Abstract
Background
Recent approval and adoption of pangenotypic direct acting antivirals (DAAs) necessitated a revision of the 2015 World Health Organization guidelines for the management of persons with hepatitis C virus (HCV) infection.
Methods
We searched MEDLINE, EMBASE, CENTRAL, and relevant conference proceedings to identify randomized and non-randomized trials, as well as prospective observational studies of DAAs. The proportions of persons with events were pooled for sustained virological response at 12 weeks post-treatment (SVR12), discontinuations due to adverse events (DAEs), serious adverse events (SAEs), and all-cause mortality. Analyses were stratified by HCV genotype and antiviral treatment experience, with subgroup analyses based on presence of cirrhosis and HIV-HCV coinfection.
Findings
The evidence base consisted of 238 publications describing 142 studies. In the overall analysis, which included all persons irrespective of treatment experience or comorbidities, the pooled proportion achieving SVR12 exceeded 0.94 for all pangenotypic regimens across genotypes 1, 2, and 4. Some heterogeneity may have led to lower SVR rates in persons with genotype 3 infection. High SVR12 (>0.90) was observed in persons with genotype 1 infection with cirrhosis, though evidence varied and was limited for genotypes 2–4. Evidence was sparse for persons with HIV–HCV coinfection. All regimens were associated with small proportions of persons with DAEs, SAEs, or all-cause mortality.
Interpretation
Based on this and other supporting evidence, the WHO issued updated guidelines with a conditional recommendation, based on moderate quality evidence, for the use of pangenotypic DAA regimens for persons with chronic HCV infection aged 18 years and older (July 2018).
Funding
This study was funded by the World Health Organization.
Keywords: Direct-acting antivirals, Hepatitis C, Pangenotypic, SVR12, Systematic review
Research in context.
Evidence before this study
All-oral, direct-acting antiviral (DAA) regimens for chronic hepatitis C virus (HCV) infection are associated with higher rates of virological cure (sustained virological response) and are better tolerated than older, interferon-based antiviral therapies.
Added value of this study
To support the World Health Organization (WHO) guidelines for the care and treatment of persons with chronic HCV infection, we present a comprehensive summary of the evidence on the pangenotypic regimens sofosbuvir-velpatasvir, sofosbuvir-daclatasvir, and glecaprevir-pibrentasvir as well as sofosbuvir-ledipasvir. Pooled analyses of trials and observational studies demonstrate high proportions of persons achieving SVR12 across genotypes 1 through 4. Findings were generally consistent across subgroups, including treatment-naïve and treatment-experienced persons, persons with cirrhosis, and persons with HIV–HCV coinfection, though evidence in some subgroups was limited. The proportions of persons with serious adverse events, treatment discontinuation due to adverse events, and all-cause mortality, were very low across treatments.
Implications of all the available evidence
The interventions recommended in the WHO's July 2018 guidelines have demonstrated high efficacy across genotypes with favourable harms profiles. Widespread adoption of pangenotypic regimens for the treatment of chronic HCV infection will simplify administration and treatment. Simple oral administrations and short treatment durations will further enhance retention and the effectiveness of public health programmes. This new era of pangenotypic DAAs is a welcome addition to the global strategy to combat HCV infection.
Alt-text: Unlabelled box
1. Introduction
In 2017, the World Health Organization established a target to eliminate chronic hepatitis C virus (HCV) infection by the year 2030. Indeed, a recent mathematical model suggests that by focusing public health programs on preventing infection in persons who do not inject drugs, providing harm reduction services to persons who inject drugs, and expanding HCV diagnosis services and treatments to 90% of infected persons, the global elimination goal is achievable by 2032 [1]. Nonetheless, challenges persist. Political barriers will need to be overcome while securing funding from national and international public health sources. This is complicated by diminishing investments in global health funding and trends toward universal health coverage and away from disease-specific programming [2]. Yet, several countries, such as Brazil and Australia, have developed innovative approaches to funding HCV programs [3,4].
Prior to 2014, HCV treatment centred on the use of interferon-based regimens with generally low cure rates, long durations of therapy, and substantial toxicity. The introduction of highly effective and well-tolerated short course oral direct-acting antiviral (DAA) therapy without interferon that can cure HCV infection with high rates of sustained virological response (SVR) within weeks transformed the treatment landscape. In 2016, the World Health Organization (WHO) updated its guidelines for the screening, care, and treatment of persons with HCV infection to recommend DAA-based regimens in place of IFN-based regimens. Since the publication of the 2016 guidelines, DAA regimens that do not require ribavirin have continued to improve and several pangenotypic regimens, which successfully resolve HCV infection in over 85% of treated individuals across all six major genotypes, were approved by regulatory bodies including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Successful resolution of chronic HCV infection is defined by the WHO, the European Association for the Study of the Liver (EASL), the US Centres for Disease Control and Prevention (CDC), and the American Association for the Study of Liver Diseases (AASLD) as SVR at 12 weeks following the end of treatment (SVR12). This outcome is associated with very low rates of virological relapse. Currently, the pangenotypic DAAs glecaprevir-pibrentasvir (8 week course), sofosbuvir-daclatasvir (12 week course), and sofosbuvir-velpatasvir (12 week course) are approved in most markets for the treatment of HCV-infected persons without cirrhosis.
The emergence of pangenotypic regimens presents new opportunities for the public health response to HCV infection, with simplified procurement, an omission of resource-intensive genotyping, and no need for frequent laboratory monitoring. Thus, the objective of this review was to identify and synthesize the evidence for the efficacy and safety of DAA regimens in adults with chronic HCV infection. Here, we present a subsection of the systematic literature review commissioned to support the WHO's Guidelines Development Group (GDG) in formulating the updated July 2018 guidelines [5]. The complete technical report has been published previously [6].
2. Methods
This systematic literature review and meta-analysis was conducted in accordance with PRISMA guidelines [7,8].
2.1. Search strategy and selection criteria
Systematic searches were conducted in MEDLINE, EMBASE, and the Cochrane Register of Controlled Trials (CENTRAL) to identify randomized controlled trials, non-randomized trials, and prospective observational studies of adults with chronic HCV infection published in English from March 2015 to July 2017. Conference proceedings from Digestive Diseases Week (DDW), the AASLD, and EASL were hand-searched. Studies included in a previous systematic literature review, commissioned by the WHO to support the April 2016 guidelines, were assessed for eligibility in the current review [9].
As the updated WHO guidelines recommend treatment with pangenotypic regimens sofosbuvir-velpatasvir, sofosbuvir-daclatasvir, glecaprevir-pibrentasvir, these regimens are the focus of this paper. We also describe the evidence for sofosbuvir-ledipasvir, a non-pangenotypic regimen commonly used in regions where only a single genotype is dominant. Given their high prevalence, this review focuses on persons with genotype 1–4 infection.
2.2. Data extraction and outcomes
All titles and abstracts, as well as the full text publications of included abstracts, were screened independently and in duplicate by two reviewers (MZ, AS). Data extraction of study characteristics and outcomes of included studies were performed independently and in duplicate by at least two reviewers (MZ, AS, RM, DZ). Outcomes extracted included the proportion of persons achieving SVR12 as well as the proportion of persons with serious adverse events (SAEs), discontinuations due to adverse events (DAEs), and all-cause mortality.
2.3. Data analysis
For each outcome, untransformed proportions of persons with events of interest were pooled to generate a point estimate with 95% confidence interval using the DerSimonian-Laird method (binary random effects model). A 0.5 correction was applied to zero-count cells [10]. Analyses were performed in OpenMeta[analyst] based on the Metafor package [11,12].
The primary analysis included all persons irrespective of treatment experience and comorbidities (all-comer analysis). Analyses were additionally stratified by treatment experience (treatment-experienced and treatment-naïve) where persons were considered treatment-experienced if they had received any prior HCV intervention, including interferon-based regimens and/or DAAs, or were classified as non-responders. Separate analyses, specified a priori, present the evidence for subgroups of persons with cirrhosis and for persons with HIV–HCV coinfection. We present analyses which include evidence from all eligible study designs.
2.4. Critical appraisal and grade
The validity of randomized trials was assessed by the Risk of Bias instrument, endorsed by the Cochrane collaboration [13]. Studies of other designs, including ‘single-arm’ trials, cohort studies, and observational studies, were evaluated using the Tool to Assess the Risk of Bias in Cohort Studies, developed by the CLARITY group at McMaster University [14].
The Grading Recommendations of Assessment, Development and Evaluation (GRADE) approach was used to assign a rating of high, moderate, low, or very low, to reflect the certainty of evidence for each outcome [15], [16], [17], [18], [19], [20]. The traditional approach was modified to suit the unique clinical and methodological characteristics of HCV research in the context of this review and to reflect the analysis approach, which combined both trial and non-trial evidence (Appendix A).
2.5. Role of the funding source
The study sponsor (World Health Organization) assisted in the conception and design of this review. However, the corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
3. Results
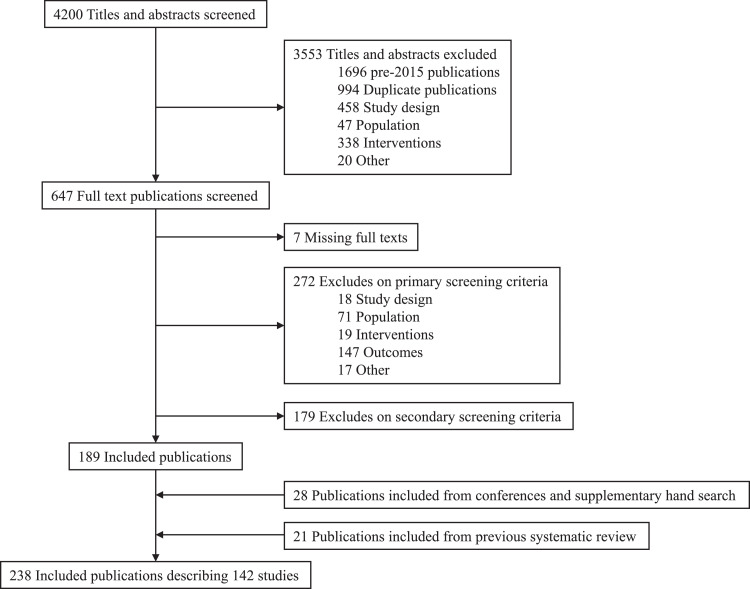
A summary of the complete review process is illustrated in Fig. 1. From the initial 4200 publications identified from the systematic searches of the bibliographic databases, 3553 publications were excluded at the abstract-screening phase with a further 458 publications excluded during full-text screening. From the review of conference proceedings and additional supplementary hand searching, 28 publications were identified and included. An additional 21 publications were retrieved from the 2016 WHO review. Thus, the complete evidence base consisted of 238 publications describing 142 unique studies. Here we summarize the evidence from 63 studies (109 publications [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129]) reporting efficacy or effectiveness outcomes for the pangenotypic regimens as well as sofosbuvir-ledipasvir.
Fig. 1.
Study selection.
Outcomes of interest in persons treated with sofosbuvir-velpatasvir, sofosbuvir-daclatasvir, glecaprevir-pibrentasvir, and sofosbuvir-ledipasvir were reported in 10, 20, 10, and 40 studies, respectively. This evidence consisted of 51 studies classified as either a randomized or other trial design (e.g. single arm trial) and 23 observational cohort or database studies. Available evidence varied by subgroup, with 61 studies reporting outcomes for persons in the all-comer analysis, 22 studies with evidence for persons with cirrhosis, and six studies for persons with HIV-HCV co-infection. Most studies consisted of mixed samples of treatment-naïve and treatment-experienced persons (n = 52, 70.3%), with 13 (17.6%) studies enrolling only persons who had no prior treatment experience, six (8.1%) studies enrolling only persons with treatment experience, and three (4.1%) studies where treatment history was unclear or not reported. Most studies were multinational with several countries represented, including the USA (n = 31, 41.9%), France (n = 13, 17.6%), Canada (n = 10, 13.5%), and Japan (n = 9, 12.2%). The majority of studies (63.5%) reported receiving at least some industry funding. Study characteristics and outcomes were only available from a conference abstract in 15 cases.
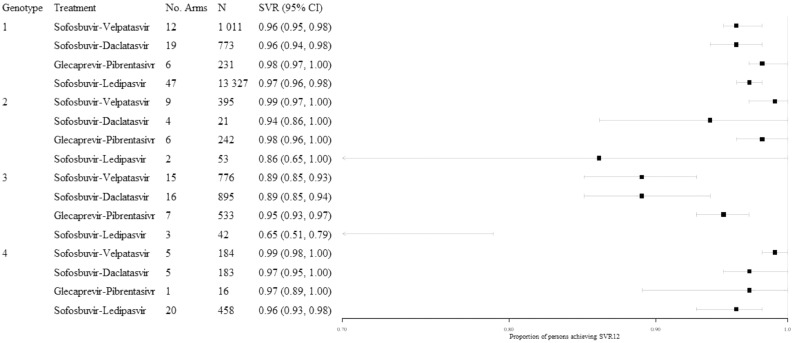
3.1. SVR12 in the all-comer population
In the all-comer analysis, which included persons irrespective of cirrhosis status or comorbidities, high SVR12 rates were observed across both treatments and genotypes, commonly with narrow confidence intervals (Fig. 2). Point estimates of pooled SVR12 proportions exceeded 0.94 for all treatments for persons with genotypes 1, 2, or 4 infection, with the exception of persons with genotype 2 infection treated with sofosbuvir-ledipasvir (0.86; 95% CI: 0.65, 1.00; N = 53). This estimate was from a single non-randomized comparative trial of sofosbuvir-ledipasvir administered for either 8 (n = 27) or 12 (n = 26) weeks, where 74% of persons treated for 8 weeks achieved SVR12 [54]. Outcomes in persons with genotype 3 HCV infection were typically characterized by wider confidence intervals, exceeding 0.05 for the pooled SVR12 proportions for sofosbuvir-velpatasvir (0.89; 95% CI: 0.85, 0.93; N = 776), sofosbuvir-daclatasvir (0.89; 95% CI: 0.85, 0.94; N = 895) and sofosbuvir-ledipasvir (0.65; 95% CI: 0.51, 0.79; N = 42). The number of persons included in the analyses by treatment varied from a high of 13 327 persons with genotype 1 infection across 47 study arms for sofosbuvir-ledipasvir to a single arm of 16 persons with genotype 4 infection treated with glecaprevir-pibrentasvir.
Fig. 2.
Illustration of findings from the all-comer analyses, stratified by genotype, all treatment experience.
In the analyses stratified by treatment experience (Table B1), the proportions of persons with genotypes 1 or 3 infection achieving SVR12 were consistent with the primary analyses. Limited or no evidence was available for persons with genotypes 2 or 4 infection, with the exception of sofosbuvir-ledipasvir in persons with genotype 4 infection which was consistent with the primary analysis.
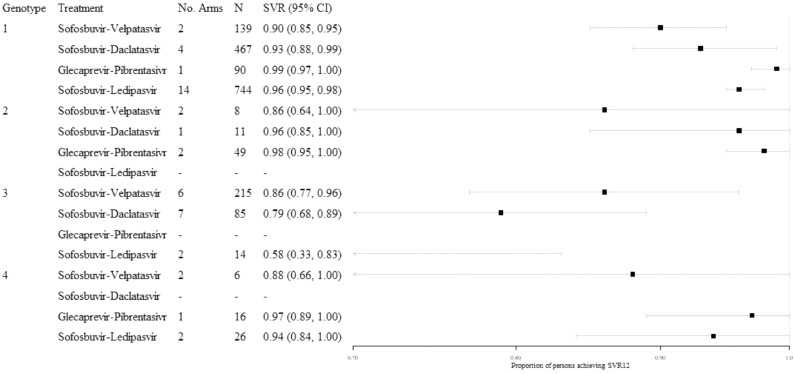
3.2. SVR12 in persons with cirrhosis
Most evidence for persons with cirrhosis came from studies of genotype 1 infection (Fig. 3). In this subpopulation, pooled SVR12 estimates exceeded 90% for all treatments, though the width of confidence intervals varied. Small pooled sample sizes were available for persons with genotype 2 or 4 infection, though the proportion of persons achieving SVR12 remained high.
Fig. 3.
Illustration of findings from the analyses of persons with cirrhosis, stratified by genotype, all treatment experience.
The proportion of persons achieving SVR12 was consistent across treatment-experience subgroups (Table B2) for sofosbuvir-ledipasvir in genotype 1 infection (treatment-experienced: 0.97 [95% CI: 0.95, 1.00], N = 175; treatment-naïve: 0.97 [95% CI: 0.93, 1.00], N = 78). For persons with genotype 3 infection, the proportion treated with sofosbuvir-velpatasvir achieving SVR12 was high in both the treatment-experienced and treatment-naïve stratifications (treatment-experienced: 0.90 [95% CI: 0.83, 0.97], N = 69; treatment-naïve: 0.97 [95% CI: 0.92, 1.00], N = 120). However, evidence by treatment experience was otherwise limited or unavailable.
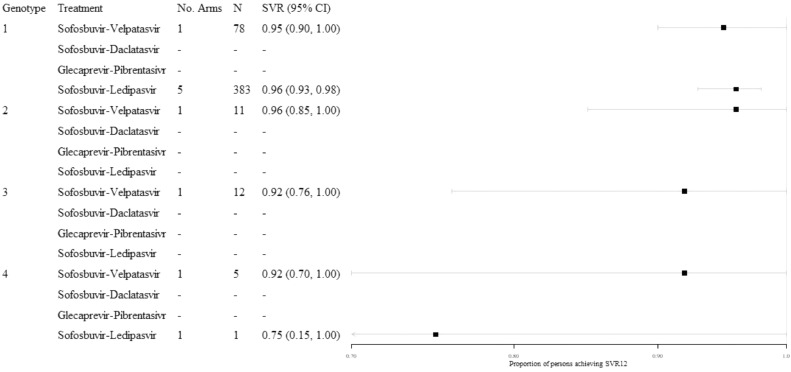
3.3. SVR12 in persons with HIV–HCV coinfection
Limited evidence was identified for persons with HIV–HCV coinfection (Fig. 4). Outcomes for persons with HCV genotype 1 infection treated with sofosbuvir-ledipasvir were the best represented in this subgroup, with a high pooled proportion achieving SVR12 (0.96; 95% CI: 0.93, 0.98; n = 383). Outcomes for sofosbuvir-velpatasvir were available from the ASTRAL-5 trial, where high SVR12 rates were observed across genotypes, though with small sample sizes. Given the limited evidence identified for this subgroup, analyses were not stratified by treatment-experience.
Fig. 4.
Illustration of findings from the analyses of persons with HIV–HCV coinfection, stratified by genotype, all treatment-experience.
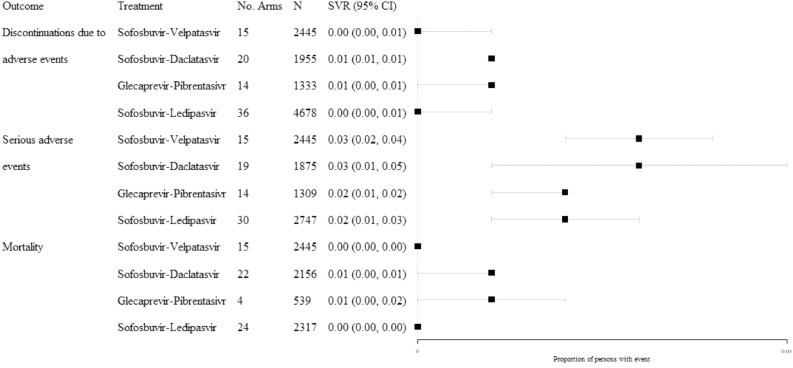
3.4. Harms
Analyses on harms data were conducted irrespective of genotype, treatment experience, and comorbidities (Fig. 5). Across the regimens studied, and based on large pooled sample sizes, the proportions of persons with DAEs, SAEs, or all-cause mortality were low. Pooled proportions of persons reporting a DAE or all-cause mortality did not exceed 0.01 and were characterized by tight confidence intervals. However, certainty around the evidence for SAE outcomes varied by regimen, with the widest confidence interval observed for sofosbuvir-daclatasvir (0.03; 95% CI: 0.01, 0.05; N = 1875).
Fig. 5.
Illustration of findings from the analyses of harms, all treatment-experience.
4. Discussion
Guidelines issued by the WHO are developed through an iterative process to address an area of uncertainty and unmet guidance need. These processes are explicit and transparent, involving consultation with several multidisciplinary stakeholders to ensure benefits and barriers are evaluated systematically and comprehensively. Importantly, the evidence used to develop these guidelines is publicly available. This review is one component of the evidence that was considered in the development of the 2018 guideline on the care and treatment of persons diagnosed with chronic HCV infection. Here we summarize SVR12 and harms outcomes for three pangenotypic DAA regimens (sofosbuvir-velpatasvir, sofosbuvir-daclatasvir, and glecaprevir-pibrentasvir) as well as sofosbuvir-ledipasvir, which were of particular relevance to decision-makers. Evidence for sofosbuvir-ledipasvir was considered as countries where HCV is largely isolated to a single genotype have had success managing care with this non-pangenotypic regimen. Across treatments, outcomes for persons with genotype 1 infection were the best represented, reflecting the high prevalence of this strain in the United States and Europe [130]. In the overall population analyses, all treatments were associated with high rates of SVR12 across genotypes 1 through 4. These findings were generally consistent across stratifications by treatment experience. While the rates of SVR12 were again high across treatments for persons with cirrhosis and genotype 1 infection, relatively limited evidence was available for genotype 2, 3, or 4 infection. The sparse evidence available for persons with HIV–HCV coinfection, both with respect to genotypes and treatments more generally, is likely a reflection that outcomes in these persons are similar to mono-infected persons. This observation has been acknowledged by international guidelines [131]. However, consideration of this sub-population is still warranted given evidence suggesting that persons with HIV–HCV coinfection are at risk of HCV treatment failure for factors such as ongoing illicit drug use and mental illness [132]. Analyses of harms suggest consistency across treatments for the very low proportions of persons with DAEs, SAEs, or all-cause mortality. This is a marked difference from previous generations of antiviral therapy for chronic HCV infection. For example, a 2013 review reported that the percentage of persons with SAEs varied from 4.7% for persons managed with pegylated interferon α−2b with ribavirin to 16% for 24 weeks of telaprevir-based triple therapy or 48 weeks of telaprevir dual therapy. Similarly, the percentage of persons with DAEs varied from 6.6% for dual therapy pegylated interferon α−2a with ribavirin to 15% for 24 weeks of telaprevir-based triple therapy [133].
The evidence described in this review was considered and applied to update current treatment guidelines for adults with chronic HCV infection. Based on this and other supporting evidence, the WHO issued a conditional recommendation, based on moderate quality of evidence, that pangenotypic DAA regimens be used for the treatment of persons with chronic HCV infection aged 18 years and above. In this recommendation, pangenotypic was defined as leading to SVR in over 85% of persons treated across all six major HCV genotypes. At the time this guidance was issued, the pangenotypic regimens available to adults without cirrhosis included sofosbuvir-velpatasvir (12 week course), sofosbuvir-daclatasvir (12 week course), and glecaprevir-pibrentasvir (8 week course). For adults with compensated cirrhosis, available regimens included sofosbuvir-velpatasvir (12 week course), glecaprevir-pibrentasvir (12 week course), sofosbuvir-daclatasvir (24 week course or 12 week course in regions where the genotype 3 prevalence is known to be less than 5%). A treatment duration of 24 weeks was recommended for sofosbuvir-daclatasvir given the lower SVR rates in persons with genotype 3 infection. For adults with or without compensated cirrhosis, glecaprevir-pibrentasvir should be used for 16 weeks for persons with genotype 3 infection who have previously received interferon and/or ribavirin.
The approach taken for this review was in accordance with WHO guideline methods and published standards. Standard literature search techniques were supplemented by contact with primary researchers, including drug manufacturers, in order to identify relevant unpublished data. Analyses were based on both randomized trials, non-randomized trials, and observational studies, with analyses stratified according to study type. In this paper we presented only analyses based on all eligible study designs. Moreover, several stratifications were planned a priori, including prior treatment experience, presence of cirrhosis, and HIV–HCV coinfection. The quality of evidence was evaluated using GRADE criteria and this approach was modified in consultation with a GRADE methodologist to suit the clinical research context of HCV.
Despite these strengths, there are limitations to this review. The scope did not encompass the complete landscape of HCV regimens and rather focused on newer, more effective pangenotypic DAAs. For example, outcomes for persons treated with ribavirin-containing regimens were not addressed here. However, some ribavirin-containing regimens were described in the full report, such as for the treatment of persons with cirrhosis and genotype 2 or 3 infection. This decision was based on the treatment burden associated with ribavirin, such as treatment-related side effects and a need for frequent laboratory monitoring; in addition, the non-ribavirin regimens summarized in this article demonstrate high efficacy. Importantly, analyses were conducted based on pooled proportions of persons with the pre-specified outcomes, irrespective of whether persons were enrolled in a multi-arm or single-arm trial or described in an observational cohort. Therefore, we cannot infer relative effects between interventions as we cannot disentangle study- and treatment-effects [134]. This limitation reflects the evidence landscape of HCV infection, where randomized trials comparing multiple active interventions are generally not feasible given the high efficacy of available interventions. However, the SVR outcome is an objective means by which to define efficacy and spontaneous SVR without antiviral therapy is very rare. For example, no patients (0/116) treated with placebo in the randomized ASTRAL-1 trial achieved SVR [118]. Important evidence may also come from study designs other than those considered here, such as retrospective studies, though these were out of scope in the current review. Finally, some unexplained statistical heterogeneity was present, which may be related to age, country, comorbidities, the use of generic drugs, treatment doses, the use of DAAs or interferon-based regimens in persons with prior treatment-experience, or treatment durations. Despite this, SVR rates were generally consistent across the included studies within each subgroup stratification. Future analyses may explore the sources of heterogeneity using patient-level, rather than study-level, evidence. Despite the strong efficacy outcomes demonstrated in the literature to date, the rapid evolution of treatments for HCV infection warrants an increased focus and scrutiny on emerging pangenotypic regimens, including the study of long-term treatment efficacy, harms, and uptake by front-line clinicians.
The benefits of curing HCV infections are far-reaching [135]. In response to emerging treatments, the WHO commissioned a systematic literature review to inform treatment guidelines for the management of persons with HCV infection. Based on the evidence, the WHO recommended the use of pangenotypic regimens to support the global campaign to eradicate HCV infection. With an oral administration, a feasibility advantage of bypassing genotype testing, and favourable tolerability profiles, the widespread adoption of pangenotypic regimens translates to a powerful and effective response to the public health threat posed by HCV infection.
Declaration of competing interest
Mr. Zoratti reports that he is a shareholder of Zoratti HEOR Consulting Inc. which was contracted to conduct this study. Ms. Siddiqua reports personal fees from Zoratti HEOR Consulting Inc. during the conduct of the study. Ms. Morassut reports personal fees from Zoratti HEOR Consulting Inc. during the conduct of the study. Ms. Zeraatkar reports personal fees from Zoratti HEOR Consulting Inc. during the conduct of the study. Dr. Chou reports serving as methodologist for the World Health Organization hepatitis C guideline, during the conduct of the study and grants from Agency for Healthcare Research and Quality outside the submitted work on hepatitis C screening and treatment. Mr. Druyts reports that he is a shareholder of Pharmalytics Consulting Group Inc. (“Pharmalytics Group”), registered in the province of British Columbia, Canada, which provides consulting services to the healthcare and pharmaceutical industries. No other author has anything to disclose.
Acknowledgements
The study sponsor (World Health Organization) assisted in the conception and design of this review. However, the corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. We would like to thank Dr. Marc Bulterys, formerly of the World Health Organization, for his contribution to this systematic literature review.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.12.007.
Contributor Information
Michael J. Zoratti, Email: michael.zoratti@zoratticonsulting.com.
Ayesha Siddiqua, Email: siddia36@mcmaster.ca.
Rita E. Morassut, Email: rmorassut2022@meds.uwo.ca.
Dena Zeraatkar, Email: zeraatd@mcmaster.ca.
Roger Chou, Email: chour@ohsu.edu.
Judith van Holten, Email: vanholtenj@who.int.
Feng Xie, Email: fengxie@mcmaster.ca.
Eric Druyts, Email: eric.druyts@pharmalyticsgroup.com.
Appendix. Supplementary materials
References
- 1.Heffernan A., Cooke G.S., Nayagam S., Thursz M., Hallett T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019 doi: 10.1016/S0140-6736(18)32277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiktor S. How feasible is the global elimination of HCV infection? Lancet. 2019 doi: 10.1016/S0140-6736(18)32750-8. [DOI] [PubMed] [Google Scholar]
- 3.da Fonseca E.M., Shadlen K., Bastos F.I. Brazil's fight against hepatitis C – universalism, local production, and patents. N Engl J Med. 2019;380(7):605–607. doi: 10.1056/NEJMp1812959. [DOI] [PubMed] [Google Scholar]
- 4.Moon S., Erickson E. Universal medicine access through lump-sum remuneration – Australia's approach to hepatitis C. N Engl J Med. 2019;380(7):607–610. doi: 10.1056/NEJMp1813728. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization; Geneva: 2018. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. [PubMed] [Google Scholar]
- 6.World Health Organization. Zoratti M. World Health Organization; 2018. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection: web annex 3.1: adult hepatitis C virus treatment systematic review. [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res Ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization; Geneva: 2016. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Updated version. [PubMed] [Google Scholar]
- 10.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 11.Viechtbauer W. Conducting meta-analyses in r with the metafor package. J Stat Softw. 2010;36(3):48. [Google Scholar]
- 12.Wallace B.C., Dahabreh I.J., Trikalinos T.A., Lau J., Trow P., Schmid C.H. Closing the gap between methodologists and end-users: r as a computational back-end. J Stat Softw. 2012;49(5):15. [Google Scholar]
- 13.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLARITY Group at McMaster University. Tools to assess risk of bias in cohort studies, case control studies, randomized controlled trials, and longitudinal symptom research studies aimed at the general population 2013. Available from: https://www.evidencepartners.com/resources/methodological-resources/.
- 15.Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt G.H., Oxman A.D., Montori V., Vist G., Kunz R., Brozek J. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G.H., Oxman A.D., Sultan S., Glasziou P., Akl E.A., Alonso-Coello P. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt G.H., Oxman A.D., Vist G., Kunz R., Brozek J., Alonso-Coello P. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias) J Clin Epidemiol. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res Ed) 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Aziz A.M., Ibrahim M.A., El-Sheikh A.A., Kamel M.Y., Zenhom N.M., Abdel-Raheim S. Effect of sofosbuvir plus daclatasvir in hepatitis c virus genotype-4 patients: promising effect on liver fibrosis. J Clin Exp Hepatol. 2017 doi: 10.1016/j.jceh.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abergel A., Metivier S., Samuel D., Jiang D., Kersey K., Pang P.S. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology. 2016;64(4):1049–1056. doi: 10.1002/hep.28706. [DOI] [PubMed] [Google Scholar]
- 23.Alqahtani S, Afdahl NH, Zeuzem S, Gordon SC, Mangia A, Kwo PY, et al. Safety ofledipasvir sofosbuvir with and without ribavirin for the treatment of patientswith chronic HCV genotype 1 infection an analysis of the phase 3 ion trials.Hepatol Int 9(1 Suppl. 1):S59–60.
- 24.Alqahtani S.A.A.N., Zeuzem S., Gordon S.C., Mangia A., Kwo P., Fried M. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: analysis of phase III ion trials. Hepatology. 2015;62(1):25–30. doi: 10.1002/hep.27890. [DOI] [PubMed] [Google Scholar]
- 25.Backus L.I., Belperio P.S., Shahoumian T.A., Loomis T.P., Mole L.A. Comparative effectiveness of ledipasvir/sofosbuvir +/- ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir +/- ribavirin in 6961 genotype 1 patients treated in routine medical practice. Alimen Pharmacol Ther. 2016;44(4):400–410. doi: 10.1111/apt.13696. [DOI] [PubMed] [Google Scholar]
- 26.Bansal A., Goyal O. Treatment of patients with chronic hepatitis genotype 3 infection with/without cirrhosis with sofosbuvir and daclatasvir therapy. Gastroenterology. 2017;152(5):S1091–S10S2. [Google Scholar]
- 27.Bourliere M., Gordon S.C., Flamm S.L., Cooper C.L., Ramji A., Tong M. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 28.Bourliere M.B.J.P., de Ledinghen V., Hezode C., Zoulim F., Mathurin P., Tran A. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15(4):397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 29.Carrieri M.P.P.C., Younossi Z., Vilotitch A., Fontaine H., Petrov-Sanchez V., Marcellin F. Health-Related quality of life in chronic HCV-Infected patients switching to pegylated-interferon-free regimens (ANRS CO20 cupic cohort study and sirius trial) Patient. 2017:1–10. doi: 10.1007/s40271-017-0232-1. [DOI] [PubMed] [Google Scholar]
- 30.Charlton M., O'Leary J., Osinusi A., Brainard D.M., McHutchison J.G., Brown R.S. Sofosbuvir/Velpatasvir for the treatment of HCV in patients with decompensated liver disease: the ASTRAL-4 study. Transplantation. 2016;100(5 Supplement 1):S102–S1S3. Conference: 22nd annual international congress of the international liver transplantation society, ILTS. 2016. South korea. Conference start: 20160504. Conference end: 20160507. [Google Scholar]
- 31.Chayama K., Suzuki F., Karino Y., Kawakami Y., Sato K., Atarashi T. CERTAIN-1: efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J. Hepatol. 2017;66:S527. [Google Scholar]
- 32.Chayama K., Suzuki F., Sato K., Atarashi T., Watanabe T., Toyoda H. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection with and without cirrhosis. J Hepatol. 2017;66:S528. doi: 10.1002/hep.29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang W.L., Chien R.N., Peng C.Y., Chang T.T., Lo G.H., Sheen I.S. Ledipasvir/sofosbuvir fixed-dose combination tablet in Taiwanese patients with chronic genotype 1 hepatitis C virus. J Gastroenterol Hepatol. 2016;31(7):1323–1329. doi: 10.1111/jgh.13305. [DOI] [PubMed] [Google Scholar]
- 34.Cornberg M.P.J., Schober A., Mauss S., Boker K.H.W., Link R., Gunther R. Real-world use, effectiveness and safety of anti-viral treatment in chronic hepatitis C genotype 3 infection. Aliment Pharmacol Ther. 2017;45(5):688–700. doi: 10.1111/apt.13925. [DOI] [PubMed] [Google Scholar]
- 35.Curry M., O'Leary J., Brown R.S., Muir A., An D., Osinusi A. Clinical benefits of successful treatment in HCV infected patients with decompensated cirrhosis treated with sofosbuvir/velpatasvir. Transplantation. 2016;100(5 Supplement 1):S136. Conference: 22nd annual international congress of the international liver transplantation society, ILTS. 2016. South korea. Conference start: 20160504. Conference end: 20160507. [Google Scholar]
- 36.Curry M.P., O'Leary J.G., Bzowej N., Muir A.J., Korenblat K.M., Fenkel J.M. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 37.Desnoyer A., Pospai D., Le M.P., Gervais A., Heurgue-Berlot A., Laradi A. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65(1):40–47. doi: 10.1016/j.jhep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Deterding K., Spinner C.D., Schott E., Welzel T.M., Gerken G., Klinker H. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis. 2017;17(2):215–222. doi: 10.1016/S1473-3099(16)30408-X. [DOI] [PubMed] [Google Scholar]
- 39.Deterding K., Spinner C.D., Schott E., Welzel T.M., Gerken G., Klinker H.H. Six weeks of sofosbuvir/ledipasvir treatment of acute hepatitis C virus genotype 1 monoinfection: final results of the the German hepnet acute HCV IV study. Hepatology. 2016;63(1 Supplement 1):416A–417A. Conference: 67th annual meeting of the american association for the study of liver diseases: the liver meeting. 2016. United states. Conference start: 20161111. Conference end: 20161115. [Google Scholar]
- 40.Everson G.T., Towner W.J., Davis M.N., Wyles D.L., Nahass R.G., Thuluvath P.J. Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis c virus infection. Ann Intern Med. 2015;163(11):818–826. doi: 10.7326/M15-1000. [DOI] [PubMed] [Google Scholar]
- 41.Feld J.J., Jacobson I.M., Hode C., Asselah T., Ruane P.J., Gruener N. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 42.Feld J.J., Maan R., Zeuzem S., Kuo A., Nelson D.R., Di Bisceglie A.M. Effectiveness and safety of sofosbuvir-based regimens for chronic HCV genotype 3 infection: results of the HCV-target study. Clin Infect Dis. 2016;63(6):776–783. doi: 10.1093/cid/ciw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fierer D.S., El Sayed A., Palaniswami P. Treatment of “acute” hepatitis C virus in human immunodeficiency virus-infected men with short-course sofosbuvir/ledipasvir. J Hepatol. 2017;66:S300. [Google Scholar]
- 44.Fontaine H.H.C., Roudot-Thoraval F., Pol S. Safety and efficacy of the combination ombitasvir/ paritaprevir/ritonavir +/- dasabuvir in HCV genotype 1-or 4-mono-infected patients from the French ANRS Co22 hepather cohort. Hepatology. 2016;63(1 Supplement 1):453A. Conference: 67th annual meeting of the american association for the study of liver diseases: the liver meeting. 2016. United states. Conference start: 20161111. Conference end: 20161115. [Google Scholar]
- 45.Forns X., Lee S., Valdes J., Lens S., Ghalib R., Aguilar H. EXPEDITION-I: efficacy and safety of glecaprevir/pibrentasvir in adults with chronic hepatitis C virus genotype 1, 2, 4, 5 or 6 infection and compensated cirrhosis. J Hepatol. 2017;66:S3. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 46.Forns X., Lee S., Valdes J.M., Lens S., Ghalib R., Aguilar H. EXPEDITION-I: efficacy and safety of glecaprevir/pibrentasvir in adults with chronic hepatitis C virus genotype 1, 2, 4, 5 or 6 infection and compensated cirrhosis. Gastroenterology. 2017;152(5):S1061. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 47.Forns X., Lee S.S., Valdes J., Lens S., Ghalib R., Aguilar H. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 48.Foster G.R., Afdhal N., Roberts S.K., Br N., Gane E.J., Pianko S. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 49.Foster G.R., Gane E., Asatryan A., Asselah T., Ruane P.J., Pol S. ENDURANCE-3: safety and efficacy of glecaprevir/pibrentasvir compared to sofosbuvir plus daclatasvir in treatment-naïve HCV genotype 3-infected patients without cirrhosis. J Hepatol. 2017;66:S34. [Google Scholar]
- 50.Foster G.R., Irving W.L., Cheung M.C.M., Walker A.J., Hudson B.E., Verma S. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 51.Gane E., Poordad F., Wang S., Asatryan A., Kwo P.Y., Lalezari J. High efficacy of ABT-493 and ABT-530 treatment in patients with HCV genotype 1 or 3 infection and compensated cirrhosis. Gastroenterology. 2016;151(4):651–659. doi: 10.1053/j.gastro.2016.07.020. e1. [DOI] [PubMed] [Google Scholar]
- 52.Gane E., Stedman C.A.M., Asselah T., Kohli A., Mir H.M., Natha M. Ledipasvir/sofosbuvir with or without ribavirin for the treatment of patients with genotype 2-6 chronic HCV infection: summary results from four phase ii studies. Am J Gastroenterol. 2015;110(16) [Google Scholar]
- 53.Gane E.J., Hyland R.H., An D., Svarovskaia E., Pang P.S., Brainard D. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015;149(6):1454–1461. doi: 10.1053/j.gastro.2015.07.063. e1. [DOI] [PubMed] [Google Scholar]
- 54.Gane E.J., Hyland R.H., Yang Y., Svarovskaia E., Stamm L.M., Brainard D.M. Efficacy of ledipasvir plus sofosbuvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2 infection. Gastroenterology. 2017;152(6):1366–1371. doi: 10.1053/j.gastro.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Gee Lim S., Patel K., Agarwal K., Han L.L., McNabb B.L., Svarovskaia E. Sofosbuvir/velpatasvir for 12 weeks results in high SVR12 rates in patients with indeterminate genotypes: an integrated analysis of efficacy from the ASTRAL-1, ASTRAL-2, and ASTRAL-3 studies. Hepatol Int. 2017;11(1 Supplement 1):S102–S1S3. Conference: 26th annual conference of the asian pacific association for the study of the liver, APASL. 2017. China. [Google Scholar]
- 56.Grebely J., Dore G.J., Zeuzem S., Aspinall R.J., Fox R., Han L. Efficacy and safety of sofosbuvir/velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: analysis of phase 3 astral trials. Clin Infect Dis. 2016;63(11):1479–1481. doi: 10.1093/cid/ciw579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grebely J.M.S., Brown A., Bronowicki J.P., Puoti M., Wyles D., Natha M. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: analysis of phase 3 ion trials. Clin Infect Dis. 2016;63(11):1405–1411. doi: 10.1093/cid/ciw580. [DOI] [PubMed] [Google Scholar]
- 58.Hezode C., Leroy V., Rosa I., Pawlotsky J.-.M., de Ledinghen V., Bronowicki J.-.P. Efficacy and safety of sofosbuvir and daclatasvir for 8 weeks in treatment-naive non-cirrhotic patients with chronic HCV genotype 3 infection. Gastroenterology. 2017;152(5):S1099. [Google Scholar]
- 59.Hezode C., Leroy V., Rosa I., Roudot-Thoraval F., Pawlotsky J.M., De Ledinghen V. Efficacy and safety of sofosbuvir and daclatasvir for 8 weeks in treatment-naïve non-cirrhotic patients with chronic hepatitis C virus genotype 3 infection. J Hepatol. 2017;66:S299. [Google Scholar]
- 60.Hlaing N.K.T., Mitrani R.A., Aung S.T., Phyo W.W., Serper M., Kyaw A.M.M. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis c virus genotypes 1-4 and 6 in Myanmar: real-world experience. J Viral Hepatol. 2017 doi: 10.1111/jvh.12721. [DOI] [PubMed] [Google Scholar]
- 61.Ide T., Eguchi Y., Harada M., Honma Y., Iwane S., Okada M. Efficacy and safety of DAAs therapy in hepatitis C: a multicenter real-world cohort of chronic hepatitis C patients. Hepatology. 2016;63(1 Supplement 1):459A. Conference: 67th annual meeting of the american association for the study of liver diseases: the liver meeting. 2016. United states. Conference start: 20161111. Conference end: 20161115. [Google Scholar]
- 62.Iio E., Shimada N., Takaguchi K., Senoh T., Eguchi Y., Atsukawa M. Clinical evaluation of sofosbuvir/ledipasvir in patients with chronic hepatitis C genotype 1 with and without prior daclatasvir/asunaprevir therapy. Hepatology Res. 2017 doi: 10.1111/hepr.12898. [DOI] [PubMed] [Google Scholar]
- 63.Ingiliz P., Christensen S., Kimhofer T., Hueppe D., Lutz T., Schewe K. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis c virus (HCV) infection in HCV-Monoinfected and HIV-HCV-Coinfected individuals: results from the German hepatitis c cohort (GECCO-01) Clin Infect Dis. 2016;63(10):1320–1324. doi: 10.1093/cid/ciw567. [DOI] [PubMed] [Google Scholar]
- 64.Isakov V., Gankina N., Salupere R., Chulanov V.P., Kozhevnikova G., Zilmer K. Ledipasvir/Sofosbuvir for 8 weeks results in high SVR rates in treatment-naive patients with chronic HCV infection and HIV/HCV co-infection. Hepatology. 2016;63(1 Supplement 1):1010A. Conference: 67th annual meeting of the american association for the study of liver diseases: the liver meeting. 2016. United states. Conference start: 20161111. Conference end: 20161115. [Google Scholar]
- 65.Jacobson I., Brau N., Bourgeois S., Mathurin P., Thuluvath P., Fessel W.J. The tolerability of SOF/VEL for 12 weeks in >1000 patients treated in the astral-1, astral-2, and astral-3 studies: an integrated safety analysis. J Hepatol. 2016;64(2 SUPPL. 1):S773–S7S4. Conference: 51st annual meeting of the european association for the study of the liver, international liver congress. 2016. Barcelona spain. Conference start: 20160413. Conference end: 20160417. Conference publication:(var.pagings) [Google Scholar]
- 66.Jacobson I.M., Lawitz E., Gane E.J., Willems B.E., Ruane P.J., Nahass R.G. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology. 2017;153(1):113–122. doi: 10.1053/j.gastro.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 67.Ji D., Chen G.F., Wang C., Wang Y.D., Shao Q., Li B. Twelve-week ribavirin-free direct-acting antivirals for treatment-experienced Chinese with HCV genotype 1b infection including cirrhotic patients. Hepatol Int. 2016;10(5):789–798. doi: 10.1007/s12072-016-9755-0. [DOI] [PubMed] [Google Scholar]
- 68.Kohli A.K.R., Sims Z., Nelson A., Sidharthan S., Lam B., Silk R. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049–1054. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohli A.O.A., Sims Z., Nelson A., Meissner E.G., Barrett L.L., Bon D. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385(9973):1107–1113. doi: 10.1016/S0140-6736(14)61228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korenaga M., Izumi N., Yokosuka O., Takehara T., Sakamoto N., Nishiguchi S. Sustained virologic response by ledipasvir/sofosbuvir reduces the incidence of hepatocellular carcinoma in Japanese patients with HCV genotype 1 infection. Comparison with simeprevir with peginterferon plus ribavirin. J Hepatol. 2017;66:S23. [Google Scholar]
- 71.Kottilil S., Wyles D., Brau N., Daar E., Workowski K., Luetkemeyer A. Sofosbuvir/velpatasvir fixed dose combination for 12 weeks in patients co-infected with HCV and HIV-1: the phase 3 ASTRAL-5 study. Hepatol Int. 2017;11(1 Supplement 1):S111. Conference: 26th annual conference of the asian pacific association for the study of the liver, APASL. 2017. China. [Google Scholar]
- 72.Kowdley K.V.N.D.R., Lalezari J.P., Box T., Gitlin N., Poleynard G., Rabinovitz M. On-treatment HCV RNA as a predictor of sustained virological response in HCV genotype 3-infected patients treated with daclatasvir and sofosbuvir. Liver Int. 2016;36(11):1611–1618. doi: 10.1111/liv.13165. [DOI] [PubMed] [Google Scholar]
- 73.Kwo P.Y., Poordad F., Asatryan A., Wang S., Wyles D.L., Hassanein T. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J Hepatol. 2017;67(2):263–271. doi: 10.1016/j.jhep.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Lawitz EZS, Stedman CA, Poordad F, Mir HM, Seyedkazemi S, Hyland RH, et al.Sofosbuvir-based regimens for patients with hepatitis C virus genotype 3 infection: summary results from the valence, lonestar-2, and electron-2 studies. Gastroenterology. 148(4 Suppl. 1):S1085–S6.
- 75.Lim Y.S., Ahn S.H., Lee K.S., Paik S.W., Lee Y.J., Jeong S.H. A phase IIIb study of ledipasvir/sofosbuvir fixed-dose combination tablet in treatment-naive and treatment-experienced Korean patients chronically infected with genotype 1 hepatitis C virus. Hepatol Int. 2016;10(6):947–955. doi: 10.1007/s12072-016-9726-5. [DOI] [PubMed] [Google Scholar]
- 76.Lionetti R., Lenci I., Siciliano M., Pompili M., Comandini U.V., Milana M. Improved virological outcomes and excellent safety profile in genotype 3 HCV-infected cirrhotic patients after an extended 24- weeks course of daclatasvir, sofosbuvir + ribavirin: insights froma real-life multicenter study. J Hepatol. 2017;66:S731. [Google Scholar]
- 77.Mangia A., Arleo A., Copetti M., Miscio M., Piazzolla V., Santoro R. The combination of daclatasvir and sofosbuvir for curing genotype 2 patients who cannot tolerate ribavirin. Liver Int. 2016;36(7):971–976. doi: 10.1111/liv.13069. [DOI] [PubMed] [Google Scholar]
- 78.McPhee F., Hernandez D., Zhou N. Effect of minor populations of NS5A and NS5B resistance-associated variants on HCV genotype-3 response to daclatasvir plus sofosbuvir, with or without ribavirin. Antivir Ther. 2017;22(3):237–246. doi: 10.3851/IMP3120. [DOI] [PubMed] [Google Scholar]
- 79.Mehta V., Mahajan R., Midha V., Narang V., Kaur K., Singh A. Impact of direct acting antiviral therapy for treatment of hepatitis c genotypes 1, 3 and 4: a real life experience from India. J Clin Exp Hepatol. 2017 doi: 10.1016/j.jceh.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizokami M., Yokosuka O., Takehara T., Sakamoto N., Korenaga M., Mochizuki H. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15(6):645–653. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]
- 81.Nehra V.T.E.M., Rizza S.A., Temesgen Z. Ledipasvir/sofosbuvir fixed-dose combination for treatment of hepatitis C virus genotype 4 infection. Drugs Today. 2016;52(2):111–117. doi: 10.1358/dot.2016.52.2.2449840. [DOI] [PubMed] [Google Scholar]
- 82.Nelson D.R.C.J.N., Lalezari J.P., Lawitz E., Pockros P.J., Gitlin N., Freilich B.F. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson D.R.C.J.N., Lalezari J.P., Lawitz E., Pockros P.J., Gitlin N., Freilich B.F. All-Oral 12-wek combination treatment with daclatasvir (DCV) and sofosbuvir (SOF) in patients infected with HCV genotype (GT) 3: aLLY-3 phase 3 study. Canad J Infect Dis Med Microbiol. 2015;26(2):e32. Conference:. 2015 CACMID-AMMI canada annual conference. Canada. [Google Scholar]
- 84.Ogawa E., Furusyo N., Nomura H., Dohmen K., Higashi N., Takahashi K. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J Gastroenterol. 2017;52(7):845–854. doi: 10.1007/s00535-016-1290-1. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa E., Furusyo N., Nomura H., Dohmen K., Higashi N., Takahashi K. Effectiveness and safety of sofosbuvir plus ledipasvir for HCV genotype 1b patients with compensated cirrhosis. Hepatol Int. 2017;11(1 Supplement 1):S1012. Conference: 26th annual conference of the asian pacific association for the study of the liver, APASL. 2017. China. [Google Scholar]
- 86.O'Leary J., Brown R.S., Reddy K.R., Fenkel J., Korenblat K., Younes Z.H. Baseline clinical and laboratory parameters associated with clinical benefits of successful hcvtreatment with sofosbuvir/velpatasvir in decompensated cirrhotic patients. J Hepatol. 2016;64(2 SUPPL. 1):S774. Conference: 51st annual meeting of the european association for the study of the liver, international liver congress. 2016. Barcelona spain. Conference start: 20160413. Conference end: 20160417. Conference publication:(var.pagings) [Google Scholar]
- 87.Osinusi A.T.K., Kohli A., Nelson A., Seamon C., Meissner E.G., Bon D. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313(12):1232–1239. doi: 10.1001/jama.2015.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearlman B., Lutchman G., Shiffman M.L., Patel J., Frazier L.M., Galati J.S. Safety and efficacy of elbasvir and grazoprevir with or without ribavirin for the treatment of hepatitis C virus genotype 1: results of the hepatitis C virus-TARGET study. J Hepatol. 2017;66:S294. [Google Scholar]
- 89.Persico M., Aglitti A., Caruso R., De Renzo A., Selleri C., Califano C. Efficacy and safety of new direct antiviral agents in HCV infected patients with diffuse large b cell non-hodgkin lymphoma. Hepatology. 2017;17:17. doi: 10.1002/hep.29364. [DOI] [PubMed] [Google Scholar]
- 90.Pianko S., Flamm S.L., Shiffman M.L., Kumar S., Strasser S.I., Dore G.J. Sofosbuvir plus velpatasvir combination therapy for treatment- Experienced patients with genotype 1 or 3 hepatitis C virus infection. Ann Intern Med. 2015;163(11):809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 91.Pol S., Bourliere M., Lucier S., Hezode C., Dorival C., Larrey D. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J Hepatol. 2016;10 doi: 10.1016/j.jhep.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Poordad F., Felizarta F., Asatryan A., Sulkowski M.S., Reindollar R.W., Landis C.S. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017 doi: 10.1002/hep.29081. (pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poordad F., Gordon S.C., Asatryan A., Felizarta F., Reindollar R.W., Landis C. High efficacy of ABT-493 and ABT-530 in HCV genotype 1 infected patients who have failed direct-acting antiviral-containing regimens: the Magellan-I study. J Hepatol. 2016;64(2 SUPPL. 1):S160–S1S1. Conference: 51st annual meeting of the european association for the study of the liver, international liver congress. 2016. Barcelona spain. Conference start: 20160413. Conference end: 20160417. Conference publication:(var.pagings) [Google Scholar]
- 94.Poordad F., Pol S., Asatryan A., Buti M., Shaw D., Hezode C. MAGELLAN-1, part 2: glecaprevir and pibrentasvir for 12 or 16 weeks in patients with chronic hepatitis C virus genotype 1 or 4 and prior direct-acting antiviral treatment failure. J Hepatol. 2017;66:S83. doi: 10.1002/hep.29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poordad F., Pol S., Asatryan A., Buti M., Shaw D.R., Hezode C. MAGELLAN-1, part 2: glecaprevir and pibrentasvir for 12 or 16 weeks in patients with chronic HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure. Gastroenterology. 2017;152(5):S1057. [Google Scholar]
- 96.Reddy K.R.L.J.K., Kuo A., Di Bisceglie A.M., Galati J.S., Morelli G., Everson G.T. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: observations through HCV-target database. Aliment Pharmacol Ther. 2017;45(1):115–126. doi: 10.1111/apt.13823. [DOI] [PubMed] [Google Scholar]
- 97.Saadoun D., Ferfar Y., Hezode C., Ahmed S.N.S., Alric L., De Saint Martin L. Sofosbuvir plus daclatasvir for hepatitis C virus-cryoglobulinemia vasculitis (HCV-CryoVas): vascuvaldic 2 study. J Hepatol. 2017;66:S56. doi: 10.1053/j.gastro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 98.Saadoun D., Pol S., Ferfar Y., Alric L., Hezode C., Si Ahmed S.N. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-Associated cryoglobulinemia vasculitis. Gastroenterology. 2017;153(1):49–52. doi: 10.1053/j.gastro.2017.03.006. e5. [DOI] [PubMed] [Google Scholar]
- 99.Shiha G., Waked I., Soliman R., Abdelrazek W., Hassany M., Fouad R. Ledipasvir/sofosbuvir for 8 or 12 weeks with or without ribavirin in HCV genotype 4 patients in Egypt. Hepatol Int. 2017;11(1 Supplement 1):S109. Conference: 26th annual conference of the asian pacific association for the study of the liver, APASL. 2017. China. [Google Scholar]
- 100.Sidharthan S.K.A., Sims Z., Nelson A., Osinusi A., Masur H., Kottilil S. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 2015;60(12):1743–1751. doi: 10.1093/cid/civ170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sulkowski M.S., Brau N., Lawitz E., Shiffman M.L., Towner W.J., Ruane P.J. A randomized controlled trial of sofosbuvir/GS-5816 fixed dose combination for 12 weeks compared to sofosbuvir with ribavirin for 12 weeks in genotype 2 HCV infected patients: the phase 3 ASTRAL-2 study. Hepatology. 2015;62(13) [Google Scholar]
- 102.Sulkowski M.S., Chuang W.L., Kao J.H., Yang J.C., Gao B., Brainard D.M. No evidence of reactivation of hepatitis b virus among patients treated with ledipasvir-sofosbuvir for hepatitis C virus infection. Clin Infect Dis. 2016;63(9):1202–1204. doi: 10.1093/cid/ciw507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Terrault N.A., Zeuzem S., Di Bisceglie A.M., Lim J.K., Pockros P.J., Frazier L.M. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis c virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151(6):1131–1140. doi: 10.1053/j.gastro.2016.08.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Townsend K.M.E.G., Sidharthan S., Sampson M., Remaley A.T., Tang L., Kohli A. Interferon-Free treatment of hepatitis c virus in HIV/hepatitis C virus-coinfected subjects results in increased serum low-density lipoprotein concentration. AIDS Res Hum Retroviruses. 2016;32(5):456–462. doi: 10.1089/aid.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Townsend K.P.T., Gordon L.A., Kohli A., Nelson A., Seamon C., Gross C. Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir. Aids. 2016;30(2):261–266. doi: 10.1097/QAD.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toyoda H., Chayama K., Suzuki F., Sato K., Atarashi T., Watanabe T. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology. 2017 doi: 10.1002/hep.29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vermehren J., Athmann C., Gunther R., Schott E., Pathil A., Boeker K.H. Use of the 6 million viral load cut-off to guide treatment duration with ledipasvir/sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection: results from the German hepatitis C-Registry (DHC-R) J Hepatol. 2017;66:S519. [Google Scholar]
- 108.Vermehren J.B.M., Pol S., Marcellin P., Hyland R.H., Jiang D., Brainard D.M. Comparison of on-treatment HCV RNA during direct antiviral therapy using two different COBAS TaqMan HCV assays. J Clin Virol. 2017;89:51–56. doi: 10.1016/j.jcv.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 109.Welzel T.M., Nelson D.R., Morelli G., Bisceglie A.D., Reddy R.K., Kuo A. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of hcv genotype 2 infection: results of the real-world, clinical practice HCV-target study. Gut. 2016;13 doi: 10.1136/gutjnl-2016-311609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilder J.M.J.L.J., Ravendhran N., Shiffman M.L., Poulos J., Sulkowski M.S., Gitlin N. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology. 2016;63(2):437–444. doi: 10.1002/hep.28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson E.M.K.S., Sidharthan S., Sims Z., Tang L., McLaughlin M., Price A. Successful retreatment of chronic HCV genotype-1 infection with ledipasvir and sofosbuvir after initial short course therapy with direct-acting antiviral regimens. Clin Infect Dis. 2016;62(3):280–288. doi: 10.1093/cid/civ874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wyles D., Brau N., Kottilil S., Daar E.S., Ruane P., Workowski K. Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis. 2017;65(1):6–12. doi: 10.1093/cid/cix260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wyles D., Brau N., Naggie S., Sulkowski M., Agarwal K., Patel K. SOF/VEL single-tablet regimen in HCV mono-infected and HIV/HCV co-infected patients: comparison of efficacy and safety data from phase 3 clinical trials. J Int AIDS Soc. Conf.: Int Congr Drug Therapy HIV Infect. 2016;19:187–188. [Google Scholar]
- 114.Yakoot M., Abdo A.M., Abdel-Rehim S., Helmy S. Response tailored protocol versus the fixed 12Weeks course of dual sofosbuvir/daclatasvir treatment in Egyptian patients with chronic hepatitis c genotype-4 infection: a randomized, open-label, non-inferiority trial. EBioMedicine. 2017;17:17. doi: 10.1016/j.ebiom.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Younossi Z., Stepanova M., Han K.H., Lim Y.S., Lee Y.J., Chuang W.L. Asian patients with hepatitis C (HCV) genotype 1 treated with ledipasvir and sofosbuvir (LDV/SOF) experience very high efficacy and improvement of health-related quality of life (HRQL) J Gastroenterol Hepatol. 2016;31(376) [Google Scholar]
- 116.Younossi Z.M., Park H., Gordon S.C., Ferguson J.R., Ahmed A., Dieterich D. Real-world outcomes of ledipasvir/sofosbuvir in treatment-naive patients with hepatitis C. Am J Manag Care. 2016;22(6 Spec No.):SP205–SP211. [PubMed] [Google Scholar]
- 117.Younossi Z.M., Stepanova M., Charlton M., Curry M.P., O'Leary J.G., Brown R.S. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1(2):122–132. doi: 10.1016/S2468-1253(16)30009-7. [DOI] [PubMed] [Google Scholar]
- 118.Younossi Z.M., Stepanova M., Feld J., Zeuzem S., Jacobson I., Agarwal K. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from ASTRAL-1 placebo-controlled trial. J Hepatol. 2016;65(1):33–39. doi: 10.1016/j.jhep.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 119.Younossi Z.M., Stepanova M., Omata M., Mizokami M., Walters M., Hunt S. Quality of life of Japanese patients with chronic hepatitis C treated with ledipasvir and sofosbuvir. Medicine. 2016;95(33):e4243. doi: 10.1097/MD.0000000000004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Younossi Z.M., Stepanova M., Sulkowski M., Foster G.R., Reau N., Mangia A. Ribavirin-free regimen with sofosbuvir and velpatasvir is associated with high efficacy and improvement of patient-reported outcomes in patients with genotypes 2 and 3 chronic hepatitis C: results from astral-2 and -3 clinical trials. Clin Infect Dis. 2016;63(8):1042–1048. doi: 10.1093/cid/ciw496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Younossi Z.M., Stepanova M., Sulkowski M., Wyles D., Kottilil S., Hunt S. Patient-reported outcomes in patients co-infected with hepatitis C virus and human immunodeficiency virus treated with sofosbuvir and velpatasvir: the ASTRAL-5 study. Liver Int. 2017 doi: 10.1111/liv.13462. [DOI] [PubMed] [Google Scholar]
- 122.Younossi Z.M.S.M., Afdhal N., Kowdley K.V., Zeuzem S., Henry L., Hunt S.L. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol. 2015;63(2):337–345. doi: 10.1016/j.jhep.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 123.Younossi Z.M.S.M., Marcellin P., Afdhal N., Kowdley K.V., Zeuzem S., Hunt S.L. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology. 2015;61(6):1798–1808. doi: 10.1002/hep.27724. [DOI] [PubMed] [Google Scholar]
- 124.Younossi Z.M.S.M., Pol S., Bronowicki J.P., Carrieri M.P., Bourliere M. The impact of ledipasvir/sofosbuvir on patient-reported outcomes in cirrhotic patients with chronic hepatitis C: the sirius study. Liver Int. 2016;36(1):42–48. doi: 10.1111/liv.12886. [DOI] [PubMed] [Google Scholar]
- 125.Younossi Z.S.M., Pol S., Bronowicki J.P., Carrieri P., Bourliere M. The impact of ledipasvir (LDV)/sofosbuvir (SOF) combination on health-related quality of life (HRQL) and patient-reported outcomes (PROS) in cirrhotic patients with chronic hepatitis C (CH-C): the sirius study. J Hepatol. 2015;62(22) [Google Scholar]
- 126.Younossi Z.S.M., Omata M., Mizokami M., Walters M., Hunt S. Health utilities using SF-6D scores in Japanese patients with chronic hepatitis C treated with sofosbuvir-based regimens in clinical trials. Health Qual Life Outcomes. 2017;15(1):25. doi: 10.1186/s12955-017-0598-8. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeng Q.L., Xu G.H., Zhang J.Y., Li W., Zhang D.W., Li Z.Q. Generic ledipasvir-sofosbuvir for patients with chronic hepatitis C: a real-life observational study. J Hepatol. 2017;66(6):1123–1129. doi: 10.1016/j.jhep.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 128.Zeuzem S., Flamm S.L., Tong M.J., Vierling J.M., Pianko S., Buggisch P. A randomized, controlled, phase 3 trial of sofosbuvir/ velpatasvir/voxilaprevir or sofosbuvir/velpatasvir for 12 weeks in direct acting antiviral-experienced patients with genotype 1-6 HCV infection: the POLARIS-4 study. Hepatology. 2016;63(1 Suppl 1):59A. Conference: 67th annual meeting of the american association for the study of liver diseases: the liver meeting. 2016. United states. Conference start: 20161111. Conference end: 20161115. [Google Scholar]
- 129.Zhdanov K., Orlova-Morozova E.A., Morozov V., Zilmer K., Abdurakmanov D., Bessonova E. Ledipasvir/sofosbuvir in treatment-Naive patients with chronic hcv infection and HIV/HCV co-infection and in SOFexperienced patients. Hepatol Int. 2017;11(1 Supplement 1):S109–SS10. Conference: 26th annual conference of the asian pacific association for the study of the liver, APASL. 2017. China. [Google Scholar]
- 130.Bruggmann P., Berg T., Ovrehus A.L., Moreno C., Brandao Mello C.E., Roudot-Thoraval F. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl(1):5–33. doi: 10.1111/jvh.12247. [DOI] [PubMed] [Google Scholar]
- 131.EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 132.Cachay E.R., Mena A., Morano L., Benitez L., Maida I., Ballard C. Predictors of hepatitis c treatment failure after using direct-acting antivirals in people living with human immunodeficiency virus. Open Forum Infect Dis. 2019;6(3):ofz070. doi: 10.1093/ofid/ofz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chou R., Hartung D., Rahman B., Wasson N., Cottrell E.B., Fu R. Comparative effectiveness of antiviral treatment for hepatitis c virus infection in adults: a systematic review. Ann Intern Med. 2013;158(2):114–123. doi: 10.7326/0003-4819-158-2-201301150-00576. [DOI] [PubMed] [Google Scholar]
- 134.Jansen J.P., Trikalinos T., Cappelleri J.C., Daw J., Andes S., Eldessouki R. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;17(2):157–173. doi: 10.1016/j.jval.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 135.Soriano V., Tefferi A. Prevention of liver cancer with new curative hepatitis C antivirals: real-world challenges. Cancer. 2018;124(8):1647–1649. doi: 10.1002/cncr.31291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.