Abstract
A string of complete genome sequences of Small ruminant morbillivirus (SRMV) have been reported from different parts of the globe including Asia, Africa and the Middle East. Despite individual genome sequence-based analysis, there is a paucity of comparative genomic and evolutionary analysis to provide overarching and comprehensive evolutionary insights. Therefore, we first enriched the existing database of complete genome sequences of SRMVs with Pakistan-originated strains and then explored overall nucleotide diversity, genomic and residue characteristics, and deduced an evolutionary relationship among strains representing a diverse geographical region worldwide. The average number of pairwise nucleotide differences among the whole genomes was found to be 788.690 with a diversity in nucleotide sequences (0.04889 ± S.D. 0.00468) and haplotype variance (0.00001). The RNA-dependent-RNA polymerase (L) gene revealed phylogenetic relationship among SRMVs in a pattern similar to those of complete genome and the nucleoprotein (N) gene. Therefore, we propose another useful molecular marker that may be employed for future epidemiological investigations. Based on evolutionary analysis, the mean evolution rate for the complete genome, N, P, M, F, H and L genes of SRMV was estimated to be 9.953 × 10–4, 1.1 × 10–3, 1.23 × 10–3, 2.56 × 10–3, 2.01 × 10–3, 1.47 × 10–3 and 9.75 × 10–4 substitutions per site per year, respectively. A recombinant event was observed in a Pakistan-originated strain (KY967608) revealing Indian strains as major (98.1%, KR140086) and minor parents (99.8%, KT860064). Taken together, outcomes of the study augment our knowledge and current understanding towards ongoing phylogenomic and evolutionary dynamics for better comprehensions of SRMVs and effective disease control interventions.
Subject terms: Genetic databases, Viral evolution
Introduction
Peste des petits ruminants (PPR), caused by Small ruminant morbillivrus (SRMV), is a contagious transboundary disease of domestic and wild ruminants1,2. Despite exhaustive vaccination, the disease is endemic across many regions/countries in Africa, Middle East and Asia, where occurrence of frequent disease outbreaks is not uncommon3–7. Currently, the PPR is threatening approximately 80% of the global population of sheep and goats with an estimated loss of USD 2.1 billion per year8.
The SRMV belongs to the genus Morbillivirus within the family Paramyxoviridae. It is a pleomorphic and enveloped virus that carries a negative sense RNA genome9 of variable length, from 15,927 to 16,058 nucleotides (NCBI database). The genome encodes six structural and two non-structural proteins in an order of 3′-N-P/C/V-M-F-HN-L-5′. Non-structural proteins (V and C) are encoded either by alternate open reading frames or mRNA editing in the phosphoprotein (P) gene. Based upon either N gene (255 bp) or F gene (322 bp), four distinct lineages of SRMVs (I-IV) are reported so far. Lineage I-II viruses are mostly reported from West African countries. Lineage III viruses seem restricted to the Middle East and East African countries. Lineage IV viruses have been reported from Asian and African countries1,10. The lineage IV is replacing prevalence of other lineages (i.e. I-III) territories and the occurrence of lineage IV is overwhelming even in Africa. These features demonstrate that lineage IV possess stronger positive selection and host-adaptation potential in a wide spectrum of hosts and geographical areas11,12.
Given the fact that genetic variations within a population of viruses could alter their pathogenicity and host spectrum, viral genetic diversity is considered a key to unleash viral evolution13. Using complete or partial sequencing of single genes (H, N or F), the clustering pattern, genomic and residue characteristics of SRMVs have widely been studied and discussed across the globe5,10,12,14. However, based upon each of these particular genes, the deduced genomic and residue characteristics may not be considered enough to predict ongoing evolutionary patterns across the whole length of the genome. In addition, many aspects of SRMVs evolution, including ancestral strain links, historical and geographic patterns of strain dispersal, divergence and time of origin remain poorly understood. These aspects are important because evolution within a single gene may not necessarily be occurring at the same rate as that of the whole genome15. Also, being RNA viruses, SRMVs are more prone to mutations during acute infection and therefore could present a polymorphic population11. Therefore, genetic diversity driven from consensus sequences of partial genomes could be far from representing the actual polymorphism across the whole length of the genome. Taken together, understanding comparative phylogenomics and evolutionary dynamics by exploiting complete genome sequence data of SRMVs facilitate better elucidate the genetic diversity, trends in its evolution and disease distribution pattern across diverse geographical regions. With this background, complimented by two complete genome sequences from Pakistan we used complete genome sequence data of SRMVs accessible in public database (until October 01, 2019) and analyzed for genetic diversity, phylogenomics and residue characteristics through different bioinformatics tools. We extend the analysis to each of the coding genes and identified potential ancestral relationship among SRMV-lineages reported from different countries during different time-period. In addition, we analyzed coding genes of all reported complete genomes to determine SRMV’s divergence time, and identified another candidate gene to be used as a phylogenetic marker. Together, the outcome will be expected to enhance our understating of phylogenetic and evolutionary dynamics of SRMVs across the globe.
Results
Comparative genome features
The comparative genomic analysis revealed a variable length of genomes as 15927, 15942, 15948, 15954, 15957 and 16058 nucleotides. Most of sequences across the globe had 15948 nucleotides (n = 39) whereas a number of Chinese strains (n = 31) and a Mongolian strain (KY888168) possessed a genome length of 15954 nucleotides. Only a single SRMV strain reported from India (KT270355) carried 15942 nucleotides. One Israeli strain (MF678816) had 15927 nucleotides. Two unusual genome lengths of 15957 (KM089831) and 16058 nucleotides (KM816619) were exclusively reported from China (Table 1). Excluding complete genomes of unusual lengths (MF678816, KM089831 and KM816619) while performing complete genome-specific analysis, the percentage for GC and AT contents was 47% and 53%, respectively. The proportion of GC content was found highest in N gene (50%) followed by P (48%), each of M, F, H (46%), L genes (43%), trailer (41%) and leader region (38%) (Table 2). The study genomes had a 52 nucleotide (nt) long leader in 3ʹ UTR at 107 nt long genome promoter region and a 73 nt long trailer at 5ʹ UTR in 109 nt long anti-genome promoter region. The total length of each of the genes varied across the whole genome: N gene (1578 nt) encoded 526 aa of 58 KDa, P gene (1530 nt) encoded 510 aa of 55 KDa, M gene (1008 nt) encoded 336 aa of 38 KDa, F gene (1641 nt) encoded 546 aa of 59 KDa, H gene (1830 nt) encoded 610 aa of 69 KDa and L gene (6552 nt) encoded 2184 aa of 247 KDa. Although all complete SRMV sequences showed variable genome length, the coding region for each of the genes was the same. The varying genome length was due to insertion of nucleotides in a non-coding region between P and M genes, and between M and F genes (Table 3). However, all genes were separated by similar conserved non-coding intergenic trinucleotide (CTT) except for one intergenic region between L gene and the trailer sequence (CTA).
Table 1.
A brief summary of dataset on SRMVs available at public database incluidng under-study Pakistan-originated strains
| Lineage | Geography | Accession number | Strain name | Genome length (nts) | Year | Host | Location |
|---|---|---|---|---|---|---|---|
| I | Africa | KP789375 | E32/1969 | 15948 | 1969 | Goat | Senegal |
| EU267273 | ICV89 | 15948 | 1989 | Goat | Nigeria | ||
| II | Africa | MF741712 | PPRV/Sierra Leone/048/2011 | 15948 | 2011 | Goat | Sierra Leone |
| KU236379 | Lib/2015 | 15948 | 2015 | Goat | Libya | ||
| KR781451 | CIV/01 P/2009 | 15948 | 2009 | Goat | Cote d’Ivoire | ||
| KM212177 | SnDk11/13 | 15948 | 2013 | Goat | Senegal | ||
| KR781449 | Benin/10/2011 | 15948 | 2011 | Sheep | Benin | ||
| KJ466104 | Ghana/NK1/2010 | 15948 | 2010 | Sheep | Ghana | ||
| KR828814 | NGKW2012–MSLN | 15948 | 2012 | Goat | Nigeria | ||
| EU267274 | Ng76/1 | 15948 | 1976 | Goat | Nigeria | ||
| KR781450 | Benin/B1/1969 | 15948 | 1969 | Goat | Benin | ||
| HQ197753* | Nigeria/75/1 | 15948 | 1975 | Goat | Nigeria | ||
| X74443* | Nig/Vaccine | 15948 | 1975 | Goat | Nigeria | ||
| III | Asia | KJ867544 | Oman 1983 | 15948 | 1983 | Goat | Oman |
| KJ867545 | UAE 1986 | 15948 | 1986 | Gazelle | UAE | ||
| Africa | KJ867540 | Ethiopia 1994 | 15948 | 1994 | Goat | Ethiopia | |
| KJ867543 | Uganda 2012 | 15948 | 2012 | Goat | Uganda | ||
| IV | Africa | KR828813 | NGYO2013–2162 | 15948 | 2013 | Goat | Nigeria |
| KJ867541 | Ethiopia 2010 | 15948 | 2010 | Goat | Ethiopia | ||
| KY885100 | S15 | 15948 | 2015 | Goat | Algeria | ||
| KC594074 | Morocco 2008 | 15948 | 2008 | Goat | Morocco | ||
| Euro-asiatic | AJ849636 | Turkey 2000 | 15948 | 2000 | Goat | Turkey | |
| Asia | MF678816 | 1008 | 15927 | 2017 | Nubian ibex | Israel | |
| MF737202 | Georgia/Tbilisi/2016 | 15948 | 2016 | Goat | Georgia | ||
| KJ867542* | Sungri 1996 MSD | 15948 | 1996 | Goat | India | ||
| KR140086 | Izatnagar/94 | 15948 | 1994 | Goat | India | ||
| KF727981* | Sungri/96 | 15948 | 1996 | Goat | India | ||
| JX217850 | Tibet/Bharal/2008 | 15948 | 2008 | Bharal | China | ||
| FJ905304 | China/Tibet/Geg/07–30 | 15948 | 2007 | Goat | China | ||
| KX421388 | China/33/2007 | 15948 | 2007 | Goat | China | ||
| JF939201 | China/Tib/07 | 15948 | 2007 | Goat | China | ||
| KM816619 | GZL-14 | 16058 | 2014 | Goat | China | ||
| KT633939 | China/XJBZ/2015 | 15954 | 2015 | Ibex | China | ||
| KY888168 | PPRV/Mongolia/9/2016 | 15954 | 2016 | Goat | Mongolia | ||
| KM089830 | CH/HNNY/2014 | 15954 | 2014 | Goat | China | ||
| KM089832 | CH/HNZM/2014 | 15954 | 2014 | Goat | China | ||
| KP868655 | CH/GDDG/2014 | 15954 | 2014 | Goat | China | ||
| KM089831 | CH/HNZK/2014 | 15957 | 2014 | Goat | China | ||
| KP260624 | China/BJ/2014 | 15954 | 2014 | Goat | China | ||
| MF443343 | ChinaJS2014 | 15954 | 2014 | Goat | China | ||
| MF443344 | ChinaJL2014 | 15954 | 2014 | Sheep | China | ||
| MF443352 | ChinaGD2014 | 15954 | 2014 | Goat | China | ||
| MF443346 | ChinaHLJ2014 | 15954 | 2014 | Goat | China | ||
| MF443337 | ChinaSX2014 | 15954 | 2014 | Goat | China | ||
| MF443353 | ChinaCQ2014 | 15954 | 2014 | Goat | China | ||
| MF443339 | ChinaSaX2014 | 15954 | 2014 | Goat | China | ||
| MF443345 | ChinaHN2014 | 15954 | 2014 | Goat | China | ||
| MF443347 | ChinaHeN2014 | 15954 | 2014 | Goat | China | ||
| MF443348 | ChinaHB2014 | 15954 | 2014 | Goat | China | ||
| MF443336 | ChinaYN2014 | 15954 | 2014 | Goat | China | ||
| MF443342 | ChinaJX2014 | 15954 | 2014 | Goat | China | ||
| MF443335 | ChinaZJ2014 | 15954 | 2014 | Goat | China | ||
| MF443338 | ChinaSC2014 | 15954 | 2014 | Goat | China | ||
| MF443340 | ChinaNX2014 | 15954 | 2014 | Sheep | China | ||
| MF443349 | ChinaGZ2014 | 15954 | 2014 | Goat | China | ||
| MF443350 | ChinaGX2014 | 15954 | 2014 | Goat | China | ||
| IV | Asia | MF443354 | ChinaAH2014 | 15954 | 2014 | Goat | China |
| KX421387 | China/5/2013 | 15954 | 2013 | Goat | China | ||
| KX354359 | PPRV-FY | 15948 | 2015 | Goat | China | ||
| MF443341 | ChinaLN2014 | 15954 | 2014 | Goat | China | ||
| MF443351 | ChinaGS2014 | 15954 | 2014 | Sheep | China | ||
| KX421384 | China/2/2013 | 15954 | 2013 | Goat | China | ||
| KX421386 | China/4/2013 | 15954 | 2013 | Goat | China | ||
| KX421385 | China/3/2013 | 15954 | 2013 | Goat | China | ||
| MG581412 | PPRV/Bangladesh/BD2/2008 | 15948 | 2008 | Goat | Bangladesh | ||
| KM091959 | China/XJYL/2013 | 15954 | 2013 | Goat | China | ||
| KT270355 | IND/TN/GIN/2014/01 | 15942 | 2014 | Goat | India | ||
| KT860063 | IND/TN/VM/2014/02 | 15948 | 2014 | Goat | India | ||
| KX033350 | IND/Delhi/2016/05 | 15948 | 2016 | Goat | India | ||
| KT860064 | IND/TN/VEL/2015/03 | 15948 | 2015 | Sheep | India | ||
| KR261605 | India/TN/Gingee/2014 | 15948 | 2014 | Goat | India | ||
| KT860065 | IND/TN/ED/2015/04 | 15948 | 2015 | Sheep | India | ||
| KY967608 | SRMV/Lahore/UVAS/Pak/2015 | 15948 | 2015 | Sheep | Pakistan | ||
| KY967609 | SRMV/Faisalabad/UVAS/Pak/2015 | 15948 | 2015 | Goat | Pakistan | ||
| KY967610 | SRMV/Layyah/UVAS/Pak/2015 | 15948 | 2015 | Goat | Pakistan |
*Vaccine strains were excluded from any of the genomic and/or residue analysis performed in this manuscript.
Table 2.
A brief descriptions on genome atlas including coding and non-coding regions of so far reported SRMVs worldwide
| Genome regions | Position | Total length | GC% | 3ʹUTR | ORF | 5ʹUTR | Coding gene amino acid* | Intergenic trinucleotide region | Molecular weight (KDa) |
|---|---|---|---|---|---|---|---|---|---|
| Leader | 1–52 | 52 | 38 | — | — | — | |||
| N | 55–1744 | 1689 | 50 | 59 | 1578 | 52 | 526 | CTT | 58 |
| P | 1748–3402 | 1655 | 48 | 66 | 1530 | 59 | 510 | CTT | 55 |
| M | 3406–4888 | 1484 | 46 | 444 | 1008 | 32 | 336 | CTT | 38 |
| F | 4892–7306 | 2410 | 46 | 136 | 1641 | 633 | 546 | CTT | 59 |
| H | 7306–9262 | 1957 | 46 | 107 | 1830 | 20 | 610 | CTT | 69 |
| L | 9266–15908 | 6643 | 43 | 69 | 6552 | 22 | 2184 | CTT | 247 |
| Trailer | 15912–15948 | 37 | 41 | — | — | — | CTA |
UTR: untranslated region, ORF: open reading frame *Including stop codon.
Table 3.
A comparative analysis for the coding genes and intergenic regions present in the whole genome of SRMVs reported from different regions of the globe.
| Regions | 15942 nt | 15948 nt | 15954 nt | 15957 nt | 16058 nt |
|---|---|---|---|---|---|
| 3ʹ UTR | 107 | 107 | 107 | 107 | 107 |
| N | 108–1685 | 108–1685 | 108–1685 | 108–1685 | 108–1685 |
| Non-coding (N-P) | 123 | 123 | 123 | 123 | 123 |
| P | 1807–3336 | 1807–3336 | 1807–3336 | 1807–3336 | 1807–3336 |
| Non-coding (P-M) | 103 | 103 | 103 | 106 | 103 |
| M | 3438–4445 | 3438–4445 | 3438–4445 | 3441–4448 | 3438–4445 |
| Non-coding (M-F) | 1076 | 1082 | 1088 | 1088 | 1192 |
| F | 5520–7160 | 5526–7166 | 5532–7172 | 5535–7175 | 5636–7276 |
| Non-coding (F-H) | 161 | 161 | 161 | 161 | 161 |
| H | 7320–9149 | 7326–9155 | 7332–9161 | 7335–9164 | 7436–9265 |
| Non-coding (H-L) | 134 | 134 | 134 | 134 | 134 |
| L | 9282–15833 | 9288–15839 | 9294–15845 | 9297–15848 | 9398–15949 |
| 5ʹ UTR | 109 | 109 | 109 | 109 | 109 |
Percentage identity of nucleotide and comparative residue analysis
We found a varying nucleotide divergence among strains representing different lineages and geographical settings. For instance, a maximum nucleotide divergence (12.7%) was observed among Mongolian, Georgian (lineage IV) and Asian strains (lineage III). This was followed by 11.9% divergence between Pakistani (lineage IV) and other Asian strains (lineage III), and 11.8% divergence between Chinese (lineage IV) and rest of Asian strains (lineage III). As high as 11.5% nucleotide divergence was observed between Asian (lineage II) and African strains (lineage III) of SRMV. Similarly, a total of 11% nucleotide divergence was observed between African strains of lineages II and III. However, a variable divergence (8.5–10.3%) was noticed between SRMVs of lineage I and IV whereas, a divergence of 1.0–4.9% was revealed among strains within lineage IV (Table 4).
Table 4.
Percntage nucleotide identities and divergence derived from complete genome consensus sequences of SRMVs strains (lineage I–IV) reported so far in the public database.
| Lineages | SRMV strains | I | II | III | IV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa /1969–89 | Africa /1969–76 | Africa /2009–15 | Asia /1983–86 | Africa /1994–2012 | India /1994–96 | India /2014–16 | China /2007–08 | China /2013–15 | Mongolia /2016 | Bangladesh /2008 | Pakistan /2015 | Georgia /2016 | Ethiopia /2010 | Morocco /2008 | ||
| I | Africa/1969–89 | 7.3 | 9.0 | 9.5 | 9.5 | 8.5 | 9.4 | 9.2 | 9.6 | 9.9 | 10.3 | 9.5 | 9.7 | 9.4 | 9.2 | |
| II | Africa/1969–76 | 92.7 | 4.1 | 10.0 | 9.7 | 6.5 | 7.5 | 7.2 | 7.4 | 7.9 | 8.3 | 7.6 | 7.8 | 7.4 | 7.3 | |
| Africa/2009–15 | 91 | 95.9 | 11.2 | 11.0 | 8.2 | 9.0 | 8.8 | 8.9 | 9.4 | 9.9 | 9.1 | 9.3 | 8.9 | 8.9 | ||
| III | Asia/1983–86 | 90.5 | 90 | 88.8 | 3.1 | 11.0 | 11.7 | 11.4 | 11.8 | 12.1 | 12.6 | 11.9 | 12.1 | 11.7 | 11.5 | |
| Africa/1994–2012 | 90.5 | 90.3 | 88.0 | 94.9 | 10.8 | 11.6 | 11.4 | 11.7 | 11.9 | 12.6 | 11.7 | 11.8 | 11.5 | 11.4 | ||
| IV | India/1994–96 | 91.5 | 93.5 | 91.8 | 88.0 | 89.2 | 2.4 | 1.9 | 2.9 | 2.9 | 3.5 | 2.4 | 2.9 | 2.4 | 2.2 | |
| India/2014–16 | 90.6 | 92.5 | 90.0 | 88.3 | 88.4 | 97.6 | 1.8 | 3.6 | 3.5 | 3.4 | 2.9 | 4.1 | 3.7 | 3.5 | ||
| China/2007–08 | 90.8 | 92.8 | 91.2 | 88.6 | 88.6 | 98.1 | 98.2 | 3.0 | 2.9 | 2.9 | 2.7 | 3.7 | 3.2 | 3.0 | ||
| China/2013–15 | 90.4 | 92.6 | 91.1 | 88.2 | 88.3 | 97.1 | 96.4 | 97 | 1.0 | 4.7 | 3.6 | 4.6 | 4.2 | 3.9 | ||
| Mongolia/2016 | 90.1 | 92.9 | 90.6 | 87.9 | 88.1 | 97.1 | 96.5 | 97.1 | 99 | 4.6 | 3.5 | 4.6 | 4.1 | 3.9 | ||
| Bangladesh/2008 | 89.7 | 91.7 | 90.1 | 87.4 | 87.4 | 96.5 | 96.6 | 97.1 | 95.3 | 95.4 | 4.1 | 5.2 | 4.8 | 4.6 | ||
| Pakistan/2015 | 90.5 | 92.4 | 90.9 | 88.1 | 88.3 | 97.6 | 97.1 | 97.3 | 96.4 | 97.5 | 95.9 | 4.2 | 3.6 | 3.5 | ||
| Georgia/2016 | 90.3 | 92.2 | 90.7 | 87.9 | 88.2 | 97.1 | 95.9 | 96.3 | 95.4 | 95.4 | 94.8 | 95.8 | 2.0 | 3.0 | ||
| Ethiopia/2010 | 90.6 | 92.6 | 91.1 | 88.3 | 88.5 | 97.6 | 96.3 | 96.8 | 95.8 | 95.9 | 95.2 | 96.4 | 98 | 2.0 | ||
| Morocco/2008 | 90.8 | 92.7 | 91.1 | 88.5 | 88.6 | 97.8 | 96.5 | 97 | 96.1 | 96.1 | 95.4 | 96.5 | 97 | 98 | ||
Comparative residue analysis of different proteins across the entire genome length revealed conserved functional and/or structural motifs; however, few substitutions were noticed in some of the studied strains. A hypervariable region of varying length was observed in each of the SRMV proteins i.e., 423–456 aa in N, 74–111 aa in P, 73–197 aa in M, 6–16 aa in F, 174–179 aa in H and 617–627 aa in L protein. The nuclear export and nuclear localization signal, and RNA binding motifs appeared conserve in N protein of all strains. In P protein, a Soyuz 1 motif was also conserved in all strains except for the consensus sequence of Africa/1994–2012 (lineage III) where a total of six substitutions (L5Q, V10N, E11K, A14E, L16I and F20K) were observed. A serine residue (151S) in the P protein and a cell membrane anchor in the M protein were conserved in all of the SRMV sequences (Table 5). The signal peptide in F protein has previously been reported to be hypervariable (Table 6); however, while comparing SRMVs of different lineages, we proposed a relatively conserved long stretch of residue (1MTRVAILTFLFLFPNVVAC19) (Fig. 1). The cleavage motif (103GRRTRR108) was conserved in the F protein of all sequences. The fusion peptide motif was conserved for 109–133 aa in all SRMV strains except for consensus sequence of China/2013–15 strain where, phenylalanine (F) was replaced by leucine (L) at 1st position of the motif. Substitutions were observed in leucin zipper domain of consensus sequence in lineage II (African/2009–15, V479I), lineage III (African/1994–2012, I463V) and lineage IV (Bangladesh/2008, A464T). All consensus strains from lineage II including China/2013–15, Mongolia/2016, Georgia/2016 and Ethiopia/2010 carried a conserved residue pattern for hydrophobic anchor membrane of F protein; however, two substitutions (A486V and G489S) were observed predominantly in sequences from lineage IV. While comparing residue type and position in the H protein, several substitutions were revealed. For strains within lineage IV, these included a substitution in the N-terminal anchor of an Indian strain (India/2014–16, A41V) and in Georgian strain (Georgia/2016, Y481H). A substitution common to all SRMV strains within lineage III was observed in SLAM binding site where tyrosine (Y) was replaced by phenylalanine (F) at position 553, whereas a substitution in asparagine N-linked glycosylation site (215NVT217) was exclusive to strains reported from Africa during 1994–2012 (Table 7). For the N protein, all functionally and structurally important motifs were conserved in strains representing lineage I-IV.
Table 5.
A summarized comparative residue analysis of important domain and motif at NP, P and M proteins of SRMVs for their structural, functional and biologic activities.
| Lineage | SRMV strains | Nucleocapsid protein | Phosphoprotein | Matrix protein | |||
|---|---|---|---|---|---|---|---|
| NES (4LLKSLALF11) | NLS (70TGVMISML77) | RNA binding motif (324FSAGAYPLLWSYAMG338) | Soyuz 1 motif (4EQAYHVNKGLECIKSLK20) | Serine 151S | Cell membrane anchor (50FMYL53) | ||
| I | Africa/1969–89 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 |
| II | Africa/1969–76 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 |
| Africa/2009–15 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| III | Asia/1983–86 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 |
| Africa/1994–2012 | 4……..11 | 70……..77 | 324……………338 | 4.L….VE..A.L...F20 | — | 50….53 | |
| IV | India/1994–96 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 |
| India/2014–16 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| China/2007–08 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| China/2013–15 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Mongolia/2016 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Bangladesh/2008 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Pakistan/2015 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Israel/2017 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Georgia/2016 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Ethiopia/2010 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
| Morocco/2008 | 4……..11 | 70……..77 | 324……………338 | 4……………..20 | — | 50….53 | |
Table 6.
A summarized comparative residue analysis of important domain and motif in the F and H proteins of SRMVs for their structural, functional and biologic activities.
| Lineage | SRMV strains | Fusion protein | Haemagglutinin protein | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal peptide (1MTRVAILAFLFLFLNAVAC19) | Cleavage site (103GRRTRR108) | Fusion peptide (109FAGAVLAGVALGVATAAQITAGVAL133) | Leucin zipper domain (459LGNAVTRLENAKELLDASDQIL480) | Hydrophobic anchor membrane (485GVPFSGNMYIALAACIGVSLGLVTLICCKGRC517) | N terminal anchor (35PYILLGVLLVMFLSLIGLLAIAG58) | Histidine 481H | SLAM binding sites (529Y, 530D, 533R, 552F, 553Y, 554P) | Asparagine N–linked glycosylation (215NVS217, 279NMS281, 395NGT397) | ||
| I | Africa/1969–89 | 1….T.T..…P…..19 | 103.……108 | 109……………………133 | 459………………….480 | 485….G..L..G……………….….517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 |
| II | Africa/1969–76 | 1…..T.V..…P.T…19 | 103.……108 | 109……………………133 | 459………………….480 | 485…………………….………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 |
| Africa/2009–15 | 1…..T.VL.…PNT…19 | 103.……108 | 109……………………133 | 459………………V.480 | 485……………………………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| III | Asia/1983–86 | 1…….TS…LT.T..S19 | 103.……108 | 109……………………133 | 459………………….480 | 485…L…L..G…………………..517 | 35…………………..58 | — | 529., 530., 533., 552., 553F, 554. | 215…217, 279…281, 395…397 |
| Africa/1994–2012 | 1..K….TS…LPNT…19 | 103.……108 | 109……………………133 | 459….I……………..480 | 485…….L..G………………R…517 | 35…………………..58 | — | 529., 530., 533., 552., 553F, 554. | 215..T217, 279…281, 395…397 | |
| IV | India/1994–96 | 1…….T..…P…..19 | 103.……108 | 109……………………133 | 459………………….480 | 485….G……………………….517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 |
| India/2014–16 | 1…….TS…LP.V…19 | 103.……108 | 109……………………133 | 459………………….480 | 485.A..G............................517 | 35……A…………….58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| China/2007–08 | 1…….T.…LP.V…19 | 103.……108 | 109……………………133 | 459………………….480 | 485.A..G………………………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| China/2013–15 | 1…….T..…P.V…19 | 103.……108 | 109L…………………133 | 459………………….480 | 485…………………….………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Mongolia/2016 | 1…….T..…P.V…19 | 103.……108 | 109……………………133 | 459………………….480 | 485…………………….………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Bangladesh/2008 | 1…….I.…LP.V…19 | 103.……108 | 109……………………133 | 459…..A…………….480 | 485.A..G……………………….517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Pakistan/2015 | 1…….TS.…P.V…19 | 103.……108 | 109……………………133 | 459………………….480 | 485.A..G……………………….517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Israel/2017 | 1…….T..…P.....19 | 103.……108 | 109……………………133 | 459………………….480 | 485….G………….F……….R…517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Georgia/2016 | 1…….K………..19 | 103.……108 | 109……………………133 | 459………………….480 | 485…………………….………517 | 35…………………..58 | 481Y | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Ethiopia/2010 | 1…….K……….19 | 103.……108 | 109……………………133 | 459………………….480 | 485…………………….………517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
| Morocco/2008 | 1…….T….S…I..19 | 103.……108 | 109……………………133 | 459………………….480 | 485….G……………………….517 | 35…………………..58 | — | 529., 530., 533., 552., 553., 554. | 215…217, 279…281, 395…397 | |
Figure 1.
WebLogo-based diversity and/or conserveness of residues in proposed stretch at fusion protein of so-far reported SRMVs
Table 7.
A summarized comparative residue analysis of important domain and motif in the L protein of SRMVs for their structural, functional and biologic activities.
| Lineage | SRMV strains | Domain interact with P protein (9VLYPEVHLDSPIV21) | RNA binding motif (540KETGRLFAKMTYKM553) | Domain I (659FITADLKKYCLNWRYCL679) | Domain II (731FIKYPMGGIEGYCQKLWTISTIPYL755) | Domain III (768SLVQGDNQTIAVTK781) | Domain IV (838YDGLLVSQSLKSIAR852) | Polymerase associated motif | ATP binding site (1766K21GEGSGSM1794) | Methyltransferase associated motifs (1766K, 1881D, 1917K, 1788GEGSGSM1974, 1809YNSG1812, 1855TWVG1858) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa/1969–89 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771QGDNQ775 | 1464GDDD1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| I | Africa/1969–76 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 |
| II | Africa/2009–15 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 |
| Asia/1983–86 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| III | Africa/1994–2012 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 |
| India/1994–96 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| IV | India/2014–16 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 |
| China/2007–08 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| China/2013–15 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Mongolia/2016 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Bangladesh/2008 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Pakistan/2015 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Israel/2017 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838…..I………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Georgia/2016 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Ethiopia/2010 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Morocco/2008 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
| Africa/1969–89 | 9………….21 | 540…………..553 | 659……………..679 | 731…………………….755 | 768…………..781 | 838……………852 | 771…..775 | 1464….1467 | 1766……..1794 | 1766., 1881., 1917., 1788…….1974, 1809….1812, 1855….1858 | |
Estimation of evolutionary and divergence rates
Using a Bayesian coalescent approach, a molecular clock analysis of the whole genome and all coding gene sequences was performed to estimate the mean rate of evolution. Based on this analysis, the mean evolution rate for the complete genome of SRMV was estimated to be 9.953 × 10–4 substitutions per site per year. Best growth model was used for individual SRMV gene dataset to estimate the TMRCA and substitution rate per site per year. A cumulative interpretation of individual gene-based analysis showed rates of evolution in N, P, M, F, H and L genes as 1.1 × 10–3, 1.23 × 10–3, 2.56 × 10–3, 2.01 × 10–3, 1.47 × 10–3 and 9.75 × 10–4 site per year, respectively. The N gene (1.1 × 10–3) showed a lesser evolution rate as compared to other genes whereas it was highest for the L gene (9.75 × 10–4).
Phylogenetic topology based on geographical pattern
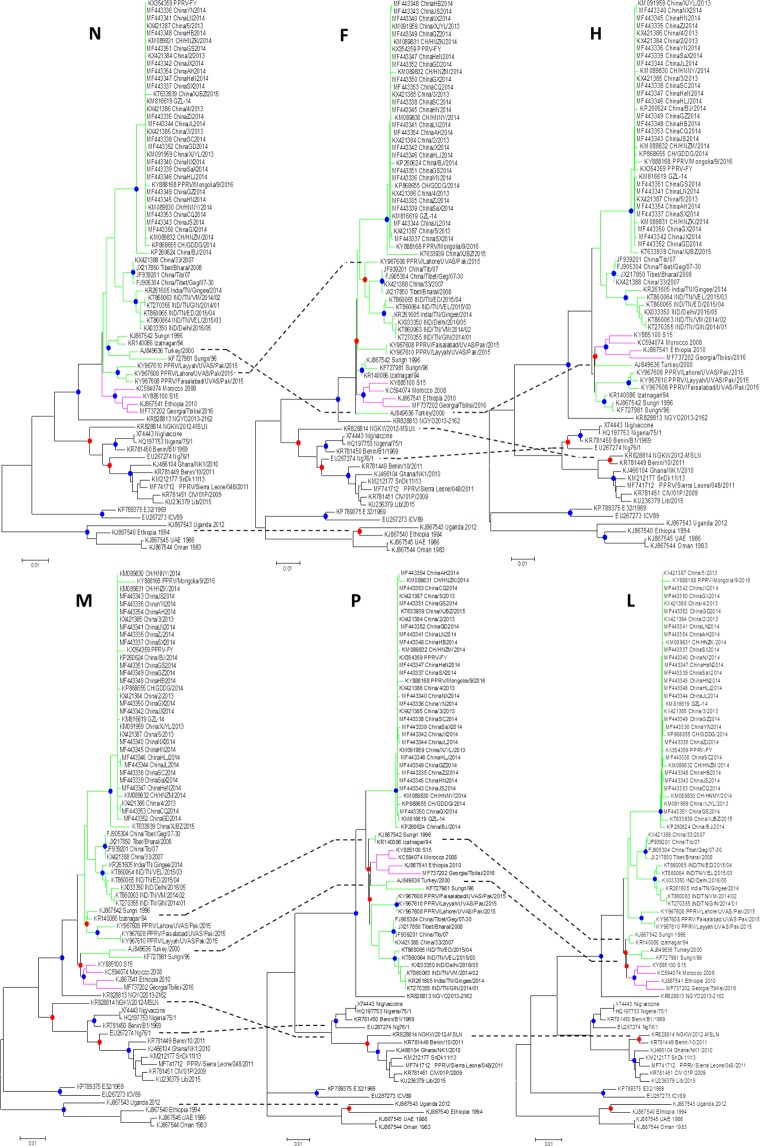
Utilizing each of the coding genes, the phylogenetic analysis of SRMV sequences revealed a distinct pattern of clustering according to the geographical locations. However, the complete N gene-based clustering pattern was more authoritative and conclusive followed by L, H, F, M and P genes (Fig. 2a,b). Within lineage II viruses, variations in clustering pattern were related to the reporting period from different regions in the African continent. In contrast, lineage III viruses from Africa showed variations in their clustering pattern on the basis of each of five gene used for analysis. For lineages IV viruses, there were significant variations in clustering pattern for each of the coding gene. The clustering pattern derived from N and L genes was similar to M, P, F and H genes. Since N and L genes-based topology of phylogenetic relationship among geographically distinct strain was found to be more precise and conclusive, the L gene is suggested to be employed in future epidemiological investigations.
Figure 2.
Individual coding gene-based phylogenetic analysis of so-far reported SRMVs revealed mismatching for monophyletic clustering of strains.
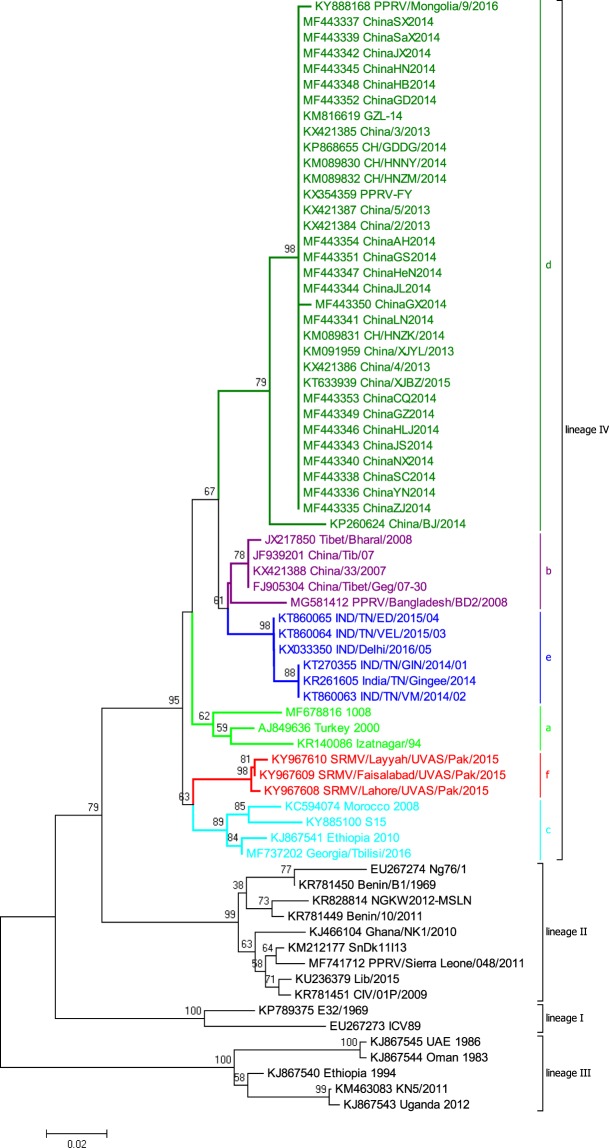
Based on the analysis of the complete N gene dataset, we proposed a geography and timeline based-classification of SRMV strains within lineage IV. A substantial analysis revealed a 6% and 2% nucleotide divegernce as a considerable cut-off criterion for the clasification of SRMV lineages and sub-lineages, respectively. Further analysis identified a total of six sub-clades (a-f) where sub-clade “a” represented strains from India, Turkey and Israel during 1994–2017, sub-clade “b” contained Chinese strains reported in 2007–08, sub-clade “c” represented strains from Africa and Georgia during 2008–2016, sub-clade “d” had Chinese strains reported during 2013–2016, sub-clade “e” possessed strains reported from India during 2014–2016, and sub-clade “f” was exclusive to Pakistan-originated strains which were reported in 2015 (Fig. 3).
Figure 3.
The complete N gene-based intra-lineage classification of strains within lineage IV.
Nucleotide diversity and selective pressure analysis
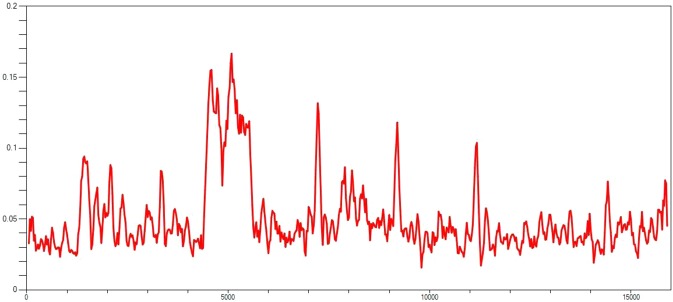
The average nucleotide diversity (Pi-value) was 0.04889 for complete genome of all SRMV strains. With a variance (0.00001) and standard deviation (0.002) for haplotype diversity (Hd = 1.000), the average nucleotide differences among all haplotypes was found to be k = 788.690. A total of 5891 mutations were observed in DnaSP analysis, where 10831 were monomorphic and 5117 were polymorphic. The polymorphic mutations consisted of 1311 singleton variable sites with 3806 parsimony informative sites. While an assessment for neutrality, the Tajima’s D value was found to be negative for all genes (p > 0.10). The reliability of the analysis, as determined by HKA test, was found to be 6.078 (X-square value) in T = 6.732 (divergence time) at a significant level (p = 0.0131) (Table 8). An analysis of the genetic diversity within the coding genes across the whole length of the genome revealed an occurrence of hotspot event (300 nt window size per ten nt overlapping steps) between 5ʹ UTR of M gene and 3ʹ UTR of F gene (Fig. 4). The nucleotide diversity across the coding genes of nucleotide sequence haplotypes was found to be highest in H gene (0.05171) followed by P (0.04527), F (0.04409), N (0.04068), L (0.03982) and M (0.03931) genes. On the other hands, the haplotype diversity (Hd) was observed to be higher in L gene (0.996) followed by F (0.926), P (0.921), H (0.920), N (0.909) and M (0.900) genes (Table 8).
Table 8.
A brief description on genome polymorphism for secletion sites in the complete genome and each of the coding regions in SRMVs.
| Parameters | Complete genome | N | P | M | F | H | L |
|---|---|---|---|---|---|---|---|
| Numbers of sites | 15948 | 1578 | 1530 | 1008 | 1641 | 1830 | 6552 |
| Monomorphic sites | 10831 | 1115 | 1031 | 738 | 1168 | 1199 | 4754 |
| Polymorphic sites | 5117 | 463 | 499 | 270 | 473 | 631 | 1798 |
| Total no. of mutation | 5891 | 496 | 532 | 288 | 497 | 649 | 1893 |
| Singleton variable sites | 1311 | 115 | 171 | 71 | 118 | 161 | 477 |
| Parsimony Informative Sites | 3806 | 348 | 328 | 199 | 355 | 470 | 484 |
| No. of haplotypes (h) | 68 | 51 | 50 | 47 | 49 | 50 | 67 |
| Haplotype diversity (Hd) | 1.000 | 0.909 | 0.921 | 0.900 | 0.926 | 0.920 | 0.996 |
| Variance of haplotype diversity | 0.00001 | 0.00096 | 0.00054 | 0.00093 | 0.00076 | 0.00036 | 0.00001 |
| Standard deviation of gene diversity | 0.002 | 0.030 | 0.023 | 0.030 | 0.028 | 0.019 | 0.003 |
| Nucleotide diversity (Pi) | 0.04889 | 0.04068 | 0.04527 | 0.03931 | 0.04409 | 0.05171 | 0.03982 |
| Standard deviation of Pi | 0.00468 | 0.00465 | 0.00497 | 0.00442 | 0.00500 | 0.00568 | 0.00454 |
| Average no. of pairwise nucleotide difference (k) | 788.690 | 64.358 | 69.279 | 39.705 | 72.406 | 94.751 | 261.289 |
| Tajima’ D | −1.23601 | −1.31743 | −1.38728 | −1.24841 | −1.10701 | −0.85748 | −1.21260 |
Note: HKA test direct mode: Divergence time T = 6.732 × –square value = 6.078, P value = 0.0131*, * = 0.01 < p < 0.05.
Figure 4.
Nucleotide diversity plotamong whole genome sequences of SRMVs derived from DnaSP.
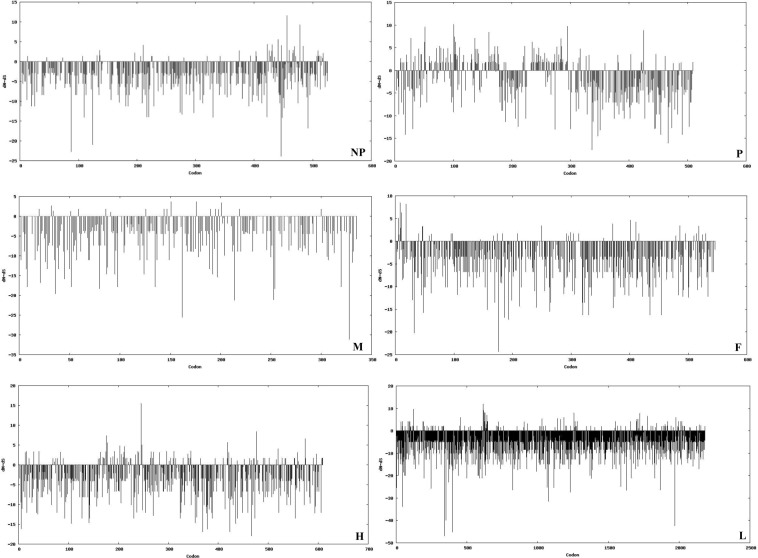
Datamonkey output for selective pressure analysis across CDS regions is summarized in Table 9. Although none of the gene carried a mean dN-dS greater than 1 at p < 0.05, it was highest for P gene (0.44679) followed by H (0.20017), N (0.12168), F (0.10253), L (0.08976) and M (0.06601) genes. At p < 0.05, analyzing through different algorithmic approaches (SLAC, FEL, IFEL, REL and MEME), revealed that the L gene showed a highest positive selection sites (96) followed by N (27), F (21), P (16), H (12) and M (2) genes. The plots against codon positions for individual genes were drawn using SLAC statistical approach based on dN-dS value (Fig. 5).
Table 9.
Data Monkey analysis based brief summary of positive and negative substitution sites in each of the coding gene of so far reported SRMVs.
| Parameters | N | P | M | F | H | L |
|---|---|---|---|---|---|---|
| Mean dN–dS | 0.12168 | 0.44679 | 0.06601 | 0.10253 | 0.20017 | 0.08976 |
| No. of duplicate sequences | 18 | 22 | 24 | 22 | 19 | 5 |
| Single Likelihood Ancestor Counting (SLAC) | ||||||
| No. of positive and negative selection sites along with codon position at 95% confidence level | 1 positive (456) and 48 negative sites | 0 positive and 13 negative sites | 0 positive and 29 negative sites | 0 positive and 48 negative sites | 1 positive (246) and 41 negative sites | 0 positive and 149 negative sites |
| Fixed Effect Likelihood (FEL) | ||||||
| No. of positive and negative selection sites along with codon position at 95% confidence level | 2 positive (456, 478) and 111 negative sites | 3 positive (52, 295, 425) and 59 negative sites | 0 positive and 72 negative sites | 1 positive (8) and 129 negative sites | 2 positive (246, 574) and 114 negative sites | 1 positive (614) and 439 negative sites |
| Internal Branch Fixed Effect Likelihood (IFEL) | ||||||
| No. of positive and negative selection sites along with codon position at 95% confidence level | 1 positive (456) and 61 negative sites | 5 positive (52, 161, 285, 295, 425) and 44 negative sites | 0 positive and 41 negative sites | 1positive (8) and 68 negative sites | 2 positive (246, 574) and 65 negative sites | 2 positive (616, 623) and 218 negative sites |
| Random Effects Likelihood (REL) | ||||||
| No. of positive and negative selection sites along with codon position at 10% confidence level | 19 positive (46, 136, 160, 11, 375, 403, 423, 425, 426, 437, 435, 441, 447, 456, 467, 478, 484, 509, 517) and 209 negative sites | 0 positive and 215 negative sites | 0 positive and 02 negative sites | 14 positive (5, 6, 8, 9, 11, 18, 46, 250, 299, 371, 411, 456, 518, 524) and 258 negative sites | 0 positive and 339 negative sites | 76 positive (35, 46, 81, 82, 93, 96, 120, 123, 124, 189, 194, 246, 279, 325, 334, 447, 455, 612, 613, 614, 617, 619, 620, 622, 623, 624, 627, 630, 631, 636, 641, 643, 645, 646, 647, 699, 720, 723, 798, 905, 928, 1004, 1031, 1116, 1185, 1257, 1264, 1280, 1375, 1390, 1401, 1547, 1551, 1649, 1655, 1660, 1698, 1700, 1710, 1722, 1725, 1747, 1783, 1840, 1918, 1976, 1980, 1995, 2010, 2029, 2135, 2142, 2144) and 160 negative sites |
| Mixed Effect Model of Episodic Selection (MEME) | ||||||
| No. of selection sites and position of codon with evidences of episodic diversifying selection at 95% confidence level | 4 sites (441, 456, 466, 478) | 8 sites (10, 20, 83, 101, 102, 137, 403, 425) | 3 sites (211, 311, 335) | 5 sites (3, 8, 11, 46, 356) | 7 sites (21, 210, 212, 288, 309, 330, 591) | 17 sites (54, 68, 230, 349, 421, 455, 614, 719, 723, 1200, 1343, 1696, 1900, 1901, 2005, 2080, 2142) |
| Fast Unbiased (FUBAR) | ||||||
| No. of false positive selection sites (Excluding to above mentioned sites) along with codon position at 95% confidence level | 202 C.I (189–211) | 293 C.I (264–312) | 144 C.I (134-153) | 208 C.I (200–222) | 261 C.I (251–280) | 779 C.I (765–802) |
Figure 5.
Differences in codon position, synonymous and non-synonymous substitutions (dN-dS values) for each of individual genes.
Recombination analysis
Lying between 5ʹ UTR of the M gene (3406–4888 bp) and 3ʹ UTR of the F gene (4892–7306 bp), a putative recombination event was observed in the complete genome (4607–5425 nts) of Pakistan-origin strain of SRMV. With a probability of MC value of 2.357 E−22, this event was found between a recombinant Pakistani strain (KY967608; SRMV/Lahore/UVAS/Pak/2015) and Indian strains (KR140086; Izatngar/94 as major parent and KT860064; IND/TN/VEL/2015/03 as minor parent) (Fig. 6). This observation was consistent in all of the seven recombination algorithm methods at p < 0.001. A detailed information on inferred breakpoint and p-value of algorithm approaches is given in Table 10.
Figure 6.
A graphical illustration of plot showing detection of recombination event.
Table 10.
Evidence of recombination events in the whole genome of Pakistan–originated SRMV strain along with breakpoint positions and significant p-values
| Detecting Methods | p-value | Breakpoint position | SRMV Strains |
|---|---|---|---|
| RDP | 4.360 × 10−23 |
Beginning breakpoint = 4607 nt Beginning breakpoint 90% C.I = 4556–4680 nt Ending breakpoint = 5425 nt Ending breakpoint 90% C.I = 5324–5504 nt Length of sequence between two breakpoint: 818 nt Binomial probability (MC corrected) = 2.357 E−22 Average ootstrap support = 89.83% |
Recombinant strain: SRMV/Lahore/UVAS/Pak/2015 (KY967608) Major Parent: Izatnagar/94 (KR140086) (98.1% nucleotide identity) Minor Parent: IND/TN/VEL/2015/03 (KT860064) (99.8% nucleotide identity) |
| GENECONV | 7.015 × 10−21 | ||
| BootScan | 2.357 × 10–22 | ||
| MaxChi | 2.278 × 10−07 | ||
| Chimaera | 1.550 × 10−06 | ||
| SiScan | 5.425 × 10−13 | ||
| 3Seq | 9.479 × 10−11 |
Discussion
We presented a comparative genetic, phylogenomic and evolutionary analysis of SRMV strains reported so far in public database. Whole genome sequences and open reading frames (ORFs) of individual genes of representative strains were used in subsequent higher-resolution bioinformatic analysis. This is because a specific gene might not evolve at the same rate as does the whole genome15 and, therefore, can provide precise information on viral evolutionary dynamics and necessary epidemiological investigations in future16. While considering the “rule of six” for whole gnome atlas, comparative complete genome analysis revealed a varying length of complete genome suggesting the potential of the virus to evolve over a period of time. A few sequences showed unsusual lengths (e.g., MF678816; 15927 bp, KM089831; 15957 bp and KM816619; 16058 bp) where, for each of these sequences, a nucleotide insertion/deletion was observed in the noncoding region between the M and F genes17,18. Interestetingly, each of these sequeunce was deriven from the next generation sequencing approach and, therefore, such an unusual length may correspond to the sequencing errors. Owing to the fact that all paramyxovirus including SRMV follow a polyhexameric genome length for the effective replication in host cells19, SRMV sequences erroneously not following the “rule of six” in genome atlas were excluded from the specific analysis.
Comparative residue analysis of viral proteins showed several conserved motifs20,21. Among these, the N protein had three conserved motifs. These included export signal, nuclear localization signal and RNA binding motif. The first two are considered responsible for transport of the N protein to nucleus of host cell, while the third one was believed to be involved in interaction of N-N monomers of RNA during genomic RNA binding and N-N self-interaction20. Developing polymerase complex with N and L proteins, the P protein plays a significant role in virus replication and RNA biosynthesis22. The protein contains a variable N-terminus whereas C-terminus is believed to be the most conserved, and is required for the interaction with L protein in synthesis of polymerase complex23. The Soyuz 1 motif and presence of 151S residue, responsible for viral transcription via altering its phosphorylation status24, were found in all study-included strains21. The M protein is a core organizer of viral morphogenesis and has the ability to interact with other proteins for maturation of viral progeny25. For all of the investigated strains, this protein carried a previously known residue pattern21 for late domain or cell membrane anchor, which has a known role for localization of cell membrane and budding activity26. An unusually long and GC rich non-coding region was observed between 3ʹ UTR-M and 5ʹ UTR-F genes in studied SRMV sequences. While no biological or functional significance is warranted, a previous study has suggested an up- and/or down-regulation of these proteins to differences in their lengths and therefore may alter cyto-pathogenicity and survival fitness of the virus in nature27.
Three motifs were also noticed in the F protein as signal peptide, cleavage site (responsible for virulence and adaptation in the environment) and a leucine zipper domain. These are known to be involved in maintenance of protein tertiary structure20,22. Since the signal peptide motif was located in a variable region28, we performed a comparative analysis to investigate the conserveness of specific residue at a specific position among all reported strains from different geographies and proposed a stretch of consensus residues at the global level. The H protein is considered responsible for attachment of the virus to host cell membrane via cleavage of sialic acid residue in cellular glycoprotein29. As observed in the current study, the protein has a hydrophobic domain at the N-terminus that acts as a signal peptide to anchor the protein into the membrane20. The findings of SLAM receptor binding sites during the analysis highlight the epitheliotropic and lymphotropic nature of SRMVs30. Herein, a high number of glycosylation sites were found in the N protein, which plays a major role in protein translocation31. The large protein (L) contributes in viral replication, transcription and polyadenylation using different domains that were found to be conserved in this study. Domain I, II and III are considered responsible for polymerase and kinase activity where GDDD and QGDNQ residues carry a prime significance32 and, as observed in a previous experimental study33, any substitution in these residues can abolish the polymerase activity of the L protein. Two highly conserved hinge regions were also observed in a pattern typically corresponding to established hinge regions of other closely related morbilliviruses34. Taken together, the potential influence of these substitutions in the functionality of corresponding proteins is scarce and, therefore, requires future investigations to determine impact of these variations in conserved domains.
The phylogenetic analysis, either based upon complete genome or each of the complete coding genes, showed a clustering pattern according to distinct geographical setting and time-period e.g., strains clustered within a distinct clade represented same country of origin within a specific time period. Therefore, while presenting a global perspective, a clustering and subsequent sub-clade grouping is proposed in the current study as an imporved and updated version of previous proposal35. This is simply becuase the previous classification proposal was limited to sub-grouping of Indian strains along with a few of those reported from the Middle East and Africa. Not only that the said proposal excluded strains reported from China and Georgia but also did not represent a well-defined evolutionary cut-off for the lowest taxonomic node (sub-lineage or sub-grouping). In addition to that, Kumar et al.35 have classified the strains into clades and subclades which contradicts previously proposed standard classification criteria for the lowest taxonomic node or sub-grouping of the viruses within a lineage or genotype36,37. Though such a classification may provide some pre-liminary assessment exclusively for Indian-origin strains, a limited geographic-pattern based classification may raise controversies for SRMV classification at global scale. Therefore, these are considered unreliable to present molecular epidemiology of SRMVs worldwide. Indeed, with a substantial increase in the number of SRMV sequences in future, following a uniform classification criterion such as presented in the current study (IVa, IVb, IVc, IVd, IVe and IVf), is necessary for a more precise clustering at the lowest taxonomic node. While comparing different coding genes (P, M, F, H and L) of SRMV strains (Fig. 2a,b), minor differences were observed in the clustering pattern indicating an influence of nucleotides in genetic diversity of SRMVs. Nevertheless, the N gene-based topography was closer to those of the L gene (RNA-dependent RNA polymerase) and complete genome sequences. Thus it (L gene) could be employed alternatively for a precise evolutionary relationship of SRMV strains originating from different geographical regions. This is important because, considering SRMV a member of the family Paramyxoviridae, L protein is now considered as a standard criterion for classification of some of the closely related members of the sub-family Avualvirinae38. The observed topology of the N gene revealed evolutionary dynamics of circulating SRMV strains consistent with observations made previously10,20. Therefore, it is suggested that complete N and L gene-based phylogeny analysis can provide an accurate evolutionary relationship of the circulating strains in particular geographical settings10,38, especially for those regions where full-genomes have not yet been reported or have limited resources.
Nucleotide diversity analysis was used to unleash the genomic variation (polymorphism) within a given dataset39 where a substitution rate is considered a prime parameter to elucidate virus evolution over a period of time. The average number of pairwise nucleotide difference among the whole genome of all SRMV sequences was found to be 788.690 with a diversity in nucleotide sequences (0.04889 ± S.D. 0.00468) and haplotype variance (0.00001). Gained observations correspond to distinct features of RNA viruses where there is a lack of proofreading activity by reverse transcriptase40. In contrast to previous observations14, a lower diversity in nucleotide and haplotype variance, and nucleotide difference in the current study may largely be ascribed to inclusion of a smaller number of complete nucleotide sequences than those employed in the current study (n = 37 vs n = 68). In addition to this, evidenced by significant nucleotide diversity over a period of time (p < 0.05), the HKA test outcome indicated an ongoing evolution or adaptation of virus in the environment.
The DnaSP based nucleotide diversity analysis revealed higher diversity in the H gene than others of SRMVs. Owing to significant roles in attachment and subsequent genome replication, the gene has been proposed to assess the evolutionary relationship of SRMV strains41. Though it ascertains further research, the substitutions in the H gene may have an influence on host adaptability and pathogenicity to susceptible host such as observed previously for SRMV14 and influenza virus42. A diverse nucleotide hotspot was obsereved between 5ʹ UTR of M and 3ʹ UTR of the F genes in the whole genome. This aligns with observations made previously where a hotspot was identified at similar position between M and F genes14, highlighting potential variations in the genome size and corresponding substitutions43 in each of the gene. An influence of these spontaneous mutations in genome was assessed by employing Tajima’s D statistics that showed a non-significant negative value for all coding genes in DnaSP analysis, suggesting a lack of influence of spontaneous mutations on the fitness of individual virus. Such observations suggest positive selection among coding region of sequences with a lower level of sequence diversity and an excess of low-frequency variants reflecting the role of natural selection in SRMV genomes. Contrary to current study findings where analysis showed negative value for each of the coding genes, positive values in F and H genes has previously been suggested14.
The non-synonymous/synonymous rate (ω = dN-dS) is an important indicator of selective pressure at the protein level where ω = 1 means neutral mutations, ω < 1 correspond to purifying selection while ω > 1 indicates diversifying positive pressure44. Herein, as reported in a previous study14, the dN-dS plot for each protein showed value not more than 1 indicating a slow genetic evolution of SRMV. Indeed, such a comparison of rates of synonymous and non-synonymous mutations provides an understanding towards the mechanisms of molecular sequence evolution. The positive selection sites were found in all coding genes (N, P, M, F, H and L) using different statistical approaches. Though these sites were found to be non-significant with a ratio less than 1 by Tajima’s D statistics, it seldom happens in structural domains of genome. However, the impact of such positive selection sites with lower level of sequence diversity may cause the emergence of variants44. According to the neutral theory of molecular evolution, such type of molecular variations, which arise via spontaneous mutations, has no influence on individual’s fitness45. However, the biological significance of these sites still remains unknown and needs to be explored in future.
The occurrence of recombination events is considered a significant source of genetic diversity for RNA viruses46. Beside rare occurrence of recombination in negative sense RNA viruses particularly SRMV, an analysis for the detection of recombination event/s is recommended as a standard component of every phylogenetic analysis to serve an important quality-control function to weed out laboratory and analytical errors47. We found recombination events among Pakistani- and Indian-origin strains which further highlight the co-existence of similar SRMV strains along with its transboundary nature of transmission48. Indicating a high resolution of prediction, the observed putative recombination event was statistically significant and was identified by more than five recombination detection algorithms. Such an interference of Indian strains as major and minor parents for Pakistan-originated recombinant strain highlight its potential to cross international borders48. Similar finding has previously been observed for another RNA virus (Yellow leaf virus) from Pakistan and India49. Potnetial reason for such a sharing of genetic material could be spectulative and may be attributed to an increased disease incidence rate and frequent disease outbreaks near borderline of these countries50,51. Though potnetial occurrence of homologous recombination in some of the negative sense RNA viruses is low52, it is not surprising because sporadic recombination in various negative-sense RNA viuses such as Hantavirus53,54, ambisense arenaviruses55,56, Newcastle disease viruses57,58 and morbilliviruses (e.g. canine distemper virus59 and measles virus60) has been evidenced. Hence, an emergence of viral variants could be anticipated that may differ antigenically and serologically and therefore may have consequences in terms of failure in diagnotics and vaccine efficacy.
Materials and Methods
Complete genome sequencing of SRMVs from Pakistan and dataset information
The complete genome sequencing of two SRMV isolates [KY967609 (SRMV/Faisalabad/UVAS/Pak/2015) and KY967610 (SRMV/Layyah/UVAS/Pak/2015)] was performed as per primers and protocols described previously5. Later, including these two strains, a total of 75 whole genome sequences of SRMVs were accessed (https://www.ncbi.nlm.nih.gov/, October 01, 2019) and processed for subsequent bioinformatic analysis. Among these 75 SRMV sequences, four were attenuated vaccine strains (KJ867542, KF727981; HQ197753, X74443) and were excluded from the dataset used in the current study. Furtermore, given the “rule of six” genome atlas or polyhexameric genome length, 03 sequences including MF678816 (15927 bp), KM089831 (15957 bp) and KM816619 (16058 bp) were also excluded from comparative whole genome-specific analysis. However, owing to length of coding region comparable to each of the protein of SRMV, only the coding regions of these sequences were included and processed further in comparative genomic and residue analsyis. All essential information related to whole genome sequences of study-included strains is presented in Table 1.
Comparative genomic analysis
The complete genome (15954 bp) dataset was aligned to equal length using ClustalW methods in BioEdit version 5.0.661 and, based upon nucleotide number and position across the whole length of the genome, different genomic features were compared among all SRMV sequences. The consensus sequences were made for those SRMV sequences that had a highest nucleotide similarity and were originated from similar geographical regions. Nucleotide identity and divergence among all consensus whole genome sequences of lineages I-IV was assessed by Pairwise Sequence Comparisons (PASC) analysis in MEGA version 6.0662. The conserved domains, functional and structural motif/s, trans-membrane regions and unique substitutions in open reading frames were predicted using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), Conserved Domain Prediction tool (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and HMMTOP program (http://www.enzim.hu/hmmtop/index.php). The potential N-glycosylation sites (N-X-T/S, where X denoted any residue except a Proline) were predicted by NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc) and accepted if the G-score was 0.5. Similarly, the diversity and/or conserveness of residues at important but hypervariable motif/s were analysed through WebLogo version 3.1 (accessible at http://weblogo.threeplusone.com/create.cgi).
Estimation of evolutionary and divergence dates
Using a Bayesian Markov Chain Monte Carlo (MCMC) approach implemented in Bayesian evolutionary analysis sampling trees (BEAST) software package version 1.8.063, the molecular evolutionary and divergence rates were co-estimated for complete genome and individual genes. For each dataset, a total of three independent runs of MCMC were conducted under a strict molecular clock model, using the Hasegawa–Kishino–Yano model of sequence evolution with a proportion of invariant sites and gamma distributed rate heterogeneity (HKY + I + C) with partitions into codon positions, and the remaining default parameters in the prior’s panel. For each gene, the MCMC run was 36107 steps long and the posterior probability distribution of the chains was sampled every 1000 steps. Convergence was assessed on the basis of an effective sampling size after 10% burn-in using Tracer software, version 1.5 (http://tree.bio.ed.ac.uk/software/tracer/). The estimations were the mean values obtained for the three runs. The mean time of the most recent common ancestor (TMRCA) and the 95% CI were calculated, and the best-fitting models were selected by a Bayes factor using marginal likelihoods implemented in Tracer64.
Phylogeography-based reconstruction of evolutionary tree
A reliability of a gene for molecular epidemiology was assessed by comparing all coding genes (N, P, M, F, H and L) extracted from whole genome sequence of SRMV and aligned separately by ClustalW methods incorporated in the BioEdit version 5.0.661. The phylogenetic trees were constructed by neighbour-joining method with best-fit substitution model for each set of sequences using MEGA version 6.0662. A 1000 replication bootstrap value was adjusted to better elucidate the probability and reliability of clustering of isolates or any change in their clustering pattern.
Nucleotide diversity and natural selective pressure analysis
Based upon variable sites for mutations, and average numbers of pairwise nucleotide differences, the nucleotide diversity among coding sequences (CDS) of complete genome sequences was assessed for genomic polymorphism by DnaSP version 5.10.01 (accessible at http://www.ub.es/dnasp). The departure from neutrality in all isolate’s sequences was tested by Tajima’s D statistical method65. Divergence time in nucleotide diversity was estimated by a direct statistical model (HKA test). Data-monkey adaptive evolution server (http://www.datamonkey.org/) was used to evaluate synonymous (dS) and non-synonymous (dN) substitution rate per codon among CDS of all sequences66. Later, the positive and negative selection sites under natural selection were determined through six different genetic algorithms including Single Likelihood Ancestor Counting (SLAC), Fixed Effect Likelihood (FEL), Internal Branch Fixed Effect Likelihood (IFEL), Random Effects Likelihood (REL), Mixed Effect Model of Episodic selection (MEME) and Fast Unbiased Bayesien Approximation (FUBAR)67.
Detection of putative recombination event
The sequences were analyzed for the identification of reliable putative breakpoints by different tools including SimPlot version 3.5.168, GARD (http://www.datamonkey.org/GARD), DAMBE version 5.2.3069 and RDP4 version 4.9570. However, owing to an enhanced accuracy, clarity and reliability of analysis, outcomes gained by RDP4 were considered conclusive for further interpretation. The RDP4 was preferred because it employs a combination of seven different algorithms named RDP, GENECONV, BootScan, MaxChi, Chimaera, SiScan and 3Seq to better unleash putative recombinant and parent isolates at p < 0.001. A putative recombination event was assumed to have occurred only when it was consistently identified by at least four of the above-mentioned algorithms at a probability threshold of 0.05.
Ethical approval and informed consent
This research did not involve human participants or animals. This article does not contain studies with animals or humans performed by any of the authors.
Acknowledgements
We thank Dr. Andrew Fielding (Lancaster University, United Kingdom) for necessary edits in improving the manuscript contents and language.
Author contributions
M.Z.S. and A.R. apprehended the idea; M.M., M.Z.S. and A.R. did analysis and manuscript write-up.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Munir M. ed. Peste des petits ruminants virus. Heidelberg, New York, Dordrecht, London: Springer. (2015).
- 2.Aziz-ul R, Wensman JJ, Abubakar M, Shabbir MZ, Rossiter P. Peste des petits ruminants in wild ungulates. Trop Anim Health Prod. 2018;50(8):1815–1819. doi: 10.1007/s11250-018-1623-6. [DOI] [PubMed] [Google Scholar]
- 3.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91(12):2885–97. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 4.Baazizi R, et al. Peste des petits ruminants (PPR): A neglected tropical disease in Maghreb region of North Africa and its threat to Europe. PloS one. 2017;12(4):e0175461. doi: 10.1371/journal.pone.0175461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shabbir MZ, Ul‐Rahman A, Zahid MN, Munir M. Genetic characterization of small ruminant morbillivirus from recently emerging wave of outbreaks in Pakistan. Transbound Emerg Dis. 2018;65(6):2032–8. doi: 10.1111/tbed.12964. [DOI] [PubMed] [Google Scholar]
- 6.Clarke BD, Islam MR, Yusuf MA, Mahapatra M, Parida S. Molecular detection, isolation and characterization of Peste des petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound Emerg Dis. 2018;65(6):1597–1604. doi: 10.1111/tbed.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhaig MM, Selim A, Mandour AS, Schulz C, Hoffmann B. Prevalence and molecular characterization of peste des petits ruminants virus from Ismailia and Suez, Northeastern Egypt, 2014–2016. Small Ruminant Res. 2018;169:94–8. doi: 10.1016/j.smallrumres.2018.07.001. [DOI] [Google Scholar]
- 8.Jones BA, et al. The economic impact of eradicating peste des petits ruminants: a benefit-cost analysis. PLoS One. 2016;11(2):e0149982. doi: 10.1371/journal.pone.0149982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maes P, et al. Taxonomy of the order Mononegavirales: second update 2018. Arch of Virol. 2019;164(4):1233–44. doi: 10.1007/s00705-018-04126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muniraju M, et al. Molecular evolution of peste des petits ruminants virus. Emerg Infect Dis. 2014;20(12):2023. doi: 10.3201/eid2012.140684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libeau G, Diallo A, Parida S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim Front. 2014;4(1):14–20. doi: 10.2527/af.2014-0003. [DOI] [Google Scholar]
- 12.Albina E, et al. Peste des petits ruminants, the next eradicated animal disease? Vet Microbiol. 2013;165(1-2):38–44. doi: 10.1016/j.vetmic.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Rouzine IM, Rozhnova G. Antigenic evolution of viruses in host populations. PLoS Pathog. 2018;14(9):e1007291. doi: 10.1371/journal.ppat.1007291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu AR, et al. Genome sequencing of an Indian peste des petits ruminants virus isolate, Izatnagar/94, and its implications for virus diversity, divergence and phylogeography. Arch Virol. 2017;162(6):1677–93. doi: 10.1007/s00705-017-3288-2. [DOI] [PubMed] [Google Scholar]
- 15.Miller PJ, Kim LM, Ip HS, Afonso CL. Evolutionary dynamics of Newcastle disease virus. Virology. 2009;391(1):64–72. doi: 10.1016/j.virol.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Valdazo-Gonzalez B, et al. Reconstruction of the transmission history of RNA virus outbreaks using full genome sequences: foot-and-mouth disease virus in Bulgaria in 2011. PLoS One. 2012;7(11):e49650. doi: 10.1371/journal.pone.0049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao J, Wang Q, et al. Evolutionary dynamics of recent peste des petits ruminants virus epidemic in China during 2013–2014. Virology. 2017;510:156–64. doi: 10.1016/j.virol.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shatar M, et al. First genetic characterization of peste des petits ruminants virus from Mongolia. Arch Virol. 2017;162(10):3157–60. doi: 10.1007/s00705-017-3456-4. [DOI] [PubMed] [Google Scholar]
- 19.Kolakofsky D, Roux L, Garcin D, Ruigrok RW. Paramyxovirus mRNA editing, the ‘rule of six’and error catastrophe: a hypothesis. Journal of general virology. 2005;86(7):1869–1877. doi: 10.1099/vir.0.80986-0. [DOI] [PubMed] [Google Scholar]
- 20.Balamurugan V, et al. Sequence and phylogenetic analyses of the structural genes of virulent isolates and vaccine strains of peste des petits ruminants virus from India. Transbound Emerg Dis. 2010;57(5):352–64. doi: 10.1111/j.1865-1682.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- 21.Chard LS, Bailey DS, Dash P, Banyard AC, Barrett T. Full genome sequences of two virulent strains of peste-des-petits ruminants virus, the Côte d’Ivoire 1989 and Nigeria 1976 strains. Virus Res. 2008;136(1-2):192–7. doi: 10.1016/j.virusres.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Lamb, R. & Parks, G. Paramyxoviridae: the viruses and their replication. In: Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B, & Straus S. E., eds Philadelphia: Lippincott Williams & Wilkins, 1449–1496 (2007).
- 23.Tuckis J, Smallwood S, Feller JA, Moyer SA. The C-terminal 88 amino acids of the Sendai virus P protein have multiple functions separable by mutation. J of Virol. 2002;76(1):68–77. doi: 10.1128/JVI.76.1.68-77.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlin D, Belshaw R. Detecting remote sequence homology in disordered proteins: discovery of conserved motifs in the N-termini of Mononegavirales phosphoproteins. PLoS One. 2012;57(3):e31719. doi: 10.1371/journal.pone.0031719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subhashri R, Shaila MS. Characterization of membrane association of Rinderpest virus matrix protein. Biochem Biophys Res Commun. 2007;355(4):1096–101. doi: 10.1016/j.bbrc.2007.02.088. [DOI] [PubMed] [Google Scholar]
- 26.Ciancanelli MJ, Basler CF. Mutation of YMYL in the Nipah virus matrix protein abrogates budding and alters subcellular localization. J Virol. 2006;80(24):12070–8. doi: 10.1128/JVI.01743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda M, Ohno S, Seki F, Nakatsu Y, Tahara M, Yanagi Y. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J Virol. 2005;79(22):14346–54. doi: 10.1128/JVI.79.22.14346-14354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison TG. Structure and function of a paramyxovirus fusion protein. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2003;1614(1):73–84. doi: 10.1016/S0005-2736(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 29.Johansson K, Bourhis JM, Campanacci V, Cambillau C, Canard B, Longhi S. Crystal structure of the measles virus phosphoprotein domain responsible for the induced folding of the C-terminal domain of the nucleoprotein. J Bio Chem. 2003;278(45):44567–73. doi: 10.1074/jbc.M308745200. [DOI] [PubMed] [Google Scholar]
- 30.Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM-or CD46-induced fusion and their localization on a new hemagglutinin structural model. J Virol. 2004;78(1):302–13. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apte-Sengupta S, et al. Base of the measles virus fusion trimer head receives the signal that triggers membrane fusion. J Bio Chem. 2012;287(39):33026–35. doi: 10.1074/jbc.M112.373308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minet C, et al. Sequence analysis of the large (L) polymerase gene and trailer of the peste des petits ruminants virus vaccine strain Nigeria 75/1: expression and use of the L protein in reverse genetics. Virus Res. 2009;145(1):9–17. doi: 10.1016/j.virusres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Schnell MJ, Conzelmann KK. Polymerase Activity ofin VitroMutated Rabies Virus L Protein. Virology. 1995;214(2):522–30. doi: 10.1006/viro.1995.0063. [DOI] [PubMed] [Google Scholar]
- 34.Ruedas JB, Perrault J. Insertion of enhanced green fluorescent protein in a hinge region of vesicular stomatitis virus L polymerase protein creates a temperature-sensitive virus that displays no virion-associated polymerase activity in vitro. J Virol. 2009;83(23):12241–52. doi: 10.1128/JVI.01273-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar KS, et al. Molecular characterisation of lineage IV peste des petits ruminants virus using multi gene sequence data. Vet Microbiol. 2014;174(1-2):39–49. doi: 10.1016/j.vetmic.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Huck B, et al. Novel human metapneumovirus sublineage. Emerg Infect Dis. 2006;12(1):147. doi: 10.3201/eid1201.050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rios L, Núñez JI, Diaz de Arce H, Ganges L, Pérez LJ. Revisiting the genetic diversity of classical swine fever virus: A proposal for new genotyping and subgenotyping schemes of classification. Transbound Emerg Dis. 2018;65(4):963–971. doi: 10.1111/tbed.12909. [DOI] [PubMed] [Google Scholar]
- 38.Rima B, et al. Problems of classification in the family Paramyxoviridae. Arch Virol. 2018;63:1395–1404. doi: 10.1007/s00705-018-3720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nei M, Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981;97(1):145–63. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nature Reviews Genetics. 2008;9(4):267. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 41.Liang Z, Yuan R, Chen L, Zhu X, Dou Y. Molecular evolution and characterization of hemagglutinin (H) in Peste des Petits ruminants virus. PloS one. 2016;11(4):e0152587. doi: 10.1371/journal.pone.0152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavakoli F, Moattari A, Shamsi SAM, Kadivar MR, Khodadad N, Pirbonyeh N, et al. Antigenic Variation of the Haemagglutinin Gene of the Influenza A (H1N1) pdm09 Virus Circulating in Shiraz, February-April 2013. Iranian J Immunol. 2015;12(3):198–208. [PubMed] [Google Scholar]
- 43.Zhu Zixiang, Zhang Xiaocui, Adili Gulizhati, Huang Jiong, Du Xiaoli, Zhang Xiangle, Li Pengfei, Zheng Xueguang, Liu Xiangtao, Zheng Haixue, Xue Qinghong. Genetic Characterization of a Novel Mutant of Peste Des Petits Ruminants Virus Isolated fromCapra ibexin China during 2015. BioMed Research International. 2016;2016:1–9. doi: 10.1155/2016/7632769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Nielsen R, Goldman N, Pedersen AM. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155(1):431–49. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fay JC, Wu CI. Sequence divergence, functional constraint, and selection in protein evolution. Annu Rev Genom Hum Genet. 2003;4:213–235. doi: 10.1146/annurev.genom.4.020303.162528. [DOI] [PubMed] [Google Scholar]
- 46.Pérez-Losada M, Arenas M, Galán JC, Palero F, González-Candelas F. Recombination in viruses: mechanisms, methods of study, and evolutionary consequences. Infect Genet Evol. 2015;30:296–307. doi: 10.1016/j.meegid.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han GZ, Worobey M. Homologous recombination in negative sense RNA viruses. Viruses. 2011;3(8):1358–1373. doi: 10.3390/v3081358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seyoum, B. & Teshome, E. Major Transboundary Disease of Ruminants and their Economic Effect in Ethiopia. Global J of Med Res (2018).
- 49.Elsayed AI, Boulila M, Odero DC, Komor E. Phylogenetic and recombination analysis of sorghum isolates of Sugarcane yellow leaf virus. Plant Pathol. 2018;67(1):221–32. doi: 10.1111/ppa.12708. [DOI] [Google Scholar]
- 50.Muthuchelvan D, et al. Molecular characterization of peste-des-petits ruminants virus (PPRV) isolated from an outbreak in the Indo-Bangladesh border of Tripura state of North-East India. Vet Microbiol. 2014;174(3-4):591–5. doi: 10.1016/j.vetmic.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 51.Aziz-ul-R. et al. Evaluation of risk factors for peste des petits ruminants virus in sheep and goats at the Wildlife-Livestock Interface in Punjab Province, Pakistan. BioMed Res Int (2016). [DOI] [PMC free article] [PubMed]
- 52.Chare ER, Gould EA, Holmes EC. Phylogenetic analysis reveals a low rate of homologous recombination in negative-sense RNA viruses. J Gen Virol. 2003;84(10):2691–703. doi: 10.1099/vir.0.19277-0. [DOI] [PubMed] [Google Scholar]
- 53.Klempa B, et al. Genetic interaction between distinct Dobrava hantavirus subtypes in Apodemus agrarius and A. flavicollis in nature. J Virol. 2003;77(1):804–809. doi: 10.1128/JVI.77.1.804-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sironen T, Vaheri A, Plyusnin A. Molecular evolution of Puumala hantavirus. J Virol. 2001;75(23):11803–11810. doi: 10.1128/JVI.75.23.11803-11810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charrel RN, de Lamballerie X, Fulhorst CF. The Whitewater Arroyo virus: natural evidence for genetic recombination among Tacaribe serocomplex viruses (family Arenaviridae) Virology. 2001;283(2):161–166. doi: 10.1006/viro.2001.0874. [DOI] [PubMed] [Google Scholar]
- 56.Archer AM, Rico-Hesse R. High genetic divergence and recombination in Arenaviruses from the Americas. Virology. 2002;304(2):274–281. doi: 10.1006/viro.2002.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang R, Wang X, Su J, Zhao J, Zhang G. Isolation and analysis of two naturally-occurring multi-recombination Newcastle disease viruses in China. Virus Res. 2010;151(1):45–53. doi: 10.1016/j.virusres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Han GZ, He CQ, Ding NZ, Ma LY. Identification of a natural multi-recombinant of Newcastle disease virus. Virology. 2008;371(1):54–60. doi: 10.1016/j.virol.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 59.Yuan C, Liu W, Wang Y, Hou J, Zhang L, Wang G. Homologous recombination is a force in the evolution of canine distemper virus. PloS one. 2017;12(4):e0175416. doi: 10.1371/journal.pone.0175416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schierup MH, Mordhorst CH, Muller CP, Christensen LS. Evidence of recombination among early-vaccination era measles virus strains. BMC Evol Biol. 2005;5(1):52. doi: 10.1186/1471-2148-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series. [London]: Information Retrieval Ltd, c1979-c2000, 95–98 (1999).
- 62.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suchard MA, Weiss RE, Sinsheimer JS. Bayesian selection of continuous-time Markov chain evolutionary models. Mol Biol Evol. 2001;18(6):1001–13. doi: 10.1093/oxfordjournals.molbev.a003872. [DOI] [PubMed] [Google Scholar]
- 65.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123(3):585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delport W, Poon AF, Frost SD, Kosakovsky PSL. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26(19):2455–7. doi: 10.1093/bioinformatics/btq429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pond SL, Frost SD. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics. 2005;21(10):2531–3. doi: 10.1093/bioinformatics/bti320. [DOI] [PubMed] [Google Scholar]
- 68.Ray, S. C. SimPlot for Windows (version 3.5.1). Baltimore, MD. Available online at: http://sray.med.som.jhmi.edu/SCRoftware/ (accessed June 2015) (2003).
- 69.Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol. 2013;30(7):1720–8. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin, D. P., Murrell, B., Golden, M., Khoosal, A. & Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol1(1) 10.1093/ve/vev003 (2015). [DOI] [PMC free article] [PubMed]