Abstract
Small heat-shock proteins (sHsps) compose the most widespread family of molecular chaperones. The human genome encodes 10 different sHsps (HspB1–10). It has been shown that HspB1 (Hsp27), HspB5 (αB-crystallin), and HspB6 (Hsp20) can form hetero-oligomers in vivo. However, the impact of hetero-oligomerization on their structure and chaperone mechanism remains enigmatic. Here, we analyzed hetero-oligomer formation in human cells and in vitro using purified proteins. Our results show that the effect of hetero-oligomer formation on the composition of the sHsp ensembles and their chaperone activities depends strongly on the respective sHsps involved. We observed that hetero-oligomer formation between HspB1 and HspB5 leads to an ensemble that is dominated by species larger than the individual homo-oligomers. In contrast, the interaction of dimeric HspB6 with either HspB1 or HspB5 oligomers shifted the ensemble toward smaller oligomers. We noted that the larger HspB1–HspB5 hetero-oligomers are less active and that HspB6 activates HspB5 by dissociation to smaller oligomer complexes. The chaperone activity of HspB1–HspB6 hetero-oligomers, however, was modulated in a substrate-specific manner, presumably due to the specific enrichment of an HspB1–HspB6 heterodimer. These heterodimeric species may allow the tuning of the chaperone properties toward specific substrates. We conclude that sHsp hetero-oligomerization exerts distinct regulatory effects depending on the sHsps involved.
Keywords: chaperone, small heat-shock protein (sHsp), protein aggregation, oligomerization, molecular chaperone, protein folding, protein–protein interaction, hetero-oligomers, HspB
Introduction
Small heat-shock proteins (sHsp)4 are a ubiquitous group of molecular chaperones. Members of this family have been found in almost all living organisms, with a few pathogenic bacteria as the exceptions (1). The common feature of sHsps is the presence of the conserved α-crystallin domain, which consists of a compact β-sheet sandwich composed of two layers of three and five anti-parallel strands, respectively, connected by a short inter-domain loop (2–4). Despite the small molecular mass of the monomers, most sHsps form large oligomeric complexes with molecular masses of up to 1 MDa (1, 5). As molecular chaperones, sHsps affect the folding and aggregation of nonnative proteins in an ATP-independent manner. They keep the substrate proteins in a state competent for later refolding by ATP-dependent chaperones (e.g. Hsp70/Hsp40) or degradation (1, 6–10). Besides the participation in protein quality control (10), sHsps are involved in the regulation of several other cellular processes like apoptosis (11–13), organization of intermediate filaments, and muscle contraction (14). Interestingly, the interaction of sHsps with the respective partner proteins in these regulatory processes often occurs already at physiological conditions as is the case for the interaction between HpsB1 with pro-caspase3 and several intermediate filament proteins (15, 16).
A striking feature of metazoa is the expansion of the number of sHsps present within a species. To date, 10 small heat-shock proteins have been described in humans (2, 17). Some of them (e.g. HspB1 (Hsp27), HspB5 (αB-crystallin), HspB6 (Hsp20), and HspB8 (Hsp22)), are expressed in many different human tissues, whereas others are tissue-specific. For example, HspB4 (αA-crystallin) is present mainly in the eye lens (18), and HspB9 (CT51) as well as HspB10 (ODFP1) are expressed only in testis (19, 20). The highest levels of sHsps were found in muscle tissue where in pectoral muscle the concentration of HspB1 can reach up to 0.3% of the total protein (21–23). HspB5 and HspB6 are also expressed at high levels in skeletal and cardiac muscles (22, 23). Interestingly, these three small heat-shock proteins were co-purified from muscle tissue, and separation of them was possible only in the presence of denaturants (21, 22). In vitro experiments showed that human sHsps can form hetero-oligomeric complexes (24–26). Based on their ability to interact, an initial two-hybrid screen divided the human sHsps into two groups. The members of the first group (HspB1, HspB5, and HspB6) interact with each other but not with members of the second group (HspB2 and HspB3) (27). Later on, HspB8 was found to interact with members of both groups (28, 29). However, more recent studies showed that recombinant HspB8 only weakly interacts with the members of the group 1 sHsps and does not form stable hetero-oligomeric complexes in vitro (25, 30). The nature of the hetero-oligomeric complexes as well as their chaperone activities have not yet been studied comprehensively. In cancer cells, the typical expression pattern of sHsp changes (31, 32). Often, the expression of HspB1 or HspB5 is increased, which leads to a higher proliferation rate and resistance of the tumor against cytotoxic compounds (33–35). The expression of HspB6 negatively correlates with malignancy in several cancer tissues (36). In this context, hetero-oligomerization of these sHsps adds to the complexity of the system and might be implicated in the proliferation decision. To close the current gap in understanding hetero-oligomerization, we focused on the interaction of these three human sHsps, which are co-expressed in several tissues and cancer cell lines, and set out to analyze the oligomeric states and function of HspB1, HspB5, and HspB6 hetero-oligomers. Two of these sHsps, HspB1 and HspB5, form large assemblies with a molecular mass of 500–800 kDa in their homo-oligomeric forms (37–39). Phosphorylation of these sHsps leads to the dissociation into smaller species, presumably tetramers or hexamers (40). Additionally, phosphorylation under most conditions increases the chaperone activity of HspB5 (41) and either activates or inactivates HspB1 depending on the experimental conditions (37, 42, 43). In vitro, HspB6 forms only dimers and does not change the oligomeric state upon phosphorylation (44). However, at least in vitro, HspB6 can form hetero-oligomers with HspB1 or HspB5, which are smaller than HspB1 or HspB5 homo-oligomers (30, 45). When HspB1, HspB5, and HspB6 were co-expressed, mixed oligomers were observed (21, 22, 26). However, the composition of the hetero-oligomers formed at physiological sHsp ratios and their respective chaperone activities are not known.
In this study, we quantified HspB1, HspB5, and HspB6 levels in five human cancer cell lines as a basis for our analysis of the consequences of hetero-oligomer formation under physiological and heat stress conditions.
Results
sHsp expression in human cell lines
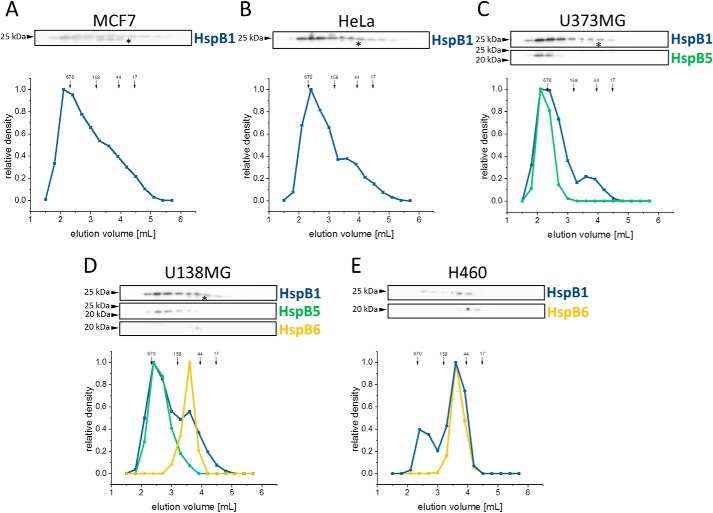
To address the significance of hetero-oligomer formation of sHsps, we focused on the human sHsps HspB1, HspB5, and HspB6 for which hetero-oligomerization in vivo is well-known (30, 45–47). Screening 11 human cell lines derived from different tissues (Table S1) under physiological conditions, we found that most of them express HspB1 (Hsp27), but the expression levels vary substantially between cell lines (Fig. 1). Interestingly, in HEK293 none of the three sHps could be detected by Western blotting. Three of the analyzed cell lines expressed more than one sHsp: U373MG (HspB1 and HspB5) and H460 (HspB1 and HspB6), whereas only U138MG expressed all three sHsp (high levels of HspB1 and HspB6 and lower amounts of HspB5).
Figure 1.
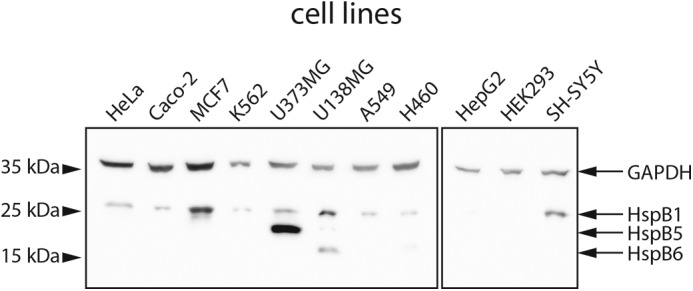
Endogenous expression of HspB1, HspB5, and HspB6 in different human cell lines. 50 μg of the soluble lysate fraction were analyzed by Western blotting using specific antibodies against HspB1, HspB5, and HspB6, respectively. GAPDH was used as a loading control.
To determine the sHsp concentrations in the cell lines under standard (ST) and heat-shock (HS) conditions, we performed quantitative Western blottings (Fig. S1) using recombinant sHsps for calibration (Table 1). Although U138MG cells did not show any significant changes in the levels of all three sHsps upon HS, in all other cell lines, the expression of HpsB1 increased upon HS. In H460 cells, HspB6 levels increased upon HS by a factor of ∼12, and HspB5 levels stayed constant in all cell lines where it is detected under all conditions tested. Thus, HspB1 is the most consistently heat-regulated sHsp in the cell lines tested. In U373MG cells, the ratio of HspB1/HspB5 was ∼1:2 under standard conditions. This ratio shifted slightly to ∼3:2 upon HS as only HspB1 was overexpressed (Table 1). For HspB1/HspB6, a 6:1 ratio was observed under ST conditions in H460 cells, which changed to a 3:2 ratio upon HS indicating a significant increase in HspB6 levels versus HspB1 levels (Table 1). The observed shifts in ratios and amounts raised the question how this influences the sizes of sHsp complexes.
Table 1.
Quantification of HspB1, HspB5, and HspB6 in five human cancer cell lines by Western blotting
sHsp amounts were determined under HS conditions as well as ST conditions. Concentrations were calculated assuming 150 mg/ml total protein concentration in the cells (46). An average of three measurements and corresponding standard deviations are stated. Not detectable sHsp amounts are labeled by n.f. (not found).
| Cell line | Protein concentration |
|||||
|---|---|---|---|---|---|---|
| HspB1 HS | HspB1 ST | HspB5 HS | HspB5 ST | HspB6 HS | HspB6 ST | |
| μm | μm | μm | μm | μm | μm | |
| HeLa | 49 ± 3 | 30 ± 2 | n.f. | n.f. | n.f. | n.f. |
| MCF7 | 50 ± 3 | 11 ± 0.7 | n.f. | n.f. | n.f. | n.f. |
| U373MG | 36 ± 0.5 | 11 ± 2 | 29 ± 3 | 29 ± 3 | n.f. | n.f. |
| U138MG | 49 ± 5 | 52 ± 5 | 7 ± 0.8 | 5 ± 0 | 33 ± 4 | 29 ± 3 |
| H460 | 7 ± 0.2 | 2 ± 0.1 | n.f. | n.f. | 6 ± 0.3 | 0.5 ± 0.2 |
Size distribution of sHsps in cancer cells
To investigate the size distribution of sHsps in cells, we separated the soluble lysate fraction by size-exclusion chromatography (SEC) and Western blot analysis. In MCF7 and HeLa cells, HspB1 eluted over a broad range (Fig. 2, A and B) with two maxima corresponding to apparent molecular masses of ∼400–600 and ∼70 kDa, respectively, as reported previously (38, 48, 49); the smaller oligomers probably represent phosphorylated species (26, 45, 50–53). Double bands for HspB1 are commonly observed (54). Both bands were included in the quantification.
Figure 2.
Size distribution of endogenously-expressed sHsps under ST conditions in five human cancer cell lines. 0.5 mg of soluble lysate fraction of different cancer cell lines was applied directly after mechanical lysis onto an SEC column. Subsequently, the fractions were analyzed by Western blotting. The relative content of the sHsp was quantified by means of densitometry blots depicted as a graph. MCF7 (A) and HeLa (B) expressing only HspB1 (blue) were used as reference. Elution profiles of sHsps from U373MG cells expressing HspB1 and HspB5 (green) (C), U138MG cells expressing HspB1, HspB5, and HspB6 (yellow) (D), and H460 cells expressing HspB1 and HspB6 (E) are shown. Positions of molecular mass standards (in kDa) are indicated with arrows. Double bands of HspB1, labeled with an asterisk, are commonly observed.
In U373MG cells, which express HspB1 and HspB5, we found a similar elution profile with two maxima at 400–600 and ∼70 kDa (Fig. 2C). Although HspB1 was again present in both peaks, HspB5 co-eluted in the 400–600-kDa peak only.
In U138MG cells, which contain HspB1, HspB5, and HspB6, the two HspB1 peaks were also observed, although HspB5 eluted in the peak corresponding to 400–600 kDa (Fig. 2D). Interestingly, HspB6 was present only in the ∼70-kDa peak. The same distribution of HspB6 was observed in H460 cells expressing only HspB1 and HspB6 (Fig. 2E). Additionally, the data indicate that the oligomer distribution of HspB1 remains unchanged in U138MG and H460 cells, despite the low level of HspB5 expression in the former and its absence in the latter case. The lack of HspB5 in H460 cells, however, causes a clear shift of the HspB1 oligomer distribution toward smaller species.
The picture emerging from these analyses is that HspB1 forms large and small oligomers in all cell lines analyzed, whereas HspB5 is present only in ensembles of large oligomers, and HspB6 is exclusively in ensembles of small oligomers. Overall, the sHsp distribution was not significantly changed upon HS within the accuracy limits of the applied methods (Fig. S2).
To further analyze whether the simultaneous expression of sHsp members leads to hetero-oligomerization, we performed co-immunoprecipitations with antibodies against HspB1, HspB5, or HspB6. In general, antibody binding could affect the equilibrium of sHsps complexes. However, in this experiment the focus is on the overall ability of different sHsps to form hetero-oligomers in cells. We found an interaction between HspB1 and HspB5 in U373MG (Fig. 3A and Fig. S3A), between HspB1 and HspB6 in H460 (Fig. 3B and Fig. S3B), and between HspB1 and HspB6 in U138MG cells (Fig. 3C and Fig. S3C). We could not detect HspB1–HspB5 complexes in the U138MG lysate due to the low endogenous level of HspB5. However, when we added recombinant HspB5 to the lysate, we readily observed the formation of hetero-oligomers (Fig. 3C).
Figure 3.
Verification of sHsp homo- and hetero-interaction by co-IP of endogenously-expressed sHsps in the soluble lysate fraction of different cancer cell lines. Independent co-IPs were performed using specific antibodies against HspB1 and HspB5 in U373MG cell lysate (A). In H460 cell lysate (B), specific antibodies against HspB1 and HspB6 were used instead, and in the case of U138MG cell lysate (C), co-IPs with α-HspB1 and α-HspB6 were performed. For HspB5 co-IPs, 10 μg of recombinant HspB5 were added to the co-IP reaction due to the low endogenous expression level. The sHsp labels indicate the respective antibody target in the experiments.
Changes in the size distribution upon hetero-oligomerization in vitro
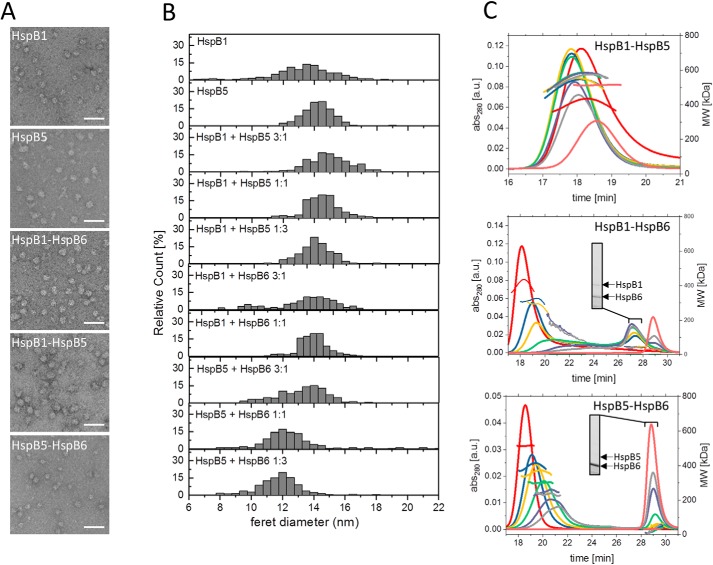
For a first estimate of the size distribution of hetero-oligomers formed in vitro, we subjected them to negative stain–transmission EM and determined the particle size distributions with the experimental limitation that only particles with diameters above 6–7 nm were included in the analyses (Fig. 4, A and B, and Fig. S4A). To cover the ratios observed in vivo, the sHsps varied from 3:1 to 1:3. As expected from earlier studies, we detected HspB1 and HspB5 homo-oligomers in heterogeneous populations of large oligomeric species with diameters between 12 and 16 nm. The mean diameter of HspB5 homo-oligomers was slightly larger than that of HspB1 homo-oligomers.
Figure 4.
Size distribution of sHsp hetero-oligomers formed at different ratios in vitro. A, representative electron micrographs of negatively-stained HspB1 and HspB5 homo-oligomers and hetero-oligomers of HspB1, HspB5, and HspB6 were formed at a 1:1 ratio. Scale bars indicate 50 nm. B, size distributions of homo-oligomers formed at different ratios determined from the Feret diameters of 10,000 negatively-stained particles. HspB6 alone and HspB1–HspB6 hetero-oligomers formed at 1:3 ratios are not included as they are too small to be discerned. C, size distributions of hetero-oligomers formed at different ratios determined by SEC-MALS. Left y axis: UV absorption at 280 nm; right y axis: molecular weight determined by MALS using RI signal. SDS-PAGE analyses of peak fractions are depicted as insets (see Fig. S4 for complete SDS-PAGE analysis). Colors indicate varying ratios of sHsps at constant total protein concentrations (red, 1:0; blue, 3:1; yellow, 2:1; green, 1:1; purple, 1:2; gray, 1:3; pink, 0:1).
Hetero-oligomers of these two proteins showed particle size distributions similar to that of HspB5 (Fig. 4, A and B, and Fig. S4A). In HspB6 samples, large oligomers were absent. The dimeric species were too small to be clearly discerned under our experimental conditions (Fig. S4A). Overall, both HspB1 and HspB5 oligomers decrease in size upon addition of HspB6, and the size distribution becomes broader. Especially hetero-oligomers formed by HspB1 and HspB6 are heterogeneous and show a high content of small species.
To gain further insight into the sizes of hetero-oligomers, we analyzed them by SEC-MALS (Fig. 4C). In agreement with our in vivo results and the EM analyses in vitro, we found that HspB1 and HspB5 form homo-oligomers of ∼400 and ∼500 kDa, respectively, which show no concentration-dependent changes within the concentration range of our experiments (Fig. S4, C and D). The apparent molecular masses of the HspB1–HspB5 hetero-oligomers were slightly larger than 500 kDa at all ratios tested and increased further at lower HspB1 to HspB5 ratios (Fig. 4C). Thus, EM and SEC-MALS analyses reveal that the sHsps HspB1 and HspB5 form large oligomers on their own and retain this property when assembled into hetero-oligomers.
Addition of dimeric HspB6 to HspB1 or HspB5 had striking effects. For HspB1 and HspB6, the ratio of the two sHsps has a strong influence on the size of the hetero-oligomeric complexes observed. In general, we found a broad peak which shifted from ∼400 to 200 kDa at increasing HspB6 concentrations, as well as two peaks representing smaller oligomers with molecular masses of 45 and 32 kDa (Fig. 4C). The 32-kDa peak represents the HspB6 homodimer, and the 45-kDa peak represents the HspB1–HspB6 heterodimer as Western blot analysis of the SEC fractions revealed that only the 45-kDa oligomers contain both proteins (Fig. 4C, inset, and Figs. S4B, S5, and S6). HspB5–HspB6 hetero-oligomerization also led to the formation of large and small oligomers. The size of the large oligomers gradually decreased with increasing amounts of HspB6 added. When HspB6 was present in excess, it was not completely integrated into the hetero-oligomers, and a peak at 32 kDa corresponding to free HspB6 dimer was detected in addition (Fig. 4C, see inset). Taken together, these results show that HspB6 decreases the size of both the HspB1 and HspB5 large oligomers in a concentration-dependent manner resulting in intermediate-sized (200–400 kDa) hetero-oligomers. Surprisingly and in contrast, HspB6 forms stable heterodimers with HspB1.
Prevention of aggregation of cellular proteins by sHsps
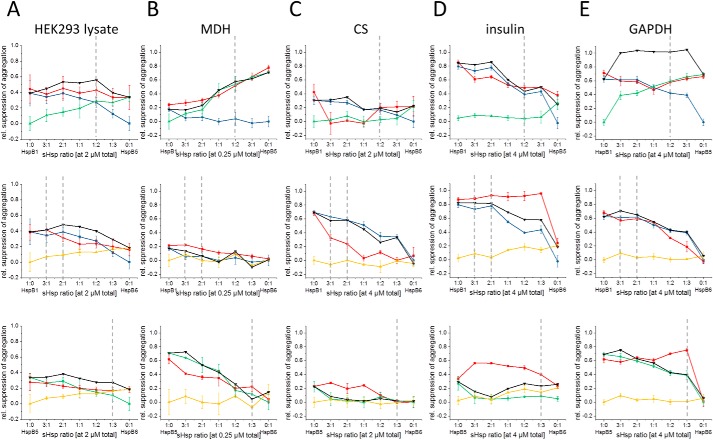
It is commonly accepted that shifting the distribution of oligomers is a key trigger for regulating the chaperone activity of sHsps. To determine whether for the chaperone activity of hetero-oligomers a shift to smaller oligomers correlates with higher activity, we tested the chaperone function of homo- and hetero-oligomeric sHsp complexes toward the soluble cytosolic proteome. To this end, we analyzed their effects on the solubility of the proteins in HEK293 lysates as these are devoid of endogenous sHsps (Fig. 5A and Fig. S7A). After incubation of lysates with sHsps at elevated temperatures, the insoluble and soluble fractions were separated by centrifugation, and the pellet fraction was analyzed by SDS-PAGE and densitometry (Fig. 5A and Fig. S8) (55). Consistent with previous data, HspB1 and HspB5 alone showed similar suppression of protein aggregation in lysates, whereas HspB6 was less active (55). For the three possible hetero-oligomers (HspB1–HspB5, HspB1–HspB6, and HspB5–HspB6), the hetero-oligomer ratios of 3:1 to 1:3 (Fig. 5A) were chosen to cover the whole range observed in vivo. The hetero-oligomers were also able to suppress the aggregation of cellular proteins. Overall, the efficiency was slightly decreased, compared with the homo-oligomeric proteins at equivalent concentration.
Figure 5.
Chaperone activity of hetero-oligomers formed at different ratios of sHsp toward HEK293 lysate and model substrates. Hetero-oligomers (HspB1–HspB5, HspB1–HspB6, or HspB5–HspB6) were formed at the constant total sHsp concentration indicated. Ratios of sHsp for hetero-oligomer formation varied from 3:1 to 1:3. Chaperone activity was measured for HEK293 lysate (A) and different model substrates like MDH (B), CS (C), insulin (D), and GAPDH (E). The color code refers to the respective hetero-oligomers: red, HspB1; blue, HspB5; green, HspB6; yellow, and theoretical activities values, black. Gray dotted lines indicate physiological ratios according to Table 1.
Chaperone activity of human sHsp hetero-oligomers on model substrates
The lack of a strong modulating effect of hetero-oligomer formation on the stress-induced aggregation of the cytosolic proteome leaves the possibility that hetero-oligomers may exhibit specific differences toward individual substrate proteins.
To test this, we performed in vitro experiments with four different model substrates: malate dehydrogenase (MDH), citrate synthase (CS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and insulin (31, 55). We kept the concentration of the model substrate as well as the total concentration of sHsp (Fig. S7) constant, but we varied the ratio of sHsps in hetero-oligomer experiments from 3:1 to 1:3. When we compared the activities of the hetero-oligomers and the homo-oligomers (Fig. 5 and Figs. S9 and S10), we found that the dimeric HspB6 is almost inactive in contrast to oligomeric HspB1 and HspB5. Among the four substrates tested, HspB6 was only able to slightly prevent insulin aggregation (Fig. 5D). The activities of HspB1 and HspB5 are also dependent on the substrate used. HspB1 effectively prevented CS, insulin, and GAPDH aggregation but showed relatively weak activity (compared with HspB5) in the MDH-aggregation assay. At the same time, the activity of HspB5 was high for CS, MDH, and GAPDH, and its activity in the insulin assay was significantly lower (Fig. 5D). In summary, these results confirm that different human sHsps selectively interact with substrate proteins, and different substrates are protected from aggregation by sHsps with different efficiencies (55). For the activities of hetero-oligomers, we calculated theoretical activity values that correspond to the sum of activities of both sHsp homo-oligomers determined independently. For HspB1–HspB5 hetero-oligomers, the measured hetero-oligomer activity was slightly lower compared with the theoretical activity in all cases tested. For substrates like CS and insulin where HspB1 and HspB5 show differences in activity, the less active sHsp seems to negatively affect the aggregation suppression of the more active one (Fig. 5). However, in the case of GAPDH, where HspB1 and HspB5 alone show similar activities, the hetero-oligomer samples exhibited strongly decreased activities compared with the theoretical values (Fig. 5).
The situation is different when HspB6 is involved. For all substrates tested, the presence of HspB6 improves the activity of HspB5 at ratios close to the physiologic scenario. Even though the influence varies between different substrates, the effect overall is consistent (Fig. 5). The influence of HspB6 on HspB1 in hetero-oligomeric complexes seems to be more substrate-dependent. Aggregation of MDH and GAPDH was prevented by the hetero-oligomer as effectively as in the absence of HspB6, and for insulin, the presence of HspB6 improved HspB1 activity and impaired HspB1 activity in the case of CS. Especially the latter effect is dominant within physiologic scenarios in our tested cell lines (Fig. 5).
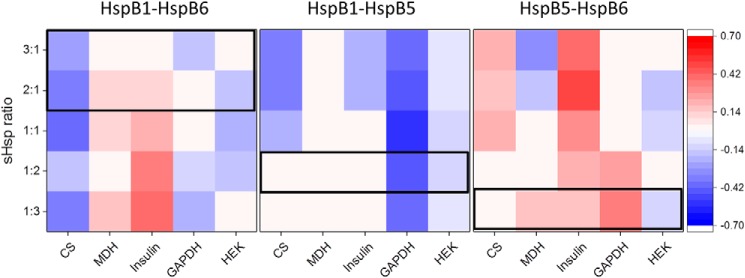
To compare the chaperone activities in a quantitative manner, we determined modulation values of the different hetero-oligomers by calculating the difference between the measured activity and the theoretical activity of hetero-oligomers (Table S2 and Fig. 6). Positive modulation values indicating an increase in activity of the hetero-oligomer are depicted in red, and the negative modulation is highlighted in blue. This analysis shows that the modulation by a sHsp depends both on the interaction partner and the substrate. For the HspB1–HspB5 hetero-oligomer, the observed decrease in activity upon hetero-oligomerization follows the common model of sHps chaperone activity (1) where an enrichment of higher oligomers leads to less activity. As hetero-oligomerization of HspB5 and HspB6 enriches smaller oligomers, a general increase in activity fits the scheme. The specific appearance of a heterodimer of HspB1–HspB6, however, renders the influence on the chaperone activity substrate-specific. Of note, at sHps ratios in the range of ST and HS situations (Fig. 6, boxes), the direction of the effect (positive or negative effect on the chaperone activity) stays the same for all substrates.
Figure 6.
Modulation of aggregation suppression ability of sHsp hetero-oligomers. The heat map shows negative modulation (indicating inhibition) in blue and positive modulation (indicating activation) in red. Modulation values equate to the difference of real activity and theoretical activity. Theoretical activity is defined as the sum of single sHsp control chaperone activities. Hence, normalized end-point aggregation values from Fig. 5 were subtracted to receive activity differences for indicated concentrations. For all experiments, the cutoff for color conditional formatting was set to 0.1, which equals the averaged standard deviation. Outlined boxes indicate physiological relevant ratios of the specific hetero-oligomers according to Table 1.
Discussion
In the current model for the regulation of the recognition of aggregation-prone cytosolic proteins by sHsp under stress conditions, sHsp activation is accompanied by a shift in the equilibrium of the oligomeric ensemble toward smaller species (1, 3, 56–59). These changes can be triggered by phosphorylation, elevated temperature, or changes in pH (38, 60–62). Hetero-oligomer formation could also affect the equilibrium and thus be a specific mechanism for regulating their chaperone activities. Alternatively, hetero-oligomerization could be a by-product of the evolution of multimember sHsp systems present in the cytosol of metazoa without functional consequences. Results supporting the view that hetero-oligomers fulfill specific functions have been mainly obtained in the context of diseases (63). An example are HpsB1 mutations linked to Marie-Tooth disease, which are affected in their ability to form HspB1–HspB6 hetero-oligomers, implying that there may be a correlation between hetero-oligomer formation and disease progression (64).
At first glance, based on the current knowledge, the simultaneous expression of different sHsps in the cytosol of a cell seems to be redundant due to a large overlap in the respective substrate spectra (55). However, it could well be that besides a general modulation of chaperone activity by hetero-oligomer formation, substrate specificity is affected. Our study, in which the chaperone activity of homo- and hetero-oligomeric sHsp complexes on the soluble, cytosolic proteome and individual model substrates was tested, allowed addressing both possibilities.
Interestingly, the expression profiles and the potential to form sHsp hetero-oligomers vary in human cells (15). In this study, we tested 11 different cell lines. All of them, except HEK293, expressed HspB1. However, only in two cell lines was HspB5 also present. We did not detect HspB5 expression in the absence of HspB1. This co-expression seems to be a general principle as similar observations were made previously (26). Strikingly, we detected additional HspB6 co-expression only in two of the 11 cancer cell lines tested. This strengthens the observations that in cancer cells mainly HspB1 and/or HspB5 are up-regulated, whereas HspB6 is often found at reduced levels (32–35, 65). However, when the tripartite sHsp system or HspB1 and HspB6 are present in the same cancer cell, the substrate-specific properties of the HspB1–HspB6 heterodimer might influence the decision between proliferation and apoptosis (33, 66, 67). Therefore, the tripartite system might serve as diagnostic biomarker or potential drug target.
Furthermore, our study shows that upon heat shock the expression pattern of sHsps in cells expressing more than one sHsp changes in a distinct way. HspB1 and HspB6 are up-regulated under heat-shock conditions, whereas changes in the levels of HspB5 are negligible. Thus, under stress conditions the activity profile of the sHsp system in cells expressing more than one sHsp changes (Figs. 6 and 7).
Figure 7.
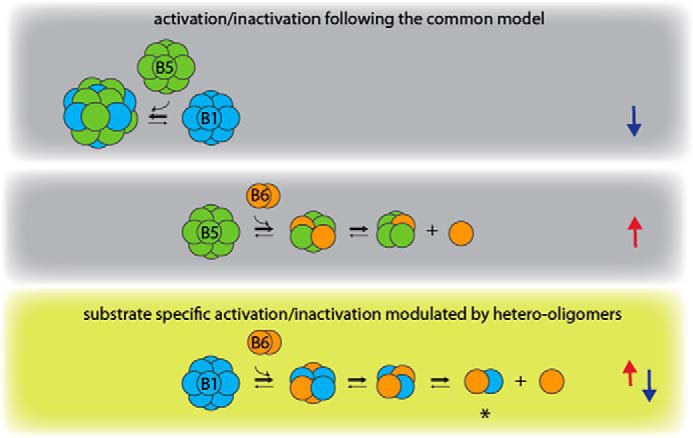
Model of activity modulation by hetero-oligomerization between HspB1, HspB5, and HspB6. The three different types of sHsp hetero-oligomers show differences in oligomeric size and chaperone activity. Arrows on the right indicate an increase (red) or decrease (blue) in chaperone activity. HspB6 activates HspB5 upon hetero-oligomer formation, whereas the large HspB1–HspB5 hetero-oligomers are inactivated. Thus, these two types of hetero-oligomers follow the common model of chaperon activity regulation (indicated by gray background) by shifting the oligomer distribution to larger oligomers (inactive species) or to smaller oligomers (active species). In contrast, HspB1–HspB6 hetero-oligomers show regulation in both directions in a substrate-dependent manner (olive green background), which seems to be a result of the specific formation of heterodimers. Circuits do not necessarily represent monomers.
In this context, the intrinsic dynamic nature of sHsps should be kept in mind. Subunit exchange is expected to occur constantly and is most likely in equilibrium under our experimental conditions. Therefore, differences in interactions observed are based on the properties of the specific interaction partners.
In general, our results show that there is not one defined effect of hetero-oligomer formation on the chaperone activity of sHsps. Rather several distinct regulatory principles become obvious (Fig. 6).
Hetero-oligomers of HspB1 and HspB5 are less active than the respective homo-oligomers in suppressing the aggregation of the cytosolic proteome. Under stress conditions when HspB1 becomes more abundant, the overall chaperone activity should be slightly higher.
Hetero-oligomer formation affects the activity of sHsps toward model substrates differentially. This suggests that one regulatory mechanism of hetero-oligomer formation may be the tuning of the chaperone activity for specific sets of substrate proteins and not a strong general regulation of chaperone activity.
HspB6 plays a special role in the context of hetero-oligomerization of sHsps. On itself, it is a largely inactive dimer. Surprisingly, however, HspB6 can act as a positive or negative modulator of the chaperone activity of a second sHsp, depending on the respective partner sHsp and the substrate. In both cases investigated here, it shifts HspB1 and HspB5 to smaller hetero-oligomeric species, but as a result, these are less or more active chaperones, respectively. Thus, HspB6 fulfills sophisticated functions within the tripartite sHsp system.
Interestingly, for HspB1–HspB6, a specific heterodimeric species of ∼45 kDa exists which is not present in the HspB5–HspB6 ensemble. This species seems to be of crucial importance for the differences in the chaperone activities of the two hetero-oligomer systems. Incorporation of HspB6 leads to the formation of small and for some substrates inactivated HspB1–HspB6 species (Fig. 7). Most likely the substrate dependence, resulting in specifically higher or lower chaperone activities, is regulated by the occurrence of the HspB1–HspB6 heterodimer in the ensemble of this hetero-oligomer system. In HspB5–HspB6 mixtures, no such heterodimeric species are formed, rather the two sHsps associate into intermediate-sized hetero-oligomers (30). These intermediate-sized hetero-oligomers significantly enhance the overall chaperone activity. Thus, the mechanism of HspB6 toward HspB1 and HspB5 is strikingly different (Fig. 7).
It should also be noted that none of our results indicates an involvement of HspB6 in the aggregation suppression of the respective hetero-oligomer. Also within the hetero-oligomer, HspB6 seems to stay largely inactive as a partner-specific regulator of assembly. However, it might have additional functions as an adaptor for the recognition of specific interaction partners like 14-3-3 proteins (68) and Bag3 (69) or for further mechanisms involving sHsps like apoptosis (11, 13), organization of intermediate filaments (14), or disaggregation (70). An additional layer of complexity is introduced by phosphorylation that is known to affect the oligomeric state of HspB1 and HspB5. Both homo-oligomers were shown to disassemble into smaller species upon phosphorylation (37, 49, 52, 71). For HspB6, it was shown that phosphorylation is required for its binding to 14-3-3 proteins (68). It should be noted that in size-distribution experiments of fresh cell lysates an undefined mixture of sHsps in different phosphorylation states is present (72), whereas our in vitro experiments were performed with nonphosphorylated sHps. Thus, further differences in size distribution and activity may be expected depending on phosphorylation in vivo. Additionally it should be noted that changes in the recognition of especially polymeric cytosolic proteins, like the cytoskeletal proteins, are not sufficiently described by the current models of sHsp function. These polymers are in the insoluble fraction of a cellular lysate and are therefore not included in our assay systems. This is of specific interest as especially larger sHsp oligomers seem to act on these polymers (14, 16, 73–75), and a regulation toward an enhanced recognition of such substrates might exist.
The finding that hetero-oligomerization between different sHsps of one organism is restricted to certain pairs or groups, as especially well-studied for plant sHsps (76), suggested that sHsp paralogs have evolved in general toward favoring specialized homo-oligomers, which have recently been demonstrated directly in a seminal study (77). After duplication of a sHsp gene, hetero-oligomers should be a natural species. Upon evolving different specificities, there could be an evolutionary pressure against hetero-oligomer formation. In this context, our observation that hetero-oligomers show modulated activities is counter-intuitive in an evolutionary setting that is restricted to avoiding hetero-oligomers (77). Thus, it can be assumed that the ability to form specific hetero-oligomers has been actively preserved throughout evolution because it is beneficial for the survival of the organism, seemingly adding another layer of regulation and functional specialization to the mechanistic repertoire of this fascinating chaperone family.
Experimental procedures
Protein purification and hetero-oligomer preparation
HspB1, HspB5, and HspB6 were expressed and purified as described previously (39, 44, 78). HspB5 was stored frozen at −20 °C in buffer B (20 mm Tris acetate, pH 7.6, 10 mm NaCl, 0.1 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, and 1 mm DTT), whereas HspB1 and HspB6 were stored in PBS, pH 7.4, with 1 mm DTT. Formation of hetero-oligomers of HspB1, HspB5, and HspB6 was performed as described elsewhere (30, 45). Briefly, sHsps were mixed pairwise (ratio from 3:1 to 1:3), reduced by a 30-min incubation with 5 mm DTT at 37°C and further incubated for 1 h at 42°C. These conditions were shown to be optimal to reach complete subunit exchange and hetero-oligomers in steady-state equilibrium (45).
Analysis of sHsp expression in different human cell lines
HeLa, MCF7, HEK293, HepG2, U373MG, U138MG, K652, SH-SY5Y, A549, H460, and Caco-2 human cell lines were grown in appropriate media (Table S1) up to 70–90% confluence, washed with PBS, and lysed with M-PER cell lysis reagent (Thermo Fisher Scientific) according to the manufacturer's protocol (suspension culture of K652 was pelleted from growth medium by centrifugation at 300 × g for 5 min prior to lysis). Total protein concentration was determined by the Bradford assay (79) using BSA as a standard, and the lysate (50 μg of total protein per pocket) was analyzed by SDS-PAGE followed by Western blotting. After SDS-PAGE, proteins were transferred by semi-dry electro-transfer onto a nitrocellulose membrane. The membrane was blocked by 30 min of incubation in PBS containing 0.1% Tween 20 (PBST) and 5% milk powder. After blocking, the membrane was incubated for 1 h at room temperature with primary antibodies (anti-HspB1 and anti-HspB5 from StressMarq; anti-HspB6 and anti-HspB8 antibodies were kindly provided by Prof. A. Katrukha, and dilution of all antibodies was 1:10,000). Then, the membrane was washed three times with PBST and incubated with secondary α-mouse IgG horseradish peroxidase-linked antibodies (1:20,000, Sigma). After three additional 10-min washing steps, the bands were visualized with WesternBright ECL spray (Advansta) and ImageQuant LAS 4000 chemiluminescence detection system (GE Healthcare).
To quantify the amount of sHsps in HeLa, MCF7, U373MG, U138MG, and H460, we loaded variable amounts of purified sHsps for calibration and a defined amount of cell lysate. The loading of equal amounts of lysate was ensured by determination of the total protein concentration using the Bradford reagent (Serva). After SDS-PAGE followed by Western blotting, we quantified integrated densities of all bands using ImageQuantTL software (GE Healthcare). Calibration curves were accepted with r2 ≥0.95.
Co-immunoprecipitation of sHsps in cell lysate
Lysates of HeLa, MCF7, U373MG, U138MG, and H460 cells were produced using M-PER as described above. As determined by the Bradford assay, about 1 mg of lysate was used for each co-IP experiment and incubated with 1 μg of specific antibody for 1 h at 4 °C. Because of the low-expression level of HspB5 in U138MG cells, the lysate was pre-incubated for 1 h with 10 μg of recombinantly-produced HspB5. After the addition of 20 μl of protein G-Sepharose fast flow beads (GE Healthcare) and further incubation for 2 h at 4 °C, the sHsp complexes were precipitated by centrifugation. Precipitate was washed three times with cold PBST and eluted in 30 μl of 0.5 m glycine, pH 3.0. Western blotting was performed as described above. About 100 ng of recombinant protein served as a positive control, and prestained peqGOLD Protein Marker IV (Peqlab) was used as molecular mass standards.
SEC and SEC-MALS
SEC-MALS was performed on a Shimadzu HPLC LC20A system equipped with a Wyatt Helios-II MALS detector using a Superdex200 Increase 10/300 GL column (GE Healthcare) equilibrated in PBS, containing 1 mm DTT. The samples (50 μl) were loaded on the column containing 50 μm protein. The column was operated at a constant flow rate of 0.5 ml/min. BSA was used for configuration purposes. All runs were carried out at room temperature.
Size distribution of sHsp in cell lines
MCF7, HeLa, U373MG, U138MG, and H460 cell lines were grown up to ≈90% confluence in a T75 flask, washed once with ice-cold PBS, scraped off in PBS, and pelleted by centrifugation at 300 × g for 5 min. The cells were lysed mechanically in PBS containing 1 mm DTT, protease inhibitor mix M (Serva), and phosphatase inhibitor mixtures 2 and 3 (Sigma) by passing them 5–6 times through a needle (diameter 0.6 mm). Cell debris was removed by centrifugation at 16,900 × g for 10 min. Cleared cell lysates (200–250 μl) containing about 0.5 mg of total protein (determined by Bradford assay) were loaded on two stacked Superdex200 5/150 columns equilibrated in PBS, pH 7.4, containing 1 mm DTT at a flow rate of 0.2 ml/min. The protein content of the collected fractions (300 μl) was precipitated by overnight incubation at 4 °C with 10% trichloroacetic acid (TCA). The pelleted proteins were dissolved in 1-fold Laemmli buffer and analyzed for sHsp content by SDS-PAGE (80) followed by Western blotting. For quantification of detected sHsp bands, ImageQuantTL software (GE Healthcare) was used. Intensities were normalized for each sHsp separately setting most intense band to 1.
Transmission EM
Prior to staining, isolated sHsps or hetero-oligomers were incubated for 10 min at 45 °C. 5-μl samples were adsorbed for 30 s onto carbon-coated grids (glow discharged). Excess protein solution was blotted off, and the samples were washed with 20 μl of water and stained with 5 μl of a 1% uranyl acetate solution. Micrographs were recorded at 0.6–0.8-μm defocus at a nominal magnification of 50,000 either on a JEOL JEM 100CX or a JEM 1400 plus transmission electron microscope operating at 100 or 120 kV, respectively. Micrographs on the JEM 1400plus microscope were collected with a JEOL Ruby CCD camera with a pixel size of 0.332 nm/px, whereas electron micrographs from the JEM 100CX were digitized using a FlexTight X5 scanner resulting in a final pixel size of 0.169 nm/px.
For size distributions, between 8,000 and 17,000 particles were selected semi-automatically from micrographs using Eman2 (81). These particles were bandpass-filtered, centered, and normalized with IMAGIC5 (82), followed by multivariate statistical analysis. Only Eigenvectors indicating size differences within the dataset were used for classification into different classes with an average of 30 particles per class. The Ferret of the class averages was measured with ImageJ (83, 84).
Analysis of model protein aggregation
Thermal aggregation of MDH (2 μm), GAPDH (1 μm), or CS (1 μm) was monitored at 45 °C in PBS containing 1 mm DTT. To determine the chaperone activity of isolated sHsp or hetero-oligomers, sHsps were added to the reaction mix prior to incubation at 45 °C. Concentrations of sHsp in 1:1 ratio assays were as follows: 0.5 or 0.25 μm (per monomer) in MDH aggregation assays, 4 μm in GAPDH aggregation assays, and 2.0 or 4.0 μm in CS aggregation assays. Chemically-induced aggregation of insulin (40 μm) was initiated by an addition of 20 mm DTT and incubation at 37 °C, and concentration of sHsps in this assay was 4.0 μm. Aggregation processes were monitored for 60–90 min following the apparent increase of light scattering induced by substrate unfolding, recording the absorbance at 360 nm in a Cary 50 UV-visible spectrophotometer (Varian) equipped with a temperature-adjustable cuvette holder. To estimate the chaperone activity of isolated sHsps or hetero-oligomers, the light-scattering values at the endpoint (60 or 90 min) were normalized to the level of spontaneous aggregation of the model substrate in the absence of sHsp. At least three measurements were averaged and processed if necessary by applying the Savitzky-Golay smoothing filter. To estimate the chaperone activity of the respective sHsps, the end-point scattering signal in the respective sample was normalized compared with the respective end-point value of spontaneous aggregation (without sHsp addition), which was set to one. The modulation of chaperone activity was achieved by subtracting these normalized values from one.
Theoretical activity values of hetero-oligomers are defined as the sum of sHsp control activity of both single homo-oligomers. At the end, modulation values were calculated by subtracting theoretical activity values from measured activity values. Hence, a negative modulation value refers to a higher activity of homo-oligomers, whereas positive modulation values refer to a higher activity of hetero-oligomers.
Prevention of aggregation of cellular proteins
Prevention of aggregation of cellular proteins was done essentially as in Ref. 55. In brief, HEK293 cells were grown up to 90–95% confluence, washed with Dulbecco's PBS (Gibco), detached from the flask by trypsinization, pelleted, and mechanically lysed in PBS buffer as described above under “Size distribution of sHsp in cell lines.” Cleared cell lysate was depleted for ATP by hexokinase treatment and stored at −80 °C. 40 μg of HEK293 cell protein was incubated for 90 min at 45 °C without or with addition of 2.0 μm isolated sHsp or pre-formed hetero-oligomers in PBS containing 1 mm DTT. After heat shock, samples were centrifuged to separate insoluble proteins. Pellets were washed twice with ice-cold PBS, then dissolved in 1-fold Laemmli buffer, and analyzed by SDS-PAGE. The average amount of insoluble proteins out of at least three experiments was quantified by densitometry using ImageQuantTL software (GE Healthcare).
Author contributions
E. V. M. and M. R. formal analysis; E. V. M., S. W., M. H., and J. B. funding acquisition; E. V. M., M. R., and C. P. investigation; M. R., S. W., M. H., and J. B. conceptualization; M. R., S. W., M. H., and J. B. writing-original draft; M. R., S. W., M. H., and J. B. writing-review and editing; J. B. project administration.
Supplementary Material
Acknowledgments
We thank Prof. N. B. Gusev (Moscow State University) for the expression construct for HspB8 and Prof. A. Katrukha (Moscow State University) for antibodies against HspB6 and HspB8. We also thank Ramona Absmeier for collecting a part of the EM micrographs and the subsequent analysis of all EM datasets. Ruby Khan is acknowledged for help with EM analysis and Anja Osterauer for technical help with several assays.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grant SFB 1035 (to J. B., M. H., and S. W.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S10 and Tables S1 and S2.
- sHsp
- small heat-shock protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ST
- standard
- SEC
- size-exclusion chromatography
- co-IP
- co-immunoprecipitation
- SEC-MALS
- size-exclusion chromatography coupled to multiangle light scattering
- MDH
- malate dehydrogenase
- CS
- citrate synthase
- HS
- heat shock
- px
- pixel.
References
- 1. Haslbeck M., Weinkauf S., and Buchner J. (2019) Small heat-shock proteins: simplicity meets complexity. J. Biol. Chem. 294, 2121–2132 10.1074/jbc.REV118.002809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kappé G., Boelens W. C., and de Jong W. W. (2010) Why proteins without an α-crystallin domain should not be included in the human small heat-shock protein family HSPB. Cell Stress Chaperones 15, 457–461 10.1007/s12192-009-0155-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delbecq S. P., and Klevit R. E. (2013) One size does not fit all: the oligomeric states of αB crystallin. FEBS Lett. 587, 1073–1080 10.1016/j.febslet.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Montfort R. L., Basha E., Friedrich K. L., Slingsby C., and Vierling E. (2001) Crystal structure and assembly of a eukaryotic small heat-shock protein. Nat. Struct. Biol. 8, 1025–1030 10.1038/nsb722 [DOI] [PubMed] [Google Scholar]
- 5. Mogk A., Ruger-Herreros C., and Bernd Bukau B. (2019) Cellular functions and mechanisms of action of small heat-shock proteins. Annu. Rev. Microbiol. 73, 89–110 10.1146/annurev-micro-020518-115515 [DOI] [PubMed] [Google Scholar]
- 6. Ecroyd H., and Carver J. A. (2009) Crystallin proteins and amyloid fibrils. Cell. Mol. Life Sci. 66, 62–81 10.1007/s00018-008-8327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narberhaus F. (2002) α-Crystallin-type heat-shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 66, 64–93 10.1128/MMBR.66.1.64-93.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehrnsperger M., Gräber S., Gaestel M., and Buchner J. (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 16, 221–229 10.1093/emboj/16.2.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee G. J., Roseman A. M., Saibil H. R., and Vierling E. (1997) A small heat-shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 16, 659–671 10.1093/emboj/16.3.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mogk A., Bukau B., and Kampinga H. H. (2018) Cellular handling of protein aggregates by disaggregation machines. Mol. Cell 69, 214–226 10.1016/j.molcel.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Voss O. H., Batra S., Kolattukudy S. J., Gonzalez-Mejia M. E., Smith J. B., and Doseff A. I. (2007) Binding of caspase-3 prodomain to heat-shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J. Biol. Chem. 282, 25088–25099 10.1074/jbc.M701740200 [DOI] [PubMed] [Google Scholar]
- 12. Pandey P., Farber R., Nakazawa A., Kumar S., Bharti A., Nalin C., Weichselbaum R., Kufe D., and Kharbanda S. (2000) Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19, 1975–1981 10.1038/sj.onc.1203531 [DOI] [PubMed] [Google Scholar]
- 13. Hu W.-F., Gong L., Cao Z., Ma H., Ji W., Deng M., Liu M., Hu X.-H., Chen P., Yan Q., Chen H.-G., Liu J., Sun S., Zhang L., Liu J.-P., Wawrousek E., and Li D. W.-C. (2012) αA- and αB-crystallins interact with caspase-3 and bax to guard mouse lens development. Curr. Mol. Med. 12, 177–187 10.2174/156652412798889036 [DOI] [PubMed] [Google Scholar]
- 14. Perng M. D., Cairns L., van den I. P., Prescott A., Hutcheson A. M., and Quinlan R. A. (1999) Intermediate filament interactions can be altered by HSP27 and αB-crystallin. J. Cell Sci. 112 2099–2112 [DOI] [PubMed] [Google Scholar]
- 15. Arrigo A. P. (2013) Human small heat-shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett. 587, 1959–1969 10.1016/j.febslet.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 16. Kayser J., Haslbeck M., Dempfle L., Krause M., Grashoff C., Buchner J., Herrmann H., and Bausch A. R. (2013) The small heat-shock protein Hsp27 affects assembly dynamics and structure of keratin intermediate filament networks. Biophys. J. 105, 1778–1785 10.1016/j.bpj.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kappé G., Franck E., Verschuure P., Boelens W. C., Leunissen J. A., and de Jong W. W. (2003) The human genome encodes 10 α-crystallin-related small heat-shock proteins: HspB1–10. Cell Stress Chaperones 8, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horwitz J. (2003) α-Crystallin. Exp. Eye. Res. 76, 145–153 10.1016/S0014-4835(02)00278-6 [DOI] [PubMed] [Google Scholar]
- 19. Kappé G., Verschuure P., Philipsen R. L., Staalduinen A. A., Van de Boogaart P., Boelens W. C., and De Jong W. W. (2001) Characterization of two novel human small heat-shock proteins: protein kinase-related HspB8 and testis-specific HspB9. Biochim. Biophys. Acta 1520, 1–6 10.1016/S0167-4781(01)00237-8 [DOI] [PubMed] [Google Scholar]
- 20. Gastmann O., Burfeind P., Günther E., Hameister H., Szpirer C., and Hoyer-Fender S. (1993) Sequence, expression, and chromosomal assignment of a human sperm outer dense fiber gene. Mol. Reprod. Dev. 36, 407–418 10.1002/mrd.1080360402 [DOI] [PubMed] [Google Scholar]
- 21. Kato K., Shinohara H., Goto S., Inaguma Y., Morishita R., and Asano T. (1992) Copurification of small heat-shock protein with αB-crystallin from human skeletal muscle. J. Biol. Chem. 267, 7718–7725 [PubMed] [Google Scholar]
- 22. Kato K., Goto S., Inaguma Y., Hasegawa K., Morishita R., and Asano T. (1994) Purification and characterization of a 20-kDa protein that is highly homologous to αB-crystallin. J. Biol. Chem. 269, 15302–15309 [PubMed] [Google Scholar]
- 23. Golenhofen N., Perng M. D., Quinlan R. A., and Drenckhahn D. (2004) Comparison of the small heat-shock proteins αB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem. Cell Biol. 122, 415–425 10.1007/s00418-004-0711-z [DOI] [PubMed] [Google Scholar]
- 24. Aquilina J. A., Shrestha S., Morris A. M., and Ecroyd H. (2013) Structural and functional aspects of hetero-oligomers formed by the small heat-shock proteins αB-crystallin and HSP27. J. Biol. Chem. 288, 13602–13609 10.1074/jbc.M112.443812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Datskevich P. N., Mymrikov E. V., and Gusev N. B. (2012) Utilization of fluorescent chimeras for investigation of hetero-oligomeric complexes formed by human small heat-shock proteins. Biochimie 94, 1794–1804 10.1016/j.biochi.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 26. Zantema A., Verlaan-De Vries M., Maasdam D., Bol S., and van der Eb A. (1992) Heat-shock protein 27 and αB-crystallin can form a complex, which dissociates by heat shock. J. Biol. Chem. 267, 12936–12941 [PubMed] [Google Scholar]
- 27. Sugiyama Y., Suzuki A., Kishikawa M., Akutsu R., Hirose T., Waye M. M., Tsui S. K., Yoshida S., and Ohno S. (2000) Muscle develops a specific form of small heat-shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J. Biol. Chem. 275, 1095–1104 10.1074/jbc.275.2.1095 [DOI] [PubMed] [Google Scholar]
- 28. Fontaine J. M., Sun X., Benndorf R., and Welsh M. J. (2005) Interactions of HSP22 (HSPB8) with HSP20, αB-crystallin, and HSPB3. Biochem. Biophys. Res. Commun. 337, 1006–1011 10.1016/j.bbrc.2005.09.148 [DOI] [PubMed] [Google Scholar]
- 29. Sun X., Fontaine J. M., Rest J. S., Shelden E. A., Welsh M. J., and Benndorf R. (2004) Interaction of human HSP22 (HSPB8) with other small heat-shock proteins. J. Biol. Chem. 279, 2394–2402 10.1074/jbc.M311324200 [DOI] [PubMed] [Google Scholar]
- 30. Mymrikov E. V., Seit-Nebi A. S., and Gusev N. B. (2012) Hetero-oligomeric complexes of human small heat-shock proteins. Cell Stress Chaperones 17, 157–169 10.1007/s12192-011-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mymrikov E. V., Seit-Nebi A. S., and Gusev N. B. (2011) Large potentials of small heat-shock proteins. Physiol. Rev. 91, 1123–1159 10.1152/physrev.00023.2010 [DOI] [PubMed] [Google Scholar]
- 32. Li F., Xiao H., Zhou F., Hu Z., and Yang B. (2017) Study of HSPB6: insights into the properties of the multifunctional protective agent. Cell. Physiol. Biochem. 44, 314–332 10.1159/000484889 [DOI] [PubMed] [Google Scholar]
- 33. Arrigo A. P., and Gibert B. (2013) Protein interactomes of three stress inducible small heat-shock proteins: HspB1, HspB5 and HspB8. Int. J. Hyperthermia 29, 409–422 10.3109/02656736.2013.792956 [DOI] [PubMed] [Google Scholar]
- 34. Assimakopoulou M., Sotiropoulou-Bonikou G., Maraziotis T., and Varakis I. (1997) Prognostic significance of Hsp-27 in astrocytic brain tumors: an immunohistochemical study. Anticancer Res. 17, 2677–2682 [PubMed] [Google Scholar]
- 35. Ciocca D. R., and Calderwood S. K. (2005) Heat-shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10, 86–103 10.1379/CSC-99r.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagasawa T., Matsushima-Nishiwaki R., Toyoda H., Matsuura J., Kumada T., and Kozawa O. (2014) Heat-shock protein 20 (HSPB6) regulates apoptosis in human hepatocellular carcinoma cells: direct association with Bax. Oncol. Rep. 32, 1291–1295 10.3892/or.2014.3278 [DOI] [PubMed] [Google Scholar]
- 37. Hayes D., Napoli V., Mazurkie A., Stafford W. F., and Graceffa P. (2009) Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J. Biol. Chem. 284, 18801–18807 10.1074/jbc.M109.011353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogalla T., Ehrnsperger M., Preville X., Kotlyarov A., Lutsch G., Ducasse C., Paul C., Wieske M., Arrigo A. P., Buchner J., and Gaestel M. (1999) Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J. Biol. Chem. 274, 18947–18956 10.1074/jbc.274.27.18947 [DOI] [PubMed] [Google Scholar]
- 39. Peschek J., Braun N., Franzmann T. M., Georgalis Y., Haslbeck M., Weinkauf S., and Buchner J. (2009) The eye lens chaperone α-crystallin forms defined globular assemblies. Proc. Natl. Acad. Sci. U.S.A. 106, 13272–13277 10.1073/pnas.0902651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ito H., Kamei K., Iwamoto I., Inaguma Y., Nohara D., and Kato K. (2001) Phosphorylation-induced change of the oligomerization state of αB-crystallin. J. Biol. Chem. 276, 5346–5352 10.1074/jbc.M009004200 [DOI] [PubMed] [Google Scholar]
- 41. Peschek J., Braun N., Rohrberg J., Back K. C., Kriehuber T., Kastenmüller A., Weinkauf S., and Buchner J. (2013) Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc. Natl. Acad. Sci. U.S.A. 110, E3780–E3789 10.1073/pnas.1308898110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lelj-Garolla B., and Mauk A. G. (2005) Self-association of a small heat-shock protein. J. Mol. Biol. 345, 631–642 10.1016/j.jmb.2004.10.056 [DOI] [PubMed] [Google Scholar]
- 43. Shashidharamurthy R., Koteiche H. A., Dong J., and McHaourab H. S. (2005) Mechanism of chaperone function in small heat-shock proteins: dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J. Biol. Chem. 280, 5281–5289 10.1074/jbc.M407236200 [DOI] [PubMed] [Google Scholar]
- 44. Bukach O. V., Seit-Nebi A. S., Marston S. B., and Gusev N. B. (2004) Some properties of human small heat-shock protein Hsp20 (HspB6). Eur. J. Biochem. 271, 291–302 10.1046/j.1432-1033.2003.03928.x [DOI] [PubMed] [Google Scholar]
- 45. Bukach O. V., Glukhova A. E., Seit-Nebi A. S., and Gusev N. B. (2009) Hetero-oligomeric complexes formed by human small heat-shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim. Biophys. Acta 1794, 486–495 10.1016/j.bbapap.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 46. Geiger T., Wehner A., Schaab C., Cox J., and Mann M. (2012) Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell. Proteomics 11, M111.014050 10.1074/mcp.M111.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bakthisaran R., Tangirala R., and Rao ChM. (2015) Small heat-shock proteins: role in cellular functions and pathology. Biochim. Biophys. Acta 1854, 291–319 10.1016/j.bbapap.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 48. Arrigo A. P., Suhan J. P., and Welch W. J. (1988) Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat-shock protein. Mol. Cell. Biol. 8, 5059–5071 10.1128/MCB.8.12.5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Behlke J., Lutsch G., Gaestel M., and Bielka H. (1991) Supramolecular structure of the recombinant murine small heat-shock protein hsp25. Fed. Eur. Biochem. Soc. 288, 119–122 10.1016/0014-5793(91)81016-2 [DOI] [PubMed] [Google Scholar]
- 50. Kato K., Hasegawa K., Goto S., and Inaguma Y. (1994) Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J. Biol. Chem. 269, 11274–11278 [PubMed] [Google Scholar]
- 51. Lambert H., Charette S. J., Bernier A. F., Guimond A., and Landry J. (1999) HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 274, 9378–9385 10.1074/jbc.274.14.9378 [DOI] [PubMed] [Google Scholar]
- 52. Bruey J. M., Ducasse C., Bonniaud P., Ravagnan L., Susin S. A., Diaz-Latoud C., Gurbuxani S., Arrigo A. P., Kroemer G., Solary E., and Garrido C. (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2, 645–652 10.1038/35023595 [DOI] [PubMed] [Google Scholar]
- 53. Thul P. J., Åkesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., Alm T., Asplund A., Björk L., Breckels L. M., Bäckström A., Danielsson F., Fagerberg L., Fall J., Gatto L., et al. (2017) A subcellular map of the human proteome. Science 356, eaal3321 10.1126/science.aal3321 [DOI] [PubMed] [Google Scholar]
- 54. Gibert B., Eckel B., Fasquelle L., Moulin M., Bouhallier F., Gonin V., Mellier G., Simon S., Kretz-Remy C., Arrigo A.-P., and Diaz-Latoud C. (2012) Knockdown of heat-shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS ONE 7, e29719 10.1371/journal.pone.0029719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mymrikov E. V., Daake M., Richter B., Haslbeck M., and Buchner J. (2017) The chaperone activity and substrate spectrum of human small heat-shock proteins. J. Biol. Chem. 292, 672–684 10.1074/jbc.M116.760413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haslbeck M., and Vierling E. (2015) A first line of stress defense: small heat-shock proteins and their function in protein homeostasis. J. Mol. Biol. 427, 1537–1548 10.1016/j.jmb.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Basha E., O'Neill H., and Vierling E. (2012) Small heat-shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem. Sci. 37, 106–117 10.1016/j.tibs.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garrido C., Paul C., Seigneuric R., and Kampinga H. H. (2012) The small heat-shock proteins family: the long forgotten chaperones. Int. J. Biochem. Cell Biol. 44, 1588–1592 10.1016/j.biocel.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 59. McHaourab H. S., Godar J. A., and Stewart P. L. (2009) Structure and mechanism of protein stability sensors: the chaperone activity of small heat-shock proteins. Biochemistry 48, 3828–3837 10.1021/bi900212j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chernik I. S., Panasenko O. O., Li Y., Marston S. B., and Gusev N. B. (2004) pH-induced changes of the structure of small heat-shock proteins with molecular mass 24/27 kDa (HspB1). Biochem. Biophys. Res. Commun. 324, 1199–1203 10.1016/j.bbrc.2004.09.176 [DOI] [PubMed] [Google Scholar]
- 61. Ehrnsperger M., Lilie H., Gaestel M., and Buchner J. (1999) The dynamics of Hsp25 quaternary structure. Structure and function of different oligomeric species. J. Biol. Chem. 274, 14867–14874 10.1074/jbc.274.21.14867 [DOI] [PubMed] [Google Scholar]
- 62. Fleckenstein T., Kastenmüller A., Stein M. L., Peters C., Daake M., Krause M., Weinfurtner D., Haslbeck M., Weinkauf S., Groll M., and Buchner J. (2015) The chaperone activity of the developmental small heat-shock protein Sip1 is regulated by pH-dependent conformational changes. Mol. Cell 58, 1067–1078 10.1016/j.molcel.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 63. Nefedova V. V., Muranova L. K., Sudnitsyna M. V., Ryzhavskaya A. S., and Gusev N. B. (2015) Small heat-shock proteins and distal hereditary neuropathies. Biochemistry 80, 1734–1747 10.1134/S000629791513009X [DOI] [PubMed] [Google Scholar]
- 64. Weeks S. D., Muranova L. K., Heirbaut M., Beelen S., Strelkov S. V., and Gusev N. B. (2018) Characterization of human small heat-shock protein HSPB1 α-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci. Rep. 8, 688 10.1038/s41598-017-18874-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ju Y. T., Kwag S. J., Park H. J., Jung E. J., Jeong C. Y., Jeong S. H., Lee Y. J., Choi S. K., Kang K. R., Hah Y. S., and Hong S. C. (2015) Decreased expression of heat-shock protein 20 in colorectal cancer and its implication in tumorigenesis. J. Cell. Biochem. 116, 277–286 10.1002/jcb.24966 [DOI] [PubMed] [Google Scholar]
- 66. Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., and Nebreda A. R. (1994) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat-shock proteins. Cell 78, 1027–1037 10.1016/0092-8674(94)90277-1 [DOI] [PubMed] [Google Scholar]
- 67. Blagosklonny M. (2002) Hsp-90–associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia 16, 455–462 10.1038/sj.leu.2402415 [DOI] [PubMed] [Google Scholar]
- 68. Sluchanko N. N., Beelen S., Kulikova A. A., Weeks S. D., Antson A. A., Gusev N. B., and Strelkov S. V. (2017) Structural basis for the interaction of a human small heat-shock protein with the 14-3-3 universal signaling regulator. Structure 25, 305–316 10.1016/j.str.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rauch J. N., Tse E., Freilich R., Mok S. A., Makley L. N., Southworth D. R., and Gestwicki J. E. (2017) BAG3 is a modular, scaffolding protein that physically links heat-shock protein 70 (Hsp70) to the small heat-shock proteins. J. Mol. Biol. 429, 128–141 10.1016/j.jmb.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mogk A., and Bukau B. (2017) Role of sHsps in organizing cytosolic protein aggregation and disaggregation. Cell Stress Chaperones 22, 493–502 10.1007/s12192-017-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehlen P., Kretz-Remy C., Briolay J., Fostan P., Mirault M. E., and Arrigo A. P. (1995) Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor α-treated T47D human carcinoma cells. Biochem. J. 312 367–375 10.1042/bj3120367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simon S., Dimitrova V., Gibert B., Virot S., Mounier N., Nivon M., Kretz-Remy C., Corset V., Mehlen P., and Arrigo A. P. (2013) Analysis of the dominant effects mediated by wild type or R120G mutant of αB-crystallin (HspB5) towards Hsp27 (HspB1). PLoS ONE 8, e70545 10.1371/journal.pone.0070545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Song S., Landsbury A., Dahm R., Liu Y., Zhang Q., and Quinlan R. A. (2009) Functions of the intermediate filament cytoskeleton in the eye lens. J. Clin. Invest. 119, 1837–1848 10.1172/JCI38277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nefedova V. V., Sudnitsyna M. V., and Gusev N. B. (2017) Interaction of small heat-shock proteins with light component of neurofilaments (NFL). Cell Stress Chaperones 22, 467–479 10.1007/s12192-016-0757-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shammas S. L., Waudby C. A., Wang S., Buell A. K., Knowles T. P., Ecroyd H., Welland M. E., Carver J. A., Dobson C. M., and Meehan S. (2011) Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys. J. 101, 1681–1689 10.1016/j.bpj.2011.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Basha E., Jones C., Wysocki V., and Vierling E. (2010) Mechanistic differences between two conserved classes of small heat-shock proteins found in the plant cytosol. J. Biol. Chem. 285, 11489–11497 10.1074/jbc.M109.074088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hochberg G. K. A., Shepherd D. A., Marklund E. G., Santhanagoplan I., Degiacomi M. T., Laganowsky A., Allison T. M., Basha E., Marty M. T., Galpin M. R., Struwe W. B., Baldwin A. J., Vierling E., and Benesch J. L. P. (2018) Structural principles that enable oligomeric small heat-shock protein paralogs to evolve distinct functions. Science 359, 930–935 10.1126/science.aam7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mymrikov E. V., Bukach O. V., Seit-Nebi A. S., and Gusev N. B. (2010) The pivotal role of the β strand in the intersubunit contacts of different human small heat-shock proteins. Cell Stress Chaperones 15, 365–377 10.1007/s12192-009-0151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 80. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 81. Ludtke S. J., Baldwin P. R., and Chiu W. (1999) EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 10.1006/jsbi.1999.4174 [DOI] [PubMed] [Google Scholar]
- 82. van Heel M., Harauz G., Orlova E. V., Schmidt R., and Schatz M. (1996) A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 10.1006/jsbi.1996.0004 [DOI] [PubMed] [Google Scholar]
- 83. Rueden C. T., Schindelin J., Hiner M. C., DeZonia B. E., Walter A. E., Arena E. T., and Eliceiri K. W. (2017) ImageJ2: imageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.