggest remedial action. Over time, trends in the healthcare system could be tracked more accurately, permitting fine-tuning of its mechanisms.
In order to investigate these challenges, EAPM would like to capitalise, through this paper, on the overview of the different countries' readiness to implement personalised healthcare and to scale up their data sharing practices. The use of a scale that touches upon the relevant aspects is therefore of central importance. Table 9 summarises the preliminary aspects that will be included in the scale. The included aspects will be taken into account when rating the readiness of a Member State regarding its capabilities of uptake regarding personalised medicine practices. Table 9 brings together these preliminary aspects.
Table 9.
Rating aspects indicating the readiness of member states to take up personalised healthcare
| Education and awareness |
|
| Practice |
|
| Regulations |
|
| Policy |
|
There is also strong support for the development of common interoperable standards and cross-border exchange of good genomic practice. Such elements are central to a coordinated approach at the European level. Although such best practices have not been shared officially, the authors of the present paper compiled a set of best practices based on the views of leading experts in the field. These are summarised in Table 10.
Table 10.
Best practices in the field of genomic sequencing at the European level (based on interviews)
| Area of interest | Best genomics practices checkpoints |
|---|---|
| Patients |
|
| Samples |
|
| Extraction procedures |
|
| Analysis |
|
| Interpretation |
|
| Databases and collaborations |
|
| Clinical applications |
|
Prospective data collection could be much more comprehensive than retrospective data collection where there may be constraints such as historical consents and issues around data ownership. And while WGS should be the principal focus in the future, existing datasets from WES and arrays are also valuable for research and should not be overlooked.
Access to genomic datasets should ideally be provided in a federated (non-centralised) network, with data that is findable, accessible, interoperable, and reusable, and there is wide support for the use of existing sequenced genomes. For newly sequenced genomes, however, the favoured approach is to minimise the variability of genomic data representations rather than address it once it arises, and suggested approaches include the use of a single sequencing technology and communication language as well as pre-agreed representation standards for genomic and phenotypic data.
A suitable governance model of cooperation for this initiative will need to acknowledge that there is heterogeneity between Member States in terms of WGS implementation, and it would be appropriate to define joint rules for genome and health data contributions. One of the main suggestions made by the survey and mapping exercise respondents was for the curation of a database with a range of comparative population genetic information that researchers and clinicians could use for identifying clinically relevant mutations at a European or global scale. The sustainability of cooperation beyond the current initiative and aims was mentioned as a point requiring further discussion and debate including the relevant stakeholders at the European level, including work at the level of special populations.
Conclusion
Normalising the widespread use of digital solutions for health and care can increase the well-being of millions of citizens and radically change the way health and care services are delivered to patients – but it is not happening. Institutions and official organisations could ease the barriers to innovation and uptake, by ensuring that health systems can access the information needed to optimise services, by countering market fragmentation, and by overcoming the current lack of interoperability. Systemic change is required to create an environment that not only allows for the deployment of new technologies, but that promotes new attitudes among citizens, patients, and professionals. So far, the progress has been minimal. Even on components as basic as electronic health records, it is hard to find much consensus emerging. Even in a country as advanced – and not large – as the Netherlands, it has proved impossible to adopt a single electronic health record format, and at EU level, the prospects for cooperation are currently dismayingly remote.
Europe possesses the skills and resources – and data – to make a success in a rapidly developing new world of digital health. But the world market is highly competitive, and the leaders, the first to market, will reap the greatest benefits. Internal EU barriers to data impede European progress. There is nothing new in the statement that barriers exist in Europe. What is new is that the stakes are higher than ever, and the path to success is, in theory at least, now much clearer. Europe's healthcare systems are strained as never before. But new thinking – on training, on tools, on validation, on innovation – could drive a transformation of European healthcare.
The call for change does not derive from comfortable theorising or commercial impatience. EAPM has taken a lead in assembling a wide coalition of practitioners from right across the healthcare sector who share the conviction that cooperation on data offers better ways of delivering for Europe's citizens – and who share an anxiety that policy discussions are still insufficiently informed of what needs to be done. EAPM has added to its own database of evidence by conducting surveys directly with the constituencies at the heart of Europe's healthcare – stakeholder groups representing basic science, translational research, the regulatory field, health systems, and patient perspectives.
Ambition is fine. Europe frequently expresses ambition. But without action and the practical steps needed to make action possible, ambition remains just that: ambition. To help turn current concepts into realities that will improve the future of healthcare, serious work is being undertaken in many parts and places of Europe – and an excellent example of this is the document drafted in the framework of the EU1MG project, which aims to capture the necessities for the Initiative.
There are multiple targets to be addressed to allow Europe to move forward effectively in realising the potential of sharing data, ranging from the political to the technical. The leap forward that the EU1MG project could provide, because it has backing from national governments, will be all the more valuable if it develops along agreed lines that can meet many of these multiple challenges, while respecting commonly agree principles.
Responding to rare diseases has been the trigger both to scientific inquiry and to institutional action, with technological advances feeding into deeper understanding of pathologies and support provided in terms of funding, incentives, and research programmes. The scope for benefiting from greater cooperation in rare diseases is a perfect example both of the needs and of the advantages of a shift in European thinking.
Ultimately, for all the technological advances, the practice of medicine is an intensely personal matter – both for the patient and for the treating professionals. That is why a successful approach to making better use of health data depends on suitably skilled professionals and a public that understands how and why healthcare is evolving.
In operational terms, data sharing is technical. This document has reviewed some of the technical moves – and impediments – to the sharing of data among Member States. At a deeper level, data sharing is political in that it depends on a commitment from governments and official bodies to reach out beyond their own national or regional borders – and on some of these aspects, this document has traced the progress, or lack of it, in Europe. But at the most fundamental level, sharing of heath data is more than technical, more than political. It transcends the customary forms of thinking in national governments and authorities about “what the patients in our country need.” This is because patients are not, and should not be seen as, a national issue. Being a patient is a personal issue, for an individual, for his or her family and entourage, and for any health professionals involved in diagnosis or treatment. The nationality of the patient or anyone else involved is secondary. What is central to the experience of ill-health – and to any response to it – is intensely personal and individual. And that applies at a level so fundamental that nationality is utterly irrelevant. What unites everyone everywhere is their common humanity, the fate that we all, as individuals, inescapably share with everyone else: life, good health, ill-health, mortality.
To the extent that there are significant common factors between patients, it is not that patient A and patient B are both French or both Spanish. It is rather that they both suffer from the same condition or have the same age or profile. At that point, there is merit in taking a view wider than the individual, because as science and technology open more doors to understanding, the experience with one other case – or a hundred similar cases – may shed light on the nature, cause, prognosis, and even treatment options. So, the sharing of data becomes not just useful; sharing is so valuable that it would be negligent not to share. The sharing should be open, neither defined nor limited by irrelevant considerations of nationality, and subject only to the norms of protecting personal privacy. This is why systems should give way to a spirit of cooperation – and to the extent that it is within the remit of a system to do so, each system should seek to maximise the sharing of data, adapting as appropriate its administrative, organisational, technical, and, yes, political arrangements accordingly.
At stake is the speed of progress in understanding human health and ill-health. That progress could also help ease the strains on Europe's healthcare systems. And it may offer Europe a head-start in the highly competitive world market place for the best care – with economic as well as health benefits. But above all, what is at stake is the well-being of Europe's patients and citizens, who stand to benefit from a more imaginative and energetic approach by Europe's policymakers and many stakeholders in the generation and processing of health data – and stand to lose if that does not happen.
Disclosure Statement
The authors declare that they have no competing interests.
Author Contributions
D.H. conceived, built and drafted the article with critical input from his co-authors, expert interviews and MEGA+ partners.
Acknowledgements
We would like to thank the members of the Million European Genome Alliance+ (MEGA+), Dr. Jan Korbel, Principal Investigator, Senior Scientist, Co-Director Molecular Medicine Partnership Unit (MMPU), European Molecular Biology Laboratory (EMBL), and Dr. Henk J. van Kranen, PhD, Center for Nutrition, Prevention and Health Services (VPZ). Also, thanks to the contribution from the consortium of DigitalHealthEurope: Support to a Digital Health and Care Innovation initiative in the context of Digital Single Market strategy.
Appendix
| Program, initiative, project | Website | Coordinating institution | Countries/Memb. | Focus area, activities, purpose | Field |
|---|---|---|---|---|---|
| 100,000 Genomes project |
https://www.england.nhs.uk/genomics/ https://www.scottishgenomespartnership.org/ http://www.wales.nhs.uk/sites3/home.cfm?orgid=525 https://www.genomicsengland.co.uk/ |
NHS | UK | Infrastructure, (clinical) cohorts, rare disease, cancer, infectious disease | Diagnostic genomics |
| Danish Strategy for Personalised Medicine | http://www.genomedenmark.dk/english/ | Ministry of HealthDK | Infrastructure, (populationbased) cohort, pathogen project | Integration of genomics into healthcare | |
| Estonian genome Project | https://www.geenivaramu.ee/en | Government | EE | Infrastructure, (populationbased) cohort | Linking GWAS data to WGS and health info |
References
- 1.Communication from the Commission to the European Parliament The Council, The European Economic and Social Committee and the Committee of the Regions on enabling the digital transformation of health and care in the Digital Single Market; empowering citizens and building a healthier society. COM/2018/233 final. Cited August 21, 2019. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2018:233:FIN.
- 2.Communication from the Commission to the European Parliament the Council, the European Economic and Social Committee and the Committee of the Regions on the Mid-Term Review on the implementation of the Digital Single Market Strategy A Connected Digital Single Market for All {SWD(2017) 155 final}) Cited August 21, 2019. Available from: https://eur-lex.europa.eu/content/news/digital_market.html
- 3.Council conclusions on “Health in the digital society – making progress in data-driven innovation in the field of health” Cited August 21, 2019. Available from: https://www.consilium.europa.eu/en/press/press-releases/2017/12/08/digital-health-council-conclusions/#.
- 4.Council conclusions on “personalised medicine for patients. Cited August 21, 2019. Available from: http://data.consilium.europa.eu/doc/document/ST-15054-2015-INIT/en/pdf
- 5.Council conclusions on innovation for the benefit of patients. (2014/C 438/06). Cited August 21, 2019. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52014XG1206(03)&from=EN
- 6.Commission Staff Working Document ‘Use of ‘-omics’ technologies in the development of personalised medicine’. Cited August 21, 2019. Available from: https://ec.europa.eu/research/health/pdf/2013-10_personalised_medicine_en.pdf.
- 7.Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10((6)):565–76. doi: 10.2217/PME.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Parliament Reprt on the proposal for a regulation of the European Parliament and of the Council on health technology assessment and amending Directive 2011/24/EU. Cited August 21, 2019. Available from: http://www.europarl.europa.eu/doceo/document/A-8-2018-0289_EN.html?redirect
- 9.Exchange of Electronic Health Records across the EU. Cited August 21, 2019. Available from: https://ec.europa.eu/digital-single-market/en/exchange-electronic-health-records-across-eu. Communication from the Commission to the European Parliament, The Council, The European Economic And Social Committee And The Committee of the Regions on enabling the digital transformation of health and care in the Digital Single Market; empowering citizens and building a healthier society COM/2018/233 final. Cited August 21, 2019. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2018:233:FIN
- 10.Report on Disruptive Innovation. Expert Panel on Effective Ways of Investing in Health (EXPH), Brussels, 2016 Cited August 21, 2019. Available from: https://ec.europa.eu/health/expert_panel/sites/expertpanel/files/012_disruptive_innovation_en.pdf
- 11.Communication from the Commission to the European Parliament. The Council, The European Economic and Social Committee and the Committee of the Regions on enabling the digital transformation of health and care in the Digital Single Market; empowering citizens and building a healthier society. COM/2018/233 final. Cited August 21, 2019. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2018:233:FIN
- 12.Directive 2011/24/Eu Of The European Parliament And Of The Council of 9 March 2011 on the application of patients' rights in cross-border healthcare. Cited August 21, 2019. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:088:0045:0065:EN:PDF
- 13.Horgan D, Romao M, Hastings R. Pulling the strands together: mega steps to drive european genomics and personalised medicine. Biomed Hub. 2017;2(Suppl. 1):169–79. doi: 10.1159/000481300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.‘Innovative Solutions For Research In Healthcare’ Developing a novel approach to deliver better precision medicine in Europe The EMA standpoint. Cited August 21, 2019. Available from: http://www.europarl.europa.eu/cmsdata/159164/Guido%20Rasi.pdf
- 15.Digital Day 2018: EU countries to commit to doing more together on the digital front. Cited August 21, 2019. Available from: https://europa.eu/rapid/press-release_IP-18-2902_en.htm
- 16.European Parliament Interest Group on Mental Health Well-being and Brain Disorders. Cited August 21, 2019. Available from: https://www.gamian.eu/wp-content/uploads/IG-Report-3.pdf
- 17. https://europa.eu/rapid/latest-press-releases.htm.
- 18.State of Health in the EU: Country Health Profiles. Cited August 21, 2019. Available from: https://ec.europa.eu/health/state/country_profiles_en
- 19.Communication from the Commission to the European Parliament. The Council, The European Economic and Social Committee and the Committee of the Regions on enabling the digital transformation of health and care in the Digital Single Market; empowering citizens and building a healthier society. COM/2018/233 final. Cited August 21, 2019. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2018:233:FIN
- 20.Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, et al. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet. 2019 Jan;104((1)):13–20. doi: 10.1016/j.ajhg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A Shared Vision For The Future Of Health In Europe: Paving The Way In 2019 And Beyond. Cited August 21, 2019. Available from: https://www.euhealthsummit.eu/wp-content/uploads/Future-of-Health-recommendations-in-full-new.pdf
- 22.COCIR welcomes European Commission's adoption of recommendation on European EHR exchange format. Cited August 21, 2019. Available from: https://www.news-medical.net/news/20190207/COCIR-welcomes-European-Commissione28099s-adoption-of-recommendation-on-European-EHR-exchange-format.aspx
- 23.Cooperation DO ‘Towards access to at least 1 million sequenced genomes in the European Union by 2022’. Available from: https://www.euapm.eu/pdf/EAPM_Declaration_Genome.pdf.
- 24.Genome sequencing: what do patients think. Cited August 21, 2019. Available from: http://www.geneticalliance.org.uk
- 25.Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) (OJ L 119, 4.5.2016,).
- 26.Siu Lillian L. “Facilitating a culture of responsible and effective sharing of cancer genome data.”. Nature medicine 22.5. 2016:464. doi: 10.1038/nm.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. https://rd-connect.eu.
- 28.Medtech sector calls on all stakeholders to support digital health interoperability standards specified by the European Commission. Cited August 21, 2019. Available from: https://ec.europa.eu/digital-single-market/en/news/medtech-sector-calls-all-stakeholders-support-digital-health-interoperability-standards
- 29.1+MG. Cited August 21, 2019. Available from: https://www.fundacionareces.es/recursos/doc/portal/2019/06/12/angel-carracedo-04-06-2019.pdf
- 30. https://icgc.org.
- 31.Interview transcripts with member states representatives, June, 2019
- 32.August C 21, 2019. Available from: https://www.eurordis.org/sites/default/files/publications/Fact_Sheet_RD.pdf
- 33.August C. 21, 2019. Orphan Drugs– to provide treatment for neglected rare diseases. Available from: http://www.neurovive.com/research-overview/orphan-drug-regulation/