Supplemental Digital Content is available in the text
Keywords: caseload, centralization, hospital volume, mortality, surgeon volume
Abstract
Accumulation of the literature has suggested an inverse association between healthcare provider volume and mortality for a wide variety of surgical procedures. This study aimed to perform meta-analysis of meta-analyses (umbrella review) of observational studies and to summarize existing evidence for associations of healthcare provider volume with mortality in major operations.
We searched MEDLINE, SCOPUS, and Cochrane Library, and screening of references.
Meta-analyses of observational studies examining the association of hospital and surgeon volume with mortality following major operations. The primary outcome is all-cause short-term morality after surgery. Meta-analyses of observational studies of hospital/surgeon volume and mortality were included. Overall level of evidence was classified as convincing (class I), highly suggestive (class II), suggestive (class III), weak (class IV), and non-significant (class V) based on the significance of the random-effects summary odds ratio (OR), number of cases, small-study effects, excess significance bias, prediction intervals, and heterogeneity.
Twenty meta-analyses including 4,520,720 patients were included, with 19 types of surgical procedures for hospital volume and 11 types of surgical procedures for surgeon volume. Nominally significant reductions were found in odds ratio in 82% to 84% of surgical procedures in both hospital and surgeon volume-mortality associations. To summarize the overall level of evidence, however, only one surgical procedure (pancreaticoduodenectomy) fulfilled the criteria of class I and II for both hospital and surgeon volume and mortality relationships, with a decrease in OR for hospital (0.42, 95% confidence interval[CI] [0.35–0.51]) and for surgeon (0.38, 95% CI [0.30–0.49]), respectively. In contrast, most of the procedures appeared to be weak or “non-significant.”
Only a very few surgical procedures such as pancreaticoduodenectomy appeared to have convincing evidence on the inverse surgeon volume-mortality associations, and yet most surgical procedures resulted in having weak or “non-significant” evidence. Therefore, healthcare professionals and policy makers might be required to steer their centralization policy more carefully unless more robust, higher-quality evidence emerges, particularly for procedures considered as having a weak or non-significant evidence level including total knee replacement, thyroidectomy, bariatric surgery, radical cystectomy, and rectal and colorectal cancer resections.
1. Introduction
Since its first introduction in the 1979 by Luft and colleagues,[1] much literature has suggested an inverse association between healthcare provider volume and mortality for a wide variety of surgical procedures. Accumulation of supportive findings has been a major driving force towards a policy of “centralization”—selective referral from a low-volume hospital to a high-volume hospital. In the UK, Canada, and the Netherlands, programmed centralization has already been implemented for complex high-risk procedures.[2–6] In the US, a national non-profit organization has advocated centralization by presenting minimum hospital and surgeon volume standards for 8 procedures.[7]
Centralization has made a great contribution to improved outcomes in complex surgical oncology represented by pancreatic resection.[2,6] However, some criticisms still linger. First, there remains controversy over whether hospital/surgeon volume can be a precise measure of quality of care.[8–11] Second, access to a high-volume hospital might be restricted especially for patients living in rural and underserved areas.[11–14] Some experts express concern that such inaccessibility might aggravate the existing health disparities between patients with high and low socioeconomic status.[2,11,15] Third, as operations are one of the crucial sources of income for hospitals, excessive centralization might plunge low-volume hospitals such as rural hospitals into financial difficulties, thereby causing serious consequences to local communities.[11]
The rationale for proponents of centralization might be based on “positive” results derived from observational studies and their meta-analyses. However, according to the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) working group classification,[16] the quality of evidence of those studies is considered “low” unless a large magnitude of effect, a dose-response gradient, or plausible confounding is certain.[17] The quality of evidence in these studies has not been evaluated to date. Furthermore, these studies, and especially the meta-analyses, were limited to one particular procedure, and it remains uncertain which procedures have a strong volume-outcome relationship and which do not.
An umbrella reviews, which is performed to review existing systematic reviews and/or meta-analyses (meta-analysis of meta-analyses), provides nearly the highest level of evidence that can be presently obtained.[18,19] The latest method of umbrella review provides a more comprehensive overview than other review methods do by using simultaneous assessment of P values, confidence intervals, prediction intervals, number of cases, largest study effects, heterogeneity, small-study effects, and excess significance bias.[18] We, therefore, conducted an umbrella review of meta-analyses of observational studies to clarify whether healthcare provider volume might be associated with decreased mortality, and if so, to what extent, or whether it might depend on methodological quality, quality of evidence, or types of surgical procedures.
2. Methods
2.1. Umbrella review methods
Meta-analysis of meta-analyses (umbrella review) was conducted according to the practical guidance published by Aromataris et al[18] and Fusar-Poli et al[19] For reanalysis of each meta-analysis from the original cohort studies, we followed the reporting guidelines for Meta-analyses Of Observational Studies in Epidemiology (MOOSE) Statement.[20] Ethical approval was not necessary because this study did not involve patient consent. The protocol for this umbrella review was registered in the University Hospital Medical Information Network in Japan (UMIN000033032).
2.2. Literature search
We searched MEDLINE, SCOPUS, and the Cochrane Library from inception through March 2018. We searched only meta-analyses that compared the mortality of patients who underwent various operations in a high-volume hospital versus a low-volume hospital or by a high-volume surgeon versus a low-volume surgeon. Each search strategy is detailed in Supplemental Content 1. Language restrictions were not applied. Unpublished studies and conference proceedings were excluded. A hand search of the references listed in eligible articles was also performed. All relevant titles and abstracts from the databases were imported into EndNote X8 (USACO Corporation, Tokyo, Japan) for further sorting. Two authors (HH, TS) independently screened the titles and abstracts. Disagreements were resolved by a third author (ZW).
2.3. Outcome measures and eligibility criteria
The primary outcome was defined as all-cause short-term mortality (30-day mortality or in-hospital mortality). The summary effect size was expressed as an odds ratio with corresponding 95% confidence interval (CI). The threshold of hospital/surgeon volume was defined according to the definition used in each original meta-analysis. Our inclusion criteria were as follows:
-
(1)
the exposure is a “high-volume hospital” and/or “high-volume surgeon”;
-
(2)
meta-analyses were conducted;
-
(3)
dichotomous outcome measures (from forest plots) were available or could be calculated from the original cohort studies;
-
(4)
effect sizes (e.g., odds ratio) with corresponding 95% CIs were available or could be derived from the original cohort studies; and
-
(5)
sample size restrictions were not applied.
If more than one meta-analysis existed on the same surgical procedure, we included the latest meta-analysis; however, if more than one meta-analysis on the same type of operation was published in the same year, we finally included only one of them after consensus was obtained and compared them in the sensitivity analysis. Systematic reviews without meta-analytic methods were excluded because we were interested mainly in summary effects sizes rather than narrative opinions. We excluded meta-analyses whose authors did not present summary effect sizes with appropriate statistical methods and for which we could not reproduce the specific data from the original cohort studies they included. The meta-analyses focusing only on long-term mortality (often referred to as 1-year or 5-year survival rate) were also excluded.
2.4. Data extraction and synthesis
Data extraction was done in a two-level fashion to avoid using data resulting from the authors’ inappropriate statistical methods (e.g., only a fixed-effects model applied) or to correct insufficient data (e.g., absence of publication bias analysis). At the first level, we extracted information from each meta-analysis including the following data: type of operation, cases (deaths), population, number of studies included, name of the first author, year of publication, type of primary outcome, and cut-off threshold of high volume per year. If dichotomous data (e.g., a 2 × 2 contingency table) were available, we used this for further data synthesis. If not (e.g., odds ratio with corresponding 95% CI only), we moved onto the second level for which we obtained all of the primary study articles that the meta-analysis included and then extracted dichotomous data from them. If this succeeded, the data was synthesized; however, if it failed, data only on the effect size with 95% CIs were used for synthesis. If we failed to even collect data on effect size with 95% CIs, we excluded the meta-analysis from our umbrella review. Data extraction was performed independently by two investigators (HH, TS), and consensus was obtained with the third investigator (ZW) if there were disagreements.
2.5. Statistical analysis
We used both fixed and DerSimonian and Laird random-effects models[21] to estimate the summary effect size (odds ratio) and the corresponding 95% CIs. We assessed the heterogeneity of effect size across studies using the Cochrane Q statistic and I2 statistic (I2 >60%: high heterogeneity; 40 to 60%: moderate heterogeneity; < 40%: low heterogeneity).
We estimated the 95% prediction intervals for the summary random effects odds ratio. The prediction interval provides information on how the true effects are distributed about the summary effect in a random-effects model.[15] For instance, if 95% prediction intervals exceed zero, the true effect in 95% of the future studies will exclude the null value. A small-study effect (publication bias) was estimated by Egger regression test.[22]
We also used the excess significance test to estimate whether the observed number of studies (O) with statistically significant results (positive studies) was different from the expected number of positive studies (E).[23] Briefly, we calculated E for each meta-analysis as the sum of the statistical power estimates for each individual study. The greater the disparity between O and E, the greater is the degree of excess significance bias.
A P value < .05 was considered significant for both the fixed- and random-effects odds ratios. A P value < .1 was considered significant for the excess significance test and Egger regression test. All the analyses were performed using STATA 15.0 (StataCorp, College Station, TX).
A sensitivity analyses was conducted when more than one meta-analysis on the same type of surgical procedures was published in the same year.
2.6. Stratification of evidence specific to an umbrella review
We performed an umbrella review-level stratification of evidence using modified criteria recommended by Fusar-Poli et al[19]:
Convincing evidence (Class I) when the number of cases (deaths) > 1000, highly significant summary associations (random-effects P < 10−6), no evidence of small-study effects, no evidence of excess significance bias, 95% prediction intervals excluding the null, and not large heterogeneity (I2 < 50%);
Highly suggestive evidence (Class II) when the number of cases > 1000, random-effects P < 10−6, and largest study with a statistically significant effect and class I criteria not met;
Suggestive evidence (Class III) when the number of cases > 1000, random-effects P < 10−3, and class I-II criteria not met;
Weak evidence (Class IV) when P < .05 and class I-III criteria not met or unclear; and
Non-significant when P > .05.
2.7. Assessment of methodological quality and quality of evidence
We assessed the methodological quality of the meta-analyses by using AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews).[24] AMSTAR 2 has adopted new evaluation system consisting of 16 items that evaluate 7 critical flaws and 9 non-critical weaknesses. Briefly, critical flaws include prior protocol registration, adequacy of the literature search, justification for excluding individual studies, risk of bias in individual studies, appropriateness of the meta-analytical methods, consideration of risk of bias, and assessment of publication bias. The final judgment by AMSTAR 2 in each meta-analysis can be categorized as “high,” “moderate,” “low,” or “critically low.”
We used the GRADE classification[16] to assess the quality of evidence for mortality in each surgical procedure included in our umbrella review. Briefly, the GRADE system downgrades the quality of evidence when risk of bias, inconsistency, indirectness, or imprecision might be certain. Conversely, the GRADE system upgrades the quality of evidence when a large magnitude of effect, dose-response gradient, or a plausible confounder is present. The final judgment of GRADE in the outcome can be categorized as “high,” “moderate,” “low,” or “very low.” AMSTAR and GRADE were assessed independently by two investigators (HH, TS). Any differences between the two investigators were resolved by consensus.
2.8. Patient and public involvement
Patients were not involved in determining research questions or outcome measures or in designing or implementing the present study. The patients were not asked for their opinions on interpreting or writing the results. The results of the present study will not be disseminated to the study participants or other relevant parties.
3. Results
3.1. Study selection and characteristics
We finally included 20 meta-analyses[25–44] with a total of 4,520,720 patients after the systematic search and selection of eligible reviews (see Fig. 1). Nineteen were written in English, and one was written in German.[41] The search yield 26 types of surgical procedures for both hospital and surgeon volume and mortality associations (19 for hospital volume and 11 for surgeon volume). The literature excluded from the full-text reviews and the reasons for doing so are listed in Supplemental content 2. The characteristics of the extracted data, calculated summary effect sizes, heterogeneity, publication bias, and excess significance are tabulated in Tables 1 and 2.
Figure 1.

PRISMA flow diagram for literature search, study screening and selection.
Table 1.
Summary of 19 meta-analyses on the association between hospital volume and mortality in the umbrella review.
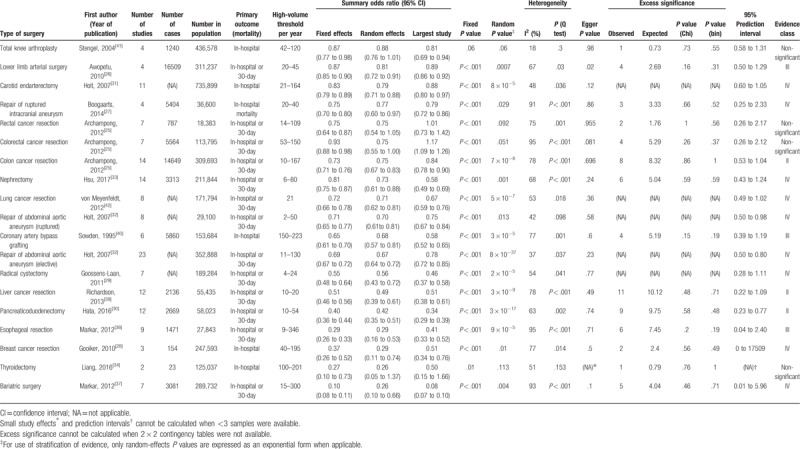
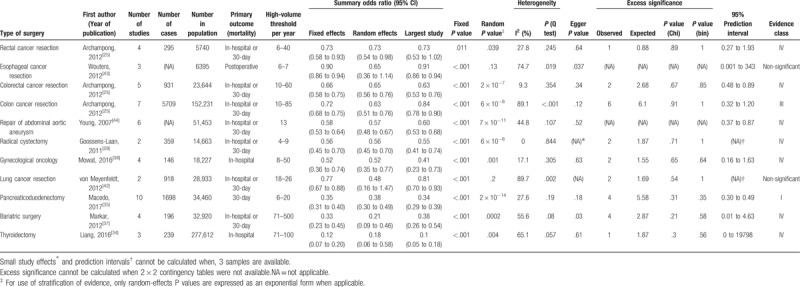
Table 2.
Summary of 11 meta-analyses on the association between surgeon volume and mortality in the umbrella review.
3.2. Summary effect size
For hospital volume and mortality associations, the summary random effects estimates were significant (P < .05) in 15 of 19 surgical procedures (79%), whereas the summary fixed effect estimates were significant in all surgical procedures (100%) (see Figs. 2 and 3). In 15 surgical procedures (84%), the effects of the largest study were significant. Regarding estimation of 95% prediction intervals, the null value was excluded in only 3 surgical procedures (repair of abdominal aortic aneurysm[32] [both elective and ruptured], and pancreaticoduodenectomy[30]).
Figure 2.

Summary random effects estimates with 95% confidence and prediction intervals from 19 meta-analyses on the association between hospital volume and mortality. AAA = abdominal aortic aneurysm; NA = not applicable.
Figure 3.

Summary random effects estimates with 95% confidence and prediction intervals from 11 meta-analyses on the association between hospital volume and mortality. AAA = abdominal aortic aneurysm; NA = not applicable.
For surgeon volume and mortality associations, the summary random effects estimates were significant in 9 of 11 surgical procedures (82%), whereas the summary fixed effects estimates were significant in all surgical procedures (100%). The effects of the largest study were significant in 10 surgical procedures (91%). Regarding estimation of 95% prediction intervals, the null value was excluded in only three surgical procedures (repair of abdominal aortic aneurysm,[44] colorectal cancer,[25] and pancreaticoduodectomy[35]).
3.3. Heterogeneity among studies
For hospital volume and mortality associations, significant heterogeneity (P < .10) was observed in 17 of 19 surgical procedures (89%). High heterogeneity (I2 > 60) was identified in 12 surgical procedures (63%), moderate heterogeneity (I2 = 40 to 60) in 5 surgical procedures (26%), and low heterogeneity (I2 < 40) in 2 surgical procedures (11%).
For surgeon volume and mortality associations, significant heterogeneity was detected in 6 of 11 surgical procedures (55%). High heterogeneity (I2 > 60) was identified in 4 surgical procedures (36%), moderate heterogeneity (I2 = 40 to 60) in 2 surgical procedures (18%), and low heterogeneity (I2 < 40) in 5 surgical procedures (45%).
3.4. Small-study effects
Small-study effects could not be calculated in one and one surgical procedure in the hospital and surgeon volume and mortality relations, respectively, due to an inadequate number of studies. For hospital volume and mortality associations, a small-study effect, as assessed using Egger test, was observed in 2 of 18 (one procedure was not applicable due to the small numbers of studies included) surgical procedures (11%). For surgeon volume and mortality associations, a small-study effect was detected in 2 of 10 (one was not applicable) surgical procedures (20%).
3.5. Excess significance
Excess significance could not be calculated in 5 and 2 surgical procedures for hospital and surgeon volume and mortality relations, respectively, because 2 × 2 contingency tables were not available. For the rest of the procedures, there was no evidence of excess significance bias for each surgical procedure in either hospital or surgeon volume and mortality associations. For hospital volume and mortality associations, among all 162 individual studies included, the O value was 66 whereas the E value was 66.9. For surgeon volume and mortality associations, among all 50 individual studies included, O was 24 whereas E was 25.1.
3.6. Stratification of evidence specific to umbrella reviews
For hospital volume and mortality associations, no surgical procedures were classified as “class I,” indicating that convincing evidence was absent. Three procedures (16%) (pancreaticoduedectomy,[30] liver cancer resection,[39] and colon cancer resection[25]) were categorized as “class II (highly suggestive).” Another three procedures (16%) were categorized as “class III (suggestive),” nine procedures (47%) as “class IV (weak),” and four procedures (21%) as “non-significant.”
For surgeon volume and mortality associations, convincing evidence (class I) was identified in one surgical procedure (9%) (pancreaticoduodenectomy[35]). No procedures were categorized as “class II”. One procedure (colon cancer resection) (9%) was categorized as “class III,” 7 procedures (64%) as “class IV,” and 2 procedures (18%) as “non-significant.”
3.7. AMSTAR 2 and GRADE classification
Figure 4 shows an overall summary of the AMSTAR 2 rating across the 20 meta-analyses. The rating of overall confidence in 1 meta-analysis[25] was judged as “high,” whereas that in the rest of the meta-analyses was judged as “critically low.” Detailed information on the results of AMSTAR 2 are shown in Supplemental Content 3. Specifically, in item 2, Prior protocol registration, only 2 meta-analyses[25,38] (10%) had evidence of registration being accomplished (e.g., Cochrane Database of Systematic Reviews[25] or PROSPERO[38]), but we could not find any information on a prespecified protocol or registration for any of the other meta-analyses. In item 4, Adequacy of the literature search, 6 meta-analyses[26,30,33,35,42,43] (30%) restricted the language to English, although no justification for this was provided, and it was unclear in seven other meta-analyses[29,31,32,36,39,40,44] (35%) whether a language restriction was applied at all. In item 11, Appropriateness of meta-analytical methods, two meta-analyses[31,44] (10%) reported the use of a fixed-effects model only. In item 16, Reporting of any potential sources of conflict of interest, including any funding the authors received for conducting the review, six meta-analyses[31,32,35,36,39,44] (30%) did not report either no competing interests or their funding sources.
Figure 4.

Results of AMSTAR 2 assessment (n = 20 meta-analyses). Among 16 items, only 7 critical domains and overall rating were indicated (see also supplemental Table 1).
For hospital volume and mortality associations, the final judgment of GRADE categorized two surgical procedures[32,41] (11%) as “low” and 17 surgical procedures (90%) as “very low.” For surgeon volume and mortality associations, the final judgment of GRADE categorized four surgical procedures[25,29,35] (36%) as “low” and seven surgical procedures (64%) as “very low.” Supplemental Content 4 shows the GRADE evidence profile representing the certainty assessment and the GRADE scores for mortality in each surgical procedure.
3.8. Sensitivity analysis
Two meta-analyses[36,43] on hospital volume and mortality association in esophageal resection were published in the same year (2012) (see Table, Supplemental Content 5). Dichotomous data were available in one meta-analysis[36] but not in the other[43]; therefore, we finally included the former meta-analysis in our umbrella review. However, the latter meta-analysis was also included in the analysis of surgeon volume and mortality association because the odds ratio and 95% CI were available. Comparison of the 2 meta-analyses is shown in supplemental Table 3. Both meta-analyses were notably different in the number of studies included, publication bias, and 95% prediction interval. The methodological quality (AMSTAR 2) and quality of evidence (GRADE) were the same in these 2 meta-analyses (“critically low” and “very low,” respectively).
4. Discussion
We found nominally significant reductions in the random-effects odds ratio in 84% of the surgical procedures in the hospital volume and mortality associations, and in 82% of the surgical procedures in the surgeon volume and morality associations. Nevertheless, the prediction intervals excluded the value of 1.0 in a few surgical procedures in both the hospital and surgeon volume relationships. This means that the true odds ratio in 95% of the future studies will not exceed the value of 1.0 for most of the surgical procedures. A low degree of heterogeneity was observed in several surgical procedures, whilst small-study effects were not observed in most of the surgical procedures, and excess significance bias was not found in any of the surgical procedures.
Summarizing the above in the context of an umbrella review-level stratification of evidence, only one surgical procedure—pancreaticoduodenectomy—fulfilled the criteria of convincing (class I) and highly suggestive (class II) evidence in both the hospital and surgeon volume and mortality relationships. That is, it is certain that pancreaticoduodenectomy performed in high-volume hospitals or by high-volume surgeons reduced all-cause short-term mortality by 58% or 62%, respectively. Strong correlations were found, and this result is in accordance with the common understanding that centralization has improved mortality in pancreaticoduodenectomy, which is representative of a surgical procedure of the highest complexity. In contrast, most of the evidence for the surgical procedures in the hospital volume- and surgeon volume-mortality relationship appeared to be weak (class IV) or “non-significant,” indicating that robust evidence on the association of healthcare provider volume and mortality was sparse in the currently available meta-analyses.
However, robust evidence is valid only when methodological flaws do not exist in each meta-analysis. Our assessment by AMSTAR 2 shows that only one meta-analysis, that registered with the Cochrane center,[25] resulted in a high rating, whereas all of the other meta-analyses were rated as “critically low.” Even pancreaticoduodenectomy could not escape from inherent methodological flaws. Notably, most of the meta-analyses did not accomplish prespecified protocol registration, implying that they are vulnerable to selective inclusion and reporting. Only 7 meta-analyses were free from language restriction. More critically, it was unclear whether language restriction was even applied at all in another 7 meta-analyses. Bias can be easily introduced when a meta-analysis is exclusively based on English-language papers alone.[45]
Furthermore, the quality of evidence as assessed by GRADE was rated as “very low” in most of the meta-analyses, and only a few were rated as “low.” A randomized controlled trial is difficult to perform for this type of the research question, probably due to ethical considerations; thus, results from observational studies may be the best evidence available at present and in the future. Basically, observational studies are categorized as “low.” A large magnitude of effect, a dose-response gradient, or plausible confounding is a prerequisite for upgrading to “high.” The meta-analyses on pacreaticoduedecotomy[30,35] could have been upgraded by strong associations (odds ratio < 0.5), but actually, they were downgraded by other factors including heterogeneity or absence of risk of bias assessment.
Our sensitivity analysis showed that the evidence level for esophageal resection in our umbrella review was “suggestive” for a hospital volume and mortality relationship.[36] Since Birkmeyer et al[46] published their paper in the early 2000s, the results of improved outcomes in esophageal resection have played a major role in pushing forward for centralization. Nevertheless, our results were quite disappointing. Furthermore, two similar meta-analyses[36,43] were published in the same year. Substantial inconsistency was present between these 2 meta-analyses with respect to heterogeneity, publication bias, and prediction interval, whilst the magnitude of the odds ratio and the AMSTAR 2 and GRADE classifications were similar. The plausible explanation for this is that each meta-analysis chose different studies. One included 9 studies,[36] whereas the other included 16 studies,[43] and more surprisingly, no studies overlapped despite the selection of similar databases and similar search periods. In any case, 6 years have passed since both were published, and an updated meta-analysis on esophageal resection is needed soon.
The strengths of our umbrella review can be appreciated from a comparison with three previously published systematic reviews of systematic reviews without meta-analytic approaches.[47–49] The strengths of our umbrella review can be appreciated from a comparison with 3 previously published systematic reviews of systematic reviews performed without applying meta-analytic approaches. Although all 3 reviews dealt with a wide variety of operations including percutaneous coronary intervention and mixed short-term and long-term outcomes were presented, the strength of our umbrella review lies in its conduction according to practical guidelines, with risk of bias and GRADE assessed with quantitative evaluations of prediction interval, excess significance, and other factors. Our study has several limitations. First, the definition of high-volume threshold varies from study to study. This might result in substantial heterogeneity in many of the meta-analyses included. It is a potential disadvantage to use provider volume as a quality indicator in this kind of study addressing the theme of volume-outcome relationships. Second, the meta-analyses included in our review spanned two decades (from 1995 to 2017) during which advancements in surgical techniques might have improved outcomes; therefore, caution is advised when discussing these meta-analyses together. Specifically, the meta-analyses published before 2010 need to be updated.
Which factor is more relevant to improving mortality, a high-volume hospital or a high-volume surgeon? This question may be more complicated by the paradox often mentioned of how do we interpret a situation in which a high-volume hospital uses low-volume surgeons or a high-volume surgeon practices in a low-volume hospital? The perception for our review is that the level of evidence for the relationship between a high-volume hospital and mortality ranked higher than that between a high-volume surgeon and mortality: however, which factor might most affect patient outcomes remains unclear. A future work using a multi-level approach (patient level, surgeon level, and hospital level) may shed some light on this question by, for instance, using a generalized linear mixed model to clarify how interactively and to what extent each factor affects an improvement in outcomes.
Policy makers and insurance companies should not expand the indications for centralization until higher-quality, more convincing evidence emerges, particularly for procedures that appeared to have a weak or non-significant evidence level such as total knee replacement, thyroidectomy, bariatric surgery, radical cystectomy, and rectal and colorectal cancer resections. However, policy makers also need to continue centralization for more complex surgical procedures such as pancreaticoduodenectomy, within a range that does cause unwanted secondary effects.
In conclusion, although healthcare provider volume and mortality have been extensively investigated over the past three decades, only a very few surgical procedures such as pancreaticoduodenectomy appear to have convincing evidence for an inverse surgeon volume-mortality relationship, and yet most surgical procedures resulted in having weak or “non-significant” evidence. Therefore, healthcare professionals and policy makers might be required to steer their centralization policy more carefully unless more robust, higher-quality evidence emerges, particularly for procedures considered as having a weak or non-significant evidence level, including total knee replacement, thyroidectomy, bariatric surgery, radical cystectomy, and rectal and colorectal cancer resections.
Acknowledgments
We thank Toshiro Tango, PhD (Center for Medical Statistics, Tokyo, Japan) for statistical consulting. We also thank George B. Powell of the firm Rise Japan for editing the manuscript.
Author contributions
Conceptualization: Toshiya Shiga.
Data curation: Hiroshi Hoshijima, Zen’ichiro Wajima, Toshiya Shiga.
Formal analysis: Hiroshi Hoshijima, Zen’ichiro Wajima, Toshiya Shiga.
Funding acquisition: Toshiya Shiga.
Software: Toshiya Shiga.
Supervision: Zen’ichiro Wajima, Hiroshi Nagasaka, Toshiya Shiga.
Validation: Hiroshi Hoshijima, Zen’ichiro Wajima.
Writing – original draft: Toshiya Shiga.
Writing – review & editing: Hiroshi Hoshijima, Zen’ichiro Wajima, Hiroshi Nagasaka, Toshiya Shiga.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, OR = odds ratio.
How to cite this article: Hoshijima H, Wajima Z, Nagasaka H, Shiga T. Association of hospital and surgeon volume with mortality following major surgical procedures. Medicine. 2019;98:44(e17712).
This research was funded in part by institutional resources from the International University of Health and Welfare.
The authors have no conflicts of interest to disclose.
References
- [1].Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364–9. [DOI] [PubMed] [Google Scholar]
- [2].Chang AC. Centralizing esophagectomy to improve outcomes and enhance clinical research: invited expert review. Ann Thorac Surg 2018;106:916–23. [DOI] [PubMed] [Google Scholar]
- [3].Dikken JL, Dassen AE, Lemmens VE, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer 2012;48:1004–13. [DOI] [PubMed] [Google Scholar]
- [4].Keong B, Cade R, Mackay S. Post-oesophagectomy mortality: the centralization debate revisited. ANZ J Surg 2016;86:116–7. [DOI] [PubMed] [Google Scholar]
- [5].van Putten M, Nelen SD, Lemmens V, et al. Overall survival before and after centralization of gastric cancer surgery in the Netherlands. Br J Surg 2018. [DOI] [PubMed] [Google Scholar]
- [6].Vonlanthen R, Lodge P, Barkun JS, et al. Toward a consensus on centralization in surgery. Ann Surg 2018;268:712–24. [DOI] [PubMed] [Google Scholar]
- [7].The Leapfrog Group. Proposed Changes to the 2018 Leapfrog Hospital Survey. http://www.leapfroggroup.org/sites/default/files/Files/LeapfrogHospitalSurvey_ProposedChanges_2018_Final_1.pdf (access date June 30, 2019). [Google Scholar]
- [8].Rodgers M, Jobe BA, O’Rourke RW, et al. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg 2007;142:829–39. [DOI] [PubMed] [Google Scholar]
- [9].Al-Sahaf M, Lim E. The association between surgical volume, survival and quality of care. J Thorac Dis 2015;7Suppl 2:S152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Postma J, Zuiderent-Jerak T. Beyond volume indicators and centralization: toward a broad perspective on policy for improving quality of emergency care. Ann Emerg Med 2017;69: 689-697 e681. [DOI] [PubMed] [Google Scholar]
- [11].Lumpkin S, Stitzenberg K. Regionalization and Its Alternatives. Surg Oncol Clin N Am 2018;27:685–704. [DOI] [PubMed] [Google Scholar]
- [12].Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol 2018;148:42–8. [DOI] [PubMed] [Google Scholar]
- [13].Macleod LC, Cannon SS, Ko O, et al. Disparities in access and regionalization of care in testicular cancer. Clin Genitourin Cancer 2018;16:e785–93. [DOI] [PubMed] [Google Scholar]
- [14].Cooke DT. Centralization of esophagectomy in the united states: might it benefit underserved populations? Ann Surg Oncol 2018;25:1463–4. [DOI] [PubMed] [Google Scholar]
- [15].Lieberman-Cribbin W, Liu B, Leoncini E, et al. Temporal trends in centralization and racial disparities in utilization of high-volume hospitals for lung cancer surgery. Medicine (Baltimore) 2017;96:e6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schunemann HJ, Cuello C, Akl EA, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132–40. [DOI] [PubMed] [Google Scholar]
- [19].Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health 2018;21:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [21].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]
- [23].Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245–53. [DOI] [PubMed] [Google Scholar]
- [24].Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Archampong D, Borowski D, Wille-Jørgensen P, et al. Workload and surgeon's specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev 2012. CD005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Awopetu AI, Moxey P, Hinchliffe RJ, et al. Systematic review and meta-analysis of the relationship between hospital volume and outcome for lower limb arterial surgery. Br J Surg 2010;97:797–803. [DOI] [PubMed] [Google Scholar]
- [27].Boogaarts HD, Van Amerongen MJ, De Vries J, et al. Caseload as a factor for outcome in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis: A systematic review. J Neurosurg 2014;120:605–11. [DOI] [PubMed] [Google Scholar]
- [28].Gooiker GA, van Gijn W, Post PN, et al. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider? Eur J Surg Oncol 2010;36Suppl 1:S27–35. [DOI] [PubMed] [Google Scholar]
- [29].Goossens-Laan CA, Gooiker GA, Van Gijn W, et al. A systematic review and meta-analysis of the relationship between hospital/surgeon volume and outcome for radical cystectomy: An update for the ongoing debate. Eur Urol 2011;59:775–83. [DOI] [PubMed] [Google Scholar]
- [30].Hata T, Motoi F, Ishida M, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: a systematic review and meta-analysis. Ann Surg 2016;263:664–72. [DOI] [PubMed] [Google Scholar]
- [31].Holt PJ, Poloniecki JD, Loftus IM, et al. Meta-analysis and systematic review of the relationship between hospital volume and outcome following carotid endarterectomy. Eur J Vasc Endovasc Surg 2007;33:645–51. [DOI] [PubMed] [Google Scholar]
- [32].Holt PJE, Poloniecki JD, Gerrard D, et al. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg 2007;94:395–403. [DOI] [PubMed] [Google Scholar]
- [33].Hsu RCJ, Salika T, Maw J, et al. Influence of hospital volume on nephrectomy mortality and complications: a systematic review and meta-analysis stratified by surgical type. BMJ Open 2017;7:e016833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liang TJ, Liu SI, Mok KT, et al. Associations of volume and thyroidectomy outcomes: a nationwide study with systematic review and meta-analysis. Otolaryngol Head Neck Surg (United States) 2016;155:65–75. [DOI] [PubMed] [Google Scholar]
- [35].Macedo FIB, Jayanthi P, Mowzoon M, et al. The impact of surgeon volume on outcomes after pancreaticoduodenectomy: a meta-analysis. J Gastrointest Surg 2017;21:1723–31. [DOI] [PubMed] [Google Scholar]
- [36].Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg 2012;16:1055–63. [DOI] [PubMed] [Google Scholar]
- [37].Markar SR, Penna M, Karthikesalingam A, et al. The impact of hospital and surgeon volume on clinical outcome following bariatric surgery. Obes Surg 2012;22:1126–34. [DOI] [PubMed] [Google Scholar]
- [38].Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol 2016;215:21–33. [DOI] [PubMed] [Google Scholar]
- [39].Richardson AJ, Pang TCY, Johnston E, et al. The volume effect in liver surgery-a systematic review and meta-analysis. J Gastrointest Surg 2013;17:1984–96. [DOI] [PubMed] [Google Scholar]
- [40].Sowden AJ, Deeks JJ, Sheldon TA. Volume and outcome in coronary artery bypass graft surgery: true association or artefact? BMJ 1995;311:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stengel D, Ekkernkamp A, Dettori J, et al. A rapid review of associations between provider volume and outcome of total knee arthroplasty. Where do the magical threshold values come from? Unfallchirurg 2004;107:967–88. [DOI] [PubMed] [Google Scholar]
- [42].Von Meyenfeldt EM, Gooiker GA, Van Gijn W, et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol 2012;7:1170–8. [DOI] [PubMed] [Google Scholar]
- [43].Wouters MWJM, Gooiker GA, Van Sandick JW, et al. The volume-outcome relation in the surgical treatment of esophageal cancer: A systematic review and meta-analysis. Cancer 2012;118:1754–63. [DOI] [PubMed] [Google Scholar]
- [44].Young EL, Holt PJ, Poloniecki JD, et al. Meta-analysis and systematic review of the relationship between surgeon annual caseload and mortality for elective open abdominal aortic aneurysm repairs. J Vasc Surg 2007;46:1287–94. [DOI] [PubMed] [Google Scholar]
- [45].The Cochrane Collaboration; 2011;Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from http://handbook.cochrane.org. (Accessed June 30, 2019). [Google Scholar]
- [46].Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
- [47].Pieper D, Mathes T, Neugebauer E, et al. State of evidence on the relationship between high-volume hospitals and outcomes in surgery: a systematic review of systematic reviews. J Am Coll Surg 2013;216: 1015-1025 e1018. [DOI] [PubMed] [Google Scholar]
- [48].Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev 2016;5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davoli M, Amato L, Colais P, et al. Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data. Epidemiol Prev 2013;372–3 Suppl 2:1–00. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


