Abstract
Our aim was to identify potential metabolomic pathway changes in the sperm cryopreservation process and to find new markers of human sperm freezability. Targeted metabolomic experiments were used to identify the quantitative metabolomic compound characterization of human sperm cryopreservation. A KEGG pathway analysis was used for these deregulated compounds. A total of 16 significantly deregulated compounds was identified between fresh and post-thawed sperm; of these, 7 were downregulated and 9 were upregulated in the frozen-thawed group. A bioinformatics analysis revealed that metabolic pathways play an important role in cryopreservation, including the citrate cycle (TCA cycle), glycolysis or gluconeogenesis, glyoxylate and dicarboxylate metabolism, pyruvate metabolism and galactose metabolism. We used immunoblotting and immunofluorescence to analyze the expression and localization of the three key enzymes in glycolysis. The glycolytic metabolic changes were noted in sperm cryopreservation. HK2 expression levels in fresh sperm were significantly higher than the levels in freeze-thawed sperm.
Keywords: Fertility preservation, sperm cryopreservation, glycolysis, targeted metabolomics, hexokinases
Introduction
Fertility preservation plays a pivotal part in reproductive medicine [1]. Sperm cryopreservation is the only effective approach used for male fertility preservation in the clinic [2]. In addition, it provides a link between sperm donors and severe infertility patients [3]. However, the sperm will undergo vigorous biochemical changes during the process of freezing and thawing; and the freeze-thaw process will induce a notable decrease in sperm motility, as well as changes in other parameters: membranes and acrosome integrity, the DNA Fragmentation index (DFI), and Reactive Oxygen Species (ROS) [4,5]. Some intracellular and extracellular factors lead to the cryodamage, including cellular dehydration, osmotic stress, the formation of ice crystals, and the toxicity of cryoprotective agents [6]. But little is known about the new aspects of cryopreserved sperm, such as epigenetic, proteomic, and metabolomic modulation. Until now the biomarkers for sperm cryodamage have not been well established at the multivariate scale.
Metabolomics can characterize small molecule metabolites and provide an overview of global biochemistry; thus, it becomes a novel method in pathophysiology. In recent years, metabolomics has seen huge advances, especially the appearance of targeted metabolomics [7]. Compared with traditional metabolomics, targeted metabolomics allows an accurate quantitative analysis of metabolites. Using mass spectrometry (MS), metabolite extraction has become a simple protocol for researchers, especially if their interest lies in common metabolic pathways, such as glycolysis, the tricarboxylic acid (TCA) cycle, the pentose-phosphate pathway, and the metabolism of amino acids. And because of its remarkable resolving power and sensitivity, MS has also played a central role by measuring multiple metabolites simultaneously [8]. It is a new area of human reproduction. These tools can provide the chance to reveal complex biological systems, including sperm motility and fertilization, and they can discover potential pathogenic mechanisms and the biomarkers with male infertility [9].
For these reasons, the aim of the current study is to compare the energy metabolism related metabolite differences between fresh and cryopreserved human sperm, using a metabolomic strategy based on MS.
Materials and methods
Ethics statement
The study was approved by the National Research Institute for Family Planning Ethics Committee on Human Subjects (2018018). Informed consents were obtained from all sperm donors at the Human Sperm Bank, National Research Institute for Family Planning in China.
Study design and sample collection
Paired design was applied to this study. Every semen sample was divided into two parts: one for the cryopreserved group and the other for the fresh group. Our focus was the difference between the cryopreserved and the fresh human sperm.
A total of 15 semen samples from 15 healthy donors were collected. Among the whole sample of each group, 10 cryopreserved and fresh samples were used for the metabolomic analysis. Then 5 semen samples were used for the Western blot and Immunofluorescence analyses.
The ages of the sperm donors were between 22 and 40 years. One ejaculate was collected from these volunteers by masturbation after 3-5 days of sexual abstinence. Routine semen analyses were performed using a computer-aided sperm analysis (CASA) (HTM-IVOS, USA), according to the World Health Organization (WHO 2010) guidelines. The initial ejaculates had the following characteristics: semen volume ≥4.0 mL; sperm concentration ≥60×106/mL; progressive motility ≥60%; normal morphology ≥4%; and round cell concentration <2%.
Semen cryopreservation protocols
After complete liquefaction, each semen sample was transferred into two, 2 ml samples: one to be used for cryopreservation and the other to be uses as a control. Glycerol-egg-yolk-citrate (GEYC) was used as a cryoprotectant. It contains 15% glycerol, 20% egg yolk, 1.3% glycine, 1.5% glucose, 1.3% sodium citrate tribasic dehydrate, and its PH ranges from 6.8-7.2. A volume of GEYC was added dropwise to two volumes of semen and then incubated for 5 minutes at 30-35°C. The slow sperm freezing method was performed according to the standardized programmable freezers (Kryo 360-1.7, Planner, United Kingdom) in our unit [6,10]. Briefly, the programmer cools the straws from 20°C to -6°C at 1.5°C/min. Then the straws are cooled to -100°C at a rate of 6°C per minute and cooled to the -100°C range for 30 minutes. Finally, the straws are transferred to liquid nitrogen.
Targeted metabolomic experiment and bioinformatics analysis
About 40×106 fresh or cryopreserved sperm were collected for the targeted metabolomic analysis. After being washed with PBS three times, the sperm samples were suspended in 800 μL of cold (-20°C) aqueous methanol (60%, v/v) to stop cell metabolism. Then the sperm were disrupted by sonication for 1 minute and frozen at -80°C for 30 minutes. Then the mixture was centrifuged at 4°C for 15 min at a rotational speed of 13000 rpm. Then 100 μl of aqueous methanol (90%, v/v) was added to the supernatant and stored in liquid nitrogen until testing. As in the previous study [11], the protein content in the residue was used to normalize the metabolite concentration for each group.
Targeted metabolomics was performed using TSQ Quantiva (Thermo, CA). C18 based reverse phase chromatography was utilized with 10 mM tributylamine, and 15 mM acetate in water and 100% methanol as mobile phase A and B respectively. The TCA cycle, the glycolysis pathway, and the pentose phosphate pathway metabolism were analyzed. In this experiment, we used a 35-minute gradient from 5% to 90%, and mobile B was set. A positive-negative ion exchange mode was used for data acquisition. The cycle time was set to 1 second and included a total of 340 ion pairs. The resolution for Q1 and Q3 were set to 0.7 FWHM. The supply voltages of the positive and negative ion mode were 3500 V and 2500 V, respectively. The scavenging gas was turned at a flow rate of 1 (ARB). A paired t-test was used as an analytical approach, and the P value was set to 0.05. All significant differences were taken as input. A KEGG pathway enrichment analysis was performed using the MetaboAnalystR (https://www.metaboanalyst.ca/) [12].
Immunofluorescence
As in a previous study [13], 100 μl of fresh or cryopreserved semen sample was diluted with 900 μl of phosphate buffered saline (PBS) and then centrifuged at 800 g for 10 min and then the washed sperm was collected, and this was repeated three times. The cleaned sperm was applied to a slide that had previously been exposed to 1% (w/v) gelatin. After being fixed with 95% (V/V) ethanol for 30 min, the sperm were incubated at 4°C overnight with primary antibody diluted 1:50 in PBS containing 3% (w/v) BSA: hexokinases (HK2), phosphofructokinase (PKFP) and pyruvate kinase (PKM) (Abclone, China). The sperm was washed three times with PBS and then incubated for 1 hour at room temperature using the corresponding secondary antibody. Subsequently, the slides were washed with PBS and observed under a confocal laser scanning microscope (LSM-710 Carl Zeiss, Germany).
Western blot
As in our previous experiment [14], the sperm protein samples were separated by 10% SDS polyacrylamide gel electrophoresis. They were then transferred to polyvinylidene fluoride membranes, blocked with 2% (w/v) skim milk for 1 hour, and incubated overnight with the primary antibody of HK2, PKM, PFKP (1:2000) (Abclone, China) at 4°C. The membranes were then washed three times with TBST and then were incubated with horseradish peroxidase (HRP) in combination with anti-IgG for 1 h at room temperature. Enhanced chemiluminescence shows immunoreactivity. The relative signal intensity of the protein bands was analyzed with Quantity One v.4.6.2.
Statistical analysis
The data were expressed as the mean ± SD and were analyzed with SPSS22.0 software (IBM, USA). A paired t-test was used as the analytical approach and P-values of 0.05 were set.
Results
Quantitative results of differential metabolomic compounds
10 samples of sperm (10 fresh + 10 cryopreservation) underwent a targeted metabolomic analysis. As a result, using the 1.5-fold or 0.67-fold change and the FDR-adjusted p-value of 0.05 as cutoffs, 16 significantly deregulated compounds were identified between the fresh and cryopreserved sperm samples: 7 were decreased and 9 were increased after the cryopreservation procedures. The details of these metabolites are shown in Table 1.
Table 1.
The content of biochemical index in fresh and cryopreserved sperm (n=10 Fresh + 10 cryopreserved)
| Names | Formula | Fold Change (Cryo/Fresh) | p-Value | KEGG CID |
|---|---|---|---|---|
| O-Phosphoethanolamine | C2H8NO4P | 0.171969405 | 0.04107734 | C00346 |
| L-Lactic acid | C3H6O3 | 0.413499863 | 0.000980779 | C00186 |
| Dihydroxyacetone phosphate | C3H7O6P | 0.437015648 | 0.007290552 | C00111 |
| Cyclic AMP | C10H12N5O6P | 0.437015648 | 0.008224171 | C00575 |
| Adenosine triphosphate | C10H16N5O13P3 | 0.46288499 | 0.047796942 | C00002 |
| Nicotinamide adenine dinucleotide (NAD+) | C21H28N7O14P2 | 0.464209346 | 0.000191267 | C00003 |
| D-Glucose | C6H12O6 | 0.620187167 | 0.001279957 | C00031 |
| D-Gluconic acid | C6H12O7 | 1.741624243 | 0.000591761 | C00257 |
| L-carnitine | C7H15NO3 | 1.961510826 | 0.04107734 | C00318 |
| Phosphoenolpyruvate | C3H5O6P | 1.961510826 | 0.001631627 | C00074 |
| cis-Aconitate | C6H6O6 | 4.781893116 | 0.000315657 | C00417 |
| 3-Phospho-DL-glycerate | C3H7O7P | 5.126680271 | 0.000796782 | C00597 |
| Isocitrate | C6H8O7 | 5.842883812 | 0.012216846 | C00311 |
| Citrate | C6H8O7 | 5.868451745 | 0.000701378 | C00158 |
| Lactose | C12H22O11 | 6.246607627 | 0.000618669 | C00243 |
| Succinate | C4H6O4 | 7.415279729 | 0.00301462 | C00042 |
KEGG pathway
A pathway enrichment analysis was performed by KEGG enrichment, in order to seek the major biochemical and signal transduction pathways. A total of 21 enriched pathways were measured in this analysis, and there were 5 pathways with significant differences (FDR<0.05), including: the citrate cycle (TCA cycle), glycolysis or gluconeogenesis, glyoxylate and dicarboxylate metabolism, pyruvate metabolism and galactose metabolism. The detailed results from the pathway analysis are shown in the Table 2. As shown in the table, the progress of cryopreservation contributes to a sharp decrease in glycolysis/gluconeogenesis, compared to the fresh sperm, but glycolysis is the predominant ATP generation pathway during sperm motility. So we focused on the glycolysis. The four differential metabolomic compounds are actually matched in this pathway: L-Lactic acid (C00186), phosphoenolpyruvic acid (C00074), D-glucose (C00031) and dihydroxyacetone phosphate (C00111).
Table 2.
KEGG analysis classification and the enrichment result
| Pathway Name | Match Status | P | -log (p) | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|
| Citrate cycle (TCA cycle) | 5/20 | 9.5363E-8 | 16.166 | 7.6291E-6 | 7.6291E-6* | 0.1911 |
| Glycolysis or Gluconeogenesis | 4/31 | 3.6833E-5 | 10.209 | 0.0029098 | 0.0014733* | 0.1035 |
| Glyoxylate and dicarboxylate metabolism | 4/50 | 2.4973E-4 | 8.2951 | 0.019479 | 0.0066594* | 0.03137 |
| Pyruvate metabolism | 3/32 | 0.0010634 | 6.8463 | 0.081884 | 0.021269* | 0.13756 |
| Galactose metabolism | 3/41 | 0.0022033 | 6.1178 | 0.16745 | 0.035253* | 0.03469 |
| Pentose phosphate pathway | 2/32 | 0.018298 | 4.001 | 1.0 | 0.24397 | 0.08639 |
| Propanoate metabolism | 2/35 | 0.021697 | 3.8306 | 1.0 | 0.24796 | 0.00134 |
| Glycerophospholipid metabolism | 2/39 | 0.026606 | 3.6266 | 1.0 | 0.26606 | 0.07129 |
| Nicotinate and nicotinamide metabolism | 2/44 | 0.033318 | 3.4017 | 1.0 | 0.29616 | 0.0015 |
| Purine metabolism | 2/92 | 0.12264 | 2.0985 | 1.0 | 0.98112 | 0.01643 |
| Alanine, aspartate and glutamate metabolism | 1/24 | 0.14857 | 1.9067 | 1.0 | 1.0 | 0.0 |
| Sphingolipid metabolism | 1/25 | 0.15429 | 1.8689 | 1.0 | 1.0 | 0.01288 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 1/27 | 0.16561 | 1.7981 | 1.0 | 1.0 | 0.0 |
| Glycerolipid metabolism | 1/32 | 0.19331 | 1.6435 | 1.0 | 1.0 | 0.0 |
| Inositol phosphate metabolism | 1/39 | 0.23064 | 1.4669 | 1.0 | 1.0 | 0.01203 |
| Butanoate metabolism | 1/40 | 0.23583 | 1.4446 | 1.0 | 1.0 | 0.01774 |
| Phenylalanine metabolism | 1/45 | 0.26134 | 1.3419 | 1.0 | 1.0 | 0.0 |
| Fructose and mannose metabolism | 1/48 | 0.27625 | 1.2864 | 1.0 | 1.0 | 0.04115 |
| Starch and sucrose metabolism | 1/50 | 0.28604 | 1.2516 | 1.0 | 1.0 | 0.01703 |
| Pentose and glucuronate interconversions | 1/53 | 0.30049 | 1.2024 | 1.0 | 1.0 | 0.0 |
Match Status = Hit/Total. The Total is the total number of compounds in the pathway; the Hits is the actually matched number. The table below shows the detailed. The P is the original p value calculated from the enrichment analysis; the Holm p is the p value adjusted by Holm-Bonferroni method; the FDR p is the p value adjusted using False Discovery Rate results from the pathway analysis.
FDR<0.05.
Glycolysis metabolic key enzymes
To further validate the the targeted metabolomic analysis and the KEGG pathway analysis results, we used Western blot and immunofluorescence to localize and quantify the three key enzymes in glycolysis: HK2, PKM, and PFKP. According to immunolocalization, the three proteins were all localized in the principal piece of sperm (Figure 1A). These protein analysis results confirmed the previous genomic analysis of the metabolomics, and the results confirmed the differential protein levels observed via 2DE (Figure 1B). The cryopreserved group had a lower level of HK2 compared with the fresh sperm, but the other proteins showed no statistical differences between the fresh and cryopreserved sperm (Figure 1Bb).
Figure 1.
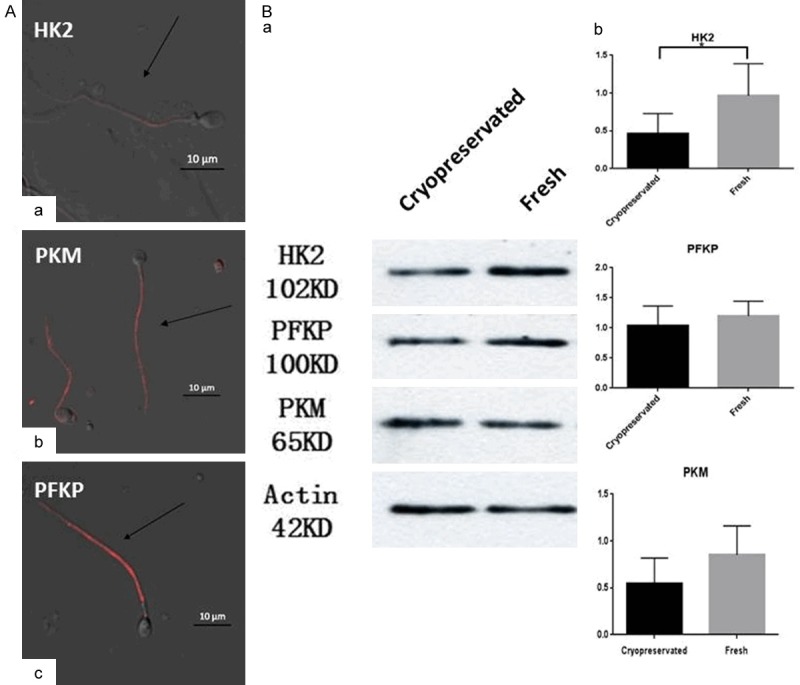
Glycolysis metabolic key enzymes analyzed with Western blot and Immunofluorescence. A. The distribution of HK2, PKM, and PFKP were detected by immunofluorescence staining in sperm. Human sperm cultured with HK2 (a), PKM (b) and PFKP (c). Scale bar: 10 μm. B. The protein levels of the three proteins were detected by Western blot analyses. Values are expressed as the mean ± SEM, n=5. Asterisks indicate a statistically significant difference from the fresh group. Statistical analysis by paired t-test. *P<0.05.
Discussion
Across the globe, the proportion of infertility caused by males ranges between 20-70% [15]; men with azoospermia or severe oligozoospermia, will benefit from sperm cryopreservation, and this service is a simple and effective way of preserving fertility potential [16]. However, after sperm cryopreservation, too many sperm lose their motility and fertility [4,17]. But, until now the biomarkers for the sperm cryodamage have not been well established at the multivariate scale. To address this question, we chose targeted metabolomic strategies for the analysis of the differences in the expression of metabolites in the progress of human sperm cryopreservation.
In recent years, many studies have shown that energy metabolism plays the most vital role in energy production in spermatozoa [18,19]. Spermatozoa are highly specialized mammalian cells; the sperm must afford enough ATP to maintain the physiological processes: motility, capacitation, hyperactivation, acrosome reaction, and fertilization, all of which are highly energy-dependent processes. Among those, glycolysis and oxidative phosphorylation (OXPHOS) are the most important and form adenosine triphosphate (ATP) to provide the energy for both the development and function of sperm. And the ATP is formed by two metabolic pathways: glycolysis and oxidative phosphorylation (OXPHOS) [19]. While many studies have shown that glycolysis is the primary source of ATP during sperm motility [19-21] and OXPHOS enables maturation and differentiation [22]. Many studies have shown that sperm motility will significantly decrease in the process of cryopreservation and that ATP is extremely downregulated in post-thawed sperm [4,23,24]. So based on the results, we chose the glycolysis pathway for the future research, and the impact index of the pathway is 0.1035 (Table 2).
Through immunofluorescence, this study found that the three key enzymes of glycolytic (HK2, PFKP, and PKM) are located in the principal sperm components (Figure 1A). The three enzymes play an important role in maintaining sperm function [25]. Among the three glycolysis key enzymes, the Western blot analysis showed that the expression levels of HK were decreased in freeze-thawed human sperm, and there were no statistical differences in the expression levels of PFKP and PKM. Hexokinase is one of the initial rate-limiting reactions of glycolysis in the glycolytic pathway. It catalyzes the phosphorylation of glucose and ATP to form glucose-6-phosphate and ADP [26]. The glucose-6-phosohate is the point of entry into glycogen synthesis and is involved in the pentose phosphate pathway and glycolysis. Several studies [27,28] have shown that the overexpression of HK2 in various cancer types may be associated with the new therapeutic target. As was found in a previous study, HK is mainly located in the principal component of sperm [29]. In 2016, Professor He’s study showed that the expression level of HK in fresh sperm is significantly higher than it is in cryopreserved sperm (P<0.001), based on a proteomics analysis [30]. However, the relationship between HK levels has not been investigated in fresh and cryopreserved sperm in humans. As one of the key enzymes in glycolysis, a decreased expression of HK2 may be associated with glycolytic pathway changes in the process of cryopreservation, and decreased ATP following freeze-thawing.
Conclusions
Human sperm cryopreservation is a simple and effective approach for the preservation of male fertility. In order to identify potential metabolomic pathway changes in this process, a targeted metabolomic experiment was conducted to identify the quantitative metabolomic compounds that characterize human sperm cryopreservation. A total of 16 significantly deregulated compounds were identified between fresh and post-thawed sperm: 7 were downregulated and 9 were upregulated in the frozen-thawed group. A bioinformatics analysis revealed that metabolic pathways play an important role in cryopreservation, including the citrate cycle (TCA cycle), glycolysis or gluconeogenesis, glyoxylate and dicarboxylate metabolism, pyruvate metabolism and galactose metabolism. We used localization and a quantitative analysis of the three key enzymes in glycolysis by immunofluorescence and immunoblotting. HK2 expression levels in cryopreserved sperm were significantly lower than those in fresh sperm. Our work will provide valuable information for future investigations and pathological studies involving sperm cryopreservation.
Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities [3332018187], Special Funds for Clinical Medical Research of Chinese Medical Association [18010310760], the National Natural Science Foundation of China [81803116], and the Basic Research Project for Medical and Health Applications of Suzhou City [SYS2018077].
Disclosure of conflict of interest
None.
References
- 1.Qiao J, Li R. Fertility preservation: challenges and opportunities. Lancet. 2014;384:1246–1247. doi: 10.1016/S0140-6736(14)61749-9. [DOI] [PubMed] [Google Scholar]
- 2.Martinez F. Update on fertility preservation from the barcelona international society for fertility preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108:407–415. e411. doi: 10.1016/j.fertnstert.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Szell AZ, Bierbaum RC, Hazelrigg WB, Chetkowski RJ. Live births from frozen human semen stored for 40 years. J Assist Reprod Genet. 2013;30:743–744. doi: 10.1007/s10815-013-9998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raad G, Lteif L, Lahoud R, Azoury J, Azoury J, Tanios J, Hazzouri M, Azoury J. Cryopreservation media differentially affect sperm motility, morphology and DNA integrity. Andrology. 2018;6:836–845. doi: 10.1111/andr.12531. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Torres MJ, Medrano L, Romero A, Fernandez-Colom PJ, Aizpurua J. Effectiveness of human spermatozoa biomarkers as indicators of structural damage during cryopreservation. Cryobiology. 2017;78:90–94. doi: 10.1016/j.cryobiol.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, Shahverdi A. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37:327–339. doi: 10.1016/j.rbmo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted metabolomics. Curr Protoc Mol Biol. 2012 doi: 10.1002/0471142727.mb3002s98. Chapter 30: Unit 30.2.1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang C, Chen L, Rabinowitz JD. Metabolomics and isotope tracing. Cell. 2018;173:822–837. doi: 10.1016/j.cell.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang B, Shang X, Qi H, Li J, Ma B, An G, Zhang Q. Metabonomic analysis of fatty acids in seminal plasma between healthy and asthenozoospermic men based on gas chromatography mass spectrometry. Andrologia. 2017;49 doi: 10.1111/and.12744. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Guo Y, Cao XF, Jia YF, Wang XW, Xu JF, Zhou F, Li H, Liang XW, Lu WH, Gu YQ. Effect of cryopreservation on DNA methylation status of imprited genes in human sperm. Journal Reproduction Medcine. 2015;24:402–408. [Google Scholar]
- 11.Wang H, Liang X, Luo G, Ding M, Liang Q. Protection effect of nicotinamide on cardiomyoblast hypoxia/re-oxygenation injury: study of cellular mitochondrial metabolism. Mol BioSyst. 2016;12:2257–2264. doi: 10.1039/c6mb00108d. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Wang G, Zhang S, Fu Y, Jiang Y, Yang X, Lin X. An integrated quantitative proteomic and metabolomics approach to reveal the negative regulation mechanism of LamB in antibiotics resistance. J Proteomics. 2018;194:148–159. doi: 10.1016/j.jprot.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Liu FJ, Liu X, Han JL, Wang YW, Jin SH, Liu XX, Liu J, Wang WT, Wang WJ. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum Reprod. 2015;30:861–869. doi: 10.1093/humrep/dev003. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wang J, Qin Y, Huang C, Archacki S, Ma J, Li D, Liu M. Identification of three mutations in the MVK gene in six patients associated with disseminated superficial actinic porokeratosis. Clin Chim Acta. 2016;454:124–129. doi: 10.1016/j.cca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahata L, Caltabellotta NM, Yeager ND, Lehmann V, Whiteside SL, O’Brien SH, Quinn GP, Gerhardt CA. Fertility perspectives and priorities among male adolescents and young adults in cancer survivorship. Pediatr Blood Cancer. 2018;65:e27019. doi: 10.1002/pbc.27019. [DOI] [PubMed] [Google Scholar]
- 17.Isaac AV, Kumari S, Nair R, Urs DR, Salian SR, Kalthur G, Adiga SK, Manikkath J, Mutalik S, Sachdev D, Pasricha R. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem Biophys Res Commun. 2017;494:656–662. doi: 10.1016/j.bbrc.2017.10.112. [DOI] [PubMed] [Google Scholar]
- 18.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 19.du Plessis SS, Agarwal A, Mohanty G, van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl. 2015;17:230–235. doi: 10.4103/1008-682X.135123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibb Z, Aitken RJ. The impact of sperm metabolism during in vitro storage: the stallion as a model. Biomed Res Int. 2016;2016:9380609. doi: 10.1155/2016/9380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hereng TH, Elgstoen KB, Cederkvist FH, Eide L, Jahnsen T, Skalhegg BS, Rosendal KR. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum Reprod. 2011;26:3249–3263. doi: 10.1093/humrep/der317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stendardi A, Focarelli R, Piomboni P, Palumberi D, Serafini F, Ferramosca A, Zara V. Evaluation of mitochondrial respiratory efficiency during in vitro capacitation of human spermatozoa. Int J Androl. 2011;34:247–255. doi: 10.1111/j.1365-2605.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell M, McClure N, Lewis SE. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17:704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 24.Jiang XP, Wang SQ, Wang W, Xu Y, Xu Z, Tang JY, Sun HY, Wang ZJ, Zhang W. Enolase1 (ENO1) and glucose-6-phosphate isomerase (GPI) are good markers to predict human sperm freezability. Cryobiology. 2015;71:141–145. doi: 10.1016/j.cryobiol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Vemuganti SA, Bell TA, Scarlett CO, Parker CE, de Villena FP, O’Brien DA. Three male germline-specific aldolase a isozymes are generated by alternative splicing and retrotransposition. Dev Biol. 2007;309:18–31. doi: 10.1016/j.ydbio.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Kawai S, Mukai T, Mori S, Mikami B, Murata K. Hypothesis: structures, evolution, and ancestor of glucose kinases in the hexokinase family. J Biosci Bioeng. 2005;99:320–330. doi: 10.1263/jbb.99.320. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Acosta P, Carrillo S, Gamboa O, Romero-Rojas A, Acosta J, Molano M, Balart-Serra J, Cotes M, Rancoule C, Magne N. Novel predictive biomarkers for cervical cancer prognosis. Mol Clin Oncol. 2016;5:792–796. doi: 10.3892/mco.2016.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18:494–504. doi: 10.2174/1568026618666180523111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura N, Miranda-Vizuete A, Miki K, Mori C, Eddy EM. Cleavage of disulfide bonds in mouse spermatogenic cell-specific type 1 hexokinase isozyme is associated with increased hexokinase activity and initiation of sperm motility. Biol Reprod. 2008;79:537–545. doi: 10.1095/biolreprod.108.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Y, Wang K, Zhao X, Zhang Y, Ma Y, Hu J. Differential proteome association study of freeze-thaw damage in ram sperm. Cryobiology. 2016;72:60–68. doi: 10.1016/j.cryobiol.2015.11.003. [DOI] [PubMed] [Google Scholar]


