Abstract
In 1969, Denis Burkitt published an article titled “Related disease-related cause?”, which became the foundation for Burkitt’s hypothesis. Working in Uganda, he noted that middle-aged people (40–60 years old) had a much lower incidence of diseases that were common in similarly aged people living in England, including colon cancer, diverticulitis, appendicitis, hernias, varicose veins, diabetes, atherosclerosis, and asthma, all of which are associated with lifestyles commonly led in high-income countries (HICs; also known as western diseases). Following Cleave’s common cause hypothesis—which suggests that if a group of diseases occur together in the same population or individual, they are likely to have a common cause—Burkitt attributed these diseases to the small quantities of dietary fibre consumed in HICs due mainly to the over-processing of natural foods. Nowadays, dietary fibre intake in HICs is around 15 g/day (well below the amount of fibre Burkitt advocated of >50 g/day—which is associated with diets from rural, southern and eastern sub-Sahalean Africa). Since Burkitt’s death in 1993, his hypothesis has been verified and extended by large-scale epidemiological studies, which have reported that fibre deficiency increases the risk of colon, liver, and breast cancer and increases all cancer mortality and death from cardiovascular, infectious, and respiratory diseases, diabetes, and all non-cardiovascular, non-cancer causes. Furthermore, mechanistic studies have now provided molecular explanations for these associations, typified by the role of short-chain fatty acids, products of fibre fermentation in the colon, in suppressing colonic mucosal inflammation and carcinogenesis. Evidence suggests that short-chain fatty acids can affect the epigenome through metabolic regulatory receptors in distant organs, and that this can reduce obesity, diabetes, atherosclerosis, allergy, and cancer. Diseases associated with high-income lifestyles are the most serious threat to health in developed countries, and public and governmental awareness needs to be improved to urge an increase in intake of fibre-rich foods. This Viewpoint will summarise the evidence that suggests that increasing dietary fibre intake to 50 g/day is likely to increase lifespan, improve the quality of life during the added years, and substantially reduce health-care costs.
The fibre hypothesis
The Lancet took a leading role in the development and dissemination of the fibre hypothesis, which was subsequently named Burkitt’s hypothesis after its major protagonist, Denis Burkitt.1,2 Many others, including Cleave, Walker, Campbell, Trowell, Painter, and Cummings, contributed to its development between 1960 and 1989.3 One of the initiating factors behind the theory was Cleave’s recognition of the association between diets in high-income countries (HICs) and the development of diabetes, obesity, coronary heart disease, constipation, diverticulosis, and colon cancer (western diseases). Diets in HICs are characterised by increased consumption of meat, fat, and refined, fibre-deficient carbohydrates. Guided by the concept that “if a group of diseases occur together in the same population, or individual, they are likely to have a common cause”,4 Cleave proposed that the fundamental cause of high-income lifestyle-associated diseases was the consumption of high quantities of refined sugar, which was and indeed still is associated with lifestyles in HICs, describing the group of diseases as the saccharine diseases.4 These views were supported by Yudkin in his book (Pure, White, and Deadly—published in 1972), which warned that the consumption of sugar was dangerous to health, increasing the risk of dental caries, obesity, diabetes, and heart attack.5 However, Burkitt, Trowell, and Walker suggested that the cause of high-income lifestyle-associated diseases was the refinement of grains and the removal of fibre during that process, which has become much more commonplace in developed countries since the Industrial Revolution (starting with the first industrial revolution in 1760 in the UK).6 Burkitt argued that sugar was an unlikely cause of colon diseases because it was absorbed before it could reach the colon. The evolution of the fibre hypothesis was complex and hindered by difficulties in the definition of what, exactly, fibre consisted of. Terminology changed from agricultural terms (such as roughage, unrefined carbohydrate, and crude fibre) to the chemical definition of fibre as a dietary carbohydrate that was resistant to digestion by human small intestinal enzymes. As highlighted by Cummings,3 the establishment of the physiological basis for fibre in the prevention of colonic diseases and non-communicable diseases associated with lifestyles in HICs was largely a consequence of the collaborative efforts of Burkitt, Trowell, and Walker.
Burkitt was a remarkable scientist with many achievements. He was one of the first people to document the association between a virus and human cancer, namely the Epstein-Barr virus and Burkitt’s lymphoma, which followed a distinct geographical distribution in Uganda, Kenya, and parts of Tanzania. Ironically, despite spending a large portion of his working life in Uganda, his research had a greater effect on the health of populations from HICs. Working in rural Uganda he documented the associations between high fibre consumption (50–120 g/day) and high colonic transit, bulky stools, and a relative absence of diseases common in HICs, best exemplified by colon cancer.2,6 He also observed that this high-fibre diet was low in red meat and animal fat but high in starch and fibre-rich foods, such as colourful fruits and vegetables, leafy greens, tubers, potatoes, beans, nuts, and whole grains. He noted that the amount of fibre consumed by the average adult in rural Uganda was around 100 g/day compared with 15 g/day in Britian.7
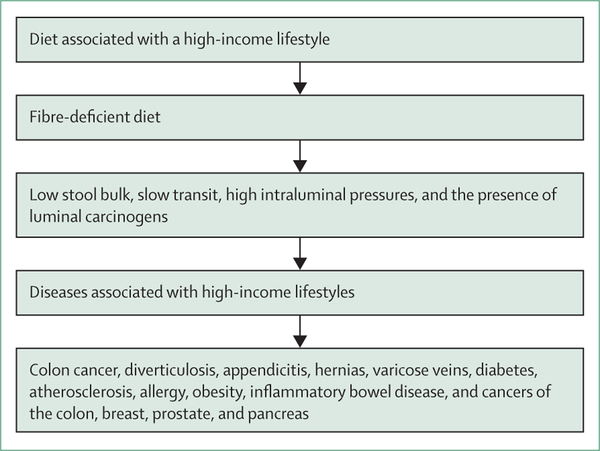
The establishment of the fibre hypothesis occurred from 1966–71 when Burkitt returned to England from Africa and was supported by the UK Medical Research Council to develop his theory. Burkitt was credited with the fibre hypothesis, despite the hypothesis being a synthesis of his experience and the research of others.3 Burkitt and Walker documented that larger, bulkier stools were passed more frequently and regularly by people living in sub-Saharan Africa, who were also noted to have shorter intestinal transit times—measured radiographically by lead pellet markers or by the passage of carmine dye.8 Burkitt and Walker proposed that the shorter transit time decreased the contact time between luminal carcinogens and the mucosa and reduced the need to strain when passing stools, avoiding excessive increase in intra-abdominal pressure. However, following the pioneering studies of Aries and colleagues,9 which showed differences in the culturable bacteria from faecal samples of English and Ugandan volunteers, Burkitt acknowledged the possibility that colonic flora (the microbiome) differences might also play a part in disease susceptibility. Burkitt proposed that the changes in stool bulk and content, bacterial flora, total transit time, and intraluminal pressures as a result of the fibre deficient HIC-diet explained the high risk of colon cancer, diverticulitis, appendicitis, hernias, varicose veins, diabetes, and atherosclerosis (figure 1).7 The contributions of Hugh Trowell are important to emphasise. Trowell was Burkitt’s senior physician colleague in Mulago hospital, Kampala, Uganda, and an acknowledged expert in protein calorie malnutrition. Influenced by Burkitt, Trowel began his own investigations into the rarity of non-infective bowel diseases in eastern sub-Sahelian Africa and became a strong advocate of the fibre hypothesis. Trowell helped Burkitt expand the fibre hypothesis to include extra-colonic diseases, specifically type 2 diabetes, cardiovascular disease, and obesity, which culminated in their joint publication of the landmark book: Refined Carbohydrate, Foods and Disease: Some Implications of Dietary Fibre—published in 1975.7
Figure 1:
Burkitt’s hypothesis
50 years on, fibre intake in HICs remains well below the greater than 50 g/day advocated by Burkitt, which is of grave concern; moreover, the number of disease cases are increasing in HICs, and, with the spread of HIC-associated diets, these diseases are making an appearance in middle-income and low-income countries around the world, including African countries (eg, Zimbabwe).10 In the UK, the average fibre intake is about 18 g/day11 and in the USA the average intake is 16 g/day.12 So, why has progress been so slow? The simple answer is that by producing and advertising tasty, low-cost, fibre-deficient fast-foods the food industry is doing a better job at influencing attitudes than health-care professionals are. Education, food security and a move towards a more plant-based diet could increase the amount of natural fibre consumed.
Concern is also growing that fibre intake recommendations are about half what they should be. The UK’s National Health Service recommendations of 30 g/day and US Department of Agriculture (USDA) recommendations of 22 g/day for women and 38 g/day for men, are well below Burkitt’s 50 g/day recommendation. In a review of fibre intake recommendations, published in 2017, across 24 European countries, the USA, and Australia and New Zealand by Stephen and colleagues,13 only the recommendations in the Netherlands came close (32–45 g/day fibre) to the proposed 50 g/day. The discrepancy can be explained by the fact that requirements in the UK were first calculated from the quantity of fibre needed to prevent constipation, but those accepted by the USDA were based on the quantity of fibre needed to prevent cardiovascular disease. At the time the guidelines were developed, the high metabolic requirements of the colonic microbiota were unappreciated. Furthermore, diseases associated with high-income lifestyles primarily affect older people, and because HIC populations are ageing, the proportion of the population at risk of the diseases is expanding. Moreover, the quality of the extended life is frequently marred by such diseases. Diseases associated with high-income lifestyles now pose the major threat to health care in the USA.
To verify Burkitt’s hypothesis and assess the optimal needs for fibre, this Viewpoint will focus on the epidemiological, human intervention, and mechanistic evidence available.
This Viewpoint will provide evidence that the current recommendations for fibre consumption are insufficient to maintain colonic health and prevent the development of diseases associated with high-income lifestyles and suggest that the recommended amount of fibre consumed daily should be closer to 50 g, as noted by Burkitt. As this is a large body of evidence, this Viewpoint will focus on robust publications to conclude with practical guidelines for better eating.
What is fibre?
Some confusion arises in the interpretation of data from dietary studies because fibre is not a specific molecule. Rather, fibre is a complex mixture of dietary residues, chiefly carbohydrates, that are not digested or absorbed by the human small intestine but are used by the colonic microbiota and are associated with health benefits. The review by Stephen and colleagues13 summarises the generally acceptable definition of fibre to include carbohydrate polymers with three or more monomeric units that are neither digested nor absorbed in the human small intestine. This includes non-starch polysaccharides from fruits and vegetables, non-digestible oligosaccharides, and resistant starch (panel). The definition usually includes associated non-carbohydrate substances, such as lignin, and cell wall components linked to polysaccharides. The definition also includes a need for any potential fibre substance to show health benefits from the polymer.
Panel: Examples of potential fibre sources13.
Non-starch polysaccharides
Cellulose
Hemicellulose
Pectin
Gums
Mucilages
Non-digestible oligosaccharides
Inulin
Fructo-oligosaccharies
Galacto-oligosaccharides
Resistant starches
Physically trapped
Resistant granules
Retrograded
The measurement of fibre content in the diet creates further challenges. The most common method is to use food composition tables, which in the UK are based on the chemical analysis of 3302 common foods.14 This approach is reasonable for assessing the content in high-fibre foods, but it does not make allowances for changes in fibre content due to cooking and preparation. An example of this is the serious underestimation of the total fibre content in cooked maize meals, which becomes enriched with resistant starch (which cannot be digested by human digestion enzymes) after cooking and reheating.15 In research studies, biochemical analysis is used where the food is incubated with digestive, pancreatic enzymes to remove the digestible complex carbohydrates and what is left is measured. This approach was developed by Southgate,16 and modified by Englyst and colleagues;17 it was extended in 2012 by McCleary’s consortium to measure all components of dietary fibre currently defined by CODEX Alimentarius.18
Fibre requirements
Developments over the past few years in high-throughput technologies have revealed that the colonic microbiota is one of the most highly metabolically active parts of the body: estimates suggest that their metabolic rate rivals that of the liver at 250–300 kcal/day.19 This caloric rate would represent the energy contained in 60–70 g of colonic carbohydrate and protein residues. However, metabolic rates are substrate dependent, and colonic energy salvage from undigested food in patients with massive small intestine losses has been estimated to increase to up to 250 g/day compared with people with complete small intestines.20,21
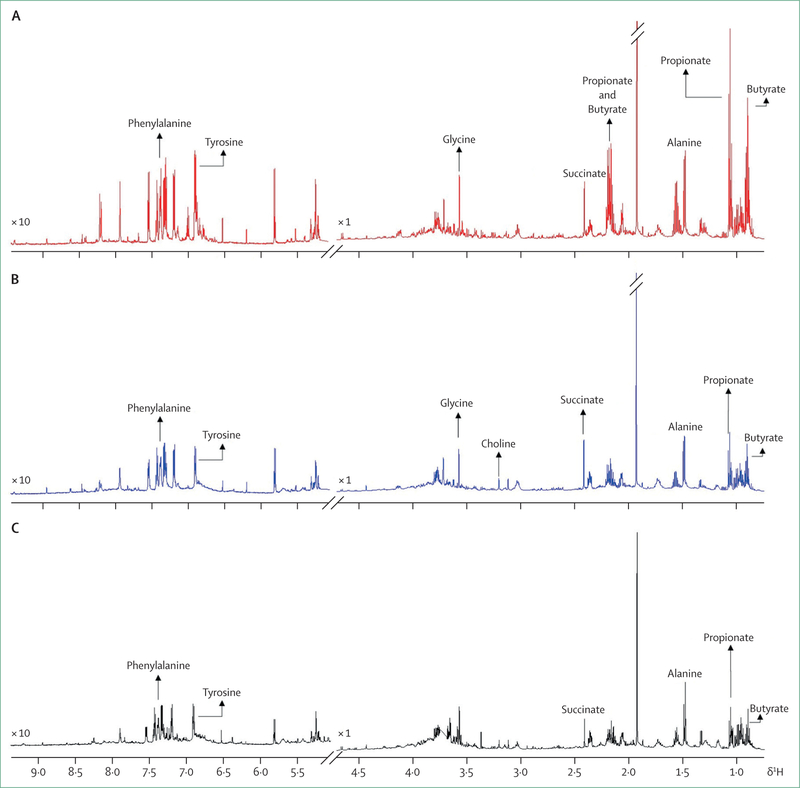
Figure 2 shows 600 MHz 1H nuclear magnetic resonance NMR spectra of faecal water extracts from three populations matched for age, sex, and weight, at variable risk of developing colon cancer: middle-aged men from rural KwaZulu-Natal, South Africa, where the incidence of colon cancer is low (<5 cases per 100 000 people per year), middle-aged African American men from Pittsburgh, PA, USA, which is a group with the highest risk of colon cancer in the continental USA (excluding Alaska Natives) (incidence about 55 cases per 100 000 people per year), and Alaska Native men, a population with the greatest reported risk of developing colon cancer in the world (incidence about 100 cases per 100 000 people per year).22 As expected, studies have shown that saccharolytic fermentation products, measured in the colon, the short-chain fatty acids acetate, propionate, and butyrate, are substantially higher in people from rural South Africa, but, perhaps surprisingly, so are proteolytic fermentation products (eg, high phenylalanine and tyrosine), possibly because of proteolytic fermentation of desquamated cells and endogenous mucoproteins.22 These metabolic and colonic functional differences raise the intriguing possibility that microbial mass and activity might be protective against colon cancer and support efforts to increase the intake of fibre-rich foods in Alaska Native groups to increase colonic metabolic activity and suppress the high risk of colon cancer. This suggestion is supported by mechanistic studies, which indicate that high fibre intake is required to provide sufficient quantities of short chain fatty acids to suppress colonic carcinogenesis through the suppression of energy balance and epigenetic inflammatory and proliferative functions.
Figure 2: Respresentative 600 MHz 1H nuclear magnetic resonance spectra of faecal water extracts from groups of middle-aged men from rural KwaZulu-Natal, South Africa (A), African Americans from Pittsburgh, PA, USA (B), and Alaskan Native people from Anchorage, AK, USA (C).
The horizontal axis is chemical shift of proton resonances in ppm (parts per million), while the vertical axis is intensity (arbitrary unit). The spectral regions of 5–9 5 ppm are magnified 10-times to better visualise the signals. Unpublished data from African, African-American, and Alaskan studies, analysed by Jia Li, Imperial College, London.
Epidemiological and observational studies
Colon cancer, all cancer, and all cause mortality
Since Burkitt died in 1993, evidence supporting the protective effects of a fibre-rich diet against colon cancer has increased dramatically. The 2010 Continuous Update Report from the World Cancer Research Fund systematic review and meta-analysis of 43 cohort or randomised controlled trials graded the evidence linking high dietary fibre with a decreased risk of colorectal cancer as convincing—the strongest grade assignable.23 From this database, a 10% increase in fibre consumption was estimated to confer a 10% reduction in cancer risk,24 which is lower than that calculated by Bingham of a 40% reduction in risk by doubling fibre intake in low intake populations.25 The Bingham estimate25 was based on data from the European Prospective Investigation into Cancer and Nutrition Study (EPIC), which included 519 978 individuals recruited from ten European countries. In support of the common cause hypothesis, a high-fibre diet has also been found to be associated with a lower risk of breast,26 liver,27 all cancer mortality,28–30 and death from other high-income lifestyle associated diseases (specifically cardiovascular, infectious, and respiratory diseases,29 diabetes, and all non-cardiovascular non-cancer30) in multinational studies. Finally, in 2019, Reynolds and colleagues31 published a systematic review and meta-analysis based on nearly 135 million person-years of data from 185 prospective studies and 58 clinical trials with 4635 adult participants that suggested a 15–30% decrease in all-cause and cardiovascular-related mortality, and incidence of coronary heart disease, stroke incidence and mortality, type 2 diabetes incidence and mortality, and colorectal cancer incidence and mortality when comparing the highest dietary fibre consumers with the lowest.31 Furthermore, they noted that fibre intakes higher than 35 g/day appeared to be even more effective than lower intakes in reducing risk cardiovascular diseases, type 2 diabetes, and colorectal, and breast cancer.
In summary, good evidence exists from epidemiological studies that suggests that high dietary fibre might not only reduce colon cancer risk and deaths, but also all-cancer deaths, and all-cause mortality, thus increasing lifespan. The reduced effect of other diseases associated with high-income lifestyles can then be expected to improve the quality of life gained.
Obesity and type 2 diabetes
Numerous studies in adults and children have now confirmed the association between low fibre and high glycaemic index diets with type 2 diabetes and obesity.32–35 Using cross-sectional weighted data from the National Health and Nutrition Examination Survey (NHANES) among adults, King and colleagues36 reported that obese participants consistently reported lower fibre intake than did individuals with a healthy weight (14·6–15·4 g/day fibre) or overweight (15·6–16·8 g/day fibre) participants. Using the same database, Albertson and colleagues37 found that high grain consumption was associated with lower bodyweights in both adults and children. Further examination of this data showed that grain consumption was strongly associated with total fibre consumption.38 In a meta-analysis of prospective studies from the EPIC-InterAct consortium, Kuijsten and colleagues39 confirmed the association between high fibre intake and low risk of type 2 diabetes. Similar inverse associations were observed for the intake of cereal and vegetable fibre, but not fruit fibre. It is of note that the associations were attenuated and no longer statistically significant after adjustment for body-mass index (BMI), indicating that the association might be explained by excess bodyweight.
Cardiovascular disease
Based on the position statement from the American Dietetic Association, Slavin concluded that AI level evidence existed to support an intake of 14 g fibre per 1000 kcal of food protects against cardiovascular disease.34 Although this finding supports the US DA requirement levels, evidence suggests that higher fibre consumption is better and associated with lower mortality. These data, based on 24 h diet questionnaires and medical histories from 9776 adults, came from NHANES. Further analyses from NHANES showed that the association was stronger for cereal fibre,40 a finding confirmed by Hajishafiee and colleagues41 in their systematic review and meta-analysis of 14 prospective cohort studies.
Allergy
Allergy, including asthma, rhinoconjuctivitis, and eczema, is a major HIC-lifestyle associated disease, affecting more than 50 million people in the USA. Concern exists that the incidence of allergic conditions is beginning to increase in less developed parts of the world.42 The epidemiological evidence connecting fibre consumption with allergy prevention is not definitive, probably because, unlike the other HIC-lifestyle-associated diseases, its incidence is highest in the young and infants, who do not usually eat fibre-rich foods. However, allergy fits into the fibre hypothesis because of the remarkable health benefits of breastfeeding. Human breast milk contains high quantities (about 10 g/L) of soluble fibre in the form of oligosaccharides: human breast milk contains more oligosaccharides, with greater oligosaccharides diversity (>200 types), than the milk of any other mammal. This might well explain the benefits of breastfeeding because it provides the main stimulus to the rapidly developing gut immune system. The study of infantile eczema reported by Saarinen and Kajosaali43 showed that breastfeeding was prophylactic against atopic disease—including atopic eczema, food allergy, and respiratory allergy—throughout childhood and adolescence.43 A comprehensive meta-analysis of 117 studies concluded that breastfeeding was protective in reducing the risk of childhood asthma and wheezing, with the strongest association in infants (aged 0–2 years).44 With regard to adults, evidence exists that suggests high fibre consumption can improve lung function in patients with diseases such as asthma and chronic obstructive pulmonary disease.45
Inflammatory bowel diseases
Crohn’s disease and ulcerative colitis are also associated with high-income lifestyles. Their incidence increased dramatically in North America and Europe during the second half of the 20th century. Although rare in less developed countries, concern exists that these diseases are becoming more common in more developed parts of Africa, including South Africa.46 Like colon cancer, diabetes, and cardiovascular disease, Crohn’s disease and ulcerative colitis are classic complex diseases generated by a combination of factors in the luminal micro-environment and genetic aberrations in epithelial responses. As reviewed by Rasmussen and Hamaker,46 numerous studies have documented low consumption of fibre-rich foods by patients with inflammatory bowel diseases (IBD), with other studies identifying common patterns of colonic microbial dysbiosis, or signatures, characterised by depletions of high butyrate producing microbes.
Human intervention studies
Randomised controlled trials
The confounding effects of other nutrients contained within a fibre-rich diet can be minimised by examining the effects of fibre supplementation alone in randomised controlled trials. Below this Viewpoint summarise the results of some of the more robust randomised controlled trials in colonic and extracolonic diseases (eg, colon cancer, cardiovascular disease, obesity, and diabetes) examining supplementation of the diet with either fibre or fibre rich foods.
Colon cancer
Most studies of fibre supplementation have been not been successful as exemplified by the 2017 Cochrane meta-analysis performed by Yao and colleagues,47 which found a small amount of evidence from randomised controlled trials ranging in duration from 2 years to 8 years for fibre supplementation to prevent adenomatous polyp recurrence. Yao and colleagues47 offered the caveat that this conclusion might be incorrect because polyps might not reflect cancer development and that longer periods of intervention would be needed for confirmation. However, the most probable explanation was that insufficient fibre was consumed to generate sufficient butyrogenesis to suppress neoplastic transformation. Importantly, as of yet no long-term studies have reported the effects of 50 g/day fibre supplementation, but we are conducting such a study using resistant starch in Alaska where tolerance to the fibre supplementation has thus far been good (NCT03028831). The Cochrane analysis was skewed by large studies, such as the Polyp Prevention Study, which randomly assigned 2079 participants with a history of polyps to receive either an advised low-fat, high-fibre diet or a standard brochure on healthy eating and assigned to follow their usual diet and found no overall difference in polyp recurrence after 4 years and 8 years between the two groups.48 However, the high-fibre group were estimated to have consumed an average of only 32 g fibre a day, and it is probable that compliance was low because biomarkers of fat (blood cholesterol) and green vegetable (vitamin A) intake were unchanged by the intervention. Most importantly, a statistical and clinical significant reduction in advanced (>1 cm, >25% villous, or high-grade dysplasia) adenomatous polyp recurrence was found in the subgroup consuming the highest quartile of high-fibre beans.49 The other large-scale clinical trials from Phoenix, Arizona, USA (13·5 g wheat bran fibre supplement),50 Europe (3·5 g ispaghula husk supplement),51 and Australia (35 g wheat bran supplement),52 were all unable to increase fibre intake to Burkitt’s 50 g/day recommendation. Many of them were randomly assigned to recieve other dietary supplements taken concurrently (eg, vitamin A) that might have confused the outcomes and conclusions. However, it is of note that the Australian study found that those given a wheat bran supplement with a low-fat diet had no large polyps (≥10 mm) detected at 24 months and 48 months (p=0·03 compared with the number of polyps less than 10 mm in size identified), leading to the conclusion that dietary modification could suppress large polyp formation. This is important because the malignant potential of large polyps is greater.52
Resistant starch, one of the insoluble fibres, has been used in many intervention studies because it is easy to consume as a drink and has been shown in controlled human studies to be strongly butyrogenic,53 and to suppress secondary bile acid production,54 proteolytic fermentation,55 and epithelial proliferation.54 Unfortunately, large-scale multicentre studies, mainly in the so-called genetic colon cancers (Lynch syndrome), have been equally unsuccessful in the prevention of polyp reccurrance, but again the supplement only provided an extra 9 g/day of fibre.56 By contrast, the dietary switch study,22 which recruited African Americans at a high risk of colon cancer, showed that an increase in fibre consumption to 50 g/day under strictly supervised conditions (ie, consumption was observed, not assumed), was associated with profound changes in microbiota and fermentation products (short-chain fatty acids and phytochemicals), accompanied by suppression of cancer risk biomarkers in the colonic mucosa within 2 weeks.22 This high fibre intake was principally the result of a high bean diet given to the participants. Additionally, Humphreys and colleagues’57 study of healthy, middle-aged (45–65 years old) Australians that showed suppression of oncogenes and mucosal proliferation markers associated with cancer risk when 40 g of butyrylated high-amylose maize starch was added daily to a high meat diet for 4 weeks. A common picture to emerge from the review of epidemiological, interventional, and mechanistic studies is that because of the complex interactions between inflammatory and anti-inflammatory foods and their metabolites, high quantities of fibre-rich foods or fibre supplements might need to be given to prevent colon cancer. The evidence points to a target of 50 g/day, or 0·7 g/kg per day, of fibre as originally advocated by Burkitt. The relative ability of different fibre sources to suppress cancer is difficult to assess. Good experimental evidence exists that shows that different fibres differ in the amount of butyrate produced and site of fermentation in the colon,58 and so weight is not the only factor. In general, the associations in observational studies were stronger for whole grains, such as oats, rye, and wheat.24 However, high butyrogenesis from a fibre source are needed to not only satisfy colonic mucosal requirements, but also systemic epigenetic regulators and free fatty acid receptors.
Type 2 diabetes and obesity
Although strong inverse associations exist between fibre intake and type 2 diabetes, conflicting evidence exists regarding the ability of fibre supplements to reverse the disease, which could be a dose-dependent effect. In a 2-year study from Germany, supplementation with 15 g insoluble fibre per day had no effect on glucose tolerance, but reduced the number of diabetes cases and HbA1c concentrations.
Other intervention studies with higher supplementation quantities have been more successful at reducing diabetes associated metabolic abnormalities. Chandalia and colleagues59 reported the results of their 6-week randomised cross-over study of a diet containing the locally recommended amount of fibre (24 g/day) compared with a diet containing 50 g/day fibre, with the fibre coming from naturally high-fibre foods in both groups. The 50 g/day fibre diet was both clinically and statistically significantly more effective at reducing plasma glucose concentrations, daily urinary glucose excretion, and at the same time lowered the area under the curve for 24 h plasma glucose and insulin concentrations. Furthermore, the high-fibre diet decreased blood cholesterol, triglyceride, and very-low-density lipoprotein cholesterol concentrations. Similar findings were reported in a randomised clinical study from China by Zhao and colleagues,60 which 43 patients followed for 84 days. Interpretation of the study by Zhao is difficult because the attribution of all the observed changes to fibre is clouded by the unusual combination of whole grains, traditional Chinese medicinal foods and prebiotics, and acarbose, a drug that blocks amylase and produces carbohydrate malabsorption. Dietary assessment suggested the supplements added 37 g of fibre to the participants usual diet, which previously consisted of 16 g of fibre a day. The differences in outcome after 3 months were impressive. HbA1c concentration, the primary outcome measure, decreased significantly (p<0·001) from baseline in a time-dependent manner in both groups; from day 28 onward. However, a greater reduction was noted in the high fibre group (p<0·05). The proportion of participants who achieved adequate glycemic control (HbA1c <7%) at the end of the intervention was also significantly higher in the high fiber group (89% vs 50% in the control group; p<0·005). Another report from Italy, which randomly assigned 56 patients with type 2 diabetes to receive either a high-fibre (the Ma-Pi 2) diet consisting of whole grains, vegetables, and legumes providing 29 g fibre/1000 kcal (estimated total 65 g/day) or a standard type 2 diabetes diet, with only 10 g fibre/1000 kcal, both prepared and given in an in-patient setting for 3 weeks. The high-fibre group showed statistically or clinically significantly greater reductions in fasting and postprandial blood glucoses, HbA1c, and lipids concentrations, and greater weight loss.61 These positive findings were not observed in other fibre supplementation studies where the intake of dietary fibre was increased by only 16 g/1000 kcal through the consumption of foods prepared in a research kitchen62 or by 14 g/day through dietary instruction.63
In 2011, Wanders and colleagues64 performed a systematic review of 102 randomised controlled trials that concluded that viscous (soluble) fibre had the most profound effect on appetite suppression.64 More recently, Thompson and colleagues65 reported their meta-analysis of 12 suitable randomised controlled trials containing 609 obese or overweight participants studied from 2 weeks to 17 weeks duration, with supplementation with a wide range of soluble fibre products, providing 3–35 g per day. Despite these profound variations in study designs, soluble fibre supplementation statistically and clinically significantly reduced BMI, body weight, body fat, fasting glucose, and insulin compared with placebo treatments. Finally, a randomised controlled trial that recruited healthy volunteers, showed that dietary supplementation with 40 g resistant starch daily led to statistically significant decreases in visceral and subcutaneous fat.66 These changes were associated with increased faecal acetate and early-phase insulin, C-peptide, and glucagon-like peptide-1 (GLP-1) secretion.
Cardiovascular diseases
An abundance of evidence from randomised controlled trials exists that suggests that increasing fibre intake can reduce systolic and diastolic blood pressure; however, the reduction is small.67 Threapleton and colleagues68 reported their meta-analysis of 24 randomised clinical trials in which they tried to differentiate the effects of soluble and insoluble fibre. They confirmed that higher consumption of fibre, insoluble fibre, and cereal-vegetable fibre, was associated with a reduction in risk of cardiovascular and coronary heart disease.69
Allergy
Great efforts have been made to humanise commercial infant milk formulae to gain some of the advantages of sustained breastfeeding. Disappointingly, large reviews and meta-analyses have not revealed sufficient evidence to recommend the addition of probiotics to milk formulae for the prevention of allergic disease or food hyper-sensitivity.69–71 However, more advanced products, such as galactooligosaccharide-polydextrose-enriched formula, were shown to protect against respiratory infections,72 possibly through their more sustained effects on colonic short-chain fatty acids production.
Inflammatory bowel disease
The association between IBD and microbiota dysbiosis has driven has driven efforts to restore the microbiota to a healthy status with fibre supplementation in patients with IBD in the hope of suppressing disease activity. The results have been variable and often disappointing, probably due to inconsistencies in study designs, and that, once again, high fibre supplementation has never been achieved. For example a maximum supplement of 15g per day was provided in the studies reviewed by Rasmussen and Hamaker46 in the form of fructoseoligosaccharides and inulin. Tolerance to nutritional supplements might also be lower because of the chronic inflammatory state and incomplete fermentation in patients with IBD.73 These findings suggest that a high-fibre diet might only be more effective in preventing rather than treating IBD.
Mechanistic studies
This Viewpoint has discussed the major advances in epidemiological and human intervention studies, which have supported Burkitt’s hypothesis. To examine the underlying mechanisms, researchers invariably depend upon the use of animal models, which might not represent the human condition. But few alternatives to animal models exist because these diseases take years to develop in humans and tissue sampling might infeasible. Consequently, studies need to be put into perspective with an orderly process of investigation starting with the human disease and ending with in-vivo models in animals or in-vitro molecular interaction investigations. Most of the biological control mechanisms in humans are shared by mammals, and the information revealed by in-vivo animal studies can certainly help determine whether epidemiological associations are likely to be cause or effect.
Short-chain fatty acids and high-income lifestyle-associated diseases
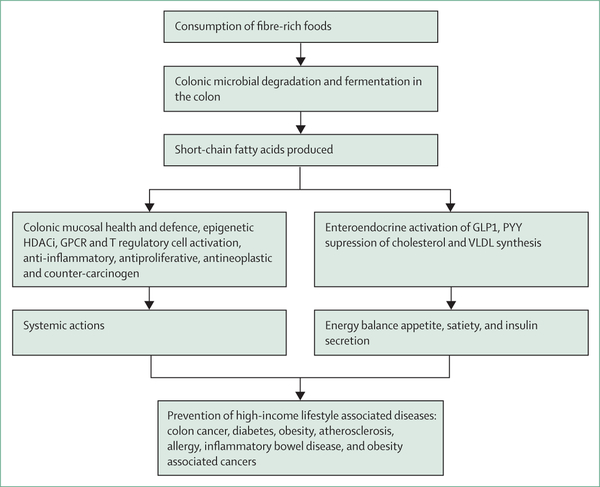
Fibre can promote colonic and whole-body health through its effects on gut transit, microbiome composition, and the microbial production of short-chain fatty acids (figure 3). The evidence connecting any specific microbe to colonic carcinogenesis is weak, but the microbiome’s ability to produce metabolites that influence carcinogenesis is strong. Perhaps the best example of mutualism in human physiology is that although all other body cells rely on glucose as their primary energy source, the colonic epithelium is unique in preferring one of the microbiota-produced short-chain fatty acids, butyrate.74 Early cultural and molecular studies showed that the most prodigious butyrate-producing bacteria belong to the Clostridium clusters IV and XlVa, notably Eubacterium rectale, Roseburia spp, and Faecalibacterium prausnitzii.75 Although a deficiency of these microbes is strongly linked with high-income lifestyle associated diseases, the evidence suggests that it is the butyrate that they produce, rather than their function, that accounts for their role in health. Butyrate inhibits colonic neoplastic transformation and progression through a number of divergent mechanisms.76 Acting through at least two pathways, short-chain fatty acids also play a pivotal role in extra-colonic energy homoeostasis, and the suppression of systemic inflammation and neoplasia.
Figure 3: Illustration of some of the major mechanisms whereby a high-fibre diet can prevent diseases associated with high-income lifestyles.
HDACi=histone deacetylase inhibitors. GPCR=G protein-coupled receptor. GLP-1=glucagon-like peptide-1. PYY=peptide YY. VLDL=very-low-density lipoprotein.
The first pathway, is the selective binding of short-chain fatty acids to mucosal G-protein coupled receptors (GPCR), alternatively known as free fatty acid receptors. In the colon, GCPRs activate regulatory T cells and promote FOXP3 and IL-10 expression, augmenting their antiproliferative functions.77 However, distal functions are generated through GCPR stimulation of secretion of gut peptides in the distal bowel. Specifically, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) are released from enteroendocrine cells,78 which enter the bloodstream and affect extracolonic organs, such as the pancreas to induce insulin secretion and the brain to promote satiety and reduce food intake.79,80 Experimentally, acetate has a direct effect on appetite regulation.81 Studies have shown the potential for high-fibre foods to reduce caloric intake and treat type 2 diabetes.60 The ability of these gut peptides to affect brain function might also explain the reports of improvement in mood, stress, anxiety, and cognitive ability associated with butyrogenic foods.82 The ability of these gut peptides to affect brain function might also explain the reports of improvement in mood, stress, anxiety, and cognitive ability associated with butyrogenic foods.83,84 This action might also support the suggestion that high-fibre foods might improve brain health and suppress autism and inflammatory diseases, such as Alzheimer’s and Parkinson’s disease.85
Alternatively, butyrate might affect distant organ function through its epigenetic role as a histone deacetylase inhibitor (HDACi) following its metabolism to acetylcholine A, which alters the expression of a wide variety of genes, some of which regulate inflammation, cell proliferation, apoptosis, and differentiation, mechanisms that are axiomatic to neoplastic transformation. The overexpression of histone deacetylase has been found in several types of cancer cells and inflammatory pathologies.86 However, strong experimental evidence exists that shows that butyrate’s HDACi properties only become active when a threshold microbial production rate is exceeded.87 This activation threshold might explain the conclusion reached by Reynold and colleagues31 that the benefits of fibre are strongest in those with high-fibre diets. High-fibre diets also increase blood concentrations of short-chain fatty acids, exposing the rest of the body to butyrate’s tumour suppressor functions.
Short-chain fatty acids also account for the protective effect of fibre in cardiovascular diseases, but through additional pathways. Because each of the three major short-chain fatty acids are produced in different quantities by the microbiota during fermentation (molar ratios of 57 acetate:22 propionate:21 butyrate),88 and because much of the butyrate production is consumed by the mucosa, variable quantities of the short-chain fatty acids will enter the bloodstream (ratio 71 acetate:21 propionate:8 butyrate) to affect lipoprotein metabolism. Acetate on its own could exacerbate hypercholesterolaemia because it is a substrate for cholesterol synthesis in the liver through acetyl-CoA. However, propionate has been shown to reduce plasma cholesterol concentrations in rodents and humans by inhibiting de-novo synthesis of cholesterol.89 Using an apolipoprotein E-deficient (apoE−/−) mouse model, Chen and colleagues90 showed that the butyrate generated from the fermentation of pectin reduced the rate of progression of atherosclerosis. A wide range of experimental studies have produced evidence that butyrate and propionate can suppress cholesterol and high-density lipoprotein metabolism through a variety of mechanisms including direct inhibition of synthesis or indirect inhibition of absorption,90 and increased bile acid secretion.91
Fibre could also reduce the risks of cardiovascular events by the systemic anti-inflammatory actions of butyrate if high quantities are consumed. Specific microbes might also play a role in the the prevention of atherosclerosis. Kasahara and colleagues92 showed that Roseburia intestinalis, a key butyrate producer, was inversely associated with atherosclerosis in a genetically diverse mouse population. Examining mechanisms in germ-free apoprotein-E deficient mice, Kasahara found evidence for this microbe’s ability to change metabolism towards an increase in fatty acid clearance in association with a reduction in systemic inflammation and atherosclerosis.
A substantial amount of evidence suggests that short-chain fatty acids affect systemic metabolism and energy balance through both GPCR activation and HDACi regulation.78,80,93,94 The consequent release of GLP-1 and PYY increase pancreatic insulin release and suppress energy intake through hypothalamic mechanisms.95 Parallel actions have been shown in mice with high blood acetate concentrations, in which acetate has been shown to cross the blood brain barrier and directly suppress appetite through central hypothalamic mechanisms involving changes in transcellular neuro-transmitter cycles.81 Confirmation that these mechanisms are active in humans was given by the study by Zhao and colleagues,60 in which many of the biochemical and dysbiotic abnormalities associated with type 2 diabetes were reversed by a high-fibre diet in association with increases in faecal butyrate and serum GLP1 and PYY. Recent microbiome-metagenome studies have identified signatures predictive of type 2 diabetes,96 characterised by low abundances of high butyrate producers. Finally, a paper, published in 2019, used bidirectional Mendelian randomisation to assert cause between gut microbial butyrate production and improved insulin response to an oral glucose-tolerance test.97
The mechanistic explanation for the ability of breast milk to prevent childhood allergy is likely to be related to the wide range of bioactive nutrients it contains, including immunoglobulins, cytokines, bacteria and their metabolites, and oligosaccharides (HMO). Although many of the components can produce immediate anti-inflammatory effects, oligosaccharides might have the unique benefit of conferring long-term tolerance through their immunomodulatory effects if consumed during the critical time of neonatal development. HMO are a form of fibre that are strongly bifidogenic and promote colonic fermentation and short-chain fatty acids production. The allergic airways response to house mite antigen was suppressed in mice by increasing the fibre content of their diet, thereby increasing Bifidobacteria and serum short-chain fatty acids.98 Additionally, propionate supplementation was shown to increase seeding in the lungs with dendritic cells of high phagocytic capacity, but impaired T helper type 2 cell allergic airway inflammation, a mechanism that was shown to be GPCR 41 dependent. Additional evidence suggests that in mice the consumption of a high-fibre diet in pregnancy might influence the development of allergy in offspring. Finally, maternal acetate generated from a high-fibre diet was shown to regulate gene expression in fetal lungs through inhibition of histone deacetylase 9. This epigenetic modification was then shown to protect offspring against the development of allergic airway disease, a model for human asthma.99 Children and adults with asthma might behave in a similar way as reported by Halnes and colleagues,100 who gave patients a high-fibre meal and noted decreased levels of several airway inflammation biomarkers 4 h after the challenge, including exhaled nitric oxide, sputum total cell, neutrophil, lymphocyte, and macrophage counts as well as sputum IL-8 protein concentration. Intriguingly, these changes correlated with increased expression of GPR41 and GPR43 in the sputum of these patients, suggesting the mechanistic basis for these beneficial changes.
Evidence also suggests that fibre fermentation products might also prevent the development of type 1 diabetes.101 Both animal and human studies have shown an association between intestinal microbiota composition, short-chain fatty acids production, and type 1 diabetes.102 The observation that the development of autoantibodies and reduced faecal and blood short-chain fatty acids preceded the expression of the disease in early life while the gut immune system was developing suggests the association might be causative.103
Finally, the suppressive effect of fibre on IBD might also involve the activation of GPCRs by short-chain fatty acids. In the dextran sulfate sodium-mouse model of ulcerative colitis, short-chain fatty acid generation from dietary fibre interacted with GPCR43 to profoundly suppress the inflammatory response, an action that was annulled in a Gpr43−/− knockout germ-free model.104 In a mouse model study of colitis, Macia and colleagues105 showed that a high-fibre diet (136 g fibre per 1000 kcal), increased short-chain fatty acids binding to GPCR43 on colonic epithelial cells and stimulated potassium efflux with hyperpolarisation, which led to NOD-like receptor protein 3 inflammasome activation, mediated by IL-18 release.105 In a GPCR 43 dependent manner, short-chain fatty acids have also been shown to regulate the size and function of the colonic T regulatory cell pool and protect against colitis in mice.77
Conclusion
One consistent observation from this Viewpoint is that higher intakes of fibre than those recommended and consumed today are needed to satisfy the needs of the human microbiome to maintain colon and whole-body health, and thus prevent the progression of disease associated with high-income lifestyle. This accords with Burkitt’s original recommendation for consumption of more than 50 g/day of fibre, which was rationalised by the value of approximately 100 g/day consumed by people living in rural areas of Uganda, and still consumed by the Hadza people in north-central Tanzania today.106 Compelling evidence exists that suggests that increased consumption of fibre-rich, plant-based foods might not only extend lifespan, but also improve the quality of the years gained by reducing the effects of diseases associated with high-income lifestyles. Consuming more fibre-rich, plant-based foods would allow a large proportion of populations in HICs to better enjoy older age and remain productive and would also go some way towards relieving the massive health-care costs associated with the management of chronic disease in the ageing population.
Acknowledgments
The comparative research studies in African Americans, Rural South Africans, and Native Alaskan people included in this article was funded by awards from the NIH through R01 CA135379, R01 CA204403. Ethical approval was obtained from the University of Pittsburgh IRB, KwaZulu-Natal Research and Ethics Committee, South Africa, Alaska Area IRB. I would like to thank Dr Jia Li, a collaborator on our African-African American-Native Alaskan studies, for their contributions to figure 2 and Faheem Bhati for help in searching for some of the initial review material.
Footnotes
Declaration of interests
I declare no competing interests.
References
- 1.Burkitt DP. Related disease—related cause? Lancet 1969; 294: 1229–31. [DOI] [PubMed] [Google Scholar]
- 2.Burkitt DP. Relationship as a clue to causation. Lancet 1970; 296: 1237–40. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JH, Engineer A. Denis Burkitt and the origins of the dietary fibre hypothesis. Nutr Res Rev 2018; 31: 1–15. [DOI] [PubMed] [Google Scholar]
- 4.Cleave TL CG. Diabetes, coronary thrombosis and the saccharine disease. Bristol: John Wright and Sons, 1966. [Google Scholar]
- 5.Winkler JT. Pure, white and deadly. Br Med J 2013; 346: e8612. [Google Scholar]
- 6.Burkitt DP, Trowell HC. refined carbohydrate foods and disease: some implications of dietary fibre. London: Academic Press, 1975. [Google Scholar]
- 7.Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer 1971; 28: 3–13. [DOI] [PubMed] [Google Scholar]
- 8.Walker AR, Walker BF. Bowel motility and colonic cancer. BMJ 1969; 3: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aries V, Crowther JS, Drasar BS, Hill MJ, Williams RE. Bacteria and the aetiology of cancer of the large bowel. Gut 1969; 10: 334–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsidzira L, Chokunonga E, Gangaidzo IT, et al. The incidence and histo-pathological characteristics of colorectal cancer in a population based cancer registry in Zimbabwe . Cancer Epidemiol 2016; 44: 96–100. [DOI] [PubMed] [Google Scholar]
- 11.NHS. How to get more fibre into your diet. Aug 1, 2018. https://www.nhs.uk/live-well/eat-well/how-to-get-more-fibre-into-your-diet (accessed Aug 1, 2018).
- 12.Institute of Medicine of the National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington DC: The National Academies Press, 2005. [Google Scholar]
- 13.Stephen AM, Champ MM, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 2017; 30: 149–90. [DOI] [PubMed] [Google Scholar]
- 14.Roe M, Pinchen H, Church S, Finglas P. McCance and Widdowson’s the composition of foods seventh summary edition and updated composition of foods integrated dataset. Nutr Bull 2015; 40: 36–39. [Google Scholar]
- 15.O’Keefe SJD, Chung D, Mahmoud N, et al. Why do African Americans get more colon cancer than native Africans? J Nutr 2007; 137: 175S–82. [DOI] [PubMed] [Google Scholar]
- 16.Southgate DA. Determination of carbohydrates in foods. II. Unavailable carbohydrates. J Sci Food Agric 1969; 20: 331–35. [DOI] [PubMed] [Google Scholar]
- 17.Englyst HN, Kingman SM, Hudson GJ, Cummings JH. Measurement of resistant starch in vitro and in vivo. Br J Nutr 1996; 75: 749–55. [DOI] [PubMed] [Google Scholar]
- 18.McCleary BV, DeVries JW, Rader JI, et al. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J AOAC Int 2012; 95: 824–4. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 2010; 92: 1369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl 1997; 32 (suppl 222): 3–9. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 1996; 31 (suppl 216): 132–48. [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nature Commun 2015; 6: 6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Cancer Research Fund. Diet, nutrition, physical activity and colorectal cancer. 2017. https://www.wcrf.org/sites/default/files/Colorectal-Cancer-2017-Report.pdf (accessed Sept 27, 2019).
- 24.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011; 343: d6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003; 361: 1496–501. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Chen Y, Ma S, et al. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget 2016; 7: 80980–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr 2014; 100 (suppl 1): 394s–98. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch Cardiovasc Dis 2016; 109: 39–54. [DOI] [PubMed] [Google Scholar]
- 29.Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med 2011; 171: 1061–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. Br Med J 2016; 353: i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019; 393: 434–5. [DOI] [PubMed] [Google Scholar]
- 32.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 2008; 87: 627–37 [DOI] [PubMed] [Google Scholar]
- 33.Kranz S, Brauchla M, Slavin JL, Miller KB. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr 2012; 3: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiber Slavin J. and prebiotics: mechanisms and health benefits. Nutrients 2013; 5: 1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahl WJ, Agro NC, Eliasson AM, et al. Health benefits of fiber fermentation. J Am Coll Nutr 2017; 36: 127–36. [DOI] [PubMed] [Google Scholar]
- 36.King DE, Mainous AG 3rd, Lambourne CA. Trends in dietary fiber intake in the United States, 1999–2008. J Acad Nutr Diet 2012; 112: 642–48. [DOI] [PubMed] [Google Scholar]
- 37.Albertson AM, Reicks M, Joshi N, Gugger CK. Whole grain consumption trends and associations with body weight measures in the United States: results from the cross sectional National Health and Nutrition Examination Survey 2001–2012. Nutr J 2016; 15: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reicks M, Jonnalagadda S, Albertson AM, Joshi N. Total dietary fiber intakes in the US population are related to whole grain consumption: results from the National Health and Nutrition Examination Survey 2009 to 2010. Nutr Res 2014; 34: 226–34. [DOI] [PubMed] [Google Scholar]
- 39.Kuijsten A, Aune D, Schulze MB, et al. Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct study and a meta-analysis of prospective studies. Diabetologia 2015; 58: 1394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Flint AJ, Qi Q, et al. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med 2015; 175: 373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajishafiee M, Saneei P, Benisi-Kohansal S, Esmaillzadeh A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 2016; 116: 343–52. [DOI] [PubMed] [Google Scholar]
- 42.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–43. [DOI] [PubMed] [Google Scholar]
- 43.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet 1995; 346: 1065–69. [DOI] [PubMed] [Google Scholar]
- 44.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol 2014; 179: 1153–67 [DOI] [PubMed] [Google Scholar]
- 45.Root MM, Houser SM, Anderson JJ, Dawson HR. Healthy eating index 2005 and selected macronutrients are correlated with improved lung function in humans. Nutr Res 2014; 34: 277–84. [DOI] [PubMed] [Google Scholar]
- 46.Rasmussen HE, Hamaker BR. Prebiotics and inflammatory bowel disease. Gastroenterol Clin North Am 2017; 46: 783–95. [DOI] [PubMed] [Google Scholar]
- 47.Yao Y, Suo T, Andersson R, et al. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2017; 1: CD003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N Engl J Med 2000; 342: 1149–55. [DOI] [PubMed] [Google Scholar]
- 49.Lanza E, Hartman TJ, Albert PS, et al. High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the polyp prevention trial. J Nutr 2006; 136: 1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. N Engl J Med 2000; 342: 1156–62. [DOI] [PubMed] [Google Scholar]
- 51.Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. Lancet 2000; 356: 1300–06. [DOI] [PubMed] [Google Scholar]
- 52.MacLennan R, Macrae F, Bain C, et al. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. J Nat Cancer Inst 1995; 87: 1760–66. [DOI] [PubMed] [Google Scholar]
- 53.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81: 1031–64. [DOI] [PubMed] [Google Scholar]
- 54.van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci 1994; 39: 834–42. [DOI] [PubMed] [Google Scholar]
- 55.Birkett A, Muir J, Phillips J, Jones G, O’Dea K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr 1996; 63: 766–72. [DOI] [PubMed] [Google Scholar]
- 56.Burn J, Bishop DT, Chapman PD, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011; 4: 655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphreys KJ, Conlon MA, Young GP, et al. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev Res (Phila) 2014; 7: 786–95. [DOI] [PubMed] [Google Scholar]
- 58.Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. M Bio 2019; published online Jan 29. DOI: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342: 1392–98. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018; 359: 1151–56. [DOI] [PubMed] [Google Scholar]
- 61.Soare A, Khazrai YM, Del Toro R, et al. The effect of the macrobiotic Ma-Pi 2 diet vs. the recommended diet in the management of type 2 diabetes: the randomized controlled MADIAB trial. Nutr Metab (Lond) 2014; 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hollenbeck CB, Coulston AM, Reaven GM. To what extent does increased dietary fiber improve glucose and lipid metabolism in patients with noninsulin-dependent diabetes mellitus (NIDDM)? Am J Clin Nutr 1986; 43: 16–24. [DOI] [PubMed] [Google Scholar]
- 63.Manhire A, Henry CL, Hartog M, Heaton KW. Unrefined carbohydrate and dietary fibre in treatment of diabetes mellitus. J Hum Nutr 1981; 35: 99–101. [DOI] [PubMed] [Google Scholar]
- 64.Wanders AJ, van den Borne JJ, de Graaf C, et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev 2011; 12: 724–39. [DOI] [PubMed] [Google Scholar]
- 65.Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2017; 106: 1514–28. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Ouyang Y, Li H, et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: a randomized crossover trial. Sci Rep 2019; 9: 4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Streppel MT, Arends LR, van ‘t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Int Med 2005; 165: 150–56. [DOI] [PubMed] [Google Scholar]
- 68.Threapleton DE, Greenwood DC, Evans CE, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Br Med J 2013; 347: f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007; 4: Cd006475. [DOI] [PubMed] [Google Scholar]
- 70.Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev 2013; 3: Cd006474. [DOI] [PubMed] [Google Scholar]
- 71.Braegger C, Chmielewska A, Decsi T, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2011; 52: 238–50. [DOI] [PubMed] [Google Scholar]
- 72.Ranucci G, Buccigrossi V, Borgia E, et al. Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: a randomized clinical trial. Nutrients 2018; published online March 1. DOI: 10.3390/nu10030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.James SL, Christophersen CT, Bird AR, et al. Abnormal fibre usage in UC in remission. Gut 2015; 64: 562–70. [DOI] [PubMed] [Google Scholar]
- 74.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982; 83: 424–29. [PubMed] [Google Scholar]
- 75.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009; 294: 1–8. [DOI] [PubMed] [Google Scholar]
- 76.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 2016; 13: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012; 61: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014; 537: 85–92. [DOI] [PubMed] [Google Scholar]
- 80.McNabney SM, Henagan TM. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017; published online Dec 12. DOI: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Commun 2014; 5: 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett 2016; 625: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarkar A, Harty S, Lehto SM, et al. The microbiome in psychology and cognitive neuroscience. Trends Cogn Sci 2018; 22: 611–36. [DOI] [PubMed] [Google Scholar]
- 84.Rea K, Dinan TG, Cryan JF. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress 2016; 4: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fibre diet improve brain health? Neurosci Lett 2016; 625: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS One 2018; 13: e0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bultman SJ. Molecular pathways: gene-environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin Cancer Res 2014; 20: 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987; 28: 1221–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvaro A, Sola R, Rosales R, et al. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life 2008; 60: 757–64. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Xu C, Huang R, Song J, Li D, Xia M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J Nutr Biochem 2018; 56: 175–82. [DOI] [PubMed] [Google Scholar]
- 91.Thandapilly SJ, Ndou SP, Wang Y, Nyachoti CM, Ames NP. Barley beta-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct 2018; 9: 3092–96. [DOI] [PubMed] [Google Scholar]
- 92.Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol 2018; 3: 1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 2008; 105: 16767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu J, Lin S, Zheng B, Cheung PCK. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr 2018; 58: 1243–49. [DOI] [PubMed] [Google Scholar]
- 95.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology 2005; 146: 879–88. [DOI] [PubMed] [Google Scholar]
- 96.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013; 498: 99. [DOI] [PubMed] [Google Scholar]
- 97.Sanna S, van Zuydam NR, Mahajan A, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 2019; 51: 600–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–66. [DOI] [PubMed] [Google Scholar]
- 99.Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6: 7320. [DOI] [PubMed] [Google Scholar]
- 100.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients 2017; published online Jan 10. DOI: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord 2015; 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Groot PF, Belzer C, Aydin O, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One 2017; 12: e0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Endesfelder D, Engel M, Davis-Richardson AG, et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 2016; 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461: 1282–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015; 6: 6734. [DOI] [PubMed] [Google Scholar]
- 106.de Vrieze J Gut instinct. Science 2014; 343: 241–43. [DOI] [PubMed] [Google Scholar]