Abstract
Near-IR fluorescence imaging is a novel modality and has great potential for detection of tumors. The goal of this study was to evaluate the value of the IR-783 dye, a near-infrared heptamethine carbocyanine, as an imaging agent for rapid detection of human cervical cancer and the underlying mechanism of IR-783 mediated imaging. The imaging was investigated in cervical cancer cells for detecting the uptake, accumulation and subcellular localization of IR-783 dye. Whole-body imaging of mice bearing human cervical cancer xenografts and freshly harvested clinical cervical cancer specimens were used for assessment of specific dye uptake and retention. Frozen tissue section was used for confirming the dye accumulation at the tissue and cellular levels. The detection of circulating tumor cells in peripheral blood spiked with cervical cancer cells was made. The results revealed that IR-783 dye could be specifically uptaken by cultured cervical cancer cells, human cervical cancer cell-spiked whole blood, human cervical cancer xenografts and freshly harvested human cervical cancer tissue, but not by normal cell tissues. In the present study, we proved that IR-783 has potential for detecting cervical cancer cells in clinical specimens and in circulating blood. It can be further developed as modality agent for deep tissue imaging of cervical cancer in clinic.
Keywords: IR-783 dye, cervical cancer, uptake, detection
Introduction
Cervical cancer has been the second most common cancer in women globally. The majority of cervical cancer related deaths occur in the developing world [1]. In recent years, cervical cancer has drawn more attention in clinical research. Accurately diagnosing is the first step toward preventing cervical cancer. Cervical Pap smear screening as a conventional imaging technology has led to a substantial reduction of cervical cancer incidence. However, it is not sufficient and has limited reproducibility and sensitivity to detect cervical cancer [2]. So, more sensitive optical imaging modalities would be useful for detection of cervical cancer.
Near-infrared fluorescence (NIRF) imaging as an attractive novel modality for cancer detection has obtained real-time pathophysiological information in many cancers. NIRF probes with emission profiles at 700 to 1,000 nm wavelength in the near-infrared region were required in this imaging technique [3-5]. These probes should meet some requirements including excellent optical characteristics, suitable biocompatibility and a cancer targeting ability [6]. IR-783 cyanine dyes have previously been described as ideal NIRF probes and they accumulate selectively at tumor sites but not in normal tissues [7]. IR-783 has been proved to have great potential in the detection of malignancies without additional conjugation with cancer-specific moieties [5]. Application of IR-783 in cervical cancer detection and diagnosis has yet to be fully explored.
To further explore their clinical value in cervical cancer, we detected IR-783 based on the specific uptake and retention of the carbocyanine dyes by cancer cells in different experimental settings. Furthermore, we tested the underlying mechanism that possibly due to the differential expression of organic anion transporting peptides (OATPs) in cancer cells. Our results suggest that this probe can be used as a sensitive contrast agent for cervical cancer visualization.
Materials and methods
Chemical reagents
IR-783 dye was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in DMSO diluted with appropriate vehicles. All materials were filtered through 0.2-μm filters and stored at 4°C before use.
Cell lines and cell culture
We used human cervical cancer cell lines including HeLa, SiHa and Caski as research objects and human normal immortalized cervical epithelial cells (H8) as control. These cell lines were all established by our laboratory, which were described in the method of other papers [8,9].
All cell lines were cultured in media containing 5% fetal bovine serum and 1 × penicillin/streptomycin at 37°C with 5% CO2.
In vitro study of IR-783 dye uptake in cultured cells and tissues
1 × 104 cells per well were seeded on four-chamber slides coated with vitronectin (NalgenNunc) and incubated with medium containing 5% fetal bovine serum for 24 hours. After removal of the medium, the slides were incubated in working solution of IR-783 dye (20 μm) at 37°C for 30 min and washed in PBS two times. Then 4% paraformaldehyde (Sigma-Aldrich) was used to fix for 10 min. The slides were covered by glass coverslips with an aqueous mounting medium (Sigma-Aldrich) after washing twice with PBS. Images were recorded by fluorescence microscope (Olympus).
Tissues collected from tumor-bearing mice and freshly harvested from patients (maximum diameter 1 mm × 2 mm × 2 mm) were used for checking imaging of IR-783. After fixation overnight, tissues were incubated in 30% sucrose solution until sink to the bottom. The tissues were then embedded in OTC medium (Leica) and frozen at -80°C, followed by histopathologic observation. The protocol was approved by the Ethics Committee of Xijing Hospital of The Fourth Military Medical University.
Evaluation subcellular localization of IR-783 dye in the cervical cancer cells
To determine the dye uptake by mitochondria and lysosomes, the molecular probes Mito Tracker Orange CMTMRos and Lyso Tracker Green DND-26 (Molecular Probes, Camarillo, CA, USA) were used to track cytoplasmic mitochondria and lysosomes respectively. The cells were seeded in live-cell imaging chambers overnight. Subsequently, cells were incubated with IR-783 dye for 30 min at 37°C and then washed twice with PBS. The cells were stained with DAPI at 37°C for 10 min. Following wash twice with PBS after DAPI staining, cells were incubated with 500 nM CMTMRos for 30 min at 37°C and 200 nM DND-26 for 60 min at 37°C respectively. Next, repeated washes and mounting were performed. Imaging was captured under a confocal microscope (OLYMPUS FV1000). The emission/excitation wavelengths for DND-26 were 554 nm/576 nm and for CMTMRos were 504 nm/511 nm.
To analyze the role of organic anion transporting peptides (OATP) in IR-783 dye uptake and accumulation in cancer cells, we added different OATP inhibitors to cervical cancer cells for 5 min before incubating the cells with IR-783 dye. These inhibitors included competitive inhibitor of OATPs bromosulfophthalein (BSP, 250 μmol/l), OATP1 inhibitor rifampicin (20 μmol/l), selective OATP1B1 inhibitor 17β-estradiol (EST, 20 μmol/l), and selective OATP1B3 inhibitor cholecystokinin octapeptide (CCK-8, 20 μmol/l). The images were observed under a confocal microscope (OLYMPUS FV1000).
Detection of cervical cancer cells in human blood samples
Blood samples were obtained from patients who suffered cervical cancer. The study was approved by the ethics committee of our hospital. Written informed patient consent was obtained from the patients. Cervical cancer cells carry the ability to metastasize to distinct organs via the circulatory system. To detect circulating tumor cells in blood, human blood samples were mixed with signal-cell suspension of cervical cancer cells pre-labeled with DAPI, and then IR-783 dye (20 μmol/l) was added. Following incubation at 37°C for 30 min, the mononuclear and cancer cells were isolated form mixture via the gradient centrifugation method using Histopaque-1077 (Sigma). After fixation of normal and cancer cells, the mixture was observed under confocal microscope to identify the cancer cells via the positive staining. Flow cytometry was used to count for the total number of cancer cells in the blood.
Uptake and accumulation of IR-783 dye in tumors of live mice
Athymic nude mice were anesthetized with ketamine (75 mg/kg) by intraperitoneal injection and kept at an anesthetized state during surgery. Then human cancer cells (1 × 106) were implanted either subcutaneously or orthotopically into athymic nude mice (n=6, respectively) following the procedures reported previously [10]. Mice were injected with IR-783 dye at a dose of 0.35 mg/kg when tumor size reached ~2-8 mm in diameter, as assessed by X-ray and by palpation. In nude mice, the tumor number could reach 4 samples, among them the maximum diameter was ~8 mm × 6 mm × 4 mm.
Athymic nude mice were obtained from the Animal Center and The Guidelines of Intramural Animal Use and Care Committee of our university were followed. The primary method of euthanasia used in this study was cervical dislocation. The mice were allowed to eat and drink ad libitum before being sacrificed.
Kodak Imaging Station 40000 MM was used to check fluorescent of whole body after 24 hours. Mice were anesthetized with diethyl ether and kept at an anesthetized state during imaging. Frozen section was obtained for immediate confocal imaging of tumor tissues. H&E staining and histological analyses were made using these frozen sections.
To assess tissue metabolic pathways of this dyes, athymic nude mice without tumor implantation were sacrificed at 0, 6, and 80 hours (n=6 each) after injection of IR-783 dye at a dose of 10 nmol/20 g. Dissected organs were subjected to IR-783 dyes for cancer imaging by a Kodak Imaging Station 4000 MM.
NIRF imaging of human cervical cancer tissues
Human cervical cancer tissue and no cancerous tissue were collected from three patients at our hospital to assess the direct IR-783 dye staining in ex vivo settings.
All samples were stained with the IVIS Lumina II imaging station (Caliper Life Sciences) for NIRF imaging. Images were captured by confocal microscopy. Then all samples were fixed and embed for frozen section. Frozen 10 μm sections were stained with DAPI and the mounted sections were analyzed with a confocal microscope to observe the NIRF signals.
Data processing and statistics
The statistical significance of data was determined by two-way analysis of variance followed by post hoc tests of Newman-Keuls multiple comparisons using GraphPad Prism software. Data were expressed as mean ± SD of the indicated number of determinations. The statistically significant difference was assigned as P < 0.05.
Results
Specific uptake of IR-783 dye by human cervical cancer cells
To detect whether the IR-783 dye was specifically uptake by cancer cells, we firstly tested the appropriate concentration and found that 20 μM was a good choice, because this concentration can give a good imaging and produces no cell proliferation effect for human cervical cancer Hela cell (data not shown). Then we assessed in human cervical cancer Hela, SiHa and Caski cells, and the results showed that IR-783 was significantly and uniformly uptake by cervical cancer cell lines but not by normal cells (Figure 1A). Mitochondrial and lysosomal tracking dyes were used for tracing the IR-783 subcellular localization. The stain was almost completely congruent with both MitoTracker and LysoTracker, suggesting that IR-783 is localized both in mitochondria (Figure 1B) and lysosomes (Figure 1C).
Figure 1.
Specific uptake of IR-783 dye by human cervical cancer cells. A. Photos of NIR imaging (NIR) showed that IR-783 dye was uptake by cervical cancer cell lines HeLa, SiHa and Caski, but not by normal cell H8. B. Co-localization of NIR and mitochondria staining in cervical cancer cell lines. C. Co-localization of NIR and lysosomes staining in cervical cancer cell lines.
Imaging human cervical cancer cells xenografts with IR-783 dye
To observe similar specificity for the uptake and retention of IR-783 by cervical tumor xenografts in vivo, Hela tumors with IR-783 dye were injected in athymic mice subcutaneous. Time-course studies showed that positive imaging was specifically detected in the tumors 24 hours after dye administration (Figure 2A). Then subcutaneous implants were used to confirm IR-783 uptake by cervical tumor cells. The frozen section revealed that clusters of cells were positive with IR-783 staining. Moreover, the presence of cervical cancer cells was also confirmed by H&E staining (Figure 2B). The main organs of mouse models were used for illustrating the metabolism of the IR-783 dye. Bio-distribution analysis indicated that IR-783 dye was primarily eliminated through the excretions of bile, urine and feces (Figure 2C).
Figure 2.

Use of IR-783 dye in detection of human cervical tumor xenografts and metabolism pathway. A. Images of subcutaneous cervical cancer. Whole-body NIR imaging and X-ray radiography were captured after 24 h administration of IR-783. B. Frozen sections of the retrieved xenografts staining with DAPI demonstrated IR-783 dye specifically uptake by cancer cells and H&E staining of retrieved xenografts confirmed the existence of cancer cells. C. Host organs were dissected and subjected to ex vivo imaging for researching metabolism of IR-783 dye.
Ex vivo IR-783 dye imaging of freshly dissected human cervical tumors
To evaluate whether IR-783 dye could be detected in cervical tumors from the patients, we checked human cervical cancer surgical specimens and found that higher staining can be detected in tumor tissues comparing with normal samples (Figure 3A). IR-783 staining in the cervical cancer was confirmed by NIR fluorescence imaging of frozen sections (Figure 3B). These data suggested that IR-783 dye could be uptake by human cervical tumors form the clinic.
Figure 3.

IR-783 dye imaging of freshly dissected human cervical tumors. A. Positive IR-783 dye signaling was detected in human cervical tumors from clinic. B. The results of NIR dye uptake by cervical cancer cells were confirmed by frozen section stain.
Detection of circulating cervical tumor cells in human blood
To assessed whether IR-783 dye could detect cervical cancer cells in peripheral blood when cancer cells were metastasized to distant organs via the systemic circulation. Fluorescence assay showed that cancer cells in blood could be visualized in sensitive signals and apparently differentiate from mononuclear cells (Figure 4A). A flow cytometric assay demonstrated that cancer cells were obviously detected in blood, even though low cell numbers were used (Figure 4B).
Figure 4.
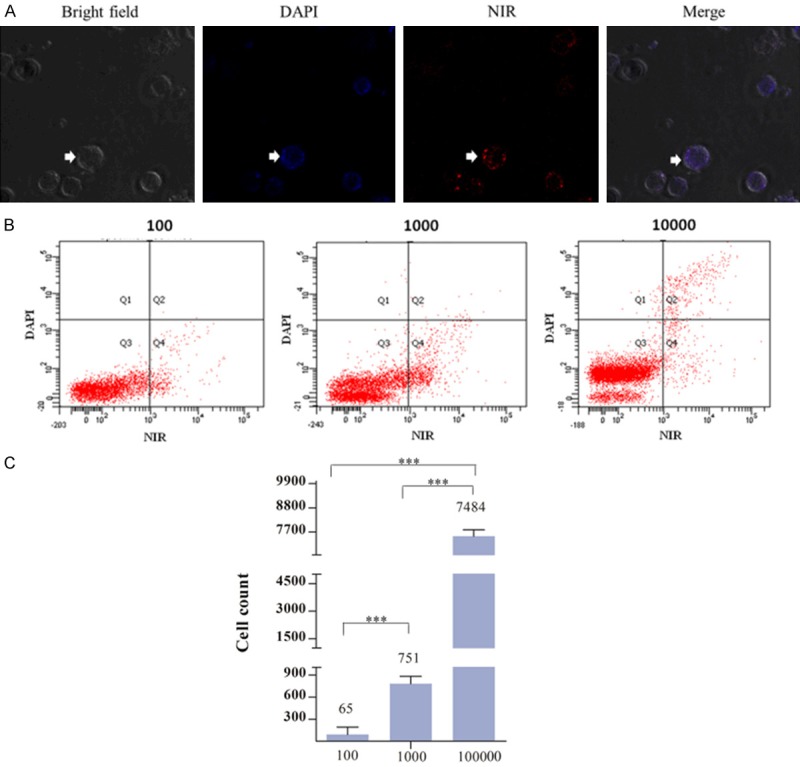
Use of IR-783 dye in detection of cervical cancer cells in human blood. A. IR-783 dye was used to stain human peripheral blood samples mixed with HeLa cervical cancer cells (showed by white arrow). B. Flow cytometric detection result confirmed the existence of cervical cancer cells in human blood. C. The number of cervical cancer cells was counted by flow cytometry.
Selective uptake of IR-783 dye in cervical cancer cells relies on the functions of OATP1B3 and OATP1
Previous studies have demonstrated that the inhibition of OATPs greatly inhibited the uptake of NIRF dyes [11,12], however, which subtype of OATPs have key role in cervical cancer remains elusive. We found that almost all the subtype of OATPs are expressed in cervical cancer cells, including OATP1, OATP1B1, OATPB3, OATP4A1 and OATP5A1. Among them, OATP4A1 and OATP5A1 had no differences between cancer cells and normal cells, but the express of OATP1, OATP1B1 and OATPB3 were decreased in cervical cancer cells (Figure 5A). BSP, as a nonspecific OATP, induced the greatest decrease in the NIRF signal, indicted by the reduction in fluorescence intensity comparing with that of the control. CCK-8 and RIF, as selective OATP1B3 and OATP1 inhibitor respectively, also significantly reduced the positive signal compared with that of the control. However, EST, as a selective OATP1B1 inhibitor, had nearly no effect on signal compared with that of the control (Figure 5B). These results indicated that selective uptake of NIRF dyes relies primarily on the transporting functions of OATP1B3 and OATP1.
Figure 5.
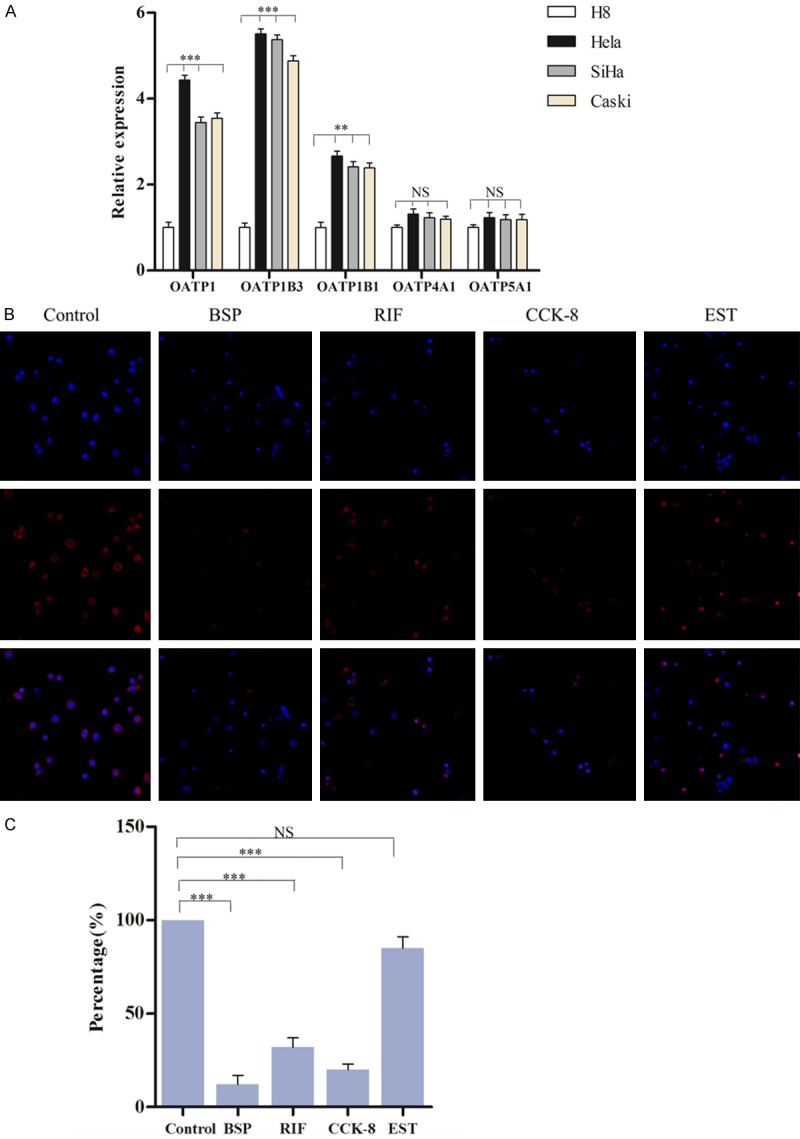
Selective uptake of IR-783 dye in cervical cancer cells relies on the functions of OATP1B3 and OATP1. A. The relative expression of OATP family in cervical cancer cells. B. BSP (inhibitor of OATP family), rifampicin (inhibitor of OATP1) and cholecystokinin octapeptide (inhibitor of OATP1B3) caused a decrease of uptake of IR-783. C. Statistical data for counting percentage of IR-783 dye positive cells.
Discussion
Human papillomavirus (HPV) cytology contesting has been recommended as the method of cervical cancer screening in clinic, which has markedly reduced mortality from squamous cell cervical cancer, which comprises 80-90% of cervical cancers [13-15]. However, it lacks specificity and cannot discriminate between common transient infections and rare prevalent pre-cancers, because atypical squamous cells of undetermined significance (ASC-US) account for the majority of abnormal cytology results [16]. So, a more effective method for imaging cervical cancer cells needs to be explored. In the present study, we found that IR-783, a heptamethine indocyanine dye, can be actively taken up and accumulated by cervical cancer cells but not by normal cells.
NIRF dyes have chemical structures of water-soluble pentamethine and heptamethinecyanine dyes have allowed for long-term tracking strategies with a satisfactory accuracy for diagnosis [17]. IR-783 dye has been proved as a powerful tool for cancer research and tumor detection in kidney cancer [7]. In this study, we demonstrated that IR-783 was taken up specifically by cervical cancer cells but not by normal cells both in vitro and in vivo. Moreover, IR-783 could be used for detecting cancer cells in blood or in tissues directly without chemical conjugation, which provides the unique advantage of identifying heterogeneous populations of cervical cancer cells in clinical specimens despite heterogeneous expression of surface markers. The surface marker, such as p16 testing for triage marker, was another strategy for improving combination of high sensitivity and high specificity for detecting cervical cancer, but has been proved to have less specificity and lower sensitivity [18-20].
The mechanism of carbocyanine dyes to be specifically taken up by malignant cells remains to be elucidated. It has been reported that IR-783 and MHI-148 uptake could be mediated by transmembrane proteins of the OATP family [21]. A further investigation which identifies the OATP member that mediates specific uptake and retention was warranted in malignant cells. IR-783 dye has the property of water-solubility which can easily diffuse across cytoplasmic membranes and co-localize with mitochondrial and lysosomal organelles in cervical cancer cells, which all reminders that OATP may be the main pathway. Indeed, differential expressions of certain OATP proteins were detected in cervical cancer cell lines. In addition, CCK-8 and RIF were used for proving that transporting functions of OATP1B3 and OATP1 were the key regulators in uptake of IR-783.
In summary, IR-783, as a heptamethine carbocyanine dye, offers an effective tool to detect cervical cancers with high sensitivity and hold great promise for novel therapeutics for future cancer imaging.
Acknowledgements
This work was supported by grants from The National Natural Science Foundation of China (81172495). This study was approved by the Ethics Committee of Xijing Hospital of The Fourth Military Medical University (Xi’an, China).
Disclosure of conflict of interest
None.
References
- 1.Lees BF, Erickson BK, Huh WK. Cervical cancer screening: evidence behind the guidelines. Am J Obstet Gynecol. 2016;214:438–443. doi: 10.1016/j.ajog.2015.10.147. [DOI] [PubMed] [Google Scholar]
- 2.Osborne JR, Akhtar NH, Vallabhajosula S, Anand A, Deh K, Tagawa ST. Prostate-specific membrane antigen-based imaging. Urol Oncol. 2013;31:144–154. doi: 10.1016/j.urolonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Licha K, Riefke B, Ntziachristos V, Becker A, Chance B, Semmler W. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem Photobiol. 2000;72:392–398. doi: 10.1562/0031-8655(2000)072<0392:hcdaca>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Shi C, Tong R, Qian W, Zhau HE, Wang R, Zhu G, Cheng J, Yang VW, Cheng T, Henary M, Strekowski L, Chung LW. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010;16:2833–2844. doi: 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi X, Wang F, Qin W, Yang X, Yuan J. Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field. Int J Nanomedicine. 2014;9:1347–1365. doi: 10.2147/IJN.S60206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Shao C, Wang R, Chu CY, Hu P, Master V, Osunkoya AO, Kim HL, Zhau HE, Chung LWK. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J Urol. 2013;189:702–710. doi: 10.1016/j.juro.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C, Sang QA, Pathak SJ, Chung LW. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A. 1996;93:15152–15157. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J Pharm Sci. 2013;102:6–28. doi: 10.1002/jps.23356. [DOI] [PubMed] [Google Scholar]
- 12.Zhang E, Luo S, Tan X, Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials. 2014;35:771–778. doi: 10.1016/j.biomaterials.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson L, Ponten J, Bergstrom R, Adami HO. International incidence rates of invasive cervical cancer before cytological screening. Int J Cancer. 1997;71:159–165. doi: 10.1002/(sici)1097-0215(19970410)71:2<159::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of minimally abnormal papanicolaou diagnoses. Obstet Gynecol. 1998;91:973–976. doi: 10.1016/s0029-7844(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 17.Licha K, Riefke B, Ebert B, Grotzinger C. Cyanine dyes as contrast agents in biomedical optical imaging. Acad Radiol. 2002;9(Suppl 2):S320–322. doi: 10.1016/s1076-6332(03)80216-7. [DOI] [PubMed] [Google Scholar]
- 18.Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6:1083–1098. doi: 10.2217/fmb.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–2545. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, Malamou-Mitsi V, Paraskevaidis E. p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis. Cancer Treat Rev. 2009;35:210–220. doi: 10.1016/j.ctrv.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]



