Abstract
Background
Monoclonal antibodies (mAbs) targeting negative regulators, or checkpoint molecules (e.g. PD1/PD-L1 & CTLA4), of anti-tumoural T cells have demonstrated clinical efficacy in treating several neoplastic diseases. While many patients enjoy remarkable responses to checkpoint inhibitors, a majority show adverse effects. Understanding how checkpoint inhibitors may augment established chemotherapy or radiotherapy regimens or other immunotherapies like oncolytic viruses may lead to better clinical outcomes measured by improved efficacy with reduced toxicity. Here, we assess how Newcastle disease virus (NDV), an oncolytic virus in clinical testing, may interact with radiotherapy to enhance checkpoint inhibitor blockade.
Methods
An immunocompetent B16-F10 murine melanoma model, generally considered to be a poorly immunogenic or “cold” tumour, was utilised to query whether combining localised radiotherapy with NDV may be more effective than either therapy alone in controlling tumours in mice treated with anti-PD1 or anti-CTLA4 monoclonal antibodies. We also investigated whether localised administration of a checkpoint inhibitor through an intratumoural injection of NDV that expresses anti-CTLA4 single-chain variable fragment (scFv) is comparable to systemic administration of anti-CTLA4 when combined with radiation in mediating its anti-tumour efficacy. Response rates were characterised by measuring tumour size over time, observation of complete tumour regression, and overall survival.
Findings
Our results show that combining NDV plus radiotherapy with checkpoint inhibitors (PD1 or CTLA4 targeted mAbs) results in significantly better complete tumour regression rates with an abscopal effect in a murine model of melanoma than either single therapy combined with checkpoint inhibitors. Finally, we also show that localised administration of a recombinant NDV expressing anti-CTLA4 plus radiation is comparable to systemic anti-CTLA4 plus radiation in mediating its anti-tumour effect as assayed by survival benefit.
Interpretation
Our results show that oncolytic NDV plus radiotherapy work together with checkpoint inhibitors to enhance tumour clearance of murine melanoma. NDV is an effective radiotherapy dose-sparing and immunotherapeutic agent capable of transgenic, in vivo expression of an anti-CTLA4 targeted scFv antibody with the potential to spare systemic exposure.
Funding
The National Institutes of Health grant HHSN272201400008C supported the work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Keywords: Oncolytic viruses, Cancer immunotherapy, Newcastle disease virus, Radiation therapy, Checkpoint inhibitors, Radiosensitiser, Radioenhancer
Research in context
Evidence before this study
Checkpoint-blockade and oncolytic immunotherapy represents the birth of a new era in the field of cancer therapy. NDV is an avian pathogen that shows great promise for use as an oncolytic agent in the clinic. It has been previously demonstrated that localised intratumoural therapy of B16-F10 melanoma with localised NDV and systemic CTLA4 blockade induced lymphocytic infiltration and anti-tumour effects in both the treated and non-treated tumour, thereby providing a rationale for combining NDV with checkpoint inhibitor therapy. In addition, it has been shown that radiotherapy enhances antigen cross-presentation that potentiates T cell infiltration into the tumours, thereby providing a rationale for combining radiotherapy with checkpoint blockade in the clinic. Finally, recent work from our lab demonstrated that intratumoural delivery of influenza A viruses engineered to express anti-CTLA4 single-chain variable fragment (scFv) potentiate an anti-tumour response in a murine melanoma model. We wanted to build on this work by demonstrating that localised administration of recombinant NDV expressing anti-CTLA4 plus radiation therapy is comparable to wild-type NDV plus radiation therapy with systemic CTLA4 blockade in promoting long-term survival.
Added value and implications of this study
To our knowledge, integrating localised oncolytic virus treatment with radiation and checkpoint blockade into a trimodality combination therapy regimen has not been evaluated for efficacy in animal models. Both oncolytic viruses and radiation therapy have the potential to activate the immune system and work synergistically with checkpoint blockade to overcome many of the barriers associated with immunologically “cold” tumours.
For this work, we have selected a well-characterised oncolytic virus to evaluate this potential anti-tumour effect. Our results show that the trimodality combination results in significantly better complete tumour regression rates in a murine model of melanoma than either single therapy combined with checkpoint inhibitors. We also demonstrate that localised administration of a recombinant NDV expressing anti-CTLA4 plus radiation is comparable to systemic anti-CTLA4 plus radiation in mediating its anti-tumour effect. The combination of NDV, checkpoint blockade, and radiation also induces an abscopal response driven primarily by the oncolytic virus.
Implications of all the available evidence
This study provides a rationale for considering targeted delivery of checkpoint blockade via a virus in combination with radiation therapy to achieve a durable response with a de-escalation of radiation dose and systemic exposure to checkpoint inhibiting antibodies. Here, we provide the first in vivo support for a novel trimodality combination of oncolytic NDV, single fraction radiotherapy, and checkpoint blockade that provides superior tumour control compared to any combination of two treatments. This may offer effective antineoplastic activity and reduced toxicities. An abscopal effect induced primarily by the virus is observed even when tumours regress rapidly when combined with single fraction radiotherapy, showing that oncolytic viruses may be effective in inducing systemic immunity when delivered to irradiated tumours.
Our results show that oncolytic NDV plus radiotherapy work with checkpoint inhibitors to enhance tumour clearance of murine melanoma. NDV is an effective radiotherapy-dose sparing and immunotherapeutic agent capable of transgenic, in vivo expression of an anti-CTLA4 targeted scFv antibody.
1. Introduction
Virchow's hypothesis linking cancer and inflammation has been realised clinically in the form of modern day immunotherapies. [1,2] The goal of any immunotherapeutic modality is to overcome the inherently immunosuppressive nature within immunologically “cold” tumour micro-environments that is mediated by a number of factors which includes, but is not limited to Tregs, [3,4] myeloid derived suppressor cells (MDSCs), [5] M2 macrophages, [6] and other inhibitory immune checkpoints and cytokines. [7] Checkpoint-blockade immunotherapy represents a paradigm shift in cancer therapy by targeting and activating the patient's own tumour-specific T cells to eradicate the tumour without directly targeting the tumour in itself. [8,9] CTLA4 and PD1 pathway blockade have produced durable responses when used as a monotherapy in about 20–40% of metastatic melanoma patients, and in up to 60% when combined. [10], [11], [12] Studies have shown that the clinical response to checkpoint inhibitors correlates with tumour immunogenicity, [13, 14] the frequency of circulating re-invigorated CD8 T cells relative to overall tumour burden [15] and PD-L1 levels in tumours [16] with about 30% of patients with immunologically “cold” tumours being refractory to checkpoint blockade. [17] The prevalence of severe immune-related adverse events with CTLA4 and PD1 pathway blockade in clinical studies ranges from 16.3 − 27.3% in monotherapy groups vs. 55% with dual checkpoint blockade. [11] Moreover, resistance to checkpoint inhibitor therapy in patients has been shown to be mediated by both tumour-cell-intrinsic and tumour-cell-extrinsic factors, such as the lack of or alterations to the MHC I antigen-presenting machinery. [18, 19] Both radiation and oncolytic immunotherapy have shown the potential to enhance MHC expression on tumour cells thereby providing an avenue to overcome this resistance mechanism. [20, 21] Therefore, combined therapies that enhance tumour immunogenicity and intratumoural T cell infiltration are desired. However, it is important to bear in mind that even when drug combinations exhibit synergy in pre-clinical models, it may be challenging to translate these results to a genetically heterogeneous patient populations with diverse tumour types. [22]
Oncolytic immunotherapy can potentially overcome the inherently immunosuppressive nature of the tumour microenvironment through direct lysis and immune-mediated cell death of tumour cells using oncolytic viruses. The first-in-class oncolytic virus talimogene laherparepvec (T-VEC) has been clinically tested in combinations with the anti-CTLA4 checkpoint inhibitor antibody, ipilimumab, and anti-PD1 checkpoint inhibitor antibody, pembrolizumab, and shown enhanced efficacy. In particular, intralesional injection of T-VEC can induce T cell infiltration in immunologically cold tumours when combined with pembrolizumab, modulating the tumour microenvironment into an inflamed or hot tumour. [23], [24], [25]
Radiotherapy (RT) is a particularly promising candidate for combinations with either oncolytic therapy or checkpoint blockade to achieve a clinically relevant abscopal response because of its well established universality and effectiveness in addition to its multipronged immunomodulatory effects. [26, 27] A number of preclinical studies have shown that local RT can potentiate a better response to checkpoint blockade by converting unresponsive tumours into responsive ones. [28], [29], [30] and dozens of clinical trials are evaluating this combination for various clinical indications (in clinicaltrials.gov).
NDV is an avian paramyxovirus with a negative sense single stranded RNA genome and is classified as the only member of the genus Avulavirus belonging to the family Paramyxoviridae. In addition to being an economically important pathogen for the poultry industry, since 1955 it has been explored as an attractive candidate for oncolytic immunotherapy. [31] Decades of research have since demonstrated the natural and selective oncolytic capabilities of NDV in different mammalian cancer cell lines, animal tumour models, and clinical trials. [32] Intratumoural delivery of NDV has been recently shown to potentiate an abscopal response by inducing increased immune infiltration into distant non-treated tumours, which can be further enhanced by intratumoural modulation of the T-cell co-stimulatory ICOS pathway with NDVs engineered to express ICOS ligands. [33, 34] Recent work from our lab has also demonstrated that intratumoural delivery of influenza A viruses engineered to express anti-CTLA4 single-chain variable fragment (scFv) potentiate an anti-tumour response in a murine melanoma model. [35]
To our knowledge, the combination of localised oncolytic virus therapy and radiation has not been evaluated for efficacy in animal models in combination with checkpoint inhibitors. Viruses are well known radiosensitisers, [36] and both oncolytic viruses and RT have the potential to activate the immune system and work synergistically with checkpoint blockade. Here we have selected a well-characterised oncolytic virus to evaluate this potential combination.
2. Materials and methods
2.1. Cell lines and antibodies
Murine melanoma cancer cell line B16-F10 (ATCC Catalogue no. CRL-6475, RRID:CVCL_0159) and Vero cells (ATCC Catalogue no. CCL-81, RRID:CVCL_0059) were obtained from ATCC. BSRT7 cells (BHK-21 cells transduced to constitutively expressing T7 RNA polymerase) were a kind gift from Dr. Benhur Lee (Icahn School of Medicine at Mount Sinai). B16-F10 cells were maintained in DMEM-F12 medium supplemented with 10% fetal bovine serum and 1% penicillin with streptomycin. BSRT7 cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin with streptomycin. Therapeutic anti-PD1 (clone RMP1-14) and anti-CTLA4 (clone 9H10) antibodies were purchased from BioXcell (Bio X Cell Catalogue no. BE0146, RRID: AB_10,949,053) and Bio X Cell Catalogue no. BE0131, RRID:AB_10,950,184).
2.2. Viruses
Recombinant lentogenic (low pathogenicity) NDV LaSota L289A strain was used for all experiments. Generation of recombinant NDV LaSota L289A viruses expressing anti-CTLA4 scFv was done by cloning DNA fragments encoding the murine anti-CTLA4 scFv into the SacII cloning site in between the P and M genes, flanked my NDV-specific transcriptional signals. The anti-CTLA4 scFv sequence was obtained from the US 20,110,044,953 A1 patent application (Inventors: Drs. James Allison and Michael Curran). Recombinant viruses were then rescued by transfecting BSRT7 cells with pNDV-LaSota-L289A-anti-CTLA4 along with the helper plasmids pTM1-L, pTM1-NP, pTM1-P and pCAGGs-T7opt as previously described. [37] Rescued viruses were grown in embryonated 9-day-old chicken eggs, and viral titers were determined by serial dilution and immunofluorescence in Vero cells. [37]
2.3. LDH cytotoxicity assay
B16-F10 cells in culture were infected with NDV at a multiplicity of infection of 3, or mock infected. Twenty-four hours later, cells were irradiated at doses of 0, 0.5, 1, 2, 4, 8, 16, 32 Gray (Gy) or mock treated. At 5 days post-infection, cells were washed with 1 ml Phosphate-Buffered Saline (PBS) (Gibco, Catalogue no. 20,012,027) and incubated with 1% Triton X-100 (Sigma-Aldrich, Catalogue no. X100-5ML) for 20 min at 37 °C. Lactate dehydrogenase (LDH) activity was measured using the CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega, Catalogue no. G1780) according to the instructions provided by the manufacturer. Mean values from 3 replicates are plotted and error bars depict standard deviations.
2.4. Mice
Female C57BL/6 mice were purchased from Jackson Laboratory. All animal experiments were performed in accordance with protocols approved by the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee. For melanoma tumour studies, 4–6 week old female mice were anaesthetised by intraperitoneal (IP) injections of ketamine/xylazine (K/X). For unilateral tumour experiments, mice were implanted intradermally with approximately 2 × 10^5 B16-F10 cells on the right flank. For bilateral tumour experiments, mice were implanted intradermally with approximately 2 × 105 and 1 × 105 B16-F10 cells on the right and left flank, respectively. On day 8–9, mice were checked for successful tumour implantation, all treated tumours were palpable and visually apparent, and randomly assigned to different treatment groups. They were then irradiated with various doses of radiation ranging from 0 Gy, 5, 10 or 20 Gy. Beginning on the day of radiation, mice were treated with intratumoural injections of 1 × 107 plaque-forming units (PFU) of NDV in PBS in a total volume of 100 ul every 2–3 days for a total of 5 injections. Checkpoint inhibiting mAbs were administered intraperitoneally with either anti-CTLA4 antibody (100 ug in 100 ul) or anti-PD1 (200 ug in 100 ul) for a total of 3 times with the first, third, and fifth NDV inoculation. The animals were euthanised following signs of distress, including ulcerated skin exposing the underlying muscle at the sight of regressed tumours, or when the total tumour volume reached 1000 mm3.
2.5. Radiation
In vitro experiments were conducted with a cesium-137 source. Mice were irradiated on the small animal radiation platform, X-RAD 320 (Precision X-RAY; North Branford, Connecticut). Mice were anaesthetised with intraperitoneally administered ketamine and xylazine. Mouse immobilisation devices and lead shielding (Partial Body Irradiation, Restrainer <25 g) were purchased from Braintree Scientific, Inc. (Braintree, MA) such that only the animal's right hind-limb with the tumour received direct radiation.
2.6. Statistical analysis
Statistical analyses were conducted in Prism software (GraphPad), and the details for these analyses are found in the figure legends.
3. Results
3.1. NDV radiosensitises B16-F10 melanoma cells in vitro
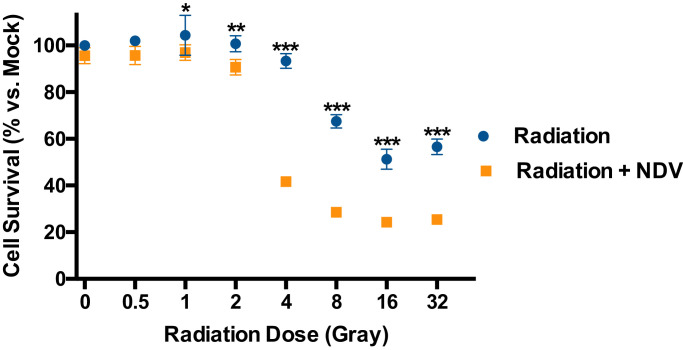
Viruses, most notably tumourigenic viruses such as human papilloma virus, are well known to interact with DNA damage repair and cell death pathways and affect cellular response to radiation. [38, 39] To assess the benefits of combining NDV and radiotherapy in vitro, B16-F10 melanoma cells were infected with NDV at multiplicity of infection of 3 and irradiated the following day with 0 to 32 Gray (Gy) delivered as a single fraction. Cell survival was assessed by lactate dehydrogenase (LDH) release assay 5 days later demonstrating that NDV is an effective radiosensitiser across a broad dose range for inducing tumour cell lysis (Fig. 1). Notably, the NDV strain LaSota is of low pathogenicity and induces little tumour cell lysis as a single agent in vitro.
Fig. 1.
Newcastle disease virusradioenhancesmelanoma cellsin vitro. B16-F10 cells in culture were infected with Newcastle disease virus (NDV) at a multiplicity of infection of 3, or mock infected. Twenty-four hours later, cells were irradiated at doses of 0, 0.5, 1, 2, 4, 8, 16, or 32 Gray (Gy). Cell viability was measured by lactate dehydrogenase assay 5 days after radiation and compared to mock treated cells. Mean values from 3 replicates are plotted and error bars depict standard deviations. Multiple t-tests were performed to show significant differences in cell survival between NDV-infected and mock-infected cells at a given radiation dose. *p < 0.05, **p < 0.01, ***p < 0.001(multiple t-tests).
3.2. NDV and radiation combined with PD1 blockade prolong survival in a metastatic murine melanoma model
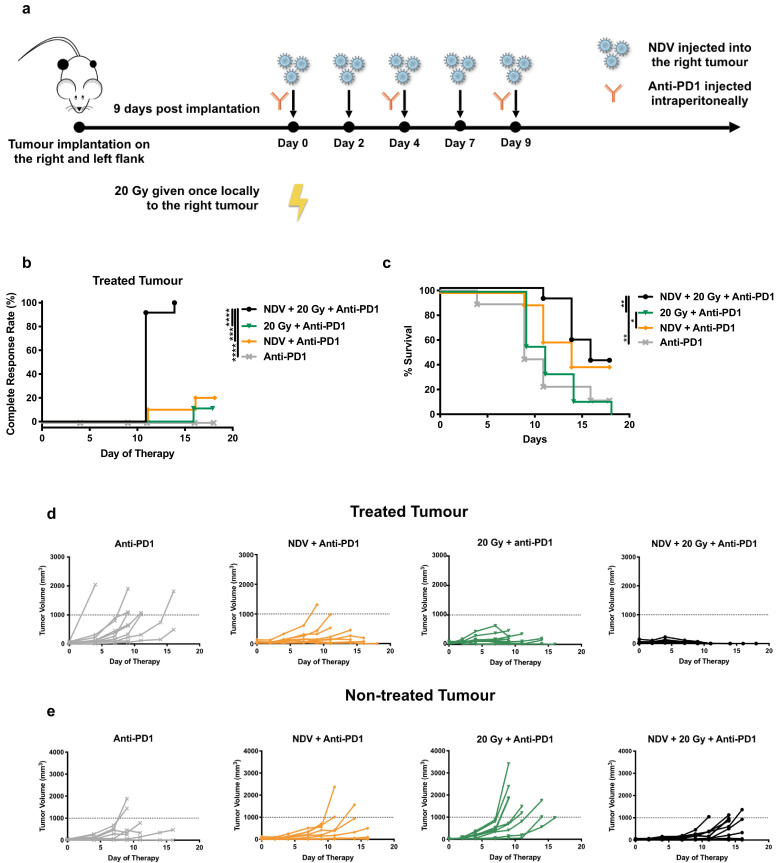
Both NDV and radiation in combination with checkpoint blockade may induce systemic anti-tumoural immunity with circulating CD8 + T cells playing a major role. [30, 33, 40] Here, we ask whether combining two localised therapies, intratumoural NDV and single fraction radiation at 20 Gy, in the setting of systemic anti-PD1 therapy will improve local tumour control and induce an abscopal effect to control systemic disease. We used a very advanced model of murine melanoma with visually apparent and palpable tumours on the bilateral flanks (Fig. 2a). The right flank was treated as indicated, while the left flank was naive to direct radiation or virus injection. All animals received systemic PD1 blockade. Animals treated with the trimodality therapy had superior local control compared to mice receiving either NDV or radiation in addition to anti-PD1 (Fig. 2b). At 18 days post-treatment, many of the irradiated animals met a humane endpoint based on ulcerated skin with exposure necessitating sacrifice. Survival was assessed at this time point, and animals receiving trimodality treatment had statistically better outcomes than mice treated with radiation + anti-PD1 or anti-PD1 alone (Fig. 2c). There was no statistically significant difference between the trimodality therapy and NDV+PD1. This suggests that the abscopal effect is driven primarily by the virus, and radiation adds superior local control (Fig. 2B) while not hampering the development of systemic immunity. Individual tumour volume progressions are shown for the treated (Fig. 2d) and non-treated (Fig. 2e) tumours. Several important observations may be made from these data. Local control is superior on the basis of complete response rates in the treated tumours in the trimodality therapy group. An abscopal effect leading to delayed tumour progression in the non-treated tumours improves the survival of mice receiving NDV plus anti-PD1 therapy. Interestingly, the addition of radiation to NDV plus anti-PD1 improves local control with rapid regression of the treated tumour, but does not significantly affect or interfere with the abscopal effect induced by NDV. Mice receiving 20 Gy + NDV had a complete response; however, long-term survival was not assessed as the mice frequently developed skin ulcerations at the site of the irradiated tumours leaving the underlying muscle exposed, requiring humane sacrifice. Because the 20 Gy radiation dose had high skin toxicity, we proceeded to perform a dose response curve to find an optimal dose of radiation in combination with NDV for local tumour control. Interestingly, we do not observe an abscopal effect with combining radiation and checkpoint blockade as observed in previous studies and this might be a consequence of the more aggressive bilateral B16 tumour model that we have used in this study as compared to previous work.
Fig. 2.
NDV and radiation combined with PD1 blockade prolong survival in a metastatic murine melanoma model:
(a) Mouse treatment scheme. 200,000 and 100,000 B16-F10 melanoma cells were implanted in the right and left flank of C57BL/6 mice (n = 9–12 mice per group). 9 days post-implantation, tumours were treated with five intratumoural administrations of NDV at 107 pfu every two days in the right flank tumour vs. mock treatment and three i.p. injections of anti-PD1 every four days. Certain cohorts of the tumour-implanted mice received 20 Gy of single fraction radiation to the right flank tumour vs. mock treatment. Tumour volumes were measured until mice reached a humane endpoint.
(b) Clinically complete regression rates, defined by the absence of a visible or palpable tumour, in response to treatment are plotted. Statistical analysis was conducted with the Mantel-Cox test. *** p < 0.001, **** p < 0.0001.
(c) Overall survival rates are also shown. Statistical analysis was conducted with the Mantel-Cox test. * p < 0.05, ** p < 0.01.
(d) Individual tumour volume progressions in the treated tumours receiving radiation and/or NDV injections (or mock treatment in the case of the anti-PD1 control).
(e) Individual tumour volume progressions in the non-treated tumours.
3.3. NDV and radiation therapy when combined with anti-PD1 improves anti-tumour efficacy in a dose-dependent manner
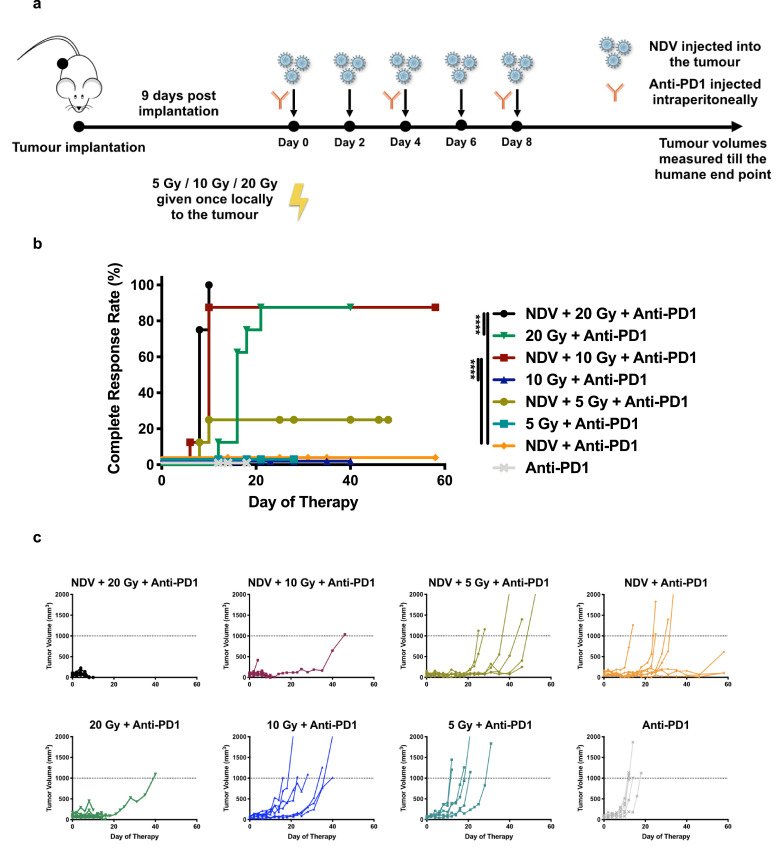
Localised NDV therapy when combined with systemic checkpoint blockade such as anti-CTLA4 potentiates an abscopal response in a bilateral B16-F10 tumour model in addition to mediating protection from tumour re-challenge. [33] Various preclinical studies have shown that PD1 blockade augments radiation when combined together in murine melanoma models. [30, 40] To assess the anti-tumour efficacy of combining three different therapeutic modalities in a single treatment regimen, B16-F10 melanoma cells were implanted in wildtype C57BL/6 mice. All mice received a checkpoint inhibitor, given evidence for efficacy with either radiation or oncolytic NDV cited above. To assay for an optimal radiation dose for both efficacy and toxicity in this combination, a radiation dose titration was performed. Tumours were treated 9 days post implantation with 0, 5, 10 or 20 Gy in one fraction. Intratumoural injections of NDV were administered every two days, three intraperitoneal injections of anti-PD1 were administered over the course in which the oncolytic virus was given, and tumour volumes were assessed (Fig. 3a).
Fig. 3.
NDV and radiation combination therapy when combined with PD1 blockade improves anti-tumour efficacy in a dose-dependent manner:
(a) Mouse treatment scheme. 200,000 B16-F10 melanoma cells were implanted in the right flank of C57BL/6 mice (n = 6–8 mice per group). 9 days post-implantation, tumours were treated with five intratumoural administrations of NDV at 107 pfu every two days vs. mock treatment and three i.p. injections of anti-PD1 every four days. Certain cohorts of the tumour-implanted mice received either 0, 5, 10 or 20 Gy of single fraction radiation. Tumour volumes were measured until mice reached a humane endpoint.
(b) Clinically complete regression rates, defined by the absence of a visible or palpable tumour, in response to treatment are plotted. Statistical analysis was conducted with the Mantel-Cox test. **** p < 0.0001.
(c) Individual tumour volume progressions.
The combination of radiation, NDV and anti-PD1 mAb significantly improved complete response rates compared to dual therapy (Fig. 3b). This is particularly evident at doses of 10 and 20 Gy. Superior complete remission rates are further demonstrated with the tumour volume progressions, with tumours treated with NDV in addition to 10 or 20 Gy and anti-PD1 demonstrating minimal growth relative to the other cohorts (Fig. 3c). Moreover, combining NDV along with 10 Gy + anti-PD1 significantly enhances the complete remission rates over both NDV + anti-PD1 and 10 Gy + anti-PD1 groups demonstrating the efficacy of combining all three modalities. The complete remission rate achieved by NDV + 10 Gy + anti-PD1 treatment group is statistically comparable to that achieved by NDV + 20 Gy + anti-PD1.
The local toxicity, namely ulcerations in the skin leaving exposed muscle requiring humane sacrifice, was substantial at 20 Gy and required humane sacrifice as per IACUC guidelines. Given the local toxicity of a single 20 Gy fraction, similar efficacy between trimodality treatment at either 10 or 20 Gy, and the strong improvement in tumour complete response rates when comparing NDV + 10 Gy (90%) vs. 10 Gy (0%) vs. NDV (0%) in addition to anti-PD1, 10 Gy appears to be an optimal dose for assessing the efficacy of the trimodality combination.
3.4. Intact immune system and checkpoint blockade is required for optimal tumour control when combining NDV and radiation
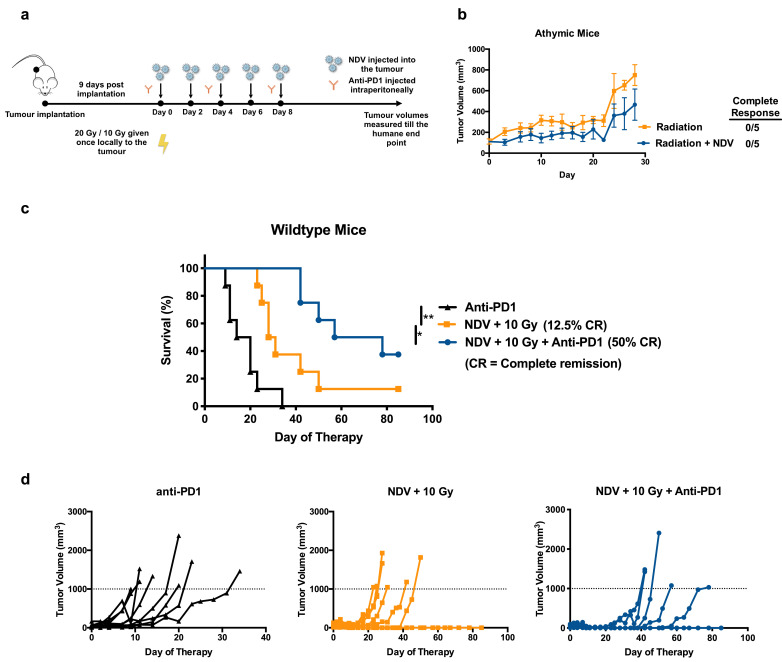
The B16-F10 melanoma in C57BL/6 mice is known to be poorly immunogenic. [41] Previous studies have shown that depletion of CD8 + T cells abrogates the therapeutic efficacy of the NDV in both the treated and non-treated tumours. [33] To initially assess the role of an intact immune system in the control of tumours treated with NDV plus RT, athymic nude mice were implanted with B16-F10 melanoma cells and treated with either a single dose of 20 Gy or 20 Gy combined with five intratumoural injections of NDV administered every two days (Fig. 4a). Average tumour volume progressions for the two treatment cohorts (Fig. 4b) over time demonstrate that the absence of any complete remissions in either groups can be attributed to the lack of an intact immune system, presumably the T cell compartment which is known to be critical in this setting for both radiotherapy or NDV when combined with checkpoint blockade, [30, 41] although the use of athymic mouse is not sufficient to conclude that a specific cell population is driving the efficacy observed in wildtype immunocompetent mice. No statistical significance was noted between the groups, and no tumour regressions were observed as opposed to in wild-type mice.
Fig. 4.
Intact immune system and checkpoint blockade is required for optimal tumour control when combining NDV and radiation:
(a) Mouse treatment scheme with average tumour volume progressions. 200,000 B16-F10 melanoma cells were implanted in the right flank of (B) athymic mice (n = 5 mice per group) or (C-E) wild-type mice (n = 7–8 mice per group).
(b) 9 days post implantation, tumours were treated with five intratumoural administrations of NDV at 107 pfu every two days vs. mock-treatment. Certain cohorts of the tumour implanted mice received radiation (20 Gy in one fraction) with or without NDV. Tumour volumes were measured until the humane end point, and the average tumour volume progressions are shown.
(c-d) Wild-type immunocompetent C57BL/6 mice were implanted with 200,000 B16-F10 melanoma cells in the right flank. Beginning 9 days post implantation, tumours were irradiated (10 Gy in one fraction), treated with five intratumoural administrations of NDV at 107 pfu every two days and three i.p. injections of anti-PD1 every 4 days and compared to mock-treated animals. (c) Overall survival and (d) the individual tumour volume progressions are shown. Statistical analyses for Kaplan-Meyer survival curves and the complete clinical response rates were conducted with the Mantel-Cox test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
To assess the importance of checkpoint blockade to this combination therapy, wild-type mice were treated with anti-PD1 alone, NDV + 10 Gy, or all three modalities in combination and assessed for long-term survival (Fig. 4c). Combining all three therapeutic modalities had the most significant effect in terms of overall survival. This is also depicted in the individual tumour volume progressions (Fig. 4d). Therefore, statistically superior long-term survival when combining single fraction radiation with oncolytic NDV is dependent upon an intact immune system and checkpoint blockade therapy.
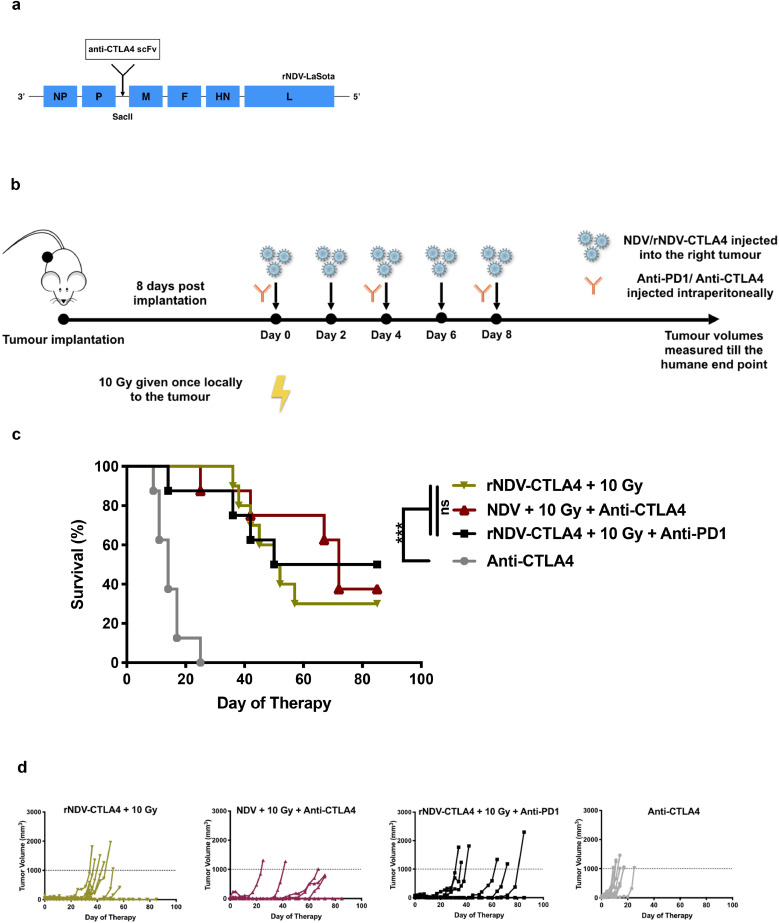
3.5. NDV expressing anti-CTLA4 is as potent in inducing an anti-tumour response as systemic anti-CTLA4 with radiation
Significant immune related adverse events limit checkpoint blockade in patients. [42] One possible way to overcome this would be tumour targeted delivery with an oncolytic virus that has been engineered to express anti-CTLA4. [35] To this end, we cloned and rescued recombinant NDV transgenically expressing anti-CTLA4 scFv (rNDV-CTLA4) (Fig. 5a). We then proceeded to assess its oncolytic effect in various combinations with RT (10 Gy single fraction) with or without additional systemic anti-PD1 mAb treatment (Fig. 5b). The overall survival rates (Fig. 5c) show that localised expression of anti-CTLA4 from intratumourally delivered NDV induces the same efficacy as systemic delivery of an anti-CTLA4 mAb when combined with wildtype NDV and RT. This is again reiterated with the individual tumour volume progressions for the different treatment cohorts (Fig. 5d). The addition of systemically delivered anti-PD1 to the combination of rNDV-CTLA4 and radiation did not improve overall survival.
Fig. 5.
NDV expressing anti-CTLA4 is as potent in inducing an anti-tumour response as systemic anti-CTLA4 with radiation
(a) Schematic representation of the rNDV-LaSota backbone showing the insertion site for the anti-CTLA4 scFv transgene in between the P and M gene's open reading frames.
(b) Mouse treatment scheme. 200,000 B16-F10 melanoma cells were implanted in the right flank of C57BL/6 mice (n = 8–10 mice per group). 8 days post implantation, tumour were treated with five intratumoural administrations of NDV or rNDV-aCTLA4 at 107 pfu every two days compared to mock-treated animals. Certain cohorts of the tumour-implanted mice received 10 Gy of radiation in a single fraction. Tumour volumes were measured until a humane endpoint.
(c) Overall survival rates are shown for the different treatment cohorts. Statistical analyses for the complete clinical response rates were conducted with the Mantel-Cox test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 or not statistically significant (ns).
(d) Individual tumour volume progressions.
4. Discussion
Oncolytic viruses represent a new paradigm in cancer therapy and include both naturally occurring and genetically modified viruses to efficiently kill cancer cells while sparing healthy tissue. RT and oncolytic viruses are both promising when combined with checkpoint inhibitors. Viruses are known radiosensitisers although oncolytic viruses have not been used as such beyond early phase clinical trials and the combination has not been established in the context of checkpoint blockade. Multimodality therapies with distinct mechanisms of action have the potential to improve cure rates will minimising side effects.
In the present study we have looked at NDV's potential as a radiosensitiser both in vitro and in vivo in combination with checkpoint inhibitor and radiation therapy in an aggressive murine model for melanoma. Previous studies have shown that NDV infection in vitro can induce significant up regulation of MHC class I, MHC class II as well as co-stimulatory molecules CD80 and CD86 in B16-F10 cells. [33] This might be mediated by virally induced type I IFN and IFNy expression from infected tumour cells, that has the added benefit of enhancing tumour immunogenicity. [43] Notably, we have utilised a very advanced tumour model in this study by beginning treatment with relatively large tumours 8–9 days after implantation. We demonstrate that NDV is a potent radiosensitiser in vitro at clinically significant doses and beyond. Moreover, the effect is seen most from 4 – 16 Gy, encompassing the optimal range reported for inducing the innate and adaptive immune response to RT. [44] This enhanced tumour lytic effect was observed in vivo in a radiation dose responsive manner when combined with NDV and a PD1 targeted mAb. Importantly, the trimodality therapy induced an abscopal effect to prolong survival in a murine model of melanoma. In the setting of checkpoint blockade, the observed abscopal effect was driven primarily by oncolytic NDV, while the addition of radiation significantly enhanced local control rates without hindering the development of systemic anti-tumoural immunity driven by the oncolytic virus. The combination of high dose RT (20 Gy) and intratumoural NDV failed to induce complete remissions in athymic nude mice that lack functional cell mediated immunity highlighting the importance of the immune system in mediating efficacy. NDV and RT enhance tumour control optimally with checkpoint blockade, indicating that they work together as immunotherapies in addition to their direct cytotoxic effects that can be observed in vitro.
Systemic toxicities and immune related adverse events that are associated with the use of checkpoint inhibitors in the clinic could potentially be reduced by targeted delivery of the therapeutic modality to the tumour compartment. We were able to achieve this with intratumoural delivery of a recombinant NDV engineered to express anti-CTLA4 scFv. Crucially, our experiments show that virally expressed anti-CTLA4 elicits statistically comparable survival as systemically administered anti-CTLA4 when combined with radiation therapy. Unexpectedly, we did not observe an added benefit with the use of dual checkpoint blockade with local, viral delivery of anti-CTLA4 when coupled with systemically administered anti-PD1. The lack of an augmented benefit may be indicative of an immune activation ceiling; however, the possible cellular mechanisms underlying this observed response remains to be elucidated and may be specific to this model as a role for combined CTLA4 and PD1 blockade is well established. [45]
This study provides a rationale for considering targeted delivery of checkpoint blockade via a virus in combination with radiation therapy to achieve a durable response without the associated side effects in the clinic. Both oncolytic viruses and RT have been used as stimulatory immunotherapies in combination with checkpoint inhibitors. Here, we provide the first in vivo support for a novel combination of cancer therapies. The trimodality combination of oncolytic NDV, single fraction RT (10 Gy), and checkpoint blockade provided superior tumour control compared to any combination of two treatments. However, a caveat and limitation to this study is the lack of a precise cellular mechanism underlying the potent ant-tumoural effects of this trimodality combination therapy and is the subject of future work. While multimodality therapies tend to have additive toxicities (e.g. traditional chemotherapies with radiation), rationally selected agents with distinct mechanisms of action may offer synergistic antineoplastic activity and reduced toxicities. In this case, an oncolytic NDV expressing anti-CTLA4 minimises systemic exposure to the checkpoint inhibitor while serving as a potent radioenhancer and immunotherapy.
Funding sources
The work was supported by the National Institutes of Health grant HHSN272201400008C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
G.V., P.H.G. and P.P. designed the research. G.V. and P.H.G. performed the research. G.V., and P.H.G. analysed data. G.V., P.H.G. and P.P. wrote the paper.
Declaration of Competing Interest
Ms. Vijayakumar has nothing to disclose, Dr. Palese has nothing to disclose, and Dr. Goff has nothing to disclose.
Acknowledgements
Thank you to Dr. Sara Cuadrado and Stephen McCroskery for assistance with mouse experiment, the Mount Sinai Hospital Department of Medicine and Dr. Salvatore Cilmi, Director of The Mount Sinai Hospital Internal Medicine Residency Program, for supporting Peter Goff. We also acknowledge Dr. Kevin Kelly, Director of the Mount Sinai Irradiator CoRE, and Dr. Jacob Kamen, Chief Radiation and Laser Safety Officer at Mount Sinai Hospital, for supporting the small animal irradiator.
References
- 1.Virchow R.L.K. J. B. Lippincott; Philadelphia: 1863. Cellular pathology as based upon physiological and pathological histology. [DOI] [PubMed] [Google Scholar]
- 2.Cooper Laurence J.N., Mittendorf Elizabeth A., Moyes Judy, Prabhakaran Sabitha. Immunotherapy in Translational. Cancer Research. 2018 [Google Scholar]
- 3.Woo E.Y., Yeh H., Chu C.S., Schlienger K., Carroll R.G., Riley J.L. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168(9):4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary B., Elkord E. Regulatory t cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016;4(3) doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer C., Cagnon L., Costa-Nunes C.M., Baumgaertner P., Montandon N., Leyvraz L. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundstedt A., O'Neill E.J., Nicolson K.S., Wraith D.C. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol. 2003;170(3):1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 8.Allison J.P. Immune checkpoint blockade in cancer therapy: the 2015 lasker-debakey clinical medical research award. JAMA. 2015;314(11):1113–1114. doi: 10.1001/jama.2015.11929. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin J., Hodi F.S., Wolchok J.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, NY) 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Liu F., Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine (Baltimore) 2017;96(18):e6369. doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A. Pembrolizumab versus chemotherapy for PD-L1–Positive non–small-cell lung cancer. New England Journal of Medicine. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 18.Marincola F.M., Jaffee E.M., Hicklin D.J., Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 19.Sucker A., Zhao F., Real B., Heeke C., Bielefeld N., Mabetaen S. Genetic evolution of T-cell resistance in the course of melanoma progression. Clin Cancer Res. 2014;20(24):6593–6604. doi: 10.1158/1078-0432.CCR-14-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am J Clin Dermatol. 2017;18(1):1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer A.C., Sorger P.K. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171(7):1678–1691. doi: 10.1016/j.cell.2017.11.009. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(22):2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119. doi: 10.1016/j.cell.2017.08.027. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36(17):1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl. Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiotto M., Fu Y.X., Weichselbaum R.R. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Science immunology. 2016;1(3) doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaria S., Kawashima N., Yang A.M., Devitt M.L., Babb J.S., Allison J.P. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 29.Dewan M.Z., Galloway A.E., Kawashima N., Dewyngaert J.K., Babb J.S., Formenti S.C. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharabi A.B., Nirschl C.J., Kochel C.M., Nirschl T.R., Francica B.J., Velarde E. Stereotactic radiation therapy augments antigen-specific PD-1-Mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flanagan A.D., Love R., Tesar W. Propagation of newcastle disease virus in ehrlich ascites cells in vitro and in vivo. Proc Soc Exp Biol Med. 1955;90(1):82–86. doi: 10.3181/00379727-90-21945. [DOI] [PubMed] [Google Scholar]
- 32.Sinkovics J.G., Horvath J.C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16(1):1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 33.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226) doi: 10.1126/scitranslmed.3008095. 226ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamarin D., Holmgaard R.B., Ricca J., Plitt T., Palese P., Sharma P. Intratumoral modulation of the inducible co-stimulator icos by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat Commun. 2017;8:14340. doi: 10.1038/ncomms14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton J.R., Vijayakumar G., Palese P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model. Cell Rep. 2018;22(1):1–7. doi: 10.1016/j.celrep.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Touchefeu Y., Vassaux G., Harrington K.J. Oncolytic viruses in radiation oncology. Radiother Oncol. 2011;99(3):262–270. doi: 10.1016/j.radonc.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 37.Vijayakumar G., Zamarin D. Design and production of newcastle disease virus for intratumoral immunomodulation. Methods Mol Biol. 2020;2058:133–154. doi: 10.1007/978-1-4939-9794-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottgens E.L., Ostheimer C., Span P.N., Bussink J., Hammond E.M. HPV, hypoxia and radiation response in head and neck cancer. Br J Radiol. 2018 doi: 10.1259/bjr.20180047. 20180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dok R., Bamps M., Glorieux M., Zhao P., Sablina A., Nuyts S. Radiosensitization approaches for HPV-positive and HPV-negative head and neck squamous carcinomas. Int J Cancer. 2019 doi: 10.1002/ijc.32558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang C., Wang X., Soh H., Seyedin S., Cortez M.A., Krishnan S. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Saffold S., Cao X., Krauss J., Chen W. Eliciting t cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161(10):5516–5524. [PubMed] [Google Scholar]
- 42.Wolchok J.D., Neyns B., Linette G., Negrier S., Lutzky J., Thomas L. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The Lancet Oncology. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 43.Zamarin D., Martinez-Sobrido L., Kelly K., Mansour M., Sheng G., Vigil A. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17(4):697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanpouille-Box C., Alard A., Aryankalayil M.J., Sarfraz Y., Diamond J.M., Schneider R.J. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duraiswamy J., Kaluza K.M., Freeman G.J., Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]