Abstract
Background and objective
Approximately 75% of diabetic patients in Saudi Arabia had poor glycaemic control. A high proportion of these patients will attend dental surgery clinics for treatment. Therefore, dentists should be well-prepared to control any complications they might arise on the dental chair during the dental procedures. Management of the associated risk factors is important to limit disease complications and improve the health of patients with diabetes.
The objectives of this review were to determine the maximum acceptable level of blood glucose for tooth removal in diabetics, show a systematic technique for the management of patients with diabetes on the dental chair. By using PRISMA guidelines, analysis of the published articles and reports across the world is considered one of the most appropriate available methods to obtain strong evidence about the acceptable levels of blood glucose where teeth extraction can be done safely.
Results
A total of 1080 studies were retrieved using the search strategy. After screening 185 titles, abstracts and 85 full-text articles, 36 studies were included. The outcome of this systematic review revealed that fasting blood glucose level of 240 mg/dl is a critical point for any dental treatment because the warning signs of diabetes start coming out. Maximum acceptable levels of blood glucose for removal of teeth in diabetics are 180 mg/dl (before meal) and 234 mg/dl (2 h after a meal). High blood glucose levels reduce the secretion of nitric oxide (powerful vasodilator) in the body which leads to poor circulation and slow-healing socket. Uncontrolled diabetics are at high risk of infection because of the high ketone levels in the blood.
Conclusion
Fasting blood glucose level of 180 mg/dl is a cut-off point for any selective dental extraction. However, Random blood glucose level of 234 mg/dl (13 mmol/l) is a cut-off point for an emergency tooth extraction. Tightly controlled diabetic patients (blood glucose level below 70 mg/dl) are susceptible to hypoglycemia.
Keywords: Management, Tooth extraction, Diabetic patients, Blood glucose cut-off points
1. Introduction
74.9% of the patients with type 2 diabetes mellitus in Saudi Arabia had poor blood glycaemic (Alzaheb & Altemani., 2018). Therefore, dental practitioners must have a better understanding of the factors affecting glycaemic control to improve the management of diabetic patients in the dental office. In the literature, there is a lack of information regarding the determination of the maximum permissible blood glucose level recommended for emergency tooth extraction in diabetic patients (Albarrak et al., 2018). A state of uncertainty exists among dental practitioners once they experience uncontrolled diabetic patient need an emergency tooth extraction. Dental practitioners become incapable of making a clinical decision to go ahead with tooth extraction because they have poor knowledge in this regard. The cut-off point of blood glucose level for an emergency tooth extraction is still not understandable (Zehani et al., 2017).
Normal fasting blood glucose is <100 mg/dl (63–99 mg/dl). Random blood glucose (2 h after meals) is <144 mg/dl (de Bedout et al., 2018). The patient is diagnosed with diabetes if fasting blood glucose ≥126 mg/dl or random blood glucose ≥200 mg/dl in addition to the presence of diabetes symptoms (American Diabetes Association, 2019a). Oral Glucose Tolerance Test (OGTT) 2-hour blood glucose ≥200 mg/dl can be used to confirm accurately the diagnosis. However, the blood test for HbA1c level is performed to determine how well the diabetes is controlled [in diabetic patient HbA1C ≥ 6.5%. Table 1] (Zehani et al., 2017, Franck et al., 2014).
Table 1.
Representing the relationship between A1C and average blood glucose.
| A1C level | Estimated average blood sugar (glucose) level |
|---|---|
| 7% | 154 mg/dl (8.6 mmol/l) |
| 8% | 183 mg/dl (10.2 mmol/l) |
| 9% | 212 mg/dl (11.8 mmol/l) |
| 10% | 240 mg/dl (13.4 mmol/l) |
If fasting blood glucose level reaches 240 mg/dl this is a sign of out of control diabetes (Bailey et al., 2016, Zehani et al., 2017, de Bedout et al., 2018, Alshareef et al., 2019, Power et al., 2019). The symptoms of high blood sugar can be mild, moderate, or severe. Mild to moderate symptoms are seen in people if their fasting blood glucose levels are 160–300 mg/dl. Symptoms at this stage include hunger, tremor or trembling, sweating, pale face, rapid heart rate, dizziness and weakness, blurred vision and confusion (American Diabetes Association, 2019b).
Severe symptoms are noticeable if fasting blood glucose levels are above 300 mg/dl. These include weight loss, dehydration, tiredness, poor concentration, irritability, and nervousness, irrational behavior, and personality changes, tingling in the mouth, and coordination problems (Alshareef et al., 2019). If blood glucose level tops 600 mg/dl (the condition is called diabetic hyperosmolar syndrome), Patient becomes confused, unconscious and ends up with diabetic coma. Coma happens as a result of a build-up of ketones (acid in the blood). People with uncontrolled diabetes are at high risk of infection and slow healing wound. The cut-off point of blood glucose level for an emergency tooth extraction is still arguable. This review provides some good explanations for the underlying causes of slow healing socket after tooth extraction in patients with uncontrolled diabetes. It also casts the light on the principles of treatment and shows a systematic technique for the management of patients with diabetes on the dental chair. In additions, cut-off points of fasting and random blood glucose levels beyond which dental extraction cannot be done, have been discussed. The main aim of this review is to give clear evidence about the most acceptable levels of blood glucose where dental practitioners can carry out the tooth extraction without hesitation or fear.
2. Material and methods
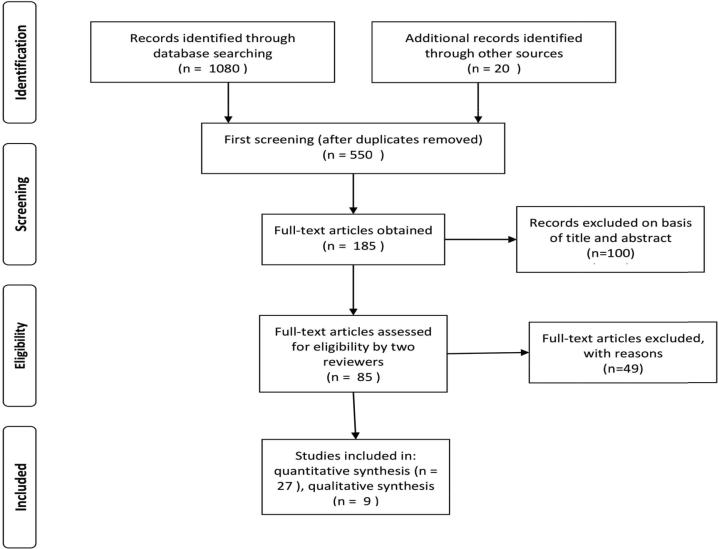
Fig. 1 represents the PRISMA flow chart of study selection. The archives of the PubMed, Scopus, Google Scholar, and Web of Science were searched for relevant literature. The review also included reports from the World Health Organization (WHO) and the American Diabetes Association (ADA). The articles were selected by reviewing their titles and abstracts as well as from the bibliography of the selected articles. Keywords used to search for relevant articles included hyperglycemia and infection, tooth mobility and bone loss, teeth extraction, slow healing socket, safe blood glucose levels for teeth extraction, principles of treatment for patients with diabetes on the dental chair, awareness, and Saudi Arabia. These terms were used individually and together to ensure an extensive literature search.
Fig. 1.
PRISMA flow chart of study selection.
3. Result
3.1. Safe blood glucose levels for an emergency tooth extraction
Based on diabetes guidelines laid down by “American College of Endocrinology (ACE), American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glucose Monitoring Consensus Statement” (American Diabetes Association, 2019c, Bailey et al., 2016, Grunberger et al., 2018), the safety scale of the preferable ranges of blood glucose levels for patients with diabetes were structured (Table 2).
Table 2.
Safety scale of blood glucose levels.
| Blood glucose level | Excellent | Good | Acceptable |
|---|---|---|---|
| Fasting (before a meal) | 72–109 mg/dl | 110–144 mg/dl | 145–180 mg/dl |
| 2 h after meal | 90–126 mg/dl | 127–180 mg/dl | 181–234 mg/dl |
On the other hand, the risk assessment for the levels of fasting blood glucose in diabetic patients was summarized in Table 3 (Bailey et al., 2016, Grunberger et al., 2018).
Table 3.
Risk assessment of fasting blood glucose levels.
| Fasting blood glucose level | Risk level assessment |
|---|---|
| ≤50 mg/dl | Dangerously low |
| 70–90 mg/dl | Low, take sugar if experiencing symptoms of low blood sugar |
| 90–120 mg/dl | Normal range |
| 120–160 mg/dl | Medium |
| 160–240 mg/dl | High, work to lower blood sugar levels |
| 240–300 mg/dl | Very high, a sign of out of control diabetes, see a doctor |
| ≥300 mg/dl | Severely high, seek immediate medical attention |
In light of the fact mentioned above, fasting blood glucose level of 240 mg/dl is a critical point for any dental treatment. When blood glucose levels reach (240 mg/dl), warning signs of diabetes start coming out (Estrich et al., 2019). These signs include tingling in hands or feet, nausea, vomiting, diarrhea, and dizziness. An emergency tooth extraction at a blood glucose level of 240 mg/dl will lead to severe infection and delay socket healing because the blood starts to build-up a high concentration of ketones.
Blood glucose levels for selective/emergency tooth extraction under LA should be considered acceptable if the dental treatment can be achieved with the minimal levels of risk or in other words with no sign of out of control diabetes. So, fasting blood glucose level of 180 mg/dl is a cut-off point for any selective dental extraction. However, random blood glucose level (2 h after a meal) of 234 mg/dl is a cut-off point for an emergency tooth extraction (Tables 2 & 3).
4. Discussion
4.1. Relationship between hyperglycemia and infection
Infection is a risk factor for uncontrolled diabetes causing an increase in the blood glucose levels. Level of stress increases when the body tries to fight off an infection. The body produces a number of stressful hormones such as cortisol and glucagon, which trigger the release of glucose from the liver; making glucose levels rise significantly (Saeb et al., 2019, Aggarwal et al., 2019).
People with diabetes are susceptible to oral candidiasis as a result of dehydration which affects the body in general and salivary glands in particular. The decrease in salivary flow rate and saliva PH promote the increase of colonization of Candida species in the oral cavity (Mohammadi et al., 2016, Sultana et al., 2018).
Zheng et al (2012) carried out a study to investigate the relationship between severe multi space infections of the oral maxillofacial region and diabetes mellitus. The outcome of this study revealed that the uncontrolled diabetics had worse infections involving more spaces, longer hospital stays, and more frequent complications than nondiabetic patients.
A study by Gholinejad Ghadi et al (2018) reported that the dental extractions may create a portal of entry for the fungal infection in the patients with uncontrolled diabetic. Fulminant mucormycosis of maxillary sinuses were detected after dental extraction in poorly controlled diabetic patients. So, the higher the blood glucose levels the greater the opportunity for predisposing conditions, such as Mucorales to grow heavily.
A systematic review investigated the relationship between diabetes and non-retention of root filled teeth. The outcome of this study revealed that the uncontrolled diabetics had a significantly higher number of extracted root-filled teeth than healthy non-diabetic subjects (Cabanillas-Balsera et al., 2019). So, uncontrolled blood glucose levels have a negative effect on the life span of teeth with root canal treatment.
A cross-sectional study was carried out in Saudi Arabia to investigate the effectiveness of blood glucose levels in the occurrence of dental caries in type 2 diabetes (Almusawi et al., 2018). The result of this study reported that patients with uncontrolled diabetes had high levels of dental caries, periodontal disease, and xerostomia. Counts of Streptococcus mutans and lactobacilli were high. The highest the levels of fasting blood glucose, salivary glucose and HbA1c are the greater the risk of dental caries (Almusawi et al., 2018).
A study by Wang et al (2018) reported that the tooth extraction in elderly patients with uncontrolled diabetes is considered as one of the triggering factors for osteonecrosis of the jaws (ONJ). So, dental extraction must be carried out with a safe range of blood glucose levels. However, dental extraction of teeth with no acute odontogenic infection does not need antibiotic prophylaxis in diabetic patients with control level of glycemic (Fernandes et al., 2015, Power et al., 2019). Moreover, plasma-rich growth factor (PRGF) application after tooth extraction in the diabetic patient does improve the healing process by accelerating socket closure (epithelialization) and tissue maturation (Mozzati et al., 2014).
Moreover, the level of high-sensitivity C-reactive protein (hs-CRP) increased in diabetic patients who had tooth extraction associated with oral infection (Lund Håheim et al., 2017). As it is known, high-sensitivity C-reactive protein is produced by the body, when blood-vessel walls are inflamed.
4.2. Tooth mobility and bone loss associated with uncontrolled diabetic patients
One possible explanation for increase the bone loss and teeth mobility in people with high blood sugar is the reduction in the blood supply for both soft and hard tissues of teeth. Poor circulation can cause blood stasis in the periodontal tissues around the teeth. Insufficient blood supply causes periodontal tissue to become starved of oxygen. A low blood oxygen level can cause over stimulation for the osteoclasts and as a consequence, the bone will get absorbed and teeth become mobile. A study by Wiener et al (2017) found that the patients with diabetes who were drinking 2 or more sugar-sweetened beverages (SSBs) per day they had 6 or more teeth extracted.
Furthermore, it was noticed that the prevalence of tooth extractions in French, population type 2 diabetic was higher than the non-diabetic population. Diabetic patients disposed to have dental extractions earlier and more often than non-diabetic individuals (Mayard-Pons et al., 2015).
4.3. Causes of delay socket healing after tooth extraction for patients with uncontrolled diabetes
The underlying causes of delayed wound healing in uncontrolled diabetes are still arguable. There are a few possible explanations might clarify the mechanism of this process. 1. Insufficient insulin level contributes to a slow-healing socket. Average daily secretion of insulin into circulation in healthy individuals about 35 units. There are also ten times this amount stored within the pancreas (Zhang et al.,2017). Tooth socket healing requires two cellular actions, for example, repair, and regeneration of tissue. This process is controlled and regulated by specific activated molecules such as transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), bone morphogenetic protein (BMP), and insulin-like growth factor (IGF). These molecules are well-conserved protein sequences involved in the initial response to injury and repair in soft and hard tissues (Younis et al., 2013). The healing of bone in uncontrolled diabetic patients prolongs because of a delay in the onset of cell proliferation and osteoblast differentiation. Insulin level has a direct effect on the expression of transforming growth factor beta TGFβ-3 and insulin-like growth factor IGF-1R, which accelerate the healing of the socket (Younis et al., 2013). 2. Patients with uncontrolled diabetes are considered immunosuppressed due to the negative effects of elevated blood sugars on the immune system. Immune defects arise from the macrophages which lose its appetite and cause a slowing down in the process of phagocytosis (Manji et al., 2019). The higher the blood glucose levels the greater the chance for infections. So, high blood sugar weakens the function of macrophages, resulting in an inability to fight the microorganisms. 3. High blood glucose levels reduce the secretion of powerful vasodilator nitric oxide (NO) in the circulatory system, cause stiffening and narrowing of blood vessels. Thus, uncontrolled diabetic patients are associated with poor circulation (Arda et al., 2019). This means a slowing down in the movement of red cells and a delay in the delivery of nutrients and oxygenated blood to the wounded or surgical site. Consequently, slow-healing socket occurs and the opportunity for secondary infection arises. 4. When fasting blood glucose levels become over 240 mg/dl, the body starts metabolizing fat at a high rate and converting fatty acids into ketones (Bailey et al., 2016, Grunberger et al., 2018). High ketones level in the blood might indicate diabetic ketoacidosis. Blood becomes acidic. Ketones bodies might interfere with the wound healing process, for example, tooth socket by either inhibiting the secretion of nitric oxide or reducing the macrophages’ appetite. A study by Noh et al., (2006) demonstrated that the blood level of nitric oxide increased by using the ketogenic diet.
4.4. Principles of treatment for patients with diabetes on the dental chair
When a patient comes to the dental clinic for tooth extraction and declares that he is a diabetic, the principles of treatment must be followed accordingly: (1) Establish if the patient is controlled with diet alone, tablets, or insulin injections. (2) Diabetic patients are immunocompromised and required early treatment of infections. (3) Controlled diabetic patients listed for standard dental extraction do not need prophylactic antibiotics. However, uncontrolled ones need antibiotic prophylaxis (Zehani et al., 2017, Power et al., 2019). (4) Hypoglycemia must be avoided as it may cause brain damage.
Based on a study by Alshareef et al (2019) reported that the clinicians should take great care with the management of diabetic patients in Saudi Arabia. Diabetic patients who visited the emergency department in Saudi Arabia hospitals had poor glycemic control. Moreover, practicing preventive diabetes care in Saudi Arabia is not sufficient, according to the diabetic standards of care recommended by the American Diabetes Association (Alshareef et al., 2019).
In the light of these facts, the dental practitioners in Saudi Arabia encounter too many uncontrolled diabetic patients in their dental surgery clinics (Alshareef et al., 2019). Therefore, dentists should be well-prepared and know how to manage and control patients with diabetes. Dental extraction sockets healing is satisfactory in diabetic patients if they are well controlled and managed (Power et al., 2019).
4.5. Management of dental extraction under local anesthesia (LA) for patients with diabetes
(1) An early morning appointment will minimize the risk of stress-induced hypoglycemia (Jia et al., 2019, Byakodi et al., 2017). (2) Check blood glucose prior to surgery by using blood glucose strips. If blood glucose is tightly controlled, the patient may become hypoglycemic during the extraction procedure. We have to bear in mind fasting blood glucose <100 mg/dl and random blood glucose <144 mg/dl (de Bedout et al., 2018, Power et al., 2019). (3) Dental treatment under LA or sedation should be arranged at least with mealtimes. The patient takes food and medications as normal. (4) Consider post-extraction antibiotics as problems can arise with wound healing and secondary infection (Zehani et al., 2017). A diabetic patient develops an infection, blood glucose levels will be higher than usual and need to increase his insulin dose (de Bedout et al., 2018). (5) The maximum permissible blood glucose level for dental extraction is 180 mg/dl (10 mmol/l) fasting blood glucose or 200 mg/dl (11 mmol/l) random blood glucose (de Bedout et al., 2018). (6) The blood glucose level of 234 mg/dl (13 mmol/l) is a cut-off point for an emergency tooth extraction (de Bedout et al., 2018, Power et al., 2019).
Emergency extraction can be carried out for a patient with blood glucose ≤234 mg/dl, has a painful mobile tooth on condition that LA without adrenaline must be administrated and a course of amoxicillin 500 mg for 5 days must be given after extraction (Gazal, 2019, Gazal et al., 2018, Byakodi et al., 2017).
5. Conclusion
Fasting blood glucose level of 180 mg/dl is a cut-off point for any selective dental extraction. However, Random blood glucose level of 234 mg/dl (13 mmol/l) is a cut-off point for an emergency tooth extraction. Tightly controlled diabetic patients (blood glucose level below 70 mg/dl) are susceptible to hypoglycemia.
Declaration of Competing Interest
The author declared that there is no conflict of interest.
Acknowledgments
I would like to acknowledge Dr. Hamdan Alghamdi, the Editor-in-Chief of Saudi Dental Journal for his suggestion to submit a short review showing most updated literature related to tooth extraction in diabetic patients.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggarwal A., Wadhwa R., Kapoor D., Khanna R. High Prevalence of genital mycotic infections with sodium-glucose co-transporter 2 Inhibitors among Indian Patients with Type 2 Diabetes. Indian J. Endocrinol. Metab. 2019;23(1):9–13. doi: 10.4103/ijem.IJEM_244_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarrak A.I., Mohammed R., Assery B., Allam D., Morit S.A., Saleh R.A., Zare'a R. Evaluation of diabetes care management in primary clinics based on the guidelines of American Diabetes Association. Int. J. Health Sci. (Qassim). 2018;12(1):40–44. [PMC free article] [PubMed] [Google Scholar]
- Almusawi M.A., Gosadi I., Abidia R., Almasawi M., Khan H.A. Potential risk factors for dental caries in Type 2 diabetic patients. Int. J. Dent Hyg. 2018;16(4):467–475. doi: 10.1111/idh.12346. [DOI] [PubMed] [Google Scholar]
- Alshareef S.M., Aldayel A.Y., AlKhathlan M.A., Alduaij K.O., Alshareef F.G., Al-Harthi M.E., Aldayel A.A., Shadid A.M., Dahmash A.B. Diabetic patients in Saudi Arabia: The evaluation of glycemic control measures based on emergency department utilization and the percentages of adherence to the recommended follow-ups for microvascular complications. Saudi Med. J. 2019;40(3):271–276. doi: 10.15537/smj.2019.3.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzaheb R.A., Altemani A.H. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab. Syndr. Obes. 2018;11:15–21. doi: 10.2147/DMSO.S156214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Older Adults: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S139–S147. doi: 10.2337/dc19-S012. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes-2019 abridged for primary care providers. Clin. Diabetes. 2019;37(1):11–34. doi: 10.2337/cd18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arda E., Ay A., Akdere H., Akdeniz E. The association of Intron 4 VNTR and Glu298Asp polymorphisms of the nitric oxide synthetase 3 gene and vasculogenic erectile dysfunction in Turkish men. Syst. Biol. Reprod. Med. 2019;12:1–7. doi: 10.1080/19396368.2019.1601792. [DOI] [PubMed] [Google Scholar]
- Bailey T.S., Grunberger G., Bode B.W., Handelsman Y., Hirsch I.B., Jovanovič L., Roberts V.L., Rodbard D., Tamborlane W.V., Walsh J. American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE), American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glycose Monitoring Consensus Statement. Endocr. Pract. 2016;22(2):231–261. doi: 10.4158/EP151124.CS. [DOI] [PubMed] [Google Scholar]
- Byakodi S., Gurjar V., Soni S. Glucose levels and hemodynamic changes in patients submitted to routine dental extraction under local Anesthesia with and without Adrenaline. J. Contemp. Dent. Pract. 2017;18(1):57–59. doi: 10.5005/jp-journals-10024-1989. [DOI] [PubMed] [Google Scholar]
- Cabanillas-Balsera D., Martín-González J., Montero-Miralles P., Sánchez-Domínguez B., Jiménez-Sánchez M.C., Segura-Egea J.J. Association between diabetes and nonretention of root filled teeth: a systematic review and meta-analysis. Int. Endod. J. 2019;52(3):297–306. doi: 10.1111/iej.13011. [DOI] [PubMed] [Google Scholar]
- de Bedout T., Kramer K., Blanchard S., Hamada Y., Eckert G.J., Maupome G., John V. Assessing the medical emergency preparedness of dental faculty, residents, and practicing periodontists: an exploratory study. J. Dent. Educ. 2018;82(5):492–500. doi: 10.21815/JDE.018.058. [DOI] [PubMed] [Google Scholar]
- Estrich C.G., Araujo M.W.B., Lipman R.D. Prediabetes and diabetes screening in dental care settings: NHANES 2013 to 2016. JDR Clin. Trans. Res. 2019;4(1):76–85. doi: 10.1177/2380084418798818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes K.S., Glick M., de Souza M.S., Kokron C.M., Gallottini M. Association between immunologic parameters, glycemic control, and postextraction complications in patients with type 2 diabetes. J. Am. Dent. Assoc. 2015;146(8):592–599. doi: 10.1016/j.adaj.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Franck S.D., Stolberg R.L., Bilich L.A., Payne L.E. Point-of-care HbA1c screening predicts diabetic status of dental patients. J. Dent. Hyg. 2014;88(1):42–52. [PubMed] [Google Scholar]
- Gazal G. Is prilocaine safe and potent enough for use in the oral surgery of medically compromised patients. Saudi Med. J. 2019;40(1):97–100. doi: 10.15537/smj.2019.1.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazal, G., 2018. Is articaine more potent than mepivacaine for use in oral surgery? J. Oral Maxillofac Res. 2018; 30;9(3): e5. [DOI] [PMC free article] [PubMed]
- Gholinejad Ghadi N., Seifi Z., Shokohi T., Aghili S.R., Nikkhah M., Vahedi Larijani L., Ghasemi M., Haghani I. Fulminant mucormycosis of maxillary sinuses after dental extraction inpatients with uncontrolled diabetic: Two case reports. J. Mycol. Med. 2018;28(2):399–402. doi: 10.1016/j.mycmed.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Handelsman Y., Bloomgarden Z.T., Fonseca V.A., Garber A.J., Haas R.A., Roberts V.L., Umpierrez G.E. American Association of Clinical Endocrinologists and American College of Endocrinology 2018 Position statement on Integration of Insulin Pumps and Continuous Glucose Monitoring in Patients with Diabetes Mellitus. Endocr. Pract. 2018;24(3):302–308. doi: 10.4158/PS-2017-0155. [DOI] [PubMed] [Google Scholar]
- Jia W., Weng J., Zhu D., Ji L., Lu J., Zhou Z., Zou D., Guo L., Ji Q., Chen L., Chen L., Dou J., Guo X., Kuang H., Li L., Li Q., Li X., Liu J., Ran X., Shi L., Song G., Xiao X., Yang L., Zhao Z. Chinese diabetes society. Standards of medical care for type 2 diabetes in China-0027. Diabetes Metab. Res. Rev. 2019;25 [Google Scholar]
- Lund Håheim L., Rønningen K.S., Enersen M., Olsen I. The predictive role of tooth extractions, oral infections, and hs-C-reactive protein for mortality in individuals with and without diabetes: a prospective cohort study of a 12 1/2-year follow-up. J. Diabetes Res. 2017;2017:9590740. doi: 10.1155/2017/9590740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji F., Lam J.C., Meatherall B.L., Church D., Missaghi B. Severe facial necrosis in a type 1 diabetic patient secondary to mucormycosis masquerading as an internal maxillary artery occlusion: a case report. BMC Infect. Dis. 2019;19(1):184. doi: 10.1186/s12879-019-3822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayard-Pons M.L., Rilliard F., Libersa J.C., Musset A.M., Farge P. Database analysis of a French type 2 diabetic population shows a specific age pattern of tooth extractions and correlates health care utilization. J. Diabetes Complications. 2015;29(8):993–997. doi: 10.1016/j.jdiacomp.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Mohammadi F., Javaheri M.R., Nekoeian S., Dehghan P. Identification of Candida species in the oral cavity of diabetic patients. Curr. Med. Mycol. 2016;2(2):1–7. doi: 10.18869/acadpub.cmm.2.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzati M., Gallesio G., di Romana S., Bergamasco L., Pol R. Efficacy of plasma-rich growth factor in the healing of postextraction sockets in patients affected by insulin-dependent diabetes mellitus. J. Oral Maxillofac. Surg. 2014;72(3):456–462. doi: 10.1016/j.joms.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Noh H.S., Kim D.W., Cho G.J., Choi W.S., Kang S.S. Increased nitric oxide caused by the ketogenic diet reduces the onset time of kainic acid-induced seizures in ICR mice. Brain Res. 2006;1075(1):193–200. doi: 10.1016/j.brainres.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Power D.J., Sambrook P.J., Goss A.N. The healing of dental extraction sockets in insulin-dependent diabetic patients: a prospective controlled observational study. Aust. Dent. J. 2019;64(1):111–116. doi: 10.1111/adj.12669. [DOI] [PubMed] [Google Scholar]
- Saeb A.T.M., Al-Rubeaan K.A., Aldosary K., Udaya Raja G.K., Mani B., Abouelhoda M., Tayeb H.T. Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb. Pathog. 2019;128:215–229. doi: 10.1016/j.micpath.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Sultana S., Jaigirdar Q.H., Islam M.A., Azad A.K. Frequency of fungal species of onychomycosis between diabetic and non-diabetic patients. Mymensingh Med. J. 2018;27(4):752–756. [PubMed] [Google Scholar]
- Wang Q., Liu J., Qi S., Liao X., Liu D., Pan J. Clinical analysis of medication related osteonecrosis of the jaws: A growing severe complication in China. J. Dent Sci. 2018;13(3):190–197. doi: 10.1016/j.jds.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener R.C., Shen C., Findley P.A., Sambamoorthi U. Tan X The association between diabetes mellitus, sugar-sweetened beverages, and tooth loss in adults: Evidence from 18 states. J. Am. Dent. Assoc. 2017;148(7):500–509.e4. doi: 10.1016/j.adaj.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis W.H., Al-Rawi N.H., Mohamed M.A., Yaseen N.Y. Molecular events on tooth socket healing in diabetic rabbits. Br. J. Oral Maxillofac. Surg. 2013;51(8):932–936. doi: 10.1016/j.bjoms.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Zehani A., Smichi I., Marrakchi J., Besbes G., Haouet S., Kchir S. Agressive infection following a dental extraction in a diabetic patient: Rhinocerebral mucormycosis. Tunis. Med. 2017;95(5):378–380. [PubMed] [Google Scholar]
- Zhang Z., Fang P., Yu M., Wang Y., Li Y., Shi M., Bo P., Gu X., Zhu Y. Serum Galanin Concentration is Increased in Subjects with Impaired Glucose Tolerance. Can. J. Diabetes. 2017;41(6):563–566. doi: 10.1016/j.jcjd.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Zheng L., Yang C., Zhang W., Cai X., Kim E., Jiang B., Wang B., Pu Y., Wang J., Zhang Z., Zhou L., Zhou J. Guan X Is there association between severe multispace infections of the oral maxillofacial region and diabetes mellitus? J. Oral Maxillofac. Surg. 2012;70(7):1565–1572. doi: 10.1016/j.joms.2011.07.010. [DOI] [PubMed] [Google Scholar]