Abstract
Background:
Medicinal cannabinoids, including medicinal cannabis, pharmaceutical cannabinoids and their synthetic derivatives, including tetrahydrocannabinol (THC) and or cannabidiol (CBD), have been suggested to have a therapeutic role for certain mental health conditions. The primary objective was to review the evidence for cannabinoids in treating symptoms of depression, anxiety, post-traumatic stress disorder, attention-deficit hyperactivity disorder, Tic/Tourette syndrome, and psychosis, either as the primary condition or secondary to other conditions. Secondary outcomes included quality of life and global functioning.
Methods:
We undertook a systematic review and meta-analysis of published and unpublished studies (1980-2018) using MEDLINE, Embase, PsycINFO, and Cochrane Central Register of Controlled Clinical Trials, clinicaltrials.gov, the EU Clinical Trials Register, and the Australian and New Zealand Clinical Trials Registry. We included randomised controlled trials (RCTs) and non-RCT treatment studies. Two independent reviewers screened all studies and performed data extraction. RCT evidence was synthesised, as odds ratios (ORs) for disorder remission and standardised mean differences (SMDs) for change in symptoms, via random-effects meta-analyses. Evidence quality was evaluated using the Cochrane Risk of Bias and GRADE approaches.
Findings:
A total of k=83 studies (k=40 RCTs, n=3067) were included: k=40 for depression (k=22 RCTs, n=2524), k=31 for anxiety (k=17 RCTs, n=605), k=8 for Tic/Tourette syndrome (k=2 RCTs, n=36), k=4 for attention-deficit hyperactivity disorder (k=1 RCT, n=30), k=12 for post-traumatic stress disorder (k=1 RCT, n=10) and k=11 for psychosis (k=6 RCTs, n=281). Pharmaceutical THC (with or without CBD) improved anxiety symptoms amongst those with other medical conditions (primarily chronic non-cancer pain and multiple sclerosis; SMD=−0.25 [95% confidence interval: −0.49:−0.01]; k=7, n=252). Pharmaceutical THC (with or without CBD) worsened negative symptoms of psychosis in a single study (SMD=0.36 [0.10:0.62]; n=24). Pharmaceutical THC (with or without CBD) did not improve any other primary outcomes but did increase adverse events (OR=1.99 [1.20:3.29]; k=10, n=1495) and withdrawals due to adverse events (OR=2.78 [1.59:4.86]; k=11, n=1621). Very few RCTs examined pharmaceutical CBD or medicinal cannabis.
Interpretation:
There is a lack of evidence that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tic/Tourette syndrome, post-traumatic stress disorder, or psychosis. There is very-low-quality evidence that pharmaceutical THC (with or without CBD) leads to a small improvement in symptoms of anxiety amongst those with other medical conditions. There remains insufficient evidence to provide guidance on the use of cannabinoids for mental health conditions within a regulatory framework. More high-quality studies examining the effect of cannabinoids on mental disorders are needed.
Review registration:
PROSPERO CRD42017059372, CRD42017059373, CRD42017059376, CRD42017064996, CRD42018102977
Funding:
Therapeutic Goods Administration, Commonwealth Department of Health; Australian National Health and Medical Research Council, NIH
Introduction
Countries are increasingly allowing cannabinoids to be made available for medicinal purposes, including for the treatment of mental disorders. In this review, based on previous agreed terminology1, we use the term ‘medicinal cannabinoids’ as an umbrella term to encompass all plant-derived and synthetic derivatives. We use ‘medicinal cannabis’ to refer to any part of the cannabis plant and plant material, such as buds, leaves or full plant extracts (e.g., cannabis sativa). We use ‘pharmaceutical cannabinoids’ to refer to pharmaceutical-grade medicinal extracts with defined and standardised THC and THC/CBD content (e.g., tetrahydrocannabinol [THC], cannabidiol [CBD] extract, or THC:CBD combinations (nabiximols)) and synthetic cannabinoid derivatives1. Given increasing interest in CBD products for a range of conditions, we also separately grouped studies using pharmaceutical CBD only.
After chronic non-cancer pain (CNCP), mental health is one of the most common reasons for accessing medicinal cannabinoids2. In terms of biological plausibility, there is a potential role of the endocannabinoid system (CB1 receptors) in reducing depressive and stress symptoms3 and the emotional and cognitive features of post-traumatic stress disorder (PTSD)4. CBD has been proposed as an effective short-term treatment for individuals experiencing social anxiety disorder5. Medicinal cannabinoids have been reported to reduce tics in Tourette Syndrome6. Many surveys report elevated rates of cannabis use among people living with depression, anxiety, PTSD, and psychosis, and self-medication of symptoms is suggested to be a driver of some of this use7,8.
Given the interest in using medicinal cannabinoids for these purposes, it is important to thoroughly review the evidence to inform policy and clinical decisions. Previous systematic reviews have been limited in their coverage of mental disorders, study designs, and use of quantitative synthesis (i.e., meta-analysis). A 2015 review by Whiting and colleagues9, which included 5 RCTs of mental disorders, found no effect on psychosis or depression, but noted low-quality evidence for some improvement in Tourette syndrome and anxiety. A 2016 review by Wilkinson and colleagues10 included 40 trials (RCTs and observational studies) of medicinal cannabinoids for PTSD, Tourette syndrome, and Alzheimer’s disease. No RCTs were identified for any condition and no meta-analysis was conducted, thus authors could not draw conclusions regarding efficacy. Crucially, highly prevalent disorders for which medicinal cannabinoids are often sought – such as depression, anxiety, and psychosis – were not included. The 2017 National Academy of Sciences (NAS) review11 reported beneficial effects for Tourette syndrome, anxiety, and PTSD, and no impact on psychosis or depression; however, this review was based largely on findings reported by Whiting and colleagues9. There remains no single review that has considered: all types of evidence; the potential differential effects of different types of medicinal cannabinoids; and the safety of using cannabinoids for mental disorders. Disentangling the evidence for different types of cannabinoids for specific mental disorders is needed to direct research efforts and provide clinical guidance.1
The aim of this systematic review and meta-analysis was to examine evidence for all types of medicinal cannabinoids and all study designs (controlled and observational) to determine:
- The impact of medicinal cannabinoids on:
- Primary outcomes including remission from and symptoms of depression, anxiety, PTSD, and psychosis; and symptoms of attention-deficit hyperactivity disorder (ADHD) and Tic/Tourette syndrome; either as the primary disorder or secondary to other disorders;
- Secondary outcomes including global functioning, quality of life, patient or caregiver impression of change; and
The safety of medicinal cannabinoids for mental health, including all-cause, serious and treatment-related adverse events and study withdrawals.
Methods
This review was registered on PROSPERO (depression: CRD42017059376; anxiety: CRD42017059373; PTSD: CRD42017064996; ADHD and Tic/Tourette syndrome: CRD42017059372; psychosis: CRD42018102977).
Search strategy
We searched MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and the Cochrane Database of Systematic Reviews via Ovid from 1980 to May 2018. Five separate searches were conducted to identify studies that evaluated the efficacy of plant-based and pharmaceutical cannabinoids in reducing or treating symptoms of depression, anxiety, PTSD, ADHD and Tic/Tourette syndrome, and psychotic disorders. The detailed search strategies for each condition are shown in Appendix A. To identify ongoing or unpublished studies, we additionally searched clinicaltrials.gov, the EU Clinical Trials Register and the Australian and New Zealand Clinical Trials Registry using keywords ‘cannabis’, ‘cannabinoids’, ‘marijuana’ and each of the five mental disorders. We also hand searched reference lists of included studies and topical reviews for potentially relevant articles. No restrictions were placed on language, publication status, or type.
Inclusion and exclusion criteria
Types of populations:
We included studies examining medicinal cannabinoids for adults aged ≥ 18 years for the purpose of treating depression, anxiety, ADHD and Tic/Tourette syndrome, PTSD and psychosis either as the primary condition or as secondary to other medical conditions (such as CNCP). We chose to review these specific conditions because they are widely cited as reasons for accessing medicinal cannabinoids2 and have onset in young adulthood and thus have impact across the lifespan12. We did not include neurocognitive disorders such as dementia as they have a markedly different pathophysiology and have onset later in life and thus warrant a separate, specific review.
Types of cannabinoids:
We considered studies examining any type and formulation of medicinal cannabinoid: tetrahydrocannabinol; cannabidiol; combination tetrahydrocannabinol + cannabidiol; cannabis sativa; and other cannabinoids e.g. tetrahydrocannabinolic acid, cannabidiolic acid, cannabidivarin, and the synthetic delta-9-tetrahydrocannabinol formulations nabilone and dronabinol. We categorised these into pharmaceutical grade THC (with or without CBD; labelled here as THC:CBD), pharmaceutical grade CBD, and medicinal cannabis.
Types of study designs:
As per existing reviews examining the efficacy of medicinal cannabinoids for CNCP13 and epilepsy14, we included both experimental and observational study designs, that is, randomised controlled trials (RCTs), non-RCTs, quasi-experimental, before and after studies, prospective and retrospective cohort studies, case control studies, analytical cross-sectional studies, observational studies, self-report, and N-of-1 studies. This approach allows researchers, clinicians, and policymakers to map current research activity and to identify knowledge gaps. For studies with a comparison group, we considered any type of comparator, including placebo, waitlist controls, and other interventions. We excluded reviews of mechanisms of cannabinoid systems, commentary articles, and clinical overviews that did not assess and synthesise individual studies.
Types of outcomes:
To be eligible for inclusion an article had to report on at least one primary outcome, that is, either mental disorder remission or change in mental disorder symptomology (see Table 1 for the full list of outcomes).
Table 1:
Primary and secondary outcomes considered for each of the disorders
| Primary Outcomes | Secondary outcomes | |
|---|---|---|
| Depression | • Remission – absence of a depressive disorder diagnosis using validated scales • Change in depressive symptoms using self-report scales or items |
• Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment |
| Anxiety | • Remission – the absence of an anxiety disorder diagnosis using validated scales • Change in anxiety symptoms using self-report scales or items |
• Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment |
| ADHD | • Change in ADHD symptom-related behaviour using standardised measures – any context • Change in ADHD symptom-related behaviour in the home using standardised measures • Change in ADHD symptom-related behaviour in school using standardised measures |
• Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment • Change in cardiovascular effects • Weight changes |
| Tic/Tourette syndrome | • Change in Tic severity measured using standardised measures | • Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment • Change in cardiovascular effects • Weight changes |
| PTSD | • Remission – the absence of PTSD diagnosis using valid and reliable clinician-rated scales • Change in severity of self-reported traumatic stress symptoms using self-report scales or items |
• Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment • Change in severity of depressive symptoms using a standardised measure • Change in severity of anxiety symptoms using a standardised measure • Change in sleep quality • Change in frequency of nightmares |
| Psychosis | • Whether patients still meet criteria for a diagnosis post-treatment • Change in positive and negative symptoms of psychosis |
• Measures of global functioning – including quality of life, patient or caregiver global impression of change, and satisfaction with treatment • Change in cognitive functioning • Measures of emotional functioning – including depression, anxiety, mood, and social skills |
| All 6 Disorders | • Adverse events (AEs) - all-cause • Serious adverse events (SAEs; as defined by authors) - all-cause • Treatment-related adverse events (TAEs) - all-cause • Study withdrawals - all-cause • Study withdrawals - due to AEs |
Study screening and selection
Two reviewers independently examined titles and abstracts using the web-based systematic review program Covidence15. Relevant articles were obtained in full and assessed for inclusion in the review independently by two authors. Inter-reviewer disagreement was resolved via discussion to reach consensus, with a third reviewer consulted where consensus could not be reached by the two initial reviewers.
Data extraction
Data were extracted by two reviewers using a pre-piloted, standardised data extraction tool in Microsoft Excel. We extracted data on details of the populations; interventions; comparisons; outcomes of significance to the mental disorder (PICO); study methods; cannabinoid dose and route of administration; placement in the therapeutic hierarchy; adverse events and study withdrawals. When data were not reported in full, we contacted authors for additional information. When authors reported multiple analyses (e.g., intention to treat [ITT], available case, or per protocol), we extracted the more conservative with a preference for ITT analyses. We reported AEs according to high-level Medical Dictionary for Regulatory Activities (MedDRA; https://www.meddra.org/) categories. We used Review Manager (RevMan) version 5.316 to perform calculations or transformation on available data to impute missing data (e.g., confidence intervals, number of cases) in order to calculate required outcome data (ORs, SMDs).
Primary and secondary outcomes
Table 1 outlines the primary and secondary outcomes for each condition. We planned to examine remission from the target mental disorder (where appropriate) and changes in symptoms of the target mental disorder as the primary outcomes. Secondary outcomes included changes in distal factors related to the mental disorder, including global functioning, cardiovascular effects, weight, and sleep (see Table 1). All-cause, serious, and treatment-related adverse events, as well as all-cause study withdrawals and study withdrawals due to adverse events were examined as secondary outcomes for all disorders.
Assessment of risk of bias and grading of evidence
For RCTs, risk of bias was assessed using the Cochrane risk of bias tool (see Appendix D for further details of the tool used as well as for risk of bias plots)17, which includes assessment of indicators of selection bias, performance bias, detection bias, attrition bias, and reporting bias. Risk of bias assessments were completed independently by two reviewers. Inter-reviewer disagreement was resolved via discussion to reach consensus, with a third reviewer consulted where consensus could not be reached by the two initial reviewers.
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to rate the quality of the evidence for each outcome18. This was conducted by one reviewer and checked by a second reviewer, with disagreements resolved via discussion with two further reviewers. In this approach, RCT evidence is allocated ‘high quality’ initially, but can be downgraded up to three levels to ‘moderate quality’, ‘low quality’, or ‘very low quality’ due to five categories of limitations. High quality indicates we are confident that the true effect is similar to the estimated effect; very low quality indicates that the true effect is likely to be substantially different to the estimated effect. Limitations considered are (1) risk of bias (i.e., whether limitations in the study design and execution would bias the effect estimate), (2) indirectness of evidence (e.g., if effects of cannabinoids on mental health disorders had to be inferred from indirect evidence amongst those without the disorder), (3) inconsistency of results (i.e., high, unexplained heterogeneity) (4) imprecision (i.e., wide confidence intervals, including potentially covering appreciable benefit and harm), and (5) publication bias (i.e., selective publication of studies leading to a systematic bias in the effect estimate).
Data analysis
All analyses were conducted using Review Manager (RevMan) version 5.316. Meta-analyses included parallel and cross-over RCTs. Continuous and dichotomous outcomes were pooled as standardised mean differences (SMD) and odds ratios (ORs), respectively, using random-effects, generic inverse variance meta-analyses. A common rule of thumb for interpreting SMDs is: 0.2, 0.5, and 0.8 represent small, medium, and large effects, respectively19. Heterogeneity was assessed using the I2 statistic. I2 values of 0-39%, 40-74%, and 75-100% can be considered unimportant, moderate/substantial, and high levels of inconsistency across studies, respectively20.
Analyses were stratified by mental health condition, cannabinoid used (pharmaceutical THC:CBD, pharmaceutical CBD, medicinal cannabis), and comparator used (active, placebo). For each of these, we first pooled the evidence from all eligible RCTs, regardless of population studied. Where applicable (depression and anxiety studies only), we then conducted sensitivity analyses restricted to only those RCTs enrolling participants with the mental health disorder. Where heterogeneity was substantial and sample sizes were sufficient, we conducted exploratory analyses to examine potential reasons for the heterogeneity. Finally, we pooled the evidence across RCTs (regardless of mental health condition) on the incidence of adverse events and withdrawals. Narrative synthesis of results from observational studies was conducted by summarising key results from each study, using the same stratification as for RCTs where possible. For the interested reader, further details on the meta-analytic approach–including methods employed to manage variations in study design and avoid unit-of-analysis errors–are provided in Appendix G.
Role of the funding source
The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report, or in the decision to submit the paper for publication.
Results
Results of searches
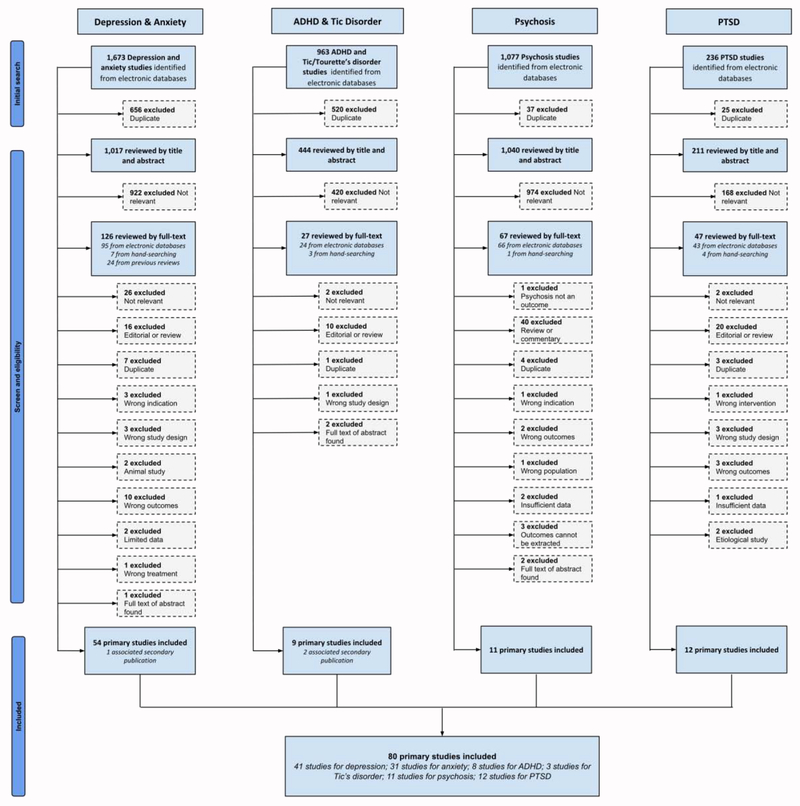
The PRISMA flowchart is shown in Figure 1, and the list of studies excluded at the full-text screening stage is listed in Appendix B. Appendix E shows the number of studies according to study designs of eligible studies for each mental health outcome and the characteristics of each individual included study. After screening, there were 83 eligible studies (40 RCTs): 42 for depression21–62 (22 RCTs), 31 for anxiety21–24,26,27,29,32–34,39,40,42–44,46,48,50,53,58,63–73 (17 RCTs), 8 for Tic/Tourette syndrome6,43,66,70,74–77 (2 RCTs), 3 for ADHD6,75,78 (1 RCT), 12 for PTSD37,71,72,79–87 (1 RCT) and 11 for psychosis88–98 (6 RCTs). Appendix C details ongoing and incomplete trials identified in the clinical trials registry.
Figure 1:
PRISMA flowchart
Description of included RCTs
Table 2 summarises the characteristics of included RCTs. By and large, medicinal cannabinoids were investigated as adjuvant medicines. The RCTs were typically very small (with median sample size between 10-39 across mental health outcomes), with short follow-up periods (median trial length was 4-5 weeks). Across disorders, the majority of RCTs examined pharmaceutical THC (with or without CBD; labelled here as THC:CBD); most commonly, these were nabiximols and nabilone. The exception was RCTs of psychosis, which primarily examined pharmaceutical CBD. Very few RCTs examined medicinal cannabis as the treatment.
Table 2:
Summary of randomised controlled trials (RCTs) of medicinal cannabinoids studies for the treatment of mental health
| Depression | Anxiety | ADHD | Tic/Tourette syndrome | PTSD | Psychosis | |
|---|---|---|---|---|---|---|
| N = 23 | N = 17 | N = 1 | N = 2 | N = 1 | N = 6 | |
| Region | ||||||
| North America | 8 | 6 | 0 | 0 | 1 | 3 |
| Western Europe | 12 | 10 | 1 | 2 | 0 | 1 |
| Other and multiple regions | 3 | 1 | 0 | 0 | 0 | 2 |
| Year of study | ||||||
| 1980-1990 | 0 | 1 | 0 | 0 | 0 | 0 |
| 1991-2000 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2001-2010 | 13 | 9 | 0 | 2 | 0 | 2 |
| 2011-onwards | 10 | 7 | 1 | 0 | 1 | 4 |
| Conflict of interest declared? | ||||||
| Yes – none | 9 | 6 | 0 | 0 | 1 | 2 |
| Yes – potential conflict | 9 | 5 | 0 | 1 | 0 | 3 |
| Not declared | 5 | 6 | 1 | 1 | 0 | 1 |
| Participant characteristics | ||||||
| Total number of participants in RCTs | 2551 | 605 | 30 | 36 | 10 | 281 |
| Median no. participants | 34 | 30 | 30 | 18 | 10 | 39 |
| Median % women | 52.8% | 50.0% | 36.7% | 14.6% | 0% | 34.6% |
| Median age | 49.8 | 47.6 | NR | 33.5 | 44 | 34.7 |
| Primary health condition of study participants | ||||||
| Depression | 0 | 0 | 0 | 0 | 0 | 0 |
| Anxiety disorder | 0 | 3 | 0 | 0 | 0 | 0 |
| Tourette syndrome | 1 | 2 | 0 | 2 | 0 | 0 |
| ADHD | 0 | 0 | 1 | 0 | 0 | 0 |
| PTSD | 0 | 0 | 0 | 0 | 1 | 0 |
| Psychotic disorder | 0 | 0 | 0 | 0 | 0 | 6 |
| Multiple sclerosis | 7 | 2 | 0 | 0 | 0 | 0 |
| Chronic non-cancer pain | 10 | 7 | 0 | 0 | 0 | 0 |
| Parkinson’s disease | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 5 | 3 | 0 | 0 | 0 | 0 |
| Primary indication | ||||||
| Depression | 2 | 1 | 0 | 0 | 0 | 0 |
| Anxiety | 1 | 4 | 0 | 0 | 0 | 2 |
| Analgesia | 14 | 9 | 0 | 0 | 0 | 0 |
| Tic severity | 1 | 2 | 0 | 2 | 0 | 0 |
| Sleep | 2 | 2 | 0 | 0 | 0 | 1 |
| ADHD symptoms | 0 | 0 | 1 | 0 | 0 | 0 |
| PTSD symptoms | 0 | 0 | 0 | 0 | 1 | 0 |
| Spasticity | 5 | 1 | 0 | 0 | 0 | 0 |
| Antipsychotic | 0 | 0 | 0 | 0 | 0 | 4 |
| % cannabinoid naïve (n studies reporting) | 38.5%/10 | 71%/7 | 33.3%/1 | 56.3%/2 | NR/1 | 17.17%/2 |
| Cannabinoid used | ||||||
| Cannabis sativa | 5 | 1 | 0 | 0 | 0 | 0 |
| THC extract | 2 | 3 | 0 | 2 | 0 | 1 |
| Nabiximols | 7 | 3 | 1 | 0 | 0 | 0 |
| THC:CBD extract | 1 | 1 | 0 | 0 | 0 | 0 |
| Cannabidiol (CBD) | 0 | 2 | 0 | 0 | 0 | 5 |
| Dronabinol | 5 | 2 | 0 | 0 | 0 | 0 |
| Nabilone | 3 | 5 | 0 | 0 | 1 | 0 |
| THC-HS | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 |
| Pharmaceutical grade | ||||||
| Yes | 18 | 15 | 1 | 2 | 2 | 5 |
| No | 4 | 1 | 0 | 0 | 0 | 0 |
| Unsure/unknown | 1 | 1 | 0 | 0 | 0 | 1 |
| Route of administration | ||||||
| Vapourised | 2 | 0 | 0 | 0 | 0 | 0 |
| Smoked | 3 | 1 | 0 | 0 | 0 | 0 |
| Oral | 10 | 12 | 0 | 2 | 1 | 3 |
| Oral mucosal spray | 8 | 4 | 1 | 0 | 0 | 0 |
| Mixed routes | 0 | 0 | 0 | 0 | 0 | 0 |
| Not recorded/unclear | 0 | 0 | 0 | 0 | 0 | 2 |
| Intravenous | 0 | 0 | 0 | 0 | 0 | 1 |
| Rectal | 0 | 0 | 0 | 0 | 0 | 0 |
| Median treatment (weeks) | 5 | 4 | 6 | 3.1 | 7 | 3.5 |
| Place in therapeutic hierarchy | ||||||
| Primary | 0 | 3 | 1 | 0 | 0 | 1 |
| Adjuvant | 20 | 12 | 0 | 2 | 1 | 5 |
| Not reported, unclear | 3 | 2 | 0 | 0 | 0 | 0 |
Note: THC – Δ-9 tetrahydrocannabinol. CBD – cannabidiol.
In most of the RCTs for depression and anxiety, the primary indication for the cannabinoid was some other medical condition, with chronic non-cancer pain (CNCP), followed by multiple sclerosis, being the most common primary conditions. In studies of other mental health conditions, the mental health outcome was the primary indication for the cannabinoid.
Risk of bias of included studies
A summary of the risk of bias of included studies is provided in Appendix D. Briefly, most RCTs reported adequate randomisation sequence generation and concealment; however, the majority were of unclear or high risk of bias for blinding of participants, personnel and outcome assessors. Most studies had other potential, albeit unclear, sources of bias, such as use of post-hoc analyses and unclear adjustment for cross-over trials.
RCT evidence on the effects of medicinal cannabinoids on symptoms of mental disorders, adverse events, and withdrawals
Results of all meta-analyses of RCTs of cannabinoids for the treatment of mental health are described below and reported in full in Tables 3 (pharmaceutical THC:CBD), Table 4 (pharmaceutical CBD), and Table 5 (medicinal cannabis). Adverse events and withdrawals for each of THC:CBD, CBD, and medicinal cannabis are described below and reported in full in Table 6. Forest plots for primary outcomes are displayed in Appendix F.
Table 3:
Summary of RCT evidence on the use of pharmaceutical THC (THC alone, or THC:CBD preparations)1 for the treatment of mental health
| Disorder | Outcome | Comparator | Studies (participants) | Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pooled SMD [95% CI]a | I2 | Favours | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in depressive symptoms† | Active | 1 (52) | Not serious | Very serious | Serious | Serious | Undetected | 0.00 [−0.17, 0.17] | NA | Neither | Very low | |
| Change in depressive symptoms† | Placebo | 12 (1656) | Not serious | Very serious | Serious | Not serious | Likely | −0.05 [−0.20, 0.11] | 67% | Neither | Very low | |
| Change in global functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Anxiety | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in anxiety symptoms† | Active | 1 (52) | Not serious | Very serious | Serious | Serious | Undetected | −0.12 [−0.30, 0.05] | NA | Neither | Very low | |
| Change in anxiety symptoms† | Placebo | 7 (252) | Serious | Serious | Serious | Serious | Likely | −0.25 [−0.49, −0.01] | 65% | THC:CBD | Very low | |
| Change in global functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| ADHD | Change in ADHD symptoms - any location† | Placebo | 1 (30) | Not serious | Not serious | Serious | Serious | Undetected | −0.67 [−1.41, 0.07] | NA | Neither | Low |
| Change in ADHD symptoms - home | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in ADHD symptoms - school | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in global functioning | Placebo | 1 (30) | Not serious | Not serious | Serious | Serious | Undetected | 0.00 [−0.72, 0.72] | NA | Neither | Low | |
| Cardiovascular effects | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Weight change | Placebo | 1 (30) | Not serious | Not serious | Serious | Serious | Undetected | 0.14 [−0.58, 0.85] | NA | Neither | Low | |
| Tic/Tourette syndrome | Change in Tic/Tourette symptoms† | Placebo | 2 (41) | Not serious | Not serious | Serious | Serious | Undetected | −0.46 [−1.32, 0.40] | 68% | Neither | Low |
| Change in global functioning | Placebo | 2 (41) | Not serious | Not serious | Serious | Very serious | Undetected | −0.84 [−2.10, 0.42] | 68% | Neither | Very low | |
| Cardiovascular effects | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Weight change | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| PTSD | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in PTSD symptoms | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | |||
| Change in global functioning | Placebo | 1 (19) | Not serious | Not serious | Serious | Serious | Undetected | −1.13 [−1.48, −0.77] | NA | THC:CBD | Low | |
| Change in depressive symptoms | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in anxiety symptoms | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in sleep quality | Placebo | 1 (19) | Not serious | Not serious | Serious | Serious | Undetected | −0.10 [−0.38, 0.18] | NA | Neither | Low | |
| Change in nightmare frequency | Placebo | 1 (19) | Not serious | Not serious | Serious | Serious | Undetected | -1.11 [−1.46, −0.76] | NA | THC:CBD | Low | |
| Psychosis | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in total symptoms | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in positive symptoms† | Placebo | 1 (24) | Not serious | Not serious | Serious | Serious | Undetected | −0.20 [−0.45, 0.06] | NA | Neither | Low | |
| Change in negative symptoms† | Placebo | 1 (24) | Not serious | Not serious | Serious | Serious | Undetected | 0.36 [0.10, 0.62] | NA | Placebo | Low | |
| Change in global functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in cognitive function | Placebo | 1 (24) | Not serious | Not serious | Serious | Serious | Undetected | 1.08 [0.71, 1.45] | NA | Placebo | Low | |
| Change in emotional functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Note:
THC:CBD refers to pharmaceutical THC + CBD combinations such as nabiximols. In all comparisons the control group (placebo/active) is the reference group.
indicates outcomes for which forest plots are available in Appendix F.
White cells are primary outcomes and shaded cells are secondary outcomes. NA = not applicable.
Table 4:
Summary of RCT evidence on the use of pharmaceutical cannabidiol (CBD) for the treatment of mental health
| Disorder | Outcome | Comparator | Studies (participants) | Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pooled SMD [95% CI]a | I2 | Favours | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Anxiety | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in anxiety symptoms† | Placebo | 2 (44) | Not serious | Not serious | Serious | Very serious | Undetected | −0.87 [−2.01, 0.27] | 85% | Neither | Very low | |
| Change in global functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| ADHD | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tic/Tourette syndrome | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| PTSD | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Psychosis | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Change in total symptoms† | Active | 1 (39) | Not serious | Not serious | Serious | Serious | Undetected | −0.02 [−0.65, 0.60] | NA | Neither | Low | |
| Change in total symptoms† | Placebo | 2 (122) | Not serious | Not serious | Serious | Serious | Undetected | 0.05 [−0.50, 0.61] | 52% | Neither | Low | |
| Change in positive symptoms† | Active | 1 (39) | Not serious | Not serious | Serious | Serious | Undetected | −0.10 [−0.73, 0.53] | NA | Neither | Low | |
| Change in positive symptoms† | Placebo | 2 (122) | Not serious | Not serious | Serious | Serious | Undetected | −0.17 [−0.69, 0.35] | 47% | Neither | Low | |
| Change in negative symptoms† | Active | 1 (39) | Not serious | Not serious | Serious | Serious | Undetected | −0.48 [−1.12, 0.16] | NA | Neither | Low | |
| Change in negative symptoms† | Placebo | 2 (122) | Not serious | Not serious | Not serious | Serious | Undetected | 0.08 [−0.27, 0.44] | 0% | Neither | Moderate | |
| Change in global functioning | Placebo | 1 (86) | Not serious | Not serious | Serious | Serious | Undetected | −0.62 [−1.14, −0.09] | NA | CBD | Low | |
| Change in cognitive function | Placebo | 3 (150) | Not serious | Not serious | Not serious | Serious | Undetected | −0.01 [−0.33, 0.32] | 0% | Neither | Moderate | |
| Change in emotional functioning | Active | 1 (39) | Not serious | Not serious | Serious | Serious | Undetected | 0.27 [−0.36, 0.90] | NA | Neither | Low | |
| Change in emotional functioning | Placebo | 2 (122) | Not serious | Not serious | Serious | Serious | Likely | 0.10 [−0.49, 0.69] | 57% | Neither | Very low |
Note:
indicates outcomes for which forest plots are available in Appendix F.
White cells are primary outcomes and shaded cells are secondary outcomes. NA = not applicable; CBD = cannabidiol. In all comparisons the control group (placebo/active) is the reference group.
Table 5:
Summary of RCT evidence on the use of medicinal cannabis for the treatment of mental health
| Disorder | Outcome | Comparator | Studies (participants) | Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias |
Pooled SMD [95% CI]a | I2 | Favours | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Remission from disorder | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Change in depressive symptoms† | Placebo | 1 (42) | Not serious | Very serious | Serious | Serious | Likely | −0.14 [−0.33, 0.05] | NA | Neither | Very low | |
| Change in global functioning | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | ||
| Anxiety | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | |
| ADHD | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Tic/Tourette syndrome | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | |
| PTSD | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Psychosis | -- | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- |
Note:
indicates outcomes for which forest plots are available in Appendix F.
White cells are primary outcomes and shaded cells are secondary outcomes. NA = not applicable. In all comparisons the control group (placebo/active) is the reference group.
Table 6:
Summary of RCT evidence on the impact of medicinal cannabinoids on adverse events and withdrawals
| Treatment | Outcome | Comparator | Studies (participants) | Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pooled OR [95% CI] | I2 | More AE/Withdrawals occurred in… | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC:CBD | Adverse events | |||||||||||
| AEs – all cause† | Active | 1 (60) | Not serious | Serious | Serious | Very serious | Undetected | 1.59 [0.57, 4.45] | NA | Neither | Very low | |
| AEs – all cause† | Placebo | 10 (1495) | Not serious | Serious | Serious | Not serious | Undetected | 1.99 [1.20, 3.29] | 59% | THC:CBD | Low | |
| SAEs – all cause | Placebo | 4 (954) | Not serious | Serious | Not serious | Serious | Undetected | 1.29 [0.94, 1.77] | 0% | Neither | Low | |
| TAEs – all cause | Placebo | 2 (385) | Not serious | Serious | Not serious | Serious | Undetected | 1.32 [0.79, 2.20] | 0% | Neither | Low | |
| Withdrawals | ||||||||||||
| Withdrawals – all cause | Placebo | 15 (2299) | Not serious | Serious | Not serious | Serious | Likely | 1.51 [0.96, 2.36] | 42% | Neither | Very low | |
| Withdrawals – due to AEs† | Active | 2 (252) | Not serious | Serious | Not serious | Serious | Undetected | 0.54 [0.17, 1.68] | 0% | Neither | Low | |
| Withdrawals – due to AEs† | Placebo | 11 (1621) | Not serious | Serious | Not serious | Not serious | Undetected | 2.78 [1.59, 4.86] | 22% | THC:CBD | Moderate | |
| CBD | Adverse events | |||||||||||
| AEs – all cause† | Placebo | 1 (88) | Not serious | Not serious | Serious | Serious | Undetected | 0.97 [0.40, 2.33] | NA | Neither | Low | |
| SAEs – all cause | Placebo | 1 (88) | Not serious | Not serious | Serious | Very serious | Undetected | 0.34 [0.01, 8.60] | NA | Neither | Very low | |
| TAEs – all cause | Placebo | 1 (88) | Not serious | Not serious | Serious | Serious | Undetected | 1.06 [0.39, 2.87] | NA | Neither | Low | |
| Withdrawals | ||||||||||||
| Withdrawals – all cause | Active | 1 (42) | Not serious | Not serious | Serious | Very serious | Undetected | 3.33 [0.32, 34.99] | NA | Neither | Very low | |
| Withdrawals – all cause | Placebo | 1 (88) | Not serious | Not serious | Serious | Very serious | Undetected | 1.61 [0.26, 10.16] | NA | Neither | Very low | |
| Withdrawals – due to AEs† | Placebo | 1 (88) | Not serious | Not serious | Serious | Very serious | Undetected | 1.05 [0.06, 17.30] | NA | Neither | Very low | |
| Cannabis | Adverse events | |||||||||||
| AEs – all cause | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| SAEs – all cause | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| TAEs – all cause | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Withdrawals | ||||||||||||
| Withdrawals – all cause | Placebo | 3 (209) | Serious | Serious | Not serious | Very serious | Undetected | 1.41 [0.51, 3.88] | 7% | Neither | Very low | |
| Withdrawals – due to AEs | -- | 0 (0) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Note:
indicates outcomes for which forest plots are available in Appendix F.
NA = not applicable. THC = Δ-9 tetrahydrocannabinol. THC:CBD includes pharmaceutical THC alone and pharmaceutical THC + CBD combinations. CBD = pharmaceutical cannabidiol. In all comparisons the control group (placebo/active) is the reference group.
Depression
Pharmaceutical THC:CBD did not significantly improve symptoms of depression, compared to either active comparators48 or placebo comparators22,25,31,39,42,43,49,50,53,55,59,61 (Table 3). The evidence GRADE was very low, in part due to indirectness because none of the included RCTs included participants with a primary diagnosis of depression; most included participants with multiple sclerosis. Following reviewer suggestion, we conducted an exploratory analysis to examine whether length of follow up contributed to the substantial heterogeneity seen (67%). One study43 administered pharmaceutical THC:CBD and assessed participants on a single day, whereas the remaining used longer treatment and follow-up periods (2-15 weeks). Removing the single shorter study made minimal difference to the effect size and heterogeneity (SMD=−0.05, 95%CI −0.22:0.13; k=11, n=1632; I2=70%).
No RCTs examining CBD for depression outcomes were identified. A single, small RCT examining medicinal cannabis for depression outcomes amongst participants with CNCP found no change in depressive symptoms compared to placebo (Table 5)57.
Anxiety
Pharmaceutical THC:CBD led to significantly greater reductions in anxiety symptoms than did placebos (SMD=−0.25, 95%CI −0.49:−0.01; k=7, n=252; I2=65%)22,39,42,43,50,53,73, with no difference seen in the single, small study that used an active comparator (Table 3)48. The evidence GRADE was very low, in part because none of the studies included participants with a primary diagnosis of anxiety; most included participants with CNCP or multiple sclerosis. Reporting bias also contributed to the very low GRADE rating; outcomes of three RCTs could not be included in this synthesis due to incomplete data reporting44,65,67. One showed a beneficial effect of pharmaceutical THC:CBD over placebo, whereas the other two showed no significant difference. Given the confidence intervals of the effect are close to zero (−0.49:−0.01), had it been possible to include these studies it is likely that the benefit of pharmaceutical THC:CBD over placebo would no longer be significant.
We conducted an exploratory analysis to check whether varying lengths of follow up contributed to the substantial heterogeneity seen in the pharmaceutical THC:CBD versus placebo comparison (65%). One study43 administered pharmaceutical THC:CBD and assessed participants on a single day, whereas the remaining used longer treatment and follow-up periods (3-12 weeks). Removing the single shorter study reduced the heterogeneity to an unimportant level and the beneficial effect of pharmaceutical THC:CBD remained significant (SMD=−0.34, 95%CI −0.53:−0.14; k=6, n=228; I2=36%).
Two studies examined the effect of CBD – both in participants with social anxiety – and did not find a significant improvement in anxiety symptoms compared to placebo (Table 4)63,64. No RCTs examined the impact of medicinal cannabis on anxiety outcomes.
ADHD
The single, small identified RCT for ADHD compared pharmaceutical THC:CBD with placebo amongst participants with ADHD78. No significant effect was seen on the primary outcome, ADHD symptoms (Table 3). Of the secondary outcomes, the study also demonstrated no significant effect of pharmaceutical THC:CBD versus placebo on global functioning or weight change. No studies examined the impact of CBD or medicinal cannabis on ADHD outcomes.
Tic/Tourette syndrome
The two identified, small RCTs for Tic/Tourette syndrome compared pharmaceutical THC:CBD with placebo amongst participants with Tic/Tourette syndrome43,70. The pooled effect from these two, small studies demonstrated no significant benefit of pharmaceutical THC:CBD compared to placebo on Tic/Tourette symptoms (Table 3). Similarly, no significant effect was seen on the secondary outcome, global functioning. No studies examined the impact of CBD or medicinal cannabis on Tic/Tourette syndrome outcomes.
PTSD
A single, small RCT with participants with PTSD was identified; this RCT did not report either of our primary outcomes82. Of the secondary outcomes, this study found a significant benefit of pharmaceutical THC:CBD compared to placebo in improving global functioning and nightmare frequency, and no significant effect on sleep quality (Table 3). No studies examined the impact of CBD or medicinal cannabis on PTSD outcomes.
Psychosis
A single, small RCT reported on the use of pharmaceutical THC:CBD amongst participants with psychosis90. This study found no significant change in positive symptoms (Table 3) but a worsening of negative symptoms (SMD=0.36, 95%CI 0.10:0.62; n=24), compared to placebo. Of the secondary outcomes, this study also found that pharmaceutical THC:CBD worsened cognitive functioning (SMD=1.08, 95%CI 0.71:1.45; n=24).
The remaining included psychosis RCTs examined CBD. Across the 1-2 studies that reported on primary outcomes, CBD did not significantly improve total symptoms, positive symptoms, or negative symptoms, compared to placebo89,96 or active94 comparators (Table 4). Of the secondary outcomes, CBD led to an improvement in global functioning compared to placebo in the single study reporting this outcome (SMD=−0.62, 95%CI −1.14:−0.09; n=86)96, but did not significantly improve cognitive or emotional functioning89,92,94,96.
No studies examined the impact of medicinal cannabis on psychosis outcomes.
Adverse events and withdrawals
We pooled adverse events and study withdrawals from all RCTs (Table 6). Pharmaceutical THC:CBD led to significantly more adverse events (OR=1.99, 95%CI 1.20:3.29; k=10, n=1495; I2=59%) and withdrawals due to adverse events (OR=2.78, 95%CI 1.59:4.86; k=11, n=1621; I2=22%) than did placebos. The evidence GRADE was low to moderate, due to inconsistency and indirectness (i.e., participants in most of the analysed studies did not have a mental disorder). It is estimated that one additional participant would experience an adverse event for every 7 (95%CI 5:25) participants treated with pharmaceutical THC:CBD (number needed to treat to harm). Further, one additional participant would withdraw due to an adverse event for every 14 (95%CI 7:39) participants treated with pharmaceutical THC:CBD. No significant differences between pharmaceutical THC:CBD and comparators were seen on serious adverse events, treatment-related adverse events, or all-cause withdrawals.
Very few RCTs examined adverse events and withdrawals due to CBD or medicinal cannabis, and these found no significant increases compared to active and placebo comparators (Table 6).
Observational evidence on the effects of medicinal cannabinoids on symptoms of mental disorders
The findings of all included observational studies are detailed in Appendix E. Here we summarise the findings of studies in which mental health was the primary indication in open-label or prospective cohorts. There were no open-label or prospective cohort studies in which depression was the primary outcome; there were 10 observational studies where depression was a secondary outcome in CNCP or multiple sclerosis patients in open-label (k=7) and prospective cohort studies (k=3). There were eight open-label and prospective cohort studies that reported on anxiety outcomes. Anxiety was a primary outcome in only one study of n=567, which found that nabilone significantly reduced anxiety. There were no open-label or observational studies for ADHD or Tic/Tourette syndrome. There were two open-label and two prospective cohort studies where PTSD was the primary outcome; three studies involved cannabis and one, THC extract. Three studies found reductions in PTSD symptoms83,85,86 and one found that PTSD symptoms worsened with cannabis use in people with PTSD and comorbid mental health disorder87. There was one open-label study where psychosis was the primary outcome, which found that CBD reduced psychosis symptoms97.
Discussion
To our knowledge this is the most comprehensive systematic review examining the available evidence for medicinal cannabinoids in treating mental disorders and symptoms. There is a notable lack of high-quality evidence where mental disorders are the primary target of treatment, and most evidence is derived from studies where mental disorders are secondary to another medical condition, commonly CNCP and multiple sclerosis. Most of the included studies were conducted among persons where depression or anxiety was secondary to another medical condition, and of these we found no impact of pharmaceutical THC (with or without CBD; THC:CBD) on depression symptoms, and a small reduction in anxiety symptoms. Of the few studies in which participants had an anxiety disorder, we did not see a significant benefit of CBD on symptoms of anxiety. Single studies found that pharmaceutical THC:CBD improved global functioning in PTSD and pharmaceutical CBD improved global functioning in psychosis. Across the small numbers of included studies, we did not find evidence that any type of cannabinoid significantly improves primary outcomes of ADHD, Tic/Tourette syndrome, PTSD, or psychosis. In fact, we found evidence that pharmaceutical THC:CBD worsened negative symptoms of psychosis.
Cannabinoids are often advocated for as a treatment of various mental health conditions. It is likely that countries that allow medicinal cannabinoid use will see increased demand for such use. Clinicians and consumers need to be aware of the limited quality and quantity of evidence on the effectiveness and the potential risk for adverse events. Most studies are based on pharmaceutical cannabinoids, rather than medicinal cannabis, but plant products are most often used by those using cannabinoids for medicinal purposes in the USA8. Although there are 16 trials underway to examine the effectiveness of pharmaceutical CBD for specific conditions, including seven in psychosis, to date there are very few or no clinical studies examining the effectiveness of CBD for depression, anxiety, Tic/Tourette syndrome or ADHD (see Appendix C).
The risk of adverse outcomes among those using medicinal cannabis products is indicated by a large body of research on the adverse effects of non-medical cannabis use. This suggests that cannabis use can increase the occurrence of depression, anxiety, and psychotic symptoms11,99–103. The evidence of cannabis’ risks is not derived solely from observational studies of people using cannabis non-medically. For example, there is experimental evidence, using a double-blind, randomised, placebo-controlled and crossover design, of the acute effects of smoked cannabis (containing 13% THC) on psychosis symptoms, which found that cannabis increased risk of acute psychotic symptoms104. Additionally, young adults (the age group at greatest risks of depression, anxiety, and psychosis) who use cannabis daily over extended periods are at risk of developing dependence upon cannabis99. These risks, and the limitations of existing evidence, need to be weighed when considering using medicinal cannabinoids to treat symptoms of common mental disorders. Those who decide to proceed should be carefully monitored for positive and negative mental health effects of using medicinal cannabinoids.
Limitations and future directions
The strengths of our review included our comprehensive search strategies (including clinical trials registries); consideration of the full range and potential distinct effects of different types of cannabinoids; and range of outcomes considered. Compared to previous reviews we identified more studies (e.g., for psychosis we identified six RCTs vs. two in a previous review9). Nonetheless our analyses and conclusions are necessarily limited by the small amount of available data, small study sizes, and heterogeneity of findings across studies. Small study sizes are of particular concern as it has previously been identified that effects are larger in small studies of medicinal cannabinoids for CNCP13. It is also important to consider that a number of independent analyses were conducted and hence may not retain significance if adjustment for multiple comparisons is made. However, there is no recommended approach for addressing multiplicity in systematic reviews, and we attempted to minimise this by: choosing few primary outcomes, keeping subgroups to a minimum, and testing effects at a single time-point only105,106. There have been few RCTs, typically of very small size, conducted to date, so the lack of significant effects for ADHD, Tic/Tourette syndrome could well reflect the limited evidence base. Studies of medicinal cannabinoids primarily for people diagnosed with depression and anxiety are lacking. It is possible that the reductions in anxiety symptoms identified in this review may have been due to improvements in the primary medical condition (CNCP or multiple sclerosis). It is crucial that future research focuses on the effectiveness of cannabinoids in patients diagnosed with primary depression and anxiety.
Conclusions
There is increasing use of pharmaceutical cannabinoids and medicinal cannabis to treat symptoms of mental disorders. This is the most comprehensive review of the evidence to date, including both randomised controlled trials and observational studies of depression, anxiety, attention-deficit hyperactivity disorder, Tic/Tourette syndrome, post-traumatic stress disorder, and psychosis. It found very little evidence on the effectiveness of pharmaceutical CBD or medicinal cannabis for the treatment of any of these mental disorders. There was some very-low-quality evidence on the use of pharmaceutical THC (with or without CBD) in treating anxiety symptoms amongst those with other medical conditions, such as chronic non-cancer pain and multiple sclerosis. We need high-quality, randomised controlled trials to properly assess the effectiveness and safety of medicinal cannabinoids, compared to placebo and standard treatments, for the treatment of mental disorders. This evidence is essential before clinical guidelines can be provided on the medicinal use of cannabinoids for these disorders. In light of the paucity of evidence and the lack of good quality evidence, and the known risk of cannabinoids, the use of cannabinoids as treatments for mental health disorders cannot be justified at this time.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed up to 12th July 2019 for reviews of cannabis use and mental health using the Medical Subject Headings (MeSH) terms (((“medical marijuana”[MeSH Terms] OR (“medical”[All Fields] AND “marijuana”[All Fields]) OR “medical marijuana”[All Fields] OR (“medical”[All Fields] AND “cannabis”[All Fields]) OR “medical cannabis”[All Fields]) AND (“mental health”[MeSH Terms] OR (“mental”[All Fields] AND “health”[All Fields]) OR “mental health”[All Fields])) AND Review[ptyp]); this led to 152 results, of which 9 were relevant reviews (or summaries of reviews, as in the case of the US National Academies of Science (NAS)) of cannabis or cannabinoids for mental health problems.
The different reviews included varied study designs to examine the impacts of cannabinoids on mental disorders; some concentrated on cross-sectional studies, others were limited to randomised controlled trials (RCTs), and some further limited this to only studies where the mental health symptoms were the primary indication for the cannabinoid. Some reviews pooled studies quantitatively on one outcome for a given mental health condition (Whiting), but other features of their eligibility criteria and date of the publication meant that there were very few studies included (e.g., zero for depression, one for anxiety, two for psychosis). All reviews agreed that the evidence was limited but in many instances some concluded that no data yet existed for some mental health outcomes (e.g., depression).
No previous reviews defined a priori both primary and secondary outcomes of cannabinoids used for different mental health symptoms, nor systematically compiled both RCT and observational study designs. Most described potential adverse outcomes of cannabinoid use by relying on evidence from studies of people with recreational cannabis use or generally pooling adverse events from any study of medicinal cannabinoids, rather than specifically extracting and pooling data on adverse events and treatment withdrawals. Reviews varied in the clarity with which the specific cannabinoids were documented and the characteristics of the study populations and the studies themselves were extracted and reported.
Added value of this study
Our study represents the most up to date and detailed review of evidence for cannabinoids for mental health symptoms. It pre-specified primary and secondary outcomes to examine for each mental health condition, we included studies where the condition was primary or secondary, we systematically collated non-RCT evidence, and we pooled all outcomes and adverse event data quantitatively wherever possible. We also made clear which cannabinoids were studied, where the data and gaps were across primary and secondary outcomes.
We concluded that there is very-low-quality evidence for the effectiveness of cannabinoids in improving symptoms of anxiety. There is a lack of evidence to suggest that cannabinoids improve depressive disorders, symptoms of depression, anxiety disorders, attention-deficit hyperactivity disorder, Tic/Tourette syndrome, post-traumatic stress disorder, or psychosis.
Implications of all the available evidence
Our findings have direct policy relevance. In countries where cannabis and cannabinoids are being made available for medicinal use, and in which mental health problems are a common reason for requesting access to cannabinoids for medicinal purposes, this review makes clear where the evidence exists and the quality of such evidence. This review also makes clear a real need for investment of high-quality research efforts to study the impact of different cannabinoids upon a range of outcomes for people with mental health disorders.
Acknowledgements
The authors would like to acknowledge Mary Kumvaj who assisted in the development of the search strategy; and Harriet Townsend, Alana Garton, Rakin Rahman, and Megan Weier who assisted with searches and screening.
Funding
Funding was received from the Health Products Regulation Group, Commonwealth Department of Health. ES, GC, and LD are supported by NHMRC research fellowships (#1104600, #1119992 and #1135991). LD is also supported by NIH grant NIDA R01DA1104470. The National Drug and Alcohol Research Centre is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
MF and LD have been investigators on untied investigator-driven educational grants funded by Reckitt Benckiser, Mundipharma and Seqirus. MF, GC, and LD have been investigators on untied investigator-driven educational grants funded by Indivior.
References
- 1.Häuser W, Finn DP, Kalso E, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. 2018; 22(9): 1547–64. [DOI] [PubMed] [Google Scholar]
- 2.Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: A survey of authorized medical cannabis patients. International Journal of Drug Policy 2017; 42: 30–5. [DOI] [PubMed] [Google Scholar]
- 3.Witkin JM, Tzavara ET, Davis RJ, Li X, Nomikos GG. A therapeutic role for cannabinoid CB 1 receptor antagonists in major depressive disorders. Trends in pharmacological sciences 2005; 26(12): 609–17. [DOI] [PubMed] [Google Scholar]
- 4.Trezza V, Campolongo P. The endocannabinoid system as a possible target to treat both the cognitive and emotional features of post-traumatic stress disorder (PTSD). Frontiers in behavioral neuroscience 2013; 7: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacology 2011; 36: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller-Vahl K, Kolbe H, Schneider U, Emrich H. Cannabinoids: possible role in pathophysiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatrica Scandinavica 1998; 98(6): 502–6. [DOI] [PubMed] [Google Scholar]
- 7.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. The American Journal of Drug and Alcohol Abuse 2014; 40(1): 23–30. [DOI] [PubMed] [Google Scholar]
- 8.Sarvet AL, Wall MM, Keyes KM, Olfson M, Cerdá M, Hasin DS. Self-medication of mood and anxiety disorders with marijuana: Higher in states with medical marijuana laws. Drug and Alcohol Dependence 2018; 186: 10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Jama 2015; 313(24): 2456–73. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. The Journal of clinical psychiatry 2016; 77(8): 1050–64. [DOI] [PubMed] [Google Scholar]
- 11.National Academies of Sciences Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 12.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current opinion in psychiatry 2007; 20(4): 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018; 159(10): 1932–54. [DOI] [PubMed] [Google Scholar]
- 14.Stockings E, Zagic D, Campbell G, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry 2018; 89(7): 741–53. [DOI] [PubMed] [Google Scholar]
- 15.Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Available at www.covidence.org. [Google Scholar]
- 16.Cochrane T Review Manager (RevMan) 5.3. Copenhagen: The Nordic Cochrane Centre 2008. [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schunemann H, Higgins JPT, Vist GE, et al. Chapter 14: Completing “Summary of findings” tables and grading the certainty of the evidence. Draft version In: Higgins J, ed. Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane; 2019. [Google Scholar]
- 19.Cohen J Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 20.Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking metaanalyses In: Higgins J, Churchill R, Chandler J, Cumpston M, eds. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane; 2017. [Google Scholar]
- 21.DI Abrams, CA Jay, SB Shade et al. Cannabis in painful HIV-associated sensory neuropathy. Neurology 2007; 68: 515–21. [DOI] [PubMed] [Google Scholar]
- 22.Aragona M, Onesti E, Tomassini V, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple clerosis: A double-blind, placebo controlled, crossover study. Clinical Neuropharmacology 2009; 32(1): 41–7. [DOI] [PubMed] [Google Scholar]
- 23.Attal N, Brasseur L, Guirimand D, Clermond-Gnamien S, Atlami S, Bouhassira D. Are oral cannabinoids safe and effective in refractory neuropathic pain? European Journal of Pain 2004; 8(2): 173–7. [DOI] [PubMed] [Google Scholar]
- 24.Bahorik AL, Leibowitz A, Sterling SA, Travis A, Weisner C, Satre DD. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. Journal of Affective Disorders 2017; 213: 168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball S, Vickery J, Hobart J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: A randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health technology assessment 2015; 19(12): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellnier T, Brown G, Ortega T, Insull R. A preliminary evaluation of the effcacy, safety, and costs associated with the treatment of chronic pain with medical marijuana in the elderly. Consultant Pharmacist 2017; 32(10): 597. [Google Scholar]
- 27.Bestard JA, Toth CC. An open-label comparison of nabilone and gabapentin as adjuvant therapy or monotherapy in the management of neuropathic pain in patients with peripheral neuropathy. Pain Practice 2011; 11(4): 353–68. [DOI] [PubMed] [Google Scholar]
- 28.Blaas K Treating depression with cannabinoids. Cannabinoids 2008; 3(2): 8–10. [Google Scholar]
- 29.Clermont-Gnamien S, Atlani S, Attal N, Le FM, Guirimand F, Brasseur L. The therapeutic use of D9-tetrahydrocannabinol (dronabinol) in refractory neuropathic pain. Presse Medicale 2002; 31:1840–5. [PubMed] [Google Scholar]
- 30.Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3): 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairhurst C The efficacy, safety and tolerability of Sativex as an adjunctive treatment to existing anti-spasticity medications in children aged 8 to 18 years with spasticity due to cerebral palsy or traumatic central nervous system injury who have not responded adequately to their existing anti-spasticity medications: A parallel group randomised, double-blind, placebo-controlled study followed by a 24-week open label extension phase. 2018. [Google Scholar]
- 32.Frank B, Serpell M, Hughes J, Matthews J, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. Bmj 2008; 336(7637): 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerardi MC, Batticciotto A, Talotta R, Ditto MC, Atzeni F, Sarzi-Puttini P. Efficacy of cannabis flos in patients with fibromyalgia: A monocentric observational study. Arthritis and Rheumatology 2016; 68: 72–4. [Google Scholar]
- 34.Gruber AJ, Pope HG, Brown ME. Do patients use marijuana as an antidepressant. Depression 1996; 4: 77–80. [DOI] [PubMed] [Google Scholar]
- 35.Hagenbach U, Luz S, Ghafoor N, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007; 45(8): 551–62. [DOI] [PubMed] [Google Scholar]
- 36.Haroutiunian S, Rosen G, Shouval R, Davidson E. Open-Label, Add-on Study of Tetrahydrocannabinol for Chronic Nonmalignant Pain. Journal of Pain & Palliative Care Pharmacotherapy 2009; 22(3): 213–7. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using Veterans with probable PTSD. J Affect Disord 2016; 190: 439–42. [DOI] [PubMed] [Google Scholar]
- 38.Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion 2012; 85(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 39.Malik Z, Bayman L, Valestin J, Rizvi-Toner A, Hashmi S, Schey R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Diseases of the Esophagus 2017; 30(12455): 1–8. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Rodriguez JE, Munteis E, Carreno M, et al. Cannabis use in Spanish patients with multiple sclerosis: fulfilment of patients’ expectations? J Neurol Sci 2008; 273(1-2): 103–7. [DOI] [PubMed] [Google Scholar]
- 41.Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. European Archives of Psychiatry and Clinical Neuroscience 1990; 240:1–4. [DOI] [PubMed] [Google Scholar]
- 42.Moreno JLLS, Caldentey JG, Cubillo PT, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J Neurol 2016; 263(7): 1390–400. [DOI] [PubMed] [Google Scholar]
- 43.Müller-Vahl KR, Koblenz A, Jobges M, Kolbe H, Emrich H, Schneider U. Influence of treatment of Tourette Syndrome with delta9-tetrahydrocannabinol (delta9-THC) on neuropsychological performance. Pharmacopsychiatry 2001; 34(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 44.Narang S, Gibson D, Wasan AD, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. The Journal of Pain 2008; 9(3): 254–64. [DOI] [PubMed] [Google Scholar]
- 45.Neff GW, O’Brien CB, Reddy KR, et al. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. The American Journal of Gastroenterology 2002; 97(8): 2117–9. [DOI] [PubMed] [Google Scholar]
- 46.Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’studies. Anaesthesia 2004; 59(5): 440–52. [DOI] [PubMed] [Google Scholar]
- 47.Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. European Journal of Neurology 2011; 18(9): 1122–31. [DOI] [PubMed] [Google Scholar]
- 48.Pini LA, Guerzoni S, Cainazzo MM, et al. Nabilone for the treatment of medication overuse headache: Results of a preliminary double-blind, active-controlled, randomized trial. The Journal of Headache and Pain 2012; 13(8): 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. The Journal of Pain 2012; 13(5): 438–49. [DOI] [PubMed] [Google Scholar]
- 50.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005; 65(6): 812–9. [DOI] [PubMed] [Google Scholar]
- 51.Rudich Z, Stinson J, Jeavons M, Brown SC. Treatment of chronic intractable neuropathic pain with dronabinol: Case report of two adolescents. Pain Research and Management 2003; 8(4): 221–4. [DOI] [PubMed] [Google Scholar]
- 52.Shah A, Craner J, Cunningham JL. Medical cannabis use among patients with chronic pain in an interdisciplinary pain rehabilitation program: Characterization and treatment outcomes. J Subst Abuse Treat 2017; 77: 95–100. [DOI] [PubMed] [Google Scholar]
- 53.Toth C, Mawani S, Brady S, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 2012; 153(10): 2073–82. [DOI] [PubMed] [Google Scholar]
- 54.van Amerongen G, Kanhai K, Baakman AC, et al. Effects on Spasticity and Neuropathic Pain of an Oral Formulation of Δ9-Tetrahydrocannabinol in Patients With Progressive Multiple Sclerosis. Clinical Therapeutics 2017. [DOI] [PubMed] [Google Scholar]
- 55.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Multiple Sclerosis Journal 2004; 10(4): 434–41. [DOI] [PubMed] [Google Scholar]
- 56.Ware MA, Wang T, Shapiro S, Collet JP, team Cs. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain 2015; 16(12): 1233–42. [DOI] [PubMed] [Google Scholar]
- 57.Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. Canadian Medical Association Journal 2010; 182(14): E694–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber J, Schley M, Casutt M, et al. Tetrahydrocannabinol (Delta 9-THC) Treatment in Chronic Central Neuropathic Pain and Fibromyalgia Patients: Results of a Multicenter Survey. Anesthesiol Res Pract 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 2010; 81(10): 1135–40. [DOI] [PubMed] [Google Scholar]
- 60.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. The Journal of Pain 2008; 9(6): 506–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. The Lancet 2003; 362(9395): 1517–26. [DOI] [PubMed] [Google Scholar]
- 62.Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. Journal of neurology, neurosurgery, and psychiatry 2005; 76(12): 1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology 2011; 36(6): 1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. Journal of Psychopharmacology 2011; 25(1): 121–30. [DOI] [PubMed] [Google Scholar]
- 65.de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H. Tetrahydrocannabinol Does Not Reduce Pain in Patients With Chronic Abdominal Pain in a Phase 2 Placebo-controlled Study. Clinical Gastroenterology and Hepatology 2017; 15(7): 1079–86.e4. [DOI] [PubMed] [Google Scholar]
- 66.Deutsch SI, Rosse RB, Connor JM, Burket JA, Murphy ME, Fox FJ. Current Status of Cannabis Treatment of Multiple Sclerosis with an Illustrative Case Presentation of a Patient with MS, Complex Vocal Tics, Paroxysmal Dystonia, and Marijuana Dependence Treated with Dronabinol. CNS Spectrums 2008; 13(05): 393–403. [DOI] [PubMed] [Google Scholar]
- 67.Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. The Journal of Clinical Pharmacology 1981; 21(S1). [DOI] [PubMed] [Google Scholar]
- 68.Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. Single-dose study of nabilone in anxious volunteers. Journal of Clinical Pharmacology 1981; 21: 383S–96S. [DOI] [PubMed] [Google Scholar]
- 69.Leehey M, Liu Y, Epstein C, et al. Open label study of cannabidiol in Parkinson’s disease. Movement Disorders 2017; 32. [Google Scholar]
- 70.Müller-Vahl KR, Schneider U, Prevedel H, et al. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. Journal of Clinical Psychiatry 2003; 64(4): 459–65. [DOI] [PubMed] [Google Scholar]
- 71.Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test Anal 2012; 4(7-8): 649–59. [DOI] [PubMed] [Google Scholar]
- 72.Shannon S, Opila-Lehman J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. The Permanente Journal 2016; 20(4): 16–005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain 2008; 9(2): 164–73. [DOI] [PubMed] [Google Scholar]
- 74.Arad S, Bar-Lev Schleider L, Knanni J, et al. Medical cannabis for the treatment of Tourette syndrome: A descriptive analysis of 24 patients. Movement Disorders 2017; 31: S309–S10. [Google Scholar]
- 75.Hasan A, Rothenberger A, Munchau A, Wobrock T, Falkai P, Roessner V. Oral delta 9-tetrahydrocannabinol improved refractory Gilles de la Tourette syndrome in an adolescent by increasing intracortical inhibition: a case report. Journal of Clinical Psychopharmacology 2010; 30(2): 190–2. [DOI] [PubMed] [Google Scholar]
- 76.Hemming M, Yellowlees PM. Effective treatment of Tourette’s syndrome with marijuana. Journal of Psychopharmacology 1993; 7(4): 389–91. [DOI] [PubMed] [Google Scholar]
- 77.Müller-Vahl KR, Schneider U, Emrich HM. Combined treatment of Tourette Syndrome with Δ9-THC and dopamine receptor antagonists. Journal of Cannabis Therapeutics 2002; 2(3-4): 145–54. [Google Scholar]
- 78.Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, Asherson P. Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial. European Neuropsychopharmacology 2017; 27(8): 795–808. [DOI] [PubMed] [Google Scholar]
- 79.Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol 2014; 34(5): 559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther 2009; 15(1): 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs 2014; 46(1): 73–7. [DOI] [PubMed] [Google Scholar]
- 82.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. 2015; 51: 585–8. [DOI] [PubMed] [Google Scholar]
- 83.Mashiah M Medical cannabis as treatment for chronic combat PTSD: Promising results in an open pilot study. Tucson, Arizona; 2012. [Google Scholar]
- 84.Quinn D, Hunter MA, Hager BW. Medicinal cannabis reduces agitation in acquired brain injury: Case study. Journal of Neurotrauma 2014; 33(13): A–80. [Google Scholar]
- 85.Reznik I Post-traumatic stress disorder and medical cannabis use: a naturalistic observational study. European Neuropsychopharmacology 2012; 22: S363–S4. [Google Scholar]
- 86.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig 2014; 34(8): 587–91. [DOI] [PubMed] [Google Scholar]
- 87.Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry 2015; 76(9): 1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhattacharyya S, Wilson R, Allen P, Bossong M, Appiah-Kusi E, McGuire P. Effect of cannabidiol on symptoms, distress and neurophysiological abnormalities in clinical high-risk for psychosis patients: A placebocontrolled study. Schizophrenia Bulletin 2018; 44: S28. [Google Scholar]
- 89.Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018: 1–10. [DOI] [PubMed] [Google Scholar]
- 90.D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 2005; 57(6): 594–608. [DOI] [PubMed] [Google Scholar]
- 91.Goswami S, Mattoo SK, Basu D, Singh G. Substance-abusing schizophrenics: Do they self-medicate. The American Journal on Addictions 2004; 13(2): 139–50. [DOI] [PubMed] [Google Scholar]
- 92.Hallak JEC, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Revista Brasileira de Psiquiatria 2010; 32(1): 56–61. [DOI] [PubMed] [Google Scholar]
- 93.Kolliakou A, Castle D, Sallis H, et al. Reasons for cannabis use in first-episode psychosis: does strength of endorsement change over 12 months? Eur Psychiatry 2015; 30(1): 152–9. [DOI] [PubMed] [Google Scholar]
- 94.Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012; 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mane A, Fernandez-Exposito M, Berge D, et al. Relationship between cannabis and psychosis: Reasons for use and associated clinical variables. Psychiatry Res 2015; 229(1-2): 70–4. [DOI] [PubMed] [Google Scholar]
- 96.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. American Journal of Psychiatry 2018; 175(3): 225–31. [DOI] [PubMed] [Google Scholar]
- 97.Zuardi AW, Crippa JAS, Hallak JEC, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. Journal of Psychopharmacology 2009; 23(8): 979–83. [DOI] [PubMed] [Google Scholar]
- 98.Zuardi AW, Hallak JEC, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. Journal of Psychopharmacology 2006; 20(5): 683–6. [DOI] [PubMed] [Google Scholar]
- 99.Organization WH. The health and social effects of nonmedical cannabis use: World Health Organization; 2016. [Google Scholar]
- 100.Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of Cannabis With Long-Term Clinical Symptoms in Anxiety and Mood Disorders: A Systematic Review of Prospective Studies. J Clin Psychiatry 2018; 79(4). [DOI] [PubMed] [Google Scholar]
- 101.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull 2005; 31(3): 608–12. [DOI] [PubMed] [Google Scholar]
- 102.Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2004; 330(7481): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val 158 Met moderation of Δ-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 2006; 31(12): 2748. [DOI] [PubMed] [Google Scholar]
- 104.Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 2006; 31(12): 2748–57. [DOI] [PubMed] [Google Scholar]
- 105.Higgins JP, Deeks JJ, Altman DG. Special topics in statistics. Cochrane handbook for systematic reviews of interventions: Cochrane book series 2008: 481–529. [Google Scholar]
- 106.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.