Abstract
Behaviors associated with reproduction are major contributors to the evolutionary success of organisms and are subject to many evolutionary forces, including natural and sexual selection, and sexual conflict. Successful reproduction involves a range of behaviors, from finding an appropriate mate, courting, and copulation, to the successful production and (in oviparous animals) deposition of eggs following mating. As a consequence, behaviors and genes associated with reproduction are often under strong selection and evolve rapidly. Courtship rituals in flies follow a multimodal pattern, mediated through visual, chemical, tactile, and auditory signals. Premating behaviors allow males and females to assess the species identity, reproductive state, and condition of their partners. Conflicts between the “interests” of individual males, and/or between the reproductive strategies of males and females, often drive the evolution of reproductive behaviors. For example, seminal proteins transmitted by males often show evidence of rapid evolution, mediated by positive selection. Postmating behaviors, including the selection of oviposition sites, are highly variable and Drosophila species span the spectrum from generalists to obligate specialists. Chemical recognition features prominently in adaptation to host plants for feeding and oviposition. Selection acting on variation in pre-, peri-, and postmating behaviors can lead to reproductive isolation and incipient speciation. Response to selection at the genetic level can include the expansion of gene families, such as those for detecting pheromonal cues for mating, or changes in the expression of genes leading to visual cues such as wing spots that are assessed during mating. Here, we consider the evolution of reproductive behavior in Drosophila at two distinct, yet complementary, scales. Some studies take a microevolutionary approach, identifying genes and networks involved in reproduction, and then dissecting the genetics underlying complex behaviors in D. melanogaster. Other studies take a macroevolutionary approach, comparing reproductive behaviors across the genus Drosophila and how these might correlate with environmental cues. A full synthesis of this field will require unification across these levels.
Keywords: Drosophila, adaptation, genetics, chemoreception, multigene family, gene–environment interaction, natural variation, selection, mating, courtship, song, postmating behaviors, fitness, pheromones, wing spots, seminal proteins, FlyBook
DROSOPHILA melanogaster, as well as other members of the genus Drosophila, has served as a model system in genetics, evolution, and development for over 100 years, and as an important model in behavioral studies for nearly as long. Some of the earliest comparative studies were done by A. H. Sturtevant (1915), and examined courtship behaviors and sexual recognition between closely related Drosophila species. Subsequent work by Dobzhansky (1946), Spieth (1947), and others further developed Drosophila as not only a model for courtship and mating behavior, but as one of the key experimental systems responsible for establishing the biological species concept (Mayr 1982), and our understanding of how species diversify and evolve (Coyne and Orr 1989, 1997).
Behaviors are more than simply the expression of the nervous system; they include complex interactions with various biotic and abiotic factors. Studies on Drosophila behavior have benefited from a diverse range of experimental approaches. For example, geneticists correlate gene expression and gene interaction studies with complex behavioral phenotypes. Ecologists elucidate the underlying biotic and abiotic stimuli giving rise to complex behaviors. Evolutionary biologists reconstruct the history of the behavior and the genes underlying those behaviors. A comprehensive approach, incorporating aspects of several disciplines, yields the most robust, and we would argue, biologically relevant and interesting results.
In this article, we focus on the evolution of reproductive behaviors in Drosophila. Classical genetic and genomic studies on D. melanogaster have had a significant impact on our understanding of behavior through powerful tools that are available to genetically dissect the molecular and neural pathways, and networks that underlie complex behaviors. The genus Drosophila is an exceptional model system for understanding the evolution of behavior, including reproductive behaviors. The melanogaster species subgroup consists of nine species, divided into four different species complexes. Two of these, the melanogaster and simulans complexes, are extensively studied models of species formation. The melanogaster complex consists of a single species, D. melanogaster, and is sister to three sibling species: D. simulans, D. mauritiana, and D. sechellia. There is extensive literature relating to behaviors, in their ecological settings, of multiple species in the genus Drosophila, allowing a comparative approach that can help determine how various behaviors may have evolved over a macroevolutionary scale spanning > 60 MY and in response to a diverse set of selection pressures (including, but not limited to, environmental conditions, host plant chemistry, predation pressures, and reproductive isolation). Sequenced genomes exist (Drosophila 12 Genomes Consortium et al. 2007; Song et al. 2011; Miller et al. 2018; Yang et al. 2018) for > 20 species, permitting comparative evolutionary studies on the genes that underlie behaviors. At the same time, the genus Drosophila contains a major genetic model system, D. melanogaster, that has been the subject of study by many laboratories for many behaviors, and their neural and genetic underpinnings, providing a framework from which to consider variation within and between Drosophila species. The community has assembled a series of resources that allow evolutionary studies at both the micro- and the macrolevel. Resources for the microlevel include the Drosophila Synthetic Population Resource, a population of recombinant inbred lines derived from an advanced intercross population of eight founder strains (King et al. 2012; Long et al. 2014), and the D. melanogaster Genetic Reference Panel (DGRP), a publicly available resource of 205 fully sequenced lines that harnesses naturally occurring variants for the dissection of complex traits, including behaviors (Mackay et al. 2012; Huang et al. 2014), and can identify candidate genes by association that can be tested genetically. The macrolevel is represented by the existence and study of many species whose phylogenic relationships are known, and for which genomes, natural biology, and descriptive studies are available. Finally, the advent of clustered regularly interspaced short palindromic repeats (CRISPR)/Cas technologies (e.g., Jinek et al. 2012; Bassett et al. 2013; Gratz et al. 2013) allows one to toggle back-and-forth between the behavioral consequences of pathways that are known in D. melanogaster and ways that they can be modulated based on results from other species.
Selective forces act on genetic variation within a population. Natural selection can change allele frequencies of mutations that improve or reduce fitness. Thus, a precondition for any behavior to evolve is that there is genetic variation for the behavior within the population. The DGRP and other resources mentioned above have revealed extensive natural variation for behavioral phenotypes (Brown et al. 2013; Harbison et al. 2013; Swarup et al. 2013; Appel et al. 2015; Arya et al. 2015; Ayroles et al. 2015; Garlapow et al. 2015; Shorter et al. 2015; Carbone et al. 2016; Rohde et al. 2017; Wu et al. 2018a), including reproductive behaviors (Turner et al. 2013; Gaertner et al. 2015). The genetic architectures underlying behaviors are often complex, allowing very fine-grained effects when selective forces result in the redistribution of allele frequencies leading to modifications of the genetic networks that orchestrate the behavior. Gene-by-gene and gene-by-environment interactions among genes or their networks are prominent hallmarks of behavioral phenotypes (Fedorowicz et al. 1998; Sambandan et al. 2006, 2008; Rollmann et al. 2007, 2008; Yamamoto et al. 2008, 2009; Kent et al. 2009; Zwarts et al. 2011; Huang et al. 2012; Swarup et al. 2012, 2013; Shorter et al. 2015; He et al. 2016).
This review leans heavily on the D. melanogaster literature for an understanding of the genetic mechanisms underlying specific reproductive behaviors and their variation. We then attempt to integrate this genetic information in a phylogenetic context to discuss how such behaviors may have arisen across the genus Drosophila. The obvious caveat here is that there is a large disconnect between what we know in D. melanogaster and the diverse behaviors that are observed across the genus. This is because behaviors, particularly those relating to reproduction, are among the most rapidly evolving traits. Accurately and completely reconstructing changes that may have taken place over relatively short time periods millions of years ago is a challenging analytical problem. Here, we present hypotheses for how divergent reproductive behaviors may have evolved, including given the genes known from studies of D. melanogaster. We will conclude with a Perspectives section summarizing the current understanding in the field and suggesting future studies.
Drosophila Reproductive Behavior
Adult Drosophila perform a series of behaviors linked to survival and fitness. Male and female Drosophila must locate mating substrates, most of which are also associated with feeding resources. Once in the mating arena, flies must identify conspecifics and, in some cases, compete for access to partners. The latter often encompasses aggressive behaviors to defend feeding and mating opportunities. Once mating has been successful, females must locate a suitable location to oviposit. Both biotic and abiotic stimuli impact reproductive behaviors. For example, social conditions can affect reproduction (Krupp et al. 2008).
Courtship and mating
A diverse array of behaviors in the genus Drosophila are associated with reproduction, including those that occur prior to mating, such as male–male aggression and various courtship displays by both males and females, and ones taking place following intromission, such as male guarding and changes in female remating behavior. Many of these behaviors often require specialized morphologies of genitalia, forelegs, mouthparts, and wings; modifications to those structures evolve with changes in those behaviors (Tanaka et al. 2009, 2015). Likewise, several molecules are associated with reproductive behaviors, including seminal proteins and pheromones, and these too evolve with the behaviors [Swanson et al. (2001) and Haerty et al. (2007); reviews include Swanson and Vacquier (2002), and Panhuis et al. (2006); the neurogenetics of female D. melanogaster reproductive behaviors has been reviewed by Laturney and Billeter (2014)]. Mating and courtship behaviors, as well as associated reproductive traits, tend to evolve rapidly, in part because of their importance in species isolation mechanisms, and in part because of sexual conflicts between males (sperm competition), and between the “interests” and strategies of females and males (Swanson and Vacquier 2002; O’Grady and Markow 2012). These behaviors include aggressive male–male competition, sexual selection acting on a range of characteristics, and sexual antagonism between conspecific males and females resulting in a coevolutionary arms race. The cost of sexual selection was demonstrated by an elegant laboratory evolution study in which single D. melanogaster females were mated with single males for 47 generations. Removal of sexual selection over many generations of forced monogamy resulted in decreased intermale aggression and increased resistance of females to male-induced postmating effects (Holland and Rice 1999).
Behaviors, because of their rapid evolution and highly plastic nature, can quickly establish barriers between species. The diversity of reproductive behaviors observed among Drosophila species is an important component of this isolation in this genus (Markow and O’Grady 2005, 2006, 2008; O’Grady and Markow 2012). Traditionally, such barriers can arise among premating, mating, postmating prezygotic, and zygotic behaviors, depending on when they occur during mating and reproduction. Premating isolation mechanisms can help individuals identify conspecifics, thus preventing matings between different species. Mating barriers are important, so individuals do not waste valuable resources (e.g., gametes and energy) on nonproductive matings. However, should interspecies matings occur, postmating prezygotic and zygotic barriers can decrease the reproductive success of the cross-species mating.
Premating and mating
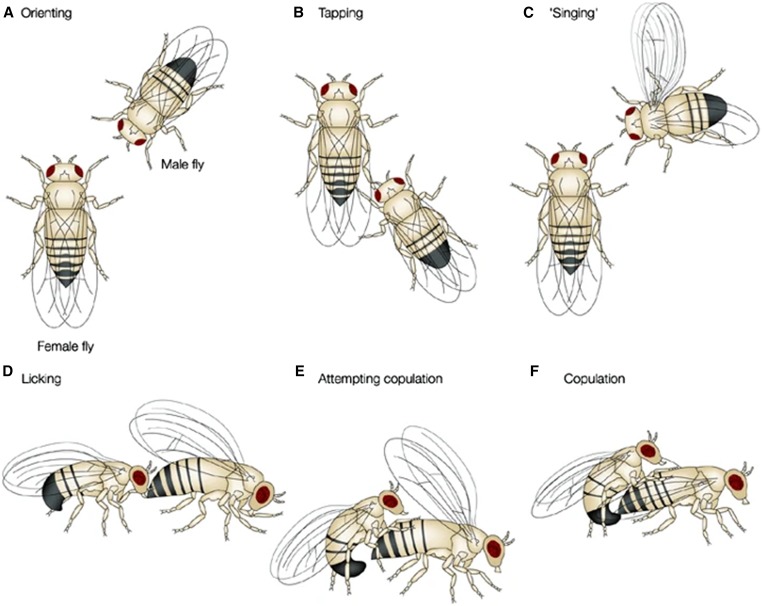
Premating behaviors allow males and females to recognize and assess partners to determine whether the species, reproductive state, and condition of the partner are appropriate for and conducive to mating. Courtship rituals in Drosophila follow a multimodal pattern, mediated through visual, chemical, tactile, and auditory signals, and consist of a sequence of orientation, genital licking, courtship song through wing vibration, and attempted copulation [Hall (1994); reviewed in Sokolowski (2001); Figure 1]. Courtship behaviors include locating a potential mate by using visual or olfactory cues, and communicating with and assessing the potential mate by olfactory, auditory, and visual cues during courtship. Behaviors during mating also are important for reproductive fitness (Markow and O’Grady 2008) and are variable. These include copulation duration as well as interactions that determine whether gametes will be present for fertilization. Although the courtship ritual of D. melanogaster males was once considered a defined stereotypical progression of behavioral elements, studies using the DGRP showed extensive heritable variation in courtship patterns (Gaertner et al. 2015). Selection acting on variation in any of the sensory inputs that drive the component behaviors of this multimodal courtship ritual can lead to a reproductive isolation barrier as a scaffold for incipient speciation.
Figure 1.
A diagram showing the sequence of behaviors during courtship in D. melanogaster. Orienting (a), tapping (b), ‘singing (c), licking (d), attempting copulation (e), and copulation (f). Reprinted, with permission, from Sokolowski (2001).
Throughout the courtship process, females assess male quality [see Laturney and Billeter (2014) for review]. For example, D. melanogaster females tend to prefer larger males (Markow and O’Grady 2006). Also, in D. melanogaster, females appear to prefer “successful” males: a male is more likely to be selected as a mate by a female who observes him mating with another female (Mery et al. 2009). Communication between flies about male quality has been suggested (Danchin et al. 2010). As a result of this assessment process, females are either receptive to mate, or they decamp and leave the courtship arena. Females can reject males by flying away or, particularly if the female has mated previously, by kicking males away or extruding the ovipositor to block the male’s access (Connolly and Cook 1973). Males persist in attempting to mate but learn from the experience: D. melanogaster male virgins who have been repeatedly rejected become less likely to mate (Siegel and Hall 1979).
Courtship behaviors in D. melanogaster [reviewed in Sokolowski (2001); Figure 1] contain all the major components of courtship seen in most other species in the genus. While courtship and mating is a continual process, and difficult to divide into discrete units, we can distinguish three broad categories of this composite behavior: mate location, display, and copulation. All species in the genus Drosophila perform these three actions during the courtship process, although the relative order, duration, and importance of each vary between species.
Mate location
D. melanogaster, and most other members of the genus Drosophila, encounter mates in close proximity to feeding resources. Therefore, mate location in most species is tied to the long-distance volatile plumes produced by microbes and associated decomposing substrates (Grosjean et al. 2011; Becher et al. 2012). This eliminates the need for species-specific volatile sex pheromones to broadcast mate location. Instead, a suite of compounds, many of which are components of decomposing host plants, evokes strong responses in specific olfactory sensory neurons (Laissue and Vosshall 2008), leading to aggregation or mate finding. This behavior is described further in the Oviposition section.
While most Drosophila species locate mates using food-related cues, with both courtship display and copulation occurring at the feeding site, many endemic Hawaiian Drosophila utilize a location separate from the feeding and oviposition substrate for courtship and copulation (Spieth 1966, 1984; Figure 2). Males aggregate to engage in competitive displays to entice visiting females that are surveying prospective mating partners. This “lek behavior” is unique to Hawaiian Drosophila. Lek behavior correlates with a high degree of male–male aggression, with competitions between males lasting > 20 min (Spieth 1984). Within some rainforest habitats, individual trees can serve as arenas for multiple Hawaiian Drosophila species, creating a spatially partitioned multispecies lek (Bell and Kipp 1994).
Figure 2.
Phylogenetic distribution of reproductive behaviors and associated morphologies in species groups of Drosophila. All terminal taxa represent species groups [for definitions see Markow and O’Grady (2005) and O’Grady and DeSalle (2018)]. AMC indicates the antopocerus-modified tarsus clade; PNA indicates the picture wing-nudidrosophila-ateledrosophila clade. Open squares denote missing information. Characters that are polymorphic within a group show multiple colors. Sexual dimorphism (present, green with horizontal line; absent, black). Dimorphic characters, observed in males, include wings (W), forelegs (L), mouthparts (M), and head broadening (H). Wing spreading (present, green with horizontal line; absent, black). Wing pigment (present, green with horizontal line; absent, black; polymorphic, both). Lek behavior (present, green with horizontal line; absent, black). Male guarding (present, green with horizontal line; absent, black). Reproductive maturity in males and females (0–5 days, red with diagonal line; 6–10 days, green with horizontal line; 11–15 days, orange with vertical line, > 15 days, blue with dot). Sperm size [< 6 mm (short), red with diagonal line; > 6 mm (giant), blue with dot]. Ovariole numbers (< 25, red with diagonal; > 25, blue with dot). Female remating frequency (frequent, red with diagonal line; infrequent, blue with dot). Copulation duration (< 20 min, red with diagonal line; 20–60 min, green with horizontal line; > 60 min, orange with vertical line). Insemination reaction (none, red with diagonal line; moderate, green with horizontal line; strong, orange with vertical line).
“Orienting” using visual and tactile displays
Once a mate is located, the first step in a successful mating is for males and females to be properly positioned so they can mate [reviewed in Sokolowski (2001); Figure 1]. This is generally referred to as orienting, where individuals of both sexes undergo a complex series of behaviors across visual, auditory, chemical, and tactile modalities to position themselves in preparation for mating. Some of these are stereotypical species-specific behaviors, while others are situational and do not necessarily occur in all mating events. Canalized, repeated display elements are considered part of the mating ritual while other, more opportunistic behaviors can be considered as a prelude to mating itself. D. melanogaster males will often chase females in their initial approach, eventually ending up in front of the female or slightly to her side [reviewed in Sokolowski (2001)]. This is primarily a visual display but can also include tactile aspects in which males use their forelegs to tap females either on their heads or forelegs. Both of these areas of the female contain high concentrations of chemosensory cells, which may sense pheromonal cues (Miyamoto and Amrein 2008). Thus, this behavior by males allows females to smell or taste potential mates (discussed below). Females, in turn, assess and respond to the male, either by being receptive to the mating display or by decamping and leaving the mating area.
While both sexes may be involved in signaling during reproduction, males are the primary signalers. Wings are important in orientation and males will often hold them out to their sides [reviewed in Sokolowski (2001)], perpendicular to the plane of the body, thus enlarging their visual footprint. Depending on the species, wings can range from completely clear (“hyaline”; e.g., D. melanogaster) to lightly shaded (“infuscated”) near the cross veins, to possessing distinct apical spots or having more extensive patterns of spots, stripes, and/or darkened areas of the wings (e.g., D. biarmipes) (Markow and O’Grady 2006; Figure 2). Males with patterned wings often wave them during visual displays to females.
Wing patterning can play an important role in species recognition. It has been suggested that it provides visual stimulation by accentuating the visibility of wing vibrations during courtship (Shevtsova et al. 2011) and, thus, may have gained an evolutionary advantage and spread to fixation in some species in which they have appeared (Fuyama 1979; Hegde et al. 2005). Indeed, many Drosophila species, including the agricultural pest D. suzukii, have independently evolved wing pigmentation spots in some males (Figure 2; True et al. 1999; Gompel et al. 2005; Prud’homme et al. 2006; Edwards et al. 2007; Werner 2015), and experiments with D. biarmipes showed that males with wing spots mate faster and have greater mating success than males without wing spots (Hegde et al. 2005). However, Roy and Gleason (2019) reported that noninvasive elimination of the wing spot of species of the suzukii group—D. biarmipes, D. suzukii, and D. subpulchrella—did not affect mating success. As noted above, different mechanisms have been used in different lineages to result in wing spots. D. biarmipes has acquired a transcription factor-binding site for the Engrailed transcriptional regulator through modification of a cis-regulatory element at the yellow locus (Gompel et al. 2005; Prud’homme et al. 2006). This results in the appearance of a male-specific pigmented spot on the wing. Multiple wing spots are present on the wings of D. guttifera as a consequence of a different regulatory mechanism that results in Wingless-mediated regulation of pigment deposition (Werner et al. 2010).
Visual displays are part of the mating behaviors of most Drosophila species. These displays include a variety of behaviors often referred to as mating “dances,” and can include wings, forelegs, and mouth parts (Markow and O’Grady 2008). Mating dances are species-specific, and distinct from the situational movements used to optimize the positions of females and males for mating. Some species, such as D. affinis, are so reliant on visual displays that courtship is abolished in darkness (McRobert and Tompkins 1987). One endemic Hawaiian species, D. clavisetae, has a particularly unique display involving both visual and chemosensory cues. Males of this species possess elongated setae on the tips of their abdomens, extending anteriorly from the cerci. These setae are used as part of the mating display: when the abdomen is everted over the top of the male’s head, the setae are extended, and a droplet of fluid is secreted and displayed in front of the female. The female then touches this droplet, obtaining taste and/or smell signals (Spieth 1966, 1974).
Another lineage of Hawaiian species, the antopocerus group, has elongated antennae that are densely packed with chemosensory setae on the dorsal surface and devoid of setae on the ventral surface. These antennae, along with modified setae on the male forelegs, are displayed to females during courtship. Spieth (1968) described the unique “lunge” behavior performed by this group. The elongated first and second antennal segments of the male forcibly spread the female’s wings, with the ventral surface of the third antennal segment sliding along the posterior surface of the female wing. These antennal modifications correlate with a dimorphism in olfactory centers of the brain, suggesting a role in host or mate location mediated by chemosensation, in addition to mediating the physical interaction during courtship (Kondoh et al. 2003).
Courtship song
Another important mating cue is the mating “song,” usually produced by the male. Drosophila mating “songs” are composed of a combination of sounds generated by vibrating or “clacking” the wings, and/or by drumming the forelegs on the substrate, rather than being a true vocalization (Fabre et al. 2012; Mazzoni et al. 2013). Different Drosophila species have evolved distinct courtship songs, and natural genetic variation in courtship song within species bears testimony to the plasticity and evolvability of this courtship component (Gleason 2005; Arthur et al. 2013; Ding et al. 2016). Males of most species sing to females, sometimes with as many as four distinct songs (Markow and O’Grady 2006).
D. melanogaster creates its courtship song through wing vibrations, which give rise to distinctive pulse song and sine song components with characteristic rhythms, frequencies, and interpulse intervals (Kyriacou and Hall 1980). While the sine song is a regularly fluctuating song, the pulse song reflects irregular bursts of sound waves. These differences are the result of how the songs are produced and have a functional role in courtship (Kyriacou and Hall 1982; Ritchie et al. 1999; Kowalski et al. 2004; Talyn and Dowse 2004; Tomaru et al. 2004). Kyriacou and Hall (1982) showed that D. melanogaster females and females of its closely related species D. simulans mate most readily when exposed to their species-specific songs, with their characteristic interpulse intervals and oscillations. Ritchie et al. (1999) confirmed that D. melanogaster females mate most quickly when stimulated by songs typical of their own species rather than songs of D. simulans or D. sechellia. Talyn and Dowse (2004) showed that the pulse song rather than the sine song stimulates female mating. Recently, Clemens et al. (2018) reported a second pulse song in D. melanogaster. They also found that visual feedback from females influenced male song choice and that male song selection had an impact on female response (e.g., receptivity vs. rejection). This male display also includes a complex visual component, with males ranging in position from directly in front of females to perpendicular to the female and vibrating the wings to produce the song [reviewed in Sokolowski (2001)]. The visual component continues as males position themselves directly behind the female.
The rhythmicity of the song appears to be regulated by genes associated with the regulation of circadian activity [see Dubowy and Sehgal (2017) for a review of circadian rhythms in Drosophila]. The per gene was one of the first loci implicated, based on analysis of mutants, in affecting the rhythmicity of the courtship song (Kyriacou and Hall 1980). In addition to circadian rhythmicity genes, genes identified in studies on other traits have been correlated with courtship song characteristics. For example, a study of 27 natural lines in Italy identified five polymorphisms at the cacophony (cac) locus that appeared to segregate under neutral selection and were associated with variation in interpulse interval, pulse amplitude, and cycles per pulse (Peixoto and Hall 1998; Peixoto et al. 2000). Finally, QTL mapping studies of courtship song qualities demonstrated that the song is a highly polygenic trait (Gleason 2005). QTL mapping studies on recombinant inbred lines of D. melanogaster showed variance that exceeded the variance observed in the parental lines, indicative of epistasis (Gleason et al. 2002). However, QTL studies have only rarely (e.g., Ding et al. 2016) identified causal polymorphisms associated with variation in courtship song parameters.
Song production requires a neural circuit that enables manifestation of the courtship song; this circuit likely evolves with the song. Male-specific neuronal differentiation of the song circuit has been attributed to genes that comprise the sex-determination pathway. Neurons in the brain that express fruitless, designated P1 and pIP10 neurons, initiate the onset of courtship song [for reviews on the fruitless-expressing neuronal circuit that controls courtship behavior, see Yamamoto and Koganezawa (2013) and Yamamoto et al. (2014)]. The descending pIP10 neuron drives activity of thoracic motor neurons to shape the courtship song (von Philipsborn et al. 2011). In addition, regulation of sexual differentiation by doublesex results in male-specific expansion of dendritic arborizations of thoracic interneurons (TN1A neurons), which drive activity of the hg1 wing motor neuron (Shirangi et al. 2016). Studies using noninvasive imaging have identified at least seven motor neurons for the generation of courtship song that are distinct from motor neurons used to power flight (O’Sullivan et al. 2018).
Like many reproductive traits, mating songs evolve rapidly. Examples of rapid mating song evolution are seen within the melanogaster species group, where females distinguish and prefer the song from their species over that of other, even closely related, species. Moreover, males of most species in this group have two distinct courtship songs. D. yakuba generates two types of pulse song, one generated by wing vibration as in D. melanogaster and the other, termed “clack song,” which results from clapping both wings together behind the back (Demetriades et al. 1999). Inhibition of the descending pIP10 neurons by tetanus toxin results in loss of the clack component of the song, and optogenetic activation of the pIP10 neuron results in the production of clack song. Production of the clack song is dependent on the level of light intensity (Ding et al. 2019). In D. yakuba, under low light only clacks are produced, but under high light intensity, clacks followed by a pulse song are observed, indicating that pIP10 neurons can access both pulse and clack song circuits in an environment-dependent manner. Whereas electrophysiological properties of the pIP10 neurons are conserved between D. yakuba and D. melanogaster, a neuroanatomical comparison between these species showed significant quantifiable differences in the song circuit, with about a twofold increase in the mesothoracic triangle of D. yakuba compared to D. melanogaster (Ding et al. 2019). The mesothoracic triangle is a triangular-shaped structure between the dorsal pro- and mesothoracic ganglia in the ventral nerve cord, which represents a neural circuit associated with courtship behavior (Yu et al. 2010). An elegant study comparing the courtship songs of the closely related species D. mauritiana and D. simulans combined a classical QTL mapping approach with CRISPR technology to identify a transposable element insertion in the slowpoke (slo) gene that accounts for differences in the sine song between these two species. This gene encodes a calcium-activated potassium channel that is expressed in neurons and muscles; molecular variation at this locus may affect muscle movements necessary to generate the courtship song (Ding et al. 2016).
Males of species in the repleta group produce either one or two songs. This shows a phylogenetic distribution: taxa with one song are in one clade and those with two are in another [reviewed in Markow and O’Grady (2005)]. The range of variation is wider in the obscura species group, where species have either one or two songs (Markow and O’Grady 2005). In yet another variation, D. subobscura has entirely lost an auditory display; it does not sing during courtship. Song characteristics in the willistoni species group are even more variable. While D. nebulosa does not sing, D. paulistorum has two distinct songs (Ritchie and Gleason 1995; Gleason and Ritchie 1998). Two other species, D. insularis and D. willistoni, utilize three songs during courtship. Interestingly, two other species in the species group, D. tropicalis and D. equinoxialis, have independently evolved a fourth song. The virilis species group also shows a pattern of independent gains of courtship song (Ritchie and Gleason 1995; Gleason and Ritchie 1998). The ancestral condition is a single song, with four unrelated species each having independently evolved a second song [reviewed in Markow and O’Grady (2005)]. The high degree of variability in auditory display is likely a reflection of how important these behaviors are, not only for females to assess male quality, but also to identify conspecific males. However, songs are constrained against being too variable. Any male with a song outside the tolerances of a conspecific female may not have the opportunity to mate (Ritchie and Kyriacou 1994; Ritchie and Gleason 1995; Klappert et al. 2007).
Evolution of courtship song requires coevolution of the production of the song by the male and receptivity to that particular song by the targeted female. Whereas most studies have focused on the production of courtship song by the male, fewer studies have focused on the female’s perception of the song. Sound is perceived by mechanosensory antennal neurons in Johnston’s organ, which project to the antennal mechanosensory and motor center (AMMC), the first synaptic relay in the brain. This relay filters incoming information to preserve the spacing of song pulses (Tootoonian et al. 2012). Recordings from central neurons that innervate the AMCC of D. melanogaster and D. simulans did not show differences in responses to their pulse songs, despite the fact that each species has evolved a unique song (Tootoonian et al. 2012), and behavioral studies show that each species responds specifically to its species-specific song (Kyriacou and Hall 1982; Ritchie et al. 1999). Thus, details of how the spectral characteristics of the courtship song are represented in the female brain and the neural basis of their species divergence that results in species-specific female behaviors in response to male songs remain to be elucidated.
Evolution of courtship song may be driven by female preference (Hoikkala et al. 1998; Yukilevich et al. 2016). Females of D. montana use courtship song to evaluate the genetic quality of the courting male and they prefer a courtship song with short sound pulses at high frequency (Hoikkala et al. 1998). Therefore, males with longer sound pulses would mate less frequently and, as a consequence, songs with such pulses would become less common in the population.
Variation in courtship song can lead to sexual isolation. This is especially evident in the striking diversity of courtship songs among the > 500 species of Drosophila that have evolved on the Hawaiian Islands. Some species have acquired aspects of their courtship songs that are reminiscent of the complex pulse rhythm observed in crickets (D. cyrtoloma) or the high carrier frequency seen in cicadas (D. fasciculisetae). Others, like D. silvestris, have songs more similar to the courtship song of D. melanogaster, but use abdominal rather than wing vibrations to generate the song (Hoy et al. 1988).
Females of some species, such as members of the virilis species group, respond to males with a song of their own, indicating receptivity to courtship (Satokangas et al. 1994; LaRue et al. 2015). While little is currently known about why female songs evolved in this group, several lines of evidence suggest that it might be a way to recognize conspecific individuals and, in closely related species, to provide a barrier to interspecific matings. Male responses to female songs in virilis species range from singing and the licking of female terminalia to completely stopping courtship. Interestingly, male genitalia in the virilis group show little variation, suggesting that morphology is most likely not the way in which conspecific individuals recognize one another, and that premating barriers are weak or lacking in this group. Evidence from forced interspecific matings in the laboratory shows that fertile hybrids are obtained between almost all species pairs (Throckmorton 1982). This suggests that there are few postmating barriers in this group. Thus, female songs in the virilis group (Satokangas et al. 1994) may have arisen to prevent interspecific matings in a morphologically homogeneous group of genetically closely related taxa.
Evolution of pheromone-mediated courtship signals
In addition to male wing displays, courtship and mating in Drosophila species are guided by chemical signals exchanged between the male and female [reviewed in Venard and Jallon (1980), Jallon (1984), Ferveur (1997, 2005), and Billeter and Wolfner (2018)].
Cuticular hydrocarbons:
Cuticular hydrocarbons protect against desiccation, and some components of the cuticle have been coopted as contact pheromones (Chung and Carroll 2015). Evolution of pheromonal communication requires coevolution of the production and perception of the pheromonal profiles of the interacting partners, and shifts in chemical communication, like changes in the courtship song, can establish reproductive barriers. Thus, evolution of differences in the hydrocarbon profile and response could lead to reproductive isolation. Male mate choice based on discrimination of female cuticular hydrocarbons has been implicated as the driving source for reproductive isolation between D. simulans and D. sechellia (Shahandeh et al. 2018). D. melanogaster, D. simulans, and D. sechellia evolved different sensitivities, and behavioral responses, to the aggregation pheromones (Z)-5-tetradecenoic acid and (Z)-7-tetradecenoic acid (Mast et al. 2014). Responses to these compounds are mediated by neurons that express ppk23 and ppk29 (Mast et al. 2014; Seeholzer et al. 2018). In adults, ion channels of the pickpocket family are expressed in fruitless (fru)-expressing neurons on the legs that respond to pheromones by mediating inhibition of courtship between males, while promoting male–female interactions (Thistle et al. 2012; Toda et al. 2012). In addition to ppk23, ppk25 also promotes conspecific courtship in D. simulans (Ahmed et al. 2019).
The cuticular hydrocarbon profile is sexually dimorphic in D. melanogaster (Antony and Jallon 1982; Jallon and David 1987; Grillet et al. 2006; Dembeck et al. 2015) and other Drosophila species, including D. sechellia (Jallon and David 1987; Gleason et al. 2009) and D. erecta (Jallon and David 1987), but sexual dimorphism in hydrocarbon profile has not been observed in their closely related sister species D. simulans (Gleason et al. 2009). D. melanogaster males produce 7-tricosene as their principal cuticular pheromone, whereas females produce 7,11-dienes, such as 7,11-heptacosadiene. Analysis of DGRP strains revealed extensive variation in cuticular hydrocarbon profiles (Dembeck et al. 2015). Variation in hydrocarbon profiles is also evident among species of the genus Drosophila. Among the three closely related sister species D. simulans, D. mauritiana, and D. sechellia, the predominant male hydrocarbon in D. sechellia is 6-tricosene (Coyne 1996a). Females of D. simulans predominantly produce 7-tricosene, the pheromone characteristic of D. melanogaster males (Coyne 1996b). The predominant compound of the cuticular hydrocarbon profile of D. sechellia females is 7,11-heptacosadiene, whereas 7-tricosene is prevalent in the cuticular hydrocarbon profile of females of D. mauritiana (Coyne and Charlesworth 1997). Thus, distinct cuticular hydrocarbon compositions have evolved among closely related species within the melanogaster group.
D. melanogaster males are stimulated by the 7,11-heptacosadiene produced by the females, but this same pheromone inhibits courtship in males of its sister species D. simulans (Coyne et al. 1994; Marcillac et al. 2005b; Seeholzer et al. 2018). Evolutionary reorganization of the neural circuit that regulates the activity of P1 neurons, which is implicated in mediating courtship song (von Philipsborn et al. 2011), is responsible for the switch in the activation and suppression of courtship in response to 7,11-heptacosadiene between D. melanogaster and D. simulans (Seeholzer et al. 2018).
QTL mapping studies on hybrids derived from closely related species, such as D. melanogaster and D. simulans, or D. mauritiana and D. sechellia, have implicated loci on the third chromosome associated with generating differences in cuticular hydrocarbon composition among species (Coyne et al. 1994; Coyne 1996a,b; Coyne and Charlesworth 1997; Gleason et al. 2005) and sensing different pheromonal signatures (McMahon et al. 2002). Subsequent studies associated members of the desaturase gene family with sex-specific expression and the evolution of differences in cuticular pheromones between Drosophila species (Marcillac et al. 2005a; Legendre et al. 2008; Bousquet et al. 2009; Shirangi et al. 2009; Keays et al. 2011; Pardy et al. 2019).
The desaturase gene family, which is located on the third chromosome in D. melanogaster, has evolved through repeated gene duplications and diversification, with evidence of purifying selection of daughter genes (Keays et al. 2011). A transposon in the Desat1 gene of D. melanogaster reduced the production of unsaturated hydrocarbons in both sexes, and mutant males could not discriminate sex pheromones of control flies and vice versa (Marcillac et al. 2005a). Expression of DesatF has been correlated with the production of long-chain dienes across the Drosophila phylogeny. Evolutionary analyses have identified multiple independent inactivations and changes in sex-specificity of this gene. These studies also identified the acquisition or loss of a functional binding site for the sex-determining transcriptional regulator Doublesex in evolutionary transitions between the acquisition and loss of sexual dimorphism of gene expression of DesatF (Shirangi et al. 2009).
African D. melanogaster show different cuticular hydrocarbon profiles from those of the cosmopolitan D. melanogaster strains that are typically used in laboratories. African D. melanogaster females produce high levels of 5,9-dienes and low levels of 7,11-heptacosadiene relative to the levels produced by their cosmopolitan counterparts (Ferveur et al. 1996; Legendre et al. 2008). Variation at the Desat2 locus has been implicated in this difference (Dallerac et al. 2000), specifically a 16-bp deletion in the 5′ regulatory region of Desat2 in African D. melanogaster relative to cosmopolitan strains (Takahashi et al. 2001). An elegant gene replacement study of Desat2 alleles between a Zimbabwe population and cosmopolitan D. melanogaster showed that the cosmopolitan Desat2 allele also contributed to cold resistance and starvation resistance, suggesting a connection between ecological adaptation and reproductive isolation (Greenberg et al. 2003). A similar demonstration connecting pheromone-producing enzymes with ecological adaptation and reproductive isolation comes from a temperature selection study of a D. melanogaster population from the Comoro islands. There, a few generations of selection at higher temperature resulted in an increase of 7-pentacosene at the expense of 7-tricosene and concomitant increased resistance to desiccation, and partial sexual isolation between selected and nonselected strains (Bontonou et al. 2013). Significant species-specific differences in cuticular hydrocarbon composition have also been documented for the closely related sibling species of the desert-dwelling cactophilic D. repleta group, D. mojavensis, D. arizonae, and D. navojoa. It has been suggested that such differences occur early in the evolution of new species (Etges and Jackson 2001).
A comprehensive survey of cuticular hydrocarbon variation in the DGRP showed that 17 DGRP lines contain the functional Desat2 allele thought to be unique to African and Caribbean D. melanogaster females, and accordingly produce a high 5,9-heptacosadiene to 7,11-heptacosadiene ratio. Thus, this Desat2 allele is also segregating among cosmopolitan D. melanogaster populations (Dembeck et al. 2015). Furthermore, genome-wide association analyses with the DGRP identified 305 and 173 genes in females and males, respectively, associated with variation in cuticular hydrocarbon profiles, of which 24 candidate genes, associated with fatty acid metabolism, were causally validated (Dembeck et al. 2015). Thus, variation in cuticular hydrocarbon composition is highly polygenic and is mediated by variation in the activities of enzymes that control cuticular hydrocarbon biosynthesis (Yew and Chung 2015). Variation in pheromonal communication can result from sexually dimorphic variation in the activity of desaturases that form double bonds (Labeur et al. 2002; Houot et al. 2010) and elongases that extend hydrocarbon chains (Chertemps et al. 2007; Ng et al. 2015).
Variation in pheromone-mediated chemical communication is in part due to the ratio of female-characteristic vs. male-characteristic cuticular hydrocarbons. Mutations in Darkener of Apricot (Doa) interfere with sex-specific splicing of doublesex pre-mRNA in D. melanogaster, resulting in the masculinization and feminization of female and male cuticular hydrocarbon profiles, respectively, along with disruption of associated courtship behavior (Fumey and Wicker-Thomas 2017).
Cuticular hydrocarbon profiles of D. melanogaster show extensive environmental plasticity (Rajpurohit et al. 2017). The composition of cuticular hydrocarbons can be modulated by time of day and social environment in D. serrata (Chenoweth et al. 2010; Gershman et al. 2014) and D. melanogaster (Kent et al. 2008; Krupp et al. 2008). Expression of cuticular hydrocarbons in males of the Australian species D. serrata increased when more females than males were present and resulted in greater male mating success (Gershman and Rundle 2017). Transcription of Desat1 in oenocytes, which are the pheromone-producing cells, is under circadian control, leading to fluctuations in the accumulation of cuticular hydrocarbons over the course of a day (Krupp et al. 2008). Cuticular hydrocarbon profiles are also modified as a result of mating and aging (Everaerts et al. 2010). This may involve the epigenetic modulation of gene expression, since aging and mating are accompanied by extensive changes in histone modifications (Zhou et al. 2014). Thus, environmental plasticity, genotype-by-environment interactions, and plasticity in the epigenetic landscape of the genome provide a rich palette for natural selection and the evolution of behavior.
cis-vaccenyl acetate:
The pheromone 11-cis-vaccenyl acetate (cVA) regulates courtship behavior and intermale aggression, and attracts females to oviposition sites (Ha and Smith 2006; Kurtovic et al. 2007; Wang and Anderson 2010). It is made in the male’s ejaculatory bulb and transferred to females during mating, where it is found in the mating plug that forms within the bursa (uterus) of the female and decreases her attractiveness. However, this inhibitory effect on attractiveness also requires transfer to the female of cuticular hydrocarbons (rubbed off from her mate during mating), particularly 7-tricosene, made by the male’s oenocytes. These two hydrocarbons are most potent as a blend: a female with reproductive tract cVA and cuticular 7-tricosene is particularly unattractive to males (Laturney and Billeter 2016). A few hours postmating, females eject their mating plugs and with it the cVA that they received from their mates. This changes their hydrocarbon blend, making them somewhat more attractive to males, as compared to immediately after mating when they have both 7-tricosene and cVA.
Odorant-binding proteins (OBPs) are a diverse group of small proteins, some of which are thought to bind hydrophobic odorants and present them to their membrane-bound chemoreceptors (Sun et al. 2018). The LUSH OBP, which was originally identified as a carrier for short-chain alcohols (Kim et al. 1998), serves as a transporter of cVA to the OR67d receptor, mediating courtship behavior and intermale aggression (Ha and Smith 2006; Kurtovic et al. 2007; Wang and Anderson 2010). The OR67d receptor is expressed in trichoid sensillae of the T1 class (van der Goes van Naters and Carlson 2007) and activation of the T1 circuitry promotes courtship behaviors (Ronderos and Smith 2010). However, cVA also interacts with the OR65a receptor suppressing intermale aggressive behavior (Liu et al. 2011) and inhibiting courtship (Lebreton et al. 2014). Thus, it appears that multiple neural circuits activated by cVA regulate intermale aggression and courtship through balanced integration between the activities of both neural projections, which could conceivably be modulated by environmental factors. OBP69a has also been implicated in mediating effects of cVA on social behavior (Bentzur et al. 2018).
Dekker et al. (2015) showed that the agricultural pest species D. suzukii does not produce cVA, and in this species the T1 neural projection has regressed. However, D. suzukii has an intact OR67d receptor, and application of cVA to D. suzukii males reduced mating (Dekker et al. 2015). In the absence of an intact T1 neural projection it is possible that this effect is mediated via the OR65a receptor.
Elimination of a functional microRNA, miR-124, which influences the sex-determination pathway in D. melanogaster by limiting expression of the female-specific form of transformer, resulted in a change in pheromone profile in males. These males produced less cVA and more pentacosenes, which are attractive to males (Siwicki et al. 2005). This altered pheromone composition promoted male–male courtship with a concomitant reduction in male reproductive success (Weng et al. 2013).
Variations in Premating and Mating Behaviors Across the Genus Drosophila
Near the end of the courtship ritual, D. melanogaster males lick the female’s genitalia with their mouthparts, prior to curling their abdomens and initiating copulation (Sokolowski 2001). Close interactions like tapping and licking may facilitate the use of cuticular hydrocarbons, or short peptides, for species recognition and mate assessment. To attempt copulation, males curl their abdomens to engage their genitalia with the female [reviewed in Sokolowski (2001)], positioning and holding females in place using a combination of “sex combs” on their forelegs and the clasper teeth (prensisetae) of the male’s genital arch; these teeth interdigitate with “vaginal teeth,” a series of bristles (setae) in the female’s external genital structures (Mattei et al. 2015). Males of melanogaster- and obscura-group species have sex combs, modified heavy bristles on their forelegs, that are used to position and hold females in place [reviewed in Sokolowski (2001)]. The degree of sexual dimorphism of anatomical features associated with courtship and mating varies across the genus (Markow and O’Grady 2006), from an absence of dimorphisms to relatively modest modifications like sex combs on the forelegs of the melanogaster and obscura species groups, to a wide array of wing, foreleg, and mouthpart structures observed in individual species of Hawaiian Drosophila (Figure 2).
Experimental removal of setae associated with male genitalia in members of the melanogaster group (D. bipectinata and D. ananassae) decreased the chance of copulation (Polak and Rashed 2010). Relatives in the melanogaster species group possess additional secondary claspers associated with the anal plate, or cerci. These “posterior lobes” are also used during positioning, interdigitating with the female’s tergites to help hold the mating pair in the appropriate position (Masly et al. 2011).
Despite the varied and intricate premating behaviors displayed by many drosophilids, some lineages lack evident courtship displays. For example, many males of species in the genus Scaptomyza do not display to females. Instead, courtship is brief, with males simply attempting to mount any females they encounter (Spieth 1966). While many Scaptomyza species lack secondary sexual dimorphism in wings, forelegs, or other structures, their males possess highly complex male genitalia. These genitalia often have additional lobes, setae, or other grasping structures, which help position and hold females in place. This investment in “locking” male genitalia may have allowed, or compensated for, a reduction in behaviors and morphologies related to premating displays.
Various aspects of mating, including copulation duration and remating frequency, are subject to selection and show a wide range of variation across Drosophila. Mating itself is a risky behavior, opening both participants to higher incidences of parasitism and predation. Longer copulations or more frequent mating would increase this risk.
Remating frequency is also highly variable across the genus Drosophila and can be an important factor in reproductive behaviors (Markow 2002). D. melanogaster females can remate roughly every 22 hr in the wild (Giardina et al. 2017). Cactophilic Drosophila remate frequently, sometimes multiple times per day (Markow 2002). Species that remate frequently have a higher incidence of sperm competition and greater variation in sperm storage structures than species that remate less frequently (Pitnick et al. 1999).
Male Postmating Behaviors
After mating, a series of behavioral changes occur in females (described below), many triggered by seminal fluid proteins [reviewed in Avila et al. (2011); see also Bath et al. (2017)], that determine the success of the first mating in terms of progeny production as well as whether the female is amenable to mating again. Finally, zygotic barriers, such as genetic incompatibilities, can further reinforce species isolation by leading to the death or sterility of heterospecific zygotes.
Evolution of male mating advantages
It is to a male’s advantage that his mate does not copulate with other males, to protect his sperm investment in the female. If the female’s attractiveness decreases after mating, the chance of the male’s sperm being competed by a rival’s also decreases. Males can use their bodies, sperm, and/or secretions from their reproductive glands to physically or chemically block the female reproductive tract, reducing the chance that another male will mate with the female prior to fertilization and the start of oviposition.
Mate-guarding behavior
Following copulation, males of most Drosophila species depart, leaving the female to feed, oviposit, or mate again. Males of two species of the cactophilic D. repleta species group, D. pegasa and D. mainlandi, have independently evolved a mate-guarding behavior (Wasserman et al. 1971). Males of these species guard females by remaining on the back of the female and riding around on her while she feeds. While genitalia are not in contact for much of this time, his presence physically blocks other males from copulation, not only allowing for time for insemination but also providing the opportunity for a second copulation by the guarding male. D. pegasa, for example, will ride on females for extended periods of time, often > 8 hr (Gronlund et al. 2002), and sometimes for as long as 14 hr. Wasserman et al. (1971) reported “chains” of up to four males riding on the backs of females.
Chemical mate guarding
In some species, males can prevent their mates from remating by chemical or physical means. This is mediated by molecules in the seminal fluid that are transferred during mating. In D. melanogaster, transfer of seminal fluids and sperm initiates at ∼5–7 min after coupling (Lung and Wolfner 1999; Gilchrist and Partridge 2000) and continues during the 15–20-min mating. Several different forms of mate guarding can occur.
First, among the seminal secretions that are transferred are components of the mating plug, a physical plug that forms within the mated female. In D. melanogaster at least, many of these components come from the male’s ejaculatory bulb, but some come from his accessory glands. The mating plug has been suggested to serve several functions in D. melanogaster, such as possibly forming a scaffold along which sperm can move (Avila et al. 2015a and b). But from the perspective of this article, which concerns reproductive behaviors, its most important function is to contain cVA, thus decreasing the female’s attractiveness as described above (Laturney and Billeter 2016).
Second, seminal secretions “guard” the female by decreasing her receptivity to remating; these are described in the next section.
Finally, whereas the two examples above concern mate guarding within the male’s own species, in certain interspecies matings insemination can result in the formation of a hard plug, the “insemination reaction” in the bursa of the female. This plug is generated by a chemical reaction between contributions of males (sperm and other seminal proteins) and females (Markow and Ankney 1988). The insemination reaction blocks the reproductive tract and prevents other males, even conspecifics, from copulating with a mated female. The hard plug formed as a result of the insemination reaction is different from a mating plug that forms in the bursa of a female during normal (intraspecific) matings.
Postmating Behavioral Changes in Drosophila Females: Competition Between the Sexes
Postmating behaviors have been well characterized in D. melanogaster. Behaviors are different between virgin and mated females. First, a mated female is less attractive to courting males. During the first hours postmating, this is due to a change in her pheromonal profile due to molecules transferred from the male, as described above. However, even after expelling the mating plug along with excess unstored sperm by grooming it off with her legs, females’ mating propensity still remains low, even though she is less unattractive in a pheromonal sense (as described above).
Rather, at this stage, a D. melanogaster female’s receptivity to remating has decreased. Mated females actively reject males, by kicking them off or extruding the ovipositor. These responses have been attributed to the action of a peptide made in the male D. melanogaster’s accessory gland and transferred in his seminal fluid (Baumann et al. 1975; Chapman et al. 2003; Liu and Kubli 2003). This 36-amino acid “sex peptide” binds to a G protein-coupled receptor called SPR (Sex Peptide Receptor; Yapici et al. 2008), and potentially to at least one more protein (Haussmann et al. 2013), to exert its action. A small number of neurons that innervate the female’s reproductive tract are sufficient for the loss of receptivity after mating (Häsemeyer et al. 2009; Rezával et al. 2012, 2014; Yang et al. 2009); at least some of these express SPR. During storage, the sex peptide is cleaved to release its C-terminal active region from the sperm. This released region binds to its receptor, keeping the female’s mating receptivity down. Viewed from the perspective of the previous section in terms of benefits to the male of decreasing the chance of his mate’s remating, the sex peptide provides the long-term chemical guarding once the male-derived hydrocarbon pheromones are gone.
In the absence of the sex peptide, copulation itself can decrease female receptivity through the activation of mechanosensory neurons (Shao et al. 2019). These neurons communicate with a pair of lateral sensory ascending neurons in the abdominal ganglion that project to neurons expressing myoinhibitory peptides in the brain. Even though sex peptide transferred from the male does not contribute to this particular aspect of the decreased female receptivity, SPR is essential for mediating the behavioral switch, suggesting that an endogenous SPR ligand is released during copulation (Shao et al. 2019).
Interestingly, D. melanogaster females normally remain less receptive to remating for 10–14 days, for as long as they contain sperm. This is because the sex peptide binds via its N-terminus to sperm, allowing it to be stored in females long-term (Peng et al. 2005). Indeed, this has been posited as a reason for an unusual feature of Drosophila sperm: they are very long in some species. Sperm size varies widely across the genus Drosophila (Pitnick et al. 1995; Gage 2012), from species with relatively short sperm of ∼1 mm in length (e.g., D. melanogaster) to species with giant sperm that approach 6 cm in length (D. bifurca; Figure 2). Peng et al. (2005) suggest that this great length has arisen because longer sperm can hold more sex peptide, permitting longer (or better) dosing of the female. While this is an attractive hypothesis, it is not clear whether receptivity-regulating seminal proteins in all species with long sperm bind to those sperm. The potential advantage of long sperm for holding sex peptide(s) is balanced by cost: it is energetically demanding to make such long sperm, so species like D. bifurca with very long sperm invest in length over sperm numbers.
Several other behaviors change in D. melanogaster females after mating, most also due to the action of the sex peptide. Many of these can be understood in terms of increased egg production by mated females. Sex peptides cause mated females to eat more (Carvalho et al. 2006), which provides more resources for their egg production; their diet also changes to more protein-rich (Ribeiro and Dickson 2010; Vargas et al. 2010) and their guts grow and increase their absorptive capacity (Cognigni et al. 2011; Apger-McGlaughon and Wolfner 2013; Reiff et al. 2015). Receipt of the sex peptide also causes a decrease in females’ siesta sleep (Isaac et al. 2010), presumably giving them more opportunities to move around and lay eggs. Finally, female–female aggression increases postmating (Bath et al. 2017), again as a result of the action of the sex peptide. Presumably this increased aggression aims to increase a female’s access to limited food resources.
Mated females also ovulate at a higher rate, but this is regulated by a different seminal protein, ovulin (Heifetz et al. 2000). Ovulin is a 264-amino acid protein (Monsma and Wolfner 1988) that is also made in the male’s accessory gland and transferred to females, where it is proteolytically processed (Park and Wolfner 1995). Through an as yet unknown receptor and cellular target, ovulin causes an increase in octopaminergic signaling in the female’s reproductive tract (Rubinstein and Wolfner 2013). In turn, this relaxes the muscles that wrap the female’s oviduct, permitting the passage of an oocyte for ovulation (Rubinstein and Wolfner 2013; Mattei et al. 2015). Another muscle-based behavior in the reproductive tract involves the storage of sperm from the mating. Drosophila sperm are long, and movement to their storage sites appears to be assisted by contractions and relaxations of the bursa or uterus (Adams and Wolfner 2007), and potentially by a scaffold provided by the mating plug (Avila et al. 2015b) that coagulates inside the female. These uterine contractions and relaxations are triggered by the action of other seminal proteins, such as the glycoprotein Acp36DE (Avila and Wolfner 2009).
Finally, mated D. melanogaster females show changes in their oviposition site behaviors (Becher et al. 2012). Oviposition site selection includes evaluating sites for ones with low likelihoods of parasites and high likelihoods of food. Females also tend to lay eggs where other females lay; cVA ejected with the mating plug can form part of the cue to attract other females to the site of oviposition (e.g., Bartelt et al. 1985; Lebreton et al. 2012; Billeter and Levine 2015). ILP7-expressing neurons are important in the selection of egg-laying sites by females (Yang et al. 2008). Also, females are attracted to acetic acid, and avoid UV light, when they are laying eggs. Their attraction to acetic acid is not triggered by mating per se but rather by the stretching of the females’ reproductive tract as eggs pass through the tract (indirectly, there can be a mating effect, as mating increases egg production and ovulation; e.g., Heifetz et al. 2000, 2001). Some ppk mechanosensory neurons that innervate the lateral and common oviducts (tract-tiling ppk neurons) are involved in sensing that eggs are present in the reproductive tract (stretching the tract); these are different from the ppk neurons that are reported to mediate the response to the sex peptide. The aversion to UV appears to be mediated by bitter-sensing neurons in the female’s proboscis, via certain isoforms of the dTRPA1 channel (Guntur et al. 2017).
Seminal proteins delivered by the male often show evidence of rapid evolution under positive selection (e.g., Haerty et al. 2007). The sex peptide is found in many, but not all Drosophila species (D. mojavensis and D. grimshawi appear to lack it) and not outside of Drosophilidae (Tsuda et al. 2015; Tsuda and Aigaki 2016). Its receptor, SPR, is found in most insects (Yapici et al. 2008), likely because of this protein’s ancestral role as a receptor for myoinhibitory peptides (Kim et al. 2010; Poels et al. 2010). The sex peptide gene occurs in more than one copy in several Drosophila species (Cirera and Aguadé 1997, 1998). Tsuda et al. (2015) showed that D. melanogaster sex peptide can induce postmating responses and binds to the oviduct in all melanogaster-group species tested, but did not induce postmating responses in females of the obscura or willistoni groups, or D. virilis, and injection of conspecific sex peptides did not either. This suggested to Tsuda and Aigaki (2016) that the induction of postmating responses via sex peptide/SPR interaction has uniquely evolved in the melanogaster group (Tsuda and Aigaki 2016). The function of the sex peptide in the other Drosophila species remains unknown.
Ovulin is also very rapidly evolving at the primary sequence level and is almost impossible to recognize, even in D. pseudoobscura (Clark et al. 1995). The seminal protein Acp36DE also shows evidence of rapid evolution (Wagstaff and Begun 2005). Such rapid evolution is also evident in mammalian seminal proteins (e.g., Dean et al. 2011) and occurs against a backdrop of conserved molecular functions in seminal fluid (Mueller et al. 2004). For example, there are proteases and protease inhibitors in the seminal fluids of all species examined (insects and mammals), but their primary sequences can be very different [reviewed in Laflamme and Wolfner (2013)]. This rapid evolution may be driven by conflicts between the interests of individual males, and/or between the reproductive strategies of males and females. In terms of the former, sperm competition occurs in D. melanogaster females, which can mate three to five times in nature (Reinhart et al. 2015). In this case, there will be selection for molecules, including better and new ones, that can improve or enhance the chance that a male’s sperm will be stored, or will compete well against sperm of other males. In terms of the latter, the changes that are caused in mated females by seminal proteins from the male may be more beneficial from his perspective than they are from hers.
Decreasing remating can be beneficial to a male in preventing competition from rivals’ sperm, but it may be less advantageous for a “choosy” female who can benefit from the chance to select among the sperm of different males. Similarly, increasing feeding and egg production, and decreasing the female’s sleep may be beneficial to the male in terms of enhancing the fertility of the mating, but it may decrease the female’s health and longevity; indeed, mating, and the sex peptide in particular, decreases the longevity of mated D. melanogaster females (Chapman et al. 1995; Wigby and Chapman 2005). Thus, there may be selection on females to develop resistance to male seminal proteins or other modulators, causing strong selection pressure for males to evolve more potent versions of those proteins, or new ones that can be coopted for their functions. In this light, it is interesting that ovulin appears to act at the very top of the ovulation-regulatory cascade, turning up octopaminergic signaling rather than tweaking the details of octopamine’s action. It is conceivably easier to evolve a new switch to turn up an existing, conserved, physiological pathway than to modify the components of that pathway (Kirschner and Gerhart 1998; Hoke et al. 2019). In a somewhat analogous argument, although the sex peptide is a novel molecule, it functions as a new ligand for a conserved, previously existing receptor; SPR is originally the receptor for myoinhibitory peptides. In addition, we can hypothesize that rapid evolution of inducers of postmating behaviors may help in species isolation. In such cases, if heterospecific matings occur despite premating isolation mechanisms, having highly species-specific molecules that induce postmating responses in females may contribute to decreasing the reproductive success of the heterospecific pair.
Oviposition
Oviposition behavior in Drosophila can be divided into two phases: (1) finding the oviposition site or “host plant selection,” and (2) the act of physically inserting the egg into the selected substrate (Jaenike 1985, 1990). The former requires chemosensory cues that are interpreted via the olfactory and gustatory system. The latter involves a series of characteristic movements leading up to and including the placement of the egg, and may be correlated with special morphological adaptations depending on the physical structure and stage of decomposition of the oviposition site.
Host plant selection
With a few exceptions, most Drosophila species are saprophagous and rely on microbes (i.e., yeasts and bacteria) to break down plant material upon which they feed. Some species, like D. melanogaster, are generalists that have been reared successfully from decomposing fruits, fungi, and flowers of many different plant species, as well as a range of other substrates (e.g., decomposing animal matter). However, the majority of species in the genus Drosophila display a degree of fidelity in oviposition site selection in or on a given host plant taxon (Throckmorton 1975; Magnacca et al. 2008). This is likely due to the fact that flies are targeting microbes that have a characteristic niche on a specific plant or group of plants (Ort et al. 2012). Interestingly, microbes are not the only drivers in this system, as it is clear that plant secondary compounds also play a role in the selection of an oviposition site, either as deterrents or as attractors [reviewed in Barker and Starmer (1982)]. For example, volatile profiles of necrotic cacti species (and many other plants) are highly attractive to some Drosophila species. However, once Drosophila arrive on a substrate, they may find that toxic plant compounds make that substrate unsuitable. Therefore, when assessing a given substrate, Drosophila must make decisions based on a complex calculation involving microbial community profile, plant species, and stage of decay of a substrate (Yang et al. 2008; Guntur et al. 2017). Since the microbial community is linked to the host plant and decay condition, we will refer to this behavior broadly as host plant selection.
Most Drosophila species fit along a spectrum from oviposition generalists, where a single species of fly may use many different plants for egg laying and development, to oviposition specialists that lay eggs on only a single plant species. O’Grady and Markow (2012) recognized four broad categories when discussing oviposition preference in Drosophila. “Broad generalists” can utilize a wide range of resources spread across feeding guilds. D. melanogaster is an example of a broad generalist and will oviposit in nearly any decomposing fruit, flower, or fungus.
“Substrate generalists” feed on a single type of host plant, but can utilize a wide range of unrelated host species of similar types. Members of the tripunctata species group fall into this category; they oviposit on fungi but, rather than lay eggs on a single fungal species, they oviposit on and develop in many different unrelated fungal species (Throckmorton 1975).
“Substrate specialists” utilize a single substrate type from a single clade of host plant. For example, the cactophilic repleta species group oviposits on only a single family of plant, Cactaceae (Oliveira et al. 2012). However, within this single family there are many different host species, and various repleta-group species may be generally attracted to and utilize many. Hawaiian Drosophila are also categorized as substrate specialists (Magnacca et al. 2008) since they oviposit on a single substrate from a single plant lineage (e.g., leaves of Araliaceae).
The most narrowly defined class of specialists are the “true specialists.” These species use only a single type of substrate from a single species of host plant. D. sechellia, which uses only the noni fruit (Morinda citrifolia) for oviposition, is an example of a specialist species (Jones 2005). D. pachea, a species that only uses senita cactus (Pachycereus schottii), is another (Lang et al. 2012).
Female ovipositor and egg morphologies often vary with preferred host sites. This has been studied extensively in the Hawaiian Drosophila and Scaptomyza (Craddock and Kambysellis 1990; Kambysellis et al. 1995). Species of Scaptomyza tend to have simple, fleshy ovipositors without many bristles or even sclerotization (the darkening of the cuticle that indicates cross-linking of proteins and structural strength). These taxa oviposit in flowers or on the surfaces of leaves, effectively “dumping” eggs, rather than inserting them into the host substrate (Kambysellis et al. 1995). The respiratory filaments of the eggs of these species are correspondingly very short or completely absent, reflecting the abundance of available oxygen and the relatively dry oviposition substrates. In contrast, females of the Drosophila picture wing species group possess large, strong ovipositors with many bristles (Kambysellis et al. 1995). These species insert their eggs deep into rotting wood or other decomposing plant tissue. Respiratory filaments in these species are elongated, so eggs can access oxygen during development. In the species just discussed, ovipositor length, oviposition depth, and respiratory filament length are highly correlated.
Attraction to or repulsion from oviposition sites
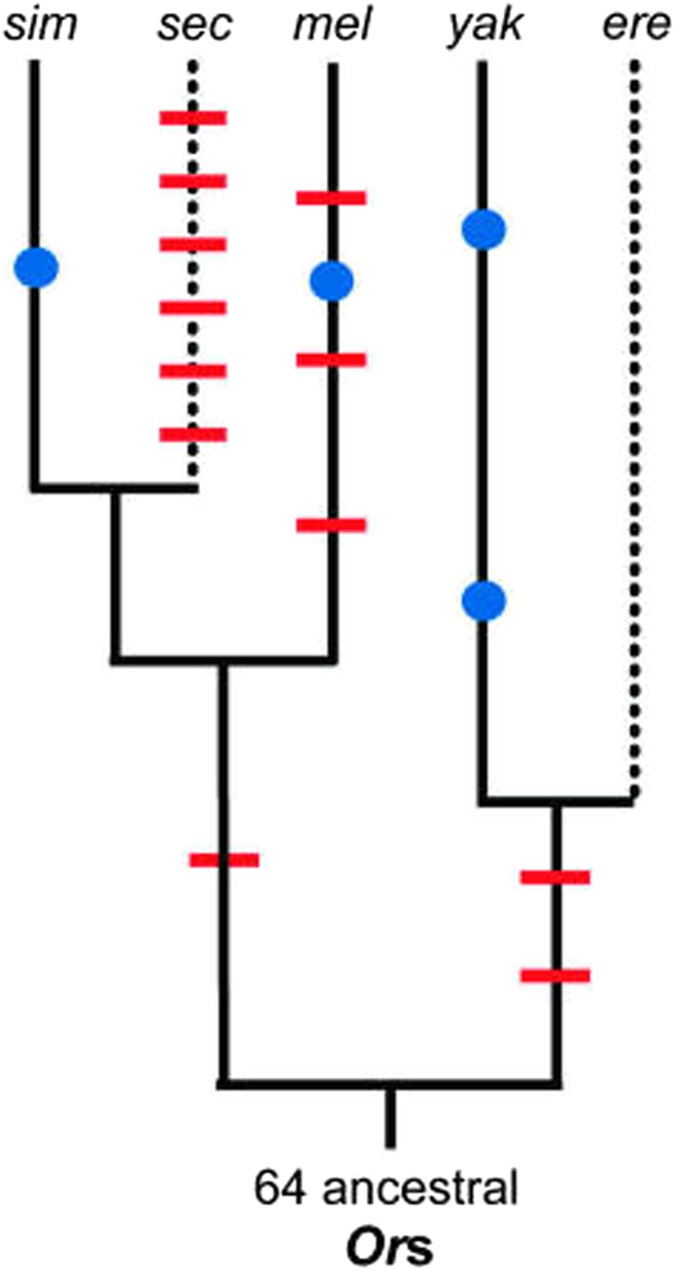
Chemical recognition features prominently in how Drosophila select host plants for feeding and oviposition. The ability to feed on a food source that is repellent to competing species reduces competition, and ensures a reliable niche for a species that is adapted to and specialized on a specific plant. For example, the noni plant, M. citrifolia, produces hexanoic and octanoic acid that are repellent to most Drosophila species (Amlou et al. 1998; Jones 2005). However, D. sechellia is attracted to Morinda and, as mentioned above, can successfully oviposit and complete development on this plant. Host plant specialization of D. sechellia has arisen through several distinct genetic adaptations, including rapid evolutionary adaptations of its chemoreceptor repertoire (McBride 2007; McBride et al. 2007; Figure 3). Adaptation to Morinda involves expression of two Obp genes, Obp57d and Obp57e, which are expressed in taste sensilla on the legs. Wild-type expression of these two OBPs results in the avoidance of hexanoic and octanoic fatty acids. Conserved cis-regulatory elements have been identified that govern expression of these OBPs (Tomioka et al. 2012). Matsuo et al. (2007) reported that a 4-bp CCAT insertion upstream of the Obp57e gene in D. sechellia prevents its expression, while its open reading frame remains intact. However, Dworkin and Jones (2009) identified a premature stop codon in the D. sechellia Obp56e gene resulting in a loss-of-function allele. It is possible that multiple Obp alleles have evolved in the D. sechellia lineage that have the same end result on host preference behavior, namely the abolition of avoidance of hexanoic and octanoic acid. Deletion of Obp57e and Obp57d in D. melanogaster also resulted in altered behavioral responses to hexanoic acid and octanoic acid (Matsuo et al. 2007). Furthermore, studies on interspecies hybrids between D. melanogaster Obp57d/e knockout flies and D. simulans or D. sechellia, shifted oviposition site preferences of the hybrid offspring to that of D. simulans or D. sechellia, respectively (Matsuo et al. 2007).
Figure 3.
Lineage-specific gene loss and gain in the Or family. Diagram that illustrates gene-loss events (red slashes) and duplications (blue dots) in the melanogaster subgroup, including D. simulans (sim), D. sechellia (sec), D. melanogaster (mel), D. yakuba (yak), and D. erecta (ere). Generalist lineages are shown by solid black lines and specialist lineages by dotted lines. The timing of events was inferred via parsimony [modified from McBride et al. (2007), with permission].
The olfactory system of D. melanogaster has been well characterized. Olfaction is mediated through odorant receptors expressed by olfactory sensory neurons in sensilla on the third antennal segment. Each neuron expresses a single odorant receptor from among the chemoreceptor repertoire and axons of olfactory sensory neurons that express the same receptor converge on output neurons in the antennal lobes, forming spherical structures of neuropil: glomeruli. Combinatorial activation of olfactory sensory neurons is translated in a spatial and temporal pattern of glomerular activity that encodes the concentration and quality of the odor [reviewed by Joseph and Carlson (2015)].
Dekker et al. (2006) reported femtogram-level sensitivity of D. sechellia antennae to the host plant odorant methyl hexanoate with an approximately threefold overrepresentation of neurons responding to this odorant, compared to D. melanogaster, and a concomitant increase in volume of the glomerulus to which they project. Ibba et al. (2010) reported that the A neuron of the ab3 sensillum responds to hexanoate esters and projects to its corresponding enlarged DM2 glomerulus, whereas ab3B neurons respond to 2-heptanone, another volatile compound from the Morinda fruit, and also project to an enlarged glomerulus. Subsequently, Prieto-Godino et al. (2017) studied acid-sensing circuitry, and found that a single amino acid change in the IR75b receptor in D. sechellia in ac3 sensilla confers sensitivity to hexanoic acid and results in attraction to this odorant. This switch in odorant response profile is associated with concomitant expansion of the DL2d glomerulus, which receives projections from IR75b-expressing neurons. However, higher-order circuits appeared unaffected (Prieto-Godino et al. 2017). In addition to the roles of OBP56e, OBP57d, OBP57e, and IR75b in mediating host preference in D. sechellia, gene expression studies identified several other genes that showed extensive upregulation in D. sechellia compared to its sister species D. simulans, including Or22a (Kopp et al. 2008) Obp50a, Or85c, and Ir84a (Shiao et al. 2015).
D. erecta, a close relative of D. melanogaster, has evolved host specialization for screw pine fruits (Pandanus sp.). Similar to the situation with D. sechellia and M. citrifolia volatiles, the number of olfactory sensory neurons that respond to 3-methyl-2-butenyl acetate, a volatile produced by the Pandanus fruits, has increased in D. erecta with corresponding enlarged glomeruli in the antennal lobe. Exposure to this odorant induces oviposition in D. erecta, but not D. melanogaster, females, providing yet another example of an exquisitely tuned olfaction-mediated host plant relationship (Linz et al. 2013).
Another well-studied example of behavioral adaptation in Drosophila comes from the cactophilic species D. mojavensis, which feeds and oviposits on decomposing cactus in Arizona, the Mojave Desert, Baja California, the Sonoran Desert, and Catalina Island off the coast of California. Different races of D. mojavensis have diversified in terms of preferences for the different types of cacti endemic at each location (Newby and Etges 1998). Electrophysiological and behavioral studies have shown olfactory adaptations to volatiles from different cacti (Date et al. 2013), and RNA-sequencing analyses have revealed differential expression of members of the Or gene family between the different populations (Crowley-Gall et al. 2016). The geographical separation and different behavioral niches among the D. mojavensis populations provide a scenario for incipient speciation (Pfeiler et al. 2009). Indeed, analyses of genome sequences between D. mojavensis and its close relatives, D. arizonae and D. navojoa, showed fixed inversion differences in three of their six chromosomes (Sanchez-Flores et al. 2016).
From an evolutionary perspective, specialization is beneficial because it reduces interspecific competition for resources. However, specialization can also be risky if a host plant or other resource becomes scarce in the future. Understanding the genetic changes behind specialization is important because it yields insight into the mechanisms of specialization and helps in understanding how flexible a given taxon might be in the face of changing environmental factors. The studies above on D. sechellia, D. erecta, and some species of cactophilic Drosophila highlight some of the recent progress made on the evolution and genetics of specialization in Drosophila.
Adaptation to fresh fruit
Unlike most Drosophila species, D. suzukii females are attracted to ripening fruit for oviposition. This adaptation has resulted in D. suzukii, often referred to as “spotted-wing Drosophila,” emerging as a major agricultural pest over the last decade as it has spread from its native range in Asia to Europe and North America (Walsh et al. 2011). Infestation of fresh fruit requires both morphological and chemical adaptations. D. suzukii females possess an enlarged, heavily serrated ovipositor relative to other taxa in the melanogaster species group. This ovipositor enables them to penetrate the skin of ripe fruit, such as strawberries, cherries, and cane fruits (Mitsui et al. 2006; Atallah et al. 2014; Cini et al. 2014).
Evolutionary changes in the olfactory receptor repertoire have also occurred that predispose D. suzukii to selecting fresh fruit as an oviposition substrate (Keesey et al. 2015). Comparisons of the Or gene repertoire of D. suzukii with those of its closely related species D. biarmipes and D. takahashii showed duplications of Or23a and Or67a in D. suzukii, with evidence for positive selection at the Or67a locus. In addition, Or74a, Or85a, and Or98b were pseudogenized in D. suzukii (Hickner et al. 2016). Furthermore, behavioral studies show that D. suzukii is attracted to the odor of ripe strawberry and that oviposition behavior on ripe fruit is reduced when the common odorant coreceptor gene, Orco, is knocked down by RNA interference or eliminated through CRISPR deletion (Karageorgi et al. 2017). However, it should be noted that in the DGRP, pseudogenes of chemoreceptors segregate in the population along with their intact counterparts apparently shielded from selection through functional redundancy; therefore, segregation of intact counterparts of pseudogenes in the D. suzukii population cannot be excluded.
Leaf mining and herbivory
Although saprophagy is the major lifestyle of most drosophilid species, a few have adapted to novel oviposition substrates. One such adaptation is the evolution of true herbivory observed in many species of the genus Scaptomyza. This involves oviposition on living plant material and the subsequent development of larvae miners in the interstitial space between the top and bottom layer of leaves. The evolution of this behavior has required a suite of morphological and chemical changes.
Scaptomyza flava oviposits, and its larvae feed, on plants of the family Brassicaceae, e.g., Arabidopsis thaliana (Whiteman et al. 2011; Goldman-Huertas et al. 2015). Evolutionary analysis of the odorant receptor repertoire of S. flava shows stepwise loss or pseudogenization of receptor genes that respond to short-chain aliphatic esters, commonly found in yeast, and duplication at the Or67b locus has resulted in three paralogs with evidence for positive selection (Goldman-Huertas et al. 2015). Interestingly, the D. melanogaster OR67b receptor also responds to the green-leaf volatile (Z)-3-hexenol (Galizia et al. 2010), suggesting a role for attraction to leaf volatiles in nonherbivorous Drosophila.
Carnivory
While most Drosophila species are attracted to rotting plant and fungal matter as oviposition substrates, there are a few with more specialized and unusual ecological niches (Ashburner 1981). Several of these are in the genus Scaptomyza, a lineage that arose on Hawaii and has since escaped to colonize all continental landmasses except Antarctica. There are endemic Hawaiian Scaptomyza in the subgenus Titanochaeta that are predators on spider eggs sacs (Wirth 1952). The female fly oviposits in the eggs and the larvae develop in the sac by eating the spider eggs, all while being guarded by the mother spider. The larval development of Titanochaeta remains unknown, but the sister lineage, the subgenus Engiscaptomyza, larviposits rather than laying eggs (Throckmorton 1975). This is quite common in some flies, particularly those that are predators or parasitoids. Members of Engiscaptomyza are suspected to be predators on endemic Hawaiian land snails, an ecological niche that might require an extremely short or nonexistent egg phase. Other parasitic drosophilid species, including members of the genus Cacoxenus, oviposit in bee nests (Ashburner 1981). Another unusual oviposition site used by three unrelated species of Drosophila is the green gland of giant land crabs (Carson 1974). This has arisen in parallel on islands of the South Pacific and Caribbean. The genetic underpinnings of the evolutionary trajectories that have given rise to these diverse ecological specializations in the genus Drosophila remain yet to be explored.
Perspectives
With its multiple sequenced and characterized species, multiple interspecies lines for examining intraspecies variation, and a vast array of publicly available resources, Drosophila offer unique opportunities for investigating the evolution of complex behaviors, such as reproductive behaviors, at both micro- and macroevolutionary scales. Much is known about the diversity of mating strategies, and ecological adaptations among members of the genus have been extensively documented. Yet, we still know little about the genetic basis of the evolutionary forces that drive these adaptations. The ability for one-way hybridization between several closely related sister species (e.g., D. melanogaster, D. simulans, D. sechellia, and D. mauritiana) provides opportunities to assess the relationship between the diversification of reproductive behaviors and speciation.
Population and quantitative genetic studies that use well-annotated whole-genome data sets to identify genes and genetic networks associated with different elements of reproductive behaviors, across multiple species, may provide insights as to how these behaviors have evolved and diverged among various lineages within the genus Drosophila. It will be important to examine the evolution of behaviors from the contexts of trade-offs, adaptations to the social and abiotic environment, and responses to different selective pressures. Since behaviors are expressions of the nervous system, neurobiological studies are necessary to identify adaptations at the level of functional neural circuits that accompany genomic adaptations. It is here of interest to note that a study of the visual and olfactory structures among 62 Drosophila species showed that expansion of visual structures occurred at the expense of the size of the third antennal segment, which mediates olfaction, and vice versa (Keesey et al. 2019). This trade-off could be accounted for by a shared larval developmental blueprint, the eye–antennal imaginal disc, and could affect bias of sensory input for the identification of hosts and mates (Keesey et al. 2019).
Comparative studies within and integrating genetic/genomic, behavioral, ecological, and neurobiological approaches across multiple Drosophila species may provide insights into the selective forces that led to the evolution of reproductive behavior, the ultimate determinant of fitness.
Acknowledgments
The authors note that the very large number of references in this field made it impossible for us to cite papers comprehensively. Therefore, we cited selected papers or reviews that can guide readers to further in-depth exploration. We apologize to the authors whose papers we were unable to cite. We are grateful for detailed helpful suggestions from anonymous reviewers. The laboratories of the authors are supported by grants from the National Institutes of Health (NIH) (GM-059469, GM-128974, AG-043490, DA-041613, and AA-016560 to R.R.H.A. and T. F. C. Mackay; HD-038921 to M.F.W.; and HD-059060 to M.F.W. and A. G. Clark). S.T.H. is supported by the Intramural Research Program at the NIH National Heart Lung and Blood Institute.
Footnotes
Communicating editor: T. Markow
Literature Cited
- Adams E. M., and Wolfner M. F., 2007. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 53: 319–331. 10.1016/j.jinsphys.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed O. M., Avila-Herrera A., Tun K. M., Serpa P. H., Peng J. et al. , 2019. Evolution of mechanisms that control mating in Drosophila males. Cell Rep. 27: 2527–2536.e4. 10.1016/j.celrep.2019.04.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlou M., Moreteau B., and David J. R., 1998. Genetic analysis of Drosophila sechellia specialization: oviposition behavior toward the major aliphatic acids of its host plant. Behav. Genet. 28: 455–464. 10.1023/A:1021689312582 [DOI] [PubMed] [Google Scholar]
- Antony C., and Jallon J.-M., 1982. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 28: 873–880. 10.1016/0022-1910(82)90101-9 [DOI] [Google Scholar]
- Apger-McGlaughon J., and Wolfner M. F., 2013. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J. Insect Physiol. 59: 1024–1030. 10.1016/j.jinsphys.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel M., Scholz C. J., Müller T., Dittrich M., König C. et al. , 2015. Genome-wide association analyses point to candidate genes for electric shock avoidance in Drosophila melanogaster. PLoS One 10: e0126986 10.1371/journal.pone.0126986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur B. J., Sunayama-Morita T., Coen P., Murthy M., and Stern D. L., 2013. Multi-channel acoustic recording and automated analysis of Drosophila courtship songs. BMC Biol. 11: 11 10.1186/1741-7007-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya G. H., Magwire M. M., Huang W., Serrano-Negron Y. L., Mackay T. F. C. et al. , 2015. The genetic basis for variation in olfactory behavior in Drosophila melanogaster. Chem. Senses 40: 233–243. 10.1093/chemse/bjv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., 1981. Entomophagous and other bizarre Drosophilidae, pp. 395–425 in The Genetics and Biology of Drosophila, Vol. 3a, edited by Ashburner M., Carson H. L., and Thompson J. N.. Academic Press, New York. [Google Scholar]
- Atallah J., Teixeira L., Salazar R., Zaragoza G., and Kopp A., 2014. The making of a pest: the evolution of fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 281: 20132840 10.1098/rspb.2013.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., and Wolfner M. F., 2009. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc. Natl. Acad. Sci. USA 106: 15796–15800. 10.1073/pnas.0904029106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Sirot L. K., LaFlamme B. A., Rubinstein C. D., and Wolfner M. F., 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56: 21–40. 10.1146/annurev-ento-120709-144823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Cohen A. B., Ameerudeen F. S., Duneau D., Suresh S. et al. , 2015a. Retention of ejaculate by Drosophila melanogaster females requires the male-derived mating plug protein PEBme. Genetics 200: 1171–1179. 10.1534/genetics.115.176669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Wong A., Sitnik J. L., and Wolfner M. F., 2015b. Don’t pull the plug! The Drosophila mating plug preserves fertility. Fly (Austin) 9: 62–67. 10.1080/19336934.2015.1120931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles J. F., Buchanan S. M., O’Leary C., Skutt-Kakaria K., Grenier J. K. et al. , 2015. Behavioral idiosyncrasy reveals genetic control of phenotypic variability. Proc. Natl. Acad. Sci. USA 112: 6706–6711. 10.1073/pnas.1503830112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. S. F., and Starmer W. T., 1982. Ecological Genetics and Evolution. The Cactus-Yeast-Drosophila Model System, edited by Barker J. S. F. and Starmer W. T.. Academic Press, Sydney. [Google Scholar]
- Bartelt R. J., Schaner A. M., and Jackson L. L., 1985. Cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11: 1747–1756. 10.1007/BF01012124 [DOI] [PubMed] [Google Scholar]
- Bassett A. R., Tibbit C., Ponting C. P., and Liu J. L., 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4: 220–228. 10.1016/j.celrep.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath E., Bowden S., Peters C., Reddy A., Tobias J. A. et al. , 2017. Sperm and sex peptide stimulate aggression in female Drosophila. Nat. Ecol. Evol. 1: 0154 10.1038/s41559-017-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Wilson K. J., Chen P. S., and Humbel R. E., 1975. The amino-acid sequence of a peptide (PS-1) from Drosophila funebris: a paragonial peptide from males which reduces the receptivity of the female. Eur. J. Biochem. 52: 521–529. 10.1111/j.1432-1033.1975.tb04023.x [DOI] [PubMed] [Google Scholar]
- Becher P. G., Flick G., Rozpedowska E., Schmidt A., Hagman A. et al. , 2012. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 26: 822–828. 10.1111/j.1365-2435.2012.02006.x [DOI] [Google Scholar]
- Bell W. J., and Kipp L. R., 1994. Drosophila percnosoma hardy lek sites: spatial and temporal distributions of males and the dynamics of their agonistic behavior (Diptera: Drosophilidae). J. Kan. Ent. Soc. 67: 267–276. [Google Scholar]
- Bentzur A., Shmueli A., Omesi L., Ryvkin J., Knapp J. M. et al. , 2018. Odorant binding protein 69a connects social interaction to modulation of social responsiveness in Drosophila. PLoS Genet. 14: e1007328 10.1371/journal.pgen.1007328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter J. C., and Levine J., 2015. The role of cVA and the Odorant binding protein Lush in social and sexual behavior in Drosophila melanogaster. Front. Ecol. Evol. 3: 1–14. 10.3389/fevo.2015.00075 [DOI] [Google Scholar]
- Billeter J. C., and Wolfner M. F., 2018. Chemical cues that guide female reproduction in Drosophila melanogaster. J. Chem. Ecol. 44: 750–769. 10.1007/s10886-018-0947-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontonou G., Denis B., and Wicker-Thomas C., 2013. Interaction between temperature and male pheromone in sexual isolation in Drosophila melanogaster. J. Evol. Biol. 26: 2008–2020. 10.1111/jeb.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet F., Houot B., Chauvel I., Dupas S., and Ferveur J. F., 2009. desat1 and the evolution of pheromonal communication in Drosophila. Ann. N. Y. Acad. Sci. 1170: 502–505. 10.1111/j.1749-6632.2009.03927.x [DOI] [PubMed] [Google Scholar]
- Brown E. B., Layne J. E., Zhu C., Jegga A. G., and Rollmann S. M., 2013. Genome-wide association mapping of natural variation in odour-guided behaviour in Drosophila. Genes Brain Behav. 12: 503–515. 10.1111/gbb.12048 [DOI] [PubMed] [Google Scholar]
- Carbone M. A., Yamamoto A., Huang W., Lyman R. A., Meadors T. B. et al. , 2016. Genetic architecture of natural variation in visual senescence in Drosophila. Proc. Natl. Acad. Sci. USA 113: E6620–E6629. 10.1073/pnas.1613833113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson H. L., 1974. Three flies and three islands: parallel evolution in Drosophila. Proc. Natl. Acad. Sci. USA 71: 3517–3521. 10.1073/pnas.71.9.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho G. B., Kapahi P., Anderson D. J., and Benzer S., 2006. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr. Biol. 16: 692–696. 10.1016/j.cub.2006.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., and Partridge L., 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373: 241–244. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T., Bangham J., Vinti G., Seifried B., Lung O. et al. , 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 100: 9923–9928. 10.1073/pnas.1631635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth S. F., Rundle H. D., and Blows M. W., 2010. Experimental evidence for the evolution of indirect genetic effects: changes in the interaction effect coefficient, psi (Psi), due to sexual selection. Evolution 64: 1849–1856. 10.1111/j.1558-5646.2010.00952.x [DOI] [PubMed] [Google Scholar]
- Chertemps T., Duportets L., Labeur C., Ueda R., Takahashi K. et al. , 2007. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 104: 4273–4278. 10.1073/pnas.0608142104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H., and Carroll S. B., 2015. Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 37: 822–830. 10.1002/bies.201500014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini A., Anfora G., Escudero-Colomar L. A., Grassi A., Santosuosso U. et al. , 2014. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 87: 559–566. 10.1007/s10340-014-0617-z [DOI] [Google Scholar]
- Cirera S., and Aguadé M., 1997. Evolutionary history of the sex-peptide (Acp70A) gene region in Drosophila melanogaster. Genetics 147: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirera S., and Aguadé M., 1998. The sex-peptide gene (Acp70A) is duplicated in Drosophila subobscura. Gene 210: 247–254. 10.1016/S0378-1119(98)00069-9 [DOI] [PubMed] [Google Scholar]
- Clark A. G., Aguadé M., Prout T., Harshman L. G., and Langley C. H., 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J., Coen P., Roemschied F. A., Pereira T. D., Mazumder D. et al. , 2018. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr. Biol. 28: 2400–2412.e6. 10.1016/j.cub.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P., Bailey A. P., and Miguel-Aliaga I., 2011. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13: 92–104. 10.1016/j.cmet.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K., and Cook R., 1973. Rejection responses by female Drosophila melanogaster: their ontogeny, causality and effects upon the behaviour of the courting male. Behaviour 44: 142–165. 10.1163/156853973X00364 [DOI] [Google Scholar]
- Coyne J. A., 1996a Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics 143: 1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., 1996b Genetics of differences in pheromonal hydrocarbons between Drosophila melanogaster and D. simulans. Genetics 143: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., and Charlesworth B., 1997. Genetics of a pheromonal difference affecting sexual isolation between Drosophila mauritiana and D. sechellia. Genetics 145: 1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., and Orr H. A., 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381. 10.1111/j.1558-5646.1989.tb04233.x [DOI] [PubMed] [Google Scholar]
- Coyne J. A., and Orr H. A., 1997. ‘Patterns of speciation in Drosophila’ revisited. Evolution 51: 295–303. [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Crittenden A. P., and Mah K., 1994. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265: 1461–1464. 10.1126/science.8073292 [DOI] [PubMed] [Google Scholar]
- Craddock E. M., and Kambysellis P. M., 1990. Vitellogenin protein diversity in the Hawaiian Drosophila. Biochem. Genet. 28: 415–432. 10.1007/BF02401429 [DOI] [PubMed] [Google Scholar]
- Crowley-Gall, A., P. Date, C. Han, N. Rhodes, P. Andolfatto et al., 2016 Population differences in olfaction accompany host shift in Drosophila mojavensis Proc. Biol. Sci. 283: 20161562 [corrigenda: Proc. Biol. Sci. 284: 20171260 (2017)]. DOI: 10.1098/rspb.2016.1562 10.1098/rspb.2016.1562 [DOI] [PMC free article] [PubMed]
- Dallerac R., Labeur C., Jallon J. M., Knipple D. C., Roelofs W. L. et al. , 2000. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97: 9449–9454. 10.1073/pnas.150243997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E., Blanchet S., Mery F., and Wagner R. H., 2010. Do invertebrates have culture? Commun. Integr. Biol. 3: 303–305. 10.4161/cib.3.4.11970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date P., Dweck H. K., Stensmyr M. C., Shann J., Hansson B. S. et al. , 2013. Divergence in olfactory host plant preference in D. mojavensis in response to cactus host use. PLoS One 8: e70027 [corrigenda: PLoS One 9 (2014)]. 10.1371/journal.pone.0070027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. D., Findlay G. D., Hoopmann M., Wu C., MacCoss M. et al. , 2011. Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics 12: 306 10.1186/1471-2164-12-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T., Ibba I., Siju K. P., Stensmyr M. C., and Hansson B. S., 2006. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr. Biol. 16: 101–109. 10.1016/j.cub.2005.11.075 [DOI] [PubMed] [Google Scholar]
- Dekker T., Revadi S., Mansourian S., Ramasamy S., Lebreton S. et al. , 2015. Loss of Drosophila pheromone reverses its role in sexual communication in Drosophila suzukii. Proc. Biol. Sci. 282: 20143018 10.1098/rspb.2014.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembeck L. M., Böröczky K., Huang W., Schal C., Anholt R. R. H. et al. , 2015. Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. Elife 4: e09861. 10.7554/eLife.09861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriades M. C., Thackeray J. R., and Kyriacou C. P., 1999. Courtship song rhythms in Drosophila yakuba. Anim. Behav. 57: 379–386. 10.1006/anbe.1998.0976 [DOI] [PubMed] [Google Scholar]
- Ding Y., Berrocal A., Morita T., Longden K. D., and Stern D. L., 2016. Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature 536: 329–332. 10.1038/nature19093 [DOI] [PubMed] [Google Scholar]
- Ding Y., Lillvis J. L., Cande J., Berman G. J., Arthur B. J. et al. , 2019. Neural evolution of context-dependent fly song. Curr. Biol. 29: 1089–1099.e7. 10.1016/j.cub.2019.02.019 [DOI] [PubMed] [Google Scholar]
- Dobzhansky Th., 1946. Complete reproductive isolation between two morphologically similar species of Drosophila. Ecology 27: 205–211. 10.2307/1932895 [DOI] [Google Scholar]
- Drosophila 12 Genomes Consortium; Clark A. G., Eisen M. B., Smith D. R., Bergman C. M., et al. , 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450: 203–218. [DOI] [PubMed] [Google Scholar]
- Dubowy C., and Sehgal A., 2017. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205: 1373–1397. 10.1534/genetics.115.185157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin I., and Jones C. D., 2009. Genetic changes accompanying the evolution of host specialization in Drosophila sechellia. Genetics 181: 721–736. 10.1534/genetics.108.093419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. A., Doescher L. T., Kaneshiro K. Y., and Yamamoto D., 2007. A database of wing diversity in the Hawaiian Drosophila. PLoS One 2: e487 10.1371/journal.pone.0000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges W. J., and Jackson L. L., 2001. Epicuticular hydrocarbon variation in Drosophila mojavensis cluster species. J. Chem. Ecol. 27: 2125–2149. 10.1023/A:1012203222876 [DOI] [PubMed] [Google Scholar]
- Everaerts C., Farine J. P., Cobb M., and Ferveur J. F., 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS One 5: e9607 10.1371/journal.pone.0009607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C. C., Hedwig B., Conduit G., Lawrence P. A., Goodwin S. F. et al. , 2012. Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr. Biol. 22: 2180–2185. 10.1016/j.cub.2012.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorowicz G. M., Fry J. D., Anholt R. R. H., and Mackay T. F. C., 1998. Epistatic interactions between smell-impaired loci in Drosophila melanogaster. Genetics 148: 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F., 1997. The pheromonal role of cuticular hydrocarbons in Drosophila melanogaster. Bioessays 19: 353–358. 10.1002/bies.950190413 [DOI] [PubMed] [Google Scholar]
- Ferveur J. F., 2005. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35: 279–295. 10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- Ferveur J. F., Cobb M., Boukella H., and Jallon J. M., 1996. World-wide variation in Drosophila melanogaster sex pheromone: behavioural effects, genetic bases and potential evolutionary consequences. Genetica 97: 73–80. 10.1007/BF00132583 [DOI] [PubMed] [Google Scholar]
- Fumey J., and Wicker-Thomas C., 2017. Mutations at the Darkener of Apricot locus modulate pheromone production and sex behavior in Drosophila melanogaster. J. Insect Physiol. 98: 182–187. 10.1016/j.jinsphys.2017.01.005 [DOI] [PubMed] [Google Scholar]
- Fuyama Y., 1979. A visual stimulus in the courtship of Drosophila suzukii. Experientia 35: 1327–1328. 10.1007/BF01963987 [DOI] [Google Scholar]
- Gaertner B. E., Ruedi E. A., McCoy L. J., Moore J. M., Wolfner M. F. et al. , 2015. Heritable variation in courtship patterns in Drosophila melanogaster. G3 (Bethesda) 5: 531–539. 10.1534/g3.114.014811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage M. J. G., 2012. Complex sperm evolution. Proc. Natl. Acad. Sci. USA 109: 4341–4342. 10.1073/pnas.1201827109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia C. G., Münch D., Strauch M., Nissler A., and Ma S., 2010. Integrating heterogeneous odor response data into a common response model: a DoOR to the complete olfactome. Chem. Senses 35: 551–563. 10.1093/chemse/bjq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlapow M. E., Huang W., Yarboro M. T., Peterson K. R., and Mackay T. F. C., 2015. Quantitative genetics of food intake in Drosophila melanogaster. PLoS One 10: e0138129 10.1371/journal.pone.0138129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman S. N., and Rundle H. D., 2017. Crowd control: sex ratio affects sexually selected cuticular hydrocarbons in male Drosophila serrata. J. Evol. Biol. 30: 583–590. 10.1111/jeb.13028 [DOI] [PubMed] [Google Scholar]
- Gershman S. N., Toumishey E., and Rundle H. D., 2014. Time flies: time of day and social environment affect cuticular hydrocarbon sexual displays in Drosophila serrata. Proc. Biol. Sci. 281: 20140821 10.1098/rspb.2014.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina T. J., Clark A. G., and Fiumera A. C., 2017. Estimating mating rates in wild Drosophila melanogaster females by decay rates of male reproductive proteins in their reproductive tracts. Mol. Ecol. Resour. 17: 1202–1209. 10.1111/1755-0998.12661 [DOI] [PubMed] [Google Scholar]
- Gilchrist A. S., and Partridge L., 2000. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution 54: 534–542. 10.1111/j.0014-3820.2000.tb00056.x [DOI] [PubMed] [Google Scholar]
- Gleason J. M., 2005. Mutations and natural genetic variation in the courtship song of Drosophila. Behav. Genet. 35: 265–277. 10.1007/s10519-005-3219-y [DOI] [PubMed] [Google Scholar]
- Gleason J. M., and Ritchie M. G., 1998. Evolution of courtship song and reproductive isolation in the Drosophila willistoni species complex: do sexual signals diverge the most quickly? Evolution 52: 1493–1500. 10.1111/j.1558-5646.1998.tb02031.x [DOI] [PubMed] [Google Scholar]
- Gleason J. M., Nuzhdin S. V., and Ritchie M. G., 2002. Quantitative trait loci affecting a courtship signal in Drosophila melanogaster. Heredity (Edinb.) 89: 1–6. 10.1038/sj.hdy.6800099 [DOI] [PubMed] [Google Scholar]
- Gleason J. M., Jallon J. M., Rouault J. D., and Ritchie M. G., 2005. Quantitative trait loci for cuticular hydrocarbons associated with sexual isolation between Drosophila simulans and D. sechellia. Genetics 171: 1789–1798. 10.1534/genetics.104.037937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason J. M., James R. A., Wicker-Thomas C., and Ritchie M. G., 2009. Identification of quantitative trait loci function through analysis of multiple cuticular hydrocarbons differing between Drosophila simulans and Drosophila sechellia females. Heredity (Edinb.) 103: 416–424. 10.1038/hdy.2009.79 [DOI] [PubMed] [Google Scholar]
- Goldman-Huertas B., Mitchell R. F., Lapoint R. T., Faucher C. P., Hildebrand J. G. et al. , 2015. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc. Natl. Acad. Sci. USA 112: 3026–3031. 10.1073/pnas.1424656112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompel N., Prud’homme B., Wittkopp P. J., Kassner V. A., and Carroll S. B., 2005. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433: 481–487. 10.1038/nature03235 [DOI] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K. et al. , 2013. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035. 10.1534/genetics.113.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. J., Moran J. R., Coyne J. A., and Wu C. I., 2003. Ecological adaptation during incipient speciation revealed by precise gene replacement. Science 302: 1754–1757. 10.1126/science.1090432 [DOI] [PubMed] [Google Scholar]
- Grillet M., Dartevelle L., and Ferveur J. F., 2006. A Drosophila male pheromone affects female sexual receptivity. Proc. Biol. Sci. 273: 315–323. 10.1098/rspb.2005.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund C. J., Deangelis M. D., Pruet-Jones S., Ward P. S., and Coyne J. A., 2002. Mate grasping in Drosophila pegasa. Behaviour 139: 545–572. 10.1163/15685390260136005 [DOI] [Google Scholar]
- Grosjean Y., Rytz R., Farine J. P., Abuin L., Cortot J. et al. , 2011. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478: 236–240. 10.1038/nature10428 [DOI] [PubMed] [Google Scholar]
- Guntur A. R., Gou B., Gu P., He R., Stern U. et al. , 2017. H2O2-Sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics 205: 749–759. 10.1534/genetics.116.195172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. S., and Smith D. P., 2006. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 26: 8727–8733. 10.1523/JNEUROSCI.0876-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerty W., Jagadeeshan S., Kulathinal R. J., Wong A., Ravi Ram K. et al. , 2007. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 177: 1321–1335. 10.1534/genetics.107.078865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. C., 1994. The mating of a fly. Science 264: 1702–1714. 10.1126/science.8209251 [DOI] [PubMed] [Google Scholar]
- Harbison S. T., McCoy L. J., and Mackay T. F. C., 2013. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics 14: 281 10.1186/1471-2164-14-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsemeyer M., Yapici N., Heberlein U., and Dickson B. J., 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61: 511–518. 10.1016/j.neuron.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Haussmann I. U., Hemani Y., Wijesekera T., Dauwalder B., and Soller M., 2013. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. Biol. Sci. 280: 20131938 10.1098/rspb.2013.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Zhou S., St Armour G. E., Mackay T. F. C., and Anholt R. R. H., 2016. Epistatic partners of neurogenic genes modulate Drosophila olfactory behavior. Genes Brain Behav. 15: 280–290. 10.1111/gbb.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S. N., Chethan B. K., and Krishna M. S., 2005. Mating success of males with and without wing patch in Drosophila biarmipes. Indian J. Exp. Biol. 43: 902–909. [PubMed] [Google Scholar]
- Heifetz Y., Lung O., Frongillo E. A. Jr., and Wolfner M. F., 2000. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10: 99–102. 10.1016/S0960-9822(00)00288-8 [DOI] [PubMed] [Google Scholar]
- Heifetz Y., Tram U., and Wolfner M. F., 2001. Male contributions to egg production: the role of accessory gland products and sperm in Drosophila melanogaster. Proc. Biol. Sci. 268: 175–180. 10.1098/rspb.2000.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickner P. V., Rivaldi C. L., Johnson C. M., Siddappaji M., Raster G. J. et al. , 2016. The making of a pest: insights from the evolution of chemosensory receptor families in pestiferous and invasive fly, Drosophila suzukii. BMC Genomics 17: 648 10.1186/s12864-016-2983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoikkala A., Aspi J., and Suvanto L., 1998. Male courtship song frequency as an indicator of male genetic quality in an insect species, Drosophila montana. Proc. Biol. Sci. 265: 503–508. 10.1098/rspb.1998.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K. L., Adkins-Regan E., Bass A. H., McCune A. R., and Wolfner M. F., 2019. Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity. J. Exp. Biol. 222: jeb190058 10.1242/jeb.190058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B., and Rice W. R., 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl. Acad. Sci. USA 96: 5083–5088. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot B., Bousquet F., and Ferveur J. F., 2010. The consequences of regulation of desat1 expression for pheromone emission and detection in Drosophila melanogaster. Genetics 185: 1297–1309. 10.1534/genetics.110.117226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy R. R., Hoikkala A., and Kaneshiro K., 1988. Hawaiian courtship songs: evolutionary innovation in communication signals of Drosophila. Science 240: 217–219. 10.1126/science.3127882 [DOI] [PubMed] [Google Scholar]
- Huang W., Richards S., Carbone M. A., Zhu D., Anholt R. R. H. et al. , 2012. Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc. Natl. Acad. Sci. USA 109: 15553–15559. 10.1073/pnas.1213423109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Massouras A., Inoue Y., Peiffer J., Ràmia M. et al. , 2014. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 24: 1193–1208. 10.1101/gr.171546.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibba I., Angioy A. M., Hansson B. S., and Dekker T., 2010. Macroglomeruli for fruit odors change blend preference in Drosophila. Naturwissenschaften 97: 1059–1066. 10.1007/s00114-010-0727-2 [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Li C., Leedale A. E., and Shirras A. D., 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 277: 65–70. 10.1098/rspb.2009.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J., 1985. Parasite pressure and the evolution of amanitin tolerance in Drosophila. Evolution 39: 1295–1301. 10.1111/j.1558-5646.1985.tb05695.x [DOI] [PubMed] [Google Scholar]
- Jaenike J., 1990. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21: 243–273. 10.1146/annurev.es.21.110190.001331 [DOI] [Google Scholar]
- Jallon J.-M., 1984. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14: 441–478. 10.1007/BF01065444 [DOI] [PubMed] [Google Scholar]
- Jallon J.-M., and David J. R., 1987. Variation in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution 41: 294–302. [DOI] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A. et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. D., 2005. The genetics of adaptation in Drosophila sechellia. Genetica 123: 137–145. 10.1007/s10709-004-2728-6 [DOI] [PubMed] [Google Scholar]
- Joseph R. M., and Carlson J. R., 2015. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 31: 683–695. 10.1016/j.tig.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambysellis M. P., Ho K. F., Craddock E. M., Piano F., Parisi M. et al. , 1995. Pattern of ecological shifts in the diversification of Hawaiian Drosophila inferred from a molecular phylogeny. Curr. Biol. 5: 1129–1139. 10.1016/S0960-9822(95)00229-6 [DOI] [PubMed] [Google Scholar]
- Karageorgi M., Bräcker L. B., Lebreton S., Minervino C., Cavey M. et al. , 2017. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 27: 847–853. 10.1016/j.cub.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keays M. C., Barker D., Wicker-Thomas C., and Ritchie M. G., 2011. Signatures of selection and sex-specific expression variation of a novel duplicate during the evolution of the Drosophila desaturase gene family. Mol. Ecol. 20: 3617–3630. [DOI] [PubMed] [Google Scholar]
- Keesey I. W., Knaden M., and Hansson B. S., 2015. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 41: 121–128. 10.1007/s10886-015-0544-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey I. W., Grabe V., Gruber L., Koerte S., Obiero G. F. et al. , 2019. Inverse resource allocation between vision and olfaction across the genus Drosophila. Nat. Commun. 10: 1162 10.1038/s41467-019-09087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C., Azanchi R., Smith B., Formosa A., and Levine J. D., 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18: 1384–1389. 10.1016/j.cub.2008.07.088 [DOI] [PubMed] [Google Scholar]
- Kent C. F., Daskalchuk T., Cook L., Sokolowski M. B., and Greenspan R. J., 2009. The Drosophila foraging gene mediates adult plasticity and gene-environment interactions in behaviour, metabolites, and gene expression in response to food deprivation. PLoS Genet. 5: e1000609 10.1371/journal.pgen.1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Repp A., and Smith D. P., 1998. LUSH odorant-binding protein mediates chemosensory responses to alcohols in Drosophila melanogaster. Genetics 150: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J., Bartalska K., Audsley N., Yamanaka N., Yapici N. et al. , 2010. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 107: 6520–6525. 10.1073/pnas.0914764107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. G., Macdonald S. J., and Long A. D., 2012. Properties and power of the Drosophila synthetic population resource for the routine dissection of complex traits. Genetics 191: 935–949. 10.1534/genetics.112.138537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., and Gerhart J., 1998. Evolvability. Proc. Natl. Acad. Sci. USA 95: 8420–8427. 10.1073/pnas.95.15.8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappert K., Mazzi D., Hoikkala A., and Ritchie M. G., 2007. Male courtship song and female preference variation between phylogeographically distinct populations of Drosophila montana. Evolution 61: 1481–1488. 10.1111/j.1558-5646.2007.00125.x [DOI] [PubMed] [Google Scholar]
- Kondoh Y., Kaneshiro K. Y., Kimura K., and Yamamoto D., 2003. Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc. Biol. Sci. 270: 1005–1013. 10.1098/rspb.2003.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Barmina O., Hamilton A. M., Higgins L., McIntyre L. M. et al. , 2008. Evolution of gene expression in the Drosophila olfactory system. Mol. Biol. Evol. 25: 1081–1092. 10.1093/molbev/msn055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski S., Aubin T., and Marin J. R., 2004. Courtship song in Drosophila melanogaster: a differential effect on male-female locomotor activity. Can. J. Zool. 82: 1258–1266. 10.1139/z04-102 [DOI] [Google Scholar]
- Krupp J. J., Kent C., Billeter J. C., Azanchi R., So A. K. et al. , 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18: 1373–1383. 10.1016/j.cub.2008.07.089 [DOI] [PubMed] [Google Scholar]
- Kurtovic A., Widmer A., and Dickson B. J., 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446: 542–546. 10.1038/nature05672 [DOI] [PubMed] [Google Scholar]
- Kyriacou C. P., and Hall J. C., 1980. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male’s courtship song. Proc. Natl. Acad. Sci. USA 77: 6729–6733. 10.1073/pnas.77.11.6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou C. P., and Hall J. C., 1982. The function of courtship song rhythms in Drosophila. Anim. Behav. 30: 794–801. 10.1016/S0003-3472(82)80152-8 [DOI] [PubMed] [Google Scholar]
- Labeur C., Dallerac R., and Wicker-Thomas C., 2002. Involvement of desat1 gene in the control of Drosophila melanogaster pheromone biosynthesis. Genetica 114: 269–274. 10.1023/A:1016223000650 [DOI] [PubMed] [Google Scholar]
- Laflamme B. A., and Wolfner M. F., 2013. Identification and function of proteolysis regulators in seminal fluid. Mol. Reprod. Dev. 80: 80–101. 10.1002/mrd.22130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue P. P., and Vosshall L. B., 2008. The olfactory sensory map in Drosophila. Adv. Exp. Med. Biol. 628: 102–114. 10.1007/978-0-387-78261-4_7 [DOI] [PubMed] [Google Scholar]
- Lang M., Murat S., Clark A. G., Gouppil G., Blais C. et al. , 2012. Mutations in the neverland gene turned Drosophila pachea into an obligate specialist species. Science 337: 1658–1661. 10.1126/science.1224829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue K. M., Clemens J., Berman G. J., and Murthy M., 2015. Acoustic duetting in Drosophila virilis relies on the integration of auditory and tactile signals. Elife 4: e07277. 10.7554/eLife.07277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laturney M., and Billeter J. C., 2014. Neurogenetics of female reproductive behaviors in Drosophila melanogaster. Adv. Genet. 85: 1–108. 10.1016/B978-0-12-800271-1.00001-9 [DOI] [PubMed] [Google Scholar]
- Laturney M., and Billeter J. C., 2016. Drosophila melanogaster females restore their attractiveness after mating by removing male anti-aphrodisiac pheromones. Nat. Commun. 7: 12322 10.1038/ncomms12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton S., Becher P. G., Hansson B. S., and Witzgall P., 2012. Attraction of Drosophila melanogaster males to food-related and fly odours. J. Insect Physiol. 58: 125–129. 10.1016/j.jinsphys.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Lebreton S., Grabe V., Omondi A. B., Ignell R., Becher P. G. et al. , 2014. Love makes smell blind: mating suppresses pheromone attraction in Drosophila females via Or65a olfactory neurons. Sci. Rep. 4: 7119 10.1038/srep07119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre A., Miao X. X., Da Lage J. L., and Wicker-Thomas C., 2008. Evolution of a desaturase involved in female pheromonal cuticular hydrocarbon biosynthesis and courtship behavior in Drosophila. Insect Biochem. Mol. Biol. 38: 244–255. 10.1016/j.ibmb.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Linz J., Baschwitz A., Strutz A., Dweck H. K., Sachse S. et al. , 2013. Host plant-driven sensory specialization in Drosophila erecta. Proc. Biol. Sci. 280: 20130626 10.1098/rspb.2013.0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., and Kubli E., 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100: 9929–9933. 10.1073/pnas.1631700100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Liang X., Gong J., Yang Z., Zhang Y. H. et al. , 2011. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci. 14: 896–902. 10.1038/nn.2836 [DOI] [PubMed] [Google Scholar]
- Long A. D., Macdonald S. J., and King E. G., 2014. Dissecting complex traits using the Drosophila synthetic population resource. Trends Genet. 30: 488–495. 10.1016/j.tig.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O., and Wolfner M. F., 1999. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem. Mol. Biol. 29: 1043–1052. 10.1016/S0965-1748(99)00078-8 [DOI] [PubMed] [Google Scholar]
- Mackay T. F. C., Richards S., Stone E. A., Barbadilla A., Ayroles J. F. et al. , 2012. The Drosophila melanogaster genetic reference panel. Nature 482: 173–178. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnacca K. N., Foote D., and O’Grady P. M., 2008. A review of the endemic Hawaiian Drosophilidae and their host plants. Zootaxa 1728: 1–58. 10.11646/zootaxa.1728.1.1 [DOI] [Google Scholar]
- Marcillac F., Grosjean Y., and Ferveur J. F., 2005a A single mutation alters production and discrimination of Drosophila sex pheromones. Proc. Biol. Sci. 272: 303–309. 10.1098/rspb.2004.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac F., Houot B., and Ferveur J. F., 2005b Revisited roles of Drosophila female pheromones. Chem. Senses 30: i273–i274. 10.1093/chemse/bjh220 [DOI] [PubMed] [Google Scholar]
- Markow T. A., 2002. Female remating, operational sex ratio, and the arena of sexual selection in Drosophila. Evolution 56: 1725–1734. 10.1111/j.0014-3820.2002.tb00186.x [DOI] [PubMed] [Google Scholar]
- Markow T. A., and Ankney P., 1988. Insemination reaction in Drosophila: a copulatory plug in species showing male contribution to offspring. Evolution 42: 1097–1100. [DOI] [PubMed] [Google Scholar]
- Markow T. A., and O’Grady P. M., 2005. Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu. Rev. Genet. 39: 263–291. 10.1146/annurev.genet.39.073003.112454 [DOI] [PubMed] [Google Scholar]
- Markow T. A., and O’Grady P. M., 2006. Drosophila: A guide to Species Identification and Use. Academic Press, London. [Google Scholar]
- Markow T. A., and O’Grady P. M., 2008. Reproductive ecology of Drosophila. Funct. Ecol. 22: 747–759. 10.1111/j.1365-2435.2008.01457.x [DOI] [Google Scholar]
- Masly J. P., Dalton J. E., Srivastava S., Chen L., and Arbeitman M. N., 2011. The genetic basis of rapidly evolving male genital morphology in Drosophila. Genetics 189: 357–374. 10.1534/genetics.111.130815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast J. D., De Moraes C. M., Alborn H. T., Lavis L. D., and Stern D. L., 2014. Evolved differences in larval social behavior mediated by novel pheromones. Elife 3: e04205 10.7554/eLife.04205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Sugaya S., Yasukawa J., Aigaki T., and Fuyama Y., 2007. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 5: e118 10.1371/journal.pbio.0050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei A. L., Riccio M. L., Avila F. W., and Wolfner M. F., 2015. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc. Natl. Acad. Sci. USA 112: 8475–8480 [corrigenda: Proc. Natl. Acad. Sci. USA 114: E5485 (2017)]. 10.1073/pnas.1505797112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E., 1982. The Growth of Biological Thought: Diversity, Evolution, and Inheritance. Belknap Press of Harvard University Press, Cambridge, MA. [Google Scholar]
- Mazzoni V., Anfora G., and Virant-Doberlet M., 2013. Substrate vibrations during courtship in three Drosophila species. PLoS One 8: e80708 10.1371/journal.pone.0080708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C. S., 2007. Rapid evolution of smell and taste receptor genes during host specialization in Drosophila sechellia. Proc. Natl. Acad. Sci. USA 104: 4996–5001. 10.1073/pnas.0608424104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C. S., Arguello J. R., and O’Meara B. C., 2007. Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics 177: 1395–1416. 10.1534/genetics.107.078683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C., Sureau G., and Ferveur J. F., 2002. Mapping of genetic loci that change pheromone discrimination in Drosophila males. Genet. Res. 79: 149–159. 10.1017/S0016672301005535 [DOI] [PubMed] [Google Scholar]
- McRobert S. P., and Tompkins L., 1987. The effect of light on the sexual behavior of Drosophila affinis. Behav. Neural Biol. 47: 151–157. 10.1016/S0163-1047(87)90257-3 [DOI] [PubMed] [Google Scholar]
- Mery F., Varela S. A., Danchin E., Blanchet S., Parejo D. et al. , 2009. Public vs. personal information for mate copying in an invertebrate. Curr. Biol. 19: 730–734. 10.1016/j.cub.2009.02.064 [DOI] [PubMed] [Google Scholar]
- Miller D. E., Staber C., Zeitlinger J., and Hawley R. S., 2018. Highly contiguous genome assemblies of 15 Drosophila species generated using nanopore sequencing. G3 (Bethesda) 8: 3131–3141. 10.1534/g3.118.200160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui H., Takahashi K. H., and Kimura M. T., 2006. Spatial distributions and clutch sizes of Drosophila species ovipositing on cherry fruits of different stages. Popul. Ecol. 48: 233–237. 10.1007/s10144-006-0260-5 [DOI] [Google Scholar]
- Miyamoto T., and Amrein H., 2008. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11: 874–876. 10.1038/nn.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma S. A., and Wolfner M. F., 1988. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 2: 1063–1073. 10.1101/gad.2.9.1063 [DOI] [PubMed] [Google Scholar]
- Mueller J. L., Ripoll D. R., Aquadro C. F., and Wolfner M. F., 2004. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc. Natl. Acad. Sci. USA 101: 13542–13547. 10.1073/pnas.0405579101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby B. D., and Etges W. J., 1998. Host preference among populations of Drosophila mojavensis (Diptera: Drosophilidae) that use different host cacti. J. Insect Behav. 11: 691–712. 10.1023/A:1022398809881 [DOI] [Google Scholar]
- Ng W. C., Chin J. S., Tan K. J., and Yew J. Y., 2015. The fatty acid elongase Bond is essential for Drosophila sex pheromone synthesis and male fertility. Nat. Commun. 6: 8263 10.1038/ncomms9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady P. M., and Markow T. A., 2012. Rapid morphological, behavioral, and ecological evolution in Drosophila: comparisons between the endemic Hawaiian Drosophila and the cactophilic repleta species group, in Rapidly Evolving Genes and Genetic Systems, edited by Singh R. S., Xu J., Kulanthial R. J.. Oxford University Press, Oxford. [Google Scholar]
- O’Grady P. M., and, DeSalle R., 2018. Phylogeny of the genus Drosophila. Genetics 209: 1–25. 10.1534/genetics.117.300583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D. C. S. G., Almeida F. C., O’Grady P. M., Armella M., Etges W. et al. , 2012. Monophyly, divergence times, and evolution of host plant use inferred from a revised phylogeny of the Drosophila repleta species group. Mol. Phylogenet. Evol. 64: 533–544. 10.1016/j.ympev.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Ort B. S., Bantay R. M., Pantoja N. A., and O’Grady P. M., 2012. Fungal diversity associated with Hawaiian Drosophila host plants. PLoS One 7: e40550 10.1371/journal.pone.0040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan A., Lindsay T., Prudnikova A., Erdi B., Dickinson M. et al. , 2018. Multifunctional wing motor control of song and flight. Curr. Biol. 28: 2705–2717.e4. 10.1016/j.cub.2018.06.038 [DOI] [PubMed] [Google Scholar]
- Panhuis T. M., Clark N. L., and Swanson W. J., 2006. Rapid evolution of reproductive proteins in abalone and Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361: 261–268. 10.1098/rstb.2005.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardy J. A., Rundle H. D., Bernards M. A., and Moehring A. J., 2019. The genetic basis of female pheromone differences between Drosophila melanogaster and D. simulans. Heredity (Edinb.) 122: 93–109. 10.1038/s41437-018-0080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., and Wolfner M. F., 1995. Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev. Biol. 171: 694–702. 10.1006/dbio.1995.1315 [DOI] [PubMed] [Google Scholar]
- Peixoto A. A., and Hall J. C., 1998. Analysis of temperature-sensitive mutants reveals new genes involved in the courtship song of Drosophila. Genetics 148: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A. A., Costa R., and Hall J. C., 2000. Molecular and behavioral analysis of sex-linked courtship song variation in a natural population of Drosophila melanogaster. J. Neurogenet. 14: 245–256. 10.3109/01677060009084501 [DOI] [PubMed] [Google Scholar]
- Peng J., Chen S., Büsser S., Liu H., Honegger T. et al. , 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15: 207–213. 10.1016/j.cub.2005.01.034 [DOI] [PubMed] [Google Scholar]
- Pfeiler E., Castrezana S., Reed L., and Markow T. A., 2009. Genetic, ecological and morphological differences among populations of the cactophilic Drosophila mojavensis from southwestern USA and northwestern Mexico, with descriptions of two new subspecies. J. Nat. Hist. 43: 923–938. 10.1080/00222930802610535 [DOI] [Google Scholar]
- Pitnick S., Markow T. A., and Spicer G. S., 1995. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl. Acad. Sci. USA 92: 10614–10618. 10.1073/pnas.92.23.10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S., Markow T. A., and Spicer G. S., 1999. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 53: 1804–1822. 10.1111/j.1558-5646.1999.tb04564.x [DOI] [PubMed] [Google Scholar]
- Poels J., Van Loy T., Vandersmissen H. P., Van Hiel B., Van Soest S. et al. , 2010. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell. Mol. Life Sci. 67: 3511–3522. 10.1007/s00018-010-0393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak M., and Rashed A., 2010. Microscale laser surgery reveals adaptive function of male intromittent genitalia. Proc. Biol. Sci. 277: 1371–1376. 10.1098/rspb.2009.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino L. L., Rytz R., Cruchet S., Bargeton B., Abuin L. et al. , 2017. Evolution of acid-sensing olfactory circuits in Drosophilids. Neuron 93: 661–676.e6. 10.1016/j.neuron.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Prud’homme B., Gompel N., Rokas A., Kassner V. A., Williams T. M. et al. , 2006. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440: 1050–1053. 10.1038/nature04597 [DOI] [PubMed] [Google Scholar]
- Rajpurohit S., Hanus R., Vrkoslav V., Behrman E. L., Bergland A. O. et al. , 2017. Adaptive dynamics of cuticular hydrocarbons in Drosophila. J. Evol. Biol. 30: 66–80. 10.1111/jeb.12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff T., Jacobson J., Cognigni P., Antonello Z., Ballesta E. et al. , 2015. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. Elife 4: e06930 10.7554/eLife.06930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart M., Carney T., Clark A. G., and Fiumera A. C., 2015. Characterizing male-female interactions using natural genetic variation in Drosophila melanogaster. J. Hered. 106: 67–79. 10.1093/jhered/esu076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C., Pavlou H. J., Dornan A. J., Chan Y. B., Kravitz E. A. et al. , 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22: 1155–1165. 10.1016/j.cub.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C., Nojima T., Neville M. C., Lin A. C., and Goodwin S. F., 2014. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 24: 725–730. 10.1016/j.cub.2013.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C., and Dickson B. J., 2010. Sex peptide receptor and neuronal TOR/S6K signalling modulate nutrient balancing in Drosophila. Curr. Biol. 20: 1000–1005. 10.1016/j.cub.2010.03.061 [DOI] [PubMed] [Google Scholar]
- Ritchie M. G., and Gleason J. M., 1995. Rapid evolution of courtship song pattern in Drosophila willistoni sibling species. J. Evol. Biol. 8: 463–479. 10.1046/j.1420-9101.1995.8040463.x [DOI] [Google Scholar]
- Ritchie M. G., and Kyriacou C. P., 1994. Genetic variability of courtship song in a population of Drosophila melanogaster. Anim. Behav. 48: 425–434. 10.1006/anbe.1999.1167 [DOI] [Google Scholar]
- Ritchie M. G., Halsey E. J., and Gleason J. M., 1999. Drosophila song as a species-specific mating signal and the behavioural importance of the Kyriacou & Hall cycles in D. melanogaster song. Anim. Behav. 58: 649–657. 10.1006/anbe.1999.1167 [DOI] [PubMed] [Google Scholar]
- Rohde P. D., Gaertner B., Ward K., Sørensen P., and Mackay T. F. C., 2017. Genomic analysis of genotype-by-social environment interaction for Drosophila melanogaster aggressive behavior. Genetics 206: 1969–1984. 10.1534/genetics.117.200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollmann S. M., Yamamoto A., Goossens T., Zwarts L., Callaerts-Végh Z. et al. , 2007. The early developmental gene Semaphorin 5c contributes to olfactory behavior in adult Drosophila. Genetics 176: 947–956. 10.1534/genetics.106.069781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollmann S. M., Edwards A. C., Yamamoto A., Zwarts L., Callaerts P. et al. , 2008. Pleiotropic effects of Drosophila neuralized on complex behaviors and brain structure. Genetics 179: 1327–1336. 10.1534/genetics.108.088435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronderos D. S., and Smith D. P., 2010. Activation of the T1 neuronal circuit is necessary and sufficient to induce sexually dimorphic mating behavior in Drosophila melanogaster. J. Neurosci. 30: 2595–2599. 10.1523/JNEUROSCI.4819-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P. R., and Gleason J. M., 2019. Assessing the use of wing ornamentation and visual display in female choice sexual selection. Behav. Processes 158: 89–96. 10.1016/j.beproc.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Rubinstein C. D., and Wolfner M. F., 2013. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc. Natl. Acad. Sci. USA 110: 17420–17425. 10.1073/pnas.1220018110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandan D., Yamamoto A., Fanara J. J., Mackay T. F. C., and Anholt R. R. H., 2006. Dynamic genetic interactions determine odor-guided behavior in Drosophila melanogaster. Genetics 174: 1349–1363. 10.1534/genetics.106.060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandan D., Carbone M. A., Anholt R. R. H., and Mackay T. F. C., 2008. Phenotypic plasticity and genotype by environment interaction for olfactory behavior in Drosophila melanogaster. Genetics 179: 1079–1088. 10.1534/genetics.108.086769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Flores A., Peñaloza F., Carpinteyro-Ponce J., Nazario-Yepiz N., Abreu-Goodger C. et al. , 2016. Genome evolution in three species of cactophilic Drosophila. G3 (Bethesda) 6: 3097–3105. 10.1534/g3.116.033779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokangas P., Liimatainen J. O., and Hoikkala A., 1994. Songs produced by the females of the Drosophila virilis group of species. Behav. Genet. 24: 263–272. 10.1007/BF01067193 [DOI] [PubMed] [Google Scholar]
- Seeholzer L. F., Seppo M., Stern D. L., and Ruta V., 2018. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559: 564–569. 10.1038/s41586-018-0322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahandeh M. P., Pischedda A., and Turner T. L., 2018. Male mate choice via cuticular hydrocarbon pheromones drives reproductive isolation between Drosophila species. Evolution 72: 123–135. 10.1111/evo.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Chung P., Wong A., Siwanowicz I., Kent C. F. et al. , 2019. A neural circuit encoding the experience of copulation in female Drosophila. Neuron 102: 1025–1036.e6. 10.1016/j.neuron.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Shevtsova E., Hansson C., Janzen D. H., and Kjærandsen J., 2011. Stable structural color patterns displayed on transparent insect wings. Proc. Natl. Acad. Sci. USA 108: 668–673. 10.1073/pnas.1017393108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao M. S., Chang J. M., Fan W. L., Lu M. Y., Notredame C. et al. , 2015. Expression divergence of chemosensory genes between Drosophila sechellia and its sibling species and its implications for host shift. Genome Biol. Evol. 7: 2843–2858. 10.1093/gbe/evv183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi T. R., Dufour H. D., Williams T. M., and Carroll S. B., 2009. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 7: e1000168 10.1371/journal.pbio.1000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi T. R., Wong A. M., Truman J. W., and Stern D. L., 2016. Doublesex regulates the connectivity of a neural circuit controlling Drosophila male courtship song. Dev. Cell 37: 533–544. 10.1016/j.devcel.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Shorter J., Couch C., Huang W., Carbone M. A., Peiffer J. et al. , 2015. Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc. Natl. Acad. Sci. USA 112: E3555–E3563. 10.1073/pnas.1510104112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. W., and Hall J. C., 1979. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc. Natl. Acad. Sci. USA 76: 3430–3434. 10.1073/pnas.76.7.3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki K. K., Riccio P., Ladewski L., Marcillac F., Dartevelle L. et al. , 2005. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn. Mem. 12: 636–645. 10.1101/lm.85605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M. B., 2001. Drosophila: genetics meets behaviour. Nat. Rev. Genet. 2: 879–890. 10.1038/35098592 [DOI] [PubMed] [Google Scholar]
- Song X., Goicoechea J. L., Ammiraju J. S., Luo M., He R. et al. , 2011. The 19 genomes of Drosophila: a BAC library resource for genus-wide and genome-scale comparative evolutionary research. Genetics 187: 1023–1030. 10.1534/genetics.111.126540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth H. T., 1947. Sexual behavior and isolation in Drosophila. I. The mating behavior of species of the willistoni group. Evolution 1: 17–31. 10.1111/j.1558-5646.1947.tb02710.x [DOI] [PubMed] [Google Scholar]
- Spieth H. T., 1966. Courtship behavior of endemic Hawaiian Drosophila. Univ. Texas Publ. 6615: 245–313. [Google Scholar]
- Spieth H. T., 1968. Evolutionary implications of the mating behavior of the species of Antopocerus Drosophilidae in Hawai’i. Univ. Texas Pub. 6818: 319–333. [Google Scholar]
- Spieth H. T., 1974. Courtship behavior in Drosophila. Annu. Rev. Entomol. 19: 385–405. 10.1146/annurev.en.19.010174.002125 [DOI] [PubMed] [Google Scholar]
- Spieth H. T., 1984. Courtship behaviors of the Hawaiian picture-winged Drosophila. Univ. Calif. Publ. Entomol. 103: 1–92. [Google Scholar]
- Sturtevant A. H., 1915. Experiments on sex recognition and the problem of sexual selection in Drosophila. J. Anim. Behav. 5: 351–366. 10.1037/h0074109 [DOI] [Google Scholar]
- Sun J. S., Xiao S., and Carlson J. R., 2018. The diverse small proteins called odorant-binding proteins. Open Biol. 8: 180208 10.1098/rsob.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W. J., and Vacquier V. D., 2002. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3: 137–144. 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Swanson W. J., Clark A. G., Waldrip-Dail H. M., Wolfner M. F., and Aquadro C. F., 2001. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. USA 98: 7375–7379. 10.1073/pnas.131568198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S., Harbison S. T., Hahn L. E., Morozova T. V., Yamamoto A. et al. , 2012. Extensive epistasis for olfactory behavior, sleep and waking activity in Drosophila melanogaster. Genet. Res. 94: 9–20. 10.1017/S001667231200002X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S., Huang W., Mackay T. F. C., and Anholt R. R. H., 2013. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc. Natl. Acad. Sci. USA 110: 1017–1022. 10.1073/pnas.1220168110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Tsaur S. C., Coyne J. A., and Wu C. I., 2001. The nucleotide changes governing cuticular hydrocarbon variation and their evolution in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98: 3920–3925. 10.1073/pnas.061465098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talyn B. C., and Dowse H. B., 2004. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim. Behav. 68: 1165–1180. 10.1016/j.anbehav.2003.11.023 [DOI] [Google Scholar]
- Tanaka K., Barmina O., and Kopp A., 2009. Distinct developmental mechanisms underly the evolutionary diversification in Drosophila: sex combs. Proc. Natl. Acad. Sci. USA 106: 4764–4769. 10.1073/pnas.0807875106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. M., Hopfen C., Herbert M. R., Schlötterer C., Stern D. L. et al. , 2015. Genetic architecture and functional characterization of genes underlying the rapid diversification of male external genitalia between Drosophila simulans and Drosophila mauritiana. Genetics 200: 357–369. 10.1534/genetics.114.174045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R., Cameron P., Ghorayshi A., Dennison L., and Scott K., 2012. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149: 1140–1151. 10.1016/j.cell.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throckmorton L., 1975. The phylogeny, ecology and geography of Drosophila, pp. 421–469 in Handbook of Genetics, edited by King R. Plenum Publishing Corporation, New York. [Google Scholar]
- Throckmorton L. H., 1982. The Drosophila virilis species group, pp. 227–297 in The Genetics and Biology of Drosophila, edited by Ashburner M., Carson H. L., and Thompson J. N.. Academic Press, London. [Google Scholar]
- Toda H., Zhao X., and Dickson B. J., 2012. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 1: 599–607. 10.1016/j.celrep.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Tomaru M., Yamada H., and Oguma Y., 2004. Female mate recognition and sexual isolation depending on courtship song in Drosophila sechellia and its siblings. Genes Genet. Syst. 79: 145–150. 10.1266/ggs.79.145 [DOI] [PubMed] [Google Scholar]
- Tomioka S., Aigaki T., and Matsuo T., 2012. Conserved cis-regulatory elements of two odorant-binding protein genes, Obp57d and Obp57e, in Drosophila. Genes Genet. Syst. 87: 323–329. 10.1266/ggs.87.323 [DOI] [PubMed] [Google Scholar]
- Tootoonian S., Coen P., Kawai R., and Murthy M., 2012. Neural representations of courtship song in the Drosophila brain. J. Neurosci. 32: 787–798. 10.1523/JNEUROSCI.5104-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- True J. R., Edwards K. A., Yamamoto D., and Carroll S. B., 1999. Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Curr. Biol. 9: 1382–1391. 10.1016/S0960-9822(00)80083-4 [DOI] [PubMed] [Google Scholar]
- Tsuda M., and Aigaki T., 2016. Evolution of sex-peptide in Drosophila. Fly (Austin) 10: 172–177. 10.1080/19336934.2016.1193655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Peyre J. B., Asano T., and Aigaki T., 2015. Visualizing molecular functions and cross-species activity of sex-peptide in Drosophila. Genetics 200: 1161–1169. 10.1534/genetics.115.177550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. L., Miller P. M., and Cochrane V. A., 2013. Combining genome-wide methods to investigate the genetic complexity of courtship song variation in Drosophila melanogaster. Mol. Biol. Evol. 30: 2113–2120. 10.1093/molbev/mst111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes van Naters W., and Carlson J. R., 2007. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17: 606–612. 10.1016/j.cub.2007.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M. A., Luo N., Yamaguchi A., and Kapahi P., 2010. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 20: 1006–1011. 10.1016/j.cub.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venard R., and Jallon J.-M., 1980. Evidence for an aphrodisiac pheromone of female Drosophila. Experientia 36: 211–213. 10.1007/BF01953737 [DOI] [Google Scholar]
- von Philipsborn A. C., Liu T., Yu J. Y., Masser C., Bidaye S. S. et al. , 2011. Neuronal control of Drosophila courtship song. Neuron 69: 509–522. 10.1016/j.neuron.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Wagstaff B. J., and Begun D. J., 2005. Comparative genomics of accessory gland protein genes in Drosophila melanogaster and D. pseudoobscura. Mol. Biol. Evol. 22: 818–832. 10.1093/molbev/msi067 [DOI] [PubMed] [Google Scholar]
- Walsh D. B., Bolda M. O. P., Goodhue R. E., Dreves A. J., Lee J. et al. , 2011. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2: G1–G7. 10.1603/IPM10010 [DOI] [Google Scholar]
- Wang L., and Anderson D. J., 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463: 227–231. 10.1038/nature08678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman M., Heller J. L., and Zombek J., 1971. Male-determined sexual discrimination in the species, Drosophila pegasa. Am. Midl. Nat. 86: 231–235. 10.2307/2423710 [DOI] [Google Scholar]
- Weng R., Chin J. S., Yew J. Y., Bushati N., and Cohen S. M., 2013. miR-124 controls male reproductive success in Drosophila. Elife 2: e00640 10.7554/eLife.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T., 2015. Leopard spots and zebra stripes on fruit fly wings. Nat. Education 8: 3. [Google Scholar]
- Werner T., Koshikawa S., Williams T. M., and Carroll S. B., 2010. Generation of a novel wing colour pattern by the Wingless morphogen. Nature 464: 1143–1148. 10.1038/nature08896 [DOI] [PubMed] [Google Scholar]
- Whiteman N. K., Groen S. C., Chevasco D., Bear A., Beckwith N. et al. , 2011. Mining the plant-herbivore interface with a leafmining Drosophila of Arabidopsis. Mol. Ecol. 20: 995–1014. 10.1111/j.1365-294X.2010.04901.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S., and Chapman T., 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15: 316–321. 10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Wirth W. W., 1952. Two new spider egg predators from the Hawaiian Islands (Diptera: Drosophilidae). Proc. Haw. Ent. Soc. 14: 415–417. [Google Scholar]
- Wolfner M. F., 2002. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity (Edinb.) 88: 85–93. 10.1038/sj.hdy.6800017 [DOI] [PubMed] [Google Scholar]
- Wu B., Ma L., Zhang E., Du J., Liu S. et al. , 2018a Sexual dimorphism of sleep regulated by juvenile hormone signaling in Drosophila. PLoS Genet. 14: e1007318 10.1371/journal.pgen.1007318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Zwarts L., Callaerts P., Norga K., Mackay T. F. C. et al. , 2008. Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 105: 12393–12398. 10.1073/pnas.0804889105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Anholt R. R., and MacKay T. F., 2009. Epistatic interactions attenuate mutations affecting startle behaviour in Drosophila melanogaster. Genet. Res. (Camb.) 91: 373–382. 10.1017/S0016672309990279 [DOI] [PubMed] [Google Scholar]
- Yamamoto D., and Koganezawa M., 2013. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 14: 681–692. 10.1038/nrn3567 [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Sato K., and Koganezawa M., 2014. Neuroethology of male courtship in Drosophila: from the gene to behavior. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200: 251–264. 10.1007/s00359-014-0891-5 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Belawat P., Hafen E., Jan L. Y., and Jan Y. N., 2008. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319: 1679–1683. 10.1126/science.1151842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Rumpf S., Xiang Y., Gordon M. D., Song W. et al. , 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61: 519–526. 10.1016/j.neuron.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Jaime M., Polihronakis M., Kanegawa K., Markow T. et al. , 2018. Re-annotation of eight Drosophila genomes. Life Sci. Alliance 1: e201800156 10.26508/lsa.201800156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N., Kim Y. J., Ribeiro C., and Dickson B. J., 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451: 33–37. 10.1038/nature06483 [DOI] [PubMed] [Google Scholar]
- Yew J. Y., and Chung H., 2015. Insect pheromones: an overview of function, form and discovery. Lipid Res. 59: 88–105. 10.1016/j.plipres.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Yu J. Y., Kanai M. I., Demir E., Jefferis G. S. X. E., and Dickson B. J., 2010. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20: 1602–1614. 10.1016/j.cub.2010.08.025 [DOI] [PubMed] [Google Scholar]
- Yukilevich R., Harvey T., Nguyen S., Kehlbeck J., and Park A., 2016. The search for causal traits of speciation: divergent female mate preferences target male courtship song, not pheromones, in Drosophila athabasca species complex. Evolution 70: 526–542. 10.1111/evo.12870 [DOI] [PubMed] [Google Scholar]
- Zhou S., Mackay T. F. C., and Anholt R. R. H., 2014. Transcriptional and epigenetic responses to mating and aging in Drosophila melanogaster. BMC Genomics 15: 927 10.1186/1471-2164-15-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts L., Magwire M. M., Carbone M. A., Versteven M., Herteleer L. et al. , 2011. Complex genetic architecture of Drosophila aggressive behavior. Proc. Natl. Acad. Sci. USA 108: 17070–17075. 10.1073/pnas.1113877108 [DOI] [PMC free article] [PubMed] [Google Scholar]