Abstract
Some of the salicylic acid-deficient Arabidopsis eds5 mutants have an unnoticed fah1-2 background mutation, which could cause salicylic acid- and EDS5-independent mutant phenotypes.
Salicylic acid (SA) is produced by the enzyme isochorismate synthase (ICS) within the chloroplast and is subsequently exported to the cytosol by the multidrug and toxic compound extrusion transporter ENHANCED DISEASE SUSCEPTIBILITY5 (EDS5; Nawrath et al., 2002; Serrano et al., 2013). The ICS pathway is the major source of SA during plant responses to various fungal and bacterial pathogens (Nawrath and Métraux, 1999; Wildermuth et al., 2001). Accordingly, Arabidopsis (Arabidopsis thaliana) mutants defective in EDS5 are impaired in the SA-dependent establishment of resistance against Pseudomonas syringae pv. tomato (Nawrath and Métraux, 1999). Several SA-deficient eds5 loss-of-function alleles have been established. The mutant alleles eds5-1 and eds5-3 (Glazebrook et al., 1996; Nawrath and Métraux, 1999) are widely used in plant pathology research, whereas eds5-2 (Volko et al., 1998) is less frequently used. We discovered that the eds5-3, syp121-1 syp122-1 eds5-3, and syp121-1 syp122-1 eds5-3 sid2-1 mutants all carried an unnoticed second-site mutation in FERULIC ACID 5-HYDROXYLASE1 (FAH1). The eds5-1 mutant lines might harbor a similar mutation in FAH1. Therefore, it is strongly recommended to test all stocks of eds5-3 and eds5-1 for the FAH1 background mutation before use.
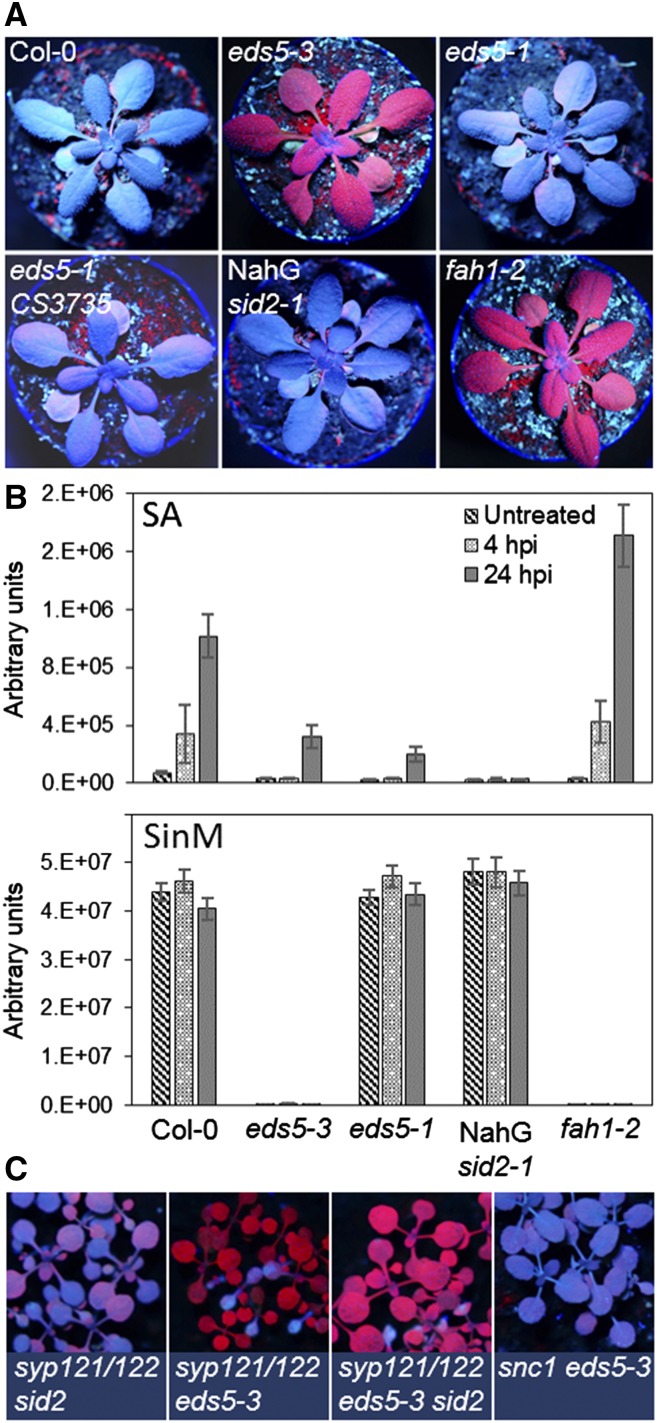
eds5 mutants do not have an obvious growth phenotype. However, under UV-A illumination, leaves of eds5-3 exhibit red chlorophyll fluorescence rather than the blue fluorescence observed in wild-type (Col-0) plants (Fig. 1A). UV-excited blue fluorescence is mainly emitted by sinapoylmalate, which is a major phenylpropanoid in vacuoles of the leaf upper epidermis, where it serves as a protective barrier against harmful UV irradiation (Fraser and Chapple, 2011). eds5-1 mutant seedlings grown from two different seed stocks (the Mario Serrano laboratory stock and the Arabidopsis Biological Resource Center stock CS3735, donated by the Frederick Ausubel laboratory) had similar levels of blue fluorescence as the wild type (Fig. 1A), suggesting that the defect in EDS5 expression did not cause the lack of sinapoylmalate in eds5-3. NahG sid2-1 plants cannot accumulate SA due to a mutation in the ICS coding gene ICS1/SID2 and transgenic expression of the SA hydroxylase gene NahG from Pseudomonas putida (Delaney et al., 1994; Supplemental Materials and Methods S1). Under UV, NahG sid2-1 was indistinguishable from wild-type plants, underpinning again that SA deficiency does not affect sinapoylmalate biosynthesis (Fig. 1A).
Figure 1.
eds5-3 carries a FAH1 mutation. A, Detection of blue fluorescent sinapoylmalate under UV-A illumination (365-nm wavelength). Mutant lines lacking sinapoylmalate emit red fluorescence under UV. B, Semiquantification of SA and sinapoylmalate (SinM) by ultra-performance liquid chromatography coupled to mass spectrometry. Plants were infected with P. syringae pv. tomato AvrRpm1, and leaf extracts were sampled at 4- and 24-h post infection (hpi). Error bars indicate the sd (n = 4–5). C, The syp121-1 syp122-1 eds5-3 and syp121-1 syp122-1 eds5-3 sid2-1 mutants carry the fah1-2 background mutation as evidenced by their red fluorescence phenotype. snc1-1 eds5-3 showed blue fluorescence under UV illumination, suggesting that the fah1-2 mutation had been crossed out. Refer to Supplemental Materials and Methods S1 for a detailed description of the mutant lines and methods.
The eds5-3 mutant had a similar UV phenotype as reported in the previously identified reduced epidermal fluorescence mutants, including the fah1-2 line (Fraser and Chapple, 2011; Fig. 1A). fah1-2 has a defect in FAH1, which encodes ferulate-5-hydroxylase, an essential enzyme for the conversion of ferulate into sinapates such as sinapoylmalate (Chapple et al., 1992; Fraser and Chapple, 2011). Ultra-performance liquid chromatography coupled to mass spectrometry (Tzin et al., 2012) was employed for molecular phenotyping of the mutants (Supplemental Materials and Methods S1). Pathogen infection with avirulent P. syringae pv tomato AvrRpm1 triggered the accumulation of SA at 4 h and more pronouncedly at 24 h after infection in wild-type and fah1-2 plants (Fig. 1B). By contrast, the SA peak was reduced in eds5-3 and eds5-1, and absent in NahG sid2-1. Sinapoylmalate levels were high and not strongly regulated upon pathogen infection in wild type, eds5-1, and NahG sid2-1. However, sinapoylmalate was not detectable in eds5-3 and fah1-2 (Fig. 1B). Collectively, these results point to sinapoylmalate biosynthesis being disrupted in eds5-3 independently of the EDS5 mutation.
This prompted us to consider whether an unknown second-site mutation caused the unexpected eds5-3 phenotype. In more recent reports, Col-0 was given as the background of eds5-3 (Nawrath et al., 2002; Serrano et al., 2013). Only the initial publications, describing the generation, identification, and characterization of eds5 mutants, provided an explanation for the sinapoylmalate deficiency of eds5-3. In these publications, it was mentioned that eds mutants were generated by EMS mutagenesis of fah1-2 rather than Col-0 seeds (Glazebrook et al., 1996; Rogers and Ausubel, 1997). The fah1-2 mutant (in the Col-0 background) was chosen because the red fluorescence phenotype is a useful marker in genetic crosses (Glazebrook et al., 1996). eds5-1 and eds5-3 both originated from the same mutant population. The fah1-2 and eds5 mutations are only 10 centimorgans apart (Reuber et al., 1998), which rendered removal of the fah1-2 mutation by backcrossing difficult due to genetic linkage. eds5-1 was successfully backcrossed with Col-0 before submission to the Arabidopsis Biological Resource Center (Fig. 1; J. Glazebrook, personal communication), whereas eds5-3 still carries the fah1-2 background mutation (Fig. 1; Nawrath and Métraux, 1999). eds5-2 originated from an independent screen, not involving fah1-2 (Volko et al., 1998).
eds5 lines were crossed with various mutants to study the interaction of SA signaling with other defense mechanisms (Zhang et al., 2008; Venugopal et al., 2009; Dong et al., 2016). Inspecting multiple mutants under UV revealed that syp121-1 syp122-1 eds5-3 and syp121-1 syp122-1 eds5-3 sid2-1 (mutants from H.T.-C.’s laboratory; Zhang et al., 2008) displayed the red fluorescence phenotype, whereas in snc1-1 eds5-3 (mutant from Xin Li’s laboratory; Dong et al., 2016) the fah1-2 mutation was seemingly outcrossed (Fig. 1C). Thus, due to genetic linkage, fah1-2 can be an unnoticed background mutation in multiple mutants containing eds5-3. Some laboratories could have stocks of the original eds5-1 line with the fah1-2 background. However, we have yet to test multiple mutants containing eds5-1, such as acd11 eds5-1 and ssi2 eds1-2 eds5-1 (Brodersen et al., 2005; Venugopal et al., 2009).
Previous studies provided evidence that the lack of sinapates and syringyl lignin in fah1-2 increased susceptibility to the fungal pathogens Botrytis cinerea and Verticillium longisporum (Lloyd et al., 2011; Demkura and Ballaré, 2012; König et al., 2014). Hence, the unnoticed fah1-2 background mutation could influence pathogen resistance of eds5-3 and possibly eds5-1 plants in a SA-independent manner, thereby leading to false conclusions on the role of EDS5 and SA in plant–pathogen interactions. For this reason, it is strongly recommended to check all lab stocks of eds5-3, eds5-1, and multiple mutants containing one of these mutations under UV-A illumination for the characteristic red fluorescence phenotype of fah1-2, followed by molecular confirmation of the genotype by PCR with FAH1-specific primers as described in Weng et al. (2010).
Supplemental Data
The following supplemental materials are available.
Supplemental Materials and Methods S1. Description of the mutant lines, materials, and methods used.
Acknowledgments
We thank Clint Chapple and Xin Li for donating the mutant lines fah1-2 and snc1 eds5-3, respectively.
Footnotes
Articles can be viewed without a subscription.
References
- Brodersen P, Malinovsky FG, Hématy K, Newman MA, Mundy J (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple CC, Vogt T, Ellis BE, Somerville CR (1992) An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell 4: 1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Demkura PV, Ballaré CL (2012) UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant 5: 642–652 [DOI] [PubMed] [Google Scholar]
- Dong OX, Tong M, Bonardi V, El Kasmi F, Woloshen V, Wünsch LK, Dangl JL, Li X (2016) TNL-mediated immunity in Arabidopsis requires complex regulation of the redundant ADR1 gene family. New Phytol 210: 960–973 [DOI] [PubMed] [Google Scholar]
- Fraser CM, Chapple C (2011) The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 9: e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König S, Feussner K, Kaever A, Landesfeind M, Thurow C, Karlovsky P, Gatz C, Polle A, Feussner I (2014) Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum. New Phytol 202: 823–837 [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, William Allwood J, Winder CL, Dunn WB, Heald JK, Cristescu SM, Sivakumaran A, Harren FJM, Mulema J, Denby K, et al. (2011) Metabolomic approaches reveal that cell wall modifications play a major role in ethylene-mediated resistance against Botrytis cinerea. Plant J 67: 852–868 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W, Ausubel FM (1998) Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J 16: 473–485 [DOI] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Wang B, Aryal B, Garcion C, Abou-Mansour E, Heck S, Geisler M, Mauch F, Nawrath C, Métraux J-P (2013) Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol 162: 1815–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin V, Malitsky S, Ben Zvi MM, Bedair M, Sumner L, Aharoni A, Galili G (2012) Expression of a bacterial feedback-insensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway in Arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol 194: 430–439 [DOI] [PubMed] [Google Scholar]
- Venugopal SC, Jeong RD, Mandal MK, Zhu S, Chandra-Shekara AC, Xia Y, Hersh M, Stromberg AJ, Navarre D, Kachroo A, et al. (2009) Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet 5: e1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volko SM, Boller T, Ausubel FM (1998) Isolation of new Arabidopsis mutants with enhanced disease susceptibility to Pseudomonas syringae by direct screening. Genetics 149: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JK, Akiyama T, Bonawitz ND, Li X, Ralph J, Chapple C (2010) Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. Plant Cell 22: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lenk A, Andersson MX, Gjetting T, Pedersen C, Nielsen ME, Newman MA, Hou BH, Somerville SC, Thordal-Christensen H (2008) A lesion-mimic syntaxin double mutant in Arabidopsis reveals novel complexity of pathogen defense signaling. Mol Plant 1: 510–527 [DOI] [PubMed] [Google Scholar]