Abstract
Background: Osteoarthritis (OA) is a common chronic degenerative disease, and the chondrocyte is reported to be a key player in OA progression. Increasing evidence has verified the regulatory role of miRNAs in OA. However, the function and underlying mechanism of miR-107 in cartilage function are still not clarified. Methods: Abundance of miR-107 and PTEN mRNA were detected by qRT-PCR. Relative protein levels of PTEN, Bcl-2, Bax, Caspase-3, aggrecan, collagen II, MMP-13, and MMP-9 were measured by western blotting (WB). A biological web server Targetscan was used to predict the putative binding sites between miR-107 and PTEN, and luciferase reporter assay was employed to further verify the true interplay between them. Cell proliferative or apoptotic activity was assessed by MTT or flow cytometry (FCM) analysis. Results: miR-107 was downregulated and PTEN was upregulated in OA tissues. PTEN could be negatively regulated by miR-107 by targeted interaction. Interference of PTEN induced proliferation of C28/I2 cells, but inhibited cell apoptosis. Restoration of PTEN reversed miR-107-stimulated cell proliferation and miR-107-inhibited apoptosis in C28/I2 cells. Furthermore, enforced abundance of miR-107 promoted the expressions of aggrecan and collagen II protein, while it attenuated MMP-13 and MMP-9 expression in C28/I2 cells, which was overturned by PTEN restoration. Conclusion: miR-107 induced chondrocyte growth and ameliorated cartilage degradation by targeting to PTEN, providing a potential therapeutic target for OA.
Keywords: Osteoarthritis, chondrocyte, miR-107, PTEN, proliferation, apoptosis
Introduction
Osteoarthritis (OA) is a chronic degenerative disease, mainly characterized by cartilage degradation and osteophytes formation, and subchondral bone thickening [1]. To data, OA has become a serious public health hazard and leads to severe physical disability with pain, stiffness, joint deformity, limited motion and disability. The high incidence of this disease is related to increased age, inflammation response, and the rising prevalence of obesity [2,3]. According to related statistics, almost 63% of OA cases are 60 years of age or older [4]. Chondrocytes, the only cells found in cartilage, can involve in the production of cartilage extracellular matrix (ECM), which is reported to play a key role in the maintenance of cartilage structure and function [5]. Growing evidence shows that enforced apoptosis of chondrocytes is correlated with the initiation and severity of cartilage degradation [6]. Thus, providing a novel strategy to improve chondrocyte function may contribute to the treatment of OA.
MicroRNAs (miRNAs) are a class of endogenous, conserved, non-protein coding RNA with approximately 20-23 nucleotide (nt) in length [7]. Mature miRNAs can bind to the 3’UTR of their target genes by the complementary nature of base pairs, leading to the degradation or translation inhibition of target mRNAs. It has been reported that miRNAs play important roles in the modulation of various life processes, including cell proliferation, apoptosis, differentiation, cycle arrest, and organ development. Recently, a large number of evidences confirm the involvement of miRNAs in OA progression and chondrocyte function. Upregulated miR-206, miR-9, miR-98 and downregulated miR-337-3p are identified in OA tissues and chondrocytes [8-10]. miR-107 is a major regulatory factor closely associated with the growth of many kinds of cells, like cancer cells [11,12] and neurons [13]. However, the role of miR-107 in chondrocyte growth and cartiliage function remains unclear.
Phosphatase and tensin homolog deleted on chromosome ten (PTEN), located on chromosome 10q23.3, is initially identified as a tumor suppressor in various cancer, including endometrial cancer [14], prostate cancer [15], and pancreatic cancer [16]. Mutant of PTEN gene is frequently observed in multiple human diseases, leading to the alteration of a series of signaling pathways, like PI3K-Akt [17], MEK/ERK [18]. Recently, the role of PTEN in OA development was confirmed and an elevated expression of PTEN was described in OA tissues and chondrocytes [19]. Intriguingly, a previous study also revealed the existence of binding sites between miR-107 and PTEN, which further suggested a functional correlation between them [20].
Accordingly, we hypothesized that miR-107 might involve in the modulation of chondrocyte growth by interacting with PTEN. Therefore, we examined the abundances of miR-107 and PTEN in OA tissues, and explored their potential roles and regulatory mechanisms in chondrocyte growth and cartilage function, aiming to provide novel insight into the pathogenesis and develop a potential molecular target for OA treatment.
Materials and methods
Preparation of cartilage tissues
OA cartilage tissues were collected from 45 patients who underwent total knee replacement at Honghui hospital, Xi’an Jiaotong University from June 2014 to July 2017. All of these patients were confirmed according to the Criteria of American Rheumatology Academy (ARA) for osteoarthritis. Normal articular cartilage specimens were obtained from 45 non-arthritic patients undergoing surgical operations for other reasons. The age and gender of all participators in OA and normal groups were not statistically significantly different. This study was approved by the Ethics Committee of Honghui hospital, Xi’an Jiaotong University, and written informed consent was signed by all donors and families.
Cell culture
Normal human cartilage C28/I2 cells obtained from American type culture collection (ATCC, Manassas, VA, USA) were cultured in high glucose DMEM/F12 medium (Gibco, Grand Island, NY, USA) in presence of 10% fetal bovine serum (FBS; Gibco). Then, cells were maintained in a humidified incubator with 5% CO2 at 37°C until the confluence achieved to 80-85%, and the medium was replaced every 48 h.
Cell transfection
The full-length PTEN sequences were inserted into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to generate PTEN-overexpressed construct (PTEN), with pcDNA3.1 vector (NC) as a negative control. miR-107 mimics (miR-107) and relative control (miR-NC), miR-107 inhibitor and inhibitor control (inhibitor-NC), small interfering RNA targeting PTEN (siPTEN) and scrambled control (siNC) were obtained from GenePharma Co., Ltd (Shanghai, China). Transfection of C28/I2 cells with miR-107, miR-107 inhibitor, si-PETN, PETN, and relevant control was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) referring to the instruction of manufacturer.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The relative expression levels of miR-107 and PTEN mRNA were determined by qRT-PCR. In brief, total RNA from articular cartilage tissues or cultured cells were extracted using TRIzol reagent (Invitrogen), followed by the analysis of RNA purity by a microspectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). 1 µg RNA was reversely transcribed into single-stranded complementary DNA (cDNA) using Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific) or High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Then, qPCR reactions were performed on the ABI 7900 (Applied Biosystems, CA, USA) using SYBR Green Master Mix (Takara, Dalian, China). The results were calculated using 2-ΔΔCt method with U6 or GAPDH as the housekeeping gene for miR-107 or PTEN. Specific primers are presented below: PTEN: 5’-ACACGACGGGAAGACAA GTT-3’ (forward) and 5’-TCCTCTGGTCCTGGTATGGAAG-3’ (reverse); GAPDH: 5’-TATGATGATATCAAGAGGGTAGT-3’ (forward) and 5’-TGTATCCAAA CTCATTGTCATAC-3’ (reverse). Primers for miR-107 and U6 were obtained from GenePharma Co., Ltd.
Western blotting (WB)
The expression levels of PTEN, Bcl-2, Bax, Caspase-3, Aggrecan, Collagen II, MMP-13, MMP-9 proteins were evaluated by WB. Total proteins in cartilage tissues and cells were extracted using RIPA lysis buffer (Thermo Fisher Scientific) containing protease inhibitor, followed by the quantification by BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein were separated by SDS-PAGE prior to being electrotranferred onto PVDF members (Millipore, Bedford, MA, USA). Then, the members were blocked with 5% non-fat milk, and incubated with primary antibodies against PTEN (1:1000) (Abcam, Cambridge, MA, USA), Bcl-2 (1:1000) (Abcam), Bax (1:2000) (Abcam), Caspase-3 (1:1000) (Abcam), aggrecan (1:100) (Abcam), collagen II (1:5000) (Abcam), MMP-13 (1:5000) (Abcam), MMP-9 (1:1000) (Abcam), β-actin (1:3000) (Abcam). After washing, the members were further developed with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10000) (Abcam). Protein blots were visualized using the enhanced chemiluminescence (ECL) system (Roche, Basel, Switzerland) and the intensity of the blots was quantified using the Image-Pro Plus software (Bio-Rad, Hercules, CA, USA).
Cell proliferation assay
The proliferative activity of transfected cells was determined using Cell Counting Kit-8 (CCK-8) (Dojindo, Tokyo, Japan) according to manufacturer’s procedure. Briefly, C28/I2 cells were seeded into 96-well microplates with 5 × 103 cells/well and cultured in an indicated incubator containing 5% CO2 at 37°C until logarithmic growth period. Then, si-PTEN, miR-107, miR-107+PTEN, or relevant control, was transfected into C28/I2 cells. About 48 h post-transfection, 10 μl of CCK-8 reagent was introduced into the medium and incubated for another 4 h. Finally, the absorbance at 450 nm was detected using a Microplate Reader (Bio-Rad, Hercules, CA, USA).
Cell apoptosis assay
The apoptotic rate of C28/I2 cells was assessed by using Annexin V-FITC Apoptosis Detection Kit (Invitrogen). Generally, transfected C28/I2 cells were rinsed twice with cold PBS buffer, and then resuspended in 300 μl 1 × Binding buffer. Next, cells were treated with 5 μl of Annexin V-FITC and 10 μl propidium iodide (PI) for 10 min in the dark, prior to the evaluation of cell apoptosis by a flow cytometer (BD Biosciences, San Jose, CA, USA).
Dual-Luciferase reporter assay
Partial 3’UTR sequences of PTEN containing putative miR-107 binding sites were amplified and cloned into the downstream end of psiCHECK-2 vector (Promega, Madison, WI, USA) to generate wild-type PTEN (PTEN WT-3’UTR). Targeted mutation of PTEN 3’UTR in miR-107 binding sites was performed using Site-Directed Mutagenesis Kit (Thermo Fisher Scientific) to generate mutant PTEN (PTEN MUT-3’UTR). Luciferase reporter containing wile-type or mutant miR-107 binding sites were transfected into C28/I2 cells along with miR-107, miR-107 inhibitor, or paired control. About 48 h after transfection, luciferase activity in each group was determined by Dual-Luciferase reporter assay system (Promega).
Statistical analysis
All experiments were repeated in triplicate. The statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Results were displayed as mean ± standard deviation (SD) in which the significant differences between groups were assessed by Student’s t-test or One-way analysis of variance (ANOVA). P values less than 0.05 were considered significant.
Results
miR-107 was downregulated and PTEN was upregulated in OA tissues
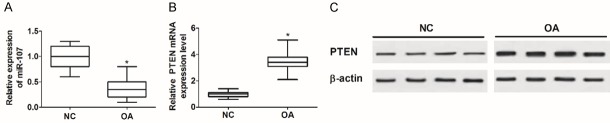
In the present study, the expression levels of miR-107 and PTEN in human cartilage tissues were first measured by qRT-PCR or WB analysis. As a result, a decreased abundance of miR-107 was observed in OA tissues compared to normal control (NC) group (Figure 1A). Inversely, elevated levels of PTEN mRNA (Figure 1B) and protein (Figure 1C) were verified in 45 OA tissues relative to NC group. These findings indicated the possible involvement of miR-107 and PTEN in OA.
Figure 1.
Dysregulation of miR-107 and PTEN in human OA tissues. A. Relative expression of miR-107 in 45 OA tissues and normal cartilage tissues were determined by qRT-PCR. B and C. The expression of PTEN at mRNA and protein levels in OA tissues and normal cartilage tissues was detected by qRT-PCR or WB analysis.
Targeted relationship between miR-107 and PTEN
A biological web server miRTarBase showed the existence of putative binding sites between miR-107 and PTEN 3’UTR (Figure 2A). To interpret the true interaction between them, luciferase reporters PTEN WT-3’UTR and PTEN MUT-3’UTR were constructed for a luciferase activity assay. The results revealed that miR-107 introduction suppressed (Figure 2B), and knockdown of miR-107 notably induced the luciferase activity of PTEN WT-3’UTR reporter in C28/I2 cells (Figure 2C). However, no significant change was observed in the luciferase activity of PTEN MUT-3’UTR reporter (Figure 2B and 2C). Next, miR-107 was overexpressed or inhibited through transfecting with miR-107 or miR-107 inhibitor into C28/I2 cells (Figure 2D), and then the regulatory effect of miR-107 on PTEN expression was performed by WB. The resulted showed that addition of miR-107 inhibited, and absence of miR-107 promoted the expression of PTEN protein (Figure 2E), hinting that miR-107 negatively modulated PTEN expression via targeted binding to PTEN.
Figure 2.
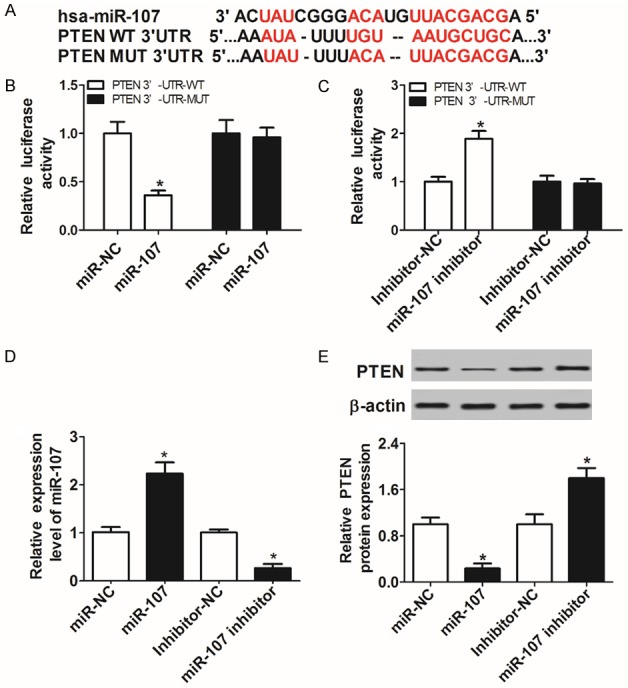
PTEN was directly targeted by miR-107 in C28/I2 cells. A. The putative binding sites between miR-107 and PTEN 3’UTR were predicted by Biological web server Targetscan. B and C. PTEN luciferase reporter containing wild-type or mutant miR-107 binding sites was transfected into C28/I2 cells along with miR-107, miR-107 inhibitor, or relative control. At 48 h post-transfection, luciferase activity in each group was detected by dual-Luciferase reporter assay. D. The abundance of miR-107 in C28/I2 cells transfected with miR-107, miR-107 inhibitor, or negative control was determined by qRT-PCR. E. The protein levels of PTEN in miR-107-overexpressed or inhibited C28/I2 cells were evaluated by WB.
Knockdown of PTEN accelerated cell proliferation and inhibited apoptosis in C28/I2 cells
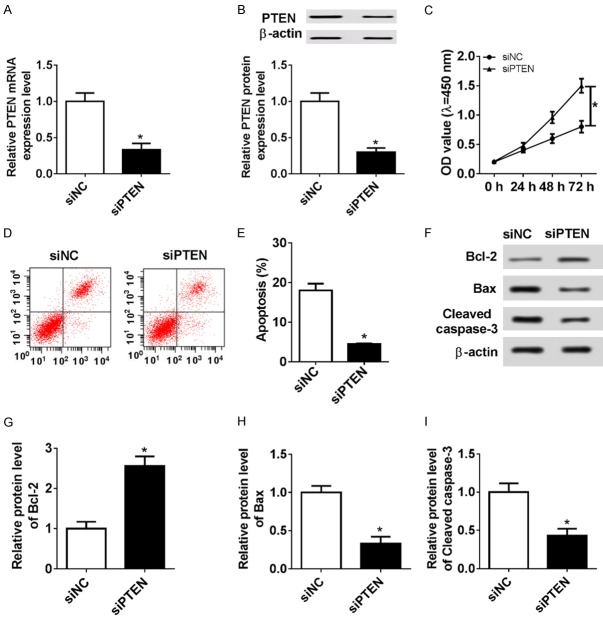
Here, we further probed the regulatory role of PTEN in chondrocyte growth. PTEN expression at mRNA (Figure 3A) and protein levels (Figure 3B) was visibly lowered in si-PETN-transfected C28/I2 cells compared with that in NC group, indicating that siPETN could be used in the following loss-of-function research. Then, MTT assay revealed that PTEN deficiency contributed to the proliferation of C28/I2 cells compared with NC group (Figure 3C). In contrast, flow cytometry (FCM) assay suggested that knockdown of PTEN led to a decreased number of apoptotic cells in the C28/I2 cells relative to NC group (Figure 3D and 3E). Then, the expression levels of apoptotic-related protein Bcl-2, Bax, and Caspase-3 were detected by WB to further explore the effect of PTEN absence on C28/I2 cell apoptosis. As the results showed in Figure 3F-I, silencing PTEN induced Bcl-2 expression, while it induced Bax and Caspase-3 expression, which further confirmed the inhibitory effect of siPTEN on C28/I2 cell apoptosis. Altogether, these findings suggested that PTEN interference stimulated the growth of C28/I2 cells by regulation of cell proliferation and apoptosis.
Figure 3.
Interference of PTEN promoted cell proliferation and inhibited apoptosis in C28/I2 cells. C28/I2 cells were transiently transfected with si-NC or si-PTEN. At 48 h after transfection, the expression of PTEN mRNA (A) and protein (B) was measured by qRT-PCR or WB analysis. (C) Cell proliferative activity was assessed by MTT assay. (D and E) Apoptotic rates of C28/I2 cells were evaluated by FCM analysis. (F-I) Relative levels of apoptosis-related proteins Bax, Bcl-2, and Caspase-3 were examined by WB.
PTEN reversed the stimulatory effect of miR-107 on cell growth in C28/I2 cells
We observed that the mRNA (Figure 4A) and protein levels (Figure 4B) of PTEN were upregulated in the PTEN-transfected C28/I2 cells relative to the NC group, showing that pcDNA-PTEN plasmid could be used to overexpress PTEN. To investigate the regulation of miR-107 and PTEN in the development of chondrocytes, C28/I2 cells were transfected with miR-NC, miR-107, miR-107+NC, or miR-107+PTEN. At 48 h after transfection, cell proliferative and apoptotic activity were determined by MTT, FCM, or WB analysis. The results showed that addition of miR-107 contributed to C28/I2 cell proliferation, which was reversed by PTEN introduction (Figure 4C). In contrast, elevated expression of miR-107 highly inhibited cell apoptosis, as reflected by the lowered apoptotic rate (Figure 4D), Bax, and Caspase-3 expression, as well as the increased Bcl-2 expression (Figure 4E-H). However, re-expression of PTEN abolished the inhibitory effect of miR-107 on the apoptosis of C28/I2 cells (Figure 4D-H). In sum, restoration of PTEN abrogated miR-107-stimulated C28/I2 cell growth, hinting that miR-107 modulated chondrocyte response by directly targeting PTEN.
Figure 4.
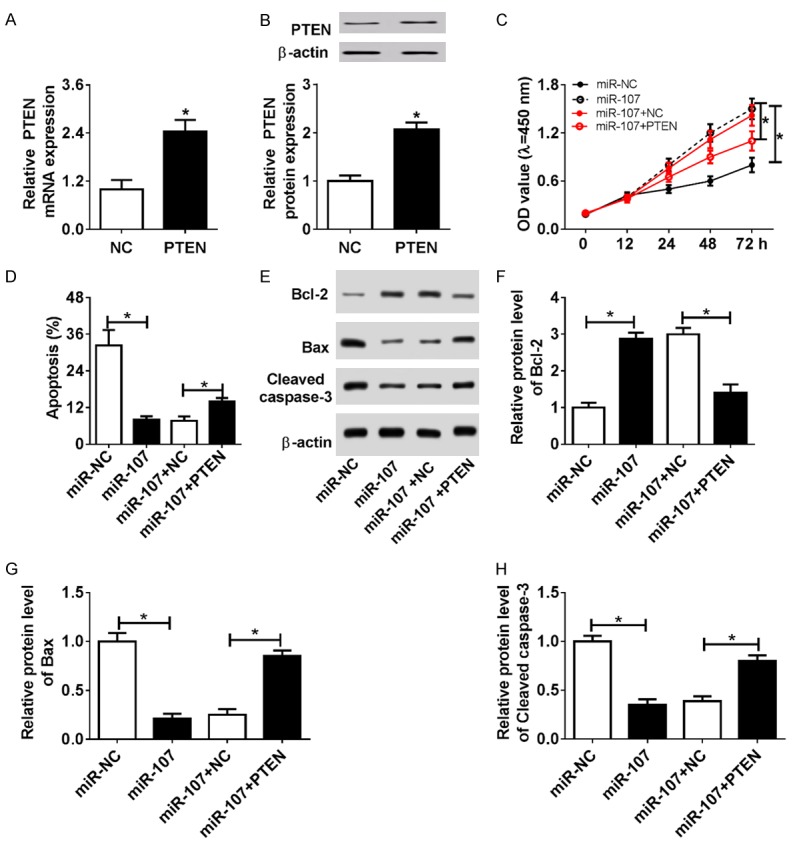
miR-107 induced cell proliferation and inhibited apoptosis by interplay with PTEN. (A and B) PTEN expression in pcDNA3.1 (NC) or pcDNA-PTEN (PTEN)-transfected C28/I2 cells. (C-H) C28/I2 cells were transiently transfected with miR-NC, miR-107, miR-107+NC, or miR-107+PTEN. At 48 h post-transfection, cell proliferative activity (C), apoptosis (D), as well as Bax, Bcl-2, and Caspase-3 expression (E-H) was detected by MTT, FCM, or WB analysis.
miR-107 alleviated cartilage dysfunction through interacting with PTEN in C28/I2 cells
Aggrecan and collagen II are known as core proteins of cartilage ECM, involved in the maintenance of cartilage function [21]. MMPs are proven to play central roles in the degradation of ECM, leading to the functional destruction of cartilage [22]. To further investigate the role of miR-107 in the regulation of cartilage function, the expressions of cartilage matrix-related proteins aggrecan and collagen II, as well as matrix-degrading enzymes MMP-9 and MMP-13 were measured by WB. As shown, enforced abundance of miR-107 highly increased the protein expressions of aggrecan and collagen II, while attenuating MMP-13 and MMP-9 expression in C28/I2 cells (Figure 5A-E). However, with the restoration of PTEN, the regulatory effects of miR-107 on aggrecan, collagen II, MMP-9, and MMP-13 were overturned (Figure 5A-E). These findings showed that miR-107 ameliorated cartilage function through negatively regulating PTEN.
Figure 5.
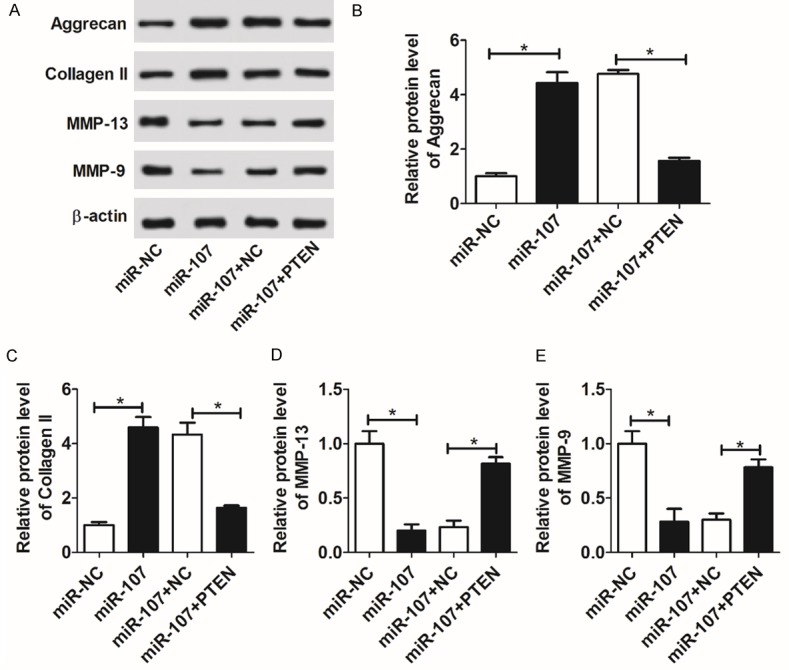
miR-107 remitted cartilage dysfunction by targeting PTEN. C28/I2 cells were transiently transfected with miR-NC, miR-107, miR-107+NC, or miR-107+PTEN. At 48 h post-transfection, the levels of cartilage matrix-related proteins aggrecan and collagen II, as well as matrix-degrading enzymes MMP-9 and MMP-13 were measured by WB (A-E).
Discussion
OA is a common functional degenerative disease, causing a heavy economic burden to family and society. Chondrocytes are reported to be involved in the initiation and development of OA. Normally, the proliferation and apoptosis of chondrocytes are in a steady state, helping the functional maintenance of articular cartilage. However, the proliferative and apoptotic activity changes notably in OA tissues. Thus, identification of related factors that associated with chondrocyte growth is necessary for OA treatment. Growing evidence indicates that miRNAs can function as major players in chondrogenesis and OA pathogenesis [23]. Borgonio Cuadra et al. measured the expression of 380 miRNAs in plasma from OA patients, in which 12 miRNAs exhibited significant upregulation, suggesting the implication of these miRNAs in OA development [24]. Zhang et al. suggested that miR-21 played a negative regulator in chondrogenesis through targeting to growth differentiation factor 5 (GDF-5) [25]. In addition, Zhang et al. reported that miR-146a induced the release of aggravated pro-inflammatory cytokines, leading to the expression inhibition of cartilage matrix-associated genes. Knockdown of miR-146a alleviated the degeneration of articular cartilage in vivo [26]. In contrast with these findings, our data indicated that introduction of miR-107 accelerated the growth of chondrocytes, and alleviated cartilage degeneration by targeted effects on PTEN, suggesting a protective role of miR-107 in human cartilage function.
miR-107 has been shown to express in various mammalian organs, especially in brain tissues [27]. The differential expression of miR-107 is observed in a variety of contexts, such as nerve damage and tumorigenesis. For example, miR-107 expression was elevated in the brain tissues and plasma from a cerebral ischemia/reperfusion (I/R) injury rat. Enhanced expression of miR-107 induced glutamate accumulation by suppression of glial glutamate transporter-1 (GLT-1) [28]. Abated expressions of miR-103a-3p and miR-107 regulated by circTCF25 could facilitate cell proliferation and migration in bladder cancer by negative modulation of CDK6 [29]. Moreover, miR-107 had lowered expression in hepatocellular carcinoma (HCC) samples. Addition of miR-107 inhibited the proliferation of HCC cells by directly targeting HMGA2 [12]. In spite of the involvement of miR-107 in multiple cellular processes, its role in chondrocyte response is still unclear. As an initial result, we pointed out that miR-107 was downregulated in OA tissues, indicating the possible involvement of miR-107 in the occurrence and development of OA. Functional analysis further certified that addition of miR-107 reduced the progression of OA by promotion of chondrogenesis.
As far as we know, PTEN exhibits elevated expression in OA tissues, and is associated with the initiation and development of OA by modulation of chondrocyte responses and ECM homeostasis [30]. Here, we also described an enforced abundance of PTEN both at mRNA and protein levels in OA tissues. Moreover, interference of PTEN triggered proliferation, but impaired apoptosis in chondrocytes, which was reflected by the decreased apoptotic rate and Bax and Caspase-3 expression, as well as increased Bcl-2 expression. As previously reported, the PTEN/AKT pathway negatively mediated by miR-337-3p inhibited the proliferation and promoted the apoptosis of OA chondrocytes [10]. Likewise, supplementation of miR-130a blocked cell proliferation and stimulated apoptosis of chondrocytes by modulation of PTEN/PI3K/Akt signaling pathway [31]. These findings demonstrated the regulatory roles of miRNAs in PTEN-associated OA progression. In present study, PTEN was identified to be a potential target of miR-107 in chondrocytes. Re-expression of PTEN abolished miR-107-stimulated chondrocyte growth and miR-107-improved cartilage function.
In summary, our data indicated that addition of miR-107 downregulated PTEN, and thus induced chondrocyte growth and ameliorated cartilage function. Our results provide a novel insight into pathogenesis of OA, and may offer a potential biologic target for OA treatment.
Acknowledgements
Xi’an health and family planning commission (No. J20161008): Study of structural changes of subchondral bone in post-traumatic arthritis in rabbits.
Disclosure of conflict of interest
None.
References
- 1.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2010;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 2.Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi: 10.1016/j.coph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. World report on disability 2011. World Health Organization. 2011;377:1977. [PubMed] [Google Scholar]
- 5.Gao Y, Liu S, Huang J, Guo W, Chen J, Li Z, Zhao B, Peng J, Wang A, Wang Y. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2015;2014:648459. doi: 10.1155/2014/648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with the initiation and severity of articular cartilage degradation. Int J Rheum Dis. 2011;14:191–198. doi: 10.1111/j.1756-185X.2010.01578.x. [DOI] [PubMed] [Google Scholar]
- 7.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Sci. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 8.Jones SW, Watkins G, Le GN, Roberts S, Murphy CL, Brockbank SM, Needham MR, Read SJ, Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Ni Z, Shang X, Tang G, Niu L. Expression of miR-206 in human knee articular chondrocytes and effects of miR-206 on proliferation and apoptosis of articular chondrocytes. Am J Med Sci. 2018;355:240–246. doi: 10.1016/j.amjms.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Zhang N, Ma W, Dai X, Liu J. MiR-337-3p promotes chondrocytes proliferation and inhibits apoptosis by regulating PTEN/AKT axis in osteoarthritis. Biomed Pharmacother. 2017;95:1194–1200. doi: 10.1016/j.biopha.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Feng L, Xie Y, Zhang H, Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29:856–863. doi: 10.1007/s12032-011-9823-1. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Chen F, Man Z, Zhe Y, Zhang S, Ye L, Gao H, Zhang X. MiR-107 suppresses proliferation of hepatoma cells through targeting HMGA2 mRNA 3’UTR. Biochem Biophys Res Commun. 2016;480:455–460. doi: 10.1016/j.bbrc.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 13.Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J, Stromberg AJ, Huang Q, Saatman KE, Nelson PT. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol. 2010;177:334–345. doi: 10.2353/ajpath.2010.091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daikoku T, Hirota Y, Tranguch S, Joshi AR, Demayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2010;120:1284–1292. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 16.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D-INDuced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Natl Acade Scie U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas SL, Alam R, Lemke N, Schultz LR, Gutiérrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro Oncol. 2010;12:941–955. doi: 10.1093/neuonc/noq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galasso O, Panza S, Santoro M, Goldring MB, Aquila S, Gasparini G. PTEN elevation, autophagy and metabolic reprogramming may be induced in human chondrocytes during steroids or nutrient depletion and osteoarthritis. J Biolog Regul Homeost Agents. 2015;29:1–14. [PubMed] [Google Scholar]
- 20.Chi H, Yang R, Zheng X, Zhang L, Jiang R, Chen J. LncRNA RP11-79H23.3 functions as a competing endogenous RNA to regulate pten expression through sponging hsa-miR-107 in the development of bladder cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazer A, Bunning RA, Thavarajah M, Seid JM, Russell RG. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-beta and interleukin-1beta. Osteoarth Cartilage. 1994;2:235–245. doi: 10.1016/s1063-4584(05)80075-5. [DOI] [PubMed] [Google Scholar]
- 22.Klatt AR, Paul-Klausch B, Klinger G, Kühn G, Renno JH, Banerjee M, Malchau G, Wielckens K. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J Orthop Res. 2010;27:65–70. doi: 10.1002/jor.20716. [DOI] [PubMed] [Google Scholar]
- 23.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumat. 2012;8:543. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgonio Cuadra VM, González-Huerta NC, Romero-Córdoba S, Hidalgo-Miranda A, Miranda-Duarte A. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PLoS One. 2014;9:e97690. doi: 10.1371/journal.pone.0097690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Jia J, Yang S, Liu X, Ye S, Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Wang C, Zhao J, Xu J, Geng Y, Dai L, Huang Y, Fu SC, Dai K, Zhang X. miR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8:e2734. doi: 10.1038/cddis.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang ZB, Zhang Z, Li TB, Lou Z, Li SY, Yang H, Yang J, Luo XJ, Peng J. Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin Sci. 2014;127:679–689. doi: 10.1042/CS20140084. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasa K, Hayashi S, Fujishiro T, Kanzaki N, Hashimoto S, Sakata S, Chinzei N, Nishiyama T, Kuroda R, Kurosaka M. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J Orthop Res. 2013;32:231–237. doi: 10.1002/jor.22506. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xu S, Huang E, Zhou H, Li B, Shao C, Yang Y. MicroRNA-130a regulates chondrocyte proliferation and alleviates osteoarthritis through PTEN/PI3K/Akt signaling pathway. Int J Mol Med. 2018;41:3699–3708. doi: 10.3892/ijmm.2018.3551. [DOI] [PubMed] [Google Scholar]