Abstract
Objectives
To evaluate the effectiveness of a collaborative goal‐setting intervention (Empowering Patients in Chronic Care [EPIC]) to improve glycaemic control and diabetesrelated distress, and implementation into routine care across multiple primary care clinics.
Design
Randomized controlled trial comparing the effectiveness of the EPIC intervention with enhanced usual care (EUC) at five clinic sites located in the greater Chicago and Houston areas. We will measure differences in haemoglobin A1c (HbA1c) and diabetes distress scale scores among study arms at post‐intervention and maintenance (6 months post‐intervention). We will evaluate implementation of the intervention across sites using the RE‐AIM framework. We will evaluate reach by comparing the per cent and characteristics of enrolled study participants among all potentially eligible participants in the given clinic population. Adoption is reflected by the characteristics of the involved providers and the number of intervention sessions conducted. Implementation of EPIC will be evaluated by number of sessions delivered, participants' evaluation of group sessions, and evaluation of quality of goal‐setting.
Patients
We randomized 280 participants with equal allocation to EPIC and enhanced usual care (EUC).
Results
At baseline, the groups were similar with the exception that EUC participants were more likely to have prior diabetes education. At baseline, participants were predominately older men who have poorly controlled diabetes (mean HbA1c = 76 mmol/mol [9.1%]) and moderate levels of diabetes distress (mean DDS = 2.43).
Conclusions
This hybrid effectiveness‐implementation protocol is designed to accelerate the translation of a patient‐centred diabetes care intervention from research to clinical practice.
Keywords: goal‐setting, group, intervention, randomized control, veteran
The Empowering Patients in Chronic Care (EPIC) intervention is an efficacious patient‐centred, goal‐oriented programme that combines group and individual approaches to help individuals with treated but uncontrolled diabetes reach their diabetic goals. The current trial is intended to examine EPIC as it transitions from laboratory setting into routine primary care.

Novelty Statement.
This protocol describes a study to evaluate the real‐world effectiveness of an innovative collaborative goal‐setting intervention and glycaemic control and patient‐reported diabetes distress.
1. INTRODUCTION
Diabetes mellitus is a global epidemic affecting more than 380 million individuals.1 Individuals with diabetes often struggle to make the behavioural and lifestyle changes necessary to prevent diabetes complications.2 Diabetes care experiences often carry a high emotional burden leading to worry and distress associated with diabetes self‐care.3 Furthermore, patients with high levels of diabetes‐related distress are significantly more likely to have poor glycaemic control, self‐care and quality of life.3 Identifying and implementing effective behavioural change interventions into routine practice is essential and requires the engagement of a coordinated, interprofessional team that supports patients.4 Goal‐setting promotes diabetes control by improving self‐management behaviours and trust in one's clinicians.5 Collaborative goal‐setting is an evidence‐based, behaviour change strategy for improving diabetes outcomes in primary care.6
Empowering Patients in Chronic Care (EPIC) is a patient‐centred intervention that uses collaborative goal‐setting to improve diabetes outcomes. EPIC uses a group‐based approach and motivational interviewing techniques to activate patients,7 guide them in setting diabetes goals and action plans,6 develop skills to communicate goals with healthcare providers,8 and negotiate action plans to achieve their goals.5 An efficacy study demonstrated that EPIC significantly improved haemoglobin A1c (HbA1c) levels compared with usual diabetes care plus diabetes and nutrition education.7 Moreover, EPIC sustained significant HbA1c improvements over 12 months, contrasting many diabetes education and self‐management interventions that experience significant regression to mean after 4 months.9
Translating behaviour change interventions into routine primary care can be challenging. Barriers to implementation include economic disincentives, administrative burdens, education and time pressures.10 The patient‐centred medical home is a model of primary care designed to address some of these barriers using behavioural and systems‐based methods to activate and empower patients and coordinate interprofessional teams to improve chronic illness care.11 Building on the prior evidence‐base for the EPIC intervention, we partnered with a large health system implementing a patient‐centred medical home model to conduct a randomized clinical effectiveness trial of the EPIC intervention embedded across several of its primary care networks.12 The aims of this trial are to evaluate: (a) the clinical effectiveness of EPIC to improve diabetes control and reduce diabetes‐related distress among adults with diabetes, and (b) the implementation of EPIC in several primary care practices. We hypothesized that patients who received EPIC would have significant improvements in HbA1c and diabetes distress levels post‐intervention and that these effects would be sustained 10‐months after enrolment.
Empowering Patients in Chronic Care is unique in that it combines group‐based intervention with individual 1:1 immediate attention following each group‐based intervention. Moving an intervention from laboratory to “real world” is a complex process. The current protocol allows for testing the effectiveness of EPIC when delivered by nonacademic providers in routine primary care across multiple clinic sites. Because each clinic has its own culture and ways of delivering primary care that may interfere with fidelity of the intervention, EPIC needs to be tested per the presented protocol to evaluate its effectiveness as it moves towards wider implementation.
2. METHODS
2.1. Study design
The overall study is a hybrid type 1 implementation/effectiveness trial of the EPIC intervention. Per Curran and Bauer,13 a hybrid trial type 1, aims to determine the effectiveness of a clinical intervention and better understand context for implementation. The current study protocol is designed to evaluate the effectiveness of EPIC on diabetes outcomes, specifically change in haemoglobin A1c and diabetes distress levels. To further understand the context of EPIC's implementation across five geographically distinct clinics consisting of a formative (phase 1) and summative (phase 2) evaluation of implementation, qualitative data collected from clinicians and participants will increase understanding of barriers and facilitators to implementation of EPIC in routine clinical practice and ultimately enhance clinical implementation. The first phase focused on evaluating providers' readiness for change at each study site and is described in detail elsewhere.12 Key informants were interviewed to identify healthcare providers' perceptions of training for conducting the EPIC programme. They also informed the research team on how best to integrate the intervention into routine clinic flow. Recommendations from this formative evaluation guided the overall implementation of the clinical trial and training of clinicians across all sites.12 The current protocol describes a randomized clinical trial comparing the EPIC intervention with enhanced usual care (EUC).
2.2. Study setting and ethics review
We recruited participants from three hospital‐based primary care clinics and two community‐based outpatient clinics. The clinics are part of two distinct regional networks of the United States Department of Veteran Affairs (VA), the largest healthcare organization in the US. The Central Institutional Review Board for the Department of Veterans Affairs (CIRB 14‐24) as well as each of the hospital‐based Research & Development committees approved this study.
2.3. Participants
2.3.1. Practice‐based health professionals
We recruited healthcare providers (HCPs) that included physicians, nurses, dieticians, pharmacists and primary care mental health providers from each intervention site. The diversity in HCP background/discipline most likely resembles conditions that EPIC will encounter as it moves from controlled laboratory setting to every day, real‐world settings. Although participation was completely voluntary, we encouraged participation from clinicians who already provided diabetes self‐management support and/or had prior training in health behaviour change methods. We enrolled three to five HCPs at each of the intervention sites. At least one HCP per site had prescribing authority which allowed him/her to adjust medications during the intervention.
The EPIC coach training was delivered by Dr Natalie Hundt, a licensed psychologist part of the main research team. EPIC coach training consisted of several modules that instructed clinician coaches on motivational interviewing, collaborative goal‐setting and action planning and how to apply these skills to behavioural management of diabetes. Additional modules covered the structure and content of the EPIC programme, as well as best practices regarding the delivery of EPIC. Training was designed to be interactive and included didactic components, audiotaped vignettes of coaches demonstrating the skills, exercises for learners to practice skills and knowledge checks. The training was iteratively developed and reviewed by team members expert in internal medicine, geriatrics, behavioural medicine, nutrition and public health. Depending on the availability of the clinicians, the training was delivered in both group and individualized format for a total of one 3‐hour session. Virtual training for clinicians outside of Houston was chosen because it would not have been financially feasible (or necessary) for Dr Hundt to travel to each distant site to deliver the training in person. In‐person training was provided for Houston clinicians, since Dr Hundt is on site at Houston. Whether delivered virtually or in person, the training structure and content were the same, the only difference being that Dr Hundt delivered the virtual training via an interactive video platform. Training objectives primarily focused on the evidence‐based behaviour change strategies of goal‐setting, action planning and motivational techniques, as well as the practical elements of how to run a group and topics covered in each setting. Based upon presurvey data and formative evaluation, we assumed all providers trained already possessed basic‐to‐advanced knowledge about diabetes itself. Providers audio‐recorded their EPIC group sessions and sent them to the trainer for review. All available sessions from the first group cohort were listened to (some recordings were unavailable due to technology issues) and a random sample of 20% of recordings after the first cohort. Session fidelity was rated on dimensions of adherence to the EPIC protocol, defined as the proportion of required EPIC treatment elements delivered, and competence or skilfulness in delivery using a previously validated rating scale for behavioural interventions by Cully et al14, 15 Scores ranged from 1 to 8, with 4‐5 considered moderately adherent/ competent and 6, 7 and 8 classified as good, very good and excellent, respectively. The trainer scored session fidelity and provided written feedback regarding fidelity, including both strengths and areas for improvement, to each team of EPIC providers as she reviewed audiotaped sessions. Average adherence was 6.3 on the 8‐point scale; average competence was 6.1, which is in the “good” range on our predetermined scale.
2.3.2. Participant recruitment
Our intervention was designed for individuals with uncontrolled diabetes despite having access to primary care and evidence‐based therapies. We started with a population‐screening approach to identify all individuals with uncontrolled diabetes within each of the clinics' known patient panels. Using limited exclusion criteria, we then attempted to enrol as many participants who met this inclusion criteria as possible. We mailed and then called all patients meeting our eligibility criteria with approval/support from clinic staff. Our recruitment strategy was selected to provide the broadest potential reach among patients meeting our eligibility criteria16 (Table 1). Study participants were recruited from five total community‐based clinics in urban, suburban and rural settings that varied in size. Patient population within these clinics is mostly older males; however, the makeup is diverse by education, race and ethnicity.
Table 1.
RE‐AIM framework applied to the empowering patients in chronic care intervention
| Dimensions | Measurement and specifications |
|---|---|
| Reach |
Participants in the EPIC study at a given site/ total population of eligible patients at the given site. Compare demographic characteristics between EPIC and EUC participants |
| Effectiveness | Evaluate for change in HbA1c and Diabetes Distress Scale Scores between EPIC and enhanced usual care study arms post‐intervention (ie. 4 mo post‐baseline) |
| Adoption | Evaluate timing and frequency of group & individual sessions at each of the participating study clinics |
| Implementation |
(1) Evaluate participate attendance at group and individual sessions (2) Rate the fidelity to EPIC programme protocol using a structured evaluation completed by an expert behavioural coach on the study team (3) Evaluate patients' perceptions of goal‐setting engagement by healthcare providers in both the intervention and EUC arms through qualitative interviewsa (4) Goal Evaluation Tool to rate the quality of the goal and action plans developed by participants |
| Maintenance | Evaluate for change in HbA1c and Diabetes Distress Scale Scores between EPIC and enhanced usual care study arms 6 mo post‐baseline (aka maintenance) |
Abbreviations: EPIC, empowering patients in chronic care; EUC, enhanced usual care; HbA1c, haemoglobin A1c; RE‐AIM, reach, effectiveness, adoption, implementation, maintenance.
Procedures and data analysis methods for the qualitative interviews about patient/clinician experiences with EPIC are delineated in an upcoming publication.24
The population‐screening approach began with the VA's electronic data warehouse which is linked to electronic medical records (EMR). Eligibility criteria included: (a) International Classification of Diseases Ninth or Tenth revision (ICD‐9,10) code for type 2 diabetes mellitus (type 2 DM) (250.xx; E11.xx, respectively), (b) primary care evaluation within the preceding 12 months, and (c) an average HbA1c level >64 mmol/mol (8.0%) in the previous 6 months. From this, we excluded individuals who: (a) were deceased; (b) did not receive primary care at one of the participating clinics; (c) had a complete hearing or vision impairment; (d) had active substance use disorder (within 1 year); (e) had active bipolar or psychotic disorder; (f) had documented dementia; (g) had contra‐indications for severe hypoglycaemia (defined as a glucagon prescription); or (h) had limited life expectancy (as identified using a validated algorithm developed in prior work).16 This criteria allows recruitment of individuals who can actively participate in group interventions and to protects those at high risk for hypoglycaemic events from taking part.
A study invitation letter was then sent to all eligible participants. Those who did not opt‐out by mail or toll‐free telephone call were contacted by research staff to determine their interest in participation. The research team screened the individuals via telephone for additional exclusion criteria, including (a) hearing or vision loss severe enough to limit their ability to participate in group discussions; (b) transportation or availability barriers; (c) significant cognitive impairment (three or more errors on the Six‐Item Screener17), or (d) active substance‐abuse (as screened by the Mini International Neuropsychiatric Interview17). Those meeting eligibility criteria and interested in participating were then consented and completed baseline data collection.
Figure 1 is the CONSORT Diagram that outlines participant recruitment. Of the 4198 individuals who received a study invitation letter, 633 completed the initial telephone screening. Of these, 19 met exclusion criteria. Half (n = 356) of those screened consented to participate in the study. Although the initial screening for eligibility to participate was a HbA1c of >64 mmol/mol (8.0%), following consent, we obtained a baseline HbA1c level and removed participants who had a HbA1c below 58 mmol/mol (7.5%) (n = 66) at baseline. The rationale for this procedure was to focus the intervention of those individuals with uncontrolled but treated diabetes and in whom clinically significant change could be observed if the intervention has a positive effect.
Figure 1.
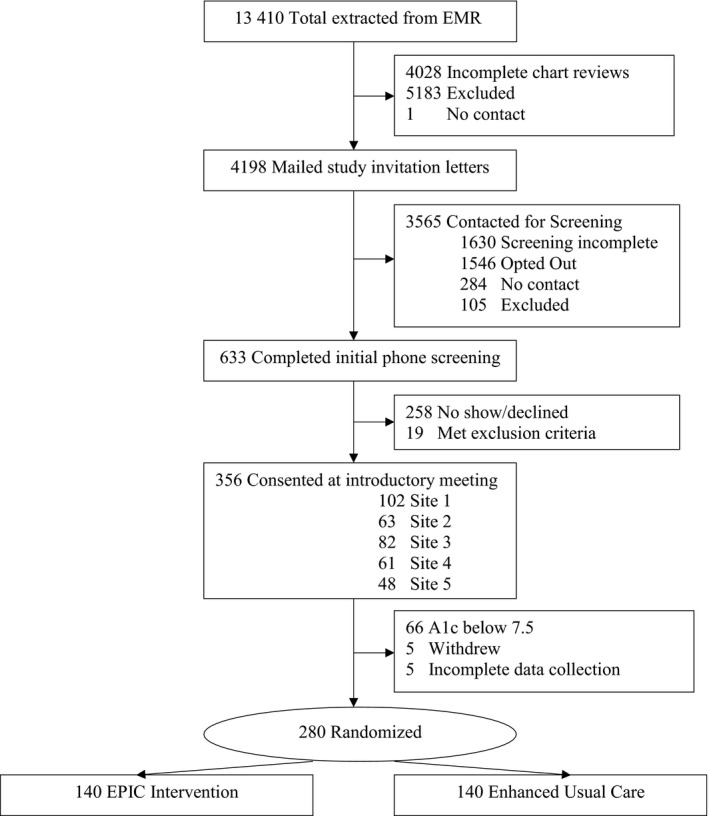
CONSORT diagram
We set a target number of participants to recruit at each of the intervention sites. Each site met their recruitment targets within the recruitment timeframe. We randomized participants in random blocks of 4, 6 or 8 equally between the study arms using SAS. Participants were clustered into groups of approximately six for delivery of the EPIC intervention. The final sample size was 280.
2.4. Procedures
2.4.1. EPIC intervention
Participants enrolled in the EPIC intervention attended six bimonthly group sessions (approximately 60 minutes each). The sessions focused on collaborative goal‐setting and patient‐centred action planning. They were led by one to three HCPs. The intervention took approximately three months to complete. Figure 2 details the six sessions' structure and themes. A 10‐minute individual session immediately follow group sessions for each participant. During the individual sessions, participants met with an EPIC‐trained HCP to discuss their personal concerns, questions, and to set and adjust goals, including medications or monitoring instructions if indicated. The research team developed a detailed guide/workbook for the EPIC‐trained HCPs and participants to use throughout the intervention.
Figure 2.
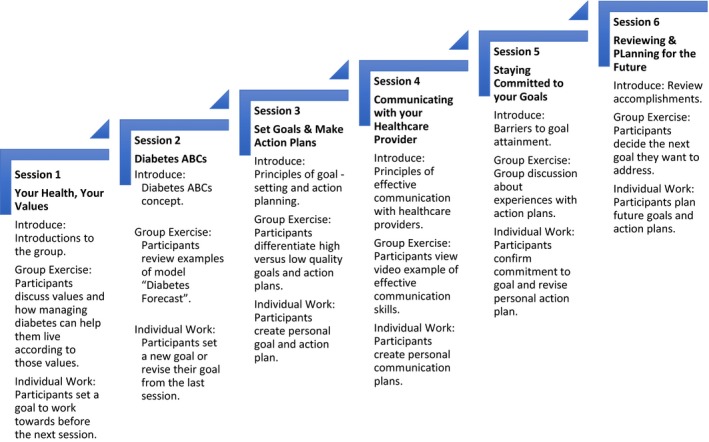
Framework for Empowering Patient in Chronic Care (EPIC), a collaborative, goal‐setting intervention
2.4.2. Enhanced usual care
Participants randomized to the EUC arm received routine care that included (a) educational materials about diabetes management, (b) an opportunity to participate in any self‐management resources routinely offered at their site, such as traditional diabetes education, nutritional counselling, medication management or weight loss support and (c) communication with their provider indicating the patient's desire for additional diabetes resources.
2.5. Primary outcome measurements
2.5.1. Effectiveness outcomes
To measure effectiveness and maintenance of the intervention, this study will examine two primary outcomes post‐intervention and 6 months post‐intervention: (a) change in HbA1c level and (b) change in the Diabetes Distress Scale (DDS) score. We measured serum HbA1c levels using clinical laboratories at the respective clinics using a standardized method of ion‐exchange liquid chromatography. Diabetes distress is an outcome of measure because it is an important patient‐reported quality of life measure for diabetes that is correlated with diabetes control. EPIC fundamentally rests on the individual with diabetes setting goals and actions plans to achieve those goals. The goals they set could address the distinct domains of diabetes‐related distress that include emotional, physician‐related, regime‐related, or interpersonal distress. Cumulatively, these add to diabetes distress. To measure distress specific to diabetes, we used the DDS, a 17‐item self‐report instrument that has high internal consistency, reliability (Cronbach's α = .93) and correlation with self‐care behaviours (r = .30, P < .001) and physical activity (r = .13, P < .01).18
2.5.2. Implementation outcomes
Outcomes for EPIC implementation will be assessed using dimensions from the RE‐AIM Framework19 as described in Table 1. Reach is the proportion of the eligible population that participated in the study. Adoption is defined as the proportion of providers who used the intervention and the number of individuals who participated in the intervention. Implementation is the extent to which the intervention is delivered as prescribed. These constructs will be operationalized by tracking the number of participants who were eligible for and participated in the study, the number and type of HCPs who took part in EPIC training, and the number of group sessions attended per patient.
2.5.3. Procedure
Blinded research staff collected data at baseline, post‐intervention, and 6 months after the intervention (ie, maintenance, occurring 10 months after baseline), with parallel time points in the EUC condition. The baseline self‐report assessments were collected in person; however, the remaining two assessments were mailed to participants. Research staff scheduled laboratory visits for HbA1c within 2 weeks of the target data collection time.
Participants received compensation of $25 after HbA1c results and self‐report assessments were collected at each time point. Study staff also performed chart abstraction to obtain clinical and service utilization data, including blood pressure, body mass index, last primary care visit date and active medications. We anticipated the rates of missing data for primary outcomes would be <20%.
2.6. Data analysis
2.6.1. Effectiveness and maintenance analysis
Primary outcome analyses at post‐intervention will be intention‐to‐treat (ITT) and will use multiple imputation procedures PROC MI and MI ANALYZE in SAS version 9.4. If intraclass correlation coefficients (ICCs) for HbA1c and DDS reveal that the degree of total variance explained by variance between cohorts and between sites is low (ie, ICCs <.05), Analysis of Covariance (ANCOVA) will be employed to examine treatment differences in outcomes immediately post‐intervention (at 4 months). However, if ICCs reveal an adequate degree of between‐cohort or between‐site variance in HbA1c or DDS (ie, ICCs >.05), we will employ multilevel modelling using PROC Mixed in SAS to account for the dependency of observations in the data. With either approach, two models will be conducted: one with HbA1c post‐intervention as the outcome and one with DDS at post‐intervention as the outcome. Models will include treatment group (ie, EPIC vs EUC) as a predictor and respective HbA1c or DDS baseline scores and any demographic or clinical variables that differed between the study arms at baseline as covariates. Treatment effect sizes will be calculated post‐intervention. If multilevel models are warranted, participants will be the level 1 unit, which will be nested within cohorts (level 2) which will be nested within sites (level 3). An unstructured covariance structure type will be specified.
Analyses for examination of maintenance of treatment effects will be similar to those for immediate treatment effects post‐intervention. Analyses will be intent‐to‐treat and either ANCOVA or multilevel models will be employed to examine treatment differences in outcomes at the maintenance assessment (6‐months post‐intervention). Two models will be conducted: one with HbA1c at 6‐months post‐intervention as the outcome and one with DDS at 6‐months post‐intervention as the outcome. Models will include treatment group (EPIC vs EUC) as a predictor and respective HbA1c or DDS scores and any demographic or clinical variables that differed between the study arms at baseline as covariates. Treatment effect sizes will be calculated at the 6 months post‐intervention.
2.6.2. Reach, adoption and implementation analyses
We will calculate descriptive statistics such as frequencies, proportions, means and standard deviations for characteristics of the overall sample and for each specific facility.
We will assess reach by comparing the per cent of enrolled study participants compared with all potentially eligible patients in the given clinic population. To assess EPIC adoption, we will use descriptive statistics including the frequency and percentage of different professional disciplines among all healthcare providers who participated in the intervention. Adoption will also include number of sessions (group and individual) that were conducted vs the number that was prescribed by site.
To examine implementation of the intervention, a behavioural coaching expert will listen to audio recordings of each site's first group and 20% of group recordings thereafter. We will assess adherence and competency of HCPs who completed training in EPIC using a previously developed and validated instrument.20 Additional measures for intervention fidelity will include an attendance ratio consisting of the total possible number of group sessions (six) as the denominator and the proportion of individual sessions attended per patient (ie 0‐6) as the numerator. The study staff also will also measure participants' self‐reported ratings of how well their group leader and individual session provider(s) engaged them in goal‐setting using a validated measure21 and ratings of goal and action plan quality using our previously validated rating Goal Evaluation Tool‐Diabetes (GET‐D) tool.22
3. RESULTS
Data analyses for effectiveness and implementation are pending. However, as indicated in Table 2, baseline data indicates that overall baseline HbA1c was 75 mmol/mol (9.08%) and the diabetes distress score was 2.43. Overall, participants were primarily men (95%) with an average age of 67 years. More than half had some college or more. Participants came from an ethnic/racial diverse background with 38% identifying as non‐Hispanic Black, 12% Hispanic, and 48% non‐Hispanic White. Approximately 2% identified themselves as being of “other” ethnicity/race. Preliminary data on the characteristics of our participants suggest that those individuals randomized to the EPIC and EUC arms of the study are not statistically significantly different from each other on key variables such as their baseline HbA1C, DDS. Participants in both groups were also similar in terms of age, race, education, income, employment level and marital status (Table 2). The only exception is that the number of participants with prior diabetes education was greater for participants in the EUC arm compared with the EPIC arm, χ 2 (1, N = 280) = 8.44, P = .00.
Table 2.
Baseline participant characteristics
| Characteristics | Total (N = 280a) | EPIC (n = 140) | EUC (n = 140) | P valueb |
|---|---|---|---|---|
| Age in years, mean ± SD | 67.2 ± 8.44 | 67.39 ± 8.57 | 66.94 ± 8.34 | .66 |
| Female sex, no. (%) | 16 (5.7) | 9 (6.4) | 7 (5.0) | .61 |
| Non‐hispanic white, no. (%) | 134 (47.9) | 70 (50.0) | 64 (45.7) | .47 |
| Education, no. (%) | ||||
| 8 grades or less | 5 (1.8) | 3 (2.1) | 2 (1.4) | .58c |
| Some high school | 7 (2.5) | 2 (1.4) | 5 (3.6) | |
| High school graduate or GED | 58 (20.7) | 32 (22.9) | 26 (18.6) | |
| Some college or trade school | 149 (53.2) | 72 (51.4) | 77 (55.0) | |
| College graduate (bachelor's degree) | 43 (15.4) | 22 (15.7) | 21 (15.0) | |
| Graduate degree | 18 (6.4) | 9 (6.4) | 9 (6.4) | |
| Lives alone, no. (%) (N = 278) | 89 (31.8) | 44 (31.7) | 45 (32.4) | .90 |
| Annual household, no. (%) (N = 258) | ||||
| <$10 000 | 48 (18.6) | 28 (21.2) | 20 (15.6) | .94d |
| $10 000‐19 999 | 32 (12.4) | 13 (9.8) | 19 (14.8) | |
| $20 000‐29 000 | 42 (16.3) | 23 (17.4) | 19 (14.8) | |
| $30 000‐39 999 | 33 (12.8) | 15 (11.4) | 18 (14.1) | |
| $40 000‐49 999 | 56 (21.7) | 28 (21.2) | 28 (21.9) | |
| $50 000‐59 999 | 18 (7.0) | 11 (8.3) | 7 (5.5) | |
| >$60 000 | 29 (11.2) | 13 (9.8) | 16 (12.5) | |
| Unemployed, no. (%) (N = 275) | 16 (5.8) | 7 (5.1) | 9 (6.6) | .60 |
| Prior diabetes education, No. (%) | 162 (57.9) | 69 (49.3) | 93 (66.4) | .004 |
| Haemoglobin (Hb) A1C, mean ± SD | 9.08 ± 1.5 | 9.11 ± 1.6 | 9.06 ± 1.3 | .75 |
| Diabetes distress scoree, mean ± SD | 2.43 ± 1.03 | 2.41 ± 1.05 | 2.45 ± 1.02 | .72 |
| Diabetes distress score ≥ 3, No. (%) | 72 (26.4) | 36 (50.0) | 36 (50.0) | .91 |
| Diabetes self‐efficacyf, mean ± SD | 5.68 ± 2.4 | 5.50 ± 2.4 | 5.86 ± 2.3 | .21 |
Unless otherwise noted.
From an unpaired t test (two‐tailed) for continuous variables, and Chi‐square tests for categorical variables.
P‐value for chi‐square test comparing the proportion of patients in each treatment group with at least some college/trade school education or beyond.
P‐value for chi‐square test comparing the proportion of patients in each treatment group with an annual household income <$40 000.
A mean of responses to 17 six‐point Likert scale items evaluating patients' emotional, physician‐related, regimen‐related and interpersonal distress. Higher scores correspond to greater distress. Scores ≥3 are considered a distress level needing clinical attention (N = 273).
A mean of eight 10‐point Likert scale questions evaluating patients' confidence performing diabetes management tasks related to diet, exercise, blood glucose monitoring, and lifestyle choices. Higher scores correspond to greater self‐efficacy (N = 275).
Table 3 highlights some characteristics of the five intervention sites, including type of recruited provider at each site, type of site and list of other diabetes services provided at each site. Prior to EPIC training, clinicians were surveyed on various items, including demographics and professional experience. Of the clinicians reported in Table 3: 65% of EPIC‐trained providers reported having 10 or more years of practice experience; 80% reported counselling and 62% reported clinical management as part of their regular activities; 62% were trained in motivational interviewing prior to EPIC participation.
Table 3.
Characteristics of intervention sites
| Characteristics | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 |
|---|---|---|---|---|---|
| EPIC‐trained healthcare providers (n = 20) | |||||
| Endocrinologist | √ √ | ||||
| Dietitian | √ | √ | √ | √ | |
| Pharmacists | √ | √ | √ | √ | |
| Nurse | √ √ | √ √ √ | |||
| Nurse educator | √ | ||||
| Nurse practitioner | √ | √ | |||
| Psychologist | √ | √ | |||
| Type of facility | |||||
| Community‐based primary care | √ | √ | |||
| Primary care clinic nested within medical center | √ | √ | √ | ||
| Diabetes‐specific services | |||||
| Nutritional counselling services | √ | √ | √ | √ | √ |
| Diabetes education | √ | √ | √ | √ | √ |
| Medication management/insulin counselling with pharmacist | √ | √ | √ | √ | |
| Weight management programme | √ | √ | |||
√ indicate the number of providers within the specific discipline that were trained to deliver EPIC.
4. DISCUSSION
The current study is a multisite, randomized clinical trial evaluating real‐world effectiveness and implementation of a collaborative goal‐setting intervention using practice‐based healthcare professionals in routine primary care settings. If evidence supports improvement in diabetic outcomes in this “real‐world” trial, then EPIC could be a viable, innovative strategy to improve the standard of care in diabetes self‐management.
Testing interventions delivered by practice‐based (not research‐based) healthcare professionals at multiple sites are logistically more complex and require significantly greater resources. We partnered with HCPs and clinic administrators to assess openness to adoption of EPIC.12 We assessed the local culture, context and experience of each clinic to develop a robust bidirectional engagement between the research and clinic teams that considered: (a) ensuring that the intervention fell within recommended guidelines and fit the clinical organization's approach to patient care, (b) developing an open communication strategy with each of the clinics, (c) identifying and addressing the needs as shared by the healthcare professionals who would be carrying out the intervention. Only after this groundwork was complete did we attempt to bring EPIC out of the research laboratory and test it in “real‐world” settings.
To facilitate the EPIC intervention, it was important to align our activities with ongoing strategic priorities of our operational partner. Thus, we structured EPIC to align with our partner clinics' implementation of shared decision‐making for HbA1c goals among their patients with diabetes. To align with our partners' focus on patient‐centredness, we also elicited patient's health‐related values that provide the motivation to control diabetes, improve health, and live according to what is most important. When healthcare decisions are aligned with patients' health values, patients are more likely to consent and adhere to those decisions.3, 23
4.1. Potential limitations
There are several potential limitations to our protocol that we will need to consider in our analysis of the trial. One potential limitation is contamination bias, especially in the smaller clinics given that they have a limited number of available providers. In effect, providers trained in EPIC could inadvertently use skills they learned to treat usual care patients and thus influence their care in a way that would make their outcomes appear similar to EPIC patients. Another potential limitation that despite the fact that we attempted to do a pragmatic clinical trial, participation by patients required informed consent and adherence to other specific clinical trial procedures. However, because patients were compensated at each of the date collection points and not for session attendance, we should have a less biased understanding of session attendance as EPIC moves further into implementation.
It is also important to acknowledge that the study took place in healthcare facilities within the Veteran Health Administration clinics. As such, the majority of the sample are men. However, there is ethnic/racial diversity within this group that adds to the generalizability. Additionally, the intervention was carried out in a number of diverse clinic settings: community‐based clinics in rural settings and clinics within larger, urban medical centres. Clinics were located in different regions within the US. Finally, the healthcare providers carrying out the intervention came from diverse professions such as nursing, dietetics, pharmacy and medicine. When considered cumulatively, EPIC appears to be able to be deliverable in diverse clinic settings by a range of healthcare providers. Moving forward, we acknowledge a need to target more women with diabetes to participate with the intervention.
Also, to ensure that participants would be able to actually participate in the group‐based intervention and prevent hypoglycaemic episodes in those at high risk for it, a number of exclusion criteria were listed. As EPIC continues down the path towards implementation, the intervention will inherently be tested under increasing less rigid environments.
4.2. Theoretical foundations
The EPIC intervention builds on a conceptual model of collaborative decision‐making among patients and their healthcare providers.7 The basis for these decisions is rooted in what matters most to patients in their health.24 The EPIC intervention begins by exploring why controlling diabetes is personally important to each participant and then focuses on behavioural changes patients are willing to make to control their diabetes, taking into account patient care preferences.7, 20 EPIC sessions also focus on understanding how the disease burden of uncontrolled diabetes can impede a patient's ability to live in accord with one's values.25
Empowering Patients in Chronic Care introduces the concept of goals and goal‐setting adapted from the organizational psychology literature.26 While patients often have vague notions of their goals, EPIC guides participants through a process of crafting specific, realistic and measurable (SMART) goals, with provider input to help patients further clarify their behavioural goals. Collaborative and proactive communication with one's healthcare providers is often an essential step in the process of developing effective health goals.8 EPIC includes a video‐guided exercise that activates patients to communicate collaboratively with their healthcare providers (ie, Speak‐Up) and teaches communication skills to improve care. Once patients define their healthcare goals, they must develop action plans to help them achieve their goals and monitor their own progress towards achieving goals. The EPIC intervention concludes with ongoing encouragement to iteratively refine and achieve one's behavioural goals by working through one or more action plans. In addition to guidance from EPIC clinicians, membership within cohorts remain static throughout the intervention, facilitating peer support for problem solving and goal attainment among group participants.7
5. CONCLUSION
As the prevalence of and sequelae of diabetes continue to rise, it is important that healthcare systems and providers integrate efficacious interventions such as EPIC into routine care. The use of a partnered research approach, as was utilized in EPIC, helped to facilitate the translation of EPIC from a research setting into clinical practice at a more rapid pace. The two phases of this study protocol allows us to understand the needs of the healthcare providers who would ultimately be responsible for delivering the intervention in the five distinct clinics within a “real‐world” setting. The results of the current study will enhance our understanding of to what extent the process of personalized, collaborative decision‐making can contribute to successful attainment of clinical and patient‐reported outcomes for diabetes control. It will also help determine the feasibility and clinical effectiveness of the collaborative goal‐setting paradigm within a practice‐based, primary care setting.
CONFLICT OF INTEREST
None of the authors of the above manuscript has declared any conflict of interest, which may arise from being named as an author on the manuscript.
AUTHOR CONTRIBUTION
LW and AN developed the study protocol and, along with NH, HSG, BH, PM and SR formulated the study design. NH, LK, EO, ES and LJ contributed in data collection. ABA conducted data analysis. LW, NK and AN drafted the manuscript. All authors participated in review and revision of the manuscript for important intellectual content.
DISCLAIMER
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
ACKNOWLEDGEMENTS
This study is supported by grants from the Department of Veterans Affairs, Health Services Research and Development Service grants CRE 12‐426, SHP 08‐182 and PPO 08‐402 and partially supported by the facilities and resources of the VA HSR&D Houston Center for Innovations in Quality, Effectiveness and Safety (CIN 13‐413), Michael E DeBakey VA Medical Center, Houston, Texas. Dr Kamdar is an advanced fellow in Health Services Research supported by the Department of Veterans Affairs Office of Academic Affiliations.
Woodard L, Kamdar N, Hundt N, et al. Empowering patients in chronic care to improve diabetes distress and glycaemic control: Protocol for a hybrid implementation‐effectiveness clinical trial. Endocrinol Diab Metab. 2020;3:e00099 10.1002/edm2.99
Trial Registration: ClinicalTrials.gov Identifier: NCT01876485.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137‐149. [DOI] [PubMed] [Google Scholar]
- 2. Association AD . Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher L, Mullan JT, Skaff MM, Glasgow RE, Arean P, Hessler D. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26(6):622‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Group TS . Health systems, patients factors, and quality of care for diabetes a synthesis of findings from the TRIAD study. Diabetes Care. 2010;33(4):940‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorig K. Action planning: a call to action. J Am Board Fam Med. 2006;19(3):324‐325. [DOI] [PubMed] [Google Scholar]
- 6. Bodenheimer T, Handley MA. Goal‐setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174‐180. [DOI] [PubMed] [Google Scholar]
- 7. Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naik AD, Kallen MA, Walder A, Street RL. Improving hypertension control in diabetes mellitus the effects of collaborative and proactive health communication. Circulation. 2008;117(11):1361‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self‐management education for adults with type 2 diabetes: a meta‐analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159‐1171. [DOI] [PubMed] [Google Scholar]
- 10. Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health‐behavior change in primary care. Am J Prev Med. 1999;17(4):275‐284. [DOI] [PubMed] [Google Scholar]
- 11. Jackson GL, Powers BJ, Chatterjee R, et al. The patient‐centered medical home: a systematic review. Ann Intern Med. 2013;158(3):169‐178. [DOI] [PubMed] [Google Scholar]
- 12. Arney J, Thurman K, Jones L, et al. Qualitative findings on building a partnered approach to implementation of a group‐based diabetes intervention in VA primary care. BMJ Open. 2018;8(1):e018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness‐implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cully JA, Mignogna J, Stanley MA, et al. Development and pilot testing of a standardized training program for a patient‐mentoring intervention to increase adherence to outpatient HIV care. AIDS Patient Care STDs. 2012;26(3):165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cully JA, Stanley MA, Petersen NJ, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. J Gen Intern Med. 2017;32(9):1014‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woodard LD, Landrum CR, Urech TH, Profit J, Virani SS, Petersen LA. Treating chronically ill people with diabetes mellitus with limited life expectancy: implications for performance measurement. J Am Geriatr Soc. 2012;60(2):193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hergueta T, Baker R, Dunbar GC. The mini‐international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM‐IVand ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):2233. [PubMed] [Google Scholar]
- 18. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes. Diabetes Care. 2005;28(3):626‐631. [DOI] [PubMed] [Google Scholar]
- 19. Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE‐AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns. 2001;44(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 20. Naik AD, Dindo LN, Van Liew JR, et al. Development of a clinically feasible process for identifying individual health priorities. J Am Geriatr Soc. 2018;66(10):1872‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med. 2003;18(11):893‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teal CR, Haidet P, Balasubramanyam AS, Rodriguez E, Naik AD. Measuring the quality of patients’ goals and action plans: development and validation of a novel tool. BMC Med Inform Decis Mak. 2012;12(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naik AD, Martin LA, Moye J, Karel MJ. Health values and treatment goals of older, multimorbid adults facing life‐threatening illness. J Am Geriatr Soc. 2016;64(3):625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naik AD, McCullough LB. Health intuitions inform patient‐centered care. Am J Bioeth. 2014;14(6):1‐3. [DOI] [PubMed] [Google Scholar]
- 25. Morrow AS, Haidet P, Skinner J, Naik AD. Integrating diabetes self‐management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72(3):418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: a 35‐year odyssey. Am Psychol. 2002;57(9):705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


