Abstract
Background
COPD increases susceptibility to sleep disturbances, which may in turn predispose to increased respiratory symptoms. The objective of this study was to evaluate, in a population-based sample, the relationship between subjective sleep quality and risk of COPD exacerbations.
Methods
Data were obtained from the Canadian Cohort Obstructive Lung Disease (CanCOLD) study. Participants with COPD who had completed 18 months of follow-up were included. Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI) and a three-factor analysis. Symptom-based (dyspnea or sputum change ≥ 48 h) and event-based (symptoms plus medication or unscheduled health services use) exacerbations were assessed. Association of PSQI with exacerbation rate was assessed by using negative binomial regression. Exacerbation-free survival was also assessed.
Results
A total of 480 participants with COPD were studied, including 185 with one or more exacerbations during follow-up and 203 with poor baseline sleep quality (PSQI score > 5). Participants with subsequent symptom-based exacerbations had higher median baseline PSQI scores than those without (6.0 [interquartile range, 3.0-8.0] vs 5.0 [interquartile range, 2.0-7.0]; P = .01), and they were more likely to have baseline PSQI scores > 5 (50.3% vs 37.3%; P = .01). Higher PSQI scores were associated with increased symptom-based exacerbation risk (adjusted rate ratio, 1.09; 95% CI, 1.01-1.18; P = .02) and event-based exacerbation risk (adjusted rate ratio, 1.10; 95% CI, 1.00-1.21; P = .048). The association occurred mainly in those with undiagnosed COPD. Strongest associations were with Factor 3 (sleep disturbances and daytime dysfunction). Time to symptom-based exacerbation was shorter in participants with poor sleep quality (adjusted hazard ratio, 1.49; 95% CI, 1.09-2.03).
Conclusions
Higher baseline PSQI scores were associated with increased risk of COPD exacerbation over 18 months’ prospective follow-up.
Key Words: acute exacerbation of chronic bronchitis, COPD, sleep medicine
Abbreviations: GOLD, Global Obstructive Lung Disease; HR, hazard ratio; PSQI, Pittsburgh Sleep Quality Index; RR, rate ratio
Sleep disorders in patients with COPD are common, including increased prevalence of insomnia, hypnotic medication use, and excessive daytime somnolence.1, 2, 3, 4 Nocturnal alterations in ventilation and symptoms such as cough and wheeze are also common.2, 3 These symptoms can result in difficulty initiating and maintaining sleep, with possible consequences of daytime somnolence, cognitive changes,5 and altered immune function,6 predisposing to acute exacerbations of COPD.7
COPD has been shown to be associated with poor sleep quality as measured by using the Pittsburgh Sleep Quality Index (PSQI).8 Sleep quality is an important predictor of health-related quality of life in COPD.7, 8, 9 Acute exacerbations of COPD are common in the course of the disease and lead to deterioration in health status and increased mortality.10 Therefore, there has been a growing interest in sleep quality in COPD and its effect on exacerbations.
The major objective of the current study was to evaluate, in a population-based sample, the relation between subjective sleep quality and risk of COPD exacerbations. We also evaluated sleep quality and risk of exacerbations according to physician-diagnosed and undiagnosed COPD. We hypothesized that individuals with COPD and poor sleep quality would be more prone to exacerbations during an 18-month follow-up period compared with individuals with good sleep quality.
Patients and Methods
Study Population
This study was embedded in the Canadian Cohort Obstructive Lung Disease (CanCOLD) trial, a prospective, multicenter, population-based cohort study. Participants were selected from a core sample of 6,592 individuals of the Canadian Obstructive Lung Disease (COLD) study identified through random digit dialing (land telephone line) from nine Canadian urban sites. CanCOLD participants underwent postbronchodilator spirometry per guidelines.11 They were classified as having either COPD, based on spirometric Global Obstructive Lung Disease (GOLD) stages I to IV; at-risk individuals (smokers with normal postbronchodilator spirometry results); or healthy control subjects (never smokers with normal spirometry results). Assessments were performed at baseline, 18 months, and 3 years following study initiation. Details of the CanCOLD study have been previously published.12 Data from participants who had completed 18 months’ follow-up were extracted from the CanCOLD database on March 12, 2018.
Measures
Self-reported sleep quality was measured by using the validated PSQI,13 which consists of 19 questions, grouped into seven components, each weighted equally on a scale of 0 to 3. Questions include subjective sleep quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Total scores range from 0 to 21. A score > 5 defines poor sleep. A validated three-factor analysis was also used, which is favored as statistically more sound over a single score.14 Factor 1, Sleep Efficiency, includes sleep duration and efficiency components (score, 0-6). Factor 2, Sleep Quality, includes the perceived sleep quality, sleep latency, and sleep medication use components (score, 0-9). Factor 3, Daily Disturbances, includes sleep disturbances (eg, bathroom use, breathing issues, pain) and sleep-related daytime dysfunction (eg, sleepiness, enthusiasm) components (score, 0-6).
Exacerbations
Exacerbations were self-reported by each participant prospectively by using a Web-based tool and also assessed by telephone interview by a study coordinator every 3 months. An exacerbation event within the 18-month follow-up was the primary outcome variable. Exacerbations were classified as either “symptom-based” or “event based.” A symptom-based exacerbation required at least one exacerbation of increased dyspnea, sputum volume, or sputum purulence lasting at least 48 h. An event-based exacerbation was defined as a symptom-based exacerbation plus utilization of antibiotics or corticosteroids, or accessing health-care services (eg, ED, unscheduled physician visit, hospitalization).15
Statistical Analysis
Descriptive data are reported as means and SDs, median and interquartile range, or number and percentage. Comparisons between groups were performed with analysis of variance or nonparametric Kruskal-Wallis or Mann-Whitney U test, Cochran-Mantel-Haenszel test for categorical variables (three groups), or χ2 test (two groups). Univariable and multivariable negative binomial regression16 models were used to estimate the association between PSQI scores and rate of exacerbations, and to calculate the exacerbation rate ratio and 95% CIs. Covariates were chosen for their association with poor sleep quality and exacerbations, based on univariable analyses or a priori. Results were calculated for all individuals with COPD, and by physician-diagnosed and undiagnosed COPD, as reported by participants.15 A Cox proportional hazards regression model was used to assess whether poor sleep quality was associated with increased risk of exacerbations. A P value < .05 was considered statistically significant. Analyses were performed by using SAS version 9.3.25 (SAS Institute, Inc.).
Results
Participants
A total of 1,556 subjects were enrolled in the CanCOLD database at the time of the current study. Of those, 1,040 had baseline PSQI data and completed the 18-month follow-up, including 480 with COPD, 324 at-risk individuals, and 236 healthy control subjects. Baseline characteristics are shown in Table 1. Characteristics of excluded subjects (239 with COPD) were similar except that 52% were male compared with 58% of those included (P = .02), and CPAP use was 2.5% vs 4.9%, respectively (P = .03). Of the 1,040 participants, 427 (41.1%) had poor quality sleep with PSQI scores > 5. There was no significant difference in median PSQI scores between those with COPD, at-risk subjects, and healthy control subjects, or among those with GOLD stages I through IV. The remainder of the results pertain to the COPD group only.
Table 1.
Baseline Characteristics and Exacerbations During Follow-up of All Subjects Who Completed the 18-Month Follow-up
| Characteristic | Total (N = 1,040) | COPD |
At-risk Control Subjects (n = 324) | Healthy Control Subjects (n = 236) | P Valueb | |||
|---|---|---|---|---|---|---|---|---|
| All (n = 480) | Diagnosed COPD (n = 141) | Undiagnosed COPD (n = 339) | P Valuea | |||||
| Age, y | 66.6 ± 9.7 | 67.5 ± 10.1 | 66.8 ± 9.2 | 67.8 ± 10.4 | .27 | 65.6 ± 9.2 | 66.3 ± 9.4 | .046c |
| Male sex | 605 (58.2) | 302 (62.9) | 71 (50.4) | 231 (68.1) | < .001c | 191 (59.0) | 112 (47.5) | < .001c |
| BMI, kg/m2 | 27.8 ± 5.4 | 27.4 ± 5.3 | 27.4 ± 5.1 | 27.4 ± 5.3 | .88 | 28.5 ± 5.7 | 27.5 ± 5.1 | .04c |
| Ever smokers | 665 (63.9) | 341 (71.0) | 119 (84.4) | 222 (65.5) | < .001c | 324 (100.0) | … | … |
| Current smokers | 161 (15.5) | 92 (19.2) | 37 (26.2) | 55 (16.2) | .01c | 69 (21.3) | … | < .001c |
| Cigarette pack-years | 16.5 ± 22.4 | 23.5 ± 25.7 | 35.7 ± 27.4 | 18.5 ± 23.2 | < .001c | 18.5 ± 18.8 | … | < .001c |
| FEV1, % predicted | 92.2 ± 20.1 | 82.6 ± 19.5 | 71.5 ± 19.6 | 87.2 ± 17.5 | < .001c | 99.7 ± 16.8 | 101.4 ± 16.7 | < .001c |
| FEV1/FVC, % | 69.8 ± 10.4 | 61.1 ± 8.3 | 56.9 ± 10.4 | 62.8 ± 6.6 | < .001c | 76.9 ± 4.6 | 77.6 ± 4.6 | < .001c |
| COPD groups | ||||||||
| GOLD stage I | 271 (26.1) | 271 (56.5) | 51 (36.2) | 220 (64.9) | < .001c | … | … | … |
| GOLD stages II and higher | 209 (20.1) | 209 (43.5) | 90 (63.8) | 119 (35.1) | < .001c | … | … | … |
| mMRC dyspnea scale score ≥ 3/5 | 54 (5.7) | 37 (8.5) | 21 (17.6) | 16 (5.0) | < .001c | 7 (3.2) | 10 (3.5) | .004c |
| CAT score | 6.6 ± 5.7 | 7.6 ± 6.5 | 11.8 ± 7.4 | 5.9 ± 5.1 | < .001c | 6.0 ± 5.3 | 5.5 ± 4.2 | < .001c |
| SGRQ-total | 12.8 ± 14.0 | 15.3 ± 15.1 | 25.5 ± 17.2 | 11.0 ± 11.8 | < .001c | 9.5 ± 11.7 | 8.2 ± 10.4 | < .001c |
| Physician-diagnosed COPD | 189 (18.2) | 141 (29.4) | 141 (100.0) | … | … | 35 (10.8) | 13 (5.5) | < .001c |
| Long-term oxygen therapy | 1 (0.1) | 1 (0.2) | 1 (0.7) | 0 | .29 | 0 | 0 | .99 |
| BPAP use | 3 (0.3) | 0 | 0 | 0 | … | 1 (0.3) | 2 (0.8) | .09 |
| CPAP use | 51 (4.9) | 23 (4.8) | 11 (7.9) | 12 (3.6) | .06 | 17 (5.3) | 11 (4.7) | .95 |
| Self-reported comorbidities | ||||||||
| Angina | 48 (4.6) | 19 (4.0) | 8 (5.7) | 11 (3.2) | .21 | 18 (5.6) | 11 (4.7) | .57 |
| Myocardial infarction | 40 (3.8) | 18 (3.8) | 8 (5.7) | 10 (2.9) | .15 | 15 (4.6) | 7 (3.0) | .59 |
| Major depression | 58 (5.6) | 36 (7.5) | 17 (12.1) | 19 (5.6) | .01c | 15 (4.6) | 7 (3.0) | .03c |
| Inhaler use | ||||||||
| LABA or LAMA | 12 (1.2) | 9 (1.9) | 8 (5.7) | 1 (0.3) | < .001c | 3 (0.9) | .07 | |
| ICS alone | 62 (6.0) | 34 (7.1) | 15 (10.6) | 19 (5.6) | .05 | 18 (5.6) | 10 (4.2) | .29 |
| ICS combined with LABA/LAMA | 119 (11.4) | 93 (19.4) | 56 (39.7) | 37 (10.9) | < .001c | 19 (5.9) | 7 (3.0) | < .001c |
| Any | 193 (18.6) | 136 (28.3) | 79 (56.0) | 57 (16.8) | < .001c | 40 (12.3) | 17 (7.2) | < .001c |
| PSQI variables | ||||||||
| Global PSQI score (scale, 0-21) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | .042c | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | .88 |
| Factor 1: Sleep Efficiency | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) | .38 | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) | .46 |
| Factor 2: Sleep Quality | 0.7 (0.3, 1.0) | 0.7 (0.3, 1.0) | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .01c | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .85 |
| Factor 3: Daily Disturbance | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | .29 | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | .45 |
| Insomnia (global PSQI score > 5) | 427 (41.1) | 203 (42.3) | 69 (48.9) | 134 (39.5) | .06 | 131 (40.4) | 93 (39.4) | .73 |
| Exacerbation frequency | ||||||||
| 0 | 686 (66.0) | 295 (61.5) | 65 (46.1) | 230 (67.8) | < .001c | 216 (66.7) | 175 (74.2) | .001c |
| 1 | 226 (21.7) | 107 (22.3) | 36 (25.5) | 71 (20.9) | .27 | 77 (23.8) | 42 (17.8) | .22 |
| 2 | 81 (7.8) | 50 (10.4) | 24 (17.0) | 26 (7.7) | .001c | 15 (4.6) | 16 (6.8) | .01c |
| ≥ 3 | 47 (4.5) | 28 (5.8) | 16 (11.3) | 12 (3.5) | < .001c | 16 (4.9) | 3 (1.3) | .01c |
Data are presented as mean ± SD, No. (%), or median (interquartile range). BPAP = bilevel positive airway pressure; CAT = COPD Assessment Test; ICS = inhaled corticosteroid; LABA = long-acting beta-agonist; LAMA = long-acting muscarinic antagonist; mMRC = modified Medical Research Council scale; PSQI = Pittsburgh Sleep Quality Index; SGRQ = St. George’s Respiratory Questionnaire.
Comparing individuals with diagnosed COPD vs undiagnosed COPD among patients with COPD.
Comparing individuals with COPD with exacerbations vs without exacerbations, at-risk control subjects, and healthy control subjects.
Statistically significant at P < .05.
The majority of subjects with COPD were undiagnosed by a physician (71%). The mean ± SD FEV1 of those with diagnosed COPD was 2.0 ± 0.7 L, compared with 2.5 ± 0.8 L in those with undiagnosed COPD (P < .001). Those with diagnosed COPD were more likely to be prescribed inhaled treatment (56% vs 17%; P < .001). This group had poorer quality sleep than those with undiagnosed COPD (PSQI score, 5.7 ± 3.3 vs 5.1 ± 3.3; P = .04) and were more likely to have at least 1 exacerbation in the follow-up period (54% vs 32%; P < .01). The overall exacerbation rate was higher in the diagnosed group than in the undiagnosed COPD group.
Baseline PSQI Scores in Those With and Without Exacerbations
Individuals with one or more symptom-based exacerbation during the 18-month follow-up period had a higher median baseline global PSQI score (6.0 vs 5.0; P = .01) and higher scores in Factors 2 and 3, compared with those without exacerbation (Table 2). They were also more likely to be poor sleepers (PSQI score > 5). Those with an event-based exacerbation during follow-up had significantly higher baseline Factor 3 scores.
Table 2.
Baseline PSQI According to Exacerbation Status During the 18-Month Follow-up Period
| PSQI Variable | All COPD (n = 480) |
|||||
|---|---|---|---|---|---|---|
| Symptom-based Exacerbation |
No Exacerbation |
P Value | Event-based Exacerbation |
No Exacerbation |
P Value | |
| (n = 185) | (n = 295) | (n = 139) | (n = 341) | |||
| Global PSQI Score (scale, 0-21), median (Q1, Q3) | 6.0 (3.0, 8.0) | 5.0 (2.0, 7.0) | .01a | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | .19 |
| Factor 1: Sleep Efficiency, median (Q1, Q3) | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .07 | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .27 |
| Factor 2: Sleep Quality, median (Q1, Q3) | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .04a | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .25 |
| Factor 3: Daily Disturbance, median (Q1, Q3) | 1.0 (0.5, 1.0) | 0.5 (0.5, 1.0) | < .001a | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | .03a |
| Poor sleeper (global PSQI score > 5), No. (%) | 93 (50.3) | 110 (37.3) | .01a | 62 (44.6) | 141 (41.3) | .51 |
| Diagnosed COPD (n = 141) |
||||||
|---|---|---|---|---|---|---|
| Symptom-based Exacerbation |
No Exacerbation |
P Value | Event-based Exacerbation |
No Exacerbation |
P Value | |
| (n = 76) | (n = 65) | (n = 66) | (n = 75) | |||
| Global PSQI Score (scale, 0-21), median (Q1, Q3) | 5.0 (3.0, 8.5) | 5.0 (3.0, 7.0) | .64 | 5.0 (3.0, 8.0) | 6.0 (4.0, 8.0) | .46 |
| Factor 1: Sleep Efficiency, median (Q1, Q3) | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .29 | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .5 |
| Factor 2: Sleep Quality, median (Q1, Q3) | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .64 | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.3) | .12 |
| Factor 3: Daily Disturbance, median (Q1, Q3) | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | .045a | 1.0 (0.5, 1.0) | 1.0 (0.5, 1.0) | .75 |
| Poor sleeper (global PSQI score > 5), No. (%) | 37 (48.7) | 32 (49.2) | .94 | 29 (43.9) | 40 (53.3) | .26 |
| Undiagnosed COPD (n = 339) |
||||||
|---|---|---|---|---|---|---|
| Symptom-based exacerbation |
No Exacerbation |
P Value | Event-based exacerbation |
No Exacerbation |
P Value | |
| (n = 109) | (n = 230) | (n = 73) | (n = 266) | |||
| Global PSQI Score (scale, 0-21), median (Q1, Q3) | 6.0 (3.0, 8.0) | 4.0 (2.0, 7.0) | .01a | 5.0 (3.0, 8.0) | 5.0 (2.0, 7.0) | .10 |
| Factor 1: Sleep Efficiency, median (Q1, Q3) | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .20 | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | .51 |
| Factor 2: Sleep Quality, median (Q1, Q3) | 0.7 (0.3, 1.3) | 0.5 (0.0, 1.0) | .02a | 0.7 (0.3, 1.3) | 0.7 (0.3, 1.0) | .05 |
| Factor 3: Daily Disturbance, median (Q1, Q3) | 1.0 (0.5, 1.5) | 0.5 (0.5, 1.0) | < .001a | 1.0 (0.5, 1.0) | 0.5 (0.5, 1.0) | .08 |
| Poor sleeper (global PSQI score > 5), No. (%) | 56 (51.4) | 78 (33.9) | .001a | 33 (45.2) | 101 (38.0) | .26 |
Data are presented as median (interquartile range) or No. (%). Comparisons were conducted by using χ2 or Mann-Whitney U tests. See Table 1 legend for expansion of abbreviation.
Statistically significant at P < .05.
When evaluating those with diagnosed and undiagnosed COPD separately, both groups reported higher baseline Factor 3 scores in association with subsequent symptom-based exacerbations. In those with undiagnosed COPD but not those with diagnosed COPD, a statistically significant association with symptom-based exacerbations was also found for global PSQI score, Factor 2 score, and PSQI score > 5; association between event-based exacerbations and Factor 2 and 3 scores did not reach statistical significance (Table 2).
Risk of Exacerbation in Relation to Baseline PSQI
Table 3 shows that in all individuals with COPD, higher baseline global PSQI score, Factor 1 and Factor 3 scores, and PSQI score > 5 were associated with a greater risk of symptom-based exacerbations during follow-up, in univariable and multivariable analyses (adjusted for age, sex, BMI, smoking status, depression, angina, baseline inhaled respiratory medications, FEV1 percent predicted, and modified Medical Research Council scale score). Risk of event-based exacerbations showed a significant association with global PSQI score and associations that trended to statistical significance with Factors 1 and 3.
Table 3.
Sleep Quality and the Risk of Exacerbation (Negative Binomial Model)
| Baseline Variable | Incidence of Symptom-based Exacerbation |
Incidence of Event-based Exacerbation |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| All COPD (n = 480) | ||||||||
| Global PSQI score (/2 points) | 1.11 (1.02-1.21) | .01a | 1.12 (1.03-1.22) | .007a | 1.10 (1.00-1.21) | .06 | 1.10 (1.00-1.21) | .048a |
| Factor 1: Sleep Efficiency (/1 point) | 1.21 (1.02-1.43) | .03a | 1.23 (1.03-1.46) | .02a | 1.20 (0.97-1.47) | .09 | 1.20 (0.98-1.47) | .07 |
| Factor 2: Sleep Quality (/1 point) | 1.16 (0.92-1.45) | .21 | 1.19 (0.95-1.49) | .13 | 1.15 (0.88-1.51) | .30 | 1.17 (0.89-1.52) | .26 |
| Factor 3: Daily Disturbance (/1 point) | 1.55 (1.14-2.11) | .005a | 1.53 (1.13-2.06) | .006a | 1.44 (1.00-2.08) | .05 | 1.42 (0.99-2.02) | .06 |
| Poor sleeper, yes vs no | 1.36 (1.02-1.81) | .04a | 1.41 (1.06-1.87) | .02a | 1.17 (0.82-1.65) | .39 | 1.18 (0.84-1.66) | .35 |
| Physician-diagnosed COPD (n = 141) | ||||||||
| Global PSQI score (/2 points) | 1.04 (0.92-1.18) | .50 | 1.09 (0.96-1.25) | .19 | 0.99 (0.86-1.14) | .88 | 1.00 (0.86-1.16) | .99 |
| Factor 1: Sleep Efficiency (/1 point) | 1.24 (0.98-1.56) | .07 | 1.28 (0.99-1.64) | .06 | 1.19 (0.91-1.56) | .19 | 1.18 (0.88-1.57) | .27 |
| Factor 2: Sleep Quality (/1 point) | 0.88 (0.63-1.22) | .45 | 1.07 (0.74-1.54) | .73 | 0.74 (0.50-1.09) | .13 | 0.83 (0.54-1.28) | .41 |
| Factor 3: Daily Disturbance (/1 point) | 1.22 (0.75-1.97) | .43 | 1.15 (0.71-1.85) | .57 | 1.02 (0.58-1.77) | .96 | 0.90 (0.52-1.56) | .71 |
| Poor sleeper, yes vs no | 0.97 (0.64-1.47) | .89 | 1.11 (0.73-1.69) | .62 | 0.79 (0.49-1.27) | .33 | 0.84 (0.52-1.36) | .48 |
| Undiagnosed COPD (n = 339) | ||||||||
| Global PSQI score (/2 points) | 1.14 (1.02-1.27) | .02a | 1.16 (1.04-1.29) | .01a | 1.17 (1.03-1.33) | .02a | 1.18 (1.04-1.35) | .01a |
| Factor 1: Sleep Efficiency (/1 point) | 1.14 (0.90-1.44) | .29 | 1.23 (0.97-1.57) | .09 | 1.14 (0.85-1.53) | .40 | 1.21 (0.91-1.63) | .19 |
| Factor 2: Sleep Quality (/1 point) | 1.30 (0.97-1.75) | .08 | 1.31 (0.97-1.76) | .08 | 1.51 (1.07-2.14) | .02a | 1.48 (1.04-2.09) | .03a |
| Factor 3: Daily Disturbance (/1 point) | 1.77 (1.21-2.59) | .003a | 1.78 (1.21-2.63) | .003a | 1.86 (1.17-2.94) | .008a | 1.87 (1.18-2.99) | .008a |
| Poor sleeper, yes vs no | 1.61 (1.10-2.34) | .01a | 1.73 (1.17-2.55) | .006a | 1.50 (0.93-2.41) | .09 | 1.63 (1.00-2.65) | .050a |
Multivariable analyses were adjusted for baseline age, sex, BMI, current smoking, angina, major depression, any inhaled respiratory medications, baseline FEV1 percent predicted, and mMRC. RR = rate ratio. See Table 1 legend for expansion of other abbreviation.
Statistically significant at P < .05.
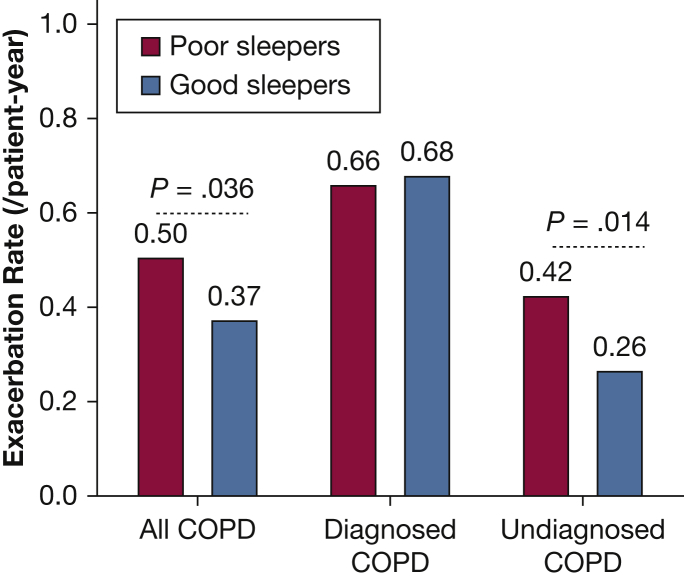
In subgroup assessments, a higher exacerbation rate was associated with poor sleep (PSQI score > 5) in the undiagnosed group only (Fig 1). In those with diagnosed COPD, the rate of exacerbations was high irrespective of sleep quality. In the undiagnosed group, the risk of exacerbation, both symptom-based and event-based, was associated with higher baseline global PSQI score, Factor 2 and 3 scores, and PSQI score > 5 (Table 3). In the subgroup of diagnosed COPD, there were no significant associations. Analyses were repeated excluding individuals with self-reported asthma (n = 320). Results were consistent with the primary models but showed a stronger effect for Factor 3 (Table 4).
Figure 1.
Symptom-based exacerbation rate in poor vs good sleepers. Poor sleepers were defined as those having a Pittsburgh Sleep Quality Index score > 5 at baseline.
Table 4.
Sleep Quality and the Risk of Exacerbation Excluding Individuals With Self-Reported Asthma (Negative Binomial Model)
| Baseline Variable | Incidence of Symptom-based Exacerbation |
Incidence of Event-based Exacerbation |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Patients with COPD (n = 321) | ||||||||
| Global PSQI Score (/2 points) | 1.10 (0.97-1.25) | .12 | 1.16 (1.02-1.31) | .02a | 1.04 (0.88-1.22) | .66 | 1.10 (0.94-1.28) | .23 |
| Factor 1: Sleep Efficiency (/1 point) | 1.06 (0.81-1.38) | .69 | 1.18 (0.91-1.53) | .21 | 0.92 (0.64-1.32) | .65 | 1.08 (0.78-1.50) | .64 |
| Factor 2: Sleep Quality (/1 point) | 1.14 (0.82-1.59) | .44 | 1.24 (0.90-1.72) | .18 | 1.03 (0.67-1.59) | .90 | 1.11 (0.74-1.67) | .61 |
| Factor 3: Daily Disturbance (/1 point) | 2.10 (1.35-3.27) | .001a | 2.23 (1.46-3.40) | < .001a | 1.76 (1.01-3.07) | .046a | 2.03 (1.19-3.46) | .01a |
| Poor sleeper, yes vs no | 1.55 (1.03-2.33) | .04a | 1.67 (1.12-2.48) | .01a | 1.18 (0.69-2.02) | .54 | 1.33 (0.80-2.20) | .27 |
| Physician-diagnosed COPD (n = 72) | ||||||||
| Global PSQI Score (/2 points) | 0.95 (0.80-1.13) | .55 | 1.24 (0.96-1.60) | .10 | 0.78 (0.62-0.99) | .04a | 0.87 (0.61-1.25) | .46 |
| Factor 1: Sleep Efficiency (/1 point) | 0.96 (0.65-1.44) | .86 | 1.42 (0.92-2.18) | .11 | 0.63 (0.35-1.13) | .12 | 1.00 (0.53-1.86) | .99 |
| Factor 2: Sleep Quality (/1 point) | 0.70 (0.44-1.11) | .13 | 1.24 (0.63-2.44) | .53 | 0.45 (0.25-0.84) | .01a | 0.58 (0.23-1.50) | .26 |
| Factor 3: Daily Disturbance (/1 point) | 1.64 (0.85-3.16) | .14 | 1.80 (0.74-4.38) | .20 | 1.14 (0.49-2.67) | .76 | 0.90 (0.30-2.70) | .85 |
| Poor sleeper, yes vs no | 0.83 (0.46-1.51) | .55 | 1.88 (0.83-4.25) | .13 | 0.46 (0.21-0.98) | .04a | 0.78 (0.27-2.23) | .64 |
| Nonphysician-diagnosed COPD (n = 249) | ||||||||
| Global PSQI Score (/2 points) | 1.20 (1.02-1.42) | .03a | 1.24 (0.96-1.60) | .10 | 1.27 (1.02-1.58) | .03a | 1.27 (1.03-1.57) | .03a |
| Factor 1: Sleep Efficiency (/1 point) | 1.12 (0.80-1.58) | .50 | 1.42 (0.92-2.18) | .11 | 1.19 (0.76-1.88) | .45 | 1.19 (0.75-1.89) | .46 |
| Factor 2: Sleep Quality (/1 point) | 1.46 (0.94-2.29) | .10 | 1.24 (0.63-2.44) | .53 | 1.78 (1.00-3.19) | .051 | 1.66 (0.95-2.91) | .08 |
| Factor 3: Daily Disturbance (/1 point) | 2.40 (1.37-4.22) | .002a | 1.80 (0.74-4.38) | .20 | 2.39 (1.18-4.84) | .02a | 3.08 (1.52-6.22) | .002a |
| Poor sleeper, yes vs no | 2.11 (1.25-3.56) | .005a | 1.88 (0.83-4.25) | .13 | 2.27 (1.12-4.59) | .02a | 1.98 (0.97-4.02) | .06 |
Time to Exacerbation
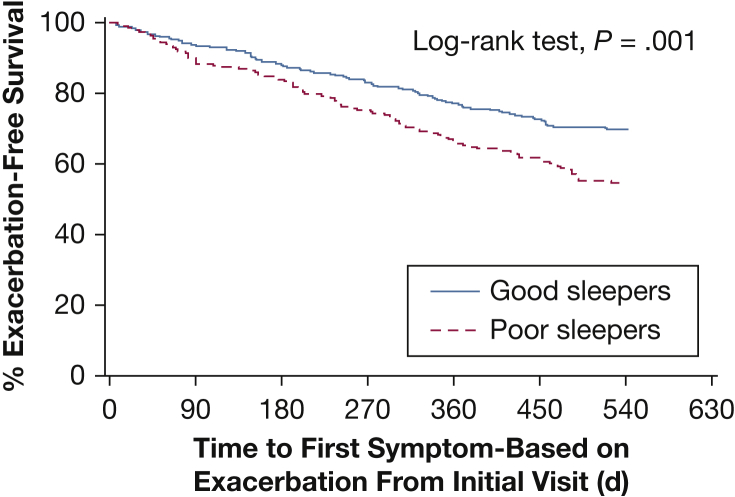
Poor baseline sleep quality was associated with a shorter time to symptom-based exacerbations (hazard ratio [HR], 1.84 [95% CI, 1.20-2.83], unadjusted; HR, 1.49 [95% CI, 1.09-2.03], adjusted for baseline age, sex, BMI, current smoking, angina, major depression, inhaled respiratory medications, FEV1 percent predicted, and modified Medical Research Council scale score). The Kaplan-Meier survival curve is presented in Figure 2. There was no statistically significant difference in time to event-based exacerbations (HR, 1.32 [95% CI, 0.78-2.23], unadjusted; HR, 1.02 [95% CI, 0.71-1.47], adjusted).
Figure 2.
Kaplan-Meier curve for exacerbation-free survival in poor vs good sleepers. Poor sleepers were defined as those having a Pittsburgh Sleep Quality Index score > 5 at baseline. Hazard ratio for symptom-based exacerbation: 1.84; 95% CI, 1.20-2.83, unadjusted. Hazard ratio, 1.49, 95% CI, 1.09-2.03, adjusted for baseline age, sex, BMI, current smoking, angina, major depression, any inhaled respiratory medications, baseline FEV1 percent predicted, and modified Medical Research Council scale score.
Discussion
The current study found that poor subjective sleep quality assessed by using the PSQI was associated with a higher risk of exacerbations in individuals with COPD. Poor sleep quality in COPD has previously been associated with reduced health-related quality of life7, 9 and reduced physical activity during the day.17 However, to our knowledge, this study is the first population-based longitudinal analysis evaluating exacerbation risk in relation to subjective sleep disturbances, and assessing previously diagnosed and undiagnosed COPD. Individuals with at least one exacerbation during the 18-month follow-up period were more likely to have had a higher baseline global PSQI score and a PSQI score > 5, the threshold defining poor sleep quality. Adjusted models found a greater risk of exacerbations in those with higher baseline PSQI scores. Participants with poor sleep quality had a shorter time to symptom-based exacerbation. Poor subjective sleep quality therefore seems to be a marker of future exacerbations.
There are several potential explanations for the association between poor sleep quality and subsequent exacerbations. Poor sleep quality can be a marker of more severe, poorly controlled disease, which would be associated with increased and more unstable respiratory symptoms. Poor respiratory function could lead to sleep disruption due to symptoms, medication use, anxiety, or other factors. Conversely, poor sleep quality might trigger changes in COPD control, increasing daytime symptoms. Sleep disruption may have detrimental effects on immune function,6 thus increasing susceptibility to infections. Sleep fragmentation augments systemic inflammation,18 which might worsen COPD control and increase exacerbation risk.19, 20 In addition, poor sleep could also lead to impaired memory and cognition, potentially fostering medication nonadherence and symptom flare-up, especially in the older COPD population.21 Poor sleep quality might also be a manifestation of OSA, which is associated with increased COPD exacerbations.22 In favor of this hypothesis is the fact that several symptoms represented by items in the PSQI (notably Factor 3) are common symptoms of OSA (eg, snoring, waking up in the middle of the night, lack of enthusiasm, daytime sleepiness). CPAP use in this cohort likely underestimates OSA burden in the study cohort because OSA remains underdiagnosed in the general population. Indeed, there is increasing interest in the link between OSA and COPD, as observational studies have found that treatment of OSA in COPD is associated with lowered mortality, exacerbations,22 and hospitalizations.23 The mechanisms whereby OSA could lead to more COPD exacerbations are currently not well understood, but exacerbation of systemic and lung inflammation has been invoked.24
In a secondary analysis of the Azithromycin for Prevention of Exacerbations of COPD study, a randomized controlled trial in 1,117 patients with moderate to severe COPD, good sleep quality (PSQI score < 5) at baseline was associated with increased time to exacerbations but not after adjusting for medications and comorbid conditions related to poor sleep quality.25 This result is in contrast to our findings, in which the association between exacerbations and PSQI remained significant following adjustments for potential confounders (eg, depression). This difference between studies could be explained by the different study populations, as these participants had generally milder COPD, and the associations were seen especially in those with undiagnosed, milder COPD. Moreover, the exacerbation rate in the azithromycin trial was considerably higher than in the current study. Thus, different mechanisms may prevail in individuals with very high exacerbation rates compared with those with lower rates.
In the current study, the association between sleep quality and exacerbations, after adjusting for potential confounders, was stronger in the undiagnosed COPD group, in whom exacerbations were related to a PSQI score > 5, higher global PSQI score, and Factor 2 and 3 scores. These associations were generally not found in diagnosed COPD. This difference between diagnosed and undiagnosed participants may be related to high prevalence of poor sleep quality and higher exacerbation rate in the diagnosed group. Sleep quality may affect exacerbation risk less in this context, as in the azithromycin trial.25 Moreover, the sample size of the diagnosed group is smaller, which may affect statistical power.
Sleep quality in the current study was related to both symptom- and event-based exacerbations. Sleep quality then seems to be related to not only propensity for transient symptom increases but also more severe exacerbations, leading to use of medication and health-care resources. This outcome likely carries a significant social and economic impact with potentially missed social activities and work and reduced health-related quality of life, in addition to an increased burden on the health-care system. The effect of sleep quality on exacerbations occurred primarily in the undiagnosed COPD group. Our findings are consistent with data from Labonté et al,15 who found that individuals with undiagnosed COPD were less symptomatic and had a better health-related quality of life than those with diagnosed COPD but similar utilization of health-care services despite fewer exacerbations. In being undiagnosed, this group may be undertreated, undereducated about their disease, and less able to recognize and self-manage symptoms. Undiagnosed COPD accounts for more than two-thirds of the total CanCOLD COPD cohort, and these individuals thus contribute greatly to the overall health-care burden of COPD.
Strengths of our study include use of the CanCOLD population-based cohort, which includes individuals with all severities of COPD (mostly GOLD stages I and II), allowing for a representative distribution of COPD severity, particularly mild disease, which is the most common yet typically underrepresented in studies. Also, a prospective evaluation of exacerbations was conducted over 18 months of follow-up.
There are potential limitations of this study. Individuals with asthma or other obstructive lung diseases could not be definitively excluded; methacholine challenges were not performed. However, analyses excluding self-reported asthma were consistent with our main results. Second, because definitions of COPD exacerbation vary among studies, comparison may be limited,26, 27 but CanCOLD used a standard definition, as recommended by GOLD.28 Our assessment of exacerbations was based on participant recall, which may be subject to recall bias, but minimized by the relatively frequent telephone assessments (ie, every 3 months). An objective sleep assessment such as polysomnography was not performed. Our results then apply to subjective sleep quality only, which is pertinent to routine clinical practice. Also, individuals more prone to exacerbations may have experienced exacerbations prior to enrollment, leading to a higher baseline PSQI score. The relationship between exacerbations and sleep quality is probably bidirectional. However, the fact that the Kaplan-Meier curves for exacerbation-free survival (symptom based) only start to diverge after the first 1 to 2 months suggests that poor baseline sleep quality is not connected to an ongoing or impending exacerbation, although it may reflect more unstable disease. Finally, the sample size may have been insufficient to show certain associations, particularly in subgroup analyses. Despite these limitations, we believe that our prospective follow-up findings suggest that poor sleep quality can be a marker of an exacerbating COPD phenotype. Further studies using objective sleep recordings are required to delineate specific sleep disturbances affecting the COPD disease process.
Conclusions
Poor subjective sleep quality in individuals with COPD was associated with increased risk of exacerbations during the 18-month follow-up, particularly in those with undiagnosed COPD. Further studies are required to explore the mechanisms of interaction between sleep disturbances and COPD exacerbations, and to determine whether interventions to improve sleep quality can modify COPD-related outcomes. Routine assessment of sleep quality may be a useful clinical predictor of exacerbation risk and a tool to identify those who might benefit from closer follow-up and intervention.
Acknowledgments
Author contributions: M. K. served as the guarantor and takes responsibility for the content of the manuscript, including the data and analysis. M. S., R. J., A. M., M. K., J. B., J. K., and N.A. designed the current study, including the objective and analysis methods. M. S., M. K., J. B., and J. K. interpreted data and participated in drafting of the manuscript. J. B., W. C. T., S. D. A., D. D. S., J. R., K. R. C., D. E. O., F. M., P. H., B. L. W., and D. M. participated in the design of the parent cohort, and coordination and supervision of data collection at their respective sites.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. B. reports grants from the Canadian Institutes of Health Research (CIHR), Canadian Respiratory Research Network, GlaxoSmithKline, Grifols, Aerocrine, Boehringer Ingelheim, Foundation of the MUHC, Novartis, and AstraZeneca, during the conduct of the study; and personal fees from GlaxoSmithKline, Grifols, Boehringer Ingelheim, Novartis, AstraZeneca, and Trudell, outside the submitted work. J. K. reports grants from CIHR, the Canadian Respiratory Research Network, GlaxoSmithKline, Grifols, Aerocrine, Boehringer Ingelheim, Foundation of the MUHC, Novartis, and AstraZeneca, during the conduct of the study; and grants from the Multiple Sclerosis Society of Canada, Philips Respironics, ResMed, and Fonds de Recherche du Québec-Santé, outside the submitted work. A. M. is funded by the National Institutes of Health; does some work with the Alfred E. Mann Foundation, which is a nonprofit; and ResMed provided a philanthropic donation to UC San Diego. W. C. T. reports personal fees from Teva and GlaxoSmithKline, outside the submitted work. D. D. S. reports grants and personal fees from AstraZeneca and Boehringer Ingelheim, grants from Merck Frosst, and personal fees from Novartis, outside the submitted work. K. R. C. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grifols, Sanofi, Genentech, Kamada, Roche, Novartis, and Merck; grants from Baxter, GlaxoSmithKline, and Amgen; and personal fees from the CIHR-GSK Research Chair in Respiratory Health Care Delivery, UHN, during the conduct of the study. F. M. reports grants from GlaxoSmithKline, Boehringer Ingelheim, Novartis, AstraZeneca, and Grifols; personal fees from Novartis, Boehringer Ingelheim, and Grifols; and personal fees from Boehringer Ingelheim and Novartis outside the submitted work. P. H. reports grants from CIHR, during the conduct of the study; personal fees from Actelion, AstraZeneca, Bayer, GlaxoSmithKline, Roche, Teva and Trudell, outside the submitted work; grants and personal fees from Boehringer Ingelheim, grants from CSL Behring, Grifols, and Prometic; and service as Secretary of Executive Committee of the Canadian Thoracic Society. B. L. W. reports grants from CIHR, AstraZeneca Canada Ltd, Boehringer Ingelheim Canada, GlaxoSmithKline Canada, and Novartis, during the conduct of the study; grants from Respiratory Health Strategic Clinical Network Alberta and personal fees from AstraZeneca, GlaxoSmithKline, and Novartis, outside the submitted work. D. M. reports grants from CIHR during the conduct of the study; grants from Boehringer-Ingelheim, GlaxoSmithKline, the Lung Association of Saskatchewan, Novartis, AstraZeneca, Canada Health Infoway, CIHR, Schering-Plough; and other from Canadian Foundation for Healthcare Improvement, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Saskatoon Health Region, Health Canada, University of Saskatchewan, outside the submitted work. M. K. reports grants from AstraZeneca Canada, Boehringer Ingelheim Canada Ltd., GlaxoSmithKline Canada Ltd., Almirall, Merck Nycomed, Pfizer Canada Ltd., Theratechnologies, Canadian Respiratory Research Network, CIHR, and Respiratory Health Network of the FRQS, during the conduct of the study; nonfinancial support from Philips Respironics and VitalAire; and grants from ResMed Corp, Fisher & Paykel, CIHR, and Weston Brain Institute outside the submitted work. None declared (M. S., R. J., N. A., S. D. A., J. R., D. E. O.).
Role of sponsors: The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
Other contributions: The authors thank the men and women who participated in the study and individuals in the CanCOLD Collaborative Research Group: Executive Committee: Jean Bourbeau, (Mcgill University, Montreal, QC, Canada); Wan C. Tan, J. Mark FitzGerald; D. D. Sin (UBC, Vancouver, BC, Canada); D. D. Marciniuk (University of Saskatoon, Saskatoon, SASK, Canada) D. E. O'Donnell (Queen's University, Kingston, ON, Canada); Paul Hernandez (University of Halifax, Halifax, NS, Canada); Kenneth R. Chapman (University of Toronto, Toronto, ON, Canada); Robert Cowie (University of Calgary, Calgary, AB, Canada); Shawn Aaron (University of Ottawa, Ottawa, ON, Canada); F. Maltais (University of Laval, Quebec City, QC, Canada); International Advisory Board: Jonathon Samet (the Keck School of Medicine of USC, CA); Milo Puhan (John Hopkins School of Public Health, Baltimore, MD); Qutayba Hamid (McGill University, Montreal, QC, Canada); James C. Hogg (UBC James Hogg Research Center, Vancouver, BC, Canada). Operations Center: Jean Bourbeau (PI), Carole Baglole, Carole Jabet, Palmina Mancino, Yvan Fortier, (University of McGill, Montreal, QC, Canada); Wan C. Tan (co-PI), Don Sin, Sheena Tam, Jeremy Road, Joe Comeau, Adrian Png, Harvey Coxson, Miranda Kirby, Jonathon Leipsic, Cameron Hague (University of British Columbia James Hogg Research Center, Vancouver, BC, Canada); Economic Core: Mohsen Sadatsafavi (University of British Columbia, Vancouver, BC, Canada); Public Health core: Teresa To, Andrea Gershon (University of Toronto, Toronto, ON, Canada); Data management and Quality Control: Wan C. Tan, Harvey Coxson (UBC, Vancouver, BC, Canada); Jean Bourbeau, Pei-Zhi Li, Jean-Francois Duquette, Yvan Fortier, Andrea Benedetti, Denis Jensen (Mcgill University, Montreal, QC, Canada), Denis O'Donnell (Queen's University, Kingston, ON, Canada; Field Centers: Wan C. Tan (PI), Christine Lo, Sarah Cheng, Cindy Fung, Nancy Ferguson, Nancy Haynes, Junior Chuang, Licong Li, Selva Bayat, Amanda Wong, Zoe Alavi, Catherine Peng, Bin Zhao, Nathalie Scott-Hsiung, Tasha Nadirshaw (UBC James Hogg Research Center, Vancouver, BC, Canada); Jean Bourbeau (PI), Palmina Mancino, David Latreille, Jacinthe Baril, Laura Labonte (McGill University, Montreal, QC, Canada); Kenneth Chapman (PI), Patricia McClean, Nadeen Audisho (University of Toronto, Toronto, ON, Canada); Brandie Walker, Robert Cowie (PI), Ann Cowie, Curtis Dumonceaux, Lisette Machado (University of Calgary, Calgary, AB, Canada); Paul Hernandez (PI), Scott Fulton, Kristen Osterling (University of Halifax, Halifax, NS, Canada); Shawn Aaron (PI), Kathy Vandemheen, Gay Pratt, Amanda Bergeron (University of Ottawa, Ottawa, ON, Canada); Denis O'Donnell (PI), Matthew McNeil, Kate Whelan (Queen's University, Kingston, ON, Canada); Francois Maltais (PI), Cynthia Brouillard (University of Laval, Quebec City, QC, Canada); Darcy Marciniuk (PI), Ron Clemens, Janet Baran (University of Saskatoon, Saskatoon, SK, Canada). The authors also thank Pei Zhi Li, MSc, for her expert data analysis.
Footnotes
FUNDING/SUPPORT: The Canadian Cohort Obstructive Lung Disease (CanCOLD) study is currently funded by the Canadian Respiratory Research Network (CRRN); industry partners: Astra Zeneca Canada Ltd; Boehringer Ingelheim Canada Ltd; GlaxoSmithKline Canada Ltd; Novartis. Researchers at RI-MUHC Montreal and Icapture Centre Vancouver lead the project. Previous funding partners are the CIHR (CIHR/ Rx&D Collaborative Research Program Operating Grants- 93326); the Respiratory Health Network of the FRQS; industry partners: Almirall; Merck Nycomed; Pfizer Canada Ltd; and Theratechnologies.
Contributor Information
Marta Kaminska, Email: marta.kaminska@mcgill.ca.
CanCOLD Collaborative Research group:
Jean Bourbeau, Wan C. Tan, J. Mark FitzGerald, D.D. Sin, D.D. Marciniuk, D.E. O'Donnell, Paul Hernandez, Kenneth R. Chapman, Robert Cowie, Shawn Aaron, F. Maltais, Jonathon Samet, Milo Puhan, Qutayba Hamid, James C. Hogg, Jean Bourbeau, Carole Baglole, Carole Jabet, Palmina Mancino, Yvan Fortier, Wan C. Tan, Don Sin, Sheena Tam, Jeremy Road, Joe Comeau, Adrian Png, Harvey Coxson, Miranda Kirby, Jonathon Leipsic, Cameron Hague, Mohsen Sadatsafavi, Andrea Gershon, Wan C. Tan, Harvey Coxson, Jean Bourbeau, Pei-Zhi Li, Jean-Francois Duquette, Yvan Fortier, Andrea Benedetti, Denis Jensen, Denis O'Donnell, Wan C. Tan, Christine Lo, Sarah Cheng, Cindy Fung, Nancy Ferguson, Nancy Haynes, Junior Chuang, Licong Li, Selva Bayat, Amanda Wong, Zoe Alavi, Catherine Peng, Bin Zhao, Nathalie Scott-Hsiung, Tasha Nadirshaw, Jean Bourbeau, Palmina Mancino, David Latreille, Jacinthe Baril, Laura Labonte, Kenneth Chapman, Patricia McClean, Nadeen Audisho, Brandie Walker, Robert Cowie, Ann Cowie, Curtis Dumonceaux, Lisette Machado, Paul Hernandez, Scott Fulton, Kristen Osterling, Shawn Aaron, Kathy Vandemheen, Gay Pratt, Amanda Bergeron, Denis O'Donnell, Matthew McNeil, Kate Whelan, Francois Maltais, Cynthia Brouillard, Darcy Marciniuk, Ron Clemens, and Janet Baran
References
- 1.Agusti A., Hedner J., Marin J.M., Barbe F., Cazzola M., Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. doi: 10.1183/09059180.00004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartman J.E., Prinzen J., van Lummel R.C., Ten Hacken N.H. Frequent sputum production is associated with disturbed night's rest and impaired sleep quality in patients with COPD. Sleep Breath. 2015;19(4):1125–1133. doi: 10.1007/s11325-014-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNicholas W.T., Verbraecken J., Marin J.M. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McSharry D.G., Ryan S., Calverley P., Edwards J.C., McNicholas W.T. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17(7):1119–1124. doi: 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 5.Banks S., Dinges D.F. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 6.Majde J.A., Krueger J.M. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Scharf S.M., Maimon N., Simon-Tuval T., Bernhard-Scharf B.J., Reuveni H., Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:1–12. doi: 10.2147/COPD.S15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes D.M., Mota R.M., de Pontes Neto O.L., Pereira E.D., de Bruin V.M., de Bruin P.F. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187(3):159–163. doi: 10.1007/s00408-009-9147-5. [DOI] [PubMed] [Google Scholar]
- 9.Zeidler M.R., Martin J.L., Kleerup E.C. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS) Sleep. 2018;41(5) doi: 10.1093/sleep/zsy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seemungal T.A., Hurst J.R., Wedzicha J.A. Exacerbation rate, health status and mortality in COPD—a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi: 10.2147/copd.s3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Bourbeau J., Tan W.C., Benedetti A. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11(2):125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 13.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psych Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Cole J.C., Motivala S.J., Buysse D.J., Oxman M.N., Levin M.J., Irwin M.R. Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 15.Labonté L.E., Tan W.C., Li P.Z. Undiagnosed COPD contributes to the burden of health care utilization: data from the CanCOLD Study. Am J Respir Crit Care Med. 2016;194(3):285–298. doi: 10.1164/rccm.201509-1795OC. [DOI] [PubMed] [Google Scholar]
- 16.Keene O.N., Calverley P.M., Jones P.W., Vestbo J., Anderson J.A. Statistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisited. Eur Respir J. 2008;32(1):17–24. doi: 10.1183/09031936.00161507. [DOI] [PubMed] [Google Scholar]
- 17.Spina G., Spruit M.A., Alison J. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax. 2017;72(8):694–701. doi: 10.1136/thoraxjnl-2016-208900. [DOI] [PubMed] [Google Scholar]
- 18.Dumaine J.E., Ashley N.T. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol. 2015;308(12):R1062–R1069. doi: 10.1152/ajpregu.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45(3):291–300. doi: 10.3109/07853890.2012.732703. [DOI] [PubMed] [Google Scholar]
- 20.Cao C., Wu Y., Xu Z. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: a systematic review and meta-analysis of observational research. Scientific Rep. 2015;5:16461. doi: 10.1038/srep16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riegel B., Weaver T.E. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009;8(5):337–344. doi: 10.1016/j.ejcnurse.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin J.M., Soriano J.B., Carrizo S.J., Boldova A., Celli B.R. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 23.Borel J.C., Pepin J.L., Pison C. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–865. doi: 10.1111/resp.12327. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Hu K., Liu K. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123–1130. doi: 10.1016/j.sleep.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Geiger-Brown J., Lindberg S., Krachman S. Self-reported sleep quality and acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:389–397. doi: 10.2147/COPD.S75840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burge S., Wedzicha J.A. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 27.Effing T.W., Kerstjens H.A., Monninkhof E.M. Definitions of exacerbations: does it really matter in clinical trials on COPD? Chest. 2009;136(3):918–923. doi: 10.1378/chest.08-1680. [DOI] [PubMed] [Google Scholar]
- 28.From the Global Strategy for the Diagnosis MaPoC, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. http://goldcopd.org. Accessed April 1, 2018.