Abstract
Secondhand tobacco smoke (SHS) exposure is a well-established risk factor for several diseases in adults. Despite the evidence that active tobacco smoke is harmful for the muscles, the association between SHS and muscle strength is still uncertain.We analyzed data from 5390 nonsmoking U.S. adults aged >30 years who participated in the National Health and Nutrition Examination Survey (NHANES) 2011-2014. Exposure to SHS was assessed with serum cotinine concentrations. Grip strength was measured using a Takei digital handgrip dynamometer, and combined grip strength was calculated as the sum of the largest reading from each hand. Median (interquartile range) serum cotinine and grip strength were 0.015 ng/mL (IQR 0.011-0.36) and 65.5 kg (IQR 53.4-86.4), respectively. After adjusting for sociodemographic, anthropometric, health-related behavioral, and clinical risk factors, the highest (0.047-9.9 ng/mL) vs lowest (≤0.011 ng/mL) quartile of serum cotinine was associated with a reduction in combined grip strength of 1.41 kg (95%CI: -2.58, -0.24), p-trend=0.02. These results were consistent across socio-demographic and clinical subgroups. In the US nonsmoking adult population, even low levels of exposure to passive smoking were associated with decreased grip strength. Despite great achievements in tobacco control, extending public health interventions to reduce SHS exposure is still needed.
Keywords: secondhand tobacco smoke, muscle strength, cotinine, musculoskeletal disease
INTRODUCTION
Exposure to secondhand tobacco smoke (SHS) is a global health problem [1]. In 2016, an estimated 33% of non-smoking women and 20% of non-smoking men were exposed to SHS worldwide [1]. SHS exposure in adult life increases the risk of coronary heart disease, stroke and lung cancer, with suggestive evidence that it may also be a risk factor of chronic obstructive pulmonary disease, asthma, impaired lung function, atherosclerosis, and cancers of the paranasal sinus, pharynx, larynx and breast [2, 3]. Only in 2016, SHS caused around 884,000 deaths worldwide [1].
Muscle strength is an important determinant of physical function, and is a good predictor of major health outcomes such as hospitalization [4], disability [5], and all-cause death [6]. Furthermore, there is some evidence suggesting that low muscle strength may increase the risk of cardiovascular disease, fractures and cognitive function decline [7, 8]. These deleterious health consequences of muscle strength declines make the identification of potential modifiable risk factors an important public health priority. Hence, for example, several epidemiological studies have studied the effects of poor nutritional status [9–11], excess body weight [12–14], physically strenuous work [12], or decreased leisure time physical activity [15] on muscle strength declines. However, and despite the evidence linking active tobacco smoke with reduced muscle strength [12, 16], increased risk of frailty [17] or physical disability [18], there are a very few studies that have addressed the potential effects of SHS on physical function or muscle strength declines, and none of these have included young adults [19, 20].
We hypothesized that SHS exposure is inversely associated with muscle strength both in middle-aged and older adults. To assess this hypothesis, we examined the association between SHS and grip strength in nonsmoking adults aged >30 years who participated in the U.S. National Health and Nutrition Examination Survey (NHANES) in 2011–2014.
RESULTS
Table 1 shows the distribution of the study population by quartiles of serum cotinine. A high proportion of subjects (48.3%) had serum cotinine values below the LOD (≤0.011 ng/mL). When compared to lower quartiles of serum cotinine, the proportion of individuals in the highest quartile (0.047-9.9 ng/mL) was higher for younger men, individuals with lower education, non-Hispanic blacks, ex-smokers, ex-drinkers, and those with poor dietary quality, obesity or cardiovascular disease.
Table 1. Number of participants (weighted percentage) across quartiles of serum cotinine concentrations, overall and stratified by participants sociodemographic and clinical characteristics.
| Serum cotinine concentration (ng/mL) | |||||
| Q1 ≤0.011 | Q2 0.015-0.02 | Q3 0.021-0.047 | Q4 0.047-9.9 | p val† | |
| Total | 2287 (48.3) | 626 (11.9) | 1193 (20.1) | 1284(19.7) | |
| Sex | |||||
| Men | 974 (44.1) | 292 (47.1) | 545 (45.6) | 643 (49.7) | |
| Women | 1313(55.9) | 334 (52.9) | 648 (54.4) | 641 (50.3) | <0.001 |
| Age, years | |||||
| 30-40 | 425 (19.0) | 127 (20.6) | 239 (21.0) | 316 (25.6) | |
| 41-50 | 470 (22.6) | 128 (22.7) | 249 (22.8) | 255 (21.5) | |
| 51-60 | 418 (21.0) | 139 (26.2) | 256 (24.8) | 259 (24.5) | |
| >60 | 974 (37.4) | 232 (30.5) | 449 (31.4) | 454 (28.4) | <0.001 |
| Educational level, | |||||
| <High School | 377 (10.0) | 107 (10.2) | 259 (13.9) | 346 (19.0) | |
| High School | 383 (15.3) | 114 (15.6) | 238 (19.5) | 330 (27.1) | |
| > High School | 1527 (74.7) | 405 (74.2) | 696 (66.6) | 608 (53.9) | <0.001 |
| Race/Ethnicity | |||||
| Non-Hispanic white | 1132 (76.9) | 232 (67.8) | 418 (65.2) | 443 (60.5) | |
| Non-Hispanic black | 292 (5.0) | 106 (7.5) | 298 (12.6) | 420 (17.6) | |
| Mexican-American | 320 (7.4) | 97 (10.0) | 122 (7.5) | 129 (7.9) | |
| Other | 543 (10.7) | 191 (14.7) | 355 (14.7) | 292 (14.0) | <0.001 |
| Country of birth | |||||
| US | 1550 (83.1) | 382 (79.7) | 713 (76.3) | 939 (82.9) | |
| Other | 737 (16.9) | 244 (20.3) | 480 (23.7) | 345 (17.1) | <0.001 |
| BMI | |||||
| Under-/ normoweight | 640 (28.4) | 177 (25.0) | 324 (24.2) | 260 (17.5) | |
| Overweight | 807 (37.0) | 205 (36.7) | 417 (36.6) | 407 (33.3) | |
| Obese | 840 (34.6) | 244 (38.3) | 452 (39.3) | 617 (49.2) | <0.001 |
| Physical activity (METs-hour/week)†† | |||||
| 1st tertile | 854 (33.3) | 239 (37.9) | 453 (34.2) | 528 (36.9) | |
| 2nd tertile | 776 (35.6) | 214 (32.2) | 382 (33.1) | 367 (29.4) | |
| 3rd tertile | 657 (31.1) | 173 (29.9) | 358 (32.7) | 389 (33.7) | 0.047 |
| Smoking, n (% weighted) | |||||
| Never | 1647 (71.8) | 449 (72.1) | 842 (66.1) | 792 (60.8) | |
| Ex-smoker | 640 (28.2) | 177 (27.9) | 351 (33.9) | 492 (39.2) | <0.001 |
| Alcohol consumption, n | |||||
| Never | 403 (13.7) | 97 (8.6) | 195 (12.6) | 195 (13.3) | |
| Ex-drinker | 483 (18.4) | 152 (21.9) | 255 (20.4) | 330 (24.4) | |
| Current | 981 (53.3) | 272 (54.7) | 473 (51.2) | 512 (47.3) | |
| Unknow | 420 (14.6) | 105 (14.8) | 270 (15.8) | 247 (15.0) | <0.001 |
| Diet quality, n | |||||
| Excellent/very good | 852 (42.6) | 214 (35.1) | 389 (34.7) | 382 (29) | |
| Good | 966 (40.6) | 266 (43.5) | 539 (45.9) | 551 (41.7) | |
| Fair/poor | 469 (16.8) | 146 (21.4) | 265 (19.4) | 351 (29.3) | <0.001 |
| Diagnosed disease, n | |||||
| Cardiovascular disease | 250 (8.7) | 64 (9.2) | 120 (8.5) | 173 (12.5) | 0.030 |
| Respiratory disease | 342 (15.6) | 98 (16.0) | 185 (15.4) | 231 (19.6) | 0.117 |
| Musculoskeletal disease | 673 (28.5) | 175 (28.5) | 330 (28.3) | 414 (31.4) | 0.063 |
| Cancer | 310 (14.3) | 69 (16.0) | 109 (11.6) | 109 (9.3) | <0.01 |
| Hypertension | 984 (38.8) | 256 (37.1) | 522 (39.5) | 585 (43.5) | 0.242 |
| Diabetes | 395 (12.5) | 110 (15.2) | 226 (14.6) | 263 (17.0) | 0.104 |
| Number of drug treatments | |||||
| None | 860 (38.0) | 219 (33.9) | 442 (37.3) | 466 (37.2) | |
| 1-2 | 286 (13.7) | 98 (16.3) | 140 (12.7) | 163 (11.6) | |
| >2 | 1141 (48.3) | 309 (49.8) | 611 (50.0) | 655 (51.2) | 0.341 |
Data are based on 5,390 adults aged >30 years from the US nonsmoking general population.
† P values are based on the chi-square test.
†† Cutoff values (men/women) were ≤12/≤4 (tertile 1), 12 to ≤48 /4.1 to ≤28 (tertile 2), and >48/28. (tertile 3) METs-hour/week.
Median (IQR) concentrations of serum cotinine and combined grip strength were 0.015 ng/mL (0.011-0.36) and 65.5 Kg (53.4-86.4), respectively. Women, individuals aged > 60 years, with lower educational level, lower BMI, never drinkers, and cardiovascular or musculoskeletal disease, showed the lowest strength (Tables 1 and 2).
Table 2. Median (interquartile range [IQR]) serum cotinine and muscle strength in adults aged >30 from the US nonsmoking general population by participants sociodemographic and clinical characteristics.
| Characteristics | n (weighted %) | Serum cotinine (ng/mL) | Combined grip strength (Kg) |
| Total | 5390 (100) | 0.015 (0.011-0.360) | 65.5 (53.4-86.4) |
| Sex | |||
| Men | 2454 (45.9) | 0.016 (0.011-0.040) | 88.5 (76.5-99.6) |
| Women | 2936 (54.1) | 0.015 (0.011-0.033) | 55.1 (47.6-62.4) |
| Age, years | |||
| 31-40 | 1107 (20.9) | 0.017 (0.011-0.046) | 72.9 (60.3-95.0) |
| 41-50 | 1102 (22.4) | 0.015 (0.011-0.035) | 72.2 (59.4-93.4) |
| 51-60 | 1072 (23.1) | 0.018 (0.011-0.038) | 66.8 (54.4-88.9) |
| >60 | 2109 (33.6) | 0.011 (0.011-0.029) | 56.2 (45.6-73.5) |
| Educational level | |||
| < High School | 1089 (12.6) | 0.023 (0.011-0.060) | 59.0 (46.8-77.1) |
| High School | 1065 (18.5) | 0.020 (0.011-0.055) | 62.3 (50.0-86.0) |
| > High School | 3236 (68.9) | 0.011 (0.011-0.029) | 67.4 (55.1-88.2) |
| Race/Ethnicity | |||
| Non-Hispanic white | 2225 (70.2) | 0.011 (0.011-0.030) | 65.1 (53.3-87.6) |
| Non-Hispanic-black | 1116 (9.3) | 0.032 (0.011-0.090) | 70.4 (58.0-88.7) |
| Mexican-American | 668 (7.8) | 0.016 (0.011-0.039) | 65.2 (53.6-87.5) |
| Other | 1381 (12.6) | 0.018 (0.011-0.041) | 62.5 (50.4-81.2) |
| BMI | |||
| Under-/ normoweight | 1401 (025) | 0.011 (0.011-0.027) | 59.9 (51.3-75.3) |
| Overweight | 1836 (36.1) | 0.015 (0.011-0.036) | 70.0 (55.1-91.1) |
| Obese | 2153 (38.9) | 0.018 (0.011-0.047) | 67.3 (53.5-89.2) |
| Physical activity (METs-hour/week) † | |||
| 1st tertile | 2074 (34.7) | 0.017 (0.011-0.039) | 62.6 (50.3-82.1) |
| 2nd tertile | 1739 (33.5) | 0.011 (0.011-0.032) | 65.8 (53.2-88.5) |
| 3rd tertile | 1577 (31.8) | 0.015 (0.011-0.039) | 69.1 (56.6-90.0) |
| Smoking | |||
| Never | 3730 (68.5) | 0.011 (0.011-0.032) | 64.6 (53.2-86.4) |
| Ex-smoker | 1660 (31.6) | 0.018 (0.011-0.047) | 67.9 (53.6-86.4) |
| Alcohol consumption | |||
| Never | 890 (12.8) | 0.011 (0.011-0.035) | 56.4 (46.1-70.4) |
| exdrinker | 1220 (20.4) | 0.017 (0.011-0.044) | 65.7 (53.6-85.4) |
| Current | 2238 (51.8) | 0.015 (0.011-0.033) | 71.7 (56.7-91.9) |
| Unknow | 1042 (15.0) | 0.015 (0.011-0.038) | 59.7 (49.3-75.2) |
| Diet quality | |||
| Excellent/very Good | 1837 (37.4) | 0.011 (0.011-0.028) | 63.7 (53.1-83.2) |
| Good | 2322 (42.2) | 0.016 (0.011-0.370) | 66.2 (53.1-88.0) |
| Fair/poor | 1231 (20.4) | 0.019(0.011-0.056) | 69.3 (54.7-90.7) |
| Diagnosed disease | |||
| Cardiovascular disease | 607 (9.5) | 0.018 (0.011-0.049) | 59.7 (44.9-78.8) |
| Respiratory disease | 856 (16.4) | 0.015 (0.011-0.042) | 61.1 (51.3-78.7) |
| Musculoskeletal disease | 1592 (29.0) | 0.016 (0.011-0.039) | 56.6 (46.7-74.4) |
| Cancer | 597 (13) | 0.011 (0.011-0.026) | 60.1 (48.2-82.3) |
| Hypertesion | 2347 (39.6) | 0.017 (0.011-0.039) | 62.3 (49.1-83.6) |
| Diabetes | 994 (14.2) | 0.018 (0.011-0.043) | 60.9 (49.1-82.5) |
| Number of drug treatments | |||
| None | 1987 (37.2) | 0.015 (0.011-0.036) | 65.6 (53.3-87.8) |
| 1-2 | 687 (13.4) | 0.015 (0.011-0.030) | 63.9 (53.6-86.4) |
| >2 | 2716 (49.4) | 0.016 (0.011-0.037) | 65.9 (53.3-85.6) |
† Cutoff values (men/women) were: ≤12/≤4 (tertile 1), 12 to ≤48 /4.1 to ≤28 (tertile 2), and >48/28 (tertile 3) METs-hour/week.
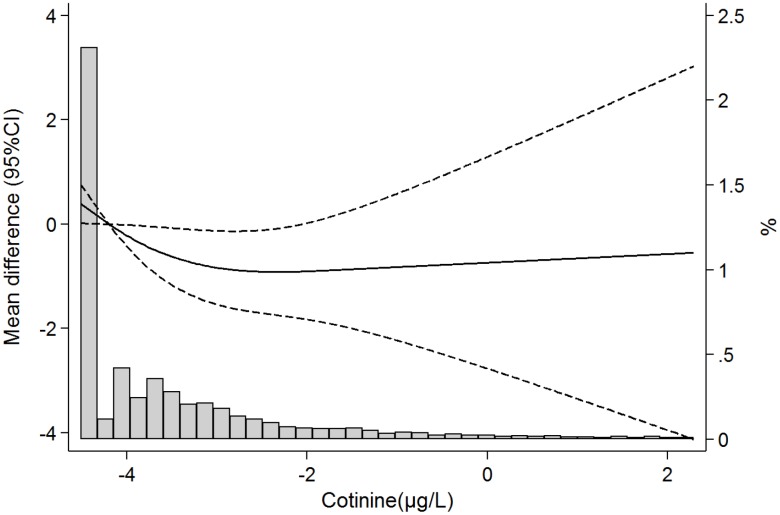
In models adjusted for sociodemographic, lifestyle and clinical risk factors (Model C), and comparing participants in the highest vs the lowest quartile of serum cotinine, combined grip strength decreased by 1.41 kg (-2.58, -0.24); p-trend across quartiles=0.02 (Table 3). Figure 1 shows the dose-response association of serum cotinine with grip strength. As observed, strength declines were observed even at very low levels of exposure, and there were no major departures from linearity (p-values for the linear and non-linear components were 0.04 and 0.11, respectively).
Table 3. Beta coefficient (95% confidence interval) for grip strength in kg, by serum cotinine concentration.
| Serum cotinine concentration quartiles (ng/mL) | |||||
| Q1 (≤0.011) | Q2 (0.015-0.02) | Q3 (0.021-0.047) | Q4 (0.048-9.9) | p-trend | |
| No. | 2275 | 627 | 1187 | 1278 | |
| Model A | 1.00 | 0.26 (-1.13, 1.65) | -0.56 (-1.79, 0.67) | -1.09 (-2.24, 0.07) | 0.0 |
| Model B | 1.00 | 0.14 (-1.25, 1.53) | -0.77 (-2.03, 0.49) | -1.32 (-2.44, -0.20) | 0.02 |
| Model C | 1.00 | 0.18 (-1.16, 1.52) | -0.86 (-2.19, 0.46) | -1.41 (-2.58, -0.24) | 0.02 |
Model A adjusted for age (continuous), sex, race-ethnicity (Non-Hispanic white, Non-Hispanic black, Mexican-American, Other), educational level (<high school, high school, > high school), and place of birth (US, other).
Model B further adjusted for tobacco smoke (never, ex-smoker), alcohol consumption (never, ex–drinker, current, unknown), diet quality (excellent/very good, good, fair/poor) and physical activity (sex-specific tertiles).
Model C further adjusted for BMI, cardiovascular disease, respiratory disease, musculoskeletal disease, cancer, hypertension, diabetes and number of drug treatments.
Figure 1.
Mean differences (95%confidence intervals) in grip strength according to serum cotinine concentrations (ng/mL) based on restricted cubic splines with knots at the 10th, 50th, and 90th percentile of serum cotinine distribution. The reference value is set at the 25th percentile of the cadmium distribution. Models are adjusted for age, sex, race/ethnicity, education, place of birth, tobacco smoke, alcohol consumption, diet quality, physical activity, BMI, cardiovascular disease, respiratory disease, musculoskeletal disease, cancer, hypertension, diabetes and number of drug treatments. Lines represent predicted values (thick line) and 95% confidence intervals (dashed lines), and vertical bars represent the histogram of log-transformed serum cotinine distribution.
Figure 2 shows results for the highest vs the lowest quartile of serum cotinine across participantʼs characteristics. Although we observed no interactions with age, sex, education, lifestyle factors or BMI, we did observe some effect modification by musculoskeletal disease. In particular, the association was only maintained among individuals without musculoskeletal disease: results for the second, third and highest versus the lowest quartile of cotinine were: -0.58 kg (-2.39- 1.23); -1.89 kg (-3.44, -0.34) and -1.37 kg (-2.80, 0.05); p-trend=0.01.
Figure 2.
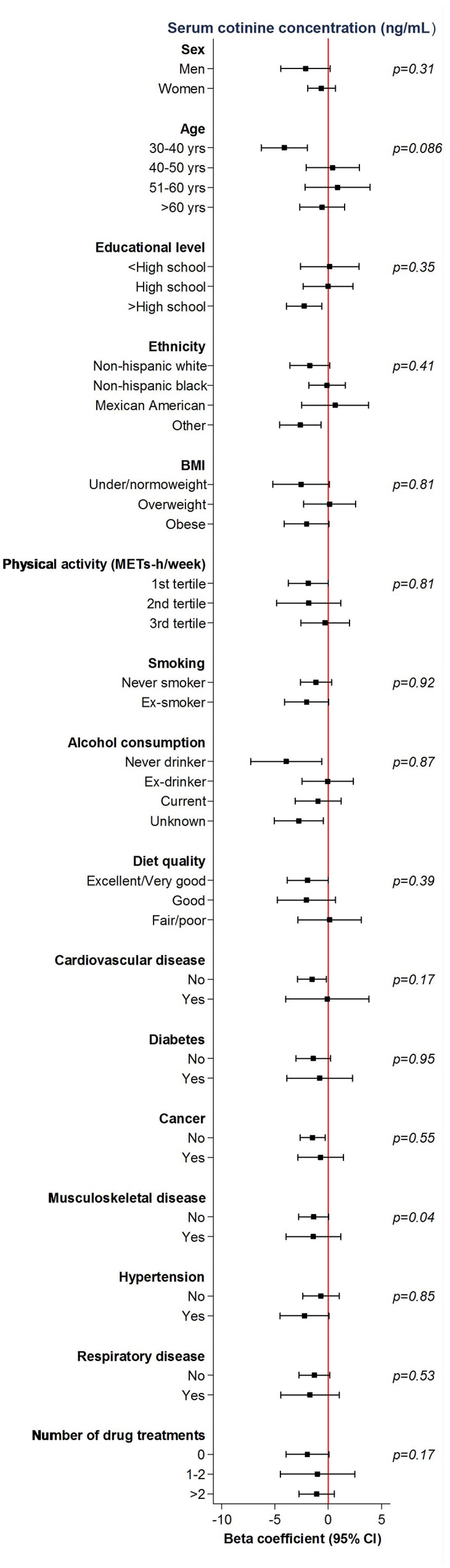
Mean differences (95%confidence intervals) in grip strength comparing the fourth to the first quartile of cotinine concentrations (ng/mL) by participant’s characteristics. Models are adjusted for age, sex, race/ethnicity, educational level, place of birth, tobacco smoke, alcohol consumption, diet quality, physical activity, BMI, cardiovascular disease, respiratory disease, musculoskeletal disease, cancer, hypertension, diabetes and number of drug treatments.
Lastly, additional adjustment for serum albumin, total protein intake, serum testosterone, and glomerular filtration rate in participants with this information available did not modify substantially our main findings (Supplementary Table 1).
DISCUSSION
In this sample of middle-aged and older non-smokers adults of the U.S. population, exposure to SHS was associated with a decrease in grip strength.
There is in vitro evidence that cigarette smoke induces skeletal muscle damage through atrophy of oxidative muscle fibers, impaired synthesis of muscle proteins, and over-expression of atrophy related genes [21–23]. Studies in vivo have also observed increased muscular oxidative stress and systemic inflammation in mice exposed to cigarette smoke [24, 25]. A comparison of skeletal muscle properties and fatigue resistance between 40 smokers and 45 non-smokers found out that, although muscle mass and contractile properties were similar in smokers and non-smokers, smokers suffered from greater peripheral muscle fatigue (measured as torque decline during a series of repetitive contractions) than non-smokers [26]. Also a longitudinal study with 963 men and women aged >30 years from the Mini-Finland health examination survey, who were followed for up to 22 years, showed that former smoking at baseline and persistent tobacco use during follow-up were associated with accelerated grip strength declines [12]. In the same line, a meta-analysis of 12 cross-sectional and case-control studies with a total of 22515 participants showed an association between self-reported active smoking and sarcopenia [27]. Despite this evidence, there is only one recent publication evaluating the potential association between SHS exposure and handgrip strength [19]; in this publication, based on the English Longitudinal Study of Aging, the authors found an inverse association between saliva cotinine concentrations and performance on several indicators of physical capacity (including declined grip strength) in older adults. Our study supports the association between SHS and declined grip strength while extends this observation to middle-aged adults (30-60 years). Moreover, it suggests that there is no apparent risk-free level of exposure to SHS.
Of interest is that the dose-response association between SHS exposure and grip strength was only observed among individuals with no arthritis or osteoarthritis. Although these are posthoc findings, and so they need to be interpreted with caution, they may reflect the fact that among individuals with musculoskeletal disease there are other more deterministic factors (i.e. pain, physical inactivity) modifying muscle strength.
Strengths of this study include the national representativeness of the sample and the high quality study protocol and laboratory methods, and the inclusion not only of old but also young adults. To ensure that only nonsmokers were included in the analyses, we applied strict criteria that excluded participants who acknowledged active smoking or whose cotinine levels were indicative of tobacco use, or who had missing responses to the smoking questions or cotinine concentrations. Additionally, our results were consistent in various sensitivity analyses.
Among the limitations of our study are the cross-sectional design and the potential for residual confounding, despite models were adjusted for many relevant variables. More importantly, a single measurement of cotinine only reflects SHS exposure over the previous 1-2 days, so it is an imperfect surrogate of long-term exposure; there is recent evidence for epigenetic biomarkers of long-term cumulative exposure, but unfortunately these are not available in NHANES [28]. Finally, due to the relatively small proportion of participants heavily exposed to SHS (with concentrations close to 10ng/mL), results are hard to interpret at these concentrations.
In the US adult population aged 30 years an over, combined grip strength declined around 1.42 kg every 2.5 years, which is similar to the observed decline in strength comparing participants in the highest vs the lowest quartile of serum cotinine. Although the clinical relevance of such a decline at the individual level is uncertain, at the population level small changes in the distribution of muscle strength, resulting from the widespread exposure to SHS, may have an important impact in functional disability. Future prospective studies should evaluate the link between SHS exposure and functional disability.
MATERIALS AND METHODS
Study population
NHANES is a program of the National Center for Health Statistics designed to assess the health and nutritional status of adults and children in the United States [29]. The present analysis used data from 8621 adults aged > 30 years who participated in NHANES 2011-2014 from whom around 90% (7723) had information about grip strength. To ensure that the sample included non-smokers only, we excluded 352 participants with missing serum cotinine data, and 1801 current smokers (reporting having smoked at least 100 cigarettes in their entire life and were active smokers at the time of the interview; or who had serum cotinine levels ≥10 ng/mL). We also excluded 180 participants with missing data on potential confounders, leaving 5390 participants for analyses.
The study was approved by the National Center for Health Statistics Research Ethics Review Board, and written informed consent was obtained from all participants.
Study variables
Grip strength
Grip strength was measured by trained personnel using a Takei Dynamometer Model T.K.K.5401 (Takei Scientific Instruments Co., Niigata, Japan). Each hand was tested three times, alternating hands with a 60-second rest between measurements. Combined grip strength was calculated by NHANES as the sum of the largest reading from each hand expressed in kg.
Tobacco smoke exposure (serum cotinine concentration)
Serum cotinine was measured using high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. The limit of detection (LOD) was 0.015ng/mL, and values under this limit were divided by the square root of 2. Serum cotinine levels were log-transformed due to their skewed distribution, and categorized into quartiles.
Other variables
We used information from a number of self-reported variables including age, sex, education (< high school, high school, > high school), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican-American, Other), physical activity (sex-specific tertiles of METs-hour/week), smoking (never smoker, ex-smoker), alcohol consumption (never, ex-drinker, current drinker, unknown), diet quality (based on the question “in general, how healthy is your overall diet?”), number of drug treatments (none, ≤2 and >2), and history of physician-diagnosed chronic conditions: cardiovascular disease (coronary heart disease, heart failure, heart attack, angina, stroke), respiratory disease (asthma, chronic bronchitis, emphysema), musculoskeletal disease (arthritis, osteoarthritis) and cancer. Hypertension was defined as a self-reported physician diagnosis, current use of anti-hypertensive medication, or a clinical blood pressure reading 140/90 mmHg. Likewise, type 2 diabetes mellitus was based as a self-reported physician diagnosis, current use of anti-diabetic medication, or fasting glucose ≥126 mg/dL. Glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation that incorporates subjects´ measures of serum creatinine, age, race and sex. Weight and height were measured in standardized conditions, and the body mass index (BMI) calculated dividing weight in kg by squared height in meters. Subjects were then classified as “underweight/ normoweight” (BMI<25 kg/m2), “overweight” (BMI ≥25 to <30 kg/m2) or “obese” (IMC≥ 30 kg/m2).
Statistical analysis
We performed all statistical analyses in Stata version 13.0, by using the survey command to account for the complex sampling design and weights in NHANES.
We first used frequency distributions to describe the distribution of participant characteristics by serum cotinine quartiles. Then, we depicted the distribution of serum cotinine and combined grip strength for participants, overall and by their main sociodemographic, lifestyle and clinical factors. To evaluate the association of serum cotinine with grip strength, we estimated the mean difference (95%confidence interval (CI)) in strength by quartiles of serum cotinine concentrations using linear regression models. Tests for trend were performed for ordinal serum quartiles in regression models using integer values (0 to 3). Next, to evaluate potential departures from linearity, we ran restricted cubic spline models with knots at the 10th (0.011 ng/mL), 50th (0.018 ng/mL), and 90th (0.0184 ng/mL) percentiles of cotinine distribution in all participants.
We built three models with progressive levels of adjustment: Model A adjusted for the main sociodemographic variables (age, sex, race/ethnicity, education, and place of birth); Model B further adjusted for lifestyle factors (physical activity, tobacco smoke, alcohol consumption, and self-reported diet quality); and Model C additionally adjusted for BMI, morbidity (cardiovascular disease, musculoskeletal disease, respiratory disease, cancer, hypertension, diabetes) and number of drug treatments.
We evaluated the consistency of our findings across categories of potential confounders using interaction models, and differences across strata were tested with interaction terms. Additionally, to reduce potential residual confounding, we performed analyses with further adjustment for nutrition factors (serum albumin and total protein intake), as these have been associated with both exposure to SHS and decreased grip strength [10, 11]. Likewise, because both passive smoking and grip strength have been associated with kidney function, we adjusted our models for glomerular filtration rate [30, 31]. Finally, since there is some evidence suggesting that cotinine may inhibit testosterone breakdown [32], and low testosterone levels are associated with decreased muscle strength [33, 34], we run models that additionally adjusted for serum testosterone concentrations [32].
Supplementary Material
ACKNOWLEDGMENTS AND FUNDING
This work has been supported by grants PI18/287 and 16/609 from the Instituto de Salud Carlos III, State Secretary of R+D+I, and FEDER/FSE.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Drope J, Schluger N, Cahn Z, Drope J, Hamill S, Islami F, Liber A, Nargis N, Stoklosa M. 2018. The Tobacco Atlas. Atlanta: American Cancer Society and Vital Strategies. https://tobaccoatlas.org/wp-content/uploads/2018/03/TobaccoAtlas_6thEdition_LoRes_Rev0318.pdf
- 2.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. 10.1037/e300842003-001 [DOI] [PubMed] [Google Scholar]
- 3.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007; 32:542–43. 10.1016/j.amepre.2007.02.026 [DOI] [PubMed] [Google Scholar]
- 4.Legrand D, Vaes B, Matheï C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. 2014; 62:1030–38. 10.1111/jgs.12840 [DOI] [PubMed] [Google Scholar]
- 5.McGrath RP, Vincent BM, Lee IM, Kraemer WJ, Peterson MD. Handgrip Strength, Function, and Mortality in Older Adults: A Time-varying Approach. Med Sci Sports Exerc. 2018; 50:2259–66. 10.1249/MSS.0000000000001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R, Ruiz JR, Ortega FB, Lee DC, Martínez-Vizcaíno V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data From Approximately 2 Million Men and Women. Arch Phys Med Rehabil. 2018; 99:2100–2113.e5. 10.1016/j.apmr.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, Hardy R, and FALCon and HALCyon Study Teams. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011; 40:14–23. 10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JM, Sattar N, Gray SR. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018; 361:k1651. 10.2337/dc17-0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Nishi M, Murayama H, Amano H, Taniguchi Y, Nofuji Y, Narita M, Matsuo E, Seino S, Kawano Y, Shinkai S. Dietary Variety and Decline in Lean Mass and Physical Performance in Community-Dwelling Older Japanese: A 4-year Follow-Up Study. J Nutr Health Aging. 2017; 21:11–16. 10.1007/s12603-016-0726-x [DOI] [PubMed] [Google Scholar]
- 10.Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M, Erkkilä AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr. 2016; 115:1281–91. 10.1017/S000711451600012X [DOI] [PubMed] [Google Scholar]
- 11.McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary Protein Intake Is Protective Against Loss of Grip Strength Among Older Adults in the Framingham Offspring Cohort. J Gerontol A Biol Sci Med Sci. 2016; 71:356–61. 10.1093/gerona/glv184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliövaara M, Impivaara O, Koskinen S. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc. 2012; 60:77–85. 10.1111/j.1532-5415.2011.03779.x [DOI] [PubMed] [Google Scholar]
- 13.de Carvalho DH, Scholes S, Santos JL, de Oliveira C, Alexandre TD. Does Abdominal Obesity Accelerate Muscle Strength Decline in Older Adults? Evidence From the English Longitudinal Study of Ageing. J Gerontol A Biol Sci Med Sci. 2019; 74:1105–11. 10.1093/gerona/gly178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Feng W, Li S, Tan Q, Zhang D, Wu Y. Impact of obesity and physical inactivity on the long-term change in grip strength among middle-aged and older European adults. J Epidemiol Community Health. 2019; 73:619–24. 10.1136/jech-2018-211601 [DOI] [PubMed] [Google Scholar]
- 15.Dodds R, Kuh D, Aihie Sayer A, Cooper R. Physical activity levels across adult life and grip strength in early old age: updating findings from a British birth cohort. Age Ageing. 2013; 42:794–98. 10.1093/ageing/aft124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan S, Jeong JY, Kim DH. The Relationship between Smoking, Socioeconomic Status and Grip Strength among Community-dwelling Elderly Men in Korea: Hallym Aging Study. Epidemiol Health. 2013; 35:e2013001. 10.4178/epih/e2013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima G, Iliffe S, Walters K. Smoking as a predictor of frailty: a systematic review. BMC Geriatr. 2015; 15:131. 10.1186/s12877-015-0134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ropponen A, Korhonen T, Svedberg P, Koskenvuo M, Silventoinen K, Kaprio J. Persistent smoking as a predictor of disability pension due to musculoskeletal diagnoses: a 23 year prospective study of Finnish twins. Prev Med. 2013; 57:889–93. 10.1016/j.ypmed.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Batty GD, Zaninotto P. Exposure to Passive Smoking and Impairment in Physical Function in Older People. Epidemiology. 2018; 29:e11–12. 10.1097/EDE.0000000000000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Esquinas E, Navas-Acien A, Rodríguez-Artalejo F. Exposure to secondhand tobacco smoke and the frailty syndrome in US older adults. Age (Dordr). 2015; 37:26. 10.1007/s11357-015-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rom O, Kaisari S, Aizenbud D, Reznick AZ. Cigarette smoke and muscle catabolism in C2 myotubes. Mech Ageing Dev. 2013; 134:24–34. 10.1016/j.mad.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernández N, Tálamo C. Peripheral muscle alterations in non-COPD smokers. Chest. 2008; 133:13–18. 10.1378/chest.07-1592 [DOI] [PubMed] [Google Scholar]
- 23.Petersen AM, Magkos F, Atherton P, Selby A, Smith K, Rennie MJ, Pedersen BK, Mittendorfer B. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007; 293:E843–48. 10.1152/ajpendo.00301.2007 [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi M, Maes K, De Vleeschauwer S, Thomas D, Verbeken EK, Decramer M, Janssens W, Gayan-Ramirez GN. Long-term nose-only cigarette smoke exposure induces emphysema and mild skeletal muscle dysfunction in mice. Dis Model Mech. 2012; 5:333–41. 10.1242/dmm.008508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreiro E, del Puerto-Nevado L, Puig-Vilanova E, Pérez-Rial S, Sánchez F, Martínez-Galán L, Rivera S, Gea J, González-Mangado N, Peces-Barba G. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012; 182:9–17. 10.1016/j.resp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Wüst RC, Morse CI, de Haan A, Rittweger J, Jones DA, Degens H. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol. 2008; 104:103–10. 10.1007/s00421-008-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Relation between cigarette smoking and sarcopenia: meta-analysis. Physiol Res. 2015; 64:419–26. 10.1186/s12877-016-0270-x [DOI] [PubMed] [Google Scholar]
- 28.Reynolds LM, Magid HS, Chi GC, Lohman K, Barr RG, Kaufman JD, Hoeschele I, Blaha MJ, Navas-Acien A, Liu Y. Secondhand Tobacco Smoke Exposure Associations With DNA Methylation of the Aryl Hydrocarbon Receptor Repressor. Nicotine Tob Res. 2017; 19:442–51. 10.1093/ntr/ntw219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC. About the National Health and Nutrition Examination Survey. 2017. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm [Google Scholar]
- 30.Hellberg M, Höglund P, Svensson P, Abdulahi H, Clyne N. Decline in measured glomerular filtration rate is associated with a decrease in endurance, strength, balance and fine motor skills. Nephrology (Carlton). 2017; 22:513–19. 10.1111/nep.12810 [DOI] [PubMed] [Google Scholar]
- 31.Jhee JH, Joo YS, Kee YK, Jung SY, Park S, Yoon CY, Han SH, Yoo TH, Kang SW, Park JT. Secondhand Smoke and CKD. Clin J Am Soc Nephrol. 2019; 14:515–22. 10.2215/CJN.09540818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Leung JY, Lin SL, Schooling CM. Cigarette smoking and testosterone in men and women: A systematic review and meta-analysis of observational studies. Prev Med. 2016; 85:1–10. 10.1016/j.ypmed.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 33.Chiang JM, Kaysen GA, Segal M, Chertow GM, Delgado C, Johansen KL. Low testosterone is associated with frailty, muscle wasting and physical dysfunction among men receiving hemodialysis: a longitudinal analysis. Nephrol Dial Transplant. 2019; 34:802–10. 10.1093/ndt/gfy252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu HT, Shih MT, Chen WL. Examining the association between grip strength and testosterone. Aging Male. 2019; 3:1–8. 10.1080/13685538.2019.1632282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.