Abstract
The innate immune sensing pathways play critical roles in the defense against pathogen infection, but their roles in cancer immunosurveillance and cancer therapies are less defined. We propose that defective innate immune sensing inside the tumor microenvironment might limit T-cell responses to immunotherapy. A recent mechanistic understanding of conventional therapies revealed that both innate immune sensing and T-cell responses are essential for optimal antitumor efficacy. T-cell-based immunotherapy, particularly immune checkpoint blockade, has achieved great success in reactivating antitumor immune responses to lead to tumor regression, but only in a small fraction of patients. Therefore, incorporating conventional therapy that can increase innate sensing and immunotherapy should lead to promising strategies for cancer patients. Here, we review the innate sensing pathways related to cancer initiation/progression and therapies, summarize the recent key findings in innate immune sensing related to conventional therapies, evaluate current combination strategies, and highlight the potential issues of combinational therapies in terms of antitumor efficacy and toxicities.
Keywords: Innate immune sensing, Conventional therapy, Immunotherapy
Subject terms: Cancer immunotherapy, Cancer microenvironment
Introduction
The innate immune system serves as the front line of host defense against invasion of pathogens by sensing pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) with pattern-recognition receptors (PRRs).1,2 Recognition of PAMPs or DAMPs by PRRs initiates the inflammatory response by activating the NF-κB, IRF3/7 or inflammasome signaling pathways and producing proinflammatory cytokines, particularly type I interferons (IFNs), which subsequently leads to the activation of adaptive immune responses that clear the pathogens.3,4 In addition to their role in infection, accumulating evidence shows that both the innate and adaptive immune systems play critical roles during tumor occurrence and progression. Evidence suggests aberrantly increased cell proliferation and cellular turnover may result in increased cell stress and release of tumor-derived DAMPs.5 These DAMPs are recognized by PRRs and trigger the innate immune system to eliminate the vast majority of incipient cancer cells. However, the activated adaptive immune system misses the weakly immunogenic variants, which are allowed to grow and form tumors.6–8 The initial innate immune sensing of tumors results in recruitment, activation, and clonal expansion of tumor-specific CD8+ T cells, which have the potential to kill cognate tumor cells and are associated with better outcomes and improved overall survival in cancer patients treated with conventional therapies or immunotherapies.9–17 Therefore, increasing the innate immune sensing of tumor cells will be a very potent strategy for improving cancer therapy.
Surgery, radiotherapy, chemotherapy, and targeted therapy are considered the major conventional anticancer therapies.18 The major antitumor effects of all these conventional therapies have been thought to reduce tumor burden though direct killing of tumor cells.19–22 Intriguingly, numerous studies in the past decade have demonstrated that conventional therapies also activate host innate immunity and adaptive immunity to cause tumors to regress, especially innate immune sensing and type I IFN production.21,23–27 However, the induction of immune responses is inconsistent and often suppressed by further prolonged treatment or high doses. A strength of conventional therapies is their high response rates because of their direct cell killing and reduction of tumor burden with potentially enhanced innate immune sensing and antitumor immunity. Their weaknesses are systemic toxicity and a short-term response to treatment because of the development of treatment resistance and/or the acquisition of adaptive immune resistance.28,29 While emerging immunotherapies, especially immune checkpoint blockade (ICB), have shown long-term responses in cancer patients, these benefits are only seen in a small fraction of patients.30–32 All these features provide potential rationale for clinically developing combinations of conventional therapy and immunotherapy to achieve a high response rate, long-lasting responses, and improved overall survival in cancer patients.23,28,33–36 Here, we will summarize the innate immune sensing pathways involved in cancer initiation/progression, conventional cancer therapies, the potential strategies and issues for integrating conventional therapies with immunotherapy.
Innate immune sensing pathways related to cancer
Toll-like receptors (TLRs) and cancer
TLRs are a family of type I integral membrane glycoproteins that play a critical role in host defense against pathogens by recognizing a variety of PAMPs or DAMPs.37 TLRs are mainly expressed on immune cells, such as macrophages, dendritic cells (DCs), monocytes, neutrophils, mast cells, eosinophils, B cells, natural killer (NK) cells, and T cells.3 These receptors localize to either the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6) to recognize lipid and protein ligands or endolysosomal compartments (TLR3, TLR7, TLR8, and TLR9) to sense nucleic acids (NAs).38 Upon ligand engagement, TLRs transmit downstream signals by recruiting adapter proteins, including myeloid differentiation factor 88 (MyD88), TIR domain-containing adapter protein, TIR domain-containing adapter inducing interferon (IFN)-β (TRIF), TRIF-related adapter molecule, and sterile α- and armadillo motif-containing protein (SARM).39,40 All TLRs, excluding TLR3, signal through MyD88 to activate the canonical NF-κB pathway to produce proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6. In addition, TLR3 and TLR4 signal through TRIF to activate TNFR-associated factor family-member-associated NF-κB activator binding kinase 1 (TBK1)/inducible IκB kinase and IRF3/7 to produce type I IFNs (Fig. 1).41–43 These signaling pathways are essential for further triggering the host innate and adaptive immune responses against infection.
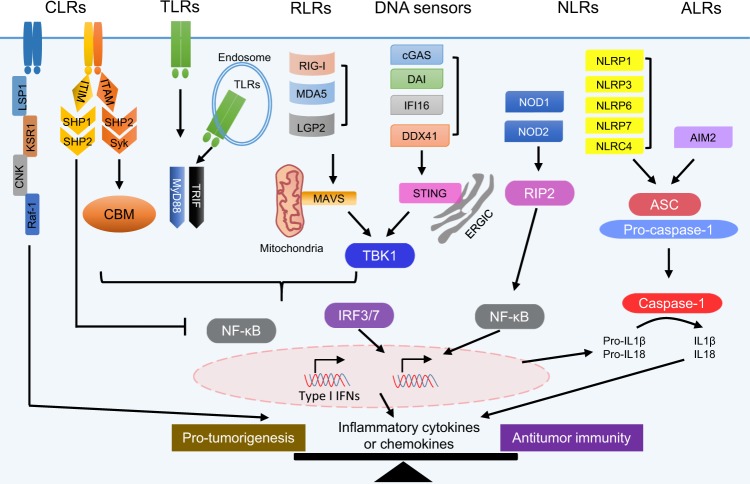
Fig. 1. Innate immune sensing pathways and cancer.
Innate immune receptors, including C-type lectin receptors (CLRs), Toll-like receptors (TLRs), RIG-like receptors (RLRs), DNA sensors, NOD-like receptors (NLRs), and AIM2-like receptors (ALRs), are expressed on and/or in various cell types. They cooperate to recognize a variety of danger-associated molecular patterns (DAMPs) from pathogens, damaged cells, or stressed cancer cells and activate downstream signaling for the production of multiple cytokines/chemokines to initiate antipathogen or antitumor immune responses. Here, we list only the key signaling pathways under the receptors upon their ligand binding. For instance, CLRs function through ITAM/Syk/CBM, ITIM/SHP-1/2, or LSP1/KSR1/CNK/Raf-1, TLRs function through MyD88 or TRIF, RLRs function through MAVS, DNA sensors function through STING, and NOD1/2 function through RIP2 to activate cells via the NF-κB and/or IRF3/7 signaling pathways and produce inflammatory cytokines and chemokines. NLRs (such as NLRP1/3/6/7 and NLRC4) and ALRs (AIM2) can form inflammasomes to activate caspase-1, which results in the release of IL-1β and IL-18. The functions of inflammatory cytokines and chemokines in tumorigenesis and antitumor immunity are still controversial, and the balance of these cytokines and chemokines might affect the outcome of activation of the innate sensing pathways in tumorigenesis and antitumor immunity.
Emerging evidence has also indicated that TLRs play important roles in cancer initiation/progression and cancer therapy. On the one hand, TLR signaling may drive cancer initiation/progression by provoking proinflammatory cytokines or antiapoptotic, proliferative, and profibrogenic signals in cells that eventually transform into tumor cells.44 TLR signaling stimulates the production of tumor-promoting inflammatory cytokines through the transcription factor NF-κB, including TNF-α, IL-1β and IL-6, which promote tumorigenesis in the intestine, liver, stomach, and skin.45 For example, in a model of adoptively transferred tumor cells, systemic lipopolysaccharide (LPS) administration increased the growth of tumor cells in a host TLR4 signaling-dependent manner. Mechanistically, the LPS-enhanced TLR4 signaling increased the systemic level of TNF-α, which in turn led to the upregulation of NF-κB-regulated antiapoptotic factors, such as B-cell lymphoma 2 (BCL-2) and inhibitor of apoptosis (IAP), in tumor cells.46,47
On the other hand, TLR signaling may drive antitumor effects by eliciting inflammatory cytokines in the tumor microenvironment (TME) to trigger antitumor immune responses or induce apoptosis and programmed necrosis of tumor cells.48 Activation of TLR signaling induces the maturation of antigen-presenting cells (APCs), including macrophages and DCs, to produce inflammatory cytokines, especially type I IFNs, and upregulates the costimulatory molecules CD80, CD86, and CD40, which further activate innate immune cells and tumor-specific T-cell responses.3,49 TLR agonists have been demonstrated to achieve potent antitumor effects in both mice and human studies. Among all the TLR agonists, the TLR7/8 agonist imiquimod is the most successful and has been approved by the Food and Drug Administration (FDA) for the treatment of basal cell carcinoma.50,51 Bacillus Calmette–Guérin, of which the antitumor effect is due to the stimulation of TLR2 and TLR4, has been approved for the treatment of bladder cancer.52 Other agonists, such as a CpG-containing TLR9 agonist, flagellin (TLR5 agonist), and poly I:C (TLR3 agonist), are still under investigation in the clinic. In addition, TLR agonists might achieve their antitumor effect through direct killing. Flagellin has been reported to induce HeLa cell death.53 Poly I:C has been reported to trigger both apoptosis and programmed necrosis in tumor cells.48,54 Therefore, targeting TLRs with agonists for cancer immunotherapy will not be a straightforward process. Further understanding the key factors of TLR signaling involved in cancer initiation and progression will guide the clinical application of TLR agonists as cancer therapeutics.
C-type lectin receptors (CLRs) and cancer
CLRs are a large superfamily of receptors that contain at least one carbohydrate-recognition domain, which is important for recognizing a variety of ligands, including galactose, N-acetylgalactosamine, carbohydrate ligands, such as β-glucan, and noncarbohydrate ligands, such as lipids and proteins.55–57 CLRs work as PRRs and are mostly expressed on myeloid cells and have been traditionally associated with fungal infection.58 According to the specific motifs in their cytoplasmic domains, CLRs can be divided into activating and inhibitory clusters. Some CLRs transduce their downstream signals through an integral immunoreceptor tyrosine-based activation motif (ITAM)-like/ITAM, which results in cellular activation, and the CLRs that function this way mainly include dectin-1/2/3, Mincle, MCL, BDCA-2, DCAR, DCAR1, DNGR-1, and mannose receptor (MR). CLRs possessing immunoreceptor tyrosine-based inhibition motifs (ITIMs) in their cytoplasmic domain usually suppress cellular activation, and the CLRs that function this way mainly include DCIR, MICL, MAgH, and Ly49Q.59–61 Upon ligand binding, activating CLRs, such as dectin-1, dectin-2, dectin-3, and mincle, initiate the phosphorylation of ITAM-like/ITAMs and further activate the Syk kinase. Subsequently, the activated CARD9–Bcl10–Malt1 (CBM) complex ultimately activates several transcription factors (such as NFAT, IRF1, IRF5, and NF-κB) to promote the production of both proinflammatory (such as TNF-α, IL-12, IL-6, and IL-1β) and anti-inflammatory cytokines (such as IL-4, TGF-β, and IL-10).62 Engagement of inhibitory CLRs, such as MICL and DCIR, results in the phosphorylation of ITIMs, which further recruit and activate SHP-1 and SHP-2 and ultimately inhibit cellular activation signaling. Interestingly, these inhibitory CLRs can also enhance cellular activation signaling by inhibiting inhibitory cellular responses.63 In addition, myeloid CLRs also contain members that do not have ITAM or ITIM domains, mainly including MMR, DEC-205, DC-SIGN, langerin, and MGL. The intracellular domains of these receptors interact with a signalosome composed of LSP1, KSR1, CNK, and the kinase Raf-1, which can modulate cytokine production and endocytic machinery for antigen processing and presentation to T cells (Fig. 1).64
Recently, CLRs have attracted increasing attention for their various functions in shaping both innate and adaptive immune responses related to cancer development and therapy. It has been demonstrated that tumor-associated carbohydrate antigens are specifically recognized by certain CLRs. For example, a well-defined tumor-associated antigen (TAA), carcinoembryonic antigen, which is overexpressed on almost all human colorectal, gastric, and pancreatic adenocarcinomas, 70% of non-small-cell lung carcinomas, and 50% of breast carcinomas, is recognized by DC-SIGN.55,65 DC-SIGN can also recognize mucin 1 with cancer-specific glycosylation changes.66 Moreover, dectin-1, a CLR for β-glucans, has been reported to recognize N-glycan structures on tumor cells. In addition to recognizing TAAs, dectin-1, DC-SIGN, and MGL have been well studied for their activity in promoting DC maturation and enhancing tumor-specific T-cell responses.67,68 On the other hand, CLRs expressed on tumor-associated macrophages (TAMs), such as MR, DC-SIGN, MGL, MICL, and DICR, can also cause TAMs to produce immunosuppressive cytokines (such as IL-10 and TGF-β) to impair antitumor T-cell responses upon ligand engagement.69,70 For instance, a study reported that activation of MR on TAMs either by tumor mucin engagement or an anti-MR antibody promoted the transition of TAMs toward a more immunosuppressive state (increased IL-10, no IL-12, and decreased CCL3).71 Due to the immunoregulatory effect of CLRs in antitumor immunity, several CLR agonists or antagonists have been considered for cancer therapy. Targeting dectin-1 with β-glucans has shown promising antitumor activity either alone or in combination with chemotherapy in both preclinical and clinical studies.72,73 Moreover, several CLRs, such as DEC-205, DNGR-1, and DC-SIGN, have been intensively studied for targeted delivery of antigens to APCs in combination with potent adjuvants for vaccine purposes.74–77
As discussed above, CLRs, especially MR and DC-SIGN, are involved in inducing both immune tolerance and eradication of tumor cells. The balance and crosstalk between different CLRs and between CLRs and other innate immune signaling pathways in the TME may affect the final outcome of cancer patients. Further understanding of how CLR signaling affects immunological outcomes, immune activation, and immune suppression will facilitate the application of CLR agonists or antagonists in the clinic to benefit patients with cancer- or CLR-related diseases.
Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and cancer
Unlike TLRs and CLRs that are commonly located on the membrane, NLRs are a large family of intracellular sensors, the members of which are characterized by sharing a C-terminal leucine-rich repeat domain, a central NOD, and a variable N-terminal effector domain.43,78 Based on their diverse functions, NLRs can be classified into three clusters: receptors (such as NOD1 and NOD2), adapters (such as NLRP3, NLRC4, and NLRP6), and regulators (such as NLRX1, NLRC3, NLRC5, and NLRP4).79 NOD1 and NOD2, receptors of PAMPs from microbes, have been well studied. Upon activation by their ligands, g-D-glutamyl-meso-diaminopimelic acid (iE-DAP) for NOD1 and muramyl dipeptide for NOD2, NOD1, and NOD2 self-oligomerize and recruit and interact with the CARD-containing adapter receptor-interacting protein kinase 2 (RIP2 or RIPK2)80 to form the signaling complex termed “the nodosome,” which subsequently leads to the activation of canonical NF-κB pathway- and MAPK pathway-mediated inflammatory responses.81 Several other NLRs, including NLRP1, NLRP3, NLRP6, NLRP7, and NLRC4, can form different types of inflammasomes.79,82 The NLRP1 inflammasome was the first complex to be identified, while the NLRP3 inflammasome is the most widely studied. Upon activation, NLRs recruit the inflammatory protease caspase-1 and the apoptosis-associated speck-like protein (ASC) to form large protein complexes termed “inflammasomes,” which further promote the secretion of IL-1β and IL-18 and induce a type of inflammatory cell death named pyroptosis (Fig. 1).83–85
As we discussed above, NLRs are involved in the regulation of inflammation, which is considered a major hallmark of cancer. Indeed, numerous studies have shown that NLR signaling is very important for cancer development and therapy. For instance, both Nod1−/− mice and Nod2−/− mice are susceptible to dextran sulfate sodium (DSS) and azoxymethane (AOM)-induced colorectal cancer (CRC).86,87 In addition, NOD2 polymorphisms are associated with increased risk and the prevalence of gastric, breast, and lung cancers.80 It has also been reported that increased expression of both NOD1 and NOD2 was observed in head and neck squamous cell carcinoma biopsies compared with healthy nasal biopsies, indicating the role of NOD signaling in enhancing head and neck cancers.88 Moreover, accumulating evidence suggests that abnormal activation of the inflammasome is closely linked to various types of human cancers. NLRP3 polymorphisms, such as mutations that render NLRP3 constitutively active, are correlated with melanoma susceptibility, CRC prognosis, and overall survival in myeloma.89 Consistently, NLRP3-deficient mice formed less pulmonary metastasis than control mice in an orthotopic transplant mammary adenocarcinoma mouse model.90 Mechanistically, NLRP3 activation increased the myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in the TME and suppressed NK and T-cell-mediated antitumor activity.91 Although all of these findings indicate the protumorigenic role of NLRP3, several studies have shown an antitumor role. NLRP3-deficient mice are more susceptible to AOM–DSS-induced mouse CRC than control mice. Similarly, the NLRP3 expression level was significantly lower in hepatic parenchymal cells in hepatocellular carcinoma biopsies than in the hepatic parenchymal cells in noncancerous samples.92 In addition to the well-studied NOD signaling, several other NLRs, such as NLRC4, NLRP6, and NLRP12, are also correlated with tumorigenesis. Mice deficient in these NLRs showed increased tumor numbers and burden upon AOM–DSS treatment. In terms of the mechanism, the cellular intrinsic role of NLRC4 in intestinal epithelial cells might be more important for tumor progression, while NLRP6 and NLRP12 mostly achieved their protective roles by regulating the NF-κB signaling pathway and its downstream proinflammatory cytokines and chemokines, such as TNF-α, IL-6, IL-1β, IL-18, CXCL12, and CXCL13.93–95 These findings highlight the potential roles of NLRs in tumorigenesis, but like the TLRs and CLRs, conflicting evidence still exist. Protumorigenic signaling is balanced by inflammasome-mediated pyroptosis, which enhances antitumor innate and adaptive immunity. Therefore, further studies focusing on understanding the precise effects of NLR signaling in tumorigenesis and discovering novel NLR ligands (agonists or antagonists) might provide potential therapeutic strategies for inflammation-related diseases and cancer.
NA-sensing pathways and cancer
In addition to TLRs, CLRs, and NLRs, cytosolic NA sensors are also important groups of PRRs in the innate immune system that can recognize cytosolic DNA or RNA. The cyclic GMP-AMP synthase (cGAS)–stimulator of IFN genes (STING) axis is the major pathway for cytosolic DNA sensing,96 while the RIG-I-like receptor (RLR)–MAVS axis is responsible for RNA sensing.97 Upon the engagement of double-stranded DNA, cGAS catalyzes the synthesis of cyclic-di-GMP-AMP (cGAMP), which in turn binds the adapter protein STING on the endoplasmic reticulum (ER) and promotes TBK1-dependent IRF3 and NF-κB activation for further production of type I IFNs, proinflammatory cytokines, and chemokines to initiate antiviral responses.98,99 In addition, several other DNA sensors, such as ZBP1, DDX41, DNA-PK, RNA polymerase III, and AIM2-like receptor family members (AIM2 and IFI16), have also been shown to detect cytosolic DNA to activate inflammasome or type I IFN signaling pathways.96,100 As RNA sensors, RIG-I preferentially recognizes 5′-triphosphate-ending (5′-3p) RNA and short dsRNA, while MDA5 detects long dsRNA. After ligand stimulation, RIG-I or MDA5 interacts with the protein MAVS on the mitochondrial membrane, which activates transcription factors such as IRF3/7 and NF-κB and thus elicits innate/adaptive immunity against viral infection (Fig. 1).97,101 The NA-sensing pathways described above have been mainly discovered and intensively studied in the field of RNA and DNA virus infection. However, mounting evidence has shown that damaged NAs released from stressed or dying cancer cells can be recognized by the cGAS–STING axis and/or RLRs in DCs to initiate innate immune responses in the TME. Subsequent type I IFN production promotes the activation and maturation of DCs to further cross-prime the tumor-specific T cells for tumor control.102,103 In addition, several human studies also indicate that NA sensors can serve as tumor suppressors and can be considered prognostic and predictive biomarkers in certain types of cancers. For instance, in human hepatocellular carcinoma, the expression of STING has been negatively correlated with advanced tumor stages and patient survival.104 Based on the role of NA sensing in antitumor immunity, cGAS-STING and RIG-I/MDA5 agonists have been developed for cancer immunotherapy. Notably, some controversial studies have also shown that inappropriate activation of STING and RIG-I signaling can contribute to the suppressive TME and promote tumor growth and metastasis.105,106 Further characterization of the outcome of activated NA-sensing pathways in the TME is required to better apply agonists of the involved proteins alone or in combination with other potential therapies to benefit cancer patients.
Type I IFN: the bridge between innate immune sensing and adaptive immunity
The generation of adaptive immune responses depends on the activation of the innate immune system. PRRs, such as TLRs, CLRs, NLRs, and NA sensors, are critical in initiating innate immune responses by activating certain key signaling pathways through which several important cytokines are produced to further trigger adaptive immune responses.43 Among these cytokines, type I IFNs are the best characterized and studied in the field of antiviral immune responses. Type I IFNs consist of a family of class II α-helical cytokines in humans and mice, including IFN-α (with different subtypes), IFN-β, IFN-ε, IFN-κ, and IFN-ω,107,108 which can be rapidly induced by certain PRRs (such as TLR3, TLR4, TLR7, TLR8, TLR9, NOD1/2, and all NA sensors) upon ligand binding.109 All type I IFNs share the same receptor, which is a heterodimer of two subunits, IFNAR1 and IFNAR2. The IFN-α receptor is expressed on almost all cells, and type I IFNs can exert direct antiviral effects through it by inhibiting viral replication and inducing apoptosis of infected cells. Type I IFNs can also stimulate the noninfected cells to express genes related to antiviral activity to prevent the virus from spreading.108,110 On the other hand, type I IFNs can also work on multiple subsets of immune cells, such as macrophages, DCs, NK cells, B cells, and T cells, to regulate host immune responses.111 This regulatory role places them as the key bridge between innate immune sensing and adaptive immunity. Type I IFNs stimulate the upregulation of both MHC I and MHC II on the cell surface, as well as the costimulatory molecules CD40, CD80, and CD86. In addition, type I IFNs promote antigen retention and cross-presentation by DCs to enhance antigen-specific CD8+ T-cell responses.112 Furthermore, type I IFNs are also one of the third signals required for human and mouse T-cell activation.113
Accumulating evidence has shown that type I IFNs play critical roles during tumorigenesis and cancer therapies. Using a methylcholanthrene (MCA)-induced carcinogenesis model and a transplantable tumor model, it has been demonstrated that endogenous type I IFNs are critical for both immunosurveillance during tumor initiation/progression and the induction of immune responses against transplanted tumors. Ifnar−/− mice were unable to reject their tumors.114,115 Mechanistically, unknown DAMPs (most likely DNA or RNA) released from MCA-treated tissues or transplanted dead tumor cells engage the PRRs to trigger the production of type I IFNs from CD11c+ DCs, which in turn promote the activation and maturation of DCs with captured antigens, especially CD8α+ DCs, to cross-prime T cells against tumor cells.115,116 Due to the important roles of type I IFNs in enhancing the host innate and adaptive immune responses, type I IFNs could be very potent therapeutics for cancer patients. Indeed, IFN-α has been used in the clinic for several human cancer types in the past few years.117,118 Several preclinical studies also demonstrated that targeted delivery of type I IFNs into tumor sites could effectively control tumor growth by enhancing the cross-priming capacity of DCs and increasing tumor-specific T-cell responses and further overcoming ICB resistance.119,120 Furthermore, the antitumor effect of conventional cancer therapies, such as chemotherapy, radiotherapy, and targeted therapies, has also been shown to depend on the type I IFN-enhanced activation of DCs and T-cell responses.121 All of these studies confirm the essential role of innate immune sensing and type I IFN production in antitumor immunity.
Targeting innate sensing with conventional therapies to improve antitumor immune responses
Conventional anticancer therapies, such as radiotherapy, chemotherapy, and targeted therapy, have been historically thought to act through direct killing of tumor cells. This concept stems from the fact that all these conventional therapies are developed to interfere with key processes of the cell cycle, such as the synthesis of DNA, RNA, and their building blocks, mitotic spindle formation, and specific oncogenic signaling pathways for cancer cells.21,25,122 However, accumulating evidence indicates that the antitumor activities of such conventional therapies also rely on host innate immunity, especially innate immune sensing, and adaptive immunity (Fig. 2).121
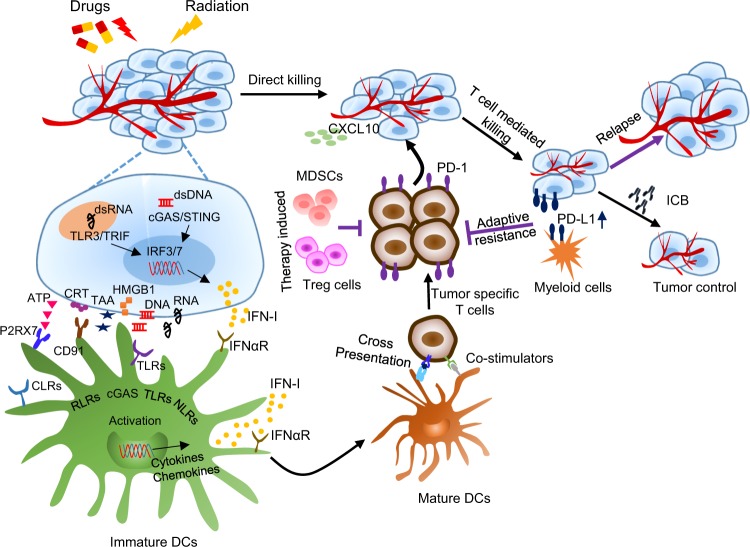
Fig. 2. Immune-based mechanisms of conventional therapies and the rationale of combinational therapy.
In addition to direct killing, conventional therapies, such as radiotherapy, chemotherapy, and targeted therapy, can active antitumor immune responses through different innate immune sensing pathways. Conventional therapies can promote the release of tumor-associated antigens (TAAs) and various types of DAMPs, including HMGB1, ATP, DNA, and RNA, and the exposure of CRT. All of these factors can activate DCs (from immature to mature) by triggering innate sensing pathways and the production of type I IFNs (IFN-I) by DCs or tumor cells and promote the cross-priming and recruitment (by increasing CXCL10) of tumor-specific T cells for additional T-cell-mediated tumor cell killing. However, conventional cancer therapies can also increase the immunosuppressive status in the tumor microenvironment (TME), via mechanisms such as upregulation of PD-L1 on both tumor cells and myeloid cells and accumulation of MDSCs and Treg cells in the TME, to cause adaptive immune resistance and result in tumor relapse. Additional immunotherapy, particularly immune checkpoint blockade, will synergize with conventional therapies to further enhance the specific immune response against tumors and achieve better tumor control.
Targeting innate sensing with radiotherapy
Radiation therapy is one of the most important components of cancer treatment, with over 50% of patients receiving radiation during their treatment.123 Canonical radiation treatment for localized tumors consists of small daily doses of ionizing radiation to reduce normal tissue toxicity. Modern advances in imaging and precision targeting have introduced stereotactic body radiation therapy using three-dimensional imaging technology to precisely map the position of the tumor and target higher doses of radiation while better sparing normal tissue.124 Radiation directly kills cancer cells by inducing DNA damage and increasing reactive oxygen species (ROS) levels, which will restrict the proliferation of irradiated cells and promote cell apoptosis.125 Intriguingly, our previous study first revealed that the adaptive immune system was required for the optimal antitumor efficacy of radiation. Lee et al. demonstrated that CD8+ T cells were essential for rapid shrinking of irradiated tumor tissue.126 Similarly, Reits et al. reported that radiation could induce the expression of MHC I on tumor cells, promote antigen presentation, and further facilitate the killing of tumor cells by CD8+ T cells.127 Furthermore, radiation has also been shown to induce immunogenic cell death (ICD) of tumor cells and promote the release of DAMPs, such as HMGB1, ATP, DNA, and RNA,26,128–130 which indicates that host innate immune sensing might play a vital role in radiation. Indeed, our previous study observed that radiation significantly increased the production of type I IFNs in the TME and demonstrated that type I IFN signaling was essential for the activation of tumor-infiltrating DCs and the cross-priming of tumor-specific T cells.131 To further investigate which innate sensing pathway is required for the antitumor effect of radiation, Deng et al. tracked various pathways and observed that radiation induced a therapeutic effect independently of MyD88 and TRIF.26 They further demonstrated that STING signaling in host cells was required for radiation-induced tumor shrinking. Mechanistically, irradiated tumor-derived dsDNA can be recognized by cGAS in DCs. This facilitates the production of type I IFNs in an autocrine fashion and increases the cytotoxic function of CD8+ T cells via cross-priming.
However, tumor cells themselves fail to produce type I IFN after radiation treatment in vivo. Thus, it is of great interest to induce functional dsDNA innate sensing in tumor cells. Harding and others reported that radiation induces the aggregation of genomic DNA in micronuclei, where cGAS can recognize such DNA fragments and activate the STING-mediated transcription of type I IFNs.132–134 They further observed that DNA-PK was essential for cGAS to recognize the DNA in micronuclei, as blocking DNA-PK with nu-7741 abolished the production of type I IFNs. Vanpouille-Box et al. found that very high-dose radiation significantly upregulated the expression of three prime repair exonuclease 1(TREX1), which degraded cytosolic DNA and restricted activation of the cGAS/STING pathway in TSA tumor cells.135 Relatively low-dose radiation treatment significantly increased the aggregation of dsDNA in the cytosol and induced the transcription of type I IFNs. Thus, they further optimized the radiation dose and observed that a hypofractionated 3 × 8 Gy schedule could synergize with anti-CTLA4 to induce a stronger abscopal effect in a tumor-derived cGAS-dependent manner. We also observed that 1 × 15 or 20 Gy was still better than 4 × 5 Gy in controlling primary tumor growth in the B16 tumor model.126 We hypothesize that the conventional 30 × 2 Gy schedule might not generate enough innate sensing and may even kill reactivated T cells. Thus, innate sensing may be dose dependent but may also depend on the tumor type. Which dose and schedules might kill more tumor cells, induce greater innate immune sensing, and generate stronger T-cell immune responses remain to be determined in the clinic. Furthermore, although radiation can induce DNA aggregation in micronuclei and enhance dsDNA innate sensing in tumor cells, only slightly increased type I IFN level was observed, which indicates that the major barrier of dsDNA sensing in tumor cells still needs to be further investigated.
In addition to the STING pathway, the inflammasome is another critical platform for recognizing cytosolic foreign or self dsDNA. AIM2 can recognize cytosolic dsDNA and assemble the AIM2 inflammasome to activate caspase-1.136 Cytosolic mtDNA and radiation-induced ROS can also activate the NLRP3 inflammasome.137,138 Previous studies have reported that AIM2 is vital for the radiation-induced death of intestinal epithelial cells and bone marrow cells,139 while radiation also induces the activation of the NLRP3 inflammasome in macrophages.140 Interestingly, there is complicated mutual regulation between STING and inflammasome sensing.141,142 As IFN-stimulated genes, AIM2 and caspase-1 are upregulated by type I IFNs,143–145 while type I IFNs also regulate the activation or suppression of the inflammasome during different treatments.142,146 Moreover, cGAS/STING can also induce K+ efflux to facilitate the activation of the NLRP3 inflammasome in a type I IFN-independent fashion.147 In contrast, the inflammasome can restrict activation of the cGAS/STING pathway in the following two ways: (1) activated caspase-1 can directly cleave cGAS;148 and (2) inflammasome-activated gasdermin D promotes pyroptosis and K+ efflux through membrane pores, which restrict the cGAS-mediated production of type I IFNs.149 These results indicate that blocking the inflammasome might increase cGAS-mediated production of type I IFNs after radiation. However, the role of the inflammasome in the therapeutic effect of radiation in cancer treatment is still largely unknown. In addition, previous studies have reported that radiation can enhance the transcription of small endogenous noncoding RNA or endogenous retrovirus elements.130,150 The induced RNA can be recognized by RIG-1 or MDA5 to facilitate type I IFN production through MAVS. However, whether and how MAVS-mediated RNA innate sensing regulates adaptive immunity after radiation treatment are still unclear.
In addition to promoting the tumor cell apoptosis, necroptosis and pyroptosis described above, radiation has been reported to induce tumor cell ferroptosis. Ferroptosis is another regulated form of cell death that results from the iron-dependent accumulation of lipid peroxide.151,152 Recently, Lang et al. revealed that radiation can trigger tumor cells to undergo ferroptosis by inducing oxidative damage and the accumulation of toxic lipid peroxidation, and also sensitize tumor cells to ferroptosis agonists.153 Moreover, they demonstrated that CD8+ T cells can regulate tumor cell ferroptosis during immunotherapy.154 Furthermore, they implicated ferroptosis as a direct link through which immunotherapy and radiotherapy cooperate to improve tumor control. Mechanistically, IFNγ derived from immunotherapy-activated CD8+ T cells synergizes with the radiation-activated ataxia telangiectasia mutated gene to suppress solute carrier family 7 member 11 (SLC7A11), resulting in enhanced tumor lipid oxidation and ferroptosis.153 However, whether ferroptosis has a negative or positive impact on radiation-induced innate sensing or immunotherapy-induced tumor-specific immune responses is still largely unknown and needs to be further investigated.
Targeting innate sensing with chemotherapy
Beyond the direct killing effect of tumor cells, numerous clinical and preclinical studies have demonstrated that chemotherapeutics can also work directly or indirectly on the immune system, which has been well reviewed by other researchers.155–157 On the one hand, some chemotherapeutics can induce tumor cell ICD, which will alert the innate immune system, followed by the activation of the host adaptive immune system. However, certain chemotherapeutics can directly affect immune cell populations via mechanisms such as inducing transient lymphodepletion or specifically reducing MDSCs or Tregs to subvert the immunosuppressive microenvironment.21 Here, we will focus on key findings related to only the innate sensing pathways activated by certain chemotherapeutics. The antitumor activity of numerous chemotherapeutic agents, such as anthracyclines and oxaliplatin, was decreased in TLR4- and MyD88-deficient mice. Mechanistically, HMGB1, which is released by chemotherapy-induced cell death, can promote activation and maturation of DCs by binding to TLR4 and activating its downstream MyD88 signaling pathway to induce antitumor T-cell responses.27,158 Similarly, loss-of-function polymorphisms affecting TLR4 are associated with decreased time to metastasis among patients with anthracycline-treated breast carcinoma.27 In addition, it has also been demonstrated that anthracyclines can stimulate the production of type I IFNs by cancer cells through the activation of TLR3, which further promoted the DC cross-priming and CXCL10 production needed for recruiting T cells against the tumor.159 Moreover, another study revealed that ATP released from dying tumor cells as a result of chemotherapy can act on P2X7 purinergic receptors (P2RX7) on DCs and trigger the NLRP3-dependent inflammasome, which is responsible for the secretion of IL-1β and enhanced tumor-specific T-cell immunity. The priming of tumor-specific T cells fails in the absence of a functional IL-1 receptor 1 and in Nlpr3-deficient or caspase-1-deficient mice unless exogenous IL-1β is provided. Accordingly, anticancer chemotherapy was ineffective against tumors established in purinergic receptor P2rx7-, NLRP3-, or caspase-1-deficient hosts. Consistently, anthracycline-treated individuals with breast cancer carrying a loss-of-function allele of P2RX7 developed metastatic disease more rapidly than individuals bearing the normal allele.90 Intriguingly, the same group also reported that another two chemotherapeutic agents, gemcitabine and 5-fluorouracil (5-FU), activated NLRP3-mediated inflammasome formation in MDSCs, followed by IL-1β production that can induce IL-17 secretion from CD4+ T cells and further impair the anticancer efficacy of chemotherapeutic drugs. Accordingly, gemcitabine and 5-FU exert increased antitumor effects in NLRP3- or caspase-1-deficient mice, and NLRP3 activation by chemotherapeutic drugs is considered a positive regulator to promote tumor growth.160 All these results indicate the pivotal and conflicting roles of innate sensing pathways in chemotherapy-induced antitumor immunity. Further investigations are needed to better understand the effects of chemotherapeutics on the immune system, which will help modulate the existing chemotherapy strategies to best benefit cancer patients.
Targeting innate sensing with targeted therapy
Cancer targeted therapies, including targeted monoclonal antibodies (mAbs) and small molecules, were previously thought to prevent tumor growth by directly blocking oncogenic signaling and inducing tumor cell apoptosis. Accumulating evidence suggests that the antitumor efficacy of these tumor-targeted therapeutics also relies on host innate and adaptive immunity. Here, we focus on summarizing the important discoveries related to targeted therapy and innate immune sensing.
Antibody-based targeted therapy
Targeted antibodies, such as trastuzumab (target ERBB2/HER2), cetuximab (target EGFR), and rituximab (target CD20), have been approved for use in patients with oncogene mutations or overexpression.161,162 In addition to blocking tumor cell growth signaling and triggering apoptosis, these mAbs engage several innate immune effector processes, including complement-dependent cytotoxicity, antibody-dependent cell cytotoxicity, and antibody-dependent cellular phagocytosis mediated by myeloid cells and NK cells.121 Previous studies with xenograft models revealed that direct growth signaling blockade or innate immune cell-mediated killing by these mAbs was sufficient to control tumors, but the antitumor efficacy was relatively limited.163–165 Whether the adaptive immune system was involved could not be determined in these xenograft models. However, later studies demonstrated that these mAbs that triggered adaptive antitumor immune responses were more efficient in controlling tumor growth and provided long-lasting protection.24,166,167 In the clinic, patients who have been treated with trastuzumab exhibit a substantial increase in CD8+ T cells and NK cells, which is correlated with improved clinical outcomes.168 Although much clinical evidence has shown this kind of correlation, the mechanisms by which the adaptive immune responses are enhanced were still largely unknown until recent studies with syngeneic tumor models in immunocompetent mice. Using a mouse mammary tumor isolated from HER2/neu transgenic mice, our previous study first demonstrated that the antitumor effect of anti-HER2/neu depended on both host innate sensing and adaptive immunity,24,169 which has been further confirmed by others.170 Mechanistically, the HER2/neu antibody induces release of the stress protein HMGB1 from treated tumor cells, which can initiate the MyD88/type I IFN innate sensing pathway and further enhance tumor-specific T-cell responses.24 A similar phenotype was also observed with EGFR (cetuximab) and CD20 (rituximab) antibodies. The EGFR antibody was shown to promote the phagocytosis of an EGFR-expressing human colon cancer cell line by DCs and to increase cross-priming of T cells through MyD88-mediated innate sensing.171 In addition, it has been reported that cetuximab, in combination with chemotherapy, fostered ICD in CRC cells via the ER stress response and an increase in phagocytosis by DCs. The authors also further confirmed the enhanced immunogenicity elicited by cetuximab in a mouse model of human EGFR-expressing CRC.172 In a preclinical B-cell lymphoma model, an anti-CD20 antibody was reported to trigger macrophages to produce type I IFNs, which in turn promoted the activation and maturation of DCs and further enhanced CD8+ T-cell responses.166 Unlike the targeted mAbs discussed above, the anti-CD47 mAb, which blocks the “don’t eat-me” molecule CD47 that is broadly expressed on multiple cancer types, is a novel and potential target agent for cancer patients.173 The antitumor effect of CD47-blocking mAbs has been considered to largely depend on the enhanced antitumor phagocytosis by macrophages in studies using xenograft tumor models in immunodeficient mice.174 Later, studies using syngeneic murine models in immunocompetent mice revealed that the therapeutic effects of the anti-CD47 mAb mainly depend on DC (but not macrophage)-mediated cross-priming of T-cell responses. The authors further demonstrated that CD47 blockade with antibody activated the innate sensing pathway related to the cytosolic DNA sensor STING in DCs, but not macrophages, and promoted the production of type I IFNs for enhanced cross-priming of tumor-specific T-cell responses.121,175
Small molecule-based targeted therapy
Unlike targeted mAbs, which were developed to target the extracellular domain of surface receptors, small molecules were always designed to target intracellular tyrosine kinase domains or other molecules essential for maintaining the malignancy of cancer cells.176 EGFR tyrosine kinase inhibitors (TKIs) are considered one of the most successful targeted therapies used for cancer patients with EGFR/HER2-driven mutations, such as patients with lung cancer, breast cancer, and colon cancer.177,178 It has been shown that EGFR TKIs show greater potency than targeted antibody therapies or existing chemotherapies.179–182 Due to a lack of syngeneic murine tumor models, studies investigating the antitumor effect of EGFR TKIs have been performed either in vitro or using xenograft tumor models in immunodeficient mice, and these studies have demonstrated that prolonged treatment can potently suppress tumor growth.183–185 These results suggest that the therapeutic effect of EGFR TKIs likely occurs through direct blockade of oncogenic signaling and induction of tumor cell apoptosis. However, recent studies have shown that EGFR TKIs might modulate tumor plasticity and enhance the tumor recognition or tumor lysis by innate NK cells and antigen-specific T cells.167,186 However, the mechanisms by which EGFR TKIs influence the host immune system are still poorly defined. Interestingly, by using EGFR TKI-sensitive mouse syngeneic tumor models, we recently observed that hypofractionated EGFR TKI treatment (a high dose with a low-frequency treatment), but not standard hyperfractionated EGFR TKI treatment (a low dose with daily treatment), could trigger great innate sensing and type I IFN and CXCL10 production through the MyD88 signaling pathway. This innate activation further enhanced tumor-specific T-cell infiltration and reactivation to prevent and limit tumor relapse. Mechanistically, we further observed that a high dose of EGFR TKI treatment rapidly induced cellular stress and apoptosis and then increased the release of DNA and RNA from tumor cells, which may promote innate sensing and type I IFN production. However, which kinds of DAMPs and TLRs are upstream of the Myd88 signaling pathway remain to be determined.23
Cabozantinib is a receptor TKI with potent activity against multiple targets, including c-MET, VEGFR2, RET, KIT, AXL, and FLT3, all of which have been associated with tumor growth and survival.187 Cabozantinib has shown striking responses across several cancer types in the clinic. A clinical study reported that cabozantinib could significantly reduce the myeloid immunosuppressive cell subsets (MDSC and TIM3+ myeloid cells) in pretreated metastatic renal cell carcinoma patients.188 Another preclinical study demonstrated that cabozantinib reduced intratumoral PMN-MDSCs by suppressing the MDSC-promoting cytokines secreted by cancer cells and enhanced the therapeutic effect of immunotherapy in a metastatic castration-resistant prostate cancer model.189 However, a recent study from Patnaik et al. reported that cabozantinib could rapidly eradicate spontaneous prostate cancer in PTEN/p53-deficient mice with increased infiltration of neutrophils. HMGB1 released from stressed tumor cells under cabozantinib treatment triggered innate sensing, and CXCL12 production resulted in robust infiltration of neutrophils into the TME for tumor clearance. Accordingly, cabozantinib-induced tumor clearance in mice was abolished by antibody-mediated granulocyte depletion, HMGB1 neutralization, or blockade of neutrophil chemotaxis with the CXCR4 inhibitor plerixafor.190
Poly (ADP-ribose) polymerase (PARP), which is responsible for DNA repair, has been demonstrated to be a promising target in cancer patients with BRCA1 or BRCA2 mutation.191,192 PARP inhibitors have shown promising clinical activities in cancer patients with BRCA mutations based on the concept of synthetic lethality between PARP inhibition and BRCA1 or BRCA2 mutation.193 The therapeutic mechanisms of PARP inhibition were recently discovered. Using a syngeneic Brca1-deficient ovarian mouse tumor model, the authors found that olaparib, a PARP inhibitor, elicited both CD4+ and CD8+ T cells against tumors. They further revealed that DCs could sense tumor-derived dsDNA fragments and/or cGAMP upon PARP inhibition and drive STING-dependent type I IFN signaling and T-cell responses.194 Another study showed that the antitumor efficacy of PARP inhibition mainly depended on tumor-specific T-cell responses in a Brac1-deficient triple-negative breast cancer model. Intriguingly, they demonstrated crosstalk between PARP inhibition and the TME related to cGAS/STING/TBK1/IRF3 pathway activation in cancer cells that governed CD8+ T-cell recruitment, activation, and antitumor efficacy.195 Therefore, these two studies revealed that in addition to synthetic lethality, the therapeutic effect of PARP inhibition in vivo is mainly caused by triggering innate sensing and eliciting host adaptive immune responses.
Cyclin-dependent kinases 4 and 6 (CDK4/6), key drivers of the cell cycle, are required for cancer initiation and progression.196 CDK4/6 inhibitors, such as abemaciclib, palbociclib, and ribociclib, have shown promising activity against several solid tumors.190 Initially, their primary antitumor effect was considered to be inhibition of the phosphorylation of the retinoblastoma tumor suppressor and induction of G1 cell cycle arrest in tumor cells.197 Recently, Goel et al. found that CDK4/6 inhibitors not only induced tumor cell cycle arrest but also promoted antitumor immunity. They revealed that the enhanced antitumor immune response had two underpinnings. On the one hand, CDK4/6 inhibitors activate transcription of endogenous retroviral elements in tumor cells and increase intracellular levels of dsRNA. This in turn stimulates the RNA innate sensing pathway and the production of type III IFNs in tumor cells for enhanced tumor antigen presentation. In addition to triggering innate sensing, CDK4/6 inhibitors also markedly suppress the proliferation of Tregs. Ultimately, these events promote tumor-specific cytotoxic T-cell responses for effective tumor clearance.198
NAD(P)H: quinone oxidoreductase 1 (NQO1) is a cytosolic two-electron oxidoreductase that is highly expressed in various human cancers.199,200 While catalase, a hydrogen peroxide (H2O2)-scavenging enzyme, has lower expression in tumor tissues than in normal tissue.201 High NQO1:catalase ratios in tumor cells offer a potential therapeutic target for NQO1 bioactivatable drugs, while low expression ratios protect normal tissues. β-lapachone, an NQO1 bioactivatable drug, can be catalyzed by NQO1 in tumor cells to generate high levels of ROS, which further causes DNA damage and cell death.202 A recent study demonstrated that β-lapachone triggered tumor-selective innate sensing, leading to T-cell-dependent tumor control. Mechanically, β-lapachone induces HMGB1 release from the oxidation-stressed tumor cells and further activates the host TLR4/MyD88/type I IFN pathway and Batf3 DC-dependent cross-priming to bridge innate and adaptive immune responses against the tumor.203
These findings reveal the critical role of innate immune sensing in the therapeutic effect of targeted therapies, showing that they are not as specific as initially designed. However, a lack of proper syngeneic tumor models restricts the characterization of the role of innate sensing in other targeted therapies. Thus, developing syngeneic murine tumor models that are sensitive to certain targeted therapies will allow us to further interrogate the relationship between the immune system and targeted therapeutic effects. This will further promote the clinical development of potential novel combinational strategies for patients with targetable cancers.
Integrating conventional cancer therapies to improve cancer immunotherapy
Cancer immunotherapy was called the “Breakthrough of the Year” by Science in 2013 due to its great success in the clinic for many forms of cancer.204,205 There are several types of immunotherapies, such as antibody-based immunotherapies (particularly ICB), adoptive cellular therapy (particularly chimeric antigen receptor (CAR) T cells), cancer vaccines, and cytokine therapy. ICB, including ipilimumab (anti-CTLA-4) and pembrolizumab (anti-PD-1), has been the most attractive form of cancer immunotherapy because of its great success in benefiting various types of cancer patients, such as those with melanoma, CRC, and non-small-cell lung cancer.206–209 CAR T-cell therapy represents another major immunotherapy because of its good responses and high complete remission rates in patients with hematologic malignancies. However, the application of CAR T-cell therapy in patients with solid tumors remains a significant challenge.210 Cancer therapeutic vaccines aim to activate the patient’s own immune system to fight cancer. Promising results from clinical trials have led to the approval of several cancer vaccines by the U.S. FDA.211 Based on the potent activity of several proinflammatory cytokines in triggering host immunity and enhancing antitumor efficacy in preclinical murine cancer models, recombinant IFN-α and IL-2 have been approved for several malignancies.212 However, although immunotherapy can improve T-cell responses against tumors to induce long-lasting responses and significantly improve the overall survival of patients, this only occurs in a small fraction of patients.213,214 A retrospective analysis of the patients who responded very well to ICB revealed that patients with highly immunogenic tumors (such as a high tumor mutation burden, high PD-L1 levels, and a high frequency of circulating Ki-67+CD8+ T cells related to the tumor) and low tumor burden were more likely to respond to ICB or other immunotherapies.215–217 Accumulating evidence shows that conventional therapies, such as radiotherapy, chemotherapy, and targeted therapies, not only can significantly reduce tumor burden through tumor-intrinsic mechanisms but also can increase tumor immunogenicity by releasing DAMPs and TAAs.218 DAMPs can trigger innate immune sensing and the production of proinflammatory cytokines and chemokines, which in turn further enhance the cross-priming and recruitment of tumor-specific T cells. Interestingly, ATP, as one of the DAMPs, has been reported to play both positive and negative roles in APC maturation and T-cell proliferation. A low concentration (~250 nM) of ATP can activate the APCs and promote T-cell proliferation, whereas a high concentration (0.1–1 mM) of ATP can induce a disordered maturation of DCs and decrease the cell proliferation ability and IL-2 production of activated T cells, even inducing T-cell death.219–221 Meanwhile, enhanced innate sensing induces immunosuppressive factors, such as high PD-L1 expression and increased immune-suppressive cell populations (MDSCs and Tregs).23,222–224 Therefore, the proper combination of immunotherapies based on an understanding of the TME after conventional therapies might achieve the maximum antitumor efficacy (Fig. 2).
Decades ago, most studies focused on the direct killing effect of radiation on tumor cells. However, emerging evidence strongly supported that the adaptive immune responses were essential for the antitumor efficacy of radiotherapy.225,226 Radiation plays dual roles in antitumor adaptive immunity. On the one hand, radiation significantly induces tumor cell death to reduce tumor burden and enhances antitumor immunity through the cGAS/STING/type I IFN innate sensing pathway.26 In addition, radiation can also induce the expression of NKG2D ligands on tumor cells, which further promotes NK and CD8+ T-cell-mediated tumor cell killing.227,228 On the other hand, radiation can also upregulate the expression of PD-L1 on both tumor and immune cells,229,230 which in turn contributes to the restriction of antitumor immunity. Although more DCs, T cells and NK cells infiltrate into the tumor tissue after radiation treatment,231,232 radiation also induces a significant increase in the number of MDSCs, Tregs, M2 macrophages, and other immune-suppressive components in the TME.229 All these immune-suppressive components are responsible for the production of immunosuppressive factors, including TGF-β, IL-10, ARG1, and IDO.233–237 Combination approaches to overcome these immunosuppressive factors have been studied to achieve optimal therapeutic antitumor immune responses. Several preclinical studies have shown that a combination of ICB or a cancer therapeutic vaccine with radiation could achieve a better therapeutic effect and generate a stronger systemic antitumor effect than a single treatment alone.135,222,238–242 Moreover, combining radiation and anti-CTLA-4 or PD-L1/PD-1 blockade has shown synergy in enhancing the abscopal effects in the clinic.243,244 Recently, clinical studies have shown that radiation can enhance CAR T-cell therapy in patients with B-cell lymphoma without increased toxicities.245 Whether radiation can increase the antitumor efficacy of CAR T-cell therapy in patients with solid tumors still needs further investigation.246 Immune stimulatory molecules, such as anti-CD137/CD40 agonists and TLRs/STING agonists, have also been reported to show synergistic antitumor effects with radiation in preclinical studies and also have the potential for clinical application.26,247–254 However, most of these agonists activate the related pathways in only host immune cells, typically in DCs, macrophages, or T cells, which may result in the activation-induced cell death of immune cells and the upregulation of immunosuppressive factors,251 all of which eventually limit antitumor immunity. Therefore, to achieve maximal antitumor efficacy in combination treatment with radiation and immunotherapies, several variables of both therapies need to be systematically assessed, including the dose range, schedule, treatment sequence for combination, and target lesion choice.35
Direct tumor cell killing is the primary goal of chemotherapy. However, it has become clear that chemotherapeutics can also enact their antitumor activity via host innate and adaptive immunity. Certain chemotherapeutic agents can initiate innate immune sensing and activate tumor-specific adaptive T-cell responses by inducing ICD of tumor cells, as discussed above. In addition, some chemotherapeutics directly affect host innate and adaptive immune cells to enact their antitumor effects. Cyclophosphamide (CTX) is one of the best characterized chemotherapeutic agents that influences host immunity against tumors directly. It was reported that low-dose CTX treatment was associated with enhanced activation of NK cells and switching of M2 macrophages to M1 macrophages, which promoted the expansion and differentiation of DC precursors in the peripheral lymphoid organs and then tumor localization, reducing and suppressing Tregs and increasing MDSCs in both clinical and preclinical studies.255–257 In addition to CTX, other commonly used chemotherapeutics, such as doxorubicin, cisplatin, 5-FU, gemcitabine, and paclitaxel (PTX), also exert similar impacts on the host immune system.21,258 All these observations indicate the feasibility of a combination of chemotherapy and immunotherapy. Indeed, combining chemotherapy and ICB (CTLA-4 blockade) has shown promising outcomes in both advanced melanoma and lung cancer patients.259,260 Previous studies from our group and others have also demonstrated that chemotherapy could significantly enhance the therapeutic effect of cancer vaccines.261 Notably, intensive chemotherapies are always preferred in the clinic to maximally reduce the tumor mass, which may also impair the host immune responses for long-term protection against tumor recurrence. How to properly use combination therapy to avoid such side effects has become an outstanding issue. Our previous study has shown that the antitumor effect of anti-HER2 treatment largely depends on host T-cell responses. Intriguingly, additional intensive chemotherapeutic drugs after antibody treatment indeed synergistically reduced the tumor burden but also impaired the anti-HER2-generated antitumor T-cell responses and resulted in tumor relapse after tumor rechallenge. Instead, when chemotherapeutic drugs were given before antibody treatment, the antibody-mediated T-cell immunity was significantly diminished, and the synergistic effect in reducing tumor burdening was abolished.24 Moreover, our group also found that when an anti-CD47 antibody was administered after chemotherapy, but not before chemotherapy, the antitumor effect was enhanced.175 Therefore, the dosing and timing of each therapy for optimal combination must be carefully considered. How chemotherapy should be combined with immunotherapy in the neoadjuvant or adjuvant setting needs further investigation.
Unlike radiotherapy and chemotherapy, which are widely used in various types of cancer patients, the applications of targeted therapies are restricted to cancer patients with driver mutations or overexpression of specific oncogenic signaling pathways that are essential for cancer cell survival. Together with their strength in reducing tumor burden, mounting evidence has shown the potential activity of targeted therapies in regulating the host immune system. In addition to the targeted therapies described above, other targeted therapies have also been reported to be associated with antitumor immune responses.122 For instance, BRAF inhibitors (vemurafenib and dabrafenib) and MEK inhibitors (trametinib and cobimetinib) have been demonstrated to increase the expression of MHC I and upregulate the presentation of TAAs on tumor cells, which in turn activate tumor-specific T cells and facilitate T-cell-mediated killing in melanomas with constitutively active BRAF isoforms (mainly the V600E substitution).35,262,263 Interestingly, MEK inhibitors have been reported to attenuate the terminal differentiation of T cells,264 while BRAF inhibitors could active the MAPK pathway in T cells.265 Moreover, BRAF inhibitors can also increase immunosuppressive cells, MDSCs, and Tregs, in the TME, whereas the addition of MEK inhibitors can counteract this suppressive effect.266 Dasatinib, an oral dual BCR/ABL and Src family TKI, has also been linked to reduced Tregs and MDSCs in the TME in a mouse melanoma model.267 Several TKIs that inhibit tumor angiogenesis through targeting vascular endothelial growth factor A (VEGFA) have been observed to modulate the TME. Sorafenib (a multiple target TKI) was shown to deplete circulating MDSCs and intratumoral Tregs both in mice and in patients with renal cell carcinoma.268,269 Sunitinib, another FDA-approved multiple target TKI, was shown to enhance the recruitment of T cells into the TME by upregulating the expression of CXCL10 and CXCL11 on tumor vessels.270 However, although targeted therapies can initially significantly reduce tumor burden with a high response rate and improve the outcome of patients with immunostimulatory effects, patients will eventually develop drug resistance, and tumors will relapse, resulting in only modest improvements in the overall survival of cancer patients. Given the advantages of immunotherapies, which can induce a long-lasting immune response against tumors and dramatically improve the overall survival of responders, the combination of targeted therapies and immunotherapies is a promising strategy to achieve both a high response rate and significantly improved overall survival in the clinic.28 Indeed, several clinical trials are ongoing to test certain targeted therapies in conjunction with immunotherapies.122 Promising synergistic antitumor efficacy has been shown in several clinical studies, whereas substantial toxicity has also increased, and some of the clinical trials have been suspended.271 Therefore, how to properly combine targeted therapies and immunotherapies to achieve optimal antitumor effects without severe side effects has become an outstanding issue. Our recent study proposed that manipulating the dosing and timing of TKIs and immunotherapy might achieve this. Intriguingly, we demonstrated that anti-PD-L1 should be combined concurrently or early after treatment with a hypofractionated EGFR TKI to obtain the maximal synergistic antitumor effect. If anti-PD-L1 was given when the tumor started to relapse and grow, almost no synergistic antitumor effect was observed. More importantly, we also found that the hypofractionated EGFR TKI/anti-PD-L1 regimen caused much fewer side effects than the hyperfractionated EGFR TKI/anti-PD-L1 regimen.23 Similarly, we have also demonstrated that an EGFR TKI could synergize with tumor-targeted IL-2 to achieve much better tumor control than either treatment alone.272 If these observations can be applied to other TKIs or targeted therapies, our findings might open new treatment avenues for targeted therapy and immunotherapy in cancer patients. Another limitation for completely investigating the immunomodulatory effects of targeted therapies or combinations with immunotherapy is the lack of syngeneic murine tumor models that are sensitive to certain targeted therapies. Developing syngeneic mouse tumor models or xenograft tumor models in humanized mice will be helpful for a deeper understanding of the impact of targeted therapies on the host immune system, which will facilitate the design of combinational therapies in clinical trials.
Conclusive remarks
Innate immune sensing pathways play critical roles in regulating host innate immunity and subsequent adaptive immunity against cancer during cancer initiation/progression and cancer therapy. It is now becoming clear that in addition to their strength in reducing tumor burden directly, all conventional therapies, including radiotherapy, chemotherapy, and targeted therapy, can deeply impact host innate and adaptive immunity by triggering certain innate sensing pathways for the production of type I IFNs. As a bridge between innate and adaptive immunity, type I IFNs enhance the capacity of DCs, especially CD8α+ DCs, to cross-prime tumor-specific T cells for tumor control. However, tumors always relapse by developing treatment/drug resistance or acquiring adaptive immune resistance. Recent advances in cancer immunotherapy offer potential combinational strategies of conventional therapies. The combination of conventional therapy and immunotherapy has shown promising clinical activity, whereas the incidence of severe toxicities also increased. A deeper understanding of the impact of conventional therapies on immune cells in terms of treatment regimen (dosing and timing) and the mechanisms of combinational therapies in inducing a synergistic antitumor effect and/or severe toxicities will help in designing potential combinational therapies to achieve maximal antitumor efficacy with minimal toxicities in the clinic.
Acknowledgements
We thank Casey Moore for helpful editing and discussions. Y.-X.F. holds the Mary Nell and Ralph B. Rogers Professorship in Immunology. This work was supported in part by Texas CPRIT grants RP180725 and RR150072 (CPRIT scholar in Cancer Research) to Y.-X.F.
Competing interests
The authors declare no competing interests.
References
- 1.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. [Pathogen recognition by innate immunity] Arerugi. 2007;56:558–562. [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 4.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 5.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases elimination, equilibrium and escape. Curr. Opin. Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid, O. et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 9, 10.1186/1479-5876-9-204 (2011). [DOI] [PMC free article] [PubMed]
- 11.Rusakiewicz S, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud SMA, et al. Tumor-infiltrating CD8(+) lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 13.Woo JW, et al. Tumour-infiltrating CD8+lymphocytes after primary systemic therapy predict clinical outcome in patients with breast cancer. Virchows Arch. 2018;473:S54–S55. [Google Scholar]
- 14.Azimi F, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 15.Mlecnik B, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 16.Pages F, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 17.Bogolyubova AV, Belousov PV. Inflammatory immune infiltration in human tumors: role in pathogenesis and prognostic and diagnostic value. Biochemistry. 2016;81:1261–1273. doi: 10.1134/S0006297916110043. [DOI] [PubMed] [Google Scholar]
- 18.Arruebo M, et al. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3:3279–3330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudino TA. Targeted cancer therapy: the next generation of cancer treatment. Curr. Drug Discov. Technol. 2015;12:3–20. doi: 10.2174/1570163812666150602144310. [DOI] [PubMed] [Google Scholar]
- 20.Padma VV. An overview of targeted cancer therapy. Biomedicine. 2015;5:19. doi: 10.7603/s40681-015-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Zhida, Han Chuanhui, Dong Chunbo, Shen Aijun, Hsu Eric, Ren Zhenhua, Lu Changzheng, Liu Longchao, Zhang Anli, Timmerman Casey, Pu Yang, Wang Yang, Chen Mingyi, Qiao Jian, Fu Yang-Xin. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Science Immunology. 2019;4(38):eaav6473. doi: 10.1126/sciimmunol.aav6473. [DOI] [PubMed] [Google Scholar]
- 24.Park S, et al. The therapeutic effect of Anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, et al. Damage to nucleic acid sensing: a strategy to enhance radiation therapy. Clin. Cancer Res. 2016;22:20–25. doi: 10.1158/1078-0432.CCR-14-3110. [DOI] [PubMed] [Google Scholar]
- 26.Deng L, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 28.Wargo JA, Cooper ZA, Flaherty KT. Universes collide: combining immunotherapy with targeted therapy for cancer. Cancer Discov. 2014;4:1377–1386. doi: 10.1158/2159-8290.CD-14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simone CB, Burri SH, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl. Lung Cancer R. 2015;4:545–552. doi: 10.3978/j.issn.2218-6751.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotwals P, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 35.Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell. 2018;33:581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert L, Ribas A, Hu-Lieskovan S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Semin. Immunol. 2016;28:73–80. doi: 10.1016/j.smim.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beutler B. Toll-like receptors: how they work and what they do. Curr. Opin. Hematol. 2002;9:2–10. doi: 10.1097/00062752-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 41.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 42.Uematsu S, Akira S. Toll-like receptors and innate immunity. J. Mol. Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 43.Cui J, Chen Y, Wang HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin Immunother. 2014;10:3270–3285. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castano-Rodriguez N, Kaakoush NO, Mitchell HM. Pattern-recognition receptors and gastric cancer. Front. Immunol. 2014;5:336. doi: 10.3389/fimmu.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27:218–224. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 46.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Harmey JH, et al. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int. J. Cancer. 2002;101:415–422. doi: 10.1002/ijc.10632. [DOI] [PubMed] [Google Scholar]
- 48.Cen X, Liu S, Cheng K. The role of Toll-like receptor in inflammation and tumor immunity. Front. Pharm. 2018;9:878. doi: 10.3389/fphar.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J. Leukoc. Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- 50.Chi H, et al. Anti-tumor Activity of Toll-like receptor 7 agonists. Front. Pharm. 2017;8:304. doi: 10.3389/fphar.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vacchelli E, et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman ES, Smith RE, Renaud RC., Jr. From the analyst's couch: TLR-targeted therapeutics. Nat. Rev. Drug Discov. 2005;4:879–880. doi: 10.1038/nrd1880. [DOI] [PubMed] [Google Scholar]
- 53.Hancz D, et al. Flagellin increases death receptor-mediated cell death in a RIP1-dependent manner. Immunol. Lett. 2018;193:42–50. doi: 10.1016/j.imlet.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Takaki H, Shime H, Matsumoto M, Seya T. Tumor cell death by pattern-sensing of exogenous RNA: Tumor cell TLR3 directly induces necroptosis by poly(I:C) in vivo, independent of immune effector-mediated tumor shrinkage. Oncoimmunology. 2017;6:e1078968. doi: 10.1080/2162402X.2015.1078968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drickamer, K. & Fadden, A. J. Genomic analysis of C-type lectins. Biochem. Soc. Symp. 69, 59–72 (2002). [DOI] [PubMed]
- 57.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 58.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Fresno C, Iborra S, Saz-Leal P, Martinez-Lopez M, Sancho D. Flexible signaling of myeloid C-type lectin receptors in immunity and inflammation. Front. Immunol. 2018;9:804. doi: 10.3389/fimmu.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drummond RA, Brown GD. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 2013;9:e1003417. doi: 10.1371/journal.ppat.1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redelinghuys P, Brown GD. Inhibitory C-type lectin receptors in myeloid cells. Immunol. Lett. 2011;136:1–12. doi: 10.1016/j.imlet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nonaka M, et al. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J. Immunol. 2008;180:3347–3356. doi: 10.4049/jimmunol.180.5.3347. [DOI] [PubMed] [Google Scholar]
- 66.Aarnoudse CA, Garcia Vallejo JJ, Saeland E, van Kooyk Y. Recognition of tumor glycans by antigen-presenting cells. Curr. Opin. Immunol. 2006;18:105–111. doi: 10.1016/j.coi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 68.Napoletano C, et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012;42:936–945. doi: 10.1002/eji.201142086. [DOI] [PubMed] [Google Scholar]
- 69.Yan H, Ohno N, Tsuji NM. The role of C-type lectin receptors in immune homeostasis. Int. Immunopharmacol. 2013;16:353–357. doi: 10.1016/j.intimp.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allavena P, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin. Dev. Immunol. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian J, et al. Beta-glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur. J. Immunol. 2013;43:1220–1230. doi: 10.1002/eji.201242841. [DOI] [PubMed] [Google Scholar]
- 73.Masuda Y, Inoue M, Miyata A, Mizuno S, Nanba H. Maitake beta-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 2009;9:620–626. doi: 10.1016/j.intimp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z, Zhou H, Wang W, Fu YX, Zhu M. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology. 2016;5:e1147641. doi: 10.1080/2162402X.2016.1147641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, et al. A novel method for synthetic vaccine construction based on protein assembly. Sci. Rep. 2014;4:7266. doi: 10.1038/srep07266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park HY, et al. Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells. NPJ Vaccines. 2017;2:31. doi: 10.1038/s41541-017-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tacken PJ, Torensma R, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology. 2006;211:599–608. doi: 10.1016/j.imbio.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 78.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 79.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Saxena M, Yeretssian G. NOD-like receptors: master regulators of inflammation and cancer. Front. Immunol. 2014;5:327. doi: 10.3389/fimmu.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 82.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 85.Agostini L, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 86.Couturier-Maillard A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Investig. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Millrud CR, et al. Nod-like receptors in head and neck squamous cell carcinoma. Acta Otolaryngol. 2013;133:1333–1344. doi: 10.3109/00016489.2013.831476. [DOI] [PubMed] [Google Scholar]
- 89.Cook GP, Savic S, Wittmann M, McDermott MF. The NLRP3 inflammasome, a target for therapy in diverse disease states. Eur. J. Immunol. 2010;40:631–634. doi: 10.1002/eji.200940162. [DOI] [PubMed] [Google Scholar]
- 90.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 91.Chow MT, et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72:5721–5732. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 92.Wei Q, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab. Investig. 2014;94:52–62. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 93.Zaki MH, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Normand S, et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl Acad. Sci. USA. 2011;108:9601–9606. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl Acad. Sci. USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoneyama M, et al. Viral RNA dedetection by RIG-I-like receptors. Curr. Opin. Immunol. 2015;32:48–53. doi: 10.1016/j.coi.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife. 2012;1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlee M. Master sensors of pathogenic RNA - RIG-I like receptors. Immunobiology. 2013;218:1322–1335. doi: 10.1016/j.imbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duewell P, et al. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8(+) T cells. Cell Death Differ. 2014;21:1825–1837. doi: 10.1038/cdd.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bu Y, Liu F, Jia QA, Yu SN. Decreased expression of TMEM173 predicts poor prognosis in patients with hepatocellular carcinoma. PLoS ONE. 2016;11:e0165681. doi: 10.1371/journal.pone.0165681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemos H, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76:2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu J, et al. Dose dependent activation of retinoic acid-inducible gene-I promotes both proliferation and apoptosis signals in human head and neck squamous cell carcinoma. PLoS ONE. 2013;8:e58273. doi: 10.1371/journal.pone.0058273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 108.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rathinam VA, Fitzgerald KA. Cytosolic surveillance and antiviral immunity. Curr. Opin. Virol. 2011;1:455–462. doi: 10.1016/j.coviro.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 111.Hervas-Stubbs S, et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 112.Lorenzi S, et al. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J. Immunol. 2011;186:5142–5150. doi: 10.4049/jimmunol.1004163. [DOI] [PubMed] [Google Scholar]
- 113.Curtsinger JM, et al. IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 114.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 115.Dunn GP, et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 116.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medrano RFV, Hunger A, Mendonca SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017;8:71249–71284. doi: 10.18632/oncotarget.19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moschos SJ, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J. Clin. Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 119.Liang Y, et al. Targeting IFNalpha to tumor by anti-PD-L1 creates feedforward antitumor responses to overcome checkpoint blockade resistance. Nat. Commun. 2018;9:4586. doi: 10.1038/s41467-018-06890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang X, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Michelle Xu M, Pu Y, Weichselbaum RR, Fu YX. Integrating conventional and antibody-based targeted anticancer treatment into immunotherapy. Oncogene. 2017;36:585–592. doi: 10.1038/onc.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu MM, Pu Y, Zhang Y, Fu YX. The Role of Adaptive Immunity in the Efficacy of Targeted Cancer Therapies. Trends Immunol. 2016;37:141–153. doi: 10.1016/j.it.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baskar R, Lee KA, Yeo R, Yeoh K-W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 2012;9:193. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoskin PJ, Bhattacharya IS. Protons and more: state of the art in radiotherapy. Clin. Med. 2014;14:s61–s65. doi: 10.7861/clinmedicine.14-6-s61. [DOI] [PubMed] [Google Scholar]
- 125.Srinivas, U. S., Tan, B., Vellayappan, B. A. & Jeyasekharan, A. D. ROS and the DNA damage response in cancer. Redox biology.25, 101084 (2018). [DOI] [PMC free article] [PubMed]
- 126.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reits EA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer. 2012;12:860. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 129.Wu Y, Wu X, Wu L, Wang X, Liu Z. The anticancer functions of RIG-I–like receptors, RIG-I and MDA5, and their applications in cancer therapy. Transl. Res. 2017;190:51–60. doi: 10.1016/j.trsl.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 130.Ranoa DRE, et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016;7:26496. doi: 10.18632/oncotarget.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burnette BC, et al. The efficacy of radiotherapy relies upon induction of type I interferon–dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harding SM, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mackenzie KJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Oliveira Mann CC, Kranzusch PJ. cGAS conducts micronuclei DNA surveillance. Trends Cell Biol. 2017;27:697–698. doi: 10.1016/j.tcb.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 135.Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shimada K, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heid ME, et al. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu B, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu YG, et al. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell Death Dis. 2017;8:e2579. doi: 10.1038/cddis.2016.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guarda G, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 143.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 145.Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS ONE. 2011;6:e27040. doi: 10.1371/journal.pone.0027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaidt MM, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–1124. e1118. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y, et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 149.Banerjee I, et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49:413–426. e415. doi: 10.1016/j.immuni.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Servomaa K, Rytömaa T. UV light and ionizing radiations cause programmed death of rat chloroleukaemia cells by inducing retropositions of a mobile DNA element (L1Rn) Int. J. Radiat. Biol. 1990;57:331–343. doi: 10.1080/09553009014552441. [DOI] [PubMed] [Google Scholar]
- 151.Yang WS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lang Xueting, Green Michael D., Wang Weimin, Yu Jiali, Choi Jae Eun, Jiang Long, Liao Peng, Zhou Jiajia, Zhang Qiang, Dow Ania, Saripalli Anjali L., Kryczek Ilona, Wei Shuang, Szeliga Wojciech, Vatan Linda, Stone Everett M., Georgiou George, Cieslik Marcin, Wahl Daniel R., Morgan Meredith A., Chinnaiyan Arul M., Lawrence Theodore S., Zou Weiping. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discovery. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang W, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Garg AD, et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front. Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 157.Zhou J, et al. Immunogenic cell death in cancer therapy: present and emerging inducers. J. Cell Mol. Med. 2019;23:4854–4865. doi: 10.1111/jcmm.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 159.Sistigu A, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 160.Bruchard M, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 161.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am. J. Transpl. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 162.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 2001;12(Suppl 1):S35–S41. doi: 10.1093/annonc/12.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 163.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 164.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 165.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N. Engl. J. Med. 2012;366:2008–2016. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 166.Ren Z, et al. CTLA-4 limits anti-CD20-mediated tumor regression. Clin. Cancer Res. 2017;23:193–203. doi: 10.1158/1078-0432.CCR-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 2016;7:e2380. doi: 10.1038/cddis.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arnould L, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Correale P, et al. Cetuximab +/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int. J. Cancer. 2012;130:1577–1589. doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 172.Pozzi C, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat. Med. 2016;22:624–631. doi: 10.1038/nm.4078. [DOI] [PubMed] [Google Scholar]
- 173.Liu J, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu X, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Arslan MA, Kutuk O, Basaga H. Protein kinases as drug targets in cancer. Curr. Cancer Drug Tar. 2006;6:623–634. doi: 10.2174/156800906778742479. [DOI] [PubMed] [Google Scholar]
- 177.Yamaoka Toshimitsu, Ohba Motoi, Ohmori Tohru. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. International Journal of Molecular Sciences. 2017;18(11):2420. doi: 10.3390/ijms18112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 179.Sequist LV, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 180.Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 181.Xu M, Xie Y, Ni S, Liu H. The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC) Ann. Transl. Med. 2015;3:96. doi: 10.3978/j.issn.2305-5839.2015.03.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl. Lung Cancer Res. 2015;4:67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Friess T, Scheuer W, Hasmann M. Erlotinib antitumor activity in non-small cell lung cancer models is independent of HER1 and HER2 overexpression. Anticancer Res. 2006;26:3505–3512. [PubMed] [Google Scholar]
- 184.Ioannou N, et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br. J. Cancer. 2011;105:1554–1562. doi: 10.1038/bjc.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cross DA, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kumai T, et al. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br. J. Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yakes FM, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 188.Verzoni, E. et al. Potent natural killer (NK) and myeloid blood cell remodeling by cabozantinib (Cabo) in pre-treated metastatic renal cell carcinoma (mRCC) patients. Ann. Oncol. 29, 10.1093/annonc/mdy283.091 (2018).
- 189.Lu X, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Patnaik A, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, other solid tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 191.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 192.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 193.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ding L, et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25:2972–2980 e2975. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pantelidou C, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative. Breast Cancer Cancer Discov. 2019;9:722–737. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Choi YJ, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 198.Goel S, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li R, Bianchet MA, Talalay P, Amzel LM. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc. Natl Acad. Sci. USA. 1995;92:8846–8850. doi: 10.1073/pnas.92.19.8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oh ET, et al. NQO1 inhibits proteasome-mediated degradation of HIF-1alpha. Nat. Commun. 2016;7:13593. doi: 10.1038/ncomms13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Doskey CM, et al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang XM, et al. Leveraging an NQO1 bioactivatable drug for tumor-selective use of poly(ADP-ribose) polymerase inhibitors. Cancer Cell. 2016;30:940–952. doi: 10.1016/j.ccell.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li, X. G. et al. NQO1 targeting prodrug triggers innate sensing to overcome checkpoint blockade resistance. Nat. Commun.10, 3251 (2019). [DOI] [PMC free article] [PubMed]
- 204.Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J. Oncol. 2019;2019:4508794. doi: 10.1155/2019/4508794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother. Sci. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 206.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 207.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 208.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 2019;94:S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 211.Guo C, et al. Therapeutic cancer vaccines: past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Berraondo P, et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 215.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qiao J, Liu Z, Fu YX. Adapting conventional cancer treatment for immunotherapy. J. Mol. Med. 2016;94:489–495. doi: 10.1007/s00109-016-1393-4. [DOI] [PubMed] [Google Scholar]
- 219.Shinohara Y, Tsukimoto M. Adenine nucleotides attenuate murine T cell activation induced by concanavalin A or T cell receptor stimulation. Front. Pharm. 2017;8:986. doi: 10.3389/fphar.2017.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.la Sala A, et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 221.Aymeric L, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 222.Deng L, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Muroyama Y, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol. Res. 2017;5:992–1004. doi: 10.1158/2326-6066.CIR-17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liang H, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017;8:1736. doi: 10.1038/s41467-017-01566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gupta A, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 227.Weiss T, et al. NKG2D-dependent antitumor effects of chemotherapy and radiotherapy against glioblastoma. Clin. Cancer Res. 2018;24:882–895. doi: 10.1158/1078-0432.CCR-17-1766. [DOI] [PubMed] [Google Scholar]
- 228.Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018;51:55–61. doi: 10.1016/j.coi.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Deng L, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dovedi SJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 231.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front. Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Filatenkov A, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat. Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Investig. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 236.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid‐derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin. Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 240.Park SS, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Luo M, et al. Synergistic STING activation by PC7A nanovaccine and ionizing radiation improves cancer immunotherapy. J. Control Release. 2019;300:154–160. doi: 10.1016/j.jconrel.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 243.Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J. Immunother. Cancer. 2018;6:46. doi: 10.1186/s40425-018-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sim Austin J., Jain Michael D., Figura Nicholas B., Chavez Julio C., Shah Bijal D., Khimani Farhad, Lazaryan Aleksandr, Krivenko Gabriel, Davila Marco L., Liu Hien D., Falchook Aaron D., Dahiya Saurabh, Rapoport Aaron P., Kim Sungjune, Locke Frederick L., Robinson Timothy J. Radiation Therapy as a Bridging Strategy for CAR T Cell Therapy With Axicabtagene Ciloleucel in Diffuse Large B-Cell Lymphoma. International Journal of Radiation Oncology*Biology*Physics. 2019;105(5):1012–1021. doi: 10.1016/j.ijrobp.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Minn I, Rowe SP, Pomper MG, Enhancing CAR. T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 2019;20:e443–e451. doi: 10.1016/S1470-2045(19)30461-9. [DOI] [PubMed] [Google Scholar]
- 247.Newcomb EW, et al. Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat. Res. 2010;173:426–432. doi: 10.1667/RR1904.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 2006;26:3445–3453. [PubMed] [Google Scholar]
- 249.Demaria S, Pilones KA, Vanpouille-Box C, Golden EB, Formenti SC. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat. Res. 2014;182:170–181. doi: 10.1667/RR13500.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Foote JB, et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol. Res. 2017;5:468–479. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sivick KE, et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 2018;25:3074–3085. e3075. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 252.Singh VK, et al. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat. Res. 2011;177:628–642. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 253.Dovedi SJ, et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood. 2013;121:251–259. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- 254.Roses RE, Xu M, Koski GK, Czerniecki BJ. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene. 2008;27:200. doi: 10.1038/sj.onc.1210909. [DOI] [PubMed] [Google Scholar]
- 255.Bryniarski K, Szczepanik M, Ptak M, Zemelka M, Ptak W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharm. Rep. 2009;61:550–557. doi: 10.1016/s1734-1140(09)70098-2. [DOI] [PubMed] [Google Scholar]
- 256.Liu P, Jaffar J, Hellstrom I, Hellstrom KE. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. 2010;33:53–59. doi: 10.1097/CJI.0b013e3181b56af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ghiringhelli F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 259.Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann. Oncol. 2013;24:1170–1179. doi: 10.1093/annonc/mds647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 261.Liu Z, et al. Coordinating antigen cytosolic delivery and danger signaling to program potent cross-priming by micelle-based nanovaccine. Cell Discov. 2017;3:17007. doi: 10.1038/celldisc.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Brea EJ, et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol. Res. 2016;4:936–947. doi: 10.1158/2326-6066.CIR-16-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boni A, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 264.Ebert PJR, et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 265.Callahan MK, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol. Res. 2014;2:70–79. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hu-Lieskovan S, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 2015;7:279ra241. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lowe DB, et al. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology. 2014;3:e27589. doi: 10.4161/onci.27589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Terme M, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 269.Desar IM, et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int. J. Cancer. 2011;129:507–512. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 270.Huang H, et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-kappaB-induced endothelial activation. FASEB J. 2015;29:227–238. doi: 10.1096/fj.14-250985. [DOI] [PubMed] [Google Scholar]
- 271.Ahn MJ, et al. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin. Drug Saf. 2017;16:465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]
- 272.Sun Z, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 2019;10:3874. doi: 10.1038/s41467-019-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]