Abstract
Aims
To assess the dose distribution among users of metformin monotherapy as well as the patterns of up‐titration following initiation of therapy in people with type 2 diabetes mellitus (T2DM).
Materials and Methods
This was a retrospective cohort study of adults with T2DM in the United Kingdom (UK). Metformin dose distribution was assessed at 0, 6 and 12 months in people initiating metformin monotherapy (new users) and cross‐sectionally in people with ongoing metformin monotherapy (prevalent users). Patterns and predictors of up‐titration were also analysed in new users. Dose distributions and treatment patterns were assessed descriptively; predictors of up‐titration were determined using multivariable logistic regressions.
Results
Totals of 6174 new users and 8733 prevalent users were included. New users initiated metformin at >0 mg to ≤500 mg (25%), >500 mg to ≤1000 mg (47%), >1000 mg to ≤1500 mg (17%) or >1500 mg to ≤2000 mg (12%) daily. This distribution did not vary over time. Prevalent users of metformin received doses of >0 mg to ≤500 mg (14%), >500 mg to ≤1000 mg (40%), >1000 mg to ≤1500 mg (15%), >1500 mg to ≤2000 mg (29%) or >2000 mg (1%) daily. Among new users of metformin, 6.7% and 10.8% had been up‐titrated at 6 and 12 months, respectively, despite the majority having glycated haemoglobin >53 mmol/mol. Predictors of up‐titration included younger age and higher HbA1c.
Conclusions
A majority of T2DM patients taking metformin received a dose ≤1000 mg/day. Up‐titration of metformin is infrequent in the first year postinitiation.
Keywords: metformin/administration and dosage, retrospective studies, treatment patterns, type 2 diabetes mellitus, United Kingdom
This manuscript describes the initial and ongoing dose distribution of metformin in adults with diabetes in the UK, as well as the patterns of up‐titration over the year after initiation. We found that most patients are receiving a metformin dose lower than the minimum recommended level and that up‐titration of the starting dose was infrequent. This suggests that patients are undergoing regimen intensification before optimizing the metformin dose.
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by a combination of insulin insensitivity in muscle and liver and suboptimal insulin production by pancreatic β‐cells.1 These insulin‐related defects result in elevated blood glucose, or hyperglycaemia, which in turn has detrimental effects on the vasculature, leading to both macrovascular complications (eg coronary artery disease) and microvascular complications (eg diabetic retinopathy).2 The primary aim of treating T2DM is to control blood glucose levels, with the goal of preventing these vascular complications.
The International Diabetes Federation estimates that 8.8% of adults worldwide have diabetes; this number ranges from 3.8% in Africa to 11.5% in North America and the Caribbean.3 As of 2014, 5.3% of people aged 16 or older in the United Kingdom had T2DM.4 International guidelines including those from the American Diabetes Association/European Association for the Study of Diabetes,5 the International Diabetes Federation6 and the National Institute for Health and Care Excellence (NICE)7 recommend metformin as the initial antihyperglycemic pharmacotherapy. Both the US Food and Drug Administration8 and the European Medicines Agency9 note the need to up‐titrate metformin, that is, to gradually increase the dose, over the first several weeks. In the NICE guidance, treatment with metformin is considered successful if, after dose optimization, the patient's glycated haemoglobin (HbA1c) is maintained at or below the desired level (typically 48 mmol/mol (6.5%) or 53 mmol/mol (7.0%)).7 If HbA1c levels are not maintained on metformin, intensification of therapy by adding a second drug or switching to another drug is recommended. Intensification occurs in over 50% of patients in the United Kingdom.10
While the patterns and consequences of intensification of T2DM therapy have been well characterized globally,10, 11, 12, 13, 14, 15, 16 studies examining up‐titration of metformin in the real‐world clinical setting are lacking. The Summary of Product Characteristics from the European Medicines Agency recommends a starting dose of 500 or 850 mg metformin 2‐3 times daily, with a maximum daily dose of 3000 mg,9, 17 but studies of intensification after metformin monotherapy generally do not report the final titrated dose of metformin before adding on or switching therapies.11, 18, 19 Since metformin has demonstrated efficacy at lowering HbA1c when given at 1500‐2000 mg/day,20 the question remains whether metformin treatment sometimes fails because of inadequate dose optimization. The objective of this study was thus to assess the dose distribution of metformin monotherapy, as well as the patterns and predictors of up‐titration following initiation of metformin, among people with T2DM in the United Kingdom.
2. MATERIALS AND METHODS
2.1. Study design and data source
This was a retrospective cohort study of people with T2DM in the United Kingdom. Data were obtained from the Clinical Practice Research Datalink (CPRD) database, which contains over 4 million active participant records (and over 11 million overall) drawn from approximately 650 primary care practices.21 The CPRD database contains anonymized longitudinal primary care medical records focusing on participant‐specific diagnoses, laboratory measurements and prescription data, many of which are linked to nonprimary care data including hospitalizations and mortality data. Data from 1 January 2012 through 31 December 2017 were accessed for the analyses of the subjects described below. Because the data were anonymized, approval by an ethics committee was not required; however, the study conformed to the principles of the Declaration of Helsinki, and access to CPRD data is subject to protocol approval by an Independent Scientific Advisory Committee (ISAC).
2.2. Selection of the population
This study included adult (aged ≥21) subjects diagnosed with T2DM during the study period (January 2012 to December 2017). The diagnosis of T2DM was based on Read/Oxford Medical Information System codes (primarily the C109 and C10F series; a complete list is available upon request), which are supported by rigorous classification techniques. Metformin use was identified in the CPRD database using British National Formulary (BNF) codes for antidiabetic medication and further by identifying generic and product names (complete list available upon request). There were two populations of interest: new users and prevalent users of metformin monotherapy.
New users of metformin monotherapy received at least one prescription for metformin between January 2013 and December 2016 (the index period for new users), with the first prescription of metformin during this period defined as the index date. No other prescriptions for antihyperglycaemic agents (including metformin) were allowed in the year prior to the index date, and the index prescription was required to continue for a minimum of 3 months. New users were also required to be enrolled in the database for at least 15 months postindex for the purposes of analysing treatment patterns over time.
Prevalent users of metformin monotherapy received at least one prescription for metformin between January and December 2017 (the index period for prevalent users), with the most recent metformin prescription defined as the index date. No prescriptions for other antihyperglycemic agents (other than metformin) were allowed in the year prior to the index date, to ensure monotherapy.
All subjects were required to have an HbA1c measurement available within 6 months prior to their index date and to have reliable dose information available at the index date. Subjects whose doses were outliers (indicating data entry errors) were excluded, as were those who used metformin in solution formulation (for which the daily dose could not be determined). Other reasons for exclusion included diagnosis codes for type 1 diabetes mellitus, gestational diabetes, other forms of secondary diabetes or polycystic ovary syndrome any time during the study period.
2.3. Study outcomes and variables
Metformin use was identified as described above. In the CPRD database, each prescription is recorded as the number of tablets to be taken each day and the tablet strength. To calculate the daily dose, the number of tablets to be taken was multiplied by the tablet strength. The daily dose was categorized as >0 mg to ≤500 mg, >500 mg to ≤1000 mg, >1000 mg to ≤1500 mg, >1500 mg to ≤2000 mg, or >2000 mg.
Treatment patterns included up‐titration, discontinuation, add‐on therapy and switching. Up‐titration was defined as progression to a metformin dose category higher than the dose category on the index date. Discontinuation of metformin was defined as having a gap of greater than 45 days from the end of one fill to the beginning of the next fill of metformin monotherapy without a prescription for another antihyperglycaemic agent at the time of discontinuation. Add‐on therapy was defined as the presence of a prescription for a second antihyperglycaemic agent in addition to a prescription for metformin or a prescription of a fixed‐dose combination that included metformin, and switching was defined as discontinuation of metformin with a recorded prescription of another antihyperglycaemic agent.
Demographic and clinical variables collected on the index date were age, sex, HbA1c level, smoking status, alcohol use, duration of T2DM, body mass index (BMI), cholesterol measurements (triglycerides, low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol (HDL‐C)), systolic and diastolic blood pressure, estimated glomerular filtration rate (eGFR), microvascular and macrovascular complications, and severe hypoglycaemia. Values not available on the index date were collected from the most recently recorded data entry prior to the index date. HbA1c levels were classified as <53 mmol/mol (7.0%), 53 to <64 mmol/mol (7.0% to <8.0%), 64 to <75 mmol/mol (8.0% to <9.0%) and ≥75 mmol/mol (9.0%). The duration of T2DM was defined as the time from the first diagnosis of T2DM in the database until the index date. BMI was measured in kg/m2 and was categorized as normal (<25), overweight (25‐29) and obesity (>29). Microvascular complications included diabetic retinopathy, nephropathy and neuropathy. Macrovascular complications included acute coronary syndrome, acute myocardial infarction, angina, arrhythmia, revascularization, heart failure, peripheral arterial disease, peripheral vascular disease and stroke/transient ischaemic attack. Severe hypoglycaemia was defined as low blood glucose levels that required assistance from another person to treat. The presence of microvascular and macrovascular complications and severe hypoglycaemia was determined using Read codes (list available upon request) and based on data from the 12 months prior to the index date.
The Charlson comorbidity index score was originally designed to predict the impact of comorbidities on the mortality of a subject. The list of comorbidities prominent in subjects with T2DM and their related Read codes were taken from Khan et al.22
2.4. Statistical analysis
Subjects' demographic and clinical characteristics on the index date were analysed descriptively and are presented as numbers and percentages for categorical variables and as means and standard deviations (or medians and interquartile ranges) for continuous variables.
The metformin dose distribution was assessed descriptively in new users at initiation (ie the index date) and again 6 and 12 months postindex. The dose distribution at initiation was also assessed by HbA1c level. In prevalent users, the index dose distribution was assessed cross‐sectionally, both overall and by HbA1c level and duration of diabetes. These data on prevalent users were included for the purpose of comparison with new users, since prevalent users were expected to have already had their metformin dose up‐titrated.
The proportion of subjects with up‐titration, defined as described above, was measured in new users at 6 and 12 months. Subject data were censored from further analyses of up‐titration if they discontinued metformin, switched to another therapy or added on other antihyperglycaemic therapy during the postindex period. For example, a subject discontinuing metformin or adding another therapy in month 7 would be included in the metformin dose distribution analysis at month 6, but would be excluded at month 12. The time to up‐titration, both overall and by HbA1c level at initiation, was measured in days (median and interquartile range) from the initial dose to the date of the highest prescribed dose within the postindex period.
Multivariable logistic regression models were used to evaluate the demographic and clinical factors associated with up‐titration in new users of metformin, adjusting for other baseline covariates. The association between subject characteristics and up‐titration was quantified at 6 and 12 months postindex as adjusted odds ratios (ORs) and 95% confidence intervals (CIs), along with P values. P values <.05 were considered statistically significant. All analyses were conducted using SAS version 9.4.
3. RESULTS
3.1. Characteristics of the study population
A total of 6,174 new users of metformin were identified during 2013‐2016 (Table 1). Their mean age was 61.7 years, and 42.7% were male. Just 17.9% of new users had an HbA1c level at the standard target of <53 mmol/mol (7.0%). Obesity was highly prevalent (67.8%), and the median duration of T2DM was 0.04 years, indicating that most of these subjects were very recently diagnosed.
Table 1.
Characteristics of new and prevalent metformin usersa
| New users (N = 6174) | Prevalent users (N = 8733) | |
|---|---|---|
| Demographic | ||
| Age, mean (SD) years | 61.7 (12.4) | 64.4 (12.6) |
| Male | 2637 (42.7%) | 4980 (57.0%) |
| Clinical | ||
| HbA1c | ||
| <53 mmol/mol (7.0%) | 1103 (17.9%) | 4455 (51.0%) |
| 53 to < 64 mmol/mol (7.0% to < 8.0%) | 2169 (35.1%) | 2630 (30.1%) |
| 64 to < 75 mmol/mol (8.0% to < 9.0%) | 1148 (18.6%) | 832 (9.5%) |
| ≥75 mmol/mol (9.0%) | 1754 (28.4%) | 816 (9.3%) |
| Currently smoke | 1081 (17.5%) | 1286 (14.7%) |
| Currently drink alcohol | 2383 (38.6%) | 3731 (42.7%) |
| Duration of T2DM, median (IQR) years | 0.04 (0‐0.84) | 3.1 (1.3‐4.9) |
| Laboratoryb | ||
| BMI, kg/m2 | ||
| Normal (<25) | 278 (6.3%) | 645 (10.6%) |
| Overweight (25‐29) | 1138 (25.9%) | 1809 (29.6%) |
| Obese (>29) | 2980 (67.8%) | 3649 (59.8%) |
| Total cholesterol, mean (SD) mmol/L | 5.04 (1.25) | 4.51 (1.12) |
| Triglycerides, mean (SD) mmol/L | 2.42 (2.03) | 2.11 (1.51) |
| LDL‐C, mean (SD) mmol/L | 2.87 (1.07) | 2.56 (1.09) |
| HDL‐C, mean (SD) mmol/L | 1.18 (0.32) | 1.23 (0.35) |
| Systolic blood pressure, mean (SD) mmHg | 135.6 (15.1) | 132.6 (13.7) |
| Diastolic blood pressure, mean (SD) mmHg | 79.6 (9.8) | 76.8 (9.1) |
| eGFR, mean (SD) mL/min/1.73 m2 | 69.7 (23.3) | 67.9 (21.2) |
| Comorbiditiesc | ||
| Macrovascular complications | 331 (5.4%) | 362 (4.2%) |
| Microvascular complications | 138 (2.2%) | 253 (2.9%) |
| Severe hypoglycaemia | 4 (0.1%) | 26 (0.3%) |
| CCI, mean (SD) | 0.73 (0.78) | 0.87 (0.79) |
BMI, body mass index; CCI, Charlson comorbidity index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Values are presented as n (%) unless otherwise indicated.
Laboratory values were available for the following percentages of prevalent users: BMI, 70%; triglycerides, 63%; LDL‐C, 68%; HDL‐C, 76%; systolic and diastolic blood pressure, 85%; eGFR, 68%. In new users the percentages were: BMI, 71%; triglycerides, 71%; LDL‐C, 78%; HDL‐C, 65%; systolic and diastolic blood pressure, 86%; eGFR, 69%.
Comorbidity rates are based on data from the 12 mo prior to the index date.
A total of 8733 prevalent users of metformin were identified during 2017 (Table 1). Their mean age was 64.4 years, and 57.0% were male. Just over half of prevalent users (51.0%) had met the HbA1C target of <53 mmol/mol (7.0%), and 59.8% were obese. The median duration of T2DM was 3.1 years.
3.2. Dose distribution and up‐titration patterns in new users
New users initiated metformin at >0 mg to ≤500 mg (25%), >500 mg to ≤1000 mg (47%), >1000 mg to ≤1500 mg (17%) or >1500 mg to ≤2000 mg (12%) daily (Figure 1A). This distribution did not vary over time (Figure 1A), but varied substantially at initiation by HbA1c: 48% of subjects with HbA1c ≥ 75 mmol/mol (9.0%) received an initial daily dose of >1000 mg, but only 14% of subjects with HbA1c < 53 mmol/mol (7.0%) did so (Figure 1B). Given this variation by HbA1c level, we assessed whether the dose distribution differed over time in subjects who had not attained HbA1c targets of <53 mmol/mol (7.0%) and <64 mmol/mol (8.0%) (Figure 1C). In the subset of subjects with HbA1c ≥ 53 mmol/mol (7.0%), 39% received a metformin dose >1000 mg at both 6 months and 12 months (compared to 31% of subjects in the combined study population at the same time points). In the subset of subjects with HbA1c ≥ 64 mmol/mol (8.0%), 48% received a metformin dose >1000 mg at 6 months and 50% did so at 12 months.
Figure 1.
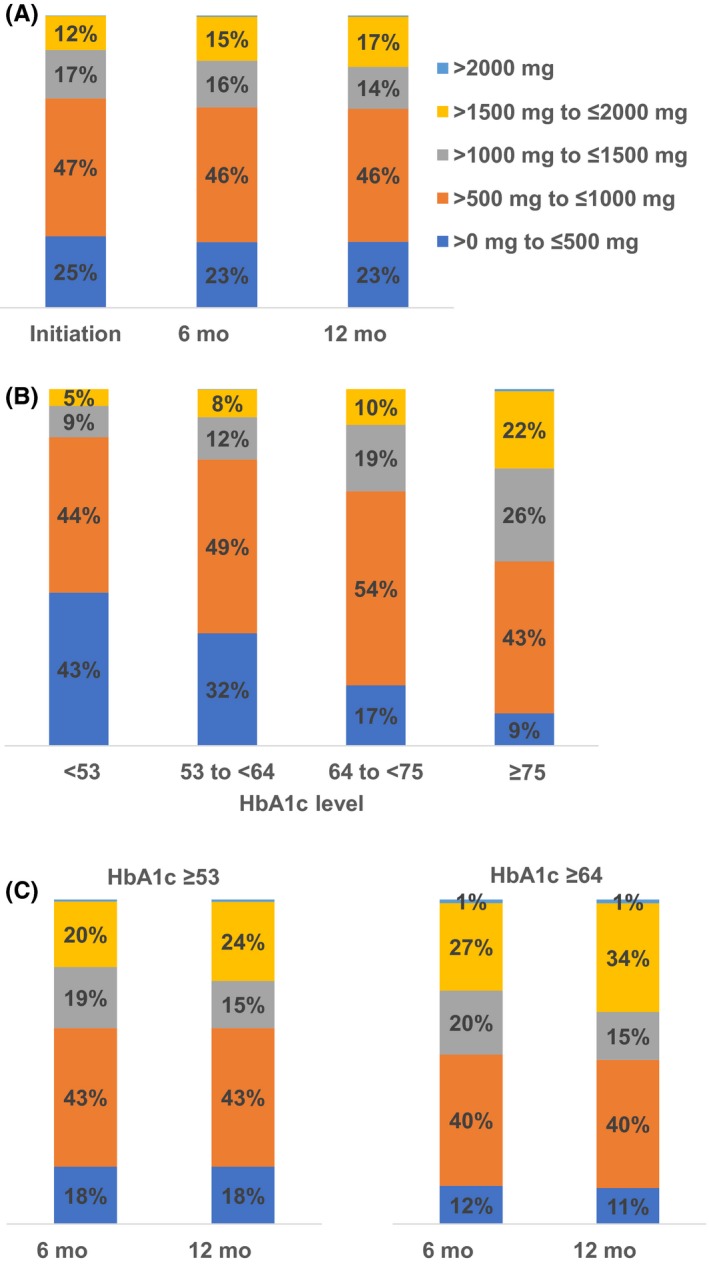
Dose distribution over time (A), at initiation by HbA1c level (B), and over time for selected HbA1c levels (C) in new users of metformin. HbA1c, glycated haemoglobin. HbA1c values are given in mmol/mol. Corresponding NGSP percentages are as follows: <53 mmol/mol (7.0%), 53 to <64 mmol/mol (7.0% to <8.0%), 64 to <75 mmol/mol (8.0% to <9.0%) and ≥75 mmol/mol (9.0%). In panel a, N = 6,174 at initiation; 4820 at 6 mo; 3592 at 12 mo. Data in panel b are at initiation. In panel c, N = 1,595 at 6 mo and 1,061 at 12 mo for HbA1c ≥ 53 mmol/mol (7.0)%; N = 527 at 6 mo and 334 at 12 mo for HbA1c ≥ 64 mmol/mol (8.0%)
At 6 and 12 months after treatment initiation, most new users had experienced no change in their treatment regimen; just 6.7% and 10.8% of new users had been up‐titrated, respectively (Figure S1). Rates of up‐titration were 5.5% in subjects with HbA1c < 53 mmol/mol (7.0%), 8.5% in those with HbA1c 53 to <64 mmol/mol (7.0% to <8.0%), 14.1% in those with HbA1c 64 to <75 mmol/mol (8.0% to <9.0%) and 14.8% in those with HbA1c ≥ 75 mmol/mol (9.0%). Of the 667 subjects up‐titrated at 12 months, 39.0% had an HbA1c measurement ≥75 mmol/mol (9.0%) at initiation, whereas just 9.2% had HbA1c < 53 mmol/mol (7.0%) (Table 2). The median time to up‐titration was 175 days, with a shallow trend towards longer times in subjects with lower HbA1c values (Table 2). Predictors of up‐titration in new users (Table 3) included younger age at 12 months postinitiation (eg OR 3.86, 95% CI 1.97‐7.58 for subjects aged <50 versus ≥70) and higher HbA1c at both 6 months and 12 months (OR 2.37, 95% CI 1.27‐4.41 at 12 months for HbA1c 64 to <75 mmol/mol (8.0% to <9.0%) vs <53 mmol/mol (7.0%); OR 2.07, 95% CI 1.11‐3.86 at 12 months for HbA1c ≥ 75 mmol/mol (9.0%) versus <53 mmol/mol (7.0%)). Triglyceride and HDL‐C levels were predictive of up‐titration at 6 months, but not at 12 months (Table 3).
Table 2.
Time to up‐titration in new metformin users, overall and by HbA1c level
| Overall | HbA1c level at initiation | ||||
|---|---|---|---|---|---|
| <53 mmol/mol (7.0%) | 53 to < 64 mmol/mol (7.0% to <8.0%) | 64 to <75 mmol/mol (8.0% to <9.0%) | ≥75 mmol/mol (9.0%) | ||
| n (%) | 667 (100.0) | 61 (9.2) | 184 (27.6) | 162 (24.3) | 260 (39.0) |
| Median, days | 175 | 179 | 177 | 175 | 173 |
| Interquartile range | 162‐348 | 163‐345 | 162‐351 | 162‐349 | 160‐342 |
Table 3.
Characteristics associated with up‐titration at 6 and 12 mo in new usersa
| 6 mo | 12 mo | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Age groups (reference, ≥70) | ||||||
| <50 | 1.06 | 0.63‐1.81 | .820 | 3.86 | 1.97‐7.58 | <.001 |
| 50 to < 60 | 1.41 | 0.92‐2.18 | .119 | 2.91 | 1.62‐5.22 | <.001 |
| 60 to < 70 | 0.79 | 0.51‐1.23 | .300 | 2.02 | 1.15‐3.55 | .014 |
| Female (reference, male) | 1.15 | 0.83‐1.60 | .393 | 1.05 | 0.71‐1.55 | .809 |
| HbA1c at initiation (reference, <53 mmol/mol (7.0%)) | ||||||
| 53 to < 64 mmol/mol (7.0% to < 8.0%) | 1.54 | 0.89‐2.67 | .125 | 1.33 | 0.75‐2.35 | .327 |
| 64 to < 75 mmol/mol (8.0% to < 9.0%) | 2.96 | 1.66‐5.28 | <.001 | 2.37 | 1.27‐4.41 | .007 |
| ≥75 mmol/mol (9.0%) | 3.71 | 2.14‐6.44 | <.001 | 2.07 | 1.11‐3.86 | .022 |
| Currently smoke | 0.96 | 0.65‐1.43 | .852 | 0.82 | 0.50‐1.35 | .433 |
| Currently drink alcohol | 1.35 | 0.99‐1.84 | .061 | 1.07 | 0.73‐1.55 | .737 |
| Diabetes duration | 1.02 | 0.95‐1.09 | .646 | 1.01 | 0.94‐1.09 | .750 |
| BMI (reference, normal) | ||||||
| Overweight | 0.95 | 0.47‐1.92 | .893 | 1.68 | 0.63‐4.48 | .304 |
| Obese | 1.03 | 0.52‐2.02 | .934 | 1.33 | 0.51‐3.49 | .562 |
| Triglycerides | 0.83 | 0.71‐0.98 | .026 | 0.99 | 0.82‐1.20 | .933 |
| LDL‐C | 1.10 | 0.94‐1.28 | .228 | 0.87 | 0.72‐1.05 | .152 |
| HDL‐C | 0.54 | 0.29‐0.98 | .041 | 1.33 | 0.68‐2.59 | .410 |
| Diastolic blood pressure | 1.01 | 0.99‐1.02 | .524 | 1.01 | 0.99‐1.03 | .646 |
| eGFR | 1.00 | 0.99‐1.01 | .740 | 1.00 | 0.99‐1.01 | .742 |
| Microvascular complicationsb | 0.68 | 0.20‐2.31 | .539 | 1.11 | 0.39‐3.18 | .850 |
| Macrovascular complicationsb | 0.99 | 0.50‐1.95 | .973 | 0.62 | 0.25‐1.51 | .289 |
| CCI | 0.92 | 0.74‐1.15 | .470 | 1.13 | 0.89‐1.44 | .303 |
BMI, body mass index; CCI, Charlson comorbidity index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Bold font indicates statistical significance.
In the 12 mo prior to the index date.
3.3. Dose distribution in prevalent users
Prevalent users of metformin received doses of >0 mg to ≤500 mg (14%), >500 mg to ≤1000 mg (40%), >1000 mg to ≤1500 mg (15%), >1500 mg to ≤ 2000 mg (29%) or >2000 mg (1%) daily (Figure 2A). Their dose distribution varied substantially by HbA1c level and duration of T2DM, with subjects with HbA1c ≥ 64 mmol/mol (8.0%) and duration of disease ≥10 years more likely to receive doses >1000 mg per day (Figure 2B, C).
Figure 2.
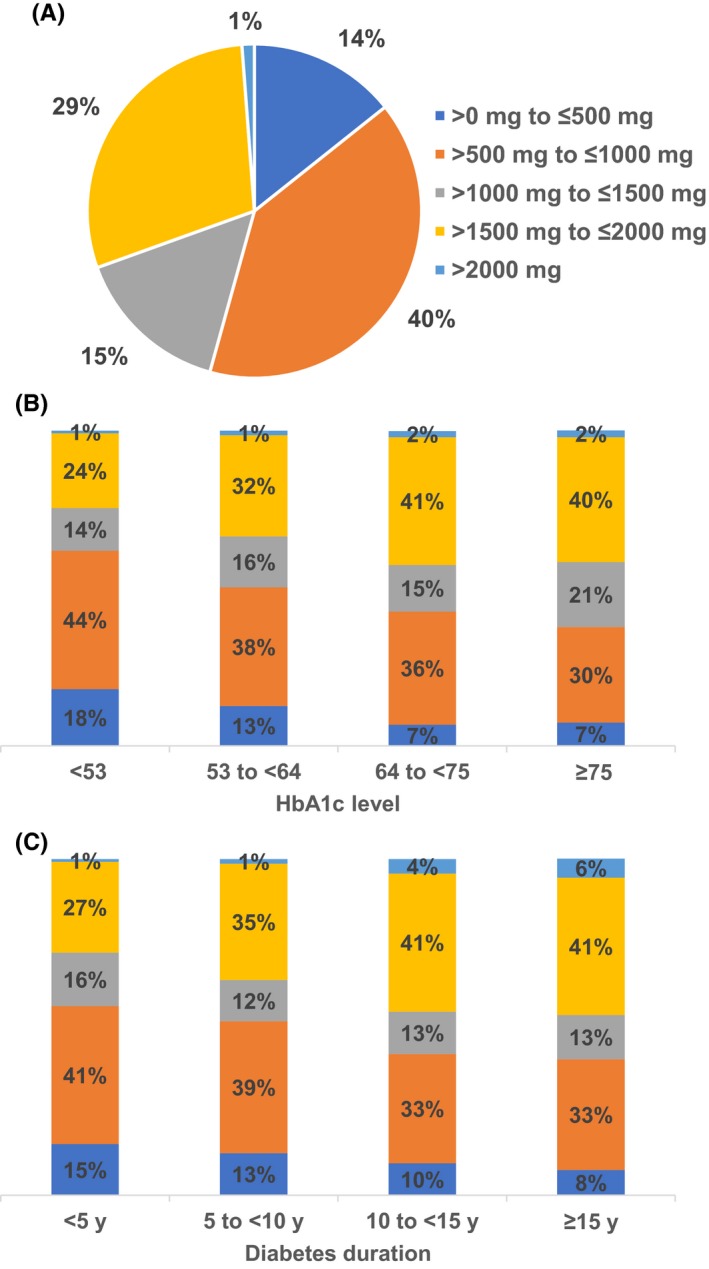
Dose distribution in prevalent users of metformin (A) overall, (B) by HbA1c level and (C) by disease duration. HbA1c, glycated haemoglobin. HbA1c values are given in mmol/mol. Corresponding NGSP percentages are as follows: <53 mmol/mol (7.0%), 53 to <64 mmol/mol (7.0% to <8.0%), 64 to <75 mmol/mol (8.0% to <9.0%) and ≥75 mmol/mol (9.0%)
4. DISCUSSION
This study showed that dosing of metformin monotherapy in people with T2DM in the United Kingdom is influenced by HbA1c levels, and in prevalent users, also by duration of disease. Up‐titration was infrequent in the first year postinitiation and was dependent on subject's age and HbA1c levels.
Metformin is widely prescribed for T2DM globally, particularly in Western countries.13, 15, 23, 24, 25 Metformin lowers blood glucose levels by reducing the amount of glucose produced and released by the liver, and by increasing insulin sensitivity.26 A major advantage of this therapy is weight stability or modest weight loss, in contrast to many other antihyperglycaemic medications. It may also reduce the risk of cardiovascular disease outcomes.27, 28 A recent US study found that up‐titration of metformin was as effective as adding a second T2DM drug in terms of the probability of glycaemic control after 6 months.29 Despite these positive features of metformin therapy, optimization of the metformin dose appears to be infrequent.
The median time to up‐titration in the current study was around 175 days, regardless of HbA1c level. This suggests that up‐titration, if it happens, generally happens within the first 6 months of treatment. This is in accordance with international guidelines that recommend dose optimization over the first several weeks.7, 8, 9 Studies of time to treatment intensification, that is, switching to another drug or adding a second drug, indicate that <40% of people on metformin monotherapy intensify their treatment within the first year,11, 18 with median times to initiation of second‐line therapy of 2‐2.3 years,18, 19 suggesting there is a sufficiently long lag time between metformin initiation and the time to intensification during which to up‐titrate metformin. In most of our study population, however, this had not been done.
Per the Summary of Product Characteristics from the European Medicines Agency, the minimum recommended starting dose of 500 mg metformin two times daily translates to 1000 mg/day.9 However, in the current study, 72% of new users were initiated at doses lower than this value, and 69% remained at this dose level 6 and 12 months later. Even among new users with suboptimal glycaemic control (HbA1c ≥ 53 mmol/mol (7.0%)), 61% remained below this dose level at 12 months. Furthermore, 54% of prevalent users of metformin were receiving less than this minimum recommended daily dose. This may explain why only 51% of prevalent metformin users had met the standard HbA1C target of <53 mmol/mol (7.0%), even after a median disease duration of 3.1 years.
The reasons for the low rates of up‐titration are speculative, but may include contraindications, intolerance and discontinuation. The NICE guidelines state that metformin is contraindicated or not tolerated in 15% of diabetes patients.7 Contraindications include renal dysfunction and recent cardiovascular events, both of which are common among people with T2DM.30 We investigated the effects of excluding patients with renal dysfunction, defined as eGFR < 30 (0.5% of new users and 1.0% of prevalent users) and found no change in the results. Intolerance of metformin may manifest as lactacidosis or gastrointestinal side effects, which sometimes lead to discontinuation.20 Our analysis showed that discontinuation was the most frequent treatment pattern (among patterns involving a change) observed in the year after initiating metformin monotherapy, suggesting that many subjects were intolerant of metformin.
Inadequate dosing of metformin limits its therapeutic efficacy. In an ethnically diverse sample of 128 T2DM patients in the United Kingdom, a lack of titration of oral antihyperglycaemic agents was identified as one of several reasons for HbA1c levels being >86 mmol/mol (10.0%).31 However, reduced efficacy may also stem from nonadherence to an optimized treatment regimen. Previous studies have found that adherence to metformin is lower than to other oral antihyperglycaemic therapies32, 33 and that poor adherence to metformin is associated with poor glycaemic control.31, 32 Thus, both clinical inertia on the part of physicians and nonadherent patient behaviours may contribute to treatment failure on metformin monotherapy. Based on our finding that up‐titration rates increased significantly across the range of HbA1c values from <53 mmol/mol (7.0%) to ≥75 mmol/mol (9.0%), physicians are attuned to the need to optimize the metformin dose to attain glycaemic control. They may also need to be reminded that the extended‐release formulation of metformin can reduce gastrointestinal side effects34 and increase patient adherence.35
4.1. Limitations
This analysis was subject to the typical limitations of electronic health data, most notably the potential for miscoding, misdiagnosis and underdiagnosis in the CPRD database. Secondly, although the database captures prescription information, prescription fills, physician instructions and medication‐taking behaviour are not recorded. As a result, the prescription data alone cannot fully explain treatment adherence. In addition, the data available in the CPRD database do not allow us to reliably track fixed‐dose combinations or determine whether the highest dose a subject had been prescribed was their true “maximum tolerable” dose of metformin. Tolerability issues due to metformin therapy, such as gastrointestinal events, were not captured (ie not available), although renal function was recorded as the eGFR and was the focus of the sensitivity analysis described above. Thirdly, most CPRD data were collected from primary care physicians and do not include diagnoses made and treatment used during hospitalizations or by specialists. As a result, serious comorbid conditions that required inpatient services or specialist care, or prescriptions written by specialists, may not have been recorded. Finally, the requirement that all subjects have an HbA1c measurement available within 6 months prior to their index date and have reliable metformin dose information available at the index date biased the population towards a well‐documented data set and limits the generalizability of the findings to the T2DM population as a whole.
5. CONCLUSIONS
In conclusion, this study of UK adults with T2DM showed that dosing of metformin monotherapy is often suboptimal and that up‐titration is infrequent. Metformin dosing and up‐titration were highly dependent on HbA1c levels, but remained suboptimal even in subjects with HbA1c levels above the recommended targets. Given the popularity of metformin as a first‐line treatment for T2DM, and its demonstrated efficacy at the recommended doses, providers should be encouraged to optimize the metformin dose, and patients should be made aware of the impact of tolerability and adherence issues on glycaemic control.
CONFLICTS OF INTEREST
Authors GF, MC and SR are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA. Author KI was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA at the time of the analysis. Authors AF and KK received honoraria from Merck & Co., Inc for participation in the study. Author BS was an external contractor whose analysis services were paid for by Merck & Co., Inc
Supporting information
ACKNOWLEDGEMENTS
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA. The authors thank Melissa Stauffer, PhD, of Scientific Editing Solutions, for medical writing assistance.
Iglay K, Sawhney B, Fu AZ, et al. Dose distribution and up‐titration patterns of metformin monotherapy in patients with type 2 diabetes. Endocrinol Diab Metab. 2020;3:e00107 10.1002/edm2.107
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from CPRD. Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowler M. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 2008;26(2):77‐82. [Google Scholar]
- 3. International Diabetes Federation . Diabetes Atlas, 7th edn http://www.diabetesatlas.org. Accessed December 5, 2017.
- 4. Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab. 2017;19(11):1537‐1545. [DOI] [PubMed] [Google Scholar]
- 5. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1‐52. [DOI] [PubMed] [Google Scholar]
- 7. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. NICE guideline. London,UK: National Institute for Health and Care Excellence (NICE) 2015. [Google Scholar]
- 8. US Food and Drug Administration . Glucophage (Metformin Hydrochloride) Product Information. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process%26ApplNo=020357. Accessed March 5, 2017.
- 9. European Medicines Agency . Metformin ‐ Summary of Product Characteristics. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Glucophage/Glucophage_Forte/Risidon/Dianben/human_referral_000096.jsp%26mxml:id=WC0b01ac05805c516f. Accessed March 5, 2017.
- 10. Desai U, Kirson NY, Kim J, et al. Time to treatment intensification after monotherapy failure and its association with subsequent glycemic control among 93,515 patients with type 2 diabetes. Diabetes Care. 2018;41(10):2096‐2104. [DOI] [PubMed] [Google Scholar]
- 11. Watson L, Das R, Farquhar R, Langerman H, Barnett AH. Consequences of delaying treatment intensification in type 2 diabetes: evidence from a UK database. Curr Med Res Opin. 2016;32(9):1465‐1475. [DOI] [PubMed] [Google Scholar]
- 12. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Overbeek JA, Heintjes EM, Prieto‐Alhambra D, et al. Type 2 diabetes mellitus treatment patterns across Europe: a population‐based multi‐database study. Clin Ther. 2017;39(4):759‐770. [DOI] [PubMed] [Google Scholar]
- 14. Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, Griffin MR. Diabetes treatment intensification and associated changes in HbA1c and body mass index: a cohort study. BMC Endocr Disord. 2016;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heintjes EM, Overbeek JA, Hall GC, et al. Factors associated with type 2 diabetes mellitus treatment choice across four European countries. Clin Ther. 2017;39(11):2296‐2310.e2214. [DOI] [PubMed] [Google Scholar]
- 16. Horii T, Iwasawa M, Shimizu J, Atsuda K. Comparing treatment intensification and clinical outcomes of metformin and dipeptidyl peptidase‐4 inhibitors in treatment naive patients with type 2 diabetes in Japan. J Diabetes Investig. 2019. 10.1002/edm2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Electronic Medicines Compendium . Glucophage 500 mg film coated tablets. https://www.medicines.org.uk/emc/product/987/smpc Accessed January, 10, 2018.
- 18. Khunti K, Godec TR, Medina J, et al. Patterns of glycaemic control in patients with type 2 diabetes mellitus initiating second‐line therapy after metformin monotherapy: retrospective data for 10 256 individuals from the United Kingdom and Germany. Diabetes Obes Metab. 2018;20(2):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilding J, Godec T, Khunti K, et al. Changes in HbA1c and weight, and treatment persistence, over the 18 months following initiation of second‐line therapy in patients with type 2 diabetes: results from the United Kingdom Clinical Practice Research Datalink. BMC Med. 2018;16(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz S, Fonseca V, Berner B, Cramer M, Chiang YK, Lewin A. Efficacy, tolerability, and safety of a novel once‐daily extended‐release metformin in patients with type 2 diabetes. Diabetes Care. 2006;29(4):759‐764. [DOI] [PubMed] [Google Scholar]
- 21. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Cao Y, Wu Y, et al. The prescription pattern of initial treatment for type 2 diabetes in Beijing from 2011 to 2015. Medicine (Baltimore). 2019;98(8):e14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clemens KK, Shariff S, Liu K, et al. Trends in antihyperglycemic medication prescriptions and hypoglycemia in older adults: 2002–2013. PLoS ONE. 2015;10(9):e0137596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States From 1999–2012. JAMA. 2015;314(17):1818‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797‐1805. [DOI] [PubMed] [Google Scholar]
- 27. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta‐analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60(9):1620‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrannini E, DeFronzo RA. Impact of glucose‐lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36(34):2288‐2296. [DOI] [PubMed] [Google Scholar]
- 29. Mahabaleshwarkar R, Liu TL, Mulder H. comparative effectiveness of metformin dosage uptitration versus adding another antihyperglycemic medication on glycemic control in type 2 diabetes patients failing initial metformin monotherapy: a retrospective cohort study. Popul Health Manag. 2019;22:457‐463. [DOI] [PubMed] [Google Scholar]
- 30. Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co‐prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243‐1252. [DOI] [PubMed] [Google Scholar]
- 31. Khan H, Lasker SS, Chowdhury TA. Exploring reasons for very poor glycaemic control in patients with Type 2 diabetes. Prim Care Diabetes. 2011;5(4):251‐255. [DOI] [PubMed] [Google Scholar]
- 32. Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose‐lowering therapies and associations with 1‐year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White AJ, Kellar I, Prevost AT, et al. Adherence to hypoglycaemic medication among people with type 2 diabetes in primary care. Prim Care Diabetes. 2012;6(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 34. Levy J, Cobas RA, Gomes MB. Assessment of efficacy and tolerability of once‐daily extended release metformin in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2010;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donnelly LA, Morris AD, Pearson ER. Adherence in patients transferred from immediate release metformin to a sustained release formulation: a population‐based study. Diabetes Obes Metab. 2009;11(4):338‐342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from CPRD. Restrictions apply to the availability of these data, which were used under license for this study.


