Supplemental Digital Content is available in the text
Keywords: cytokine, hepatitis C, HIV, innate immunity, opioid-use disorder
Abstract
Background:
Opioid-use disorders (OUD) and hepatitis C or B co-infection (HEP) are common among people living with HIV (PLHIV). The impact of OUD on innate and adaptive immunity among PLHIV with and without HEP is unknown.
Objectives:
To investigate the impact of OUD on monocyte and T-cell phenotypes, cytokine responses to lipopolysaccharide (LPS) and phytohemagglutinin (PHA), and plasma inflammatory markers, among PLHIV with and without HEP.
Methods:
Cross-sectional study enrolling PLHIV receiving ART, with and without OUD. Flow cytometry determined monocyte and T-cell phenotypes; LPS and PHA-induced cytokine production was assessed following LPS and PHA stimulation by multiplex cytokine array; plasma IL-6, soluble CD163, and soluble CD14 were measured by ELISA.
Results:
Twenty-two PLHIV with OUD and 37 PLHIV without OUD were included. PLHIV with OUD exhibited higher frequencies of intermediate (CD14++CD16+) and nonclassical (CD14dimCD16+) monocytes when compared with PLHIV without OUD (P = 0.0025; P = 0.0001, respectively), regardless of HEP co-infection. Soluble CD163 and monocyte cell surface CD163 expression was increased among PLHIV with OUD and HEP, specifically. Regardless of HEP co-infection, PLHIV with OUD exhibited reduced production of IL-10, IL-8, IL-6, IL-1alpha, and TNF-alpha in response to LPS when compared with PLHIV without OUD; PHA-induced production of IL-10, IL-1alpha, IL-1beta, IL-6, and TNF-alpha were also reduced among individuals with OUD.
Conclusion:
OUD among PLHIV are associated with altered monocyte phenotypes and a dysregulated innate cytokine response. Defining underlying mechanisms of opioid-associated innate immune dysregulation among PLHIV should be prioritized to identify optimal OUD treatment strategies.
Introduction
Chronic opioid use among people living with HIV (PLHIV) is a public health crisis, with 25--57% of PLHIV dependent on opioids [1]. PLHIV with opioid-use disorders (OUD) have a higher risk of death and progression to AIDS, as compared with PLHIV without a substance use disorder (SUD), even after adjustments for comorbidities and adherence to antiretroviral therapy (ART) are considered [1–3]. Among PLHIV with OUD, rates of hepatitis C co-infection continue to rise, and the impact of OUD on host immunity among co-infected individuals has not been characterized [4]. Chronic immune activation is associated with progression to AIDS, as well as excessive non-AIDS-related morbidity and mortality [5–9]. It remains unknown if OUD impact the burden of chronic immune activation among PLHIV with and without hepatitis C or B (HEP) co-infection.
During untreated HIV-infection, markers of T-cell activation and exhaustion are highly correlated with progression to AIDS and death [10–14]. However, among PLHIV on ART, monocytes and their plasma mediators are significant drivers of inflammation, and biomarkers reflective of innate immune activation independently predict mortality [15–17]. Opioids may exacerbate HIV-associated innate immune activation by accelerating intestinal epithelial damage to promote systemic translocation of lipopolysaccharide (LPS) [18–20], resulting in dysregulation of innate immunity [5,7]. Altered monocyte phenotype has been observed among individuals with AIDS, PLHIV with poorly controlled viral load, and SUD [21–23]. We hypothesized that PLHIV on ART with OUD would exhibit alterations in monocyte phenotype and functional responses, and a higher burden of immune activation and systemic inflammation, when compared with PLHIV without OUD. To address this, we compared ex-vivo monocyte and T-cell phenotypes, soluble indicators of systemic inflammation, and cytokine responses to LPS and the mitogen phytohemagglutinin (PHA), among PLHIV, with and without HEP co-infection and OUD (OUD+/HEP+; OUD+/HEP−), and a reference population of PLHIV without OUD (OUD−/HEP−).
Materials and methods
Participant recruitment and ethics statement
PLHIV with OUD were recruited through CTN-0055 CHOICES study from 2014 to 2015 (clinicaltrials.gov NCT01908062). CTN-0055 was an open-label, randomized, pilot trial of extended release naltrexone versus treatment-as-usual for treatment of OUD, alcohol use disorders (AUD), and mixed OUD/AUD in PLHIV [24]. Preintervention blood samples from CTN-0055 participants with OUD or mixed OUD/AUD were used for this analysis. CTN-0055 was conducted by the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) and approved by Institutional Review Boards (IRB) at Oregon Health & Science University (OHSU) and pilot sites. PLHIV without OUD (OUD−/HEP−) were recruited from OHSU HIV primary care clinic (2014–2016) through an independent, OHSU-approved IRB protocol. To be eligible for the OUD−/HEP− cohort, individuals were 18--65 years of age, not pregnant, and denied current or recent (past 12 months) use of: opioids (including opioid-containing medications), cocaine, methamphetamines, daily cannabis, and daily alcohol. Relevant demographic and medical information including age, sex, ethnicity, tobacco use, CD4+ T-cell count, HIV viral load, hepatitis B and hepatitis C serostatus, ART, diagnosis of OUD, SUD, or AUD in past 12 months, was obtained from participant medical records. All study participants provided written, informed consent.
PBMC and plasma processing and storage
Up to 32 ml of peripheral blood was collected into CPT Vacutainer tubes (BD Biosciences, Franklin Lakes, New Jersey, USA) at a single time point. Samples were centrifuged within 2 h of collection, and PBMC and plasma mixed by inversion. Samples from CTN-0055 CHOICES participants were shipped at room temperature, and plasma and PBMC processing completed within 24 h of collection. PBMC were cryopreserved in 10% DMSO (Sigma-Aldrich, St Louis, Missouri, USA) in fetal bovine serum with 0.1% Gentamicin; undiluted plasma was stored at −80 °C. Plasma and PBMC from OUD-/HEP- participants were collected, processed, and stored following an identical protocol, including a 24 h delay following centrifugation to replicate conditions for CTN-0055 CHOICES samples. The number of participants included in each assay are shown in Supplemental Figure 1.
Flow cytometry
The following reagents were utilized: cell viability (Live/Dead Fixable Green or Aqua; Invitrogen, Thermo-Fisher Scientific, Waltham, Massachusetts, USA), anti-CD3 [APC-H7, BD Biosciences, clone SK7; PerCP, BioLegend (San Diego, California, USA), clone UCHT1], anti-HLA-DR (BV421, BioLegend, clone L243), anti-CD14 (BV605, BD Biosciences, clone M5E2; BV510, BioLegend, clone M5E2), anti-PD-1 (BV650 BioLegend, clone EH12.2H7), anti-CD38 (BV711, BioLegend, clone HIT2), anti-CD4 (BV785, BioLegend, clone OKT4), anti-CD8 (FITC, BioLegend, clone RPA-T8), anti-CD57 (APC, BioLegend, clone HNK-1), anti-CD28 (PE-Cy7, BioLegend, clone 28.2), anti-CD19 (PerCP, BioLegend, clone SJ25C1), anti-CD56 (PerCP, BioLegend, clone HCD56), anti-CD16 (APC, BioLegend, clone 3G8), and anti-CD163 (PE, BioLegend, clone GHI/61). Isotypes assessed for nonspecific binding; FMOs were used to set gates for positive and negative staining. Cells were acquired using a BD Fortessa or Symphony and analyzed using FlowJo (v 9.8.5). Boolean gating calculated co-expression of markers.
Plasma ELISA
Plasma cytokine quantifications were performed using commercial kits according to the manufacturers’ instructions: IL-6 (BioLegend), sCD14 (R&D Systems, Minneapolis, Minnesota, USA) and sCD163 (Invitrogen).
PBMC stimulation and measurement of cytokine production
PBMC were cultured in 96-well plates (105 cells/well) in serum-free medium (XVNS-15; Lonza), with or without ultrapure LPS from Salmonella enterica serovar minnesota mutant R595 (10 ng/ml; Invitrogen) or PHA (10 μg/ml; Sigma-Aldrich). Cell-free tissue supernatants were collected at 18 h and stored at −20 °C for batched analysis. Cytokine quantifications in cell-free culture supernatants at 1 : 2 dilution were performed using a customized multiplex cytokine array, including: IFN-α2, IFN-γ, IL-10, IL-12p70, IL-13, IL-15, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, MCP-1, and TNF-α (Luminex 200 System, EMD-Millipore-Sigma). IL-8 levels were above the highest standard in the multiplex array, and retested using aliquoted culture supernatant by commercial ELISA (BioLegend) at a 1 : 20 (rest) or 1 : 40 (LPS/PHA) dilution according to manufacturer's instructions.
Statistical analysis
Quantitative variables were presented as medians with interquartile range (IQR) for comparison of demographic and clinical characteristics, and chi-squared or Fisher's exact testing applied (Table 1). Comparison of nonnormally distributed data between all CTN-0055 CHOICES participants (regardless of HEP co-infection) and our PLHIV reference group, was performed using a Mann–Whitney U-test (Supplemental Tables 1–3; Figs. 1 and 3). Due to concern that HEP co-infection could be confounding, a three-group comparison (OUD+/HEP+; OUD+/HEP−; OUD−/HEP−) was performed using the Kruskall--Wallis test (Supplemental Tables 1–3; Fig. 2). To assess associations between immunologic variables and participant characteristics, Gamma regression was employed in a multivariate model with and without adjustment for HIV viral load, age, and sex (Table 2). Spearman Rank analysis assessed for correlations between immunologic variables. Due to restricted sample size and exploratory nature of this pilot, correction for multiple comparisons was not performed and the individual error rate is considered rather than the family-wise error rate. The level of significance alpha = 0.05 was set for each test. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Table 1.
Demographic and clinical characteristics of study participants.
| CTN-0055 CHOICES | PLHIV reference | |||
| OUD+/HEP+, n = 12 | OUD+/HEP−, n = 10 | OUD−/HEP−, n = 37 | P value | |
| Age (median/IQR) | 53.5 (42–56) | 46 (31–49) | 49 (37–55) | 0.292a |
| Gender (female) | 7 (58.3%) | 5 (50%) | 3 (8.1%) | 0.0004b |
| Mixed OUD/AUD | 4 (33.3%) | 5 (50%) | 0 | 0.66c |
| Hepatitis B | 1 (8.3%) | 0 | 0 | 0.3729c |
| Hepatitis C | 11 (91.7%) | 0 | 0 | <0.0001c |
| On ART | 12 (100%) | 10 (100%) | 37 (100%) | NA |
| CD4+ (cells/μl) (median/IQR) | 682 (302–975) | 537 (372–631) | 596 (393–855) | 0.665a |
| Undetectable Viral load | 7 (58.3%) | 6 (60%) | 33 (89.2%) | 0.0262b |
| HIV viral loadd (median/IQR) | 530 (267–1738) | 1270 (365–3712) | 41 (41–60.5) | 0.0105a |
AUD, alcohol-use disorders; ART, antiretroviral therapy; HEP, hepatitis C or B co-infection; IQR, interquartile range; OUD, opioid-use disorders.
aKruskal--Wallis.
bChi-squared.
cFisher's exact between OUD+/HEP+ and OUD+/HEP−.
dAmong individuals with a detectable HIV viral load.
Fig. 1.
Opioid-use disorder among people living with HIV is associated with altered monocyte phenotype.
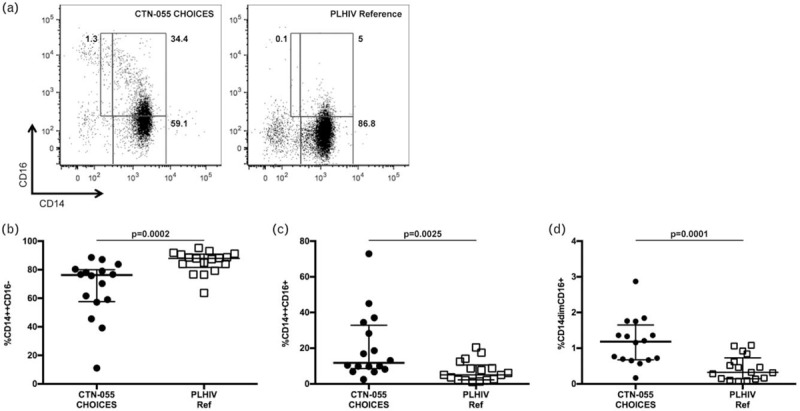
Shown are representative flow cytometry plots of PBMC from a CTN-0055 CHOICES participant (a, left) and PLHIV reference group participant (a, right). Lower gate in each plot denotes CD14++CD16− monocytes (classical); upper right gate denotes CD14++CD16+ monocytes (intermediate); upper left gate denotes CD14dimCD16+ monocytes (nonclassical). Gate frequencies were calculated from total live CD3−CD19−CD56− monocytes (see Supplemental Figure 2 for gating strategy). Shown are unadjusted medians with interquartile ranges of classical, intermediate, and nonclassical monocytes (b--d). P values to assess for differences between CTN-0055 CHOICES participants and the PLHIV reference group, were calculated using the Mann--Whitney test (also see Supplemental Table 1). Adjusted analysis examining differences between OUD+/HEP+ vs. OUD−/HEP−, OUD+/HEP− vs. OUD−/HEP−, and OUD+/HEP+ vs. OUD+/HEP−, are shown in Table 2. HEP, hepatitis C or B co-infection; OUD, opioid-use disorders; PBMC, peripheral blood mononuclear cells.
Fig. 3.
Altered pattern of cytokine production in response to lipopolysaccharide and phytohaemagglutinin among people living with HIV with opioid-use disorder.
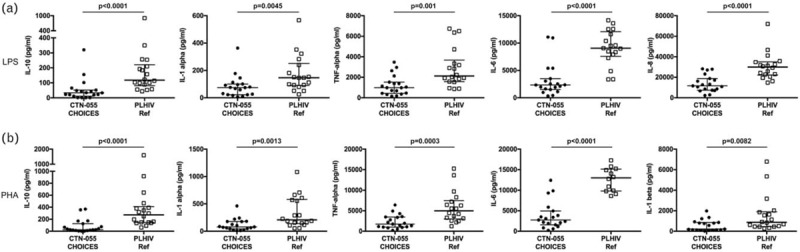
PBMC from CTN-0055 CHOICES participants and the PLHIV reference group were thawed and stimulated for 18 h with LPS (a) or PHA (b), and cell culture supernatants collected and assayed using a customized multiplex cytokine array (IFN-α2, IFN-γ, IL-10, IL-12p70, IL-13, IL-15, IL-1α, IL-1β, IL-2, IL-4, IL-6, MCP-1, TNF-α) or single-plex ELISA (IL-8). Data in both (a) and (b) are displayed as medians with interquartile ranges. P values to assess for differences between CTN-0055 CHOICES participants and the PLHIV reference group, were calculated using the Mann–Whitney test (also see Supplemental Tables 2 and 3). Adjusted analysis examining differences between OUD+/HEP+ vs. OUD−/HEP−, OUD+/HEP− vs. OUD−/HEP−, and OUD+/HEP+ vs. OUD+/HEP− are shown in Table 2. HEP, hepatitis C or B co-infection; OUD, opioid-use disorders; PHA, phytohaemagglutinin.
Fig. 2.
Cell surface and soluble CD163 are significantly increased among people living with HIV with opioid-use disorders and hepatitis C or B co-infection.
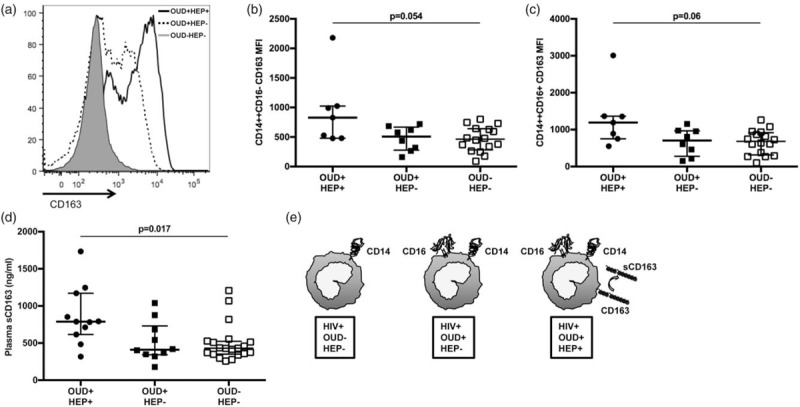
CD163 expression was analyzed on CD14++CD16−, CD14++CD16+ and CD14dimCD16+ monocytes using flow cytometry among individuals in OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− cohorts. Data from three representative individuals are displayed as a histogram with mean fluorescence intensity (MFI) of CD163 on CD14++CD16− monocytes, on the x-axis (a). Shown are unadjusted medians with interquartile ranges of CD163 MFI on classical (b) and intermediate (c) monocytes. Soluble CD163 (sCD163; ng/ml) in the plasma of OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− participants was quantified using a standard ELISA (medians with interquartile ranges displayed; d). P values to assess for differences among three cohorts were calculated using Kruskal--Wallis test. Adjusted analysis examining differences between OUD+/HEP+ vs. OUD−/HEP−, OUD+/HEP− vs. OUD−/HEP−, and OUD+/HEP+ vs. OUD+/HEP−, are shown in Table 2. A model illustrating the phenotypic differences among monocytes from OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− participants is shown in panel (e). HEP, hepatitis C or B co-infection; OUD, opioid-use disorders.
Table 2.
Comparison of immune phenotypes and cytokine responses among cohorts using multivariate model with and without adjustment for HIV viral load, age, and sex.
| CTN-0055 CHOICES | PLHIV reference | Comparisons between cohorts | ||||||
| Cell phenotype | OUD+/HEP+ mean (SD) | OUD+/HEP− mean (SD) | OUD−/HEP− mean (SD) | 95% CI (unadjusted) | P value (unadjusted) | 95% CI (adjusteda) | P value (adjusteda) | |
| CD8CD38+ (%) | 29.1 (5.63) | 27.5 (5.3) | 14.8 (2.26) | OUD+/HEP+ vs. OUD-/HEP- | (1.21–3.2) | 0.006 | (0.81–2.1) | 0.29 |
| OUD+/HEP- vs. OUD-/HEP- | (1.14–3.01) | 0.012 | (0.68–1.83) | 0.67 | ||||
| OUD+/HEP+ vs. OUD+/HEP- | (0.62–1.81) | 0.83 | (0.72–1.87) | 0.55 | ||||
| CD8CD38+HLADR+ (%) | 8.62 (1.90) | 8.4 (1.85) | 4.46 (0.83) | OUD+/HEP+ vs. OUD−/HEP- | (1.1–3.40) | 0.023 | (0.76–2.44) | 0.3 |
| OUD+/HEP− vs. OUD−/HEP− | (1.07–3.31) | 0.023 | (0.78–2.9) | 0.22 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.56–1.89) | 0.93 | (0.48–1.72) | 0.76 | ||||
| CD14++CD16− (%) | 69.85 (7.19) | 63.90 (6.58) | 85.38 (6.03) | OUD+/HEP+ vs. OUD−/HEP- | (0.64–1.045) | 0.11 | (0.66–1.17) | 0.38 |
| OUD+/HEP- vs. OUD−/HEP- | (0.59–0.96) | 0.02 | (0.6–1.13) | 0.23 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.82–1.45) | 0.54 | (0.79–1.45) | 0.65 | ||||
| CD14++CD16+ (%) | 19.09 (5.35) | 22.19 (6.22) | 7.10 (1.37) | OUD+/HEP+ vs. OUD−/HEP- | (1.38–5.23) | 0.0036 | (1.1–4.27) | 0.026 |
| OUD+/HEP− vs. OUD−/HEP− | (1.61–6.08) | 0.0008 | (1.01–5.07) | 0.047 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.4–1.87) | 0.70 | (0.41–2.2) | 0.92 | ||||
| CD14dimCD16++ (%) | 0.84 (0.2) | 1.52 (0.36) | 0.45 (0.072) | OUD+/HEP+ vs. OUD−/HEP− | (1.08–3.31) | 0.026 | (1.03–3.42) | 0.039 |
| OUD+/HEP− vs. OUD−/HEP− | (1.95–5.96) | <0.0001 | (1.79–6.86) | 0.0002 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.29–1.07) | 0.077 | (0.27–1.07) | 0.076 | ||||
| CD14++CD16− CD163 MFI | 929.7 (175.25) | 472.62 (83.34) | 463.42 (57.78) | OUD+/HEP+ vs. OUD−/HEP− | (1.29–3.12) | 0.0021 | (1.28–3.11) | 0.0023 |
| OUD+/HEP− vs. OUD−/HEP− | (0.67–1.56) | 0.93 | (0.58–1.64) | 0.94 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (1.19–3.26) | 0.0088 | (1.22–3.42) | 0.0069 | ||||
| CD14++CD16+CD163 MFI | 1302.0 (277.62) | 665.7 (132.8) | 659.7 (93.04) | OUD+/HEP+ vs. OUD−/HEP− | (1.2–3.26) | 0.0078 | (1.101–3.09) | 0.019 |
| OUD+/HEP− vs. OUD−/HEP− | (0.63–1.63) | 0.97 | (0.44–1.48) | 0.49 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (1.10–3.47) | 0.022 | (1.25–4.21) | 0.0077 | ||||
| LPS cytokine response (pg/ml) | CTN-0055 CHOICES | PLHIV reference | Comparisons between cohorts | |||||
| OUD+/HEP+ mean (SD) | OUD+/HEP− mean (SD) | OUD−/HEP− mean (SD) | 95% CI (unadjusted) | P value (unadjusted) | 95% CI (adjusteda) | P value (adjusteda) | ||
| IL-10 | 53.26 (17.85) | 51.67 (18.25) | 185.9 (46.43) | OUD+/HEP+ vs. OUD−/HEP− | (0.13–0.65) | 0.0028 | (0.14–0.85) | 0.022 |
| OUD+/HEP− vs. OUD−/HEP− | (0.12–0.65) | 0.0031 | (0.12–0.85) | 0.023 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.4–2.68) | 0.95 | (0.38–3.03) | 0.9 | ||||
| IL-8 | 12296 (1968) | 15379 (2594) | 30737 (3667) | OUD+/HEP+ vs. OUD−/HEP− | (0.27–0.59) | <0.0001 | (0.27–0.64) | <0.0001 |
| OUD+/HEP− vs. OUD−/HEP− | (0.33–0.75) | 0.0008 | (0.34–0.91) | 0.019 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.51–1.26) | 0.34 | (0.46–1.22) | 0.24 | ||||
| IL-6 | 2981 (613.8) | 3491 (757.7) | 9163 (1446) | OUD+/HEP+ vs. OUD-/HEP- | (0.2–0.54) | <0.0001 | (0.22–0.70) | 0.0017 |
| OUD+/HEP− vs. OUD−/HEP− | (0.225–0.65) | 0.0003 | (0.23–0.83) | 0.011 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.48–1.54) | 0.6 | (0.48–1.59) | 0.66 | ||||
| IL-1α | 98.3 (24.51) | 66.5 (17.47) | 174.9 (32.5) | OUD+/HEP+ vs. OUD−/HEP− | 0.31–1.03 | 0.064 | (0.37–1.38) | 0.31 |
| OUD+/HEP− vs. OUD−/HEP− | 0.20–0.71 | 0.0027 | 0.21–0.81 | 0.011 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | 0.73–3.0 | 0.28 | 0.85–3.54 | 0.13 | ||||
| TNF-α | 1083 (239.6) | 1349 (314.6) | 2911 (480) | OUD+/HEP+ vs. OUD−/HEP− | (0.22–0.64) | 0.0003 | (0.24–0.81) | 0.0084 |
| OUD+/HEP− vs. OUD−/HEP− | (0.26–0.81) | 0.0071 | (0.26–0.93) | 0.028 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.43–1.51) | 0.49 | (0.47–1.71) | 0.73 | ||||
| PHA cytokine response (pg/ml) | CTN-0055 CHOICES | PLHIV reference | Comparisons between cohorts | |||||
| OUD+/HEP+ mean (SD) | OUD+/HEP− mean (SD) | OUD−/HEP− mean (SD) | 95% CI (unadjusted) | P value (unadjusted) | 95% CI (adjusteda) | P-value (adjusteda) | ||
| IL-10 | 84.02 (8.6) | 80.2 (28.7) | 372.2 (94.3) | OUD+/HEP+ vs. OUD−/HEP− | (0.098–0.52) | 0.0004 | (0.15–0.91) | 0.031 |
| OUD+/HEP− vs. OUD−/HEP− | (0.09–0.51) | 0.0005 | (0.092–0.66) | 0.0050 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.4–2.76) | 0.92 | (0.51–4.32) | 0.47 | ||||
| IL-1α | 128.9 (34) | 103.6 (28.8) | 343 (67.4) | OUD+/HEP+ vs. OUD−/HEP− | (0.2–0.77) | 0.0029 | (0.24–0.96) | 0.037 |
| OUD+/HEP− vs. OUD−/HEP− | (0.16–0.59) | 0.0004 | (0.16–0.68) | 0.0028 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.59–2.64) | 0.57 | (0.69–3.13) | 0.32 | ||||
| IL-1β | 673.1 (203) | 412 (131) | 1612 (362.6) | OUD+/HEP+ vs. OUD−/HEP− | (0.20–0.87) | 0.02 | (0.27–1.26) | 0.17 |
| OUD+/HEP− vs. OUD−/HEP− | (0.12–0.55) | 0.0005 | (0.12–0.57) | 0.0008 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.69–3.86) | 0.26 | (0.98–5.1) | 0.055 | ||||
| IL-8 | 12681 (3304) | 15899 (4367) | 21536 (4183) | OUD+/HEP+ vs. OUD−/HEP− | (0.31–1.11) | 0.10 | (0.29–1.22) | 0.16 |
| OUD+/HEP− vs. OUD−/HEP− | (0.38–1.43) | 0.37 | (0.38–2.02) | 0.74 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.38–1.68) | 0.55 | (0.30–1.57) | 0.38 | ||||
| IL-6 | 3373 (730) | 4236 (966) | 12625 (2494) | OUD+/HEP+ vs. OUD−/HEP− | (0.15–0.48) | <0.0001 | (0.16–0.59) | 0.0004 |
| OUD+/HEP− vs. OUD−/HEP− | (0.19–0.61) | 0.0003 | (0.2–0.78) | 0.0071 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.43–1.48) | 0.47 | (0.41–1.48) | 0.44 | ||||
| TNF-alpha | 1787 (369) | 2825 (615.4) | 5839 (899) | OUD+/HEP+ vs. OUD−/HEP− | (0.18–0.51) | <0.0001 | (0.18–0.58) | 0.0001 |
| OUD+/HEP− vs. OUD−/HEP− | (0.29–0.82) | 0.0065 | (0.27–0.89) | 0.0201 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (0.35–1.14) | 0.13 | (0.36–1.24) | 0.2 | ||||
| Plasma soluble CD163 (ng/ml) | CTN-0055 CHOICES | PLHIV reference | Comparisons between cohorts | |||||
| OUD+/HEP+ mean (SD) | OUD+/HEP− mean (SD) | OUD−/HEP− mean (SD) | 95% CI (unadjusted) | P value (unadjusted) | 95% CI (adjusteda) | P value (adjusteda) | ||
| sCD163 | 863.5 (113.5) | 517.9 (71.4) | 499.9 (48.8) | OUD+/HEP+ vs. OUD-/HEP− | (1.25–2.38) | 0.0008 | (1.54–3.14) | <0.0001 |
| OUD+/HEP− vs. OUD−/HEP− | (0.74–1.44) | 0.83 | (0.84–1.67) | 0.33 | ||||
| OUD+/HEP+ vs. OUD+/HEP− | (1.15–2.42) | 0.0073 | (1.3–2.64) | 0.0007 | ||||
CI, confidence interval; HEP, hepatitis C or B co-infection; IQR, interquartile range; OUD, opioid-use disorders.
aAdjustment for HIV viral load, age, and sex.
Results
Characteristics of study participants
Twenty-two PLHIV with OUD enrolled in CTN-0055 CHOICES study [24], and a reference population of 37 PLHIV without OUD (OUD−/HEP−), were included. Of 22 PLHIV with OUD, all met DSM-V criteria for untreated, moderate-to-severe OUD, with 7 of 22 classified as mixed OUD/AUD (Table 1). HEP co-infection was common among CTN-0055 participants, whom were also more likely to have a detectable HIV viral load and to be female, as compared with the OUD−/HEP− cohort (Table 1). Age and CD4+ cell count were similar between populations, and all individuals were prescribed ART.
Opioid-use disorder is associated with altered monocyte phenotype among people living with HIV
To determine if OUD was associated with altered monocyte phenotype among PLHIV, monocytes were identified in PBMC by flow cytometry based on light scatter properties and expression of CD14+ and CD16+, and classified as exhibiting classical (CD14++CD16−), intermediate (CD14++CD16+), and nonclassical (CD14dimCD16+) phenotypes (Supplemental Figure 2) [25]. In unadjusted analysis, we compared results among all CTN-0055 CHOICES participants regardless of HEP co-infection (OUD+/HEP+ and OUD+/HEP−) to our PLHIV reference population (OUD−/HEP−; Supplemental Table 1). Here we found significant differences in frequencies of classical, intermediate, and nonclassical monocytes between populations with and without OUD (P = 0.0002, P = 0.0025, and P = 0.0001, respectively; Fig. 1). As HEP co-infection could confound results, subsequent analysis was performed after assigning individuals to three cohorts based on HEP co-infection and OUD (Supplemental Table 1; Table 2). When adjusted for HIV viral load, age, and sex, in our multivariate model (Table 2), frequencies of intermediate and nonclassical monocytes were significantly higher in the OUD+/HEP+ as compared with the OUD−/HEP− cohort (P = 0.026 and 0.039, respectively). Frequencies of intermediate and nonclassical monocytes were also significantly higher in OUD+/HEP− as compared with OUD−/HEP− (P = 0.047 and 0.0002, respectively) cohort (Table 2). Frequencies of classical, intermediate, and nonclassical monocytes did not differ significantly between OUD+/HEP+ and OUD+/HEP− cohorts (Table 2).
The proportion of CD4+ and CD8+ T cells expressing activation markers CD38 and/or HLA-DR, exhaustion marker PD-1, and molecules associated with immunosenescence (CD28-CD57+ T cells), were compared among OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− cohorts. Although unadjusted analysis revealed evidence for differences in the burden of activated CD8+ T cells among OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− cohorts (Supplemental Table 1), following adjustments for HIV viral load, age, and sex, these differences were no longer significant (Table 2).
Hepatitis C co-infection is associated with increased cell surface and soluble CD163 among people living with HIV with opioid-use disorders
CD163 is a cell-surface glycoprotein scavenger receptor highly expressed on monocytes and tissue macrophages, that serves as a receptor for hemoglobin--haptoglobin complexes [26]. CD163 is shed into plasma upon monocyte recognition of LPS via TLR-4 [27], and plasma sCD163 is considered a marker of HIV disease activity, and monocyte/macrophage-mediated inflammation [17,28,29]. In unadjusted, three-group analysis, we observed a trend towards significant differences in cell surface expression of CD163 on classical and intermediate monocytes among OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− cohorts (Fig. 2; Supplemental Table 1). Following adjustment for HIV viral load, age, and sex (Table 2), we found that the OUD+/HEP+ cohort exhibited significantly higher cell surface CD163 expression on classical and intermediate monocytes, when compared with either OUD−/HEP− or OUD+/HEP− cohorts. Soluble CD163 also differed among OUD+/HEP+, OUD+/HEP−, and OUD−/HEP− cohorts in unadjusted analysis (P = 0.017; Fig. 2; Supplemental Table 1); in our multivariate model, the OUD+/HEP+ cohort exhibited significantly higher plasma sCD163 when compared with either OUD−/HEP− or OUD+/HEP− cohorts (Table 2). We did not find evidence for differences in plasma IL-6 or soluble CD14 (sCD14) among cohorts (Supplemental Table 1).
Opioid-use disorder is associated with dysregulated innate cytokine production among people living with HIV
Animal models and in-vitro studies suggest that opioid exposure alters cytokine production from mitogen or LPS-stimulated PBMC [30–32]. We compared production of 14 cytokines by PBMC stimulated with either the TLR-4 ligand LPS, or mitogen PHA, between CTN-0055 CHOICES participants and our PLHIV reference population (Supplemental Table 2). In unadjusted analysis, LPS-induced production of IL-10, IL-1α, IL-6, IL-8, and TNF-α, was significantly different among individuals with and without OUD (Fig. 3; Supplemental Table 2). Following cohort assignment based on HEP co-infection and adjustment for HIV viral load, age, and sex (Table 2), LPS-induced production of IL-10, IL-1α, IL-6, IL-8, and TNF-α remained significantly reduced in both OUD+/HEP+ and OUD+/HEP− cohorts, as compared with the OUD−/HEP− reference. We observed no significant differences in LPS-induced cytokine production between OUD+/HEP+ and OUD+/HEP− cohorts (Table 2). Thus, a dysregulated cytokine response to LPS is observed among PLHIV with OUD, regardless of HEP co-infection.
In unadjusted analysis, PHA-induced production of IL-10, IL-1α, IL-1β, IL-6, and TNF-α, was significantly different between CTN-0055 CHOICES participants and our PLHIV reference population (Fig. 3; Supplemental Table 3). Following cohort assignment based on HEP co-infection and adjustment for HIV viral load, age, and sex, in our multivariate model (Table 2), differences in PHA-induced production of IL-10, IL-1α, IL-6, and TNF-α remained significantly reduced between OUD+/HEP+ and OUD+/HEP− cohorts, and the OUD−/HEP− reference group. PHA-induced production of IL-1β was significantly reduced when comparing results between OUD+/HEP− and OUD−/HEP− cohorts, specifically, in our adjusted analysis (P = 0.008; Table 2). There were no significant differences in PHA-induced cytokine production between OUD+/HEP+ and OUD+/HEP− cohorts (Table 2). There were no significant differences in PHA-induced production of IL-2, IFN-γ, or IL-4 among cohorts (Supplemental Table 3).
Monocyte phenotype is strongly correlated with lipopolysaccharide-induced cytokine production
We performed Spearman Rank correlation analysis to examine relationships between frequencies of classical and intermediate monocytes, and LPS-induced production of IL-6, IL-10, IL-8, TNF-α, MCP-1, IL-1α, and IL-1β. Correlations between frequency of intermediate monocytes and plasma sCD14 and sCD163, plasma sCD163 and plasma IL-6, and intermediate monocyte CD163+ MFI versus plasma sCD163 were also assessed. The frequency of classical monocytes was significantly correlated with production of IL-6 (rs = 0.713; P < 0.0001), IL-10 (rs = 0.625; P < 0.0001), and IL-8 (rs = 0.520; P = 0.0019). Negative associations between the frequency of intermediate monocytes and production of TNF-α (rs = −0.446; P = 0.0092), IL-1α (rs = −0.548; P = 0.0010), and IL-1β (rs = −0.452; P = 0.0083), were observed. No other significant correlations were identified (Supplemental Table 4).
Discussion
Here we report that OUD in PLHIV is associated with increased frequencies of CD16+ intermediate and nonclassical monocytes, and reduced production of both proinflammatory and anti-inflammatory innate cytokines in response to LPS and PHA, regardless of HEP co-infection. We have identified a higher burden of cell surface and soluble CD163 among PLHIV with OUD and HEP co-infection specifically, indicating an increased burden of monocyte activation in this population (see model, Fig. 2). In contrast, CD4+ and CD8+ T-cell phenotypes, and production of T-cell cytokines were comparable between PLHIV with and without OUD once differences in HIV viral load were considered. Thus, among PLHIV receiving ART, OUD is associated with dysregulation of innate immunity that is further exacerbated by HEP co-infection.
Monocytes that express CD16 are more susceptible to HIV infection, promote viral replication, and have distinctive cytokine responses to molecules, such as LPS [25,33,34]. Increased circulating CD14++CD16+ monocytes were noted among individuals with AIDS-dementia in the pre-ART era [21], and CD14++CD16+ monocytes are key mediators of chronic neuroinflammation that are capable of crossing the blood–brain barrier (BBB) to deliver HIV into the CNS [23,35–37]. Despite the success of ART, the prevalence of HIV-associated neurologic disorder (HAND) continues to increase [38] and there remains a strong association between CD16+ monocytes and HAND [21,22,35,37,39–42]. Indeed, CD16+ monocytes that co-express CCR2 preferentially cross an in-vitro BBB model in response to CCL2/MCP-1 [36,40]. Although we did not observe significant differences in CCL2/MCP-1 production (Supplemental Tables 2 and 3) among our cohorts, our findings cannot be compared with prior studies illustrating elevated levels of CCL2/MCP-1 in brain tissue and CSF of individuals with HAND as we did not have access to CNS samples for our current study. Notably, the investigational dual CCR2/CCR5 inhibitor cenicriviroc improved cognitive performance among PLHIV, and this impact is thought to be because of its association with reduced monocyte activation [43]. It is also reported that buprenorphine, a partial mu-opioid receptor agonist and complete antagonist of the kappa-opioid receptor, interferes with CCL2-mediated transmigration of human CD14+CD16+ monocytes across an in-vitro BBB system [35]. Our findings combined with prior reports, support that monocyte phenotype is modulated by opioids, and that manipulation of opioid receptors using partial agonists or antagonists could provide a novel therapeutic option to temper the consequences of chronic innate immune dysregulation among PLHIV.
There are a limited number of studies examining peripheral blood monocyte phenotype and function among PLHIV with OUD or other SUD. Calderon et al.[23], reported an increased frequency of CD16+ monocytes among PLHIV with mixed SUD as compared with PLHIV without SUD; however, only a small proportion of their participants (7.1%) were reported to be exposed to opioids, and potential confounders including HEP co-infection, and HIV viral load, were not included in the analysis. Meijerink et al.[44], reported that treatment-naïve PLHIV with heroin exposure produced reduced amounts of IL-1β, IL-6, TNF-α, and IFN-γ in whole blood in response to LPS when compared with PLHIV without heroin exposure. We have extended findings from these prior reports using a well characterized cohort of PLHIV with moderate-to-severe OUD [24], to demonstrate that OUD is associated with both altered monocyte phenotypes and dysregulated innate cytokine responses, and that these associations remain significant after important biologic confounders are considered.
Intermediate monocytes are primary producers of pro-inflammatory cytokines TNF-α and IL-1β in response to bacterial products recognized by TLR-4 and TLR-2; whereas classical monocytes are the main producers of CCL2 (MCP-1), IL-10, IL-8, and IL-6 in response to these stimuli. When examining correlations between LPS-induced cytokine production and monocyte phenotypes, we found expected positive correlations between frequency of classical monocytes and IL-6, IL-10, and IL-8 production. We identified a negative correlation between LPS-induced TNF-α or IL-1β production and frequency of intermediate monocytes. Prior reports associating monocyte phenotypes with functional responses were performed using either purified monocyte populations, in-vitro differentiated cells, or intracellular flow cytometry [45–47]. In our current ex-vivo study, cytokine responses were measured in unfractionated PBMC; thus, LPS-responsive effector cells other than monocytes [such as B cells, dendritic cells, and natural killer (NK) cells] may have contributed to dysregulated cytokine production among PLHIV with OUD. We also consider that HIV directly modulates LPS-induced immune responses independent from changes in monocyte phenotype [48], and that there are several mechanisms through which OUD may alter innate immune responses. For example, opioids can directly interact with endogenous opioid receptors expressed by monocytes [32,35,49,50], modulate TLR-4 regulation [51], and may damage intestinal mucosa leading to increased microbial translocation, chronic innate immune stimulation, and immunoparalysis [18–20,52]. Given the complexity of the human cohorts supporting our investigations and differences in experimental design, it is not surprising that we have identified patterns of LPS-induced cytokine production related to monocyte phenotype that do not precisely replicate those previously published.
Phytohaemagglutinin is a mitogen that requires accessory cells, such as monocytes, to elicit T-cell activation, proliferation, and production of IL-2 [53]. We observed reduced IL-10, IL-6, TNF-α, and IL-1α production in response to PHA among PLHIV with OUD, as compared with PLHIV without OUD. Combined with our observation that PHA-induced production of IL-2, IFN-γ, and IL-4 were comparable among all cohorts (Supplemental Table 3), our findings support that among PLHIV receiving ART with preserved CD4+ T-cell counts, OUD primarily impacts production of innate cytokines, whereas T-cell cytokine production remains intact. This defect in innate cytokine production is not specific to immune responses mediated by TLR-4, and reflects a more complex disruption of monocyte functional responses than previously recognized.
Our study examined the impact of OUD on immune phenotype and function of PLHIV with and without HEP co-infection. We identified a significant increase in CD16+ monocyte surface expression of CD163, as well as sCD163 in plasma of co-infected individuals, when compared with HIV-monoinfected individuals with or without OUD. Stimulation of monocytes through TLR-4, as well as TLR-2 and TLR-5, triggers shedding of CD163 from the surface of monocytes [27] and sCD163 interferes with T-cell activation and proliferation. Thus, shedding of CD163 from activated monocytes and macrophages may reflect a regulatory response to limit immune activation triggered by bacterial stimuli. Our findings are consistent with those of other groups reporting a significantly higher burden of sCD163 among HIV/HEP co-infected individuals as compared to those with HIV monoinfection [54,55].
Our study has several limitations. The restricted sample size limited the power of our statistical model to assess for differences in immunologic outcomes among cohorts while accounting for all potential biologic confounders (e.g. ART adherence; OUD versus mixed OUD/AUD) and to correct for multiple comparisons. However, the associations identified within our generalized linear model between OUD, monocyte phenotype, and cytokine production, remained robust when differences in HIV viral load, age, and sex were considered. Our assessment for opioid exposure, and current SUD or AUD, among our PLHIV reference population was based on self-report and review of medical records, and testing for recent use of drugs-of-abuse was not performed. Although it is possible that some individuals in our reference population did have recent exposure to opioids or other drugs of abuse, this is highly unlikely as all individuals in this cohort were established patients recruited from a single-provider's HIV care clinic (PTK) who volunteered to participate after careful review of the study's goals and eligibility criteria with the principle investigator (CL). Although the collection, processing, and storage of PBMC and plasma was performed identically for all participants, we cannot exclude the possibility that subtle differences in processing time or sample handling between recruitment sites impacted immunologic outcomes.
In conclusion, OUD among PLHIV who are receiving ART is associated with altered monocyte phenotype characterized by expression of CD16, as well as dysregulated innate cytokine responses to LPS and PHA. Among HIV/HEP co-infected individuals with OUD, there is additional evidence for advanced monocyte activation as illustrated by significant elevations in cell surface and sCD163. Innate immune activation is strongly correlated to risk of death, AIDS, and non-AIDS-related morbidities among PLHIV, and CD16+ monocytes are key mediators of HAND. Thus, in addition to access to treatment for OUD, novel therapies to reduce the burden of innate immune activation and restore populations of classical monocytes, should be prioritized among PLHIV suffering from OUD.
Acknowledgements
We would like to thank all study participants for their valuable contribution to this work. The study was funded by the US National Institutes of Health, National Institute on Drug Abuse (NIDA: UG1DA015815, UG1DA013732, R03DA039731 and R01DA046229), the Oregon Clinical and Translational Research Institute, and the Collins Medical Trust. We are grateful to CTN for their technical assistance and EMMES for CTN data management. We acknowledge HIV clinics in Vancouver, BC and Chicago, Illinois, USA for recruitment and data collection. We would like to thank the CTN Network, staff of the OHSU HIV Care clinic, and Erin Merrifield for supporting participant recruitment. We thank Drs Marcel Curlin and Ruth Napier for their assistance with editing this manuscript.
Author Contribution: Guarantor of the article is C.L.L. M.L.U. collected and analyzed data, wrote the manuscript. T.N. analyzed data, critically reviewed the manuscript. L.S.U. analyzed data, wrote the manuscript. L.K. collected data, critically reviewed the manuscript. P.T.K. designed the study, collected data, critically reviewed the manuscript. C.L.L. designed the study, collected and analyzed data, wrote the manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Flores J, Liang Y, Ketchum NS, Turner BJ, Bullock D, Villarreal R, et al. Prescription opioid use is associated with virologic failure in people living with HIV. AIDS Behav 2017; 22:1323–1328. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg DF, Gordon KS, Barry DT, Becker WC, Crystal S, Edelman EJ, et al. Long-term prescription of opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. J Acquir Immune Defic Syndr 2015; 69:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronald PJRJ, ELton RA. Continued drug use and other cofactors for progression to AIDS among injecting drug users. AIDS 1994; 8:339–343. [DOI] [PubMed] [Google Scholar]
- 4.Perlman DC, Jordan AE. The syndemic of opioid misuse, overdose, HCV, and HIV: structural-level causes and interventions. Curr HIV/AIDS Rep 2018; 15:96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Boer RJ, Borghans JA. Immune activation and collateral damage in AIDS pathogenesis. Front Immunol 2013; 4:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlini E, Tincati C, Biasin M, Saulle I, Cazzaniga FA, d’Arminio Monforte A, et al. Stimulation of PBMC and monocyte-derived macrophages via toll-like receptor activates innate immune pathways in HIV-infected patients on virally suppressive combination antiretroviral therapy. Front Immunol 2016; 7:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasi M, Pinti M, Mussini C, Cossarizza A. Persistent inflammation in HIV infection: established concepts, new perspectives. Immunol Lett 2014; 161:184–188. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis 2016; 7:e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859–870. [DOI] [PubMed] [Google Scholar]
- 11.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003; 17:1881–1888. [DOI] [PubMed] [Google Scholar]
- 12.Carbone J, Gil J, Benito JM, Navarro J, Munoz-Fernandez A, Bartolome J, et al. Increased levels of activated subsets of CD4 T cells add to the prognostic value of low CD4 T cell counts in a cohort of HIV-infected drug users. AIDS 2000; 14:2823–2829. [DOI] [PubMed] [Google Scholar]
- 13.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18:332–340. [DOI] [PubMed] [Google Scholar]
- 15.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as Regulators of Inflammation and HIV-Related Comorbidities during cART. J Immunol Res 2014; 2014:569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, et al. Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy (SUN Study) Investigators. Monocyte-Activation Phenotypes Are Associated With Biomarkers of Inflammation and Coagulation in Chronic HIV Infection. J Infect Dis 2014; 210:1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng J, Sindberg GM, Roy S. Disruption of gut homeostasis by opioids accelerates HIV disease progression. Front Microbiol 2015; 6:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 2013; 8:e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sindberg GM, Sharma U, Banerjee S, Anand V, Dutta R, Gu CJ, et al. An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol 2015; 10:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet 1997; 349:692–695. [DOI] [PubMed] [Google Scholar]
- 22.Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol 2004; 157:93–98. [DOI] [PubMed] [Google Scholar]
- 23.Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, et al. Dopamine increases CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: implications for substance abuse and HIV neuropathogenesis. J Neuroimmune Pharmacol 2017; 12:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korthuis PT, Lum PJ, Vergara-Rodriguez P, Ahamad K, Wood E, Kunkel LE, et al. CTN-0055 CHOICES Investigators. Feasibility and safety of extended-release naltrexone treatment of opioid and alcohol use disorder in HIV clinics: a pilot/feasibility randomized trial. Addiction 2017; 112:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 26.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature 2001; 409:198–201. [DOI] [PubMed] [Google Scholar]
- 27.Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 2007; 81:663–671. [DOI] [PubMed] [Google Scholar]
- 28.Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom 2005; 63:16–22. [DOI] [PubMed] [Google Scholar]
- 29.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after antiretroviral therapy. J Infect Dis 2011; 204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabati S, Asadikaram G, Arababadi MK, Shahabinejad G, Rezaeian M, Mahmoodi M, et al. The plasma levels of the cytokines in opium-addicts and the effects of opium on the cytokines secretion by their lymphocytes. Immunol Lett 2013; 152:42–46. [DOI] [PubMed] [Google Scholar]
- 31.Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ, Jr, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol 2000; 68:723–728. [PubMed] [Google Scholar]
- 32.Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF. Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J Clin Invest 1987; 80:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 2007; 178:6581–6589. [DOI] [PubMed] [Google Scholar]
- 34.Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, et al. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 2006; 344:267–276. [DOI] [PubMed] [Google Scholar]
- 35.Jaureguiberry-Bravo M, Lopez L, Berman JW. Frontline science: buprenorphine decreases CCL2-mediated migration of CD14(+) CD16(+) monocytes. J Leukoc Biol 2018; 104:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol 2011; 267:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 2014; 12:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaton RK, Franklin DR, Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. CHARTER Group. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7:528–541. [DOI] [PubMed] [Google Scholar]
- 40.Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio 2017; 8: pii: e01280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm 2014; 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, et al. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 2013; 8:e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr 2018; 79:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meijerink H, Indrati A, Utami F, Soedarmo S, Alisjahbana B, Netea MG, et al. Heroin use is associated with suppressed pro-inflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS One 2015; 10:e0122822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002; 168:3536–3542. [DOI] [PubMed] [Google Scholar]
- 46.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadadi E, Zhang B, Baidzajevas K, Yusof N, Puan KJ, Ong SM, et al. Differential IL-1beta secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability. Sci Rep 2016; 6:39035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yim HC, Li JC, Lau JS, Lau AS. HIV-1 Tat dysregulation of lipopolysaccharide-induced cytokine responses: microbial interactions in HIV infection. AIDS 2009; 23:1473–1484. [DOI] [PubMed] [Google Scholar]
- 49.Borner C, Stumm R, Hollt V, Kraus J. Comparative analysis of mu-opioid receptor expression in immune and neuronal cells. J Neuroimmunol 2007; 188:56–63. [DOI] [PubMed] [Google Scholar]
- 50.Cornwell WD, Lewis MG, Fan X, Rappaport J, Rogers TJ. Effect of chronic morphine administration on circulating T cell population dynamics in rhesus macaques. J Neuroimmunol 2013; 265:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, et al. Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci Rep 2013; 3:1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kern JA, Reed JC, Daniele RP, Nowell PC. The role of the accessory cell in mitogen-stimulated human T cell gene expression. J Immunol 1986; 137:764–769. [PubMed] [Google Scholar]
- 54.Shmagel KV, Saidakova EV, Shmagel NG, Korolevskaya LB, Chereshnev VA, Robinson J, et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med 2016; 17:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lidofsky A, Holmes JA, Feeney ER, Kruger AJ, Salloum S, Zheng H, et al. Macrophage activation marker soluble CD163 is a dynamic marker of liver fibrogenesis in human immunodeficiency virus/hepatitis C virus coinfection. J Infect Dis 2018; 218:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


