Abstract.
Host seeking is an essential process in mosquito reproduction. Field releases of modified mosquitoes for population replacement rely on successful host seeking by female mosquitoes, but host-seeking ability is rarely tested in a realistic context. We tested the host-seeking ability of female Aedes aegypti mosquitoes using a semi-field system. Females with different Wolbachia infection types (wMel-, wAlbB-infected, and uninfected) or from different origins (laboratory and field) were released at one end of a semi-field cage and recaptured as they landed on human experimenters 15 m away. Mosquitoes from each population were then identified with molecular tools or through minimal dusting with fluorescent powder. Wolbachia-infected and uninfected populations had similar average durations to landing and overall recapture proportions, as did laboratory and field-sourced Ae. aegypti. These results indicate that the host-seeking ability of mosquitoes is not negatively affected by Wolbachia infection or long-term laboratory maintenance. This method provides an approach to study the host-seeking ability of mosquitoes in a realistic setting, which will be useful when evaluating strains of mosquitoes that are planned for releases into the field to suppress arbovirus transmission.
INTRODUCTION
The occurrence of arboviral diseases such as dengue, Zika, Japanese encephalitis, and West Nile fever is increasing, especially in tropical and subtropical areas.1–3 These viruses require blood-feeding mosquitoes to complete their life cycle,4 and an effective way to control their transmission is to suppress the vector mosquito populations. The sterile insect technique (SIT),5 incompatible insect technique (IIT),6 and the release of insects carrying a dominant lethal gene (RIDL)7 are promising noninsecticidal approaches, where wild-type females that mate with the released “modified” males have few viable offspring, decreasing the population size.
An alternative approach aims to decrease the ability of mosquitoes to transmit viruses by introducing endosymbiotic Wolbachia bacteria.8,9 Wolbachia are transmitted maternally and can invade natural populations through cytoplasmic incompatibility and/or direct fitness benefits.10,11 When introduced into mosquitoes from other insect species, some Wolbachia strains reduce their capacity to transmit viruses.8,12 Aedes aegypti mosquitoes infected with the wMel Wolbachia strain have been introduced into field populations, with the first releases taking place in Cairns, Australia, in 2011.13 In locations in Australia where Wolbachia have established, there have been no confirmed locally transmitted cases of dengue occurring within the release areas.14,15
Population replacement and suppression strategies ideally should be preceded by investigations to assess their potential for success, address safety concerns,16 and performing community engagement.14,17 When using Wolbachia to block arbovirus transmission, fitness costs18–20 due to Wolbachia infection and variation in cytoplasmic incompatibility21 and maternal transmission22 must be considered. Such effects mean that Wolbachia must exceed a threshold frequency to spread in natural populations.13,23,24 Sterile insect technique, IIT, and RIDL programs are simpler in that the only concern is male fitness but still require the released males to have a high competitiveness to ensure successful mating with wild females.25,26 Populations reared in the laboratory can adapt to the artificial conditions which may reduce field performance.27,28 For instance, laboratory maintenance can lead to the loss of pesticide resistance,29 greatly reducing fitness in release areas with heavy pesticide use.17
Fitness assays are usually carried out in the laboratory to detect fitness costs, but during releases, mosquitoes must locate hosts or mates under variable environmental conditions. Performance under laboratory conditions often does not translate into performance in the field.30–33 For example, males from the transgenic OX3604C strain of Ae. aegypti successfully suppressed laboratory populations34 but were much less effective under semi-field conditions because of a strong mating disadvantage.35 Successful host seeking is a key to population replacement programs because female mosquitoes require blood for reproduction. In a field context, host seeking relies on the detection of olfactory cues over a long range and heat, moisture, and visual contrast over a shorter distance, as well as flight ability.36–38
In this article, we tested the host-seeking ability of female Ae. aegypti using a semi-field cage in North Queensland, Australia,39 to simulate an outdoor setting. Females were released at one end of the semi-field cage and then recaptured by two experimenters seated at the other end. This method allows for a direct comparison of host-seeking ability between different mosquito strains in a common environment. To test the method, we compared mosquitoes with the wMel and wAlbB Wolbachia strains, which are now being released into the field in disease control programs,13,40 against uninfected counterparts. To evaluate whether laboratory adaptation could affect host seeking as demonstrated in laboratory experiments previously,41 we also compared a laboratory population with a population collected recently from the field.
MATERIAL AND METHODS
Mosquito strains and maintenance.
Aedes aegypti mosquitoes in this study were reared at 26–28°C in a controlled temperature room at James Cook University, Cairns, using methods described previously.42 We performed two sets of experiments to compare the effects of Wolbachia infection and laboratory maintenance on host-seeking ability, respectively. To test for the effects of Wolbachia infection, we used wMel- and wAlbB-infected Ae. aegypti derived from lines transinfected previously.8,43 The wMel population was collected from Cairns, Australia, in May 2013 from regions that had been invaded 2 years earlier,13,44 whereas the wAlbB population was crossed to an Australian background for at least six generations and maintained in the laboratory for at least 50 generations.45 The uninfected population for the experimental control was established from Ae. aegypti (Wolbachia-uninfected) eggs collected in Cairns, Queensland, Australia, in November 2015.46 Before the experiments, mosquitoes were screened using qPCR to confirm infection status, and females from all Wolbachia-infected lines were backcrossed for three generations to the uninfected males to obtain a similar genetic background.19
To test for the effects of laboratory maintenance, we compared the host-seeking ability of laboratory and field populations. The laboratory population was identical to the uninfected population described earlier and had been maintained in the laboratory for 27 generations. The field population of Ae. aegypti (Wolbachia-uninfected) was collected in September 2018 from the same location as the laboratory population and was a mix of the first and second laboratory generations at the time of experiments. All populations maintained in the laboratory were blood fed on human arms each generation, as approved by the Human Research Ethics Committee, James Cook University (approval H4907), and the University of Melbourne Human Ethics Committee (approval 0723847). All adult subjects provided informed consent (no children were involved).
For each release, the compared colonies were hatched synchronously, provided with TetraMin® fish food tablets (Tetra, Melle, Germany) ad libitum, and the larval density was controlled to 150 in 1 L water to ensure matched eclosion. After pupation, approximately 80 pupae were selected with a mix of 80% females and 20% males and left to emerge as adults in one cage (BugDorm-4M1515 Insect Rearing Cage; MegaView Science Co., Ltd., Taichung City, Taiwan). Each cage was provided with a cup of 10% sucrose and water and left for at least 4 days to allow females to mature and mate but not blood feed. One day before the release, sugar cups were removed with only water cups remaining to starve the females because sugar feeding may affect host-seeking behavior.47,48 The released females were 5-day-old in both the Wolbachia infection comparison and the laboratory maintenance comparison.
Release–recapture method.
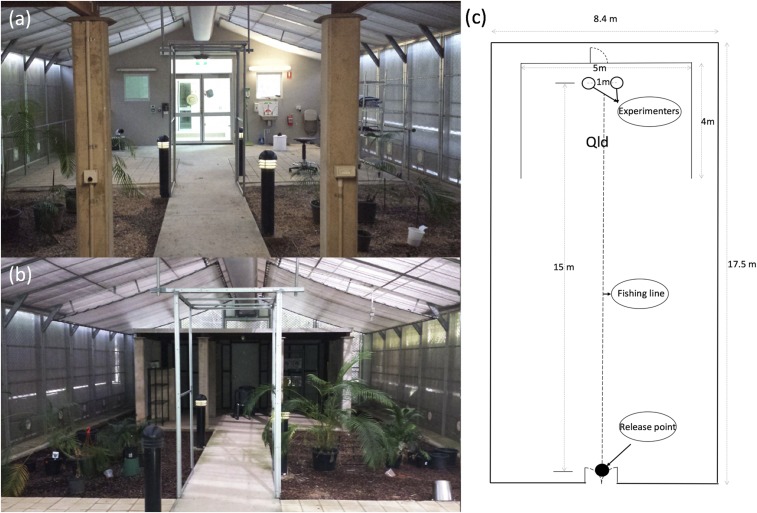
We used a semi-field system (17.5 × 8.4 m) at James Cook University, Cairns, Australia, containing soil, vegetation, a “Queenslander” house structure (Qld), and a ventilation system to match outside ambient temperatures to simulate natural conditions (Figure 1).39 Mosquitoes were released near the door side from a box with a mesh lid, whereas two experimenters were seated within the Qld structure to attract mosquitoes from the other end 15 m away (Figure 1). This distance was chosen because of the size limitations of the semi-field cage. Two temperature loggers (Thermochron; 1-Wire, iButton.com, Dallas Semiconductors, Sunnyvale, CA) were placed near the release point and two were placed under the Qld structure to monitor temperatures during experiments (Supplemental Table 1). All human subjects gave informed consent to participate in the semi-field experiments, which were approved by the JCU Institutional Biosafety Committee and Human Research Ethics Committee (ethics approval H4450).
Figure 1.
Interior of the semi-field cage. (A) View of the door from inside the Queenslander (Qld). (B) View of the Qld from the door. (C) Schematic diagram of the cage showing the release point and the location of two experimenters. This figure appears in color at www.ajtmh.org.
Females from all populations in the comparison were aspirated into a single release box (Supplemental Figure 1) and placed in the semi-field cage to acclimate for at least 30 minutes before experiments commenced. For the Wolbachia infection comparison, 50 uninfected, 50 wMel-infected, and 50 wAlbB-infected females were released into the box. For the laboratory maintenance comparison, 50 laboratory and 50 field source females were released. Females that were damaged during handling were replaced.
Two experimenters wore bug net mesh hats and long-sleeved shirts and shorts, exposing only their lower legs to restrict the area where mosquitoes could land. The same two experimenters undertook all experiments. Experimenters sat on the floor within the Qld structure 1 m apart (Figure 1) with an electronic timer, mechanical aspirators (Model 2809C; BioQuip Products, Inc., Rancho Dominguez, CA), and 15 collection vials nearby. The experiment commenced by pulling the fishing line to remove the mesh lid from the box to release the mosquitoes (Supplemental Figure 1), after which the timer was immediately started. Females landing on exposed skin were collected with mechanical aspirators as they landed. Collection vials were replaced with empty vials at 3-minute intervals until 42 minutes had elapsed. After 42 minutes, both experimenters moved to the opposite end of the cage to capture mosquitoes that did not land during the experiment. Collections occurred until no more mosquitoes were detected after a thorough search of the semi-field cage. Between experiments, two Biogents Sentinel traps (Biogents AG, Regensburg, Germany) were placed inside the semi-field cage to assist in the capture of any remaining mosquitoes. At least 1 hour before each experiment commenced, the experimenters searched the semi-field cage and used an electric mosquito swatter to kill any mosquitoes found. All mosquitoes collected or killed between experiments were discarded and excluded from the analyses.
Wolbachia infection comparison.
The host-seeking experiment was repeated seven times with 50 uninfected, 50 wMel-infected, and 50 wAlbB-infected females that were 5 days old. Females collected from each replicate and time interval were stored in absolute ethanol at 4°C for wing length measurements, DNA extraction, and Wolbachia screening. One replicate was discarded from other analyses because of the loss of samples during wing dissection. An additional experiment was performed with 20-day-old females with identical methods; this was repeated four times.
Field-collected Ae. aegypti are smaller and more variable in size than laboratory-reared Ae. aegypti.49 Because host-seeking females collected from the field in a previous experiment tended to be larger than non–host-seeking females,50 we tested whether host-seeking speed and successful host seeking within 42 minutes were affected by size. We measured the wing length of females from two experimental replicates to obtain an indication of their body size.51 Intact wings were dissected from individual females and fixed under a 10-mm circular coverslip (Menzel-Gläser, Braunschweig, Germany) using Hoyer’s solution52 for further observation and measurement with an NIS Elements BR imaging microscope (Nikon Instruments, Tokyo, Japan).20
DNA extraction and Wolbachia screening were conducted according to the methods of Lee et al.53 DNA from whole mosquitoes was extracted using 200 µL of 5% Chelex-100 resin (Bio-Rad Laboratories, Hercules, CA) and 3 μL of proteinase K (20 mg/mL, Bioline Australia Pty Ltd., Alexandria, Australia). Extractions were diluted by 1/10, pipetted into four positions of a 384-well plate, and amplified with mosquito-specific (mRpS6) primers, Ae. aegypti–specific (aRpS6) primers, Wolbachia wMel-specific (w1) primers, and Wolbachia wAlbB-specific (wAlbB) primers44,45,54,55 using a LightCycler 480 system (Roche Applied Science, Indianapolis, IN). Robust and similar amplification of mRpS6 and aRpS6 (within one cycle) was expected for each individual. Uninfected Ae. aegypti were expected to show no amplification and, therefore, no crossing point (Cp) value with both w1 and wAlbB primers. Aedes aegypti were classified as wMel infected when they exhibited no amplification with wAlbB primers, low Cp values (< 28), and a Tm within the expected range for w1 primers based on wMel-infected laboratory controls. The wAlbB-infected Ae. aegypti tested positive for wAlbB, mRpS6, and aRpS6 primers but also showed late amplification (Cp > 28) with w1 primers. Individuals were, therefore, classified as wAlbB infected when they exhibited a low Cp value (< 28) with wAlbB primers, a Tm within the expected range for wAlbB primers, and an amplification curve shape consistent with wAlbB-infected laboratory control values (Supplemental Figure 2). At least two consistent technical replicates were obtained for each individual.
Laboratory maintenance comparison.
In this experiment, laboratory and field populations were marked with different colors of fluorescent powder (DayGlo, Barnes Products Pty Ltd., Moorebank, Australia) before release because the two populations could not be distinguished by molecular assays. Orange, blue, and yellow colors were used and were cycled between replicates. To reduce potential negative effects of marking, we used a minimal but visually identifiable amount (Supplemental Figure 3) by weighing powder on a microbalance (Sartorius BP 210 D, Sartorius AG, Goettingen, Germany). One hour before the release, 50 females from each population were aspirated into two separate 70-mL specimen cups containing approximately 0.4 mg of fluorescent powder in different colors. The cups were shaken gently to coat the mosquitoes evenly in powder before placing them in the release box (Supplemental Figure 1). Recaptured females were killed by freezing at −20°C for 30 minutes and identified under a microscope using a ultraviolet flashlight. This experiment was repeated six times.
In the absence of molecular tools, visual marking is needed to distinguish between populations in the same experiment. However, overapplication of fluorescent powder may affect longevity and behavioral responses, with effects depending on the method and the color used for marking.56–58 We, therefore, compared the average landing times of uninfected, unmarked females from the Wolbachia comparison with the uninfected, marked females from the laboratory maintenance comparison.
Data analyses.
Data visualization and analysis of variance (ANOVA) were conducted using R studio (R Studio Inc., Boston, MA) with the packages Rmisc,59 plyr,60 and ggplot2.61 Mosquitoes were captured at 3-minute intervals and assigned a value based on the median time of each catching interval for average landing time calculations. We analyzed the proportion of mosquitoes caught within 3, 21, and 42 minutes as well as average landing time; those caught after 42 minutes were considered as not landing. We ran Shapiro–Wilk tests in IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL) to show that average landing times and proportions were normally distributed. Because we ran analyses on four variables in the same experiment, significance levels were adjusted with the Bonferroni method. A two-way ANOVA analyzed differences in average landing time of the mosquitoes and the proportion of females that landed at different time points by treating population as a fixed factor and experimental replicate as a random factor. Linear regression was used to test for an association between wing length and time to landing.
RESULTS
Wolbachia infection comparison.
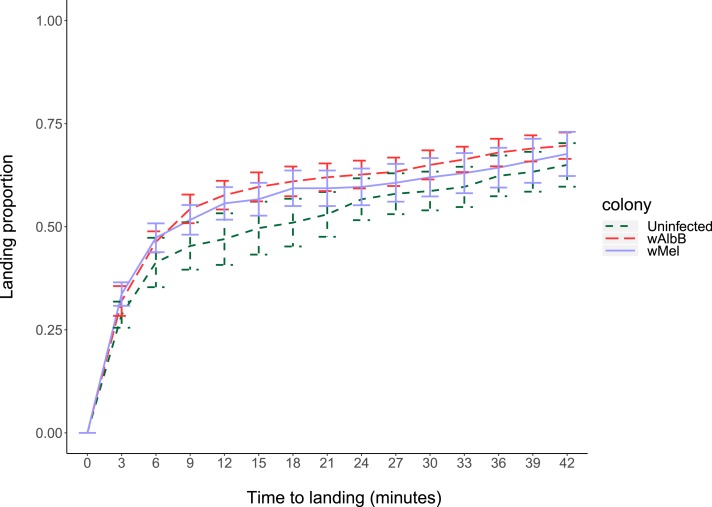
We compared the host-seeking ability of uninfected, wMel-infected, and wAlbB-infected females when released simultaneously in a semi-field cage. On average, more than 30% of the mosquitoes were captured during the first 3 minutes of the experiment, with approximately 70% landing over the course of 42 minutes (Figure 2). We compared the proportion of females from each population that had landed on humans at 3, 21, and 42 minutes as an indicator of host-seeking success. The wMel-infected females were quicker to land than uninfected females at the beginning of the experiment, with a higher proportion landing within the first 3 minutes (two-way ANOVA: F1,5 = 19.74, P = 0.007). However, there were no significant differences between the proportion of wMel-infected and uninfected females landing by 21 minutes or by the end of the experiment (all P > 0.05). There was no significant effect of experimental replicate for comparisons between wMel-infected and uninfected females except at 3 minutes (F1,5 = 27.67, P = 0.001). The wAlbB-infected females had a similar landing rate to uninfected females at 3 and 42 minutes (all P > 0.05) but had a significantly higher proportion landing at 21 minutes (F1,5 = 7.500, P = 0.04). In this comparison, there was also a significant effect of replicate at 42 minutes (F1,5 = 7.505, P = 0.023) but not at the other time points (all P > 0.05). Note that these replicate and population effects for the wAlbB comparison are no longer significant after Bonferroni correction.
Figure 2.
Host-seeking ability of 5-day-old wMel-infected, wAlbB-infected, and uninfected Aedes aegypti females in a semi-field cage. Cumulative landing proportions of females on human experimenters are shown across all replicates. Lines represent means and error bars represent standard errors. This figure appears in color at www.ajtmh.org.
The average time to landing of each population was used as an estimate of host-seeking speed. Average time to landing (Shapiro–Wilk test: uninfected: P = 0.989; wMel-infected: P = 0.501; and wAlbB-infected: P = 0.091) did not differ significantly between uninfected (mean ± SE: 9.5 ± 0.9 minutes), wMel-infected (7.6 ± 0.6 minutes), and wAlbB-infected (7.5 ± 0.3 minutes) females (two-way ANOVA: wMel: uninfected: F1,5 = 2.503, P = 0.174 and wAlbB: uninfected: F1,5 = 6.434, P = 0.052). There was also no significant effect of replicate on average time to landing in either comparison (wMel uninfected: F5,5 = 0.617, P = 0.696; wAlbB: uninfected: F5,5 = 2.009, P = 0.231).
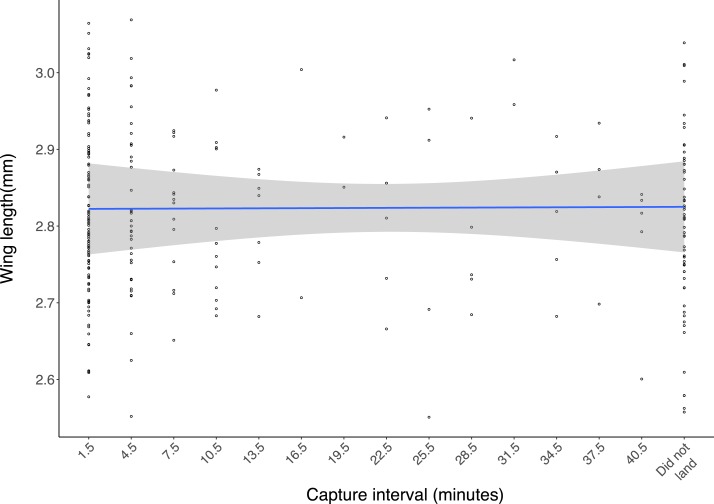
Females from two replicates of the Wolbachia infection comparison were measured for wing length (Figure 3). We found no relationship between wing length and time to landing through a linear model (R2 = 0.003, F1,264 = 0.863, P = 0.354). Females landing within the first 3 minutes (2.81 ± 0.02 mm, n = 102) did not differ in size from females collected after 42 minutes had elapsed (2.80 ± 0.03 mm, n = 57), indicating no difference in size between fast host-seeking females and non–host seekers (F1,80 = 1.311, P = 0.256).
We also ran experiments with wMel, wAlbB-infected, and uninfected Ae. aegypti females that were 20 days old and found no significant differences between populations (Supplemental Figure 4). Although mosquitoes of different ages were not compared in the same experiment, we found that 20-day-old females had slower average times to landing (two-way ANOVA: ages: F1,24 = 8.567, P = 0.007; colonies: F2,24 = 1.407, P = 0.264) but higher landing proportions (ages: F1,24 = 5.802, P = 0.024; colonies: F2,24 = 0.461, P = 0.636) than 5-day-old females by treating mosquito age and colony as fixed factors. Host-seeking ability may be influenced by mosquito age but direct comparisons between ages in the same experiment are needed to confirm this finding.
Laboratory adaptation comparison.
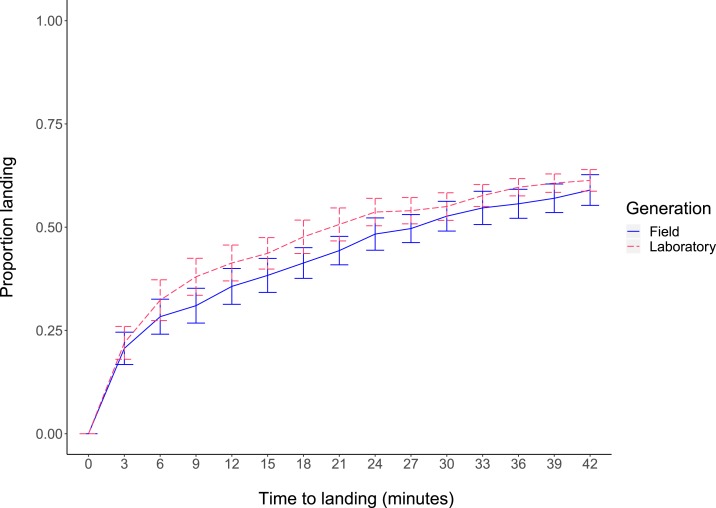
In comparisons of laboratory and field Ae. aegypti females, approximately 25% of released mosquitoes were caught within the first 3 minutes and 60% were caught over the duration of the experiments (Figure 4). Cumulative landing proportions at 3, 21, and 42 minutes did not differ between females from the laboratory and field populations (two-way ANOVA: 3 minutes: F1,5 = 0.122, P = 0.741; 21 minutes: F1,5 = 5.918, P = 0.059; 42 minutes: F1,5 = 0.745, P = 0.428). There was also no significant effect of replicate at any time point (all P > 0.05). The average time to landing (Shapiro–Wilk test: laboratory: P = 0.572; field: P = 0.332) did not differ significantly between field (mean ± SE: 12.3 ± 1.1 minutes) and laboratory (10.5 ± 1.3 minutes) females (two-way ANOVA: F1,5 = 2.346, P = 0.186, Figure 4B), with no significant effect of replicate (F5,5 = 2.876, P = 0.136). These results indicate that laboratory maintenance does not affect host-seeking ability.
Figure 4.
Host-seeking ability of field and laboratory Aedes aegypti females in a semi-field cage. Cumulative landing proportions of females on human experimenters are shown across all replicates. Lines represent means and error bars represent standard errors. This figure appears in color at www.ajtmh.org.
Figure 3.
Wing lengths of female Aedes aegypti collected during two replicates of the Wolbachia infection host-seeking experiment. Points represent wing lengths of individual females collected across each 3-minute interval of the experiment. Wing lengths of females captured concluded after the experiment are also presented. This figure appears in color at www.ajtmh.org.
We compared marked and unmarked females from the different experiments to test for effects of marking on host seeking. No significant differences were found between marked and unmarked uninfected laboratory females in terms of average arrival time (one-way ANOVA: F1,10 = 0.388, P = 0.547) and proportion landing at 42 minutes (F1,10 = 0.387, P = 0.548). Although the two sets of experiments were conducted at different times, the minimal amount of fluorescent powder used for marking does not appear to affect host-seeking ability.
Effects of experimental replicate and power analysis.
We observed effects of experimental replicate on host-seeking traits that could potentially be explained by environmental factors.62–64 We found no effect of temperature or time of releases (Supplemental Table 1) on average time to landing or proportion landing (Spearman’s rank correlation, P > 0.05), indicating that temperature and time of day did not substantially influence host seeking. We also ran a power analysis using an online calculator (http://powerandsamplesize.com/Calculators/Compare-2-Means/2-Sample-Equality) with a 80% power test using the average times and SDs of Wolbachia-infected and uninfected colonies. To detect a 20% difference in average time to landing, at least 15 experimental replicates would have been needed, whereas a difference of 30% could have been detected with six replicates.
DISCUSSION
Suppressing the transmission of dengue and other arboviruses by releasing Wolbachia-infected mosquitoes is becoming increasingly popular, with releases taking place in at least 12 countries (https://www.worldmosquitoprogram.org/; https://www.nea.gov.sg/corporate-functions/resources/research/wolbachia-aedes-mosquito-suppression-strategy/project-wolbachia-singapore; https://www.imr.gov.my/wolbachia/). For releases to succeed, the strain intended for deployment needs to have comparable fitness to wild-type mosquitoes, which should be tested before large-scale field release. The semi-field cage setting is widely used as an intermediate step between laboratory studies and open-field releases.65–67 Semi-field experiments have been used to test the mating success and invasive ability of Wolbachia infections8,68,69 and for evaluating the efficacy of novel mosquito traps and pesticides.67,70,71 Although host seeking is critical to the success of Wolbachia-based population replacement programs, the strains used in field releases have only been tested in laboratory studies72 and not in a realistic context.
We compared the host-seeking ability of female Ae. aegypti with different Wolbachia infection types and from laboratory and field origins in a semi-field cage. Our method was similar to the method developed by McMeniman et al.73 In their study, the host-seeking ability of wild-type and Gr3 mutant females lacking a response to CO2 was compared by releasing mosquitoes in the middle of the cage and leaving them to disperse naturally for 5 hours before the experiment. In our design, female mosquitoes were released simultaneously at a single release point 15 m away from the experimenters, thus standardizing the distance over which host seeking is tested. This is the first time that a semi-field approach has been used to evaluate the host-seeking ability of mosquitoes with Wolbachia strains intended for field deployment.
We found weakly significant differences between Ae. aegypti with different Wolbachia infection types on host-seeking ability, with higher landing rates of Wolbachia-infected females at an early time point in the experiment. However, overall landing proportions and average times to landing did not differ between populations. In general, females infected with the wMel or wAlbB strains should, therefore, not be at a disadvantage in terms of host seeking if released into the field. Although a study with a Puerto Rican Ae. aegypti population indicated that laboratory maintenance altered attraction to human odors,41 no significant differences were found in overall host seeking between laboratory and field populations in our semi-field experiments. Therefore, our laboratory maintenance protocol42 should not lead to compromised host-seeking ability in the field, although other factors that can coincide with laboratory maintenance such as inbreeding may reduce fitness.74 Different rearing procedures, such as the use of membrane feeders, nonhuman blood or small cages may also affect host-seeking ability if adaptation occurs.75
Many factors can influence mosquito attraction to humans, including environmental temperature and humidity, in addition to the CO2, skin emanations, body heat, and moisture of the host.62–64 Although we found no clear association between temperature or time of day and host seeking, environmental factors likely contribute to the observed variation between experimental replicates (Supplemental Tables 2 and 3, Supplemental Figure 5). This variability highlights the importance of comparing populations in a common environment at the same time.
In addition to studying the host-seeking ability of females, it may be possible to extend this method to male mosquitos. For SIT, IIT, and RIDL approaches, testing the competitiveness of males before the release is essential.25,33 A previous semi-field cage study showed that Wolbachia infection does not reduce the competitiveness of Ae. aegypti males.69 However, in nature, adult female densities will not be as high as in semi-field cage tests; males will typically locate and fly around a human host first before detecting female flight tones and initiating courtship behaviour.76–78 In a pilot experiment where we released males into the semi-field cage, we found that Ae. aegypti males exhibited a similar host-seeking response to females (Supplemental Figure 6), but this requires further testing.
In conclusion, we demonstrate that Wolbachia infection and long-term laboratory rearing have minimal effects on Ae. aegypti host-seeking ability in a semi-field cage. Comparisons of host-seeking ability using this approach will be informative when evaluating mosquito strains for field release. This method can also be used to compare other factors such as age and rearing conditions, which can help to better understand the host-seeking behavior of mosquitoes.
Supplemental tables and figures
Acknowledgments:
We thank Qiong Yang and Ashley Callahan of the Pest and Environmental Adaptation Research Group for LightCycler technical assistance and Edward Tsyrlin for helping with taking mosquito photos. We also thank Chris Paton, Michael Townsend, and Kyran Staunton in James Cook University for collecting the field population and providing space and facility support for mosquito rearing and semi-field cage experiments.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Gould EA, Higgs S, 2009. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 103: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter P, 2001. Climate change and mosquito-borne disease. Environ Health Perspect 109: 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, 2013. The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler DJ, 2002. The global emergence/resurgence of arboviral diseases as public health problems. Med Res Arch 33: 330–342. [DOI] [PubMed] [Google Scholar]
- 5.Benedict MQ, Robinson AS, 2003. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol 19: 349–355. [DOI] [PubMed] [Google Scholar]
- 6.Mains JW, Brelsfoard CL, Rose RI, Dobson SL, 2016. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci Rep 6: 33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, Oviedo MN, Lacroix R, Naish N, Morrison NI, 2012. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol 30: 828–830. [DOI] [PubMed] [Google Scholar]
- 8.Walker T, et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–453. [DOI] [PubMed] [Google Scholar]
- 9.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP, 2018. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog 14: e1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson SL, Fox CW, Jiggins FM, 2002. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc Lond B Biol Sci 269: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL, 2009. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann AA, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, Greenfield M, Durkan M, Leong Y, Dong Y, 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, Dong Y, Kenny N, Paton CJ, Ritchie SA, 2018. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie SA, 2018. Wolbachia and the near cessation of dengue outbreaks in northern Australia despite continued dengue importations via travellers. J Travel Med 25: tay084. [DOI] [PubMed] [Google Scholar]
- 16.Benedict M, D’Abbs P, Dobson S, Gottlieb M, Harrington L, Higgs S, James A, James S, Knols B, Lavery J, 2008. Guidance for contained field trials of vector mosquitoes engineered to contain a gene drive system: recommendations of a scientific working group. Vector Borne Zoonotic Dis 8: 127–166. [DOI] [PubMed] [Google Scholar]
- 17.de Azambuja Garcia G, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JBP, Petersen MT, Lourenço-de-Oliveira R, Shadbolt MF, Rašić G, 2019. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis 13: e0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang Y-F, O’Neill SL, 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. [DOI] [PubMed] [Google Scholar]
- 19.Yeap HL, et al. 2010. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross PA, Endersby NM, Hoffmann AA, 2016. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl Trop Dis 10: e0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross PA, Ritchie SA, Axford JK, Hoffmann AA, 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13: e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt TL, Filipovic I, Hoffmann AA, Rasic G, 2018. Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity (Edinb) 120: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock PA, White VL, Callahan AG, Godfray CH, Hoffmann AA, Ritchie SA, 2016. Density‐dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia. J Appl Ecol 53: 785–793. [Google Scholar]
- 24.Hu L, Tang M, Wu Z, Xi Z, Yu J, 2019. The threshold infection level for Wolbachia invasion in random environments. J Differ Equ 266: 4377–4393. [Google Scholar]
- 25.Chambers EW, Hapairai L, Peel BA, Bossin H, Dobson SL, 2011. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl Trop Dis 5: e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisen W, 2003. Lessons from the past: an overview of studies by the University of Maryland and the University of California, Berkeley. Dordrecht, The Netherlands: Springer. Ecological Aspects for Application of Genetically Modified Mosquitoes 2: 25–32. [Google Scholar]
- 27.Hoffmann AA, Ross PA, 2018. Rates and patterns of laboratory adaptation in (mostly) insects. J Econ Entomol 111: 501–509. [DOI] [PubMed] [Google Scholar]
- 28.Maclean H, Kristensen T, Sørensen J, Overgaard J, 2018. Laboratory maintenance does not alter ecological and physiological patterns among species: a Drosophila case study. J Econ Entomol 31: 530–542. [DOI] [PubMed] [Google Scholar]
- 29.Grossman MK, Uc-Puc V, Rodriguez J, Cutler DJ, Morran LT, Manrique-Saide P, Vazquez-Prokopec GM, 2018. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biol Lett 14: 20180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristensen TN, Hoffmann AA, Overgaard J, Sorensen JG, Hallas R, Loeschcke V, 2008. Costs and benefits of cold acclimation in field-released Drosophila. Proc Natl Acad Sci USA 105: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann AA, 2009. Drosophila and selection in nature: from laboratory fitness components to field assessments. van der Werf J, Graser H-U, Frankham R, Gondro C, eds. Adaptation and Fitness in Animal Populations: Evolutionary and Breeding Perspectives on Genetic Resource Management. Dordrecht, The Netherlands: Springer Netherlands, 169–182. [Google Scholar]
- 32.Calisi RM, Bentley GE, 2009. Lab and field experiments: are they the same animal? Horm Behav 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 33.Aldersley A, Pongsiri A, Bunmee K, Kijchalao U, Chittham W, Fansiri T, Pathawong N, Qureshi A, Harrington LC, Ponlawat A, 2019. Too “sexy” for the field? Paired measures of laboratory and semi-field performance highlight variability in the apparent mating fitness of Aedes aegypti transgenic strains. Parasit Vectors 12: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Valdez MRW, Nimmo D, Betz J, Gong H-F, James AA, Alphey L, Black WC, 2011. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci USA 108: 4772–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchinelli L, Valerio L, Ramsey JM, Gould F, Walsh RK, Bond G, Robert MA, Lloyd AL, James AA, Alphey L, 2013. Field cage studies and progressive evaluation of genetically-engineered mosquitoes. PLoS Negl Trop Dis 7: e2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dekker T, Geier M, Cardé RT, 2005. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol 208: 2963–2972. [DOI] [PubMed] [Google Scholar]
- 37.Cardé R, 2015. Multi-cue integration: how female mosquitoes locate a human host. Curr Biol 25: R793–R795. [DOI] [PubMed] [Google Scholar]
- 38.van Breugel F, Riffell J, Fairhall A, Dickinson MH, 2015. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol 25: 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritchie SA, Johnson PH, Freeman AJ, Odell RG, Graham N, Dejong PA, Standfield GW, Sale RW, O’Neill SL, 2011. A secure semi-field system for the study of Aedes aegypti. PLoS Negl Trop Dis 5: e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazni WA, et al. 2019. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark GG, Bernier UR, Allan SA, Kline DL, Golden FV, 2011. Changes in host-seeking behavior of Puerto Rican Aedes aegypti after colonization. J Med Entomol 48: 533–537. [DOI] [PubMed] [Google Scholar]
- 42.Ross PA, Axford JK, Richardson KM, Endersby-Harshman NM, Hoffmann AA, 2017. Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J Vis Exp 126: e56124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xi ZY, Khoo CCH, Dobson SL, 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, Montgomery B, Turley AP, O’Neill SL, 2014. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8: e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA, 2016. Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg 94: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA, 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13: e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dittmer J, Alafndi A, Gabrieli P, 2019. Fat body–specific vitellogenin expression regulates host-seeking behaviour in the mosquito Aedes albopictus. PLoS Biol 17: e3000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attardo GM, Hansen IA, Raikhel AS, 2005. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 35: 661–675. [DOI] [PubMed] [Google Scholar]
- 49.Yeap HL, Endersby NM, Johnson PH, Ritchie SA, Hoffmann AA, 2013. Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am J Trop Med Hyg 89: 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasci RS, 1986. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J Am Mosq Control Assoc 2: 61–62. [PubMed] [Google Scholar]
- 51.Briegel H, 1990. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol 36: 165–172. [Google Scholar]
- 52.Anderson LE, 1954. Hoyer’s solution as a rapid permanent mounting medium for bryophytes. Bryologist 57: 242–244. [Google Scholar]
- 53.Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM, 2012. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol 78: 4740–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DHT, Hoang NLT, Chau NVV, Iturbe-Ormaetxe I, Simmons CP, O’Neill SL, 2016. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog 12: e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou W, Rousset F, O’Neill S, 1998. Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B Biol Sci 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhulst NO, Loonen JA, Takken W, 2013. Advances in methods for colour marking of mosquitoes. Parasit Vectors 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickens BL, Brant HL, 2014. Effects of marking methods and fluorescent dusts on Aedes aegypti survival. Parasit Vectors 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagler JR, Jackson CG, 2001. Methods for marking insects: current techniques and future prospects. Annu Rev Entomol 46: 511–543. [DOI] [PubMed] [Google Scholar]
- 59.Hope RM, 2013. Rmisc: Ryan Miscellaneous. R Package Version 1.5. Available at: https://cran.r-project.org/web/packages/Rmisc/index.html. [Google Scholar]
- 60.Wickham H, 2009. plyr: tools for splitting, applying and combining data. R Package Version 0.1 9: 651. [Google Scholar]
- 61.Wickham H, 2016. ggplot2: Elegant Graphics for Data Analysis: New York, NY: Springer, 224. [Google Scholar]
- 62.Zwiebel L, Takken W, 2004. Olfactory regulation of mosquito–host interactions. Insect Biochem Mol Biol 34: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takken W, 1991. The role of olfaction in host-seeking of mosquitoes: a review. Int J Trop Insect Sci 12: 287–295. [Google Scholar]
- 64.Bowen M, 1991. The sensory physiology of host-seeking behavior in mosquitoes. Annu Rev Entomol 36: 139–158. [DOI] [PubMed] [Google Scholar]
- 65.Madakacherry O, Lees RS, Gilles JRL, 2014. Aedes albopictus (Skuse) males in laboratory and semi-field cages: release ratios and mating competitiveness. Acta Trop 132: S124–S129. [DOI] [PubMed] [Google Scholar]
- 66.Mancini MV, Spaccapelo R, Damiani C, Accoti A, Tallarita M, Petraglia E, Rossi P, Cappelli A, Capone A, Peruzzi G, 2016. Paratransgenesis to control malaria vectors: a semi-field pilot study. Parasit Vectors 9: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Darbro JM, Johnson PH, Thomas MB, Ritchie SA, Kay BH, Ryan PA, 2012. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am J Trop Med Hyg 86: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, Ritchie SA, Hoffmann AA, 2014. Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segoli M, Hoffmann AA, Lloyd J, Omodei GJ, Ritchie SA, 2014. The effect of virus-blocking Wolbachia on male competitiveness of the dengue vector mosquito, Aedes aegypti. Plos Negl Trop Dis 8: e3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Lima Santos ND, da Silva Paixão K, Napoleão TH, Trindade PB, Pinto MR, Coelho LC, Eiras ÁE, Navarro DM, Paiva PM, 2014. Evaluation of Moringa oleifera seed lectin in traps for the capture of Aedes aegypti eggs and adults under semi-field conditions. Parasitol Res 113: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 71.Johnson BJ, Ritchie SA, 2016. The Siren’s song: exploitation of female flight tones to passively capture male Aedes aegypti (Diptera: Culicidae). J Med Entomol 53: 245–248. [DOI] [PubMed] [Google Scholar]
- 72.Turley A, Smallegange R, Takken W, Zalucki M, O’neill S, McGraw E, 2014. Wolbachia infection does not alter attraction of the mosquito Aedes (Stegomyia) aegypti to human odours. Med Vet Entomol 28: 457–460. [DOI] [PubMed] [Google Scholar]
- 73.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB, 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156: 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross PA, Endersby‐Harshman NM, Hoffmann AA, 2019. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol Appl 12: 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross PA, Lau M-J, Hoffmann AA, 2019. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS One 14: e0224268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartberg W, 1971. Observations on the mating behaviour of Aedes aegypti in nature. Bull World Health Organ 45: 847–850. [PMC free article] [PubMed] [Google Scholar]
- 77.Stone CM, Tuten HC, Dobson SL, 2013. Determinants of male Aedes aegypti and Aedes polynesiensis (Diptera: Culicidae) response to sound: efficacy and considerations for use of sound traps in the field. J Med Entomol 50: 723–730. [DOI] [PubMed] [Google Scholar]
- 78.Cator LJ, Arthur BJ, Ponlawat A, Harrington LC, 2011. Behavioral observations and sound recordings of free-flight mating swarms of Ae. aegypti (Diptera: Culicidae) in Thailand. J Med Entomol 48: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.