Abstract
Background:
Opioid use disorder (OUD) is a significant public health problem for which a substantial amount of treatment exists. The degree to which methadone and buprenorphine are administered in different treatment modalities is not clear but critical to understanding treatment success rates and service development strategies.
Methods:
Data from the national Treatment Episode Dataset for Admissions and Discharges (TEDS-A[N=4,070,264] and TEDS-D [832,731], respectively) were used to determine the likelihood patients initiating detoxification and outpatient OUD treatment between 2006-2015 were expected to receive opioid agonist treatment. Joinpoint regression evaluated significant trends and a generalized linear model with logit link function identified characteristics associated with receiving an agonist during detoxification. TEDS-D informed the percent of patients leaving detoxification against medical advice who did/did not receive an opioid agonist.
Results:
Though agonist use in outpatient settings increased by 60% during 2012-2015, agonist use in detoxification was lower than outpatient treatment, decreased significantly by 26% from 2009-2015, and never exceeded 16% of detoxification admissions during 2006-2015. In 2015, persons who were under 25, homeless, had co-occurring psychiatric problems, utilized Medicare, Medicaid, or had no insurance, and had no prior OUD treatment or were high treatment utilizers were the least likely to receive an agonist during detoxification.
Conclusions:
Efforts to expand opioid agonist access has been successful for outpatient but not detoxification settings. Improving detoxification outcomes is a potentially high impact way for the US to expand efficacious OUD treatment access in the US.
Keywords: opioid, withdrawal, buprenorphine, methadone, TEDS
1. Introduction
Opioid use disorder (OUD) is a significant public health crisis that is leading to excessive rates of morbidity and mortality and directly decrementing overall life expectancy in the United States (Dowell et al., 2017) . Administration of an opioid agonist (i.e., methadone) or partial agonist (i.e., buprenorphine) is considered the gold standard method of treating OUD. Opioid agonist treatment is effective, in part, because it can suppress opioid withdrawal symptoms and the associated cravings that drive continued opioid misuse and abuse (Hutcheson, Everitt, Robbins, & Dickinson, 2001; Negus & Banks, 2018) . Opioid agonists can be administered chronically in a maintenance paradigm, or used to taper patients to abstinence in a detoxification paradigm. Patients who are able to achieve abstinence during a taper have the option of being maintained on the opioid antagonist naltrexone, which blunts the effects of exogenous opioids and serves as a relapse prevention medication.
Over the past decade, great efforts to increase opioid agonist treatment availability have been made (Larney & Hall, 2019), with the primary focus being the expansion of maintenance opportunities. This has been motivated, in part, by evidence that providers were not utilizing maintenance medications as frequently as expected (Huhn & Dunn, 2017; Stein et al., 2016; Thomas et al., 2017) , as well as data suggesting patients in maintenance were more likely to be retained in treatment relative to those who undergo detoxification (Fiellin et al., 2014). Evidence has also suggested that the risk of experiencing a fatal opioid-related overdose increases after detoxification (Degenhardt et al., 2011; Ravndal & Amundsen, 2010) , whereas maintenance has been associated with a complementary decreased risk of overdose (Schwartz et al., 2013). Yet, despite the momentum that has built around opioid maintenance treatments, detoxification remains a mainstay of the OUD treatment continuum, for several possible reasons. First, the scale of the opioid crisis continues to outpace maintenance treatment slots, and as a result more patients receiving treatment for OUD undergo detoxification than maintenance (Jones, Campopiano, Baldwin, & McCance-Katz, 2015). Second, detoxification is supported by a vast provider and payer infrastructure that is unlikely to change quickly and is highly prevalent in rural areas where maintenance options are the least available, making it the most feasible form of treatment for many individuals (Peterson, Xu, Mikosz, Florence, & Mack, 2018). Third, individuals who are seeking treatment for the first time may be unfamiliar with maintenance and preferentially seek out detoxification opportunities (Huhn, Tompkins, & Dunn, 2017). Finally, maintenance remains highly stigmatized in many regions (Wakeman & Rich, 2018), which may prompt patients to seek out detoxification for a briefer course of treatment.
There are significant ideological concerns about whether detoxification should be retained within the OUD treatment continuum, based largely on concerns of opioid relapse and overdose (Tetrault & Fiellin, 2018). However, there have been few direct comparisons of maintenance and detoxification outcomes and the data that do exist are largely from randomized controlled trials rather than real-world treatment settings. One study that randomly assigned patients to either maintenance or detoxification reported that detoxification participants were likely to leave treatment prematurely (Fiellin et al., 2014). Although informative, this randomized controlled comparison may not generalize directly into real-world heterogeneous treatment settings. For instance, evidence has suggested that patients are detoxified to abstinence (Chutuape, Jasinski, Fingerhood, & Stitzer, 2001) or prior to induction onto naltrexone for relapse prevention treatment (Lee et al., 2018; Tanum et al., 2017) are often able to abstain from opioids. Identifying why patients do poorly in real-world detoxification settings is critical to improving outcomes. This research would also support efforts to match patients to treatment type and determine the optimal allocation of treatment resources, particularly in resource-scare areas.
Data from randomized trials have repeatedly suggested that detoxifications that are conducted with methadone or buprenorphine produce results that are superior to detoxifications with non-opioid medications (i.e., clonidine) or placebo (Amato et al., 2013; Gowing et al., 2017). Therefore, this study sought to determine the likelihood that an individual entering detoxification or outpatient treatment for OUD would receive these standard-of-care medications, to determine whether medication access might contribute to the differential outcomes observed between real-world detoxification and outpatient treatments. This was accomplished by examining past ten-year trends in the U.S. Treatment Episode Dataset for Admissions (TEDS-A) for 2006-2015. Since persons entering detoxification were observed to have a low likelihood of receiving treatment with an opioid pharmacotherapy, data from the most recent year (2015) were further evaluated to determine whether any patient-level characteristics differentially predicted access to methadone or buprenorphine in that treatment context.
2. Methods
2.1. Data Source:
Data were taken from the publicly available Treatment Episode Dataset for Admissions (TEDS-A). The TEDS-A database is collected on an annual basis by the Substance Abuse and Mental Health Services Administration (SAMHSA). TEDS-A provides detailed reporting on individual, de-identified, patient-level demographic (e.g., age, sex, race), drug use (e.g., primary substance of abuse, route of administration), and treatment characteristics (e.g., service setting at admission, receiving medication-assisted treatment) from all admissions of persons aged 12 or older who were treated in a state-licensed or certified substance abuse treatment program that received public funds.
These analyses focused on TEDS-A data from 2006-2015. Although data from 2016 are available, the maintenance medication category could not be compared to previous years because naltrexone was collapsed with methadone and buprenorphine for the first time and it was not possible to distinguish between medication types for analyses. Evaluations were restricted to admissions for which an opioid (defined as either heroin, non-prescription methadone, or other opiates or synthetics, based upon TEDS definitions) was identified within the TEDS-A data as the primary substance of abuse, representing a total sample of 4,070,264 for the entire evaluated period. Treatment type was collapsed into two mutually exclusive groups that were conceptualized as representing “detoxification” (defined in TEDS as “Detox 24-hr Hospital Inpatient”; “Detox 24-hr Free-standing Residential”, or “Ambulatory Detoxification”; Total N=1,607,557) or “outpatient” (“Ambulatory Intensive Outpatient” or “Ambulatory Non-intensive Outpatient”, Total N= 2,462,707) treatment. TEDS-A also indicates whether the treatment facility planned to administer the opioid agonists methadone or buprenorphine to the patient as part of his or her treatment. Thus, admissions were further dichotomized based upon whether treatment with the opioid agonists methadone or buprenorphine was planned (yes/no).
2.3. Data Analysis:
Trends in planned opioid agonist utilization in detoxification settings between 2006-2015 were assessed using Joinpoint regression analyses (version 4.6.0) (Martinez-Beneito, García-Donato, & Salmerón, 2011; Yu, Barrett, Kim, & Feuer, 2007). Joinpoint regression was designed specifically to assess unique trends over time in cross-sectional data by optimizing the number of regression lines that best fit the shape of a curve (National Cancer Institute, Bethesda, MD). Results are reported here as the duration/percent change for the time periods when agonist use was increasing/decreasing in treatment settings, as informed by objective regression analyses, and were considered significant at p-trend <0.05 (Table 1; Figure 1). Given the low utilization of opioid agonists in detoxification settings, a generalized linear model with logit link function was also used to assess patient characteristics that impacted the likelihood of receiving an agonist medication during detoxification in the most recent year available (2015). Predictor variables included sex (female/male), age (25 and under, 26-54, 55 and older), race (white, black/African American, all other), homelessness (yes/no), co-occurring psychiatric problem (yes/no), prior treatment episodes (none, 1-3, 4 or more), and health insurance (private, Medicare, Medicaid, or none). Pairwise comparisons with Bonferroni correction were used to identify differences within categorical variables and results were considered significant at p<0.05.
Table 1.
Admission Rates as a Function of Treatment Type and Planned Opioid Agonist Treatment
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Admissions (Numbers) | ||||||||||
| Total | 286849 | 291589 | 322145 | 343175 | 344510 | 381008 | 360348 | 374032 | 392518 | 435463 |
| Outpatient | 165181 | 169417 | 195645 | 208156 | 215311 | 233453 | 220522 | 228285 | 244564 | 291676 |
| Detoxification | 121668 | 122172 | 126500 | 135019 | 129199 | 147555 | 139826 | 145747 | 147954 | 143787 |
| Opioid Agonist Planned | ||||||||||
| Total | 93319 | 92316 | 101524 | 104089 | 101700 | 104916 | 100920 | 104809 | 118927 | 171827 |
| Outpatient | 79874 | 78294 | 85892 | 84245 | 82758 | 86054 | 82749 | 87393 | 101364 | 156080 |
| Detoxification | 13445 | 14022 | 15632 | 19844 | 18942 | 18862 | 18171 | 17416 | 17563 | 15747 |
| Admissions (Percentages) | ||||||||||
| Outpatient | 57.6 | 58.1 | 60.7 | 60.7 | 62.5 | 61.3 | 61.2 | 61.0 | 62.3 | 67.0 |
| Detoxification | 42.4 | 41.9 | 39.3 | 39.3 | 37.5 | 38.7 | 38.8 | 39.0 | 37.7 | 33.0 |
| Opioid agonist planned, % treatment type | ||||||||||
| Outpatient | 48.4 | 46.2 | 43.9 | 40.5 | 38.4 | 36.9 | 37.5 | 38.3 | 41.4 | 53.5 |
| Detoxification | 11.1 | 11.5 | 12.4 | 14.7 | 14.7 | 12.8 | 13.0 | 11.9 | 11.9 | 11.0 |
| Opioid Agonist Planned, % of all admissions | ||||||||||
| Outpatient | 27.8 | 26.9 | 26.7 | 24.5 | 24.0 | 22.6 | 23.0 | 23.4 | 25.8 | 35.8 |
| Detoxification | 4.7 | 4.8 | 4.9 | 5.8 | 5.5 | 5.0 | 5.0 | 4.7 | 4.5 | 3.6 |
Admissions data based on Treatment Episode Dataset-Admissions. Data present number of admissions treated for primary heroin, non-prescribed methadone, or opiate/synthetics use disorder. Total represents all admissions treated in detoxification or outpatient settings. Detoxification represents 24-hr hospital-based, 24-free standing, or ambulatory detoxifications. Outpatient represents ambulatory non-intensive or intensive outpatient treatment. Opioid agonist treatment indicates whether methadone or buprenorphine was planned (TEDS-A) as part of treatment.
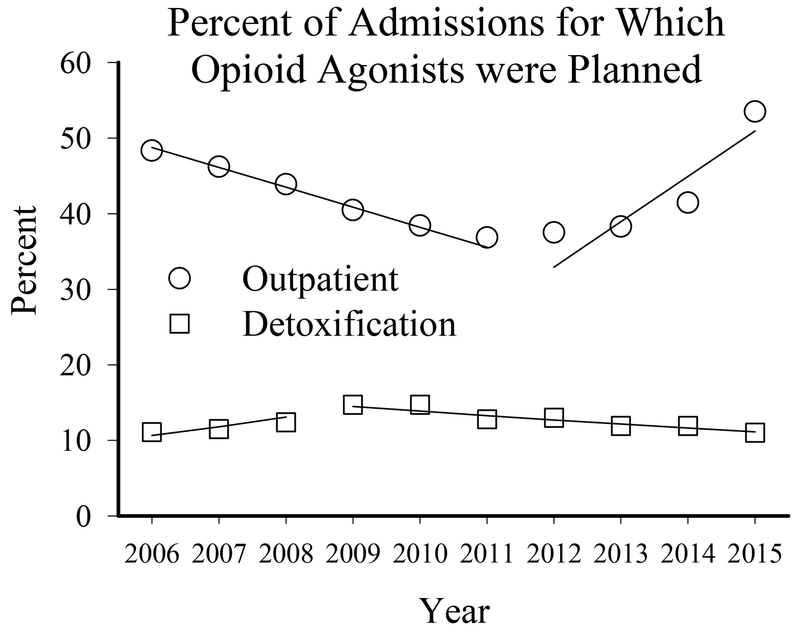
Figure 1.
Percent of persons admitted for primary dependence on heroin, non-prescribed methadone, or opiates/synthetics, as reported to the Treatment Episode Dataset-Admissions, for which treatment with the opioid agonists methadone or buprenorphine was planned as a function of outpatient (circles; TEDS categories “ambulatory non-intensive” or “intensive outpatient”) or detoxification (squares; TEDS categories “24-hr hospital based”, “free-standing”, or “ambulatory”) treatment, between 2006 and 2015. Lines represent significant joinpoint trends within each treatment domain over time.
3. Results
3.1. Participants:
Table 1 provides the number of admissions treated each year in detoxification or outpatient treatments for primary opioid use and for which opioid agonist treatment was planned.
3.2. TEDS-Admission Outcomes:
Patients were less likely to receive an opioid agonist in a detoxification versus outpatient treatment setting during every year examined (Figure 1; Table 1). Although opioid agonist use in detoxification settings increased by 34% between 2006 and 2009 (p-trend=0.007), it decreased by 26% between 2009 and 2015 (p-trend=0.002) and opioid agonist use during detoxification never exceeded 16% of all detoxification admissions or 5.8% of all treatment admissions during the entire evaluated period. In contrast, planned opioid agonist use decreased in outpatient settings by 31% from 2006 to 2012 (p-trend=0.001) and then increased by 60% from 2012 to 2015 (p-trend=0.004).
3.3. Correlates of Receiving an Agonist During Detoxification:
In 2015, 11.0% and 53.5% of admissions undergoing detoxification and outpatient treatment were expected to receive opioid agonists, respectively. As shown in Table 2, patients in detoxification had lower odds of receiving an opioid agonist if they were homeless (AOR=0.53, 95% CI, 0.47-0.59, p<0.001), had a co-occurring psychiatric problem (AOR=0.59, 95% CI, 0.53-0.63, p<0.001), had Medicaid (AOR=0.41, 95% CI, 0.36-0.47, p<0.001), Medicare (AOR=0.28, 95% CI, 0.24-0.37, p<0.001), or no insurance (AOR=0.39, 95% CI, 0.34-0.45, p<0.001) relative to private insurance, and or had no prior treatment episodes (AOR=0.63, 95% CI, 0.56-0.71, p<0.001) or ≥4 prior treatment episodes (AOR=0.56, 95% CI, 0.51-0.62, p<0.001) relative to persons with 1-3 prior treatment episodes. Patients in detoxification had higher odds of receiving an opioid agonist if they were aged 26-54 (AOR=1.19. 95% CI, 1.06-1.32, p=0.002) or 55 and older (AOR=1.51, 95% CI, 1.19-1.91, p=0.001), relative to patients 25 and under, and if they were white (AOR=2.82, 95% CI, 2.30-3.46, p<0.001) or black/African American (AOR=2.56, 95% CI, 2.01-3.30, p<0.001) relative to patients that were any other race.
Table 2.
Characteristics Associated with Planned Opioid Agonist Treatment (OAT) During Detoxification, 2015
| Admission Characteristics | OAT Planned (%) | No OAT Planned (%) | AOR | 95% CI | Wald Chi-square | p-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 10.4 | 89.6 | Ref | Ref | - | - |
| Female | 12.2 | 87.8 | 1.01 | 0.93-1.11 | 0.06 | 0.814 |
| Age | ||||||
| 25 and Under | 10.0 | 90.0 | Ref | Ref | - | - |
| 26-54 | 10.5 | 89.5 | 1.19* | 1.06-1.32 | 9.23 | 0.002 |
| 55 and Older | 21.0 | 79.0 | 1.51* | 1.19-1.91 | 11.42 | 0.001 |
| Race | ||||||
| White | 10.4 | 89.6 | 2.82* | 2.30-3.46 | 100.42 | <0.001 |
| Black/African American | 13.0 | 87.0 | 2.56* | 2.01-3.30 | 56.65 | <0.001 |
| All Other | 12.9 | 87.1 | Ref | Ref | - | - |
| Housing Status | ||||||
| Dependent/Independent | 12.3 | 87.7 | Ref | Ref | - | - |
| Homeless | 6.7 | 93.3 | 0.53* | 0.47-0.59 | 117.94 | <0.001 |
| Psychiatric Comorbidity | ||||||
| No | 17.5 | 82.5 | Ref | Ref | - | - |
| Yes | 12.4 | 87.6 | 0.59* | 0.53-0.63 | 159.52 | <0.001 |
| Prior Treatment Episodes | ||||||
| 0 | 17.1 | 82.9 | 0.63* | 0.56-0.71 | 58.41 | <0.001 |
| 1-3 | 18.4 | 81.6 | Ref | Ref | - | - |
| 4 or more | 10.3 | 89.7 | 0.56* | 0.51-0.62 | 141.34 | <0.001 |
| Insurance | ||||||
| Private Insurance | 21.6 | 78.4 | Ref | Ref | - | - |
| Medicaid | 8.7 | 91.3 | 0.41* | 0.36-0.47 | 179.24 | <0.001 |
| Medicare | 6.6 | 93.4 | 0.28*,a | 0.24-0.37 | 197.56 | <0.001 |
| No Insurance | 6.8 | 93.2 | 0.39* | 0.34-0.45 | 179.72 | <0.001 |
Results represent the percent of patients admitted to detoxification centers in the U.S. in 2015 for which methadone or buprenorphine was planned as part of their treatment regimen. Outcomes are presented according to Treatment Episode Data Set - Admissions (TEDS-A) response options. A generalized linear model with logit link function was used to assess patient characteristics that impacted the odds of having methadone or buprenorphine treatment planned as part of detoxification.
denotes significant AOR (p<0.05). AOR < 1.00 suggests patients had lower odds of receiving medication. 95% CI=95% Confidence Interval; AOR=adjusted odds ratio; OAT=opioid agonist treatment; Ref=reference variable within each category.
Significantly different from all other insurance types after within-category Bonferroni correction
4. Discussion
This brief report used the Treatment Episode Dataset to evaluate the degree to which the opioid agonists methadone and buprenorphine were planned for use in patients being admitted to either outpatient or detoxification treatment for OUD. Results highlighted significant disparities in the use of opioid agonists by these two types of OUD treatment programs. Specifically, between 2006 and 2015, treatment with an opioid agonist was unlikely for 85-89% of the patients undergoing detoxification for OUD. In addition, the likelihood that someone entering detoxification would be treated with an opioid agonist actually decreased between 2009 and 2015 despite a recognized increase in the rate of opioid misuse and patients entering OUD treatment during that period. The low levels of opioid agonist use in detoxification contrasts starkly with the fact that 46-62% of patients entering outpatient treatment for OUD were expected to receive opioid agonist treatment and the fact that agonist use in outpatient settings was observed to increase coincident with the growing opioid crisis over the past several years. These upward trends are consistent with the efforts that have been made to increase maintenance availability (Jones et al., 2015) , though it is clear they have also not yet met the scale of treatment needed (Larney & Hall, 2019) .
The fact that methadone and buprenorphine have not been well integrated into detoxification settings at a level comparable to outpatient treatments may be one reason that patients leave detoxification settings more frequently than outpatient treatments. Given that the US is in the midst of an opioid crisis that has reached the level of a national emergency (Gostin, Hodge, & Noe, 2017) and originates from a highly complex and heterogeneous array of circumstances that make addressing it a slow process (Dasgupta, Beletsky, & Ciccarone, 2018; Gallagher, 2018) , all efforts to improve outcomes from existing treatment infrastructures should be examined.
To our knowledge, this is the first study to show that efforts to increase adoption of opioid agonist treatments in the US that have been working for outpatient settings may not have been adequately targeting detoxification settings. The discrepancy in agonist treatment between detoxification and outpatient treatments may reflect differences in treatment ideologies, and/or geographic differences in agonist access and resources for prescribing physicians (Abraham et al., 2017; Rosenblatt, Andrilla, Catlin, & Larson, 2015; Wickramatilake et al., 2017) . It is also possible that providers shifted their treatment approach from detoxification to extended outpatient strategies as they became waivered to prescribe buprenorphine. The present evaluation of characteristics that predicted agonist access during detoxification in 2015 suggested that persons who were homeless, had co-occurring psychiatric conditions, were being treated for OUD for the first time, were high treatment utilizers, were older than 25 years of age, were white or black/African American, and were receiving Medicare, Medicaid, or no insurance were particularly disadvantaged with regard to agonist treatment. Public health efforts focused on the expansion of opioid agonist treatment in outpatient/maintenance settings should expand to include detoxification settings, as evidence suggests that detoxification with an opioid agonist results in greater treatment induction and retention relative to non-opioid comparators (Amato et al., 2013; Gowing et al., 2017).
These data are limited by their correlational nature and goal of providing brief overviews of the opioid treatment system rather than in-depth patient-level analyses; it is possible that results may vary as a function of specific demographic, drug use, or geographic characteristics in important but undetermined ways. Nevertheless, the rate of opioid agonist adoption among detoxification settings has not yet been well-delineated, and these data highlight an important opportunity for treatment expansion that can be targeted to help combat the existing opioid epidemic.
In summary, detoxification treatment programs are a primary part of the OUD care continuum and there is increasing use of this modality of service in recent years. Efforts to understand the lack of agonist use and increase such use during detoxification are needed. Improving detoxification outcomes is a potentially high impact and efficient way to expand efficacious OUD treatment access in the U.S.
References
- Abraham AJ, Andrews CM, Grogan CM, Pollack HA, D’Aunno T, Humphreys K, et al. (2017). State-targeted funding and technical assistance to increase access to medication treatment for opioid use disorder. Psychiatric Services, 69(4), 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Ferri MM, & Ali R (2003). Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews, (2). CD003409. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, & Stitzer ML (2001). One-, three-, and six-month outcomes after brief inpatient opioid detoxification. The American Journal of Drug and Alcohol Abuse, 27(1), 19–44. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Beletsky L, & Ciccarone D (2018). Opioid crisis: No easy fix to its social and economic determinants. American Journal of Public Health, 108(2), 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. (2011). Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction (Abingdon, England), 106(1), 32–51. [DOI] [PubMed] [Google Scholar]
- Dowell D, Arias E, Kochanek K, Anderson R, Guy GP, Losby JL, et al. (2017). Contribution of opioid-involved poisoning to the change in life expectancy in the united states, 2000-2015. Jama, 318(11), 1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, & O’Connor PG (2014). Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: A randomized clinical trial. JAMA Internal Medicine, 174(12), 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R (2018). Opioid-related harms: Simplistic solutions to the crisis ineffective and cause collateral damage. Health Services Insights, 11, 1178632918813321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin LO, Hodge JG, & Noe SA (2017). Reframing the opioid epidemic as a national emergency. Jama, 318(16), 1539–1540. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM, & Mbewe D (2017). Buprenorphine for managing opioid withdrawal. Cochrane Database of Systematic Reviews, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, & Dunn KE (2017). Why aren’t physicians prescribing more buprenorphine? Journal of Substance Abuse Treatment, 78, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Tompkins DA, & Dunn KE (2017). The relationship between treatment accessibility and preference amongst out-of-treatment individuals who engage in non-medical prescription opioid use. Drug and Alcohol Dependence, 180, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson D, Everitt B, Robbins T, & Dickinson A (2001). The role of withdrawal in heroin addiction: Enhances reward or promotes avoidance? Nature Neuroscience, 4(9), 943. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E (2015). National and state treatment need and capacity for opioid agonist medication-assisted treatment. Journal Information, 105(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larney S, & Hall W (2019). A major expansion of opioid agonist treatment is needed to reduce overdose deaths in the USA. The Lancet Public Health, [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): A multicentre, open-label, randomised controlled trial. The Lancet, 391(10118), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Beneito MA, García-Donato G, & Salmerón D (2011). A bayesian joinpoint regression model with an unknown number of break-points. The Annals of Applied Statistics, 5(3), 2150–2168. [Google Scholar]
- Negus SS, & Banks ML (2018). Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development. Pharmacology Biochemistry and Behavior, 164, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Xu L, Mikosz CA, Florence C, & Mack KA (2018). US hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011–2015. Journal of Substance Abuse Treatment, 92, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravndal E, & Amundsen EJ (2010). Mortality among drug users after discharge from inpatient treatment: An 8-year prospective study. Drug and Alcohol Dependence, 108(1-2), 65–69. [DOI] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CH, Catlin M, & Larson EH (2015). Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of Family Medicine, 13(1), 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, O’Grady KE, Sharfstein JM, Warren G, Olsen Y, et al. (2013). Opioid agonist treatments and heroin overdose deaths in baltimore, maryland, 1995-2009. American Journal of Public Health, 103(5), 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Sorbero M, Dick AW, Pacula RL, Burns RM, & Gordon AJ (2016). Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. Jama, 316(11), 1211–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Benth JŠ, Opheim A, Sharma-Haase K, Krajci P, et al. (2017). Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry, 74(12), 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, & Fiellin DA (2018). More beds or more chairs? using a science-based approach to address the opioid epidemic. Annals of Internal Medicine, 168(1), 73–74. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Doyle E, Kreiner PW, Jones CM, Dubenitz J, Horan A, et al. (2017). Prescribing patterns of buprenorphine waivered physicians. Drug and Alcohol Dependence, 181, 213–218. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, & Rich JD (2018). Barriers to medications for addiction treatment: How stigma kills. Substance use & Misuse, 53(2), 330–333. [DOI] [PubMed] [Google Scholar]
- Wickramatilake S, Zur J, Mulvaney-Day N, Klimo M. C. v., Selmi E, & Harwood H (2017). How states are tackling the opioid crisis. Public Health Reports, 132(2), 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Barrett MJ, Kim H, & Feuer EJ (2007). Estimating joinpoints in continuous time scale for multiple change-point models. Computational Statistics & Data Analysis, 51(5), 2420–2427. [Google Scholar]