Abstract
Background
There is insufficient evidence on the effect of statins, particularly high‐intensity statins, in patients with acute ischemic stroke and atrial fibrillation. We investigated the impact of statins on the outcomes in these patients, including those who might be vulnerable to statin therapy and those without clinical atherosclerotic cardiovascular diseases.
Methods and Results
A total of 2153 patients with acute ischemic stroke and atrial fibrillation were enrolled in the present nationwide, multicenter, cohort study. The primary composite end point was the occurrence of net adverse clinical and cerebral events (NACCE; death from any cause, stroke, acute coronary syndrome, or major bleeding) over a 3‐year period based on statin intensity. NACCE rates were lower in patients receiving low‐ to moderate‐intensity (adjusted hazard ratio 0.64; 95% CI: 0.52‐0.78) and high‐intensity statins (hazard ratio 0.51; 95% CI 0.40‐0.66) than in those not receiving statin therapy. High‐intensity statins were associated with a lower risk for NACCE than low‐ to moderate‐intensity statins (hazard ratio 0.76; 95% CI 0.59‐0.96). Subgroup analyses showed that the differences in hazard ratio for 3‐year NACCE favored statin use across all subgroups, including older patients, those with low cholesterol levels, patients receiving anticoagulants, and patients without clinical atherosclerotic cardiovascular diseases. Magnified benefits of high‐intensity statins compared with low‐ to moderate‐intensity statins were observed in patients who underwent revascularization therapy and those under 75 years of age.
Conclusions
Statins, particularly high‐intensity statins, could reduce the risk for NACCE in patients with acute ischemic stroke and atrial fibrillation; this needs to be further explored in randomized controlled trials.
Keywords: atrial fibrillation, ischemic stroke, NACCE, outcome, statin
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Quality and Outcomes
Clinical Perspective
What Is New?
Statin therapy, particularly high‐intensity statin therapy, was associated with reduced risk of net adverse clinical and cerebral events and increased the probability of favorable outcomes compared with no statin therapy in patients with acute ischemic stroke and atrial fibrillation.
The benefits of statin therapy were consistent across various subgroups, including patients who may be vulnerable to statin therapy and those without atherosclerotic cardiovascular diseases.
High‐intensity statins were more effective than low‐ to moderate‐intensity statins in reducing the risk for net adverse clinical and cerebral events in these patients, particularly in patients aged ≤75 years and those who underwent revascularization therapy.
What Are the Clinical Implications?
Statin therapy may lead to a better prognosis than no statin therapy in patients with acute ischemic stroke and atrial fibrillation, including the subgroup of patients who may be vulnerable to statin therapy and those without atherosclerotic cardiovascular diseases, with high‐intensity statin use leading to more favorable outcomes.
This study may add to the body of evidence as to which starting statin, particularly high‐intensity statin, will improve net adverse clinical and cerebral events in stroke patients with atrial fibrillation.
Prospective randomized controlled trials are necessary to assess the effectiveness and safety of various statin dose regimens in patients with acute ischemic stroke and atrial fibrillation.
Introduction
With the aging population and the development of diagnostic methods, the incidence of atrial fibrillation (AF) in patients with acute ischemic stroke (AIS) is increasing.1, 2 AF is a major risk factor for the recurrence of stroke, and cardioembolic stroke can be disabling and fatal.3, 4 Therefore, attempts have been made to improve the prognosis of patients with AIS and AF.5, 6 Statins, particularly high‐intensity statins, are one of the most potent treatments for improving the prognosis of stroke.7, 8 Research indicates that high‐intensity statin use is associated with improved outcomes and survival in patients with a noncardioembolic AIS of presumed atherosclerotic origin.9, 10 Therefore, current guidelines recommend high‐intensity statins for patients aged under 75 years who have atherosclerotic cerebrovascular disease.6, 11
Statin therapy in patients with AIS and AF has been associated with improved survival rates and reduced risk for future cerebrovascular and cardiovascular events in previous studies.12, 13, 14 However, there is no evidence on the benefits of statins based on the different intensities in risk reduction of net adverse clinical events in stroke patients with AF. Furthermore, there is insufficient evidence for the benefits of statin therapy, particularly high‐intensity statins, in patients who may be vulnerable to statin therapy and those without clinical atherosclerotic cardiovascular diseases (ASCVD). There are also no recommendations for the current guidelines concerning the use and intensity of statin therapy for patients with AIS and AF.6, 15
Therefore, we systematically investigated the impact of statin therapy, emphasizing high‐intensity statin therapy, on the occurrence of net adverse clinical events in a nationwide population with AIS and AF. We also examined the effect of statin therapy on clinical outcomes in subgroups of patients who may be vulnerable to statin therapy and those without clinical ASCVD.
Materials and Methods
Data Availability Statement
Any data not published within the article are available in a public repository and include digital object identifiers, accession numbers to the data sets, and anonymized data. These data will be shared on reasonable request from any qualified investigator.
Subjects
The present study used data from a prospective, multicenter, national registry, K‐ATTENTION (the Korean Atrial Fibrillation Evaluation Registry in Ischemic Stroke Patients). K‐ATTENTION is a database of consecutive patients with AIS and AF admitted to 11 medical centers in South Korea between January 2013 and December 2015. Among the 3213 patients registered in the database, we identified the patients with AIS and AF over the age of 20 years who experienced neurologic symptoms or signs and had a diagnosis of AIS confirmed using brain imaging (computed tomography or magnetic resonance imaging). The exclusion criteria included incomplete data for statin intensity, patients who died during hospitalization, incomplete data for the constituents of the clinical outcomes over a 3‐year period, and follow‐up less than 3 months.
The study subjects were divided into 3 groups based on whether a statin medication was prescribed on discharge, according to the American Heart Association/American College of Cardiology guidelines: no statin, low‐ to moderate‐intensity statin, and high‐intensity statin therapy.15 We assessed high‐intensity statins (atorvastatin [40 or 80 mg] and rosuvastatin [20 or 40 mg]), which typically lower low‐density lipoprotein cholesterol (LDL‐C) levels by more than 50%. All other statins were considered low to moderate intensity. Additionally, study participants were divided into 2 subgroups based on the presence of clinical ASCVD. Based on current guidelines clinical ASCVD included acute coronary syndromes, history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral arterial disease presumed to be of atherosclerotic origin.15 We analyzed the effect of statin therapy in patients who survived hospitalization and received statin treatment during both hospitalization and at discharge.
The present study was approved by the institutional review boards of all participating centers. The boards waived the necessity to obtain patient consent because of the retrospective nature of the study. All clinical and laboratory investigations described were conducted in accordance with the Declaration of Helsinki.
Protocol and Outcome Measures
The primary outcome measure was the occurrence of net adverse clinical and cerebral events (NACCE; a composite of death from any cause, stroke, acute coronary syndrome, or major bleeding) according to statin intensity over a 3‐year period.16 The key secondary outcomes included the constituents of NACCE over the 3‐year period and favorable outcomes after 3 months. We evaluated functional clinical outcomes using the modified Rankin Scale (mRS), which consists of 7 levels ranging from 0 (no symptoms) to 6 (death). Favorable outcomes were defined as functional independence with an mRS score of 0 to 2. Acute coronary syndrome was defined as unstable angina requiring urgent revascularization or myocardial infarction.
Information regarding clinical outcomes after discharge was prospectively obtained from all patients during routine clinic visits or via telemedicine with patients or caregivers over a 3‐year period. Thromboembolic risk and bleeding risk were assessed using the CHA2DS2‐VASc (Congestive heart failure, Hypertension, Age over 75 years [doubled], Diabetes mellitus, Prior Stroke or TIA or Thromboembolism [doubled], Vascular disease, Age 65‐74 years, Sex category) and HAS‐BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INR, Age under 65 years, Drugs or alcohol) scores, respectively. Medication adherence was assessed through interviews 3 months after index AIS using the 6‐item modified Morisky Medication Adherence Scale at the largest center.17 High adherence was defined as motivation and knowledge domain scores greater than 2. Subgroups of patients who may be vulnerable to statin therapy included older patients; those with high baseline National Institute of Health Stroke Scale (NIHSS) score, high HAS‐BLED score, or low baseline LDL; and patients receiving revascularization or anticoagulant therapies.
Statistical Analyses
The differences between the groups based on statin use (no statin versus low‐ to moderate‐intensity versus high‐intensity) were analyzed using either an analysis of variance or the Kruskal‐Wallis test for continuous variables. The chi‐squared or Fisher exact tests were used to analyze categorical variables and the distribution of mRS scores. Unadjusted associations of statin intensity, the risk for NACCE, and the constituents of NACCE were obtained using Kaplan‐Meier curves over the entire study period to account for censoring. Cox proportional hazards regression models were then constructed to calculate the adjusted hazard ratios (HR) and 95% CIs for NACCE and its constituents with respect to statin intensity categories. Adjustments were performed for the following variables based on their clinical significance and the results of previous studies: age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking, congestive heart failure, prior history of stroke or TIA, prior mRS scores, trial of Org 10 172 in acute stroke treatment classification, and initial NIHSS scores. Multiple logistic regression analysis was used to calculate the adjusted odds ratio for favorable outcomes at 3 months after AIS. A 2‐tailed test was used with a significance level of P<0.05.
A subgroup analysis for heterogeneity of the statin effect was performed, with subgroups defined according to age (≤75 or >75 years), sex, baseline NIHSS score (<12 or ≥12), baseline LDL level (≤70 mg/dL or >70 mg/dL), revascularization therapy (“yes” or “no”), oral anticoagulant treatment (“yes” or “no”), baseline CHA₂DS₂‐VASc score (<5 or ≥5), baseline HAS‐BLED score (<3 or ≥3), trial of Org 10 172 in acute stroke treatment classification (cardioembolism or undetermined ≥2 etiology: cardioembolism and more), and clinical ASCVD (“yes” or “no”). Interactions were tested by entering the selected multiplicative interaction terms between the subgroups and statin treatment effect into the proportional hazard models. Statistical analyses were performed using SPSS (version 23.0; IBM, Armonk, NY), SAS (version 9.4; SAS Institute, Cary, NC), and R software (version 3.3.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Our sample comprised 2153 patients with AIS and AF (Figure S1). Among these, 1100 (51.3%) were previously diagnosed with AF before index AIS, and 1053 (48.7%) were first diagnosed with AF during hospitalization. The median follow‐up duration was 17.3 (interquartile range: 4.3‐31.3) months. The clinical and biochemical characteristics of the patients who received no statin ( n=574, 26.7%), low‐ to moderate‐intensity statin (n=1013, 47.0%), and high‐intensity statin therapy (n=566, 26.3%) are shown in Table.
Table 1.
Baseline Clinical and Biochemical Characteristics According to Statin Intensity
| Nonstatin (n=574) | Low‐ to Moderate‐Intensity Statins (n=1013) | High‐Intensity Statins (n=566) | Total (n=2153) | P Valuea | |
|---|---|---|---|---|---|
| Age, y (mean±SD) | 72.7±11.0 | 73.6±9.1 | 73.2±9.8 | 73.2±9.8 | 0.428 |
| Male, n (%) | 322 (56.1) | 513 (50.6) | 285 (50.4) | 1120 (52.0) | 0.073 |
| Nonvalvular AF, n (%) | 557 (97.0) | 1001 (98.8) | 559 (98.8) | 2117 (98.3) | 0.019 |
| History of risk factors, n (%) | |||||
| Hypertension | 395 (68.8) | 700 (69.1) | 409 (72.3) | 1504 (69.9) | 0.346 |
| Diabetes mellitus | 156 (27.2) | 269 (26.6) | 165 (29.2) | 590 (27.4) | 0.535 |
| Dyslipidemia | 98 (17.1) | 269 (26.6) | 135 (23.9) | 502 (23.3) | <0.001 |
| Coronary artery disease | 61 (10.6) | 127 (12.5) | 79 (14.0) | 267 (12.4) | 0.230 |
| Congestive heart failure | 26 (4.5) | 26 (2.6) | 29 (5.1) | 81 (3.8) | 0.020 |
| Current smoking | 84 (14.6) | 117 (11.5) | 102 (18.0) | 303 (14.1) | 0.002 |
| Peripheral artery disease | 4 (0.7) | 15 (1.5) | 4 (0.7) | 23 (1.1) | 0.214 |
| Prior stroke or TIA | 203 (35.4) | 298 (29.4) | 197 (34.8) | 698 (32.4) | 0.019 |
| Biochemical variables (mean±SD) | |||||
| Total‐C, mg/dL | 159.7±35.0 | 160.5±36.9 | 169.2±41.1 | 162.6±37.7 | <0.001 |
| LDL‐C, mg/dL | 94.6±30.9 | 97.1±32.2 | 104.8±37.6 | 98.6±33.7 | <0.001 |
| Triglyceride, mg/dL | 98.0±76.2 | 91.9±48.9 | 96.2±51.0 | 94.7±57.7 | 0.658 |
| HDL‐C, mg/dL | 47.8±16.1 | 47.1±15.5 | 49.7±24.3 | 48.0±18.5 | 0.087 |
| Glycated hemoglobin, % | 6.1±1.1 | 6.0±1.1 | 6.2±1.2 | 6.1±1.1 | 0.256 |
| Admission glucose, mg/dL | 141.1±57.2 | 138.9±95.5 | 141.5±56.9 | 140.2±77.4 | 0.926 |
| Prestroke mRS 0 to 2, n (%) | 485 (84.5) | 814 (80.4) | 493 (87.1) | 1792 (83.2) | 0.002 |
| Initial NIHSS, median (IQR) | 8 (2;16) | 7 (2;14) | 7 (2;14) | 7 (2;14) | 0.037 |
| Intravenous alteplase, n (%) | 142 (24.7) | 230 (22.7) | 140 (24.7) | 512 (23.8) | 0.481 |
| Mechanical thrombectomy, n (%) | 54 (9.4) | 111 (11.0) | 70 (12.4) | 235 (10.9) | 0.276 |
| CHA₂DS₂‐VASc score, median (IQR) | 5 (4; 6) | 5 (4; 6) | 5 (4; 6) | 5 (4; 6) | 0.054 |
| CHA₂DS₂‐VASc score ≥5, n (%) | 343 (59.8) | 632 (62.4) | 367 (64.8) | 1342 (62.3) | 0.208 |
| HAS‐BLED score, median (IQR) | 2 (1; 3) | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 0.017 |
| HAS‐BLED score ≥3, n (%) | 214 (37.3) | 435 (42.9) | 248 (43.8) | 897 (41.7) | 0.043 |
| Cerebral atherosclerosis, n (%) | 250 (51.5) | 536 (57.1) | 263 (52.7) | 1049 (54.6) | 0.087 |
| TOAST classification | |||||
| CE | 499 (86.9) | 846 (83.5) | 448 (79.2) | 1793 (83.3) | 0.002 |
| UD (2 or more) | 75 (13.1) | 167 (16.5) | 118 (20.8) | 360 (16.7) | |
| Clinical ASCVDb, n (%) | 129 (22.5) | 276 (27.2) | 180 (31.8) | 585 (27.2) | 0.002 |
| Anticoagulation, n (%) | |||||
| Warfarin | 311 (54.2) | 679 (67.0) | 355 (62.7) | 1345 (62.5) | <0.001 |
| NOAC | 95 (16.6) | 114 (11.3) | 107 (18.9) | 316 (14.7) | |
AF indicates atrial fibrillation; ASCVD, atherosclerotic cardiovascular disease; CE, cardioembolism; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke (double weight) score; HAS‐BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INR, Age under 65 years, Drugs or alcohol; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile ranges; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NOAC, non–vitamin K antagonist oral anticoagulant; TIA, transient ischemic attack; TOAST, trial of Org 10 172 in acute stroke treatment classification; Total‐C, total cholesterol; UD, stroke of undetermined etiology; VASc, vascular.
Continuous variables were compared between the groups using 1‐way analysis of variance (ANOVA) or Mann‐Whitney U tests. The chi‐squared test was used for noncontinuous variables.
Clinical ASCVD includes acute coronary syndromes, history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral arterial disease presumed to be of atherosclerotic origin
Compared with those in the no‐statin group, the patients in the statin groups were more likely to have nonvalvular AF, history of dyslipidemia, higher total cholesterol and LDL‐C levels, lower initial NIHSS score, higher baseline HAS‐BLED score, clinical ASCVD, and 2 or more stroke etiologies and were more likely to be taking anticoagulant drugs after discharge. Compared with other groups, the patients in the low‐ to moderate‐intensity statin group were less likely to have a history of congestive heart failure, current smoking, and prior stroke or TIA. No significant differences were observed between the groups in the age, the number of undergone acute revascularization therapies (intravenous alteplase and mechanical thrombectomy), or history of hypertension and diabetes mellitus. Information on medication adherence after 3 months was available for the largest center, and high adherence to the same intensity statin therapy was observed in 96.3% (602/625) of the patients.
Clinical Outcomes
Overall, 514 patients were identified to have had a NACCE during follow‐up. Figure 1 presents the Kaplan‐Meier analysis showing that statin medication use (high‐ and low‐ to moderate‐intensity statins) resulted in a better overall NACCE‐free survival than not using statin medication. The crude incidence rates of the primary outcome in patients with AIS and AF were 11.92 NACCE per 100 person‐years for high‐intensity statin users, 15.52 NACCE per 100 person‐years for low‐ to moderate‐intensity statin users, and 24.56 NACCE per 100 person‐years for the patients in the no‐statin group. The unadjusted risks using the Cox proportional hazards modeling showed that patients with AIS and AF in the low‐ to moderate‐intensity statin group had a 35% lower risk of NACCE (HR 0.65; 95% CI 0.53‐0.78), whereas those in the high‐intensity statin group had a 51% lower risk of NACCE (HR 0.49; 95% CI 0.38‐0.63) than the patients in the no‐statin group (Table S1). The use of high‐intensity statins was associated with a 25% lower risk of NACCE risk in AIS patients with AF compared with the use of low‐ to moderate‐intensity statins (HR 0.75; 95% CI 0.59‐0.96; Figure 1 and Table S1).
Figure 1.
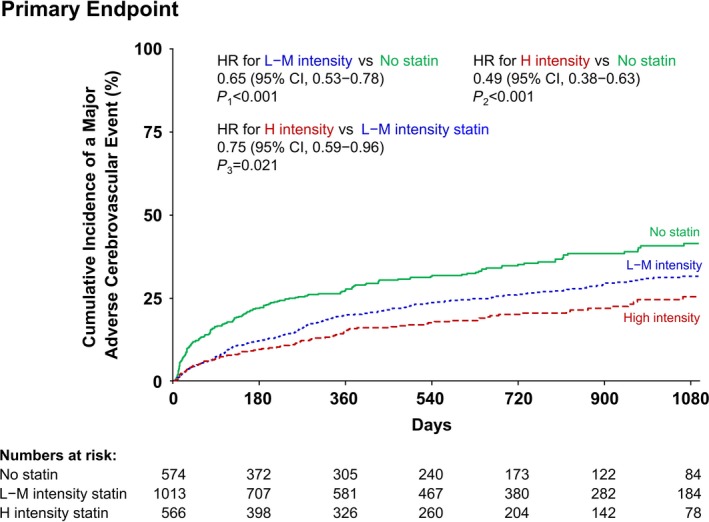
Kaplan‐Meier curves for the primary composite outcome according to statin intensity. Adjusted variables: age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking, congestive heart failure, prior history of stroke or transient ischemic attack, prior mRS scores, TOAST classification, and initial NIHSS scores. H intensity statin indicates high‐intensity statin; L‐M intensity statin, low‐ to moderate‐intensity statin; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; TOAST, trial of Org 10 172 in acute stroke treatment.
Regarding the key secondary outcomes, the rate of mortality was lower in the low‐ to moderate‐intensity statin and high‐intensity statin groups than in the no‐statin group (Figure 2A and Table S1). The rates of the individual end points of recurrent stroke (Figure 2B) and acute coronary syndrome (Figure 2C) were not significantly different among the 3 groups (Table S1). The rate of major bleeding was significantly lower in the low‐ to moderate‐intensity statin users than among the no‐statin users (Figure 2D and Table S1). The risk for major bleeding was also lower in the high‐intensity statin group than in the no‐statin group, but it did not reach statistical significance (Figure 2D and Table S1).
Figure 2.
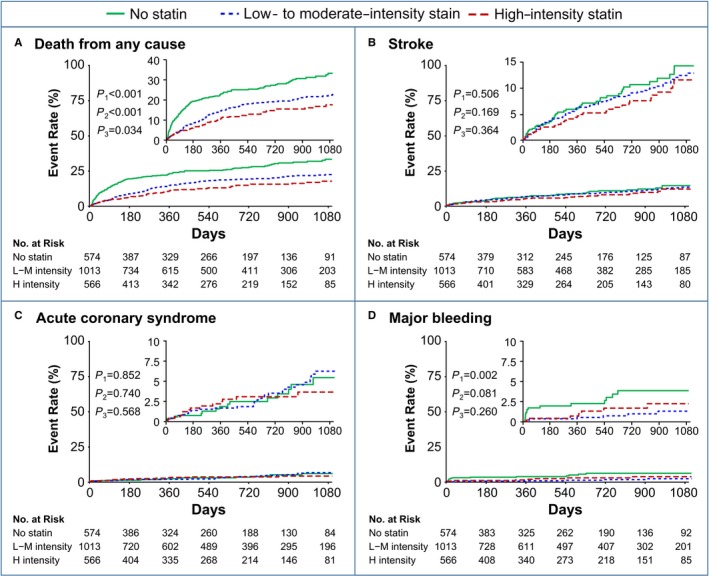
Kaplan‐Meier curves for (A) death from any cause, (B) recurrent stroke, (C) acute coronary syndrome, and (D) major bleeding according to the statin intensity. P 1, no statin vs low‐ to moderate‐intensity statins; P 2, no statin vs high‐intensity statins; P 3, low‐ to moderate‐intensity statins vs high‐intensity statins. H intensity indicates high intensity; L‐M intensity, low to moderate intensity.
To better identify the effect of statin therapy on risk for a NACCE and its constituents in patients with AIS and AF, we adjusted for confounds. In the multivariable Cox regression analyses, compared with patients who did not receive statin, those who received low‐ to moderate‐intensity statins had a significantly lower risk for NACCE (HR 0.64; 95% CI 0.52‐0.78; P<0.001), mortality (HR 0.61; 95% CI 0.48‐0.77; P<0.001), and major bleeding (HR 0.27; 95% CI 0.11‐0.69; P=0.006; Figure 3A). Similarly, those who received high‐intensity statins had a lower risk for NACCE (HR 0.51; 95% CI 0.40‐0.66; P<0.001) and mortality (HR 0.47; 95% CI 0.35‐0.63; P<0.001; Figure 3B). The risks for recurrent stroke and major bleeding were lower in the high‐intensity statin group than in the no‐statin group but were not statistically significant (HR 0.64; 95% CI 0.39‐1.06; P=0.087 and HR 0.45; 95% CI 0.18‐1.12; P=0.088, respectively; Figure 3B). Among the patients receiving statins, those in the high‐intensity statin group had lower risks for NACCE (HR 0.76; 95% CI 0.59‐0.96; P=0.025) and mortality (HR 0.70; 95% CI 0.53‐0.94; P=0.018) than those in the low‐ to moderate‐intensity statin group (Figure 3C). There were no differences in the risks for recurrent stroke, acute coronary syndrome, or major bleeding between the 2 statin therapy groups (Figure 3C).
Figure 3.
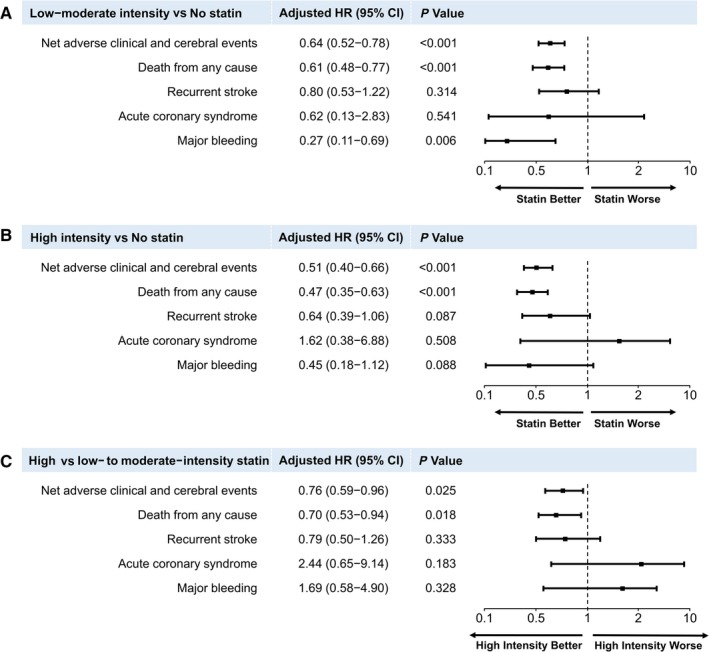
Cox proportional hazards regression analyses for primary and secondary outcomes. Adjusted hazards ratios for low‐ to moderate‐ (A) and high‐ (B) intensity statins compared with no statin. C, Comparison of the effect of statins with different intensities among statin users. Adjusted variables were age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking, congestive heart failure, prior history of stroke or transient ischemic attack, prior mRS scores, TOAST classification, and initial NIHSS scores. HR indicates hazards ratio; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; TOAST, trial of Org 10 172 in acute stroke treatment.
Next, we analyzed the other key secondary outcome, favorable outcomes at 3 months after AIS. The chi‐squared test revealed that as the statin intensity increased, the number of patients with favorable outcomes increased significantly (44.3% versus 50.4% versus 57.4% in the no‐statin, low‐ to moderate‐intensity statin, and high‐intensity statin groups, respectively; Figure 4A). After adjustment for confounds, the use of high‐intensity statins was still associated with favorable outcomes compared with the use of low‐ to moderate‐intensity statins in the multiple logistic regression analysis (odds ratio 1.37; 95% CI 1.05‐1.78; P=0.020) and no‐statin use (odds ratio 1.81; 95% CI 1.35‐2.44; P<0.001; Figure 4B).
Figure 4.
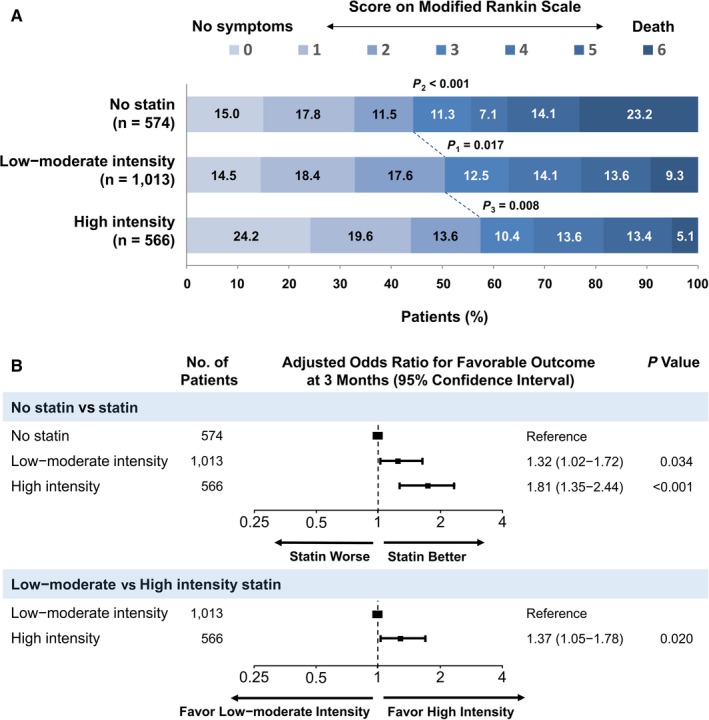
Functional outcome according to the statin intensity after 3 months. A, Distribution of the modified Rankin Scale (mRS) scores according to statin intensity. Blue lines indicate differences in mRS categories between the groups for favorable (mRS scores 0‐2 vs 3‐6) outcomes. B, The adjusted odds ratio for favorable outcome 3 months after an acute ischemic stroke according to the statin intensity. P 1, no statin vs low‐ to moderate‐intensity statins; P 2, no statin vs high‐intensity statins; P 3, low‐ to moderate‐intensity statin vs high‐intensity statins. Adjusted variables are age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking, congestive heart failure, prior history of stroke or transient ischemic attack, prior mRS scores, TOAST classification, and initial NIHSS scores. NIHSS indicates National Institutes of Health Stroke Scale; TOAST, trial of Org 10 172 in acute stroke treatment classification.
In the subgroup analyses the beneficial effect of the statin therapy on the risk for NACCE was uniform across all subgroups examined, including the patients who may be vulnerable to statin therapy and those without clinical ASCVD (Figure 5A). Among the patients receiving statins, the treatment effect heterogeneity for NACCE that showed a magnified benefit of the high‐intensity statins compared with the low‐ to moderate‐intensity statins was observed in patients treated with revascularization therapy (HR 0.52; 95% CI 0.32‐0.84; P interaction=0.042; Figure 5B) and in relatively younger patients, aged ≤75 years (HR 0.57; 95% CI 0.39‐0.86; P interaction=0.044; Figure 5B). High‐intensity statin use was associated with a significantly lower risk of NACCE compared with low‐ to moderate‐intensity statins in patients without clinical ASCVD (HR 0.72; 95% CI 0.54‐0.95; P=0.024; Figure 5B).
Figure 5.
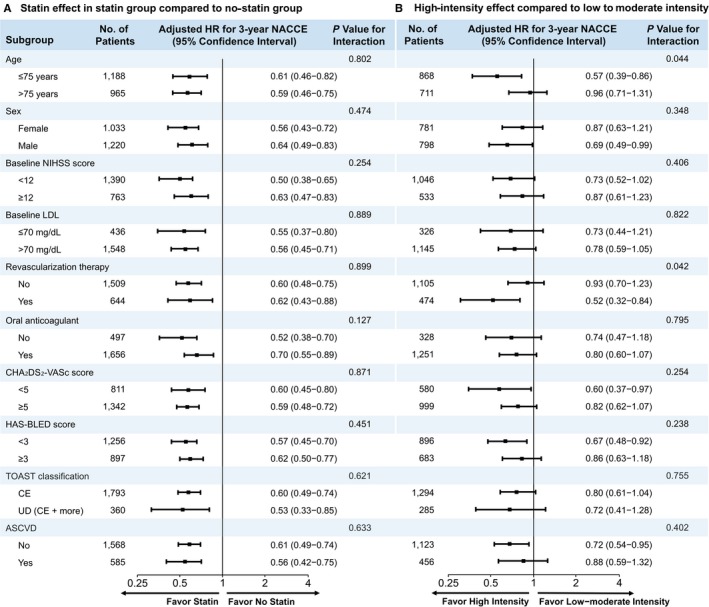
Subgroup analyses of the primary outcome. The forest plot shows the differences in the hazard ratios (HR) for net adverse clinical and cerebral events (NACCE) over a 3‐year period in the subgroups. Adjusted variables are age, sex, hypertension, dyslipidemia, diabetes mellitus, current smoking, congestive heart failure, prior history of stroke or transient ischemic attack, prior mRS scores, TOAST classification, and initial NIHSS scores. ASCVD indicates atherosclerotic cardiovascular disease; CE, cardioembolism; HR, hazards ratio; LDL, low‐density lipoprotein; NIHSS, National Institute of Health Stroke Scale; TOAST, trial of Org 10 172 in acute stroke treatment; UD, stroke of undetermined etiology.
Discussion
The present study highlights the beneficial effect of statin therapy, particularly with high‐intensity statins, in reducing the risk for NACCE in patients with AIS and AF. This was a nationwide study from a prospective multicenter registry that investigated whether different statin intensities had a significant effect on the incidence of NACCE and functional outcomes in patients with AIS and AF. Notably, the risk for NACCE during the 3‐year period in patients who were receiving high‐intensity statin therapy decreased by 50% compared with those not receiving statin therapy, and by 25% compared with those receiving low‐to‐moderate‐intensity statin therapy. This is despite patients in the high‐intensity statin group having a higher prevalence of risk factors, such as congestive heart failure, current smoking, and prior stroke or TIA, than those in the low‐ to moderate‐intensity statin group. The beneficial effect of statin treatment in reducing the risk for NACCE was driven by the reduction in mortality.
Previous studies have demonstrated that statin therapy has been associated with reduced mortality or reduced number of future cardiovascular events in AF patients with or without stroke.12, 13, 14, 18 However, net adverse clinical events and functional outcomes according to the different intensities of statins in AF patients with AIS were not previously systematically investigated. Furthermore, it was unclear whether statin therapy, particularly high‐intensity statin therapy, was beneficial in reducing the risk of NACCE in these patients with vulnerability to statin therapy or without clinical ASCVD. The present study involved a large cohort of patients with AIS and AF, who were prescribed statins in real‐world practice. Our population‐based study showed that high‐intensity statin therapy was associated with a reduced risk for NACCE, reduced mortality rates, and increased probability of favorable functional outcomes compared with no‐statin therapy and low‐ to moderate‐intensity statin therapy in patients with AIS and AF.
Current guidelines recommend high‐intensity statins for patients who have clinical ASCVD presumed to be of atherosclerotic origin.6, 15 Since patients with AF were excluded from a previous trial that was the foundation for these recommendations, it is still unclear whether statin therapy is effective in AF patients with AIS, especially those without clinical ASCVD.10 We investigated the effects of statins in AF patients without clinical ASCVD presumed to be of atherosclerotic origin and considered the various etiologies, such as atherosclerotic disease, that can accompany AF. Notably, statin therapy was associated with a significantly reduced risk of NACCE, and high‐intensity statins were found to be more effective than the low‐to‐moderate‐intensity statins in reducing this risk in AF patients without clinical ASCVD. Moreover, we demonstrated that statin therapy was effective in reducing the risk for NACCE not only in patients with 2 or more etiologies including large‐artery atherosclerosis and cardioembolism but also in patients with pure cardioembolic stroke due to AF. There was no treatment effect heterogeneity of the high‐intensity statin therapy for NACCE between the patients with pure cardioembolic stroke due to AF and those with 2 or more etiologies (large‐artery atherosclerosis and cardioembolism).
In addition, we investigated the effect of statins in patients who may be vulnerable to statin therapy. There is a concern that statin therapy may increase the risk of intracerebral hemorrhage.19 Therefore patients who are older, undergoing anticoagulant treatment, have a low baseline LDL‐C, with high initial NIHSS or HAS‐BLED scores, and who underwent revascularization therapy may be at a higher risk for intracerebral hemorrhage when using statins, particularly high‐intensity statins.20, 21, 22, 23, 24 Moreover, patients in Asian populations may benefit more from low‐ to moderate‐intensity statins and may be more vulnerable to the side effects of high‐intensity statin therapy compared with those in Western populations.25, 26 Therefore, there may be a concern about the use of high‐intensity statins among Asian patients with AIS and AF, particularly those who may be considered to be susceptible to statins.
Our present study demonstrated that statin therapy could provide a better prognosis than no‐statin therapy in all subgroups of patients who may be vulnerable to statin therapy. Furthermore, we found that statins, both high and low to moderate intensity, might not significantly increase the risk for major extracranial bleeding and intracerebral hemorrhage, even in patients with higher baseline HAS‐BLED scores. Instead, the patients who were taking statins had fewer major hemorrhages during their follow‐up than those who were not taking statins. Safety assessments revealed that there were no significant differences between the high‐ and low‐to‐moderate‐ intensity groups in the incidence of major hemorrhages. High‐intensity statin therapy showed a better prognosis in terms of NACCE than low‐to‐moderate‐intensity statin therapy. Our results suggest that high‐intensity statins may be beneficial in younger patients (age ≤75 years) with AF and AIS treated with revascularization therapy.
There are several aspects of the benefits of statins in patients with AIS and AF that should be considered. The benefits of statin therapy in the patients with atherosclerotic cerebro/cardiovascular disease result from the reduction in the risk for cerebral infarction, which may be mainly attributable to the reduction in LDL‐C levels and stabilization of the atherosclerotic plaques.10, 27, 28 However, the incidence of recurrent vascular events was not different between the statin groups in patients with AF‐associated stroke in our study and a previous study.12 Conversely, the survival rates during a 3‐year period and the functional outcome after 3 months were significantly better in the patients treated with statins with a dose‐response relationship. Therefore, these results highlight the importance of the pleiotropic effect (neuroprotective, collateral circulation promoting, anti‐inflammatory, and antioxidant effects) of statins that may have a more significant positive impact on functional recovery and long‐term survival than preventive effects against recurrent vascular events in patients with AIS and AF.7, 8, 28, 29, 30 The greater efficacy of high‐intensity statins could also be related to the improved collateral flow, arteriogenesis, and restoration of the endothelial dysfunction induced by statins.30, 31, 32
Our study has several limitations; therefore, the findings must be interpreted with caution. First, because this was a cohort study with a retrospective analysis of prospectively registered data with no randomization, our data are prone to possible residual bias, confounding, and important methodologic shortcomings. Second, the compliance with prescribed statin therapy and the duration for which the statin was used were not ascertained using a pill count or during direct interview. In addition, information on medication adherence after 3 months was available only for the study population in the largest center; however, ≈96% of the patients whose medication adherence information was available reported a high adherence with the same intensity statin therapy. Because patients followed up for less than 3 months, who may have low adherence, were excluded, high adherence can be expected in all of the study population. Third, we did not collect detailed information on the change in LDL‐C levels and the associated treatment dose during the follow‐up period. Fourth, the effect of statins in reducing the risk of NACCE could be related to the impact of statins on the risk derived from the AIS event rather than from the AF. Fifth, although this study was a large, nationwide study, the patient cohort was restricted to a South Korean population; studies in other races or ethnic groups are needed so that the results can be generalized. However, the present study may be necessary for that; it was conducted among Asians in whom statins may have a different pharmacokinetic effect compared with that in Westerners.
Conclusions
Our findings suggest that statin therapy, particularly high‐intensity statin therapy, could be associated with a reduced risk for NACCE, reduced mortality rates, and increased probability of favorable functional outcomes compared with no‐statin therapy in patients with AIS and AF. These benefits of statin therapy were consistent across various subgroups of patients, including those who are older patients, had low baseline LDL‐C levels, receiving anticoagulant treatment, and without clinical ASCVD. Among the statins, high‐intensity statins were more effective than low‐ to moderate‐intensity statins in reducing the risk for NACCE in these patients, particularly in patients aged under 75 years and those being treated with revascularization therapy. Our results support the beneficial effect of statins, particularly high‐intensity statins, in patients with AIS and AF, including the subgroup of patients who may be vulnerable to statin therapy and those without clinical ASCVD. These findings require further investigation and confirmation in prospective randomized controlled trials.
Sources of Funding
This study was supported by a grant from the National Research Foundation of Korea, funded by the Korean Government (NRF‐2019M3A9E8020261, Choi) and by a grant from the Korean Neurological Association (KNA‐17‐MI‐10, Seo) and the National Research Foundation of Korea (2019R1A2C2008788, Seo).
Disclosures
Seo received honoraria for lectures from Pfizer, Sanofi‐Aventis, Otsuka Korea, Dong‐A Pharmaceutical Co, Ltd, Bayer, Daewoong Pharmaceutical Co, Ltd, Daiichi Sankyo Korea Co, Ltd, and Boryung Pharmaceutical; study grants from Daiichi Sankyo Korea Co, Ltd; and a consulting fee from OBELAB Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Event Rates and Association Estimates From Cox Proportional Hazards Modeling According to Statin Intensity
Figure S1. Study population enrollment flow.
Acknowledgments
We thank Professor Ji‐Sung Lee (Clinical Research Center, Department of Biostatistics, ASAN Medical Center, Seoul, Korea) for her help with the statistical analysis.
(J Am Heart Assoc. 2019;8:e013941 DOI: 10.1161/JAHA.119.013941.)
Contributor Information
Woo‐Keun Seo, Email: mcastenosis@gmail.com.
Man‐Seok Park, Email: mspark@chonnam.ac.kr.
References
- 1. Jung KH, Lee SH, Kim BJ, Yu KH, Hong KS, Lee BC, Roh JK; Korean Stroke Registry Study Group . Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean Stroke Registry Study (secular trends in Korean stroke). Circ Cardiovasc Qual Outcomes. 2012;5:327–334. [DOI] [PubMed] [Google Scholar]
- 2. Yiin GSC, Li L, Bejot Y, Rothwell PM. Time trends in atrial fibrillation‐associated stroke and premorbid anticoagulation. Stroke. 2019;50:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winter Y, Wolfram C, Schaeg M, Reese JP, Oertel WH, Dodel R, Back T. Evaluation of costs and outcome in cardioembolic stroke or TIA. J Neurol. 2009;256:954–963. [DOI] [PubMed] [Google Scholar]
- 4. Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population‐based study. Stroke. 2005;36:1115–1119. [DOI] [PubMed] [Google Scholar]
- 5. Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48:2142–2149. [DOI] [PubMed] [Google Scholar]
- 6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Committee . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 7. Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta‐analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. [DOI] [PubMed] [Google Scholar]
- 8. Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208–213. [DOI] [PubMed] [Google Scholar]
- 9. Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group . Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions. Lancet. 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 10. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 11. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi JY, Seo WK, Kang SH, Jung JM, Cho KH, Yu S, Oh K. Statins improve survival in patients with cardioembolic stroke. Stroke. 2014;45:1849–1852. [DOI] [PubMed] [Google Scholar]
- 13. Ntaios G, Papavasileiou V, Makaritsis K, Milionis H, Manios E, Michel P, Lip GY, Vemmos K. Statin treatment is associated with improved prognosis in patients with AF‐related stroke. Int J Cardiol. 2014;177:129–133. [DOI] [PubMed] [Google Scholar]
- 14. Wu YL, Saver JL, Chen PC, Lee JD, Wang HH, Rao NM, Lee M, Ovbiagele B. Effect of statin use on clinical outcomes in ischemic stroke patients with atrial fibrillation. Medicine (Baltimore). 2017;96:e5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 16. Feres F, Costa RA, Abizaid A, Leon MB, Marin‐Neto JA, Botelho RV, King SB III, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr, Nicolela EL Jr, Perin MA, Devito FS, Labrunie A, Salvadori D Jr, Gusmao M, Staico R, Costa JR Jr, de Castro JP, Abizaid AS, Bhatt DL, OPTIMIZE Trial Investigators . Three vs twelve months of dual antiplatelet therapy after zotarolimus‐eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 17. Kelly MS, Moczygemba LR, Gatewood SS. Concordance of pharmacist assessment of medication nonadherence with a self‐report medication adherence scale. J Pharm Pract. 2016;29:194–198. [DOI] [PubMed] [Google Scholar]
- 18. Proietti M, Laroche C, Nyvad O, Haberka M, Vassilikos VP, Maggioni AP, Boriani G, Lip GYH; EORP‐AF Pilot Investigators . Use of statins and adverse outcomes in patients with atrial fibrillation: an analysis from the EURObservational Research Programme Atrial Fibrillation (EORP‐AF) general registry pilot phase. Int J Cardiol. 2017;248:166–172. [DOI] [PubMed] [Google Scholar]
- 19. Goldstein LB, Amarenco P, Szarek M, Callahan A III, Hennerici M, Sillesen H, Zivin JA, Welch KM; SPARCL Investigators . Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 20. Tikkanen MJ, Holme I, Cater NB, Szarek M, Faergeman O, Kastelein JJ, Olsson AG, Larsen ML, Lindahl C, Pedersen TR; Incremental Decrease through Aggressive Lipid Lowering Investigators . Comparison of efficacy and safety of atorvastatin (80 mg) to simvastatin (20 to 40 mg) in patients aged <65 versus ≥65 years with coronary heart disease (from the Incremental DEcrease through Aggressive Lipid Lowering [IDEAL] study). Am J Cardiol. 2009;103:577–582. [DOI] [PubMed] [Google Scholar]
- 21. Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–2283. [DOI] [PubMed] [Google Scholar]
- 22. Antoniou T, Macdonald EM, Yao Z, Hollands S, Gomes T, Tadrous M, Mamdani MM, Juurlink DN; Canadian Drug Safety and Effectiveness Research Network . Association between statin use and ischemic stroke or major hemorrhage in patients taking dabigatran for atrial fibrillation. CMAJ. 2017;189:E4–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez‐Luna D, Rubiera M, Ribo M, Coscojuela P, Pagola J, Pineiro S, Ibarra B, Meler P, Maisterra O, Romero F, Alvarez‐Sabin J, Molina CA. Serum low‐density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke. 2011;42:2447–2452. [DOI] [PubMed] [Google Scholar]
- 24. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85–94. [DOI] [PubMed] [Google Scholar]
- 25. Kong SH, Koo BK, Moon MK. Efficacy of moderate intensity statins in the treatment of dyslipidemia in Korean patients with type 2 diabetes mellitus. Diabetes Metab J. 2017;41:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. HPS2 Collaborative Group . HPS2‐THRIVE randomized placebo‐controlled trial in 25 673 high‐risk patients of ER niacin/laropiprant: trial design, pre‐specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amarenco P, Lavallee P, Touboul PJ. Stroke prevention, blood cholesterol, and statins. Lancet Neurol. 2004;3:271–278. [DOI] [PubMed] [Google Scholar]
- 28. Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med. 2019;29:451–455. [DOI] [PubMed] [Google Scholar]
- 29. Calabro P, Yeh ET. The pleiotropic effects of statins. Curr Opin Cardiol. 2005;20:541–546. [DOI] [PubMed] [Google Scholar]
- 30. Lee MJ, Bang OY, Kim SJ, Kim GM, Chung CS, Lee KH, Ovbiagele B, Liebeskind DS, Saver JL. Role of statin in atrial fibrillation‐related stroke: an angiographic study for collateral flow. Cerebrovasc Dis. 2014;37:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG‐CoA reductase inhibitor simvastatin activates the protein kinase AKT and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Event Rates and Association Estimates From Cox Proportional Hazards Modeling According to Statin Intensity
Figure S1. Study population enrollment flow.
Data Availability Statement
Any data not published within the article are available in a public repository and include digital object identifiers, accession numbers to the data sets, and anonymized data. These data will be shared on reasonable request from any qualified investigator.


