Visual Abstract
Keywords: sickle cell disease, metabolic acidosis, fasting urine osmolality, adult, sickle cell anemia, homeostasis, acidosis, urinary tract, body fluids, fludrocortisone, furosemide, glomerular filtration rate, ammonium compounds, human EGFR protein, epidermal growth factor receptor, France, renal insufficiency
Abstract
Background and objectives
Metabolic acidosis is a frequent manifestation of sickle cell disease but the mechanisms and determinants of this disorder are unknown. Our aim was to characterize urinary acidification capacity in adults with sickle cell disease and to identify potential factors associated with decreased capacity to acidify urine.
Design, setting, participants, & measurements
Among 25 adults with sickle cell disease and an eGFR of ≥60 ml/min per 1.73 m2 from a single center in France, we performed an acute acidification test after simultaneous administration of furosemide and fludrocortisone. A normal response was defined as a decrease in urinary pH <5.3 and an increase in urinary ammonium excretion ≥33 µEq/min at one or more of the six time points after furosemide and fludrocortisone administration.
Results
Of the participants (median [interquartile range] age of 36 [24–43] years old, 17 women), 12 had a normal and 13 had an abnormal response to the test. Among these 13 participants, nine had normal baseline plasma bicarbonate concentration. Plasma aldosterone was within the normal range for all 13 participants with an abnormal response, making the diagnosis of type 4 tubular acidosis unlikely. The participants with an abnormal response to the test were significantly older, more frequently treated with oral bicarbonate, had a higher plasma uric acid concentration, higher hemolysis activity, lower eGFR, lower baseline plasma bicarbonate concentration, higher urine pH, lower urine ammonium ion excretion, and lower fasting urine osmolality than those with a normal response. Considering both groups, the maximum urinary ammonium ion excretion was positively correlated with fasting urine osmolality (r2=0.34, P=0.002), suggesting that participants with sickle cell disease and lower urine concentration capacity have lower urine acidification capacity.
Conclusions
Among adults with sickle cell disease, impaired urinary acidification capacity attributable to distal tubular dysfunction is common and associated with the severity of hyposthenuria.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_12_10_CJN07830719.mp3
Introduction
Kidney involvement is commonly found in sickle cell disease and includes a broad spectrum of manifestation related to a large variety of tubular and/or glomerular disorders (1,2). The underlying pathophysiologic process involved in sickle cell disease nephropathy is multifactorial, including chronic hemolysis–related endothelial dysfunction, chronic exposure of the kidneys to cell-free hemoglobin, and ischemia/reperfusion injury promoted by the sickled cells leading to a hypoxic state of the kidney parenchyma (2–7).
The major feature of tubular injury in sickle cell disease is an impairment of urinary concentrating ability, leading to hyposthenuria and a higher subsequent risk of vaso-occlusive crisis. Additionally, Maurel et al. (8) recently reported a high prevalence (42%) of metabolic acidosis among adults with sickle cell disease without CKD. This metabolic acidosis is assumed to be related to a defective urinary acidification capacity (9–11), as highlighted by impaired urinary acidification after ammonium chloride or calcium chloride administration (11,12). Moreover, impaired potassium tubular secretion without hyperkalemia (10) and impaired hydrogen and potassium tubular secretion, leading to hyperkalemic distal kidney tubular acidosis in the setting of mild to moderate CKD (13), have been described in adults with sickle cell disease.Chronic metabolic acidosis may have several clinical consequences such as bone demineralization and fractures (14), a higher risk of accelerated GFR decline, and ESKD (15). Moreover, metabolic acidosis favors hemoglobin S (HbS) polymerization (16) and may subsequently increase the risk of acute and chronic complications of sickle cell disease.
In this study, we sought to better characterize urinary acidification capacity early in the course of sickle cell disease, when GFR is still normal, by using the furosemide/fludrocortisone test (17) with the aim of identifying potential factors that are predictive of an abnormal response to this dynamic test.
Materials and Methods
Patient Recruitment
Our study was approved by our local ethics committee (Comité de Protection des Personnes Ile-de-France IV, reference 2013/29NICB) and conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent. We conducted this prospective monocentric study between January 2013 and December 2017. Participants were all recruited at the Adult Sickle-Cell Referral Center of the Henri Mondor Hospital (Paris, France) and referred to the Department of Physiology at the European Georges Pompidou University Hospital (Paris, France), where the furosemide/fludrocortisone test was performed.
We included adults (aged ≥18 years) with sickle cell disease (SS or S-β0 thalassemia) with normal or low plasma bicarbonate (HCO3−) concentration. For inclusion, participants had to be at steady state, defined as >1 month after vaso-occlusive crisis or acute chest syndrome episode or infection and >3 months after blood transfusion. The exclusion criteria were ongoing pregnancy and breastfeeding, an eGFR <60 ml/min per 1.73 m2 (18), use of diuretics, psychiatric condition, previous history of stroke, left ventricular ejection fraction <50%, and no healthcare coverage. Hematologic parameters were assessed in all participants including red blood cell density and percentage of dense red blood cells (Supplemental Methods, Supplemental References) (19,20). The analysis was performed with hematologic parameters at inclusion for participants not treated with hydroxyurea. For participants treated with hydroxyurea, baseline values were the steady-state hematologic parameters collected from the referral center before treatment.
Furosemide/Fludrocortisone Test
The furosemide/fludrocortisone test, which assesses urine acidification capacity, has previously been described (Supplemental Figure 1, Supplemental Methods) (17). Oral treatment with sodium bicarbonate was discontinued 48 hours before the test, and inhibitors of the renin-angiotensin system were stopped 10 days before the test. Before enrolment of adults with sickle cell disease, this test was validated in a historical group of six healthy subjects (five white males, median age [interquartile range] of 28.1 [23–33] years) (Supplemental Figures 2 and 3). Methods for biologic analysis are provided in Supplemental Methods.
Statistical Analyses
Data were presented as medians±interquartile range (first and third quartiles) or as a percentage for quantitative and categoric variables, respectively. Values were compared by Mann–Whitney or chi-squared tests using Prism version 7.0b for MacOSX (GraphPad Software) when appropriate. We evaluated the discrimination ability by calculating the area under the receiver operating characteristic curve (AUROC) for each significant variable. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated only for parameters with AUROC>0.800. We calculated the coefficient of determination r2 by linear regression (goodness of fit) between urine osmolality and urine ammonium concentration. For comparisons between groups and time points, two-way ANOVA tests were performed followed by Tukey multiple comparison tests. P<0.05 was considered a significant difference.
Results
Description of the Population
A total of 30 participants were included but the furosemide/fludrocortisone test was not performed in five participants due to poor vascular access at the time of the investigation. Consequently, 25 participants with sickle cell disease (17 women, median age [interquartile range] of 36 [24–43] years) were studied: 23 with the HbSS genotype and two with S-β0 thalassemia. Table 1 summarizes the characteristics of the participants before the furosemide/fludrocortisone test.
Table 1.
Characteristics of study participants with sickle cell disease recruited from Henri Mondor Hospital in Paris, France
| Characteristics | All Participants | Normal Response to the Test | Abnormal Response to the Test | P |
|---|---|---|---|---|
| Number of participants | 25 | 12 | 13 | |
| Demographic data | ||||
| Women | 17 (68) | 8 (67) | 9 (69) | 0.99 |
| Age (yr) | 36 (24–43) | 27 (24–38) | 40 (34–46) | 0.04a |
| BMI (kg/m2) | 21.3 (19.6–23.1) | 20.4 (18.7–21.9) | 21.5 (20.4–24.4) | 0.09 |
| No. of comorbidities | ||||
| Hypertension | 1 (4) | 0 (0) | 1 (8) | 0.99 |
| Diabetes mellitus | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Treatments | ||||
| ACE inhibitor use | 4 (16) | 0 (0) | 4 (31) | 0.10 |
| Hydroxyurea | 15 (60) | 6 (50) | 9 (75) | 0.40 |
| Exchange transfusion | 0 (0) | 0(0) | (0) | 1.00 |
| Sodium bicarbonate use | 12 (48) | 2 (17) | 10 (77) | 0.005a |
| Specific sickle cell disease data | ||||
| Genotype | ||||
| Sickle-β thalassemia (HbSβ+ thalassaemia) | 2 (8) | 1 (8) | 1 (8) | 0.99 |
| Homozygous sickle cell disease (HbSS) | 23 (92) | 11 (92) | 12 (92) | 0.99 |
| Hemoglobin (g/dl) | 8.4 (7.7–9.3) | 9.0 (8.4–9.7) | 8.0 (6.8–9.1) | 0.02a |
| HbF (%) | 6.9 (4.9–8.4) | 7.8 (5.0–11.0) | 5.6 (3.5–8.1) | 0.22 |
| HbS (%) | 82.6 (79.1–85.3) | 82.3 (78.3–85.3) | 83.9 (80.1–89.4) | 0.46 |
| Reticulocyte count, 109/L | 193 (169–236) | 183 (157–193) | 216 (181–261) | 0.08 |
| D50d | 1.095 (1.092–1.097) | 1.092 (1.091–1.096) | 1.096 (1.095–1.101) | 0.03a |
| Percentage of dense red blood cellse | 11 (6–17) | 6 (4–11) | 17 (11–30) | 0.02a |
| Plasma total bilirubine (µM) | 40 (28–56) | 40 (17–75) | 41 (30–54) | 0.73 |
| Plasma lactate dehydrogenase (UI/L) | 455 (307–587) | 361 (249–470) | 525 (372–660) | 0.04a |
| Plasma uric acid (µM)b | 307 (205–413) | 229 (195–307) | 396 (336–472) | 0.03a |
| History of | ||||
| Acute chest syndrome | 15 (60) | 7 (58) | 8 (62) | 0.99 |
| Vaso-occlusive crisisc | 12 (48) | 5 (42) | 7 (54) | 0.70 |
| Pulmonary hypertension | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Osteonecrosis | 2 (8) | 1 (8) | 1 (8) | 0.99 |
| Leg ulcer | 3 (12) | 1 (8) | 2 (15) | 0.99 |
| Retinopathy | 14 (56) | 4 (33) | 10 (77) | 0.05 |
| Priapism | 6 (24) | 2 (17) | 4 (31) | 0.64 |
Qualitative data are expressed as n (%), quantitative data as mean (±SD) or median (interquartile range) as appropriate. P values represent the comparison between participants with an abnormal response to the test and participants with a normal response to the test. BMI, body mass index; ACE, angiotensin converting enzyme; HbF, fetal hemoglobin; HbS, hemoglobin S.
P<0.05.
Data missing for three participants.
Vaso-occlusive crisis refers to crisis requiring a hospitalization during the year preceding the test.
Dense red blood cells should have the note (density >1.11 mg/mLD50 is the density for which cells below/(cells below + cell above) was equal to 0.5.
Dense red blood cells are cells with a density >1.11 mg/mL.
Response to the Furosemide/Fludrocortisone Test
Based on the results of the previously published furosemide/fludrocortisone test (17) and from the study of our historical control group regarding urinary ammonium ion (NH4+) excretion during this test (Supplemental Methods), 12 participants (48%) had a normal response and 13 (52%) had an abnormal response (Supplemental Figures 2 and 3, Supplemental Table 1). Baseline demographic, clinical, and hematologic data of these participants are listed in Table 1. Among the 13 participants with an abnormal response to the test, urinary pH did not decrease below 5.3 in seven participants (54%), meaning that the secretion of hydrogen ions (H+) by the α-intercalated cells of the collecting duct is impaired, and NH4+ excretion did not increase above 33 mEq/min despite a decrease in urinary pH below 5.3 in six participants (46%), meaning that the capacity of the α-intercalated cells to secrete H+ is preserved but the medullary availability of ammonia (NH3) is decreased.
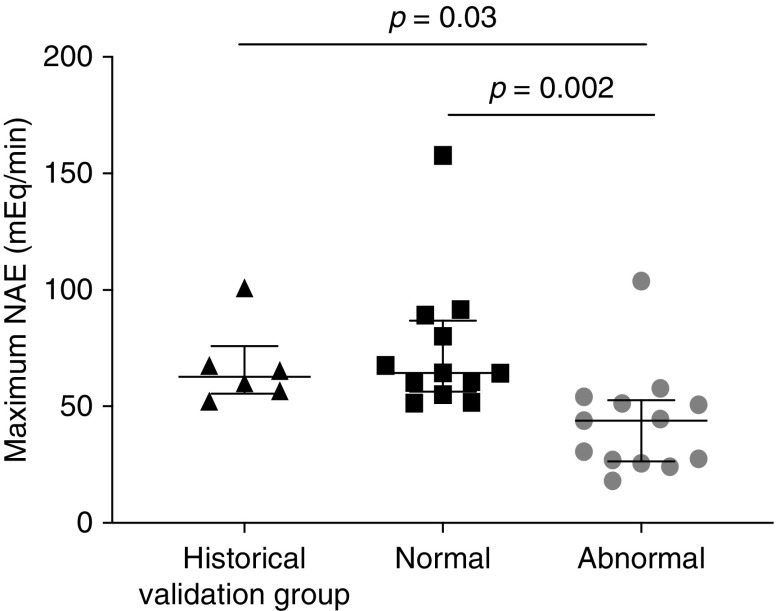
The maximum net acid excretion reached during the test was similar between our validation group of six healthy subjects and the participants with sickle cell disease who had a normal response to the test, but was significantly lower in participants with sickle cell disease who had an abnormal response to the test (P=0.002; Figure 1). Among participants with an abnormal response to the test, nine (69%) had a normal plasma HCO3− concentration, whereas four (31%) had low basal plasma HCO3− concentrations.
Figure 1.
Maximum net acid excretion (mEq/min) reached during the furosemide/fludrocortisone test is lower in sickle cell disease participants with an abnormal response to the test compared to subjects of the historical validation group or to sickle cell disease participants with a normal response to the test. The validation group (n=6) were healthy subjects tested in our department before testing of the participants with sickle cell disease. The normal response group (n=12) of participants with sickle cell disease had a normal response to the test. The abnormal response group (n=13) were participants with sickle cell disease who had an abnormal response to the test. Net acid excretion (NAE) was calculated as the sum of ammonium ions (NH4+) and titratable acid excretions, of which bicarbonate (HCO3−) excretion was removed.
Factors Associated with an Abnormal Response to the Furosemide/Fludrocortisone Test
The furosemide/fludrocortisone test is not widely available, so we sought to identify parameters predictive of an abnormal response to this test and thus an impaired capacity of the kidney to properly acidify urine. Tables 1 and 2 show the characteristics of the participants according to their response to the test. Compared with participants with sickle cell disease who had a normal responses to the test, those with abnormal responses were significantly older, more frequently treated with oral sodium bicarbonate, had a lower eGFR, a higher plasma uric acid concentration, a higher hemolysis activity (reflected by lower hemoglobin level and higher plasma lactate dehydrogenase concentration), and higher percentage of dense red blood cells and D50 (Tables 1 and 2). Just before the test, participants with an abnormal response displayed a lower fasting urine osmolality (i.e., a lower capacity to concentrate urine) compared with participants with a normal response (Table 2). The participants with an abnormal response to the test also had a lower baseline plasma HCO3− concentration, a higher fasting urine pH, a lower fasting and daily urine net acid excretion, and a lower fasting and daily urine NH4+ excretion than participants with a normal response (Table 2). Of note, participants with an abnormal response to the test more frequently exhibited sickle cell disease retinopathy than participants who responded normally (P=0.05; Table 1).
Table 2.
Kidney function, metabolic parameters, and acid-base status before the furosemide/fludrocortisone test
| Characteristics | Normal Response to the Test | Abnormal Response to the Test | P |
|---|---|---|---|
| Number of participants | 12 | 13 | |
| Blood | |||
| eGFR (ml/min per 1.73 m2) | 131 (124–140) | 114 (93–127) | 0.03a |
| Plasma sodium (mEq/L) | 140 (138–141) | 138 (138–140) | 0.22 |
| Plasma potassium (mEq/L) | 3.9 (3.6–4.1) | 4.1 (3.8–4.6) | 0.06 |
| Plasma chloride (mEq/L) | 107 (105–108) | 107 (105–108) | 0.80 |
| Plasma anion gap (mEq/L)b | 14 (12–16) | 13 (13–15) | 0.78 |
| Serum ionized calcium (md/dl)c | 4.93 (4.85–5.00) | 4.97 (4.85–5.17) | 0.45 |
| Plasma phosphate (mg/dl)d | 3.6 (3.1–3.9) | 3.7 (3.3–3.8) | 0.95 |
| Plasma magnesium (mg/dl)e | 1.9 (1.8–2.0) | 1.7 (1.6–2.0) | 0.22 |
| Plasma glucose (mg/dl)f | 89 (76–93) | 86 (80–91) | 0.93 |
| Blood venous pH (UpH) | 7.39 (7.37–7.40) | 7.38 (7.36–7.39) | 0.28 |
| Blood venous pCO2 (mm Hg) | 427 (39–43) | 39 (36–42) | 0.08 |
| Blood venous bicarbonate (mEq/L) | 24 (23–25) | 23 (20–24) | 0.02a |
| Plasma renin (mU/L) | 20 (11–44) | 19 (6–42) | 0.72 |
| Plasma aldosteroneg | 0.11 (0.07–0.16) | 0.13 (0.08–0.23) | 0.53 |
| Plasma osmolality (mOsm/kg water) | 295 (293–296) | 296 (294–301) | 0.32 |
| Urine | |||
| Daily urinary sodium excretion (mEq/d)d | 135 (61–152) | 112 (86–172) | 0.65 |
| Daily urinary potassium excretion (mEq/d)h | 39 (26–45) | 31 (22–42) | 0.38 |
| Daily urinary chloride excretion (mEq/d)h | 127 (73–153) | 103 (74–163) | 0.61 |
| Daily urinary creatinine excretion (mg/kg per day)h,i | 19.2 (14.7–22.6) | 14.7 (13.6–19.2) | 0.14 |
| Daily urinary calcium excretion (mg/d)c | 51 (34–86) | 38 (11–98) | 0.51 |
| Daily urinary phosphate excretion (mg/d)d | 543 (381–651) | 443 (273–521) | 0.27 |
| Daily urinary magnesium excretion (mg/d)e | 59 (43–83) | 57 (60–78) | 0.86 |
| Daily urinary uric acid excretion (mg/d)h,j | 471 (437–622) | 437 (303–588) | 0.31 |
| Fasting urine pH (UpH) | 5.45 (5.26–5.71) | 5.76 (5.52–6.40) | 0.02a |
| Fasting urinary net acid excretion (mEq/min) | 29 (19–36) | 10 (7–26) | 0.02a |
| Fasting urinary NH4+ (mEq/L) | 21 (17–35) | 12 (7–15) | 0.006a |
| Fasting urinary NH4+ (mEq/min) | 23 (14–26) | 10 (7–17) | 0.01a |
| Fasting urinary titratable acid excretion (mEq/min) | 6.9 (5.5–13.2) | 5.0 (0.7–12.5) | 0.17 |
| Daily urinary net acid excretion (mEq/d)h | 38 (32–42) | 259 (14–35) | 0.01a |
| Daily urinary NH4+ excretion (mEq/d)h | 25 (23–32) | 17 (13–22) | <0.001a |
| Daily urinary anion gap (mEq/d)h | 22 (9–32) | 33 (29–39) | 0.04a |
| Urinary protein/creatinine ratio (mg/mmol) | 16 (9–18) | 15 (11–61) | 0.49 |
| Urinary albumin/creatinine ratio (mg/mmol) | 4 (1–4) | 3 (1–35) | 0.79 |
| Daily glycosuriak | 0 (0) | 0 (0) | 0.99 |
| Fasting urine osmolality (mOsm/kg water) | 438 (422–452) | 383 (359–434) | 0.05 |
| Daily urine output (L/d)h | 1.67 (1.42–1.97) | 1.51 (1.10–2.50) | 0.92 |
Data are expressed as n (%), mean (±SD), or median (interquartile range). P values represent the comparison between participants with an abnormal response to the test and the participants with a normal response to the test. All plasma parameters were assessed in fasting conditions, and 24-h urine samples were collected the day before the test. Urinary fasting parameters are based on fasting morning urine collected upon arrival of the patient in the Physiology Department at 8 am after 12 h of strict fasting and correspond to second fasting morning urine. pCO2, partial pressure of carbon dioxide; NH4+, ammonium ions.
P<0.05.
Plasma anion gap=(sodium ions + potassium ions) − (chloride ions − bicarbonate ions). Normal range: 12–20 mEq/L.
To convert calcium concentrations to mmol/L, divide values in mg/dl by 4; to get values in mmol/d, divide values in mg/d by 40.
To convert phosphate concentrations to mmol/L, divide values in mg/dl by 3.1; to get values in mmol/d, divide values in mg/d by 31.
To convert magnesium concentrations to mmol/L, divide values in mg/dl by 2.43; to get values in mmol/d, divide values in mg/d by 24.3.
To convert glucose concentrations to mmol/L, multiply values in mg/dl by 0.055.
Three different assays were used to measure plasma aldosterone concentration during the study so results are reported as the ratio to the upper limit of the normal range.
Data missing for four participants.
To get creatinine values in mmol/kg per day, divide values in mg/kg per day by 113.12.
To get uric acid excretion values in mmol/d, divide values in mg/d by 168.11.
Data missing for eight participants. Glycosuria was defined as >0.3 mmol/d.
Plasma aldosterone was within the normal range for all 13 participants with an abnormal response, making the diagnosis of type 4 tubular acidosis unlikely (Table 2). None of the participants tested had signs of proximal tubular defects (i.e., tubular proteinuria, glycosuria, hypophosphatemia, hypokalemia, or low plasma uric acid levels; Tables 1 and 2).
Among the parameters showing a significant difference between the two groups of participants, we retained those with an AUROC of ≥0.800 for discrimination of participants with a normal response from those with an abnormal response to the test. These parameters were the percentage of dense red blood cells, daily urinary net acid excretion, daily NH4+ excretion, fasting NH4+ excretion, and fasting urine osmolality. Table 3 shows specificity, sensitivity, and positive and negative predictive values of these five parameters. A fasting urine osmolality <391 mOsm/kg water (H2O) had a 100% positive predictive value, meaning a 100% probability that there will be an abnormal response to the test if fasting urine osmolality is below this threshold. A fasting urine osmolality <449 mOsm/kg H2O had a 100% negative predictive value, meaning a 100% probability of a normal response to the test if fasting urine osmolality is above this threshold.
Table 3.
Contingency table for parameters with an area under the receiver operating characteristic curve above 0.800 to discriminate between participants with a normal response to the furosemide/fludrocortisone test from those with an abnormal response
| Parameters | Cut-off | True Positive (n) | False Positive (n) | True Negative (n) | False Negative (n) | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|---|---|---|---|
| Percentage of dense red blood cells | >9 | 10 | 3 | 8 | 0 | 100 | 73 | 77 | 100 |
| >18.8 | 4 | 0 | 11 | 6 | 40 | 100 | 100 | 65 | |
| Daily ammonium excretion (mmol/d)a | <25.8 | 7 | 6 | 1 | 0 | 100 | 14 | 54 | 100 |
| <22.5 | 6 | 0 | 7 | 1 | 86 | 100 | 100 | 88 | |
| Daily net acid excretion (mEq/d)a | <51.2 | 11 | 6 | 1 | 0 | 100 | 14 | 65 | 100 |
| <27.8 | 7 | 0 | 7 | 4 | 64 | 100 | 100 | 64 | |
| Fasting NH4+ (mEq/L) | <33.1 | 13 | 9 | 3 | 0 | 100 | 25 | 59 | 100 |
| <8.3 | 6 | 1 | 11 | 7 | 46 | 92 | 86 | 61 | |
| Fasting urine osmolality (mOsm/kgH2O) | <449 | 13 | 8 | 4 | 0 | 100 | 33 | 62 | 100 |
| <391 | 7 | 0 | 12 | 6 | 54 | 100 | 100 | 67 |
Values are expressed as numbers (n) or percentages (%). Among the parameters showing a significant difference between the two groups, we retained those with an AUROC superior or equal to 0.800 to discriminate between participants with a normal or an abnormal response to the furosemide/fludrocortisone test: (1) all of the 13 participants with an abnormal response to the test had a fasting urine osmolality value <449 mOsm/kg H2O (i.e., the sensitivity equals 100%); (2) among the four participants who did not have a fasting urine osmolality value <449 mOsm/kg H2O, all had a normal response to the test (i.e., negative predictive value equals 100%); (3) of the 12 participants who did not have a fasting urine osmolality value <391 mOsm/kg H2O, all had a normal response to the test (i.e., the specificity for this threshold equals 100%); and (4) of seven participants who had fasting urine osmolality <391 mOsm/kgH2O, all had an abnormal response to the test (i.e., the positive predictive value for this threshold equals 100%). NH4+, ammonium ions; AUROC, area under the receiver operating characteristic curve.
Data missing for five participants.
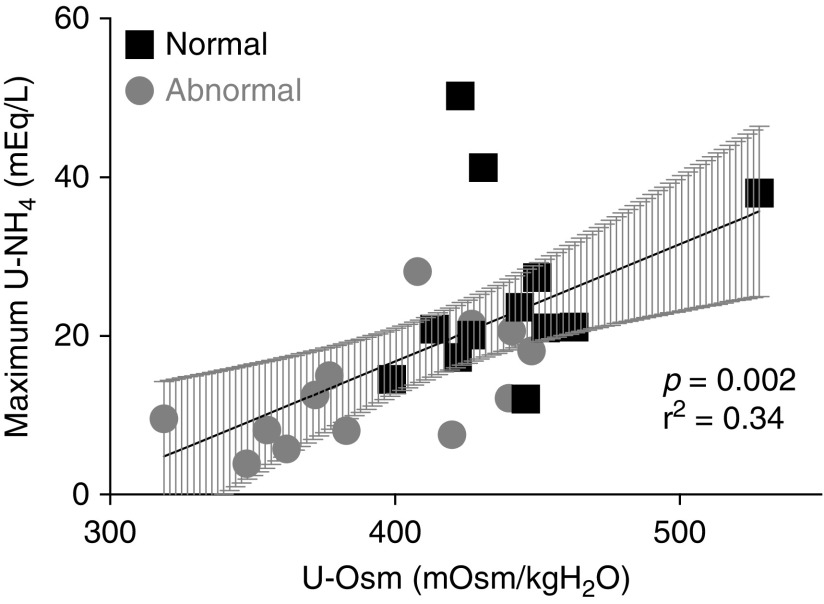
All participants, regardless of test results, showed an impaired ability to concentrate urine as highlighted by a urine osmolality <800 mOsm/kg H2O after 12 hours of water restriction (the maximum value was 528 mOsm/kg H2O), but participants with an abnormal response to the test had a significantly lower fasting urine osmolality compared with participants with a normal response (Table 2). In both groups, the maximum urinary NH4+ excretion was positively correlated with fasting urine osmolality, suggesting that participants with lower urine concentration capacity have lower urine acidification capacity (r2=0.34, P=0.002; Figure 2).
Figure 2.
Significant positive correlation between maximum urinary NH4+ excretion reached during the furosemide/fludrocortisone test and fasting urinary osmolality. Maximum urinary ammonium (U-NH4+) excretion reached during the test plotted versus fasting urine osmolality (U-Osm) on the day of the test for participants with a normal response to the test (black squares) and those with an abnormal response to the test (gray circles). The gray shadow represents the 95% confidence interval. r2, Pearson coefficient.
Discussion
We report here for the first time the response of adults with sickle cell disease to the furosemide/fludrocortisone test, a dynamic urine acidification test that determines the capacity of the distal parts of the kidney tubule to acidify urine without inducing systemic acidification. We show that half of the participants had an abnormal response to this test. Moreover, we demonstrate that some parameters, among them fasting urine osmolality, are predictive of an abnormal response to this test. As previously shown by the analysis of urinary acidification after ammonium chloride or calcium chloride administration (11,12), our study confirms that a defect in the capacity to acidify urine is a frequent and early complication of sickle cell disease in adults.
The furosemide/fludrocortisone test assesses the capacity of the collecting duct to excrete protons either in free form (reflected by a decrease in urinary pH) or linked to a buffer system (mainly reflected by an increase in urinary NH4+ excretion) but does not investigate the proximal tubule functions. If kidney acidosis is due to a proximal tubular defect, the decreased capacity of the proximal tubular cells to reabsorb HCO3− is always associated with a low plasma HCO3− concentration, whereas tubular acidosis of distal origin can be associated with low or normal plasma HCO3− concentration. In our study, four of the 25 participants had a low plasma HCO3− concentration at the time of the test, and each of these four participants had an abnormal response to the test, and consequently distal tubular acidosis. The other nine participants with an abnormal response to the test had normal baseline plasma HCO3− concentrations, ruling out a proximal tubular acidosis. Consistent with this, none of the participants in our cohort had proximal tubular defects, indicating that the participants in our cohort have no overt proximal tubular dysfunction. In contrast to a previous report (21), our results do not support the hypothesis of a hyporeninemic hypoaldosteronism as the primary process to elucidate this tubular acidosis because plasma renin and aldosterone concentrations were within the normal range for all of the participants. It was previously reported that adults with sickle cell disease without overt metabolic acidosis (i.e., with normal baseline plasma HCO3− concentrations) are likely to have an impaired urinary acidification capacity after ammonium chloride infusion (9,21). We confirmed this finding in our cohort by showing that, among participants with an abnormal response to the furosemide/fludrocortisone test, 69% had normal baseline plasma HCO3− concentrations.
In addition to the confirmation of the kidney origin of the acidosis in sickle cell disease and the exclusion of a proximal tubular defect, our study highlights a potential mechanism involved in impaired kidney acid excretion. A higher lactate dehydrogenase concentration has been reported in adults with sickle cell disease with overt metabolic acidosis (8) compared with those without overt metabolic acidosis. Likewise, we found that participants with an abnormal response to the test had a stronger hemolytic activity than those who responded normally. In addition, we found that these participants had a higher percentage of dense red blood cells, which was associated with a higher hemolysis rate (19). Dense red blood cells are characterized by a higher mean corpuscular hemoglobin concentration due to intracellular dehydration that leads to HbS polymerization and by an increased rigidity and an impairment in oxygen transport (19,20). A high percentage of dense red blood cells and/or high hemolysis levels are associated with chronic organ dysfunction including glomerular damage, pulmonary hypertension, leg ulcers, and priapism (19,22–25).
Our results are consistent with the hypothesis that sustained hemolytic activity could result in medullary ischemia due to vasa recta obstruction and/or rarefaction especially deep in the kidney medulla, which can lead to distal nephron dysfunction (2,3). This medullary hypoxia may impair the capacity of the α-intercalated cells to secrete H+ and/or may decrease the medullary NH3 availability, which limits urinary NH4+ excretion but also impairs the corticopapillary gradient and thus the urine concentration capacity, which is reflected by the high prevalence of hyposthenuria in sickle cell disease (26). In our study, this hypothesis is supported by the positive association observed between impaired urine concentration ability (reflected by fasting urinary osmolality) and impaired urine acidification ability (reflected by the maximum urinary NH4+ concentration reached during the test). Moreover, fasting urine osmolality and the percentage of dense red blood cells are both strong predictors of the response to the furosemide/fludrocortisone test, suggestive of a close pathophysiologic relationship between these two variables and the ability of the kidney to acidify urine. In other words, insufficient medullary NH3 availability may represent the limiting step for the distal urinary acidification in some cases. This finding is consistent with the previous report by Maurel et al. which showed that fasting and 24-hour urinary NH4+ excretions were lower than expected in adults with sickle cell disease with metabolic acidosis (8).
A chronic positive acid balance, as suggested by the impaired response to the furosemide/fludrocortisone test, is relevant to clinical practice. It is associated with bone buffer system mobilization, which increases the risk of bone demineralization and fractures (14). Decreased urinary NH4+ excretion is associated with a higher risk of ESKD and with a rapid GFR decline, suggesting the inability to excrete the daily acid load is deleterious to kidney outcomes (15). Finally, adults with sickle cell disease with a higher hemolysis rate and percentage of dense red blood cells are prone to metabolic acidosis, which in turn favors HbS polymerization, so that metabolic acidosis may increase the risk of complications in sickle cell disease (16). For these reasons, identification of patients with a urine acidification defect is crucial to reduce the risk of clinical complications related to metabolic acidosis. In our study, acute and chronic complications related to sickle cell disease were no more frequent in participants with an abnormal response to the test than in participants with a normal response. However, despite the small number of participants included, we showed that retinopathy was more frequently reported in participants with an abnormal response to the test.
The furosemide/fludrocortisone test is not widely available; therefore, we sought to identify surrogate markers predictive of an abnormal response to this test. Five laboratory parameters discriminated participants with sickle cell disease who had normal responses from those who had abnormal responses to the test. Among these parameters, fasting urine osmolality can be conveniently obtained because it does not require a 24-hour urine collection and could therefore represent a simple method to detect patients with an impaired urine acidification capacity. We found that a fasting urine osmolality <391 mOsm/kg H2O has a 100% positive predictive value to identify participants with an abnormal response to the test, whereas a fasting urine osmolality <449 mOsm/kg H2O has a 100% negative predictive value to rule out an abnormal response to the test. If fasting urine osmolality is between 391 and 449 mOsm/kg H2O, the assessment of a potential urinary acidification defect using the furosemide/fludrocortisone test would be warranted.
As previously reported (27), plasma HCO3− concentrations showed intraindividual variations that could be due to acute respiratory modifications. To properly interpret plasma HCO3− concentration, an arterial gas analysis would be required, which is difficult to implement in clinical practice. Moreover, plasma HCO3− concentration analyses suffer from wide interassay variations (28). This variability could explain the finding that plasma HCO3− was not the best variable to discriminate between participants with or without a defect in the ability to acidify urine in our study.
We must acknowledge that our study has some limitations. Although the furosemide/fludrocortisone test was validated in our department in a small historical control group of healthy white subjects, we could not study an optimal control group with the same racial/ethnic background as the participants due to difficulty in obtaining approval from the ethics committee to perform an invasive test in healthy subjects. However, this limitation did not affect the results or interpretation of the data because NH4+ excretion was markedly different between participants with normal and abnormal responses to the test. It has been suggested that abnormality in the furosemide/fludrocortisone test could be confirmed by ammonium chloride testing (29). This test was not performed on participants in our cohort given the potential risk of increasing HbS polymerization (16). Consequently, we acknowledge there may be a potential risk of false positive for the furosemide/fludrocortisone test. Lastly, we did not assess adults with sickle cell disease with a mild genotype or with a sickle cell trait who are also at risk for development of CKD (30,31).
In conclusion, our study confirmed that adults with sickle cell disease have a higher risk of impaired urinary acidification capacity due to a distal tubular dysfunction. We propose the use of fasting urine osmolality to detect those at high risk of kidney acidosis. Further studies will be needed to confirm prospectively these preliminary results and to determine whether therapeutic interventions such as sodium bicarbonate or potassium citrate reduce the consequences of chronic metabolic acidosis in adults with sickle cell disease.
Disclosures
Dr. Audard reports receiving personal fees from Addmedica outside of the submitted work. Dr. Cornière reports receiving grants from Foundation PHILANCIA and from l’association pour l’utilisation du rein artificiel à la Réunion (AURAR) and travel support from Sanofi-Genzyme, outside of the submitted work. Dr. Courbebaisse reports receiving grants from Advicenne, Amgen, BioHealth Laboratory, Crinex, and Kyowa Kirin outside of the submitted work. Dr. Prot-Bertoye reports receiving personal and other fees from BioHealth outside of the submitted work. All other authors have nothing to disclose.
Funding
The authors report no funding was received for this study.
Supplementary Material
Acknowledgments
We would like to acknowledge all of the clinicians involved in the medical care of the patients, and the patients who participated in this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07830719/-/DCSupplemental.
Supplemental Methods. Furosemide and fludrocortisone test and biological analyses.
Supplemental Table 1. Results of the furosemide/fludrocortisone test.
Supplemental Figure 1. Principle of the furosemide/fludrocortisone test.
Supplemental Figure 2. Results of the furosemide/fludrocortisone test.
Supplemental Figure 3. Data validating the furosemide/fludrocortisone test.
References
- 1.Scheinman JI: Sickle cell disease and the kidney. Nat Clin Pract Nephrol 5: 78–88, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Nath KA, Hebbel RP: Sickle cell disease: Renal manifestations and mechanisms. Nat Rev Nephrol 11: 161–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath KA, Katusic ZS: Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol 23: 781–784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe CC, Thein SL: Sickle cell nephropathy - a practical approach. Br J Haematol 155: 287–297, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Saraf SL, Zhang X, Kanias T, Lash JP, Molokie RE, Oza B, Lai C, Rowe JH, Gowhari M, Hassan J, Desimone J, Machado RF, Gladwin MT, Little JA, Gordeuk VR: Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol 164: 729–739, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deux JF, Audard V, Brugières P, Habibi A, Manea E-M, Guillaud-Danis C, Godeau B, Galactéros F, Stehlé T, Lang P, Grimbert P, Audureau E, Rahmouni A, Bartolucci P: Magnetic resonance imaging assessment of kidney oxygenation and perfusion during sickle cell vaso-occlusive crises. Am J Kidney Dis 69: 51–59, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Merle NS, Grunenwald A, Rajaratnam H, Gnemmi V, Frimat M, Figueres ML, Knockaert S, Bouzekri S, Charue D, Noe R, Robe-Rybkine T, Le-Hoang M, Brinkman N, Gentinetta T, Edler M, Petrillo S, Tolosano E, Miescher S, Le Jeune S, Houillier P, Chauvet S, Rabant M, Dimitrov JD, Fremeaux-Bacchi V, Blanc-Brude OP, Roumenina LT: Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3: 96910, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurel S, Stankovic Stojanovic K, Avellino V, Girshovich A, Letavernier E, Grateau G, Baud L, Girot R, Lionnet F, Haymann JP: Prevalence and correlates of metabolic acidosis among patients with homozygous sickle cell disease. Clin J Am Soc Nephrol 9: 648–653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens JP, Statius van Eps LW, Schouten H, Giterson AL: Incomplete renal tubular acidosis in sickle cell disease. Clin Chim Acta 41: 149–156, 1972 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Taufield PA, Black H, McPhedran P, Cooke CR: Impaired renal tubular potassium secretion in sickle cell disease. Ann Intern Med 90: 310–316, 1979 [DOI] [PubMed] [Google Scholar]
- 11.Ho PK, Alleyne GA: Defect in urinary acidification in adults with sickle-cell anaemia. Lancet 2: 954–955, 1968 [DOI] [PubMed] [Google Scholar]
- 12.Silva Junior GB, Vieira AP, Couto Bem AX, Alves MP, Meneses GC, Martins AM, Sanches TR, Andrade LC, Seguro AC, Libório AB, Daher EF: Renal tubular dysfunction in sickle cell disease. Kidney Blood Press Res 38: 1–10, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA: Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 72: 188–192, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Deutschmann HA, Weger M, Weger W, Kotanko P, Deutschmann MJ, Skrabal F: Search for occult secondary osteoporosis: Impact of identified possible risk factors on bone mineral density. J Intern Med 252: 389–397, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P; NephroTest Cohort Study group : Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Chatel B, Messonnier LA, Bendahan D: Do we have to consider acidosis induced by exercise as deleterious in sickle cell disease? Exp Physiol 103: 1213–1220, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Walsh SB, Shirley DG, Wrong OM, Unwin RJ: Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: An alternative to ammonium chloride. Kidney Int 71: 1310–1316, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Arlet JB, Ribeil JA, Chatellier G, Eladari D, De Seigneux S, Souberbielle JC, Friedlander G, de Montalembert M, Pouchot J, Prié D, Courbebaisse M: Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: A prospective observational cohort study. BMC Nephrol 13: 83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartolucci P, Brugnara C, Teixeira-Pinto A, Pissard S, Moradkhani K, Jouault H, Galacteros F: Erythrocyte density in sickle cell syndromes is associated with specific clinical manifestations and hemolysis. Blood 120: 3136–3141, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Di Liberto G, Kiger L, Marden MC, Boyer L, Poitrine FC, Conti M, Rakotoson MG, Habibi A, Khorgami S, Vingert B, Maitre B, Galacteros F, Pirenne F, Bartolucci P: Dense red blood cell and oxygen desaturation in sickle-cell disease. Am J Hematol 91: 1008–1013, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Yoshino M, Amerian R, Brautbar N: Hyporeninemic hypoaldosteronism in sickle cell disease. Nephron 31: 242–244, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Kato GJ, Gladwin MT, Steinberg MH: Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37–47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP: Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 886–895, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, Maitre B, Yaïci A, Hajji L, O’Callaghan DS, Clerson P, Girot R, Galacteros F, Simonneau G: A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365: 44–53, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Bartolucci P, Habibi A, Stehlé T, Di Liberto G, Rakotoson MG, Gellen-Dautremer J, Loric S, Moutereau S, Sahali D, Wagner-Ballon O, Remy P, Lang P, Grimbert P, Audureau E, Godeau B, Galacteros F, Audard V: Six months of hydroxyurea reduces albuminuria in patients with sickle cell disease. J Am Soc Nephrol 27: 1847–1853, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaniran KO, Eneanya ND, Nigwekar SU, Vela-Parada XF, Achebe MM, Sharma A, Thadhani RI: Sickle cell nephropathy in the pediatric population. Blood Purif 47: 205–213, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, Minchinela J, Perich C, Simón M: Current databases on biological variation: Pros, cons and progress. Scand J Clin Lab Invest 59: 491–500, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Kraut JA, Madias NE: Re-evaluation of the normal range of serum total CO2 concentration. Clin J Am Soc Nephrol 13: 343–347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhayat NA, Gradwell MW, Pathare G, Anderegg M, Schneider L, Luethi D, Mattmann C, Moe OW, Vogt B, Fuster DG: Furosemide/fludrocortisone test and clinical parameters to diagnose incomplete distal renal tubular acidosis in kidney stone formers. Clin J Am Soc Nephrol 12: 1507–1517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, Grove ML, Bick AG, Fontanillas P, Rich SS, Smith JD, Boerwinkle E, Rosamond WD, Ito K, Lanzkron S, Coresh J, Correa A, Sarto GE, Key NS, Jacobs DR, Kathiresan S, Bibbins-Domingo K, Kshirsagar AV, Wilson JG, Reiner AP: Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA 312: 2115–2125, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derebail VK, Ciccone EJ, Zhou Q, Kilgore RR, Cai J, Ataga KI: Progressive decline in estimated GFR in patients with sickle cell disease: An observational cohort study. Am J Kidney Dis 74: 47–55, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.