Abstract
Background
The emergence and spread of HIV-1 drug resistance may compromise HIV control globally. In response to HIV/AIDS epidemic, China launched national HIV/AIDS treatment program in 2003, and started to accumulate drug resistance data since 2001. In this study we aimed to assess the level, trend and distribution of HIV-1 drug resistance during a period of 17 years from 2001 to 2017, and to characterize crucial drug resistance mutations.
Methods
We systematically reviewed 4737 studies published between January 1, 2001 and March 31, 2019 in PubMed, Embase, China National Knowledge Infrastructure (CNKI), WanFang Database, Web of Science, conference abstracts from the Chinese Medical Association and the Chinese AIDS Academic Conferences, and selected 170 studies that met our study criteria. To assess the prevalence of drug resistance in whole country or a local region, we performed pooled analyses of raw data. The transformed proportions were pooled using the inverse variance fixed effects methods or the DerSimonian-Laired random effects methods. The temporal trend of transmitted drug resistance (TDR) was determined using generalized additive model implemented in the Mgcv version 1.8 package. HIV-1 genotypic resistance was analyzed using the Stanford HIVdb algorithm.
Findings
We assembled 218 datasets from 170 selected studies (129 in Chinese and 41 in English), covering 21,451 ART-naïve and 30,475 ART-treated individuals with HIV-1 infection. The pooled prevalence of TDR was 3.0% (95%CI: 2.8–3.2), including 0.7% (95%CI: 0.4–1.0), 1.4% (95%CI: 1.3–1.6) and 0.5% (95%CI: 0.4–0.6) for nucleoside reverse transcriptase inhibitor (NRTI), non-NRTI (NNRTI) and protease inhibitor (PI) resistance, respectively. The acquired drug resistance (ADR) prevalence was 44.7% (95%CI: 39.3–50.2), including 31.4% (95%CI: 28.2–34.6), 39.5% (95%CI: 35.6–43.5) and 1.0% (95%CI: 0.8–1.2) for NRTI, NNRTI and PI resistance, respectively. TDR and ADR prevalence had characteristic regional patterns. The worst prevalence of drug resistance occurred in Central China, and higher ADR prevalence occurred in South China than North China. TDR in whole country has risen since 2012, and this rise was driven mainly by NNRTI resistance. One NRTI-associated (M184V/I) and three NNRTI-associated (K103N/S, Y181C/I and G190A/S) mutations had high percentages in ART-naïve and ART-treated individuals, and these mutations conferred high-level resistance to 3TC, EFV and/or NVP.
Interpretation
These findings suggest that the current available first-line ART regimens containing 3TC and/or EFV or NVP need to be revised. In addition, scale-up of multiple viral load measurements per year and drug resistance testing prior to ART initiation are recommended. Furthermore, implementation of pre-treatment education and counseling to improve patient adherence to ART is encouraged.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81672033, U1302224, and 81271888) and Open Research Fund Program of the State Key Laboratory of Virology of China (2019IOV002).
Keywords: HIV-1, Drug resistance, China, Antiretroviral therapy (ART), Meta-analysis, Drug resistance mutation
Research in context.
Evidence before this study
We searched PubMed/Medline and Chinese literature databases up until March 31, 2019 for meta-analysis papers on HIV-1 drug resistance in China using the search terms used are (“HIV” OR “human immunodeficiency virus” OR “AIDS” OR “acquired immunodeficiency syndrome”), AND (“drug resistance” OR “drug resistant”) AND “China” AND “meta-analysis”. Six meta-analysis papers that contained original studies conducted before 2014 were found. This paper is the most comprehensive systematic review and meta-analysis that contained 170 original studies (129 in Chinese and 41 in English) and covered the data from 2001 to 2017.
Added value of this study
We present the overall prevalence of acquired (ADR) and transmitted (TDR) drug resistances in China, as well as the national trend of TDR from 2001 to 2017, and report the region-specific prevalence pattern of HIV-1 drug resistance in China. In addition, we report high percentages of one NRTI (M184V/I) and three NNRTI (K103N/S, Y181C/I and G190A/S) mutations in ART-naïve and ART-treated individuals, which confer high-level resistance to 3TC, EFV and/or NVP. The results provide first-hand information for non-Chinese researchers to include China data into the estimate of global prevalence trend and evolution of HIV-1 drug resistance.
Implications of all available evidence
Region-specific prevalence of HIV-1 drug resistance and SDRM profile provide new insights into the design of region-specific first-line and second-line ART regimens. Increased prevalence of HIV-1 drug resistance suggests that a number of strategies, including scale-up of multiple viral load measurements per year, optimization of ART regimens, and early counseling and pre-treatment education to improve ART adherence should be implemented in China in order to achieve the WHO's third 90% goal by 2020 and to eliminate AIDS epidemic by 2030.
Alt-text: Unlabelled box
1. Introduction
HIV/AIDS epidemic still remains a huge public health burden especially to low-income and middle-income countries (LMICs) [1]. In response to the global HIV/AIDS epidemic, WHO and UNAIDS launched in 2014 the “90–90–90” target to be achieved by 2020 [2], and set a global goal in 2016 to end the AIDS epidemic as a public health threat by 2030 [1]. The AIDS epidemic in China started among injection drug users (IDUs) in southwestern region (e.g., Yunnan province) of China in 1989 [3,4]. Since the mid-1990s, the large HIV/AIDS outbreak among former plasma donors (FPDs) in Central China (e.g., Henan province) and the rapid spread of HIV-1 infections to most provinces of the country raised the public attention and concern from the Chinese government. In response to the national HIV/AIDS epidemic, the Chinese government initiated the “Four Frees and One Care” policy to fight against HIV/AIDS in 2003, more than ten years earlier than the announcement of the “90–90–90” target by WHO and UNAIDS [4,5]. The policy includes 4 “Frees” (i. Free treatment, ii. Free voluntary counseling and testing, iii. Free prevention of mother to child transmission (MTCT), and, iv. Free schooling for AIDS orphans) and one “Care” (provision of social relief for AIDS patients) [4]. The costs were paid by government. By Oct. 30, 2019, the scale-up of antiretroviral therapy (ART) for the treatment of HIV/AIDS had reached 829,628 individuals, accounting for 86.6% of 958,000 estimated HIV-1 infections in China [6]. As a country with the largest population, the Chinese efforts on the prevention and control of HIV/AIDS are contributing substantially to the global goal of ending the AIDS epidemic as a public health threat by 2030.
ART is the most effective tool to prevent and control HIV/AIDS pandemic in the absence of an effective AIDS vaccine [7]. The national scale-up of ART for the treatment of HIV/AIDS has markedly reduced HIV/AIDS-related mortality from 39.3% in 2002 to 14.2% in 2009 in China [8]. However, long-term ART brought about a huge concern on the emergence of HIV-1 drug resistance [9]. Data showed that about 4.6%–57.1% of patients receiving first-line ART regimens in China had virological failure (VF: defined as having viral load >1000 copies/ml) after 12 months of treatment, and 39–%81.3% of the patients with VF carried drug-resistant HIV-1 variants, resulting in a primary drug resistance rate of 3.1–%47.1% [10], [11], [12], [13], [14], [15]. Because there are only a few free antiretroviral drugs (7 free drugs since 2014) for first-line and second-line ART regimens in China (Appendix 1), and HIV-1 genetic diversity is becoming more complex, increased prevalence of acquired HIV-1 drug resistance (ADR) and consequential transmission of drug-resistant virus to newly infected people have further increased the VF rate at a national level [16].
Some previous studies showed that the rates of VF and drug resistance varied in different cohorts and different provinces and regions of China, with the worst situation being observed in Henan province [[10], [11], [12], [13],17]. However, prevalence trend and pattern of HIV-1 drug resistance in treated and untreated individuals with HIV-1 are rarely systematically assessed at the national level since the rollout of ART in China in 2003. In this study, we aimed to comprehensively assess the levels, molecular epidemiological trends, and distributions of both ADR and transmitted drug resistance (TDR) by a meta-analysis. Additionally, we characterized the crucial drug resistance mutations that are responsible for ADR and TDR in different regions.
2. Methods
2.1. Search strategy and selection criteria
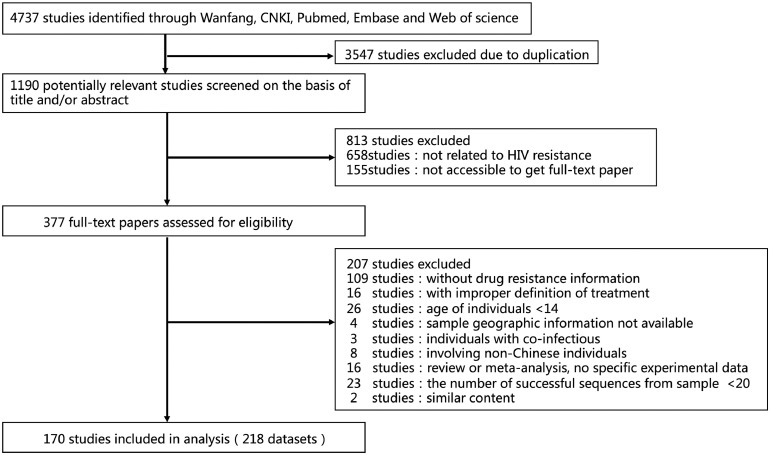
This study is a meta-analysis that systematically reviewed the regional and temporal trends of HIV-1 drug resistance among ART-naïve and ART-treated adult patients in China (Fig. 1). We conducted the research and reported the findings in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (Appendix 2) [18]. We searched for studies published between Jan. 1, 2001 to Mar. 31, 2019 in PubMed (Appendix 3), Embase, China National Knowledge Infrastructure (CNKI), WanFang Database, Web of Science, and conference abstracts from the Chinese Medical Association and the Chinese AIDS Academic Conferences. The searches were restricted to studies in English and Chinese using the search terms (“HIV” OR “AIDS”) AND “antiretroviral therapy” AND (“transmitted drug resistance” OR “naïve” OR “acquired drug resistance” OR “prevalence analysis” OR “infectious diseases surveillance”) AND “China”. We included studies of HIV-1-infected adults (aged >14 years) who were eligible for initiation of the first-line ART in China. The studies that used the WHO-recommended methods to assess resistance in ART-naïve patients and reported at least twenty HIV-1 pol sequences were included. We excluded studies focusing on non-Chinese patients, mother-to-child transmission patients, and co-infected patients. The studies that did not report detailed ART status (receiving or not receiving), information on drug resistance and/or geographical origin were excluded. The review papers and conference abstracts were also excluded from the analysis. We did not contact study authors for publically unavailable data.
Fig. 1.
Study selection.
Two reviewers LZ and HL individually assessed study quality and eligibility, and performed data extraction. Study quality was assessed according to the optimal Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [19,20] (Appendix 4). Any discrepancies were decided by LZ. The following data were extracted: the information of geographical origin (e.g., province and city), sampling year, receiving or non-receiving ART, duration of ART, risk group, transmission route, study type, CD4+ cell count, viral load (VL), numbers of patients with and without any surveillance drug resistance mutation (SDRM), nucleoside reverse transcriptase inhibitor (NRTI) mutations, non-nucleoside reverse transcriptase inhibitor (NNRTI) mutations, and protease inhibitor (PI) mutations.
2.2. Statistical analysis
To investigate the prevalence of TDR and ADR in different geographical regions of China and characterize the trend of TDR over time, we grouped the patients according to drug resistance types (i.e., NRTI, NNRTI and PI), sampling years and geographical locations. Based on the website of the Area Division Department, Ministry of Civil Affairs of China (http://www.xzqh.org.cn/), China contains seven major regions: North (Beijing, Hebei, Inner Mongolia, Shanxi, and Tianjin provinces/municipalities/autonomous region), East (Anhui, Fujian, Jiangsu, Jiangxi, Shanghai, Shandong, and Zhejiang provinces/municipality), Central (Henan, Hubei, and Hunan provinces), Northwest (Gansu, Qinghai, Ningxia, Shaanxi, and Xinjiang provinces/autonomous regions), South (Guangdong, Guangxi, and Hainan provinces), Southwest (Chongqing, Guizhou, Sichuan, Tibet, and Yunnan provinces/municipality/autonomous region), and Northeast China (Heilongjiang, Jilin, and Liaoning provinces) (Appendix 5). To assess the prevalence of drug resistance, we estimated pooled proportions of sequences with any SDRM (r) in all sequences (n). For studies including sequences with no mutation, the proportion was estimated as: 1 ÷ (4 × n). To minimize the variance of drug resistance rate across selected studies, the rate was transformed using the Freeman-Turkey double arcsine square root transformation [21].
The heterogeneity between studies was assessed using the I-squared statistic of the meta-analysis [22]. Because relatively few datasets were available for certain regions, and high heterogeneity was observed in the seven major regions, we performed further subgroup analysis according to North, South and Central China (Fig. 2). North China covers North, Northeast, and Northwest China. South China covers East, South, and Southwest China. We also performed subgroup analyses according to languages of studies and the duration time of ART to assess potential causes of heterogeneity (Appendix 6–8). We pooled the transformed proportions using the inverse variance fixed effects methods if I-squared <75%, or the DerSimonian-Laired random effects methods if I-squared >75% [22]. We also assessed publication bias using Egger test (Appendix 9–10). Meta-analysis was performed using Stata version 14.1. To assess the temporal trend of HIV-1 drug resistance prevalence in ART-naïve individuals in China or a specified region, we used generalized additive model (GAM) implemented in the Mgcv version 1.8 package to fit the trend data. The correlations between drug resistance and various characteristics (e.g., subtypes and regions) were performed using SPSS Statistics 23.0. The nonparametric comparison was performed using the Kruskal–Wallis method. Other comparisons were made using Chi squared test with Yates' correction or Fisher's exact test.
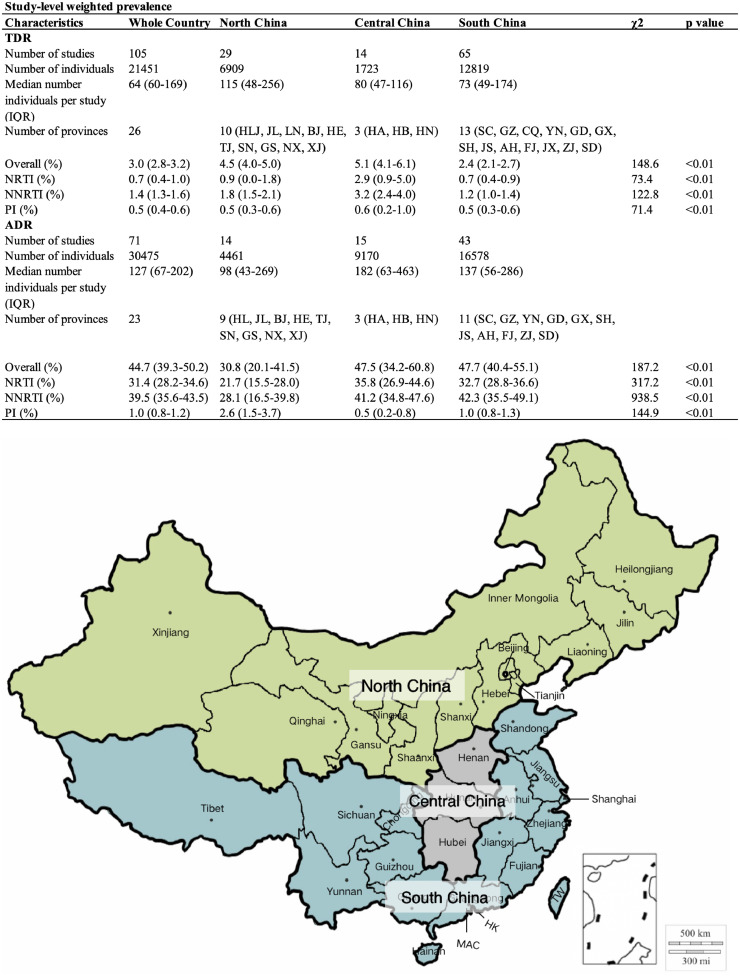
Fig. 2.
Prevalence of HIV-1 drug resistance by three major regions of China. North, Central and South China contain 14, 3, and 13 provinces, respectively. The three major regions are highlighted by different colors. NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. HLJ, Heilongjiang; JL, Jilin; LN, Liaoning; BJ, Beijing; TJ, Tianjing; SX, Shanxi; HE, Hebei; NM, Inner Mongoria IM; SN, Shaanxi; GS, Gansu; QH, Qinghai; NX, Ningxia; XJ, Xinjiang; GD, Guangdong; GX, Guangxi; HI, Hainan; SH, Shanghai; JS, Jiangsu; AH, Anhui; FJ, Fujian; JX, Jiangxi; SD, Shandong; SC, Sichuan; GZ, Guizhou; YN, Yunnan; CQ, Chongqing; XZ, Tibet; HA, Henan; HB, Hubei; HN, Hunan; HK, Hong Kang; MAC, Macao; TW, Taiwan.
2.3. Sequence analyses
To analyze HIV-1 SDRMs, we downloaded all available HIV-1 pol sequences in China from HIV database (https://www.hiv.lanl.gov) with a choice of “YES” or “NO” using advanced search term “drug naïve”. The mutations were determined according to the 2009 WHO SDRM list using the online tool (http://cpr.stanford.edu/cpr.cgi) [23]. HIV-1 genotypic resistance of each sequence with one or more SDRMs to antiretroviral drugs was analyzed using the HIVDB genotypic resistance interpretation system (HIVdb version 8.9:https://hivdb.stanford.edu/hivdb/by-mutations/) [24]. The percentage of a mutation was estimated by calculating its proportion after pooling the numbers of the sequences with this mutation and those with any SDRM.
2.4. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, and writing of the report.
3. Results
We initially identified 4737 study records from 5 electronic databases according to our search strategy. After removing records not meeting our eligibility criteria, 218 datasets from 170 studies (129 in Chinese and 41 in English) were subjected to our meta-analysis (Fig. 1). 105 studies focused on TDR and covered 21,451 individuals from 2001 to 2017 and 71 studies focused on ADR and covered 30,475 individuals from 2003 to 2016 were included. Among these studies, 51,660 sequences were used to determine drug resistance, with a median of 96 (IQR: 49-229) for each study. The median number of involved individuals per study was 64 (IQR: 60-169) for TDR and 127 (IQR: 67-202) for ADR. The involved specimens were collected from 2001 to 2017, and covered almost all provinces of China excluding Inner Mongolia (IM), Tibet (XZ), Qinghai (QH), Hainan (HaiN), Taiwan (TW), Hong Kong (HK) and Macao (MAC).
3.1. Estimated prevalence of HIV-1 drug resistance in China
The pooled TDR prevalence in China was 3.0% (95%CI: 2.8–3.2), including 0.7% (95%CI: 0.4–1.0) NRTI, 1.4% (95%CI: 1.3–1.6) NNRTI and 0.5% (95%CI: 0.4–0.6) PI resistance (Fig. 2). The ADR prevalence was 44.7% (95%CI: 39.3–50.2), including 31.4% (95%CI: 28.2–34.6) NRTI, 39.5% (95%CI: 35.6–43.5) NNRTI and 1.0% (95%CI: 0.8–1.2) PI resistance (Fig. 2). It was not surprising that the TDR prevalence was substantially lower than ADR prevalence, and NNRTI resistance was more prevalent than NRTI resistance. To avoid publication bias (Appendix 9 and 10), we re-calculated the national prevalence of drug resistance by re-analyzing all the raw data from included studies. The re-estimated prevalence of TDR and ADR was 4.5% and 35.9%, respectively, slightly different from model-estimated prevalence (4.5% vs. 3.8% for TDR, and 35.9% vs. 44.7% for ADR).
The spread of HIV-1 in China was generally from South to North [25,26]. Furthermore, a unique HIV/AIDS epidemic occurred mainly among FPDs in Central China [27]. To estimate the regional prevalence of HIV-1 drug resistance, we analyzed the data from Central, North and South China. The TDR prevalence had I-squared values of 42.3%–77.2% for three main regions, indicating a low degree of heterogeneity (Appendix 11–14). The pooled TDR prevalence was 4.5% (95%CI: 4.0–5.0), 5.1% (95%CI: 4.1–6.1) and 2.4% (95%CI: 2.1–2.7) in North, Central and South China, respectively (Fig. 2). A relatively high degree of heterogeneity was observed for ADR with I-squared values of 99.5%–99.8% in three main regions (Appendix 15–18). The pooled ADR prevalence was 30.8% (95%CI: 20.1–41.5), 47.5% (95%CI: 34.2–60.8) and 47.7% (95%CI: 40.4–55.1) in North, Central and South China, respectively (Fig. 2). Central China had the highest drug resistance prevalence regardless of TDR or ADR. North China appeared to have higher prevalence of TDR but lower ADR than South China (Fig. 2). Of particular importance is that PI resistance had a prevalence rate of 1.5% in ART-naïve HIV-1 infected individuals, similar to 1.7% in ART-treated individuals.
The difference in geographic prevalence of drug resistance was observed between Central China and other regions, as well as between South and North China. Similar trend of HIV-1 drug resistance prevalence in geographic regions was also observed when seven main regions of China were taken into account (Appendix 5).
3.2. Temporal trend of HIV-1 drug resistance prevalence in ART-naïve individuals in China
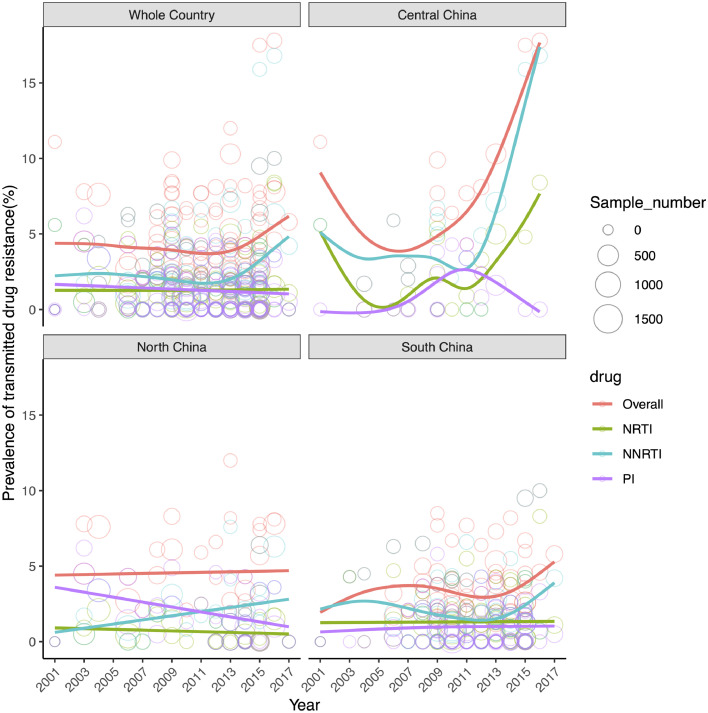
China has an overall rising trend of HIV-1 drug resistance in ART-naïve individuals from 2001 to 2017 (Fig. 3). A slow decrease of TDR from 4.75% to 3.75% was observed during 2001–2011, followed by a rapid increase from 3.75% in 2012 to 6.25% in 2017. The trend of resistance to NNRTI decreased from 2.25% in 2001 to 1.75% in 2012, and then increased from 1.75% in 2012 to 5.0% in 2017; whereas the trends of NRTI and PI remained stable. The trend of NNRTI resistance was well consistent with the overall trend of HIV-1 drug resistance, indicating that HIV-1 drug resistance was mainly driven by NNRTI resistance.
Fig. 3.
Temporal trend of drug resistance prevalence in ART-naive individuals with HIV by three major regions of China since 2001. Every circle represents a study and the size of the circle is proportional to the number of samples successfully sequenced in that study. The colored lines showed the predicted prevalence trends. The abbreviations of NRTI, NNRTI, and PI are provided in Fig. 2.
The prevalence of three classes of drug resistance in ART-naïve individuals appeared to be more serious and fluctuant in Central China than in South and North China (Fig. 3 and Appendix 19). In Central China, TDR showed a rising prevalence trend from 2001 to 2017. The dynamics showed a rapid decrease from 9.0% in 2001 to 4.0% in 2007, followed by a steep increase from 4.0% in 2007 to 17.75% in 2017 at an average growth of 1.4% per year. The prevalence trends of NNRTI and NRTI resistances were similar to the overall pattern that had a first decrease and then an increase. A difference was that the prevalence of PI resistance was relatively stable from 2005 to 2017 with a small peak (2.75%) occurring in 2011. In South China, HIV-1 drug resistance showed a rising trend from 2001 to 2017 with an episode of decrease from 2007 to 2012 (Fig. 3). In North China, the overall prevalence of HIV-1 drug resistance remained fairly stable from 2001 to 2017 (Fig. 3). The prevalence of NNRTI appeared to slowly increase from 0.5% in 2001 to 3.0% in 2017, accompanied by a slow decrease in PI resistance from 3.75% to 1.75%.
3.3. HIV-1 SDRMs in China
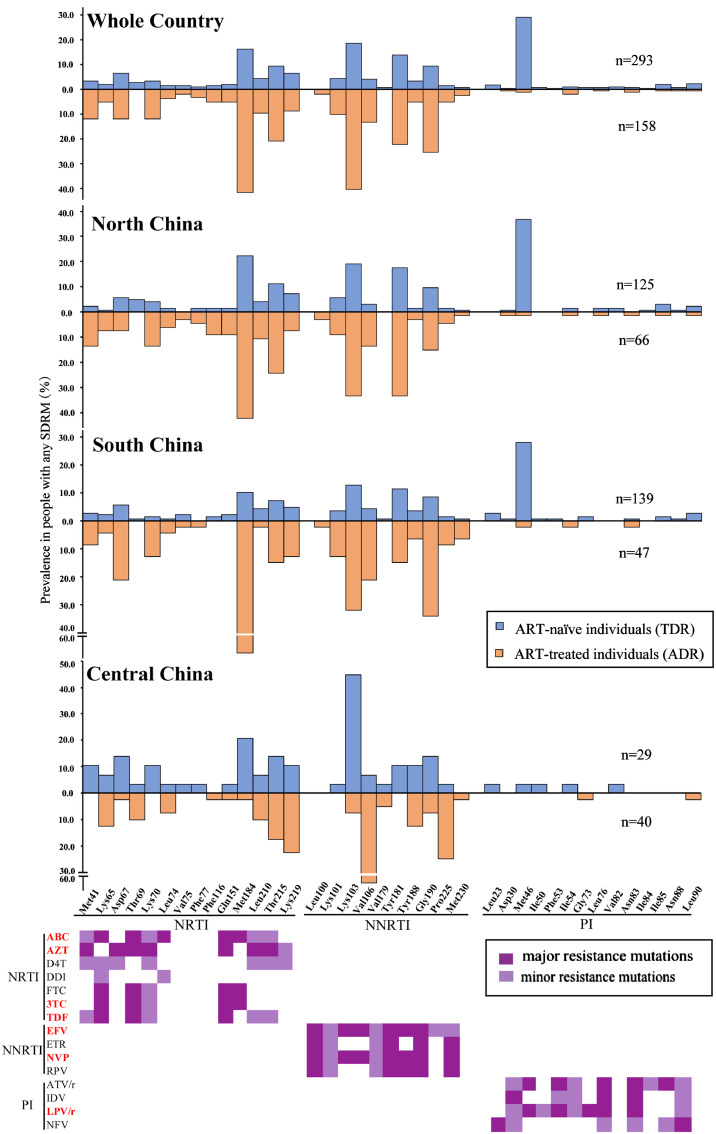
Having described the overall prevalence of HIV-1 drug resistance, we went on to examine which mutations contributed to drug resistance. From HIV database (https://www.hiv.lanl.gov), we downloaded 9010 available HIV-1 pol sequences from China, including 8439 and 571 sequences from ART-naïve and ART-treated individuals, respectively. There were 293 (3.5%) and 158 (27.7%) sequences carrying at least one SDRM to be identified from ART-naïve and ART-treated individuals, respectively. The SDRM profile appeared to be very different between ART-naïve and ART-treated individuals at the national level, especially for PI mutations (Fig. 4). In spite of this, the most common NRTI and NNRTI mutations observed in ART-naïve and ART-treated individuals were well consistent. The top NRTI mutations identified in ART-naïve and ART-treated individuals were M184V/I (16.3% and 41.8%) and T215I/Y/S/D/F (20.9% and 9.5%), and the top NNRTI mutations were K103N/S (18.7% and 40.5%), Y181C/I (14.0% and 22.2%), and G190A/S (9.5% and 22.2%, respectively). Furthermore, we found a very high proportion (29.4%) of PI mutation M46I/L in ART-naïve individuals, significantly higher than that in ART-treated individuals (1.3%) (Fig. 4). The most commonly observed SDRM are involved in HIV-1 resistance to 10 antiretroviral drugs (including NRTIs, NNRTIs and PIs) available in China, including 5 free drugs (i.e., AZT, 3TC, EFV, NVP and LPV/r) used in first- and/or second-line regimens (Fig. 4 and Appendix 1).
Fig. 4.
Estimated prevalence of HIV-1 drug resistance mutations in ART-naïve and ART-treated individuals with any SDRM. The major and minor resistance mutations are highlighted by dark and light violets, respectively. ABC, abacavir; AZT, zidovudine; D4T, stavudine; DDI, didanosine; FTC, emtricitabine; 3TC, lamivudine; TDF, tenofovir; DOR, doracirine; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; RPV, rilpivirine; ATV, atazanavir; IDV, indinavir; LPV/r, lopinavir/ritonavir; NFV, nelfinavir.
The SDRM profile (especially several most commonly observed SDRMs) varied largely in different regions of China (Fig. 4). Although the SDRM profiles in North and South China were similar to each other and to the national ones, lower percentages of NRTI and NNRTI mutations were observed in ART-naïve individuals in South China than in North China. Another difference was that a very high proportion of M184V/I (NRTI mutation) occurred in ART-treated individuals in South China (66.0%), higher than that in North China (42.4%). Dramatically distinct from other regions, Central China had a unique mutation profile. The two most commonly observed SDRMs M184V/I (NRTI) and M46I/L (PI) in North and South China were rarely found in Central China (Fig. 4). Importantly, a very high percentage of K103N/S (18.71%) was observed in ART-naïve, but rarely seen in ART-treated individuals in Central China. In contrast, a high percentage of this mutation occurred in ART-treated but rarely observed in ART-naïve individuals in other regions. Furthermore, a higher percentage of V106A/M (13.29%) in ART-treated individuals was observed in Central China than other regions.
3.4. Drug sensitivity of sequences with SDRMs
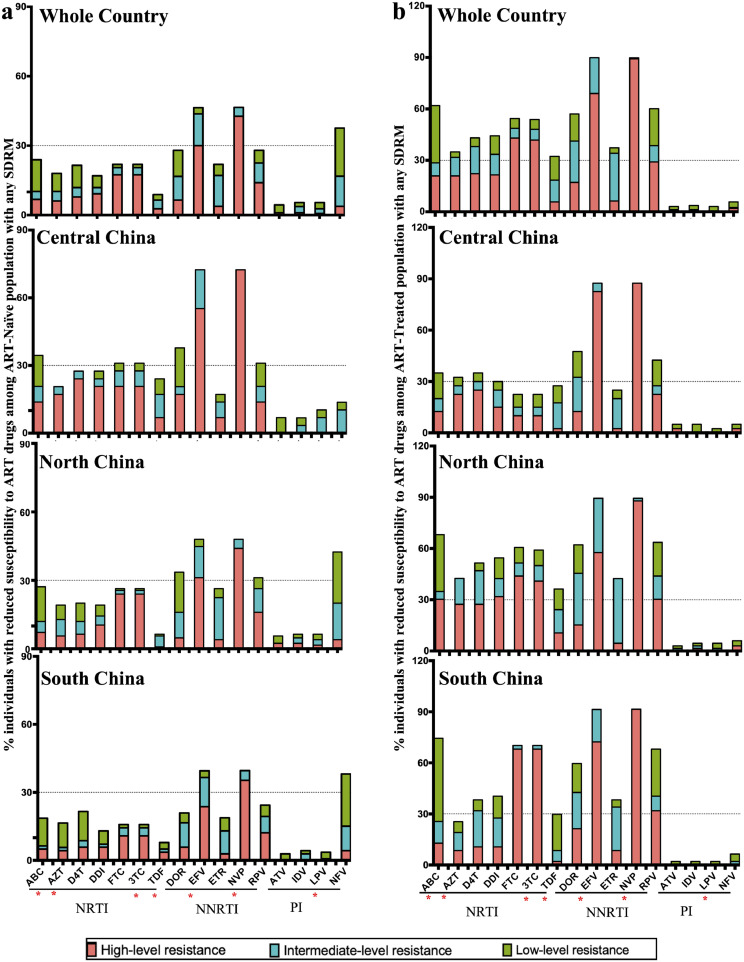
We further analyzed the resistance levels of sequences with any SDRM to 13 NRTI, NNRTI and PI drugs available in China (Fig. 5). In ART-naïve individuals, the proportion of sequences with any SDRM ranged from 2.7% (TDF) to 17.1% (3TC/FTC) for seven NRTI drugs, from 3.75% (ETR) to 42.7% (NVP) for five NNRTI drugs, and 0.7% (LPV/r) to 3.8% (NFV) for PI drugs (Fig. 5(a)). Although TDR-associated SDRM profile to antiretroviral drugs varied in different regions, resistance to two free NNRTIs EFV and NVP was very common at the national level with the worst in Central China. TDR to NFV was common in North (4.0%) and South (4.3%) China, but rare in Central China. Of particular importance is that almost all resistant strains to EFV and NVP showed intermediate- and high-level of resistance (Fig. 5(a)).
Fig. 5.
Estimated levels of predicted genotypic drug resistance for samples successfully sequenced with any SDRM. (a) ART-naïve individuals with HIV; (b) ART-treated individuals with HIV. The abbreviations of antiretroviral drugs are provided in Fig. 4 and Appendix 1.
In ART-treated individuals, the proportion of sequences with any SDRM ranged from 5.7% (TDF) to 43.0% (FTC) for NRTI drugs, and from 6.3% (ETR) to 89.2% (NVP) for NNRTI drugs (Fig. 5(b)). Over 50% resistance strains showed resistance to three NRTI drugs (ABC, FTC and 3TC) and four NNRTI drugs (DOR, EFV, NVP and RPV) in North and South China. Intermediate- and high-level resistance to two NNRTI drugs EFV (88%) and NVP (90%), as well as two NRTI drugs 3TC (48.1%) and FTC (48.7%) was common in the whole country. Resistance to PI drugs was rarely found in ART-treated individuals (0–1.9%). Central China had relatively lower percentage of resistance to NNRTI and NRTI drugs, except for EFV (82.5%) and NVP (87.5%).
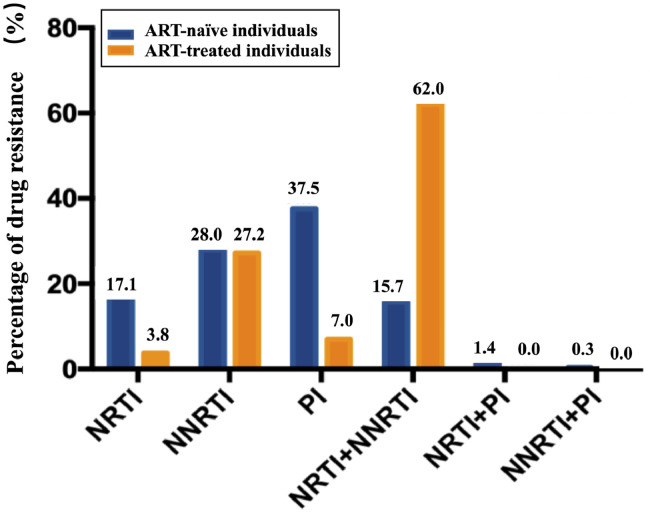
We further evaluated the percentages of TDR- and ADR-associated SDRMs to three classes of antiretroviral drugs. The NNRTI resistance was common in both ART-naïve (28.0%) and ART-treated (27.2%) individuals (Fig. 6). NRTI (17.1% vs. 3.8%) and PI (37.5% vs. 7.0%) resistance was more common in ART-naïve individuals than ART-treated individuals. A significantly higher percentage of NRTI/NNRTI dual-class resistance was observed in ART-treated individuals (62.0%) than ART-naïve individuals (15.7%) (Fig. 6).
Fig. 6.
Prevalence of HIV drug resistance in ART-naïve and ART-treated individuals with HIV by drug class. The abbreviations of NRTI, NNRTI, and PI are provided in Fig. 2.
3.5. Correlation between SDRMs and virus subtypes
There are 76 (25.9%) subtype B, 151 (51.5%) CRF01_AE, 27 (9.2%) CRF07_BC, and 39 (13.3%) other subtypes in 293 HIV-1 pol sequences with any SDRM in ART-naïve individuals. The SDRM profile was very different in subtype B, CRF01_AE and CRF07_BC sequences (Appendix 20). Four mutations T69D (NRTI), K103N/S (NNRTI), M46I/L (PI) and L90M (PI) showed significantly different percentages in different subtypes (p < 0.05) (Table 1). Low percentages of NRTI and NNRTI mutations, but a very high percentage (47.7%) of PI mutation (M46I/L) occurred in CRF01_AE; in contrast, high percentages of NRTI (M184I/V) and NNRTI (K103N/S and Y181C/I) mutations were observed in subtype B. For CRF07_BC, NNRTI mutations appeared to be more frequent than NRTI and PI mutations.
Table 1.
HIV drug resistance mutation profiles in ART-naïve individuals by subtypes.
| Mutation Proportion and (No.) (%) |
B n = 76 | CRF01_AE n = 151 | CRF07_BC n = 27 | CRF08_BC n = 6 | Others n = 33 | Total n = 293 | X2 | P |
|---|---|---|---|---|---|---|---|---|
| Resistance to nucleoside reverse transcriptase inhibitors (NRTIs) | ||||||||
| M41L | 7.9 (6) | 1.3 (2) | 3.7 (1) | 3 (1) | 3.4 (10) | 6.47 | 0.11 | |
| K65R | 2.6 (2) | 1.3 (2) | 3.7 (1) | 3 (1) | 2.0 (6) | 2.769 | 0.551 | |
| D67G/N/E/H/T | 10.5 (8) | 4.6 (7) | 12.1 (4) | 6.5 (19) | 6.114 | 0.147 | ||
| T69D/I | 5.3 (4) | 0.7 (1) | 16.7 (1) | 6.1 (2) | 2.7 (8) | 10.105 | 0.019 | |
| K70R/E | 7.9 (6) | 2.7 (4) | 3.4 (10) | 5.022 | 0.224 | |||
| L74I/V | 2.6 (2) | 1.3 (2) | 1.4 (4) | 2.092 | 0.858 | |||
| V75M/T/A/S/I | 2.6 (2) | 1.3 (2) | 1.4 (4) | 2.092 | 0.858 | |||
| F77L | 4.0 (3) | 1.0 (3) | 6.92 | 0.131 | ||||
| F116Y | 4.0 (3) | 0.7 (1) | 1.4 (4) | 4.316 | 0.312 | |||
| G151M | 5.3 (4) | 1.3 (2) | 2.0 (6) | 4.135 | 0.31 | |||
| M184V/I | 26.3 (20) | 11.9 (18) | 11.1 (3) | 21.2 (7) | 16.4 (48) | 8.859 | 0.053 | |
| L210W | 6.6 (5) | 4.6 (7) | 3 (1) | 4.4 (13) | 1.713 | 0.743 | ||
| T215F/I/Y/S | 14.5 (11) | 6.6 (10) | 7.4 (2) | 16.7 (1) | 12.1 (4) | 9.6 (28) | 5.011 | 0.251 |
| K219E/Q/N/R | 9.2 (7) | 5.3 (8) | 7.4 (2) | 6.1 (2) | 6.5 (19) | 1.621 | 0.787 | |
| Resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) | ||||||||
| K101E/Q/H/P | 6.6 (5) | 2 (3) | 6.1 (2) | 3.4 (10) | 4.648 | 0.265 | ||
| K103N/S | 39.5 (30) | 7.3 (11) | 37 (10) | 12.1 (4) | 18.8 (55) | 40.268 | 0 | |
| V106A/M | 5.3 (4) | 3.3 (5) | 3.7 (1) | 6.1 (2) | 4.1 (12) | 1.515 | 0.808 | |
| V179F/D/E | 1.3 (1) | 0.7 (1) | 0.7 (2) | 3.004 | 1 | |||
| Y181C/V/I/F | 18.4 (14) | 9.9 (15) | 11.1 (3) | 27.3 (9) | 14.0 (41) | 8.189 | 0.069 | |
| Y188C/L/H | 6.6 (5) | 2 (3) | 7.4 (2) | 3.4 (10) | 5.464 | 0.175 | ||
| G190A/S/E | 15.8 (12) | 7.3 (11) | 15.2 (5) | 9.6 (28) | 8.32 | 0.061 | ||
| P225H | 1.3 (2) | 7.4 (2) | 1.4 (4) | 6.28 | 0.138 | |||
| M230L | 1.3 (1) | 0.7 (1) | 0.7 (2) | 3.004 | 1 | |||
| Resistance to protease inhibitors (PIs) | ||||||||
| L23I | 1.3 (1) | 1.3 (2) | 7.4 (2) | 1.7 (5) | 4.854 | 0.264 | ||
| D30N | 0.7 (1) | 0.3 (1) | 4.457 | 1 | ||||
| M46I/L | 5.3 (4) | 47.7 (72) | 7.4 (2) | 33.3 (2) | 18.2 (6) | 29.4 (86) | 58.792 | 0 |
| I50V/L | 1.3 (1) | 0.7 (1) | 0.7 (2) | 3.004 | 1 | |||
| F53L/Y | 0.7 (1) | 0.3 (1) | 4.457 | 1 | ||||
| I54V/I | 1.3 (1) | 6.1 (2) | 1.0 (3) | 8.041 | 0.052 | |||
| G73S | 0.7 (1) | 3 (1) | 0.7 (2) | 4.672 | 0.467 | |||
| L76V | 1.3 (2) | 0.7 (2) | 3.03 | 0.732 | ||||
| V82A/F | 1.3 (1) | 6.1 (2) | 1.0 (3) | 8.041 | 0.052 | |||
| N83D | 1.3 (1) | 0.7 (1) | 0.7 (2) | 3.004 | 1 | |||
| I84V | 0.7 (1) | 0.3 (1) | 4.457 | 1 | ||||
| I85V | 2.6 (2) | 1.3 (2) | 6.1 (2) | 2.0 (6) | 3.816 | 0.386 | ||
| N88D/S | 1.3 (1) | 0.7 (1) | 0.7 (2) | 3.004 | 1 | |||
| L90M | 2.6 (2) | 7.4 (2) | 9.1 (3) | 2.4 (7) | 12.917 | 0.004 | ||
The vast majority of the M46I/L mutation occurred in CRF01_AE in ART-naïve individuals (Table 1), suggesting that this mutation was likely a consequence of natural polymorphism of HIV-1. To address this issue, we performed phylogenetic analysis using all available sequences with any SDRM. The phylogenetic tree showed that the majority of M46I/L-carrying CRF01_AE strains formed genetic clusters with a few dispersed within the strains without this mutation (Appendix 21), suggesting that most M46I/L-carrying CRF01_AE strains were more likely transmitted rather than generated by natural polymorphism. In contrast, most M46I/L variants in other subtypes were scattered within the strains without this mutation, implying natural polymorphism of HIV-1. Furthermore, very high percentages of M184I/V (26.3%) and K103N/S (39.5%) were found in subtype B strains in ART-naïve individuals (Table 1), and some of them were scattered within the strains without the mutations, implying natural polymorphism of HIV-1 (Appendix 22). Because the majority of these strains were isolated from Central China where there was a long history of ART, a few of them were not excluded due to undisclosed ART or prior exposures to ART.
Among 158 sequences with any SDRM in ART-treated individuals, 77 (48.7%) belonged to subtype B, 40 (25.3%) CRF01_AE, 15 (9.5%) CRF07_BC, 16 (10.1%) CRF08_BC and 10 (6.3%) other subtypes. The percentages of four mutations K65R (NRTI), M184I/V (NRTI), G190A/S (NNRTI) and M230L (NNRTI) were significantly correlated with subtypes (p < 0.05) (Table 2). Subtype B had an obviously different ADR mutation profile from other subtypes (e.g. CRF01_AE, CRF07_BC and CRF08_BC) (Appendix 20). In NRTI mutations, T215I/Y/S/N/F/E had the highest percentage (31.2%) in subtype B, whereas M184I/V had the most predominant percentage (43.8–73.3%) in CRF01_AE and other subtypes (Table 2 and Appendix 20). In NNRTI mutations, K103N/S and G190N/S were the two most common mutations in almost all subtypes (Table 2). Furthermore, Y181C/I was predominant in B, CRF01_AE and other subtypes, whereas V106aA/M was predominant in CRF07_BC. There was very low percentage of PI mutations in all subtypes in ART-treated individuals.
Table 2.
HIV drug resistance mutation profiles in ART-treated individuals by subtypes.
| Mutation Proportion and (No.) (%) |
B n = 77 | CRF01_AE n = 40 | CRF07_BC n = 15 | CRF08_BC n = 16 | Others n = 10 | Total n = 158 | X2 | P |
|---|---|---|---|---|---|---|---|---|
| Resistance to nucleoside reverse transcriptase inhibitors (NRTIs) | ||||||||
| M41L | 16.9 (13) | 10.0 (4) | 6.7 (1) | 10.0 (1) | 12 (19) | 3.769 | 0.408 | |
| K65R | 1.3 (1) | 7.5 (3) | 20.0 (3) | 10.0 (1) | 5.1 (8) | 9.538 | 0.019 | |
| D67G/N/E/H/T | 10.4 (8) | 15.0 (6) | 13.3 (2) | 6.3 (1) | 20.0 (2) | 12 (19) | 1.998 | 0.75 |
| K70R/E | 11.7 (9) | 15.0 (6) | 13.3 (2) | 6.3 (1) | 10.0 (1) | 12 (19) | 0.932 | 0.947 |
| L74I/V | 2.6 (2) | 2.5 (1) | 20.0 (3) | 3.8 (6) | 6.953 | 0.073 | ||
| V75M/T/A/S/I | 1.3 (1) | 5.0 (2) | 1.9 (3) | 2.575 | 0.705 | |||
| F77L | 2.6 (2) | 7.5 (3) | 3.2 (5) | 2.4 | 0.608 | |||
| F116Y | 7.8 (6) | 5.0 (2) | 5.1 (8) | 1.581 | 0.81 | |||
| G151M | 7.8 (6) | 5.0 (2) | 5.1 (8) | 1.581 | 0.81 | |||
| M184V/I | 19.5 (15) | 67.5 (27) | 73.3 (11) | 43.8 (7) | 60.0 (6) | 41.8 (66) | 34.954 | 0 |
| L210W | 16.7 (13) | 5.0 (2) | 9.5 (15) | 7.412 | 0.074 | |||
| T215F/I/Y/S | 31.2 (24) | 12.5 (5) | 6.7 (1) | 12.5 (2) | 10.0 (1) | 20.9 (33) | 8.635 | 0.059 |
| K219E/Q/N/R | 7.8 (6) | 7.5 (3) | 13.3 (2) | 12.5 (2) | 10.0 (1) | 8.9 (14) | 1.692 | 0.82 |
| Resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) | ||||||||
| L100I | 20.0 (3) | 1.9 (3) | 6.185 | 0.127 | ||||
| K101E/Q/H/P | 9.1 (7) | 17.5 (7) | 12.5 (2) | 10 (16) | 4.312 | 0.31 | ||
| K103N/S | 40.3 (31) | 32.5 (13) | 60.0 (9) | 37.5 (6) | 50.0 (5) | 41 (64) | 3.876 | 0.427 |
| V106A/M | 9.1 (7) | 17.5 (7) | 26.7 (4) | 6.3 (1) | 20.0 (2) | 13.3 (21) | 5.346 | 0.216 |
| V179F/D/E | ||||||||
| Y181C/V/I/F | 27.3 (21) | 25.0 (10) | 6.3 (1) | 30.0 (3) | 22.2 (35) | 8.986 | 0.051 | |
| Y188C/L/H | 3.9 (3) | 5.0 (2) | 6.7 (1) | 6.3 (1) | 10.0 (1) | 5.1 (8) | 2.182 | 0.669 |
| G190A/S/E | 19.5 (15) | 32.5 (13) | 6.7 (1) | 50 (8) | 30.0 (3) | 25.3 (40) | 10.169 | 0.031 |
| P225H | 2.6 (2) | 5.0 (2) | 13.3 (2) | 6.3 (1) | 10.0 (1) | 5.1 (8) | 4.728 | 0.235 |
| M230L | 10.0 (4) | 2.5 (4) | 7.925 | 0.037 | ||||
| Resistance to protease inhibitors (PIs) | ||||||||
| L23I | ||||||||
| D30N | 1.3 (1) | 0.6 (1) | 3.609 | 1 | ||||
| M46I/L | 1.3 (1) | 2.5 (1) | 1.3 (2) | 2.21 | 1 | |||
| I50V/L | ||||||||
| F53L/Y | ||||||||
| I54V/I | 2.6 (2) | 6.3 (1) | 1.9 (3) | 3.074 | 0.536 | |||
| G73S | ||||||||
| L76V | 10.0 (1) | 0.6 (1) | 7.691 | 0.063 | ||||
| V82A/F | ||||||||
| N83D | 1.3 (1) | 6.3 (1) | 1.3 (2) | 4.043 | 0.516 | |||
| I84V | ||||||||
| I85V | 2.5 (1) | 0.6 (1) | 4.918 | 0.513 | ||||
| N88D/S | 1.3 (1) | 0.6 (1) | 3.609 | 1 | ||||
| L90M | 1.3 (1) | 0.6 (1) | 3.609 | 1 | ||||
4. Discussion
We systemically investigated the overall prevalence of HIV-1 drug resistance in ART-naïve and ART-treated HIV-infected individuals in China, and analyzed the spatio-temporal trend of TDR from 2001 to 2017 by a system review and meta-analysis. Three important observations were presented here. First, TDR and ADR prevalence had region-specific patterns with the worst in Central China. Second, TDR was rapidly rising in China in recent years (typically since 2012), and this rise was mainly driven by NNRTI resistance. Third, two NRTI (M184V/I and K65R), three NNRTI (K103N/S, Y181C/I and G190A/S) and one PI (M46I/L) mutations had higher prevalence than other SDRMs in China. These observations have crucial implications for policy formulation and clinical decision-making for the prevention and control of HIV/AIDS in China.
The regional difference in HIV-1 drug resistance was mainly ascribed to various coverage rates and durations of ART since its introduction. China had a complex HIV-1 genetic diversity and experienced a distinct history of HIV-1 epidemic from other countries/regions [25,26,28]. Early HIV-1 epidemic occurred mainly among IDUs in southwestern border regions [29]. The virus spread to Central China and other regions of China with different subtypes via different transmission routes [25,26,28,30]. In general, the national spread of HIV-1 was from South to North [25,26]. Relative to North China, South China had an earlier and higher prevalence of HIV-1; therefore started ART program earlier [31]. Variable coverage rates and durations of ART may cause the difference in prevalence of HIV-1 drug resistance between South and North China and provide an explanation for higher prevalence of ADR in South China than North China.
The national free ART program started from Central China [32]. In the early-mid 1990s, many small unlicensed commercial plasma collection stations were established in rural areas of Central China (especially Henan province), and tens of thousands of plasma donors were paid for blood donation. After removing plasma, pooled red blood cells from blood type-matched donors were re-infused into plasma donors to prevent anemia. The unsanitary practices (e.g. returning of pooled red blood cells from different donors) together with the reuse of contaminated instruments caused thousands of FPDs to be infected by HIV-1 until illegal blood donation (IBD) was banned in 1996 [33,34]. Besides, many people who received contaminated blood transfusions/blood products were also infected by HIV-1. In late 1990s and early 2000s, the vast majority of HIV-infected FPDs in Central China developed clinical AIDS, which raised huge concern from Chinese government and facilitated the implementation of free ART program for AIDS patients with low-income or living in rural areas. The earliest free ART program was initially piloted in late 2002 in Shangcai County, Henan Province, one of the worst areas affected by illegal blood donation (IBD)-associated HIV-1 infection [16]. The free ART program was rapidly rolled out to all affected areas in Central China after the implementation of the national “Four Frees and One Care” policy in 2003 [5]. Rapid expansion of ART inevitably led to the emergence and transmission of HIV-1 drug resistance. Compared to Central China, South and North China initiated the free ART program later. For example, Yunnan, another worst hit province by HIV/AIDS launched the free ART program in 2004, one to two years later than Henan [35]. Furthermore, Central China had higher ART coverage compared with South and North China [36]. Higher coverage rate and longer duration of access to ART might explain the highest ADR and TDR prevalence in Central China [17,37,38]. Another potential explanation is that the majority of ART-treated individuals in Central China live in rural areas, and have relatively lower adherence to treatment, which facilitates the emergence and transmission of HIV-1 drug resistance [39].
To guide the national free ART Program, four editions of the China Free ART Manual had been released from 2004 to 2016. Accordingly, available antiretroviral drugs increased from 8 in 2004 to 17 in 2019 (Appendix 1), of which 7 drugs, including 4 NRTI (AZT/ZDV, 3TC, TDF and ABC), 2 NNRTI (EFV and NVP) and 1 PI (LPV/r) drugs, were free for use in the first- and second-line ART regimens. Accompanied with the increase of individuals receiving ART from 80,000 in 2003 to 154,620 in 2012 [40], the virological suppression rate increased from 78% in 2004–2005 to 90% in 2013 [41], and accordingly the TDR prevalence declined from 4.75% in 2001 to 3.75% in 2012. During the same period, there was only 11 available antiretroviral drugs, of which just 5–8 free drugs were used in the first- and second-line ART regimens. Furthermore, only AIDS patients with CD4+ T cell counts less than 200 and 350 cells/μL are eligible to free ART before 2008 and 2013, respectively [42]. Long duration of few antiretroviral drugs resulted in the accumulation of resistance mutations, which drives the rise of ADR, especially in some rural areas of Central China [17,39]. Furthermore, drugs out of supply and treatment interruptions also contributed to the emergence of ADR [43], [44], [45].
As a crucial component, NNRTI drugs are recommended to be used in the first-line ART regimens in the low-income and middle-income countries by WHO in 2016 [46]. EFV and NVP are two widely-used NNRTI drugs in the first-line ART regimens especially in LMICs. It was well demonstrated that a single point mutation can confer HIV-1 resistance to EFV and NVP, and affect the efficacy of ART regimens containing NNRTI [47,48]. In China, EFV or NVP was used in the first-line ART regimens since 2004, and retained today, in spite of two additional NNRTI drugs were recently introduced into China (as self-paid drugs). Strong selection pressure by long-term use of limited number of NNRTI drugs facilitated the generation and spread of NNRTI-associated drug resistance [49]. High prevalence of NNRTI mutations (e.g. K103N, Y181C/I and G190S/A) conferred high percentages (88–90%) of high- and intermediate-level resistance to EFV and NVP in ART-treated individuals across the country. Of particular importance is that a high prevalence of NNRTI resistance to EFV and NVP also appeared in HIV-1 naïve individuals, leading to an increase of NNRTI-associated TDR since 2012. The most serious TDR to EFV and NVP occurred in Central China where they conferred over 50% high- and intermediate-level resistance and contributed to more than 10% NNRTI resistance. The dynamics decrease of TDR in Central China during 2001 to 2007 might be explained by a dramatic decrease of new HIV-1 infections, benefitted from massive scale-up of ART, whereas the sharp rise of TDR after 2012 might be mainly due to primary infection by HIV-1 drug resistant strains from ART-treated individuals [50,51].
Another point of concern is the free NRTI drug 3TC, which was listed and used in almost all the first- and second-line ART regimens during the past almost two decades in China. The M184V/I mutation confers a high proportion of high- and intermediate-level resistance to 3TC, as well as FTC (self-paid NRTI drug) [48,52], and has a high fitness cost for virus by reducing the replicative capacity of reverse transcriptase (RT) [53]. Although there was still a controversy on whether continued use of 3TC provides actual benefit, some studies demonstrated that maintaining 3TC treatment in the presence of the M184V mutation resulted in a greater risk for virologic failure, compared with switching to other NRTI drugs [54,55]. The observation of a high percentage of M184V/I in ART-treated and ART-naïve individuals across the country suggests revision in the treatment regimens. Similarly, another free NRTI drug AZT, which was often used in the first-line ART regimens, also faced an increased resistance by high percentage of mutation T215D/S/C among both ART-treated and ART-naïve individuals. In particular, the NRTI/NNRTI dual-class resistances accounted for 62.0% of ART-treated individuals with emerging VF. Such a high percentage of NRTI/NNRTI dual-class resistance highlights an urgent need to revise the current ART regimens in China.
Current available antiretroviral drugs in China contain 6 NRTIs, 4 NNRTIs, 3 PIs, 4 integrase inhibitors (INSTIs), one co-receptor antagonist, and one fusion inhibitor, as well as various combination of them. The free drugs contain 4 NRTIs (AZT, 3TC, TDF and ABC), 2 NNRTIs (EFV and NVP) and 1 PI (LPV/r). Five of them are currently used in the first- and second-line ART regimens. HIV-1 drug resistance profile appeared to vary largely in ART-naïve and ART-treated individuals in different regions. Development of region-specific ART regimens based on local SDRM profile may help to reduce drug resistance prevalence and VF rate, and therefore are strongly encouraged [21,56,57]. Since 2016, all newly diagnosed HIV-infected individuals are required to receive ART. By October 2019, 829,628 individuals have received ART [6]. The massive scale-up of ART will inevitably increase the threat of TDR to the national AIDS elimination effort. Our results showed that NNRTI-associated TDR had exceeded 10% in Central China since 2015, a warning threshold recommended by WHO to take urgent actions to counter the threat from TDR [58]. New first-line ART regimens without 3TC and/or EFV or NVP are recommended to be developed and used in Central China as a pilot. For other regions, the resistance mutations to 3TC, EFV and NVP should be closely monitored. In particular, drug-resistance testing prior to ART initiation should be performed to guide the first-line ART regimen selection before initiating ART. On the other hand, relative to developed countries, far fewer antiretroviral drugs are available in China. Introduction of more antiretroviral drugs should be considered, and development of new long-acting antiretroviral drugs with independent intellectual property rights should be especially encouraged in China [59].
Previous studies had showed that HIV-1 subtypes had different TDR resistance characteristics [41]. Here, we found that various subtypes exhibited variable resistance mutation profiles in ART-naïve and ART-treated individuals. Three major subtypes B, CRF01_AE and CRF07_BC had high percentages of several high-level NNRTI resistance mutations regardless of in ART-naïve and ART-treated individuals. Distinct from subtype B that had higher percentage of NRTI mutation M184V/I (26.3% vs. 11.9% and 11.1%) in ART-naïve individuals than in ART-treated individuals, CRF01_AE and CRF07_BC had higher percentage of this mutation (67.5% and 73.3% vs. 19.5%) in ART-treated individuals than in ART-naïve individuals. Furthermore, high percentage of PI mutation M46I/L (2.4%) was observed in CRF01_AE strains in ART-naïve individuals, which was substantially higher than those (0.6–1.7%) observed in the surrounding countries (e.g. Thailand and Vietnam) [60,61]. M46I/L is a major mutation conferring resistance to PIs individually or together with other mutations [62,63]. Although natural polymorphism of M46I was reported in multiple HIV-1 subtypes (especially subtypes F and G) in ART-naïve individuals, such high percentage of this mutation in CRF01_AE strains in Chinese ART-naïve individuals was less likely a consequence of natural polymorphisms of the virus. This hypothesis was supported by the phylogenetic analysis that showed that most M46I/L-carrying CRF01_AE strains formed genetic clusters. Currently, China is experiencing a sharp transition of HIV-1 molecular epidemiology with a rapidly increasing prevalence of CRF01_AE and CRF07_BC, accompanied with a sharply decreasing trend of subtype B among all high-risk groups (especially sexual contact population, such as men who have sex with men (MSM)) [64], [65], [66], [67]. On-going monitoring of HIV-1 drug resistance in predominant HIV-1 subtypes (e.g. CRF01_AE and CRF07_BC) will benefit the treatment of individuals involved. With respect to high prevalence of M184V/I in subtype B in ART-naïve individuals, the reasons are possibly related to acquisition of this mutation from patients failing treatment with resistant strains, prior exposures to ART, or undisclosed ART [56,[68], [69], [70], [71]].
On-going effort by China during the past two decades have made great stride towards achieving the UNAIDS “90–90–90” targets by 2020. However, accompanied with increased use of ART, a rising prevalence of HIV-1 drug resistance at the national level poses a significant challenge to the third 90% goal and the elimination of AIDS epidemic from the population by 2030. The VF rate remained at about 10% in patients receiving over 12 months of the first-line ART treatment [39,72,73]. In addition to drug resistance, treatment interruptions and low adherence to ART regimens are two other important factors interfering with the success of ART. Treatment interruptions are almost invariably associated with a rebound of plasma HIV-1 RNA levels and facilitate the emergence of drug resistance mutations (especially for M184V) [74,75]. In particular, treatment interruptions more than 48 h were demonstrated to be associated with an increased risk of the emergence of drug resistance [76]. In China, the ART adherence rate was estimated to be about 76.6% [77], and the patients living in rural areas have relatively lower adherence than those living in urban areas [78]. Furthermore, MSM have become the most predominant risk group for HIV-1 infection [79,80]. Although the vast majority of MSM live in urban areas [67], the high-mobility feature of MSM population might affect their adherence to therapy, which may facilitate the emergence and transmission of drug resistance. To improve the adherence rate to treatment, a pre-treatment education on the relationship between ART effectiveness and adherence should be especially encouraged for all HIV-1 infected individuals, especially for MSM and patients living in rural areas [81]. On the other hand, easy and flexible access to ART (e.g. one-pill-a-day formulated single tablet regimens and/or long-acting antiretroviral drugs), harmonious doctor-patient relationship, reduced drug side-effect, and humanized multidisciplinary care may help to improve adherence of all patients and reduce drug resistance rate [82].
VF is often associated with emerging drug resistance. Timely measurement of VL are crucial for evaluating treatment outcomes and monitoring VF. Although the routine detection of VL is required for national HIV-1 treatment programmes, low frequency of this assay once per year is not enough to timely monitor VF or rebound of VL and then further to identify SDRMs via drug resistance testings [83]. Therefore, three to four times of VL assays per year are encouraged [42]. For most regions, especially the rural areas, the biggest barrier to VL was the high cost of the assay and the lack of sophisticated laboratories and experienced professionals. Given the complex of HIV-1 genetic diversity and specific SDRM profile in China, cheaper domestic alternatives for VL assay should be developed.
Our study has several limitations. First, all data used in the analysis were retrieved from literatures, rather than from the raw data from Chinese Center for Disease Control and Prevention; so there may have a small bias in data selection. Because most studies in Chinese did not deposit their sequences in GenBank, HIV database or other public databases, the sequence size for resistance mutation analysis was relatively small (especially for subtypes of CRF07_BC and CRF08_BC). Second, only limited studies involving ART-treated individuals had available information on duration times of ART, therefore limiting the relationship analysis between ADR rate and ART duration. Third, we did not analyze the correlation of drug resistance with transmission routes and demographic characteristics since these information were also not always available in most studies.
In summary, this is the largest meta-analysis of Chinese data on HIV-1 drug resistance that presents the overall prevalence of drug resistance and national trend of TDR in China from 2001 to 2017. The finding that a few NRTI-associated (M184V/I) and NNRTI-associated (K103N/S, Y181C/I and G190A/S) SDRMs were responsible for most cases of acquired and transmitted drug resistance suggests that the current available first-line ART regimens containing to 3TC and/or EFV or NVP should be urgently amended, or promptly switched to the second-line regimens if individuals who are identified as carrying these mutations. The rising trend of TDR in recent years highlights the importance of routine drug resistance screening prior to ART initiation, especially in the region with the highest prevalence of TDR such as Central China. In view of these findings, scale-up of multiple VL measurements per year, optimized ART regimens, and implementation of effective strategies such as early counseling and pre-treatment education to improve patient adherence to ART will benefit China to eliminate AIDS epidemic by 2030.
Declaration of competing interest
We declare that we have no conflicts of interest.
Acknowledgments
Acknowledgments
We thank Dr. Jin Zhao at Shenzhen Center for Disease Control and Prevention for her valuable suggestions on this work, and Miss Rong Xu at IPS, CAS for her technical support.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81672033 and U1302224), and the Open Research Fund Program of the State Key Laboratory of Virology of China (2019IOV002).
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, and writing of the manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100238.
Appendix. Supplementary materials
References
- 1.WHO. Global AIDS update 2016. https://www.who.int/hiv/pub/arv/global-AIDS-update-2016_en.pdf?ua=1. (accessed August 3, 2019).
- 2.UNAIDS. Ending AIDS progress towards the 90–90–90 targets. 2017. https://reliefweb.int/report/world/ending-aids-progress-towards-90-90-90-targets. (accessed August 3, 2019).
- 3.Xiao Y., Kristensen S., Sun J., Lu L., Vermund S.H. Expansion of HIV/AIDS in China: lessons from Yunnan province. Soc Sci Med. 2007;64(3):665–675. doi: 10.1016/j.socscimed.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao Y. AIDS epidemic at age 25 and control efforts in China. Retrovirology. 2006;3:87. doi: 10.1186/1742-4690-3-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F.J., Pan J., Yu L., Wen Y., Zhao Y. Current progress of China's free art program. Cell Res. 2005;15(11–12):877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 6.Wang BY, Lin MM, Bao XJ. 958,000 survivors were reported in China, and the AIDS epidemic is at a low level. 2019. http://www.xinhuanet.com/politics/2019-12/01/c_1125295336.htm. (accessed December 5, 2019).
- 7.WHO. ART in prevention of HIV and TB. 2011. https://www.who.int/hiv/topics/tb/faq_art_prevention_hiv_and_tb_revised_april_2011.pdf. (accessed August 3, 2019).
- 8.Zhang F., Dou Z., Ma Y. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–524. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 9.Pennings P.S. HIV drug resistance: problems and perspectives. Infect Dis Rep. 2013;5(Suppl 1):e5. doi: 10.4081/idr.2013.s1.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Z., Liang S., Sun X. Drug resistance and virological failure among HIV-Infected patients after a decade of antiretroviral treatment expansion in eight provinces of China. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0166661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoohela T., Naerkezi A., Wang L. A survey of the distribution of HIV subtypes and drug resistance in HIV-infected patients receiving antiretroviral treatment in YUi prefecture of Xinjiang, China. Chin J Mierobiol Immunol. 2015;35(12):905–909. in Chinese. [Google Scholar]
- 12.Sun X.G., Lin B., Su S.L. Mutation of drug resistant gene in HIV/AIDS patients with antiretroviral therapy in Shandong province in 2011. Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46(11):982–986. in Chinese. [PubMed] [Google Scholar]
- 13.Huang X., Zeng H., Wang X., Xu X. Drug resistance in HIV-infected persons receiving HIV antiviral therapy from 2010 to 2015. Parasit Infect Dis. 2018;16(2):6. in Chinese. [Google Scholar]
- 14.Yuan Y., Xing H., Wang X.Y. The prevalence of HIV-1 drug resistance and associated factors in AIDS patients receiving HAART in Zhecheng county, Henan province. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(7):619–624. in Chinese. [PubMed] [Google Scholar]
- 15.Li J.J., Liu W. HIV-1drug resistant strains in some areas of guangxi in 2011:a cros-sectional survey. Chin J AIDS STD. 2015;21(2):4. in Chinese. [Google Scholar]
- 16.Zhang F., Ma Y. Progress and challenges in China's free art programme. Lancet HIV. 2019;6(1):e8–e9. [Google Scholar]
- 17.Li J.Y., Li H.P., Li L. Prevalence and evolution of drug resistance HIV-1 variants in Henan, China. Cell Res. 2005;15(11–12):843–849. doi: 10.1038/sj.cr.7290356. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sobanski V., Dauchet L., Lefevre G. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol. 2014;66(2):407–417. doi: 10.1002/art.38219. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R.K., Gregson J., Parkin N. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melsen W.G., Bootsma M.C., Rovers M.M., Bonten M.J. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 23.Bennett D.E., Camacho R.J., Otelea D. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T.F., Shafer R.W. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takebe Y., Liao H., Hase S. Reconstructing the epidemic history of HIV-1 circulating recombinant forms CRF07_BC AND CRF08_BC in east Asia: the relevance of genetic diversity and phylodynamics for vaccine strategies. Vaccine. 2010;28(Suppl 2) doi: 10.1016/j.vaccine.2009.07.101. B39-44. [DOI] [PubMed] [Google Scholar]
- 26.An M., Han X., Xu J. Reconstituting the epidemic history of HIV strain CRF01_AE among men who have sex with men (MSM) in Liaoning, northeastern China: implications for the expanding epidemic among MSM in China. J Virol. 2012;86(22):12402–12406. doi: 10.1128/JVI.00262-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao Y., Tian D., Zheng Y.Y. Characteristics of HIV-1 natural drug resistance-associated mutations in former paid blood donors in Henan province, China. PLoS ONE. 2014;9(2):e89291. doi: 10.1371/journal.pone.0089291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyrer C., Razak M.H., Lisam K., Chen J., Lui W., Yu X.F. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14(1):75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X., Tian C., Choi K.H. Injecting drug use and HIV infection in southwest China. AIDS. 1994;8(8):1141–1147. doi: 10.1097/00002030-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y., Li Z., Shangde Z. People living with HIV in China are found to be infected with HIV at first time.(in chinese) Chin J Epidemioly. 1990;11:2. [Google Scholar]
- 31.Zhao Y., Sun X., He Y. Progress of the national pediatric free antiretroviral therapy program in China. AIDS Care. 2010;22(10):1182–1188. doi: 10.1080/09540121003615129. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F., Haberer J.E., Wang Y. The chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl 8):S143–S148. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 33.Dou Z., Chen R.Y., Wang Z. HIV-infected former plasma donors in rural central China: from infection to survival outcomes, 1985–2008. PLoS ONE. 2010;5(10):e13737. doi: 10.1371/journal.pone.0013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N., Wang Z., Sun D. HIV among plasma donors and other high-risk groups in Henan, China. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):S41–S47. doi: 10.1097/QAI.0b013e3181c7d717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao F.39th Meeting of the unaids programme coordinating board. 2016. http://www.unaids.org/sites/default/files/media/documents/PCB-39_Yunnan-Province-vice-governor_en.pdf. (accessed March 2, 2018)
- 36.Dou Z., Chen R.Y., Xu J. Changing baseline characteristics among patients in the China national free antiretroviral treatment program, 2002–09. Int J Epidemiol. 2010;39(Suppl 2):ii56–ii64. doi: 10.1093/ije/dyq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Sun G., Liang S. Different distribution of HIV-1 subtype and drug resistance were found among treatment naive individuals in Henan, Guangxi, and Yunnan province of China. PLoS ONE. 2013;8(10):e75777. doi: 10.1371/journal.pone.0075777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J., Wu Y., Yang W. Population-based human immunodeficiency virus 1 drug resistance profiles among individuals who experienced virological failure to first-line antiretroviral therapy in Henan, China during 2010-2011. AIDS Res Ther. 2015;12:22. doi: 10.1186/s12981-015-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y., Zhao D., Yu L. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50(2):264–271. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC, NCSTD Progress in the national AIDS and STD epidemic situation and major prevention and control work in the third quarter of 2012. Chin J AIDS STD. 2012;18(11) in Chinese. [Google Scholar]
- 41.Zhao S., Feng Y., Hu J. Prevalence of transmitted hiv drug resistance in antiretroviral treatment naive newly diagnosed individuals in China. Sci Rep. 2018;8(1):12273. doi: 10.1038/s41598-018-29202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y., Dou Z., Guo W. The human immunodeficiency virus care continuum in China: 1985–2015. Clin Infect Dis. 2018;66(6):833–839. doi: 10.1093/cid/cix911. [DOI] [PubMed] [Google Scholar]
- 43.Oyugi J.H., Byakika-Tusiime J., Ragland K. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 44.Marcellin F., Boyer S., Protopopescu C. Determinants of unplanned antiretroviral treatment interruptions among people living with HIV in Yaounde, Cameroon (EVAL survey, ANRS 12-116) Trop Med Int Health. 2008;13(12):1470–1478. doi: 10.1111/j.1365-3156.2008.02170.x. [DOI] [PubMed] [Google Scholar]
- 45.Moon S., Van Leemput L., Durier N. Out-of-pocket costs of aids care in China: are free antiretroviral drugs enough? AIDS Care. 2008;20(8):984–994. doi: 10.1080/09540120701768446. [DOI] [PubMed] [Google Scholar]
- 46.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach—second edition. 2013. https://www.who.int/hiv/pub/arv/arv-2016. (accessed August 3, 2019). [PubMed]
- 47.Clutter D.S., Jordan M.R., Bertagnolio S., Shafer R.W. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avila-Rios S., Garcia-Morales C., Matias-Florentino M. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 who survey. Lancet HIV. 2016;3(12):e579–ee91. doi: 10.1016/S2352-3018(16)30119-9. [DOI] [PubMed] [Google Scholar]
- 49.Gilks C.F., Crowley S., Ekpini R. The who public-health approach to antiretroviral treatment against HIV in resource-limited settings. The Lancet. 2006;368(9534):505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Xu W., He C. The prevalence of primary HIV-1 drug resistance in newly reported HIV infections in Henan. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(5):643–647. doi: 10.3760/cma.j.issn.0254-6450.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Yan J.Z., Sun D.Y., Wang Z. The study of HIV-1 drug resistance transmision among newly reported HIV in fections in Henan 2015. HENAN Med Res. 2018;27(4):598–601. [Google Scholar]
- 52.Gallant J.E. Antiretroviral drug resistance and resistance testing. Top HIV Med. 2005;13(5):138–142. [PubMed] [Google Scholar]
- 53.Quiñones-Mateu M.E., Weber J., Rangel H.R., Chakraborty B. HIV-1 fitness and antiretroviral drug resistance. AIDS Rev. 2001;3:223–242. [Google Scholar]
- 54.Winters M.A., Bosch R.J., Albrecht M.A., Katzenstein D.A., Team ACTGS Clinical impact of the M184V mutation on switching to didanosine or maintaining lamivudine treatment in nucleoside reverse-transcriptase inhibitor-experienced patients. J Infect Dis. 2003;188(4):537–540. doi: 10.1086/377742. [DOI] [PubMed] [Google Scholar]
- 55.Rusconi S., La Seta Catamancio S., Citterio P. Virological response in multidrug-experienced HIV-1-infected subjects failing highly active combination regimens after shifting from lamivudine to didanosine. Antivir Ther. 2001;6(1):41–46. [PubMed] [Google Scholar]
- 56.Rhee S.Y., Blanco J.L., Jordan M.R. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med. 2015;12(4) doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta R.K., Jordan M.R., Sultan B.J. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380(9849):1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yerly S., Calmy A. Time to overcome pretreatment HIV drug resistance. Lancet Infect Dis. 2018;18(3):239–240. doi: 10.1016/S1473-3099(17)30709-0. [DOI] [PubMed] [Google Scholar]
- 59.Nyaku A.N., Kelly S.G., Taiwo B.O. Long-acting antiretrovirals: where are we now? Curr HIV/AIDS Rep. 2017;14(2):63–71. doi: 10.1007/s11904-017-0353-0. [DOI] [PubMed] [Google Scholar]
- 60.Manosuthi W., Thongyen S., Nilkamhang S., Manosuthi S., Sungkanuparph S. HIV-1 drug resistance-associated mutations among antiretroviral-naive Thai patients with chronic HIV-1 infection. J Med Virol. 2013;85(2):194–199. doi: 10.1002/jmv.23452. [DOI] [PubMed] [Google Scholar]
- 61.Phan T.T., Ishizaki A., Phung D.C., Bi X., Oka S., Ichimura H. Characterization of HIV type 1 genotypes and drug resistance mutations among drug-naive HIV type 1-infected patients in Northern Vietnam. AIDS Res Hum Retroviruses. 2010;26(2):233–235. doi: 10.1089/aid.2009.0206. [DOI] [PubMed] [Google Scholar]
- 62.Kantor R., Katzenstein D. Polymorphism in HIV-1 non-subtype B protease and reverse transcriptase and its potential impact on drug susceptibility and drug resistance evolution. AIDS Rev. 2003;5(1):25–35. [PubMed] [Google Scholar]
- 63.Johnson V.A., Brun-Vezinet F., Clotet B. Update of the drug resistance mutations in HIV-1: spring 2008. Top HIV Med. 2008;16(1):62–68. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 64.Li X., Li W., Zhong P. Nationwide trends in molecular epidemiology of HIV-1 in China. AIDS Res Hum Retroviruses. 2016;32(9):851–859. doi: 10.1089/AID.2016.0029. [DOI] [PubMed] [Google Scholar]
- 65.Shang H., Xu J., Han X., Spero Li J., Arledge K.C., Zhang L. HIV prevention: bring safe sex to China. Nature. 2012;485(7400):576–577. doi: 10.1038/485576a. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L., Chow E.P., Jing J. HIV prevalence in china: integration of surveillance data and a systematic review. The Lancet Infect Dis. 2013;13(11):955–963. doi: 10.1016/S1473-3099(13)70245-7. [DOI] [PubMed] [Google Scholar]
- 67.Zhao J., Chen L., Chaillon A. The dynamics of the hiv epidemic among men who have sex with men (MSM) from 2005 to 2012 in Shenzhen, China. Sci Rep. 2016;6:28703. doi: 10.1038/srep28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gandhi R.T., Wurcel A., Rosenberg E.S. Progressive reversion of human immunodeficiency virus type 1 resistance mutations in vivo after transmission of a multiply drug-resistant virus. Clin Infect Dis. 2003;37(12):1693–1698. doi: 10.1086/379773. [DOI] [PubMed] [Google Scholar]
- 69.Harrison L., Castro H., Cane P. The effect of transmitted HIV-1 drug resistance on pre-therapy viral load. AIDS. 2010;24(12):1917–1922. doi: 10.1097/QAD.0b013e32833c1d93. [DOI] [PubMed] [Google Scholar]
- 70.Frontiers of retrovirology 2011, Amsterdam, the Netherlands, 3-5 october 2011. meeting abstracts. Retrovirology. 2011;8(Suppl 2):O1–P89. doi: 10.1186/1742-4690-8-S2-O1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onywera H., Maman D., Inzaule S. Surveillance of HIV-1 pol transmitted drug resistance in acutely and recently infected antiretroviral drug-naive persons in rural western Kenya. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0171124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang P., Tan J., Ma W. Outcomes of antiretroviral treatment in HIV-infected adults: a dynamic and observational cohort study in Shenzhen, China, 2003–2014. BMJ Open. 2015;5(5) doi: 10.1136/bmjopen-2014-007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong K., Ye L., Leng Y. Prevalence of HIV-1 drug resistance among patients with antiretroviral therapy failure in Sichuan, China, 2010–2016. Tohoku J Exp Med. 2019;247(1):1–12. doi: 10.1620/tjem.247.1. [DOI] [PubMed] [Google Scholar]
- 74.Yerly S., Fagard C., Gunthard H.F., Hirschel B., Perrin L., Swiss HIVCS Drug resistance mutations during structured treatment interruptions. Antivir Ther. 2003;8(5):411–415. [PubMed] [Google Scholar]
- 75.Martinez-Picado J., Morales-Lopetegi K., Wrin T. Selection of drug-resistant HIV-1 mutants in response to repeated structured treatment interruptions. AIDS. 2002;16(6):895–899. doi: 10.1097/00002030-200204120-00009. [DOI] [PubMed] [Google Scholar]
- 76.Graham S.M., Jalalian-Lechak Z., Shafi J. Antiretroviral treatment interruptions predict female genital shedding of genotypically resistant HIV-1 RNA. J Acquir Immune Defic Syndr. 2012;60(5):511–518. doi: 10.1097/QAI.0b013e31825bd703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huan Z., Fuzhi W., Lu L., Min Z., Xingzhi C., Shiyang J. Comparisons of adherence to antiretroviral therapy in a high-risk population in China: a systematic review and meta-analysis. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0146659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y.Y., Jin Y., Chen C. Meta-analysis of adherence to highly active antiretroviral therapy in patients with HIV infection in China. AIDS Care. 2019;31(8):913–922. doi: 10.1080/09540121.2018.1554238. [DOI] [PubMed] [Google Scholar]
- 79.UNAIDS. Country progress report – china global aids monitoring 2018. 2018. https://www.unaids.org/sites/default/files/country/documents/CHN_2018_countryreport.pdf. (accessed August 3, 2019).
- 80.NCAIDS, NCSTD, CDC Update on the AIDS/STD epidemic in China the third quarter of 2018. Chin J AIDS STD. 2018;24(11) in Chinese. [Google Scholar]
- 81.Hegazi A., Bailey R.L., Ahadzie B., Alabi A., Peterson K. Literacy, education and adherence to antiretroviral therapy in the Gambia. AIDS Care. 2010;22(11):1340–1345. doi: 10.1080/09540121003693514. [DOI] [PubMed] [Google Scholar]
- 82.Atreja A., Bellam N., Levy S.R. Strategies to enhance patient adherence: making it simple. MedGenMed. 2005;7(1):4. [PMC free article] [PubMed] [Google Scholar]
- 83.Shen Z., Zhu Q., Tang Z. Effects of CD4 cell counts and viral load testing on mortality rates in patients with HIV infection receiving antiretroviral treatment: an observational cohort study in rural southwest china. Clin Infect Dis. 2016;63(1):108–114. doi: 10.1093/cid/ciw146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.