Abstract
APE1 is essential in cancer cells due to its central role in the Base Excision Repair pathway of DNA lesions and in the transcriptional regulation of genes involved in tumor progression/chemoresistance. Indeed, APE1 overexpression correlates with chemoresistance in more aggressive cancers, and APE1 protein-protein interactions (PPIs) specifically modulate different protein functions in cancer cells. Although important, a detailed investigation on the nature and function of protein interactors regulating APE1 role in tumor progression and chemoresistance is still lacking. The present work was aimed at analyzing the APE1-PPI network with the goal of defining bad prognosis signatures through systematic bioinformatics analysis. By using a well-characterized HeLa cell model stably expressing a flagged APE1 form, which was subjected to extensive proteomics analyses for immunocaptured complexes from different subcellular compartments, we here demonstrate that APE1 is a central hub connecting different subnetworks largely composed of proteins belonging to cancer-associated communities and/or involved in RNA- and DNA-metabolism. When we performed survival analysis in real cancer datasets, we observed that more than 80% of these APE1-PPI network elements is associated with bad prognosis. Our findings, which are hypothesis generating, strongly support the possibility to infer APE1-interactomic signatures associated with bad prognosis of different cancers; they will be of general interest for the future definition of novel predictive disease biomarkers. Future studies will be needed to assess the function of APE1 in the protein complexes we discovered. Data are available via ProteomeXchange with identifier PXD013368.
Subject terms: Cancer genomics, Cancer genomics, Gene regulatory networks, Gene regulatory networks
Introduction
Alteration of DNA repair mechanisms is an important hallmark of cancer cells, and plays a role both in the onset of an initial cancerous phenotype and in tumor progression. Tumor cells can develop drug resistance through repair mechanisms that counteract the DNA damage induced by chemotherapy or radiotherapy1,2. Thus, specific DNA repair inhibitors are often combined with DNA-damaging agents to improve therapy efficacy. Emerging evidences in tumor biology suggest that: i) protein-protein interactions (PPIs) specifically modulate both canonical and non-canonical roles of DNA repair enzymes; ii) RNA processing pathways participate in DNA-Damage Response (DDR); iii) defects in the above-mentioned regulatory mechanisms are associated with cancer genomic instability3. Very recent studies clearly show that many DNA repair proteins are associated with those involved in RNA metabolism, proving a role of their interactome network in undertaking non-canonical functions affecting gene expression in tumors. In addition, novel studies have shown that interaction of DDR components and miRNA biogenesis process is linked to cancer development2. In the context of these emerging lines, we already demonstrated the crucial role that enzymes belonging to the base excision DNA repair (BER) pathway play4. In particular, we showed that the essential BER enzyme apurinic/apyrimidinic endonuclease 1 (APE1), which is encoded by the APEX1 gene, contributes to the regulation of oxidative stress responses and to the expression of chemoresistance genes via unsuspected functions in RNA metabolism4–8. The involvement of this protein in RNA processing events9–11, including miRNA expression, was recently unraveled by our group using a limited unbiased functional proteomic approach4. However, the reduced characterization of APE1 interaction with proteins involved in miRNA processing, e.g. NPM1, hnRNPU, PRP19, SFPQ and p5312, strongly limited our complete understanding of this phenomenon and of its functional relevance in cancer biology, thus hampering a further translation of these findings into therapy.
It is well known that the different functions of APE1 may depend on its interacting partners13,14; for example, an alteration of its interaction networks has been reported to play a significant role in BER impairment. A recent hypothesis also suggests that APE1 functional dysregulation may impact on the RNome expression and, thus, on the expression of target genes playing a relevant role in the pathology onset4. Since several cancer-associated APE1 variants present mutations in the endonuclease domain, exhibiting only mild nuclease defects in vitro15–17, we hypothesize that APE1 non-canonical functions associated with its overexpression and/or an altered expression of its protein interacting partners should be related to cancer promotion. A proof of concept for the relevance of APE1 PPIs in cancer biology is represented by the paradigmatic example of the APE1-nucleophosmin 1 (NPM1) interaction, as ascertained by us18. NPM1 is a multifunctional protein that controls cell growth and genome stability through a mechanism either involving nucleolar-cytoplasmic shuttling or a fine modulation of the whole BER pathway11,19. Recently, an important role for NPM1 in miRNA biology, associated with cancer development, was outlined20–22. Abnormal cytoplasmic APE1 and NPM1 levels were associated with the oncogenic progression and chemoresistance of HGSC (high-grade serous ovarian adenocarcinoma), a prediction of a poor prognosis therein23,24, and a dysregulation of the BER and miR-221/222 processing pathways in AML cells18,25. The efficacy of novel APE1/NPM1 interaction inhibitors, which sensitize cancer cells to chemotherapy agents, supports the translational importance of these findings26. These results further support the hypothesis that an alteration of other APE1 PPIs may be causally involved in cancer development and chemoresistance.
Prompted by these observations, the present work was aimed at: i) implementing the already known APE1-PPI network using a more efficient functional proteomics approach, and ii) defining the association of the APE1-PPI network with the modulation of tumor progression and chemoresistance through a systematic bioinformatics analysis of the Cancer Genome Atlas (TCGA) datasets. To this purpose, we used HeLa cells as a general and well-characterized cancer cell model to generate novel disease hypotheses and molecular diagrams. Having a well-characterized cell model is essential when using unbiased strategies, such as genomics and proteomics, in order to easily interpret data and generate novel assumptions. In this context, few years ago, we developed a specific HeLa cell line stably expressing a flagged tagged APE1 form for selective and efficient protein complex immunocapture4,17,26,27. This cell line, and its corresponding products, were characterized in both cancer and genotoxic damage response contexts by means of different holistic approaches, including genomics, transcriptomics and proteomics4,12. In this study, we wanted to improve our previous interactomic analyses by taking advantage of more sensitive mass spectrometry technologies, which were here applied to the analysis of extracts from different subcellular compartments. Resulting data were analysed by bioinformatics in a dedicated translational perspective. Our findings support the possibility to infer APE1 interactomic signatures associated with bad prognosis of different cancers and will be of general interest for the definition of novel predictive biomarker signatures of cancers.
Results
Proteomic characterization of the APE1-PPI network
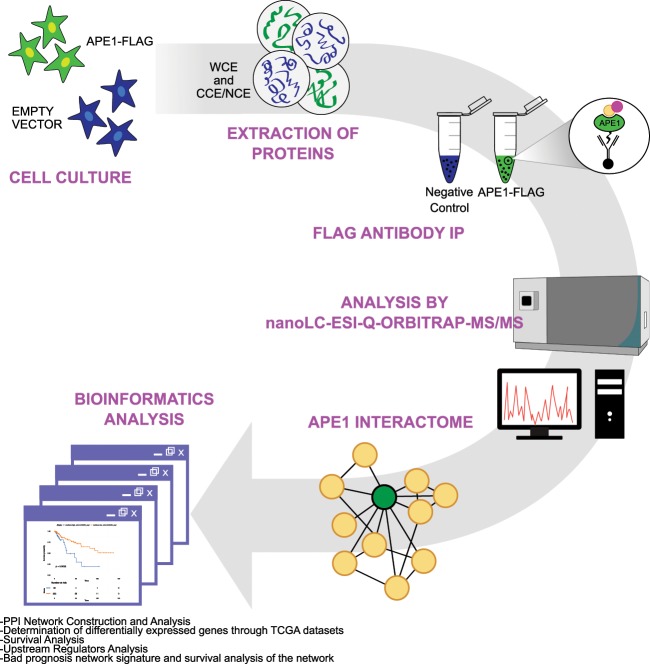
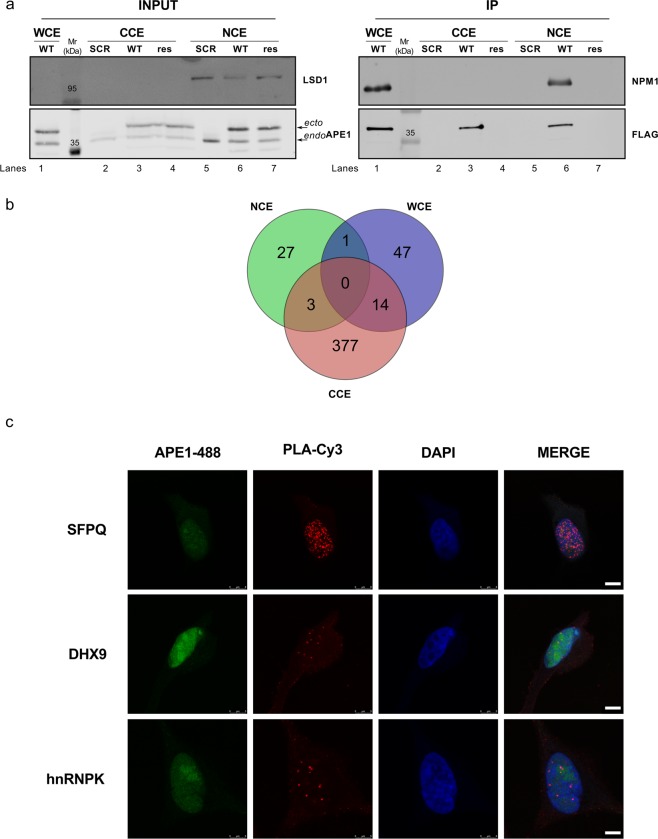
With this work, we wanted to study the relevance of APE1-PPIs in cancer using an unbiased functional proteomic approach in order to expand the number of known APE1 PPIs, as derived from studies from this and other groups4,12. To this purpose, we used HeLa cells stably expressing the APE1 FLAG-tagged protein12 (WT), which were here managed to optimize the isolation of APE1-PPI complexes through co-immunoprecipitation experiments (Figs. 1 and 2A). Differently from our previous investigations12, APE1-interacting protein complexes were isolated from either the whole cell lysate, or nuclear- and cytoplasmic-enriched subcellular fractions. Then, resulting protein mixtures were resolved by SDS-PAGE, and corresponding gel lanes were cut into parallel gel portions that were further subjected to proteomic analysis. As control experiments, we applied the same proteomic procedure to a cell clone stably transfected with the empty scramble vector (SCR) (Figs. 1 and 2A). As additional negative control experiments, nuclear and cytoplasmic cell extracts from HeLa cells expressing APE1 FLAG-tagged were co-immunoprecipitated with a resin lacking the anti-FLAG antibody to exclude any additional background (Fig. 2A, res). Western blotting analysis showed an APE1 enrichment in co-immunoprecipitated material from nuclear, cytoplasmic and total cell extracts of a HeLa cell clone stably expressing the flagged protein (Fig. 2A). In order to check for the quality of the immunoprecipitated materials, nucleophosmin 1 (NPM1) was used as a known APE1 interactor prior to further proteomic analysis12,18,19,28. By using this approach, a number of proteins were identified in APE1-FLAG co-immunoprecipitates from the above-mentioned whole-cell lysate and corresponding subcellular fractions (Supplementary Table S1 and Table S2). After careful filtration for false positives identified in the corresponding control (SCR and res) samples, 62, 31 and 394 proteins were identified as potential APE1 interactors in the whole-cell lysate, nuclear fraction and cytoplasmic fractions, respectively, which accounted for 455 non-redundant proteins. A poor overlapping of components from different extracts was observed, qualitatively confirming the preparation specificity (Fig. 2B). Various molecules (i.e. FEN1, hnRNPK, NPM1, PABPC1, SFPQ and XRCC1) were already described as APE1-interacting partners in other studies from this4,12 and other groups24,29,30, confirming the good quality of our analysis. The following proximity ligation assays were then successfully carried out to validate the identified APE1-interacting proteins within cells: i) SFPQ (splicing factor, proline- and glutamine-rich), DHX9 (DEAH-box helicase 9) and hnRNPK (heterogeneous nuclear ribonucleoprotein K) in HeLa cells (Fig. 2C and Supplementary Figs. S1 and S2); ii) hnRNPA2/B1 in the JHH-6 hepatocellular carcinoma cell line31 (Supplementary Fig. S1B); iii) SFPQ in the A549 lung cancer cell line (Supplementary Fig. S1C)32. The above-mentioned APE1-binding partners list was then added with additional proteins (n = 80) deriving from previous APE1-focused interactomic investigations4 to yield a final list of APE1-PPI elements (n = 535), which were associated with 531 non-redundant genes. The large size of this inventory (containing direct as well as indirect protein-binding partners) was rationalized based on the multiple biological functions/activities in which APE1 has been associated (redox control, transcriptional activity, DNA and RNA metabolism), and its localization in various subcellular districts. This condition is similar to the one recently reported for two APE1 protein interactors, namely XRCC6 and XRCC5, which have been similarly demonstrated to bind both to RNA/DNA as well as to about 300 proteins33. Other examples of proteins having hundreds of interactors (as deduced by a single immunocapture experiment) are already present in the scientific literature34–37. On the other hand, this final list, of direct or indirect APE1-binding partners, contained about 100 proteins whose capability to interact (directly or indirectly) with APE1 was already ascertained by different experimental approaches (Supplementary Table S3). The above-mentioned protein inventory was then subjected to bioinformatics analysis to establish a PPI-network associated with APE1 (Fig. 1); then, we linked this analysis to additional cancer/biological databases with the aim to provide a more complete picture of the APE1 biological roles in both cancer and cellular biology.
Figure 1.
Schematic experimental pipeline used in this study. Schematic representation of the workflow for ascertaining APE1-interacting proteins by proteomic experiments. Whole-cell lysates (WCE), or nuclear (NCE) and cytoplasmatic (CCE) extracts were prepared from the 3 × APE1 FLAG-tagged expressing cells or control cells transformed with an empty vector (SCR). M2 antibodies against the FLAG peptide were used for co-immunoprecipitation of the above cell lysates. As additional control experiment, identical cell extracts from HeLa cells expressing 3 x APE1 FLAG-tagged were also co-immunoprecipitated with a resin lacking the FLAG antibody (res). The resulting bound proteins were digested with trypsin and analyzed by nanoLC-ESI-Q-Orbitrap-MS/MS. By comparison with proteins identified in the control co-immunoprecipitation experiments, we removed contaminant proteins from components identified in WCE, NCE and CCE; the resulting APE1-interacting partners were further added of additional proteins binding to APE1 (n = 80) from previous studies4, and finally subjected to bioinformatics analysis.
Figure 2.
Proteomic characterization of the APE1 interactome. (A) Representative Western blotting to confirm APE1 pulldown in the co-immunoprecipitation experiment. Western blotting analysis was performed on total HeLa cell clone extracts (INPUT) and on co-immunoprecipitated material (IP) with specific antibodies for APE1 and FLAG. The endogenous (endo) and ectopic (ecto) form of the APE1 protein is visible. The resulting material was tested for the occurrence of NPM1, a known APE1 interactor. LSD1 was used to probe nuclear enrichment. SCR, HeLa cell clone transfected with empty vector; WT, HeLa cell clone expressing APE1-FLAG tagged protein; res, co-immunoprecipitated with a resin lacking the FLAG antibody; WCE, whole cell extract; NCE, nuclear cell extract; CCE, cytoplasmic cell extract. (B) Venn diagram showing APE1-interacting partners identified in whole-cell lysates (WCE), or nuclear (NCE) and cytoplasmatic (CCE) extracts. (C) Nucleoplasmic interaction between APE1 and three identified interactors. HeLa cells were seeded on a glass coverslip and the PLA reaction was carried out using anti-APE1, anti-SFPQ, anti-DHX9 and anti-hnRNPK antibodies. APE1 localization was detected by using an anti-APE1 antibody and visualized in green. Confocal microscopy analysis highlighted the presence of distinct fluorescent red dots (PLA signals) indicating the occurrence of in vivo interaction between APE1 and its protein partners. DAPI staining was used as a reference for the nuclei. See also Supplementary Figs. S1 and S2 for negative controls. Bars, 8 µM.
APE1-PPI network construction and analysis
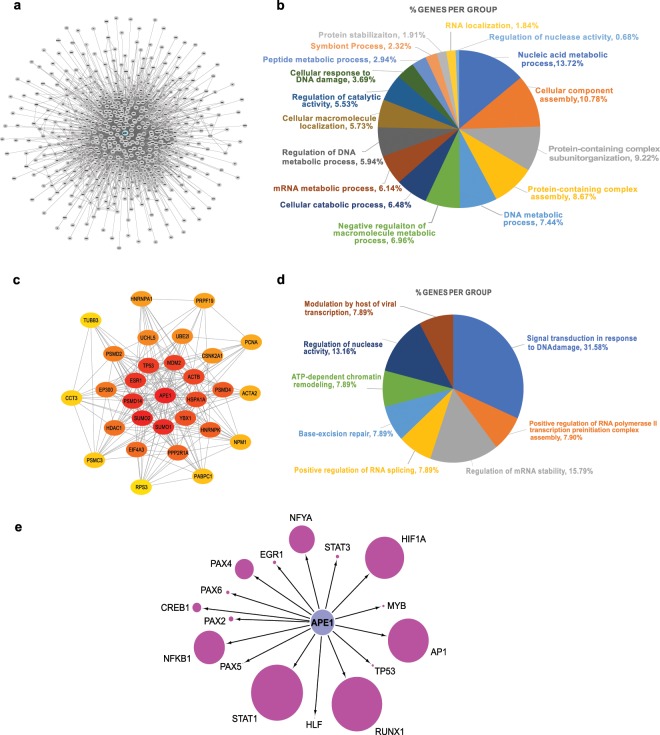
The APE1-interacting partners from this and other investigations (n = 535) were used to establish the APE1-PPI network. Direct and/or indirect interactions between these molecules were retrieved by the InWeb_InBioMap web tool, which is a large data compendium for high-quality PPI networks. Afterwards, the undirected PPI network, representing the interactome of APE1, was constructed with 511 nodes (24 proteins were not recognized by the tool) and 3934 edges (Fig. 3A). The resulting network was visualized and analyzed by using the Cytoscape software and its packages38. The initial analysis of the network was carried out by performing functional enrichment analysis for terms belonging to the “Gene Ontology - Biological Process” database, using the ClueGO tool with standard parameters to identify enriched pathways on the basis of the network’s gene frequency in each pathway (n = 383, 75%). Based on this analysis, 109 genes were enriched in the group of pathways called “DNA metabolic process” (7.4% genes per group), 90 genes were enriched in the group of pathways called “mRNA metabolic process” (6.1% genes per group), 54 genes were enriched in the group of pathways called “DNA damage response” (3.7% genes per group) and 27 genes were enriched in the group of pathways called “RNA localization” (1.8% genes per group) (Fig. 3B and Supplementary Table S4). These results clearly confirmed the involvement of APE1 and its interacting partners in processes involved in RNA (with particular emphasis on mRNA), DNA and protein metabolism/stability, supporting our previous findings4,12.
Figure 3.
Bioinformatics characterization of the APE1 interactome. (A) Global APE1 Protein-Protein Interaction Network. (B) Functional annotation of the global network based on Gene Ontology - Biological Process terms (p < 0.05). In the pie chart, the percentage of the proteins/genes enriched in the group of pathways is shown. (C) Top 30 hubs of the APE1-PPI network, based on global metric, betweenness centrality. Color shades represent the significance of the hub, with red color as the most significant and yellow color as the least. (D) Functional annotation of the Top 30 hubs based on Gene Ontology - Biological Process terms (p < 0.05). In the pie chart, the percentage of the genes enriched in the group of pathways is shown. (E) Transcriptional regulatory network of the APE1 interactome. Node size represents the number of putative binding sites identified by the LASAGNA-Search 2.0 tool in the promoters (−2500, -1nt from the TSS) of the APE1 interactome genes for 16 transcription factors that are modified by APE1 redox activity or use APE1 as a co-factor.
The complex PPI-network containing 511 nodes was then studied in order to focus on its most important elements; this was done through a hub analysis based on global metric, betweenness centrality. In graph theory, betweenness centrality is a measure of centrality in a graph based on the shortest paths; therefore, it represents the degree to which nodes stand between each other. As a result, the top 30 hub nodes were identified and extracted from the main APE1-PPI network as hub-subnetworks (Fig. 3C). This hub module was then analyzed with ClueGO to understand the specific roles of these genes in biological processes (Fig. 3D and Supplementary Table S4). The predominant enrichment was observed for: i) 12 hub genes involved in the group of pathways named “signal transduction in response to DNA damage” (31.6% genes per group); ii) 6 hub genes enriched in the group of pathways called “regulation of mRNA stability” (15.8% genes per group); iii) 3 central hub genes (HSPA1A, PRPF19 and PSMD4) involved in the group of pathways related to “positive regulation of RNA splicing”, confirming previous interactomic results4. Globally, these results indicate the significant involvement of the APE1-PPI network in RNA and DNA metabolism, showing that APE1 acts as the central hub connecting different subnetworks with diverse functions.
Role of APE1 in the transcriptional regulation of the APE1-PPI network
Among its multifunctional biological roles, APE1 is also known as transcriptional and post-transcriptional regulator. On the one hand, APE1 can exert its nuclear redox control function on several transcription factors (TFs), modulating their activity and, hence, gene expression39. On the other hand, it can also act at the RNA level through its ability in binding to transcripts, thus affecting corresponding stability and processing4,10. Therefore, we can hypothesize that APE1 may contribute to gene expression regulation of elements of its own PPI. In order to check whether APE1 could be involved in the former activity, we performed a TFBS motif discovery analysis with the LASAGNA-Search 2.0 tool40 on the promoters of the above-mentioned 531 genes representing the APE1 interactome. This allowed to verify and quantify the presence of enriched putative binding sites for more than a hundred TFs (Supplementary Table S5). In particular, we identified putative sites for 16 TFs that are known to be stimulated by the APE1 redox activity or to regulate gene expression by using APE1 as a co-factor (e.g. AP1, NF-κB1, HIF1α and members of the STAT family) (Fig. 3E). Some of these TF binding sites (e.g. PAX5 sites) were not highly abundant in the obtained results but, since they are known to regulate gene expression in a tissue-specific manner, we believe that the HeLa cell model used in the present work could not represent the most suitable one to study their effect. We also compared the list of APE1-PPI partners identified in the present work with that of the RNA molecules bound by APE1 (n = 1015), which we previously had defined through RIP-seq experiments4,12; this analysis demonstrated that 42 genes, that interact with APE1 at the transcript level, are also part of the APE1-PPI (p-value = 0.01, Supplementary Fig. S3). We finally integrated these data with the results of a microarray differential gene expression profiling analysis performed in siAPE1 HeLa cells12. Interestingly, we found that 55 genes, belonging to the APE1-PPI network, were differentially expressed (absolute log fold change ≥1, adj. p-value ≤ 0.05), as well as 45 other transcripts originating from the RIP-seq experiment, globally accounting for 95 unique genes (p-value = 0.0001, Supplementary Fig. S3). Altogether, these results strongly support the possibility that APE1 may contribute to the regulation of the expression of the majority of its own PPI genes.
Correlation between APE1 and its protein-binding partners at the gene expression level
Correlated expression of differently associated genes is often observed being a common feature and an important booster of the transformation process41, even though a perfect correlation of gene expression at mRNA and protein levels is not always observed42. However, data described in the previous section provided interesting evidences suggesting that the integration of proteomic and transcriptomic data, correlated by common biological functions, may highlight the potential involvement of subsets of genes in neoplastic transformation.
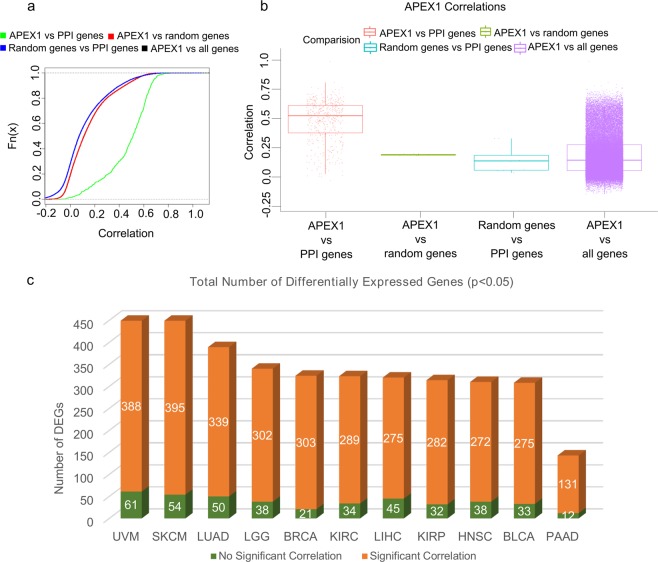
In order to understand if a relationship between APE1 and its protein interaction partners may be observed also at the gene expression level, we analyzed the corresponding correlations in the Genomic Data Commons (GDC) RNA-Seq tumor database. In more than 11,000 tumor sample datasets, we observed that the gene expression correlation between APE1 and its PPI-network elements is higher (p-value < 1015) than that with respect to: (i) all the genes, (ii) random-gene datasets (PPI size) or iii) random genes vs the APE1-PPI network (Fig. 4A,B). By using the same dataset, gene expression data for the genes coding the 531 interactors of APE1 were obtained for 33 different tumor types described in TCGA (Supplementary Table S6) as well as for the associated normal tissues. Further analyses were performed across the top 11 TCGA cancer datasets having the highest number of bad prognostic genes (see below) that were up- and down-regulated (p < 0.05, absolute log fold change >1) (Figs. 4C, 5 and Supplementary Table S6). According to the results of the differential gene expression analysis, more than half of the APE1 interactors were found to be differentially expressed in many datasets (Fig. 4). The gene expression of APE1 across these datasets was used to calculate the Pearson correlation existing between APE1 and its interactors. A very high percentage of differentially expressed genes (DEGs) in those datasets was found to have significant (p < 0.05) correlation with APE1 gene expression (Fig. 4C). Altogether, these results demonstrated the existence of a very strong orchestration in the expression of APE1 and of its interacting proteins in the aforementioned cancer types, suggesting the existence of common pathways of transcriptional regulation for APE1-PPIs in cancer development.
Figure 4.
Differential gene expression and correlation analysis of APE1-interacting partners in the TCGA datasets. (A) Empirical cumulative distribution function (ECDF). Empirical cumulative distribution curves of the correlation of the gene expression profiles of APEX1 and APEX1-PPI network genes in the TCGA datasets or in the control groups. Green line: APEX1 expression versus the PPI network genes; red line: APEX1 expression versus 100 sets with the same size of the PPI gene set composed by random genes; blue line: 100 random genes expression versus the PPI network genes; black line: APEX1 expression versus all genes. Note that the black line is nearly superimposed to the red line and, for this reason, almost hidden by it. (B) Box-plots of the same data. The average correlations for all the control groups are statistically significantly different (p < 0.005) with respect to the APEX1-PPI correlation. (C) Total number of differentially expressed genes (p < 0.05, absolute log fold change difference > 1) across the top 11 TCGA datasets having the highest number of up- and down-regulated genes. In the bar chart, the number of genes having significant correlation (absolute (PCC) >0.6, p < 0.05) with the expression of APE1 is shown in orange, while the others are shown in green.
Figure 5.
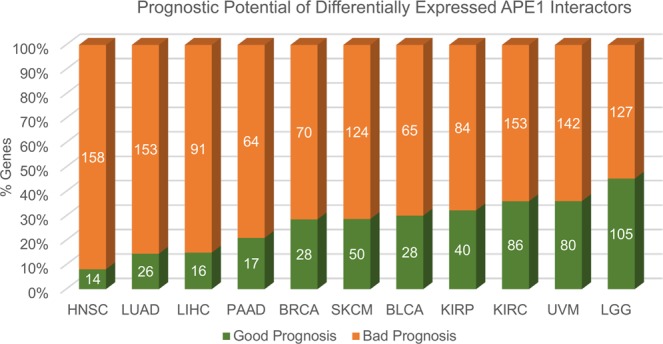
Survival analysis of APE1-interacting partners in the TCGA datasets. For each cancer type, bar plots represent the percentages and the total number of genes having significant (p < 0.05) bad or good prognosis, shown in orange and green color, respectively.
Upstream regulators analysis
With the aim of ascertaining the role of these gene modules in more detail, an upstream regulators analysis was performed using the TRANSPATH tool within the geneXplain 4.11 web platform. For each TCGA cancer dataset, the list of significant DEGs was analyzed, and the three molecules having the lowest Ranks sum were retained (Table 1) as the ones having the best probability of being the master regulators of that network. These results strengthened, on the one hand, the general role of the modules in RNA and DNA repair mechanisms. In fact, XRCC6 (master regulator of 5 datasets) and DDB1 (master regulator of 3 datasets) are proteins associated with the DNA repair process43,44. YBX1, another highly represented master regulator (KIRC, KIRP and PAAD), is known to act as both an RNA- and a DNA-binding protein, as well as being involved in miRNA processing45. However, what really equated all these regulators was their role in the apoptotic, proliferative and resistance pathways, as shown in Table 1. We also evaluated the correlation of the expression profiles of APEX1 and of these regulators in each bad prognostic network (Fig. 6 and Supplementary Figs. S4–S11, data is represented according to a color code).
Table 1.
APE1-PPI bad prognostic signatures top regulators analysis.
| Dataset | Top1 | Top1 Bibliography | Gene Symbol Description | Top2 | Top2 Bibliography | Gene Symbol Description | Top3 | Top3 Bibliography | Gene Symbol Description |
|---|---|---|---|---|---|---|---|---|---|
| HNSC | XRCC6 | (1) (2) (3) | X-ray repair cross-complementing protein 6 | PRKDC | (1) (2) (3) | Protein kinase, DNA-activated, catalytic subunit | TERF2 | (1) (2) (3) | Telomeric repeat binding factor 2 |
| KIRC | PRKCD | (1) (2) (3) | Protein kinase C delta type | PPP1CB | (2) | Protein phosphatase 1 catalytic subunit beta | YBX1 | (1) (2) (3) | Y-Box binding protein 1 |
| LUAD | PRKN | (2) | Parkin RBR E3 ubiquitin protein ligase | HDAC2 | (1) (2) (3) | Histone deacetylase 2 | XRCC6 | (1) (2) (3) | X-ray repair cross-complementing protein 6 |
| UVM | SMYD2 | (1) (2) (3) | SET and MYND domain containing 2 | KDM1A | (1) (2) (3) | Lysine demethylase 1 A | XRCC5 | (1) (2) | X-ray repair cross-complementing protein 5 |
| LGG | SETD7 | (1) (2) (3) | SET domain containing 7, histone lysine Methyltransferase | KDM1A | (1) (2) (3) | Lysine demethylase 1A | CUL1 | (1) (2) | Cullin-1 |
| SKCM | DDB1 | (1) (2) (3) | Damage specific DNA binding protein 1 | PTP4A3 | (1) (2) (3) | Protein tyrosine phosphatase 4A3 | XRCC6 | (1) (2) (3) | X-ray repair cross-complementing protein 6 |
| LIHC | PRKN | (2) | Parkin RBR E3 ubiquitin protein ligase | HDAC2 | (1) (2) (3) | Histone deacetylase 2 | XRCC6 | (1) (2) (3) | X-ray repair cross-complementing protein 6 |
| KIRP | YBX1 | (1) (2) (3) | Y-Box binding protein 1 | PTP4A3 | (1) (2) (3) | Protein tyrosine phosphatase 4A3 | TCF21 | (1) (2) (3) | Transcription factor 21 |
| BRCA | DDB1 | (1) (2) (3) | Damage specific DNA binding protein 1 | XRCC6 | (1) (2) (3) | X-ray repair cross-complementing protein 6 | IDE | (1) (2) | Insulin-degrading enzyme |
| BLCA | DDB1 | (1) (2) (3) | Damage specific DNA binding protein 1 | XRCC5 | (1) (2) | X-ray repair cross-complementing protein 5 | TRIM28 | (1) (2) (3) | Tripartite motif containing 28 |
| PAAD | YBX1 | (1) (2) (3) | Y-Box binding protein 1 | ACTL6A | (1) (2) | Actin-like protein 6A | HNRNPK | (1) (2) (3) | Heterogeneous nuclear ribonucleoprotein K |
GeneXplain identification of the Top 3 putative master regulators (ranked by ascending Ranks sum) of bad prognostic genes in the 11 selected TCGA cancer datasets. Numbers in brackets refer to the presence of bibliographic evidence associating upstream regulators with proliferation (1), apoptosis (2) and resistance (3), respectively. For TCGA abbreviations, see Supplementary Table S6; for all the references Supplementary Table S7.
Figure 6.
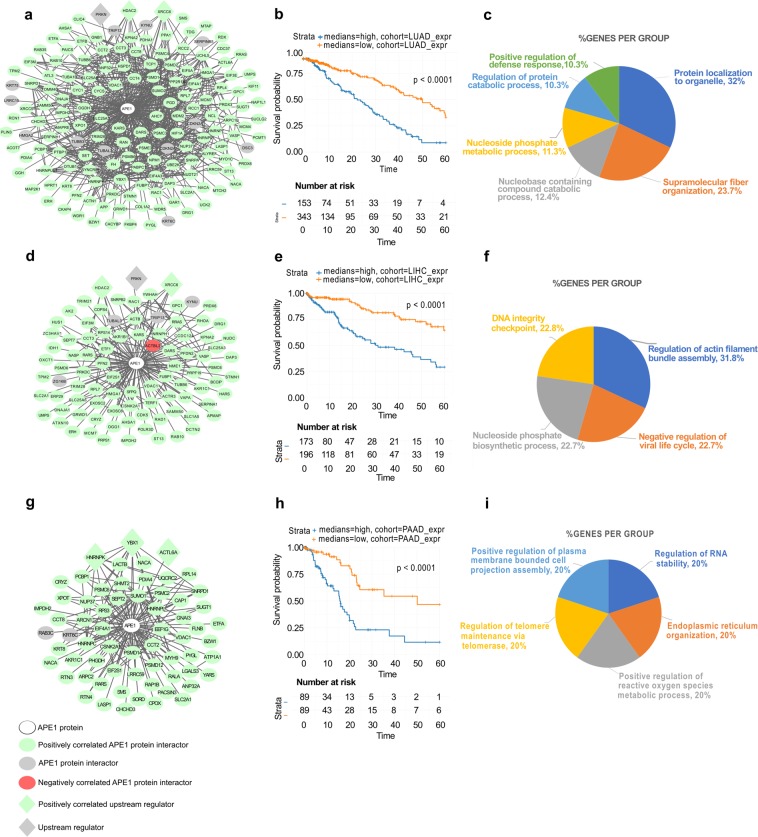
The most relevant cancer-specific prognostic subnetworks in the APE1 interactome. (A,D,G) Bad Prognosis networks of the LUAD (A), LIHC (D) and PAAD (G) datasets formed by the protein interactors of APE1. Circular nodes represent the interactors of APE1, while diamond nodes represent the top 3 upstream regulators of the network. APE1 is symbolized with the white color as the central node. Significant negatively correlated nodes are symbolized with the red color (PCC < −0.6), while the nodes having significant positive correlation (PCC >0.6) are symbolized with the green color. Upstream regulators are symbolized with the purple color and the rest of the nodes are symbolized with the grey color. (B,E,H) Survival probability of the patients having high and low median expression of the gene signatures forming the overall networks, as represented by Kaplan-Meier plots. (C,F,I) Functional annotation of the Bad Prognosis networks based on Gene Ontology - Biological Process terms (p < 0.05). In the pie chart, the percentage of the genes enriched in the pathways is given next to the enriched terms.
Afterwards, upstream regulators analysis was performed also on the global APE1-PPI network to widen our understanding of its functions. The resulting top 10 master regulators are provided in Supplementary Table S8. Interestingly, we observed that some of these master regulators (i.e. NUAK1, KDM1A, UBE2D1, RBX1 and UBE2M) were known to be associated with the p53 signaling pathway46–51.
Definition of a bad-prognostic APE1 signature in cancers
With the aim of evaluating the real impact of APE1-interacting partners in TCGA cancer datasets, survival analyses were performed using the RTCGA Bioconductor package. The genes that were differentially expressed in a statistically significant manner (p < 0.05, absolute log fold change >1) between tumor and normal tissues were analyzed; Kaplan-Meier plots were obtained for each gene in each dataset, allowing to define good and bad prognosis gene signatures on a per cancer basis (Supplementary Table S4). The distribution of the genes with respect to significant good or bad prognosis (p < 0.05) in those cancer datasets is represented in Fig. 5.
In order to focus our attention on the most relevant cancer types, we based our bioinformatic analysis on the eleven datasets having the highest number of bad prognostic genes. Among those, we were interested in particular in liver (LIHC)52–56, lung (LUAD)57–60 and pancreatic (PAAD)61–65 cancer datasets, as the essential role of APE1 in the tumorigenic processes of these cancer types is already well established. Notably, the LUAD dataset was the third having the highest number of bad prognostic genes (n = 153). We then represented these genes as a subnetwork of the APE1-PPI network having correlation information with the following color code: green for significant positive correlation (PCC >0.6), red for significant negative correlation (PCC <−0.6) and grey for no correlation (Fig. 6A). In the network, the top 3 master regulators were also represented with diamond-shaped nodes. Afterwards, we specifically studied the survival outcomes of patients with high or low expression of the bad prognosis signature, using the median log fold change value of the genes in the network. The Kaplan-Meier plot (Fig. 6B) clearly shows that high expression of the genes in the bad prognostic network was associated with significant (p < 0.0001) lower survival probability. The biological processes dominantly enriched in this network were as follows: nucleic acid metabolism, protein transporters and arrangements to form complex subunits that have polymerized to generate fiber-shaped structures (Fig. 6C and Supplementary Table S4).
Because of the strong interest in APE1 as a prognostic factor in liver52–56 and pancreatic cancers61–63, we applied the same representation also to the LIHC (Fig. 6D–F) and PAAD datasets (Fig. 6G–I) with a total number of 91 and 64 bad prognostic genes, respectively. The bad prognostic signatures in these networks highlighted again a significant involvement of DNA- and RNA-related pathways (Figs. 6F–I; Supplementary Table S4). In general, the results of survival analysis emphasized the importance of the APE1-PPI network for real clinical data. Specifically, 84.3% of the genes (n = 451) present in the APE1-PPI network was associated with bad prognosis in one or more cancer datasets.
APE1-inhibitors sensitize cancer cells to mitochondrial toxicant Rotenone
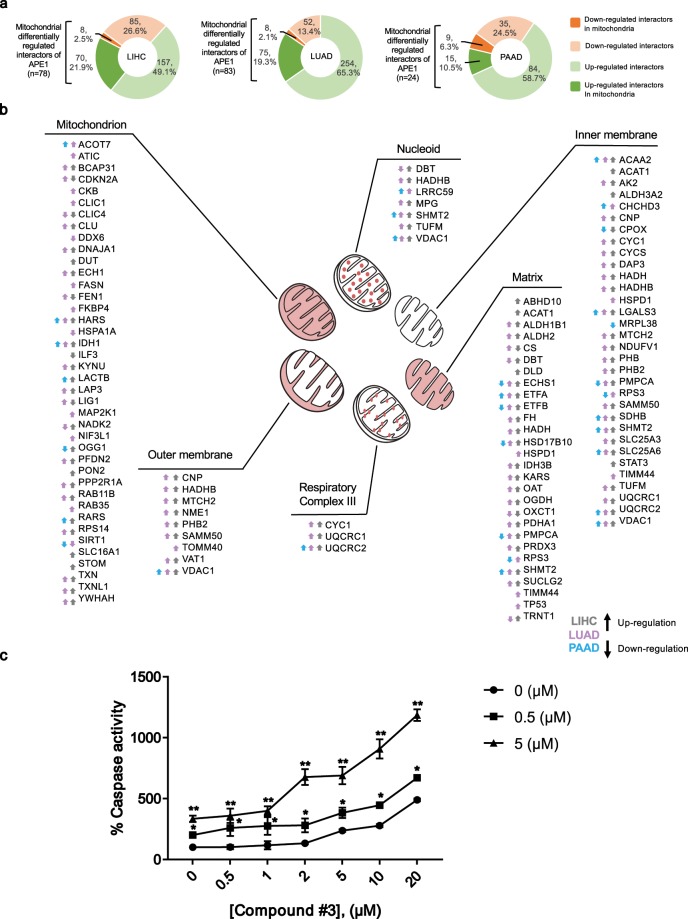
In order to refine our study and to provide an additional level to the functional characterization of APE1-PPI, we finally performed a comparative bioinformatics analysis to define the cellular localization of DEGs, as obtained from the LIHC, LUAD and PAAD datasets. As shown in Fig. 7A, 24.4% of the DEGs in LIHC, 21.4% of the DEGs in LUAD and 16.8% of the DEGs in PAAD represented APE1-PPIs that can also localize to mitochondria. Interestingly, 89.7%, 90.4% and 62.5% of these DEGs were observed to be upregulated in the corresponding TCGA datasets. The mitochondrial compartments and expression trends associated with these DEGs are shown in Fig. 7B.
Figure 7.
Differentially expressed APE1-PPIs in LIHC, LUAD and PAAD datasets point to the relevance of mitochondrial pathways impairment in cancer. (A) Differentially expressed APE1 interactors with mitochondrial localization. The colour codes are as follows: bright green colour for up-regulated DEGs that can localize to mitochondria, bright orange colour for down-regulated DEGs that can localize to mitochondria, light green and light orange colours for up- and down- regulated DEGs that do not localize to mitochondria, respectively. (B) Localization of DEGs in the mitochondrial compartments. Specific mitochondrial sub-compartmentalization of DEGs is shown. For each location, the global expression trend and dataset information is given with arrow and colour codes. Upside arrow represents up-regulation, while downside arrow represents down-regulation. The colour of the arrows represents the datasets as follows: grey colour for LIHC, purple colour for LUAD and blue colour for PAAD. (C) Inhibition of APE1 endonuclease activity in combination with treatment with rotenone sensitizes HeLa cells to apoptosis. Cells were treated with Compound #3 in the absence or presence of rotenone (0.5 and 5 µM), for 24 h. The Apo-ONE assay was used to quantify relative levels of apoptosis. The activities of caspases 3/7 were examined using a fluorescence-based assay. Data were normalized on untreated cells and represent the means ± SD of three independent experiments. Asterisks represent a significant difference with respect to cells treated with Compound #3 alone. Data were evaluated statistically by two-tails Student t-test.
With the aim to support APE1 involvement in mitochondrial functionality, we performed a caspase activity assay upon treatment with Compound #3, an APE1 endonuclease inhibitor66, in combination with rotenone, a well-known mitochondrial respiratory chain inhibitor67. HeLa cells were treated with different concentrations of Compound #3 in the presence/absence of rotenone (at the doses of 0.5 μM and 5 μM) for 24 h (Fig. 7C) (Supplementary Fig. S12). HeLa cells treated with the combination of both compounds resulted more sensitive to apoptosis, demonstrating the existence of a synthetic lethality relation between APE1 and mitochondrial activity, which may be further explored for designing novel anticancer strategies.
Discussion
The multifunctional DNA repair protein APE1 is a central enzyme in the BER pathway, and it has also been involved in the regulation of cellular response to genotoxic damage via direct and indirect mechanisms. In addition to the primary roles of APE1 in DNA damage repair, emerging evidences indicate that APE1 may also control RNA metabolism processes and thus gene expression4,13,68. In order to better clarify these novel functional aspects, we used well-established systems biology methods as optimal procedures to dissect/identify newly arising roles and possible mechanisms involving APE1. In this study, we focused on interactomic information obtained from previous studies4,12 as well as from novel experiments, which were performed using sensitive mass spectrometry technologies applied to the analysis of subcellular compartments. This allowed defining a final list containing 535 APE1-PPI elements that were finally subjected to a deep bioinformatics investigation.
Starting from this comprehensive list of proteins, the identification of direct and/or indirect interactions among APE1-binding partners was deduced, and a direct/indirect PPI network representing the global interactome of APE1 was constructed. This network, which was composed of 511 nodes and 3934 edges, retained a level of complexity that hampered the full understanding of the specific properties of each node. Therefore, a hub analysis was accomplished to define the top 30 nodes that were crucial for the communication of the whole network. Specifically, hub analysis was performed on betweenness centrality, a global metric that determines the involvement of each node in the information flow within the network. As a consequence, this method allowed the identification of the most informative proteins (hubs) for optimized therapeutic targeting in specific tumors (based on further bioinformatic analyses reported below). Among the most important hubs, APE1, SUMO1, SUMO2, TP53, ESR1, MDM2, PSMD4 and ACTB are worth mentioning (Fig. 3C). The resulting hub module was then analyzed with the Cytoscape plugin ClueGO to understand the role of these proteins in biological processes; the obtained results pointed to the involvement of these proteins mainly in DNA damage, mRNA stability and RNA splicing (Fig. 3D and Supplementary Table S4), indicating how these pathways were intertwined through the central role of APE1.
These data corroborated an emerging evidence in tumor biology; in fact, many DNA repair proteins are associated with those involved in RNA metabolism, thus proving a substantial role of the corresponding interactome networks in determining their non-canonical functions, which impact on gene expression in tumor cells3. These considerations emphasized the importance of understanding the regulatory behavior of APE1-interacting partners in various cancer datasets. To achieve this, the differential expression profiles and the gene expression correlations of APE1 and its interacting partners were calculated through the analysis of 33 TCGA datasets. The top 11 datasets with the highest number of differentially expressed genes, together with their correlation information, are represented as a bar chart in Fig. 4C. A very high percentage of differentially expressed genes was observed to have a significant prognostic potential, which was indicative for the relevance of these gene sets for related cancers (Fig. 5). These 11 gene sets were then subjected to survival analysis for a better understanding of their role according to a clinical perspective. LIHC, LUAD and PAAD networks, which were composed of bad prognostic genes in high percentages, confirmed previous studies on the important role of APE1 in these cancers52–55,57–65. Kaplan-Meier estimation was used to calculate survival probability associated with these genes; the ones having significantly lower survival prognosis (p < 0.05) were used to create cancer-specific PPI subnetworks (Fig. 6 and Supplementary Figs. S4–S11). These cancer-specific bad prognostic network signatures unveiled potential protein interactor targets to be further probed by novel therapeutic approaches.
In addition, the top 11 datasets were used to perform the upstream regulators analysis for wider understanding of the regulatory events acting on the gene sets and the top 3 master regulator genes, as well as their involvement in the apoptotic, proliferative and resistance pathways, were characterized. Out of 11 datasets, XRCC6 was found to be one of the top 3 master regulators in 5 datasets (i.e. HNSC, LUAD, LIHC, SKCM and BRCA), together with its interacting partner XRCC5, which was the master regulator of 2 datasets (UVM and BLCA). XRCC6 and XRCC5 are coding the proteins Ku70 and Ku80, which form a molecular heterodimer involved in the initial step of the non-homologous end joining (NHEJ) pathway. Ring-shaped Ku complex directs DNA-dependent protein kinase catalytic subunit (DNA PKcs coded by PRKDC/XRCC7) to the DNA ends, and triggers its kinase activity for DNA repair69. Interestingly, PRKDC was found among APE1 interactors as a component having a high correlation and bad survival prognosis in various datasets. It was previously reported that genetic variants of PRKDC play an important role in splicing regulation, causing mRNA instability70. PRKDC and XRCC6 play an important role in the suppression of chromosomal rearrangements and in the maintenance of genome integrity, along with a significant function in the recognition and repair of double strand breaks70. Therefore, their roles were studied in various cancers, such as breast71,72, glioma73, renal74, hepatocellular75,76, digestive77, bladder78 and lung70 cancer. On the other hand, Ku complex is known to interact with RECQL4 and to form a macromolecular assembly promoting NHEJ. A well-known RECQL4-binding partner, namely DDB179, was found to be one of the top 3 master regulators of 3 datasets (SKCM, BLCA and BRCA). DDB1 has been reported to be involved in the damage recognition step of the BER pathway80, and to be correlated with a high risk when down-regulated in head and neck squamous cell carcinoma81. The oncogenic transcriptional factor of RECQL4, namely YB1 (coded by YBX1), was here recognized as an interacting partner of APE1 and a master regulator observed in KIRC, KIRP and PAAD datasets. YB1 was found to be overexpressed in various cancer types and frequently associated with poor outcome and chemotherapy resistance82,83. It has also been reported to act as both an RNA- and DNA-binding protein, and as a component involved in miRNA processing45,84. These findings underline the important involvement of APE1-centered prognostic networks mainly in DNA repair, with the association of RNA metabolism in various cancer types.
Among the remaining top regulators identified in this study, we also found some important genes that might give some clues about the general involvement of the APE1 interactome in the p53 signaling pathway. In particular, some of the top 10 master regulators that were associated with the p53 signaling pathway (i.e. NUAK1, KDM1A, UBE2D1, RBX1 and UBE2M) deserved particular attention. For example, NUAK1 is known to be involved in DNA damage response by phosphorylating p53 and participating in the transcriptional regulation of the CDKN1A promoter46. Since NUAK1 is phosphorylated by AKT47, it has been hypothesized that this protein could be involved in acting between ATR and CDKN1A in response to low doses of UV irradiation48. Analogously, the master regulator KDM1A (LSD1) was hypothesized to be a potential therapeutic target for the estrogen-regulated type I endometrial cancer because of its crucial role in the LSD1/cyclin D1/PI3K/AKT feedback loop49. On the other hand, Zhou and colleagues recently reported that UBE2D1 facilitated the growth of hepatocellular carcinoma in vitro and in vivo by decreasing the p53 protein level in an ubiquitin-dependent manner50. Interestingly, the master regulators UBE2M and RBX1 were also reported to have up-regulation together with other neddylation enzymes to highlight overexpression of the neddylation pathway in HCC51. In conclusion, our analysis of the global network indicated the relation of APE1 and its interactors with DNA repair mechanisms, and the possible involvement of the p53 signaling pathway, as confirmed by the identification of p53 binding sites among the significantly enriched TFBS located in the promoters of APE1-PPI genes. However, what associated the highest number of top regulators was their role in the apoptotic, proliferative and resistance pathways.
In order to further characterize the LIHC, LUAD and PAAD TCGA datasets, the corresponding DEGs were examined also considering their subcellular locations. As a result, 24.4% of DEGs in LIHC, 21.4% of DEGs in LUAD and 16.8% of DEGs in PAAD datasets pointed to the involvement of APE1-PPIs in mitochondria functionality (Fig. 7). For example, the bad prognostic gene VDAC1 (voltage dependent anion channel 1), which is a multifunctional mitochondrial protein and an important regulator of cancer cell fate through its metabolic and energetic functions85,86, was commonly up-regulated in all 3 datasets. Similarly, many other mitochondria-resident APE1-PPIs were found to be commonly up-regulated in the analyzed datasets. Among these, it is worth mentioning: (i) SHMT2 (serine hydroxymethyltransferase), that mainly localizes to the matrix, nucleoid and inner membranes, and is known to be targeted by c-myc for cell survival, with various studies confirming its bad prognostic power in different cancer types87–91; (ii) pro-apoptotic protein SLC25A6 (mitochondrial ADP/ATP carrier-3, AAC3)92,93; (iii) ROS regulating respiratory complex III protein UQCRC2 (ubiquinol-cytochrome c reductase complex core protein 2)94; (iv) respiratory complex II protein SDHB (succinate dehydrogenase B)95; v) fatty acid β- oxidation proteins ETFA (electron transfer flavoprotein subunit alpha)96,97 and ACAA2 (acetyl-CoA acyltransferase 2)98; vi) bad prognostic multifunctional LGALS3 (galectin-3) protein99; (vii) autoimmunity protein HARS (histidyl-tRNA synthetase)100; (viii) oxidative damage control protein IDH1 (isocitrate dehydrogenase 1)101. Additional matrix proteins were found being up-regulated in LIHC and LUAD, while down-regulated in PAAD; they included fatty acid β-oxidation proteins ECHS1 (enoyl coenzyme A hydratase short chain 1)102 and ETFB (electron transfer flavoprotein subunit beta)103, multifunctional protein 17β-HSD10 (17β-hydroxysteroid dehydrogenase type 10, encoded by HSD17B10)104 and mitochondrial protein processor PMPCA (mitochondrial-processing peptidase subunit alpha)105. The relevance of APE1 function within mitochondria in tumor cells has recently been highlighted by our106 and other publications107, emphasizing a pivotal role for APE1 in mitochondrial-mediated signalling in cancer cells, thus opening new perspectives in cancer therapy.
Testing on a limited set of APE1-interacting partners, we previously observed that the APE1-interactome is dynamically regulated during genotoxic stress conditions4 with an enrichment of proteins involved in BER, while losing interaction with typical proteins involved in RNA metabolism. It would be interesting to extend this study to the whole APE1-PPI network described here. On the same track, it would be interesting to specifically evaluate the contribution of DNA and RNA molecules in modulating the APE1-PPI network dynamics. Information on the latter aspects may be obtained by characterizing the APE1-interactome before and upon the enzymatic removal of RNA and DNA. In conclusion and for the sake of clarity, we must state that this work is hypothesis generating and future studies will be needed to assess the function of APE1 in the protein complexes we discovered. Our current work is actually focused along these lines.
Materials and Methods
Cell line and materials
Inducible HeLa cell clones silenced for endogenous APE1 and reconstituted with the ectopic FLAG-tagged APE1 form were used11. HeLa cell clones were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Monza, Italy) supplemented with 10% v/v fetal bovine serum (Euroclone, Milan, Italy), 100 U/ml penicillin, 10 μg/ml streptomycin sulphate, 3 μg/ml blasticidin, 100 μg/ml zeocine, 400 μg/ml geneticin (Invitrogen), and cultured in a humidified incubator containing a 5% CO2 atmosphere, at 37 °C. For inducible APE1-shRNA experiments, doxycycline (1 μg/ml) (Sigma-Aldrich, St. Louis, MO) was added to the cell culture medium and cells were grown for 10 days. JHH-6 cells (undifferentiated hepatocellular carcinoma)31 were cultured in William’s medium E (Sigma-Aldrich), while A549 (adenocarcinomic human alveolar basal epithelial cells)32 cells were cultured in RPM1 (Euroclone); both cell cultures were supplemented with 10% v/v fetal bovine serum, 100 U/ml penicillin, 10 μg/ml streptomycin sulphate. All cell lines were tested for mycoplasma contamination (N-GARDE Mycoplamsa PCR Reagent, Euroclone).
Preparation of cell extracts and co-immunoprecipitation
Immunoprecipitation studies were carried out with whole cell extracts, and nuclear or cytoplasmatic subfractions of HeLa cell clones as already reported11,108 (see Supplementary Material and Methods for details).
Immunofluorescence confocal and Proximity Ligation analyses
Immunofluorescence procedures and Proximity Ligation Assay (PLA) (Duolink, Sigma-Aldrich) were carried out as described earlier4. PLA was performed following the manufacturer’s instructions. Cells were visualized through a Leica TCS SP8 confocal system (Leica Microsystems GmbH, Germany). See Supplementary Material and Methods for the list of the antibodies used.
Antibodies used and Western blotting analysis
For Western blotting analyses, cell lysates were resolved on 12% T SDS-PAGE, transferred onto nitrocellulose membranes (AmershamTM ProtranTM, GE Healthcare) and probed with the indicated antibodies (see Supplementary Material). The corresponding secondary antibodies labeled with IR-Dye (anti-rabbit IgG IRDye 680 and anti-mouse IgG IRDye 800) were used. Detection was performed with the Odyssey CLx Infrared imaging system (LI-COR GmbH, Germany). Protein bands were quantified using Odyssey software (Image Studio 5.0). Original uncropped images of western blots used in this study can be found in Supplementary Fig. S13.
Proteomic analysis
Immunopurified proteins from whole, nuclear and cytoplasmic cell extracts of HeLa cell clones expressing ectopic APE1 FLAG-tagged protein or stably transfected with the empty vector12 (SCR) were analyzed in parallel by 12% T SDS-PAGE. As additional control experiment, identical cell extracts from HeLa cells expressing APE1 FLAG-tagged were also co-immunoprecipitated with a resin lacking the FLAG antibody (res). After staining with colloidal Coomassie blue, whole gel lanes from all samples were cut into 12 slices, minced and washed with water. Corresponding proteins were separately in-gel reduced, S-alkylated with iodoacetamide and digested with trypsin, as previously reported109 and subjected to mass spectrometry analysis as detailed in Supplementary Material and Methods. A careful filtration for false positives ascertained in control samples (SCR and res) from whole, nuclear or cytoplasmic cell extracts allowed identifying APE1-binding proteins in APE1-FLAG co-immunoprecipitates from the corresponding cell extracts.
APE1-PPI network construction
The gene list of APE1-interacting partners was used to construct the corresponding PPI network by defining the interactions between the partners using the InWeb_InBioMap tool, applying the suggested parameters110. The APE1-PPI network was represented as an undirected graph (i.e., nodes and edges symbolize proteins and interactions between them, respectively), and it was visualized via Cytoscape (v3.6.1)38. The network enrichment analysis was performed using the ClueGO tool, using standard parameters111. The hubs of the network were obtained by using the Cytohubba tool based on the global metric, betweenness centrality112.
Transcription factor binding sites discovery
The FASTA-formatted sequences corresponding to the promoters (−2500,−1 nt from the TSS) of the 531 genes of the APE1 interactome were recovered using the “getfasta” command of the BEDTools toolset113. Sequences were analyzed using the LASAGNA-Search 2.0 tool to identify the presence of enriched transcription factor binding sites (TFBS); the matrix-derived model that used JASPAR CORE matrices was selected and the positions of the 145 TF models available were mapped for every promoter (Cutoff p-value: 0.001). For each promoter, the top10 results were then identified and used to provide the global counts of each TFBS in the analyzed dataset. Finally, the putative binding sites for TFs that underwent APE1 redox activity or that played their regulatory activity using APE1 as co-factor were represented as a network using Cytoscape, with node size corresponding to the number of identified binding sites.
Tumor datasets and differential gene expression analysis
The differential gene expression results from TCGA and normal datasets (GTEX data) for the genes encoding the proteins present in the APE1-PPI network were obtained via the GDC data portal hub (https://portal.gdc.cancer.gov/, last accessed July 2018). The RUVSeq package inside the R/Bioconductor environment was used to eliminate the batch effect coming from the combination of two data sources114. In order to better estimate the differentially expressed genes between the tumor and the normal corresponding datasets, we obtained “in-silico empirical” negative controls, i.e., the least significantly DE genes based on a first-pass DE analysis performed prior to RUVg normalization114. Empirical Distribution analysis, Pearson correlations and Kolmogorov-Smirnov analyses were performed between the gene expression profiles of APE1 and APE1-PPI elements in cancer patients or in the control groups using the stats package inside the R/Bioconductor environment. In particular, we compared the correlations of APE1 expression vs the PPI network genes expression with respect to: a) APE1 expression vs 100 sets with the same size of the PPI genes set composed by random genes; b) 100 random genes expressions vs the PPI network genes; c) APE1 expression vs all genes.
The analyses of the differentially expressed genes based on GO-CC (Gene Ontology-Cellular Component) was performed using the DAVID annotation tool115.
Survival analysis
For each TCGA dataset, differentially expressed genes (multiple correction adjustment using the Benjamini-Hochberg method, p < 0.05; absolute log fold change difference ≥1) corresponding to an interacting partner of APE1 were used to perform survival analysis. Kaplan-Meier plots were drawn using the RTCGA Bioconductor package116, which uses maximally selected rank statistics (maxstat) to determine the optimal cutpoint for continuous variables. Division of the samples was done within the 30–70% percentile range of gene expression by the optimal cutpoint value. The Benjamini-Hochberg method was used for p-value correction of Kaplan-Meier plots.
Upstream regulators analysis
The set of genes corresponding to APE1 interactors having significant differential expression and significant bad survival prognosis was used to define cancer specific bad prognostic subnetworks of APE1 for the top 11 TCGA datasets. For each subnetwork, putative master regulators were identified by the TRANSPATH database (5.1.1.1)117 through the geneXplain platform (geneXplain web edition 4.11)118. Identified regulators (max radius: 4; Score cutoff: 0.2; FDR cutoff: 0.05; Z-score cutoff: 1.0) were sorted ascendingly based on the Ranks sum, reflecting a combination of sorting by Score and by Z-score. Upon sorting by Score from the biggest values to the lowest, a rank was assigned to the molecules (the molecule with the highest Score had rank 1). Upon independent sorting by Z-Score from the biggest values to the lowest, a rank was assigned to the molecules (the molecule with the highest Z-score had rank 1). Afterwards, for each molecule, the ranks upon sorting by Score and upon sorting by Z-Score were summed up, and the Ranks Sum was generated. The lower the Ranks sum, the more interesting the candidate molecule was, with good Score and good Z-score values. The same analysis was also repeated on the APE1-PPI network regardless of any differential expression analysis, survival probability and cancer type.
Caspases activity assay
Caspase 3/7 activity levels were measured in a fluorescence-based assay using the Apo-One® Homogeneous Caspase 3/7 assay (Promega Corp., WI, USA). Assays were performed according to the manufacturer’s recommendations. Four-thousands cells were plated onto black 96-well plates and the day after cells were treated with Compound #366 in the presence/absence of rotenone (R8875, Sigma-Aldrich) (0.5 μM and 5 μM), for 24 h. Fluorescence was measured at 521 nm by using a multi-well plate reader (Enspire 2300 Multilabel Reader, PerkinElmer). The values were standardized to wells containing media alone.
Supplementary information
Acknowledgements
Authors thank Gianfranco Novi for technical assistance, Prof. Alexander Kel and the geneXplain team for the generous help in the upstream analysis, and Prof. Alessandro Quattrone for the support from the CIBIO Core Facilities scientific research. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG19862) to G. Tell and from Regione Campania, PO FESR 2014‐2020 – Obiettivo specifico 1.2 ‐ “Manifestazione di interesse per la realizzazione di Technology Platform nell’ambito della lotta alle patologie oncologiche” for the project “Sviluppo di approcci terapeutici innovativi per patologie neoplastiche resistenti ai trattamenti (SATIN)” to AS. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG19862) to G. Tell, and Programma Operativo Regionale Campania FESR 2014–2020, asse 1 OO.SS. 1.2.2/1.1.2 (SATIN - Sviluppo di approcci terapeutici innovativi per patologie neoplastiche resistenti ai trattamenti) to A. Scaloni.
Author contributions
G.T. designed and conceived the study and supervised the experiments; G.A. performed most of the wet lab experiments, analyzed the data and critically contributed to the interpretation of the results; G.M. performed the PLA analysis on JHH6 cell line; G.A., C.D.A. and A.S. carried out the proteomic analysis; D.A., E.D., S.P. performed the bioinformatics analysis of all the proteomics and genomics datasets; G.T., D.A., S.P. and G.A. mainly wrote the manuscript; E.D. and A.S. provided critical comments and suggestions and contributed to the interpretation of the results and writing of the manuscript. All authors critically read and approved the final version of the manuscript.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE119 partner repository with the dataset identifier PXD013368. Reviewer account details: Username: reviewer03955@ebi.ac.uk Password: Rh2r9CaX.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dilara Ayyildiz, Giulia Antoniali and Chiara D’Ambrosio.
Contributor Information
Gianluca Tell, Email: gianluca.tell@uniud.it.
Silvano Piazza, Email: silvano.piazza@unitn.it.
Supplementary information
is available for this paper at 10.1038/s41598-019-56981-z.
References
- 1.Hein N, Hannan KM, George AJ, Sanij E, Hannan RD. The nucleolus: an emerging target for cancer therapy. Trends Mol. Med. 2013;19:643–54. doi: 10.1016/j.molmed.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Wickramasinghe VO, Venkitaraman AR. RNA Processing and Genome Stability: Cause and Consequence. Mol. Cell. 2016;61:496–505. doi: 10.1016/j.molcel.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniali G, et al. Mammalian APE1 controls miRNA processing and its interactome is linked to cancer RNA metabolism. Nat. Commun. 2017;8:797. doi: 10.1038/s41467-017-00842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfatti MC, et al. Abasic and oxidized ribonucleotides embedded in DNA are processed by human APE1 and not by RNase H2. Nucleic Acids Res. 2017;45:11193–11212. doi: 10.1093/nar/gkx723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vohhodina J, Harkin DP, Savage KI. Dual roles of DNA repair enzymes in RNA biology/post-transcriptional control. Wiley Interdiscip. Rev. RNA. 2016;7:604–619. doi: 10.1002/wrna.1353. [DOI] [PubMed] [Google Scholar]
- 7.Jobert L, Nilsen H. Regulatory mechanisms of RNA function: emerging roles of DNA repair enzymes. Cell. Mol. Life Sci. 2014;71:2451–2465. doi: 10.1007/s00018-014-1562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbotts R, et al. Targeting human apurinic/apyrimidinic endonuclease 1 (APE1) in phosphatase and tensin homolog (PTEN) deficient melanoma cells for personalized therapy. Oncotarget. 2014;5:3273–3286. doi: 10.18632/oncotarget.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berquist BR, McNeill DR, Wilson DM. Characterization of abasic endonuclease activity of human Ape1 on alternative substrates, as well as effects of ATP and sequence context on AP site incision. J. Mol. Biol. 2008;379:17–27. doi: 10.1016/j.jmb.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes T, et al. Identification of Apurinic/apyrimidinic endonuclease 1 (APE1) as the endoribonuclease that cleaves c-myc mRNA. Nucleic Acids Res. 2009;37:3946–3958. doi: 10.1093/nar/gkp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vascotto C, et al. APE1/Ref-1 interacts with NPM1 within nucleoli and plays a role in the rRNA quality control process. Mol. Cell Biol. 2009;29:1834–54. doi: 10.1128/MCB.01337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vascotto C, et al. Genome-wide analysis and proteomic studies reveal APE1/Ref-1 multifunctional role in mammalian cells. Proteomics. 2009;9:1058–74. doi: 10.1002/pmic.200800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniali G, Lirussi L, Poletto M, Tell G. Emerging roles of the nucleolus in regulating the DNA damage response: the noncanonical DNA repair enzyme APE1/Ref-1 as a paradigmatical example. Antioxid. Redox Signal. 2014;20:621–639. doi: 10.1089/ars.2013.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tell G, Demple B. Base excision DNA repair and cancer. Oncotarget. 2015;6:584–585. doi: 10.18632/oncotarget.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W-C, et al. Characterization of the endoribonuclease active site of human apurinic/apyrimidinic endonuclease 1. J. Mol. Biol. 2011;411:960–971. doi: 10.1016/j.jmb.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illuzzi JL, et al. Functional assessment of population and tumor-associated APE1 protein variants. PLoS One. 2013;8:e65922. doi: 10.1371/journal.pone.0065922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lirussi L, et al. APE1 polymorphic variants cause persistent genomic stress and affect cancer cell proliferation. Oncotarget. 2016;7:26293–26306. doi: 10.18632/oncotarget.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vascotto C, et al. Functional regulation of the apurinic/apyrimidinic endonuclease 1 by nucleophosmin: impact on tumor biology. Oncogene. 2014;33:2876–2887. doi: 10.1038/onc.2013.251. [DOI] [PubMed] [Google Scholar]
- 19.Poletto M, et al. Role of the unstructured N-terminal domain of the hAPE1 (human apurinic/apyrimidinic endonuclease 1) in the modulation of its interaction with nucleic acids and NPM1 (nucleophosmin) Biochem. J. 2013;452:545–557. doi: 10.1042/BJ20121277. [DOI] [PubMed] [Google Scholar]
- 20.Zou Q, et al. The human nucleophosmin 1 mutation A inhibits myeloid differentiation of leukemia cells by modulating miR-10b. Oncotarget. 2016;7:71477–71490. doi: 10.18632/oncotarget.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havelange V, et al. Implications of the miR-10 family in chemotherapy response of NPM1-mutated AML. Blood. 2014;123:2412–2415. doi: 10.1182/blood-2013-10-532374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Londero AP, et al. Expression and prognostic significance of APE1/Ref-1 and NPM1 proteins in high-grade ovarian serous cancer. Am. J. Clin. Pathol. 2014;141:404–414. doi: 10.1309/AJCPIDKDLSGE26CX. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, et al. The expression profile and prognostic value of APE/Ref-1 and NPM1 in high-grade serous ovarian adenocarcinoma. APMIS. 2017;125:857–862. doi: 10.1111/apm.12733. [DOI] [PubMed] [Google Scholar]
- 25.Rommer A, et al. Overexpression of primary microRNA 221/222 in acute myeloid leukemia. BMC Cancer. 2013;13:364. doi: 10.1186/1471-2407-13-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poletto M, et al. Inhibitors of the apurinic/apyrimidinic endonuclease 1 (APE1)/nucleophosmin (NPM1) interaction that display anti-tumor properties. Mol. Carcinog. 2016;55:688–704. doi: 10.1002/mc.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barchiesi A, Wasilewski M, Chacinska A, Tell G, Vascotto C. Mitochondrial translocation of APE1 relies on the MIA pathway. Nucleic Acids Res. 2015;43:5451–5464. doi: 10.1093/nar/gkv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantini D, et al. Critical lysine residues within the overlooked N-terminal domain of human APE1 regulate its biological functions. Nucleic Acids Res. 2010;38:8239–56. doi: 10.1093/nar/gkq691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristini A, Groh M, Kristiansen MS, Gromak N. RNA/DNA Hybrid Interactome Identifies DXH9 as a Molecular Player in Transcriptional Termination and R-Loop-Associated DNA Damage. Cell Rep. 2018;23:1891–1905. doi: 10.1016/j.celrep.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farg MA, Konopka A, Soo KY, Ito D, Atkin JD. The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2017;26:2882–2896. doi: 10.1093/hmg/ddx170. [DOI] [PubMed] [Google Scholar]
- 31.Fujise K, et al. Integration of hepatitis B virus DNA into cells of six established human hepatocellular carcinoma cell lines. Hepatogastroenterology. 1990;37:457–460. [PubMed] [Google Scholar]
- 32.Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998;243:359–366. doi: 10.1006/excr.1998.4172. [DOI] [PubMed] [Google Scholar]
- 33.Abbasi S, Schild-Poulter C. Mapping the Ku Interactome Using Proximity-Dependent Biotin Identification in Human Cells. J. Proteome Res. 2019;18:1064–1077. doi: 10.1021/acs.jproteome.8b00771. [DOI] [PubMed] [Google Scholar]
- 34.Shchepachev V, et al. Defining the RNA interactome by total RNA-associated protein purification. Mol. Syst. Biol. 2019;15:e8689. doi: 10.15252/msb.20188689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira PI, et al. Nucleic acid oxidation in Alzheimer disease. Free. Radic. Biol. Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Li Yang, Merkel Chelsea D., Zeng Xuemei, Heier Jonathon A., Cantrell Pamela S., Sun Mai, Stolz Donna B., Watkins Simon C., Yates Nathan A., Kwiatkowski Adam V. The N-cadherin interactome in primary cardiomyocytes as defined using quantitative proximity proteomics. Journal of Cell Science. 2019;132(3):jcs221606. doi: 10.1242/jcs.221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arifuzzaman M, et al. Large-scale identification of protein-protein interaction of Escherichia coli K-12. Genome Res. 2006;16:686–691. doi: 10.1101/gr.4527806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Wilson DM. Human apurinic/apyrimidinic endonuclease 1. Antioxid. Redox Signal. 2014;20:678–707. doi: 10.1089/ars.2013.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C, Huang C-H. LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. BioTechniques. 2013;54:141–153. doi: 10.2144/000113999. [DOI] [PubMed] [Google Scholar]
- 41.Maurizio E, et al. Translating Proteomic Into Functional Data: An High Mobility Group A1 (HMGA1) Proteomic Signature Has Prognostic Value in Breast Cancer. Mol. Cell Proteom. 2016;15:109–123. doi: 10.1074/mcp.M115.050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wittschieben BO, Iwai S, Wood RD. DDB1-DDB2 (xeroderma pigmentosum group E) protein complex recognizes a cyclobutane pyrimidine dimer, mismatches, apurinic/apyrimidinic sites, and compound lesions in DNA. J. Biol. Chem. 2005;280:39982–9. doi: 10.1074/jbc.M507854200. [DOI] [PubMed] [Google Scholar]
- 44.Sugasawa K. Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair. (Amst.) 2016;44:110–117. doi: 10.1016/j.dnarep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Wu S-L, et al. Genome-wide analysis of YB-1-RNA interactions reveals a novel role of YB-1 in miRNA processing in glioblastoma multiforme. Nucleic Acids Res. 2015;43:8516–8528. doi: 10.1093/nar/gkv779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou X, et al. A new role of NUAK1: directly phosphorylating p53 and regulating cell proliferation. Oncogene. 2011;30:2933–2942. doi: 10.1038/onc.2011.19. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki A, et al. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- 48.Esteve-Puig R, et al. A mouse model uncovers LKB1 as an UVB-induced DNA damage sensor mediating CDKN1A (p21WAF1/CIP1) degradation. PLoS Genet. 2014;10:e1004721. doi: 10.1371/journal.pgen.1004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, et al. LSD1 sustains estrogen-driven endometrial carcinoma cell proliferation through the PI3K/AKT pathway via di-demethylating H3K9 of cyclin D1. Int. J. Oncol. 2017;50:942–952. doi: 10.3892/ijo.2017.3849. [DOI] [PubMed] [Google Scholar]
- 50.Zhou C, Bi F, Yuan J, Yang F, Sun S. Gain of UBE2D1 facilitates hepatocellular carcinoma progression and is associated with DNA damage caused by continuous IL-6. J. Exp. Clin. Cancer Res. 2018;37:290. doi: 10.1186/s13046-018-0951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu, J. et al. Overactivated neddylation pathway in human hepatocellular carcinoma. Cancer Med (2018). [DOI] [PMC free article] [PubMed]
- 52.Cheng T-L, et al. Induction of apurinic endonuclease 1 overexpression by endoplasmic reticulum stress in hepatoma cells. Int. J. Mol. Sci. 2014;15:12442–12457. doi: 10.3390/ijms150712442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z, Zhao J. Effect of APE1 and XRCC1 gene polymorphism on susceptibility to hepatocellular carcinoma and sensitivity to cisplatin. Int. J. Clin. Exp. Med. 2015;8:9931–9936. [PMC free article] [PubMed] [Google Scholar]
- 54.Di Maso V, et al. Transcriptional Up-Regulation of APE1/Ref-1 in Hepatic Tumor: Role in Hepatocytes Resistance to Oxidative Stress and Apoptosis. PLoS ONE. 2015;10:e0143289. doi: 10.1371/journal.pone.0143289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Z, et al. Differential expression of APE1 in hepatocellular carcinoma and the effects on proliferation and apoptosis of cancer cells. Biosci. Trends. 2018;12:456–462. doi: 10.5582/bst.2018.01239. [DOI] [PubMed] [Google Scholar]
- 56.Pascut D, et al. Serum AP-endonuclease 1 (sAPE1) as novel biomarker for hepatocellular carcinoma. Oncotarget. 2018;10:383–394. doi: 10.18632/oncotarget.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, et al. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients. Oncotarget. 2016;7:77482–77494. doi: 10.18632/oncotarget.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu S-N, et al. Evaluation of Prediction of Polymorphisms of DNA Repair Genes on the Efficacy of Platinum-Based Chemotherapy in Patients With Non-Small Cell Lung Cancer: A Network Meta-Analysis. J. Cell. Biochem. 2017;118:4782–4791. doi: 10.1002/jcb.26147. [DOI] [PubMed] [Google Scholar]
- 59.Thakur S, Dhiman M, Mantha AK. APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Mol. Cell. Biochem. 2018;441:201–216. doi: 10.1007/s11010-017-3186-7. [DOI] [PubMed] [Google Scholar]
- 60.Lu G-S, Li M, Xu C-X, Wang D. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis. 2018;9:1111. doi: 10.1038/s41419-018-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Y, Zhou S, Sandusky GE, Kelley MR, Fishel ML. Reduced expression of DNA repair and redox signaling protein APE1/Ref-1 impairs human pancreatic cancer cell survival, proliferation, and cell cycle progression. Cancer Invest. 2010;28:885–95. doi: 10.3109/07357907.2010.512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cardoso AA, et al. APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3 dual-targeting effectively inhibits pancreatic cancer cell survival. PLoS One. 2012;7:e47462. doi: 10.1371/journal.pone.0047462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Logsdon DP, et al. Regulation of HIF1α under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual Targeting in Patient-Derived 3D Pancreatic Cancer Models. Mol. Cancer Ther. 2016;15:2722–2732. doi: 10.1158/1535-7163.MCT-16-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah F, et al. APE1/Ref-1 knockdown in pancreatic ductal adenocarcinoma - characterizing gene expression changes and identifying novel pathways using single-cell RNA sequencing. Mol. Oncol. 2017;11:1711–1732. doi: 10.1002/1878-0261.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logsdon DP, et al. Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1 dramatically affects pancreatic cancer cell survival. Sci. Rep. 2018;8:13759. doi: 10.1038/s41598-018-32034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rai G, et al. Synthesis, biological evaluation, and structure-activity relationships of a novel class of apurinic/apyrimidinic endonuclease 1 inhibitors. J. Med. Chem. 2012;55:3101–12. doi: 10.1021/jm201537d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Höglinger GU, et al. Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J. Neurochem. 2003;86:1297–1307. doi: 10.1046/j.1471-4159.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- 68.Tell G, Wilson DM, 3rd, Lee CH. Intrusion of a DNA repair protein in the RNome world: is this the beginning of a new era? Mol. Cell Biol. 2010;30:366–71. doi: 10.1128/MCB.01174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karran P. DNA double strand break repair in mammalian cells. Curr. Opin. Genet. Dev. 2000;10:144–150. doi: 10.1016/S0959-437X(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 70.Singh A, Singh N, Behera D, Sharma S. Role of polymorphic XRCC6 (Ku70)/XRCC7 (DNA-PKcs) genes towards susceptibility and prognosis of lung cancer patients undergoing platinum based doublet chemotherapy. Mol. Biol. Rep. 2018;45:253–261. doi: 10.1007/s11033-018-4158-z. [DOI] [PubMed] [Google Scholar]
- 71.He W, et al. The Ku70 -1310C/G promoter polymorphism is associated with breast cancer susceptibility in Chinese Han population. Mol. Biol. Rep. 2012;39:577–583. doi: 10.1007/s11033-011-0773-7. [DOI] [PubMed] [Google Scholar]
- 72.Nasiri M, Saadat I, Omidvari S, Saadat M. Genetic variation in DNA repair gene XRCC7 (G6721T) and susceptibility to breast cancer. Gene. 2012;505:195–197. doi: 10.1016/j.gene.2012.04.065. [DOI] [PubMed] [Google Scholar]
- 73.Wang L-E, et al. Polymorphisms of DNA repair genes and risk of glioma. Cancer Res. 2004;64:5560–5563. doi: 10.1158/0008-5472.CAN-03-2181. [DOI] [PubMed] [Google Scholar]
- 74.Hirata H, et al. Polymorphisms of DNA repair genes are associated with renal cell carcinoma. Biochem. Biophys. Res. Commun. 2006;342:1058–1062. doi: 10.1016/j.bbrc.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh Y-H, et al. DNA double-strand break repair gene XRCC7 genotypes were associated with hepatocellular carcinoma risk in Taiwanese males and alcohol drinkers. Tumour Biol. 2015;36:4101–4106. doi: 10.1007/s13277-014-2934-5. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, et al. Genetic polymorphism of DNA base-excision repair genes (APE1, OGG1 and XRCC1) and their correlation with risk of lung cancer in a Chinese population. Arch. Med. Res. 2011;42:226–34. doi: 10.1016/j.arcmed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Yang M-D, et al. Predictive role of XRCC5/XRCC6 genotypes in digestive system cancers. World J. Gastrointest. Oncol. 2011;3:175–181. doi: 10.4251/wjgo.v3.i12.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S-Y, et al. Genetic variants of the XRCC7 gene involved in DNA repair and risk of human bladder cancer. Int. J. Urol. 2008;15:534–539. doi: 10.1111/j.1442-2042.2008.02049.x. [DOI] [PubMed] [Google Scholar]
- 79.Lu H, et al. Cell cycle-dependent phosphorylation regulates RECQL4 pathway choice and ubiquitination in DNA double-strand break repair. Nat. Commun. 2017;8:2039. doi: 10.1038/s41467-017-02146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spivak G. Nucleotide excision repair in humans. DNA Repair. (Amst.) 2015;36:13–18. doi: 10.1016/j.dnarep.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han P, et al. Reduced mRNA expression of nucleotide excision repair genes in lymphocytes and risk of squamous cell carcinoma of the head and neck. Carcinogenesis. 2017;38:504–510. doi: 10.1093/carcin/bgx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein U, et al. YB-1 facilitates basal and 5-fluorouracil-inducible expression of the human major vault protein (MVP) gene. Oncogene. 2005;24:3606–3618. doi: 10.1038/sj.onc.1208386. [DOI] [PubMed] [Google Scholar]
- 83.Wu J, Stratford AL, Astanehe A, Dunn SE. YB-1 is a Transcription/Translation Factor that Orchestrates the Oncogenome by Hardwiring Signal Transduction to Gene Expression. Transl. Oncogenomics. 2007;2:49–65. [PMC free article] [PubMed] [Google Scholar]
- 84.Suresh PS, Tsutsumi R, Venkatesh T. YBX1 at the crossroads of non-coding transcriptome, exosomal, and cytoplasmic granular signaling. Eur. J. Cell Biol. 2018;97:163–167. doi: 10.1016/j.ejcb.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta. 2015;1848:2547–2575. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 86.Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Mol. Ther. Nucleic Acids. 2014;3:e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woo CC, Chen WC, Teo XQ, Radda GK, Lee PTH. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget. 2016;7:53005–53017. doi: 10.18632/oncotarget.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, et al. Prognostic and therapeutic value of mitochondrial serine hydroxyl-methyltransferase 2 as a breast cancer biomarker. Oncol. Rep. 2016;36:2489–2500. doi: 10.3892/or.2016.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang B, et al. Mitochondrial serine hydroxymethyltransferase 2 is a potential diagnostic and prognostic biomarker for human glioma. Clin. Neurol. Neurosurg. 2017;154:28–33. doi: 10.1016/j.clineuro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Lee GY, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74:3114–3126. doi: 10.1158/0008-5472.CAN-13-2683. [DOI] [PubMed] [Google Scholar]
- 91.Antonov A, et al. Bioinformatics analysis of the serine and glycine pathway in cancer cells. Oncotarget. 2014;5:11004–11013. doi: 10.18632/oncotarget.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zamora M, Granell M, Mampel T, Viñas O. Adenine nucleotide translocase 3 (ANT3) overexpression induces apoptosis in cultured cells. FEBS Lett. 2004;563:155–160. doi: 10.1016/S0014-5793(04)00293-5. [DOI] [PubMed] [Google Scholar]
- 93.Gallerne C, et al. The fourth isoform of the adenine nucleotide translocator inhibits mitochondrial apoptosis in cancer cells. Int. J. Biochem. Cell Biol. 2010;42:623–629. doi: 10.1016/j.biocel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 94.Aguilera-Aguirre L, et al. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saxena Neetu, Maio Nunziata, Crooks Daniel R., Ricketts Christopher J., Yang Youfeng, Wei Ming-Hui, Fan Teresa W-M., Lane Andrew N., Sourbier Carole, Singh Anamika, Killian J. Keith, Meltzer Paul S., Vocke Cathy D., Rouault Tracey A., Linehan W. Marston. SDHB-Deficient Cancers: The Role of Mutations That Impair Iron Sulfur Cluster Delivery. Journal of the National Cancer Institute. 2015;108(1):djv287. doi: 10.1093/jnci/djv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 97.Toogood HS, Leys D, Scrutton NS. Dynamics driving function: new insights from electron transferring flavoproteins and partner complexes. FEBS J. 2007;274:5481–5504. doi: 10.1111/j.1742-4658.2007.06107.x. [DOI] [PubMed] [Google Scholar]
- 98.Miltiadou D, et al. Variants in the 3’ untranslated region of the ovine acetyl-coenzyme A acyltransferase 2 gene are associated with dairy traits and exhibit differential allelic expression. J. Dairy. Sci. 2017;100:6285–6297. doi: 10.3168/jds.2016-12326. [DOI] [PubMed] [Google Scholar]
- 99.Wang H, et al. LGALS3 Promotes Treatment Resistance in Glioblastoma and Is Associated with Tumor Risk and Prognosis. Cancer Epidemiol. Biomarkers Prev. 2019;28:760–769. doi: 10.1158/1055-9965.EPI-18-0638. [DOI] [PubMed] [Google Scholar]
- 100.Freist W, Verhey JF, Rühlmann A, Gauss DH, Arnez JG. Histidyl-tRNA synthetase. Biol. Chem. 1999;380:623–646. doi: 10.1515/BC.1999.079. [DOI] [PubMed] [Google Scholar]
- 101.Khurshed, M. et al. IDH1-mutant cancer cells are sensitive to cisplatin and an IDH1-mutant inhibitor counteracts this sensitivity. FASEB J. fj201800547R (2018). [DOI] [PMC free article] [PubMed]
- 102.Zhu X-S, et al. Attenuation of enoyl coenzyme A hydratase short chain 1 expression in gastric cancer cells inhibits cell proliferation and migration in vitro. Cell. Mol. Biol. Lett. 2014;19:576–589. doi: 10.2478/s11658-014-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiz-Pinto S, et al. Exome array analysis identifies ETFB as a novel susceptibility gene for anthracycline-induced cardiotoxicity in cancer patients. Breast Cancer Res. Treat. 2018;167:249–256. doi: 10.1007/s10549-017-4497-9. [DOI] [PubMed] [Google Scholar]
- 104.Yang S-Y, et al. Roles of 17β-hydroxysteroid dehydrogenase type 10 in neurodegenerative disorders. J. Steroid Biochem. Mol. Biol. 2014;143:460–472. doi: 10.1016/j.jsbmb.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Joshi M, et al. Mutations in the substrate binding glycine-rich loop of the mitochondrial processing peptidase-α protein (PMPCA) cause a severe mitochondrial disease. Cold Spring Harb. Mol. Case Stud. 2016;2:a000786. doi: 10.1101/mcs.a000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Codrich M, et al. Inhibition of APE1-endonuclease activity affects cell metabolism in colon cancer cells via a p53-dependent pathway. DNA Repair. (Amst.) 2019;82:102675. doi: 10.1016/j.dnarep.2019.102675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li M, et al. Human AP endonuclease/redox factor APE1/ref-1 modulates mitochondrial function after oxidative stress by regulating the transcriptional activity of NRF1. Free. Radic. Biol. Med. 2012;53:237–248. doi: 10.1016/j.freeradbiomed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Antoniali G, Marcuzzi F, Casarano E, Tell G. Cadmium treatment suppresses DNA polymerase δ catalytic subunit gene expression by acting on the p53 and Sp1 regulatory axis. DNA Repair. (Amst.) 2015;35:90–105. doi: 10.1016/j.dnarep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Spreafico A, et al. A proteomic study on human osteoblastic cells proliferation and differentiation. Proteomics. 2006;6:3520–3532. doi: 10.1002/pmic.200500858. [DOI] [PubMed] [Google Scholar]
- 110.Li T, et al. A scored human protein-protein interaction network to catalyze genomic interpretation. Nat. Methods. 2017;14:61–64. doi: 10.1038/nmeth.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bindea G, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chin C-H, et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014;8:S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 116.Kosinski, M. & Biecek, P. RTCGA: The Cancer Genome Atlas Data Integration. R package version 1.8.0, https://rtcga.github.io/RTCGA.
- 117.Krull M, et al. TRANSPATH: an information resource for storing and visualizing signaling pathways and their pathological aberrations. Nucleic Acids Res. 2006;34:D546–551. doi: 10.1093/nar/gkj107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kolpakov F, Poroikov V, Selivanova G, Kel A. GeneXplain — Identification of Causal Biomarkers and Drug Targets in Personalized Cancer Pathways. J. Biomol. Tech. 2011;22:S16. [Google Scholar]
- 119.Perez-Riverol Y, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE119 partner repository with the dataset identifier PXD013368. Reviewer account details: Username: reviewer03955@ebi.ac.uk Password: Rh2r9CaX.