Abstract
Objective:
To conduct a systematic review to evaluate exercise and structured physical activity for people living with Progressive Supranuclear Palsy.
Data sources:
AMED, CINAHL, Cochrane, EMBASE, Informit, MEDLINE, PEDro, PsycINFO, PubMed and SportDiscus were searched until 18 August 2019. Reference lists of included studies were hand-searched.
Methods:
Cochrane guidelines informed review methods. English language peer-reviewed studies of any design, in any setting, were included. Method quality was appraised with the Physiotherapy Evidence Database scale and Joanna Briggs Institute instruments. Data were extracted for study design, sample characteristics and therapy content. Effectiveness was calculated where possible.
Results:
Eleven studies were included. Method appraisal showed moderate to high risk of bias. Research designs included three randomized controlled trials, two quasi-experimental studies, one cohort study, four case studies and one case series. Sample sizes ranged from 1 to 24. Exercise interventions included supported and robot-assisted gait training, gaze training, balance re-education and auditory-cued motor training. Dosage ranged from two to five sessions per week over four to eight weeks. End-of-intervention effect sizes were small (6-minute walk test: –0.07; 95% confidence interval (CI): –0.87, 0.73) to moderate (balance: –0.61; 95% CI: –1.40, 0.23; Timed Up and Go: 0.42; 95% CI: –0.49, 1.33) and statistically non-significant. Function, quality of life and adverse events were inconsistently reported.
Conclusions:
For people with Progressive Supranuclear Palsy, robust evidence was not found for therapeutic exercises. Reported improvements in walking were derived from two clinical trials. The effects of structured physical activity for people with advanced Progressive Supranuclear Palsy are not known.
Keywords: Progressive Supranuclear Palsy, atypical Parkinsonism, exercise, rehabilitation, systematic review
Introduction
Progressive Supranuclear Palsy is a debilitating and rapidly progressing form of atypical Parkinson’s disease.1–4 Although diagnosis can be difficult, the International Parkinson and Movement Disorder Society (MDS-PSP) clinical criteria have provided expert guidance and increased the diagnostic sensitivity and specificity.5–7 Reports have emerged that physiotherapy, some forms of exercise and high-intensity physical activities might be of benefit for people with Progressive Supranuclear Palsy.8 For idiopathic Parkinson’s disease, high-dosage sustained exercises and select movement strategies appear to be beneficial in the short term.9–16 In the early stages of disease progression, therapeutic exercises can improve mobility17–21 and prevent falls.12,13 There is also growing evidence that exercise can modify disease progression in early Parkinson’s disease.15,16 Whether the same applies to Progressive Supranuclear Palsy remains open to question.8
For Progressive Supranuclear Palsy, the literature on exercise, movement rehabilitation and physical activity is fragmented and lacks a theoretical framework.22–24 This possibly relates to the difficulties associated with diagnostic certainty of this neurological condition.25 There is a need to define the optimal content, dosage and scheduling of exercises, physical activities and physical therapies for people living with Progressive Supranuclear Palsy. The feasibility, effectiveness, efficacy and economic costs of therapeutic exercises also need clarification. As a first step to defining optimal therapy for Progressive Supranuclear Palsy, it is informative to review the published literature.
There was one systematic review on Progressive Supranuclear Palsy that searched databases up until 2014,22,23 and a narrative review that searched up until 2017.24 The authors reported preliminary evidence that physiotherapy rehabilitation programmes, including supported treadmill training and balance exercises, may be beneficial for quality of life and falls reduction. Further research with appropriate statistical methods, larger sample sizes and longer follow-up times were advised before clinical practice recommendations could be made.22–24 In addition, these prior reviews did not include comprehensive risk of bias assessments, method quality assessments or quantitative data analysis. An updated systematic review and meta-analysis of the contemporary literature is needed, and will inform the delivery of therapeutic exercises, physiotherapy and structured physical activities for people living with Progressive Supranuclear Palsy.
To address this gap, the overall aims are to (1) critically evaluate the literature on exercise, movement rehabilitation, physiotherapy and structured physical activity for people living with Progressive Supranuclear Palsy and (2) make recommendations for the design of exercise programmes and future trials for people living with this rapidly deteriorating neurological condition.
Method
The review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42018103845).26 The review methods were informed by Cochrane guidelines.27 The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.28,29
The eligibility criteria form that was applied to each study is listed in Supplemental Appendix 1. Studies were included if they were on Progressive Supranuclear Palsy, peer-reviewed in English language, and available in full text. They could be of any design and conducted in any setting. Studies were included if participants were adults who were stated to have a primary diagnosis of probable or possible Progressive Supranuclear Palsy or where the international criteria were applied.25 The interventions could include exercise, physical activity, physical therapies, physiotherapy, movement strategies, therapeutic movement, gait, mobility, compensatory strategies, dance, music-cued exercise, rehabilitation and movement rehabilitation. For inclusion, studies had to report data measured at baseline, together with data obtained within or following the therapy intervention period. The trial reports needed to include quantitative raw data enabling statistical analysis. Trials/studies that reported raw data or statistics relating to movement disorders and non-motor disorders, impairments, disability, overall health, well-being, quality of life, and social, vocational, leisure and community engagement were included.
A three-step search strategy was used to identify possible studies from international electronic databases. The initial search consisted of nine electronic databases from inception up until 18 August 2019: AMED, CINAHL, Cochrane, EMBASE, Informit, MEDLINE, PEDro, PsycINFO, PubMed and SportDiscus by title and abstract with PSP synonyms. The second search used all identified keywords and index terms. The third was a hand-search of reference lists of the included studies, and citation tracking and consultation with content experts to identify additional papers. The search used key search terms that included the target condition and target interventions. The MEDLINE search strategy is included in Supplemental Appendix 2. The MEDLINE strategy was adapted to the other databases, and these are available from the first author on request.
Findings from the search were transferred into a bibliographic database. Following deletion of duplicate titles, one reviewer (S.C.S.) screened each title using previously determined eligibility criteria. Two researchers (S.C.S. and M.E.M.) then independently screened the remaining abstracts and then read the full text to identify studies that fulfilled the eligibility criteria. Agreement was reached by discussion without the need of an independent arbiter.
Two reviewers (S.C.S. and M.E.M.) independently evaluated the eligible studies for method quality and reached consensus by discussion without a third reviewer/arbiter. The Physiotherapy Evidence Database (PEDro) scale30,31 was used for randomized or quasi-randomized controlled trials. We selected a valid instrument for other study designs (non-randomized experimental studies) such as case studies from the Joanna Briggs Institute Critical Appraisal Tools database32 (Supplemental Appendix 3). The reviewers then assigned a risk of bias as low, medium or high according to the scoring matrix of each instrument.
Two independent reviewers (S.C.S. and M.E.M.) extracted data into a pre-tested standardized data spreadsheet. For studies that included exercise, physiotherapy or rehabilitation, the Consensus on Exercise Reporting Template (CERT) was used to determine the exercise programme details.33,34 The CERT is an internationally endorsed reporting guideline designed specifically for exercise interventions. The independently extracted data were merged and checked for accuracy. Disagreements and discrepancies were discussed to reach agreement.
Data that were descriptive, textual, nominal, categorical (including dichotomous and other), ordinal, ratio or metric (continuous) in nature and pertained to patients, caregivers, professionals, the health system, economic outcomes and other outcomes were extracted. Summary statistics were used for all data. For the categorical data, the relative risks and the odds ratios, with 95% confidence intervals (CIs), were calculated if possible. For continuous data, mean difference scores with 95% CIs were calculated if included studies used identical outcome measurement tools for like outcomes.
Continuous outcome data were also analysed using the standardized mean difference if studies reported the same outcome yet with alternative measurement tools (e.g. for pain, disability or quality of life). Meta-analysis was performed when quantitative data could be pooled and was considered appropriate. Where pooling of data was not possible, the findings were described in a narrative format. In the absence of statistical data, a narrative, thematic or content analysis of the results occurred. The corresponding authors of each study were contacted to obtain the relevant data when it was not reported. If missing data could not be obtained, an imputation method was considered.27When there were insufficient data to enter into meta-analysis, even after contacting the authors, the results were reported qualitatively.24
The overall quality of evidence for each outcome was determined by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and the GRADE guidelines.35,36 Factors that can reduce the quality of evidence include study type and risk of bias, inconsistency of results, lack of generalizability, imprecise data and other reporting bias. The GRADEpro calculator was used to generate summary tables.37
Results
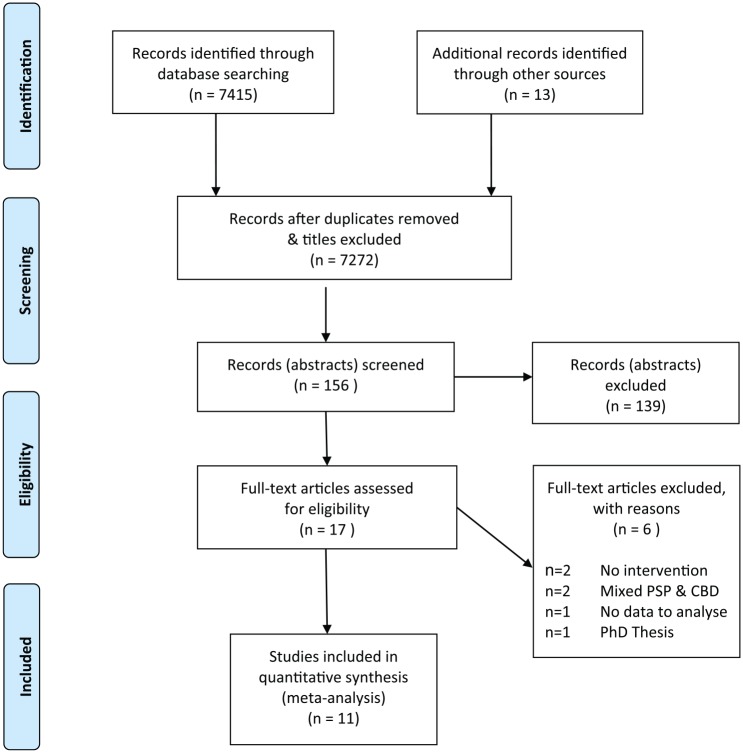
The results are presented in text format, with a flowchart, summary tables, statistical analysis and narrative summaries. From a yield of 7415 titles, finally 11 studies were included38–48 (Supplemental Appendix 4). Figure 1 contains a PRISMA-compliant flowchart of search results and selection into the review. The final excluded studies and reasons for their exclusion are listed in Supplemental Appendix 5. The two independent reviewers were able to reach consensus at all stages of the selection process without arbitration from the third reviewer. Meta-analysis could not be conducted due to data heterogeneity. Subgroup analysis was not performed due to lack of data.
Figure 1.
PRISMA flowchart for inclusion into the review.
Method quality appraisal indicated that the risk of bias was low,43–45 medium38,42,45,47 and high39–41,46 (Supplemental Table S1). Study designs included three randomized controlled and quasi-randomized controlled trials,38–40 two quasi-experimental studies,43,44 one cohort study,41 four case studies42,45,46,48 and one case series.47 The study designs, small sample sizes and method quality appraisals demonstrated that the elements of chance were generally not well controlled for in the reported results (Supplemental Table S2). The sources of bias included sampling, selection, performance and measurement biases.
The sample sizes ranged from 1 to 24 people diagnosed with Progressive Supranuclear Palsy. The interventions consisted of treadmill training,38,41,42,48 robot-assisted walking,38,44 balance training,39,40 virtual gaming45 and auditory cueing.43,47 Frequency of therapy ranged from two to five sessions per week, and intervention duration from four to eight weeks. Outcome measures were predominantly related to locomotion and postural stability, which are prominent in Progressive Supranuclear Palsy. Motor and non-motor functions were inconsistently reported. Medications, comorbidities, adverse events, quality-of-life function, disability and burden of disease or care were usually not reported (Supplemental Table S2). Details of the intervention elements were extracted using the CERT and will be reported in a subsequent publication.
The corresponding authors of the studies with missing data were contacted for these data via the email details provided in the published reports, and one author responded.38 Imputations were made from the graphs presented in the published manuscripts, and effects were then estimated.39,40 Where means and standard deviations were provided, or could be calculated or retrieved from the study results,41–47 between-groups analyses were conducted with an effect size calculator.49 Intervention effects were typically small to moderate on outcomes that included balance and gait and were statistically non-significant. Immediate post-intervention outcomes were always measured; short-term follow-up was measured in three trials,41–43 but there were no data on medium- to long-term effects. The overall absence of raw data and measurement precision resulted in less than definitive estimations of effect. The results from data analyses are presented in Table 1.
Table 1.
Data analysis results.
| First author, year | Outcome measures | Data/data analysis | Time-points | |
|---|---|---|---|---|
|
Clerici 2017 Randomized controlled trial (RCT) n = 24 Supported treadmill versus Lokomat robotic treadmill gait training |
PSPRS (Progressive Supranuclear Palsy Rating Scale) PSPRS LIMB PSPRS GAIT BBS (Berg Balance Scale: 0–56) 6MWT (6-minute walk test – metres) FALLS |
Effect size (ES) 0.55 0.46 0.42 –0.61 –0.07 0.00 |
95% CI –0.28, 1.35 –0.36, 1.26 –0.40, 1.22 –1.40, 0.23 –0.87, 0.73 –0.80, 0.80 |
Baseline to end of intervention (six weeks) |
|
Di Pancrazio 2013 Cohort study n = 10 Body-weight supported treadmill gait training |
BBS (Berg Balance Scale) |
Mean 37.7 (baseline (BL)) 47.6 (end of intervention (EI)) |
SD 12.1 (BL) 9.2 (EI) |
BL EI |
|
Irons 2015 Single case study n = 1 Body-weight supported treadmill gait training |
6MWT FOG-Q (Freezing of Gait Questionnaire) SSC (self-selected comfortable treadmill speed (m/min)) OXYGEN COST of SSC walk speed |
BL 129.2 12 21.5 0.44 |
EI 212.1 12 21.5 0.41 |
Intervention period: eight weeks |
|
Nicolai 2010 Uncontrolled quasi-experimental study n = 8 Balance exercises (sitting, standing, stepping) |
BBS (higher score is better) TUG (Timed Up and Go – seconds) (higher score – better) PDQ-39 (Parkinson’s Disease Questionnaire) (lower score – better) ABC (Activities-Specific Balance Confidence) (higher score – better) |
BL 35 (6–50) 24.5 (19.3, 50.7) 36.2 (28.6, 55.4) 13.8 (1.3, 28.1) |
EI 44 (9–50) 22.5 (15.3, 66.7) 26.7 (22.3, 44.0) 6.9 (0.0, 21.3) |
Intervention period: six weeks. Measures – median (range) |
|
Sale 2014 Pre/post–quasi-experimental design n = 5 Robotic gait training |
Gait velocity (metres/sec) Gait cadence (steps/minute) Step length, left (mm) Step length, right (mm) |
Mean (SD) BL 0.54 (0.17) 83.00 (9.62) 363.20 (94.77) 421.00 (98.83) |
Mean (SD) EI 0.67 (0.16) 93.60 (15.44) 429.80 (67.57) 466.40 (105.74) |
BL EI |
|
Seamon 2017 Single case study n = 1 Xbox Kinect virtual exer-gaming |
BBS (higher score is better) TUG (higher score is better) PDQ-39 (lower score is better) 10MWT (10-metre walking test – metres) |
BL 49 11.65 41 1.16 |
EI 49 11.11 52 1.05 |
BL EI Intervention period: six weeks |
|
Suteerawattananon 2002 Single case study n = 1 Body-weight supported treadmill gait training |
TUG (higher score is better) BBS (higher score is better) 15.2 m (50 ft) walk test – seconds (lower score is better) Functional Reach Test – cm (higher score is better) |
Mean (SD) BL 12.80 (1.74) 45/56 17.02 (1.45) 23.93 (3.35) |
Mean (SD) EI 13.50 (2.47) 47/56 12.63 (0.64) 27.51 (6.53) |
BL EI Intervention period: eight weeks |
|
Wallace 2013 Single case study n = 1 Weighted vest |
Descriptive analysis – the authors reported that when the weighted vest was donned there was a reduction in gait irregularity (entropy). For forward, lateral and vertical movement, the reduction was 4.5, 2.18 and 5.15 MDCs (minimal detectable change) respectively. When the weighted vest was removed there was an increase in gait irregularity (entropy). For forward, lateral and vertical movement, the increase was 33.1, 2.55 and 14.4 MDCs respectively |
Intervention period: 120 days Measure: MDC |
||
|
Wittwer 2018 Case series study n = 5 Music & auditory-cued gait training |
ACE-III (Addenbrooke’s Cognitive Examination–III) score (0–100 – lower is better) GDS (Geriatric Depression Scale) (0–15) (score >5 indicates depression) Satisfaction survey (0–55) (higher score is better) |
Mean (SD) BL 89.4 (7.1) 6.6 (4.3) Not measured |
Mean (SD) EI 90.2 (8.6) 6.4 (3.9) 50.2 (3.8) |
BL EI Intervention period: eight weeks |
|
Zampieri 2008 Quasi RCT n = 19 Balance + eye movement training compared to balance alone for gait |
Stance duration Step length 8FWT (8-foot walk test – seconds) TUG (higher score – better) |
ES –0.72 –0.48 0.80 0.42 |
95% CI –1.65 to 0.21 –1.39 to 0.44 –0.13 to 1.74 –0.49 to 1.33 |
Intervention period: six weeks |
|
Zampieri 2009 Quasi RCT n = 19 Balance + eye movement training compared to balance alone for gaze control |
Vertical Gaze Fixation Score Gaze Error Index |
ES 0.00 0.47 |
95% CI –0.90 to 0.90 –0.44 to 1.38 |
Intervention period: six weeks |
CI: confidence interval.
The overall quality of evidence was appraised and presented in Supplemental Table S3. There was heterogeneity of the variables and outcome measurement instruments and the interventions that were tested. The sample sizes were small (ranged from 1 to 24) and resulted in underpowered studies. Study designs generally did not control for risk of bias.
Discussion
This systematic review showed the existing literature on exercise, physical therapies, movement rehabilitation and physical activity for people with Progressive Supranuclear Palsy to lack methodological rigour and statistical power. This concurs with the findings of two previous reviews. Also, the therapeutic exercises used in the included studies may not represent the full range of interventions and assessment methods available in contemporary clinical practice such as progressive resistive strength training, movement strategies or community walking. The interventions were not informed by progressive resistance exercise or physical activity principles recommended by the American College of Sports Medicine Position Stands.50–52 The interventions also did not appear to utilize international clinical practice guidelines for Parkinson’s disease.53 The exercise therapies were not reported to be augmented by motivation and adherence strategies, and guidance was not provided for intervention progression. Many of the interventions in the reviewed studies used complex, research clinic-based equipment rather than easy-to-access exercise equipment that is available to most people. The latter highlights a problem for translation into clinical practice and implementation at the community level.
The small sample sizes, low statistical power and comparatively low methodological rigour introduce risk of bias and limit believability of the results of the included trials. Due to the frequent absence of control groups, inability to pool data and the low method quality, caution is advised when interpreting the GRADE recommendations. It is recommended that readers consider the interventions at the individual study level. No multisite or intercountry trials were reported. No replication studies were evident.
Several investigations used therapies that were designed for individuals with idiopathic Parkinson’s disease or stroke. These therapies included music cueing,43,47 balance and eye movement training,39,40 harness-supported treadmill training,38,41,42,48 weighted vests during ambulation,46 virtual reality and gaming45 and robot-assisted gait training.38,44 Of these, balance exercise39,40 and gait training38,41,42–44,48 indicated potential benefit and music-cued walking demonstrated participant satisfaction with this model of care.47
The review confirmed the need for a core set of outcome measures to evaluate effects of exercise in people living with Progressive Supranuclear Palsy. A minimum core set of three outcome measures has been recommended for use in Progressive Supranuclear Palsy research. These are the Clinical Rating Scale for PSP, the Unified Parkinson’s Disease Rating Scale and the Frontal Assessment Battery.54 Seven additional scales are desirable: Natural History and Neuroprotection in Parkinson Plus Syndromes–Parkinson Plus Scale, Supranuclear Palsy Quality of Life scale, Neuropsychiatric Inventory, Mini-Mental State Examination, Dementia Rating Scale, Modified Hoehn & Yahr Scale and EuroQol generic health index.54 Only a few of these measures were used in the reviewed literature. The overall inconsistency of tested variables suggested that a more comprehensive core set of outcome measures could be developed to include, for example, movement disorders, gait, balance, falls, function, burden of care, and non-motor symptoms such as fatigue, anxiety and cognitive impairment. Adverse events were seldom mentioned, and it is not possible to state whether they were not reported or did not occur.
This systematic review highlighted that clinicians and people living with a diagnosis of Progressive Supranuclear Palsy may have difficulty in determining the content and dosage of an exercise programme for outcomes including strengthening, falls prevention, gait training, movement rehabilitation and participation in activities of daily living. There was no evidence of consideration of personal preferences in exercise and activity prescription. The strengths of this review were the inclusion of a comprehensive search of the literature and application of rigorous literature review methods. The limitation was an inability to review literature in languages other than English and initial title screening by one author.
The review findings reinforce the recommendations of two previous reviews that future research will require large scale, well-controlled randomized controlled trials evaluating the outcomes of different Progressive Supranuclear Palsy–specific physical therapies on movement disorders, balance, falls, ambulation, oculomotor function, cognition, well-being and life quality. The challenges will be recruitment and the need for a consortium or international collaboration to increase the sample size to provide robust statistical evidence.
Whether progressive resistance strength training is beneficial for people with Progressive Supranuclear Palsy has not been reported, even though it has strong evidence of benefit in other chronic neurological conditions such as stroke,55,56 multiple sclerosis,57 traumatic brain injury58 and Parkinson’s disease.10–21 The effectiveness of falls education for people with Progressive Supranuclear Palsy, and their caregivers, has not been documented despite established benefit for people living with Parkinson’s disease.12,13,20 Likewise, there is also a need for randomized controlled trials that systematically evaluate the effects of auditory and visual cues on movement and the performance of structured physical activities for people with Progressive Supranuclear Palsy. Whether or not walking programmes, aquatic therapy and cycling are beneficial in Progressive Supranuclear Palsy is not known. In a similar manner, there is a need to understand if complementary therapies such as music therapy, dancing, boxing, tai chi, Pilates and yoga are helpful. Future trials need to control for Progressive Supranuclear Palsy medications and stage of disease progression when evaluating the relative benefits of exercise therapies as well as explore the facilitators and barriers to activity engagement.
For individuals in the early stages of Progressive Supranuclear Palsy, conclusive evidence was not found to demonstrate strong or sustained effects of therapeutic exercises. Some gains in walking speed and quality were associated with music-cued movement rehabilitation, weight-supported treadmill training and robotic gait training, yet supporting data were derived from only two clinical trials. The important elements of exercise and physical activity programmes for people with Progressive Supranuclear Palsy at all levels of disease severity cannot be derived from the current published literature. Whether exercises demonstrated to be effective for people living with idiopathic Parkinson’s disease generalize to those with Progressive Supranuclear Palsy also awaits verification.
Clinical messages.
Weight-supported treadmill training, music-cued movement rehabilitation and robotic-assisted gait training may be beneficial early in Progressive Supranuclear Palsy.
There is insufficient evidence to know benefits in advanced PSP or whether therapeutic exercises designed for people living with idiopathic Parkinson’s disease benefit those with Progressive Supranuclear Palsy.
Supplemental Material
Supplemental material, Supplemental_Material for Exercise and physical activity for people with Progressive Supranuclear Palsy: a systematic review by Susan C Slade, David I Finkelstein, Jennifer L McGinley and Meg E Morris in Clinical Rehabilitation
Acknowledgments
We are grateful for the support of Parkinson’s Victoria and the Argyrou family.
Footnotes
Author contributions: S.C.S. and M.E.M. conceived the idea for the study. All authors were responsible for the study design, study implementation, writing and manuscript revisions. All authors have read and approved the final manuscript. The corresponding author guarantees that the authorship statement is correct.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that the submitted work was carried out in the absence of any personal, professional or financial relationships that could be construed as a conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: S.C.S. and M.E.M. are supported by Parkinson’s Victoria, the Argyrou Family Fellowship, Healthscope and La Trobe University. The funders had no role in systematic review protocol development or conduct.
ORCID iD: Susan C Slade  https://orcid.org/0000-0001-6325-2705
https://orcid.org/0000-0001-6325-2705
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ali F, Josephs K. The diagnosis of progressive supranuclear palsy: current opinions and challenges. Expert Rev Neurother 2018; 18(7): 603–616. [DOI] [PubMed] [Google Scholar]
- 2. Stamelou M, de Silva R, Arias-Carrion O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133(Pt 6): 1578–1590. [DOI] [PubMed] [Google Scholar]
- 3. Stamelou M, Hoeglinger GU. Atypical parkinsonism: an update. Current Opinion Neurol 2013; 26(4): 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bluett B, Litvan I. Pathophysiology, genetics, clinical features, diagnosis and therapeutic trials in progressive supranuclear palsy. Expert Opin Orphan Drugs 2015; 3(3): 253–265. [Google Scholar]
- 5. Stamelou M. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord 2019; 34(8): 1087–1088. [DOI] [PubMed] [Google Scholar]
- 6. Grimm MJ, Respondek G, Stamelou M, et al. How to apply the movement disorder society criteria for progressive supranuclear palsy. Mov Disord 2019; 34(8): 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali F, Martin PR, Botha H, et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord 2019; 34(8): 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashok C, Kumari AA, Shekhar PKC. Physiotherapy management for progressive supranuclear palsy. Internat Journal Physiother Res 2013(2): 41–45. [Google Scholar]
- 9. Nieuwboer A, Kwakkel G, Rochester L. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2006; 78(2): 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung CL, Thilarajah S, Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil 2016; 30(1): 11–23. [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson CL, Herd CP, Clarke CE, et al. Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Syst Rev 2014; 6: CD002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris ME, Menz HB, McGinley JL, et al. Randomized controlled trial to reduce falls in people with Parkinson’s disease. Neurorehabil Neural Repair 2015; 29(8): 777–785. [DOI] [PubMed] [Google Scholar]
- 13. Shen X, Wong-Yu ISK, Mak MKY. Effects of exercise on falls, balance and gait ability in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair 2015; 30(6): 512–527. [DOI] [PubMed] [Google Scholar]
- 14. Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair 2015; 29(2): 123–131. [DOI] [PubMed] [Google Scholar]
- 15. Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol 2018; 75(2): 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva-Batista C, Corcos DM, Kanegusuku H, et al. Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait Posture 2018; 61: 90–97. [DOI] [PubMed] [Google Scholar]
- 17. Van Ooteghem K, Frank JS, Horak FB. Postural motor learning in Parkinson’s disease: the effect of practice on continuous compensatory postural regulation. Gait Posture 2017; 57: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison EC, McNeely ME, Earhart GM. The feasibility of singing to improve gait in Parkinson disease. Gait Posture 2017; 53: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rocha P, Aguiar L, McClelland JA, et al. Dance therapy for Parkinson’s disease: a randomised controlled trial. Internat J Ther Rehabil 2018; 25(2): 64–72. [Google Scholar]
- 20. Canning CG, Sherrington C, Lord SR, et al. Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Neurology 2015; 84(3): 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carroll LM, Volpe D, Morris ME, et al. Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil 2017; 98(4): 631–638. [DOI] [PubMed] [Google Scholar]
- 22. Tilley E, White S, Peters MD. The effectiveness of allied health therapy in the symptomatic management of progressive supranuclear palsy: a systematic review protocol. JBI Database System Rev Implement Rep 2014; 12(7): 119–137. [DOI] [PubMed] [Google Scholar]
- 23. Tilley E, McLoughlin J, Koblar SA, et al. Effectiveness of allied health therapy in the symptomatic management of progressive supranuclear palsy: a systematic review. JBI Database System Rev Implement Rep 2016; 14(6): 148–195. [DOI] [PubMed] [Google Scholar]
- 24. Intiso D, Bartolo M, Santamato A, et al. The role of rehabilitation in patients with progressive supranuclear palsy: a narrative review. PM&R 2018; 10(6): 636–645. [DOI] [PubMed] [Google Scholar]
- 25. Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017; 32(6): 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. http://www.crd.york.ac.uk/prospero
- 27. Higgins J, Green S. (eds). Cochrane handbook for systematic reviews of interventions. Version 5.3.0 (Updated October 2015). London: The Cochrane Collaboration, 2015. [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 29. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating methodological quality of randomized controlled trials. Phys Ther 2003; 83(8): 713–727. [PubMed] [Google Scholar]
- 31. De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009; 55(2): 129–133. [DOI] [PubMed] [Google Scholar]
- 32. Joanna Briggs institute critical appraisal tools database, http://joannabriggs.org/research/critical-appraisal-tools.html
- 33. Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): a modified Delphi study. Phys Ther 2016; 96(10): 1514–1524. [DOI] [PubMed] [Google Scholar]
- 34. Slade SC, Dionne CE, Underwood M, et al. The Consensus on Exercise Reporting Template (CERT): explanation and elaboration statement. Brit J Sports Med 2016; 50(23): 1428–1437. [DOI] [PubMed] [Google Scholar]
- 35. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4): 383–394. [DOI] [PubMed] [Google Scholar]
- 36. http://www.gradeworkinggroup.org/
- 37. https://gradepro.org/
- 38. Clerici I, Ferrazzoli D, Maestri R, et al. Rehabilitation in progressive supranuclear palsy: effectiveness of two multidisciplinary treatments. PLoS ONE 2017; 12(2): e0170927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zampieri C, Di Fabio RP. Balance and eye movement training to improve gait in people with progressive supranuclear palsy: quasi-randomized clinical trial. Phys Ther 2008; 88(12): 1460–1473. [DOI] [PubMed] [Google Scholar]
- 40. Zampieri C, Di Fabio RP. Improvement of gaze control after balance and eye movement training in patients with progressive supranuclear palsy: a quasi-randomized controlled trial. Arch Phys Med Rehabil 2009; 90(2): 263–270. [DOI] [PubMed] [Google Scholar]
- 41. Di Pancrazio L, Bellomo RG, Franciotti R, et al. Combined rehabilitation program for postural instability in progressive supranuclear palsy. Neurorehabilitation 2013; 32(4): 855–860. [DOI] [PubMed] [Google Scholar]
- 42. Irons SL, Brusola GA, Buster TW, et al. Novel motor-assisted elliptical training intervention improves 6-minute walk test and oxygen cost for an individual with progressive supranuclear palsy. Cardiopulmonary Phys Ther J 2015; 26(2): 36–41. [Google Scholar]
- 43. Nicolai S, Mirelman A, Herman T, et al. Improvement of balance after audio-biofeedback. A 6-week intervention study in patients with progressive supranuclear palsy. Z Gerontol Geriatr 2010; 43(4): 224–228. [DOI] [PubMed] [Google Scholar]
- 44. Sale P, Stocchi F, Galafate D, et al. Effects of robot assisted gait training in progressive supranuclear palsy (PSP): a preliminary report. Front Hum Neurosci 2014; 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seamon B, DeFranco M, Thigpen M. Use of the Xbox Kinect virtual gaming system to improve gait, postural control and cognitive awareness in an individual with progressive supranuclear palsy. Disabil Rehabil 2017; 39(7): 721–726. [DOI] [PubMed] [Google Scholar]
- 46. Wallace R, Abbott C, Gibson-Horn C, et al. In-home measurement of the effect of strategically weighted vests on ambulation. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 949–952. [DOI] [PubMed] [Google Scholar]
- 47. Wittwer JE, Winbolt M, Morris ME. A home-based, music-cued movement program is feasible and may improve gait in progressive supranuclear palsy. Front Neurol 2019; 10: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suteerawattananon M, MacNeill B, Protas EJ. Supported treadmill training for gait and balance in a patient with progressive supranuclear palsy. Phys Ther 2002; 82(5): 485–495. [PubMed] [Google Scholar]
- 49. https://www.cem.org/effect-size-calculator
- 50. Ratamess NA, Alvar BA, Evetoch TK. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009; 41(3): 687–708. [DOI] [PubMed] [Google Scholar]
- 51. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009; 41(7): 1510–1530. [DOI] [PubMed] [Google Scholar]
- 52. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43(7): 1334–1359. [DOI] [PubMed] [Google Scholar]
- 53. Keus SHJ, Munneke M, Graziano M, et al. European physiotherapy guidelines for Parkinson’s disease. Nijmegen: KNGF/ParkinsonNet, 2014. [Google Scholar]
- 54. Hall DA, Forjaz MJ, Golbe LI, et al. Scales to assess clinical features of progressive supranuclear palsy: MDS task force report. Mov Disord Clin Pract 2015; 2(2): 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. English C, Hillier SL, Lynch EA. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst Rev 2017; 6: CD007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dorsch S, Ada L, Alloggia D. Progressive resistance training increases strength after stroke but this may not carry over to activity: a systematic review. J Physiother 2018; 64(2): 84–90. [DOI] [PubMed] [Google Scholar]
- 57. Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson’s disease. Medicine 2015; 94(4): e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams G, Clark RA, Hansson J, et al. Feasibility of ballistic strengthening exercises in neurologic rehabilitation. Am J Phys Med Rehabil 2014; 93(9): 828–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Exercise and physical activity for people with Progressive Supranuclear Palsy: a systematic review by Susan C Slade, David I Finkelstein, Jennifer L McGinley and Meg E Morris in Clinical Rehabilitation