Abstract
Epidemiological studies have indicated that blood vitamin D levels are linked to cancer. Here we conducted a dose–response meta-analysis based on published observational studies to evaluate the association of vitamin D intake and blood vitamin D levels with breast cancer susceptibility. PubMed, EMBASE, and Web of Science databases were searched up to January 2019. The pooled odds ratio (OR) and 95% confidence intervals (CIs) were extracted to estimate the risk. We identified 70 relevant studies on blood vitamin D levels (50 studies) and vitamin D intake (20 studies), respectively. Linear and nonlinear trend analyses were performed and showed that an increase in blood vitamin D levels by 5 nmol/l was associated with a 6% decrease in breast cancer risk (OR = 0.94, 95% CI = 0.93–0.96). Similar results were obtained for premenopausal (OR = 0.96, 95% CI = 0.93–0.99) and postmenopausal women (OR = 0.96, 95% CI = 0.94–0.98). The pooled OR of breast cancer risk for a 400IU/day increase in vitamin D intake was 0.97 (95% CI = 0.92–1.02). In conclusion, we found that breast cancer risk was inversely related to blood vitamin D levels; however, no significant association was observed in vitamin D intake.
Keywords: vitamin D, dose-response, breast cancer risk, menopause, meta-analysis
INTRODUCTION
Breast cancer is the most common form of malignancy and the main cause of cancer-related death among women worldwide [1]. Epidemiological studies revealed that the incidence of breast cancer has been increasing globally since the end of the 1970s [2]. According to a recent study conducted by the American Cancer Society, the most three commonly diagnosed cancer in 2019 are breast, lung, and colorectal cancer. Furthermore, breast cancer accounts for 30% of the all newly diagnosed cancer cases in women [3].
Vitamin D is a steroid derivative, and plays a key role in promoting bone growth. Epidemiological studies have shown that low serum vitamin D levels was linked to a higher risk of colon and bladder cancer, and higher circulating concentration of 25(OH)D decreased the risk of renal cell carcinoma [4–6]. Additionally, an anti-cancer effect on vitamin D against breast, prostate, and colorectal cancers have been reported [7]. The review has clarified that calcitriol, the product of vitamin D, was involved in the proliferation, apoptosis, differentiation, inflammation, invasion, angiogenesis and metastasis of tumor by regulating various signaling pathways, which may affect the development and growth of tumor [8]. Previous experimental studies has revealed that the active metabolite of vitamin D, 1, 25(OH)2D, inhibited breast cancer progression and metastasis by inducing apoptosis, reducing cell growth and angiogenesis [9]. Since the 1970s, numerous observational studies have discussed the relationship between vitamin D and the risk of breast cancer [10]; however, the results of individual studies do not show a similar association. Although recently published meta-analysis and reviews have focused on the relationship between blood vitamin D levels and vitamin D intake with breast cancer risk [11–15], the findings remain controversial. Therefore, based on prospective cohort and case-control studies, we conducted a dose-response meta-analysis to systematically assess the relationship of vitamin D intake and vitamin D levels with the risk of breast cancer.
RESULTS
Literature selection and study characteristics
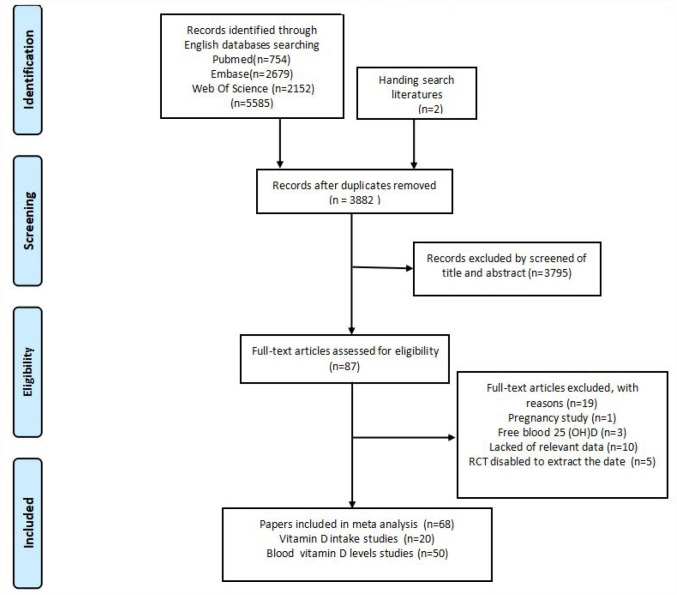
The flow chart of the selection of publications from the existing literature is shown in Figure 1. Firstly, 5587 articles were searched through the databases as well as through hand searching. Next, 1705 articles were excluded for duplication; 3795 articles were excluded after reading the titles and abstracts for the lack of relevance; 10 articles did not contain the relevant data; three articles measured blood vitamin D levels in pg/ml or pmol/l; five articles disabled the extraction data; and one article was associated with pregnancy. Additional there are two studies, O’ Brien et al [16] and Fedirko et al [17], provide data on blood vitamin D and vitamin D intake. So we went through the full texts of 68 articles. Finally, a total of 68 articles that contained 70 observational studies (case-control or cohort) were eligible for the analysis. The characteristics of the 68 selected publications are summarized in Table 1 and Supplementary Table 1.
Figure 1.
Flowchart of included studies for the meta-analysis.
Table 1. Characteristics of prospective studies included in the meta-analysis of vitamin D intake and breast cancer risk.
| Author | Country | Study type | Follow-up period (year) | Age (year) | No. of cases/controls/ persons | Vitamin D Intake (IU/day)a | Adjusted OR(95%CI)b | Adjustment factors |
| O’Brien et al, 2017 | USA | Cohort | 5 | 35-74 | 1699/49044 | Total ≥600 vs <200 | 0.90 (0.78–1.05) | Age, BMI, race, education, menopausal status, current birth control use, physical activity, hormone therapy type, current alcohol use, osteoporosis, total energy intake, parity, and a BMI× menopausal status interaction term |
| Abbas et al, 2013 | Europe | Cohort | 8.8 | 50.2 | 7760/319985 | Dietary ≥218.4 vs <74 | 1.04 (0.94–1.14) | No-fat, no-alcohol energy, fat, alcohol consumption, weight, height, smoking status, menopausal status, physical activity, age at menarche, education level and current use of contraceptives or hormones |
| Rollison et al, 2012 | USA | Case- control | 1999-2004 | 24-79 | 2318/2521 | Dietary 7.0-122.5 vs 308.6-1362.7 | 1.28 (1.09-1.5) | Age |
| Fedirko et al, 2012 | Mexico | Case- control | 2004-2007 | 35-69 | 570/638 | Dietary >111.8 vs ≤65 | 0.69 (0.47–1.00) | SES, BMI, alcohol consumption, height, parity/number of children born alive, age at first full term pregnancy, family history of breast cancer, breast feeding, use of hormone for menopause, physical activity index, total energy intake, and menopausal status |
| Edvardsen et al, 2011 | Norway | Cohort | 1997-2007 | 40-70 | 844/41758 | Total ≥832 vs ≤108 | 1.07 (0.87–1.32) | Age at entry, BMI, height, menopausal status, HRT use, use of oral contraceptives, mothers’ history of breast cancer, frequency of mammography, combined parity and age at first birth and daily intake of alcohol. |
| Kawase et al, 2010 | Japan | Case- control | 2001-2005 | 20-79 | 1803/3606 | Dietary 266-1400 vs 80-114 | 0.76 (0.63–0.9) | Age, BMI, menopausal status, smoking habit, drinking habit, physical activity, family history of breast cancer in a first degree relative, age at menarche, parity, hormone use, total nonalcohol energy, and referral pattern |
| Anderson et al, 2010 | Canada | Case- control | 2002-2003 | 25-74 | 3101/3471 | Total ≥600 vs <100 | 0.99 (0.78-1.26) | Age, BMI, education, age at menarche, age at first live birth, parity, menopausal status, smoking, relative energy intake, breast cancer in first degree, moderate physical activity, time spent outdoors, total calcium intake, and total vitamin D intake |
| Lee et al, 2010 | China | Case- control | 2004-2005 | Cases 52.5 Controls 48.9 | 200/200 | Total 428-1148 vs 6.8-125.6 | 0.52 (0.25–1.07) | Age, BMI, education, parity, use of HRT, total energy intake, sunlight exposure, menopausal status, and homocysteine. |
| Engel et al, 2010 | French | Cohort | 10.4 | 41.8-72 | 2871/67721 | Dietary >113 vs <80 | 0.94 (0.86–1.03) | BMI, age at menopause, age at menarche, physical activity, parity, use of menopausal, use of HRT, alcohol intake, daily calcium intake, calcium supplement, energy intake without alcohol, university degree, previous family history of breast cancer, previous personal history of benign breast disease, previous history of mammographic exam, sun burn resistance, menopausal status, and skin complexion. |
| Kuper et al, 2009 | Sweden | cohort | 1991-2003 (12.9) | 30-49 | 840/41889 | Dietary Q4 vs Q1 | 0.90 (0.80–1.10) | BMI, parity, age at first birth, age at menarche, use of hormonal contraceptives, consumption of alcohol, breast-feeding, education, family history of breast cancer, physical activity, and smoking. |
| Rossi et al, 2008 | Italy | Case- control | 1991-1994 | 23-74 | 2569/2588 | Total >190.4 vs <60.4 | 0.76 (0.58–1.00) | age, parity, age at menarche, study center, education, total energy intake, menopausal status, vegetable and fruit consumption, calcium, b-carotene, vitamin E, flavones, and flavonol intake |
| Abbas et al, 2007 | German | Case- control | 1992-1995 | 24-50 | 278/666 | Dietary ≥200 vs < 80 | 0.50 (0.26–0.96) | BMI, age at menarche, energy intake, duration of breast feeding, first-degree family history, number of births, nonalcohol, alcohol consumption, and mineral and vitamin supplements |
| Robien et al, 2007 | USA | Cohort | 1986-2004 | 50-70 | 2440/34321 | Total ≥800 vs <400 | 0.90 (0.78–1.04) | Age, BMI, smoking status, age at menarche, age at menopause, first degree relative with breast cancer, estrogen use, age at first live birth, number of live births, education category, activity level, live on a farm, mammogram history, and daily energy, fat, and alcohol intake. |
| Lin et al, 2007 | USA | Cohort | 10 | 55.2 | Cases Pre276/Post743 Persons Pre10578/Post20909 | Total ≥548 vs <162 | Pre 0.65 (0.42-1.00) Post 1.30 (0.97-1.73) | Age, BMI, randomized treatment assignment, physical activity, family history of breast cancer in a first-degree relative, history of benign breast disease, age at menarche, parity, age at first birth, multivitamin use, smoking status, alcohol consumption, total energy intake, age at menopause, and baseline postmenopausal hormone therapy. |
| McCullough et al,2005 | USA | Cohort | 1992-2001 | 50-74 | 2855/68567 | Total ≥700 vs ≤100 | 0.94 (0.80-1.10) | Age, energy, history of breast cyst, family history of breast cancer, height, weight gain since age 18, alcohol use, race, age at menopause, age at first birth and number of live births, education, mammography history, and hormone therapy. |
| Frazier et al, 2004 | USA | Cohort | 1989-1998 | 34-51 | 361/47517 | Total 591 vs 159.6 | 0.92 (0.66-1.27) | Age, BMI, time period, height, parity and age at first birth, age at menarche, family history of breast cancer, history of benign breast disease, menopausal status, alcohol intake, energy, oral contraceptive use, and weight gain since age 18. |
| Shin et al, 2002 | USA | Cohort | 1980-1996 | 46.7 | Pre827/Post2345/ 88691 | Total >500 vs ≤150 | Pre 0.89 (0.68-1.15) Post 0.93 (0.80-1.08) | Age, BMI, time period, physical activity, history of benign breast disease, family history of breast cancer, height, weight change, age at menarche, parity, age at first birth, alcohol intake, total energy intake, total fat intake, glycemic index, β-carotene intake, and total active vitamin E intake. |
| Levi et al, 2000 | Switzerland | Case- control | 1993-1999 | 23-74 | 289/442 | Total 108000 vs 56000 | 1.43 (0.90–2.26) | Age, BMI, education, parity, menopausal status, total energy intake, and alcohol drinking |
| John et al, 1999 | USA | Cohort | 1971-1992 (17.3) | 25-74 | 179/4747 | Dietary ≥200 vs <100 | 0.85 (0.59–1.24) | Age, BMI, education, age at menarche, age at menopause, frequency of alcohol consumption, physical activity, and calcium intake |
| Potischman et al,1999 | USA | Case- control | 1990-1992 | 20-44 years | 568/1451 | Total ≥400 vs 0 | 0.98 (0.8–1.2) | Age at diagnosis, study site, ethnicity, combination age at first birth and parity, of oral contraceptive use, smoking, education and alcohol consumption. |
Abbreviations: OR=odds ratio; CI=confidence interval; BMI=body mass index (kg/m^2); HRT=hormone replacement therapy; HT=hormone therapy; POST=postmenopausal; PRE=premenopausal.
a. Vitamin D intake levels in ug/day were converted to IU/day using the conversion factor, 1ug/d=40IU/day.
b. The ORs of all studies used the lowest category of vitamin D intake levels as a reference in the meta-analysis.
For the association of vitamin D intake with breast cancer risk, there are 20 relevant studies, including 11 prospective cohort studies [16, 18–27] consisting 24040 cases, and 9 case-control studies [17, 28–35] consisting 11696 cases and 15583 controls. Among these, nine studies were performed in the US [16, 18–21, 25, 26, 28, 33], seven in Europe [22–24, 27, 29, 34, 35], two in Asia [31, 32], one in Canada [30] and one in Mexico [17]. There were seven studies [17, 18, 21, 22, 24, 31, 32] that provided risk estimates which were stratified by menopausal status in the 20 studies. The risk estimates of most of the studies were adjusted for potential confounders, including age, body mass index (BMI), education level, and physical activity. The adjusted confounding factors are shown in Table 1.
We identified 50 prospective studies on the association of blood vitamin D levels and breast cancer risk; the six cohort studies [36–41] consisting of 2257 incident cases, and 44 case-control studies [16, 17, 42–83] consisting 29095 cases and 53060 controls were included. Among these, 16 studies were conducted in the Europe [36, 38–40, 43, 44, 47, 50–57], fifteen in the US [16, 19, 37, 41, 42, 45, 46, 48, 58–63, 82], twelve in Asia [64–75], two in Canada [76, 77], one each in Austria [78], Mexico [17], and Brazil [79]. In addition, two studies [80, 81] contained mixed population in Europe and in the US. The menopausal status was categorized as premenopausal, postmenopausal, or mixed. Overall, nine studies [16, 17, 48, 50–53, 62, 64] assessed risk estimates based on participants’ menopausal status and twelve studies [43, 50, 57, 59, 65, 68, 71, 72, 77–79, 83] provided unadjusted results. The majority of the studies were adjusted for potential confounders including age, BMI, race, education level and time at blood collection.
Overall analyses
Vitamin D intake and breast cancer risk
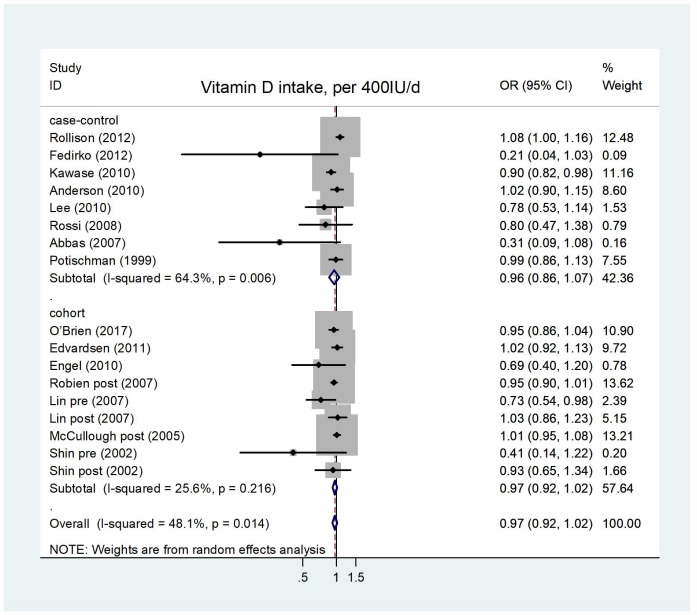
The pooled OR of breast cancer risk for the highest versus lowest category of vitamin D intake was 0.94 (95% CI = 0.88–1.00), with an evidence of heterogeneity I2 = 57.2%, P = 0.000 (Supplementary Figure 1). No publication bias was found after visual inspection of the funnel the plot (Supplementary Figure 3). The summary of estimations for case-control studies were OR = 0.89, 95% CI = 0.73–1.08, I2=77.0%, P = 0.228, and for cohort studies were OR=0.95, 95% CI = 0.90–1.00, I2 = 16.1%, P = 0.055. Eight case-control studies and seven cohort studies were eligible for the dose-response analysis of the association of vitamin D intake and breast cancer risk. As shown in Figure 2, the random-effects model was used and showed that a 400 IU/day increment in vitamin D intake had no significant effect on occurrence of breast cancer, and the pooled OR were 0.97 (95% CI = 0.92–1.02, I2 = 25.6%, P = 0.222) and 0.96 (95% CI = 0.86–1.07, I2 = 64.3%, P = 0.427) for cohort studies and case-control studies, respectively. Heterogeneity among studies was statistically significant (I2 = 48.1%, P = 0.014). There existed significant publication bias according to Begg's test (P = 0.009) and Egger's test (P = 0.007) (Supplementary Figure 5). In sensitivity analyses, a single study had no influence on the results, and the stable OR in the overall analysis ranged from 0.93–0.98 (Supplementary Figure 7). We failed to identify a significant dose-response relationship between vitamin D intake and breast cancer risk. When the subgroup analysis was stratified by menopausal status, study type, geographical location, and follow-up years, the results were stable; except for Asian studies (OR = 0.97, 95% CI = 0.95–0.99, Table 2) with no significant heterogeneity (I2 = 0.0%, P = 0.472), and studies related to premenopuase (OR = 0.79, 95% CI = 0.64–0.96, Table 2) with a significant heterogeneity (I2 = 56.1%, P = 0.026). This result suggests that higher vitamin D intake could reduce the risk of breast cancer in Asian women and premenopausal women.
Figure 2.
Forest plot of meta-analysis of the association between vitamin D intake increment (per 400IU/d) and breast cancer risk. Abbreviations: OR, odds ratio; CI, confidence interval.
Table 2. Subgroup analyses of vitamin D intake and breast cancer.
| Analysis specification | No. of studies | OR(95% CI) | P | Heterogeneity | |
| I2 | p | ||||
| Highest vs lowest | |||||
| All studies | 20 | 0.94(0.88-1.00) | 0.063 | 57.2% | 0 |
| Case-control | 9 | 0.89(0.73-1.08) | 0.228 | 77.0% | 0 |
| Cohort | 11 | 0.95(0.90-1.00) | 0.055 | 16.1% | 0.281 |
| Increment of 400 IU/d | |||||
| All studies | 15 | 0.97(0.92-1.02) | 0.201 | 48.1% | 0.014 |
| Case-control | 8 | 0.96(0.86-1.07) | 0.427 | 64.3% | 0.006 |
| Cohort | 7 | 0.97(0.92-1.02) | 0.222 | 25.6% | 0.216 |
| Menopausal status | |||||
| Premenopause | 8 | 0.79(0.64-0.96) | 0.021 | 56.1% | 0.026 |
| Postmenopausal | 8 | 0.98(0.94-1.02) | 0.243 | 0 | 0.631 |
| Geographic location | |||||
| Europe | 4 | 0.83(0.60-1.15) | 0.257 | 48.3% | 0.122 |
| America | 7 | 0.99(0.93-1.04) | 0.599 | 47.3% | 0.056 |
| Asia | 2 | 0.89(0.82-0.97) | 0.008 | 0 | 0.472 |
| Follow-up duration | |||||
| <10 years | 9 | 0.96(0.88-1.05) | 0.358 | 60.9% | 0.009 |
| ≥10 years | 5 | 0.95(0.88-1.03) | 0.245 | 32% | 0.183 |
| Source vitamin D | |||||
| Dietary | 5 | 0.90(0.73-1.10) | 0.308 | 78.9% | 0.001 |
| Dietary+Supplement | 10 | 0.98(0.94-1.01) | 0.185 | 5.3% | 0.393 |
Blood vitamin D levels and breast cancer risk
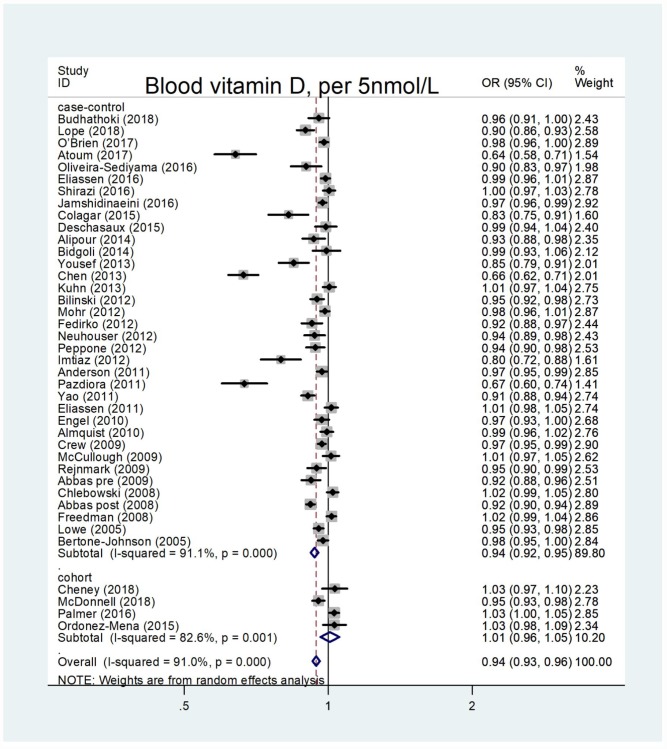
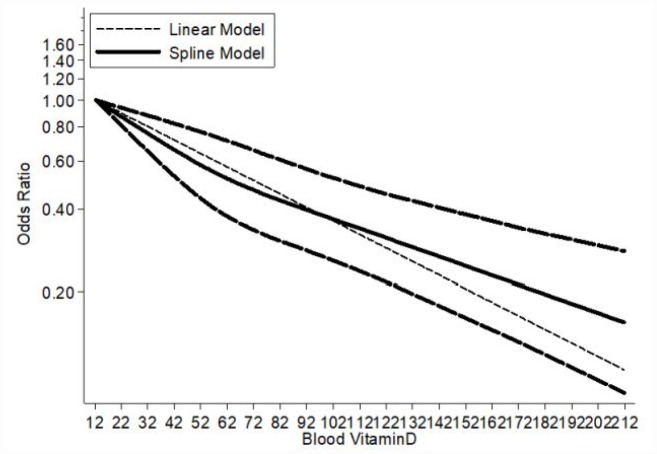
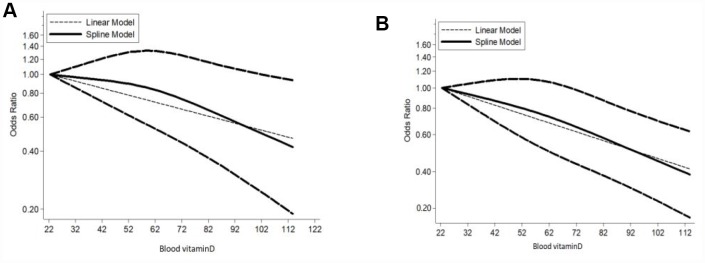
The summary OR of breast cancer for the highest versus lowest category of blood vitamin D levels was 0.61 (95% CI = 0.53–0.70), with an evidence of heterogeneity I2 = 89.3%, P = 0.000 (Supplementary Figure 2). A significant publication bias was observed through the funnel plot (Supplementary Figure 4). The pooled OR for case-control studies was 0.57 (95% CI = 0.48–0.66; I2 = 89.9%, P = 0.000), and for cohort studies was 1.17 (95% CI = 0.92–1.48; I2 = 31.6%, P = 0.192). Overall, 36 case-control studies and four cohort studies were eligible for the dose-response analysis of the association on blood vitamin D levels with breast cancer risk. The pooled OR for breast cancer risk for a 5 nmol/l increase in blood vitamin D levels was 0.94 (95% CI = 0.93–0.96), with a significant heterogeneity among all studies (I2 = 91.0%, P = 0.000) (Figure 3). The summary OR for case-control studies was 0.94 (95% CI = 0.92–0.95) and for cohort studies was 1.01 (95% CI = 0.96–1.05). Begg’s test (P = 0.004) and Egger’s test (P = 0.004) showed a significant publication bias and the funnel plot was asymmetrical (Supplementary Figure 6). The sensitivity analyses indicated that the ORs ranged from 0.96–0.97, and our results were statistically stable (Supplementary Figure 8). 36 eligible case-control studies showed an evidence of a linear association between blood vitamin D levels and breast cancer risk (Pnonlinearity = 0.1893) (Figure 4). Subgroup analysis based on menopausal status (Figure 5), showed linear relationship between blood vitamin D levels and breast cancer risk for premenopausal and postmenopausal women (Pnonlinearity = 0.2140 and Pnonlinearity =0.4900, respectively). The results of subgroup are presented in Table 3. A 5 nmol/l increase in blood vitamin D corresponded to a 16% decrease in breast cancer risk in Asian women.
Figure 3.
Forest plot of meta-analysis of the association between blood vitamin D increment (per 5nmol/L) and breast cancer risk. Abbreviations: OR, odds ratio; CI, confidence interval.
Figure 4.
Dose–response meta-analysis of blood vitamin D and breast cancer risk (linear and nonlinear models).
Figure 5.
Dose-response meta-analysis of blood vitamin D and breast cancer risk stratified by menopausal status (linear and nonlinear models). Note: (A) Premenopause; (B) Postmenopause.
Table 3. Subgroup analyses of blood vitamin D and breast cancer.
| Analysis specification | No. of studies | OR(95% CI) | P | Heterogeneity | |
| I^2 | p | ||||
| Highest vs lowest | |||||
| All studies | 50 | 0.61(0.53-0.70) | 0 | 89.3% | 0 |
| Case-control | 44 | 0.57(0.48-0.66) | 0 | 89.9% | 0 |
| Cohort | 6 | 1.17(0.92-1.48) | 0.192 | 31.6% | 0.198 |
| Increment of 5 nmol/l | |||||
| All studies | 40 | 0.94(0.93-0.96) | 0 | 91.0% | 0 |
| Case-control | 36 | 0.94(0.92-0.95) | 0 | 91.1% | 0 |
| Cohort | 4 | 1.01(0.96-1.05) | 0.734 | 82.6% | 0.001 |
| Menopausal status | |||||
| Premenopause | 11 | 0.96(0.93-0.99) | 0.011 | 68.2% | 0 |
| Postmenopausal | 15 | 0.96(0.94-0.98) | 0.001 | 86.4% | 0 |
| Geographic location | |||||
| Europe | 13 | 0.95(0.92-0.98) | 0 | 89.1% | 0 |
| America | 14 | 0.98(0.96-0.99) | 0.034 | 82.4% | 0 |
| Asia | 9 | 0.84(0.77-0.92) | 0 | 95.7% | 0 |
| Follow-up duration | |||||
| <10 years | 24 | 0.94(0.91-0.96) | 0 | 92.4% | 0 |
| ≥10 years | 10 | 0.99(0.98-1.00) | 0.051 | 26.7% | 0.198 |
| Serum or Plasm | |||||
| Serum | 31 | 0.93(0.91-0.97) | 0 | 92.80% | 0 |
| Plasm | 9 | 0.97(0.96-0.98) | 0 | 50.50% | 0.04 |
DISCUSSION
Vitamin D is known to be associated with the risk of human cancers, [7, 8, 84] and vitamin D deficiency has been reported to be correlated with colorectal cancer and prostate cancer [85–88]. The recent case-control study suggested that higher serum 25(OH) D level was significantly inversely correlated with melanoma in Italy population [89]. Nevertheless, the evidence of the association of vitamin D with breast cancer risk remains controversial. Lowa [43] shown that low plasma vitamin D levels were related to higher breast cancer risk in the Caucasian population in the United Kingdom. However, McCullough [49] demonstrated that serum vitamin D level was not associated with the occurrence of breast cancer. Therefore, our meta-analysis aimed to explore the correct relationship between vitamin D and breast cancer risk.
In the current study, we performed a meta-analysis of 70 observational studies. No significant association of a 400 IU/day increment in vitamin D intake and breast cancer risk was observed. However, vitamin D intake might decrease the risk of breast cancer in Asian and premenopause women. In addition, the result of case-control studies indicated that there was an underlying linear relationship between blood vitamin D levels and the risk of breast cancer; the overall risk decreased by 6% for each 5 nmol/l increase in blood vitamin D.
The human body obtains a relatively small quantity of vitamin D through limited dietary sources; the major source, however, is endogenous production of vitamin D. Although the exact mechanism by which vitamin D is linked to breast cancer risk remains unclear, experimental studies reported an anti-proliferative effect of 1,25(OH)2D3 on malignant melanoma cells and a pro-differentiating effects on myeloid leukemia cells [90]. In addition, the recent experimental study indicated that 1,25(OH)2D3 played a long-lasting anti-inflammatory and anti-proliferation effect in synoviocytes of rheumatoid arthritis and osteoarthritis [91]. Furthermore, the ability of 1, 25(OH)2D3 to induce apoptosis and inhibit angiogenesis in cancer cells has been confirmed [9]. The experimental study has also demonstrated that 1, 25(OH)2D3 deficiency promotes tumorigenesis by increasing oxidative stress and DNA damage of malignant cells, and activating oncogenes and inactivating tumor suppressor genes, therefore enhancing cancer cells proliferation [92].
An anterior meta-analysis [13] showed that vitamin D intake exceeding 400IU/day was associated with a 8% reduction in breast cancer risk. However, a previous meta-analysis [93] including 10 prospective studies showed no association between vitamin D intake and breast cancer risk. The latest multicenter randomized double-blind placebo-controlled study also does not support the use of vitamin D supplementation in premenopausal women for breast cancer risk reduction [94]. In our meta-analysis of additional 9 studies [16, 17, 28–34], we received the same conclusion that the pooled OR of vitamin D intake 400 IU/day increment for breast cancer was 0.97 (95% CI = 0.92–1.02). However, when we stratified by geographical location, it tended to show a middle inverse association (OR = 0.89, 95%CI = 0.82–0.97) among Asian women and a strong opposite association (OR = 0.79, 95% CI = 0.64–0.96) for premenopausal women. Hence, our result did not support that vitamin D intake prevents breast cancer, except for Asian and premenopausal populations. Kawase [31] and Lee [32] both indicated that vitamin D intake decreased breast cancer risk in premenopausal women, and they also found that the anti-cancer mechanism of vitamin D centered on reproductive hormone and the higher level of serum reproductive hormone in premenopausal women may explain the relationship between vitamin D intake and premenopausal breast cancer risk. There may be specific vitamin D receptor gene polymorphisms associated with breast cancer risk in Asian women [95–97].
The recent meta-analysis [93] based on 24 observational studies about dietary and blood 25-hydroxyvitamin D (25(OH)D) included 31867 breast cancer cases showed that the RRs were 0.95 (95% CI = 0.88–1.01) for vitamin D intake and 0.92 (95% CI = 0.83–1.02) for blood 25(OH)D levels. An initial case-control study [10] showed a negative (OR = 1.00, 95% CI = 0.20–3.40) association between breast cancer risk and serum 1,25(OH)2 D levels > 51 pg/ml. However, a study [98] conducted in the Nurses' Health Study II, which researched circulating free 25(OH)D and risk of breast cancer, concluded the estimated risk of breast cancer associated with the high 25(OH)D level (OR = 3.2, 95% CI = 1.7–6.0). A recent large study conducted by Vojdeman [99], in 217244 individuals from Primary Health Care in Denmark, indicated that there was no association between an increment of 10nmol/l blood vitamin D levels and the incidence of breast cancer. In this meta-analysis, we have added more full studies, then came to an intuitive conclusion (OR = 0.94, 95% CI = 0.93–0.96) for blood vitamin D 5 units increases and low risk of breast cancer, and the dose-response curve shown a linear change (Pnonlinearity = 0.1893).
Significant heterogeneity was observed in this meta-analysis. Sensitivity analyses suggested that the stable pooled OR was not significantly affected by any single study. In addition, we performed subgroup analysis stratified by confounding factors to identify the sources of heterogeneity. Differences in baseline characteristics of the study population and vitamin D cut-off values may have also resulted in the observed heterogeneity.
The study had several limitations. Firstly, as most of the studies were designed as case-control, recall bias and selection bias were inevitable and affected the results. Secondly, our results showed a greater heterogeneity. We found distinct sources through subgroup analysis. The heterogeneity could be caused by the presence of different races, outdoor physical activities, season of blood collection, and the method for measuring blood vitamin D levels. Thirdly, breast cancer is a heterogeneous disease, and vitamin D may affect only some sub-types of breast cancer. However, there are only few studies on this topic and hence could not be included in the current meta-analysis. Lastly, there was an obvious publication bias, possibly because positive results are more likely to be published than negative results.
Compared with former studies, our meta-analysis included a greater number of single studies to increase the sample size. Hence, the results of this meta-analysis could be considered closer to reality. In addition, we used the exact dose-response to access the relation of blood vitamin D levels or vitamin D intake with breast cancer risk. This method gives us a more intuitive analysis of relationship between vitamin D and breast cancer.
In conclusion, our study supports the hypothesis that a higher blood vitamin D status is related to a lower risk of breast cancer. However, we cannot draw the same conclusion regarding the association between vitamin D intake and breast cancer risk. Further well-designed studies are needed to prove the results and to clarify the role of vitamin D against the pathogenesis of breast cancer.
MATERIALS AND METHODS
Literature retrieval
Relevant English publications up to January 2019 were searched in PubMed, EMBASE, and Web of Science using the search terms “Vitamin D,”, “25-hydroxyvitamin D,”, “25(OH)D,”, “breast neoplasms,”, “breast cancer,”, “incidence,” and “risk”. We also searched for relevant studies in the reference lists of the eligible meta-analysis and reviews. Two authors independently read the retrieved literature, screened the relevant publications according to the exclusion criteria, and then removed any duplication literature. Disagreement between two authors was resolved by discussion.
Inclusion and exclusion criteria
Publications were screened according to the following inclusion criteria: (1) an original article; (2) a prospective cohort or case-control study in design; (3) the exposure factor was blood vitamin D levels or vitamin D intake; (4) the outcome of interest was the incidence of breast cancer; (5) availability of statistical parameters including the relative risk ratio (RR), hazard ratio (HR) or odds ratio (OR) with the corresponding 95% confidence intervals (CIs); alternatively, availability of sufficient data to calculate the aforementioned parameters; (6) ≥ three categories of exposure were provided. The exclusion criteria were the following: (1) a duplicate publication; (2) a publication which is not an original research such as reviews and systematic reviews; (3) low quality of research and poor reliability.
We chose total vitamin D intake when the study reported results on both dietary and total vitamin D intake. The RR, HR, with the corresponding 95% CI were recalculated when the low category of vitamin D was not the reference category in the original study.
Data extraction and quality assessment
From each selected publication, the following variables were collected: name of the first author, year of publication, geographical location where the study was conducted, type of study (case-control or cohort), follow-up periods, age of the study population, number of cases and controls, person-time, source of measurement of vitamin D levels (serum or plasma), source of vitamin D intake (dietary or dietary and supplement), blood vitamin D levels (nmol/l), vitamin D intake (IU/day), HR, RR or OR, 95% CI, and confounding factors that were adjusted. If a study provided several risk estimates, we used the estimate from the major multivariable model, which included a greater number of adjusted confounders. To facilitate comparison, the value of blood vitamin D levels and vitamin D intake that were expressed in conventional units (ng/ml and ug/d, respectively) were converted to SI units (nmol/l and IU/day, respectively).
The quality of each selected publication was assessed independently by two authors according to the Newcastle-Ottawa Quality Assessment Scale (NOS) [100]. The content of the study was evaluated for four major aspects: selection, comparability, exposure, and results, and thereafter, categorized into high, medium, and low quality. A study with a score > 6 was considered to be well quality.
Statistical analysis
We used the Q or I2 statistics to assess the heterogeneity among the studies [101]. P < 0.1 or I2 > 50% indicated significant heterogeneity. The random-effects model was used to estimate the pooled ORs and 95% CIs for a 5 -unit increment in blood vitamin D levels and a 100 -unit increment in vitamin D intake [102]. We evaluated the mean of the natural logarithm of the ORs, and weighted the OR of each study by the reciprocal of its variance. All statistical tests were two-sided, P < 0.05 was considered significance.
In all studies on the three categories of data on vitamin D, we assigned a median value of vitamin D to each category. For the open-ended upper category, we assumed that it had the same amplitude as the previous one. The generalized least square for tend estimation was used to transfer category-specific risk values to the OR related to every 5nmol/l and 400IU/day increase in blood vitamin D levels and vitamin D intake, respectively [103].
We used a two-stage hierarchical regression model to examine the possible linear dose-response association between blood vitamin D levels or vitamin D intake and breast cancer risk [104]. We analyzed data using the random-effects restricted cubic spline and four knots models. The covariance of multivariate adjusted OR was estimated by using the methods of Greenland and Longnecker [105].
We conducted subgroup analyses based on menopausal status, study type, geographical location, vitamin D source and follow-up duration. Sensitivity analyses were performed to assess the effect of individual study on the results. Potential publication bias was assessed by using Egger’s test and Begg’s test. All data were analyzed using the Stata 12 software.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of our study team for their whole-hearted cooperation and the original authors of the included studies for their wonderful work.
Abbreviations
- OR
odds ratio
- RR
relative risk
- HR
hazard ratio
- CI
confidence interval
- NOS
Newcastle-Ottawa Scale
- SI
standard international
Footnotes
AUTHOR CONTRIBUTIONS: All authors read, critically reviewed and approved the final manuscript. SDL, DYJ and LK conducted the database searches, screened titles, abstracts and full-texts for eligibility, performed study quality assessments. DZJ planed and designed the research; ZLH and LN provided methodological support/advice; ZY tested the feasibility of the study; HQ, SY and YW extract data; ZZ performed the statistical analysis; SDL wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare that there are no competing interests associated with the manuscript.
FUNDING: National Natural Science Foundation of China, Grant/Award Number: 81471670.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002; 162:1985–93. 10.1001/archinte.162.17.1985 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.Okazaki R. [Vitamin D and cancer]. Clin Calcium. 2014; 24:1193–99. [PubMed] [Google Scholar]
- 5.Dunn JA, Jefferson K, MacDonald D, Iqbal G, Bland R. Low serum 25-hydroxyvitamin D is associated with increased bladder cancer risk: A systematic review and evidence of a potential mechanism. J Steroid Biochem Mol Biol. 2019; 188:134–40. 10.1016/j.jsbmb.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Li F, Zhao H, Hou L, Ling F, Zhang Y, Tan W. A higher circulating concentration of 25-hydroxyvitamin-D decreases the risk of renal cell carcinoma: a case-control study. Int Braz J Urol. 2019; 45:523–30. 10.1590/s1677-5538.ibju.2018.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005; 16:83–95. 10.1007/s10552-004-1661-4 [DOI] [PubMed] [Google Scholar]
- 8.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014; 14:342–57. 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- 9.Vanoirbeek E, Krishnan A, Eelen G, Verlinden L, Bouillon R, Feldman D, Verstuyf A. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best Pract Res Clin Endocrinol Metab. 2011; 25:593–604. 10.1016/j.beem.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiatt RA, Krieger N, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N. Prediagnostic serum vitamin D and breast cancer. J Natl Cancer Inst. 1998; 90:461–63. 10.1093/jnci/90.6.461 [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010; 46:2196–205. 10.1016/j.ejca.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Tian C, Zhang X. Dietary calcium intake, vitamin D levels, and breast cancer risk: a dose-response analysis of observational studies. Breast Cancer Res Treat. 2012; 136:309–12. 10.1007/s10549-012-2172-8 [DOI] [PubMed] [Google Scholar]
- 13.Gissel T, Rejnmark L, Mosekilde L, Vestergaard P. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008; 111:195–99. 10.1016/j.jsbmb.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T. Vitamin D exposure and Risk of Breast Cancer: a meta-analysis. Sci Rep. 2018; 8:9039. 10.1038/s41598-018-27297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore). 2013; 92:123–31. 10.1097/MD.0b013e3182943bc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien KM, Sandler DP, Taylor JA, Weinberg CR. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ Health Perspect. 2017; 125:077004. 10.1289/EHP943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedirko V, Torres-Mejía G, Ortega-Olvera C, Biessy C, Angeles-Llerenas A, Lazcano-Ponce E, Saldaña-Quiroz VA, Romieu I. Serum 25-hydroxyvitamin D and risk of breast cancer: results of a large population-based case-control study in Mexican women. Cancer Causes Control. 2012; 23:1149–62. 10.1007/s10552-012-9984-z [DOI] [PubMed] [Google Scholar]
- 18.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002; 94:1301–11. 10.1093/jnci/94.17.1301 [DOI] [PubMed] [Google Scholar]
- 19.McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005; 14:2898–904. 10.1158/1055-9965.EPI-05-0611 [DOI] [PubMed] [Google Scholar]
- 20.Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer Causes Control. 2007; 18:775–82. 10.1007/s10552-007-9020-x [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007; 167:1050–59. 10.1001/archinte.167.10.1050 [DOI] [PubMed] [Google Scholar]
- 22.Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Joint effects of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2011; 20:187–98. 10.1158/1055-9965.EPI-10-1039 [DOI] [PubMed] [Google Scholar]
- 23.Edvardsen K, Veierød MB, Brustad M, Braaten T, Engelsen O, Lund E. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int J Cancer. 2011; 128:1425–33. 10.1002/ijc.25463 [DOI] [PubMed] [Google Scholar]
- 24.Abbas S, Linseisen J, Rohrmann S, Chang-Claude J, Peeters PH, Engel P, Brustad M, Lund E, Skeie G, Olsen A, Tjønneland A, Overvad K, Boutron-Ruault MC, et al. Dietary intake of vitamin D and calcium and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Nutr Cancer. 2013; 65:178–87. 10.1080/01635581.2013.752018 [DOI] [PubMed] [Google Scholar]
- 25.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999; 8:399–406. [PubMed] [Google Scholar]
- 26.Frazier AL, Li L, Cho E, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control. 2004; 15:73–82. 10.1023/B:CACO.0000016617.57120.df [DOI] [PubMed] [Google Scholar]
- 27.Kuper H, Yang L, Sandin S, Lof M, Adami HO, Weiderpass E. Prospective study of solar exposure, dietary vitamin D intake, and risk of breast cancer among middle-aged women. Cancer Epidemiol Biomarkers Prev. 2009; 18:2558–61. 10.1158/1055-9965.EPI-09-0449 [DOI] [PubMed] [Google Scholar]
- 28.Potischman N, Swanson CA, Coates RJ, Gammon MD, Brogan DR, Curtin J, Brinton LA. Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer. Int J Cancer. 1999; 82:315–21. [DOI] [PubMed] [Google Scholar]
- 29.Rossi M, McLaughlin JK, Lagiou P, Bosetti C, Talamini R, Lipworth L, Giacosa A, Montella M, Franceschi S, Negri E, La Vecchia C. Vitamin D intake and breast cancer risk: a case-control study in Italy. Ann Oncol. 2009; 20:374–78. 10.1093/annonc/mdn550 [DOI] [PubMed] [Google Scholar]
- 30.Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010; 91:1699–707. 10.3945/ajcn.2009.28869 [DOI] [PubMed] [Google Scholar]
- 31.Kawase T, Matsuo K, Suzuki T, Hirose K, Hosono S, Watanabe M, Inagaki M, Iwata H, Tanaka H, Tajima K. Association between vitamin D and calcium intake and breast cancer risk according to menopausal status and receptor status in Japan. Cancer Sci. 2010; 101:1234–40. 10.1111/j.1349-7006.2010.01496.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MS, Huang YC, Wahlqvist ML, Wu TY, Chou YC, Wu MH, Yu JC, Sun CA. Vitamin D decreases risk of breast cancer in premenopausal women of normal weight in subtropical taiwan. J Epidemiol. 2011; 21:87–94. 10.2188/jea.JE20100088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollison DE, Cole AL, Tung KH, Slattery ML, Baumgartner KB, Byers T, Wolff RK, Giuliano AR. Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern U.S. Breast Cancer Res Treat. 2012; 132:683–91. 10.1007/s10549-011-1885-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbas S, Linseisen J, Chang-Claude J. Dietary vitamin D and calcium intake and premenopausal breast cancer risk in a German case-control study. Nutr Cancer. 2007; 59:54–61. 10.1080/01635580701390223 [DOI] [PubMed] [Google Scholar]
- 35.Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast-cancer risk. Int J Cancer. 2001; 91:260–63. [DOI] [PubMed] [Google Scholar]
- 36.Cheney CP, Thorand B, Huth C, Berger K, Peters A, Seifert-Klauss V, Kiechle M, Strauch K, Quante AS. The Association between Serum 25-Hydroxyvitamin D and Cancer Risk: Results from the Prospective KORA F4 Study. Oncol Res Treat. 2018; 41:117–21. 10.1159/000485512 [DOI] [PubMed] [Google Scholar]
- 37.Palmer JR, Gerlovin H, Bethea TN, Bertrand KA, Holick MF, Ruiz-Narvaez EN, Wise LA, Haddad SA, Adams-Campbell LL, Kaufman HW, Rosenberg L, Cozier YC. Predicted 25-hydroxyvitamin D in relation to incidence of breast cancer in a large cohort of African American women. Breast Cancer Res. 2016; 18:86. 10.1186/s13058-016-0745-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ordóñez-Mena JM, Schöttker B, Fedirko V, Jenab M, Olsen A, Halkjær J, Kampman E, de Groot L, Jansen E, Bueno-de-Mesquita HB, Peeters PH, Siganos G, Wilsgaard T, et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol. 2016; 31:311–23. 10.1007/s10654-015-0040-7 [DOI] [PubMed] [Google Scholar]
- 39.Skaaby T, Husemoen LL, Thuesen BH, Pisinger C, Jørgensen T, Roswall N, Larsen SC, Linneberg A. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol Biomarkers Prev. 2014; 23:1220–29. 10.1158/1055-9965.EPI-14-0007 [DOI] [PubMed] [Google Scholar]
- 40.Ordóñez-Mena JM, Schöttker B, Haug U, Müller H, Köhrle J, Schomburg L, Holleczek B, Brenner H. Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2013; 22:905–16. 10.1158/1055-9965.EPI-12-1332 [DOI] [PubMed] [Google Scholar]
- 41.McDonnell SL, Baggerly CA, French CB, Baggerly LL, Garland CF, Gorham ED, Hollis BW, Trump DL, Lappe JM. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs 50 nmol/L): pooled analysis of two randomized trials and a prospective cohort. PLoS One. 2018; 13:e0199265. 10.1371/journal.pone.0199265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14:1991–97. 10.1158/1055-9965.EPI-04-0722 [DOI] [PubMed] [Google Scholar]
- 43.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005; 41:1164–69. 10.1016/j.ejca.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 44.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer—results of a large case-control study. Carcinogenesis. 2008; 29:93–99. 10.1093/carcin/bgm240 [DOI] [PubMed] [Google Scholar]
- 45.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, et al. , and Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008; 100:1581–91. 10.1093/jnci/djn360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008; 17:889–94. 10.1158/1055-9965.EPI-07-2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbas S, Chang-Claude J, Linseisen J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2009; 124:250–55. 10.1002/ijc.23904 [DOI] [PubMed] [Google Scholar]
- 48.Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL, Neugut AI, Santella RM. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila). 2009; 2:598–604. 10.1158/1940-6207.CAPR-08-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009; 11:R64. 10.1186/bcr2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, Mosekilde L. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2009; 18:2655–60. 10.1158/1055-9965.EPI-09-0531 [DOI] [PubMed] [Google Scholar]
- 51.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int J Cancer. 2010; 127:2159–68. 10.1002/ijc.25215 [DOI] [PubMed] [Google Scholar]
- 52.Engel P, Fagherazzi G, Boutten A, Dupré T, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010; 19:2341–50. 10.1158/1055-9965.EPI-10-0264 [DOI] [PubMed] [Google Scholar]
- 53.Lope V, Castelló A, Mena-Bravo A, Amiano P, Aragonés N, Fernández-Villa T, Guevara M, Dierssen-Sotos T, Fernandez-Tardón G, Castaño-Vinyals G, Marcos-Gragera R, Moreno V, Salas-Trejo D, et al. Serum 25-hydroxyvitamin D and breast cancer risk by pathological subtype (MCC-Spain). J Steroid Biochem Mol Biol. 2018; 182:4–13. 10.1016/j.jsbmb.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 54.Shirazi L, Almquist M, Borgquist S, Malm J, Manjer J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case-control study. Breast. 2016; 28:184–90. 10.1016/j.breast.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 55.Deschasaux M, Souberbielle JC, Latino-Martel P, Sutton A, Charnaux N, Druesne-Pecollo N, Galan P, Hercberg S, Le Clerc S, Kesse-Guyot E, Ezzedine K, Touvier M. Weight Status and Alcohol Intake Modify the Association between Vitamin D and Breast Cancer Risk. J Nutr. 2016; 146:576–85. 10.3945/jn.115.221481 [DOI] [PubMed] [Google Scholar]
- 56.Kühn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, Dossus L, Tjønneland A, Olsen A, Overvad K, Chang-Claude J, Lukanova A, Buijsse B, et al. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: a nested case-control study. Int J Cancer. 2013; 133:1689–700. 10.1002/ijc.28172 [DOI] [PubMed] [Google Scholar]
- 57.Pazdiora P, Svobodova S, Fuchsova R, Kucera R, Prazakova M, Vrzalova J, Narsanska A, Strakova M, Treskova I, Pecen L, Treska V, Holubec L Jr, Pesek M, et al. Vitamin D in colorectal, breast, prostate and lung cancer: a pilot study. Anticancer Res. 2011; 31:3619–21. [PubMed] [Google Scholar]
- 58.Eliassen AH, Warner ET, Rosner B, Collins LC, Beck AH, Quintana LM, Tamimi RM, Hankinson SE. Plasma 25-Hydroxyvitamin D and Risk of Breast Cancer in Women Followed over 20 Years. Cancer Res. 2016; 76:5423–30. 10.1158/0008-5472.CAN-16-0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, Wingard DL, Horst R, Garland CF. Serum 25-hydroxyvitamin D and breast cancer in the military: a case-control study utilizing pre-diagnostic serum. Cancer Causes Control. 2013; 24:495–504. 10.1007/s10552-012-0140-6 [DOI] [PubMed] [Google Scholar]
- 60.Neuhouser ML, Manson JE, Millen A, Pettinger M, Margolis K, Jacobs ET, Shikany JM, Vitolins M, Adams-Campbell L, Liu S, LeBlanc E, Johnson KC, Wactawski-Wende J. The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin D with risk of colorectal and breast cancer in postmenopausal women. Am J Epidemiol. 2012; 175:673–84. 10.1093/aje/kwr350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peppone LJ, Rickles AS, Janelsins MC, Insalaco MR, Skinner KA. The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann Surg Oncol. 2012; 19:2590–99. 10.1245/s10434-012-2297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao S, Sucheston LE, Millen AE, Johnson CS, Trump DL, Nesline MK, Davis W, Hong CC, McCann SE, Hwang H, Kulkarni S, Edge SB, O’Connor TL, Ambrosone CB. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case-control and a case-series study. PLoS One. 2011; 6:e17251. 10.1371/journal.pone.0017251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011; 13:R50. 10.1186/bcr2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Budhathoki S, Hidaka A, Yamaji T, Sawada N, Tanaka-Mizuno S, Kuchiba A, Charvat H, Goto A, Kojima S, Sudo N, Shimazu T, Sasazuki S, Inoue M, et al. , and Japan Public Health Center-based Prospective Study Group. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ. 2018; 360:k671. 10.1136/bmj.k671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colagar AH, Firouzjah HM, Halalkhor S, Vitamin D. Vitamin D Receptor Poly(A) Microsatellite Polymorphism and 25-Hydroxyvitamin D Serum Levels: Association with Susceptibility to Breast Cancer. J Breast Cancer. 2015; 18:119–25. 10.4048/jbc.2015.18.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yousef FM, Jacobs ET, Kang PT, Hakim IA, Going S, Yousef JM, Al-Raddadi RM, Kumosani TA, Thomson CA. Vitamin D status and breast cancer in Saudi Arabian women: case-control study. Am J Clin Nutr. 2013; 98:105–10. 10.3945/ajcn.112.054445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen P, Li M, Gu X, Liu Y, Li X, Li C, Wang Y, Xie D, Wang F, Yu C, Li J, Chen X, Chu R, et al. Higher blood 25(OH)D level may reduce the breast cancer risk: evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS One. 2013; 8:e49312. 10.1371/journal.pone.0049312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atoum MF, Al-Khatib YM. Association between Serum 25-hydroxy Vitamin D Concentration and TaqI Vitamin D Receptor Gene Polymorphism among Jordanian Females with Breast Cancer. Chin Med J (Engl). 2017; 130:1074–78. 10.4103/0366-6999.204933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jamshidinaeini Y, Akbari ME, Abdollahi M, Ajami M, Davoodi SH. Vitamin D Status and Risk of Breast Cancer in Iranian Women: A Case-Control Study. J Am Coll Nutr. 2016; 35:639–46. 10.1080/07315724.2015.1127786 [DOI] [PubMed] [Google Scholar]
- 70.Alipour S, Hadji M, Hosseini L, Omranipour R, Saberi A, Seifollahi A, Bayani L, Shirzad N. Levels of serum 25-hydroxy-vitamin d in benign and malignant breast masses. Asian Pac J Cancer Prev. 2014; 15:129–32. 10.7314/APJCP.2014.15.1.129 [DOI] [PubMed] [Google Scholar]
- 71.Bidgoli SA, Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case control study in Sabzevar, Iran. Asian Pac J Cancer Prev. 2014; 15:3391–96. 10.7314/APJCP.2014.15.8.3391 [DOI] [PubMed] [Google Scholar]
- 72.Imtiaz S, Siddiqui N, Raza SA, Loya A, Muhammad A. Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J Endocrinol Metab. 2012; 16:409–13. 10.4103/2230-8210.95684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sofi NY, Jain M, Kapil U, Seenu V, Kamal VK, Pandey RM. Nutritional risk factors and status of serum 25(OH)D levels in patients with breast cancer: A case control study in India. J Steroid Biochem Mol Biol. 2018; 175:55–59. 10.1016/j.jsbmb.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 74.Sofi NY, Jain M, Kapil U, Seenu V, R L, Yadav CP, Pandey RM, Sareen N. Reproductive factors, nutritional status and serum 25(OH)D levels in women with breast cancer: A case control study. J Steroid Biochem Mol Biol. 2018; 175:200–04. 10.1016/j.jsbmb.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 75.Park S, Lee DH, Jeon JY, Ryu J, Kim S, Kim JY, Park HS, Kim SI, Park BW. Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among Korean women: a case-control study. Breast Cancer Res Treat. 2015; 152:147–54. 10.1007/s10549-015-3433-0 [DOI] [PubMed] [Google Scholar]
- 76.Anderson LN, Cotterchio M, Kirsh VA, Knight JA. Ultraviolet sunlight exposure during adolescence and adulthood and breast cancer risk: a population-based case-control study among Ontario women. Am J Epidemiol. 2011; 174:293–304. 10.1093/aje/kwr091 [DOI] [PubMed] [Google Scholar]
- 77.Amir E, Cecchini RS, Ganz PA, Costantino JP, Beddows S, Hood N, Goodwin PJ. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat. 2012; 133:1077–88. 10.1007/s10549-012-2012-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bilinski K, Boyages J. Association between 25-hydroxyvitamin D concentration and breast cancer risk in an Australian population: an observational case-control study. Breast Cancer Res Treat. 2013; 137:599–607. 10.1007/s10549-012-2381-1 [DOI] [PubMed] [Google Scholar]
- 79.Oliveira Sediyama CM, Dias MM, Pessoa MC, Queiroz AR, Suhett LG, Freitas RN, De Paula SO, Peluzio MD. Lifestyle and vitamin D dosage in women with breast cancer. Nutr Hosp. 2016; 33:584. 10.20960/nh.584 [DOI] [PubMed] [Google Scholar]
- 80.Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, Arslan AA, Chen Y, Hallmans G, Lundin E, Rinaldi S, Toniolo P, Shore RE, Zeleniuch-Jacquotte A. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2013; 15:R15. 10.1186/bcr3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y, Sarkissyan M, Clayton S, Chlebowski R, Vadgama JV. Association of Vitamin D3 Level with Breast Cancer Risk and Prognosis in African-American and Hispanic Women. Cancers (Basel). 2017; 9:E144. 10.3390/cancers9100144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, Maskarinec G, Hernandez BY, Le Marchand L, Henderson BE, Kolonel LN, Goodman MT. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multiethnic cohort study. BMC Cancer. 2014; 14:29. 10.1186/1471-2407-14-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veldhuis S, Wolbers F, Brouckaert O, Vermes I, Franke HR. Cancer prevalence in osteoporotic women with low serum vitamin D levels. Menopause. 2011; 18:319–22. 10.1097/gme.0b013e3181f81ad5 [DOI] [PubMed] [Google Scholar]
- 84.Grant WB. A Review of the Evidence Supporting the Vitamin D-Cancer Prevention Hypothesis in 2017. Anticancer Res. 2018; 38:1121–36. 10.21873/anticanres.12331 [DOI] [PubMed] [Google Scholar]
- 85.Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, Kemp JP, Donovan JL, Hamdy FC, Neal DE, Lane JA, Smith GD, Lathrop M, Martin RM. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control. 2015; 26:205–18. 10.1007/s10552-014-0500-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandler PD, Buring JE, Manson JE, Giovannucci EL, Moorthy MV, Zhang S, Lee IM, Lin JH. Circulating Vitamin D Levels and Risk of Colorectal Cancer in Women. Cancer Prev Res (Phila). 2015; 8:675–82. 10.1158/1940-6207.CAPR-14-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, Eliassen AH, Zeleniuch-Jacquotte A, Agnoli C, Albanes D, Barnett MJ, Buring JE, Campbell PT, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst. 2019; 111:158–69. 10.1093/jnci/djy087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao J, Wei W, Wang G, Zhou H, Fu Y, Liu N. Circulating vitamin D concentration and risk of prostate cancer: a dose-response meta-analysis of prospective studies. Ther Clin Risk Manag. 2018; 14:95–104. 10.2147/TCRM.S149325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cattaruzza MS, Pisani D, Fidanza L, Gandini S, Marmo G, Narcisi A, Bartolazzi A, Carlesimo M. 25-Hydroxyvitamin D serum levels and melanoma risk: a case-control study and evidence synthesis of clinical epidemiological studies. Eur J Cancer Prev. 2019; 28:203–11. 10.1097/CEJ.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 90.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981; 108:1083–86. 10.1210/endo-108-3-1083 [DOI] [PubMed] [Google Scholar]
- 91.Huhtakangas JA, Veijola J, Turunen S, Karjalainen A, Valkealahti M, Nousiainen T, Yli-Luukko S, Vuolteenaho O, Lehenkari P. 1,25(OH)2D3 and calcipotriol, its hypocalcemic analog, exert a long-lasting anti-inflammatory and anti-proliferative effect in synoviocytes cultured from patients with rheumatoid arthritis and osteoarthritis. J Steroid Biochem Mol Biol. 2017; 173:13–22. 10.1016/j.jsbmb.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 92.Chen L, Yang R, Qiao W, Yuan X, Wang S, Goltzman D, Miao D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int J Cancer. 2018; 143:368–82. 10.1002/ijc.31317 [DOI] [PubMed] [Google Scholar]
- 93.Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014; 110:2772–84. 10.1038/bjc.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crew KD, Anderson GL, Hershman DL, Terry MB, Tehranifar P, Lew DL, Yee M, Brown EA, Kairouz SS, Kuwajerwala N, Bevers T, Doster JE, Zarwan C, et al. Randomized Double-Blind Placebo-Controlled Biomarker Modulation Study of Vitamin D Supplementation in Premenopausal Women at High Risk for Breast Cancer (SWOG S0812). Cancer Prev Res (Phila). 2019; 12:481–90. 10.1158/1940-6207.CAPR-18-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Li B, Jiang Q, Zhang Y, Liu A, Wang H, Zhang J, Qin Q, Hong Z, Li BA. Do genetic polymorphisms of the vitamin D receptor contribute to breast/ovarian cancer? A systematic review and network meta-analysis. Gene. 2018; 677:211–27. 10.1016/j.gene.2018.07.070 [DOI] [PubMed] [Google Scholar]
- 96.Shi J, Grundy A, Richardson H, Burstyn I, Schuetz JM, Lohrisch CA, SenGupta SK, Lai AS, Brooks-Wilson A, Spinelli JJ, Aronson KJ. Genetic variation in vitamin D-related genes and risk of breast cancer among women of European and East Asian descent. Tumour Biol. 2016; 37:6379–87. 10.1007/s13277-015-4417-8 [DOI] [PubMed] [Google Scholar]
- 97.Shahbazi S, Alavi S, Majidzadeh-A K, Ghaffarpour M, Soleimani A, Mahdian R. BsmI but not FokI polymorphism of VDR gene is contributed in breast cancer. Med Oncol. 2013; 30:393. 10.1007/s12032-012-0393-7 [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Eliassen AH, Spiegelman D, Willett WC, Hankinson SE. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer Causes Control. 2014; 25:819–27. 10.1007/s10552-014-0383-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vojdeman FJ, Madsen CM, Frederiksen K, Durup D, Olsen A, Hansen L, Heegaard AM, Lind B, Tjønneland A, Jørgensen HL, Schwarz P. Vitamin D levels and cancer incidence in 217,244 individuals from primary health care in Denmark. Int J Cancer. 2019; 145:338–46. 10.1002/ijc.32105 [DOI] [PubMed] [Google Scholar]
- 100.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–05. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 101.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 102.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015; 45:139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135:1301–09. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 104.Liu Q, Cook NR, Bergström A, Hsieh CC. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Comput Stat Data Anal. 2009; 53:4157–67. 10.1016/j.csda.2009.05.001 [DOI] [Google Scholar]
- 105.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012; 175:66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.