Analogous to exercise training, time-restricted eating may rescue some of the deleterious effects on metabolic health induced by our modern-day lifestyle.
Key Words: time-restricted eating, exercise, circadian biology, meal timing, metabolic health
Abstract
This Perspective for Progress provides a synopsis for the potential of time-restricted eating (TRE) to rescue some of the deleterious effects on circadian biology induced by our modern-day lifestyle. We provide novel insights into the comparative and potential complementary effects of TRE and exercise training on metabolic health.
Key Points
Our modern lifestyle incorporating round-the-clock access to energy-dense food and low levels of habitual physical activity perturbs the circadian clock, increasing the risk for metabolic diseases.
Eating over a prolonged period (>12 h⋅d−1) may be deleterious for health outcomes in “at-risk” individuals.
Time-restricted eating (in which energy intake is restricted to 8–10 h⋅d−1) improves markers of metabolic health, but if the recommended quantity and quality of exercise are performed, meal timing may be less important.
INTRODUCTION
Numerous metabolic and physiological processes are underpinned by 24-h biological oscillations that are under the control of a central circadian clock, present in all mammalian cells (1). Synchronization of the expression of circadian clock genes in the suprachiasmatic nucleus (SCN) of the hypothalamus is primarily governed by the light-dark cycle (1). However, other environmental and behavioral time cues, termed “zeitgebers,” such as the timing of meals and exercise, along with sleep-wake cycles, can “fine-tune” the central clock (2). These nonphotic cues can reset or induce time-phase shifts in circadian oscillations through mechanisms independent of the SCN (3,4). Indeed, our prevailing modern lifestyle (round-the-clock access to energy-dense food, low levels of habitual physical activity accompanied by periods of prolonged sitting, and inadequate quality/quantity of sleep) interacts with underlying biology to create an environment in which circadian rhythms are disrupted, often resulting in a plethora of metabolic conditions (3–5). This was not always the case.
Throughout human evolution, lifestyle and energy availability were inextricably linked to the periodic cycles of feasts and famines. During these natural cycles, specific genes evolved to regulate efficient storage of endogenous fuel stores, so-called thrifty genes (6). During the early hunter-gatherer period, there was also the selection of genes and traits to support a “physical activity cycle” (7,8), and under these constraints, most of the present human genome evolved. Today, those alleles and traits that evolved for energy storage and locomotion are exposed to a host of unfavorable environment cues over an extended lifespan, perturbing the intrinsic circadian clock and increasing the risk of many lifestyle-induced metabolic diseases (3–5). In this Perspective for Progress, we provide a synopsis of the efficacy of diet and exercise interventions to rescue many of the deleterious effects on circadian biology induced by our modern-day lifestyle. We describe new insights into the comparative and potential complementary effects of exercise training and a novel dietary intervention that encourages a longer daily duration of fasting to improve human metabolic health, but argue that exercise still remains the optimal strategy to improve the majority of lifestyle-induced disorders in metabolism.
STRATEGIES TO IMPROVE METABOLIC HEALTH
The benefit of improving dietary quality combined with undertaking regular exercise is undoubtedly the best approach to prevent/treat noncommunicable diseases (9). However, adherence to lifestyle modifications is poor, and such behavioral changes have met with limited success at the population level (10). Consequently, diet and exercise strategies that focus on more socially acceptable and achievable interventions (e.g., exercise “breaks” after meals, changing the timing of eating, high-intensity sprint interval training) may be more effective for improving metabolic health (11–13). The interactions between the timing of exercise and meals is complex: changes in energy and/or macronutrient intake rapidly alter the concentration of blood-borne substrates and hormones causing marked perturbations in the storage profile of skeletal muscle and other insulin-sensitive tissues (14). In turn, the energy status of muscle exerts marked effects on resting fuel metabolism and patterns of fuel use during exercise (15), influencing acute regulatory processes underlying gene expression and cell signaling (16). Although it is generally accepted that adaptations to exercise training result from the cumulative and chronic effect of the transient increases in mRNA transcripts that encode for various proteins after each successive exercise bout (17), the chronic effects of shifts in meal timing are, as yet, unknown.
Diet Interventions
In the past decade, evidence has accumulated to suggest that timing of meals affects a wide variety of physiological functions, including the sleep/wake cycle, core body temperature, athletic performance, and mental alertness (2). Furthermore, the time of meals has a profound effect on skeletal muscle insulin sensitivity and whole-body metabolic health (18) and can be manipulated to help prevent/treat a number of lifestyle-related disease states. This has been termed “chrono-nutrition” and reflects a new appreciation that the timing of meals in addition to the energy content and macronutrient composition of food is critical for the well-being of an organism. Hence, chrono-nutrition refers to the synchronization of eating with the body's entrained circadian rhythms (2).
Numerous diet strategies have been proposed to curb the soaring prevalence of obesity and lifestyle-induced metabolic disorders. A feature common to most of these diets is the manipulation of the feeding-fasting cycle. In this regard, time-restricted eating (TRE), in which energy intake is limited to a “window” of less than 10 h⋅d−1, has emerged as a practical intervention to increase the length of time spent fasting, by reducing the time over which energy is consumed (Fig. 1). Gill and Panda (11) were the first to show that 16 wk of TRE in overweight humans (body mass index (BMI), >25 kg⋅m−2) induced a modest weight loss (~3% body mass) after decreasing the eating window from more than 14 h to less than or equal to 10 h⋅d−1. This weight loss was maintained for 12 months, suggesting TRE may be a practical strategy for weight maintenance over the long term. Although the participants in that study (11) were not asked to change nutritional quality or quantity, reducing daily eating duration led to 20% reduction in energy intake (11). With small subject numbers (five males and three females) and no control group, wider interpretation of the results of this study is limited.
Figure 1.
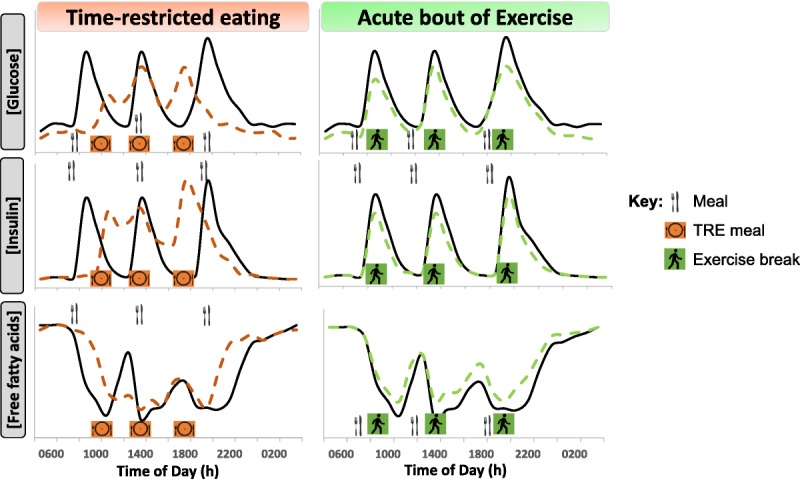
Proposed perturbations to glucose, insulin, and free fatty acid (FFA) concentrations over a 24-h period with time-restricted eating (TRE) (orange, dashed lines) or “exercise snacks” (~10 min walking; green, dashed lines) compared with typical circulating metabolite patterns in response to three meals consumed during waking hours.
Other studies since that of Gill and Panda (11) confirm that TRE induces modest weight loss (19–21), although these interventions have been short term (≤12 wk) with no follow-up. For example, Gabel et al. (21) reported that 23 individuals with obesity who adhered to 12 wk of TRE (1000–1800 h) for ~5–6 d⋅wk−1 had a loss of 2.6% body mass. Others have also observed small reductions in energy intake when implementing TRE of 8–10 h⋅d−1 (11,21,22), making it difficult to attribute weight loss solely to the timing of energy intake. Of note, energy-restricted dietary interventions, without an exercise stimulus, typically induce a loss of ~250–300 g of lean tissue for every kilogram of weight lost (23), and although no measures of body composition have been made in any TRE study to date, such losses in lean mass would be expected to be similar to dietary fasting interventions (24).
The results of a recent study suggest that the health benefits of TRE may be independent of weight loss (25). In the first supervised trial of early TRE (eTRE), in which all meals were provided to participants, Sutton et al. (25) studied eight men with prediabetes who were randomized to eTRE (meals consumed within 6 h, last energy intake before 1500 h) or an unrestricted eating pattern (meals consumed over 12 h, from 0800 to 2000 h) for 5 wk. No measures of body composition or physical activity were assessed. Compared with unrestricted eating, eTRE improved insulin sensitivity and B-cell responsiveness, blood pressure, and oxidative stress, although selected measures of appetite were also modified (25). The precise mechanism(s) of how TRE improves health outcomes without enforced energy restriction is currently unknown.
The circadian clock affects hormonal metabolism, with the timing of meals fine-tuning endocrine biology with regard to glycemic control (26). An influx of glucose from cortisol-stimulated hepatic glucose production occurs around 0800 h, when cortisol levels typically peak after waking (27). Delaying (not skipping) breakfast to late morning (~1000 h) and missing the circadian-related release in hepatic glucose (28) could improve postprandial glycemic control (Fig. 1). Insulin secretion and sensitivity also are under circadian regulation: these parameters are increased early in the day and drop in the evening (28), even when there are equidistant 12-h fasts between meals (29). Thus, the reduction in insulin sensitivity in the evening explains the impaired glucose tolerance measured in response to late-night dinner consumption (30,31).
An important question for both health outcomes and the practicality of implementing TRE interventions is whether the window of meal timing throughout the day, as well as the start/finish time of meals, is associated with the magnitude of improvement in health markers. The eTRE intervention by Sutton et al. (25) revealed hyperinsulinemia was reduced when daily eating was completed by 1500 h, but such a strict eating protocol is not likely to be practical or socially acceptable at a population level. Studies of “late” TRE, or when total energy intake was restricted to meals consumed after 1600 h, have resulted in impaired fasting glucose, lowered glucose tolerance, and increased ratings of hunger (32,33). Studies that have commenced TRE in the middle of the day have shown either no effect (21) or tended to be beneficial with regard to glycemic control (19,22). In the only comparative time window TRE study to date, Hutchison et al. (34) compared 1 wk of eTRE (0800–1700 h) with delayed TRE (1200–2100 h) in men at risk of developing type 2 diabetes (T2D). Both protocols improved glycemic control in response to a test meal, but only eTRE improved overnight fasting glucose levels (34). Clearly, there is a trade-off between the feasibility of undertaking TRE and adherence to an optimal TRE window that aligns with healthy circadian rhythms. Accordingly, future studies should determine whether it is the placement of the eating window or the duration spent in the fasted state over each day that induces many of the improvements in metabolic health.
Satiety-inducing hormones glucagon-like peptide-1 (GLP-1), glucose-inhibitory peptide (GIP), and peptide YY (PYY) are increased, and the hunger-inducing hormone ghrelin is suppressed in response to meals. These gut hormones play a critical role in modulating appetite and the rate of gastric emptying (which slows in the evening), and, therefore, the glycemic response to meals. Secretion of these hormones is under circadian regulation (35). For example, ghrelin release peaks at mid evening (~2000 h) and is lowest upon waking (36), explaining, in part, the strong biological drive to eat at night. As such, diets that promote greater energy intake in the morning and lower energy intake (or longer fasting) in the evening are likely to produce sustained weight loss (37,38). Perhaps surprisingly, subjective ratings of appetite are not increased by TRE, at least when measured during the day (11,25). TRE initiated from 0800 to 1700 h or from 1200 to 2100 h did not alter the postprandial suppression of ghrelin or the rise in PYY, GLP-1, or GIP response, and there were no differences in subjective appetite ratings during a meal test (34).
Reducing the time spent in a postprandial and postabsorptive state improves glycemia (39) while concomitantly shifting patterns of substrate use. Prolonged (>24 h) fasting increases the production of ketone bodies in humans (40) and rates of whole-body fat oxidation. Extended fasting periods are likely to upregulate fat oxidation via an increase in lipolysis and circulating free fatty acids (FFAs). Throughout a typical day of eating (i.e., three meals plus snacks), circulating FFA and triglyceride (TG) concentrations are suppressed (41), and prolonged energy consumption (>14 h⋅d−1) limits lipolysis and rates of FFA oxidation at rest. An extended overnight fast with TRE augments the increases in FFAs and TGs, resulting in elevated fasting lipid profiles (25). Whether such a lipid profile is beneficial for human health over the long term remains to be established. To date, the results from animal studies reveal that hepatic fat stores are reduced with TRE (42), but further investigations in humans are needed to understand the effect of TRE on whole-body and hepatic lipid metabolism.
In terms of sustainability, TRE seems to offer a practical advantage over stricter energy-restricted diet interventions, given there is no specific instruction around energy restriction or discretionary food choices. However, the types of foods we consume often are aligned closely to distinct times of the day; alcohol typically is consumed at the end of the day, as are sweet (refined sugar) foods such as ice cream (11). A reduction in food intake later in the day may not only reduce total energy intake but also curtail discretionary food intake and improve overall dietary quality (37,43–45). However, it also is possible that placing time restrictions on eating could result in poorer food choices in some individuals.
Exercise Interventions
TRE extends the length of time in the fasted state (Fig. 1), inducing several responses that are similar to those observed after exercise training (Fig. 2). However, when compared with dietary interventions, both the amplitude and extent of exercise training‐induced responses/adaptations are likely to be greater in a head-to-head comparison (Fig. 2). Evidence for such a premise comes from epidemiological data demonstrating that the association between low cardiometabolic fitness (i.e., maximum oxygen uptake) and all-cause mortality is stronger than that of obesity (i.e., high BMI) (46). Exercise training delays the onset of at least 40 chronic metabolic conditions/diseases (see (47,48) for reviews). However, getting people to comply with even the minimal recommended quantity and quality of exercise required to confer health benefits has proven difficult (10).
Figure 2.
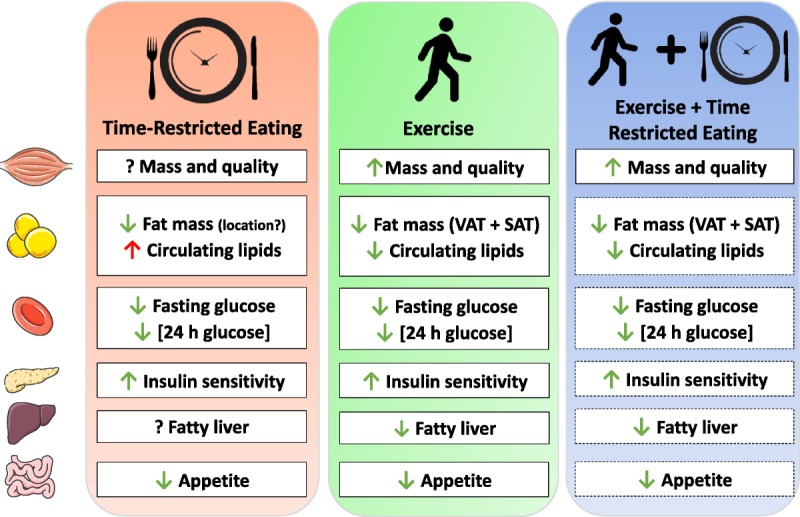
Effects of time-restricted eating (TRE) and exercise training on metabolic health in humans (known, solid box; proposed, dashed box). Green arrows, positive change; red arrows, negative change; question mark, unknown effect. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Exercise training has a significant impact on body composition, reducing both subcutaneous and visceral fat (49) while preserving lean mass (23). Aerobic-based exercise of sufficient duration/intensity promotes beneficial changes in whole-body metabolism and reduces fat mass (50), whereas resistance exercise preserves or increases lean (muscle) mass (51,52). Energy-restricted diets in isolation are effective short-term strategies for rapid weight loss but result in a reduction in both fat and muscle mass, predisposing one to an unfavorable body composition and poor health prognosis. Exercise typically results in only a modest increase in total daily energy expenditure but has minimal effects on long-term weight loss, depending on the volume/intensity of exercise performed (23). As such, the performance of regular and appropriate exercise is the only mechanism to improve body composition (i.e., lose fat mass while maintaining lean mass). For an individual solely focused on changes in weight, the short-term reduction in body mass with TRE may initially exceed that from exercise, masking any long-term exercise-induced changes to body composition. Long-term studies of TRE that incorporate body composition measures are urgently needed to understand the effects induced by the longer duration of daily fasting.
Although TRE interventions have the potential to change habitual dietary practices, there may also be changes to physical activity patterns (53,54). The effect of increasing the amount of exercise on dietary intake, however, appears equivocal; increases in physical activity have either had no effect (55,56) or resulted in an improvement (57) on dietary quality. In older adults with prediabetes, performing regular resistance-based exercise reduced self-reported intake of carbohydrate, sugar, sweets, and desserts, with little effect on protein intake (58). Such changes to total energy and macronutrient intake may be related to changes in the regulation of appetite with exercise. Acute physical activity transiently represses appetite in both lean and obese individuals (59) via a suppression of ghrelin and increases in PYY and GLP-1 (60). However, the effects of longer-term exercise training on the control of appetite are equivocal (61,62). Exercise training may balance appetite responses by an increased satiety response to a meal despite an increased drive to eat (63,64). Exercise training also reduces circulating leptin concentrations, reducing fat mass, and positively affects appetite and body composition (65,66). Therefore, exercise has the ability to reduce appetite and improve overall dietary intake. To date, there have been no interventions of TRE in combination with an exercise training program compared with TRE alone.
A single bout of exercise increases skeletal muscle glucose uptake (67). However, this “insulin-sensitizing” effect is short-lived and dissipates after ~48 h (68). In contrast, repeated physical activity (i.e., exercise training) results in a persistent increase in insulin action in skeletal muscle from health individuals and people with insulin resistance and obesity (69). Exercise training also improves glucose tolerance (70). The precise volume of exercise required to induce a clinically meaningful change in glycemia is a contentious issue, but small in relation to the time spent awake. Dempsey et al. (12,71) reported that less than 40 min of walking or body weight resistance exercises (12 × 3-min bouts undertaken between 0900 and 1500 h) improved both waking and nocturnal glucose concentrations in individuals with T2D. Breaking up exercise bouts (“exercise snacking”) into 3 × 10-min bouts after meals improved daily glycemic control in individuals with T2D to a greater magnitude than a continuous 30-min walk (72). In individuals with prediabetes, three “exercise snacks” (6 × 1 min of high-intensity activity before each meal) improved daily glycemia on both the day of exercise and over the subsequent 24 h (13). As such, adequate physical activity no matter how it is accumulated across a day is effective in attenuating postprandial glucose and insulin concentrations (Fig. 1).
Regular exercise increases postexercise rates of whole-body fat oxidation and improves metabolic flexibility (the ability to respond to changes in hormonal milieu and switch between fuel sources in response to the prevailing metabolic demand) (73)). Exercise also has a positive effect on circulating lipid profiles, with decreased fasting and postprandial FFA concentrations, increased uptake of FFA by the muscle, and lower uptake of FFA to the liver (74), contributing to improvement in nonalcoholic fatty liver disease (75). A meta-analysis of aerobic-based exercise training programs performed for 12 wk or longer revealed reduced circulating TGs and higher high-density lipoprotein (HDL), low-density lipoprotein, and total cholesterol (76). Reductions in cholesterol and circulating lipids have also been observed both in response to a single bout of resistance-based exercise (77) and after resistance training (78), although the magnitude of reduction is typically less than that observed after aerobic exercise training. Even short bouts of physical activity accumulated for ~40 min across a day improve the postprandial handling of lipids (79).
The optimal timing of exercise to maximize health benefits is currently unknown and likely to be confounded by a number of variables (health status of the individual, entrained waking time, circadian phenotype, mode and duration/intensity of exercise, and meal timing). Acute performance of aerobic-based exercise (i.e., continuous or high-intensity interval training) in the afternoon/evening improves glycemic control (72,80), likely due to the timing in relation to both circadian-related insulin resistance and the postprandial state (81). Resistance exercise performed in the morning, afternoon, or evening improves force generation (i.e., muscle strength) (82,83). However, there is no clear consensus regarding the merits of performing either morning or evening exercise with regard to superior improvements in aerobic capacity (84) or resistance training/strength adaptations (85). Although it has recently been proposed that time of day is a major modifier of exercise responses/adaptations and associated metabolic pathways (86) and that there is a day-night rhythm in mRNA expression of molecular clock genes in human skeletal muscle (87), we urge caution with regard to recommendations on the “optimal time of day” to exercise for optimal health benefits: individual health status (i.e., known cardiovascular disease/hypertension), personal exercise goals, and feasibility should all be considered.
TRE and Exercise Training: Some Considerations
The discovery of muscle “cross-talk” with other organs, including adipose tissue, liver, pancreas, bone, and the brain, provides a framework for understanding how exercise mediates many of its beneficial whole-body effects (88). Although several acute responses to TRE are similar to those attained after exercise training (Fig. 2), exercise evokes widespread and extensive remodeling of almost every organ/tissue in the body (i.e., increased bone mineral density, improved cardiovascular dynamics and blood flow, increases in muscle oxidative capacity and capillarization, increases in muscle cross-sectional area, etc.). Indeed, it is the very complexity and multiplicity of networks involved in exercise responses that make it unlikely that such whole-body effects could be induced by TRE.
Whether adding exercise training to a TRE regimen or supplementing an exercise program with TRE confers any additional benefits above and beyond either intervention in isolation has not been systematically investigated. To date, only two TRE interventions in humans have included exercise as an adjunct to changes to dietary timing (19,89). Moro et al. (19) studied healthy males during 8 wk of resistance training who were assigned either to a group who undertook alternate days of late TRE (1300–2000 h) or to a group who were unrestricted in their eating (0800–2000 h) but matched for energy intake. Although both groups increased muscle mass after the intervention, only the TRE group decreased total body mass and fat mass, with small but concomitant positive improvements in several metabolic health markers (improved glucose, HDL, and TG profiles) (19). Tinsley et al. (89) examined the effects of 8 wk of resistance training (3 d⋅wk−1) with or without late TRE. The TRE protocol consisted of consuming all meals within a 4-h period between 1600 h and midnight on nontraining days (4 d⋅wk−1), with no limitations on quantities or types of foods chosen. A “control” condition consisted of resistance training with no restrictions on energy intake on the nonworkout days. Late TRE resulted in a 10% reduction in energy intake compared with the control group but did not confer any additional improvements in body composition, and markers of metabolic health were not measured (89). Post hoc tests revealed a small positive effect of the resistance training on the accretion of lean tissue mass in the control group only, which was likely due to the greater (1.4 vs 1.0 g⋅kg−1 body mass) protein intake in the control compared with the TRE group. Thus, the additive effects of TRE to exercise training may be dependent on the effects that TRE has on both total daily energy and protein intake, and the timing of protein ingestion relative to when the exercise is performed.
In other interventions of TRE, participants have been instructed “not to change physical activity levels” (20,21,34) or there has been no control of physical activity (11,22). There are no studies investigating the potential for an additive benefit of exercise training to a TRE dietary regimen compared with TRE alone. As dietary quality and quantity are not markedly improved when individuals undertake regular physical activity, complementing exercise training with TRE may help modify behavioral patterns of eating, (i.e., reduced end-of-day snacking/alcohol consumption) and enhance overall health benefits. For individuals with medical conditions where physical activity cannot be performed, TRE offers a feasible strategy to improve or maintain metabolic health. However, we propose that for most “healthy” individuals, adding TRE to a program of regular physical activity would impart minimal additive effects on a range of health-related outcomes.
FUTURE PERSPECTIVES
Optimal cardiometabolic health for individuals at risk of chronic lifestyle-related diseases results from interventions in which dietary intake is reduced and/or quality is improved, and exercise of sufficient mode, duration, and intensity is performed (23,90). However, adherence to changes in habitual dietary patterns is often considered more arduous than medical therapy (91), and the majority of individuals report “a lack of time” as the major reason for not undertaking regular exercise (92). As such, the debate becomes “what priority should be given to modifying diet versus implementing exercise training for improving health outcomes?” As energy, via food, is required to sustain life, it is perhaps no surprise that dietary modifications are often the first in line of attack in the arsenal of lifestyle interventions to prevent/treat many metabolic diseases. Although there is an extensive menu of dietary options available to improve metabolic health outcomes, their success/failure, as with any exercise intervention, depends on long-term adherence (93). We believe that exercise training undertaken in accordance with national and international guidelines imparts greater whole-body and tissue-specific metabolic health benefits than any current dietary intervention. Whether TRE in humans confers additive benefits to disordered metabolism above and beyond those induced by exercise training remains to be determined experimentally.
Acknowledgments
The authors were funded by the following grants while writing this article: ESPEN Early Career Fellowship 2018 (E.B.P.), ACURF grant (ACURF2016000353; J.A.H. and E.B.P.), and Novo Nordisk Foundation Challenge grant (NNF14OC0011493; J.A.H. and E.B.P.).
Footnotes
Editor: Roger M. Enoka, Ph.D.
References
- 1.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol. Rev. 2013; 93:107–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challet E. The circadian regulation of food intake. Nature Reviews Endocrinology [Internet]. [Cited 2019 May 9]. Available from: 10.1038/s41574-019-0210-x. [DOI] [PubMed]
- 3.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015; 161:84–92. [DOI] [PubMed] [Google Scholar]
- 4.Panda S. Circadian physiology of metabolism. Science. 2016; 354:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriel BM, Zierath JR. Circadian rhythms and exercise—re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019; 15:197–206. [DOI] [PubMed] [Google Scholar]
- 6.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962; 14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 2002; 93:3–30. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 2004; 96:3–10. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015; 25:1–72. [DOI] [PubMed] [Google Scholar]
- 10.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health. 2018; 6:e1077–86. [DOI] [PubMed] [Google Scholar]
- 11.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015; 22:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J. Hypertens. 2016; 34:2376–82. [DOI] [PubMed] [Google Scholar]
- 13.Francois ME, Baldi JC, Manning PJ, et al. “Exercise snacks” before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014; 57:1437–45. [DOI] [PubMed] [Google Scholar]
- 14.Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J. Appl. Physiol. 2011; 110:834–45. [DOI] [PubMed] [Google Scholar]
- 15.Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem. J. 1920; 14:290–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojtaszewski JF, MacDonald C, Nielsen JN, et al. Regulation of 5'AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003; 284:E813–22. [DOI] [PubMed] [Google Scholar]
- 17.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010; 588(Pt. 23):4795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders: maintenance of metabolic rhythms reduce cardiometabolic disorders. J. Physiol. 2017; 595:3691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith ST, LeSarge JC, Lemon PWR. Time-restricted eating in women—a pilot study. West Undergrad. Res. J. Health Nat. Sci. 2017; 8:1–6. [Google Scholar]
- 21.Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging. 2018; 4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of Nutritional Science [Internet]. 2018 [cited 2018 Dec 7];7. Available from: https://www.cambridge.org/core/product/identifier/S2048679018000137/type/journal_article.
- 23.Parr EB, Coffey VG, Hawley JA. “Sarcobesity”: a metabolic conundrum. Maturitas. 2013; 74:109–13. [DOI] [PubMed] [Google Scholar]
- 24.Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015; 73:661–74. [DOI] [PubMed] [Google Scholar]
- 25.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018; 27:1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014; 10:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bierwolf C, Kern W, Mölle M, Born J, Fehm H. Rhythms of pituitary-adrenal activity during sleep in patients with Cushing's disease. Exp. Clin. Endocrinol. Diabetes. 2000; 108:470–9. [DOI] [PubMed] [Google Scholar]
- 28.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J. Clin. Investig. 1991; 88:934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J. Diabetes Complications. 2014; 28:836–43. [DOI] [PubMed] [Google Scholar]
- 30.Imai S, Kajiyama S, Hashimoto Y, et al. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: a randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2017; 129:206–12. [DOI] [PubMed] [Google Scholar]
- 31.Sato M, Nakamura K, Ogata H, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011; 5:e220–8. [DOI] [PubMed] [Google Scholar]
- 32.Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007; 56: 1729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007; 85:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchison AT, Regmi P, Manoogian ENC, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019; 27:724–32. [DOI] [PubMed] [Google Scholar]
- 35.Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. U. S. A. 2015; 112:E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian J, Morris CJ, Caputo R, Garaulet M, Scheer FAJL. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. (Lond). 2019; 43:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. (Lond). 2013; 37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013; 21:2504–12. [DOI] [PubMed] [Google Scholar]
- 39.Monnier L. Is postprandial glucose a neglected cardiovascular risk factor in type 2 diabetes? Eur. J. Clin. Invest. 2000; (Suppl. 2):3–11. [PubMed] [Google Scholar]
- 40.Brandhorst S, Choi IY, Wei M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015; 22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlierf G, Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J. Clin. Investig. 1973; 52:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012; 15:848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J. Hum. Nutr. Diet. 2014; 27:255–62. [DOI] [PubMed] [Google Scholar]
- 44.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011; 19:1374–81. [DOI] [PubMed] [Google Scholar]
- 45.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr. Res. 2014; 34:930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am. J. Clin. Nutr. 1999; 69:373–80. [DOI] [PubMed] [Google Scholar]
- 47.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J. Appl. Physiol. 2000; 88:774–87. [DOI] [PubMed] [Google Scholar]
- 48.Ruegsegger GN, Booth FW. Health benefits of exercise. Cold Spring Harb. Perspect. Med. 2018; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int. J. Obes. (Lond). 2006; 30:1211–6. [DOI] [PubMed] [Google Scholar]
- 50.Ballor DL, Keesey RE. A meta-analysis of the factors affecting exercise-induced changes in body mass, fat mass and fat-free mass in males and females. Int. J. Obes. 1991; 15:717–26. [PubMed] [Google Scholar]
- 51.Ballor DL, Katch VL, Becque MD, Marks CR. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am. J. Clin. Nutr. 1988; 47:19–25. [DOI] [PubMed] [Google Scholar]
- 52.McGlory C, van Vliet S, Stokes T, Mittendorfer B, Phillips SM. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J. Physiol. 2019; 597:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarma EA, Moyer A, Messina CR, et al. Is there a spillover effect of targeted dietary change on untargeted health behaviors? Evidence from a dietary modification trial. Health Educ. Behav. 2019; 46:569–81. [DOI] [PubMed] [Google Scholar]
- 54.Johannessen KB, Oettingen G, Mayer D. Mental contrasting of a dieting wish improves self-reported health behaviour. Psychol. Health. 2012; (Suppl. 2):43–58. [DOI] [PubMed] [Google Scholar]
- 55.Dutton GR, Napolitano MA, Whiteley JA, Marcus BH. Is physical activity a gateway behavior for diet? Findings from a physical activity trial. Prev. Med. 2008; 46:216–21. [DOI] [PubMed] [Google Scholar]
- 56.Rhew I, Yasui Y, Sorensen B, et al. Effects of an exercise intervention on other health behaviors in overweight/obese post-menopausal women. Contemp. Clin. Trials. 2007; 28:472–81. [DOI] [PubMed] [Google Scholar]
- 57.Fleig L, Lippke S, Pomp S, Schwarzer R. Intervention effects of exercise self-regulation on physical exercise and eating fruits and vegetables: a longitudinal study in orthopedic and cardiac rehabilitation. Prev. Med. 2011; 53:182–7. [DOI] [PubMed] [Google Scholar]
- 58.Halliday TM, Davy BM, Clark AG, et al. Dietary intake modification in response to a participation in a resistance training program for sedentary older adults with prediabetes: findings from the Resist Diabetes study. Eat. Behav. 2014; 15:379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douglas JA, King JA, Clayton DJ, et al. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int. J. Obes. Relat. Metab. Disord. 2017; 41:1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schubert MM, Sabapathy S, Leveritt M, Desbrow B. Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med. 2014; 44:387–403. [DOI] [PubMed] [Google Scholar]
- 61.Dorling J, Broom D, Burns S, et al. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: the modulating effect of adiposity, sex, and habitual physical activity. Nutrients. 2018; 10:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King NA, Horner K, Hills AP, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br. J. Sports Med. 2012; 46:315–22. [DOI] [PubMed] [Google Scholar]
- 63.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009; 90:921–7. [DOI] [PubMed] [Google Scholar]
- 64.Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metabol. 2010; 95:1609–16. [DOI] [PubMed] [Google Scholar]
- 65.Guelfi KJ, Donges CE, Duffield R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism. 2013; 62:235–43. [DOI] [PubMed] [Google Scholar]
- 66.Pil-Byung C, Shin-Hwan Y, Il-Gyu K, et al. Effects of exercise program on appetite-regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men. J. Sports Med. Phys. Fitness. 2011; 51:654–63. [PubMed] [Google Scholar]
- 67.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013; 93:993–1017. [DOI] [PubMed] [Google Scholar]
- 68.Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017; 2:e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf). 2008; 192:127–35. [DOI] [PubMed] [Google Scholar]
- 70.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006; 29:2518–27. [DOI] [PubMed] [Google Scholar]
- 71.Dempsey PC, Blankenship JM, Larsen RN, et al. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia. 2017; 60:499–507. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds AN, Mann JI, Williams S, Venn BJ. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study. Diabetologia. 2016; 59:2572–8. [DOI] [PubMed] [Google Scholar]
- 73.Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017; 25:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brouwers B, Hesselink MKC, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia. 2016; 59:2068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shojaee-Moradie F, Cuthbertson DJ, Barrett M, et al. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J. Clin. Endocrinol. Metab. 2016; 101:4219–28. [DOI] [PubMed] [Google Scholar]
- 76.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc. 2001; 33:S502–15; discussion S528-529. [DOI] [PubMed] [Google Scholar]
- 77.Lira FS, Yamashita AS, Uchida MC, et al. Low and moderate, rather than high intensity strength exercise induces benefit regarding plasma lipid profile. Diabetol. Metab. Syndr. 2010; 2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prabhakaran B, Dowling EA, Branch JD, Swain DP, Leutholtz BC. Effect of 14 weeks of resistance training on lipid profile and body fat percentage in premenopausal women. Br. J. Sports Med. 1999; 33:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grace MS, Dempsey PC, Sethi P, et al. Breaking up prolonged sitting alters the postprandial plasma lipidomic profile of adults with type 2 diabetes. J. Clin. Endocrinol. Metab. 2017; 102:1991–9. [DOI] [PubMed] [Google Scholar]
- 80.Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019; 62:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heden TD, Kanaley JA. Syncing exercise with meals and circadian clocks. Exerc. Sport Sci. Rev. 2019; 47:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guette M, Gondin J, Martin A. Time-of-day effect on the torque and neuromuscular properties of dominant and non-dominant quadriceps femoris. Chronobiol. Int. 2005; 22:541–58. [DOI] [PubMed] [Google Scholar]
- 83.Martin A, Carpentier A, Guissard N, van Hoecke J, Duchateau J. Effect of time of day on force variation in a human muscle. Muscle Nerve. 1999; 22:1380–7. [DOI] [PubMed] [Google Scholar]
- 84.Brooker PG, Gomersall SR, King NA, Leveritt MD. The feasibility and acceptability of morning versus evening exercise for overweight and obese adults: a randomized controlled trial. Contemp Clin Trials Commun. 2019; 14:100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grgic J, Lazinica B, Garofolini A, Schoenfeld BJ, Saner NJ, Mikulic P. The effects of time of day-specific resistance training on adaptations in skeletal muscle hypertrophy and muscle strength: a systematic review and meta-analysis. Chronobiol. Int. 2019; 36:449–60. [DOI] [PubMed] [Google Scholar]
- 86.Ezagouri S, Zwighaft Z, Sobel J, et al. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab. 2019. [DOI] [PubMed] [Google Scholar]
- 87.van Moorsel D, Hansen J, Havekes B, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016; 5:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014; 159:738–49. [DOI] [PubMed] [Google Scholar]
- 89.Tinsley GM, Forsse JS, Butler NK, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 2017; 17:200–7. [DOI] [PubMed] [Google Scholar]
- 90.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011; 364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vijan S, Stuart NS, Fitzgerald JT, et al. Barriers to following dietary recommendations in type 2 diabetes. Diabet. Med. 2005; 22:32–8. [DOI] [PubMed] [Google Scholar]
- 92.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med. Sci. Sports Exerc. 2002; 34:1996–2001. [DOI] [PubMed] [Google Scholar]
- 93.Gibson AA, Sainsbury A. Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behav Sci (basel). 2017; 7:pii: E44. [DOI] [PMC free article] [PubMed] [Google Scholar]


