Abstract
Introduction
Multinutrient approaches may produce more robust effects on brain health through interactive qualities. We hypothesized that a blood-based nutritional risk index (NRI) including three biomarkers of diet quality can explain cognitive trajectories in the multidomain Alzheimer prevention trial (MAPT) over 3-years.
Methods
The NRI included erythrocyte n-3 polyunsaturated fatty acids (n-3 PUFA 22:6n-3 and 20:5n-3), serum 25-hydroxyvitamin D, and plasma homocysteine. The NRI scores reflect the number of nutritional risk factors (0–3). The primary outcome in MAPT was a cognitive composite Z score within each participant that was fit with linear mixed-effects models.
Results
Eighty percent had at lease one nutritional risk factor for cognitive decline (NRI ≥1: 573 of 712). Participants presenting without nutritional risk factors (NRI=0) exhibited cognitive enhancement (β = 0.03 standard units [SU]/y), whereas each NRI point increase corresponded to an incremental acceleration in rates of cognitive decline (NRI-1: β = −0.04 SU/y, P = .03; NRI-2: β = −0.08 SU/y, P < .0001; and NRI-3: β = −0.11 SU/y, P = .0008).
Discussion
Identifying and addressing these well-established nutritional risk factors may reduce age-related cognitive decline in older adults; an observation that warrants further study.
Keywords: Nutrient biomarkers, Metabolomics, Biomarkers of diet quality, Omega-3 fatty acids, DHA, EPA, Homocysteine, Vitamin D, Aging, Elderly, Cognitive decline
Highlights
-
•
Multi-nutrient approaches may produce more robust effects through interactive properties
-
•
Nutritional risk index can objectively quantify nutrition-related cognitive changes
-
•
Optimum nutritional status associated with cognitive enhancement over 3-years
-
•
Suboptimum nutritional status associated with cognitive decline over 3-years
-
•
Optimizing this nutritional risk index may promote cognitive health in older adults
1. Introduction
Randomized controlled trials of dietary supplements to slow cognitive decline in older adults with and without Alzheimer's disease or mild cognitive impairment have been disappointing [[1], [2], [3], [4], [5]] with some notable exceptions [3,[6], [7], [8]]. These well-executed trials have raised important questions as to how robust epidemiological and preclinical evidence can meet inconclusive trial results. Two possible explanations include supplementation in the general population, irrespective of the individuals' nutritional status, and the lack of insight into the potential interactive qualities among nutrients. Approaches to better understand nutritional status and nutrient interaction may help advance the field. This notion has emerged in trials of homocysteine (HCy)-lowering B vitamins to slow cognitive decline and reduce total brain atrophy in people with hyperhomocysteinemia and higher baseline n-3 polyunsaturated fatty acid (PUFA) in circulation [7,9]. These findings are consistent with several other nutritional interventions in older adults that targeted those with suboptimum nutritional status in primary [6] or secondary analyses [10] or determined a posteriori that baseline nutritional status dictated efficacy [2,3,8].
The literature on food and nutrient interaction is limited and fragmented across disciplines [9,[11], [12], [13], [14]]. More recent studies of nutrient biomarker patterns do capture the interactive properties among nutrients and may explain significant variance in domain-specific cognitive functions [15], long-term risk for dementia [16], and more favorable neuroimaging parameters [15,17]. These US and French studies across different populations by different groups implicate n-3 PUFA, HCy-lowering B vitamins, and D as potential nutritional requirements for healthy cognitive aging. However, more longitudinal studies are needed to establish thresholds for biomarkers of diet quality in terms of cognitive health. Although each of these nutrients have been or are currently undergoing trial testing [18] (clinicaltrials.gov: NCT03613116), their combination may ultimately prove most beneficial through a variety of interactive mechanisms. For instance, the n-3 PUFAs have known anti-inflammatory and synaptogenic properties, and the HCy-lowering B vitamins may improve blood-brain barrier integrity [19,20], one carbon [21,22] and tau metabolism [21], whereas D may promote neurogenesis by maintaining day-to-day clearance of soluble amyloid-beta (Aβ) oligomers from the central nervous system (CNS) into the periphery [[23], [24], [25], [26], [27], [28]].
Nutrient biomarkers that include blood-based nutrients, subsequent metabolites, or metabolic indicators thereof can allow the objective assessment of diet quality and nutrient interaction in relation to cognitive decline and dementia risk. In this study, a blood-based nutritional risk index (NRI) is proposed that captures n-3 PUFA, D, and HCy at baseline in participants of the multidomain Alzheimer prevention trial (MAPT). Cutoffs for each biomarker were prespecified using the existing literature forming an NRI score of 0–3 for each participant depending on the number of suboptimum nutrient biomarker concentrations identified. This way of investigating nutritional risk for cognitive decline can support or refute a role for nutrient interaction and nutritional status in conferring risk for cognitive decline. Given that the MAPT reported null results, we hypothesized that the NRI explains 3-year cognitive trajectories in these older adults at risk for dementia.
2. Methods
2.1. Study population
The full detailed methods for MAPT are described elsewhere [29,30]. The MAPT was a multicenter, randomized, and placebo-controlled trial with four treatment arms, including (1) multidomain intervention (MDI) plus placebo, (2) MDI plus n-3 PUFA, (3) supplementation with n-3 PUFA without MDI, and (4) placebo-only group followed for more than 3 years. The MDI included nutritional counseling, physical activity, and cognitive training. Individualized preventive outpatient visits also explored possible risk factors for cognitive decline at baseline, 1 year, and 2 years [31]. Small group training sessions in six to eight participants for more than twelve 120-minute sessions lasted the first 2 months (two sessions a week for the first month and one session a week the second month). Each session includes 60 minutes of cognitive training, 45 minutes of physical training, and 15 minutes of nutritional counseling. Starting on the third month, 60-minute sessions each month throughout the 3-year intervention period were conducted to reinforce the key messages of the program and increase compliance. Details on the cognitive and physical training and the nutritional counseling are described in detail elsewhere [29].
The MAPT was conducted in 13 memory centers across France and Monaco by experts in the diagnosis and management of cognitive impairment and dementia. Community-dwelling elders aged 70 and older meet at least one of three criteria placing them at risk for cognitive decline: subjective memory complaint expressed to their physician, limitation in one instrumental activity of daily living, or gait speed recorded at ≤0.8 m/s or >5 seconds to walk 4 m. Participants with a Mini-Mental State Examination score below 24, dementia, difficulty in basic activities of daily living, and those taking n-3 PUFA supplements were excluded.
Blood draw and banking of biospecimens yielded 729 volunteers with baseline blood samples available for nutrient biomarker analysis, of which, 712 had complete data to carry out hypothesis testing. The demographic and clinical characteristics of our analytic sample (n = 712) and that of the MAPT participants missing baseline biospecimens (n = 968) were similar in age (mean age, 75.6 vs. 75.1, P = .17 via t test), cognitive composite Z scores (−0.028 vs. −0.011, P = .14 via t test), and education (no diploma: 23.6% vs. 21.8%; secondary education: 34.3% vs. 33.2%; high-school diploma: 14.6% vs. 14.8%; and university level: 27.5% vs. 30.2%, P = .63 via chi-square test). Our study had a marginally higher prevalence of women (67.4% vs. 62.8%, P = .049 chi-squared test).
2.2. Ethical approval
The trial protocol was approved by the French Ethics Committee located in Toulouse and authorized by the French Health Authority. The MAPT/Data Sharing Alzheimer (DSA) group approved the present study protocol permitting availability of tissue and existing data to carry out the specific research aims.
2.3. Neuropsychological assessments
The MAPT cognitive composite Z score was derived from the following four tests: (1) free and total recall of the free and cued selective reminding test [32]; (2) 10 Mini-Mental State Examination orientation items; (3) the digit symbol substitution test score from the Wechsler Adult Intelligence Scale–Revised [33]; and (4) the verbal fluency category test (i.e., 2 minutes of category fluency in animals) [34]. Baseline mean and standard deviation for each test were used to calculate baseline and longitudinal cognitive Z scores [30].
2.4. Nutrient biomarker assays
2.4.1. Erythrocyte n-3 PUFA
The percentage of eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) was quantified using gas chromatography coupled with a flame ionization detector (GC-FID) and denoted as n-3 PUFA. Erythrocytes are separated from plasma by centrifugation and washed three times before lipid extraction by the Folch method including a mixture of hexane and isopropanol after acidification. Margaric acid is added as an internal standard (Sigma). Total lipid extracts were saponified and methylated. Fatty acid methyl esters were extracted with pentane and analyzed by GC using an Agilent Technologies 6890N gas chromatograph with a split injector, a bonded silica capillary column (BPX 70, 60 m × 0.25 mm; 0.25 μm of film thickness) and an FID. Helium was used as a carrier gas, and the column temperature program started at 150°C, increased by 1.3°C/min to 220°C and held at 220°C for 10 minutes [35]. Identification of fatty acid methyl esters was based on retention times obtained from fatty acid standards. The area under the curve was determined using ChemStation software (Agilent). EPA and DHA concentrations are calculated using internal standards and expressed as micrograms/grams of erythrocytes. Fatty acid methyl ester is quantifiable after transmethylation using a GC 2100 Gas Chromatograph equipped with a silica capillary column (Shimadzu). Programmed temperature spray injector and an FID calculated the percentage of EPA and DHA of total fatty acids identified.
2.4.2. Serum 25-hydroxyvitamin D
An electrochemiluminescence binding assay was used for the in vitro determination of total D (Cobas 8000; Roche). This assay used vitamin D-binding protein (VDBP) to capture and quantify total serum D, which includes 25-hydroxyvitamin D3 and D2 [36]. The sample is incubated with a pretreatment reagent for 9 minutes that denatures the natural VDBP in the sample and releases the bound D. The sample is then further incubated with a recombinant ruthenium-labeled VDBP to form a complex of D and the ruthenylated VDBP. The addition of a biotinylated D creates a compound of the ruthenium-labeled VDBP and the biotinylated D. The entire complex becomes bound to the solid phase by the interaction of biotin and streptavidin-coated microparticles, which are captured on the surface of the electrode. The unbound substances are removed. Adding voltage to the electrode induces a chemiluminescent and quantifiable emission by a photomultiplier. Results are determined via an instrument-specific calibration curve generated by a two-point calibration and master curve provided by the reagent barcode yielding units expressed in nanogram/milliliter.
2.4.3. Plasma homocysteine
Total plasma HCy was measured using a commercially available enzymatic cycling assay (Cobas 8000; Roche) [37]. The concentration of total plasma HCy was measured in plasma samples against a standard curve. Oxidized HCy was first reduced and then reacted with S-adenosylmethionine to form methionine and S-adenosyl HCy (SAH) in the presence of HCy S-methyl transferase. SAH is then assessed by coupled enzymatic reactions where SAH is hydrolyzed into adenosine and HCy by SAH hydrolase, and HCy is cycled back into the HCy conversion reaction that serves to amplify the detection signal. The formed adenosine is hydrolyzed into inosine and ammonia. Glutamate dehydrogenase catalyzes the reaction of ammonia with 2-oxoglutarate and nicotinamide adenine dinucleotide to form NAD+. The concentration of HCy is then directly proportional to the amount of nicotinamide adenine dinucleotide converted to NAD+, which is read at an absorbance of 340 nm. This yields units expressed as μmol/L.
2.5. Construction of an NRI for cognition using biomarkers of diet quality
This NRI was conceptualized as an objective and quantitative measure of the nutrition-related risk for age-related cognitive decline and dementia. Three nutrient biomarkers were selected on the basis of having validated and readily available bioanalytical assays, plausible mechanisms of action relevant to the pathogenesis of Alzheimer dementia or vascular dementia, and modifiable through diet and supplementation. The nutrient biomarkers included the following: (1) erythrocyte n-3 PUFA [[38], [39], [40], [41]]; (2) serum total D [[42], [43], [44],65], and (3) plasma total HCy, a sulfur-containing amino acid and metabolic indicator of one-carbon metabolism sensitive to B vitamin intake (B6, B9, B12, and betaine) [21,45].
The NRI indicates the number of nutritional risk factors for cognitive decline using a relatively simple and readily available blood test. The operationalized cutoffs for nutritional risk include the following: n-3 PUFA (≤4.82 wt% = 1) [30,46,47], serum D (≤20 ng/mL = 1) [48,65], and plasma HCy (≥14 μmol/L = 1) [49]. NRI scores range from 0 (optimumor clear of nutritional risk) to 3 (maximum nutritional risk). Because three nutrient biomarkers were included there were a total of eight possible nutritional risk profiles. Sensitivity analyses explored alternative cutoff criteria based on the MAPT population distribution. Those included serum D (≤15 ng/mL vs. ≤20) and total plasma HCy (≥18.1 μmol/L vs. ≥14), whereas n-3 PUFA remained at the 25th percentile (Supplementary Fig. 1 and Supplementary Table 1).
2.6. Analytical approach and hypothesis testing
2.6.1. Descriptive statistics
Demographic and clinical characteristics and their distributions were calculated for each nutrient biomarker. Histograms illustrate the frequency and spread of each nutrient biomarker.
2.6.2. Statistical analysis
Cognitive composite Z score trajectories during 3 years as a function of baseline NRI scores were fit using linear mixed-effects models. The initial construction of the NRI scores assumed that the estimated magnitude of effect of each nutritional risk factor on cognition was homogeneous. Therefore, an NRI-1 is achieved by suboptimum concentration of any one of the three biomarkers. NRI-2 did not discriminate on which two nutrient biomarkers met the suboptimum criteria. Then the same models were constructed assuming that each nutritional risk factor estimated that magnitude of effect on cognition was heterogenous. This approach discretely defined nutritional risk by labeling the NRI-1 and NRI-2 scores depending on the specific nutrients or combinations meeting the suboptimum concentration criteria (i.e., NRI-1 [O3]; NRI-1 [D]; and NRI-1 [HCy]; NRI-2 [O3-HCy]; NRI-2 [O3-D]; and NRI-2 [HCy-D]). Covariates in the mixed-effects models included time measured as years from baseline, the age of participants in years, gender, education in years, trial arm allocation (placebo, MDI, MDI plus n-3 PUFA supplementation, and n-3 PUFA supplementation), and trial arm × time interaction with intercept and slope as random effects (model 1). Nonlinear effects including time squared and time quadratic effects were examined in preliminary analyses but failed to improve the goodness of fit of the model. Therefore, linear mixed-effects models are reported. We further adjusted for apolipoprotein E (APOE) ε4 (carrier vs. not) and baseline clinical dementia rating (CDR) (CDR = 0 vs. CDR = 0.5). After completing the primary analysis, two sensitivity analyses were conducted: (1) redefining the suboptimum cutoffs for nutrient biomarkers using the MAPT population percentiles (e.g., lowest 25th percentile for n-3 PUFA and 25-hydroxyvitamin D and highest 25th percentile for HCy) and (2) restriction of the analysis to the MAPT participants naive to the n-3 PUFA supplementation (i.e., the placebo and MDI arms only).
3. Results
Nutrient biomarker analysis included 43% of the total MAPT participants (n = 729 of 1679) and yielded an analytical cohort of 712 representing approximately 25% of the total population within each trial arm (Table 1). The mean concentrations for n-3 PUFA, D, and HCy were 5.8 wt%, 23.7 ng/mL, and 15.8 μmol/L, respectively (Table 1). The distribution of their concentrations (i.e., 0, 25th, median, 75th, and 100th percentiles) was 2.3, 4.8, 5.7, 6.7, 11.8 wt% (n-3 PUFA); 3, 15, 22, 31, and 123 ng/mL (D); and 5.6, 12.2, 14.9, 18.1, and 49.3 μmol/L (HCy) (Supplementary Fig. 1 and Supplementary Table 1).
Table 1.
Baseline characteristics of the MAPT analytical cohort (n = 712)
| Total | Omega-3 PUFA |
Serum 25-OH-D |
Plasma homocysteine |
||||
|---|---|---|---|---|---|---|---|
| Optimum (n = 524) | Suboptimum ≤4.82 wt% (n = 188) | Optimum (n = 390) | Suboptimum ≤20 ng/mL (n = 322) | Optimum (n = 292) | Suboptimum ≥14 μmol/L (n = 420) | ||
| Age, y; mean (SD)∗ | 75.6 (4.5) | 75.5 (4.5) | 76.1 (4.5) | 75.0 (4.2) | 76.3 (4.8) | 74.6 (4.0) | 76.3 (4.7) |
| Women, n (%) | 480 (67.4) | 356 (67.9) | 124 (66.0) | 266 (68.2) | 214 (66.5) | 231 (79.1) | 249 (59.3) |
| Education | |||||||
| No diploma/primary school | 168 (23.6) | 104 (19.9) | 64 (34.0) | 71 (18.2) | 97 (30.1) | 66 (22.6) | 102 (24.3) |
| Secondary education | 244 (34.3) | 184 (35.1) | 60 (31.9) | 148 (37.9) | 96 (29.8) | 98 (33.5) | 146 (34.8) |
| High-school diploma | 104 (14.6) | 82 (15.7) | 22 (11.7) | 52 (13.3) | 52 (16.1) | 48 (16.4) | 56 (13.3) |
| University level | 196 (27.5) | 154 (29.4) | 42 (22.3) | 119 (30.5) | 77 (23.9) | 80 (27.4) | 116 (27.6) |
| MMSE | 28.0 (1.6) | 28.1 (1.6) | 27.8 (1.8) | 28.1 (1.6) | 27.9 (1.7) | 27.9 (1.6) | 28.1 (1.6) |
| Cognitive Z score | −0.029 (0.70) | 0.015 (0.66) | −0.15 (0.78) | 0.049 (0.66) | −0.12 (0.73) | 0.07 (0.63) | −0.10 (0.74) |
| APOE ε4 available, n (%) | 624 (87.6) | 472 (90.1) | 152 (80.9) | 346 (88.7) | 278 (86.3) | 261 (89.4) | 363 (86.4) |
| APOE ε4 carrier, n (%) | 129 (20.7) | 97 (20.6) | 32 (21.1) | 74 (21.4) | 55 (19.8) | 50 (19.2) | 79 (21.8) |
| Treatment arm | |||||||
| O3 | 180 (25.3) | 126 (24.1) | 54 (28.9) | 96 (24.6) | 84 (26.1) | 67 (22.9) | 113 (26.9) |
| MDI | 176 (24.7) | 141 (26.9) | 34 (18.2) | 94 (24.1) | 82 (25.5) | 77 (26.4) | 99 (23.6) |
| O3 + MDI | 177 (24.9) | 126 (24.1) | 51 (27.3) | 100 (25.6) | 77 (23.9) | 71 (24.3) | 106 (25.2) |
| Placebo | 179 (25.1) | 131 (25.0) | 48 (25.7) | 100 (25.6) | 79 (24.5) | 77 (26.4) | 102 (24.3) |
| Omega-3 PUFA, wt% | 5.8 (1.5) | 6.4 (1.2) | 4.0 (0.6) | 5.9 (1.5) | 5.6 (1.4) | 6.2 (1.5) | 5.5 (1.4) |
| Serum 25-OH-D, ng/mL | 23.7 (12.3) | 24.1 (12.5) | 22.6 (11.7) | 31.7 (10.4) | 13.7 (4.3) | 24.0 (12.9) | 23.5 (11.8) |
| Plasma homocysteine, μmol/L | 15.8 (5.3) | 15.3 (5.3) | 17.1 (5.3) | 15.6 (5.0) | 16.0 (5.7) | 11.4 (1.7) | 18.8 (4.9) |
Abbreviations: MAPT, multidomain Alzheimer prevention trial; PUFA, polyunsaturated fatty acid; Serum 25-OH-D, serum 25-hydroxy vitamin D; SD, standard deviation; MMSE, Mini-Mental State Examination; APOE, apolipoprotein E; O3, omega-3 PUFA intervention; MDI, multidomain intervention.
Mean (SD) or number (% of total).
Nutritional risk was identified in 80.4% of the population (NRI ≥1 = 573 of 712), and 40.8% had multiple nutritional risk factors (NRI ≥2 = 291 of 712). Prevalence of suboptimum n-3 PUFA, D, and HCy was 26.4%, 45.2%, and 58.9%, respectively. There were 19.5% (n = 139) participants without nutritional risk (i.e., NRI-0), 39.6% (n = 282) with one nutritional risk factor (i.e., NRI-1), 31.6% (n = 225) with two nutritional risk factors (NRI-2), and 9.3% (n = 66) with maximum nutritional risk (i.e., NRI-3) (Table 2).
Table 2.
The Prevalence and Nature of Nutritional Risk in Older Adults using Nutrient Biomarkers in the MAPT (n = 712)∗
| NRI | RBC n-3 PUFA |
Serum 25-OH D |
Plasma homocysteine |
Sample size |
|||
|---|---|---|---|---|---|---|---|
| Optimum | Suboptimum | Optimum | Suboptimum | Optimum | Suboptimum | n (%) | |
| 0 | |||||||
| NRI-0 | 139 (19.5) | ||||||
| 1 | |||||||
| NRI-1 (HCy) | 156 (21.9) | ||||||
| NRI-1 (D) | 104 (14.6) | ||||||
| NRI-1 (O3) | 22 (3.1) | ||||||
| 2 | |||||||
| NRI-2 (HCy + D) | 125 (17.6) | ||||||
| NRI-2 (O3 + D) | 27 (3.8) | ||||||
| NRI-2 (O3 + HCy) | 73 (10.3) | ||||||
| 3 | |||||||
| NRI-3 (O3 + D + HCy) | 66 (9.3) | ||||||
| Total prevalence | 26.4% (188/712) | 45.2% (322/712) | 58.9% (420/712) | ||||
Abbreviations: MAPT, multidomain Alzheimer prevention trial; NRI, nutritional risk index; PUFA, polyunsaturated fatty acid; Serum 25-OH D, serum 25-hydroxyvitamin D; HCy, homocysteine; D, vitamin D; O3, omega-3 PUFA intervention.
Suboptimum status requires one of the following: omega-3 PUFA (O3) ≤4.82 wt%, total serum 25-OH-D (D) ≤20 ng/mL, or plasma total HCy ≥14 μmol/L.
3.1. NRI and 3-year age-related cognitive trajectories in older adults
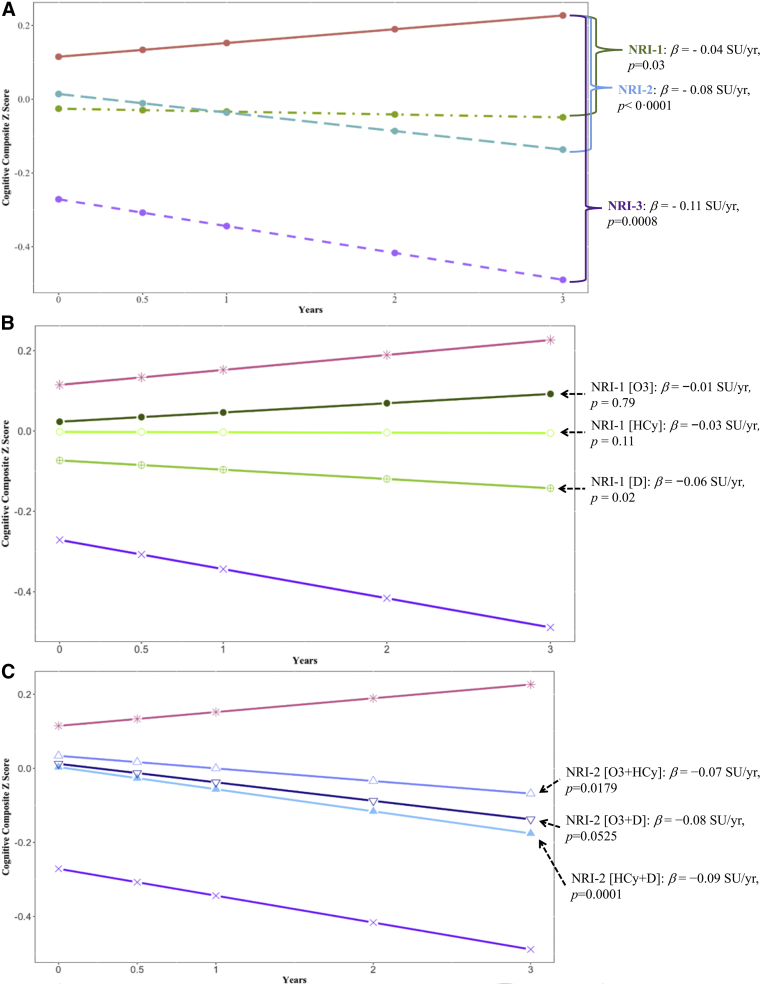
In adjusted mixed-effects models, participants without nutritional risk (NRI-0) had superior baseline cognitive performance (Fig. 1A; Table 3). The annual rate of decline on the cognitive composite Z score across all participants was −0.008 standard units (SU)/y. Fig. 1 illustrates the cognitive enhancement observed during 3 years in older adults without nutritional risk (NRI-0: β = 0.0168 SU/y; β = 0.05 SU after 3 years). Each additional nutritional risk factor detected and identified at baseline corresponded with an incremental worsening in the cognitive trajectory (e.g., NRI-1: β = −0.0451 SU/y; NRI-2: β = −0.0875 SU/y; and NRI-3: β = −0.1101 SU/y) (Fig. 1A; Table 3). The estimated difference in rates of cognitive decline between those with NRI-0 and NRI-3 was on the order of magnitude expected with approximately 4 years of aging (−0.11 [NRI-3]/−0.03 [age] = 3.6).
Fig. 1.
Mean 3-year cognitive trajectories by baseline NRI in the multidomain Alzheimer prevention trial (n = 712). NRI increases by one point for the following: omega-3 polyunsaturated fatty acid(EPA+DHA) ≤4.82 wt%; serum 25-hydroxyvitamin D ≤20 ng/mL; plasma homocysteine ≥14 μmol/L. (A) Mean 3-year cognitive composite Z score trajectories by baseline NRI 0, 1, 2, and 3. (B) Mean 3-year cognitive composite Z score trajectories by each distinct NRI-1 profile with NRI-0 and NRI-3 included for comparison. (C) Mean 3-year cognitive composite Z score trajectories by each distinct NRI-2 profile with NRI-0 and NRI-3 included for comparison. Abbreviations: NRI, nutritional risk index; SU, standard units; HCy, homocysteine.
Table 3.
Mean differences in cognitive trajectories according to the NRI scores in the MAPT (n = 712)
| characteristic | ß coefficient estimate | SE | Pr > |t| |
|---|---|---|---|
| Intercept | 2.4787 | 0.4296 | <0.0001 |
| Age, y | −0.0329 | 0.0055 | <0.0001 |
| Time, y | 0.0168 | 0.0211 | 0.4256 |
| Gender, men compared with women | −0.1869 | 0.0532 | 0.0005 |
| Education | |||
| No diploma/primary school | 0 | — | — |
| Secondary education | 0.1004 | 0.0665 | 0.1317 |
| High-school diploma | 0.4657 | 0.0828 | <0.0001 |
| College or more | 0.4671 | 0.0706 | <0.0001 |
| NRI, baseline | |||
| 0 (reference group) | 0 | — | — |
| 1 | −0.1408 | 0.0686 | 0.0403 |
| 2 | −0.1010 | 0.0722 | 0.1621 |
| 3 | −0.3864 | 0.1004 | 0.0001 |
| NRI × time (y) | |||
| 0 (reference group) | 0 | — | — |
| 1 | −0.0451 | 0.0211 | 0.0331 |
| 2 | −0.0875 | 0.0219 | <0.0001 |
| 3 | −0.1101 | 0.0327 | 0.0008 |
NOTE. Model adjusted for baseline age, gender, education, trial arm, and trial arm × time interaction; primary outcome measure is the cognitive composite Z score. NRI increases by one point for each of the following: omega-3 PUFA (O3) ≤4.82 wt%; serum 25-OH D ≤20 ng/mL; and plasma homocysteine ≥14 μmol/L.
Abbreviations: NRI, nutritional risk index; MAPT, multidomain Alzheimer prevention trial; SE, standard error.
Because of the potential heterogeneity in the effect size of each nutritional risk factor, we explored each distinct six additional NRI profiles (e.g., NRI-1 due to subotimum n-3 PUFA (NRI-1 [O3]), NRI-1 [D], NRI-1 [HCy], and NRI-2 due to suboptimum n-3 PUFA and vitamin D[O3 + D], NRI-2 [O3 + HCy], NRI-2 [D + HCy]) (Table 2). NRI-1 [D] was the only nutritional risk factor that independently associated with cognitive decline (β = −0.06 SU/y) (Fig. 1B; Table 4). However, any two nutritional risk factors combined was associated with acceleration in rates of cognitive decline ranging from −0.07 SU/y (NRI-2 [O3 + HCy]) to −0.09 SU/y (NRI-2 p[HCy + D]) (Fig. 1C; Table 4). Further adjustment for baseline CDR (0 vs. 0.5), APOE ε4 carrier versus not and their interaction with time produced no material changes to the estimated effects (data not shown).
Table 4.
Mean differences in cognitive trajectories according to the baseline NRI in the MAPT (n = 712)
| characteristic | Omega-3 PUFA | Serum 25-OH-D | Plasma HCy | ß coefficient estimate | SE | P |
|---|---|---|---|---|---|---|
| NRI, baseline | ||||||
| 0 | 0 | 0 | 0 | 0 | — | — |
| 1 (HCy) | 0 | 0 | 1 | −0.1168 | 0.0775 | .1320 |
| 1 (D) | 0 | 1 | 0 | −0.1880 | 0.0863 | .0296 |
| 1 (O3) | 1 | 0 | 0 | −0.0917 | 0.1544 | .5526 |
| 2 (HCy + D) | 0 | 1 | 1 | −0.1114 | 0.0828 | .1785 |
| 2 (O3 + HCy) | 1 | 0 | 1 | −0.0809 | 0.0949 | .3943 |
| 2 (O3 + D) | 1 | 1 | 0 | −0.1025 | 0.1412 | .4680 |
| 3 (O3 + D + HCy) | 1 | 1 | 1 | −0.3859 | 0.1006 | .0001 |
| NRI × time (y) | ||||||
| 0 | 0 | 0 | 0 | 0 | — | — |
| 1 (HCy) | 0 | 0 | 1 | −0.0383 | 0.0240 | .1121 |
| 1 (D) | 0 | 1 | 0 | −0.0603 | 0.0267 | .0243 |
| 1 (O3) | 1 | 0 | 0 | −0.0142 | 0.0538 | .7924 |
| 2 (D + HCy) | 0 | 1 | 1 | −0.0969 | 0.0253 | .0001 |
| 2 (O3 + HCy) | 1 | 0 | 1 | −0.0712 | 0.0300 | .0179 |
| 2 (O3 + D) | 1 | 1 | 0 | −0.0872 | 0.0449 | .0525 |
| 3 (O3 + D + HCy) | 1 | 1 | 1 | −0.1098 | 0.0328 | .0008 |
NOTE. Model adjusted for baseline age, gender, education, trial arm, trial arm × time interaction; primary outcome is MAPT cognitive composite Z score. NRI increases by one point for each of the following: omega-3 PUFA (O3) ≤4.82 wt%; serum 25-OH-D (D) ≤20 ng/mL; and plasma HCy ≥14 μmol/L.
Abbreviations: NRI, nutritional risk index; MAPT, multidomain Alzhemier prevention trial; PUFA, polyunsaturated fatty acid; Serum 25-OH-D, serum 25-hydroxyvitamin D; HCy, homocysteine; SE, standard error; D, vitamin D; O3, omega-3 PUFA intervention.
The sensitivity analysis that redefined the nutrient biomarker cutoffs using MAPT population quartiles (Supplementary Table 1) produced consistent results (e.g., NRI-1 = −0.04 SU/y, P = .0213; NRI-2 = −0.06 SU/y, P = .006; and NRI-3 = −0.17 SU/y, P = .009) (Supplementary Table 2). The sensitivity analysis restricted to MAPT participants naive to n-3 PUFA supplementation also produced consistent results (placebo and MDI arms, n = 355: NRI-1 = −0.08 SU/y, P = .006; NRI-2 = −0.09 SU/y, P = .002; and NRI-3 = −0.19 SU/y, P = .0004) (Supplementary Table 3).
4. Discussion
Nutritional risk for cognitive decline was identified in more than 80% of the population, and 40% had multiple nutritional risk factors. Participants without nutritional risk exhibited learning effects on repeat cognitive testing, whereas each additional nutritional risk factor corresponded with a stepwise incremental acceleration in rates of cognitive decline. These estimated effects were unattributable to age, gender, education, trial treatment allocation, baseline cognitive status, or APOE ε4 genotype. A blood-based NRI may facilitate more individualized and conclusive nutritional strategies for prevention of cognitive decline in the future.
The observations in the study are biologically plausible. Both in vitro and in vivo studies have demonstrated that the n-3 PUFA and DHA can promote neurogenesis [50], increase synaptic activity [51,52], reduce Aβ production and accumulation [53], and preserve memory and learning [54,55]. The n-3 PUFA precursor to DHA, EPA, has anti-inflammatory properties such as the downregulation of soluble intercellular adhesion molecule-1 that modulates the migration of neutrophils into the CNS across the blood-brain barrier [20,56,57]. Total n-3 PUFA in peripheral circulation is associated with cerebral cortical tissue concentrations in older adults at autopsy supporting the notion that the brain meets its nutritional demands via peripheral circulation measured in our study [58]. Hyperhomocysteinemia as one indicator of disturbed one-carbon metabolism can induce the overexpression of tau kinases (e.g., Cdk5 kinase), rarefaction of the cerebral capillaries, and impairment of nitric oxide and endothelial cell-mediated vasodilation [21,59,60]. Hyperhomocysteinemia may indirectly obstruct the methylation of phosphatidylethanolamine to phosphatidylcholine by phosphatidylethanolamine N-methyltransferase (PEMT) [61]. The PEMT pathway may be essential to the liberation of hepatic DHA into circulation for delivery to the brain in support of synaptic structure and function [62,63]. The PEMT pathway may explain our observation that n-3 PUFA and HCy were not independently associated with cognitive decline but when combined acceleration in cognitive decline ensued [64]. On the other hand, suboptimum D was associated with cognitive decline in the setting with n-3 PUFA and HCy optimum. This may be explained by experiments showing that depleted D induces cortical amyloid deposition indirectly by downregulating the low-density lipoprotein receptor-related protein-1 involved in clearance of soluble CNS Aβ oligomers [[23], [24], [25], [26], [27], [28],65]. Another contributing factor may have been the prevalence of D deficiency in the population (>45%) and therefore more power to observe the association. Together, these results suggest that older adults with n-3 PUFA >4.82 wt%, D > 20 ng/mL, and HCy <14 μmol/L can prevent cognitive decline during 3 years. These may represent actionable targets for experimental modulation and further empirical research in larger more diverse populations.
The concept of an NRI for cognition is attractive because it offers a reliable, objective, and quantitative measure of the nutrition-related risk for cognitive decline. The nutrient biomarker selection was based on their broad and potentially interactive biological activity together with their sensitivity to diet and supplementation. The cutoffs were defined a priori using an admittedly fragmented evidence base where distinct nutritional requirements for cognitive health do not currently exist. Therefore, it was necessary and important to explore opposing cutoffs in this initial hypothesis-driven study. n-3 PUFA was maintained at the lowest quartile in the study population (≤4.82 wt%) because the evidence base for optimum/suboptimum is in its infancy. However, both serum D (≤15 vs. ≤20 ng/mL) and HCy (≥18.1 vs. ≥14 μmol/L) were set at more extreme suboptimum cutoffs defined by the MAPT population distribution. The estimated effects of the NRI on cognitive decline was essentially unchanged with perhaps one exception; NRI-3 was higher in magnitude (−0.11 SU/y to −0.17 SU/y). This increase in estimated effect size was encouraging and offers one indication of a potential dose effect of more nutritional risk corresponding to more rapid cognitive decline (Supplementary Table 3 vs. Table 3).
The MAPT was deemed null by the investigators [30]. We leveraged this as an opportunity to explore the NRI concept with trial arm allocation adjustment statistically as a covariate in our models. Adding further assurance, analysis was restricted to participants naive to n-3 PUFA supplementation, and the results were consistent with the possible exception of greater effects of NRI on rates of cognitive decline (e.g., NRI-1: β = −0.08 vs. −0.04 SU/y; NRI-3: β = −0.19 vs. −0.11 SU/y). In an exploratory analysis using a three-way interaction term in the model (trial arm × NRI × time), we determined that the MAPT interventions remained null regardless of baseline NRI (data not shown). The limited sample size and the fact that a multinutrient combination (n-3 PUFA, D, and HCy-lowering B vitamins) was not included in MAPT may explain these findings and prevent any firm conclusions at this point. The consistent results regardless of the approach to these data are remarkable and encourage support for the robustness of the primary findings.
The nutrient biomarker concentrations are comparable with those seen in other studies with some exceptions. The mean plasma HCy in the MAPT population was 15.8 μmol/L. This is somewhat higher than what has been observed in older US adults (70 years and older) in both the prefolate and postfolate fortification eras (~11–14μmol/L) [66,67]. There are several possible explanations, including differences in the B vitamin nutriture, renal status of the populations, and duration of time before the blood spin down. However, it is perhaps important to note that hyperhomocysteinemia in isolation was not associated with cognitive decline unless it was accompanied by suboptimum D, n-3 PUFA, or both. Another point of consideration is that all nutritional risk groups (NRI ≥1) were compared with a reference group considered as optimum nutritional status in the setting of cognitive performance (NRI-0). There are other nutritional interventions showing greater effects of HCy-lowering B vitamins on slowing cognitive decline in older adults with hyperhomocysteinemia and higher n-3 PUFA at baseline [9]. Otherwise, the distributions observed for D [65,68] and n-3 PUFA [69,70] were similar to older European and US adults.
Despite the substantial evidence in support of large randomized controlled trials of high-dose nutrient supplementation, most have produced disappointing results calling into question whether nutrition can indeed reduce the risk of cognitive decline and dementia, including Alzheimer's disease. Many of these well-executed interventions may have underappreciated nutritional status and nutrient interaction in their design and interpretations. Many participants in most single or few nutrient supplement trials for prevention of cognitive decline have included significant proportions of people already replete in the nutrient understudy [2,3,6,8,71]. Perhaps the NRI concept will facilitate more definitive nutritional interventions in the future through a more individualized approach in identifying people in the general population at nutritional risk for cognitive decline. Operational challenges exist, and the deployment of a blood-based NRI is not trivial requiring unique expertise and resources. The next generation of nutritional interventions should inform on the utility and effectiveness of incorporating the NRI concept for subject enrichment (i.e., those at nutritional risk), monitoring adherence and describing dose responses (clinicaltrials.gov: NCT01953705 and NCT03080675).
In conclusion, optimizing n-3 PUFA, D, and HCy to promote cognitive health is a biologically plausible and testable hypothesis that warrants experimental and further observational study. Expanding the application of nutrient biomarkers may enable the establishment of distinct nutritional requirements for healthy brain aging that currently do not exist.
Research In Context.
-
1.
Systematic review: The omega-3 PUFAs (n-3 PUFA), vitamin D, and homocysteine are each independently associated with cognitive decline, yet clinical trials of high-dose dietary supplements have been mostly disappointing raising questions as to whether nutrition indeed has a role in prevention of age-related cognitive decline and dementia. However, most of these studies have focused on supplementation in the general population in which many participants are already replete in the nutrient understudy. We tested the hypothesis that an NRI including biomarkers of diet quality (erythrocyte n-3 PUFA, serum 25-hydroxyvitamin D, and plasma homocysteine) explains the 3-year cognitive trajectories in older adults at risk for dementia in the multidomain Alzheimer prevention trial.
-
2.
Interpretation: Participants with optimum nutritional status (NRI-0) exhibited cognitive enhancement during the 3 years of follow up. However, each additional NRI point increase was associated with a stepwise incremental acceleration in rates of cognitive decline.
-
3.
Future directions: A blood-based NRI may help identify subsets of the general population well suited for the evidence-based nutritional strategies that promote cognitive health. The simultaneous optimization of these biomarkers of diet quality may prevent age-related cognitive decline. The broader utilization of biomarkers in nutrition studies may help raise attention to the nutritional requirements for optimum brain health with aging.
Acknowledgments
MAPT/DSA group refers to MAPT Study Group: Principal investigator: Bruno Vellas (Toulouse); Coordination: Sophie Guyonnet; Project leader: Isabelle Carrié; Clinical research assistant: Lauréane Brigitte; Investigators: Catherine Faisant, Françoise Lala, Julien Delrieu, and Hélène Villars; Psychologists: Emeline Combrouze, Carole Badufle, and Audrey Zueras; Methodology, statistical analysis, and data management: Sandrine Andrieu, Christelle Cantet, and Christophe Morin; Multidomain group: Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland (physical and nutritional components), Céline Caillaud, Pierre-Jean Ousset (cognitive component), and Françoise Lala (preventive consultation). The cognitive component was designed in collaboration with Sherry Willis from the University of Seattle, and Sylvie Belleville, Brigitte Gilbert, and Francine Fontaine from the University of Montreal. Coinvestigators in associated center: Jean-François Dartigues, Isabelle Marcet, Fleur Delva, Alexandra Foubert, and Sandrine Cerda (Bordeaux); Marie-Noëlle-Cuffi and Corinne Costes (Castres); Olivier Rouaud, Patrick Manckoundia, Valérie Quipourt, Sophie Marilier, and Evelyne Franon (Dijon); Lawrence Bories, Marie-Laure Pader, Marie-France Basset, Bruno Lapoujade, Valérie Faure, Michael Li Yung Tong, Christine Malick-Loiseau, and Evelyne Cazaban-Campistron (Foix); Françoise Desclaux and Colette Blatge (Lavaur); Thierry Dantoine, Cécile Laubarie-Mouret, Isabelle Saulnier, Jean-Pierre Clément, Marie-Agnès Picat, Laurence Bernard-Bourzeix, Stéphanie Willebois, Iléana Désormais, and Noëlle Cardinaud (Limoges); Marc Bonnefoy, Pierre Livet, Pascale Rebaudet, Claire Gédéon, Catherine Burdet, Flavien Terracol (Lyon), Alain Pesce, Stéphanie Roth, Sylvie Chaillou, and Sandrine Louchart (Monaco); Kristelle Sudres, Nicolas Lebrun, and Nadège Barro-Belaygues (Montauban); Jacques Touchon, Karim Bennys, Audrey Gabelle, Aurélia Romano, Lynda Touati, Cécilia Marelli, and Cécile Pays (Montpellier); Philippe Robert, Franck Le Duff, Claire Gervais, and Sébastien Gonfrier (Nice); Yannick Gasnier and Serge Bordes, Danièle Begorre, Christian Carpuat, Khaled Khales, Jean-François Lefebvre, Samira Misbah El Idrissi, Pierre Skolil, and Jean-Pierre Salles (Tarbes). Magnetic resonance imaging group: Carole Dufouil (Bordeaux), Stéphane Lehéricy, Marie Chupin, Jean-François Mangin, and Ali Bouhayia (Paris); Michèle Allard (Bordeaux); Frédéric Ricolfi (Dijon); Dominique Dubois (Foix); Marie Paule Bonceour Martel (Limoges); François Cotton (Lyon); Alain Bonafé (Montpellier); Stéphane Chanalet (Nice); Françoise Hugon (Tarbes); Fabrice Bonneville, Christophe Cognard, and François Chollet (Toulouse). Positron emission tomography scan group: Pierre Payoux, Thierry Voisin, Julien Delrieu, Sophie Peiffer, and Anne Hitzel (Toulouse); Michèle Allard (Bordeaux); Michel Zanca (Montpellier); Jacques Monteil (Limoges); Jacques Darcourt (Nice). Medicoeconomics group: Laurent Molinier, Hélène Derumeaux, and Nadège Costa (Toulouse). Biological sample collection: Sylvie Caspar-Bauguil, Bertrand Perret, and Claire Vinel (Toulouse). Safety management: Pascale Olivier-Abbal. DSA group: Sandrine Andrieu, Christelle Cantet, and Nicola Coley.
Funding: Nutrient biomarker and statistical analysis was supported by the Nestle Institute of Health Sciences. The MAPT study was supported by grants from the Gérontopôle of Toulouse, the French Ministry of Health (PHRC 2008, 2009), Pierre Fabre Research Institute (manufacturer of the n-3 supplement), Exhonit Therapeutics SA, and Avid Radiopharmaceuticals Inc. The promotion of this study was supported by the University Hospital Center of Toulouse. The data sharing activity was supported by the Association Monegasque pour la Recherche sur la maladie d'Alzheimer and the UMR 1027 Unit INSERM––University of Toulouse III.
Contributions: G.L.B.: conceptualized, designed the study, secured the funding, selected the biomarkers of diet quality and analytical assays, composed the statistical analysis plan, interpreted the data, and drafted and revised the manuscript; H.H.D.: concept and design, composed and conducted the statistical analysis plan, interpreted the data, and revised the manuscript; N.Z.: performed statistical analysis; S.G.: literature review and revised the manuscript; J.D.: literature review and revised the manuscript; A.B.: contributed to the management and coordination of the statistical analysis; J.S.: literature review and revised the manuscript; C.H.: literature review and revised the manuscript; T.B.; S.A.; and B.V.: concept and design, interpreted the data, and revised the manuscript.
Footnotes
Disclosures: G.L.B. reports US National Institutes of Health funding; is an unpaid Scientific Advisory Board member of the PROPAG-AGEING EU Horizon 2020 project; H.H.D. reports US National Institutes of Health funding; N.Z. reports no disclosures; S.G. reports no disclosures; the Nestle Institute of Health Sciences employs J.D.; the Nestle Institute of Health Sciences employs A.B.; Nestle Research Center employs J.S.; C.H. reports no disclosures; T.B. reports no disclosures; S.A. reports grants from JPND program, EU-FP7 program, Beaufour Ipsen Pharma, personal fees from Beaufour Ipsen Pharma, Pierre Fabre, Lilly, Nestlé, Sanofi, Servier, MSD, and nonfinancial support from Biogen, Pfizer, Icon, grants from France Alzheimer Association, AMPA Association outside the submitted work; B.V. reports grants from Pierre Fabre, Avid, Exonhit, AbbVie, Lilly, Lundbeck, MSD, Otsuka, Regeneron, Sanofi, Roche, Astra Zeneca, LPG Systems, Nestlé, and Alzheon.
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.11.004.
Contributor Information
Gene L. Bowman, Email: drgenebowman@gmail.com.
MAPT/DSA Study Group:
Bruno Vellas, Sophie Guyonnet, Isabelle Carrié, Lauréane Brigitte, Catherine Faisant, Françoise Lala, Julien Delrieu, Hélène Villars, Emeline Combrouze, Carole Badufle, Audrey Zueras, Sandrine Andrieu, Christelle Cantet, Christophe Morin, Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland, Céline Caillaud, Pierre-Jean Ousset, and Françoise Lala
Supplementary data
References
- 1.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 2.Kang J.H., Cook N., Manson J., Buring J.E., Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 3.Kang J.H., Cook N., Manson J., Buring J.E., Albert C.M., Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88:1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aisen P.S., Schneider L.S., Sano M., Diaz-Arrastia R., van Dyck C.H., Weiner M.F. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durga J., van Boxtel M.P., Schouten E.G., Kok F.J., Jolles J., Katan M.B. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 7.Smith A.D., Smith S.M., de Jager C.A., Whitbread P., Johnston C., Agacinski G. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5:e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker J.G., Batterham P.J., Mackinnon A.J., Jorm A.F., Hickie I., Fenech M. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms––the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2012;95:194–203. doi: 10.3945/ajcn.110.007799. [DOI] [PubMed] [Google Scholar]
- 9.Oulhaj A., Jerneren F., Refsum H., Smith A.D., de Jager C.A. Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J Alzheimers Dis. 2016;50:547–557. doi: 10.3233/JAD-150777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diet and health: implications for reducing chronic disease risk. National Academies Press (US); 1989. Washington (DC); 1989. [PubMed] [Google Scholar]
- 12.Hurrell R.F., Lynch S., Bothwell T., Cori H., Glahn R., Hertrampf E. Enhancing the absorption of fortification iron. A SUSTAIN Task Force report. Int J Vitam Nutr Res. 2004;74:387–401. doi: 10.1024/0300-9831.74.6.387. [DOI] [PubMed] [Google Scholar]
- 13.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 14.Jerneren F., Elshorbagy A.K., Oulhaj A., Smith S.M., Refsum H., Smith A.D. Brain atrophy in cognitively impaired elderly: the importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015;102:215–221. doi: 10.3945/ajcn.114.103283. [DOI] [PubMed] [Google Scholar]
- 15.Bowman G.L., Silbert L.C., Howieson D., Dodge H.H., Traber M.G., Frei B. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–249. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amadieu C., Lefevre-Arbogast S., Delcourt C., Dartigues J.F., Helmer C., Feart C. Nutrient biomarker patterns and long-term risk of dementia in older adults. Alzheimers Dement. 2017;13:1125–1132. doi: 10.1016/j.jalz.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Zwilling C.E., Talukdar T., Zamroziewicz M.K., Barbey A.K. Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. Neuroimage. 2019;188:239–251. doi: 10.1016/j.neuroimage.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Bowman G.L., Silbert L.C., Dodge H.H., Lahna D., Hagen K., Murchison C.F. Randomized trial of marine n-3 polyunsaturated fatty acids for the prevention of cerebral small vessel disease and inflammation in aging (PUFA Trial): rationale, design and baseline results. Nutrients. 2019;11 doi: 10.3390/nu11040735. 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann M., Regland B., Blennow K., Gottfries C.G. Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16:145–150. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- 20.Bowman G.L., Dayon L., Kirkland R., Wojcik J., Peyratout G., Severin I.C. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14:1640–1650. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 21.Dayon L., Guiraud S.P., Corthesy J., Da Silva L., Migliavacca E., Tautvydaite D. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: homocysteine and beyond. Alzheimers Res Ther. 2017;9:43. doi: 10.1186/s13195-017-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith R.G., Hannon E., De Jager P.L., Chibnik L., Lott S.J., Condliffe D. Elevated DNA methylation across a 48-kb region spanning the HOXA gene cluster is associated with Alzheimer's disease neuropathology. Alzheimers Dement. 2018;14:1580–1588. doi: 10.1016/j.jalz.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briones T.L., Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation. 2012;9:244. doi: 10.1186/1742-2094-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nissou M.F., Guttin A., Zenga C., Berger F., Issartel J.P., Wion D. Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J Alzheimers Dis. 2014;42:789–799. doi: 10.3233/JAD-140411. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y.N., Ho Y.J., Lai C.C., Chiu C.T., Wang J.Y. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J Neuroinflammation. 2015;12:147. doi: 10.1186/s12974-015-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y.X., He L.Y., Zhang M., Wang F., Liu F., Peng W.X. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. doi: 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y., Li D., Zhao J., Song J., Zhao Y. The role of the low-density lipoprotein receptor-related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Rev Neurosci. 2016;27:623–634. doi: 10.1515/revneuro-2015-0069. [DOI] [PubMed] [Google Scholar]
- 28.Morello M., Landel V., Lacassagne E., Baranger K., Annweiler C., Feron F. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer's disease. Mol Neurobiol. 2018;55:6463–6479. doi: 10.1007/s12035-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vellas B., Carrie I., Gillette-Guyonnet S., Touchon J., Dantoine T., Dartigues J.F. MAPT study: a multidomain approach for preventing Alzheimer's disease: design and baseline data. J Prev Alzheimers Dis. 2014;1:13–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Rouch L., Cestac P., Cool C., Helmer C., Dartigues J.F., Berr C. Effectiveness of a standardized and specific follow-up in memory centers in patients with Alzheimer's disease. Curr Alzheimer Res. 2017;14:255–267. doi: 10.2174/1567205013666161108114850. [DOI] [PubMed] [Google Scholar]
- 31.Gillette-Guyonnet S., Andrieu S., Dantoine T., Dartigues J.F., Touchon J., Vellas B. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer's disease. Alzheimers Dement. 2009;5:114–121. doi: 10.1016/j.jalz.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Grober E., Buschke H., Crystal H., Bang S., Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Psychological Corp; New York: 1981. Wechsler Adult Intelligence Scale—Revised. [Google Scholar]
- 34.Cardebat D., Doyon B., Puel M., Goulet P., Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90:207–217. [PubMed] [Google Scholar]
- 35.Legrand P., Schmitt B., Mourot J., Catheline D., Chesneau G., Mireaux M. The consumption of food products from linseed-fed animals maintains erythrocyte omega-3 fatty acids in obese humans. Lipids. 2010;45:11–19. doi: 10.1007/s11745-009-3376-5. [DOI] [PubMed] [Google Scholar]
- 36.Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dou C., Xia D., Zhang L., Chen X., Flores P., Datta A. Development of a novel enzymatic cycling assay for total homocysteine. Clin Chem. 2005;51:1987–1989. doi: 10.1373/clinchem.2005.053421. [DOI] [PubMed] [Google Scholar]
- 38.Bowman G.L., Shannon J., Ho E., Traber M.G., Frei B., Oken B.S. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis Assoc Disord. 2011;25:49–57. doi: 10.1097/WAD.0b013e3181f333d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowman G.L., Dodge H.H., Mattek N., Barbey A.K., Silbert L.C., Shinto L. Plasma omega-3 PUFA and white matter mediated executive decline in older adults. Front Aging Neurosci. 2013;5:92. doi: 10.3389/fnagi.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flock M.R., Skulas-Ray A.C., Harris W.S., Etherton T.D., Fleming J.A., Kris-Etherton P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flock M.R., Harris W.S., Kris-Etherton P.M. Long-chain omega-3 fatty acids: time to establish a dietary reference intake. Nutr Rev. 2013;71:692–707. doi: 10.1111/nure.12071. [DOI] [PubMed] [Google Scholar]
- 42.Ross A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 43.Miller J.W., Harvey D.J., Beckett L.A., Green R., Farias S.T., Reed B.R. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol. 2015;72:1295–1303. doi: 10.1001/jamaneurol.2015.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwill A.M., Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc. 2017;65:2161–2168. doi: 10.1111/jgs.15012. [DOI] [PubMed] [Google Scholar]
- 45.Gao X., Yao M., McCrory M.A., Ma G., Li Y., Roberts S.B. Dietary pattern is associated with homocysteine and B vitamin status in an urban Chinese population. J Nutr. 2003;133:3636–3642. doi: 10.1093/jn/133.11.3636. [DOI] [PubMed] [Google Scholar]
- 46.Tan Z.S., Harris W.S., Beiser A.S., Au R., Himali J.J., Debette S. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hooper C., De Souto Barreto P., Coley N., Cantet C., Cesari M., Andrieu S. Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J Nutr Health Aging. 2017;21:988–993. doi: 10.1007/s12603-017-0957-5. [DOI] [PubMed] [Google Scholar]
- 48.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D'Agostino R.B. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 50.Kawakita E., Hashimoto M., Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto M., Hossain S., Shimada T., Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 52.Ma Q.L., Teter B., Ubeda O.J., Morihara T., Dhoot D., Nyby M.D. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer's disease (AD): relevance to AD prevention. J Neurosci. 2007;27:14299–14307. doi: 10.1523/JNEUROSCI.3593-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim G.P., Calon F., Morihara T., Yang F., Teter B., Ubeda O. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calon F., Lim G.P., Yang F., Morihara T., Teter B., Ubeda O. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moriguchi T., Greiner R.S., Salem N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y., Lu N., Chen D., Meng L., Zheng Y., Hui R. Effects of n-3 PUFA supplementation on plasma soluble adhesion molecules: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:972–980. doi: 10.3945/ajcn.111.025924. [DOI] [PubMed] [Google Scholar]
- 57.Bowman G.L., Dayon L., Severin I., Tautvydaite D., Henry H., Oikonomidi A. A neuroinflammatory biomarker signature of blood-brain barrier impairment in older adults. Alzheimers Dement. 2016;12:P670. [Google Scholar]
- 58.Tanprasertsuk J., Mohn E.S., Matthan N.R., Lichtenstein A.H., Barger K., Vishwanathan R. Serum carotenoids, tocopherols, total n-3 polyunsaturated fatty acids, and n-6/n-3 polyunsaturated fatty acid ratio reflect brain concentrations in a cohort of centenarians. J Gerontol A Biol Sci Med Sci. 2019;74:306–314. doi: 10.1093/gerona/gly125. [DOI] [PubMed] [Google Scholar]
- 59.Troen A.M., Shea-Budgell M., Shukitt-Hale B., Smith D.E., Selhub J., Rosenberg I.H. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008;105:12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai W.K., Kan M.Y. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 61.Selley M.L. A metabolic link between S-adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer's disease. Neurobiol Aging. 2007;28:1834–1839. doi: 10.1016/j.neurobiolaging.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Watkins S.M., Zhu X., Zeisel S.H. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr. 2003;133:3386–3391. doi: 10.1093/jn/133.11.3386. [DOI] [PubMed] [Google Scholar]
- 63.Yan J., Ginsberg S.D., Powers B., Alldred M.J., Saltzman A., Strupp B.J. Maternal choline supplementation programs greater activity of the phosphatidylethanolamine N-methyltransferase (PEMT) pathway in adult Ts65Dn trisomic mice. FASEB J. 2014;28:4312–4323. doi: 10.1096/fj.14-251736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Astarita G., Jung K.M., Berchtold N.C., Nguyen V.Q., Gillen D.L., Head E. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feart C., Helmer C., Merle B., Herrmann F.R., Annweiler C., Dartigues J.F. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement. 2017;13:1207–1216. doi: 10.1016/j.jalz.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Jacques P.F., Rosenberg I.H., Rogers G., Selhub J., Bowman B.A., Gunter E.W. Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 1999;69:482–489. doi: 10.1093/ajcn/69.3.482. [DOI] [PubMed] [Google Scholar]
- 67.Ganji V., Kafai M.R. Population reference values for plasma total homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr. 2006;84:989–994. doi: 10.1093/ajcn/84.5.989. [DOI] [PubMed] [Google Scholar]
- 68.Goodwill A.M., Campbell S., Simpson S., Jr., Bisignano M., Chiang C., Dennerstein L. Vitamin D status is associated with executive function a decade later: data from the Women's Healthy Ageing Project. Maturitas. 2018;107:56–62. doi: 10.1016/j.maturitas.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Heude B., Ducimetiere P., Berr C., Study E.V.A. Cognitive decline and fatty acid composition of erythrocyte membranes––the EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 70.Stark K.D., Van Elswyk M.E., Higgins M.R., Weatherford C.A., Salem N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016;63:132–152. doi: 10.1016/j.plipres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Smith A.D., Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211–239. doi: 10.1146/annurev-nutr-071715-050947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.