Abstract
Age-related hearing loss (ARHL) is one of the most common disorders affecting elderly individuals. There is an urgent need for effective preventive measures for ARHL because none are currently available. Cockayne syndrome (CS) is a premature aging disease that presents with progressive hearing loss at a young age, but is otherwise similar to ARHL. There are two human genetic complementation groups of CS, A and B. While the clinical phenotypes in patients are similar, the proteins have very diverse functions, and insight into their convergence is of great interest. Here, we use mouse models for CS (CSA−/− and CSBm/m) that recapitulate the hearing loss in human CS patients. We previously showed that NAD+, a key metabolite with various essential functions, is reduced in CS and associated with multiple CS phenotypes. In this study, we report that NAD+ levels are reduced in the cochlea of CSBm/m mice and that short-term treatment (10 days) with the NAD+ precursor nicotinamide riboside (NR), prevents hearing loss, restores outer hair cell loss, and improves cochlear health in CSBm/m mice. Similar, but more modest effects were observed in CSA−/− mice. Remarkably, we observed a reduction in synaptic ribbon counts in the presynaptic zones of inner hair cells in both CSA−/− and CSBm/m mice, pointing to a converging mechanism for cochlear defects in CS. Ribbon synapses facilitate rapid and sustained synaptic transmission over long periods of time. Ribeye, a core protein of synaptic ribbons, possesses an NAD(H) binding pocket which regulates its activity. Intriguingly, NAD+ supplementation rescues reduced synaptic ribbon formation in both CSA−/− and CSBm/m mutant cochleae. These findings provide valuable insight into the mechanism of CS- and ARHL-associated hearing loss, and suggest a possible intervention.
Subject terms: Neurodegenerative diseases, Genome
Introduction
Hearing loss is one of the most prominent age-associated conditions. Its prevalence almost doubles every decade starting from adult life and affects up to 80% of individuals over the age of 85.1 There are two major types of hearing loss: conductive and sensorineural. Any damage or obstruction that prevents sound from being conducted into the inner ear is considered conductive hearing loss. Sensorineural hearing loss derives from deficits in sensory cells or auditory nerves of the inner ear. A common type of sensorineural hearing loss is age-related hearing loss (ARHL), which occurs progressively in individuals as they age.2 Despite its high prevalence and cost, there are no interventions that prevent ARHL.
Cockayne syndrome (CS) is a premature aging disorder with prominent sensorineural hearing loss, similar to ARHL.3,4 CS is primarily caused by mutations in CSA and CSB proteins that participate in various biological processes including DNA repair, transcription, and mitochondrial functions.5 Hearing loss is a cardinal clinical symptom of CS, affecting up to 80% of CS patients by age 10.4 Hearing loss in CS resembles ARHL as both are bilateral, sensorineural, and progressive. Mouse models of CS (CSA−/− and CSBm/m) recapitulate the progressive hearing loss and manifest sensory hair cell degeneration seen in the patients.3,4,6 Notably, hearing loss progression is slower in CSA−/− than in CSBm/m mice, reflecting the situation in CS patients.4,6
Our lab recently reported that the reduced abundance of nicotinamide dinucleotide (NAD+) in CS cells (patient-derived fibroblasts) and mice are associated with key CS phenotypes.7 NAD+ is a critical cofactor for several enzymes involved in mitochondrial biogenesis, mitophagy, and energy metabolism.8 It is likely that persistent DNA damage, observed in CS, constitutively activates poly-ADP ribose polymerase 1 (Parp1), which in turn depletes the cellular pool of NAD+.7 NAD+ declines with age and treatment with exogenous NAD+ improves mitochondrial function and life span in mice.9–12 Here, we investigate the relationship between NAD+ levels and hearing loss in CS mice. CS mice had lower levels of NAD+ so they were dosed with nicotinamide riboside (NR), an NAD+ precursor. NR has been shown to elevate cellular NAD+ levels in the cochlea and reduce neurite degeneration in auditory cells in mice following noise-induced damage.13
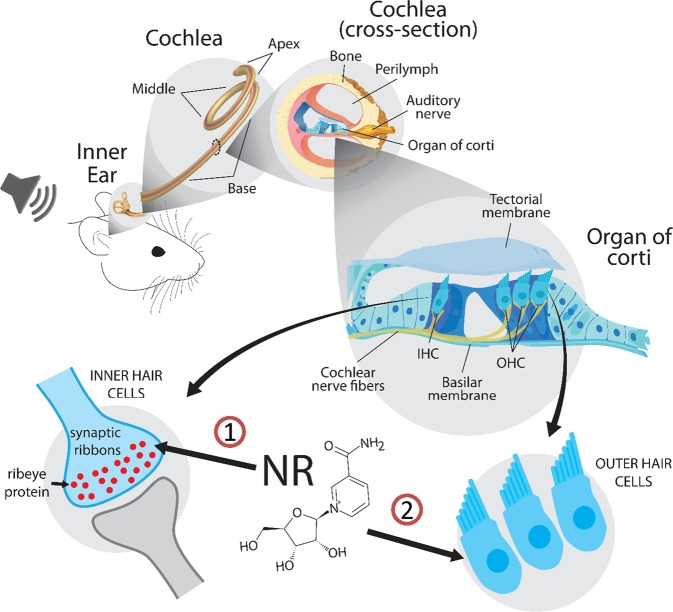
Hearing-related outcome measures used in this study included auditory brainstem response (ABR) and distortion product otoacoustic emission (DPAOE). ABR measures the fluctuation in voltage reflecting a neuronal response to sound, and DPOAE quantifies the electromotility of outer hair cells in the cochlea. The cochlea is the auditory portion of the inner ear and is composed of several cell types including inner and outer hair cells.14 Inner hair cells transduce sound vibration into electrical activity to be relayed into auditory nerve cells, while outer hair cells mechanically amplify low-level sound.
We find that NAD+ levels were reduced in the cochlea of CSBm/m mice and that a brief intervention with NR (only 10 days) rescues progressive high-frequency hearing loss, improves outer hair cell survival, and normalizes DPAOEs in CSBm/m mice. We observed similar but more modest effects on hearing loss in CSA−/− mice following NAD+ intervention. Remarkably, we detected reduced numbers of synaptic ribbons in inner hair cells in both CSA−/− and CSBm/m mice, which were normalized after NAD+ supplementation. The assembly and function of synaptic ribbons in inner hair cells, which facilitate high vesicle turnover,15 are modulated by NAD+ and NADH.16 Therefore, these results provide insight into a converging mechanism underlying hearing loss in CSA−/− and CSBm/m mice, which may be similar to the mechanism underlying hearing loss in humans.
Results
Hearing in NR-treated CS mouse genotypes
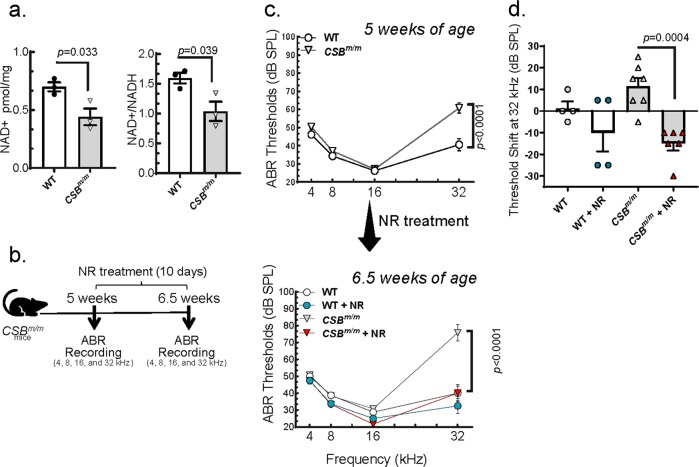
We previously reported that reduced NAD+ levels are associated with CS pathology.7,17 Given that hearing loss is a cardinal symptom of CS, we assayed NAD+ levels in the cochlea of CSBm/m mice. We found that total NAD+ and relative NAD+/NADH levels were lower in the cochlea of the CSBm/m mice compared to WT (Fig. 1a). In this study, CS mice were dosed with NR, an NAD+ precursor, to assess the effect of NAD+ supplementation on progressive hearing loss in CS mouse genotypes. We began treating mice with NR just after 5 weeks of age and assessed hearing capacity by measuring ABRs (see Methods for details) in response to sound at multiple frequencies (Fig. 1b). We observed that CSBm/m mice at 6.5 weeks of age develop a progressive hearing impairment and have a hearing loss of more than 35 dB at 32 kHz compared to WT (Fig. 1c and Supplementary Fig. 1). Strikingly, only 10 days of exposure to NR (12 mM) significantly prevented the hearing loss in CSBm/m mice and reduced the high-frequency hearing threshold from 77 to 40 dB (Fig. 1c, compare top and lower panels). We then compared the hearing threshold shift in individual mice in NR-treated and non-treated groups. We observed that non-treated CSBm/m mice at 6.5 weeks of age, on average, lost 12 dB over 10 days and the treatment with NR not only prevented hearing loss, but improved hearing by an average of 15 dB (Fig. 1d).
Fig. 1. NAD+ supplementation prevents progressive hearing loss in CSBm/m mice.
a Total NAD+ levels per mg of the cochlea and relative NAD+/NADH levels were measured in the cochlea of CSBm/m and WT (6-month-old) mice (N = 3). Two-tailed t-tests were used to determine significant difference. b Outline for NR treatment and ABR recordings in CSBm/m mice. c ABR thresholds for CSBm/m and WT mice following a 10-day treatment with NR (12 mM) delivered via drinking water. A total of 21 mice (CSBm/m (N = 7); CSBm/m + NR (N = 6); WT (N = 4); WT + NR (N = 4)) were tested by ABR at 5 weeks of age and again following the 10-day treatment (6.5 weeks of age). Multiple graphs are used to visualize error bars on each dataset. Two-way ANOVA with Tukey’s post-hoc test was used to determine significant difference. d Treatment with NR (12 mM) prevents the increased threshold shift observed in CSBm/m mice between 5 weeks and 6.5 weeks at 32 kHz. Note: ABR data in c at 5 and 6.5 weeks of age were used to calculate the hearing shift. Two-way ANOVA with uncorrected Fisher's LSD post-hoc test was used to determine significant difference.
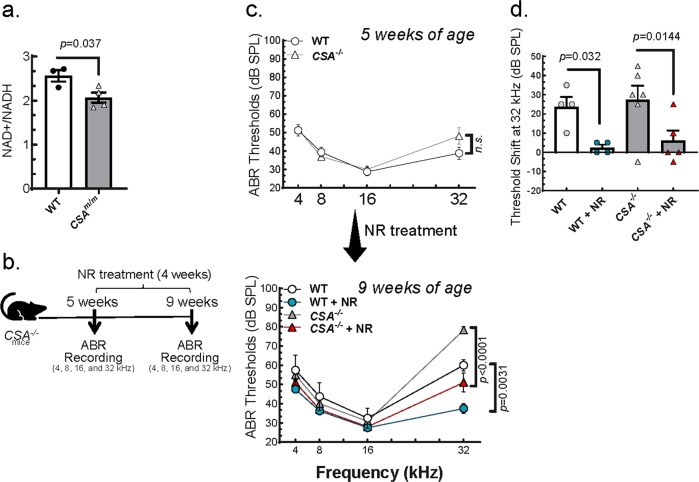
Like CSBm/m, CSA−/− mice also manifested progressive hearing loss although less severe6 (Supplementary Fig. 2a). This less severe phenotype has also been reported in CSA patients.4,6 We found that NAD+/NADH levels were also lower in the cochlea of CSA−/− mice (Fig. 2a) and tested the effect of NAD+ supplementation on the hearing defect in CSA−/− mice (Fig. 2b). Initial treatment with 12 mM dose of NR did not attenuate the hearing loss in these mice (Supplementary Fig. 2b) and thus we increased the dose of NR (24 mM) over a 4-week period from 5 to 9 weeks of age. After exposure to 24 mM NR, the ABR threshold at 32 kHz for CSA−/− decreased by ~25 dB (Fig. 2c, compare top and lower panels). During this 4-week period, non-treated CSA−/− mice developed an average of 28 dB of hearing loss while the hearing threshold in NR-treated CSA−/− mice was elevated only 6 dB, which is not considered as hearing loss (<10 dB) (Fig. 2d). These results collectively suggest that NAD+ supplementation strongly reduces hearing loss in both CSA−/− and CSBm/m mice.
Fig. 2. NAD+ supplementation prevents progressive hearing loss in CSA−/− mice.
a NAD+/NADH levels were measured in CSA−/− (7-month-old) mice (N = 3). Two-tailed t-tests were used to determine significant difference. b Outline for NR treatment and ABR recordings in CSA−/− mice. c ABR thresholds of CSA−/− and WT mice following 4 weeks of NR treatment (24 mM) in their drinking water. Total of 19 mice (CSA−/− (N = 6); CSA−/− + NR (N = 5); WT (N = 4); WT + NR (N = 4)) were used to measure ABR at the age of 5 weeks (before NR treatment) and 9 weeks (after NR treatment). CSA−/− mice do not show a significant threshold shift at any frequency at 5 weeks of age. However, at 9 weeks, CSA−/− mice show an approximately 20-dB shift by comparison with WT. Multiple graphs are used to visualize error bars on each dataset. Two-way ANOVA with Tukey’s post-hoc test was used in to determine significant difference. d A significant threshold shift is present in CSA−/− mice and WT at 32 kHz. Treatment with NR (24 mM) induced a significant rescue of the age-related threshold shift in CSA−/− and WT mice. ABR data in c at 5 and 9 weeks of age were used to calculate the hearing shift. Two-way ANOVA with uncorrected Fisher's LSD post-hoc test was used to determine significant difference.
Outer hair cells in NR-treated CS mouse genotypes
To gain more insight into the mechanisms by which NR reduces hearing loss in CS mouse genotypes, we assessed auditory capacity with DPOAE assay, an approach that provides information about cochlear integrity and outer hair cell function. The results show that CSBm/m mice at 12 weeks of age have lower DPOAE signals than WT mice at 16 kHz (Fig. 3a, left panel), but not at 10 and 12 kHz (Supplementary Fig. 3a). This decrease at 16 kHz was normalized by exposure to NR (Fig. 3a, middle panel), suggesting that NAD+ supplementation improves outer hair cell function in CSBm/m mice at 16 kHz. In the CSA−/− mice at 9 weeks of age, the DPOAE response was similar to WT mice for all frequencies tested, suggesting that outer hair cell function in 9-week-old CSA−/− mice are intact and functional (Fig. 3b and Supplementary Fig. 3b).
Fig. 3. Short-term NR supplementation restores reduced DPOAE levels in CSBm/m mice at 16 kHz.
a NR intervention (12 mM of NR for 7 weeks) corrects reduced DPOAEs in CSBm/m mice at 12 weeks of age at 16 kHz (CSBm/m (N = 5); CSBm/m + NR (N = 3); WT (N = 7); WT + NR (N = 6)). Multiple graphs are used to visualize error bars in each dataset. b DPOAE levels in CSA−/− and WT at 9 weeks of age (CSA−/− (N = 6); CSA−/− + NR (N = 5); WT (N = 3); WT + NR (N = 4)) were recorded at 16 kHz following 4 weeks of NR treatment (24 mM) in their drinking water. Mean ± S.E. is shown in all graphs. Multiple graphs are used to visualize error bars on each dataset. The input in dB/output in dB functions in the range from 50 dB SPL to 60 dB SPL input were used to perform statistical analysis on groups, followed by a linear mixed-effect model on repeated-measures of DPOAEs in each group.
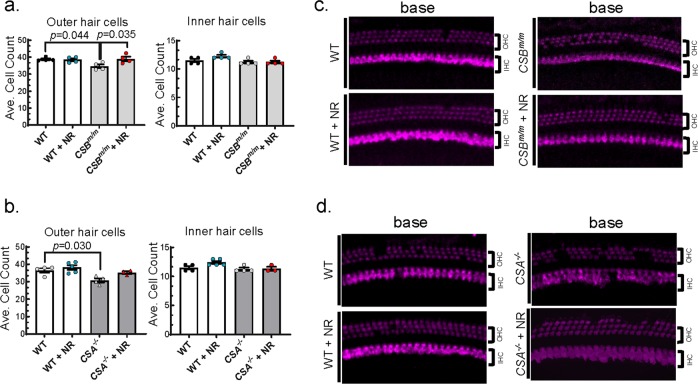
Sensorineural hearing loss arises specifically from degenerative and functional changes in cochlear hair and auditory nerve cells, and loss of their synaptic connections.18,19 Next, we assessed the degenerative changes in cochlear hair cells in CSBm/m and CSA−/− mice via cochlear histology analysis. The cochlea is composed of base, middle, and apex regions; the base region senses high-frequency and the apex region senses low-frequency sounds. Since hearing loss in CS mice was restricted to high frequencies (Fig. 2), we focused on the basal region of the cochlea, although middle and apical regions were also analyzed (see Methods for details). Hair cell numbers in each area correlate with the hearing capacity for specific frequency ranges. To investigate the influence of NR on hair cell number and function, cochleae were isolated and stained with an antibody to Myo7a, a hair cell marker. There were significantly fewer outer hair cells in the basal cochlear region in CSBm/m mice than in WT (Fig. 4a, b), while middle and apex regions were largely unaffected (Supplementary Fig. 4). This observation is consistent with the ABR data showing high-frequency hearing loss in CSBm/m mice. Exposure to NR normalized outer hair cell numbers in the base cochlear region in CSBm/m mice (Fig. 4a, b). The outer hair cell number was also reduced in the base cochlear region in CSA−/− mice but did not increase significantly after treatment with NR (Fig. 4c, d). As was the case for CSBm/m, the numbers of outer hair cells in the apical and middle regions of the cochleae in CSA−/− mice were not affected, which is consistent with our DPOAE and ABR results at lower frequencies (Supplementary Fig. 5). These results suggest that NAD+ supplementation restores outer hair cell number specifically in CSBm/m mice.
Fig. 4. Outer hair cells are reduced in CSA−/− and CSBm/m mice, and short-term NR supplementation rescues outer hair cell loss in CSBm/m mice.
a Summary data for the average number of outer (left) and inner (right) hair cells per 100 μm in the basal region of the cochlea in WT or CSBm/m mice with and without NR treatment (12 mM in drinking water for 10 days) at 6.5 weeks of age. The decreased number of outer hair cells observed in CSBm/m mice is prevented by NR treatment. In contrast, no changes in the number of inner hair cells were observed between conditions. N = 4; mean ± S.E.; two-way ANOVA with Tukey’s post-hoc test was used to determine significant difference. b Representative images of outer and inner hair cells in the cochlear base region of CSBm/m and WT mice following NR treatment as described in a. Myo7a staining is used to identify hair cells. c Outer hair cells were reduced in base region in CSA−/− mice (CSA−/− (N = 4); CSA−/− + NR (N = 3); WT (N = 4); WT + NR (N = 5)). Mean ± S.E.; two-way ANOVA with Tukey’s post-hoc test was used to determine significant difference. d Representative images of base outer and inner hair cells of CSA−/− and WT mice following NR treatment as described in c. Myo7a staining is used to identify hair cells.
Ribbon synapses in NR-treated CS mouse genotypes
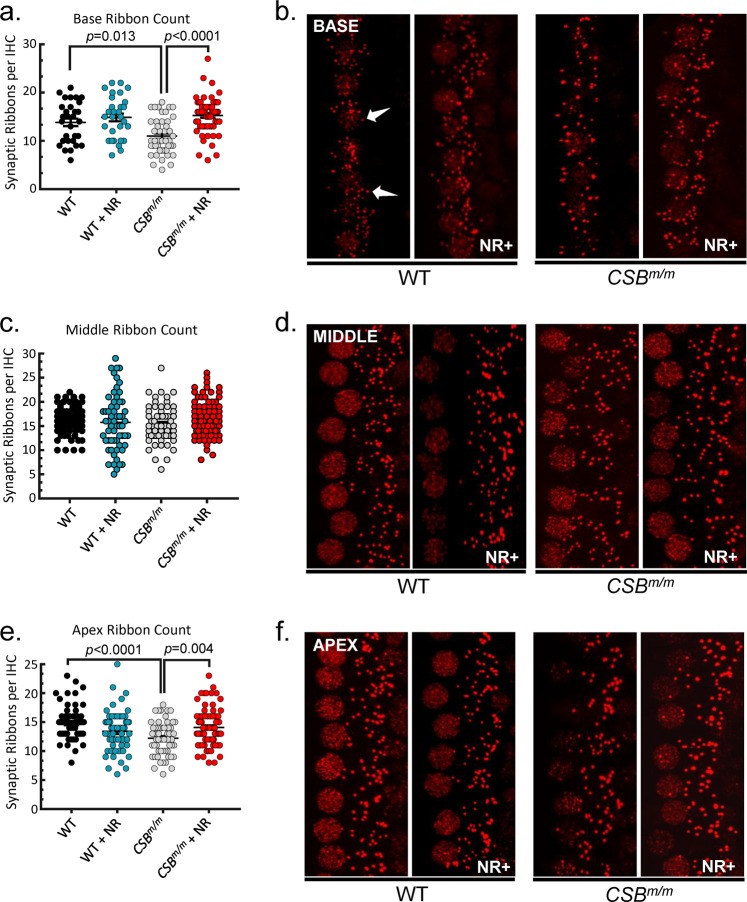
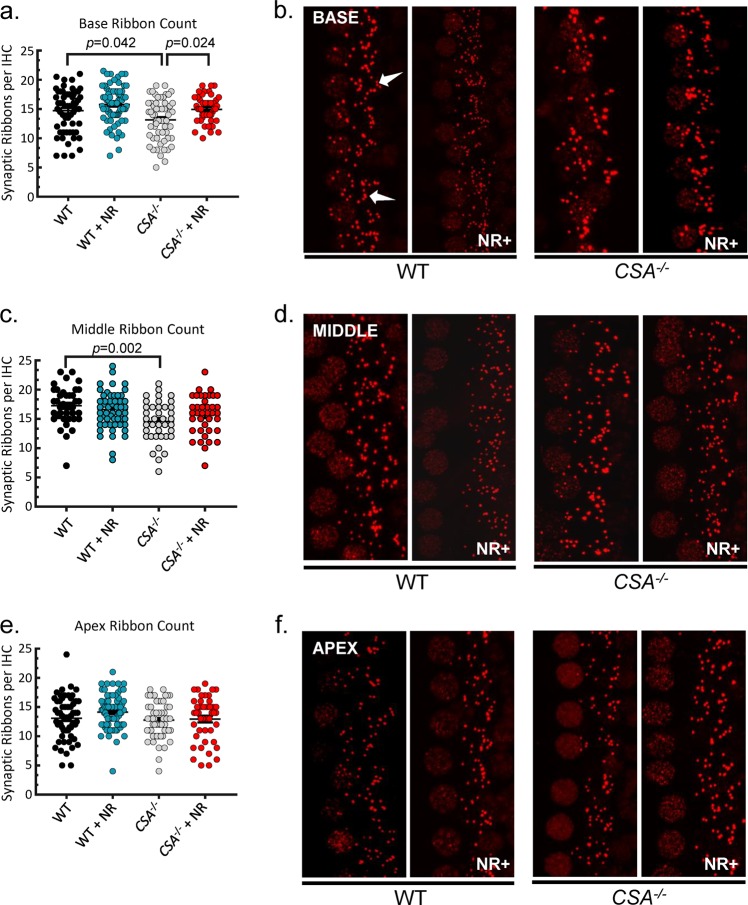
The number of inner hair cells in all regions of the cochlea was similar in WT, CSA−/−, and CSBm/m mice (Fig. 4a, c, right panels). We therefore next investigated the integrity of synaptic transmission between the inner hair cells and afferent neurons affecting the hearing loss in CS mouse genotypes. Inner hair cells form a specialized synapse, ribbon synapse, which provides rapid and sustained synaptic transmission.15,20,21 The function of ribbon synapses relies on the formation of unique electron-dense structures called synaptic ribbons, which are regarded as an accurate metric of inner hair cell afferent innervation.15,20,22 Interestingly, the major component of the synaptic ribbons is the protein Ribeye, which is important for the function and physical integrity of ribbon synapses, and it possesses a binding pocket for NAD(H).23 It has been shown that NAD(H) binding to Ribeye modulates the dimerization of Ribeye monomers to build the synaptic ribbon.16,24 Therefore, we tested whether NAD+ supplementation impacts ribbon formation in CS. We isolated cochleae and stained them with anti-Ctbp2 (Ribeye). Immunolabeling was quantified by counting ribbon-forming puncta in inner hair cells of WT, CSA−/−, and CSBm/m mice. Compared to WT mice, CSA−/− and CSBm/m mice exhibited a significant reduction in ribbon counts of inner hair cells in the basal region of the cochlea, suggesting a defect in synaptic transmission in both genotypes of CS mouse (Figs. 5a, b and 6a, b). Remarkably, treatment with NR normalized ribbon counts in the same cochlear region of CSA−/− and CSBm/m mice (Figs. 5a, b and 6a, b). Synaptic ribbons were also reduced in the middle and apex cochlear regions in CSA−/− and CSBm/m mice, respectively (Figs. 5e, f and 6c, d). After intervention with NR, synaptic ribbons were normalized in the apex region in CSBm/m but not in CSA−/− mice. NR had no impact on the middle cochlear ribbons in either mouse strain (Figs. 5c, d and 6c, d). Although NR reduced hearing loss in WT mice (Fig. 2d), intervention with NR did not alter inner hair cell number/synaptic ribbon count in WT mice (Fig. 6a, b). Collectively, these results suggest that NR intervention restores impaired ribbon synapse formation, thereby improving synaptic transmission during the cochlear response to auditory stimuli to rescue high-frequency hearing loss in both genotypes of CS mouse.
Fig. 5. NR enhances synaptic ribbon count per inner hair cell in the base and apex regions in CSBm/m mice cochlea.
a, c, e The average synaptic ribbon count per inner hair cell in the cochlea base region (a), middle region (c), and apex region (e). The average number of ribbons is reduced in the base turn of the cochlea in CSBm/m mice relative to WT at 6.5 weeks of age. However, this effect is prevented by NR treatment. A similar change is observed in the apical turn but not in the middle region of the cochlea. b, d, f Representative image of immunostaining for synaptic ribbons (red, anti-Ctbp2) of cochlear base segments (b), middle segments (d), and apex segments (f). A magnification of 40× was used for all images that are oriented such that the base of each hair cell is located on the right side of the image. Hair cell nuclei, which are also labeled with anti-Ctbp2, are located on the left side. Each individual puncta represents a single synaptic ribbon (arrows). Fifteen cells per region per mouse are used for quantification; 12 mM NR in drinking water for 10 days was used for intervention (CSBm/m (N = 4); CSBm/m + NR (N = 5); WT (N = 4); WT + NR (N = 4); mean ± S.E.; two-way ANOVA with Tukey’s post-hoc test was used to determine significant difference).
Fig. 6. NR enhances synaptic ribbon count per inner hair cell in the base region in CSA−/− mice cochlea.
a, c, e The average synaptic ribbon count per inner hair cell in the cochlea base region (a), middle region (c), and apex region (e). The average number of ribbons is reduced in the base turn of the cochlea in CSA−/− mice relative to WT at 9 weeks of age. However, this effect is prevented by NR treatment. b, d, f Representative image of immunostaining for synaptic ribbons (red, anti-Ctbp2) of cochlear base segments (b), middle segments (d), and apex segments (f). A magnification of 40× was used for all images that are oriented such that the base of each hair cell is located on the right side of the image. Hair cell nuclei, which are also labeled with anti-Ctbp2, are located on the left side. Each individual puncta represents a single synaptic ribbon (arrows). Fifteen cells per region per mouse are used for quantification; 24 mM NR in drinking water for 4 weeks was used for intervention (CSA−/− (N = 4); CSA−/− + NR (N = 4); WT (N = 4); WT + NR (N = 5); mean ± S.E.; two-way ANOVA with Tukey’s post-hoc test was used to determine significant difference).
Discussion
Hearing loss is a major feature in CS patients. Here we sought to quantify hearing changes in CS mouse models and interrogate the potential utility of NAD+ supplementation. The results support the following conclusions: (1) Hearing loss progresses in CSA−/− and CSBm/m mice at high frequencies and short-term exposure to NR normalizes the ABR threshold at 32 kHz in CSA−/− and CSBm/m mice; (2) fewer outer hair cells are detected in the basal region of cochleae from CSA−/− and CSBm/m mice than in WT cochlea, and NR normalizes this phenotype in CSBm/m mice; (3) the abundance of synaptic ribbons are lower in the base cochlear region from CSA−/− and CSBm/m mice than in control WT mice, and NR normalizes this phenotype in CSA−/− and CSBm/m mice. Although the hearing loss phenotype and its response to NR in WT and CS mice are complex, the data presented here support the idea that NAD+ depletion plays an important role in the etiology of age-dependent hearing loss in these mice.
CSA patients generally have a milder form of the disease than CSB patients.5,6 This is also recapitulated in the CSA mouse model which has less severe features than the CSB mouse.6 Importantly, both CSA−/− and CSBm/m mice show a dose-dependent reduction/normalization of hearing loss after exposure to NR (Supplementary Figs. 2b and 6). We next examined if there is a change in cochlear histology along with hearing loss prevention in NR-treated CS mice. To that end, we found that reduced DPOAEs in CSBm/m mice at 16 kHz at 12 weeks of age were restored with NR (Fig. 3a). In line with the DPOAE results, we observed a reduction in the number of outer hair cells in the base turn of CSBm/m cochlea at 6.5 weeks of age, and NR treatment rescued this abnormality (Fig. 4a, b). NAD+ supplementation corrects multiple aspects of mitochondrial abnormalities in the cerebellum of CSBm/m mice.7 Perhaps, the same phenomenon occurs in CSBm/m cochlea as well, enhancing outer hair cell survival via improving mitochondrial homeostasis. Interestingly, although there is a significant reduction of outer hair cells in the base region of CSA−/− cochlea, this defect was not reflected in DPOAE levels in CSA−/− mice (Figs. 3b and 4c, d). It is possible that loss of outer hair cells in CSA−/− mice was compensated with increased function of nearby outer hair cells, thereby elevating DPOAEs. Nevertheless, unlike in CSBm/m mice, NR treatment did not have a significant impact either on outer hair cells or on DPOAEs in CSA−/− mice, suggesting that hearing benefits of NR treatment in this genotype does not include outer hair cell function or survival.
Despite the dramatic hearing loss, the number of inner hair cells was largely unaffected in CS mice cochleae. Therefore, we assessed synaptic connectivity between inner hair cells and innervating afferent neurons. We observed that there were reduced synaptic ribbon (Ribeye) counts in the base turn of CSA−/− and CSBm/m cochlea compared to WT mice, suggesting that defects in the ribbon synapses might be a converging mechanism leading to hearing loss in CS. The reduction of synaptic ribbon count in inner hair cells was 11% in CSA−/− mice by the age of 9 weeks and 21% in CSBm/m mice by the age of 6.5 weeks at high frequencies (Figs. 5a and 6a). This amount of reduction in the synaptic ribbon counts occurs at approximately 32 and 80 weeks of age in mice with normal aging, respectively.25 Remarkably, NR intervention significantly corrected the reduced ribbon counts in both CSA−/− and CSBm/m cochlear inner hair cells (Figs. 5a, b and 6a, b). We conclude that depleted NAD+ levels due to deficiency of CS proteins disrupt synaptic ribbon assembly and function in inner hair cells. Thus, NAD+ supplementation may improve inner hair cell function via affecting ribbon synapses. This scenario is also consistent with the dramatic benefits of NR on hearing loss with short treatment duration (only 10 days) as replenishing NAD+ might exert its beneficial effect rapidly on synaptic ribbons and improve hearing. It is worth noting that synaptic ribbons are also present in cells of the retina.26 Interestingly, retinal dystrophy is another clinical feature of CS4,27, and NAD+ intervention protects against retinal degeneration in mice.28 Collectively, our results suggest that NAD+ supplementation via NR has distinct targets including synaptic ribbon formation in inner hair cells and the rescue of outer hair cell loss in CS cochlea (Fig. 7).
Fig. 7. Figure demonstrating that NR intervention enhances synaptic ribbon formation in inner hair cells of CSA−/− and CSBm/m mice (arrow #1), and prevents outer hair cell loss in CSBm/m mice (arrow #2).
OHC stands for outer hair cells. IHC stands for inner hair cells.
We previously reported that NAD+ supplementation can activate Sirt1 and rescue CS-related phenotypes.7 Sirt1 is a NAD+-dependent protein deacetylase and is known to have protective effects against aging-associated degeneration.29 Notably, Sirt1 expression also declines in the mouse cochlea during aging and its overexpression protects cochlear hair cells and delays early-onset ARHL. Given that Sirt1 activation is attenuated in CSBm/m patient fibroblasts,7 the benefits of NAD+ supplementation on hearing in CS might also be mediated by Sirt1 activation. Indeed, Sirt1 activation by resveratrol treatment prevents outer hair cell loss and rescues the reduced synaptic ribbon counts in inner hair cells upon aging.30,31 Future studies exploring the CSB-Sirt1-NAD axis in the context of hearing loss are warranted as the role of Sirt1 on cochlear function is still a subject of debate.32
NR is generally recognized as safe for use in dietary supplements and well-tolerated up to 1000 g daily in humans for 6 weeks.33 Recent human clinical reports suggested potential benefits of NR intervention on improving redox homeostasis and exercise performance while reducing systolic blood pressure and arterial stiffness.33,34 Our study provides a rationale for exploring NR as a potential therapeutic approach for both accelerated and normal sensorineural hearing loss in humans. Hearing loss in CS resembles ARHL as both are sensorineural and progressive rather than congenital. Therefore, NAD+ supplementation might provide protective effects on hearing loss during aging as well. Indeed, the results presented here show that NR (24 mM dose) protects against hearing loss above 20 dB in WT mice (Fig. 2d). With the increasing prevalence of hearing loss in the aging population and the lack of therapeutic approaches for its treatment, the results of this study could ultimately, once successfully translated to the clinical arena, have a large public health impact. However, given that the cellular bioavailability of NAD+ declines upon aging,11,12 chronic NR supplementation may be necessary to achieve its full benefits. Notably, the hearing defect and beneficial results of the intervention are seen in both CSA−/− and CSBm/m mice. The proteins have very different functions that converge in our studies and thus may explain fundamental issues related to the CS disorder.
Methods
Animals
Mouse models of CSA−/− (knockout mice),35 CSBm/m (mice carrying a premature stop codon in exon 5 to mimic the K337 stop truncation mutation from CS patient (CS1AN)),36 and wild-type (WT) on a C57BL/6J background in the range of 5–12 weeks of age were used for the auditory and histology experiments. Six- or 7-month-old mice were used for quantifying NAD+ levels in the cochlea. The NR treatment started at the age of 5 weeks for both CS mice models right after auditory assessment. All animal protocols were approved by the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Aging, in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
NAD+ supplementation
Unless stated otherwise, CSBm/m mice were given NR at 3.5 mg/ml (12 mM) for 10 days and CSA−/− mice were given NR at 7 mg/ml (24 mM) for 4 weeks in their drinking water, while the non-treated control groups received only drinking water. The drinking water supply was replenished/changed twice per week. A total of 1000 mg of NR daily supplementation for 6 weeks at this dose is well-tolerated in humans and effectively increases blood cellular NAD+ concentrations.33 The average 6 ml daily water intake of C57BL/6J mouse at an early age corresponds to the average of 21 mg (0.51 mg/kg) or 42 mg (1.02 mg/kg) daily NR intake in CSBm/m and CSA−/− mice, respectively.37 Given the difference in weight/surface area between human and mice,38 the range of 0.51–1.02 mg/kg of NR in mice corresponds to the range of 2.48–4.96 mg/kg of NR in humans, which is under the tolerated levels for NR consumption and is higher than the concentration needed to increase whole-blood NAD+.33,39
NAD+ quantification
Following dissection in cold PBS, cochleae were weighed and placed in NADH/NAD Extraction Buffer (Abcam, ab65348). Following homogenization with a micro pestle, an NAD/NADH Assay Kit (Abcam, ab65348) was used to quantify NAD+ and NADH levels. Two technical replicates were performed on n = 3 mice per condition; data were normalized to cochlear weight.
Audiometry
The ABR assay is a quantitative assessment of the neurological response, measured as evoked potential, detected within 10 ms of an auditory stimulus. ABR assays were performed on mice anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via an intraperitoneal (i.p.) injection, placed on a heating pad 7 cm from the sound source, an MF1 Multi-Field Magnetic Speaker, in a soundproof chamber. Needle electrodes were inserted sub-dermally (vertex–ventrolateral to pinna). Tone burst stimuli (5 ms duration with a 0.1-ms rise–fall time) were presented at variable volume (10–90 dB SPL) in 5 dB steps at 4, 8, 16, and 32 kHz. The outcome measure is the minimum volume threshold (in dB) that evokes a response at each frequency tested. For each frequency, the threshold was determined by an average of 512 responses. ABR threshold was determined by visual inspection of the averaged waveforms. The threshold was considered to be the lowest stimulus level at which at least one wave was considered to be present. The threshold was the lowest dB stimulus that evoked at least one response wave. ABR assays were performed using an RZ6 system (Tucker Davis Technologies) with Biosig software (Tucker Davis Technologies).
DPOAE quantifies the electromotility of outer hair cells in the cochlea. DPOAEs were measured by inserting an ear-plug containing a small microphone (ER-10B+) and two speakers (MF1 Multi-Field Magnetic Speaker), in the outer ear canal of each mouse. A series of auditory stimuli were delivered, each composed of two tones at equal decibel level but distinct frequencies, f1 and f2, where f2 > f1, f2/f1 = 1.2) at f0 = 10, 12, 16, and 32 kHz (f0 = (f1 × f2)1/2). Decibel level of both tones varied over the range 80 dB SPL to 10 dB SPL in 5-dB steps. The cubic distortion product at the frequency 2f1 − f2 was recorded as an average of 512 responses at each frequency tested. DPOAE measurements were made using the RZ6 system (Tucker Davis Technologies) with Biosig software.
Whole-mount cochlea immunostaining
Entire otic capsules were dissected and decalcified in a 0.25 M EDTA solution that was changed daily for 5–7 days. Subsequently, cochleae were dissected and divided into the apex, middle, and basal turns. Following decalcification and dissection, apex, middle, and basal turns of cochlea were blocked for 1 h with gentle agitation at room temperature in PBS, 20% triton X-100 (1:200), 10% normal donkey serum, and donkey anti-mouse fab fragments (Jackson ImmunoResearch Laboratories Inc., catalog #715-007-003, 1:200) with agitation. The primary antibody was added and incubated overnight at 4 °C. Primary antibodies were Myo7a (rabbit, Proteus Biosciences Inc., catalog #25-6790, 1:2000), and Ctbp2 (mouse, BD Biosciences, catalog #612044). After washing, the secondary antibody was added, and samples were incubated for 1 h at room temperature. Donkey secondary antibodies conjugated to Alexa Fluor fluorophores (Fisher Scientific, 1:1000) were used with phalloidin-Atto 390 (Sigma Aldrich, catalog #50556, 1:200) to stain filamentous actin or DAPI (4',6-diamidino-2-phenylindole, dihydrochloride, catalog #D1306, Thermo Fisher Scientific, 1:10,000) to label nuclei. Samples were mounted on glass slides in Vectashield antifade mounting medium (Vector Labs, catalog# H-1000), then imaged on a Zeiss 710 confocal microscope.
The total cochlear length was measured using ZEISS ZEN Black 2.3 SP1 software. Apex, middle, and base midpoints were assigned as loci at 25%, 50%, and 75% of the total length of the cochlea, respectively. Hair cell counts were quantified in 100-μm segments of the total cochlea for all regions (manual counting). For segments with damaged or immeasurable tissue, the nearest 150 µm of intact tissue was used. For synaptic counts, the number of presynaptic terminals in 15 inner hair cells was determined.
Statistical analysis
Two-way ANOVA with Tukey’s post-hoc test was used to determine significant differences across multiple samples with two groups unless indicated otherwise. Two-way ANOVA with uncorrected Fisher's LSD post-hoc test was used to determine the significant difference in the hearing threshold when a comparison between groups was not involved. One-way ANOVA with Tukey’s post-hoc test was used to determine significant differences across multiple samples in more than two groups. Two-tailed t-tests were used to compare single groups. Statistical analyses were performed with GraphPad Prism (GraphPad Software, Inc.). The area under the curve across the whole dB SPL (f2) above the noise levels and a linear mixed-effect model for repeated-measures of DPOAE with multiple comparisons at the range of 50–60 dB SPL (f2) were employed to determine significant analysis for DPOAE levels between groups and both methods provided the same results.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Dr. Yujun Hou for her help in the initial cardiac perfusion experiments. This research was supported by the Intramural Research Program of NIA, NIH (V.A.B.), the NIDCD (M.W.K., DC000059), NIH Bench-to-Bedside (BtB) Program (V.A.B.), ChromaDex (V.A.B. has a CRADA), and The Office of Dietary Supplements (ODS).
Author contributions
M.N.O., M.W.K., and V.A.B. designed the experiments. M.N.O. wrote the manuscript. B.M., S.H., and K.E.-H. performed whole-mouth cochlear staining. M.N.O., B.M., D.L.C., R.K., and T.F. performed data analysis. M.N.O., J.T., and W.O. performed the ABR and DPOAE analyses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
V.A.B. receives nicotinamide riboside from Chromadex Corp.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41514-019-0040-z.
References
- 1.Yamasoba T, et al. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear. Res. 2013;303:30–38. doi: 10.1016/j.heares.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TS, Chung JW. Evaluation of age-related hearing loss. Korean J. Audiol. 2013;17:50–53. doi: 10.7874/kja.2013.17.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shemen LJ, Mitchell DP, Farkashidy J. Cockayne syndrome–an audiologic and temporal bone analysis. Am. J. Otol. 1984;5:300–307. [PubMed] [Google Scholar]
- 4.Wilson BT, et al. The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care. Genet. Med. 2016;18:483–493. doi: 10.1038/gim.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karikkineth AC, Scheibye-Knudsen M, Fivenson E, Croteau DL, Bohr VA. Cockayne syndrome: clinical features, model systems and pathways. Ageing Res. Rev. 2017;33:3–17. doi: 10.1016/j.arr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagtegaal AP, et al. Cockayne syndrome group B (Csb) and group a (Csa) deficiencies predispose to hearing loss and cochlear hair cell degeneration in mice. J. Neurosci. 2015;35:4280–4286. doi: 10.1523/JNEUROSCI.5063-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheibye-Knudsen M, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 10.Schultz MB, Sinclair DA. Why NAD(+) declines during aging: it's destroyed. Cell Metab. 2016;23:965–966. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massudi H, et al. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown KD, et al. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins K. Deafness in cochlear and auditory nerve disorders. Handb. Clin. Neurol. 2015;129:479–494. doi: 10.1016/B978-0-444-62630-1.00027-5. [DOI] [PubMed] [Google Scholar]
- 15.Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J. Membr. Biol. 2006;209:153–165. doi: 10.1007/s00232-005-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magupalli VG, et al. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J. Neurosci. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheibye-Knudsen M, et al. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J. Exp. Med. 2012;209:855–869. doi: 10.1084/jem.20111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisina RD, Ding B, Zhu X, Walton JP. Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging (Albany NY) 2016;8:2081–2099. doi: 10.18632/aging.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu NC, Friedman RA. Age-related hearing loss: unraveling the pieces. Laryngoscope Investig. Otolaryngol. 2018;3:68–72. doi: 10.1002/lio2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safieddine S, El-Amraoui A, Petit C. The auditory hair cell ribbon synapse: from assembly to function. Annu. Rev. Neurosci. 2012;35:509–528. doi: 10.1146/annurev-neuro-061010-113705. [DOI] [PubMed] [Google Scholar]
- 21.Khimich D, et al. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- 22.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/S0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 24.Thio SS, Bonventre JV, Hsu SI. The CtBP2 co-repressor is regulated by NADH-dependent dimerization and possesses a novel N-terminal repression domain. Nucleic Acids Res. 2004;32:1836–1847. doi: 10.1093/nar/gkh344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J. Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgels TG, et al. Retinal degeneration and ionizing radiation hypersensitivity in a mouse model for Cockayne syndrome. Mol. Cell. Biol. 2007;27:1433–1441. doi: 10.1128/MCB.01037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin JB, et al. NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elibol, B. & Kilic, U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front. Endocrinol. (Lausanne)9, 614 (2018). [DOI] [PMC free article] [PubMed]
- 30.Xiong H, et al. Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiol. Aging. 2019;79:30–42. doi: 10.1016/j.neurobiolaging.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Pang JQ, et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol. Aging. 2019;80:127–137. doi: 10.1016/j.neurobiolaging.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Han C, et al. Sirt1 deficiency protects cochlear cells and delays the early onset of age-related hearing loss in C57BL/6 mice. Neurobiol. Aging. 2016;43:58–71. doi: 10.1016/j.neurobiolaging.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat. Commun.9, 1286 (2018). [DOI] [PMC free article] [PubMed]
- 34.Dolopikou, C. F. et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur. J. Nutr. 10.1007/s00394-019-01919-4 (2019). [DOI] [PubMed]
- 35.van der Horst GT, et al. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair. 2002;1:143–157. doi: 10.1016/S1568-7864(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 36.van der Horst GT, et al. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/S0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- 37.Bachmanov AA, Reed DR, Beauchamp GD, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002;32:435–443. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 39.Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 2019;9:9772. doi: 10.1038/s41598-019-46120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.